Abstract
Learning to associate cues or contexts with potential threats or rewards is adaptive and enhances survival. Both aversive and appetitive memories are therefore powerful drivers of behaviour, but the inappropriate expression of conditioned responding to fear‐ and drug‐related stimuli can develop into anxiety‐related and substance abuse disorders respectively. These disorders are associated with abnormally persistent emotional memories and inadequate treatment, often leading to symptom relapse. Studies show that cannabidiol, the main non‐psychotomimetic phytocannabinoid found in Cannabis sativa, reduces anxiety via 5‐HT1A and (indirect) cannabinoid receptor activation in paradigms assessing innate responses to threat. There is also accumulating evidence from animal studies investigating the effects of cannabidiol on fear memory processing indicating that it reduces learned fear in paradigms that are translationally relevant to phobias and post‐traumatic stress disorder. Cannabidiol does so by reducing fear expression acutely and by disrupting fear memory reconsolidation and enhancing fear extinction, both of which can result in a lasting reduction of learned fear. Recent studies have also begun to elucidate the effects of cannabidiol on drug memory expression using paradigms with translational relevance to addiction. The findings suggest that cannabidiol reduces the expression of drug memories acutely and by disrupting their reconsolidation. Here, we review the literature demonstrating the anxiolytic effects of cannabidiol before focusing on studies investigating its effects on various fear and drug memory processes. Understanding how cannabidiol regulates emotion and emotional memory processing may eventually lead to its use as a treatment for anxiety‐related and substance abuse disorders.
Linked Articles
This article is part of a themed section on Pharmacology of Cognition: a Panacea for Neuropsychiatric Disease? To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.19/issuetoc
Abbreviations
- BNST
bed nucleus of the stria terminalis
- CBD
cannabidiol
- CS
conditioned stimulus
- dlPAG
dorsolateral periaqueductal gray
- EPM
elevated plus‐maze
- IL
infralimbic
- PAG
periaqueductal gray
- PL
prelimbic
- PTSD
post‐traumatic stress disorder
- THC
Δ9‐tetrahydrocannabinol
- US
unconditioned stimulus
Tables of Links
| TARGETS | |
|---|---|
| Other protein targets a | Voltage‐gated ion channels d |
| Fatty acid binding proteins (FABPs) | TRPA1 |
| GPCRs b | TRPM8 |
| 5‐HT1A receptor | TRPV1 |
| A1 receptor | TRPV2 |
| CB1 receptor | Nuclear hormone receptors e |
| CB2 receptor | PPARγ |
| D4 receptor | Catalytic receptors f |
| GPR55 | TrkB |
| Ligand‐gated ion channels c | Enzymes g |
| GluA1 receptor | Fatty acid amide hydrolase (FAAH) |
| LIGANDS |
|---|
| Adenosine |
| Anandamide |
| Cannabidiol |
| Cocaine |
| Morphine |
| THC |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,d,e,f,gAlexander et al., 2015a,b,c,d,e,f,g).
Introduction
Anxiety (e.g. generalized and social anxiety, panic and phobias), trauma‐related [i.e. post‐traumatic stress disorder (PTSD)] and substance abuse disorders are serious forms of mental illness associated with a significant lifetime prevalence. These disorders pose an enormous social and financial burden as they are often chronic in nature and inadequately treated (Di Luca et al., 2011). Certain anxiety‐related disorders (e.g. phobias and PTSD) and addiction are characterized by aberrant and persistent emotional memories of fear‐ and drug‐related stimuli. These discrete or contextual cues can trigger the emergence of symptoms or even their re‐emergence after treatment, highlighting the limited effectiveness of the psychological and pharmacological therapies currently available to curtail symptom relapse over the long‐term (Tronson and Taylor, 2013; Everitt, 2014; Kindt, 2014; Singewald et al., 2015). Moreover, there is also significant co‐morbidity between substance abuse disorders and PTSD, which can further complicate how PTSD develops and is treated. For example, the learning and memory processes involved in the psychological therapies that are used for treating PTSD can be adversely affected by different drugs of abuse, which may also have complex drug–drug interactions with pharmacological treatments for PTSD (Tipps et al., 2014). Thus, there is an urgent need to improve the treatment of these disorders.
An area of real promise in this field involves the use of existing or novel medications as adjuncts to psychological therapies to enhance the efficacy of treatment. Cannabidiol (CBD) is one such drug that shows therapeutic potential in a broad range of neurological and psychiatric diseases (Campos et al., 2012b). This phytocannabinoid is the main non‐psychotomimetic constituent of the Cannabis sativa plant, and mounting evidence indicates that CBD has anxiolytic properties (Blessing et al., 2015). Emerging preclinical and clinical evidence also indicates that CBD regulates different aversive and appetitive memory processes (Prud'homme et al., 2015; Jurkus et al., 2016), in keeping with the findings of recent studies showing a role for CBD in modulating other types of memory, such as novel object and social recognition, in cognitively‐impaired animals (Fagherazzi et al., 2012; Cheng et al., 2014). In this paper, we begin with a brief historical account of the discovery of CBD and touch on the first studies that investigated its behavioural effects in rodents and humans. We then review the literature on CBD regulation of anxiety and the pharmacological and brain mechanisms involved. The bulk of the paper focuses on discussing the findings from the growing number of studies, mostly preclinical, that have examined the regulation of learned fear and, more recently, addictive drug memory processing by CBD. Importantly, these studies have used experimental procedures with clinical relevance for understanding the psychological and neurobiological mechanisms involved in the pathophysiology and treatment of anxiety‐related and substance abuse disorders.
CBD discovery and initial studies on its behavioural effects
The C. sativa plant contains more than 100 chemically related terpenephenol components called phytocannabinoids (Izzo et al., 2009; Gould, 2015). Since the seminal work of Raphael Mechoulam's group in the 1960s, Δ9‐tetrahydrocannabinol (THC) is considered the main component responsible for the pharmacological effects of the plant (Gaoni and Mechoulam, 1964). The second major component of most samples of C. sativa is CBD. Originally isolated by Adams and co‐workers in 1940 (Adams et al., 1940), its structure was elucidated by Mechoulam and Shvo (1963). Although the CBD molecule is similar to THC, it has a distinct spatial conformation that could help to explain their different pharmacological properties. Whereas THC has a planar conformation, CBD presents a ‘bent’ structure with two rings at a right angle to each other (Burstein, 2015).
Initial studies performed in the 1970s, mostly in Brazil, indicated that CBD could block some effects induced by THC in rodents (Karniol and Carlini, 1973; Russo and Guy, 2006). Following these initial studies, Zuardi and collaborators investigated if CBD could prevent the effects of high doses of THC in healthy human volunteers. They found that it attenuates the psychotomimetic and anxiogenic effects of THC (Zuardi et al., 1982). Although the mechanisms of action of these two drugs were completely unknown at that time, the fact that not all effects of THC were blocked by CBD indicated that the latter was not simply an antagonist of a putative THC receptor. On the contrary, the study suggested that CBD possesses its own antipsychotic and anxiolytic properties (Zuardi et al., 1982).
Laboratory animal tests used to assess the anxiolytic properties of CBD
The potential anxiolytic effect of CBD was initially investigated in preclinical studies. Several animal tests have been employed to explore the effects of putative anxiolytic drugs and the neurobiology of anxiety, which can be defined separately from fear as the emotional response to potential or anticipated (as opposed to actual and present) threat (Tovote et al., 2015). These tests are based on the measurement of defensive behaviours (either active or inhibitory) expressed in response to a threatening or unpleasant stimulus (Campos et al., 2013a). The initial preclinical studies investigating the possible anxiolytic‐like effects of CBD were performed in learning‐based models and produced mixed results. These apparently conflicting results were later explained by Guimarães et al. (1990) using the elevated plus‐maze (EPM). This is a commonly used test to investigate anxiety‐like behaviour in preclinical studies and is based on the natural aversion that rodents show to open spaces (Handley and Mithani, 1984; Pellow et al., 1985; Treit et al., 1993; Carobrez and Bertoglio, 2005).
Using the EPM and performing a full dose–response curve in rats, Guimarães and co‐workers showed that acute systemic administration of CBD produces a typical ‘bell‐shaped’ dose–response curve, being anxiolytic at low and intermediate doses but not at high doses. Although some contradictory results exist in the literature, most studies using unlearned or operant conditioning models of anxiety have confirmed these initial findings, and the studies investigating CBD effects in classical (Pavlovian) conditioning models also go in the same direction, which will be discussed separately below (summarized in Tables 1 and 2). Moreover, these anxiolytic effects of CBD in animals have been replicated in human studies using healthy subjects exposed to anxiety‐provoking stimuli or situations (Zuardi et al., 1982, 1993; Crippa et al., 2004; Fusar‐Poli et al., 2009, 2010) and in patients with anxiety, and possibly also substance abuse, disorders (Bergamaschi et al., 2011, Crippa et al., 2011; Hurd et al., 2015; Shannon and Opila‐Lehman, 2016; summarized in Table 3).
Table 1.
CBD effects on anxiety‐like behaviour in male animals
| Reference | Test used | Strain, species, effective dose, and route/site of administration | Effect | Pharmacological mechanism |
|---|---|---|---|---|
| Guimarães et al. (1990) | EPM | Wistar rats, 2.5–10 mg·kg−1, i.p. | Anxiolytic (bell‐shaped dose–response curve) | Not tested |
| Onaivi et al. (1990) | EPM | ICR mice, 1 and 10 mg·kg−1, i.p. | Anxiolytic (bell‐shaped dose–response curve) | BZD (blocked by flumazenil) |
| Guimarães et al. (1994) | EPM | Wistar rats, 5 mg·kg−1, i.p. | Anxiolytic | Not tested |
| Bitencourt et al. (2008) | Fear‐potentiated EPM | Wistar rats, 6.4 nmol, i.c.v. | Anxiolytic | Not tested |
| Campos and Guimarães (2008) | EPM | Wistar rats, 30 nmol, intra‐dlPAG | Anxiolytic (bell‐shaped dose–response curve) | 5‐HT1A receptor activation |
| Campos and Guimarães (2009) | EPM | Wistar rats, 30 nmol, intra‐dlPAG (60 nmol effective when combined with a TRPV1 channel antagonist) | Anxiolytic | Lack of anxiolytic effect of high doses associated with TRPV1 channel activation |
| Malone et al. (2009) | THC‐induced decrease in social interaction | Sprague Dawley rats, 20 mg·kg−1, i.p. | Anxiolytic | Not tested |
| Resstel et al. (2009) | Restraint stress, autonomic changes, delayed (24 h) anxiogenic effect in EPM | Wistar rats, 10 mg·kg−1, i.p. | Anti‐stress | 5‐HT1A receptor activation |
| Casarotto et al. (2010) | MBT | C57BL/6 mice, 15–60 mg·kg−1, i.p. | Anti‐compulsive | Indirect CB1 receptor activation |
| Soares Vde et al. (2010) | ETM, electrical stimulation of dlPAG | Wistar rats, 15–60 nmol, intra‐ dlPAG | Anxiolytic/panicolytic | 5‐HT1A receptor activation |
| Long et al. (2010) | Open field and light–dark tests | C57BL/6 mice, 1 mg·kg−1 (light–dark test) and 50 mg·kg−1 (open‐field), i.p., daily for 21 days | Anxiolytic | Not tested |
| Gomes et al. (2011) | EPM | Wistar rats, 30 nmol, intra‐BNST | Anxiolytic | 5‐HT1A receptor activation |
| Granjeiro et al. (2011) | Restraint stress, autonomic reactivity, delayed (24 h) anxiogenic effect in EPM | Wistar rats, 30 nmol, intra‐cisterna magna | Anti‐stress | Not tested |
| Campos et al. (2012a) | EPM after predator (cat) exposure | Wistar rats, 5 mg·kg−1, i.p., daily for 7 days | Anxiolytic | 5‐HT1A receptor activation |
| Deiana et al. (2012) | MBT | Swiss mice, 120 mg·kg−1, orally or i.p. | Anticompulsive | Not tested |
| Long et al. (2012) | Open field and light–dark tests | C57BL/6 Arc mice, 1 and 100 mg·kg−1, i.p. daily for 13 days | Anxiolytic (open‐field only) | Not tested |
| Uribe‐Mariño et al. (2012) | Snake exposure | Swiss mice, 0.3–30 mg·kg−1, i.p. | Panicolytic | Not tested |
| Hsiao et al. (2012) | Repeated EPM and open‐field | Wistar rats, 3.2 nmol, intra‐central amygdaloid nucleus | Anxiolytic | Not tested |
| Campos et al. (2013a,b) | EPM and NSF | C57BL/6 mice, 30 mg·kg−1, daily for 14 days (CUS‐exposed animals) | Anti‐stress | CB1 receptor‐mediated facilitation of hippocampal neurogenesis |
| O'Brien et al. (2013) | Light–dark test | Sprague Dawley rats, 2.5 mg·kg−1, i.p. for 14 days | No effect | Not tested |
| Twardowschy et al. (2013) | Snake exposure | Swiss mice, 3.0 mg·kg−1, i.p. | Panicolytic | 5‐HT1A receptor activation |
| Almeida et al. (2013) | Social interaction test | Wistar and SHR rats, 1 mg·kg−1, i.p. | Increased social interaction (Wistar rats only) | Not tested |
| Cheng et al. (2014) | EPM | C57BL/6 J mice, 20 mg·kg−1, i.p. daily for 21 days | No effect | Not tested |
| Fogaça et al. (2014) | EPM | Wistar rats, 30 nmol, intra‐PL cortex | Anxiogenic (bell‐shaped dose‐response curve), anxiolytic 24 h after restraint stress | 5‐HT1A receptor activation |
| Nardo et al. (2014) | MBT | Swiss mice, 30 mg·kg−1, i.p. | Attenuated mCPP‐induced increase in marble‐burying (bell‐shaped dose response curve) | Indirect CB1 receptor activation |
| Marinho et al. (2015) | EPM | Wistar rats, 15–30 nmol, intra‐IL cortex | Anxiolytic (bell‐shaped dose response curve), no effect 24 h after restraint stress | 5‐HT1A receptor activation |
| Todd and Arnold (2016) | Open‐field | C57BL/6 mice, 10 mg·kg−1, i.p. | Prevented THC‐ induced anxiogenesis | Not tested |
| Schiavon et al. (2016) | EPM | Swiss mice, 3 mg·kg−1, i.p. | Anxiolytic | Not tested |
BZD, benzodiazepine; CUS, chronic unpredictable stress; ETM, elevated T‐maze; ICR, Institute of Cancer; MBT, marble burying test; NSF, novelty suppressed feeding; SHR, spontaneously hypertensive rats.
Table 2.
CBD effects on learned fear processing in male animals
| Reference | Test used | Strain, species, effective dose and route/site of administration | Effect | Pharmacological mechanism |
|---|---|---|---|---|
| Studies conducted in operant conditioning paradigms | ||||
| Silveira Filho and Tufik, 1981 | Geller‐Seifter conflict test | Wistar rats, 100 mg·kg−1, i.p. | No effect | Not tested |
| Musty et al. (1985) | Vogel punished licking test | Sprague–Dawley rats, 5–10 mg·kg−1, i.p. | Anxiolytic (bell‐shaped dose–response curve) | Not tested |
| Moreira et al. (2006) | Vogel punished licking test | Wistar rats, 10 mg·kg−1, i.p. | Anxiolytic | Not blocked by BZD antagonism (flumazenil) |
| Gomes et al. (2011) | Vogel punished licking test | Wistar rats, 30–60 nmol, intra‐BNST | Anxiolytic | 5‐HT1A receptor activation |
| Studies conducted in classical (Pavlovian) conditioning paradigms | ||||
| Zuardi and Karniol, 1983 | AFC | Wistar rats, 10 mg·kg−1, i.p. | Anxiolytic (decreased fear expression) | Not tested |
| Resstel et al. (2006) | CFC | Wistar rats, 10 mg·kg−1, i.p. | Anxiolytic (decreased fear expression) | Not tested |
| Bitencourt et al. (2008) | CFC | Wistar rats, 6.4 nmol, i.c.v. | Facilitated fear memory extinction | Indirect CB1 receptor activation |
| Lemos et al. (2010) | CFC | Wistar rats, 10 mg·kg−1, i.p. | Anxiolytic (decreased fear expression) | Not tested |
| Lemos et al. (2010) | CFC | Wistar rats, 30 nmol, intra‐PL cortex | Anxiolytic (decreased fear expression) | Not tested |
| Lemos et al. (2010) | CFC | Wistar rats, 30 nmol, intra‐IL cortex | Anxiogenic (increased fear expression) | Not tested |
| ElBatsh et al. (2012) | CFC | Lister‐hooded rats, 10 mg·kg−1, i.p. daily for 14 days | Anxiogenic (increased fear expression) | Decreased hippocampal BDNF and TrkB, reduced frontal cortex phospho‐ERK1/2 expression |
| Gomes et al. (2012) | CFC | Wistar rats, 30–60 nmol, intra‐BNST | Anxiolytic (decreased fear expression) | 5‐HT1A receptor activation |
| Levin et al. (2012) | CFC | Wistar and SHR rats, 1–15 mg·kg−1, i.p. | Anxiolytic (decreased fear expression) and/or disrupted fear memory formation (Wistar rats only) | Not tested |
| Stern et al. (2012) | CFC | Wistar rats, 3–30 mg·kg−1, i.p. | Disrupted fear memory reconsolidation (bell‐shaped dose response curve) | Indirect CB1 receptor activation |
| Do Monte et al. (2013) | CFC | Long–Evans hooded rats, 1.3 nmol, intra‐IL cortex | Facilitated fear memory extinction | Indirect CB1 receptor activation |
| Cheng et al. (2014) | AFC | C57BL/6 J mice, 20 mg·kg−1, i.p. daily for 21 days | No effect | Not tested |
| Fogaça et al. (2014) | CFC | Wistar rats, 30 nmol, intra‐PL cortex | Anxiolytic (decreased fear expression) | 5‐HT1A receptor activation |
| Gazarini et al. (2015) | CFC | Wistar rats, 10 mg·kg−1, i.p. | Disrupted fear memory reconsolidation | Not tested |
| Stern et al. (2014) | CFC | Wistar rats, 10 mg·kg−1, i.p. | Disrupted fear memory reconsolidation | Indirect CB1 receptor activation in PL cortex |
| Marinho et al. (2015) | CFC | Wistar rats, 30 nmol, intra‐IL cortex | Anxiogenic (increased fear expression) | 5‐HT1A receptor activation |
| Stern et al. (2015) | CFC | Wistar rats, 1 mg·kg−1 + THC 0.1 mg·kg−1, i.p. | Disrupted fear memory reconsolidation | Not tested |
| Norris et al. (2016) | OFC | Sprague Dawley rats, 0.03–0.32 nmol, intra‐nucleus accumbens shell | Disrupted fear memory formation (acquisition) | 5‐HT1A receptor activation |
| Song et al. (2016) | CFC | Lister hooded rats, 10 mg·kg−1, i.p., before extinction (after weak or strong conditioning) | Impaired or enhanced extinction after weak or strong conditioning, respectively | Not tested |
| Jurkus et al. (2016) | AFC | Lister hooded rats, 5–20 mg·kg−1, i.p. | Anxiolytic (decreased fear expression) at highest dose, no effect on extinction | Not tested |
| Stern et al. (2016) | CFC | Wistar rats, 10–30 mg·kg−1, i.p. | Disrupted fear memory consolidation | Indirect CB1 or CB2 receptor activation |
AFC, auditory fear conditioning; BZD, benzodiazepine; CFC, contextual fear conditioning; OFC, olfactory fear conditioning.
Table 3.
CBD effects on anxiety in humans
| Reference | Subjects and test(s) used | Effective dose and route of administration | Effect | Possible pharmacological or neural mechanism |
|---|---|---|---|---|
| Zuardi et al. (1982) | Healthy subjects, THC‐induced anxiety | ~70 mg (1 mg·kg−1) orally | Prevented the anxiogenic effects of THC | Not tested |
| Zuardi et al. (1993) | Healthy subjects, simulated public speaking‐induced anxiety | 300 mg orally | Prevented public speaking‐induced increase in anxiety | Not tested (effects similar to the 5‐HT1A receptor partial agonist ipsapirone) |
| Crippa et al. (2004) | Healthy subjects, SPECT | 400 mg orally | Anxiolytic | Decreased blood flow in medial temporal structures and posterior cingulate gyrus |
| Fusar‐Poli et al. (2009, 2010) | Healthy subjects, fearful faces, fMRI | 600 mg orally | Anxiolytic (trend) | Decreased blood flow in amygdala and anterior cingulate cortex that correlated with a reduced SCR to fearful faces |
| Bergamaschi et al. (2011) | Social anxiety disorder patients, simulated public speaking‐induced anxiety | 600 mg orally | Anxiolytic | Not tested |
| Crippa et al. (2011) | Generalized anxiety disorders patients, SPECT | 400 mg orally | Decreased subjective anxiety | Altered blood flow in limbic and paralimbic brain areas |
| Hurd et al. (2015) | Abstinent heroin abusers, heroin cue‐induced anxiety | 400 or 800 mg orally | Decreased subjective anxiety (preliminary data) | Not tested |
| Shannon and Opila‐Lehman, 2016 | A 10 year‐old girl with PTSD (case report) | At least 25 mg daily for 5 months | Reduced anxiety and improved sleep | Not tested |
fMRI, functional magnetic resonance imaging; SCR, skin conductance response; SPECT, single‐photon emission computed tomography.
Pharmacological mechanisms and brain sites involved in the anxiolytic effects of CBD
The potential therapeutic effects of CBD have been related to multiple pharmacological mechanisms, including the agonism of 5‐HT1A receptors, inhibition of reuptake and/or metabolism of the endocannabinoid anandamide (resulting indirectly in cannabinoid receptor activation), activation of transient receptor potential vanilloid 1 (TRPV1) channels, inhibition of adenosine reuptake, antagonism of GPR55, agonism of PPARγ receptors, intracellular Ca2+ increase, and anti‐oxidative effects, among others (summarized in Figure 1). These pharmacological mechanisms have been discussed recently in several reviews (Izzo et al., 2009; Campos et al., 2012a; Ibeas Bih et al., 2015; McPartland et al., 2015), to which the reader is referred. So far, however, only two of these mechanisms – 5‐HT1A receptor activation and indirect potentiation of endocannabinoid transmission – have been implicated in the attenuation of defensive responses to threatening or stressful stimuli.
Figure 1.
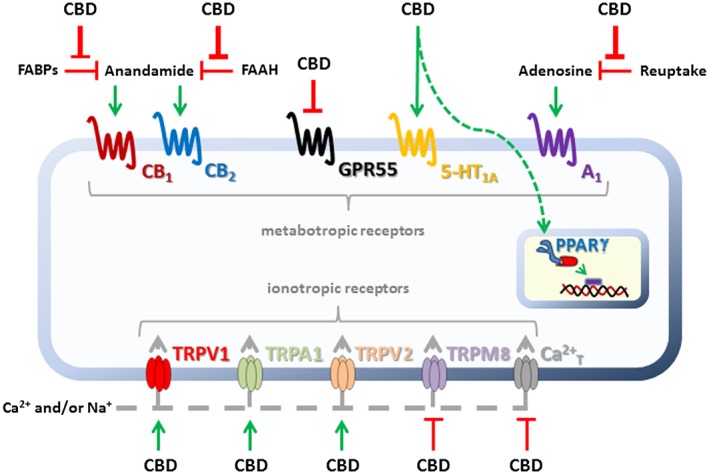
The main molecular targets and potential mechanisms of action of CBD. This drug inhibits both FAAH, the enzyme which metabolizes anandamide, and FABPs, which mediate the transport of anandamide to FAAH; both mechanisms ultimately result in the indirect activation of CB1 and/or CB2 receptors. CBD also activates the 5‐HT1A receptor, PPARγ and the transient receptor potential channels TRPV1, TRPA1 and TRPV2. Finally, CBD inhibits adenosine reuptake and antagonizes GPR55, TRPM8 and T‐type Ca2+ channels. 5‐HT1A and (indirect) cannabinoid receptor activation are the mechanisms that have been implicated in the anxiolytic effects of CBD to date (see Ibeas Bih et al. (2015) and McPartland et al. (2015) for further details).
Two primary brain systems organize defensive responses to threatening stimuli: one responsive to innate threats and the other responsible for the association between neutral and aversive stimuli, although the neural circuit mechanisms underlying the regulation of anxiety and learned fear show considerable overlap (for reviews, see McNaughton and Corr, 2004; Canteras et al., 2010; Gross & Canteras, 2012; Tovote et al., 2015). The brain areas implicated in the anxiolytic effects of cannabidiol include certain medial prefrontal cortical subregions [e.g. prelimbic (PL) and infralimbic (IL) cortex], the bed nucleus of the stria terminalis (BNST), periaqueductal gray (PAG) and amygdala. This evidence comes from preclinical studies and functional imaging studies in humans, which have confirmed the involvement of some of these brain areas. For example, CBD reduced amygdala activation in both mice and humans (Todd and Arnold, 2016; Crippa et al., 2004). Activity in and functional connectivity between the amygdala and anterior cingulate cortex, the homologous region to the rodent dorsomedial prefrontal cortex, were both also decreased by CBD when viewing fearful facial expressions (Fusar‐Poli et al., 2009; 2010).
In an initial preclinical study using the EPM test, Campos and Guimarães (2008) showed that the anxiolytic‐like effects of CBD injected into the dorsolateral PAG (dlPAG) were prevented by local treatment with the 5‐HT1A receptor antagonist WAY100635. Even if this drug can also activate D4 receptors (Chemel et al., 2006), the anti‐aversive effects of CBD were similar to other 5‐HT1A receptor agonists infused into the dlPAG (Graeff, 2002). The involvement of the 5‐HT1A receptor in the acute anxiolytic/anti‐stress effect of CBD was further demonstrated in other relevant brain regions, including the BNST (Gomes et al., 2011) and IL cortex (Marinho et al., 2015). Moreover, systemic treatment with 5‐HT1A receptor antagonists was also able to prevent this CBD‐induced anxiolysis (see Table 1).
In the marble‐burying test and after repeated administration, however, CBD effects on anxiety seem to depend on CB1 receptors rather than 5‐HT1A receptors (Casarotto et al., 2010; Campos et al., 2013a; Nardo et al., 2014). Even if the (+)‐CBD enantiomer shows affinity for CB1 receptors, the naturally occurring (−) CBD does not bind to these receptors (Hanuš et al., 2005), indicating that the CB1 receptor‐mediated anti‐aversive effects of CBD are probably indirect. Bisogno et al. (2001) showed that CBD blocked the reuptake and metabolism of anandamide in vitro. Correspondingly, using embryonic hippocampal cells, Campos et al. (2013b) showed that the increase in cell proliferation induced by CBD is prevented by antagonism of either CB1 or CB2 receptors, as well as by overexpression of fatty acid amide hydrolase (FAAH), the enzyme responsible for anandamide metabolism. More recently, Dale Deutsch's group demonstrated that CBD binds to fatty acid‐binding proteins (FABPs) necessary for the transport of anandamide from the plasma membrane to intracellular FAAH, which might be a primary mechanism by which CBD decreases anandamide uptake/metabolism (Elmes et al., 2015). Consistent with these in vitro studies, the anti‐stress (in mice) and antipsychotic (in humans) effects of repeated CBD administration were associated with increased hippocampal and serum levels, respectively, of anandamide (Leweke et al., 2012, Campos et al., 2013b).
Emotional learning and memory processing
We will first summarize the psychological mechanisms involved in classical conditioning, a type of associative learning whereby discrete cues or contexts come to predict the occurrence of threatening or rewarding stimuli, before reviewing the evidence demonstrating a role for CBD in regulating different fear and drug memory processes. During conditioning, an innocuous conditioned stimulus (CS), which can be a discrete cue (e.g. sound, light or odour) or a context (e.g. testing chamber/arena), becomes associated with an aversive (e.g. footshock) or appetitive (e.g. drug reward availability) unconditioned stimulus (US). After conditioning, the CS–US association undergoes consolidation into long‐term memory, and later presentation of or re‐exposure to the CS alone initially elicits conditioned fear (e.g. freezing/avoidance) or drug‐seeking (e.g. lever pressing/place preference) responses (Peters et al., 2009). Retrieval of the CS can make emotional memories labile by destabilizing the memory trace, which allows for these memories to be maintained or updated through the process of reconsolidation (Lee, 2009). Repeated presentations of or prolonged exposure to the CS causes the extinction of emotional memories, resulting in the formation of a new CS–no US association which competes with the original emotional memory to suppress conditioned responding to the CS (Peters et al., 2009). Understanding how behavioural and/or pharmacological interventions can attenuate conditioned responding, disrupt memory reconsolidation and/or enhance extinction has clinical relevance given that all of these mechanisms are potential therapeutic strategies for alleviating the symptoms of PTSD (i.e. pathological fear) and addiction (i.e. drug craving) (Tronson and Taylor, 2013; Everitt, 2014; Kindt, 2014; Singewald et al., 2015).
CBD effects on fear memory processing
As alluded to above, there is growing evidence indicating that CBD also regulates learned fear (see Table 2). Systemic CBD administration has been shown to reduce the expression of fear memory when given acutely (Zuardi and Karniol, 1983; Resstel et al., 2006; Lemos et al., 2010; Jurkus et al., 2016). CBD has also been reported to impair the acquisition of fear learning; acute systemic administration before fear conditioning resulted in attenuated fear expression during later memory retrieval testing (Levin et al., 2012). In contrast, there are few reported effects of repeated CBD administration on fear memory expression and those that exist are conflicting. In one study, daily injections of CBD for 14 days prior to conditioning enhanced fear expression during retrieval testing, suggesting that chronic CBD facilitated fear learning (ElBatsh et al., 2012), whereas another study showed no effect of CBD on fear conditioning when it was administered for 21 days (Cheng et al., 2014).
The results of several studies indicate that CBD also modulates the extinction and reconsolidation of conditioned fear, leading to lasting effects on learned fear expression. I.c.v. infusions of CBD given before three extinction sessions resulted in enhanced contextual fear extinction (Bitencourt et al., 2008). Systemic administration of CBD given acutely before extinction has been shown to affect contextual fear extinction depending on the strength of fear conditioning beforehand. CBD impaired extinction after weak conditioning but enhanced extinction after strong conditioning (Song et al., 2016). However, CBD given systemically before auditory fear extinction reduced fear expression acutely without affecting extinction memory (Jurkus et al., 2016). Interestingly, a study in humans also showed that CBD had no effect on the extinction of visual fear memory when given before extinction, but it did enhance extinction memory when given immediately after extinction (Das et al., 2013).
Contrary to the reported facilitatory effects of CBD on fear extinction, this drug has been shown to disrupt the reconsolidation of contextual fear memory after its brief retrieval (Stern et al., 2012; 2015; Gazarini et al., 2015), although these contrasting effects of CBD on fear extinction and memory reconsolidation both result in a lasting reduction of learned fear expression. The disruptive effect of systemic CBD administration on reconsolidation required that it was given immediately after memory retrieval as CBD had no effect if it was given without, or 6 h after, retrieval. CBD was also able to disrupt the reconsolidation of both newer and older fear memories. Moreover, the subsequent reduction of learned fear expression lasted for over 21 days and was not reinstated by later shock presentation, indicating that the effects of CBD were due to disrupted memory reconsolidation and not enhanced extinction (Stern et al., 2012).
In another study, CBD given immediately after retrieval disrupted the reconsolidation of an abnormally persistent fear memory when the partial NMDA receptor agonist D‐cycloserine was first administered before retrieval to facilitate memory destabilization. Fear memory was strengthened pharmacologically by enhancing adrenergic transmission immediately after conditioning, resulting in generalized fear expression and impaired fear suppression by extinction (Gazarini et al., 2015). Understanding the mechanisms underlying reconsolidation disruption of such fear memories is important because there is evidence indicating that strong fear memories can show resistance to pharmacological disruption of reconsolidation (Lee, 2009), which has implications for using this potential therapeutic approach to weaken traumatic memories in the treatment of PTSD.
Pharmacological mechanisms and brain sites involved in the effects of CBD on learned fear
Just as the anxiolytic effects of CBD involve a direct effect on 5‐HT1A receptors and an indirect effect on cannabinoid receptors via elevated endocannabinoid levels, so too do its effects on different fear memory processes. Similarly, there is overlap in the neural circuitry involved in mediating the effects of CBD on anxiety and learned fear. The reduction in conditioned fear expression induced by CBD was accompanied by attenuated c‐Fos expression in the PL and IL cortices and the BSNT. Moreover, CBD infusion into the BNST or PL cortex reduced fear memory expression, although infusing CBD into the IL cortex enhanced the expression of learned fear (Lemos et al., 2010). This discrepancy between the effects of CBD infused into the PL or IL cortex is probably due to these medial prefrontal cortical subregions exerting opposing influences on learned fear, with the former facilitating its expression and the latter being involved in its suppression and/or extinction (Fenton et al., 2014; Giustino and Maren, 2015). The regulation of conditioned fear expression by CBD in these brain areas was shown to be dependent on 5‐HT1A receptors (Gomes et al., 2012; Fogaça et al., 2014; Marinho et al., 2015). The inhibitory effect of CBD on the acquisition of fear conditioning has also been shown to depend on 5‐HT1A receptor activation in the nucleus accumbens shell (Norris et al., 2016).
In contrast to the acquisition and expression of fear memory, the reconsolidation and extinction of learned fear involve (indirect) cannabinoid receptor activation. The facilitatory effect of i.c.v. CBD infusion on fear extinction was inhibited by prior CB1 receptor antagonism but not TRPV1 channel blockade (Bitencourt et al., 2008). CBD was shown to act in the IL cortex to facilitate fear extinction as infusing CBD into this region enhanced extinction, an effect which also depended on CB1 receptors (Do Monte et al., 2013). The disruptive effect of CBD on fear memory reconsolidation was blocked by pretreatment with a CB1 receptor antagonist given systemically or infused into the PL cortex, whereas prior 5‐HT1A receptor antagonism had no effect on the disruption of reconsolidation by CBD (Stern et al., 2012; 2014).
CBD effects on addictive drug memory processing
In contrast to the study of fear memories, to date there has been a much more limited exploration of the effects of CBD on addictive drug‐related memories. This necessitates a narrative review of the relevant literature, which follows below. Moreover, the small number of studies has been conducted across a variety of experimental paradigms and with different drugs of abuse. These drugs can elicit sensitized responses with intermittent repeated administration, which is context‐dependent and thereby reliant upon context‐drug associations. Similarly, the acquisition and expression of conditioned place preference behaviour depends upon the integrity of context‐drug and/or cue‐drug associations. Finally, cue‐drug associations can precipitate cue‐induced relapse of drug seeking in rodents previously trained to self‐administer a drug (Aguilar et al., 2009; Steketee and Kalivas, 2011). Each of these paradigms can be studied using stimulants (e.g. cocaine and amphetamine), opiates (e.g. heroin and morphine), and other drugs (e.g. alcohol and nicotine).
Unlike THC, studies have shown that CBD lacks any rewarding effects of its own given that it fails to induce conditioned place preference or enhance the reinforcing effects of electrical brain self‐stimulation (Parker et al., 2004; Vann et al., 2008; Katsidoni et al., 2013). In a study of amphetamine‐induced locomotor sensitization, infusions of CBD (100 ng) into the shell subregion of the nucleus accumbens attenuated the development of locomotor sensitization (Renard et al., 2016). While this might suggest that CBD impaired the formation of an amphetamine memory that supports locomotor sensitization, these findings were within the context of mesolimbic mechanisms involved in the potential antipsychotic action of CBD. Moreover, even though the attenuation of locomotor sensitization was paralleled by modulation of cellular mechanisms of synaptic plasticity, it remains a challenge to distinguish learning‐related behavioural effects from modulation of drug reward (cf. Katsidoni et al., 2013; Prud'homme et al., 2015), which would impact upon reward‐dependent learning. The non‐mnemonic interpretation is supported by a failure of CBD to prevent the acquisition of amphetamine place preference (Parker et al., 2004). However, while it appears that CBD does not disrupt the formation of amphetamine‐related memories, this does not rule out potential effects on memories formed in relation to other drugs of abuse.
Subsequent to their acquisition, CBD might affect the expression of drug memories. Here there appears to be a disparity depending upon the drug reward under study. Acute administration of CBD (5 and 10 mg·kg−1) did not alter cocaine self‐administration or cue‐induced relapse to cocaine seeking (Mahmud et al., 2016) and so failed to replicate an earlier study of heroin self‐administration (Ren et al., 2009). While CBD (5 and 20 mg·kg−1) similarly did not alter heroin self‐administration, it did have an effect on cue‐induced relapse to heroin seeking (Ren et al., 2009), a measure of cue‐heroin memory expression. CBD (5 mg·kg−1) reduced responding in a cue‐induced relapse test but only when given 24 h, and not 30 min, prior to the test. This long‐lasting effect on the expression of the cue‐heroin memory was even more persistent (up to 14 days) when three consecutive daily injections of 5 mg·kg−1 CBD were given. This ability of CBD to have such long‐lasting effects may be mediated by an up‐regulation of AMPA GluA1 receptors in the nucleus accumbens (Ren et al., 2009).
The impaired expression of cue‐heroin relapse in response to CBD administration in animals suggests that this drug might have anti‐relapse properties in opiate addiction in humans. This has been explored in a preliminary study of heroin addicts, in which participants were given daily doses of CBD (400 or 800 mg) or placebo for 3 days (Hurd et al., 2015). CBD reduced craving both 24 h and 7 days later, mirroring the preclinical rodent study (Ren et al., 2009). This beneficial effect of CBD may not be limited to opiate addiction as a conceptually similar, albeit more modest, effect has also been observed in tobacco smokers (Morgan et al., 2013). In this small week‐long study, smokers were instructed to inhale a metered dose of CBD (400 μg) or placebo when they felt like smoking. CBD acutely reduced the number of cigarettes smoked, but this effect was not maintained after the cessation of CBD administration. Interestingly, and in contrast to the heroin study, CBD did not alter craving, either acutely or persistently. Therefore, it is not clear whether CBD has generalized effects on the expression of cue‐drug memory to elicit craving and precipitate relapse, or whether its effects are specific to certain classes of addictive drugs.
For the maintenance (i.e. reconsolidation) of drug‐related memories, there is a single study on morphine and cocaine conditioned place preference. When the place preference memory was briefly reactivated in order to trigger reconsolidation, CBD administration (10 mg·kg−1) immediately thereafter led to an impairment in the subsequent maintenance of both cocaine and morphine memories to reduce place preference at test (de Carvalho and Takahashi, 2016). This was a long‐lasting effect, which is usually evidence for reconsolidation impairments. However, the study lacked a true non‐reactivation control, and so the long‐lasting impairment, especially for morphine place preference, is not dissimilar to the aforementioned persistent reduction in the expression of cue‐heroin memories in the self‐administration setting (Ren et al., 2009). Therefore, it is still unclear whether CBD indeed impairs the reconsolidation of drug memories. Nevertheless, there are indications from the comparison between the place preference and self‐administration studies to suggest that their results might be underpinned by qualitatively different processes. For example, while the CBD‐induced impairment failed to ameliorate heroin‐primed reinstatement of drug seeking (Ren et al., 2009), post‐reactivation CBD did prevent morphine‐primed reinstatement of place preference (de Carvalho and Takahashi, 2016). Moreover, the contrasting effects of post‐reactivation CBD and acute CBD treatment on the subsequent expression of cocaine memories (de Carvalho and Takahashi, 2016) suggest that the impairment in cocaine place preference is not simply explained by long‐lasting modulation of drug memory expression.
Similarly, there is a single study on the effect of CBD on drug memory extinction. Injection of CBD (5 mg·kg−1) prior to an extinction trial enhanced the subsequent reduction in cocaine and amphetamine place preference (Parker et al., 2004). Despite the lack of a no‐extinction control, the observation that CBD reduces the expression of stimulant‐induced place preference again suggests that such a reduction was, at least in part, due to the concomitant extinction trial. Interestingly, the ability of CBD to reduce cocaine and amphetamine place preference in this extinction study (Parker et al., 2004) is similar to the previous observation that CBD impairs the reconsolidation of morphine and cocaine memories in the same place preference setting (de Carvalho and Takahashi, 2016). Indeed, while there was a difference in the timing of CBD administration between the two studies, the single behavioural trial that served to extinguish (Parker et al., 2004) or destabilize (de Carvalho and Takahashi, 2016) the drug memory did not differ greatly. The extinction trial was 15 min in duration, compared with a 10 min reactivation trial, although the former was confined to the drug‐paired chamber, whereas the latter was a test. Moreover, the conditioning parameters were similar across the two studies, and also to previous studies of reconsolidation that have used 30 min confined reactivation trials for amphetamine place preference (Sakurai et al., 2007), 20 min confined reactivation trials for cocaine place preference (Valjent et al., 2006) and 10 min confined reactivation trials for morphine and nicotine place preference (Wang et al., 2008; Fang et al., 2011). Also, given that the parameters of appetitive memory reconsolidation and extinction are usually well distinguished, such that they are each typically defined by very different durations of context re‐exposure or numbers of cue presentations (Flavell and Lee, 2013), it is unclear if CBD both enhances extinction and impairs reconsolidation of drug memories. It is perhaps more likely that the ability of CBD to reduce later drug place preference observed in these two studies (Parker et al., 2004; de Carvalho and Takahashi, 2016) instead reflects qualitatively similar processes. By simply considering the parametric comparisons presented above, we conclude that there is stronger evidence for CBD impairing drug memory reconsolidation than there is for it enhancing drug memory extinction. Furthermore, given that pharmacological enhancement of extinction is usually dependent upon appreciable extinction‐mediated memory reduction (Weber et al., 2007; Bouton et al., 2008), and there was no evidence for any such reduction in the CBD study (Parker et al., 2004), it remains unclear if CBD actually enhances drug memory extinction.
Concluding remarks and future directions
Converging lines of evidence have established that acute CBD treatment is anxiolytic in both animals and humans. A growing number of preclinical studies also indicate that this drug reduces fear memory expression when given acutely. Importantly, CBD produces an enduring reduction in learned fear expression when given in conjunction with fear memory reconsolidation or extinction by disrupting the former and facilitating the latter. This makes CBD a potential candidate for testing as a pharmacological adjunct to psychological therapies or behavioural interventions used in treating PTSD and phobias. These effects of CBD are mediated at least in part by 5‐HT1A receptors and indirectly via endocannabinoid‐mediated action on cannabinoid receptors, although the involvement of other possible pharmacological mechanisms has not yet been investigated. Studies have begun to elucidate the neural circuit mechanisms underlying the effects of CBD on anxiety and learned fear. The recent functional imaging studies in humans, which examined the alterations in brain activity that accompany the anxiolytic effects of CBD, may inform future preclinical and clinical studies investigating the wider neural circuitry involved in mediating its effects on learned fear. In contrast to anxiety and learned fear, research into the effects of CBD on addictive drug memory processing is still in its infancy. Therefore, further studies are needed to determine the psychological, pharmacological, and brain mechanisms involved in the attenuation of drug memory expression by CBD in relation to different classes of abused drugs. Given the significant co‐morbidity between anxiety‐related and substance abuse disorders, CBD should also be investigated as a common treatment for such disorders. One outstanding issue that needs to be addressed is determining the effects of chronic CBD treatment on different emotional memory processes. For example, one potential therapeutic strategy is to use CBD chronically to reduce symptoms by dampening fear and/or drug memory expression. However, CBD given acutely during the psychological therapy session aimed at impairing memory reconsolidation or enhancing extinction might be sufficient to facilitate this effect. Another important consideration is how CBD would be delivered for treating these disorders. Most of the recreationally used cannabis available today contains low levels of CBD and high levels of THC, which can exacerbate symptoms; however, cannabis strains containing a more favourable CBD : THC ratio might be an option (Hurd et al., 2015). Similarly, novel formulations of CBD containing only trace amounts of other phytocannabinoids have recently become available for the putative treatment of childhood epileptic disorders (e.g. Epidiolex, GW Pharmaceuticals; Gofshteyn et al., 2016). In summary, this line of research may lead to the development of a formulation of CBD for use as a treatment for anxiety‐related and substance abuse disorders in the future.
Conflict of interest
F.S.G. is co‐inventor of the patent ‘Fluorinated CBD compounds, compositions and uses thereof. Pub. No.: WO/2014/108899. International Application No.: PCT/IL2014/050023’; Def. US no. Reg. 62193296; 29/07/2015; INPI in 19/08/2015 (BR1120150164927).
Acknowledgements
F.S.G., J.L.C.L. and C.W.S. were funded jointly by a FAPESP‐University of Birmingham‐University of Nottingham pump‐priming award (2012/50896‐8). L.J.B. was funded by a Brazilian CNPq research fellowship (307895/2013‐0). The funders had no other involvement in any aspect of this work.
Lee, J. L. C. , Bertoglio, L. J. , Guimarães, F. S. , and Stevenson, C. W. (2017) Cannabidiol regulation of emotion and emotional memory processing: relevance for treating anxiety‐related and substance abuse disorders. British Journal of Pharmacology, 174: 3242–3256. doi: 10.1111/bph.13724.
References
- Adams R, Hunt M, Clark JH (1940). Structure of cannabidiol, a product isolated from the marihuana extract of minnesota wild hemp. J Am Chem Soc 62: 196–200. [Google Scholar]
- Aguilar MA, Rodríguez‐Arias M, Miñarro J (2009). Neurobiological mechanisms of the reinstatement of drug‐conditioned place preference. Brain Res Rev 59: 253–277. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Ligand‐gated ion channels. Br J Pharmacol 172: 5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Voltage‐gated ion channels. Br J Pharmacol 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015e). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015f). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015g). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida V, Levin R, Peres FF, Niigaki ST, Calzavara MB, Zuardi AW et al. (2013). Cannabidiol exhibits anxiolytic but not antipsychotic property evaluated in the social interaction test. Prog Neuropsychopharmacol Biol Psychiatry 41: 30–35. [DOI] [PubMed] [Google Scholar]
- Bergamaschi MM, Queiroz RH, Chagas MH, de Oliveira DC, De Martinis BS, Kapczinski F et al. (2011). Cannabidiol reduces the anxiety induced by simulated public speaking in treatment‐naïve social phobia patients. Neuropsychopharmacology 36: 1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I et al (2001). Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 134: 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitencourt RM, Pamplona FA, Takahashi RN (2008). Facilitation of contextual fear memory extinction and anti‐anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur Neuropsychopharmacol 18: 849–859. [DOI] [PubMed] [Google Scholar]
- Blessing EM, Steenkamp MM, Manzanares J, Marmar CR (2015). Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics 12: 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Vurbic D, Woods AM (2008). D‐cycloserine facilitates context‐specific fear extinction learning. Neurobiol Learn Mem 90: 504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein S (2015). Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg Med Chem 23: 1377–1385. [DOI] [PubMed] [Google Scholar]
- Campos AC, de Paula SV, Carvalho MC, Ferreira FR, Vicente MA, Brandão ML et al. (2013a). Involvement of serotonin‐mediated neurotransmission in the dorsal periaqueductal gray matter on cannabidiol chronic effects in panic‐like responses in rats. Psychopharmacology (Berl) 226: 13–24. [DOI] [PubMed] [Google Scholar]
- Campos AC, Ferreira FR, Guimarães FS (2012a). Cannabidiol blocks long‐lasting behavioral consequences of predator threat stress: possible involvement of 5HT1A receptors. J Psychiatr Res 46: 1501–1510. [DOI] [PubMed] [Google Scholar]
- Campos AC, Guimarães FS (2009). Evidence for a potential role for TRPV1 receptors in the dorsolateral periaqueductal gray in the attenuation of the anxiolytic effects of cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry 33: 1517–1521. [DOI] [PubMed] [Google Scholar]
- Campos AC, Guimarães FS (2008). Involvement of 5HT1A receptors in the anxiolytic‐like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology (Berl) 199: 223–230. [DOI] [PubMed] [Google Scholar]
- Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS (2012b). Multiple mechanisms involved in the large‐spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos Trans R Soc Lond B Biol Sci 367: 3364–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AC, Ortega Z, Palazuelos J, Fogaça MV, Aguiar DC, Díaz‐Alonso J et al. (2013b). The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: involvement of the endocannabinoid system. Int J Neuropsychopharmacol 16: 1407–1419. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Resstel LB, Bertoglio LJ, Carobrez Ade P, Guimarães FS (2010). Neuroanatomy of anxiety. Curr Top Behav Neurosci 2: 77–96. [DOI] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ (2005). Ethological and temporal analyses of anxiety‐like behavior: the elevated plus‐maze model 20 years on. Neurosci Biobehav Rev 29: 1193–1205. [DOI] [PubMed] [Google Scholar]
- Casarotto PC, Gomes FV, Resstel LB, Guimarães FS (2010). Cannabidiol inhibitory effect on marble‐burying behaviour: involvement of CB1 receptors. Behav Pharmacol 21: 353–358. [DOI] [PubMed] [Google Scholar]
- Chemel BR, Roth BL, Armbruster B, Watts VJ, Nichols DE (2006). WAY‐100635 is a potent dopamine D4 receptor agonist. Psychopharmacology (Berl) 188: 244–251. [DOI] [PubMed] [Google Scholar]
- Cheng D, Low JK, Logge W, Garner B, Karl T (2014). Chronic cannabidiol treatment improves social and object recognition in double transgenic APPswe/PS1∆E9 mice. Psychopharmacology (Berl) 231: 3009–3017. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Zuardi AW, Garrido GE, Wichert‐Ana L, Guarnieri R, Ferrari L et al. (2004). Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology 29: 417–426. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Derenusson GN, Ferrari TB, Wichert‐Ana L, Duran FL, Martin‐Santos R et al. (2011). Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol 25: 121–130. [DOI] [PubMed] [Google Scholar]
- Das RK, Kamboj SK, Ramadas M, Yogan K, Gupta V, Redman E et al. (2013). Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology (Berl) 226: 781–792. [DOI] [PubMed] [Google Scholar]
- Deiana S, Watanabe A, Yamasaki Y, Amada N, Arthur M, Fleming S et al. (2012). Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9‐tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive–compulsive behaviour. Psychopharmacology (Berl) 219: 859–873. [DOI] [PubMed] [Google Scholar]
- de Carvalho CR, Takahashi RN (2016). Cannabidiol disrupts the reconsolidation of contextual drug‐associated memories in Wistar rats. Addict Biol. doi:10.1111/adb.12366. [DOI] [PubMed] [Google Scholar]
- Di Luca M, Baker M, Corradetti R, Kettenmann H, Mendlewicz J, Olesen J et al. (2011). Consensus document on European brain research. Eur J Neurosci 33: 768–818. [DOI] [PubMed] [Google Scholar]
- Do Monte FH, Souza RR, Bitencourt RM, Kroon JA, Takahashi RN (2013). Infusion of cannabidiol into infralimbic cortex facilitates fear extinction via CB1 receptors. Behav Brain Res 250: 23–27. [DOI] [PubMed] [Google Scholar]
- ElBatsh MM, Assareh N, Marsden CA, Kendall DA (2012). Anxiogenic‐like effects of chronic cannabidiol administration in rats. Psychopharmacology (Berl) 221: 239–247. [DOI] [PubMed] [Google Scholar]
- Elmes MW, Kaczocha M, Berger WT, Leung K, Ralph BP, Wang L et al. (2015). Fatty acid‐binding proteins (FABPs) are intracellular carriers for Δ9‐tetrahydrocannabinol (THC) and cannabidiol (CBD). J Biol Chem 290: 8711–8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ (2014). Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories – indications for novel treatments of addiction. Eur J Neurosci 40: 2163–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagherazzi EV, Garcia VA, Maurmann N, Bervanger T, Halmenschlager LH, Busato SB et al. (2012). Memory‐rescuing effects of cannabidiol in an animal model of cognitive impairment relevant to neurodegenerative disorders. Psychopharmacology (Berl) 219: 1133–1140. [DOI] [PubMed] [Google Scholar]
- Fang Q, Li FQ, Li YQ, Xue YX, He YY, Liu JF et al. (2011). Cannabinoid CB1 receptor antagonist rimonabant disrupts nicotine reward‐associated memory in rats. Pharmacol Biochem Behav 99: 738–742. [DOI] [PubMed] [Google Scholar]
- Fenton GE, Pollard AK, Halliday DM, Mason R, Bredy TW, Stevenson CW (2014). Persistent prelimbic cortex activity contributes to enhanced learned fear expression in females. Learn Mem 21: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell CR, Lee JLC (2013). Reconsolidation and extinction of an appetitive pavlovian memory. Neurobiol Learn Mem 104: 25–31. [DOI] [PubMed] [Google Scholar]
- Fogaça MV, Reis FM, Campos AC, Guimarães FS (2014). Effects of intra‐prelimbic prefrontal cortex injection of cannabidiol on anxiety‐like behavior: involvement of 5HT1A receptors and previous stressful experience. Eur Neuropsychopharmacol 24: 410–419. [DOI] [PubMed] [Google Scholar]
- Fusar‐Poli P, Allen P, Bhattacharyya S, Crippa JA, Mechelli A, Borgwardt S et al. (2010). Modulation of effective connectivity during emotional processing by delta 9‐tetrahydrocannabinol and cannabidiol. Int J Neuropsychopharmacol 13: 421–432. [DOI] [PubMed] [Google Scholar]
- Fusar‐Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin‐Santos R et al. (2009). Distinct effects of {delta}9‐tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry 66: 95–105. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R (1964). Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc 86: 1646. [Google Scholar]
- Gazarini L, Stern CA, Piornedo RR, Takahashi RN, Bertoglio LJ (2015). PTSD‐like memory generated through enhanced noradrenergic activity is mitigated by a dual step pharmacological intervention targeting its reconsolidation. Int J Neuropsychopharmacol 18. doi:10.1093/ijnp/pyu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustino TF, Maren S (2015). The role of the medial prefrontal cortex in the conditioning and extinction of fear. Front Behav Neurosci 9: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gofshteyn JS, Wilfong A, Devinsky O, Bluvstein J, Charuta J, Ciliberto MA et al. (2016). Cannabidiol as a potential treatment for febrile infection‐related epilepsy syndrome (FIRES) in the acute and chronic phases. J Child Neurol 32: 35–40. [DOI] [PubMed] [Google Scholar]
- Gomes FV, Reis DG, Alves FH, Corrêa FM, Guimarães FS, Resstel LB (2012). Cannabidiol injected into the bed nucleus of the stria terminalis reduces the expression of contextual fear conditioning via 5‐HT1A receptors. J Psychopharmacol 26: 104–113. [DOI] [PubMed] [Google Scholar]
- Gomes FV, Resstel LB, Guimarães FS (2011). The anxiolytic‐like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5‐HT1A receptors. Psychopharmacology (Berl) 213: 465–473. [DOI] [PubMed] [Google Scholar]
- Gould J (2015). The Cannabis crop. Nature 525: S2–S3. [DOI] [PubMed] [Google Scholar]
- Graeff FG (2002). On serotonin and experimental anxiety. Psychopharmacology (Berl) 163: 467–476. [DOI] [PubMed] [Google Scholar]
- Granjeiro EM, Gomes FV, Guimarães FS, Corrêa FM, Resstel LB (2011). Effects of intracisternal administration of cannabidiol on the cardiovascular and behavioral responses to acute restraint stress. Pharmacol Biochem Behav 99: 743–748. [DOI] [PubMed] [Google Scholar]
- Gross CT, Canteras NS (2012). The many paths to fear. Nat Rev Neurosci 13: 651–658. [DOI] [PubMed] [Google Scholar]
- Guimarães FS, Chiaretti TM, Graeff FG, Zuardi AW (1990). Antianxiety effect of cannabidiol in the elevated plus‐maze. Psychopharmacology (Berl) 100: 558–559. [DOI] [PubMed] [Google Scholar]
- Guimarães FS, de Aguiar JC, Mechoulam R, Breuer A (1994). Anxiolytic effect of cannabidiol derivatives in the elevated plus‐maze. Gen Pharmacol 25: 161–164. [DOI] [PubMed] [Google Scholar]
- Handley SL, Mithani S (1984). Effects of alpha2‐adrenoceptor agonists and antagonists in a maze‐exploration model of fear‐motivated behavior. Naunyn Schmiedebergs Arch Pharmacol 327: 1–5. [DOI] [PubMed] [Google Scholar]
- Hanuš LO, Tchilibon S, Ponde DE, Breuer A, Fride E, Mechoulam R (2005). Enantiomeric cannabidiol derivatives: synthesis and binding to cannabinoid receptors. Org Biomol Chem 3: 1116–1123. [DOI] [PubMed] [Google Scholar]
- Hsiao YT, Yi PL, Li CL, Chang FC (2012). Effect of cannabidiol on sleep disruption induced by the repeated combination tests consisting of open field and elevated plus‐maze in rats. Neuropharmacology 62: 373–384. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Yoon M, Manini AF, Hernandez S, Olmedo R, Ostman M et al. (2015). Early phase in the development of cannabidiol as a treatment for addiction: opioid relapse takes initial center stage. Neurotherapeutics 12: 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibeas Bih C, Chen T, Nunn AV, Bazelot M, Dallas M, Whalley BJ (2015). Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics 12: 699–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R (2009). Non‐psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci 30: 515–527. [DOI] [PubMed] [Google Scholar]
- Jurkus R, Day HL, Guimarães FS, Lee JL, Bertoglio LJ, Stevenson CW (2016). Cannabidiol regulation of learned fear: implications for treating anxiety‐related disorders. Front Pharmacol 7: 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karniol IG, Carlini EA (1973). Pharmacological interaction between cannabidiol and delta‐9‐tetrahydrocannabinol. Psychopharmacologia 33: 53–70. [DOI] [PubMed] [Google Scholar]
- Katsidoni V, Anagnostou I, Panagis G (2013). Cannabidiol inhibits the reward‐facilitating effect of morphine: involvement of 5‐HT1A receptors in the dorsal raphe nucleus. Addict Biol 18: 286–296. [DOI] [PubMed] [Google Scholar]
- Kindt M (2014). A behavioural neuroscience perspective on the aetiology and treatment of anxiety disorders. Behav Res Ther 62: 24–36. [DOI] [PubMed] [Google Scholar]
- Lee JL (2009). Reconsolidation: maintaining memory relevance. Trends Neurosci 32: 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JI, Resstel LB, Guimarães FS (2010). Involvement of the prelimbic prefrontal cortex on cannabidiol‐induced attenuation of contextual conditioned fear in rats. Behav Brain Res 207: 105–111. [DOI] [PubMed] [Google Scholar]
- Levin R, Almeida V, Peres FF, Calzavara MB, da Silva ND, Suiama MA et al. (2012). Antipsychotic profile of cannabidiol and rimonabant in an animal model of emotional context processing in schizophrenia. Curr Pharm Des 18: 4960–4965. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C et al. (2012). Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2: e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long LE, Chesworth R, Huang XF, McGregor IS, Arnold JC, Karl T (2010). A behavioural comparison of acute and chronic delta9‐tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. Int J Neuropsychopharmacol 13: 861–876. [DOI] [PubMed] [Google Scholar]
- Long LE, Chesworth R, Huang XF, Wong A, Spiro A, McGregor IS et al. (2012). Distinct neurobehavioural effects of cannabidiol in transmembrane domain neuregulin 1 mutant mice. PLoS One 7: e34129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmud A, Gallant S, Sedki F, D'Cunha T, Shalev U (2016). Effects of an acute cannabidiol treatment on cocaine self‐administration and cue‐induced cocaine seeking in male rats. J Psychopharmacol. doi:10.1177/0269881116667706. [DOI] [PubMed] [Google Scholar]
- Malone DT, Jongejan D, Taylor DA (2009). Cannabidiol reverses the reduction in social interaction produced by low dose delta(9)‐tetrahydrocannabinol in rats. Pharmacol Biochem Behav 93: 91–96. [DOI] [PubMed] [Google Scholar]
- Marinho AL, Vila‐Verde C, Fogaça MV, Guimarães FS (2015). Effects of intra‐infralimbic prefrontal cortex injections of cannabidiol in the modulation of emotional behaviors in rats: contribution of 5HT₁A receptors and stressful experiences. Behav Brain Res 286: 49–56. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ (2004). A two‐dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav Rev 28: 285–305. [DOI] [PubMed] [Google Scholar]
- McPartland JM, Duncan M, DI Marzo V, Pertwee RG (2015). Are cannabidiol and Δ(9) ‐tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol 172: 737–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Shvo Y (1963). 1. Structure of cannabidiol. Tetrahedron 19: 2073–2078. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Guimarães FS (2006). Anxiolytic‐like effect of cannabidiol in the rat Vogel conflict test. Prog Neuropsychopharmacol Biol Psychiatry 30: 1466–1471. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Das RK, Joye A, Curran HV, Kamboj SK (2013). Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict Behav 38: 2433–2436. [DOI] [PubMed] [Google Scholar]
- Musty RE, Conti LH, Mechoulam R (1985). Anxiolytic properties of cannabidiol In: Harvey D. (ed). Marihuana '84. IRL Press: Oxford, pp. 713–719. [Google Scholar]
- Nardo M, Casarotto PC, Gomes FV, Guimarães FS (2014). Cannabidiol reverses the mCPP‐induced increase in marble‐burying behavior. Fundam Clin Pharmacol 28: 544–550. [DOI] [PubMed] [Google Scholar]
- Norris C, Loureiro M, Kramar C, Zunder J, Renard J, Rushlow W et al. (2016). Cannabidiol modulates fear memory formation through interactions with serotonergic transmission in the mesolimbic system. Neuropsychopharmacology 41: 2839–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien LD, Wills KL, Segsworth B, Dashney B, Rock EM, Limebeer CL et al. (2013). Effect of chronic exposure to rimonabant and phytocannabinoids on anxiety‐like behavior and saccharin palatability. Pharmacol Biochem Behav 103: 597–602. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Green MR, Martin BR (1990). Pharmacological characterization of cannabinoids in the elevated plus maze. J Pharmacol Exp Ther 253: 1002–1009. [PubMed] [Google Scholar]
- Parker LA, Burton P, Sorge RE, Yakiwchuk C, Mechoulam R (2004). Effect of low doses of delta9‐tetrahydrocannabinol and cannabidiol on the extinction of cocaine‐induced and amphetamine‐induced conditioned place preference learning in rats. Psychopharmacology (Berl) 175: 360–366. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M (1985). Validation of open:closed arm entries in an elevated plus‐maze as a measure of anxiety in the rat. J Neurosci Methods 14: 149–167. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ (2009). Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem 16: 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud'homme M, Cata R, Jutras‐Aswad D (2015). Cannabidiol as an intervention for addictive behaviors: a systematic review of the evidence. Subst Abuse 9: 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Whittard J, Higuera‐Matas A, Morris CV, Hurd YL (2009). Cannabidiol, a nonpsychotropic component of cannabis, inhibits cue‐induced heroin seeking and normalizes discrete mesolimbic neuronal disturbances. J Neurosci 29: 14764–14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard J, Loureiro M, Rosen LG, Zunder J, de Oliveira C, Schmid S et al. (2016). Cannabidiol counteracts amphetamine‐induced neuronal and behavioral sensitization of the mesolimbic dopamine pathway through a novel mTOR/p70S6 kinase signaling pathway. J Neurosci 36: 5160–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LB, Joca SR, Moreira FA, Corrêa FM, Guimarães FS (2006). Effects of cannabidiol and diazepam on behavioral and cardiovascular responses induced by contextual conditioned fear in rats. Behav Brain Res 172: 294–298. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Tavares RF, Lisboa SF, Joca SR, Corrêa FM, Guimarães FS (2009). 5‐HT1A receptors are involved in the cannabidiol‐induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br J Pharmacol 156: 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E, Guy GW (2006). A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses 66: 234–246. [DOI] [PubMed] [Google Scholar]
- Sakurai S, Yu L, Tan SE (2007). Roles of hippocampal N‐methyl‐D‐aspartate receptors and calcium/calmodulin‐dependent protein kinase II in amphetamine‐produced conditioned place preference in rats. Behav Pharmacol 18: 497–506. [DOI] [PubMed] [Google Scholar]
- Schiavon AP, Bonato JM, Milani H, Guimarães FS, Weffort de Oliveira RM (2016). Influence of single and repeated cannabidiol administration on emotional behavior and markers of cell proliferation and neurogenesis in non‐stressed mice. Prog Neuropsychopharmacol Biol Psychiatry 64: 27–34. [DOI] [PubMed] [Google Scholar]
- Shannon S, Opila‐Lehman J (2016). Effectiveness of cannabidiol oil for pediatric anxiety and insomnia as part of posttraumatic stress disorder: a case report. Perm J 20: 108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira Filho NG, Tufik S (1981). Comparative effects between cannabidiol and diazepam on neophobia, food intake and conflict behavior. Res Comm Psychol Psychiatr Behav 6: 25–66. [Google Scholar]
- Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ (2015). Pharmacology of cognitive enhancers for exposure‐based therapy of fear, anxiety and trauma‐related disorders. Pharmacol Ther 149: 150–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares Vde P, Campos AC, Bortoli VC, Zangrossi H Jr, Guimarães FS, Zuardi AW (2010). Intra‐dorsal periaqueductal gray administration of cannabidiol blocks panic‐like response by activating 5‐HT1A receptors. Behav Brain Res 213: 225–229. [DOI] [PubMed] [Google Scholar]
- Song C, Stevenson CW, Guimarães FS, Lee JL (2016). Bidirectional effects of cannabidiol on contextual fear memory extinction. Front Pharmacol 7: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW (2011). Drug wanting: behavioral sensitization and relapse to drug‐seeking behavior. Pharmacol Rev 63: 348–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CA, Gazarini L, Takahashi RN, Guimarães FS, Bertoglio LJ (2012). On disruption of fear memory by reconsolidation blockade: evidence from cannabidiol treatment. Neuropsychopharmacology 37: 2132–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CA, Gazarini L, Vanvossen AC, Zuardi AW, Guimaraes FS, Takahashi RN et al. (2014). Involvement of the prelimbic cortex in the disruptive effect of cannabidiol on fear memory reconsolidation. Eur Neuropsychopharmacol 24: S322. [DOI] [PubMed] [Google Scholar]
- Stern CA, Gazarini L, Vanvossen AC, Zuardi AW, Galve‐Roperh I, Guimaraes FS et al. (2015). Δ9‐Tetrahydrocannabinol alone and combined with cannabidiol mitigate fear memory through reconsolidation disruption. Eur Neuropsychopharmacol 25: 958–965. [DOI] [PubMed] [Google Scholar]
- Tipps ME, Raybuck JD, Lattal KM (2014). Substance abuse, memory, and post‐traumatic stress disorder. Neurobiol Learn Mem 112: 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd SM, Arnold JC (2016). Neural correlates of interactions between cannabidiol and Δ(9)‐tetrahydrocannabinol in mice: implications for medical cannabis. Br J Pharmacol 173: 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A (2015). Neuronal circuits for fear and anxiety. Nat Rev Neurosci 16: 317–331. [DOI] [PubMed] [Google Scholar]
- Treit D, Menard J, Royan C (1993). Anxiogenic stimuli in the elevated plus‐maze. Pharmacol Biochem Behav 44: 463–469. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR (2013). Addiction: a drug‐induced disorder of memory reconsolidation. Curr Opin Neurobiol 23: 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twardowschy A, Castiblanco‐Urbina MA, Uribe‐Mariño A, Biagioni AF, Salgado‐Rohner CJ, Crippa JA et al. (2013). The role of 5‐HT1A receptors in the anti‐aversive effects of cannabidiol on panic attack‐like behaviors evoked in the presence of the wild snake Epicrates cenchria crassus (Reptilia, Boidae). J Psychopharmacol 27: 1149–1159. [DOI] [PubMed] [Google Scholar]
- Uribe‐Mariño A, Francisco A, Castiblanco‐Urbina MA, Twardowschy A, Salgado‐Rohner CJ, Crippa JA et al. (2012). Anti‐aversive effects of cannabidiol on innate fear‐induced behaviors evoked by an ethological model of panic attacks based on a prey vs the wild snake Epicrates cenchria crassus confrontation paradigm. Neuropsychopharmacology 37: 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corbille AG, Bertran‐Gonzalez J, Herve D, Girault JA (2006). Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci U S A 103: 2932–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann RE, Gamage TF, Warner JA, Marshall EM, Taylor NL, Martin BR et al. (2008). Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of delta(9)‐tetrahydrocannabinol. Drug Alcohol Depend 94: 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XY, Zhao M, Ghitza UE, Li YQ, Lu L (2008). Stress impairs reconsolidation of drug memory via glucocorticoid receptors in the basolateral amygdala. J Neurosci 28: 5602–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Hart J, Richardson R (2007). Effects of D‐cycloserine on extinction of learned fear to an olfactory cue. Neurobiol Learn Mem 87: 476–482. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Cosme RA, Graeff FG, Guimarães FS (1993). Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharmacol 7 (1 Suppl): 82–88. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Karniol IG (1983). Changes in the conditioned emotional response of rats induced by 9‐THC, CBD and mixture of the two cannabinoids. Arq Biol Tecnol 26: 391–397. [Google Scholar]
- Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG (1982). Action of cannabidiol on the anxiety and other effects produced by delta 9‐THC in normal subjects. Psychopharmacology (Berl) 76: 245–250. [DOI] [PubMed] [Google Scholar]


