Abstract
Allergen-specific immunotherapy has been proposed as an attractive strategy to actively treat food allergy using the following three different immunotherapy routes: oral (OIT), sublingual (SLIT) and epicutaneous (EPIT) immunotherapy. Regulatory T cells (Tregs) have been shown to have a pivotal role in the mechanisms of immunotherapy. The aim of this study was to compare the phenotype and function of Tregs induced in peanut-sensitized BALB/c mice using these three routes of treatment. We show that although EPIT, OIT and SLIT were all able to effectively desensitize peanut-sensitized mice, they induced different subsets of Tregs. Foxp3+ Tregs were induced by the three treatment routes but with greater numbers induced by EPIT. EPIT and OIT also increased the level of LAP+ Tregs, whereas SLIT induced IL-10+ cells. The suppressive activity of EPIT-induced Tregs did not depend on IL-10 but required CTLA-4, whereas OIT acted through both mechanisms and SLIT was strictly dependent on IL-10. Moreover, the three routes influenced the homing properties of induced Tregs differently, with a larger repertoire of chemokine receptors expressed by EPIT-induced Tregs compared with OIT- and SLIT- induced cells, resulting in different protective consequences against allergen exposure. Furthermore, whereas OIT- or SLIT-induced Tregs lost their suppressive activities after treatment was discontinued, the suppressive activities of EPIT-induced Tregs were still effective 8 weeks after the end of treatment, suggesting the induction of a more long-lasting tolerance. In summary, EPIT, OIT and SLIT mediated desensitization through the induction of different subsets of Tregs, leading to important differences in the subsequent protection against allergen exposure and the possible induction of tolerance.
Keywords: allergy, immunotherapy, mechanisms, regulatory T cells
Introduction
Food allergy affects approximately 15 million Americans and 17 million Europeans, most being young children.1 At present, there is no approved treatment outside of dietary avoidance and the availability of self-injectable epinephrine. Allergen-specific immunotherapy is an attractive strategy to actively treat food allergy, and three different routes are being explored: oral (OIT), sublingual (SLIT) and epicutaneous immunotherapy (EPIT). Allergen-specific immunotherapy aims at reducing (desensitization) or even abrogating (tolerance) the sensitivity to an allergen. Immunotherapy implies decreased allergen-specific IgE and increased IgG4 levels, reduced responses of effector CD4+ T cells and the induction of regulatory T cells (Tregs).2 Tregs have been shown to have a pivotal role in maintaining immune tolerance, whereas Treg cell deficiencies are implicated in the development of allergies. Recently, we demonstrated that EPIT, in a mouse model of peanut allergy, acts through the induction of Tregs rather than by switching cells from a Th2 to a Th1 pattern.3 In an OIT clinical trial, Syed et al.4 found that when tolerant, participants had a higher Treg number with greater suppressive function than non-tolerant and control participants, further demonstrating the role of Tregs in tolerance in humans.
Differences in the route of administration for OIT, SLIT and EPIT also involve different allergen doses, clinical outcomes5 and, most likely, the induction of different Treg subsets with diverse suppressive capacity. An OIT trial suggested that the percentages of Tr1 and Foxp3+ cells are increased in patients receiving therapy.4 SLIT induces IL-10 production and increases the frequency of IL-10+ regulatory cells.6 No data regarding Treg induction in patients treated by EPIT are available at this time, but in a murine model, IL-10-independent Foxp3+ Tregs were induced by EPIT.3 However, no comparative studies of the three treatments, either in humans or animal models, have been conducted to date in comparable settings.
The comparison of Treg populations should encompass several features. Appropriate trafficking is of particular importance to mediating efficient immune responses in vivo.7 Moreover, tissue-specific Tregs and tissue-resident Tregs could have unique functions and participate in the regulation of a plethora of human diseases.8 The differential expression of adhesion molecules and chemokine receptors determines the specific migration of leukocytes into distinct tissues and microenvironments, and Tregs could display variable efficacy in vivo based on their expression of tissue-specific homing molecules.9 Foxp3+ T cells have been shown to upregulate their expression of many homing receptors that correspond to Th1 cells (CXCR3, CCR5 and CXCR6), Th2 cells (CCR4 and CCR8) and Th-17 cells (CCR4, CCR6, CCR2 and CXCR3) as well as specific homing receptors for the gut (CCR9 and α4β7 integrin) or skin (cutaneous lymphocyte antigen (CLA)), CCR4 and CCR10).10 Given that chemokine receptors expressed by T cells could be identified by their initial tissue site of priming, different routes of allergen immunotherapy could lead to the induction of Tregs with different homing properties and, consequently, distinct efficacies in vivo.
In addition, the ultimate goal of allergen immunotherapy is the induction of immune tolerance that persists for years after treatment discontinuation. However, few studies have addressed the question of maintenance of the clinical effects of OIT when treatment is discontinued, with 21–65% of children maintaining sustained tolerance, and no data are available for SLIT.11 Recently, it was proposed that the induction of Tregs that persist after the discontinuation of treatment could participate in long-term tolerance.4 It is therefore of great interest to compare the long-lasting effect of different treatments on the suppressive activities of Tregs.
Here we show that although OIT, SLIT and EPIT are all able to efficiently desensitize peanut-sensitized mice, they induce different subsets of Tregs. Moreover, the three treatment routes influence the homing properties of induced Tregs differently, and the suppressive activities of EPIT, but not OIT or SLIT, are maintained for a prolonged period after discontinuation.
Materials and methods
Animals and study design
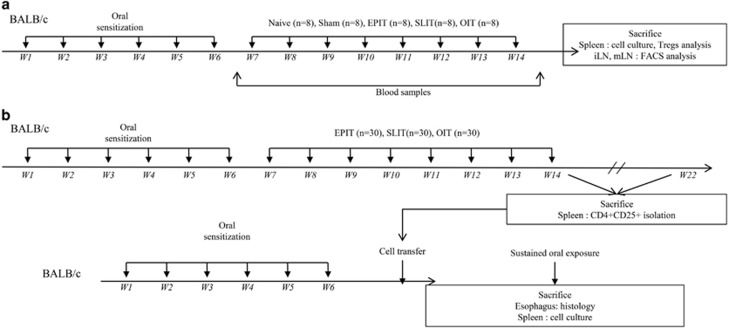
Three-week-old female BALB/c mice (Charles River, Lyon, France) were purchased and housed under standard animal husbandry conditions. The mice were acclimated for 1 week before starting experiments (Figure 1).
Figure 1.
Study design of experiments. (a) BALB/c mice were sensitized to peanut proteins. Then, the mice were treated by EPIT (100 μg), OIT (1 mg the first week, 2 mg the second week and 5 mg the five following weeks), SLIT (100 μg) or placebo (sham) for 8 weeks. Naive mice remained unsensitized and untreated. Following treatment, the blood was recovered for sIgE and sIgG2a measurement, and the mice were killed for spleen and lymph node recovery for cell culture and FACS analysis. (b) BALB/C mice were sensitized and treated as described above (donor mice). After treatment (n=15 for each group) or 8 weeks after the end of treatment (n=15 for each group), the donor mice were killed, the CD4+CD25+ T cells were sorted from spleen cells and transferred into peanut-sensitized non-treated mice. Three days after the transfer, the mice were submitted to a 10-day sustained oral exposure to peanuts. The day after the last challenge, the mice were killed for organ recovery for esophagus histology and spleen cell culture.
Immunotherapy efficacy and characterization of regulatory T cells induced by different immunotherapies
After a phase of sensitization with peanut protein extract (PPE) validated by an increase in specific IgE (sIgE), the mice were randomly allocated into the following four groups of eight animals and treated for 8 weeks: EPIT (100 μg), OIT (1 mg the first week, 2 mg the second week and 5 mg the five following weeks), SLIT (100 μg) and sham-treated mice (Figure 1a). Eight naive mice (not sensitized and not treated) were also included. Following treatment, blood was recovered to measure sIgE and sIgG2a, and the mice were killed for spleen and lymph node recovery. The spleen and lymph node cells were isolated, and allergen-specific responses were analyzed by the production of cytokines following in vitro restimulation with PPE. The spleen and lymph node Treg phenotypes were analyzed by flow cytometry.
Transfer of protection by Tregs. After a phase of sensitization, mice were randomly allocated into the following six groups of 15 animals and treated for 8 weeks: two groups were treated by EPIT, two groups were treated by SLIT and two groups were treated by OIT (Figure 1b). Following treatment or 8 weeks after the end of treatment, one group treated using each form of immunotherapy was killed for spleen cell recovery and CD4+CD25+ cell sorting. The cells were then transferred into peanut-sensitized, non-treated mice (n=8 per groups). Three days after the transfer, the mice were orally exposed to peanuts daily for 10 days, leading to eosinophilic infiltration of the esophagus, as previously described.12 This eosinophilic infiltration of the esophagus is used as a biologic marker to evaluate the potential protective activities of Tregs in eosinophilic esophagitis after oral peanut ingestion. Eight sensitized mice not receiving transferred cells and eight naive mice (not sensitized and not treated) were also included. At the end of the 10-day feeding period, the esophagi were harvested for histological analysis and isolation of spleen cells for in vitro restimulation.
The skin preparations before applying the epicutaneous delivery system or sublingual administration were all performed under general anesthesia with Ketamine (Imalgen1000, Merial) (100 mg/kg body weight) and Xylazine (Rompun, Bayer, Shawnee Mission, KS, USA) (10 mg/kg body weight).
All experiments have been repeated independently two times and were performed according to the European Community rules on animal care with permission 00574.01 from the French Authorities.
Sensitization
Sensitization was induced by means of six intra-gastric gavage feedings of peanut proteins, 1 mg mixed with 10 μg of Cholera Toxin (CT) (Servibio, Biological Laboratories, inc., Campbell, CA, USA) once a week for 6 weeks, as previously described.12
Treatment
The treatment protocols are meant to mimic the techniques utilized in human immunotherapy. For OIT, a dose escalation protocol was validated. For EPIT and SLIT treatment, a previous dose effect study provided information to determine the doses used in these experiments.
EPIT and SLIT treatment were performed once a week for 8 weeks.
EPIT was performed using a patented epicutaneous delivery system, Viaskin (DBV Technologies, Paris, France).13 The patch loaded with the indicated quantity of peanut protein was applied for 48 h to the back of mice who had been previously prepared by removing the hair on the application area.
OIT treatment was performed every day for 8 weeks. The mice received 200 μl of a homogeneous suspension of 1 mg of PPE for the first week, 2 mg of PPE for the second week and 5 mg from weeks 3 to 8 by oral gavage.
The mice in the SLIT group received 5 μl of a homogeneous, viscous preparation containing the indicated quantity of peanut protein in phosphate-buffered saline administered under the tongue, as previously described.14 To increase the viscosity, the sublingual solutions were prepared with 1.2% carboxymethylcellulose (w/v) (Sigma, Saint Quentin Fallavier, France). To prevent the animals from swallowing the solution, weekly administrations were all performed under anesthesia with ketamine and xylazine.
Cell sorting and adoptive transfer
Cell sorting and adoptive transfer were performed as previously described.3 The spleens were prepared in a single-cell suspension and washed in RPMI-1640 (Gibco, Life Technologies SAS, Saint Aubin, France). After red blood cell lysis, the splenocytes were washed three times in RPMI-1640. CD4+CD25+ T cells were sorted using a CD4+CD25+ Regulatory T Cell Isolation Kit (MiltenyiBiotec, Paris, France), according to the manufacturer’s instructions. The cells were counted and adjusted to inject 5 × 105 cells in 100 μl of phosphate-buffered saline intravenously into the tail vein of the mice. The sorting purity was ascertained by flow cytometry and was greater than 90%.
Oral allergen exposure and histological analysis of the esophagus
The 10-day sustained oral exposure to peanuts, leading to eosinophilic infiltration in the esophagus as previously described,12 consisted of exclusive feeding with peanut kernels instead of standard mouse food for four consecutive days. The animals then received peanut kernels mixed into standard food for the following 6 days, and daily intra-gastric administration of a solution containing 10 mg of peanut protein for the last 3 days.
At the end of 10 days, the esophagi were harvested, fixed in 4% neutral buffered formalin, embedded in paraffin wax, transversally cut into 5-μm thick sections, fixed to positively charged slides and stained using a routine hematoxylin–eosin–safranin staining method. Three sections of the esophagus were analyzed by blind reading. Image analysis was performed on sections of esophagus using a digital camera (Leïca DFC 420C, Nanterre, France) combined with image-analysis software (Leïca LAS Software). Six high-powered fields were randomly selected around the esophageal lumen, and the eosinophil counts were expressed as the number of eosinophils/mm2.
Blood-specific IgE and IgG2a
Blood was collected from the retro-orbital venous plexus following sensitization and after 8 weeks of treatment under isoflurane (IsofluraneBelamont, Nicholas Piramal India). Specific antibodies were quantified using a quantitative enzyme-linked immunosorbent assay developed in-house according to the 2001 FDA guidelines, as previously published.13 Briefly, plasma samples were incubated in microtiter plates coated with peanut protein extract. The presence of specific IgE and IgG2a were detected by the addition of an anti-mouse IgE or IgG2a antibody labeled with alkaline phosphatase (Serotec, Oxford, England). The reagent (pNPP—Sigma) was used as an enzyme substrate and the optical density was measured at 405 nm.
Cytokine production
Following treatment, the spleens were prepared in a single-cell suspension and washed three times in RPMI-1640 (Gibco). The cells were counted and 2 × 106 cells were incubated in a 24-well microtiter plate (Nunc) in 1 ml of medium alone or medium with peanut protein (100 μg/ml). In some experiments, mouse-specific anti-IL-10 or anti-CTLA-4 blocking antibodies (4 μg/ml) were added to the culture to assess the respective contribution of these pathways to the suppression of allergen-specific responses. The supernatants were harvested after 72 h and analyzed for the presence of cytokines (IL-5, IL-13, IL-10 and IFN-γ) using a Bio-Plex system (Bio-Rad, Marnes-la-Coquette, France), according to the manufacturer’s instructions.
Regulatory T-cell phenotypes
For Treg analysis, spleen cells were stained with different combinations of the following antibodies: anti-mouse CD4-FITC (clone GK1.5), CD4-PerCP-Cy5.5 (clone RM4-5), CD25-FITC and CD25-PE-Cy7 (clone PC61), IL10-PE (clone JES5-16E3), CD62L-APC (clone MEL-14), CD44-APC-Cy7 (clone IM7) (all from BD Biosciences, Le Pont de Claix, France), purified anti-CCR4 (Clinisicence, Nanterre, France), donkey anti Rabbit-PE, CTLA-4-PE, CCR9-PerCP-Cy5.5 (clone eBioCW-1.2), CXCR3-PerCP-Cy5.5 (clone CXCR3-173), CLA-efluo660 (clone HECA-452), latency-associated peptide (LAP)-PercP-efluor710 (clone TW7-16B4), Foxp3-PE and Foxp3-APC (clone FJK-16s) (from e-Bioscience, Paris, France), CCR10-PE (clone 248918), CCR6-PE (clone 140706), CCL1 biotinylated fluorokine kit (R&D Systems, Lille, France), CCR3-PerCP-Vio700 (clone REA122) (MiltenyiBiotec, Paris, France) or control isotypes. Intracellular staining was performed after fixation and permeabilization, using a Cytofix/cytoperm kit (BD Bioscience) for IL-10 staining or a Foxp3 Perm Kit (e-Bioscience) for Foxp3 staining. Flow cytometry was performed on a Guava easyCyte 8HT cytometer and analyzed using FlowJo software. For the analysis of Tregs, the cells were gated on lymphocytes using FCS/SSC after the exclusion of doublets, and the percentages of CD4+CD25+Foxp3+, CD4+CD25+IL-10+ or CD4+LAP+ cells were measured. The proportions of CD44hi/CD62L−, CD44lo/CD62L+, CTLA-4+ and chemokine receptor expressing cells were analyzed in CD4+CD25+Foxp3+ cells.
Statistical analysis
GraphPad Prism Software 5.0 (San Diego, CA, USA) was used for statistical analysis. The results are expressed as the medians with the range. For histological analyses, antibody measurements, cytokine responses and FACS analysis, the overall statistical significance comparing different sets of mice was determined using a Kruskal–Wallis test before performing the Mann–Whitney test for pairwise comparisons.
Results
Immunotherapy-induced desensitization of peanut-sensitized mice
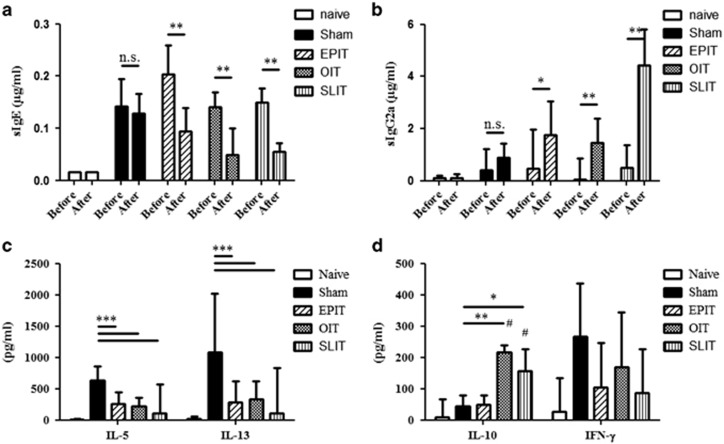
The desensitization of mice sensitized to peanuts was monitored by PPE-sIgE, sIgG2a and PPE-specific cytokine production by splenocytes. In sham-treated mice, sIgE and sIgG2a did not vary over time. EPIT, OIT and SLIT significantly decreased the sIgE levels and increased sIgG2a (Figures 2a and b). The production of Th2 cytokines (IL-5 and IL-13) by PPE-activated splenocytes was significantly decreased by the three treatments, with no difference between them (P<0.001 versus sham) (Figure 2c). Splenocytes from OIT- and SLIT-treated mice produced higher levels of IL-10 than the sham mice (P<0.01 and P<0.05, respectively) and EPIT-treated mice (P<0.001 and P<0.05, respectively) (Figure 2d). The IFN-γ levels were not significantly modified by any treatment route (Figure 2d).
Figure 2.
Immunotherapies induced the desensitization of peanut-sensitized mice. Sensitized mice were EPIT-, OIT-, SLIT- or sham-treated for 8 weeks. The quantities of peanut-specific IgE (a) and IgG2a (b) were measured for each group and before treatment after sensitization (before) and after the treatment period (after). The production of Th2 cytokines (IL-5 and IL-13) (c) and IL-10 and IFN-γ (d) were measured after in vitro stimulation with peanuts for 72 h. Cytokine levels were measured using Bioplex. The data are shown as the median with range from three independent experiments with n=8 for each group of mice. *P<0.05; **P<0.01; ***P<0.001; #P<0.05 compared with EPIT.
Different phenotypes of Tregs induced by EPIT, OIT or SLIT
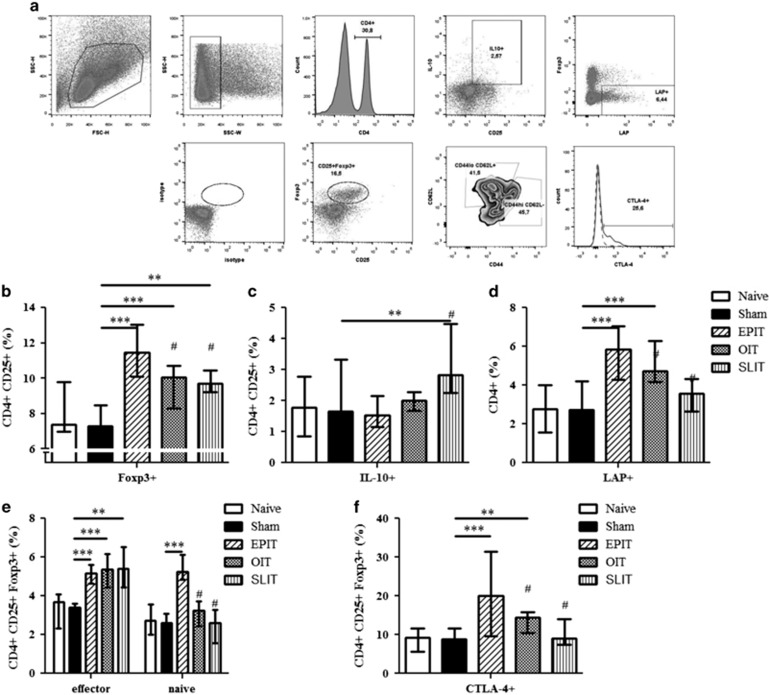
The desensitization of mice sensitized to peanuts was accompanied by a significant increase in CD4+CD25+Foxp3+ Tregs in EPIT-, OIT- and SLIT-treated mice (P<0.001, P<0.001 and P<0.01 versus sham, respectively; Figure 3b). This increase was significantly greater for EPIT compared with OIT and SLIT (P<0.01). The proportion of CD4+CD25+IL-10+ Tr1 cells increased only for SLIT compared with the sham group (P<0.01; Figure 3c). Moreover, EPIT and OIT induced a significant increase in CD4+LAP+ cells (Th3) compared with the sham group (P<0.001); this increase was significantly higher for EPIT compared with OIT (Figure 3d). Following EPIT, both naive (CD44lo/CD62L+) and effector (CD44hi/CD62−) Foxp3+ Tregs were increased significantly compared with the sham group (P<0.001; Figure 3e), whereas only effector (CD44hi/CD62−) Foxp3+ Tregs were increased significantly after OIT and SLIT (P<0.01 versus EPIT). EPIT and OIT induced a higher level of CTLA-4+ Tregs compared with the sham (P<0.001 and P<0.01, respectively) and SLIT groups (P<0.01; Figure 3f).
Figure 3.
Different phenotypes of Tregs induced by EPIT, OIT or SLIT. Sensitized mice were EPIT-, OIT-, SLIT- or sham-treated for 8 weeks. At the end of treatment, the spleen cells were harvested for flow cytometry analysis. (a) Gating strategies: the cells were gated on CD4+ cells among the lymphocytes identified by FSC/SSC after doublet exclusion; the percentages of CD25+Foxp3+ (b), CD25+IL10+ (c) or LAP+ cells (d) and the percentages of CD25+Foxp3+CD44hiCD62L− or CD25+Foxp3+CD44loCD62L+ cells (e) were analyzed. (f) Using the same gating strategies, the cells were gated on CD4+CD25+Foxp3+ cells and the percentage of cells expressing CTLA-4 was analyzed. The data are shown as the median with range from three independent experiments with n=6 for each group of mice. *P<0.05; **P<0.01; ***P<0.001; #P<0.01 compared with EPIT.
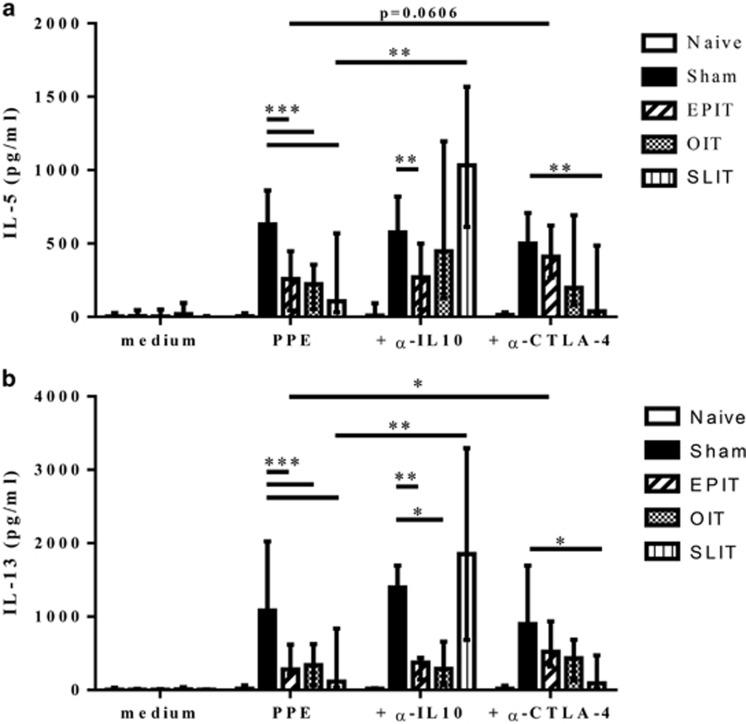
The mechanisms of suppression by Tregs were analyzed using the in vitro re-stimulation of splenocytes in the presence of anti-IL-10 or anti-CTLA-4 blocking antibodies. Consistent with previous results, the suppression of Th2 cytokine production with EPIT was not affected by anti-IL-10 compared with the sham group but was completely blocked by anti-CTLA-4 (Figures 4a and b). Furthermore, the production of IL-5 by splenocytes from EPIT-treated mice had a tendency to increase (P=0.0606), and IL-13 significantly increased in the presence of anti-CTLA-4 (P<0.05). The suppressive effect of OIT compared with sham on IL-5 production but not IL-13 production was blocked by anti-IL-10, and both were partially blocked by anti-CTLA-4 (Figures 4a and b); however, the difference in cytokine production with or without blocking antibodies was not significant due to high variability. In contrast, Th2 inhibition observed with SLIT was completely blocked by anti-IL-10 but not by anti-CTLA-4 with a significant increase in cytokine production (P<0.01; Figures 4a and b). No alterations were observed for the production of IFN-γ (data not shown).
Figure 4.
Mechanism of suppression of allergen-specific Th2 production. Sensitized mice were EPIT-, OIT-, SLIT- or sham-treated for 8 weeks. At the end of the treatment, the spleen cells were harvested and stimulated with peanut in the presence or absence of anti-IL-10 or anti-CTLA-4 blocking antibodies for 72 h. The IL-5 (a) and IL-13 (b) levels in the supernatant were measured using Bioplex. The data are shown as the median with range from three independent experiments with n=6 for each group of mice. *P<0.05; **P<0.01; ***P<0.001; #P<0.05 compared with EPIT.
EPIT induced higher levels of CD4+CD25+Foxp3+ cells compared with OIT or SLIT. This difference is due to an increase in naive Tregs in EPIT because the induction of effector Tregs was similar in EPIT, OIT and SLIT, and only EPIT induced naive Tregs. Moreover, EPIT-induced Tregs induced higher levels of CTLA-4 compared with OIT-induced cells. The mechanisms of suppression differed between the IL-10-independent, CTLA-4-mediated action of EPIT-induced Tregs, IL-10-dependent and CTLA-4-independent action of SLIT-induced Tregs and IL-10- and CTLA-4-dependent mechanism in OIT.
Greater expression of Treg homing receptors with EPIT
The differential expression of adhesion molecules and chemokine receptors determines the specific migration of leukocytes into distinct tissues and microenvironments. Because Tregs display distinct efficacy in vivo based on their expression of tissue-specific homing molecules, we investigated the level of representative homing receptors on Tregs induced by EPIT, OIT or SLIT.
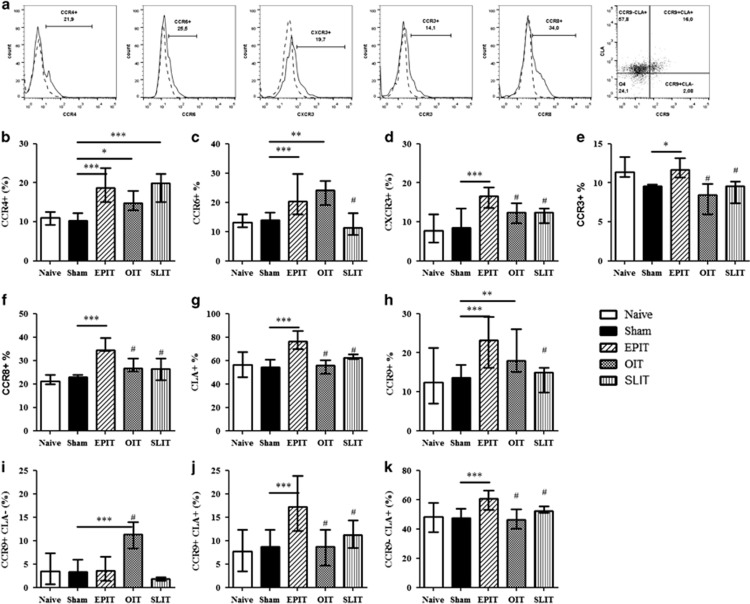
In the spleen, CCR4 expression increased significantly on Tregs induced by the three treatment routes compared with the sham group (Figure 5b). CCR6 increased on the Tregs induced by EPIT and OIT but not SLIT (Figure 5c), whereas CXCR3 was induced by EPIT only (Figure 5d). CCR3 expression on Tregs was decreased in sensitized mice compared with naive mice, but this decrease was counteracted by EPIT, resulting in the significantly higher expression of CCR3 on EPIT-induced Tregs compared with the sham, OIT and SLIT groups (P<0.05; Figure 5e). Due to the increased level of Tregs induced by EPIT, these data imply an increase in the level of Tregs expressing CCR3 in the EPIT group only. The expression of the Th2 homing receptor CCR8 increased in Tregs induced by EPIT but not by OIT or SLIT (Figure 5f). Concerning skin and gut homing, CLA-expressing cells increased only with EPIT (Figure 5g), and CCR9 was induced by both EPIT and OIT but not SLIT (Figure 5h). Notably, among the EPIT-induced CLA+ Tregs, both CCR9− and CCR9+ Tregs were observed. Indeed, EPIT-induced Tregs expressing CCR9 also expressed CLA, whereas CCR9+ Tregs in the OIT group did not, suggesting that some of the Tregs induced through the skin acquired gut homing properties (Figures 5i–k). Moreover, following EPIT, 70.5±3.8% of the CCR9+ Tregs were naive (CD44lo/CD62L+), resulting in a significant increase in CCR9+ naive Tregs (P<0.001) but not in CCR9+ effector Tregs compared with the sham treatment (data not shown). No alterations in CCR10 expression was observed (data not shown).
Figure 5.
Expression of homing receptors on Tregs in the spleen. Sensitized mice were EPIT-, OIT-, SLIT- or sham-treated for 8 weeks. At the end of treatment, the spleen cells were harvested for flow cytometry analysis. Using the same gating strategies, the cells were gated on CD4+CD25+Foxp3+ and the percentages of expression of homing receptors were analyzed, as shown by a representative histogram and dot plot (a). The percentages of cells expressing CCR4 (b), CCR6 (c), CXCR3 (d), CCR3 (e), CCR8 (f), CLA (g) or CCR9 (h) were analyzed. Double staining was used for the expression of CLA and CCR9 to analyze the percentage of CD4+CD25+Foxp3+ cells expressing either only CLA (CCR9-CLA+) (i), both CCR9 and CLA (CCR9+CLA+) (j) or only CCR9 (CCR9+CLA−) (k). The data are shown as the median with range from three independent experiments with n=6 for each group of mice. *P<0.05; **P<0.01; ***P<0.001; #P<0.05 compared with EPIT.
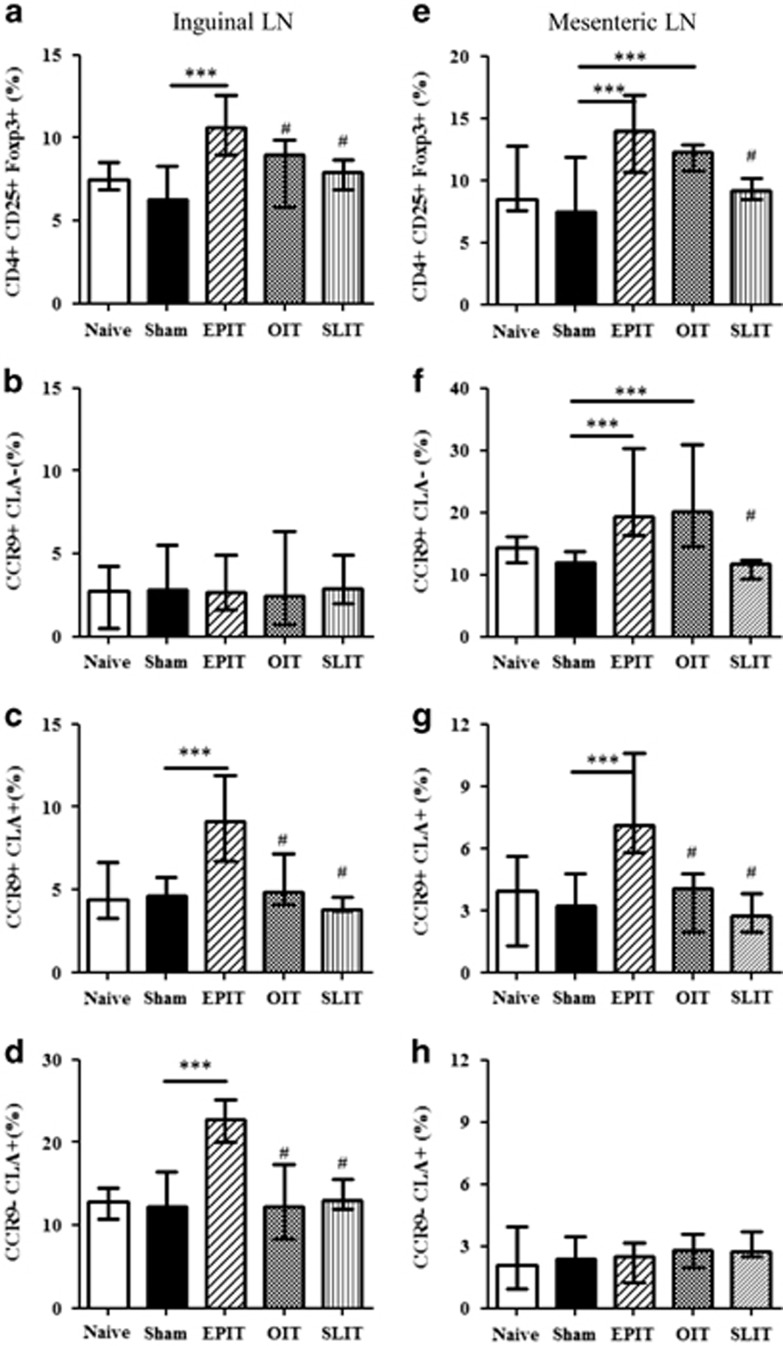
In the inguinal lymph nodes (iLN), EPIT significantly increased the level of Foxp3+ Tregs compared with sham treatment, OIT or SLIT (Figure 6a). In the iLN, EPIT increased CLA+ but not CLA− Tregs (Figures 6b–d). Among the CLA+ Tregs, both CLA+CCR9− and CLA+CCR9+ Tregs increased (Figures 6c and d).
Figure 6.
Induction of Tregs and their expression of homing receptors in the iLN and mLN. Sensitized mice were EPIT-, OIT-, SLIT- or sham-treated for 8 weeks. At the end of treatment, the iLN (a–d) and mLN (e–h) cells were harvested for flow cytometric analysis. The cells were gated on CD4+ among the lymphocytes identified by FSC/SSC after doublet exclusion, and the percentages of CD25+Foxp3+ (a, e), the percentage of CD4+CD25+Foxp3+ cells expressing either only CCR9 (CCR9+CLA−) (b, f), both CCR9 and CLA (CCR9+CLA+) (c, g) or only CLA (CCR9-CLA+) (d, h) were analyzed. The data are shown as the median with range from three independent experiments, with n=6 for each group of mice. *P<0.05; **P<0.01; ***P<0.001; #P<0.01 compared with EPIT.
In the mesenteric lymph nodes (mLN), the level of Tregs increased with EPIT and OIT, but not with SLIT (Figure 6e), consistent with the expression of CCR9 on spleen Tregs. Indeed, OIT increased the expression of CCR9 but not CLA, whereas EPIT induced both CCR9+CLA− and CCR9+CLA+ Treg expression (Figures 6f and g). No induction of CCR9- Tregs was observed (Figure 6h). The presence of a significant level of CCR9+CLA− Tregs in the mLN following EPIT suggested that either some of the EPIT-induced Tregs lose CLA after migration to the mLN or CLA+CCR9+ Tregs induced Tregs locally that expressed only CCR9 but not CLA.
Level and phenotype of Tregs persisted after discontinuation of EPIT but not after SLIT or OIT
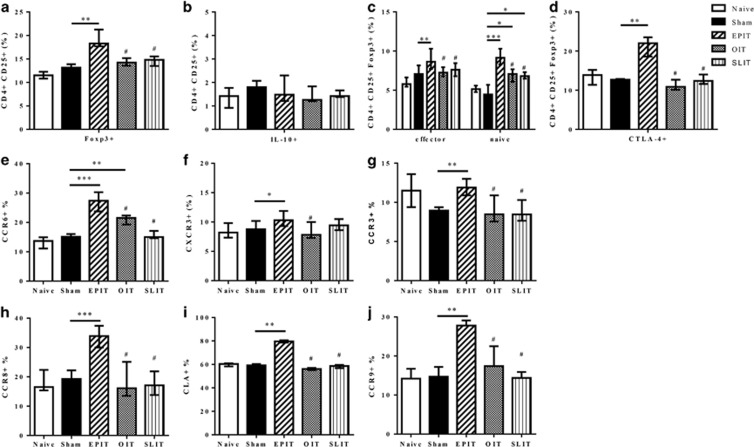
To analyze the persistence of the induced Tregs after the discontinuation of immunotherapy, the level and phenotype of Tregs were analyzed 8 weeks after the termination of EPIT, OIT and SLIT. Higher percentages of CD4+CD25+Foxp3+ Tregs persisted after the discontinuation of EPIT but not after OIT or SLIT (Figure 7a). An increase in the proportion of CD4+CD25+IL-10+ Tr1 cells was no longer observed after SLIT was discontinued (Figure 7b). Following the discontinuation of EPIT, both naive (CD44lo/CD62L+) and effector (CD44hi/CD62−) Foxp3+ Tregs persisted (P<0.01 and P<0.001, respectively, compared with sham), whereas this was not observed with OIT and SLIT (Figure 7c). The higher level of CTLA-4+ Tregs observed at the end of EPIT and OIT was maintained after the discontinuation of EPIT only but not OIT (P<0.01; Figure 7d).
Figure 7.
Sustainability of different Tregs induced by EPIT, OIT or SLIT. Sensitized mice were EPIT-, OIT-, SLIT- or sham-treated for 8 weeks and left untreated for 8 more weeks. The spleen cells were harvested for flow cytometry analysis. The percentages of CD25+Foxp3+ (a), CD25+IL10+ (b) and CD25+Foxp3+CD44hiCD62L− or CD25+Foxp3+CD44loCD62L+ (c) were analyzed. (d) Using the same gating strategies, the cells were gated on CD4+CD25+Foxp3+ and the percentage of cells expressing CTLA-4 was analyzed. The percentages of CD4+CD25+Foxp3+ cells expressing CCR6 (e), CXCR3 (f), CCR3 (g), CCR8 (h), CLA (i) or CCR9 (j) was analyzed. The data are shown as the median with range from three independent experiments with n=6 for each group of mice. *P<0.05; **P<0.01; ***P<0.001; #P<0.01 compared with EPIT.
Concerning the expression of homing receptors in the spleen, CCR6 was maintained on the Tregs induced by EPIT, and to a lesser extent by OIT, 8 weeks after the discontinuation of treatment (P<0.001 and P<0.01, respectively compared with sham; P<0.05 between EPIT and OIT; Figure 7e). CXCR3 was decreased 8 weeks after discontinuing EPIT compared with the end of treatment (Figure 7f). The CCR3 expression on Tregs was similar 8 weeks after the end of immunotherapy compared with the end of treatment, with significantly greater expression of CCR3 on EPIT-induced Tregs compared with the sham, OIT and SLIT groups (P<0.05; Figure 7g). The expression of the Th2 homing receptor CCR8 was maintained on the Tregs after the discontinuation of EPIT but not after the discontinuation of OIT or SLIT (Figure 7h). The expression of the skin homing receptor CLA was also maintained on EPIT-induced Tregs 8 weeks after the end of treatment (Figure 7i). Concerning gut homing, CCR9 was maintained on Tregs induced by EPIT but not OIT (Figure 7j).
Immunotherapy-induced Tregs displayed in vivo suppressive activities persisting after the discontinuation of EPIT but not after the discontinuation of SLIT or OIT
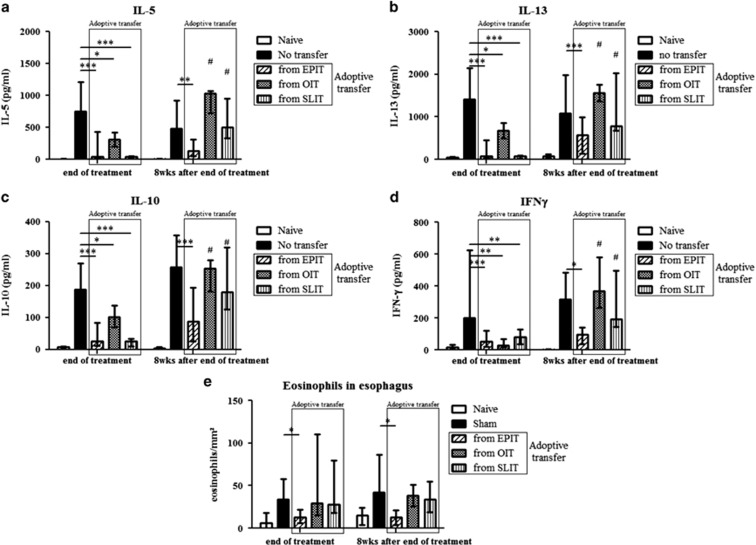
To assess the in vivo suppressive activities of Tregs induced by EPIT, OIT and SLIT, CD4+CD25+ cells were isolated from the spleens of mice after discontinuing treatment and were transferred into PPE-sensitized mice and compared with non-transferred sensitized mice. The PPE-specific production of Th2 (IL-5 and IL-13), Th1 (IFN-γ) as well as the IL-10 cytokine by in vitro reactivated splenocytes was significantly decreased by the transfer of Tregs isolated from the three groups of mice just after the discontinuation of treatment (Figures 8a–d, left panels). This demonstrates that the Tregs induced by EPIT, OIT or SLIT displayed suppressive activities at the end of treatment.
Figure 8.
Immunotherapy-induced Tregs displayed in vivo suppressive activities that persist after the discontinuation of EPIT but not SLIT or OIT. Mice were sensitized and administered CD4+CD25+ T cells isolated just after or 8 weeks after the end of EPIT, OIT and SLIT. These mice were then compared with non-transferred sensitized mice and naive mice. Splenocytes were stimulated with peanut for 72 h. The concentrations of IL-5 (a), IL-13 (b), IL-10 (c) and IFN-γ (d) were measured in the supernatants using Bioplex. (e) Mice were exposed to oral peanut for 10 days, and the esophagi were harvested. Formalin-fixed, paraffin-embedded tissue sections were stained with hematoxylin–eosin–safranin, and eosinophil infiltration of the esophagus was measured by a double-blind reading in a × 40-powered field. The results are expressed as the median of the number of eosinophils per mm2 with range for each group of mice. The data are shown as the median with range from three independent experiments, with n=6 for each group of mice. *P<0,05; **P<0,01; ***P<0,001; #P<0,05 compared with EPIT.
To analyze the persistence of these suppressive activities following the discontinuation of treatment, CD4+CD25+ cells were isolated from the spleens of mice 8 weeks after the end of treatment and transferred into PPE-sensitized mice. The transfer of Tregs isolated 8 weeks after completing OIT or SLIT did not decrease allergen-specific cytokine production, whereas Tregs isolated 8 weeks after the discontinuation of EPIT did (Figures 8a–d, right panels), suggesting that only EPIT induced persisting Tregs.
To investigate the potential protection offered by Tregs against allergen exposure, recipient mice were subjected to peanut oral exposure, which induced inflammation and the infiltration of eosinophils into the esophagus. Although OIT- and SLIT-induced Tregs isolated just after the end of treatment decreased Th2 cytokine responses, they were unable to protect sensitized mice from the infiltration of eosinophils, whereas EPIT-induced Tregs blocked eosinophil infiltration (Figure 8e). The protective effect of EPIT-induced Tregs was still present when the cells were isolated 8 weeks following the end of treatment, confirming the persistence of Treg suppressive activity.
Discussion
Three forms of allergen-specific immunotherapy are currently being explored to treat food allergy, that is, oral, sublingual and epicutaneous immunotherapy, with the induction of Tregs playing a pivotal role.3, 4 The different routes are likely to induce different Treg subsets, with differing homing properties and consequently distinct efficacy and maintenance capabilities in vivo. In this study, we show that although OIT, SLIT and EPIT were initially able to effectively desensitize peanut-sensitized mice, they induced different subsets of Tregs. Moreover, the three immunotherapeutic routes activated different homing properties of induced Tregs, with subsequent differences in suppressive activities that persisted for a prolonged period after the discontinuation of EPIT but not OIT or SLIT.
Among the different subsets of Tregs induced by immunotherapy, the three most relevant subsets seem to be the IL-10-producing Tr1 cells, the TGF-β-producing Th3 cells (LAP+) and the CD4+CD25+ Tregs.2 Depending on the tissue, origin and stimulatory conditions, the subsets differ in cytokine production and surface marker expression as well as in the suppression of immune responses. In general, specific immunotherapy induces the production of IL-10 and TGF-β and increases the frequency of IL-10+ regulatory cells6, 15 and Foxp3+ cells.3, 4 In our model, EPIT and OIT increased the numbers of Foxp3+ and LAP+ Tregs, whereas SLIT increased the numbers of CD4+CD25+IL10+ Tregs. This clearly suggests different mechanisms of desensitization for the three routes; the suppressive activity of EPIT-induced Tregs did not depend on IL-10 but required CTLA-4, OIT acted through both mechanisms, and SLIT was strictly dependent on IL-10, as previously described.6, 16 The production of IL-10 by activated splenocytes was increased after OIT and SLIT but not after EPIT, although EPIT induced a higher level of Tregs. We previously observed that despite clinical protection, EPIT did not induce allergen-specific IL-10 production by re-stimulated splenocytes and did not increase IL-10 levels in the bronchoalveolar lavage fluid of aerosol-challenged mice or in the esophagus of peanut-sensitized mice orally exposed to peanuts.12, 13 These results emphasize the IL-10-independent mechanism of suppression by EPIT-induced Tregs. Moreover, in sensitized mice receiving Tregs, IL-10 production was decreased compared with mice not receiving transferred cells, suggesting that the IL-10 observed in these mice was secreted by Th2 cells, which were inhibited by the transferred Tregs, further suggesting the induction of IL-10-independent Tregs in EPIT.
A major difference in the phenotype of EPIT-induced Tregs was the induction of both effector/memory (measured by the expression of CD44hi and CD62L−) and naive (CD44lo and CD62L+) cells, whereas OIT and SLIT induced only effector/memory Tregs. CD62L, a marker utilized to differentiate naive cells from effector cells, is a crucial lymphoid homing molecule. Although CD4+CD25+CD62L+ and CD4+CD25+CD62L– Tregs have similar suppressive capabilities in vitro, the CD4+CD25+CD62L+ subset seems more efficient in vivo in some pathologic immune states, such as graft-versus-host disease and diabetes17, 18, which is probably due to distinct lymphoid homing capacity (CD62L+ Tregs migrate into the LN more efficiently than CD62L− Tregs). However, effector Tregs have also been shown to induce greater suppressive activity in vivo than naive cells.19, 20 In our model, we did not see any superiority in the decrease of Th2 cytokines in response to transferred EPIT-Tregs compared with OIT- or SLIT-induced cells when they were isolated at the end of treatment. As the proportion of naive Tregs in EPIT is significantly greater than in OIT or SLIT, it seems that the presence of naive Tregs is not associated with greater suppressive activity. However, it could be part of a mechanism of long-term maintenance, as only EPIT Tregs displayed sustained suppression after the discontinuation of treatment, possibly because the naive Tregs proliferate more efficiently than effector Tregs, at least in vitro.19, 20
The suppression of immune responses in lymphoid organs or in diverse tissues requires the appropriate trafficking of Tregs according to their expression of various homing receptors. In the mLN, Tregs acquire the expression of CCR9 to mediate the correct migration toward the intestine.21 Similarly, the delayed accumulation of Tregs in the mLN was observed in a model of inflammatory bowel disease if the cells were deficient in CCR4.22 In a model of gastric mucosal inflammation, Cook et al.23 demonstrated that Tregs in gastric biopsies are predominantly CCR6+ and respond to CCL20 expression during infection, suggesting a role for CCR6 in the migration of Tregs toward the gut. In our model, both EPIT and OIT increased the levels of CCR4, CCR6 and CCR9 on spleen-derived Tregs, whereas SLIT increased only CCR4. These results suggest that EPIT- and OIT-induced Tregs would be more likely to migrate toward the mLN and gut mucosa to locally suppress the immune response. CCR4 is also upregulated in Tregs that accumulate in the lungs of allergic mice and is necessary for the attenuation of allergic inflammation.24 SLIT has been utilized extensively in the treatment of allergy to aero-allergens with good efficacy; the increased expression of CCR4 but not CCR6 or CCR9 by SLIT-induced Tregs could indicate that SLIT may be more effective for treating lung inflammation than food allergy. Based on this murine model, EPIT and OIT appear to be a better approach for food allergy. However, only EPIT-induced Tregs protected sensitized mice from esophageal inflammation following oral peanut exposure, even though the Tregs induced by the three treatments had suppressive activity against Th2 cytokines in splenocytes. This could result from different migratory properties, with EPIT- but not OIT- or SLIT-induced Tregs being able to migrate toward the esophagus to dampen local inflammation. Indeed, only EPIT-induced Tregs increased the expression of CCR8, a well-known Th2 homing receptor, and CCR3, an eotaxin receptor, which is implicated in esophageal infiltration by eosinophils. In humans, the de novo onset of eosinophilic esophagitis occurs in ~2.7% of patients with OIT for IgE-mediated food allergy,25 suggesting that desensitization with OIT does not prevent inflammation of the esophagus and may even aggravate it. This is in accordance with the absence of protection observed after the transfer of OIT-induced Tregs in our model.
The major skin homing receptor for T cells is CLA, but skin tropic Tregs may also express CCR4, CCR10, CCR8 and CCR6.26 EPIT-induced Tregs had significantly higher levels of CLA and CCR8 than OIT. This is probably due to the route of administration; EPIT selectively targets skin dendritic cells.27 Effector/memory Foxp3+ Tregs were shown to migrate from the skin toward the draining lymph nodes at the steady state as well as during inflammation and can return to the skin following antigen exposure.28 The presence of increased levels of CLA+CCR9+ Tregs in the iLN as well as in the spleen and mLN following EPIT clearly suggests that EPIT induces Tregs in the skin or in draining lymph nodes after Langerhans cell migration. Some of these Tregs also expressed CCR9 and were thus able to migrate toward the mLN and gut mucosa. On the other hand, OIT-induced Tregs expressed CCR9 but not CLA, and no increase in the number of Tregs was observed in the iLN of mice treated by OIT, suggesting that they are induced in the mLN and maintain gut homing properties only.
The large repertoire of homing receptors expressed by EPIT-induced Tregs suggests that Tregs are able to migrate to various sites of allergen exposure to induce protection from Th2-induced inflammation and to suppress local responses to allergen stimulation. Indeed, EPIT actually proved efficacious following different routes of allergen administration, including bronchial hyper-responsiveness,13, 29 eosinophil recruitment in the skin27 and upon peanut-induced gut inflammation.3, 12 This suggests the induction of global tolerance rather than local desensitization.
The aim of allergen immunotherapy is the induction of immune tolerance; that is, persistent desensitization following the discontinuation of treatment. In patients treated with OIT, certain participants can withstand a short interruption of OIT and remain tolerant, but most do not develop long-term tolerance. No study on long-term tolerance is available for SLIT.11 Recently, it was proposed that the induction of Tregs that persist following the discontinuation of treatment could participate in long-term tolerance.4 Our study shows that EPIT-induced Tregs retained their suppressive capacity for a prolonged period of time following the end of treatment, whereas OIT- and SLIT-induced cells did not, suggesting that EPIT but not OIT or SLIT can induce long-term tolerance, at least in our model. The basis of the maintenance of tolerance following different routes of immunotherapy can be summarized as follows. SLIT induced a transient increase in Tr-1 cells followed by immune deviation toward Th1 responsiveness.6 During EPIT, sensitized mice presented a general increase in Foxp3+ Tregs of both the naive and effector phenotypes, whereas OIT and SLIT induced Tregs of the effector phenotype only. The naive Treg subset has been reported to preferentially proliferate in vitro, while effector Tregs are prone to die and expand poorly in vitro.19, 20 This difference in the induction of naive Tregs by EPIT compared with OIT and SLIT could, at least in part, explain the differences in the long-term maintenance of Tregs.
In summary, the induction of Tregs during allergen-specific immunotherapy, which could be central to the induction of long-term tolerance, is clearly different between EPIT, OIT and SLIT. Indeed, EPIT modulates the allergen-specific T-cell response via a mechanism that seems to differ from other specific immunotherapy routes. EPIT was able to induce a high level of gut homing Tregs similar to OIT, strongly suggesting its relevance in food allergy, and a greater repertoire of homing receptors on Tregs than SLIT or OIT, suggesting a possible application in various forms of allergy. Moreover, whereas OIT- or SLIT-induced Tregs lose their suppressive activity after the discontinuation of treatment, the suppressive activities of EPIT-induced Tregs were still present at 8 weeks after the end of treatment, suggesting the induction of a more long-lasting tolerance.
Acknowledgments
We would like to thank the staff of the animal facility of Paris XI University, School of Pharmacy, for animal care. We acknowledge Muriel Andrieu and Karine Labroquere of the Cochin Immunobiology Facility and Maryline Favier and Corinne Lesaffre of the Cochin Histology Facility.
Footnotes
This study was supported by DBV Technologies, the developer and owner of VIASKIN. Vincent Dioszeghy, Lucie Mondoulet, Veronique Dhelft, Mélanie Ligouis, Emilie Puteaux, Camille Plaquet and Pierre-Henri Benhamou are DBV Technologies employees. Christophe Dupont received honoraria and/or compensation with regard to the study, as investigators, coordinators or experts, in relation with the time spent on the study.
References
- Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010; 126 (6 Suppl): S1–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol 2014; 133: 621–631. [DOI] [PubMed] [Google Scholar]
- Dioszeghy V, Mondoulet L, Dhelft V, Ligouis M, Puteaux E, Dupont C et al. The regulatory T cells induction by epicutaneous immunotherapy is sustained and mediates long-term protection from eosinophilic disorders in peanut-sensitized mice. Clin Exp Allergy 2014; 44: 867–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J Allergy Clin Immunol 2014; 133: 500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Burks AW, Dupont C. State of the art on food allergen immunotherapy: oral, sublingual, and epicutaneous. J Allergy Clin Immunol 2014; 133: 318–323. [DOI] [PubMed] [Google Scholar]
- Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol 2007; 120: 707–713. [DOI] [PubMed] [Google Scholar]
- Siegmund K, Feuerer M, Siewert C, Ghani S, Haubold U, Dankof A et al. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood 2005; 106: 3097–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Tang J, Cao H, Fan H, Li B. Tissue resident regulatory T cells: novel therapeutic targets for human disease. Cell Mol Immunol 2015; 12: 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol 2008; 9: 970–980. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kang SG, Kim CH. FoxP3+ T cells undergo conventional first switch to lymphoid tissue homing receptors in thymus but accelerated second switch to nonlymphoid tissue homing receptors in secondary lymphoid tissues. J Immunol 2007; 178: 301–311. [DOI] [PubMed] [Google Scholar]
- Wang J, Sampson HA. Oral and sublingual immunotherapy for food allergy. Asian Pac J Allergy Immunol 2013; 31: 198–209. [PubMed] [Google Scholar]
- Mondoulet L, Dioszeghy V, Larcher T, Ligouis M, Dhelft V, Puteaux E et al. Epicutaneous immunotherapy (EPIT) blocks the allergic esophago-gastro-enteropathy induced by sustained oral exposure to peanuts in sensitized mice. PLoS One 2012; 7: e31967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondoulet L, Dioszeghy V, Ligouis M, Dhelft V, Dupont C, Benhamou PH. Epicutaneous immunotherapy on intact skin using a new delivery system in a murine model of allergy. Clin Exp Allergy 2010; 40: 659–667. [DOI] [PubMed] [Google Scholar]
- Mondoulet L, Dioszeghy V, Ligouis M, Dhelft V, Puteaux E, Dupont C et al. Epicutaneous immunotherapy compared with sublingual immunotherapy in mice sensitized to pollen (Phleum pratense). ISRN Allergy 2012; 2012: 375735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis JN, James LK, Paraskevopoulos G, Wong C, Calderon MA, Durham SR et al. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol 2008; 121: 1120–1125. [DOI] [PubMed] [Google Scholar]
- Mascarell L, Lombardi V, Louise A, Saint-Lu N, Chabre H, Moussu H et al. Oral dendritic cells mediate antigen-specific tolerance by stimulating TH1 and regulatory CD4+ T cells. J Allergy Clin Immunol 2008; 122: 603–609. [DOI] [PubMed] [Google Scholar]
- Ermann J, Hoffmann P, Edinger M, Dutt S, Blankenberg FG, Higgins JP et al. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood 2005; 105: 2220–2226. [DOI] [PubMed] [Google Scholar]
- Szanya V, Ermann J, Taylor C, Holness C, Fathman CG. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. J Immunol 2002; 169: 2461–2465. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Eder R, Boeld TJ, Doser K, Piseshka B, Andreesen R et al. Only the CD45RA+ subpopulation of CD4+CD25 high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood 2006; 108: 4260–4267. [DOI] [PubMed] [Google Scholar]
- Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med 2004; 199: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med 2007; 204: 1765–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Bromley SK, Means TK, Jones KJ, Hayashi F, Bhan AK et al. CCR4-dependent regulatory T cell function in inflammatory bowel disease. J Exp Med 2007; 204: 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook KW, Letley DP, Ingram RJ, Staples E, Skjoldmose H, Atherton JC et al. CCL20/CCR6-mediated migration of regulatory T cells to the Helicobacter pylori-infected human gastric mucosa. Gut 2014; 63: 1550–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustino L, da Fonseca DM, Takenaka MC, Mirotti L, Florsheim EB, Guereschi MG et al. Regulatory T cells migrate to airways via CCR4 and attenuate the severity of airway allergic inflammation. J Immunol 2013; 190: 2614–2621. [DOI] [PubMed] [Google Scholar]
- Lucendo AJ, Arias A, Tenias JM. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Ann Allergy Asthma Immunol 2014; 113: 624–629. [DOI] [PubMed] [Google Scholar]
- Islam SA, Luster AD. T cell homing to epithelial barriers in allergic disease. Nat Med 2012; 18: 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dioszeghy V, Mondoulet L, Dhelft V, Ligouis M, Puteaux E, Benhamou PH et al. Epicutaneous immunotherapy results in rapid allergen uptake by dendritic cells through intact skin and downregulates the allergen-specific response in sensitized mice. J Immunol 2011; 186: 5629–5637. [DOI] [PubMed] [Google Scholar]
- Tomura M, Honda T, Tanizaki H, Otsuka A, Egawa G, Tokura Y et al. Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. J Clin Invest 2010; 120: 883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondoulet L, Dioszeghy V, Vanoirbeek JA, Nemery B, Dupont C, Benhamou PH. Epicutaneous immunotherapy using a new epicutaneous delivery system in mice sensitized to peanuts. Int Arch Allergy Immunol 2011; 154: 299–309. [DOI] [PubMed] [Google Scholar]