Abstract
In attaining UNAIDS targets of 90-90-90 to achieve epidemic control, understanding who the current utilizers of HIV treatment services are will inform efforts aimed at reaching those not being reached. A retrospective chart review of CAPRISA AIDS Treatment Program (CAT) patients between 2004 and 2013 was undertaken. Of the 4043 HIV-infected patients initiated on ART, 2586 (64.0%) were women. At ART initiation, men, compared to women, had significantly lower median CD4+ cell counts (113 vs 131 cells/mm3, p <0.001), lower median body mass index (BMI) (21.0 vs 24.2 kg/m2, p<0.001), higher mean log viral load (5.0 vs 4.9 copies/ml, p<0.001) and were significantly older (median age: 35 vs. 32 years, p<0.001). Men had higher mortality rates compared to women, 6.7 per 100 person-years (p-y), (95% CI: 5.8–7.8) vs. 4.4 per 100 p-y, (95% CI: 3.8–5.0); mortality rate ratio: 1.54, (95% CI: 1.27–1.87), p <0.001. Age-standardised mortality rate was 7.9 per 100 p-y (95% CI: 4.1–11.7) for men and 5.7 per 100 p-y (95% CI: 2.7 to 8.6) for women (standardised mortality ratio: 1.38 (1.15 to 1.70)). Mean CD4+ cell count increases post-ART initiation were lower in men at all follow-up time points. Men presented later in the course of their HIV disease for ART initiation with more advanced disease and experienced a higher mortality rate compared to women.
Introduction
In South Africa, an estimated 6.2 million people are currently living with HIV infection [1]. South Africa has been successful in implementing the largest anti-retroviral programme in the world with more than 3 million HIV infected people currently on treatment [2]. Despite this, high HIV incidence rates continue to occur, and HIV related morbidity and mortality dominates as the main cause of hospitalization and premature death for the last 2 decades [3].
In order to achieve the UNAIDS 90-90-90 goals for epidemic control and enhance individual and population level impact of public access anti-retroviral therapy (ART), detailed understanding of gaps in access to treatment services and factors that contribute to poor HIV treatment outcomes including ongoing mortality, is imperative. Data from other countries are inconsistent and contradictory, with some studies demonstrating gender imbalances in access to HIV treatment services and in clinical outcomes among patients on ART [4,5], and others demonstrating poorer clinical outcomes in women [6–8] or no gender difference in immunological response or mortality [9].
Notwithstanding the higher burden in women and 5–7 year earlier acquisition of HIV infection in adolescent girls and young women between the ages of 15–24 years [10], men are known to be poor utilizers of health care services [11] and characteristically present at a late stage of disease [12]. Women, are the predominant users of public sector ART [13], with reports from South Africa indicating that up to 60% of eligible women received ART compared to 41% of eligible men by mid-2011 [14]. This reflects the reality that infected women are linked to care through routinely offered HIV testing when utilizing antenatal and other sexual reproductive health services; an option that is not available for men.
A South African study assessing the sustainability of task shifting in a rural primary care clinic demonstrated that 68.8% of those accessing ART services between 2004 and 2010 (p < 0.05) were women [15], and those men that access ART are less likely to be retained in care [16].
In a country with the largest ART rollout program, it is important to understand who the current utilizers of HIV treatment services are, as this will help inform efforts aimed at reaching those currently not being reached. This study sought to investigate whether there were gender and age differences in those accessing HIV care services and clinical outcomes in those initiated on ART among urban and rural patients utilizing a free HIV treatment program in KwaZulu-Natal, South Africa.
Methods
Study design
We undertook a retrospective chart review of ambulant HIV infected, ART naïve adult patients enrolled into the PEPFAR-funded CAPRISA AIDS Treatment Programme (CAT) between June 2004 and August 2013. Patients were enrolled from two different catchment populations in KwaZulu-Natal; a TB clinic in the urban eThekwini district and a rural primary health care clinic in Vulindlela, rural KwaZulu-Natal. ART eligibility criteria, and definitions of immunologic and virological response to ART, defined as <400 copies/ml was as per current South African Government HIV/AIDS treatment guidelines [17]. Following ART initiation, patients presented for clinical review and adherence assessment weekly for the first two weeks, then monthly for the first 6 months, and every 3 months thereafter unless clinically indicated.
Routine demographic and clinical data were recorded at baseline and at follow-up visits. Laboratory safety assessments and CD4+ cell counts and viral loads were conducted at baseline and every 6 months or as clinically indicated. Patients were regarded as lost to follow-up if they missed 3 consecutive scheduled visits and if all attempts to track them telephonically and physically had failed. No lost to follow-up patients re-engaged in care. Patients provided written informed consent for participation in the CAT programme and for the use of their medical records. Approval for data collection and analysis was obtained from the University of KwaZulu-Natal, Biomedical Research Ethics Committee (Ref: E248/05).
Data analysis
We used unpaired t-test, and Wilcoxon rank sum test for continuous data and Fisher’s exact test for categorical data to compare demographics and clinical characteristics between men and women. Data was censored at 72 months of follow-up. Kaplan-Meier was used to construct survival curves and compare them using the log-rank test. Poisson approximations and F-tests were used to construct 95% confidence intervals (CI) for mortality rate, TB incidence rate and rate ratios, respectively. We calculated age-standardised mortality rates using 5-year age band. The province of KwaZulu-Natal was used as a standard population. Predictors of mortality were assessed through both univariate and multivariate proportional hazards regression. Proportionality was assessed by fitting time dependent covariates in a model created by interacting baseline variables with survival time.
The association between gender and CD4+ cell count was determined through linear mixed models. All models were adjusted for baseline covariates such as clinic site, age, tuberculosis status at baseline, past history of TB, WHO stage, CD4+ cell count and viral load. We conducted sensitivity analysis evaluating the impact of ART programme maturity on the effect of gender on mortality during ART initiation periods of 2004–2008 and 2009–2013.
We calculated attributable risk in order to assess the relative contribution and impact of specific covariates on mortality. The effect of loss to follow-up on mortality was determined through sensitivity analysis. P values less than 0.05 were considered statistically significant. All analyses were conducted using SAS, version 9.4 (SAS Institute INC., Cary) and R version 3.2.2.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Patient baseline clinical characteristics
A total of 4043 ambulant HIV infected patients, 1457 (36%) men and 2586 women (64%), 14 years and older, were initiated on ART between June 2004 and August 2013. Baseline clinical characteristics are presented in Table 1. Women comprised 59.4% and 40.6%, (p <0.001) of the study cohort at the urban and rural sites, respectively. Men were older, (median age of 35 vs 32 years), p<0.001, presented more often with WHO stage 4 disease (14.3% vs 9.7%), p<0.001 and were more likely to have had a past history of tuberculosis (TB) (34.4% vs 26.3%), p <0.001, (Table 1). At ART initiation, men had lower median CD4+ cell counts (113 vs131 cells/mm3), p <0.001, a lower median body mass index (BMI) (21.0 vs 24.2 kg/m2), p<0.001 and higher mean log viral loads (5.0 vs 4.9 copies/ml), p<0.001 (Table 1). The overall retention rate at 72 months was 91.8%, with 89.9% of men compared to 91.8% of women (p = 0∙07) retained in care. Men and women were loss to follow-up at a median (IQR) of 9.7 (2.7–23.7) and 12.6 (4.4–27.2) months respectively (p = 0.118). Rates of viral suppression, (89.5% vs 96%, p = 0.094) and proportion diagnosed with TB after ART initiation, (3.8% vs 4.2%, p = 0.620), did not differ significantly between men and women. In contrast, ART regimen change; 7∙1% vs 9.5% p = 0.012 was significantly different between men and women (Table 1).
Table 1. Baseline and follow-up characteristics of men and women initiated on ART.
| Variable | Men (N = 1457) |
Women (N = 2586) |
p-value |
|---|---|---|---|
| Characteristics at ART initiation | |||
| Overall age (years), median(IQR) | 35 (30–41) | 32 (28–38) | <0.001 |
| Age group: n (%) | |||
| <24 | 45 (3.1) | 232 (9.0) | <0.001 |
| 24–34 | 655 (45.0) | 1350 (52.3) | |
| 35+ | 757 (52.0) | 1001 (38.8) | |
| BMI(kg/m2), median(IQR) | 21 (19.0–3.2) | 24.2 (21.1–28.1) | <0.001 |
| CD4+ cell count (cells/mm3), median(IQR)a | 113 (47–177) | 131 (68–189) | <0.001 |
| CD4+ cell count, cells/mm3: n (%) | |||
| <50 | 344 (25.9) | 423 (18.3) | <0.001 |
| 50–200 | 783 (58.9) | 1428 (61.6) | |
| >200 | 203 (15∙3) | 466 (20.1) | |
| Log VL, mean(SD)b | 5∙0 (0.9) | 4.9 (0.9) | <0.001 |
| Rural site, n (%) | 692 (47.5) | 1536 (59.4) | <0.001 |
| Urban site, n (%) | 765 (52.5) | 1050 (40.6) | |
| WHO stage 1–3, n (%)c | 1244 (85.7) | 2315 (90.3) | <0.001 |
| WHO stage 4, n (%)c | 208 (14.3) | 250 (9.7) | |
| Previous history of TB, n (%)d | 483 (34.4) | 661 (26.3) | <0.001 |
| Prevalence of TB, n (%) | 467 (32.1) | 505 (19.5) | <0.001 |
| Follow-up | |||
| Number initiated on second line ART, n (%) | 104 (7.1) | 245 (9.5) | 0.012 |
| Lost to follow-up | 161 (11.1) | 239 (9.2) | 0.070 |
a396 patents had missing baseline CD4+ count,
b496 missing VL,
c26 missing WHO stage,
d121 missing previous history TB
Mortality
Deaths occurred among 173 men and 239 women, over 8033.71 person-years of follow-up, (median of 16.3 months [interquartile range (IQR), 8.1 to 35]), (Fig 1). Overall mortality rates were 5.1 per 100 person-years (p-y) (95% CI: 4.6–5.6) and 6.7 per 100 p-y (95% CI: 5.8–7.8) in men and 4.4 per 100 p-y (95% CI: 3.8–5.0) among women (mortality rate ratio: 1.54 (95% CI: 1.29–1.91, p< 0.001), (Fig 1). Age-standardised mortality rate was 7.9 per 100 p-y (95% CI: 4.1–11.7) for men and 5.7 per 100 p-y (95% CI: 2.7 to 8.6) for women (standardised mortality ratio: 1.38 (1.15 to 1.70). Mortality rates observed for men and women were significantly different at all follow-up time points up to 72 months; men had higher mortality rates throughout follow-up; peaking in the first 6 months post ART initiation at 15.2 (95% CI: 12.3–18.4) and 10.2 (95% CI: 8.5–12.2), between men and women respectively, p = 0.004 (Fig 1).
Fig 1. Kaplan-Meier estimates of cumulative annualised mortality rates between men and women accessing ART.
Mortality rates were highest in young men between the ages of 20 and 24 years at 11.8 per 100 p-y, (95% CI: 5.7–21.8) vs 3.9 per 100 p-y (95% CI: 2.4–5.9) in women of the same age, mortality rate ratio: 3.06 (95% CI: 1.45–6.46), p = 0.003 (Table 2). Among those ≥ 25 years, mortality rates among men were significantly different compared to women: 6.6 per 100 p-y (95% CI 5.6–7.7) vs 4.4 per 100 p-y (95% CI 3.9–5.1), mortality rate ratio: 1.49 (95% CI: 1.21–1.83), p <0.001 (Table 2). The mortality rate between men and women stratified by baseline CD4+ cell count was 10.7 (95% CI 8.3–13.6) and 8.2 (95% CI 6.5–10.1) for patients with baseline CD4+ cell counts <50 cells/mm3, p = 0.098; 5.4 (95% CI 4.3–6.7) and 3.8 (95% CI 3.2–4.6) for baseline CD4+ cell counts of 50–200 cells/mm3, p = 0.012 and 5.2 (95% CI 2.8–8.9) and 2.1 (95% CI 1.2–3.3). Mortality rate ratio stratified by baseline CD4+ cell count comparing men and women was 1.31 (95% CI 0.95–1.8) for baseline CD4+ cell counts < 50 cells/mm3, p = 0.098; 1.42 (95% CI 1.08–1.87) for baseline CD4+ cell counts of 50–200 cells/mm3, p = 0.012 and 2.1 (95% CI 1.22–5.26) for baseline CD4+ cell counts of > 200 cells/mm3 (Table 2).
Table 2. Mortality rates for men and women stratified by baseline CD4+ cell count and age.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | No of deaths | Person-years | Mortality rate (95% CI) | No of deaths | Person-years | Mortality rate (95% CI) | Rate ratio (95% CI) |
p-value |
| Age group (years) | ||||||||
| <20 | 1 | 16.32 | 6.1 (0.2–34.1) | 3 | 86.31 | 3.5 (0.7–10.2) | 1.76 (0.18–16.92) | 0.624 |
| 20–24 | 10 | 84.42 | 11.8 (5.7–21.8) | 22 | 568.07 | 3.9 (2.4–5.9) | 3.06 (1.45–6.46) | 0.003 |
| ≥25 | 162 | 2460.88 | 6.6 (5.6–7.7) | 213 | 4808.60 | 4.4 (3.9–5.1) | 1.49 (1.21–1.83) | <0.001 |
| Baseline CD4+ count (cells/mm3) | ||||||||
| <50 | 67 | 626.59 | 10.7 (8.3–13.6) | 85 | 1039.12 | 8.2 (6.5–10.1) | 1.31 (0.95–1.8) | 0.098 |
| 50–200 | 85 | 1563.29 | 5.4 (4.3–6.7) | 128 | 3343.48 | 3.8 (3.2–4.6) | 1.42 (1.08–1.87) | 0.012 |
| >200 | 13 | 250.01 | 5.2 (2.8–8.9) | 16 | 777.17 | 2.1 (1.2–3.3) | 2.53 (1.22–5.26) | 0.013 |
Treatment response
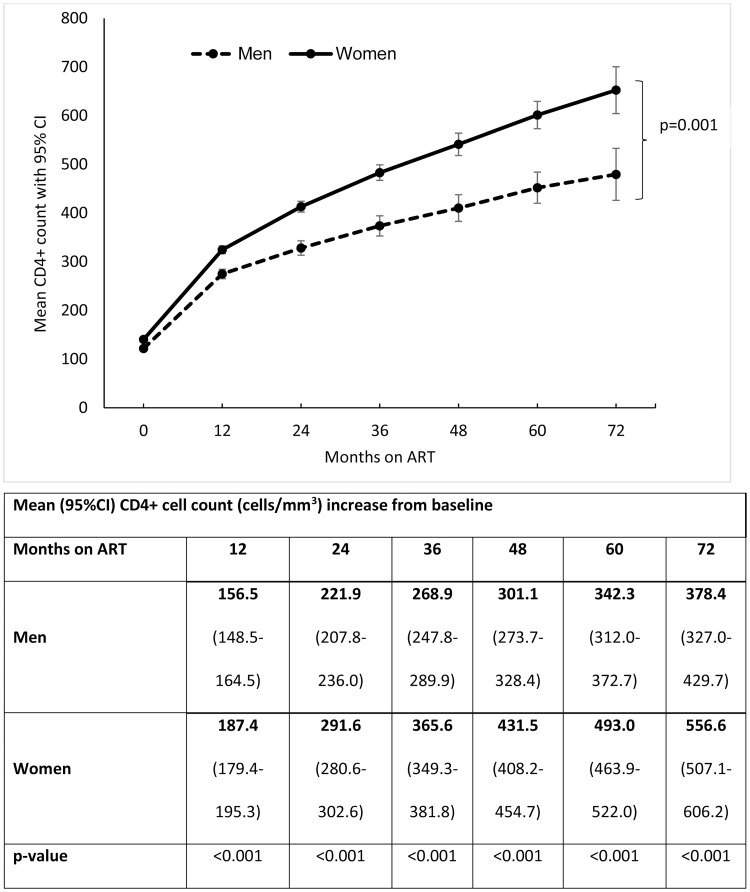
Women demonstrated a better immunological response during ART follow-up showing consistently higher mean CD4+ cell counts at each clinical assessment (Fig 2). Mean CD4+ cell count increases post-ART initiation were lower in men at all follow-up time points (Fig 2). At 72 months of ART, mean CD4+ cell count gain was 378.4 cells/mm3 and 556.6 cells/mm3, p = 0.001 among men and women respectively (Fig 2). Multivariate linear mixed model showed a significant interaction between gender and follow-up time suggesting significantly different CD4+ cell count recovery between men and women; p = 0.001 (Fig 2).
Fig 2. Mean CD4+ cell count change from baseline over time.
Adjusted proportional hazards regression analysis showed that men had a 29% higher risk of death compared to women (adjusted hazard ratio (aHR): 1.29, 95% CI: 1.05–1.58, p = 0.016 (Table 3). Risk of mortality varied by pre-ART CD4+ cell count. Compared to patients with baseline CD4+ cell counts >200 cells/mm3, there was a 4-fold higher risk of death among patients with a baseline CD4+ cell count <50 cells/mm3 (aHR: 3.86, 95% CI: 2.55–5.84), p<0.0001, and a two-fold higher risk of death among patients with a baseline CD4+ cell count between 50–200 cells/mm3, (aHR: 1.94, 95%CI: 1.30–2.91), p = 0.001, (Table 3).
Table 3. Analysis of factors associated with mortality.
| Variable at ART initiation | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Gender (ref: women) | Male | 1.41 (1.16–1.72) | 0.001 | 1.29 (1.05–1.58) | 0.016 |
| Age (per 5 year increase) | 1.03 (0.97–1.08) | 0.364 | 1.04 (0.98–1.10) | 0.182 | |
| Site (ref: rural) | Urban | 0.82 (0.67–1.01) | 0.061 | 0.90 (0.70–1.16) | 0.410 |
| Baseline CD4+ cell count (ref:>200), cells/mm3 | <50 | 4.10 (2.75–6.10) | <.0001 | 3.86 (2.55–5.84) | <.0001 |
| 50–200 | 1.89 (1.28–2.79) | 0.001 | 1.94 (1.30–2.91) | 0.001 | |
| Viral load (log10 copies/ml) | 1.52 (1.31–1.76) | <0.001 | 1.30 (1.11–1.52) | 0.001 | |
| Tuberculosis (ref: Yes) | No | 1.26 (0.98–1.61) | 0.0711 | 1.74 (1.33–2.28) | <.0001 |
| WHO stage (ref: 1–3) | Stage 4 | 2.49 (1.97–3.15) | <.0001 | 2.32 (1.80–2.99) | <.0001 |
| Past history of TB (ref: No) | Yes | 1.20 (0.97–1.47) | 0.092 | 1.04 (0.81–1.33) | 0.759 |
In addition, patients presenting with no TB at ART initiation or a higher mean log viral load or WHO stage 4 disease had significantly higher risk of death (Table 3).
We found that the attributable risk of death among patients initiating ART was 24.3% (95% CI 17.3–30.5) for patients with a low baseline CD4+ cell count and 10.2% (95% CI 2.7–16.9) for male patients (Table 4).
Table 4. Impact of different risk factors on mortality.
| Variable | Attributable risk (95% CI) |
|---|---|
| Male | 10.2% (2.7–16.9) |
| CD4+ cell count <50cells/mm3 | 24.3% (17.3–30.5) |
| WHO stage 4 | 13.2% (9.0–18.1) |
| No TB prevalence | 24.2% (7.0–40.8) |
Of note, sensitivity analysis evaluating the impact of ART programme maturity on the effect of gender on mortality showed that mortality rates between men and women was consistently two-fold higher and significantly different at all follow-up time-points between 2009–2013. This finding did not hold for the period 2004–2008 during the period of ART initiation (Table 5).
Table 5. Sensitivity analysis: Mortality rate by gender.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Follow-up | No of deaths | Person-years | Mortality rate (95% CI) | No of deaths | Person-years | Mortality rate (95% CI) | Mortality Rate ratio (95% CI) | p-value |
| Scenario† | 334 | 2566.73 | 13.0 (11.7–14.5) |
478 | 5466.98 | 8.7 (8.0–9.6) |
1.49 (1.30–1.71) |
<0.001 |
| Scenario‡ | 236 | 2566.73 | 9.2 (8.1–10.4) |
316 | 5466.98 | 5.8 (5.2–6.5) |
1.59 (1.34–1.88) |
<0.001 |
† Assumes all patients who were loss to follow-up died
‡ Assumes all patients who were loss to follow-up within 6 months of ART initiation died
Discussion
We provide evidence of gender disparities between HIV infected men and women in access and response to antiretroviral therapy. Clinical outcomes differed by gender; with men demonstrating higher overall mortality rates and suboptimal immunologic recovery.
Mortality
The bulk of deaths in both men and women occurred in the first 24 months of follow-up concurring with findings from other studies [18,19]. Despite ART access, all patients showed unacceptably high mortality rates with men having 50% higher mortality rate compared women throughout follow-up and their mortality was consistently higher irrespective of baseline CD4+ cell counts. Results from sensitivity analyses, which assumed that all patients who were lost to follow-up died, and that all patients who were lost to follow-up within six months of ART initiation died, were consistent with the main findings (S1 Table).
After removing the confounding effect of age, mortality rates observed in men and women in our study was lower than the age-standardised mortality rates. Interestingly, mortality rates among men and women in the study population closely approximated age-standardised mortality when we assumed that patients who were loss to follow-up within 6 months of ART initiation died.
In contrast to the epidemiological data of the evolving epidemic in southern Africa wherein HIV acquisition does not occur in men until their late twenties [20,21] and we found that the highest mortality rates were in young men between the ages of 20–24 years. However, we had a very small number of men less than 24 years of age enrolled into the programme.
Conversely, studies from developed countries show differing results, finding no gender differences in clinical, virological and immunological outcomes among HIV infected men and women initiating ART [22,23]. A multi-site cohort study found higher rates of virological rebound in women [7]. Studies conducted elsewhere in SSA also demonstrate poor outcomes among men on ART with respect to higher rates of mortality and loss-to-follow-up [24,25]. Published literature from South Africa support our findings and demonstrates that while life expectancy was not significantly different pre-ART between genders; in 2011 women were 27% less likely to die from HIV than men [26].
Risk factors for poor clinical outcomes
Poor prognostic markers of ART response were apparent in men at baseline, who were older, had lower CD4+ cell counts and higher viral loads compared to women. This finding concurs with published South African literature demonstrating that compared to men, women were more likely to access HIV testing, and among those testing HIV positive, women were younger with higher CD4+ cell counts than their male counterparts [27].
This study also showed that the late presentation of men was associated with poor immunological response to ART with higher risk of morbidity and mortality [27]. Our findings of significant correlation between baseline characteristics, virological suppression rates, immunologic recovery and mortality concur with previously published studies conducted in South Africa [28,29]. There were no significant differences in virologic suppression in follow-up between men and women. Notwithstanding the greater than 90% virologic suppression rate on ART, men remained at higher risk of dying, emphasising the impact of advanced HIV disease at baseline on subsequent antiretroviral treatment response. We found significantly different CD4+ cell count recovery between men and women with men consistently demonstrated significantly smaller CD4+ cell count increases compared to women during longitudinal follow-up. Given that viral load suppression was similar between sexes, women however had higher CD4+ cell counts indicating that there may be underlying genetic differences in ART response.
We show that during follow up overall mortality rate halved among men and women with baseline CD4+ cell counts of 50–200 cells/mm3 in comparison with those patients that presented with baseline CD4+ cell counts of less than 50 cells/mm3. Furthermore, we found that the mortality risk among patients initiating ART was attributable to both low baseline CD4+ cell count and male gender, corroborating published literature showing that baseline CD4+ cell count is an important factor impacting CD4+ cell count increase and recovery on treatment [30]. In settings that are still guided by CD4+ cell count thresholds for ART initiation consideration should be given for men to access ART at higher CD4+ cell counts.
Our findings demonstrate that the absence of a diagnosis of prevalent TB increased risk of mortality. Published literature has cited that undiagnosed TB is higher among patients accessing ART than in the general population; with the majority of incident TB diagnosed in the early weeks of ART initiation being TB prevalent but missed at baseline screening [19,31,32]. It is possible that undiagnosed TB at ART initiation also contributed to higher risk of mortality in our study. The magnitude of the TB epidemic, burden of disease and varied presentation of TB in HIV infected individuals may have contributed to missed TB diagnosis.
Programmatic outcomes
Men had higher lost to follow-up rates in our cohort in keeping with reports from published literature [33,34]. Adverse clinical outcomes notably death can be masked by LTFU resulting in the underestimation of actual mortality rates within ART programmes [35]. Previous South African studies have documented gender differences in service uptake within the HIV care cascade; women have higher rates of HIV testing [27] and linkage to care than men [13]. Once initiated on ART, men have poorer adherence [36] and lower retention in care [28,35]. Govindasamy et al in a systematic review of 42 publications from 12 countries showed high attrition of men from pre-ART and ART programmes [37]. These studies did not however describe mortality differentials between the genders. Overall, the design and results of this article are similar to those found by Cornell et al [28].
Limitations
As this was a secondary analysis of routinely collected programme data, missing data limited our ability to comment on various correlations e.g. the impact of socio-economic and demographic variables on clinical outcomes. Treatment adherence was not measured and its impact on outcomes could be underestimated. We have not investigated other biological and behavioural factors that may have contributed to poor ART outcomes in men. While the vast majority of patients enrolled into this cohort were women, we had very few pregnancies and are therefore unable to comment on the impact of pregnancy in women on mortality in this cohort. Additional studies that explore potential barriers that South African men experience when seeking HIV care, and optimal strategies to link and engage men in ART services, is warranted.
Conclusion
While heterosexual transmission is the primary mode of HIV transmission in sub Saharan Africa, the impact of free ART provision on access and clinical outcomes in men and women is unequal. Men presented late for ART initiation with more advanced disease and a higher mortality rate compared to women in this six-year ART program. The increased feminization of the HIV epidemic in sub Saharan Africa has rightfully demanded that greater focus be given to women with respect to HIV prevention, transmission, treatment and care. However, in a predominantly heterosexual epidemic, greater programmatic effort should be directed toward encouraging men to test early and frequently. As we prepare to scale-up “Test and Treat” in all HIV infected patients, innovative strategies are needed to ensure that men and women that test HIV positive have access to effective linkage to care for early ART initiation to achieve the goal of reducing mortality and increasing AIDS free survival.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
This work was supported by the US President’s Emergency Plan for AIDS Relief (PEPFAR), the Global Fund to fight AIDS, Tuberculosis and Malaria and the National Institutes of Health Comprehensive International Program of Research on AIDS (UM1AI069469). K.N. and N.P. were supported by the Columbia University-South Africa Fogarty AIDS International Training and Research Program (AITRP, Grant D43 TW000231).
KN is currently an Executive Director, Board of Control, Centre for the AIDS Program of Research in South Africa (CAPRISA). NP is currently Deputy Director, Centre for the AIDS Program of Research in South Africa (CAPRISA) and QAK and SAK are directors for Centre for the AIDS Program of Research in South Africa (CAPRISA). SAK is a Director at the DST-NRF Centre of Excellence in HIV Prevention.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the US President’s Emergency Plan for AIDS Relief (PEPFAR), the Global Fund to fight AIDS, Tuberculosis and Malaria and the National Institutes of Health Comprehensive International Program of Research on AIDS (UM1AI069469). K.N. and N.P. were supported by the Columbia University-South Africa Fogarty AIDS International Training and Research Program (AITRP, Grant D43 TW000231). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.STATISTICS SOUTH AFRICA, ‘Midyear population estimates 2015’; online: https://www.statssa.gov.za/publications/P0302/P03022015.pdf acessed: 17 May 2016.
- 2.UNAIDS report 2015 accessed online: http://www.unaids.org/sites/default/files/media_asset/JC2702_GARPR2015guidelines_en.pdf accessed 01 April 2016
- 3.Norheim OF, Jha P, Admasu K, Godal T, Hum RJ, et al. (2015) Avoiding 40% of the premature deaths in each country, 2010–30: review of national mortality trends to help quantify the UN Sustainable Development Goal for health. The Lancet 385: 239–252. [DOI] [PubMed] [Google Scholar]
- 4.Takarinda KC, Harries AD, Shiraishi RW, Mutasa-Apollo T, Abdul-Quader A, et al. (2015) Gender-related differences in outcomes and attrition on antiretroviral treatment among an HIV-infected patient cohort in Zimbabwe: 2007–2010. International Journal of Infectious Diseases 30: 98–105. doi: 10.1016/j.ijid.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menzaghi B, Ricci E, Vichi F, De Sociod G, Carenzi L, et al. (2014) Gender differences in HIV infection: is there a problem? Analysis from the SCOLTA cohorts. Biomedicine & Pharmacotherapy 68: 385–390. [DOI] [PubMed] [Google Scholar]
- 6.Rosin C, Elzi L, Thurnheer C, Fehr J, Cavassini M, et al. (2015) Gender inequalities in the response to combination antiretroviral therapy over time: the Swiss HIV Cohort Study. HIV Med 16: 319–325. doi: 10.1111/hiv.12203 [DOI] [PubMed] [Google Scholar]
- 7.Cescon A, Patterson S, Chan K, Palmer AK, Margolese S, et al. (2013) Gender differences in clinical outcomes among HIV-positive individuals on antiretroviral therapy in Canada: a multisite cohort study. PLoS One 8: e83649 doi: 10.1371/journal.pone.0083649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saunders P, Goodman A, Smith C, Marshall N, O'Connor J, et al. (2016) Does gender or mode of HIV acquisition affect virological response to modern antiretroviral therapy (ART)? HIV medicine 17: 18–27. doi: 10.1111/hiv.12272 [DOI] [PubMed] [Google Scholar]
- 9.Lee MP, Zhou J, Messerschmidt L, Honda M, Ditangco R, et al. (2015) Impact of Gender on Long-Term Treatment Outcomes of Highly Active Antiretroviral Therapy (HAART) in the TREAT Asia HIV Observational Database. AIDS patient care and STDs 29: 229–231. doi: 10.1089/apc.2014.0232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karim QA, Sibeko S, Baxter C (2010) Preventing HIV Infection in Women: A Global Health Imperative. Clinical Infectious Diseases 50: S122–S129. doi: 10.1086/651483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muula AS, Ngulube TJ, Siziya S, Makupe CM, Umar E, et al. (2007) Gender distribution of adult patients on highly active antiretroviral therapy (HAART) in Southern Africa: a systematic review. BMC public health 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornell M, McIntyre J, Myer L (2011) Men and antiretroviral therapy in Africa: our blind spot. Trop Med Int Health 16: 828–829. doi: 10.1111/j.1365-3156.2011.02767.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassett IV, Regan S, Chetty S, Giddy J, Uhler LM, et al. (2010) Who starts antiretroviral therapy in Durban, South Africa?… not everyone who should. AIDS (London, England) 24: S37–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornell M (2013) Gender inequality: Bad for men's health. South Afr J HIV Med 14: 12–14. doi: 10.7196/SAJHIVMED.894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naranbhai V, Karim QA, Naidoo K, Yende-Zuma N, Karim SSA (2012) Sustainability of task-shifting for antiretroviral treatment. The Lancet 380: 1907–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, et al. (2010) Timing of initiation of antiretroviral drugs during tuberculosis therapy. New England Journal of Medicine 362: 697–706. doi: 10.1056/NEJMoa0905848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(2004) South African Anti-Retroviral Guidelines 2004 http://www0.sun.ac.za/ruralhealth/ukwandahome/rudasaresources2009/DOH/1.%20Antiretroviral.pdf accessed: 20 April 2016.
- 18.Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, et al. (2006) Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. The Lancet 367: 1335–1342. [DOI] [PubMed] [Google Scholar]
- 19.Etard J-F, Ndiaye I, Thierry-Mieg M, Gueye NFN, Gueye PM, et al. (2006) Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. Aids 20: 1181–1189. doi: 10.1097/01.aids.0000226959.87471.01 [DOI] [PubMed] [Google Scholar]
- 20.Harling G, Newell M-L, Tanser F, Kawachi I, Subramanian S, et al. (2014) Do age-disparate relationships drive HIV incidence in young women? Evidence from a population cohort in rural KwaZulu-Natal, South Africa. Journal of acquired immune deficiency syndromes (1999) 66: 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaidi J, Grapsa E, Tanser F, Newell M-L, Bärnighausen T (2013) Dramatic increases in HIV prevalence after scale-up of antiretroviral treatment: a longitudinal population-based HIV surveillance study in rural kwazulu-natal. AIDS (London, England) 27: 2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorsteinsson K, Ladelund S, Jensen-Fangel S, Larsen MV, Johansen IS, et al. (2012) Impact of gender on the risk of AIDS-defining illnesses and mortality in Danish HIV-1-infected patients: a nationwide cohort study. Scand J Infect Dis 44: 766–775. doi: 10.3109/00365548.2012.684220 [DOI] [PubMed] [Google Scholar]
- 23.Giles ML, Zapata MC, Wright ST, Petoumenos K, Grotowski M, et al. (2016) How do outcomes compare between women and men living with HIV in Australia? An observational study. Sexual health 13: 155–161. doi: 10.1071/SH15124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor-Smith K, Tweya H, Harries A, Schoutene E, Jahn A (2010) Gender differences in retention and survival on antiretroviral therapy of HIV-1 infected adults in Malawi. Malawi Med J 22: 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikuabe PO, Ebuenyi ID, Harry TC (2015) Limited elevations in antituberculosis drug-induced serum alanine aminotransferase (ALT) levels in a cohort of Nigerians on treatment for pulmonary tuberculosis and HIV infection in Yenagoa. Niger J Med 24: 103–107. [PubMed] [Google Scholar]
- 26.Bor J, Rosen S, Chimbindi N, Haber N, Herbst K, et al. (2015) Mass HIV Treatment and Sex Disparities in Life Expectancy: Demographic Surveillance in Rural South Africa. PLoS Med 12: e1001905; discussion e1001905. doi: 10.1371/journal.pmed.1001905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramkissoon AMP, Beksinska MMP, Searle CMA, Govender TD, Smit JBMP, et al. (2011) HIV Counseling and Testing in an Urban Reproductive Primary Health Clinic in South Africa: Gender Differences Among Clients. JAIDS Journal of Acquired Immune Deficiency Syndromes 58: e138–e140. doi: 10.1097/QAI.0b013e3182381171 [DOI] [PubMed] [Google Scholar]
- 28.Cornell M, Schomaker M, Garone DB, Giddy J, Hoffmann CJ, et al. (2012) Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: a multicentre cohort study. PLoS Med 9: e1001304 doi: 10.1371/journal.pmed.1001304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maskew M, Brennan AT, Westreich D, McNamara L, MacPhail AP, et al. (2013) Gender differences in mortality and CD4 count response among virally suppressed HIV-positive patients. J Womens Health (Larchmt) 22: 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nash D, Katyal M, Brinkhof MW, Keiser O, May M, et al. (2008) Long-term immunologic response to antiretroviral therapy in low-income countries: Collaborative analysis of prospective studies: The Antiretroviral Therapy in Lower Income Countries (ART-LINC) Collaboration of the International epidemiological Databases to Evaluate AIDS. AIDS (London, England) 22: 2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawn SD, Gupta A, Wood R (2012) Assessing the impact of prevalent tuberculosis (TB) on mortality among ART initiators: accurate TB diagnosis is essential. AIDS (London, England) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naidoo K, Karim QA, Bhushan A, Naidoo K, Yende-Zuma N, et al. (2014) High rates of tuberculosis in patients accessing HAART in rural South Africa. Journal of acquired immune deficiency syndromes (1999) 65: 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brinkhof MW, Pujades-Rodriguez M, Egger M (2009) Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PloS one 4: e5790 doi: 10.1371/journal.pone.0005790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fox MP, Rosen S (2010) Patient retention in antiretroviral therapy programs up to three years on treatment in sub‐Saharan Africa, 2007–2009: systematic review. Tropical medicine & international health 15: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yende-Zuma N, Naidoo K (2016) The effect of timing of initiation of ART on loss to follow up in HIV-TB co infected patients in South Africa: An open label randomized controlled trial. Journal of acquired immune deficiency syndromes (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boulle C, Kouanfack C, Laborde-Balen G, Boyer S, Aghokeng AF, et al. (2015) Gender Differences in Adherence and Response to Antiretroviral Treatment in the Stratall Trial in Rural District Hospitals in Cameroon. J Acquir Immune Defic Syndr 69: 355–364. doi: 10.1097/QAI.0000000000000604 [DOI] [PubMed] [Google Scholar]
- 37.Govindasamy D, Ford N, Kranzer K (2012) Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. Aids 26: 2059–2067. doi: 10.1097/QAD.0b013e3283578b9b [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper.