Abstract
By rapidly modulating neuronal excitability, neurosteroids regulate physiological processes, such as responses to stress and development. Excessive stress affects their biosynthesis and causes an imbalance in cognition and emotions. The progesterone derivative, allopregnanolone (Allo) enhances extrasynaptic and postsynaptic inhibition by directly binding at GABAA receptors, and thus, positively and allosterically modulates the function of GABA. Allo levels are decreased in stress‐induced psychiatric disorders, including depression and post‐traumatic stress disorder (PTSD), and elevating Allo levels may be a valid therapeutic approach to counteract behavioural dysfunction. While benzodiazepines are inefficient, selective serotonin reuptake inhibitors (SSRIs) represent the first choice treatment for depression and PTSD. Their mechanisms to improve behaviour in preclinical studies include neurosteroidogenic effects at low non‐serotonergic doses. Unfortunately, half of PTSD and depressed patients are resistant to current prescribed ‘high’ dosage of these drugs that engage serotonergic mechanisms. Unveiling novel biomarkers to develop more efficient treatment strategies is in high demand. Stress‐induced down‐regulation of neurosteroid biosynthesis and changes in GABAA receptor subunit expression offer a putative biomarker axis to develop new PTSD treatments. The advantage of stimulating Allo biosynthesis relies on the variety of neurosteroidogenic receptors to be targeted, including TSPO and endocannabinoid receptors. Furthermore, stress favours a GABAA receptor subunit composition with higher sensitivity for Allo. The use of synthetic analogues of Allo is a valuable alternative. Pregnenolone or drugs that stimulate its levels increase Allo but also sulphated steroids, including pregnanolone sulphate which, by inhibiting NMDA tonic neurotransmission, provides neuroprotection and cognitive benefits. In this review, we describe current knowledge on the effects of stress on neurosteroid biosynthesis and GABAA receptor neurotransmission and summarize available pharmacological strategies that by enhancing neurosteroidogenesis are relevant for the treatment of SSRI‐resistant patients.
Linked Articles
This article is part of a themed section on Pharmacology of Cognition: a Panacea for Neuropsychiatric Disease? To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.19/issuetoc
Abbreviations
- 3α‐HSD
3α‐hydroxysteroid dehydrogenase
- 3β‐HSD
3β‐hydroxysteroid dehydrogenase
- AEA
anandamide
- Allo
allopregnanolone
- AlloS
allopregnanolone sulphate
- CB1
cannabinoid receptor type 1
- dMPFC
dorsal medial prefrontal cortex
- GABAARs
GABAA receptors
- ODNs
oligonucleotides
- PAS
pregnanolone sulphate
- PEA
palmitoylethanolamide
- PES
pregnenolone sulphate
- PMS
premenstrual syndrome
- PTSD
post‐traumatic stress disorder
- PXR
pregnane xenobiotic receptor
- SBSSs
selective brain steroidogenic stimulants
- SPS
single prolonged stress
- SSRIs
selective serotonin reuptake inhibitors
- StAR
steroidogenic acute regulatory protein
- THDOC
tetrahydrodeoxycorticosterone
- THIP
4,5,6,7‐tetrahydroisoxazolo(5,4‐c)pyridin‐3‐ol
- TSPO
18 kDa translocator protein
Introduction
Neurosteroids, including 5alpha‐dihydroprogesterone (5alpha‐DHP), allopregnanolone (Allo) and its stereoisomers, for example, pregnanolone (PA), are directly synthesized in the central nervous system by brain neurons (glutamatergic and GABAergic long‐projecting neurons) (Baulieu and Robel, 1990; Agís‐Balboa et al., 2006, 2007) and act not only at classical steroid hormone receptors (that regulate gene expression and have long‐lasting effects) but also rapidly modulate neuronal excitability by binding to membrane receptors and ion channels. For example, they are potent modulators of GABAA receptors (GABAARs) (Belelli and Lambert, 2005). Progesterone or its neuroactive metabolite, Allo, when administered to rodents or humans, induces anxiolytic, sedative, anaesthetic, analgesic and anticonvulsant effects (Belelli et al., 2009) by potently increasing Cl− ion flux induced by GABA binding at GABAARs. Both neurosteroid levels and GABAAR expression are subjected to physiological changes during pregnancy and the ovarian cycle (Concas et al., 1998; Maguire et al., 2005), or in pathological conditions caused by protracted or traumatic stress, such as anxiety, depression, and post‐traumatic stress disorder (PTSD) (Rasmusson et al., 2006; Romeo et al., 1998; Uzunova et al., 1998).
The upstream synthesis of Allo starts with the cholesterol's transport into the inner mitochondrial membrane of glial cells by the activity of the steroidogenic acute regulatory protein (StAR) and the 18 kDa translocator protein (TSPO) (Papadopoulos et al., 2006), formerly called the peripheral mitochondrial benzodiazepine receptor (Costa and Guidotti, 1991). The enzyme cytochrome p450scc converts cholesterol into pregnenolone, the precursor for all neurosteroids. TSPO in glial cells is important in neurosteroidogenesis and is activated by selective ligands that initiate a cascade of neurosteroid biosynthetic events in several brain areas (Rupprecht et al., 2009). Although this view has been suggested by an extensive number of studies, recent reports also suggest that TSPO is not essential for steroid hormone biosynthesis or viability (Selvaraj and Stocco, 2015; Selvaraj et al., 2016). Pregnenolone can then be sulphated to pregnenolone sulphate (PES) and act not only at NMDA and AMPA receptors (reviewed in Smith et al., 2014) but also at GABAARs (Shen et al., 1999) or can be taken up by neurons where it is further metabolized by the enzyme 3β‐hydroxysteroid dehydrogenase (3β‐HSD) into progesterone. Then progesterone can be converted by 5α‐reductase type I and 3α‐hydroxysteroid dehydrogenase (3α‐HSD) into Allo (Figure 1). Allo and its GABAAR‐active stereoisomer, PA can be further sulphated into Allo sulphate (AlloS) and PA sulphate (PAS) that act as inhibitors of NMDA receptors neurotransmission (Smith et al., 2014).
Figure 1.
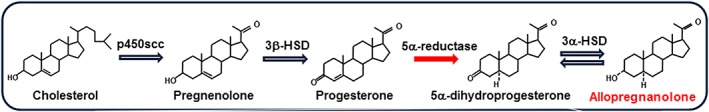
Biosynthesis of allopregnanolone. Neurons can synthesized allopregnanolone ex novo starting from pregnenolone, the precursor of all neurosteroids. Pregnenolone is metabolized into progesterone by 3β‐HSD and progesterone can be further converted by the enzyme 5α‐reductase into 5α‐dihydroprogesterone (5α‐pregnan‐3,20‐dione, 5α‐DHP), and, finally, 5α‐DHP is reduced into allopregnanolone (3α‐hydroxy‐5α‐pregnan‐20‐one or 3α,5α‐tetrahydroprogesterone) by the 3α‐HSD enzyme.
The enzymes implicated in the biosynthesis of neurosteroids are not homogeneously expressed in the brain but are region‐ and neuron‐specific (Agís‐Balboa et al., 2006). For example, 5α‐reductase and 3α‐HSD are highly expressed and co‐localized in pyramidal neurons and granular cells in the cortex and hippocampus, and in pyramidal‐like neurons in the basolateral amygdala (Agís‐Balboa et al., 2007). It has been suggested that Allo, synthesized in glutamatergic cortical or hippocampal pyramidal neurons or in granular cells of the dentate gyrus may be secreted by: (i) a paracrine mechanism, which would allow newly‐synthesized Allo to exert an effect on GABAARs located in the synaptic membranes of distal glutamatergic neurons, (ii) an autocrine mechanism, that allows Allo to bind to postsynaptic as well as to extrasynaptic GABAARs located in the synaptic membranes of the same neurons in which it was produced, or (iii) Allo may diffuse into synaptic boutons of the cell bodies or dendrites of neurons in which it is synthesized, so that it reaches GABAARs via intracellular pathways (Akk et al., 2004; Agís‐Balboa et al., 2007). These mechanisms, by which neurosteroids are produced, secreted, and act at postsynaptic and extrasynaptic GABAARs, have been supported by more recent studies in which the neural location of Allo has been explored using specific antibodies (Cook et al., 2014).
Neurosteroidogenesis is altered in various neuropsychiatric disorders. An alteration in the concentrations of Allo, tetrahydrodeoxycorticosterone (THDOC), PA and progesterone has been identified in the serum, and CSF of patients affected by depression, PTSD, premenstrual syndrome (PMS), and addiction (Romeo et al., 1998; Uzunova et al., 1998; Pinna et al., 2006a; Rasmusson et al., 2006). Administration of selective serotonin reuptake inhibitors (SSRIs), such as the antidepressants fluoxetine, fluvoxamine, paroxetine, and sertraline, the serotonin‐norepinephrine reuptake inhibitor, venlafaxine, or the norepinephrine reuptake inhibitor, reboxetine, and the atypical antidepressant, mirtazapine improve depressive symptoms by normalizing brain, plasma, and CSF levels of Allo in psychiatric disorders (Uzunov et al., 1996; Romeo et al., 1998; Uzunova et al., 1998; Agís‐Balboa et al., 2014; Schüle, 2014). Brain Allo levels are also decreased in mouse models of anxiety, depression and PTSD (Pinna et al., 2004, 2006a; Uzunova et al., 2006). In stressed rodents, we have found that 1/10 of the concentration of SSRIs that engage serotonin mechanisms, is needed to up‐regulate Allo levels and improve behaviour (Pinna et al., 2003, 2004). In patients, SSRIs are associated with high non‐response rates (Block and Nemeroff, 2014; Otte et al., 2016) when administered at doses that engage serotonergic mechanisms, while it remains unknown whether these ‘high’ doses still stimulate neurosteroid biosynthesis. Thus, one may wonder whether, when administering an SSRI, ‘less is more’ and a low dosage may offer a pharmacological advantage over a high dosage in the treatment of PTSD and depression. Hence, new clinical trials are required to test this hypothesis. Whereas treatment strategies specifically for the SSRI non‐responder subpopulation have recently been suggested (Fava et al., 2016), new therapies and novel biomarkers to guide selection of treatments for stress‐induced psychiatric disorders are urgently required. Neurosteroid biosynthesis can be stimulated by several diverse receptors and neural systems, including the pregnane xenobiotic receptor (PXR) and the endocannabinoid system (Frye et al., 2012; Vallée et al., 2014; Vallée, 2016). The use of synthetic analogues of Allo also represents a strategy to reinstate functional GABAergic neurotransmission in PTSD or other neuropsychiatric disorders with altered neurosteroidogenesis.
This report reviews the neurophysiological role of neurosteroids acting as positive or negative modulators of GABAARs, including when several GABAAR subunit expression is changed due to stress. The down‐regulation of neurosteroid levels found in PTSD patients can be modelled in rodent models of stress, including the socially isolated (SI) mouse and the single prolonged stress (SPS) mouse. This translational approach provides the ground for exploiting several neurosteroidogenic targets with drugs that improve emotional behaviour by enhancing Allo levels and thereby offer promising therapeutic tools for novel PTSD treatment. The emerging therapeutic role of sulphated pregnane steroids in neuropsychiatric disorders is also reviewed as a possible additional mechanism for neurosteroidogenic drugs to improve both emotional and cognitive behaviour. Taken together, deficits in Allo biosynthesis interacting with stress‐induced changes in GABAAR subtypes may represent a valuable biomarker axis for use in predicting, preventing, treating and monitoring symptoms of stress‐induced disorders, including PTSD and depression.
Neurosteroid and stress modulation of GABAARs
GABAARs are probably the principal target of neurosteroids, including the endogenous neurosteroids, Allo and THDOC (Reddy, 2003; Belelli and Lambert, 2005; Belelli et al., 2009). Allo plays a pivotal neurophysiological role by fine‐tuning GABAARs to agonists and allosteric modulators (Pinna et al., 2000) by binding at two distinct residues on GABAARs; a potentiation site is formed in a cavity within α‐subunits and an activation site is located at the interface of α and β subunits (Hosie et al., 2006). By this mechanism, Allo may also play an important role in the regulation of emotional behaviour (Pinna et al., 2009).
There are two types of GABAARs that differ by subneuronal distribution, function, structure and pharmacology: synaptic and extrasynaptic receptors (Belelli and Lambert, 2005; Carver and Reddy, 2013). Synaptic GABAARs contain mainly α1, α2 and α3 subunits, along with β subunit variants and the γ2 subunit (Fritschy and Panzanelli, 2014), and are located at the synaptic membrane (Somogyi et al., 1996; Essrich et al., 1998), where they mediate a rapid and transitory inhibition (phasic current) following the intermittent release of high (millimolar) concentrations of GABA from the presynaptic terminals (Farrant and Nusser, 2005); importantly, these receptors are sensitive to benzodiazepines as well as Allo (Hájos et al., 2000). GABAARs that contain α4 and α6 subunits, often along with the δ subunit (instead of the γ2 subunit) are located at the peri‐ and extra‐synaptic membranes (Nusser et al., 1998; Wei et al., 2003; Tretter and Moss, 2008; Fritschy and Panzanelli, 2014). Extrasynaptic GABAARs containing α4/β/δ mediate a persistent inhibition (tonic current) under conditions of low (nanomolar) concentrations of ambient GABA (Mody, 2001; Semyanov et al., 2004; Glykys and Mody, 2007). Deletion of the δ subunit (and the concomitant loss of α4 expression) significantly reduces tonic receptor activation in granule cells of the dentate gyrus (Peng et al., 2002; Stell et al., 2003). Reports also suggest that efficacy of δ‐containing extrasynaptic receptors for GABA can be increased by neurosteroids (Stell et al., 2003; Shu et al., 2012). Remarkably, GABAARs incorporating α4, α6 and δ subunits are highly sensitive to Allo (Farrant and Nusser, 2005; Belelli et al., 2009). The modulation of GABAARs subtypes by Allo is thus pharmacologically distinct from benzodiazepines, which fail to activate GABAARs containing α4 and α6 subunits or extrasynaptic GABAARs receptors containing δ subunits (Costa and Guidotti, 1996). Thus, these features make them an attractive pharmacological target after stimulation of neurosteroid biosynthesis, following administration of an Allo analogue, such as ganaxolone (3α‐hydroxy‐3β‐methyl‐5α‐pregnan‐20‐one), or the GABAAR agonist, 4,5,6,7‐tetrahydroisoxazolo(5,4‐c)pyridin‐3‐ol (THIP), which shows selectivity for δ‐containing receptors (Gulinello et al., 2003; Belelli and Lambert, 2005; Herd et al., 2013).
Extrasynaptic receptors containing α5 subunits are also involved in the modulation of tonic inhibition in the hippocampus, olfactory bulb and cerebral cortex (Caraiscos et al., 2004; Glykys and Mody, 2006; Serwanski et al., 2006; Bonin et al., 2007; Glykys et al., 2008; Fritschy and Panzanelli, 2014; Perez‐Sanchez et al., 2017). In fact, α5‐containing extrasynaptic GABAARs, similarly to δ‐containing GABAARs, are highly sensitive to low, persistent, ambient concentrations of GABA. Moreover, a study conducted on recombinant α5β2γ2 and α1β2γ2 GABAARs in Xenopus oocytes, using a two electrodes voltage‐clamp technique, showed a significantly lower level of desensitization in the α5‐containing receptors. In addition, the potencies of Allo and THDOC to increase GABA response were significantly higher in the α5‐containing receptors; however, their efficacies among the two receptors was not changed. In both α1‐ and α5‐containing receptors, the efficacy of THDOC was higher than that of Allo (Rahman et al., 2006). Using patch clamp recording in the perirhinal cortex, Schwabe et al. (2005) showed that THDOC prolonged the time course and the amplitude of mono‐ and biphasic miniature inhibitory postsynaptic currents in rats that overexpress α2, α3 and α5 subunits. By comparison, THDOC was much more effective than Allo in inducing a tonic current in these rats.
The α5‐containing GABAAR subtypes in the hippocampus play an important role in cognition, learning and memory (Caraiscos et al., 2004; Soh and Lynch, 2015). KO mice presenting a deletion of α5 subunit in the dentate gyrus are characterized by reduced tonic inhibition of granule cells without any change in fast phasic inhibition. As a result, α5‐KO mice show impairments in cognitive tasks (Engin et al., 2015). Recently, α5 positive selective allosteric modulators have been synthesized and they improved hippocampal‐dependent memory in aged rats with identified cognitive impairment (Koh et al., 2013).
Importantly, GABAARs can also be negatively modulated by steroids that are sulphated at the C3 position, including PES and dehydroepiandrosterone sulphate (DHEAS). Although the precise site for sulphated steroids has not been established, it appears that PES acts at GABAARs via a membrane sensitive site rather than a lock and key site positioned in the receptor complex (Shen et al., 1999; Akk et al., 2001). This view supports two distinct binding sites for neuroactive steroids that mediate positive (e.g. Allo, PA and THDOC) and negative (e.g. PES and DHEAS) GABAAR modulation (Rahman et al., 2006; Wang et al., 2008). The inhibitory site for sulphated steroids is distinct from the site where picrotoxin acts and sulphated steroids seem to induce inhibition of GABA action by enhancing GABAAR sensitization (Eisenman et al., 2003).
Furthermore, the 3β‐hydroxysteroids, including (3β,5α)‐3‐hydroxypregnan‐20‐one, (3β5β)‐3‐hydroxypregnan‐20‐one) and (3β5β)‐THDOC can noncompetitively antagonize the potentiation on GABAARs induced by their 3α‐diastereomers, including Allo (3α,5α)‐3‐hydroxypregnan‐20‐one, PA (3α,5β)‐3‐hydroxypregnan‐20‐one, or 3α5α‐THDOC (3α,5α)‐3,21‐dihydroxypregnan‐20‐one and 3α5β‐THDOC (3α,5β)‐3,21‐dihydroxypregnan‐20‐one that positively modulate GABAARs (Wang et al., 2002). The 3β‐hydroxysteroids are also able to significantly reduce the potentiation induced by high concentrations of barbiturates. The profile of block is similar to that exhibited by sulfated steroids that block GABAARs (Wang et al., 2002).
Collectively, these observations are highly significant in designing therapeutic strategies to counteract cognitive and emotional deficits resulting from a GABA signal transduction dysfunction observed after protracted stress, as well as behavioural impairment related to excessive GABA‐mediated inhibition.
Depression and PTSD and other stress‐related disorders are accompanied by changes in GABAergic neurotransmission. For example, patients with major depression show decreases in GABA levels (Luscher et al., 2011). Postmortem studies show reduced number of GABAergic interneurons in the cortex and hippocampus of patients with mood disorders (Benes et al., 2008). Up‐regulation of genes governing expression of GABAAR subunits, such as α5 and δ, was also shown in prefrontal cortex subregions of depressed patients (Choudary et al., 2005; Klempan et al., 2009). A study of the brains of depressed suicide victims has demonstrated a marked up‐regulation of GABRA5 (α5) and GABRG2 (γ2) genes in the prefrontal cortex and inferior temporal cortex while GABRG1 (γ1) was downregulated in the prefrontal cortex (Sequeira et al., 2009). Altered abundance of α1, α3, α4 and δ subunit mRNAs in the frontopolar cortex of suicide victims was also shown (Merali et al., 2004).
Furthermore, a PET and a structural MRI study of male Dutch veterans showed a significant reduction of [13C]flumazenil binding in the cerebral cortex, hippocampus, and hypothalamus of PTSD patients (Geuze et al., 2008). This is in accord with a previous study showing reduced [123I]iomazenil uptake in Brodman's Area 9 in male Viet Nam veterans with PTSD (Bremner et al., 2000). This evidence points to possible stress‐induced changes in the expression of GABAAR subunits at which benzodiazepines bind and act. Hence, identification and further characterization of these deficits could be valuable in selecting treatments to improve symptoms of PTSD and depression.
In preclinical studies, chronic cold stress in male rats induced a reduction of GABA levels, GAD expression, and [3H]GABA binding in the cerebral cortex, olfactory bulb, hypothalamus and striatum (Acosta et al., 1993). Numerous studies report altered GABAAR subunit expression following chronic stress. In male mice, repeated swim stress induces a decrease of hippocampal α1 subunit expression (Montpied et al., 1993). Chronic unpredictable stress decreases hippocampal δ subunit and increases hippocampal α5 subunit expression in male rats (Verkuyl et al., 2004). Protracted social isolation decreases α1, α2 and γ2, and increases α4 and α5 GABAA subunit expression in the hippocampus and frontal cortex (Pinna et al., 2006b; Matsumoto et al., 2007). These changes are associated with altered pharmacological response to GABAAR agonists, such as resistance to the sedative and anxiolytic effects of diazepam and zolpidem (Pinna et al., 2006b; Nin et al., 2011a), which bind with high affinity to α1, α2, α3 and α5 (diazepam) and α1 (zopidem) subunits. The binding site of benzodiazepines is located at the interface of α and γ2 subunits (Figure 2), and GABAARs devoid of γ2 subunits show lower sensitivity to the pharmacological action of these drugs (Rudolph et al., 1999). Thus, resistance to the sedative and anxiolytic effects of diazepam and zolpidem in SI mice could relate to down‐regulation of α1, α2 and γ2 subunits and to up‐regulation of GABAAR subtypes containing α4 and δ subunits, which are insensitive to benzodiazepines (Rudolph et al., 1999; Serra et al., 2006; Nin et al., 2011a). This is further supported by the finding that social isolation increases the expression of specific GABAAR subunits mainly expressed extrasynaptically, like α4 and α5 subunits (Belelli et al., 2009; Fritschy and Panzanelli, 2014; Deprez et al., 2016). A study conducted in SI rats also showed up‐regulation of hippocampal α4 and δ subunits, which confers higher sensitivity to alcohol (Serra et al., 2006). Whether stress directly causes alterations in the expression of GABAAR subunits or whether these changes are the effect of altered neurosteroid biosynthesis remains to be elucidated.
Figure 2.
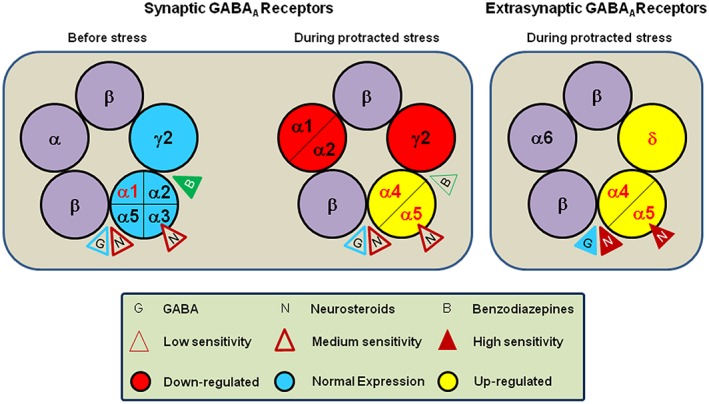
Schematic representation of the GABAAR. Structurally, GABAAR is a macromolecular complex composed by five subunits that delimit a central chloride‐permeable ion channel. The most expressed subunit configuration in the brain under basal condition includes two α, two β and one γ subunit (see figure on the left). GABA, by acting at two binding sites located between α and β subunits (Smith and Olsen, 1995; Baumann et al., 2002), induces the channel opening, allows the chloride ion influx, and, ultimately, permits the inhibitory action on cellular membranes. Moreover, the GABAAR has two different residues important for neurosteroid (e.g. allopregnanolone) action; the first is located between α and β subunits, and the second is formed by a cavity exclusively on α subunits (Hosie et al., 2006). The binding sites for benzodiazepine are located between α and γ subunits (Sigel, 2002). Importantly, the different pharmacological profiles of benzodiazepines depend on the specific α subunit included in the GABAAR. For example, the α1 subunits mediate the sedative, amnesic and partially anticonvulsant effects inducted by benzodiazepines (McKernan et al., 2000); the α2 subunits are responsible for the anxiolytic effects (Löw et al., 2000), and α3 subunits induce muscle relaxant and, partially, anxiolytic effects (Whiting, 1999). The α5 subunits are involved in the amnesic effects and tolerance induced by benzodiazepines, while GABAARs including α4 and α6 subunits are benzodiazepine‐insensitive (Wisden et al., 1991). Thus, different receptor configurations are associated to different pharmacological and functional properties. In particular, α/β/γ GABAAR subtype, the most prevalent configuration expressed in the synaptic membrane region (mediating inhibitory phasic currents), is highly sensitive to benzodiazepines, and, in parallel, shows lower sensitivity both to GABA and neurosteroids (Nusser and Mody, 2002). On the other hand, the α/β/δ GABAAR subtype, located in the extrasynaptic region (mediating inhibitory tonic currents), is not sensitive to benzodiazepines, and show low efficacy for GABA, and neurosteroids increase its agonist efficacy (Stell et al., 2003; Shu et al., 2012). The efficacy of neurosteroids is greatly enhanced for this receptor combination (Brown et al., 2002; Nusser and Mody, 2002; Wohlfarth et al., 2002) (see figure on the right). The physiological and pharmacological relevance of neurosteroid action on the different GABAAR subtypes has been studied in relation with stress and several neuropsychiatric disorders. Importantly, the expression of specific subunits of GABAARs changes after prolonged stress. For instance, the α4, α5 and δ subunit expression is markedly increased, and the α1, α2 and γ2 is significantly decreased, both in the frontal cortex and hippocampus of SI mice, a putative mouse model of PTSD (Pinna et al., 2006b; Pibiri et al., 2008). Moreover, these effects are strongly related to a decreased pharmacological response to benzodiazepines (Pinna et al., 2006b; Nin et al., 2011a). On the other hand, the altered GABAAR subunit composition, which results from protracted stress, favours high sensitivity for neurosteroids, neurosteroid‐like molecules (e.g. the synthetic allopregnanolone analogue, ganaxolone) or alcohol (Pinna et al., 2006b; Serra et al., 2006). Collectively, these findings suggest that treatments that stimulate allopregnanolone biosynthesis may offer a therapeutic advantage for stress‐induced psychiatric disorders, in which benzodiazepines are ineffective or in patients who show a poor or no response to SSRI antidepressants.
Altered expression and function of GABAARs were observed after ethanol exposure that changes Allo levels in different brain regions (Cagetti et al., 2003; Suryanarayanan et al., 2011; Lindemeyer et al., 2014). The expression of specific subunits of the GABAAR also changes following fluctuations in neurosteroid levels induced by oestrous cycle, pregnancy, after delivery or by treatment with synthetic steroids, including oral contraceptives and anabolic androgenic steroids (Concas et al., 1998; Maguire et al., 2005; Pibiri et al., 2006; Porcu et al., 2012; Modol et al., 2014; Locci et al., 2017). GABAARs containing α4 subunits are overexpressed during progesterone withdrawal (Smith et al., 1998) and after inhibition of 5α‐reductase, which decreases brain Allo levels. Also a switch in the expression of extrasynaptic δ subunits and synaptic γ2 GABAA subunits is observed across the oestrous cycle (Maguire et al., 2005) and in pregnancy (Concas et al., 1998; Sanna et al., 2009). Other findings suggest rapid, continuous and dynamic changes in the localization and distribution of synaptic and extrasynaptic GABAARs in the hippocampus (Jacob et al., 2008). Thus, in response to stress and hormonal state, dynamic changes in the expression of GABAARs and related alterations in GABAAR function and pharmacology mediated by inhibitory phasic versus tonic GABA currents is conceivable. Administration of molecules with high sensitivity for extrasynaptic receptors, like Allo, its analogues, or THIP – rather than administration of benzodiazepines – may be indicated when such switches in brain GABAAR subtypes occur. Such agents that target subunits mainly expressed in extrasynaptic GABAARs, in fact, show anxiolytic effects in SI mice, whereas benzodiazepines are ineffective (Pinna et al., 2006b; Nin et al., 2011a).
These converging findings suggest that mouse stress models with altered GABAAR subunit composition can be used to study drugs that target extrasynaptic GABAARs that have been up‐regulated in response to stress. Drugs acting at such receptors would be expected to alter GABAergic tonic inhibition in a manner that improves cognitive capacities, which are critical to the reprocessing of traumatic events and recovery from PTSD and depression.
Neurosteroid down‐regulation: From mouse models to psychiatric disorders
Deficits in neurosteroid levels are strongly associated with major depression and PTSD (for a review please see Zorumski et al., 2013). Patients with depression show serum, plasma, CSF, and brain reductions of Allo levels and/or biosynthesis (Romeo et al., 1998; Uzunova et al., 1998; van Broekhoven and Verkes, 2003; Agís‐Balboa et al., 2014; Porcu et al., 2016). Likewise, depression during pregnancy and postpartum is associated with altered Allo levels (Nemeroff, 2008). Progesterone levels are subjected to a rapid decline immediately after parturition; thus, progesterone (and Allo) ‘withdrawal’ may result in anxiety and depressive symptoms (Smith et al., 2007). Also, patients who take drugs that inhibit endogenous Allo levels, for example, 5α‐reductase inhibitor, finasteride, can exhibit cognitive dysfunction, depressive symptoms and suicidality (Altomare and Capella, 2002; Caruso et al., 2015; Welk et al., 2017). The concentration of Allo in the CSF is 40–60% lower in patients affected by unipolar major depression and premenopausal women with PTSD, respectively, with the lowest levels found in the PTSD patients with comorbid depression (Uzunova et al., 1998; Rasmusson et al., 2006). Chronic treatment with fluoxetine or fluvoxamine restored plasma and CSF Allo levels in association with improvements in depressive symptoms (Romeo et al., 1998; Uzunova et al., 1998). Thus, the normalization of Allo may mediate the anxiolytic and antidysphoric pharmacological effects of SSRIs. Patients with PMS show alterations in progesterone‐derived neurosteroid plasma levels during the luteal phase (Rapkin et al., 1997; Sundström et al., 1997; Bicíková et al., 1998; Girdler et al., 2001), and like depression, anxiety and mood swings in PMS are improved by increasing Allo concentrations (Bäckström et al., 2003). Smith et al. (1998) have suggested that PMS may be explained by progesterone withdrawal at the end of the luteal phase; however, symptoms are often present earlier in the luteal phase, suggesting that deficits in Allo synthesis, as observed among women with PTSD, may be responsible for such symptomatology.
Studies conducted on the oestrous cycle of female rats have demonstrated that the drastic decrease of progesterone concentrations during diestrous is associated with an overexpression of extrasynaptic α4β1δ GABAARs in the periaqueductal grey matter, which mediates the anxiolytic and mood regulating effects of Allo in this oestrous phase (Lovick, 2006). Increased anxiety‐like behaviour during the diestrous phase in rodents is prevented by short‐term non‐serotonergic low dose treatment with fluoxetine, which may counteract the rapid physiological fall in Allo (Lovick, 2013).
Brain levels of Allo are reduced in male rodent models of stress‐induced behavioural dysfunction, including the SI mouse (Pibiri et al., 2008) and SPS (Zhang et al., 2014), which are suitable preclinical translational approaches to model the behavioural alterations observed in patients affected by anxiety, depression and PTSD (Pinna, 2010; Nin et al., 2011b). These models allow investigations into the molecular mechanisms of GABAAR neurotransmission dysfunction that results from: (i) stress‐induced changes in GABAARs and (ii) the reduction of Allo brain concentrations (Pinna, 2010). In SI male mice, the reduction of Allo is a result of decreased expression of 5α‐reductase type I in the pyramidal‐like neurons of the basolateral amygdala, in the hippocampal CA3 pyramidal neurons and the glutamatergic granular cells of the dentate gyrus, as well as in cortical pyramidal neurons of layers V‐VI (Agís‐Balboa et al., 2007). The expression of the enzyme 3α‐HSD, as well as that of TSPO was not altered in SI mice (Dong et al., 2001).
This evidence thus suggests that social isolation in male mice specifically alters the p450 enzyme pathway involved in Allo biosynthesis. Allo biosynthesis was not affected by social isolation alone in female mice, unless they were ovariectomized and replaced with testosterone (Pinna et al., 2005). These findings are strikingly consistent with the sex specificity of the Allo synthetic pathway dysfunction in humans. In premenopausal women with PTSD, a synthesis blockade of Allo was found at 3α‐HSD (Rasmusson et al., 2006). New CSF data from trauma‐exposed men with and without PTSD reveals a PTSD‐related p450 enzyme blockade at 5α‐reductase (Rasmusson et al., 2016). Of note, Gillespie et al. (2013) reported an association between PTSD risk in males and a polymorphism in the 5α‐reductase 2 gene. Although focus in the field of neuropsychiatry has been, for the most part, on brain 5α‐reductase 1, 5α‐reductase 2 is highly expressed peripherally and specifically in the zona glomerulosa of the adrenal cortex (Eicheler et al., 1994), a possible significant source of another GABAergic neuroactive steroid that changes during stress and accesses the brain, THDOC (Purdy et al., 1991). Thus, the extent of involvement and manner by which deficient expression of 5α‐reductase 1 and maybe 2 contribute to neuropsychiatric disorders with a down‐regulation of Allo and other neurosteroid (i.e. THDOC) levels (e.g. in PTSD) are yet to be clarified.
To elucidate the hypothesis that Allo represents a putative biomarker for stress‐induced PTSD (Dong et al., 2001; Pinna, 2010), we established in male mice the impact of social isolation on reducing brain levels of Allo and on several emotional behaviours (Pibiri et al., 2008; Nelson and Pinna, 2011). These mice show an increase in aggressive behaviour, anxiety‐like behaviour and exaggerated contextual fear responses compared to group‐housed mice (Pibiri et al., 2008; Nin et al., 2011b). Interestingly, a down‐regulation of corticolimbic Allo levels associated with behavioural deficits, such as enhanced anxiety‐like behaviour and exaggerated contextual fear responses, were also observed in the SPS mouse model of PTSD. These PTSD‐like behaviours were improved by normalizing cortical Allo levels after stimulation of TSPO (Zhang et al., 2014, 2016).
Using SI mice, we similarly observed that systemic administration of Allo or equimolar concentrations of its analogue, ganaxolone reduces behavioural dysfunction related to corticolimbic Allo deficits (Pinna and Rasmusson, 2014). In addition, administration of SSRI antidepressants that at low doses, devoid of serotonergic mechanisms, act as selective brain steroidogenic stimulants (SBSSs) and up‐regulate Allo levels, improve behaviour in Allo deficient rodents, whereas it shows no steroidogenic effects in group‐housed mice (Pinna et al., 2003, 2009). While it is not completely clear why SSRI treatment is associated with low pharmacological response rate at doses that one would expect to enhance Allo levels, some speculations can be drawn. First, SSRI treatment resistant depression may not be a result of a simple serotonin deficit but rather by an excess of midbrain peri‐raphe serotonin and resulting deficits at the level of frontolimbic projection sites, which ultimately may compromise serotonin‐mediated neuroplasticity (Coplan et al., 2014). Second, it is also conceivable that too high doses of SSRIs may even mask beneficial drug effects that are often associated with severe side effects included in the spectrum of serotonin syndrome and that include agitation, mental confusion, increased reflexes, tremor, sweating and even seizures in the worst cases (Volpi‐Abadie et al., 2013; Coplan et al., 2014; Ferri, 2016). Third, the neurosteroidogenic effect of these drugs at low doses that correlate with improved behaviour (Pinna et al., 2003, 2004; Uzunova et al., 2006; Lovick, 2013) makes one wonder whether 5‐HT reuptake inhibition may even be necessary or sufficient to explain SSRIs pharmacological action to improve dysphoria, anxiety and other mood disorders in depressed patients (reviewed in Pinna et al., 2006a, 2009). Finally, it is important to elucidate in clinical trials whether neurosteroidogenic low SSRIs doses offer a pharmacological advantage over large SSRIs doses in the treatment of PTSD and depression. Due to lack of controlled clinical trials to resolve this issue, these findings have inspired studies in which low, non‐serotonergic but neurosteroidogenic doses of fluoxetine have been suggested for the treatment of PMS (Lovick, 2013). Moreover, administration of low fluoxetine doses may be valuable to treat depression in teens, as it may avoid the increased rate of suicide observed when depressed adolescents are administered higher serotonergic doses of SSRIs (Rahn et al., 2015).
Resistance to currently prescribed antidepressant drugs
Mental and substance use disorders are major contributors to the global burden of disease (Whiteford et al., 2013), and major depressive disorders represent a public problem affecting approximately 16% of adults in the United States. Several SSRIs and other antidepressants can counteract depressive symptoms. However, in spite of the rapid evolution of pharmacological therapies, epidemiological evidence demonstrates that only 50% of treated depressed patients respond positively to first‐line therapy with antidepressants, while more than one third develop resistance to antidepressants (Kemp et al., 2008). Moreover, half of patients treated with SSRI antidepressants fail to reach a full remission (Golden et al., 2002; Rush et al., 2006; Kemp et al., 2008). The causes of resistance to antidepressants can be multiple and include pharmacokinetic factors or comorbidity with other mental disorders (El‐Hage et al., 2013; Willner et al., 2013). Another major negative aspect of these therapies is their delayed onset of therapeutic action (4–6 weeks) (Belzung, 2014).
PTSD is another prevalent disorder with no specific treatment. The efficacy of the SSRIs, sertraline and paroxetine, the only drugs currently approved by the FDA for the treatment of PTSD, rarely exceed 40–60%; further, only 20–30% of SSRI‐treated PTSD patients do not relapse. Exposure to combat situations results in a particularly severe form of PTSD that tends to be chronic, disabling and heavily comorbid with depression and suicide (Prigerson et al., 2001). Veterans affected by PTSD are generally resistant to SSRI therapy (van der Kolk et al., 1994), leaving limited available options for their treatment. Studies suggest that certain types of trauma or traumas in a crucial period of an individual's life can predict which subpopulation of PTSD and/or depressed patients will respond successfully to treatment with SSRI antidepressants. Recent studies in the field have found, for instance, that a history of early‐life trauma may predict a failure to respond to antidepressants during adulthood. Williams and collaborators, as part of the international Study to Predict Optimized Treatment for Depression, a randomized clinical trial with enrolment from December 2008 to January 2012 at eight academic and nine private clinical settings in five countries, have conducted a study to establish the role of early‐life trauma as a predictor of acute response to antidepressants. They found that exposure to several types of traumatic events before the age of 18 (overall trauma ‘load’ and specific type of abuse) was significantly more likely to result in major depression. Abuse in general but – most notably – abuse occurring at 7 years of age or younger predicted a lower response to 8 weeks of SSRI antidepressants. Hence, specific types of early‐life trauma and in particular physical, emotional and sexual abuse when they occurred at 7 years of age or before may dictate a patient's future response to antidepressant therapy (Williams et al., 2016).
High resistance to SSRIs invites the development of new approaches for the treatment of non‐responsive patients. In preclinical studies of stress‐induced PTSD and depression, it would be important to determine variability among animals or groups that are more responsive from others that are less responsive to the beneficial effects of SSRIs. The non‐responders or less responsive may offer an important subpopulation in which to explore new pharmacological agents that may improve behaviour acting at different neurosteroidogenic receptors (e.g. TSPO and cannabinoid receptors) than SSRIs'. These animals may also be studied using agents that may act directly and selectively at specific GABAAR subunits (e.g. selective agonists for α4 or α5), given that stress appears to up‐regulate these subunits.
Neurosteroidogenesis: A putative target for development of new pharmacotherapies
Numerous preclinical studies have suggested a number of brain neurosteroidogenic targets for new anxiolytic and PTSD treatments (Rupprecht et al., 2009; Pinna, 2014). One of the best characterized is probably the TSPO (Costa et al., 1994; Papadopoulos et al., 2006), that, according to traditional views, serves as the upstream starting point for the neurosteroidogenic cascade. Transfer of cholesterol from the outer to the inner mitochondrial membrane occurs through the ‘transduceosome’ complex. This requires the cytosolic StAR to interact with the outer mitochondrial membrane proteins, TSPO, and the voltage‐dependent anion channel (VDAC). Cholesterol transfer occurs at contact sites that are peculiar for bridging the membranes of VDAC and inner mitochondrial membrane adenine nucleotide translocase. Immunoblot and mass spectrometry analyses revealed that the 800‐kDa complex is responsible for steroid production. The 800‐kDa complex offers the micro‐environment needed for cytochrome P450 enzyme CYP11A1 activity and for which the StAR mobilizes the cholesterol bound at the 800‐kDa complex, which results in increased steroidogenesis. Thus, a multimeric protein complex, which extends from the outer to the inner mitochondrial membrane is implicated for the hormone‐induced import, segregation, targeting, and, finally, metabolism of cholesterol (Rone et al., 2012).
New molecules that bind and potently stimulate TSPO are able to induce a downstream increase in Allo concentrations in mouse hippocampus and cortex. TSPO activating drugs, such as AC‐5216/XBD173 and etifoxine are able to exert significant anxiolytic effect in animal models of PTSD (Rupprecht et al., 2010). Importantly, the behavioural effects induced by these drugs seem to be directly related to increases in brain Allo levels; indeed, pretreatment with finasteride drastically suppresses their anxiolytic effect (Schüle et al., 2011). These findings have been demonstrated also in studies conducted with anxious patients (Rupprecht et al., 2010; Schüle et al., 2011). Chronic administration with YA‐IPA08, a selective TSPO activator, induced a marked reduction of anxiety and contextual fear resulting from exposure to the SPS, an effect inhibited by pre‐injection with the TSPO antagonist, PK11195 (Zhang et al., 2014). In addition, the administration of YA‐IPA08 normalized Allo levels in the prefrontal cortex and serum of PTSD‐mice in whom Allo levels were decreased by SPS exposure, an effect that was completely blocked by PK11195 (Zhang et al., 2014).
The PXR represents another neurosteroidogenic target with potential to modulate emotional behaviour. PXR is a ubiquitous nuclear receptor that acts as a classical transcription factor that enhances the expression of several major families of genes such as the cytochrome p450 enzymes (Frye et al., 2014a). PXR mediates the production or metabolism of various neurobiological factors (Kliewer et al., 2002). The expression of PXR changes during the oestrous cycle in the adult female rat; indeed, the abundance of both PXR mRNA and protein is higher during proestrous than in diestrous 1 or in male rats (Frye et al., 2012). A recent study has demonstrated that infusion of the PXR antisense oligonucleotides (ODNs) in the VTA, a brain area in which this receptor is highly expressed, induces a drastic reduction of time spent in the open arms of the elevated plus maze; of note, infusion of the antisense ODNs targeted against PXR outside the VTA does not produce the same effect. Thus, specific down‐regulation of the activity of PXR in the VTA is associated with an increase in anxiety‐like behaviour (Frye et al., 2012). Interestingly, Allo binds directly at PXR. In addition, antagonizing TSPO with PK11195, which significantly decreases Allo in the VTA, inhibits sexual behaviour, an effect reversed by Allo administration, but not by Allo co‐administered with PXR antisense ODNs (Frye et al., 2014b). This evidence suggests that PXR, acting upstream of TSPO, may modulate neurosteroid‐mediated behavioural effects.
The endocannabinoid system is a newly discovered target for stimulation of neurosteroidogenesis. Δ9‐tetrahydrocannabinol, the most important psychotropic compound found in Cannabis sativa, markedly increases the synthesis of pregnenolone by the direct activation of the cannabinoid receptor type 1 (CB1) (Vallée et al., 2014; Vallée, 2016). There are several similarities between the endocannabinoid and neurosteroid systems. Levels of both Allo and anandamide (AEA) are decreased in a variety of animal models of anxiety and depression and in patients with depression and PTSD (Romeo et al., 1998; Uzunova et al., 1998; van Broekhoven and Verkes, 2003; Rasmusson et al., 2006; Hill et al., 2008, 2013). Expression of CB1 receptors is high in targets for neurosteroidogenesis (e.g. pyramidal neurons) in brain areas involved in emotional behaviours such as hippocampus, frontal cortex and amygdala (Katona, 2009). The role of the endocannabinoid system in the modulation of emotional behaviours, and specifically in the attenuation of exaggerated fear responses, is underscored by the extensive literature addressing the therapeutic effects of AEA or other CB1 agonists (Lisboa et al., 2010; Jenniches et al., 2016). Moreover, recent studies have demonstrated that cannabidiol, another main active compound of cannabis, blocks reconsolidation of recent and older contextual fear memories by a CB1 receptor‐mediated mechanism (Stern et al., 2012). Similar effects were observed when reconsolidation of reactivated memories was blocked with midazolam, a benzodiazepine that, importantly, acts at TSPO to induce Allo biosynthesis (Stern et al., 2012). The endocannabinoid, N‐palmitoylethanolamide (PEA) also stimulates Allo biosynthesis by binding to intracellular PPARα, a class of nuclear hormone receptor involved in various cellular and molecular mechanisms (Sasso et al., 2012). Administration of PEA induces antidepressant effects in a similar manner to the SSRI/SBSS, fluoxetine (Yu et al., 2011). This evidence thus highlights the existence of a fine network of relationships between the neurosteroid and the endocannabinoid systems; and the development of cannabinoid‐like molecules with neurosteroidogenic effects could lead to new depression, anxiety and PTSD therapeutics.
Finally, in patients for whom the administration of an SBSS (i.e. a molecule that induces steroidogenesis) is ineffective because neurosteroidogenesis is greatly impaired, an alternative therapeutic strategy may be the use of Allo ‘substitutes’ (Gulinello et al., 2003), such as ganaxolone, or other molecules such as topiramate that directly activate extrasynaptic GABAARs (Berlant, 2004). For example, treatment with pregnenolone appears to have the potential to improve emotional regulation by increasing Allo levels. Sripada et al. (2013) showed that intravenous administration of pregnenolone reduced activation of brain areas involved in negative emotions and increased activity in the dorsal medial prefrontal cortex (dMPFC). It also enhanced connectivity between the amygdala and dMPFC in association with reduced self‐reported anxiety and improved performance on an emotion regulation task. Remarkably, activities such as brief but vigorous acute exercise has been shown to raise Allo levels among responsive individuals (Scioli‐Salter et al., 2015) to levels shown by Sripada et al. (2013) to enhance amygdala‐prefrontal cortical coupling in association with improved emotion regulation. After contextual fear conditioning in SI mice, a single dose of ganaxolone (an Allo analogue) administered after the first session of extinction training steeply and significantly reduced behavioural freezing on the following day. Freezing remained low in these mice over the remaining extinction training sessions and did not return via ‘spontaneous recovery’ after the passage of time, as it did in vehicle‐treated rodents that extinguished slowly to the same low freezing endpoint (Pinna and Rasmusson, 2014). This study suggests that Allo administered to Allo‐deficient mice prevents reconsolidation of fear and/or enhances extinction retention. Hence, the use of Allo or synthetic analogues of Allo, acting preferentially at extrasynaptic GABAARs, may represent a valid means of augmenting exposure based cognitive treatments for PTSD. Remarkably, a rapid remission of post‐partum depression achieved at 60 h and mainted at 30 day follow‐up was recently induced in 70% of patients treated with a two‐day course of intravenous Allo (SAGE‐547) compared to 10% who received placebo (Herper, 2016; Kanes et al., 2016; Meltzer‐Brody 2016).
Together this evidence suggests that alterations in Allo biosynthesis and changes in GABAAR subunit expression contribute to disorders of mood and cognition during stress and changes in reproductive state. Behaviour can be improved by administration of neurosteroids or drugs that may stimulate neurosteroidogenesis.
Emerging therapeutic role of sulphated pregnane steroids in neuropsychiatric disorders
Upon its production, following the translocation of cholesterol into the inner mitochondrial membrane, pregnenolone can be further converted into a number of pregnane steroids that ultimately produce Allo. It can be also converted into PES by sulphation of the C3 hydroxyl group by the enzymes sulphotransferase and sulphatase, which are expressed in various rodent and human brain structures. PES is expressed in the human and rodent brain and serum and its concentrations reach levels in the nanomolar range, (Reviewed by Gibbs et al., 2006; Smith et al., 2014). Whether these concentrations are consistent with a neurophysiological role for this neurosteroid as a putative endogenous neuromodulator remains a matter of debate and future studies are required to carefully examine this issue. Micromolar concentrations of PES negatively modulate GABAAR, and based on receptor subunit composition, PES negatively or positively affects NMDA receptor‐mediated neurotransmission (Majewska et al., 1988; Jo et al., 1989; Wu et al., 1991; Malayev et al., 2002; Smith et al., 2014). PES preferentially potentiates NMDA receptors containing NR2A and NR2B subunits, whereas it elicits a negative modulation of NR2C and NR2D‐containing receptors (Malayev et al., 2002). Importantly, PES shows affinity for an inhibitory site, which likely is also a binding site for other sulphated pregnane steroids (e.g. PAS) within the NMDA receptors (Horak et al., 2006). Recent investigation on the role of PES at glutamate receptors has established its allosteric inhibitory modulation at AMPA receptors by binding both GluA2 ATD and S1S2 domains (Cameron et al., 2012). PES binding at the GluA2 subunit suggests a different binding domain from that of other sulphated neurosteroids. By such mechanisms, pharmacologically induced PES levels play an important neuromodulatory role at glutamate receptors in nanomolar to low micromolar concentrations. These are consistent with an agonist influence on LTP and memory. PES has reportedly showed neuroprotective effects on dentate gyrus granular cells (Yang et al., 2012), enhances neurite outgrowth, reverses Aβ‐induced toxicity, improves spatial memory (Xu et al., 2012) and decreases immobility time in the tail suspension test (Dhir and Kulkarni, 2008). The effects of PES as a cognitive enhancer underline its therapeutic role in improving cognition deficits in schizophrenia and depression (Marx et al., 2009).
Importantly, the inhibitory role at NMDA receptors has recently emerged for the endogenous neuroactive steroid, PAS. PAS has been shown to accumulate in plasma membranes, which provides the preferential route by which this neurosteroid accesses its binding sites located in NMDA receptors (Borovska et al., 2012). PAS is particularly potent at inhibiting tonic rather than synaptically activated NMDA receptors. Synaptic activation of NMDA receptors is important for synaptic plasticity, learning and memory, and synaptogenesis, whereas tonic NMDA receptor activation is generally associated with excitotoxicity. Consistently, PAS's negative regulation of tonic NMDA neurotransmission has recently been reported to provide neuroprotection in vivo in a manner that is devoid of unwanted psychotomimetic effects (Vyklicky et al., 2016). This feature is highly relevant for developing a novel class of steroid‐based NMDA‐inhibitor therapeutics, which lack the side effects that plague the use of classical NMDA receptor inhibitors, including ketamine and congeners. Based on this mechanism of action, a number of PAS analogues have been recently synthesized, including the amide‐based analogues (Adla et al., 2016) and pregnanolone hemipimelate that has no action at phasic and synaptic NMDA receptors but shows a strong selectivity (several folds higher than PAS) in inhibiting tonic‐activated receptors (Vyklicky et al., 2016).
The question of whether PES and even PAS or AlloS reach neurophysiologically significant brain levels has been matter of recent debate and Smith et al. (2014) examined this issue in terms of supporting a role for these sulphated steroids as endogenous neuromodulators. Precise quantification of PES has been difficult because of the methodology used in the past, which yielded a wide range of concentrations in the serum and brain. In the hippocampus, for example, in studies employing a direct detection by HPLC–MS/MS, nano‐LC‐ES or LC–MS, concentrations varied from under the threshold of detection and the low concentrations of ~50 pM to 26 000 pM (i.e. 10.25 ng·g−1) (Liu et al., 2003; Jäntti et al., 2010; Rustichelli et al., 2013; reviewed in Smith et al., 2014). Other methods including enzyme‐linked immunoassorbent assay, avoiding solvolysis, found brain concentrations less than 0.15 ng·g−1 (Higashi et al., 2003). In our laboratory we have established methods for the serum, plasma, saliva and brain quantification of neuroactive steroids (Uzunov et al., 1996; Pinna et al., 2000). Determination of sulphated steroids is performed indirectly after solvolysis, HPLC purification and separation of the steroids, which is followed by HFBA derivatization and gas‐chromatography MS (GC–MS) quantification after adding deuterated internal standard for the steroids examined. We found that the mouse frontal cortex concentrations of PES, DHEAS, PAS, and AlloS were in the low ng/g range (2–20 ng·g−1). Whereas PES levels were about 25% higher than pregnenolone levels in the frontal cortex, DHEAS, PAS and AlloS concentrations appeared to be 4, 2.5 and 1.5 times higher than those of the non‐sulphated parental neuroactive steroids, respectively. Hence, endogenous PAS, DHEAS, PES and AlloS are highly measurable in the mouse frontal cortex. However, it remains to be clarified whether this expression level reaches neurophysiologically relevant concentrations to modulate NMDA receptor neurotransmission and by doing so, whether these sulphated neuroactive steroids are capable of improving cognitive functions in mouse models of neuropsychopathologies. Given that pregnenolone is preferentially metabolized into Allo and its parental neurosteroids or is converted into a sulphated class of steroids, the upstream activation of neurosteroidogenesis by targeting PXR, PPARα and CB1 receptors by increasing pregnenolone levels may also give rise to increased levels of PES, PAS and AlloS. This mechanism of pregnenolone's metabolism may have an impact on NMDA receptor neurotransmission. Altogether, we can draw a picture by which Allo and pregnanolone on the one hand and their sulphated congeners on the other may work in concert, by enhancing tonic inhibition with activation of extrasynaptic GABAAR (Allo and PA) or by inhibiting tonic NMDA receptor neurotransmission (sulphated congeners), which may have an important role in improving cognitive and emotional deficits in various neuropsychiatric disorders. Research on the levels and biosynthetic regulation of PES, AlloS, and PAS during stress‐induced neuropathologies and in rodent stress models may be helpful to understand new neuronal targets by which these neuroactive steroids can be elevated to improve symptoms.
Conclusions
Stress‐induced down‐regulation of Allo biosynthesis and changes in GABAAR subtypes that create a receptor conformation with higher sensitivity to Allo invite the consideration of low Allo levels or enzymatic blocks in the Allo synthetic pathway as putative biomarkers for PTSD. The advantage of Allo biosynthesis as a biomarker for stress‐induced disorders is that it can be strategically targeted by addressing several neurosteroidogenic mechanisms, thus limiting the odds of an unsuccessful treatment. It is plausible that neuroactive steroids themselves or drugs that stimulate neurosteroid biosynthesis upstream offer a valid therapeutic alternative for counteracting or even correcting biochemical alterations related to PTSD and depressive symptoms. This therapeutic approach may be a useful option for patients who show: (i) compromised Allo biosynthesis, (ii) altered GABAergic neurotransmission, and (iii) relative resistance and/or the inability to respond to SSRI treatment. Perhaps then, diagnosis and therapeutic approaches could be guided by pretreatment verification of Allo levels, or identification of Allo biosynthesis blocks and changes in GABAAR subunit expression as biomarkers of GABAergic function. Recognizing such dysfunction may not only increase treatment efficacy, but also prevent relapse – or even potentially prevent development of these stress‐related disorders in the first place.
The use of pregnenolone or drugs that stimulate its levels is emerging as a key therapeutic approach that may originate both a downstream increase of PA and Allo, which by stimulation of GABAAR may improve emotional behaviour, and at the same time may increase the sulphated forms of these steroids, including PAS and AlloS, that by inhibiting NMDA receptor tonic neurotransmission may provide neuroprotection and cognitive benefits in neuropsychiatric disorders.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a,b,c,d,e).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This review was supported by the United States Department of Defense Grant W81XWH‐15‐1‐0521 and Veteran Affairs Grant VA241‐15‐D‐0041 (to G. Pinna).
Locci, A. , and Pinna, G. (2017) Neurosteroid biosynthesis down‐regulation and changes in GABAA receptor subunit composition: a biomarker axis in stress‐induced cognitive and emotional impairment. British Journal of Pharmacology, 174: 3226–3241. doi: 10.1111/bph.13843.
References
- Acosta GB, Otero Losada ME, Rubio MC (1993). Area‐dependent changes in GABAergic function after acute and chronic cold stress. Neurosci Lett 154: 175–178. [DOI] [PubMed] [Google Scholar]
- Adla SK, Slavikova B, Smidkova M, Tloustova E, Svoboda M, Vyklicky V et al. (2016). Physicochemical and biological properties of novel amide‐based steroidal inhibitors of NMDA receptors. Steroids pii: S0039‐128X (16) 30106‐4. [DOI] [PubMed] [Google Scholar]
- Agís‐Balboa RC, Guidotti A, Pinna G (2014). 5α‐reductase type I expression is downregulated in the prefrontal cortex/Brodmann's area 9 (BA9) of depressed patients. Psychopharmacology (Berl) 231: 3569–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agís‐Balboa RC, Pinna G, Pibiri F, Kadriu B, Costa E, Guidotti A (2007). Down‐regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation‐induced behavior in mice. Proc Natl Acad Sci U S A 104: 18736–18741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agís‐Balboa RC, Pinna G, Zhubi A, Maloku E, Costa E, Guidotti A (2006). Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci U S A 103: 14602–14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Bracamontes J, Steinbach JH (2001). Pregnenolone sulfate block of GABA(A) receptors: mechanism and involvement of a residue in the M2 region of the alpha subunit. J Physiol 532: 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Bracamontes JR, Covey DF, Evers A, Dao T, Steinbach JH (2004). Neuroactive steroids have multiple actions to potentiate GABAA receptors. J Physiol 558 (Pt 1): 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015d). The concise guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015e). The concise guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015a). The concise guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015b). The concise guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E et al. (2015c). The concise guide to PHARMACOLOGY 2015/16: Ligand‐gated ion channels. Br J Pharmacol 172: 5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altomare G, Capella GL (2002). Depression circumstantially related to the administration of finasteride for androgenetic alopecia. J Dermatol 29: 665–669. [DOI] [PubMed] [Google Scholar]
- Bäckström T, Andreen L, Birzniece V, Björn I, Johansson IM, Nordenstam‐Haghjo M et al. (2003). The role of hormones and hormonal treatments in premenstrual syndrome. C N S Drugs 17: 325–342. [DOI] [PubMed] [Google Scholar]
- Baulieu EE, Robel P (1990). Neurosteroids: a new brain function? J Steroid Biochem Mol Biol 37: 395–403. [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E (2002). Forced subunit assembly in alpha1beta2gamma2 GABAA receptors. Insight into the absolute arrangement. J Biol Chem 277: 46020–46025. [DOI] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW (2009). Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci 29: 12757–12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ (2005). Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci 6: 565–575. [DOI] [PubMed] [Google Scholar]
- Belzung C (2014). Innovative drugs to treat depression: did animal models fail to be predictive or did clinical trials fail to detect effects? Neuropsychopharmacology 39: 1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Subburaju S, Walsh JP (2008). Circuitry‐based gene expression profiles in GABA cells of the trisynaptic pathway in schizophrenics versus bipolars. Proc Natl Acad Sci U S A 105: 20935–20940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlant JL (2004). Prospective open‐label study of add‐on and monotherapy topiramate in civilians with chronic nonhallucinatory posttraumatic stress disorder. B M C Psychiatry 4: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicíková M, Dibbelt L, Hill M, Hampl R, Stárka L (1998). Allopregnanolone in women with premenstrual syndrome. Horm Metab Res 30: 227–230. [DOI] [PubMed] [Google Scholar]
- Block SG, Nemeroff CB (2014). Emerging antidepressants to treat major depressive disorder. Asian J Psychiatr 12: 7–16. [DOI] [PubMed] [Google Scholar]
- Bonin RP, Martin LJ, MacDonald JF, Orser BA (2007). Alpha5GABAA receptors regulate the intrinsic excitability of mouse hippocampal pyramidal neurons. J Neurophysiol 98: 2244–2254. [DOI] [PubMed] [Google Scholar]
- Borovska J, Vyklicky V, Stastna E, Kapras V, Slavikova B, Horak M et al. (2012). Access of inhibitory neurosteroids to the NMDA receptor. Br J Pharmacol 166: 1069–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Innis RB, Southwick SM, Staib L, Zoghbi S, Charney DS (2000). Decreased benzodiazepine receptor binding in prefrontal cortex in combat‐related posttraumatic stress disorder. Am J Psychiatry 157: 1120–1126. [DOI] [PubMed] [Google Scholar]
- van Broekhoven F, Verkes RJ (2003). Neurosteroids in depression: a review. Psychopharmacology (Berl) 165: 97–110. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA (2002). Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol 136: 965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW (2003). Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol 63: 53–64. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Pinna G, Guidotti A, Baicy K, Olsen RW (2004). Chronic intermittent ethanol (CIE) administration in rats decreases levels of neurosteroids in hippocampus, accompanied by altered behavioral responses to neurosteroids and memory function. Neuropharmacology 46: 570–579. [DOI] [PubMed] [Google Scholar]
- Cameron K, Bartle E, Roark R, Fanelli D, Pham M, Pollard B et al. (2012). Neurosteroid binding to the amino terminal and glutamate binding domains of ionotropic glutamate receptors. Steroids 77: 774–779. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You‐Ten KE, Cheng VY, Belelli D, Newell JG et al. (2004). Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit‐containing gamma‐aminobutyric acid type A receptors. Proc Natl Acad Sci U S A 101: 3662–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso D, Abbiati F, Giatti S, Romano S, Fusco L, Cavaletti G et al. (2015). Patients treated for male pattern hair with finasteride show, after discontinuation of the drug, altered levels of neuroactive steroids in cerebrospinal fluid and plasma. J Steroid Biochem Mol Biol 146: 74–79. [DOI] [PubMed] [Google Scholar]
- Carver CM, Reddy DS (2013). Neurosteroid interactions with synaptic and extrasynaptic GABA(A) receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology (Berl) 230: 151–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP et al. (2005). Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A 102: 15653–15658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M et al. (1998). Role of brain allopregnanolone in the plasticity of gamma‐aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci U S A 95: 13284–13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JB, Dumitru AM, O'Buckley TK, Morrow AL (2014). Ethanol administration produces divergent changes in GABAergic neuroactive steroid immunohistochemistry in the rat brain. Alcohol Clin Exp Res 38: 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan JD, Gopinath S, Abdallah CG, Berry BR (2014). A neurobiological hypothesis of treatment‐resistant depression – mechanisms for selective serotonin reuptake inhibitor non‐efficacy. Front Behav Neurosci 8: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E, Auta J, Guidotti A, Korneyev A, Romeo E (1994). The pharmacology of neurosteroidogenesis. J Steroid Biochem Mol Biol 49: 385–389. [DOI] [PubMed] [Google Scholar]
- Costa E, Guidotti A (1991). Diazepam binding inhibitor (DBI): a peptide with multiple biological actions. Life Sci 49: 325–344. [DOI] [PubMed] [Google Scholar]
- Costa E, Guidotti A (1996). Benzodiazepines on trial: a research strategy for their rehabilitation. Trends Pharmacol Sci 17: 192–200. [DOI] [PubMed] [Google Scholar]
- Deprez F, Vogt F, Floriou‐Servou A, Lafourcade C, Rudolph U, Tyagarajan SK et al. (2016). Partial inactivation of GABAA receptors containing the α5 subunit affects the development of adult‐born dentate gyrus granule cells. Eur J Neurosci 44: 2258–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir A, Kulkarni S (2008). Involvement of sigma (sigma1) receptors in modulating the anti‐depressant effect of neurosteroids (dehydroepiandrosterone or pregnenolone) in mouse tail‐suspension test. J Psychopharmacol 22: 691–696. [DOI] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H et al. (2001). Brain 5alpha‐dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci U S A 98: 2849–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicheler W, Tuohimaa P, Vilja P, Adermann K, Forssmann WG, Aumüller G (1994). Immunocytochemical localization of human 5 alpha‐reductase 2 with polyclonal antibodies in androgen target and non‐target human tissues. J Histochem Cytochem 42: 667–675. [DOI] [PubMed] [Google Scholar]
- Eisenman LN, He Y, Fields C, Zorumski CF, Mennerick S (2003). Activation‐dependent properties of pregnenolone sulfate inhibition of GABAA receptor‐mediated current. J Physiol 550: 679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Hage W, Leman S, Camus V, Belzung C (2013). Mechanisms of antidepressant resistance. Front Pharmacol 4: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin E, Zarnowska ED, Benke D, Tsvetkov E, Sigal M, Keist R et al. (2015). Tonic inhibitory control of dentate gyrus granule cells by α5‐containing GABAA receptors reduces memory interference. J Neurosci 35: 13698–13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Lüscher B (1998). Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci 1: 563–571. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z (2005). Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6: 215–229. [DOI] [PubMed] [Google Scholar]
- Fava M, Memisoglu A, Thase ME, Bodkin JA, Trivedi MH, de Somer M et al. (2016). Opioid modulation with buprenorphine/samidorphan as adjunctive treatment for inadequate response to antidepressants: a randomized double‐blind placebo‐controlled trial. Am J Psychiatry 173: 499–508. [DOI] [PubMed] [Google Scholar]
- Ferri FF (2016). Ferri's clinical advisor 2017: 5 Books in 1. Elsevier Health Sciences : 1154–1155 ISBN 9780323448383. [Google Scholar]
- Fritschy JM, Panzanelli P (2014). GABAA receptors and plasticity of inhibitory neurotransmission in the central nervous system. Eur J Neurosci 39: 1845–1865. [DOI] [PubMed] [Google Scholar]
- Frye CA, Koonce CJ, Walf AA (2014a). Novel receptor targets for production and action of allopregnanolone in the central nervous system: a focus on pregnane xenobiotic receptor. Front Cell Neurosci 8: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Koonce CJ, Walf AA (2014b). The pregnane xenobiotic receptor, a prominent liver factor, has actions in the midbrain for neurosteroid synthesis and behavioral/neural plasticity of female rats. Front Syst Neurosci 8: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Walf AA, Rusconi JC (2012). Effects and mechanisms of 3α,5α,‐THP on emotion, motivation, and reward functions involving pregnane xenobiotic receptor. Front Neurosci 5: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze E, van Berckel BN, Lammertsma AA, Boellaard R, de Kloet CS, Vermetten E et al. (2008). Reduced GABAA benzodiazepine receptor binding in veterans with post‐traumatic stress disorder. Mol Psychiatry 13: 74–83 3. [DOI] [PubMed] [Google Scholar]
- Gibbs TT, Russek SJ, Farb DH (2006). Sulfated steroids as endogenous neuromodulators. Pharmacol Biochem Behav 84: 555–567. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Almli LM, Smith AK, Bradley B, Kerley K, Crain DF et al. (2013). Sex dependent influence of a functional polymorphism in steroid 5‐α‐reductase type 2 (SRD5A2) on post‐traumatic stress symptoms. Am J Med Genet B Neuropsychiatr Genet 162B: 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL (2001). Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry 49: 788–797. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I (2008). Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci 28: 1421–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I (2006). Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABA A receptor alpha5 subunit‐deficient mice. J Neurophysiol 95: 2796–2807. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I (2007). The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol 582 (Pt 3): 1163–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden RN, Nemeroff CB, McSorley P, Pitts CD, Dubé EM (2002). Efficacy and tolerability of controlled‐release and immediate‐release paroxetine in the treatment of depression. J Clin Psychiatry 63: 577–584. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Smith SS (2003). Progesterone withdrawal increases the anxiolytic actions of gaboxadol: role of alpha4betadelta GABA(A) receptors. Neuroreport 14: 43–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Nusser Z, Rancz EA, Freund TF, Mody I (2000). Cell type‐ and synapse‐specific variability in synaptic GABAA receptor occupancy. Eur J Neurosci 12: 810–818. [DOI] [PubMed] [Google Scholar]
- Herd MB, Foister N, Chandra D, Peden DR, Homanics GE, Brown VJ et al. (2013). Inhibition of thalamic excitability by 4,5,6,7‐tetrahydroisoxazolo[4,5‐c]pyridine‐3‐ol: a selective role for delta‐GABA(A) receptors. Eur J Neurosci 29: 1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herper (2016). Experimental drug eliminates postpartum depression in 7 out of 10 patients, Forbes. Available at: http://www.forbes.com/sites/matthewherper/2016/07/12/experimental‐drug‐dramatically‐reduces‐post‐partum‐depression‐in‐small‐study/#4f6f8eab7aa3 (accessed 2017).
- Higashi T, Sugitani H, Yagi T, Shimada K (2003). Studies on neurosteroids XVI. Levels of pregnenolone sulfate in rat brains determined by enzyme‐linked immunosorbent assay not requiring solvolysis. Biol Pharm Bull 26: 709–711. [DOI] [PubMed] [Google Scholar]
- Hill MN, Bierer LM, Makotkine I, Golier JA, Galea S, McEwen BS et al. (2013). Reductions in circulating endocannabinoid levels in individuals with post‐traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology 38: 2952–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Miller GE, Ho WS, Gorzalka BB, Hillard CJ (2008). Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry 41: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak M, Vlcek K, Chodounska H, Vyklicky L Jr (2006). Subtype‐dependence of N‐methyl‐D‐aspartate receptor modulation by pregnenolone sulfate. Neuroscience 137: 93–102. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG (2006). Endogenous neurosteroids regulate GABAA receptors through two discrete transmem brane sites. Nature 444: 486–489. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R (2008). GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci 9: 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäntti SE, Tammimäki A, Raattamaa H, Piepponen P, Kostiainen R, Ketola RA (2010). Determination of steroids and their intact glucuronide conjugates in mouse brain by capillary liquid chromatography‐tandem mass spectrometry. Anal Chem 82: 3168–3175. [DOI] [PubMed] [Google Scholar]
- Jenniches I, Ternes S, Albayram O, Otte DM, Bach K, Bindila L et al. (2016). Anxiety, stress, and fear response in mice with reduced endocannabinoid levels. Biol Psychiatry 79: 858–868. [DOI] [PubMed] [Google Scholar]
- Jo DH, Abdallah MA, Young J, Baulieu EE, Robel P (1989). Pregnenolone, dehydroepiandrosterone, and their sulfate and fatty acid esters in the rat brain. Steroids 54: 287–297. [DOI] [PubMed] [Google Scholar]
- Kanes S, Colquhoun H, Gunduz‐Bruce H, Doherty J, Jonas J, Rubinow D et al. (2016). SAGE‐547 for the treatment of severe postpartum depression. Neuropsychopharmacology 41: S76 https://doi.org/10.1038/npp.2016.240. [Google Scholar]
- Katona I (2009). Endocannabinoid receptors: CNS localization of the CB1 cannabinoid receptor. Curr Top Behav Neurosci 1: 65–86. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Gordon E, Rush AJ, Williams LM (2008). Improving the prediction of treatment response in depression: integration of clinical, cognitive, psychophysiological, neuroimaging, and genetic measures. C N S Spectr 13: 1066–1086. [DOI] [PubMed] [Google Scholar]
- Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, ffrench‐Mullen J et al. (2009). Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol Psychiatry 14: 175–189. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Goodwin B, Willson TM (2002). The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev 23: 687–702. [DOI] [PubMed] [Google Scholar]
- Koh MT, Rosenzweig‐Lipson S, Gallagher M (2013). Selective GABA(A) α5 positive allosteric modulators improve cognitive function in aged rats with memory impairment. Neuropharmacology 64: 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kolk BA, Dreyfuss D, Michaels M, Shera D, Berkowitz R, Fisler R et al. (1994). Fluoxetine in posttraumatic stress disorder. J Clin Psychiatry 55: 517–522. [PubMed] [Google Scholar]
- Lindemeyer AK, Liang J, Marty VN, Meyer EM, Suryanarayanan A, Olsen RW et al. (2014). Ethanol‐induced plasticity of GABAA receptors in the basolateral amygdala. Neurochem Res 39: 1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisboa SF, Reis DG, da Silva AL, Corrêa FM, Guimarães FS, Resstel LB (2010). Cannabinoid CB1 receptors in the medial prefrontal cortex modulate the expression of contextual fear conditioning. Int J Neuropsychopharmacol 13: 1163–1173. [DOI] [PubMed] [Google Scholar]
- Liu S, Sjövall J, Griffiths WJ (2003). Neurosteroids in rat brain: extraction, isolation, and analysis by nanoscale liquid chromatography‐electrospray mass spectrometry. Anal Chem 75: 5835–5846. [DOI] [PubMed] [Google Scholar]
- Locci A, Porcu P, Talani G, Santoru F, Berretti R, Giunti E et al. (2017). Neonatal estradiol exposure to female rats changes GABAA receptor expression and function, and spatial learning during adulthood. Horm Behav 87: 35–46. [DOI] [PubMed] [Google Scholar]
- Lovick T (2013). SSRIs and the female brain‐‐potential for utilizing steroid‐stimulating properties to treat menstrual cycle‐linked dysphorias. J Psychopharmacol 27: 1180–1185. [DOI] [PubMed] [Google Scholar]
- Lovick TA (2006). Plasticity of GABAA receptor subunit expression during the oestrous cycle of the rat: implications for premenstrual syndrome in women. Exp Physiol 91: 655–660. [DOI] [PubMed] [Google Scholar]
- Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA et al. (2000). Molecular and neuronal substrate for the selective attenuation of anxiety. Science 290: 131–134. [DOI] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N (2011). The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry 16: 383–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I (2005). Ovarian cycle‐linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci 8: 797–804. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Mienville JM, Vicini S (1988). Neurosteroid pregnenolone sulfate antagonizes electrophysiological responses to GABA in neurons. Neurosci Lett 90: 279–284. [DOI] [PubMed] [Google Scholar]
- Malayev A, Gibbs TT, Farb DH (2002). Inhibition of the NMDA response by pregnenolone sulphate reveals subtype selective modulation of NMDA receptors by sulphated steroids. Br J Pharmacol 135: 901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx CE, Keefe RS, Buchanan RW, Hamer RM, Kilts JD, Bradford DW et al. (2009). Proof‐of‐concept trial with the neurosteroid pregnenolone targeting cognitive and negative symptoms in schizophrenia. Neuropsychopharmacology 34: 1885–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Puia G, Dong E, Pinna G (2007). GABA(A) receptor neurotransmission dysfunction in a mouse model of social isolation‐induced stress: possible insights into a non‐serotonergic mechanism of action of SSRIs in mood and anxiety disorders. Stress 10: 3–12. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR et al. (2000). Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci 3: 587–592. [DOI] [PubMed] [Google Scholar]
- Meltzer‐Brody (2016). ClinicalTrials.gov identifier: NCT02614547 70.M.
- Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO et al. (2004). Dysregulation in the suicide brain: mRNA expression of corticotropin‐releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci 24: 1478–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modol L, Casas C, Navarro X, Llidó A, Vallée M, Pallarés M et al. (2014). Neonatal finasteride administration alters hippocampal α4 and δ GABAAR subunits expression and behavioural responses to progesterone in adult rats. Int J Neuropsychopharmacol 17: 259–273. [DOI] [PubMed] [Google Scholar]
- Mody I (2001). Distinguishing between GABA(A) receptors responsible for tonic and phasic conductances. Neurochem Res 26: 907–913. [DOI] [PubMed] [Google Scholar]
- Montpied P, Weizman A, Weizman R, Kook KA, Morrow AL, Paul SM (1993). Repeated swim‐stress reduces GABAA receptor alpha subunit mRNAs in the mouse hippocampus. Brain Res Mol Brain Res 18: 267–272. [DOI] [PubMed] [Google Scholar]
- Nelson M, Pinna G (2011). S‐norfluoxetine microinfused into the basolateral amygdala increases allopregnanolone levels and reduces aggression in socially isolated mice. Neuropharmacology 60: 1154–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB (2008). Understanding the pathophysiology of postpartum depression: implications for the development of novel treatments. Neuron 59: 185–186. [DOI] [PubMed] [Google Scholar]
- Nin MS, Martinez LA, Thomas R, Nelson M, Pinna G (2011a). Allopregnanolone and S‐norfluoxetine decrease anxiety‐like behavior in a mouse model of anxiety/depression. Trabajos del Instituto Cajal 83: 215–216. [Google Scholar]
- Nin MS, Martinez LA, Pibiri F, Nelson M, Pinna G (2011b). Neurosteroids reduce social isolation‐induced behavioral deficits: a proposed link with neurosteroid‐mediated upregulation of BDNF expression. Front Endocrinol (Lausanne) 2: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Mody I (2002). Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol 87: 2624–2628. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P (1998). Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci 18: 1693–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M et al. (2016). Major depressive disorder. Nat Rev Dis Primers 2: 16065. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P et al. (2006). Translocator protein (18kDa): new nomenclature for the peripheral‐type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 27: 402–409. [DOI] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW et al. (2002). GABA(A) receptor changes in delta subunit‐deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J Comp Neurol 446: 179–197. [DOI] [PubMed] [Google Scholar]
- Perez‐Sanchez J, Lorenzo LE, Lecker I, Zurek AA, Labrakakis C, Bridgwater EM et al. (2017). α5GABAA receptors mediate tonic inhibition in the spinal cord dorsal horn and contribute to the resolution of hyperalgesia. J Neurosci Res 95: 1307–1318. [DOI] [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Carboni G, Pinna G (2006). Neurosteroids regulate mouse aggression induced by anabolic androgenic steroids. Neuroreport 17: 1537–1541. [DOI] [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G (2008). Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: a model relevant for posttraumatic stress disorder. Proc Natl Acad Sci U S A 05: 5567–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G (2010). In a mouse model relevant for post‐traumatic stress disorder, selective brain steroidogenic stimulants (SBSS) improve behavioral deficits by normalizing allopregnanolone biosynthesis. Behav Pharmacol 21: 438–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G (2014). Targeting neurosteroidogenesis as therapy for PTSD. Front Pharmacol 4: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Agis‐Balboa RC, Zhubi A, Matsumoto K, Grayson DR, Costa E et al. (2006b). Imidazenil and diazepam increase locomotor activity in mice exposed to protracted social isolation. Proc Natl Acad Sci U S A 103: 4275–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A (2004). Fluoxetine and norfluoxetine stereospecifically facilitate pentobarbital sedation by increasing neurosteroids. Proc Natl Acad Sci U S A 101: 6222–6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A (2005). Changes in brain testosterone and allopregnanolone biosynthesis elicit aggressive behavior. Proc Natl Acad Sci U S A 102: 2135–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A (2006a). Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5‐HT reuptake. Psychopharmacology (Berl) 186: 362–372. [DOI] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A (2009). SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5‐HT reuptake. Curr Opin Pharmacol 9: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A (2003). In socially isolated mice, the reversal of brain allopregnanolone down‐regulation mediates the anti‐aggressive action of fluoxetine. Proc Natl Acad Sci U S A 100: 2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Rasmusson AM (2014). Ganaxolone improves behavioral deficits in a mouse model of post‐traumatic stress disorder. Front Cell Neurosci 8: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Uzunova V, Matsumoto K, Puia G, Mienville JM, Costa E et al. (2000). Brain allopregnanolone regulates the potency of the GABA(A) receptor agonist muscimol. Neuropharmacology 39: 440–448. [DOI] [PubMed] [Google Scholar]
- Porcu P, Barron AM, Frye CA, Walf AA, Yang SY, He XY et al. (2016). Neurosteroidogenesis today: novel targets for neuroactive steroid synthesis and action and their relevance for translational research. J Neuroendocrinol 28: 12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, Mostallino MC, Sogliano C, Santoru F, Berretti R, Concas A (2012). Long‐term administration with levonorgestrel decreases allopregnanolone levels and alters GABA(A) receptor subunit expression and anxiety‐like behavior. Pharmacol Biochem Behav 102: 366–372. [DOI] [PubMed] [Google Scholar]
- Prigerson HG, Maciejewski PK, Rosenheck RA (2001). Combat trauma: trauma with highest risk of delayed onset and unresolved posttraumatic stress disorder symptoms, unemployment, and abuse among men. J Nerv Ment Dis 189: 99–108. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH Jr, Paul SM (1991). Stress‐induced elevations of gamma‐aminobutyric acid type A receptor‐active steroids in the rat brain. Proc Natl Acad Sci U S A 88: 4553–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M, Zhu D, Lindblad C, Johansson IM, Holmberg E, Isaksson M et al. (2006). GABA‐site antagonism and pentobarbital actions do not depend on the alpha‐subunit type in the recombinant rat GABA receptor. Acta Physiol (Oxf) 187: 479–488. [DOI] [PubMed] [Google Scholar]
- Rahn KA, Cao YJ, Hendrix CW, Kaplin AI (2015). The role of 5‐HT1A receptors in mediating acute negative effects of antidepressants: implications in pediatric depression. Transl Psychiatry 5: e563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh VB (1997). Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol 90: 709–714. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, King M, Gregor K, Scioli‐Salter E, Pineles S, Valovski I et al. (2016). Sex differences in the enzyme site at which GABAergic neuroactive steroid synthesis is blocked in PTSD: implications for targeting of ptsd therapeutics. In symposium: Sex Specificity in Posttraumatic Stress Disorder: From Biological Mechanisms to Treatment Response (Fellingham K: Chair; Jovanovich T: Discussant). 32nd Annual Meeting, International Society for Traumatic Stress Studies Dallas, TX, November 10–12, 2016.
- Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D et al. (2006). Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry 60: 704–713. [DOI] [PubMed] [Google Scholar]
- Reddy DS (2003). Pharmacology of endogenous neuroactive steroids. Crit Rev Neurobiol 15: 197–234. [DOI] [PubMed] [Google Scholar]
- Romeo E, Ströhle A, Spalletta G, di Michele F, Hermann B, Holsboer F et al. (1998). Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry 155: 910–913. [DOI] [PubMed] [Google Scholar]
- Rone MB, Midzak AS, Issop L, Rammouz G, Jagannathan S, Fan J et al. (2012). Identification of a dynamic mitochondrial protein complex driving cholesterol import, trafficking, and metabolism to steroid hormones. Mol Endocrinol 26: 1868–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy JM et al. (1999). Benzodiazepine actions mediated by specific gamma‐aminobutyric acid(A) receptor subtypes. Nature 401: 796–800. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N et al. (2010). Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov 9: 971–988. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Rammes G, Eser D, Baghai TC, Schüle C, Nothdurfter C et al. (2009). Translocator protein (18 kD) as target for anxiolytics without benzodiazepine‐like side effects. Science 325: 490–493. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Bernstein IH, Trivedi MH, Carmody TJ, Wisniewski S, Mundt JC et al. (2006). An evaluation of the quick inventory of depressive symptomatology and the hamilton rating scale for depression: a sequenced treatment alternatives to relieve depression trial report. Biol Psychiatry 59: 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustichelli C, Pinetti D, Lucchi C, Ravazzini F, Puia G (2013). Simultaneous determination of pregnenolone sulphate, dehydroepiandrosterone and allopregnanolone in rat brain areas by liquid chromatography‐electrospray tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 930: 62–69. [DOI] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Murru L, Carta M, Talani G, Zucca S et al. (2009). Changes in expression and function of extrasynaptic GABAA receptors in the rat hippocampus during pregnancy and after delivery. J Neurosci 29: 1755–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasso O, Russo R, Vitiello S, Raso GM, D'Agostino G, Iacono A et al. (2012). Implication of allopregnanolone in the antinociceptive effect of N‐palmitoylethanolamide in acute or persistent pain. Pain 153: 33–41. [DOI] [PubMed] [Google Scholar]
- Schüle C (2014). Chronic depression‐‐epidemiological data and therapeutic options. Fortschr Neurol Psychiatr 82: 155–171. [DOI] [PubMed] [Google Scholar]
- Schüle C, Eser D, Baghai TC, Nothdurfter C, Kessler JS, Rupprecht R (2011). Neuroactive steroids in affective disorders: target for novel antidepressant or anxiolytic drugs? Neuroscience 191: 55–77. [DOI] [PubMed] [Google Scholar]
- Schwabe K, Gavrilovici C, McIntyre DC, Poulter MO (2005). Neurosteroids exhibit differential effects on mIPSCs recorded from normal and seizure prone rats. J Neurophysiol 94: 2171–2181. [DOI] [PubMed] [Google Scholar]
- Scioli‐Salter ER, Forman DE, Otis JD, Gregor K, Valovski I, Rasmusson AM (2015). The shared neuroanatomy and neurobiology of comorbid chronic pain and PTSD: therapeutic implications. Clin J Pain 31: 363–374. [DOI] [PubMed] [Google Scholar]
- Selvaraj V, Stocco DM (2015). The changing landscape in translocator protein (TSPO) function. Trends Endocrinol Metab 26: 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj V, Tu LN, Stocco DM (2016). Crucial role reported for tspo in viability and steroidogenesis is a misconception. Commentary: conditional steroidogenic cell‐targeted deletion of tspo unveils a crucial role in viability and hormone‐dependent steroid formation. Front Endocrinol (Lausanne) 7: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA (2004). Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci 27: 262–269. [DOI] [PubMed] [Google Scholar]
- Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V et al. (2009). Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One 4: e6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M, Mostallino MC, Talani G, Pisu MG, Carta M, Mura ML et al. (2006). Social isolation‐induced increase in alpha and delta subunit gene expression is associated with a greater efficacy of ethanol on steroidogenesis and GABA receptor function. J Neurochem 98: 122–133. [DOI] [PubMed] [Google Scholar]
- Serwanski DR, Miralles CP, Christie SB, Mehta AK, Li X, De Blas AL (2006). Synaptic and nonsynaptic localization of GABAA receptors containing the alpha5 subunit in the rat brain. J Comp Neurol 499: 458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Mennerick S, Zorumski EC, Covey DF, Zorumski CF (1999). Pregnenolone sulfate and dehydroepiandrosterone sulfate inhibit GABA‐gated chloride currents in Xenopus oocytes expressing picrotoxin‐insensitive GABA(A) receptors. Neuropharmacology 38: 267–271. [DOI] [PubMed] [Google Scholar]
- Shu HJ, Bracamontes J, Taylor A, Wu K, Eaton MM, Akk G et al. (2012). Characteristics of concatemeric GABA(A) receptors containing α4/δ subunits expressed in Xenopus oocytes. Br J Pharmacol 165: 2228–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E (2002). Mapping of the benzodiazepine recognition site on GABA(A) receptors. Curr Top Med Chem 2: 833–839. [DOI] [PubMed] [Google Scholar]
- Smith CC, Gibbs TT, Farb DH (2014). Pregnenolone sulfate as a modulator of synaptic plasticity. Psychopharmacology (Berl) 231: 3537–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GB, Olsen RW (1995). Functional domains of GABAA receptors. Trends Pharmacol Sci 16: 162–168. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench‐Mullen JM, Li X (1998). GABA(A) receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 392: 926–930. [DOI] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X (2007). Neurosteroid regulation of GABA(A) receptors: Focus on the alpha4 and delta subunits. Pharmacol Ther 116: 58–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh MS, Lynch JW (2015). Selective Modulators of α5‐Containing GABAA Receptors and their Therapeutic Significance. Curr Drug Targets 16: 735–746. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Fritschy JM, Benke D, Roberts JD, Sieghart W (1996). The gamma 2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the alpha 1 and beta 2/3 subunits in hippocampus, cerebellum and globus pallidus. Neuropharmacology 35: 1425–1444. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, Marx CE, King AP, Rampton JC, Ho SS, Liberzon I (2013). Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits. Biol Psychiatry 73: 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I (2003). Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit‐containing GABAA receptors. Proc Natl Acad Sci U S A 100: 14439–14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CA, Gazarini L, Takahashi RN, Guimarães FS, Bertoglio LJ (2012). On disruption of fear memory by reconsolidation blockade: evidence from cannabidiol treatment. Neuropsychopharmacology 37: 2132–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström I, Nyberg S, Bäckström T (1997). Patients with premenstrual syndrome have reduced sensitivity to midazolam compared to control subjects. Neuropsychopharmacology 17: 370–381. [DOI] [PubMed] [Google Scholar]
- Suryanarayanan A, Liang J, Meyer EM, Lindemeyer AK, Chandra D, Homanics GE et al. (2011). Subunit compensation and plasticity of synaptic GABA(A) receptors induced by ethanol in α4 subunit knockout mice. Front Neurosci 5: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Moss SJ (2008). GABA(A) receptor dynamics and constructing GABAergic synapses. Front Mol Neurosci 1: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunov DP, Cooper TB, Costa E, Guidotti A (1996). Fluoxetine‐elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc Natl Acad Sci U S A 93: 12599–12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Sampson L, Uzunov DP (2006). Relevance of endogenous 3alpha‐reduced neurosteroids to depression and antidepressant action. Psychopharmacology (Berl) 186: 351–361. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E et al. (1998). Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A 95: 3239–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallée M (2016). Neurosteroids and potential therapeutics: focus on pregnenolone. J Steroid Biochem Mol Biol 160: 78–87. [DOI] [PubMed] [Google Scholar]
- Vallée M, Vitiello S, Bellocchio L, Hébert‐Chatelain E, Monlezun S, Martin‐Garcia E et al. (2014). Pregnenolone can protect the brain from cannabis intoxication. Science 343: 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkuyl JM, Hemby SE, Joëls M (2004). Chronic stress attenuates GABAergic inhibition and alters gene expression of parvocellular neurons in rat hypothalamus. Eur J Neurosci 20: 1665–1673. [DOI] [PubMed] [Google Scholar]
- Volpi‐Abadie J, Kaye AM, Kaye AD (2013). Serotonin syndrome. Ochsner J 13: 533–540. [PMC free article] [PubMed] [Google Scholar]
- Vyklicky V, Smejkalova T, Krausova B, Balik A, Korinek M, Borovska J et al. (2016). Preferential inhibition of tonically over phasically activated NMDA receptors by pregnane derivatives. J Neurosci 36: 2161–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, He Y, Eisenman LN, Fields C, Zeng CM, Mathews J et al. (2002). 3beta ‐hydroxypregnane steroids are pregnenolone sulfate‐like GABA(A) receptor antagonists. J Neurosci 22: 3366–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MD, Borra VB, Strömberg J, Lundgren P, Haage D, Bäckström T (2008). Neurosteroids 3beta, 20 (R/S)‐pregnandiols decrease offset rate of the GABA‐site activation at the recombinant GABA A receptor. Eur J Pharmacol 586: 67–73. [DOI] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I (2003). Perisynaptic localization of delta subunit‐containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci 23: 10650–10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welk B, McArthur E, Ordon M, Anderson KK, Hayward J, Dixon S (2017). Association of suicidality and depression with 5α‐reductase inhibitors. JAMA Intern Med 177: 683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE et al. (2013). Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382: 1575–1586. [DOI] [PubMed] [Google Scholar]
- Whiting PJ (1999). The GABA‐A receptor gene family: new targets for therapeutic intervention. Neurochem Int 34: 387–390. [DOI] [PubMed] [Google Scholar]
- Williams LM, Debattista D, Duchemin A‐M, Schatzberg AF, Nemeroff CB (2016). Childhood trauma predicts antidepressant response in adults with major depression: data from the randomized international study to predict optimized treatment for depression. Transl Psychiatry 6: e799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Scheel‐Krüger J, Belzung C (2013). The neurobiology of depression and antidepressant action. Neurosci Biobehav Rev 37 (10 Pt 1): 2331–2371. [DOI] [PubMed] [Google Scholar]
- Wisden W, Herb A, Wieland H, Keinänen K, Lüddens H, Seeburg PH (1991). Cloning, pharmacological characteristics and expression pattern of the rat GABAA receptor alpha 4 subunit. FEBS Lett 289: 227–230. [DOI] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL (2002). Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J Neurosci 22: 1541–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FS, Gibbs TT, Farb DH (1991). Pregnenolone sulfate: a positive allosteric modulator at the N‐methyl‐D‐aspartate receptor. Mol Pharmacol 40: 333–336. [PubMed] [Google Scholar]
- Xu B, Yang R, Chang F, Chen L, Xie G, Sokabe M et al. (2012). Neurosteroid PREGS protects neurite growth and survival of newborn neurons in the hippocampal dentate gyrus of APPswe/PS1dE9 mice. Curr Alzheimer Res 9: 361–372. [DOI] [PubMed] [Google Scholar]
- Yang R, Chen L, Wang H, Xu B, Tomimoto H, Chen L (2012). Anti‐amnesic effect of neurosteroid PREGS in Aβ25‐35‐injected mice through σ1 receptor‐ and α7nAChR‐mediated neuroprotection. Neuropharmacology 63: 1042–1050. [DOI] [PubMed] [Google Scholar]
- Yu HL, Deng XQ, Li YJ, Li YC, Quan ZS, Sun XY (2011). N‐palmitoylethanolamide, an endocannabinoid, exhibits antidepressant effects in the forced swim test and the tail suspension test in mice. Pharmacol Rep 63: 834–839. [DOI] [PubMed] [Google Scholar]
- Zhang LM, Qiu ZK, Chen XF, Zhao N, Chen HX, Xue R et al. (2016). Involvement of allopregnanolone in the anti‐PTSD‐like effects of AC‐5216. J Psychopharmacol 30: 474–481. [DOI] [PubMed] [Google Scholar]
- Zhang LM, Qiu ZK, Zhao N, Chen HX, Liu YQ, Xu JP et al. (2014). Anxiolytic‐like effects of YL‐IPA08, a potent ligand for the translocator protein (18 kDa) in animal models of post‐traumatic stress disorder. Int J Neuropsychopharmacol 17: 1659–1669. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Paul SM, Izumi Y, Covey DF, Mennerick S (2013). Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev 37: 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]


