Abstract
Aims
The objective of this study was to examine factors that predict antiretroviral therapy (ART) access among eligible, HIV-positive methadone maintenance treatment (MMT) clients. We also tested the hypothesis that sustained MMT participation increases the likelihood of accessing ART.
Design
A nationwide cohort study conducted from March 1, 2004 to December 31, 2011.
Setting
MMT clients were followed from the time of their enrollment in China's national MMT program until their death or the study end date.
Participants
Our cohort was composed of 7,111 ART-eligible, HIV-positive MMT clients, 49.2% of whom remained ART-naïve and 50.8% of whom received ART.
Measurements
Demographic variables, drug use history, MMT program participation, and HIV-related clinical characteristics of study participants who remained naïve to ART and those who accessed ART were compared by univariate and multivariable analysis.
Findings
Predictors of accessing ART among this cohort included being retained in MMT at the time of first meeting ART eligibility (AOR=1.84, CI: 1.55-2.21, p<0.001) compared to meeting ART eligibility before entering MMT (AOR=0.98, CI:0.80-1.21, p=0.849) or previously entering MMT and dropping out before meeting ART eligibility. Additional predictors were CD4 >200 cells/μL when ART-eligibility requirement was first met (AOR=1.94, CI: 1.73-2.19, p<0.001 compared to CD4=200-350 cells/μL), and being in a stable partner relationship (married/cohabitating: AOR=1.14, CI: 1.01-1.28, p=0.029).
Conclusions
Retained participation in methadone maintenance treatment (MMT) increases the likelihood that eligible clients will access antiretroviral therapy (ART). These results highlight the potential benefit of co-localization of MMT and ART services in a “one-stop-shop” model.
Keywords: HIV/AIDS, drug user, methadone maintenance treatment, heroin, antiretroviral therapy, China
Introduction
Globally, more than 33 million people were estimated to be living with HIV/AIDS in 2009,1 3 million of whom were drug users (DUs).2 China is thought to have the largest DU population in the world, with an estimated 2.35 million people abusing a broad variety of illicit substances by various methods,2, 3 and China's HIV epidemic is well-known to have originated and spread rapidly within this population, particularly among injection drug users (IDUs), as a result of unsafe drug use behaviors (i.e., sharing needles). Compared to these global figures, DUs represent a much larger proportion of those infected with HIV in China—approximately 28.4% of the estimated 780,000 HIV cases nationwide by the end of 2011,4 or roughly 222,000 HIV-infected DUs.
Unfortunately, despite rapid expansion in recent years of treatment coverage for both drug dependence and HIV/AIDS by China's National Methadone Maintenance Treatment (MMT) Program and National Free Antiretroviral Treatment Program (NFATP), respectively, only a relatively small proportion of HIV-positive DUs have accessed treatment for either drug dependence or HIV infection, and even fewer are receiving treatment for both.5, 6 Although China's MMT program has now become the largest opioid-replacement treatment network in the world,7 having served more than 300,000 clients cumulatively since its initiation in 2004, HIV-positive clients appear to be under-represented, composing only approximately 6% of the total.8 Similarly, DUs represented a mere 15% of the nearly 67,000 HIV-positive patients who began antiretroviral therapy (ART) between 2002 and 2009 through China's NFATP.9 Low treatment coverage among DUs is not surprising, given the relative youth of these programs and the enormous challenges inherent in scaling-up national programs in a large, developing, and rapidly industrializing country. China is not unique in this situation; estimates of drug addiction treatment coverage and ART coverage among eligible HIV-infected DUs are low worldwide.5
This problem of how treatment engagement can be promoted in the DU population is complex. Drug users often have a general distrust of the healthcare system in China, and they may fear that seeking drug treatment may lead to encounters with police. In many cases, these individuals have other serious comorbid conditions such as psychiatric disorders (i.e., post-traumatic stress disorder, schizophrenia, anxiety, or depression),10 or hepatitis C virus (HCV) infection, which is thought to affect an estimated 1.6 million DUs in China.11 It is thus not hard to imagine that the barriers to treatment faced by DUs newly diagnosed with HIV in China are significant. These barriers are further exaggerated in light of the stigma still surrounding HIV infection in China, which makes some individuals reluctant to come forward to even be tested, much less to engage in treatment.
New evidence from other countries has suggested that enrollment in MMT itself independently promotes engagement in, and increased subsequent adherence to, ART for eligible HIV-positive MMT clients.12-14 The primary proposed mechanism is that MMT is effective in stabilizing clients and offering opportunities for medical treatment intervention.12 Enrollment in MMT brings HIV-infected DU into regular contact with the health care system and is associated with regular monitoring of CD4 count, which is a crucial factor in determining ART eligibility.15, 16 The daily treatment with methadone also allows for directly observed therapy options for ART by providers.17
While the effectiveness of China's national MMT program has been evaluated via a variety of measures (i.e., reductions in ongoing drug abuse, drug-related crime, heroin consumption and trade, and new HIV infections),10, 18, 19 its effect on promoting access of its HIV-positive clients to life-saving ART has not been previously assessed. In this study, we aimed to identify factors associated with accessing ART among eligible, HIV-positive, MMT clients and to test the hypothesis that long-term enrollment in MMT promotes ART engagement.
Methods
Study design and participants
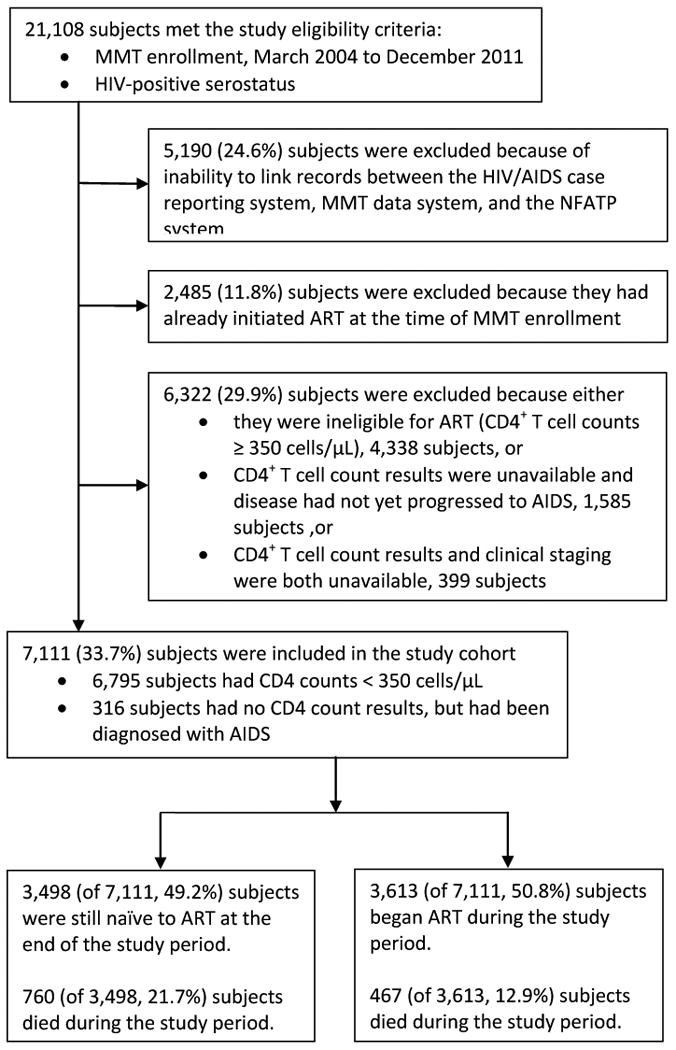
This study was designed to examine a nationwide cohort of HIV-positive, ART-eligible, MMT clients; specifically, we aimed to compare those who accessed ART and those who did not over the study period (March 2004 to December 2011) and to identify predictors for accessing ART. Eligibility criteria were 1) enrollment in MMT during the study period, and 2) HIV-positive serostatus as reported on client MMT data system records. HIV-positive serostatus is determined by positive results on at least two ELISA-based screening assays and one Western blot confirmatory test. Subjects were excluded from the study if 1) it was not possible to link their records in the HIV/AIDS case reporting system and the MMT data system, 2) they had already initiated ART at the time of MMT enrollment (based on existing records in the NFATP data system), or 3) they were ineligible for ART, their CD4 count results were unavailable, or their clinical staging was unavailable. In accordance with World Health Organization recommendations,20 the eligibility criteria for ART in China are a CD4 <350 cells/μL (expanded from the <200 cells/μL eligibility guidelines in 200821) or a clinical diagnosis of AIDS. Recommended first-line ART regimens for all eligible HIV-positive patients involve combinations of zidovudine, stavudine, lamivudine, nevirapine and efavirenz.22 A flow chart depicting the cohort development is shown in Figure 1. All subjects included in the cohort were followed until the study endpoint or until death, whichever came first.
Figure 1. Development of the study cohort.
Data collection
For this study, we extracted data from three independent, ongoing, observational databases, each of which form a part of the national online data collection system managed by the Chinese National Center for AIDS/STD Control and Prevention (NCAIDS): 1) the HIV/AIDS case reporting system,23 2) the MMT data system,10 and 3) the National Free Antiretroviral Treatment Program (NFATP) data system.24, 25 Providers upload case report information to the databases daily, and updates can be viewed by NCAIDS staff in real-time. Provincial-level Center for Disease Control and Prevention (CDC) staff assess the databases for duplicate records, errors, and omissions on a regular basis. For the purposes of this study, datasets were downloaded from these three systems on January 31, 2012. The data included national identification number, demographic information, drug use history, HIV-related clinical information including ART status, and HCV serostatus. Datasets were linked by clients' national identification numbers.
HIV/AIDS Case Reporting System
The national HIV/AIDS case reporting system was launched in 1985, with the first confirmed case of HIV infection in a foreigner visiting China. This system has since been upgraded to a real-time, web-based data system in which all hospitals and local CDC offices that have certified HIV testing laboratories are required to report discovery of new HIV/AIDS cases within 24 hours. Records created in this system include identification and demographic information, health status information, HIV testing results, ART engagement status, and other HIV-related clinical information, and self-reported transmission route.23
MMT Data System
Upon enrollment in MMT, new clients are required to provide identification and demographic information and detailed drug use history. This initial baseline survey information is updated with information collected from follow-up surveys conducted after six months and then annually. Clients are also tested at survey times for HIV, HCV, and syphilis, and results of these tests are also recorded in this data system. Methadone treatment dose and other treatment-related information for each client is recorded and uploaded daily.10
NFATP Data System
Upon initiation of ART by an eligible, HIV-positive patient, a record is created in this data system that includes information related to the ongoing clinical management of the HIV-positive patient and all ART-related information (i.e., drugs, doses, CD4 counts, viral load, etc.) as well as standard identification and demographic information.24, 25
Statistical analysis
Categorical variables are presented as number and percentages. Continuous variables are split into categories and presented as number and percentages and are also described using median and interquartile-range (IQR). ART-naïve and ART-treated subgroup characteristics were compared using Wilcoxon Rank-Sum test for continuous variables and Chi-Square Test for categorical variables. Univariate analysis was conducted to produce unadjusted odds ratios (ORs), confidence intervals (CIs), and p-values. All variables, regardless of whether they had statistically significant p-values in univariate analysis, were included in our multivariable logistic regression model. Coding of variables for the multivariable analysis was based on the distribution of data and experience with this cohort. Coding was performed in multiple ways (e.g. different age range subgroups) and presented in the format that was most informative.. Missing values received no special treatment, but rather were excluded from the analyses. All CIs reported are 95%, and all p-values are two-sided. P-values less than 0.05 were considered statistically significant. All statistical analyses were performed using SAS software (Version 9.1.3, SAS Institute Inc., Cary, NC, USA).
Ethical approval
The Institute of Review Board (IRB) of National Center for AIDS/STD Control and Prevention (NCAIDS) reviewed and approved the study. This study employed data routinely collected as a part of China's HIV/AIDS case monitoring efforts and NFATP and MMT program participation. As a part of these public programs, informed consent is required to be collected from each subject at the beginning of participation. A request of waver of written informed consent for inclusion in this study was reviewed and approved by NCAIDS IRB. All personal identifiers were removed from the final dataset to preserve anonymity.
Results
Study cohort
The development of our study cohort is depicted in the form of a flow diagram in Figure 1. In summary, 21,108 HIV-positive MMT clients met the eligibility criteria. Of these subjects, 5,190 (24.6%) were excluded due to inability to link their records across the three data systems, and 6,322 (29.9%) were excluded due to ART ineligibility (i.e., CD4 ≥350 and no clinical AIDS diagnosis or no available information). A further 2,485 (11.8%) individuals were excluded because they had already begun ART prior to MMT enrollment; the demographic and clinical characteristics of these excluded persons were statistically similar to the enrolled study cohort. The average age was 35 years (IQR: 31-39), 13.65% were female, and 39.49% were married. The average time from initiating ART to entering MMT was 0.9 years (IQR: 0.3-2.0), and the baseline CD4 count was 193 (IQR:110-269).
Figure 2 presents the annual cumulative enrollment numbers in MMT and ART from 2004 to 2011. Prior to 2006, drug users were not covered under the NFATP. The final study cohort was composed of 7,111 subjects, 3,498 (49.2%) of whom remained ART-naïve by the end of the study period, and 3,613 (50.8%) of whom started ART during the study period. Prior to the end of the study, 760 (21.7%) subjects within the ART-naïve subgroup and 467 (12.9%) subjects in the ART-treated group had died. Figure 3 describes the possible pathways between MMT entry, ART-eligibility, and ART initiation (if applicable) for observed individuals in our study cohort and the associated duration of each period in months (median and IQR).
Figure 2. Cumulative annual enrollment of MMT and ART participants in China, 2004-2011.
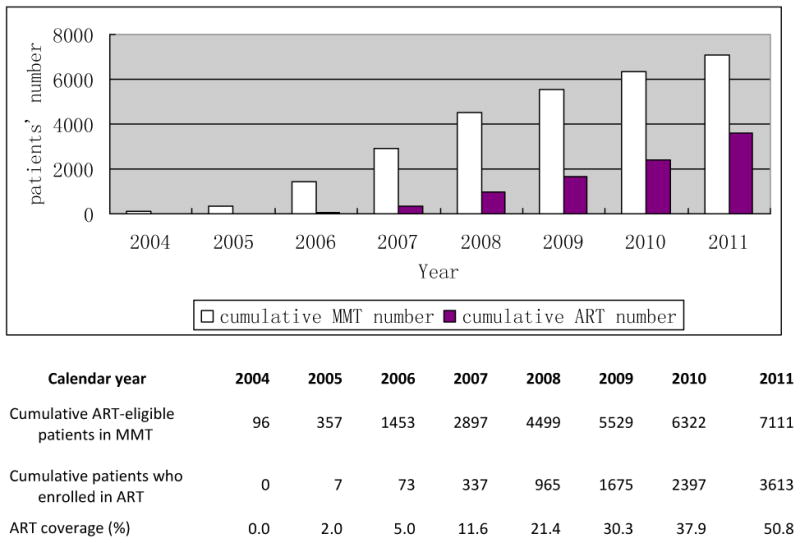
Figure 3. Duration of observed pathways between MMT entry, ART eligibility, and ART initiation.
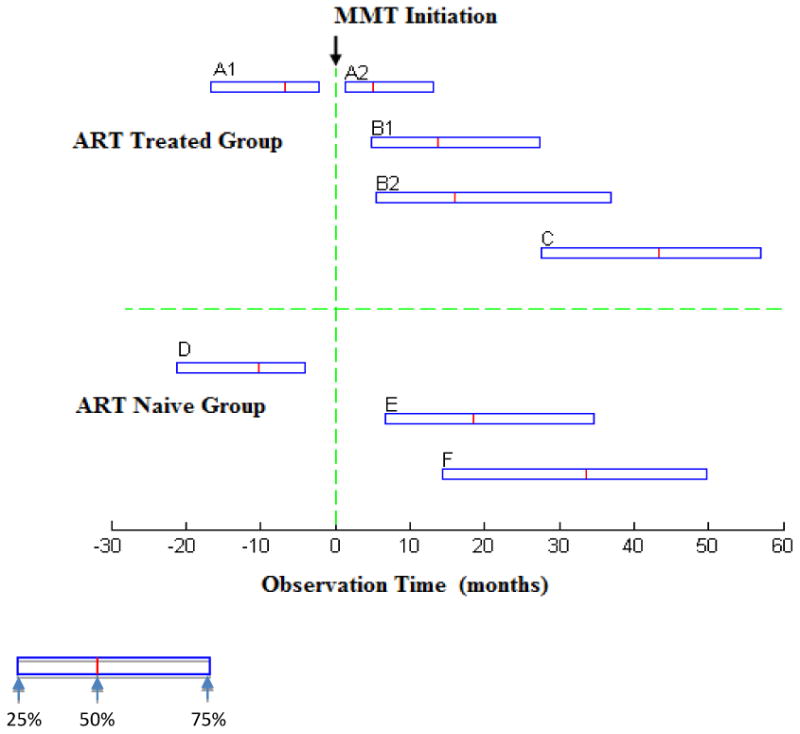
A, B, and C describes patients who received ART. (A: N=807; B: N=2806; C: N=3613).
A describes patients who met ART eligibility criteria before entering MMT. A1 is the period from first meeting ART eligibility criteria to entering MMT (A1: Median=6.74 months, IQR: 2.20-16.59). A2 is the period from entering MMT to starting ART (A2: Median= 4.99 months, IQR: 1.31-13.08).
B describes patients who met ART criteria after entering MMT. B1 is the period from entering MMT to first meeting ART eligibility criteria (B1: Median=13.70 months, IQR: 4.83-27.47). B2 is the period from first meeting ART criteria to starting ART (B2: Median=2.33 months, IQR: 0.72-9.56).
C is the period from MMT initiation to the study endpoint for all ART-treated patients (N=3613, C: Median = 43.43 months, IQR: 27.56-56.94). Due to drop-outs and re-entries, actual median retention time in MMT was 34.8 months (IQR 13.2-51.6).
D, E, and F describes ART-naïve patients (D: N=955; E N=2538; F N=3498).
D describes patients who met ART eligibility criteria before entering MMT. D is the period between first meeting ART eligibility criteria to entering MMT (D: Median=10.12 months, IQR: 3.75-21.09.
E describes patients who met ART criteria after entering MMT. E is the period between entering MMT to first meeting ART eligibility criteria.(E: Median=18.43 months, IQR: 6.74- 34.66).
F is the period from MMT initiation to the study endpoint for all ART-naive patients (N=3498, F: Median= 33.64 months, IQR: 14.39-49.74), Due to drop-outs and re-entries, actual median retention time at MMT was 19.2 months (IQR: 6.0-42.0).
Patients who never received ART were observed in the study for a median period of 18 months (CI: 7-31) from initial treatment-eligible CD4+ tests to their death or the end of the study (December 2011). In comparison, patients who ultimately received ART were observed for 6 months (CI: 1-18) from their initial treatment-eligible CD4 tests to ART initiation.
Table 1 summarizes the demographic, drug use history, and clinical characteristics of the study cohort. Upon MMT enrollment, the median age of study participants was 33 years (IQR: 29-38). The majority of subjects were male (85.4%), had a middle school-level education or greater (69.7%), were Han Chinese (66.4%), were unemployed (67.8%), and were single, divorced, or widowed (61.7%). Most subjects had used drugs for ≥10 years (62.6%) and had had a history of drug rehabilitation (91.7%) prior to MMT enrollment. The majority of subjects had CD4 between 200-350 at time of first meeting ART-eligibility requirements (68.9%, 4,681/6,795) and first met ART-eligibility criteria in 2008-2009 (39.7%) or 2010-2011 (42.0%) and. Over half (60.9%) of the study cohort had entered MMT and were retained in MMT at the time of meeting ART eligibility, compared to 14.3% who had previously entered and dropped out of MMT and 24.8% who were ART-eligible at the time of MMT entry. Most participants in the study were also HCV-positive (84.1%, 4,977/5,918).
Table 1. Demographic characteristics, drug use history, and clinical characteristics among eligible MMT clients upon MMT program enrollment, China, 2004-2011.
| Characteristics | All Subjects N (%) |
ART-naïve Subjects N (%) |
ART-treated Subjects N (%) |
Value of Statistic (X2/T-test) |
P-value*§ |
|---|---|---|---|---|---|
| Overall | 7,111 (100%) | 3,498 (100%) | 3,613 (100%) | ||
|
| |||||
| Demographic Characteristics | |||||
|
| |||||
| Age (years) | |||||
| ≤ 29 | 1,802 (25.3) | 897 (25.6) | 905 (25.0) | 14.28 | 0.003 |
| 30 - 34 | 2,233 (31.4) | 1,039 (29.7) | 1,194 (33.0) | ||
| 35 - 39 | 1,904 (26.8) | 938 (26.8) | 966 (26.7) | ||
| ≥ 40 | 1,172 (16.5) | 624 (17.8) | 548 (15.2) | ||
| Median (IQR) | 33 (29-38) | 34 (29-38) | 33 (29-37) | 1.95 | 0.052 |
| Gender | |||||
| Male | 6,075 (85.4) | 3,011 (86.1) | 3,064 (84.8) | 2.31 | 0.128 |
| Female | 1,036 (14.6) | 487 (13.9) | 549 (15.2) | ||
| Education level | |||||
| ≤ Primary school | 2,151 (30.3) | 1,095 (31.3) | 1,056 (29.2) | 4.57 | 0.102 |
| ≥ Middle school | 4,959 (69.7) | 2,403 (68.7) | 2,556 (70.8) | ||
| Ethnicity | |||||
| Han | 4,719 (66.4) | 2,307 (66.0) | 2,412 (66.8) | 1.51 | 0.470 |
| Other | 2,391 (33.6) | 1,191 (34.0) | 1,200 (33.2) | ||
| Employment status | |||||
| Unemployed | 4,821 (67.8) | 2,372 (67.8) | 2,249 (67.8) | 0.97 | 0.616 |
| Employed | 2,289 (32.2) | 1,126 (32.2) | 1,163(32.2) | ||
| Marital status | |||||
| Single/divorced/widowed | 4,384 (61.7) | 2,215 (63.3) | 2,169 (60.0) | 9.02 | 0.011 |
| Married/cohabitating | 2,726 (38.3) | 1,283 (36.7) | 1,443 (40.0) | ||
|
| |||||
| Drug Use History | |||||
|
| |||||
| History of drug use (years) | |||||
| < 5 | 888 (12.5) | 470 (13.4) | 418 (11.6) | 27.24 | <0.001 |
| 5 - 9 | 1,766 (24.8) | 822 (23.5) | 944 (26.1) | ||
| 10 - 14 | 2,758 (38.8) | 1,305 (37.3) | 1,453 (40.2) | ||
| ≥ 15 | 1,690 (23.8) | 899 (25.7) | 791 (21.9) | ||
| Missing | 9 (0.1) | 2 (0.1) | 7 (0.2) | ||
| Median (IQR) | 11.4 (8.0-14.7) | 11.6 (7.7-15.1) | 11.4 (8.1-14.5) | 2.29 | 0.022 |
| History of drug rehabilitation prior to MMT | |||||
| No | 587 (8.3) | 286 (8.2) | 301 (8.3) | 0.06 | 0.812 |
| Yes | 6,524 (91.7) | 3,212 (91.8) | 3,312 (91.7) | ||
|
| |||||
| Clinical Characteristics | |||||
|
| |||||
| CD4 count (cells/μL) when first meeting ART-eligibility criteria | |||||
| < 200 | 2,114 (29.7) | 825 (23.6) | 1,289 (35.7) | 145.98 | <0.001 |
| 200 - 350 | 4,681 (65.8) | 2,464 (70.4) | 2,217 (61.4) | ||
| Missing | 316 (4.4) | 209 (6.0) | 107 (3.0) | ||
| Median (IQR) | 252 (177-306) | 267 (199-314) | 238 (159-297) | 11.95 | <0.001 |
| Calendar year of first meeting ART criteria | 223.97 | <.0001 | |||
| 2004-2007 | 1301 (18.3) | 513 (14.7) | 788 (21.8) | ||
| 2008-2009 | 2823 (39.7) | 1207 (34.5) | 1616 (44.7) | ||
| 2010-2011 | 2987 (42.0) | 1778 (50.8) | 1209 (33.5) | ||
| Relationship between MMT and first meeting ART eligibility | 156.45 | <0.001 | |||
| Entered MMT and retained in MMT when meeting ART eligibility | 4330 (60.9) | 1893 (54.1) | 2437 (67.5 | ||
| Entered MMT and dropped-out of MMT before meeting ART eligibility | 1019 (14.3) | 650 (18.6) | 369 (10.2) | ||
| Met ART eligibility before entering MMT | 1762 (24.8) | 955 (27.3) | 807 (22.3) | ||
| HCV serostatus | |||||
| Positive | 4,977 (70.0) | 2,435 (69.6) | 2,542 (70.4) | 1.81 | 0.405 |
| Negative | 941 (13.2) | 482 (13.8) | 459 (12.7) | ||
| Missing | 1,193 (16.8) | 581 (16.6) | 612 (16.9) | ||
IQR: inter-quartile range.
Subgroups were compared (ART-naïve vs. ART-treated) using the Wilcoxon Rank-Sum test for continuous variables and Chi-Square Test for categorical variables to generate p-values listed.
All p-values presented are two-sided.
Missing data analyses were undertaken, but did not change the pattern of findings. There were very few pieces of missing data for all categories except for CD4 T-Cell count (4.4%), and HCV serostatus(16.8%). Thus, the missing data values are not presented in this table.
Comparison of two subgroups: ART-naïve and ART-treated
Upon comparison of the demographic, drug use history, and HIV-related clinical characteristics of the two subgroups, ART-naïve and ART-treated, several statistically significant differences were observed (Table 1). ART-treated subjects were more likely to be younger (p=.003), have a shorter history of drug use (p<0.001), and married (p=0.011). Additionally, ART-treated subjects tended to have a lower CD4 count when first meeting ART-eligibility requirements (p<0.001) and to have first met ART eligibility in earlier years (2004-2007 and 2008-09, p<0.001). Subjects were had entered MMT and were retained in MMT at the time of becoming ART eligible were more likely to have initiated ART (p<0.001). No other statistically significant differences between ART-naïve and ART-treated subgroups were detected.
Factors associated with ART initiation
In univariate analysis (Table 2), younger age (15-29 years: unadjusted OR=1.15, CI: 0.99-1.33, p=0.065, 30-34 years: unadjusted OR=1.31, CI: 1.14-1.51, p<0.001, and 35-39 years: unadjusted OR=1.17, CI: 1.01-1.36, p=0.032), being married or cohabitating with a partner (married/cohabitating: unadjusted OR=1.15, CI: 1.04-1.26, p=0.005), having a 5-14 year history of drug use (10-14 years: unadjusted OR=1.25, CI: 1.08-1.46, p=0.004 and 5-9 years: unadjusted OR=1.29, CI: 1.10-1.52, p=0.002), first meeting ART eligibility in 2004-09 (2004-07: unadjusted OR=2.26, CI:1.98-2.58, p<0.001 and 2008-09: unadjusted OR=1.97, CI:1.77-2.19, p<0.001), being retained in MMT at the time of first meeting ART eligibility (unadjusted OR=2.27, CI: 1.97-2.61, p<0.001) or meeting ART eligibility before entering MMT (unadjusted OR=1.49, CI:1.27-1.74, p<0.001), and having lower CD4 count results when first meeting ART-eligibility criteria (<200 cells/μL: unadjusted OR=1.74, CI: 1.56-1.93, p<0.001) were all associated with accessing ART.
Table 2. Unadjusted and adjusted odds ratios for accessing ART among eligible MMT clients based on demographic characteristics, drug use history, and clinical characteristics of study subjects upon MMT program enrollment, China, 2004-2011.
| Characteristics | Unadjusted OR (CI)†§ |
P-value†§ | Adjusted OR (CI)‡§ |
P-valueठ|
|---|---|---|---|---|
| Demographic Characteristics | ||||
|
| ||||
| Age (years) | ||||
| ≤29 | 1.15 (0.99-1.33) | 0.065 | 1.02(0.84-1.23) | 0.135 |
| 30 - 34 | 1.31 (1.14-1.51) | <0.001 | 1.26(1.07-1.50) | 0.003 |
| 35 - 39 | 1.17 (1.01-1.36) | 0.032 | 1.15(0.97-1.36) | 0.412 |
| ≥40 | 1.00 | 1.00 | ||
| Gender | ||||
| Male | 0.90 (0.79-1.03) | 0.129 | 0.96(0.82-1.12) | 0.601 |
| Female | 1.00 | 1.00 | ||
| Education level | ||||
| ≤ Primary school | 0.91 (0.82-1.00) | 0.058 | 1.00(0.89-1.13) | 0.983 |
| ≥ Middle school | 1.00 | 1.00 | ||
| Ethnicity | ||||
| Han | 1.00 | 1.00 | ||
| Other | 0.96 (0.87-1.06) | 0.461 | 0.92(0.81-1.04) | 0.177 |
| Employment status | ||||
| Unemployed | 1.00 (0.90-1.10) | 0.994 | 0.92(0.82-1.04) | 0.206 |
| Employed | 1.00 | 1.00 | ||
| Marital status | ||||
| Single/divorced/widowed | 1.00 | 1.00 | ||
| Married/cohabitating | 1.15 (1.04-1.26) | 0.005 | 1.14(1.01-1.28) | 0.030 |
|
| ||||
| Drug Use History | ||||
|
| ||||
| History of drug use (years) | ||||
| < 5 | 1.00 | 1.00 | ||
| 5 - 9 | 1.29 (1.10-1.52) | 0.002 | 1.21(1.00-1.47) | 0.135 |
| 10 - 14 | 1.25 (1.08-1.46) | 0.004 | 1.25(1.03-1.50) | 0.017 |
| ≥ 15 | 0.99 (0.84-1.16) | 0.898 | 1.04(0.85-1.29) | 0.207 |
| History of drug rehabilitation prior to MMT | ||||
| No | 1.00 | 1.00 | ||
| Yes | 0.98 (0.83-1.16) | 0.812 | 0.84 (0.69-1.03) | 0.096 |
|
| ||||
| Clinical Characteristics | ||||
|
| ||||
| CD4 count (cells/μL) when first meeting ART-eligibility criteria | ||||
| < 200 | 1.74 (1.56-1.93) | <0.001 | 1.81(1.61-2.05) | <0.001 |
| 200 - 350 | 1.00 | 1.00 | ||
| Calendar year of first meeting ART criteria | ||||
| 2004-2007 | 2.26(1.98-2.58) | <0.001 | 3.22(2.71-3.83) | <0.001 |
| 2008-2009 | 1.97(1.77-2.19) | <0.001 | 2.03(1.80-2.30) | 0.039 |
| 2010-2011 | 1.0 | |||
| Relationship between MMT and first meeting ART eligibility | ||||
| Met ART eligibility before entering MMT | 1.49(1.27-1.74) | <0.001 | 0.98(0.80-1.21) | 0.849 |
| Entered MMT and retained in MMT when meeting ART eligibility | 2.27(1.97-2.61) | <0.001 | 1.84(1.54-2.21) | <0.001 |
| Entered MMT and dropped-out of MMT before meeting ART eligibility | 1.0 | |||
| HCV serostatus | ||||
| Positive | 1.10 (0.95-1.26) | 0.196 | 1.04(0.89-1.21) | 0.628 |
| Negative | 1.00 | 1.00 | ||
OR: odds ratio, CI: confidence interval,
Unadjusted odds ratios and p-values generated via univariate analysis.
Adjusted odds ratios and p-values resulted from multivariate analysis using a logistic regression model. All variables in univariate analysis were included in the multivariable model.
All CI presented are 95% CI and all p-values presented are two-sided.
Missing data analyses were undertaken, but did not change the pattern of findings. There were very few pieces of missing data for all categories except for CD4 count (4.4%), and HCV serostatus(16.8%). Thus, the missing data values are not presented in this table.
All variables were further analyzed using a multivariable model (Table 2). We found that first meeting ART eligibility in 2004-09 (2004-07: AOR=3.22, CI:2.71-3.83, p<0.001 and 2008-09: AOR=2.03, CI:1.80-2.30, p=0.039) and being retained in MMT at the time of first meeting ART eligibility (AOR=1.84, CI: 1.54-2.21, p<0.001) were predictors of accessing ART. Additionally, CD4 count when first meeting ART-eligibility criteria (<200 cells/μL: AOR=1.94, CI: 1.73-2.19, p<0.001) and marital status (AOR=1.14, CI: 1.01-1.28, p=0.029) were both predictors of ART initiation.
Discussion
China can draw upon the experiences of other countries in identifying effective strategies to manage treatment for DUs. Successful linkage between MMT and ART services improves health outcomes for DUs including initiation of ART, adherence and virological response.12, 14, 15, 26-28 Thus, many international experts, including American, Australian, French, and World Health Organization researchers, have strongly recommended the inclusion and expansion of opioid substitution treatment in combination treatment.12-14, 28-30 Clinical studies and modeling studies from other countries have also documented the effect of MMT and ART integration in terms of improving treatment and preventing transmission.14, 31-35
We hypothesized that stable MMT participation by ART-eligible, HIV-positive MMT clients in China would be a significant predictor of accessing ART treatment. Therefore, in this nationwide, cohort study, we sought to examine demographic, drug use history, and HIV-related clinical characteristics of ART-eligible, HIV-positive MMT clients, comparing those who remained naïve to ART and those who initiated ART treatment during the study period.
Our main finding was that being retained in MMT program enrollment at the time of meeting ART eligibility was associated with an increased likelihood of accessing ART treatment. This is consistent with a growing literature that documents the positive effect of drug addiction treatment on initiation and adherence to ART.12, 14, 15, 26-28 It has been proposed that MMT program participation brings clients into regular contact with the healthcare system, which promotes regular monitoring of CD4 counts, prompt referral to ART programs upon fulfillment of eligibility criteria, education regarding ART adherence, reduced distrust of health care providers, and intervention opportunities to address psychosocial barriers such as mental health issues and homelessness.14, 15, 28 Substitution treatments for drug addictions such as MMT are also known to help to stabilize patients and promote their social rehabilitation,30 both of which increase the potential of ART-eligible, HIV-positive, MMT clients to access and to adhere to ART regimens.
We found that nearly half of the eligible clients in China's MMT program did not access ART. Clients who reached ART eligibility criteria in earlier calendar years were more likely to have initiated ART because they had a longer time period of ART-eligibility. Since 2003, the Chinese government has implemented a policy of free antiretroviral therapy for HIV-positive patients.36, 37 Unfortunately, treatment coverage is inconsistent across populations at high-risk for HIV; coverage is lower in IDUs compared with those infected through sexual transmission or through receipt of contaminated blood products.38 In this study, clients who did not access ART had a median period of 18 months between meeting treatment criteria and the study end date or their death. This suggests that they are unlikely to access ART in the future because clients who accessed ART did so within a median of 6 months. This is a point of concern because many studies have noted that early initiation of ART leads to lower mortality rates.39, 40 The literature suggests that factors discouraging DUs from receiving ART include lack of medical insurance and family support, insufficient capacity of health facilities, poor treatment compliance, treatment separated by specialties, discrimination by health care providers, police harassment, and interruption of HIV treatment in detention centers.29
In our study cohort, several baseline demographic variables were associated with ART access: age 30-34, being married, and a history of drug use for 10-14 years. We suggest that age 30-34 may be significant for ART initiation because familial responsibilities are increased during this stage. Additional research is needed to understand this factor. Age 30-44 is correlated with a drug use history of 10-14 years because the average age at first drug use in China is approximately 23 years.41 Marriage or a stable partnership is associated with overall improved ART coverage in China.21
An initial CD4<200 cells/μL was a factor strongly associated with accessing ART, which is consistent across all modes of transmission.40 Clients with CD4 <200 are considered to have progressed to AIDS, which motivates clients to seek ART. For patients with initial CD4 counts ranging from 200-350 cells/μL, the likelihood of treatment initiation was reduced due to fewer presenting symptoms of advanced disease. Also, prior to 2008, patients with CD4 >200 were not eligible for free antiretroviral therapy.21
It is frequently recommended that routine HIV screening of populations at high risk is crucial because early detection is the first step to initiation of treatment.40 In China, DUs are likely to be identified with HIV infections earlier than other high-risk populations because of routine testing upon entry into detoxification centers and MMT clinics. There are also active monitoring programs focused on IDUs who engage in risky behavior such as sharing injecting equipment. However, while IDUs have significantly higher CD4 counts at HIV diagnosis, they experience much longer delays to receiving ART compared to those infected through other transmission routes.40, 42 Although there are great challenges in promoting ART initiation among DUs in China, we suggest that improving the capacity of healthcare providers and driving stronger integration of different departments are ways to address this issue.
Another issue facing DUs is a high prevalence of HCV infection. In our study, 84.1% of the clients tested positive for HCV. Because of shared transmission pathways, HCV and HIV co-infection is common. Providing treatment for DU patients with HIV and HCV co-infection is particularly difficult due to the complicated management of concurrent diseases.43-45 Patients are also discouraged by the high cost of medical services associated with HCV treatment, which unlike ART, is not covered by the Chinese government. The ability to provide assessment and treatment services for HCV and HIV lags far behind the number of DUs who could benefit from it.
MMT provides a good entry for HIV testing and effective HIV-treatment and is a critical venue for promoting HIV-prevention among DUs. The well-documented benefits of MMT have led the World Health Organization to call for the integration of opioid substitution therapies in its HIV treatment guidelines for DUs.46 There are strategies that can be undertaken to address some issues brought up by this study.
Our study showed that sustained engagement in MMT promoted the initiation of ART. Encouraging a higher frequency of cross-referrals between ART and MMT services can increase concurrent engagement. Expansion of MMT services should be strongly advocated to improve access to ART among DUs. In most situations, MMT clients must seek HIV treatment from an additional medical setting outside of the MMT clinic. Published research studies have observed and strongly suggested that clients would benefit greatly if ART and MMT services could be simultaneously provided in one location.29, 47 Providing services at a centralized clinic to address the multiple health issues facing HIV-positive DUs would promote increased enrollment and would cut down on time and costs. Future analyses should be conducted in China to assess the feasibility of combining ART and substance abuse treatment to optimize health outcomes. In addition, care providers should be understanding and empathetic to the unique needs of DUs and provide a welcoming environment for those seeking treatment for both drug dependence and HIV infection.48, 49
Our study had several limitations. Firstly, nearly 25% HIV-positive MMT clients were excluded because of inability to link records between the MMT data system and the HIV case-reporting system. This is the strongest potential source of bias. We also excluded cases due to incomplete records, missing CD4 count results and/or clinical staging. Secondly, the present study is an observational study, so no inferences about causality can be made. Thirdly, under the logistic regression analysis, the unequal client distributions in the categories can affect the size of odd ratios and thus, the interpretation of results. Since the overall sample size is very large and there are sufficient numbers of subjects in each category of variables, we believe that this influence should be minimum. Finally, the accuracy and reliability of the data used in this study is dependent on truthful responses and self-reporting by subjects and on the performance of local care providers. Subject responses may be influenced by recall or social desirability biases. Although ART and MMT providers follow national guidelines published by China's Ministry of Health, clinical decisions are not standardized due to providers' potentially biased opinions and professional judgments, as well as other factors. Care providers may subscribe to stigmatized beliefs that DUs are less likely to adhere to ART treatment plans, leading to reluctance to prescribe HIV treatment.46, 50 We attempted to reduce the role of confounding variables by using a multivariable analysis model, but as in all observational studies, there still remains a residual risk of confounding. Despite these limitations, we believe our findings to have high generalizability and reproducibility due to the large participant cohort drawn from the national treatment programs.
The Chinese national MMT and ART programs have developed and expanded rapidly in recent years. In summary, our findings demonstrate that stable maintenance in MMT can increase the likelihood of accessing ART for DUs. However, challenges still exist among IDUs in receiving ART, even if they have engaged in MMT. We recommend that access to MMT services and integration of MMT with HIV treatment should be increased to improve patient outcomes.
Acknowledgments
The authors would like to thank Enwu Liu who manages the national MMT database providing help in checking the MMT data.
Funding: This study was supported by the China National AIDS Program, the China national technical support and operational research for HIV/AIDS prevention, treatment and care project (131-11-0001-0501), the National Science and Technology Major Project on Prevention and Treatment of Major Infectious Diseases including AIDS and Viral Hepatitis (2012ZX10001-007), and by the Fogarty International Center and the National Institute on Drug Abuse at the U.S. National Institutes of Health (China ICOHRTA2, NIH Research Grant number is U2RTW06918). The funding organizations had no role in the development of study design, collection, analysis, and interpretation of data, or the final decision to submit the manuscript for publication.
Footnotes
Declarations of interest: All authors have no interests to declare.
Disclaimer: The opinions expressed herein reflect the collective views of the co-authors and do not necessarily represent the official position of the National Center for AIDS/STD Control and Prevention, Chinese Center for Diseases Control and Prevention.
References
- 1.Joint United Nations Programme on HIV/AIDS. UNAIDS Report on the Global AIDS Epidemic 2010. Geneva: Joint United Nations Programme on HIV/AIDS; 2010. [Google Scholar]
- 2.Mathers B, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee S, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372(9651):1733–45. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 3.National Narcotics Control Commission. Annual Report on Drug Control in China 2011. Beijing: Ministry of Public Security; 2011. [Google Scholar]
- 4.Ministry of Health, Joint United Nations Programme on HIV/AIDS, World Health Organization. 2011 Estimates for the HIV/AIDS Epidemic in China. Geneva: World Health Organization; 2011. [Google Scholar]
- 5.Mathers B, Degenhardt L, Ali H, Wiessing L, Hickman M, Mattick R, et al. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet. 2010;375(9719):1014–28. doi: 10.1016/S0140-6736(10)60232-2. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Wang N, Wang L, Li D, Jia M, Gao X, et al. The 2007 Estimates for People at Risk for and Living With HIV in China: Progress and Challenges. J Acquir Immune Defic Syndr. 2009;50(4):414–8. doi: 10.1097/QAI.0b013e3181958530. [DOI] [PubMed] [Google Scholar]
- 7.Metzger D, Zhang Y. Drug treatment as HIV prevention: expanding treatment options. Curr HIV/AIDS Rep. 2010;7(4):220–5. doi: 10.1007/s11904-010-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao X, Wu Z. Methadone maintenance treatment as an HIV prevention strategy: a research update (in Chinese) Chin J AIDS STD. 2011;17(2):196–9. [Google Scholar]
- 9.Dou Z, Chen R, Xu J, Ma Y, Jiao J, Durako S, et al. Changing baseline characteristics among patients in the China National Free Antiretroviral Treatment Program, 2002-09. Int J Epidemiol. 2010;39(Suppl 2):56–64. doi: 10.1093/ije/dyq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin W, Hao Y, Sun X, Gong X, Li F, Li J, et al. Scaling up the national methadone maintenance treatment program in China: achievements and challenges. Int J Epidemiol. 2010;39(Suppl 2):29–37. doi: 10.1093/ije/dyq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson P, Mathers B, Cowie B, Hagan H, Des Jarlais D, Horyniak D, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378(9791):571–83. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roux P, Carrieri M, Villes V, Dellamonica P, Poizot-Martin I, Ravaux I, et al. The impact of methadone or buprenorphine treatment and ongoing injection on highly active antiretroviral therapy (HAART) adherence: evidence from the MANIF2000 cohort study. Addiction. 2008;103(11):1828–36. doi: 10.1111/j.1360-0443.2008.02323.x. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan L, Metzger D, Fudala P, Fiellin D. Decreasing international HIV transmission: the role of expanding access to opioid agonist therapies for injection drug users. Addiction. 2005;100(2):150–8. doi: 10.1111/j.1360-0443.2004.00963.x. [DOI] [PubMed] [Google Scholar]
- 14.Uhlmann S, Milloy M, Kerr T, Zhang R, Guillemi S, Marsh D, et al. Methadone maintenance therapy promotes initiation of antiretroviral therapy among injection drug users. Addiction. 2010;105:907–13. doi: 10.1111/j.1360-0443.2010.02905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg K, Mouriz J, Li X, Duggan E, Goldberg U, Arnsten J. Rationale, design, and sample characteristics of a randomized controlled trial of directly observed antiretroviral therapy delivered in methadone clinics. Contemp Clin Trials. 2009;30(5):481–9. doi: 10.1016/j.cct.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood E, Kerr T, Zhang R, Guillemi S, Palepu A, Hogg R, et al. Poor adherence to HIV monitoring and treatment guidelines for HIV-infected injection drug users. HIV Med. 2008;9(7):503–7. doi: 10.1111/j.1468-1293.2008.00582.x. [DOI] [PubMed] [Google Scholar]
- 17.Lucas G, Mullen B, Weidle P, Hader S, McCaul M, Moore R. Directly administered antiretroviral therapy in methadone clinics is associated with improved HIV treatment outcomes, compared with outcomes among concurrent comparison groups. Clin Infect Dis. 2006;42(11):1628–35. doi: 10.1086/503905. [DOI] [PubMed] [Google Scholar]
- 18.Pang L, Hao Y, Mi G, Wang C, Luo W, Rou K, et al. Effectiveness of first eight methadone maintenance treatment clinics in China. AIDS. 2007;21(Suppl 8):103–7. doi: 10.1097/01.aids.0000304704.71917.64. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Lin C, Wan D, Zhang L, Lai W. Concurrent heroin use among methadone maintenance clients in China. Addict Behav. 2012;37(3):264–8. doi: 10.1016/j.addbeh.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. Geneva: World Health Organization; 2010. 2010 revision. [PubMed] [Google Scholar]
- 21.Zhang F, Dou Z, Ma Y, Zhang Y, Zhao Y, Zhao D, et al. Effect of earlier initiation of antiretroviral treatment and increased treatment coverage on HIV-related mortality in China: a national observational cohort study. Lancet Infect Dis. 2011;11(7):516–24. doi: 10.1016/S1473-3099(11)70097-4. Epub 2011/05/24. [DOI] [PubMed] [Google Scholar]
- 22.Ministry of Health Working Group on Clinical AIDS Treatment. China Free Antiretroviral Therapy Manual (in Chinese) Beijing: People's Medical Publishing House; 2012. [Google Scholar]
- 23.Mao Y, Wu Z, Poundstone K, Wang C, Qin Q, Ma Y, et al. Development of a unified web-based national HIV/AIDS information system in China. Int J Epidemiol. 2010;39(Suppl 2):79–89. doi: 10.1093/ije/dyq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma Y, Zhang F, Zhao Y, Zang C, Zhao D, Dou Z, et al. Cohort profile: the Chinese national free antiretroviral treatment cohort. Int J Epidemiol. 2010;39(4):973–9. doi: 10.1093/ije/dyp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang F, Haberer JE, Wang Y, Zhao Y, Ma Y, Zhao D, et al. The Chinese free antiretroviral treatment program: challenges and responses. AIDS. 2007;21(Suppl 8):S143–8. doi: 10.1097/01.aids.0000304710.10036.2b. [DOI] [PubMed] [Google Scholar]
- 26.Clarke S, Delamere S, McCullough L, Hopkins S, Bergin C, Mulcahy F. Assessing limiting factors to the acceptance of antiretroviral therapy in a large cohort of injecting drug users. HIV Med. 2003;4(1):33–7. doi: 10.1046/j.1468-1293.2003.00130.x. [DOI] [PubMed] [Google Scholar]
- 27.Lert F, Kazatchkine MD. Antiretroviral HIV treatment and care for injecting drug users: an evidence-based overview. Int J Drug Policy. 2007;18(4):255–61. doi: 10.1016/j.drugpo.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spire B, Lucas G, Carrieri M. Adherence to HIV treatment among IDUs and the role of opioid substitution treatment (OST) Int J Drug Policy. 2007;18(4):262–70. doi: 10.1016/j.drugpo.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Wolfe D, Carrieri M, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet. 2010;376(9738):355–66. doi: 10.1016/S0140-6736(10)60832-X. [DOI] [PubMed] [Google Scholar]
- 30.Lawrinson P, Ali R, Buavirat A, Chiamwongpaet S, Dvoryak S, Habrat B, et al. Key findings from the WHO collaborative study on substitution therapy for opioid dependence and HIV/AIDS. Addiction. 2008;103(9):1484–92. doi: 10.1111/j.1360-0443.2008.02249.x. [DOI] [PubMed] [Google Scholar]
- 31.Palepu A, Tyndall M, Joy R, Kerr T, Wood E, Press N, et al. Antiretroviral adherence and HIV treatment outcomes among HIV/HCV co-infected injection drug users: the role of methadone maintenance therapy. Drug Alcohol Depend. 2006;84(2):188–94. doi: 10.1016/j.drugalcdep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Weber R, Huber M, Rickenbach M, Furrer H, Elzi L, Hirschel B, et al. Uptake of and virological response to antiretroviral therapy among HIV-infected former and current injecting drug users and persons in an opiate substitution treatment programme: the Swiss HIV Cohort Study. HIV medicine. 2009;10:407–16. doi: 10.1111/j.1468-1293.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 33.Tran B, Ohinmaa A, Duong A, Do N, Nguyen L, Mills S, et al. Cost-effectiveness of methadone maintenance treatment for HIV-positive drug users in Vietnam. AIDS Care. 2012;24(3):283–90. doi: 10.1080/09540121.2011.608420. [DOI] [PubMed] [Google Scholar]
- 34.Xing Y, Sun J, Cao W, Lee L, Guo H, Li H, et al. Economic evaluation of methadone maintenance treatment in HIV/AIDS control among injecting drug users in Dehong, China. AIDS Care. 2012;(6):756–62. doi: 10.1080/09540121.2011.630359. [DOI] [PubMed] [Google Scholar]
- 35.Alistar S, Owens D, Brandeau M. Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: a modeling analysis for Ukraine. PLoS medicine. 2011;8(3):e1000423. doi: 10.1371/journal.pmed.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Z, Sullivan S, Wang Y, Rotheram-Borus M, Detels R. Evolution of China's response to HIV/AIDS. Lancet. 2007;369(9562):679–90. doi: 10.1016/S0140-6736(07)60315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang F, Dou Z, Ma Y, Zhao Y, Liu Z, Bulterys M, et al. Five-year outcomes of the China National Free Antiretroviral Treatment Program. Ann Intern Med. 2009;151(4):241–51. doi: 10.7326/0003-4819-151-4-200908180-00006. [DOI] [PubMed] [Google Scholar]
- 38.Zhang F, Dou Z, Ma Y, Zhang Y, Zhao Y, Zhao D, et al. Effect of earlier initiation of antiretroviral treatment and increased treatment coverage on HIV-related mortality in China: a national observational cohort study. Lancet Infect Dis. 2011;11(7):516–24. doi: 10.1016/S1473-3099(11)70097-4. [DOI] [PubMed] [Google Scholar]
- 39.Mocroft A, Ledergerber B, Katlama C, Kirk O, Reiss P, Monforte AdA, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362(9377):22–9. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Lu L, Li HQ, Liu W, Tang ZR, Fang H, et al. Engaging HIV-infected patients in antiretroviral therapy services: CD4 cell count testing after HIV diagnosis from 2005 to 2009 in Yunnan and Guangxi, China. Chin Med J (Engl) 2011;124(10):1488–92. [PubMed] [Google Scholar]
- 41.Yang X, Latkin C, Celentano D, Luo H. Prevalence and correlates of HIV risk behaviors among drug users in China. AIDS Behav. 2006;10(1):71–81. doi: 10.1007/s10461-005-9028-8. [DOI] [PubMed] [Google Scholar]
- 42.Rodríguez-Arenas M, Jarrín I, del Amo J, Iribarren J, Moreno S, Viciana P, et al. Delay in the initiation of HAART, poorer virological response, and higher mortality among HIV-infected injecting drug users in Spain. AIDS Res Hum Retroviruses. 2006;22(8):715–23. doi: 10.1089/aid.2006.22.715. [DOI] [PubMed] [Google Scholar]
- 43.Grebely J, Tyndall M. Management of HCV and HIV infections among people who inject drugs. Curr Opin HIV AIDS. 2011;6(6):501–7. doi: 10.1097/COH.0b013e32834bcb36. [DOI] [PubMed] [Google Scholar]
- 44.Dieterich D. Hepatitis C virus and human immunodeficiency virus: clinical issues in coinfection. Am J Med. 1999;107(6B):79S–84S. doi: 10.1016/s0002-9343(99)00390-3. [DOI] [PubMed] [Google Scholar]
- 45.Verucchi G, Calza L, Mandredi R, Chiodo F. Human immunodeficiency virus and hepatitis C virus coinfection: epidemiology, natural history, therapeutic options and clinical management. Infection. 2004;32(1):33–46. doi: 10.1007/s15010-004-3063-7. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization, United Nations Office on Drugs and Crime, Joint United Nations Programme on HIV/AIDS. Technical guide for countries to set targets for universal access to HIV prevention, treatment and care for injecting drug users. Geneva: World Health Organization; 2009. [Google Scholar]
- 47.Celentano D, Vlahov D, Cohn S, Shadle V, Obasanjo O, Moore R. Self-reported antiretroviral therapy in injection drug users. JAMA. 1998;280(6):544–6. doi: 10.1001/jama.280.6.544. [DOI] [PubMed] [Google Scholar]
- 48.Krüsi A, Wood E, Montaner J, Kerr T. Social and structural determinants of HAART access and adherence among injection drug users. Int J Drug Policy. 2010;21(1):4–9. doi: 10.1016/j.drugpo.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Lin C, Li L, Wan D, Wu Z, Yan Z. Empathy and avoidance in treating patients living with HIV/AIDS (PLWHA) among service providers in China. AIDS Care. 2012;24(11):1341–8. doi: 10.1080/09540121.2011.648602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wood E, Hogg R, Kerr T, Bonner S, Strathdee S, Palepu A, et al. Rates of inappropriate antiretroviral prescription among injection drug users. Harm Reduct J. 2007;4:2. doi: 10.1186/1477-7517-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


