Abstract
Objective:
Brown fat can exhibit high uptake of fluorine-18 fludeoxyglucose (18F-FDG) on positron emission tomography (PET) and interferes with interpretation of the scan. The goal of this study was to identify factors that may influence brown adipose tissue (BAT) activation.
Methods:
A retrospective study of 18F-FDG PET scans was performed using a database of 15,109 PET/CT reports. BAT activation reported by nuclear medicine physicians and factors influencing BAT activation were gathered. The data were analyzed using in-house software.
Results:
The total reported BAT activation was 3.6%. BAT activation was reported significantly more often in patients who were female (p < 0.0001), younger (p < 0.0001), with lower body mass index (p < 0.0001), with lower blood glucose levels (p = 0.01), indicated for breast cancer (p = 0.004), not administered chemotherapy recently before the scan (p < 0.0001) and shown to have BAT activation in a previous scan (p < 0.0001). BAT activation was also reported significantly more for lower outdoor temperatures (p < 0.0001) and for late morning scans than for afternoon (p = 0.005) and early morning (p = 0.001) scans.
Conclusion:
This retrospective study of 15,109 scans highlights multiple factors contributing to BAT activation on 18F-FDG PET. The identification of new factors influencing BAT and confirmation of previously identified factors with a larger data set can be used to more accurately identify patients at risk for BAT activation so that prevention strategies can be implemented.
Advances in knowledge:
This study presents new factors associated with higher incidence of BAT activation, such as time of day, previous BAT activation and breast cancer. Conversely, recent chemotherapy was associated with reduced incidence of BAT activation.
INTRODUCTION
Positron emission tomography (PET)/CT with fluorine-18 fludeoxyglucose (18F-FDG) is currently a standard diagnostic tool for identifying tumours and their metastases as well as infectious or inflammatory lesions.1–3 However, it is not tumour specific.4–6 High physiological uptake of 18F-FDG is found in organs with high glucose metabolism, such as the brain and heart. One particular tissue with high 18F-FDG uptake that can interfere with scan interpretation is brown fat.7
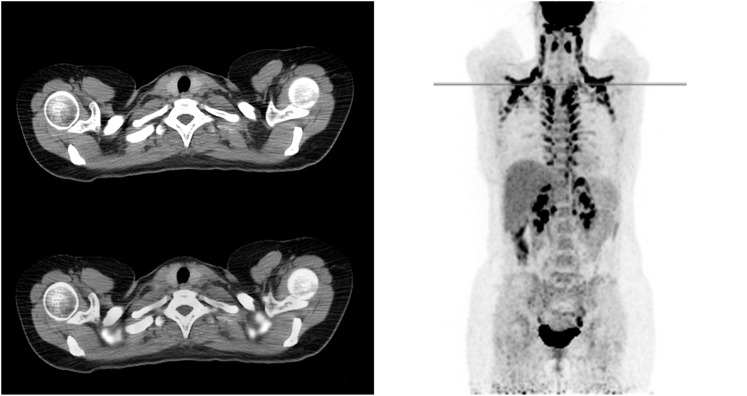
Brown fat, or brown adipose tissue (BAT), is involved in non-shivering thermogenesis and creates heat through glucose metabolism. It is found mostly in the cervical, supraclavicular and thoracic paravertebral regions,8 and some is also found in the axillae, mediastinum and abdomen.9 Although it is usually simple for nuclear medicine physicians to recognize BAT uptake through its typical pattern and from the low attenuation in the CT scan (Figure 1), high uptake in BAT can still be mistaken for a malignant lesion or cause false-negative results by obscuring small metastases and decrease diagnostic confidence of the professional reading the scan. BAT has a preferential distribution over the body and a preferential occurrence in female, younger and thinner patients. It mainly interferes with scan interpretation in tumours that occurs at younger age and is located in or metastasize to the head, neck and periclavicular/axillar regions, such as melanoma of the upper body, breast cancer, and head and neck cancers, but it can also cause problems in lung cancer and other cancers.10,11
Figure 1.
An example of a fluorine-18 fludeoxyglucose positron emission tomography/CT scan of a patient with high brown adipose tissue (BAT) uptake in the neck, interscapular, paravertebral and perinephric regions is shown. BAT can usually be distinguished from pathological uptake by its typical pattern and low attenuation in the CT scans.
Currently, it is not known exactly why BAT activation only occurs in some patients and not others, or why some patients who exhibited BAT activation previously do not show activation in subsequent PET/CT scans.12 However, it is known that BAT activation is related to age, sex and weight, therefore the people who are most likely to show BAT activation are younger females with a low body mass index (BMI).13 It is also known that patients who feel cold are more likely to have BAT activation, and some researchers have found significantly more patients with BAT activation when outdoor temperatures were low.13,14
A few protocols are used to prevent BAT activation in patients receiving 18F-FDG PET/CT scans, such as preventing the patient from getting cold and/or the administration of beta-blockers such as propranolol.15–17 Another drug given to prevent BAT activation is diazepam, a benzodiazepine that is used as treatment for several conditions such as anxiety, insomnia and muscle spasms. The use of diazepam to reduce BAT activation was originally conceived by Barrington et al18 when they discovered abnormally high uptake in the neck during PET scans. The authors originally believed that this pattern was due to skeletal muscle uptake resulting from muscle tension and proposed the use of diazepam to reduce the unwanted uptake. Subsequent studies have had mixed results regarding the use of diazepam, with some authors claiming that diazepam reduced BAT activation19,20 and some claiming that it did not have a significant effect.21,22 In our institute, we generally give diazepam for the reduction of unwanted muscle or BAT uptake to patients in whom cervical BAT or muscle uptake would interfere with scan interpretation. This mainly applies to patients with tumours in the head, neck and breast. We also give diazepam to patients who are nervous for the scan to help them relax.
In this study, we examined the effect of various factors on BAT activation by retrospective examination of patient reports over several years.
METHODS AND MATERIALS
A retrospective study of 15,109 18F-FDG PET/CT scans of 10,432 patients (5035 males and 5397 females, BMI 25 ± 5 kg m−2, age 61 ± 13 years) was performed over a 7-year period from January 2008 to October 2014. All 18F-FDG PET/CT scans were performed on whole-body PET/CT scanners (Gemini TF or Gemini TF Big Bore; Philips, Eindhoven, Netherlands) 60 ± 10 min after the injection of 190 ± 30 MBq of 18F-FDG. The patients were instructed to fast for at least 6 h before administration of 18F-FDG. Diazepam, if given, was administered orally approximately 5–10 min before the administration of 18F-FDG. Patients under 60 years referred for breast cancer, head and neck cancer or lymphoma were indicated for diazepam. However, many patients who were indicated for diazepam did not receive diazepam, and many patients not indicated for diazepam received it, such as nervous patients to help them relax. Doses of 5 or 10 mg were given depending on the weight of the patient. Overall, diazepam was given to the patients prior to 3599 of the 15,109 scans (23.8%). After injection of 18F-FDG, patients were instructed to lie still on a bed under a blanket in a warm room (23 °C) in order to reduce BAT activation and muscular activity. In our hospital, all PET/CT scans are read and reported by experienced nuclear medicine physicians with several years of experience in hybrid imaging, including correlation of PET images with low-dose CT and diagnostic contrast-enhanced CT.
Information regarding age, sex, injected dose, time and date of injection, BMI and blood glucose levels prior to 18F-FDG injection, the presence of diabetes mellitus, administration of diazepam and clinical indication were retrieved from the departmental prospectively maintained PET database. Information regarding the presence of BAT and the recent administration of chemotherapy was extracted from the PET report. The hourly temperatures for the day of examination were retrieved from the Royal Netherlands Meteorological Institute (De Bilt, Netherlands). The temperature for each examination was determined by using the temperature reading 1 h before injection.
Software for analyzing the electronic medical record database was developed in house using the Python programming language (Python Software Foundation).
Statistical analysis
Statistical analysis was performed in the Python programming language using the statsmodels package (http://statsmodels.sourceforge.net/). In order to determine the variables that contribute to BAT activation, univariate analysis of the influence of each variable on BAT activation was performed by testing significance with a z-test for proportions. Cut-offs for univariate analysis were set to the median values of the distribution. The variables included in the model were age, sex, time of injection, outdoor temperature, BMI, blood glucose levels, diazepam usage, the presence of diabetes, indication for breast cancer, recent chemotherapy and whether the patient had BAT activation in a previous scan. Chemotherapy was considered recent if the patient received the PET scan for a response assessment, in which case patients had typically received chemotherapy treatment less than 4 to a maximum of 6 weeks prior to the PET/CT scan. Several variables were tested for significance in the univariate analysis but not included in the final model due to the lack of significant difference. These variables include recent radiotherapy and most tumour types (colon, head and neck, lung, lymphoma and melanoma); only breast cancer was included in the multivariate analysis. Although chemotherapy was insignificant overall in the univariate analysis, it was significant among breast cancer patients, therefore it was included in the multivariate analysis. Multivariate analysis was performed, and a logistic regression with the Newton–Raphson method was performed. For both the univariate and multivariate analysis, the variables were considered significant with a p-value <0.05.
RESULTS
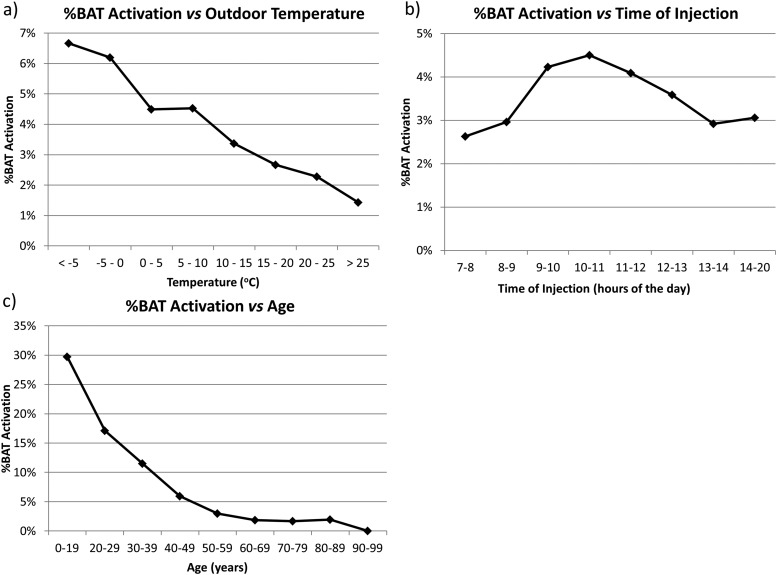
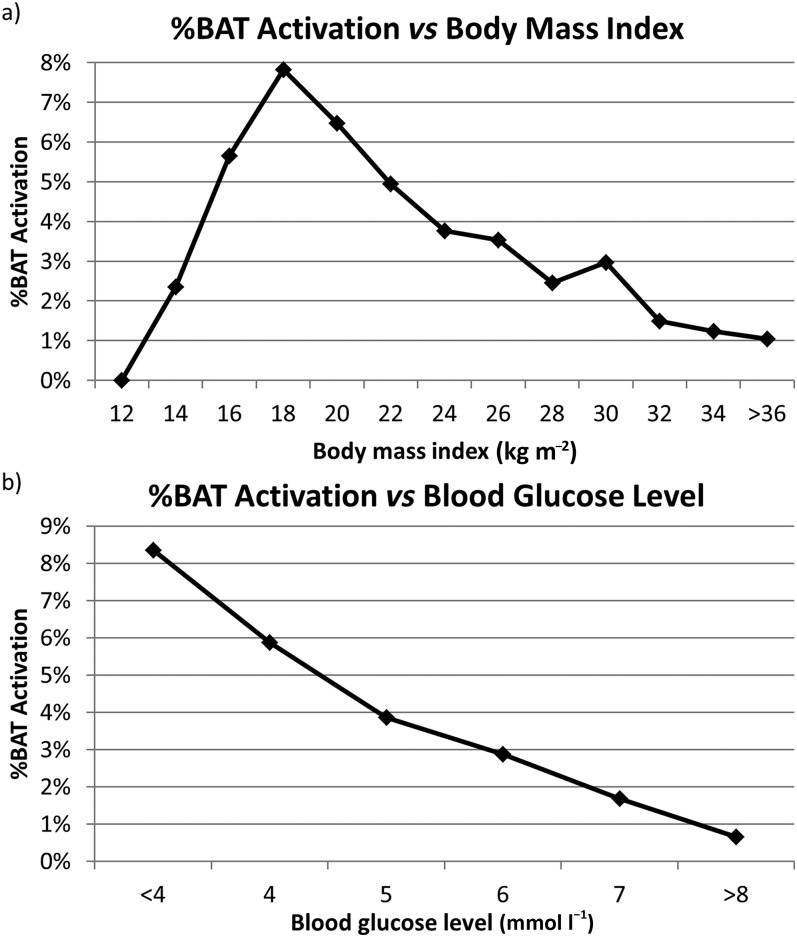
Overall, of the 15,109 18F-FDG PET/CT scans analyzed, 540 reports (3.6%) mentioned the presence of BAT activation. Of the 540 scans with BAT activation, there were 76 cases (14.1%) where the nuclear medicine physician specifically stated uncertainty in the assessment due to BAT activation. The univariate analysis identified several factors that were associated with higher BAT activation (Table 1, Figures 2–5). As is shown in Figure 2, BAT activation was the highest when outdoor temperatures were low and patients were young. BAT activation was less frequent in the early morning (7:00–9:00) than in the late morning (9:00–12:00) (2.9% vs 4.3%, p = 0.001), and BAT activation was less frequent in the afternoon than the late morning (3.4% vs 4.3%, p = 0.005). As is shown in Figure 3, BAT activation was significantly more frequent at BMI <25 than >25 kg m−2 (4.6% vs 2.4%, p < 0.0001). BAT activation in the 95 patients with a very low BMI <16 kg m−2 was much less frequent, but the difference between patients with BMI <16 kg m−2 compared with those between 18 and 21 kg m−2 did not reach significance (p = 0.07). As can be expected given outside temperatures, BAT activation most frequently occurred during the winter and least frequently during the summer (Figure 4). However, the late summer experienced more frequent BAT activation than what would be expected by temperature alone.
Table 1.
The results of the univariate and multivariate analysis using the logistic regression analysis with BAT activation as a dependent variable is shown. The univariate analysis was based on median values [11:07 AM, 61 years, 12.7 °C, body mass index (BMI) 24.7 and blood glucose 5.4 mmol l−1]. The coefficient indicates the increase or decrease in possibility of brown adipose tissue (BAT) activation per unit change of each parameter [e.g. for a patient 1 year older, the %BAT activation is reduced by approximately 4.3% (e−0.044 = 0.957)]
| Parameter | Univariate | Multivariate |
Higher BAT activation | |
|---|---|---|---|---|
| p-value | Coefficient | p-value | ||
| Intercept | – | 2.7 | <0.0001 | – |
| Age (years) | <0.0001 | −0.044 | <0.0001 | Younger |
| BAT previously | <0.0001 | 1.3 | <0.0001 | BAT activation in previous scan |
| Blood glucose (mmol l−1) | <0.0001 | −0.15 | 0.01 | Lower blood glucose level |
| BMI (kg m−2) | <0.0001 | −0.070 | <0.0001 | Lower BMI |
| Breast cancer | <0.0001 | 0.34 | 0.004 | Scanned for breast cancer |
| Chemotherapy | 1.0 | −0.58 | <0.0001 | No chemotherapy |
| Diabetes | <0.0001 | −0.53 | 0.2 | – |
| Diazepam | <0.0001 | −0.0091 | 0.4 | – |
| Sex | <0.0001 | −0.95 | <0.0001 | Female |
| Temperature (°C) | <0.0001 | −0.047 | <0.0001 | Lower outdoor temperatures |
| Time (hours) | 0.07 | −0.026 | 0.2 | – |
Figure 2.
Percentage of fluorine-18 fludeoxyglucose positron emission tomography/CT scans with brown adipose tissue (BAT) activation vs outdoor temperatures, time of injection and age is shown.
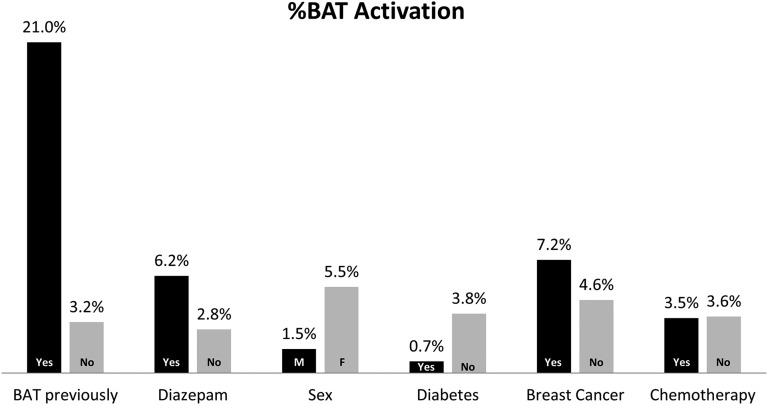
Figure 5.
Percentage of patients with brown adipose tissue (BAT) activation in different subgroups is shown. F, female; M, male.
Figure 3.
Percentage of fluorine-18 fludeoxyglucose positron emission tomography/CT scans with brown adipose tissue (BAT) activation vs body mass index and blood glucose levels is shown.
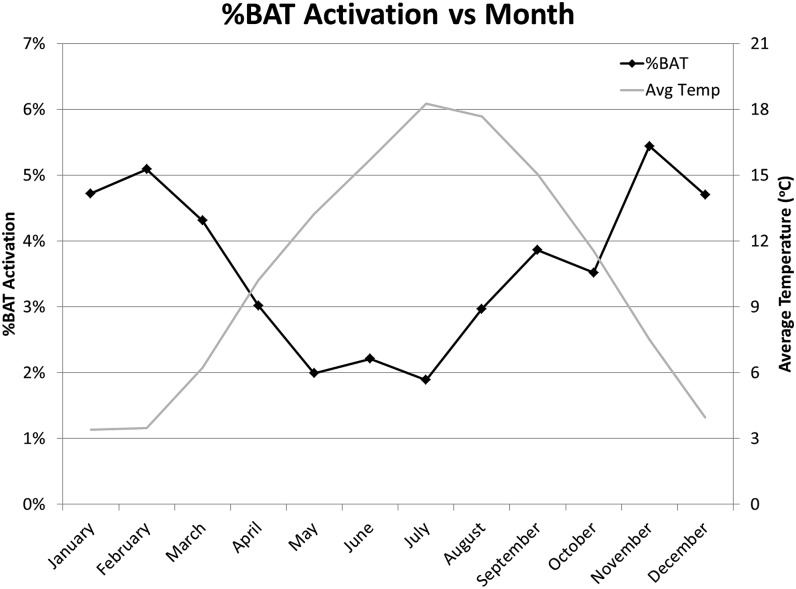
Figure 4.
The percentage of patients with brown adipose tissue (BAT) activation by month is shown. The average (Avg) daily outdoor temperatures (Temp) for each month are displayed as an overlay.
Some other factors that had an effect on BAT activation are shown in Figure 5. Patients who had received diazepam prior to the scan showed BAT activation significantly more frequently than patients who had not received diazepam (6.2% vs 2.8%, p < 0.0001). However, when differentiating between patients who had been prescribed diazepam because of high risk of BAT activation and those who only got it for anxiety, the effect of diazepam disappeared. BAT activation among those who were prescribed diazepam and took it vs those who did not take it was 10.1% and 8.1%, respectively (not significant). Also, BAT activation among those who were not prescribed diazepam but took it and those who did not take it was 2.7% and 2.5%, respectively (not significant). Females with breast cancer were more likely to have BAT activation reported than other females (7.2% vs 4.6%, p < 0.0001). There was no difference between patients who recently received chemotherapy and those who did not receive chemotherapy (3.5% vs 3.6%, p = 0.8). However, among breast cancer patients, there was a significantly reduced percentage of BAT activation in those who had recently received chemotherapy compared with those who had not received chemotherapy (3.8% vs 8.2%, p < 0.0001).
Using a logistic regression model, the significance of the variables was analyzed. The results of the multivariate analysis are shown alongside the univariate analysis in Table 1. The coefficients indicate that reported BAT activation was increased with younger age, BAT activation in previous scan, lower BMI, breast cancer, female sex, lower blood glucose level and lower outdoor temperature. Diabetes was not significant in the multivariate analysis despite being significant in the univariate analysis, which was likely due to the inclusion of fasting blood glucose levels in the regression model. Chemotherapy was highly significant in the multivariate analysis despite being insignificant in the univariate analysis, and diazepam was insignificant in the multivariate analysis despite being significant in the univariate analysis.
DISCUSSION
The purpose of this study was to analyze factors influencing BAT activation in patients undergoing 18F-FDG PET/CT scans. These statistics may be used to take measures to reduce BAT activation in patients who are most likely to show activation. Our study showed an association between BAT activation and factors such as sex, age, BMI and outdoor temperatures, which is in agreement with other publications.23–26 Reducing BAT activation is important since activated BAT can interfere with scan interpretation by obscuring small metastases or by mimicking metastases. We found that in 76 of the 540 cases of BAT activation (14.1%), the nuclear medicine physician specifically stated that there was uncertainty in scan assessment due to BAT activation. In the remaining cases, uncertainty was not specifically mentioned, but this does not rule out the possibility of reduced accuracy of scan interpretation.
Administration of diazepam prior to the scan had no effect on BAT activation in the multivariate analysis, despite being significantly associated with higher BAT activation in the univariate analysis. The significance in the univariate analysis was due to diazepam being prescribed specifically to patients at high risk of BAT activation. There was no significant difference when comparing patients who were prescribed diazepam and took it with patients who were prescribed diazepam and did not take it (e.g. patients with contraindications against diazepam, such as those having to drive after the PET examination). There was also no significant difference between those who were not prescribed diazepam and did not take it with patients who were not prescribed diazepam and took it (e.g. for anxiety). Thus, we can confirm that diazepam has no significant effect on BAT activation, which is in agreement with the results of other studies.26,27
Our study showed significance between BAT activation and other factors that have not been analyzed in a large multivariate study. One such factor was the time of injection, which had not been studied in previous reports. We found significantly more patients with BAT activation in the morning than in the afternoon. This could not be explained entirely by the lower outdoor temperatures in the morning, since the time of injection remained a significant factor in the multivariate analysis where hourly temperatures were taken into account. Despite the lower outdoor temperatures in the morning, patients who were injected in the early morning before 9 AM were less likely to exhibit BAT activation. This may be explained by the naturally lower core body temperatures found in the morning,28,29 by the length of the fasting period or by the higher cortisol levels in the morning. However, while some researchers state that cortisol inhibits BAT function,30 others have demonstrated that cortisol has a stimulatory effect.31,32
It was also observed that there were seasonal variations of BAT activation. Although BAT activation occurred most often during the winter and least in the summer, the results could not be explained entirely by differences in outdoor temperatures. For example, the percentage of patients with BAT activation in August was 57% higher than in July despite similar average temperatures, and the same as in April despite 7.5 °C higher average temperatures. As Au-Yong et al33 observed, the presence of BAT was more closely related to the photoperiod than outdoor temperatures, which is consistent with our results.
Another interesting observation is the low rate of BAT activation in underweight patients. The highest rate of BAT activation occurred in patients with a BMI of 18–21 kg m−2, and the rate of BAT activation declined with higher BMI as expected. However, BAT activation showed a trend towards reduction for patients with BMI <16.0 kg m−2. This is in contrast to other studies that reported increased BAT activity among underweight patients. However, in the study of Hany et al,34 the lowest BMI was 16.8 kg m−2. The study of Pasanisi et al35 found that all seven patients with constitutional leanness (mean BMI 16.2 ± 1.0 kg m−2, age 21.7 ± 3.6 years) but none of the seven patients with anorexia nervosa (mean BMI 15.5 ± 0.8 kg m−2, age 23.4 ± 4.5 years) showed BAT activation. The article shows that BAT function was reduced in severely underweight patients, consistent with our results, but only in the patients with anorexia nervosa. Bauwens et al36 showed that while there is a high prevalence of BAT post-mortem in cachexic cancer patients, there was no evidence for either increased or decreased BAT activity in living cachexic patients. In our study, we lack sufficient information to ascertain whether the patients with very low BMI had anorexia nervosa, cancer cachexia or constitutional leanness.
It was also found that females with breast cancer were much more likely to have BAT activation reported than other females. After taking potential dependent variables such as age into account using multivariate analysis, breast cancer was found to be a highly significant variable. The result is in agreement with Cao et al,37 who showed much higher prevalence of BAT activation in females with breast cancer than in females with other cancers. A possible explanation for this result may be that nuclear medicine physicians tend to mention BAT activation in clinical reports more often when it is considered relevant, such as in nodal staging of breast cancer.
For patients who had received chemotherapy, univariate analysis showed no difference in BAT activation, but multivariate analysis showed a highly significant reduction in BAT activation after recent chemotherapy. The reason for the difference between the univariate and multivariate results is that the subset of patients who underwent chemotherapy was younger than the subset of those who did not undergo chemotherapy (median age: 54 vs 62 years). The reduction in BAT activation suggests that some chemotherapy drugs may inhibit BAT function. The lower incidence of BAT activation after chemotherapy is consistent with the results of Gadea et al,38 who showed a decrease in 18F-FDG activity in BAT for breast cancer patients who had received chemotherapy. The exact mechanism of interference of chemotherapy with BAT activation remains to be elucidated, but it probably depends on the type of chemotherapy, dosage, number of cycles and time between the last cycle of chemotherapy and the 18F-FDG PET scan.
Patients who had shown BAT activation in a previous 18F-FDG PET scan were also at increased risk to have BAT activation again. This confirms the work of Lee et al,39 although our study showed a much higher percentage of BAT activation (21% vs 13%). Since the effect remained significant in the multivariate model, patients who had BAT activation in a previous scan probably had some unknown intrinsic characteristic not included in the regression model. The variable “BAT previously” was included in the regression model in order to minimize errors in the fit due to clustering of patients receiving multiple scans.
Based on the results, we can offer some recommendations for reducing the number of patients with BAT activation. First, the patients who are at highest risk of BAT activation need to be identified (i.e. young, females, low BMI, indicated for breast cancer and had BAT activation in a previous scan). Patients at highest risk of BAT activation may be scanned either in the early morning or later in the afternoon. Ideally, the patients with the highest risk should either be the first or last patient to be scanned. Furthermore, previously described methods to reduce BAT activation may be used to prepare these patients for the scan, such as staying in a warm room and administering propranolol.
Limitations of our study include its retrospective nature and the fact that scans were not reviewed blindly to score BAT activation. Since BAT activation was not scored blindly, it is possible that some patients had BAT activation not mentioned in the reports or had only minimal BAT activation that was mentioned in the reports. This introduces a possible bias due to increased reporting of BAT activity in patients with tumours in areas where BAT interferes most or in patients who had BAT activation in a previous scan.
CONCLUSION
This large retrospective study identifies new factors that influence BAT activation, such as the time of day and recent chemotherapy. It also confirms that BAT activation occurs more often at lower outdoor temperatures, in females, in younger people and in people with a lower BMI. There were a few new findings, such as the low frequency of BAT activation in the early morning and among breast cancer patients who had recently received chemotherapy. Better understanding of factors influencing BAT activation may help in the design of interventions to reduce or promote BAT activation, and it can be used to identify patients at risk of BAT activation during 18F-FDG PET examinations.
Acknowledgments
ACKNOWLEDGMENTS
The authors thank Vincent van der Noort from the Department of Scientific Statistics at Netherlands Cancer Institute, Amsterdam, Netherlands, for his assistance in reviewing the statistical methods and results presented in the article.
Contributor Information
Jeffrey D Steinberg, Email: j.steinberg@nki.nl.
Wouter Vogel, Email: w.vogel@nki.nl.
Erik Vegt, Email: e.vegt@nki.nl.
REFERENCES
- 1.Bar-Shalom R, Yefremov N, Guralnik L, Gaitini D, Frenkel A, Kuten A, et al. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med 2003; 44: 1200–9. [PubMed] [Google Scholar]
- 2.Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med 2000; 41: 1369–79. [PubMed] [Google Scholar]
- 3.Kwee TC, Kwee RM. Combined FDG-PET/CT for the detection of unknown primary tumors: systematic review and meta-analysis. Eur Radiol 2009; 19: 731–44. doi: https://doi.org/10.1007/s00330-008-1194-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mankoff DA, Bellon JR. Positron-emission tomographic imaging of cancer: glucose metabolism and beyond. Semin Radiat Oncol 2001; 11: 16–27. doi: https://doi.org/10.1053/srao.2001.18100 [DOI] [PubMed] [Google Scholar]
- 5.Mankoff DA, Eary JF, Link JM, Muzi M, Rajendran JG, Spence AM, et al. Tumor-specific positron emission tomography imaging in patients: [18F] fluorodeoxyglucose and beyond. Clin Cancer Res 2007; 13: 3460–9. doi: https://doi.org/10.1158/1078-0432.ccr-07-0074 [DOI] [PubMed] [Google Scholar]
- 6.Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology 2004; 231: 305–32. doi: https://doi.org/10.1148/radiol.2312021185 [DOI] [PubMed] [Google Scholar]
- 7.Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J Nucl Med 2003; 44: 170–6. [PubMed] [Google Scholar]
- 8.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360: 1509–17. doi: https://doi.org/10.1056/nejmoa0810780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heaton JM. The distribution of brown adipose tissue in the human. J Anat 1972; 112(Pt 1): 35–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Truong MT, Erasmus JJ, Munden RF, Marom EM, Sabloff BS, Gladish GW, et al. Focal FDG uptake in mediastinal brown fat mimicking malignancy: a potential pitfall resolved on PET/CT. AJR Am J Roentgenol 2004; 183: 1127–32. doi: https://doi.org/10.2214/ajr.183.4.1831127 [DOI] [PubMed] [Google Scholar]
- 11.Reddy MP, Ramaswamy MR. FDG uptake in brown adipose tissue mimicking an adrenal metastasis: source of false-positive interpretation. Clin Nucl Med 2005; 30: 257–8. doi: https://doi.org/10.1097/01.rlu.0000156798.59230.2e [DOI] [PubMed] [Google Scholar]
- 12.Rousseau C, Bourbouloux E, Campion L, Fleury N, Bridji B, Chatal JF, et al. Brown fat in breast cancer patients: analysis of serial (18)F-FDG PET/CT scans. Eur J Nucl Med Mol Imaging 2006; 33: 785–91. doi: https://doi.org/10.1007/s00259-006-0066-x [DOI] [PubMed] [Google Scholar]
- 13.Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab 2011; 96: 192–9. doi: https://doi.org/10.1210/jc.2010-0989 [DOI] [PubMed] [Google Scholar]
- 14.Cohade C, Mourtzikos KA, Wahl RL. “USA-Fat”: prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. J Nucl Med 2003; 44: 1267–70. [PubMed] [Google Scholar]
- 15.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007; 293: E444–52. doi: https://doi.org/10.1152/ajpendo.00691.2006 [DOI] [PubMed] [Google Scholar]
- 16.Parysow O, Mollerach AM, Jager V, Racioppi S, San Roman J, Gerbaudo VH. Low-dose oral propranolol could reduce brown adipose tissue F-18 FDG uptake in patients undergoing PET scans. Clin Nucl Med 2007; 32: 351–7. doi: https://doi.org/10.1097/01.rlu.0000259570.69163.04 [DOI] [PubMed] [Google Scholar]
- 17.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009; 360: 1500–8. doi: https://doi.org/10.1056/nejmoa0808718 [DOI] [PubMed] [Google Scholar]
- 18.Barrington SF, Maisey MN. Skeletal muscle uptake of fluorine-18-FDG: effect of oral diazepam. J Nucl Med 1996; 37(7): 1127–9. [PubMed] [Google Scholar]
- 19.Aukema TS, Vogel WV, Hoefnagel CA, Valdes Olmos RA. Prevention of brown adipose tissue activation in 18F-FDG PET/CT of breast cancer patients receiving neoadjuvant systemic therapy. J Nucl Med Technol 2010; 38: 24–7. doi: https://doi.org/10.2967/jnmt.109.065557 [DOI] [PubMed] [Google Scholar]
- 20.Rakheja R, Ciarallo A, Alabed YZ, Hickeson M. Intravenous administration of diazepam significantly reduces brown fat activity on 18F-FDG PET/CT. Am J Nucl Med Mol Imaging 2011; 1: 29–35. [PMC free article] [PubMed] [Google Scholar]
- 21.Tatsumi M, Engles JM, Ishimori T, Nicely O, Cohade C, Wahl RL. Intense (18)F-FDG uptake in brown fat can be reduced pharmacologically. J Nucl Med 2004; 45: 1189–93. [PubMed] [Google Scholar]
- 22.Gelfand MJ, O'Hara SM, Curtwright LA, Maclean JR. Pre-medication to block [(18)F]FDG uptake in the brown adipose tissue of pediatric and adolescent patients. Pediatr Radiol 2005; 35: 984–90. doi: https://doi.org/10.1007/s00247-005-1505-8 [DOI] [PubMed] [Google Scholar]
- 23.Huang YC, Chen TB, Hsu CC, Li SH, Wang PW, Lee BF, et al. The relationship between brown adipose tissue activity and neoplastic status: an (18)F-FDG PET/CT study in the tropics. Lipids Health Dis 2011; 10: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Persichetti A, Sciuto R, Rea S, Basciani S, Lubrano C, Mariani S, et al. Prevalence, mass, and glucose-uptake activity of (1)(8)F-FDG-detected brown adipose tissue in humans living in a temperate zone of Italy. PLoS One 2013; 8: e63391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfannenberg C, Werner MK, Ripkens S, Stef I, Deckert A, Schmadl M, et al. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes 2010; 59: 1789–93. doi: https://doi.org/10.2337/db10-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pace L, Nicolai E, D'Amico D, Ibello F, Della Morte AM, Salvatore B, et al. Determinants of physiologic 18F-FDG uptake in brown adipose tissue in sequential PET/CT examinations. Mol Imaging Biol 2011; 13: 1029–35. doi: https://doi.org/10.1007/s11307-010-0431-9 [DOI] [PubMed] [Google Scholar]
- 27.Sturkenboom MG, Hoekstra OS, Postema EJ, Zijlstra JM, Berkhof J, Franssen EJ. A randomised controlled trial assessing the effect of oral diazepam on 18F-FDG uptake in the neck and upper chest region. Mol Imaging Biol 2009; 11: 364–8. doi: https://doi.org/10.1007/s11307-009-0207-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgess HJ, Trinder J, Kim Y, Luke D. Sleep and circadian influences on cardiac autonomic nervous system activity. Am J Physiol 1997; 273(4 Pt 2): H1761–8. [DOI] [PubMed] [Google Scholar]
- 29.Waterhouse J, Fukuda Y, Morita T. Daily rhythms of the sleep-wake cycle. J Physiol Anthropol 2012; 31: 5. doi: https://doi.org/10.1186/1880-6805-31-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab 2010; 11: 268–72. doi: https://doi.org/10.1016/j.cmet.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 31.Ramage LE, Akyol M, Fletcher AM, Forsythe J, Nixon M, Carter RN, et al. Glucocorticoids acutely increase brown adipose tissue activity in humans, revealing species-specific differences in UCP-1 regulation. Cell Metab 2016; 24: 130–41. doi: https://doi.org/10.1016/j.cmet.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson LJ, Law JM, Symonds ME, Budge H. Brown adipose tissue activation as measured by infrared thermography by mild anticipatory psychological stress in lean healthy females. Exp Physiol 2016; 101: 549–57. doi: https://doi.org/10.1113/EP085642 [DOI] [PubMed] [Google Scholar]
- 33.Au-Yong IT, Thorn N, Ganatra R, Perkins AC, Symonds ME. Brown adipose tissue and seasonal variation in humans. Diabetes 2009; 58: 2583–7. doi: https://doi.org/10.2337/db09-0833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, von Schulthess GK. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging 2002; 29: 1393–8. doi: https://doi.org/10.1007/s00259-002-0902-6 [DOI] [PubMed] [Google Scholar]
- 35.Pasanisi F, Pace L, Fonti R, Marra M, Sgambati D, De Caprio C, et al. Evidence of brown fat activity in constitutional leanness. J Clin Endocrinol Metab 2013; 98: 1214–18. doi: https://doi.org/10.1210/jc.2012-2981 [DOI] [PubMed] [Google Scholar]
- 36.Bauwens M, Wierts R, van Royen B, Bucerius J, Backes W, Mottaghy F, et al. Molecular imaging of brown adipose tissue in health and disease. Eur J Nucl Med Mol Imaging 2014; 41: 776–91. doi: https://doi.org/10.1007/s00259-013-2611-8 [DOI] [PubMed] [Google Scholar]
- 37.Cao Q, Hersl J, La H, Smith M, Jenkins J, Goloubeva O, et al. A pilot study of FDG PET/CT detects a link between brown adipose tissue and breast cancer. BMC Cancer 2014; 14: 126. doi: https://doi.org/10.1186/1471-2407-14-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gadea E, Thivat E, Merlin C, Paulon R, Kwiatkowski F, Chadeyras JB, et al. Brown adipose tissue activity in relation to weight gain during chemotherapy in breast cancer patients: a pilot study. Nutr Cancer 2014; 66: 1092–6. doi: https://doi.org/10.1080/01635581.2014.948212 [DOI] [PubMed] [Google Scholar]
- 39.Lee P, Greenfield JR, Ho KK, Fulham MJ. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2010; 299: E601–06. doi: https://doi.org/10.1152/ajpendo.00298.2010 [DOI] [PubMed] [Google Scholar]