Summary
Background
Reoperation rates are high after surgery for hip fractures. We investigated the effect of a sliding hip screw versus cancellous screws on the risk of reoperation and other key outcomes.
Methods
For this international, multicentre, allocation concealed randomised controlled trial, we enrolled patients aged 50 years or older with a low-energy hip fracture requiring fracture fixation from 81 clinical centres in eight countries. Patients were assigned by minimisation with a centralised computer system to receive a single large-diameter screw with a side-plate (sliding hip screw) or the present standard of care, multiple small-diameter cancellous screws. Surgeons and patients were not blinded but the data analyst, while doing the analyses, remained blinded to treatment groups. The primary outcome was hip reoperation within 24 months after initial surgery to promote fracture healing, relieve pain, treat infection, or improve function. Analyses followed the intention-to-treat principle. This study was registered with ClinicalTrials.gov, number NCT00761813.
Findings
Between March 3, 2008, and March 31, 2014, we randomly assigned 1108 patients to receive a sliding hip screw (n=557) or cancellous screws (n=551). Reoperations within 24 months did not differ by type of surgical fixation in those included in the primary analysis: 107 (20%) of 542 patients in the sliding hip screw group versus 117 (22%) of 537 patients in the cancellous screws group (hazard ratio [HR] 0.83, 95% CI 0.63–1.09; p=0.18). Avascular necrosis was more common in the sliding hip screw group than in the cancellous screws group (50 patients [9%] vs 28 patients [5%]; HR 1.91, 1.06–3.44; p=0.0319). However, no significant difference was found between the number of medically related adverse events between groups (p=0.82; appendix); these events included pulmonary embolism (two patients [<1%] vs four [1%] patients; p=0.41) and sepsis (seven [1%] vs six [1%]; p=0.79).
Interpretation
In terms of reoperation rates the sliding hip screw shows no advantage, but some groups of patients (smokers and those with displaced or base of neck fractures) might do better with a sliding hip screw than with cancellous screws.
Funding
National Institutes of Health, Canadian Institutes of Health Research, Stichting NutsOhra, Netherlands Organisation for Health Research and Development, Physicians’ Services Incorporated.
Introduction
Worldwide, 4.5 million people per year become disabled after sustaining a hip fracture, with the number living with disability due to hip fracture expected to increase to 21 million in the next 40 years.1,2 Despite surgical intervention, the need for reoperation remains high (10.0–48.8%), has remained largely unchanged in the past 30 years,3,4 and is associated with substantial morbidity, mortality, and costs.5 The high proportion of reoperations has generated controversy about the optimum approach for fixing femoral neck fractures.6
Biomechanical and laboratory studies7 suggest that although a single large screw at a fixed angle with a side-plate (ie, a sliding hip screw) provides greater biomechanical stability, especially in displaced and unstable fracture types, multiple cancellous screws, which is the present standard of care, are less invasive and better preserve blood supply. Previous small trials6 did not find a difference in the effect of the two fixation approaches on outcomes important to patients, particularly reoperations, leaving uncertainty among surgeons about the optimum approach for fixing femoral neck fractures.
We did the Fixation using Alternative Implants for the Treatment of Hip fractures (FAITH) trial to examine the effect of a sliding hip screw versus cancellous screws on the risk of reoperation and other key outcomes during 24 months.
Methods
Study design and participants
FAITH was an international, multicentre, allocation concealed, randomised controlled trial assessing the effects of a sliding hip screw versus cancellous screws on reoperation rates over a 24 month follow-up in patients with a low-energy femoral neck fracture. A previous report8 details the trial objectives and methods. All participating centres obtained ethics approval.
We enrolled patients with a low-energy fracture of the hip requiring fracture fixation across 81 clinical centres in the USA, Canada, Australia, the Netherlands, Norway, Germany, the UK, and India. Eligible patients were those aged 50 years or older with a low-energy femoral neck fracture requiring operative fixation. Complete eligibility criteria are provided in the appendix. All patients provided written informed consent.
Randomisation and masking
Patients were randomly assigned by minimisation to receive a sliding hip screw or cancellous screws, with a centralised computer system to ensure allocation concealment and balanced prognosis between intervention groups for fracture displacement, age, prefracture living status, prefracture function, American Society for Anesthesiologists class, and centre.9 The study computer programmer generated the minimisation algorithm. Local research personnel at each site performed randomisation by minimisation using the centralised computer system. Surgeons and patients were not masked but the data analyst, while doing the analyses, remained masked to treatment groups.
Procedures
Participants allocated to the sliding hip screw group received a single large-diameter (8.0 mm), partly threaded screw affixed to the proximal femur with a side plate (with a minimum of two holes and a maximum of four holes) and no supplemental fixation. Patients allocated to the cancellous screws group received multiple threaded screws, with a minimum of two screws and diameter of 6.5 mm. All participating surgeons had done at least 25 hip fracture fixation procedures during their career, and at least five fracture fixation procedures in the year before participation.
Surgeons chose the manufacturer, the reduction technique, whether to do a capsulotomy or aspiration of intracapsular haematoma, and the final screw position; injectable bone substitutes were not permitted. The protocol specified perioperative antibiotics, thrombo-prophylaxis, and weight-bearing regimens, but left to the surgeons’ discretion patient positioning, fracture reduction, and surgical exposure in the operating room. We provided surgeons with specific criteria for acceptability of postfixation radiographic fracture alignment.
Participants returned for follow-up at 1 and 10 weeks, and at 6, 9, 12, 18, and 24 months after surgery. Additional details of the trial intervention and standardisation of perioperative care, surgeon expertise, and the follow-up processes are provided in the appendix.
Outcomes
The primary endpoint was reoperation, defined as surgery that occurred subsequent to the initial procedure and within 24 months to promote fracture healing, relieve pain, treat infection, or improve function. An independent Central Adjudication Committee, adjudicated all primary and key secondary outcomes (mortality, fracture healing, and fracture complications, including avascular necrosis, non-union, implant failure, and infections). Health-related quality of life was measured by the Short Form-12, the EuroQol-5 Dimensions, and the Western Ontario and McMaster Universities Arthritis Index. Details of the primary outcomes, secondary outcomes, and adjudication processes are provided in the appendix.
All adverse events were reported to local ethics boards as per local requirements. An independent Data and Safety Monitoring Board monitored the safety of the trial and reviewed all serious adverse events.
Statistical analysis
The sample size calculation was based on the primary outcome of hip reoperation and used the Cox proportional hazards model as described by Collett.10 Originally we determined that enrolment of 1500 patients would give the trial 81.5% power to detect a hazard ratio (HR) of 0.75 in the sliding hip screw group, at a two-sided alpha level of 0.05, on the assumption that the percentage of the primary outcome in the cancellous screws group would be 25% (appendix).8
The Data and Safety Monitoring Board met in January, 2014, after 589 patients completed follow-up. They provided the Steering Committee with overall event rates to inform a revised power analysis that balanced feasibility of completing recruitment within an acceptable timeframe and supporting plausible hypotheses of treatment effect and baseline event rates. On the basis of the number of events in the first 589 patients, we estimated a 24 month primary event rate of 27.2%; a 24 month mortality rate of 18.2%; a 24 month 5.9% incidence of loss to follow-up; and a combined 6.8% crossover rate. With these estimates, a sample size of 500 patients per group would provide 95.7% power to detect a relative risk reduction of 35%. On the basis of these data, we targeted a sample size of 1100 patients. A methods paper8 published previously presents details of the sample size calculations and rationale.
Analyses followed the intention-to-treat principle and included all patients in the groups to which they were randomly assigned. Patients who did not complete the 24 month follow-up were censored at their last documented follow-up. After assessment of its appropriateness, the primary analysis used a Cox proportional hazards model stratified by clinical site. We report the treatment effects as HRs and 95% CIs. An analysis adjusting for death as a competing risk provided a sensitivity analysis. The analyses of treatment effect on fracture-related adverse events and mortality also relied on a stratified Cox proportional hazards regression.
We analysed the health-related quality-of-life outcomes at 24 months using a multiple linear regression model with treatment and pre-injury quality of life (obtained at 1 week) included as independent variables. Results are reported as mean differences with corresponding 95% CIs and p values.
At the trial onset we specified a single a-priori subgroup analysis investigating fracture displacement as a possible effect modifier, anticipating that sliding hip screw relative to multiple cancellous screws would do better in displaced versus non-displaced fractures.10 At the completion of the trial, but before unblinding and as described in our statistical analysis plan, we prespecified an additional five subgroup analyses that investigated a possible effect modification by location of fracture line, body-mass index, verticality of the fracture line, smoking status, and quality of fracture reduction. We did an additional post-hoc subgroup analysis assessing the possible effect modification of patient age. We did tests of interaction for individual subgroups and, when three provide significant results, an analysis that simultaneously considered all their possible interactions. We used multiple criteria to consider the credibility of any possible subgroup effects.11,12 Details for postulated subgroup effects are provided in the appendix. All analyses were done with SAS version 9.4 (Cary, NC, USA). This study was registered with ClinicalTrials.gov, number NCT00761813.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
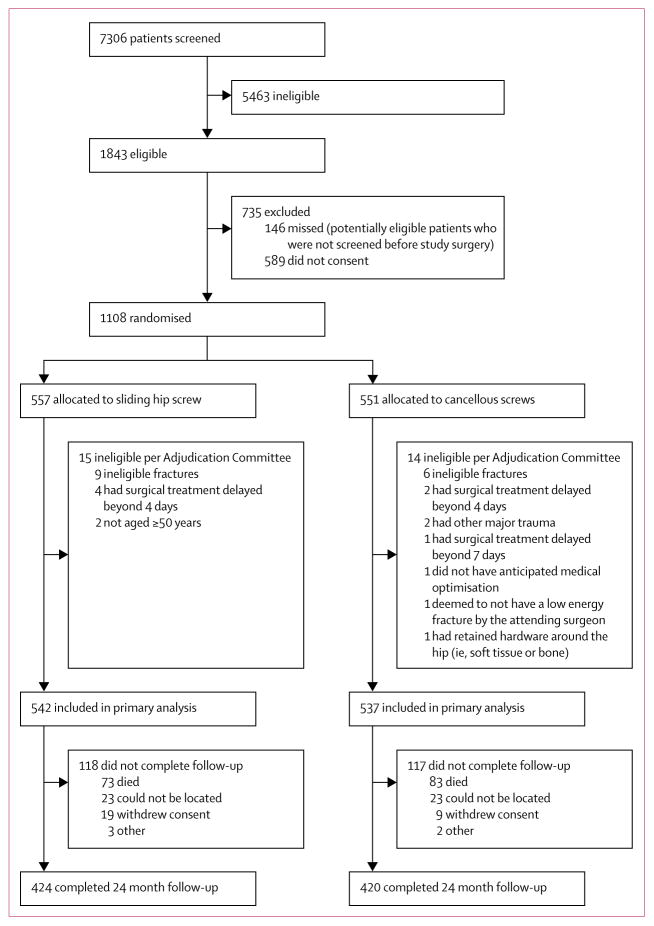
Between March 3, 2008, and March 31, 2014, we randomly assigned 1108 patients to receive a sliding hip screw (n=557) or cancellous screws (n=551; figure 1). The Adjudication Committee determined that 29 patients were ineligible, most as a result of an ineligible fracture type, or surgical treatment delayed beyond 4 days in patients with displaced fractures, leaving 1079 patients in the final analyses. The rationale for post-randomisation exclusions is in the appendix. Of the 923 patients alive at 24 months, we achieved 24 month follow-up for 844 (91%; figure 1, appendix). The mean length of follow-up was 633 days (SD 208).
Figure 1.
Trial profile
Typical patients were women aged 70–80 years who had fallen and sustained an isolated, non-displaced fracture of the femoral neck; group characteristics were similar (tables 1, 2). Acceptable reduction (open and closed) was achieved in nearly all patients (99%; table 2). In 549 patients with displaced fractures, acceptable reduction was achieved in 545 (99%). Those who underwent cancellous screw fixation typically received three parallel screws in a triangular configuration, whereas those in the sliding hip screw group received a single large compression screw in the centre–centre head position with a two-hole side plate; perioperative management was similar across groups (tables 1, 2; appendix).
Table 1.
Baseline characteristics
| Sliding hip screw (n=542) | Cancellous screws (n=537) | Total (n=1079) | |
|---|---|---|---|
| Age (years) | 72.2 (12.0) | 72.0 (12.3) | 72.1 (12.2) |
|
| |||
| Sex | |||
| Male | 212/535 (40%) | 210/535 (39%) | 422/1070 (39%) |
| Female | 323/535 (60%) | 325/535 (61%) | 648/1070 (61%) |
|
| |||
| Ethnic origin | |||
| Native | 1/533 (<1%) | 3/535 (1%) | 4/1068 (<1%) |
| South Asian | 65/533 (12%) | 65/535 (12%) | 130/1068 (12%) |
| East Asian | 6/533 (1%) | 4/535 (1%) | 10/1068 (1%) |
| Black | 22/533 (4%) | 18/535 (3%) | 40/1068 (4%) |
| Hispanic | 3/533 (1%) | 1/535 (<1%) | 4/1068 (<1%) |
| White | 436/533 (82%) | 444/535 (83%) | 880/1068 (82%) |
|
| |||
| Smoking history | |||
| Never smoked | 268/533 (50%) | 276/532 (52%) | 544/1065 (51%) |
| Current smoker | 101/533 (19%) | 100/532 (19%) | 201/1065 (19%) |
| Former smoker | 164/533 (31%) | 156/532 (29%) | 320/1065 (30%) |
|
| |||
| Current drugs | |||
| None | 170/535 (32%) | 179/534 (34%) | 349/1069 (33%) |
| NSAIDS | 86/535 (16%) | 64/534 (12%) | 150/1069 (14%) |
| General cardiac | 167/535 (31%) | 167/534 (31%) | 334/1069 (31%) |
| Opioid analgesics | 43/535 (8%) | 56/534 (10%) | 99/1069 (9%) |
| Pulmonary drugs | 58/535 (11%) | 69/534 (13%) | 127/1069 (12%) |
| Anti-hypertension drugs | 244/535 (46%) | 252/534 (47%) | 496/1069 (46%) |
| Osteoporosis drugs | 67/535 (13%) | 73/534 (14%) | 140/1069 (13%) |
|
| |||
| BMI | |||
| Underweight (BMI <18.5) | 37/530 (7%) | 33/528 (6%) | 70/1058 (7%) |
| Normal weight (18.5–24.9) | 276/530 (52%) | 300/528 (57%) | 576/1058 (54%) |
| Overweight (25–29.9) | 159/530 (30%) | 148/528 (28%) | 307/1058 (29%) |
| Obese (30–39.9) | 58/530 (11%) | 47/528 (9%) | 105/1058 (10%) |
|
| |||
| Fractured hip | N=535 | N=535 | N=1070 |
| Left | 280/535 (52%) | 281/535 (53%) | 561/1070 (52%) |
| Right | 255/535 (48%) | 254/535 (47%) | 509/1070 (48%) |
|
| |||
| Mechanism of injury | |||
| Fall | 515/533 (97%) | 521/534 (98%) | 1036/1067 (97%) |
| Spontaneous | 13/533 (2%) | 6/534 (1%) | 19/1067 (2%) |
| Other low energy trauma | 5/533 (1%) | 7/534 (1%) | 12/1067 (1%) |
|
| |||
| History of surgery to affected hip | |||
| Yes | 3/535 (1%) | 0/535 (0%) | 3/1070 (<1%) |
| No | 532/535 (99%) | 535/535 (100%) | 1067/1070 (100%) |
|
| |||
| Additional injuries | |||
| Yes | 67/535 (13%) | 72/535 (13%) | 139/1070 (13%) |
| No | 468/535 (87%) | 463/535 (87%) | 931/1070 (87%) |
Data are mean (SD) or n/N (%). For some subgroups, the numbers of patients analysed are smaller than that of the overall group number because data are missing for some variables. NSAIDS=non-steroidal anti-inflammatory drugs. BMI=body-mass index.
Table 2.
Fracture characteristics
| Sliding hip screw (n=542) | Cancellous screws (n=537) | Total (n=1079) | |
|---|---|---|---|
| Level of the fracture line | |||
|
| |||
| Subcapital | 331/535 (62%) | 351/536 (65%) | 682/1071 (64%) |
| Midcervical | 159/535 (30%) | 154/536 (29%) | 313/1071 (29%) |
| Basal | 45/535 (8%) | 31/536 (6%) | 76/1071 (7%) |
|
| |||
| Garden classification13 | |||
|
| |||
| Undisplaced | 360/542 (66%) | 369/537 (69%) | 729/1079 (68%) |
| Garden I | 257/542 (47%) | 277/537 (52%) | 534/1079 (49%) |
| Garden II | 99/542 (18%) | 92/537 (17%) | 191/1079 (18%) |
| Displaced | 182/542 (34%) | 168/537 (31%) | 350/1079 (32%) |
| Garden III | 121/542 (22%) | 128/537 (24%) | 249/1079 (23%) |
| Garden IV | 58/542 (11%) | 39/537 (7%) | 97/1079 (9%) |
|
| |||
| Pauwel’s classification14,15 | |||
|
| |||
| Type I | 59/535 (11%) | 59/536 (11%) | 118/1071 (11%) |
| Type II | 398/535 (74%) | 394/536 (74%) | 792/1071 (74%) |
| Type III | 78/535 (15%) | 83/536 (15%) | 161/1071 (15%) |
|
| |||
| Preoperative traction | |||
|
| |||
| Skin traction | 75/535 (14%) | 76/535 (14%) | 151/1070 (14%) |
| Skeletal traction | 7/535 (1%) | 3/535 (1%) | 10/1070 (1%) |
| None | 453/535 (85%) | 456/535 (85%) | 909/1070 (85%) |
|
| |||
| Type of reduction | |||
|
| |||
| None | 210/531 (40%) | 237/528 (45%) | 447/1059 (42%) |
| Closed | 287/531 (54%) | 277/528 (52%) | 564/1059 (53%) |
| Acceptable | 286/287 (100%) | 275/277 (99%) | 561/564 (99%) |
| Unacceptable | 1/287 (<1%) | 2/277 (1%) | 3/564 (1%) |
| Open | 34/531 (6%) | 14/528 (3%) | 48/1059 (5%) |
| Acceptable | 32/34 (94%) | 14/14 (100%) | 46/48 (96%) |
| Unacceptable | 2/34 (6%) | 0/14 (0%) | 2/48 (4%) |
Data are n/N (%).
The overall crossover was 2.0%, with significant differential crossovers between treatment groups (p=0.03). Surgeon compliance for the initial allocated surgical fixation approach was 97% (n=16) for sliding hip screw and 99% (n=6) for cancellous screws (appendix).
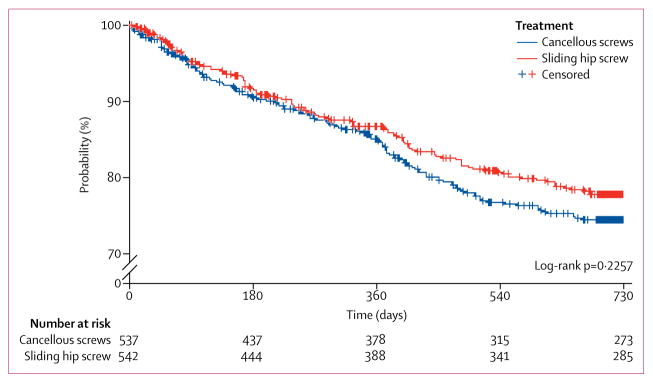
The primary study endpoint, hip reoperation within 24 months, did not differ by type of surgical fixation (table 3, figure 2). A competing risk sensitivity analysis adjusting for death yielded similar results for the primary endpoint (0.89, 0.69–1.16).
Table 3.
Study outcomes by treatment group
| Overall (n=1079) | Sliding hip screw (n=542) | Cancellous screws (n=537) | Hazard ratio (95% CI) | p value | |
|---|---|---|---|---|---|
| Reoperation | |||||
| Any reoperation | 224 (21%) | 107 (20%) | 117 (22%) | 0.83 (0.63–1.09) | 0.18 |
| Implant removal | 74 (7%) | 25 (5%) | 49 (9%) | 0.42 (0.25–0.70) | 0.0009 |
| Implant exchange: total hip arthroplasty | 104 (10%) | 64 (12%) | 40 (7%) | 1.51 (1.00–2.27) | 0.0494 |
| Implant exchange: hemiarthroplasty | 55 (5%) | 26 (5%) | 29 (5%) | 0.89 (0.52–1.51) | 0.66 |
| Implant exchange: internal | 16 (1%) | 2 (<1%) | 14 (3%) | 0.14 (0.03–0.62) | 0.0024 |
|
| |||||
| Fixation | |||||
| Implant exchange: spacer | 3 (<1%) | 1 (<1%) | 2 (<1%) | 0.50 (0.05–5.45) | 0.56 |
| Soft tissue procedure | 6 (1%) | 4 (1%) | 2 (<1%) | 1.98 (0.36–10.77) | 0.42 |
| Proximal femoral osteotomy | 1 (<1%) | 1 (<1%) | 1 (<1%) | 0.99 (0.06–15.80) | 0.99 |
|
| |||||
| Avascular necrosis | 78 (7%) | 50 (9%) | 28 (5%) | 1.91 (1.06–3.44) | 0.0319 |
|
| |||||
| Non-union | 66 (6%) | 33 (6%) | 33 (6%) | 0.92 (0.48–1.75) | 0.80 |
|
| |||||
| Implant failure | 87 (8%) | 42 (8%) | 45 (8%) | 0.95 (0.61–1.48) | 0.81 |
|
| |||||
| Infection | |||||
| Any infection | 19 (2%) | 10 (2%) | 9 (2%) | 1.10 (0.45–2.69) | 0.83 |
| Superficial infection | 8 (1%) | 4 (1%) | 4 (1%) | 0.99 (0.25–3.94) | 0.99 |
| Deep infection | 11 (1%) | 6 (1%) | 5 (1%) | 1.19 (0.37–3.87) | 0.77 |
|
| |||||
| Fracture healing* | |||||
| Healed by month 24 | 532/795 (67%) | 262/398 (66%) | 270/397 (68%) | .. | 0.71 |
| Not healed by month 24 | 3/795 (<1%) | 2/398 (1%) | 1/397 (<1%) | .. | |
| Not healed at time of last visit | 260/795 (33%) | 134/398 (34%) | 126/397 (32%) | .. | |
|
| |||||
| Fracture shortening >5 mm (n=532)† | 146/532 (27%) | 69/262 (26%) | 77/270 (29%) | 0.92 (0.70–1.22) | 0.57 |
|
| |||||
| Mortality | 156 (14%) | 73 (13%) | 83 (15%) | 0.81 (0.58–1.12) | 0.20 |
Data are n (%). Relative risk was calculated where the total number of events is less than 50.
795 patients were included in the fracture healing analysis. 284 patients did not have radiograph available for fracture healing adjudication, and therefore were not included in the denominator.
532 patients were included in the shortening analysis on the basis of the number of healed fractures with shortening data.
Figure 2.
Kaplan-Meier curves for reoperation
Number of deaths did not differ between groups (table 3). Avascular necrosis occurred in about 7% of patients overall and differed by fixation group, with more occurring in the sliding hip screw group than in the cancellous screws group. Of these, 54 (69%) patients required an operation: 38 patients in the sliding hip screw group and 16 in the cancellous screws group (p=0.002). Implant removal took place significantly less frequently in the sliding hip screw group than in the cancellous screws group (table 3). Implant exchange to revise to another internal fixation also occurred less frequently with sliding hip screws than cancellous screws (table 3). Alternatively, implant exchange to a total hip replacement was more common in the sliding hip group.
Non-unions, implant failures, infections, fracture shortening, and fracture healing did not differ by surgical fixation approach (table 3). Health-related quality of life did not differ between patients assigned to sliding hip screws and multiple cancellous screws at 12 month and 24 month follow-up (table 4).
Table 4.
Health-related quality of life by treatment groups without interaction of displacement
| Sliding hip screw | Cancellous screws | n | Adjusted mean difference (95% CI) | p value for differences between groups | |||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| n | Mean (SD) | n | Mean (SD) | ||||
| 12 months | |||||||
|
| |||||||
| SF-12 PCS16 | 235 | 40.8 (11.1) | 224 | 41.9 (10.7) | 435 | −0.02 (−1.79 to 1.74) | 0.98 |
| WOMAC17 | 240 | 44.69 (19.08) | 226 | 41.32 (16.73) | 438 | 1.98 (−1.13 to 5.09) | 0.21 |
| EQ-5D Index18 | 249 | 0.77 (0.20) | 238 | 0.80 (0.17) | 460 | −0.02 (−0.05 to 0.02) | 0.33 |
|
| |||||||
| 24 months | |||||||
|
| |||||||
| SF-12 PCS16 | 207 | 41.6 (10.9) | 181 | 41.4 (11.8) | 358 | 0.50 (−1.61 to 2.61) | 0.64 |
| WOMAC17 | 205 | 40.97 (16.33) | 183 | 39.75 (17.09) | 355 | 0.35 (−3.03 to 3.74) | 0.84 |
| EQ-5D Index18 | 232 | 0.79 (0.19) | 207 | 0.80 (0.19) | 406 | −0.01 (−0.04 to 0.02) | 0.51 |
SF-12 PCS=Short Form-12. PCS=Physical component score. WOMAC=Western Ontario and McMaster Universities Arthritis Index. EQ-5D=EuroQol-5 Dimensions.
Medically related adverse events did not differ by treatment group (p=0.82; table 5).
Table 5.
Medically related adverse events
| Sliding hip screw (n=542) | Cancellous screws (n=537) | p value | |
|---|---|---|---|
| Pneumonia | 23 (4%) | 24 (4%) | 0.86 |
| Deep vein thrombosis | 6 (1%) | 8 (1%) | 0.58 |
| Pulmonary embolism | 2 (<1%) | 4 (1%) | 0.41 |
| Sepsis | 7 (1%) | 6 (1%) | 0.79 |
| Myocardial infarction | 9 (2%) | 7 (1%) | 0.63 |
| Congestive heart failure | 7 (1%) | 6 (1%) | 0.79 |
| Other cardiovascular adverse events | 43 (8%) | 43 (8%) | 0.96 |
| Pulmonary | 24 (4%) | 16 (3%) | 0.21 |
| Decreased cognitive ability | 14 (3%) | 23 (4%) | 0.12 |
| Neurological | 10 (2%) | 10 (2%) | 0.98 |
| Digestive | 17 (3%) | 18 (3%) | 0.84 |
| Blood | 17 (3%) | 12 (2%) | 0.36 |
| Renal | 8 (1%) | 10 (2%) | 0.62 |
| Urinary | 23 (4%) | 20 (4%) | 0.66 |
| Multiple organ complications | 3 (1%) | 0 | 0.08 |
| Total | 122 (23%) | 124 (23%) | 0.82 |
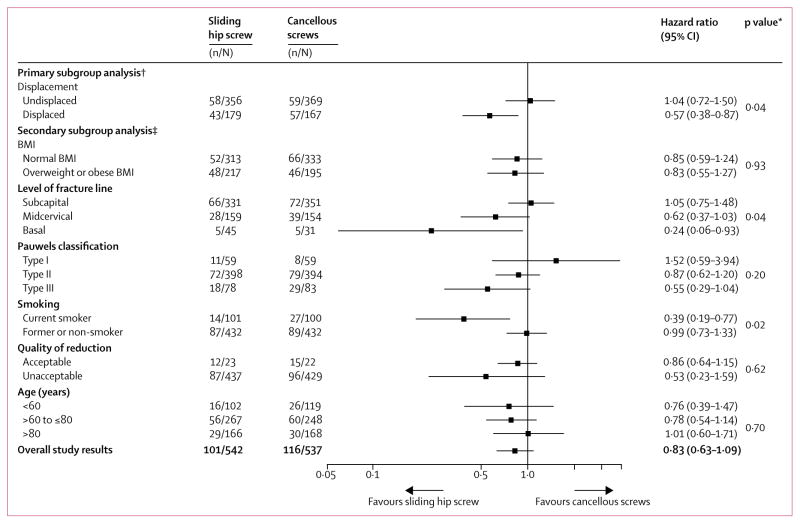
Subgroup analyses favoured sliding hip screw in patients with displaced fractures, fractures at the base of the femoral neck, and in those who were current smokers (appendix, figure 3). Only smoking status remained significant when these three subgroups were entered into a single analysis (figure 3). Sliding hip screw was superior in current smokers, but not in former or non-smokers (figure 3, appendix).
Figure 3. Subgroup analyses of surgical fixation primary endpoint (reoperation).
BMI=body-mass index.*Interaction p value for group comparison. †Defined at study start. ‡Defined at study close (before unblinding).
Discussion
We have shown a similar risk of hip reoperation in patients with low-energy femoral neck fractures randomly assigned to sliding hip screw as in those assigned to cancellous screws at 24 months; avascular necrosis occurred more frequently in patients allocated to sliding hip screw. Subgroup analyses of low-to-moderate credibility, suggested sliding hip screws reduced reoperations in patients with displaced fractures, fractures at the base of the femoral neck, and in current smokers.
Although the frequency of reoperations was similar between the two treatment groups, there were differences in the component outcomes of reoperations. Patients in the sliding hip screw group, compared with those in the cancellous screws group, had a lower frequency of a reoperation for an implant removal and an implant exchange based on an internal fixation approach, but had a higher frequency of reoperation for an implant exchange with a total hip arthroplasty approach. Patients undergoing total hip arthroplasty after failed internal fixation compared with those undergoing primary total hip arthroplasty could be at higher risk of complications.19 As such, the number of arthroplasties in the cancellous screws group suggests a potential advantage of the treatment over a sliding hip screw. Although the decision to choose one approach to implant exchange over another was left to the surgeon’s discretion, the choice might also indicate surgeon preference.
Looking separately at possible effect modifiers, displaced versus non-displaced fractures, fracture site, and smoking status, interaction p values all reached conventional statistical significance, all suggesting benefits of sliding hip screws over cancellous screws in a subpopulation of patients (figure 3). All were a-priori hypotheses with a biological rationale that led to a correct predicted direction of effect. In particular, the greater biomechanical stability of sliding hip screws might offer advantages in fracture with displacement and in smokers, who have greater risk of osteoporosis and diminished bone density than do non-smokers.7,20–23 Sliding hip screws remain the standard of care in patients with inter-trochanteric fractures, a region in close proximity to the base of the femoral neck,24 providing a biological rationale for the finding that sliding hip screws reduced reoperations in the subgroup of patients with base of femoral neck fractures. Furthermore, the displacement hypothesis was originally our sole hypothesis and a minimisation variable.11,12
However, when all three potential effect modifiers were considered together, only smoking retained a low, although not extremely low, p value (figure 3). Additionally, we tested multiple hypotheses, and did not find similar effects in health-related quality of life. As such, in view of all these issues, the apparent subgroup effects have only modest credibility.
Strengths of FAITH include safeguards against risk of bias (concealed randomisation, centralised and independent outcome adjudication, blinded analysis of data); high compliance with study procedures; broad inclusion criteria with a large number of centres in countries with diverse health-care systems; a focus on outcomes of importance to both patients and the health-care system (ie, reoperation, health-related quality of life); and rigorous investigation of subgroup effects, with due attention to their credibility.11,12
Our study has limitations. Surgeons and patients were not blinded. However, we did minimise the associated risk of bias with central and independent, although unblinded, radiographic adjudication of the primary endpoint. Furthermore, reoperation is an objective endpoint and a major procedure; surgeons will seldom decide to reoperate in the absence of a compelling indication. Follow-up at 24 months was less than complete (844 [91%] of 923 patients who were alive); our success in following up patients was consistent and in most cases better than in previous smaller trials.3 Unavoidable heterogeneity related to the variables that were not standardised also existed; these variables were patient positioning, fracture reduction, surgical exposure, use of operative traction, surgical delay, type of anaesthetic, physiotherapy, and rehabilitation programmes.
In relation to previous research, a meta-analysis of small trials suggested a non-significant difference in reoperations favouring sliding hip screw (relative risk 0.86, 95% CI 0.70–1.05; p=0.13).19 An updated pooled analysis of reoperation including small trials with our FAITH results (eight trials, 1913 patients) reported a 95% CI that was narrower than our study’s but was still consistent, with no difference between fixation methods (0.91, 0.76–1.08; p=0.27, I2=7%).25–31
With respect to avascular necrosis, our results differ importantly from a previous systematic review19 of small trials, which suggested that a sliding hip screw, in comparison with cancellous screws, might reduce the risk of avascular necrosis. The addition of another small trial to a previous meta-analysis results in a significant reduction in the risk of avascular necrosis with sliding hip screws (77 events; relative risk 0.64, 95% CI 0.43–0.97; p=0.04, I2=0%).19,25
Despite the previous results, increased risk of avascular necrosis from our results has a plausible biological rationale. Hip fractures can disrupt the retinacular vessels, which are crucial for the vascular supply of the femoral head.32 A randomised trial33 of 104 patients with femoral neck fractures using bone scintigraphy showed reduced vascularity in patients receiving a sliding hip screw compared with those receiving cancellous screws (35% vs 11%, p<0.01). Furthermore, suboptimum positioning of large implants, such as sliding hip screws, risks damage to the blood supply to the femoral head.34 In terms of the importance of avascular necrosis, observational studies have shown that many patients remain asymptomatic, with only one in five requiring further surgery.35,36
Our results, in the context of previous results, suggest that the choice of procedure for internal fixation is a matter of discretion. Findings favouring cancellous screws were the non-significant difference in the likelihood of reoperation between procedures, and the higher likelihood of avascular necrosis and subsequent total arthroplasties in patients receiving a sliding hip screw. However, our finding of increased avascular necrosis with a sliding hip screw, compared with cancellous screws, is inconsistent with other studies and did not result in more operations or poorer quality of life in the total population. Moreover, findings across all trials remain consistent, with overall decreased reoperations in patients receiving a sliding hip screw, and subgroup analyses of low credibility suggesting that patients with displaced fractures, smokers, and patients with base of neck fractures might do better with a sliding screw than with cancellous screws.
Supplementary Material
Research in context.
Evidence before this study
We searched the computerised databases Medline and PubMed for randomised clinical trials published in English between Jan 1, 1969, and June 1, 2002, using the search terms “femoral neck fracture” AND “arthroplasty”, as well as “femoral neck fracture” AND “internal fixation”. In addition, bibliographies were searched for relevant studies. We also identified additional studies through hand searches of major orthopaedic journals, bibliographies of major orthopaedic textbooks, and personal files. Of 140 citations initially identified, 14 met all eligibility criteria. Three investigators independently graded study quality and abstracted relevant data, including information on revision and mortality rates. An international survey of orthopaedic surgeons identified that most surgeons preferred to fix femoral neck hip fractures with multiple cancellous screws rather than a sliding hip screw. Despite the popularity of cancellous screws for hip fracture fixation, biological investigations have suggested that the sliding hip screw is a more biomechanically stable construct compared with cancellous screws. Moreover, a previous Cochrane meta-analysis of small trials reported a non-significant reduction in reoperations with a sliding hip screw compared with multiple cancellous screws (relative risk 0.86, 95% CI 0.70–1.05), and that the risk of avascular necrosis with a sliding hip screw was significantly reduced. The small sample sizes and resultant imprecise estimates, and methodological limitations, of previous work left the issue in doubt.
Added value of this study
Our trial enrolled more than 1000 patients across multiple countries, providing improved precision and generalisability to both high-income countries and low-to-middle-income countries. Our trial also addresses the dearth of health-related quality-of-life data for patients who had surgical fixation for femoral neck fractures.
Implications of all the available evidence
Although our findings, consistent with those of previous randomised controlled trials, did not show a difference in the likelihood of reoperation in patients randomly assigned to a sliding hip screw versus cancellous screws, health-related quality of life was similar across both interventions. However, we did show a significant increase in the likelihood of avascular necrosis with a sliding hip screw compared with cancellous screws. Nonetheless, this finding is not only inconsistent with previous results, but also did not result in an overall increase in reoperations or a decrement in health-related quality of life. Moreover, our results raise the possibility that a sliding hip screw, relative to cancellous screws, might reduce reoperations in patients with displaced fractures, in smokers, and in patients with base of neck fractures.
Acknowledgments
This study was funded by research grants from the Canadian Institutes of Health Research (MOP-106630 and MCT-87771), National Institutes of Health (1R01AR055267-01A1), Stichting NutsOhra (SNO-T-0602-43), The Netherlands Organisation for Health Research and Development (80-82310-97-11032), Physicians’ Services Incorporated, and Stryker GmBH. MB was also funded, in part, through the Early Research Award Program, which provided funding for the present study, and by a Canada research chair in musculoskeletal trauma, which is unrelated to the present study (McMaster University, Hamilton, ON, Canada). Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR055267-01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Research reported in this publication was also supported by The County Durham & Tees Valley Comprehensive Local Research Network, which operates as part of the National Institute for Health Research Comprehensive Clinical Research Network in England.
Footnotes
Contributors
Writing committee: Mohit Bhandari (Chair, McMaster University), P J Devereaux (McMaster University), Gordon Guyatt (McMaster University), Lehana Thabane (McMaster University), Stephen D Walter (McMaster University), Martin J Heetveld (Spaarne Gasthuis, Haarlem), Kyle J Jeray (Greenville Health System), Susan Liew (The Alfred), Emil H Schemitsch (University of Western Ontario), Paul Tornetta III (Boston University Medical Center), Gregory J Della Rocca (Duke University), Richard E Buckley (Foothills Medical Centre), Robert McCormack (Royal Columbian Hospital/Fraser Health Authority/University of British Columbia), Todd M Oliver (Boone Hospital Center—Columbia Orthopaedic Group), Michiel J M Segers (St Antonius Ziekenhuis), Amar Rangan (The James Cook University Hospital), Martin Richardson (University of Melbourne), Sheila Sprague (McMaster University), Gerard P Slobogean (University of Maryland, Baltimore), Taryn Scott (McMaster University), Julie Agel (University of Minnesota), Alisha Garibaldi (McMaster University), Qi Zhou (McMaster University), Diane Heels-Ansdell (McMaster University), Helena Viveiros (McMaster University), Stephanie M Zielinski (Erasmus MC, University Medical Center Rotterdam), Esther M M Van Lieshout (Erasmus MC, University Medical Center Rotterdam), Herman Johal (McMaster University), Birgit C Hanusch (The James Cook University Hospital), and Marc Swiontkowski (Co-Chair, University of Minnesota)
The Clinical Advances through Research and Information Translation (CLARITY) Research Group at McMaster University coordinated the trial. The CLARITY Research Group was responsible for the trial randomisation, maintenance of the database, data validation, data analyses, and study-centre coordination. The University of Minnesota (Minneapolis, MN, USA) assisted in coordination of sites in the USA; Erasmus MC, University Medical Centre (Rotterdam, Netherlands) assisted in coordination of sites in the Netherlands; and James Cook University Hospital (North Yorkshire, UK) assisted in coordination of sites in the UK. The Steering Committee designed the trial, prespecified the statistical analysis plan, and vouch for the completeness and accuracy of the data and analyses. MB, the first author and chair of the writing committee, wrote the first draft of the manuscript; the writing committee made revisions.
Declaration of interests
MB reports grants from Canadian Institutes of Health Research, the National Institutes of Health, Stichting NutsOhra, The Netherlands Organisation for Health Research and Development, Physicians’ Services Incorporated and Stryker GmBH, and the Early Research Award Program during the conduct of the study; and personal fees from Sanofi, Pendopharm, Ferring, DJO, and Stryker, and grants from Pluristem and Amgen, outside the submitted work. MJH reports grants from Stichting NutsOhra and The Netherlands Organization for Health Research and Development during the conduct of the study. PJD reports grants from Abbott Diagnostics, Boehringer Ingelheim, Covidien, Octopharma, Roche Diagnostics, and Stryker, outside the submitted work. KJJ reports grants from the National Institutes of Health and Canadian Institutes of Health Research during the conduct of the study; and personal fees from Zimmer, outside the submitted work. EHS reports personal fees from Stryker, Smith & Nephew, Zimmer, Acumed, Amgen, Sanofi, and Bioventus, and other from Elsevier, outside the submitted work. PT III reports personal fees from Lippicott Williams & Wilkins, outside the submitted work, and has a patent (US8888824 B2) with royalties paid by Smith & Nephew. GJDR reports grants from the University of Minnesota during the conduct of the study; and grants from DePuy-Synthes, personal fees from Bioventus and DePuy-Synthes, other from Amedica, The Orthopaedic Implant Company, LuminCare, Mergenet Medical, and Intellectual ventures, outside the submitted work. RM reports personal fees from Sanofi and Pendopharm, outside the submitted work. AR reports grants from the Canadian Institutes of Health Research, during the conduct of the study; and grants from the National Institute for Health Research (UK), Orthopaedic Research UK, and DePuy, outside the submitted work. GPS reports personal fees from Zimmer Biomet and grants from the Canadian Institutes of Health Research, outside the submitted work. SS reports other from McMaster University and Global Research Solutions, and personal fees from the University of Maryland, outside the submitted work. JA reports grants from the National Institutes of Health during the conduct of the study. SMZ reports grants from Stichting NutsOhra (SNO-T-0602-43) and The Netherlands Organisation for Health Research and Development (80-82310-97-11032), during the conduct of the study. EMMVL reports grants from Stichting NutsOhra and The Netherlands Organization for Health Research and Development during the conduct of the study. MS reports grants from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, during the conduct of the study; and other from The Journal of Bone & Joint Surgery Editor, outside the submitted work. MJMS reports personal fees from DePuy Synthes and AO Trauma Europe, outside the submitted work. GG, LT, SDW, SL, REB, TMO, MR, TS, AG, QZ, DH-A, HV, HJ, and BCH declare no competing interests.
References
- 1.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7:407–13. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- 2.Parker M, Johansen A. Hip fracture. BMJ. 2006;333:27–30. doi: 10.1136/bmj.333.7557.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhandari M, Devereaux PJ, Swiontkowski MF, et al. Internal fixation compared with arthroplasty for displaced fractures of the femoral neck. A meta-analysis. J Bone Joint Surg Am. 2003;85–A:1673–81. doi: 10.2106/00004623-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Mundi S, Pindiprolu B, Simunovic N, Bhandari M. Similar mortality rates in hip fracture patients over the past 31 years. Acta Orthop. 2014;85:54–59. doi: 10.3109/17453674.2013.878831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zielinski SM, Bouwmans CA, Heetveld MJ, et al. The societal costs of femoral neck fracture patients treated with internal fixation. Osteoporos Int. 2014;25:875–85. doi: 10.1007/s00198-013-2487-2. [DOI] [PubMed] [Google Scholar]
- 6.Bhandari M, Devereaux PJ, Tornetta P, 3rd, et al. Operative management of displaced femoral neck fractures in elderly patients. An international survey. J Bone Joint Surg Am. 2005;87:2122–30. doi: 10.2106/JBJS.E.00535. [DOI] [PubMed] [Google Scholar]
- 7.Hoshino CM, O’Toole RV. Fixed angle devices versus multiple cancellous screws: what does the evidence tell us? Injury. 2015;46:474–77. doi: 10.1016/j.injury.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 8.FAITH Investigators. Fixation using alternative implants for the treatment of hip fractures (FAITH): design and rationale for a multi-centre randomized trial comparing sliding hip screws and cancellous screws on revision surgery rates and quality of life in the treatment of femoral neck fractures. BMC Musculoskelet Disord. 2014;15:219. doi: 10.1186/1471-2474-15-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott NW, McPherson GC, Ramsay CR, Campbell MK. The method of minimization for allocation to clinical trials. A review. Control Clin Trials. 2002;23:662–74. doi: 10.1016/s0197-2456(02)00242-8. [DOI] [PubMed] [Google Scholar]
- 10.Collett D. Modelling survival data in medical research. 1. London, UK: Chapman and Hall; 1994. [Google Scholar]
- 11.Sun X, Briel M, Busse JW, et al. Credibility of claims of subgroup effects in randomised controlled trials: systematic review. BMJ. 2012;344:e1553. doi: 10.1136/bmj.e1553. [DOI] [PubMed] [Google Scholar]
- 12.Study to Prospectively Evaluate Reamed Intramedullary Nails in Tibial Fractures (SPRINT) Investigators. Sun X, Heels-Ansdell D, et al. Is a subgroup claim believable? A user’s guide to subgroup analyses in the surgical literature. J Bone Joint Surg Am. 2011;93:e8. doi: 10.2106/JBJS.I.01555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garden RS. Low-angle fixation in fractures of the femoral neck. J Bone Joint Surg. 1961;43B:647. [Google Scholar]
- 14.Pauwels F. Ein mechaizisches Problem. Stuttgart: F Enke; 1935. Der Schenkelhalsbruch. [Google Scholar]
- 15.Bartonícek J. Pauwels’ classification of femoral neck fractures: correct interpretation of the original. J Orthop Trauma. 2001;15:358–60. doi: 10.1097/00005131-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 16.McHorney CA, Ware JE, Jr, Rogers W, et al. The validity and relative precision of MOS short- and long-form health status scales and Dartmouth COOP charts. Results from the Medical Outcomes Study. Med Care. 1992;30:MS253–65. doi: 10.1097/00005650-199205001-00025. [DOI] [PubMed] [Google Scholar]
- 17.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 18.Brooks R, Rabin R, de Charro F. The measurement and valuation of health status using EQ-5D: a European perspective. Dordrecht, Netherlands: Kluwer Academic Publishers; 2003. [Google Scholar]
- 19.Parker MJ, Gurusamy KS. Internal fixation implants for intracapsular proximal femoral fractures in adults. Cochrane Database Syst Rev. 2001;4:CD001467. doi: 10.1002/14651858.CD001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deneka DA, Simonian PT, Stankewich CJ, Eckert D, Chapman JR, Tencer AF. Biomechanical comparison of internal fixation techniques for the treatment of unstable basicervical femoral neck fractures. J Orthop Trauma. 1997;11:337–43. doi: 10.1097/00005131-199707000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Blair B, Koval KJ, Kummer F, Zuckerman JD. Basicervical fractures of the proximal femur. A biomechanical study of 3 internal fixation techniques. Clin Orthop Relat Res. 1994;306:256–63. [PubMed] [Google Scholar]
- 22.Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ. 1997;315:841–46. doi: 10.1136/bmj.315.7112.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19:151–57. doi: 10.1097/00005131-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Parker MJ, Handoll HH. Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2010;9:CD000093. doi: 10.1002/14651858.CD000093.pub3. [DOI] [PubMed] [Google Scholar]
- 25.Watson A, Zhang Y, Beattie S, Page RS. Prospective randomized controlled trial comparing dynamic hip screw and screw fixation for undisplaced subcapital hip fractures. ANZ J Surg. 2013;83:679–83. doi: 10.1111/j.1445-2197.2012.06256.x. [DOI] [PubMed] [Google Scholar]
- 26.Benterud JG, Husby T, Nordsletten L, Alho A. Fixation of displaced femoral neck fractures with a sliding screw plate and a cancellous screw or two Olmed screws. A prospective, randomized study of 225 elderly patients with a 3-year follow-up. Ann Chir Gynaecol. 1997;86:338–42. [PubMed] [Google Scholar]
- 27.Kuokkanen H, Korkala O, Antti-Poika I, Tolonen J, Lehtimäki MY, Silvennoinen T. Three cancellous bone screws versus a screw-angle plate in the treatment of Garden I and II fractures of the femoral neck. Acta Orthop Belg. 1991;57:53–57. [PubMed] [Google Scholar]
- 28.Madsen F, Linde F, Andersen E, Birke H, Hvass I, Poulsen TD. Fixation of displaced femoral neck fractures. A comparison between sliding screw plate and four cancellous bone screws. Acta Orthop Scand. 1987;58:212–16. doi: 10.3109/17453678709146468. [DOI] [PubMed] [Google Scholar]
- 29.Harper WM. Treatment of intracapsular proximal femoral fractures. Doctoral thesis. University of Leicester; 1994. A prospective randomised trial comparing multiple parallel cannulated screws with a sliding hip screw and plate. [Google Scholar]
- 30.Sørensen JL, Varmarken JE, Bømler J. Internal fixation of femoral neck fractures. Dynamic hip and Gouffon screws compared in 73 patients. Acta Orthop Scand. 1992;63:288–92. doi: 10.3109/17453679209154784. [DOI] [PubMed] [Google Scholar]
- 31.Paus A, Gjengedal E, Hareide A, Jørgensen JJ. Dislocated fractures of the femoral neck treated with von Bahr screws or hip compression screw. Results of a prospective, randomized study. J Oslo City Hosp. 1986;36:55–61. [PubMed] [Google Scholar]
- 32.Swiontkowksi MF. Intracapsular fractures of the hip. J Bone Joint Surg. 1994;76A:129–38. doi: 10.2106/00004623-199401000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Linde F, Andersen E, Hvass I, Madsen F, Pallesen R. Avascular femoral head necrosis following fracture fixation. Injury. 1986;17:159–63. doi: 10.1016/0020-1383(86)90322-0. [DOI] [PubMed] [Google Scholar]
- 34.Brodetti A. The blood supply of the femoral neck and head in relation to the damaging effects of nails and screws. J Bone Joint Surg. 1960;42B:794–801. [Google Scholar]
- 35.Barnes R, Brown JT, Garden RS, Nicoll EA. Subcapital fractures of the femur. A prospective review. J Bone Joint Surg Br. 1976;58:2–24. doi: 10.1302/0301-620X.58B1.1270491. [DOI] [PubMed] [Google Scholar]
- 36.Nikolopoulos KE, Papadakis SA, Kateros KT, et al. Long-term outcome of patients with avascular necrosis, after internal fixation of femoral neck fractures. Injury. 2003;34:525–28. doi: 10.1016/s0020-1383(02)00367-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.