Abstract
Objective
To examine the impact of skilled nursing facility (SNF) use on hospitalizations among heart failure (HF) patients and to examine predictors of hospitalization among HF patients admitted to a SNF.
Patients and Methods
Olmsted County, MN residents with first-ever HF from 1/1/2000-12/31/2010 were identified and clinical data were linked to SNF utilization data from CMS. Andersen-Gill models were used to determine the association between SNF use and hospitalizations and to determine predictors of hospitalization.
Results
Among 1,498 incident HF patients (mean age 75±14, 45% male), 605 (40.4%) were admitted to a SNF after HF diagnosis (median (min, max) follow-up 3.6 (0-13.0) years). Among those with a SNF admission, 225 (37%) had 2 or more admissions. After adjustment for age, sex, ejection fraction and comorbidities, being in a SNF was associated with a 50% increased risk of hospitalization compared to not being in a SNF (adjusted HR, 95% CI: 1.52, 1.31-1.76). Among SNF users, arrhythmia, asthma, chronic kidney disease, and the number of activities of daily living (ADLs) requiring assistance were independently associated with an increased risk of hospitalization.
Conclusion
Approximately 40% of HF patients were admitted to a SNF at some point after diagnosis. Compared to patients not in a SNF, SNF users were more likely to be hospitalized. Characteristics associated with hospitalization among the SNF users were mostly non-cardiovascular, including reduced ability to perform ADLs. These findings underscore the impact of physical functioning on hospitalizations among HF patients in SNFs and the importance of strategies to improve physical functioning.
Introduction
Heart failure (HF) is a major clinical and public health problem as it affects more than 5 million individuals in the U.S., and more than 550,000 new cases of HF are diagnosed each year.1,2 The number of patients living with HF continues to rise, and it is estimated that more than 8 million Americans will have HF by the year 2030.3 HF disproportionately affects the elderly and is prevalent in an estimated 20%-37.4% of the 1.5 to 2 million persons living in a skilled nursing facility (SNF) in the United States.4
Although HF is common among persons in SNFs, data on the outcomes for HF patients in SNFs is sparse. Indeed, a recent scientific statement from the American Heart Association underscores that ‘further studies that provide longitudinal data regarding the range of patient experiences after hospital discharge to a SNF are needed’.4 Addressing this recognized information gap requires comprehensive knowledge of clinical data that can be linked to SNF data in order to obtain the requisite complete longitudinal information and reconstruct the patient experience. These steps are conceptually straightforward but operationally complex and hence seldom executed. Yet, generating such datasets is important to study hospitalizations, a key indicator of HF management. Indeed, while referrals to SNF could be envisioned as a way to reduce hospitalizations, a “revolving door” phenomenon after SNF admission has been hypothesized to drive hospital readmissions,5 and there is evidence that mortality and hospital readmissions are increased for hospitalized older adults with HF discharged to SNFs compared to those discharged to other sites.6 The urgent need to study the impact of SNF utilization on hospitalizations in HF was recently emphasized6,7 but has yet to be carefully examined.
To address these gaps in knowledge, we assembled a community cohort of patients with validated incident (first-ever) HF who were not in a SNF immediately prior to diagnosis. We then created a comprehensive linked dataset including clinical, medical-care use, and SNF information to examine SNF utilization after HF diagnosis, study the impact of SNF use on hospitalizations and examine the predictors of hospitalization among patients admitted to a SNF.
Methods
Study Setting
Our study was conducted among residents in Olmsted County, Minnesota from January 1, 2000 to December 31, 2010. Olmsted County (2010 population: 144,248) is representative of the state of Minnesota and the Upper Midwest region of the US8 with similar age, sex and ethnic characteristics. Additionally, age- and sex-specific mortality rates are similar for Olmsted County, the state of Minnesota and the entire United States.8
Longitudinal, population-based epidemiologic studies in Olmsted County are possible because only a few providers (Mayo Clinic, Olmsted Medical Center and a few private providers) deliver nearly all health care to the local residents.9,10 The provider-linked medical records from each institution are indexed through the Rochester Epidemiology Project (REP), resulting in the linkage of all detailed clinical (including medical-care use) and demographic information from nearly all sources of care.8 Thus, the REP constitutes a unique infrastructure for epidemiologic and outcomes studies because the population of Olmsted County is served by a unified medical system with comprehensive clinical records.9 The data architecture consists of diagnostic and procedure codes that enable case finding to direct medical record review and data collection. Thus, through the REP, HF cases were identified and validated, and clinical data (including hospitalizations) were abstracted from the complete medical record.
Identification of Heart Failure Patients
All Olmsted County in- and outpatients with a diagnosis assigned an International Classification of Diseases- 9th revision, Clinical Modification (ICD-9-CM) code 428 from January 1, 2000 to December 31, 2010 were identified.11 A random sample of 50% of the HF diagnoses between 2000 and 2006 and 100% of HF diagnoses from 2007 to 2010 were manually reviewed. HF diagnoses were validated by experienced nurse abstractors using the Framingham criteria,12 which is highly reliable.11 The event was classified as incident if no history of prior HF was found upon review of all sources of information.
Patient Characteristics
We selected the 20 conditions recently identified as a public health priority by the US Department of Health and Human Services13,14 to classify comorbidities.15,16 These conditions were ascertained electronically by retrieving ICD-9-CM codes from both inpatient and outpatient encounters at all providers indexed in the REP. As described previously,15 two occurrences of a code (either the same code or two different codes within the code set for a given disease) separated by more than 30 days and occurring within 5 years prior to the incident HF date were required for diagnosis. Since all patients had HF, this condition was not included. Conditions that were absent (autism, human immunodeficiency virus) or quite infrequent (hepatitis N=9) were also not included, leaving 16 chronic conditions: coronary artery disease (CAD), arrhythmia, stroke, hypertension, hyperlipidemia, diabetes, arthritis, osteoporosis, asthma, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), cancer, depression, dementia, schizophrenia and substance abuse.
Body mass index (BMI) (kg/m2) was calculated using height at the time of HF diagnosis and weight from the last outpatient visit prior to HF diagnosis. Marital status was ascertained from the medical record.
Echocardiographic examinations were performed within 90 days of HF diagnosis17 following the recommendations of the American Society of Echocardiography.18 Ejection fraction (EF) was used to categorize HF into preserved (≥50%) or reduced (<50%) systolic function.17,19
Skilled Nursing Facility Data
SNF utilization was obtained through 12/31/2012 from the Centers for Medicare and Medicaid Services (CMS) Long-Term Care Minimum Data Set (MDS) 2.0 and 3.0 assessments.20 This data set includes all nursing home use regardless of level of care or payer. Consistent with the terminology used in a recent AHA scientific statement, we used the term skilled nursing facility to include facilities traditionally called nursing home.4 Thus, we included both post-acute care and long-term use in our analysis of SNF utilization. If a patient was in a SNF and was hospitalized and returned immediately back to the same SNF after discharge, it was counted as one SNF stay, not two. Patients were excluded from the analysis if they were in a SNF within 30 days before their first HF date.
Ability to perform activities of daily living (ADLs) was ascertained from the MDS assessments. Patients are assessed at SNF admission, regularly during their stay, when they experience a change in status and at discharge. Patients are evaluated as to whether they have difficulty performing the following activities on their own: feeding themselves, dressing, using the toilet, housekeeping, bathing and locomotion on the unit (walking or self-sufficient wheeling). Each activity is rated on a scale from 1-5 (1=independent, 2=supervision, 3=limited assistance, 4=extensive assistance, 5=total dependence). We included all assessments on patients in our cohort; thus, we analyzed multiple assessments per person.
Hospitalization Ascertainment
Data regarding all hospitalizations through 12/31/2012 were retrieved from REP resources which, as described previously, include information on all hospital care delivered to Olmsted County residents within the county. In-hospital transfers or transfers between hospitals were counted as a single hospitalization.
Data Management and Statistical Analysis
A longitudinal dataset was generated by linking the comprehensive community medical records of the cohort to the SNF data (Figure 1). Doing so required the creation of timelines that enabled the construction of the chronological time course after diagnosis for each patient, including multiple outpatient clinical evaluations, hospitalizations, and SNF admissions. Patient characteristics, overall and among patients with a SNF admission after HF diagnosis, are presented as frequencies (percent), mean (SD) or median (25th, 75th percentile) as appropriate. Number of SNF admissions for reduced vs. preserved EF was compared with a chi-square test. Age and sex-adjusted rates of time spent in a SNF, expressed as rate of days per person-year, were calculated overall, by age (adjusted for sex), by sex (adjusted for age), and by reduced vs. preserved EF. Differences in the rates were tested with negative binomial regression. Unadjusted and adjusted Andersen-Gill models, a form of the Cox model that models multiple outcomes per person, were used to examine associations between SNF use, modeled as a time-dependent variable, and hospitalization. Models were adjusted for age, sex, EF and comorbidities (modeled as time-dependent variables). Predictors of hospitalization among patients in SNFs were examined using Andersen-Gill models. Potential predictors included age, sex, BMI, EF, marital status, comorbidities (modeled as time-dependent variables) and ADLs. All ADLs that were recorded as part of the SNF stay were obtained and modeled as a time-dependent variable. When analyzing individual ADLs, ratings of “independent” and “supervision” were grouped together as were “extensive assistance” and “total dependence” for ease of interpretation. When analyzing number of ADLs requiring assistance, since few patients did not require any assistance with ADLs (N=89), we grouped together those who required assistance with 0 or 1 ADL. We also grouped together those who required assistance with 2-4 and 5-6 ADLs because of comparable hazard ratios when modeled individually. Models were run for each predictor, adjusted for age and sex; a fully adjusted model including all possible predictors was also run. The proportional hazards assumption was tested using the scaled Schoenfeld residuals and found to be valid.
Figure 1.
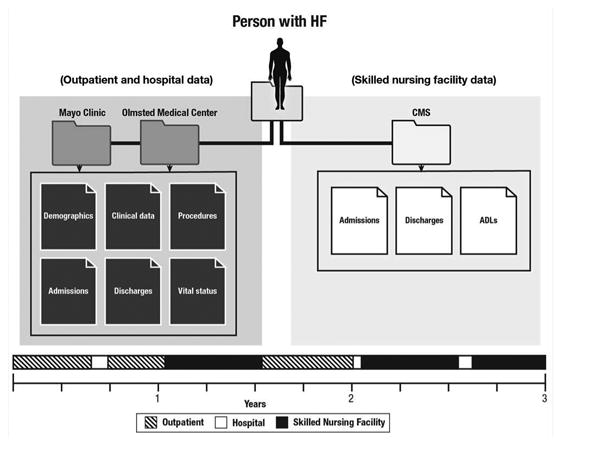
Linkage of comprehensive community medical records to skilled nursing facility data.
EF was missing in 17% of the cases, so multiple imputation21 was employed. Five datasets were created with missing values replaced by imputed values based on a model incorporating various demographic and clinical variables and an indicator for HF along with the cumulative baseline hazard of HF approximated by the Nelson-Aalen estimator.22 The results of these datasets were then combined using Rubin's rules.21 All analyses were performed using SAS statistical software, version 9.3 (SAS Institute Inc., Cary, NC). This study was approved by the appropriate Institutional Review Boards.
Results
Patient Characteristics
Among 1,727 patients identified with incident HF from 1/1/2000 to 12/31/2010, 36 (2%) died during their incident HF hospitalization and 193 (11%) were in a SNF at the time of HF or within the 30 days prior. These patients were excluded from further analyses, resulting in a final community cohort of 1,498 patients (mean [SD] age 75.1 [13.7] years, 45% male; 52% preserved EF; Table 1).
Table 1. Clinical Characteristics of Incident Heart Failure Patients Overall and among Those with an Admission to A Skilled Nursing Facility after Heart Failure Diagnosis, Olmsted County, 2000-2010.
| Overall | Skilled Nursing Facility | |
|---|---|---|
| (N=1498) | (N=605) | |
| Male | 680 (45.4) | 210 (34.7) |
| Age, mean (SD) | 75.1 (13.7) | 81.3 (9.3) |
| Marital status | ||
| Unknown, n | 7 | 0 |
| Not married | 754 (50.6) | 367 (60.7) |
| Married | 737 (49.4) | 238 (39.3) |
| Body mass index category | ||
| Unknown, n | 1 | 1 |
| Underweight | 33 (2.2) | 11 (1.8) |
| Normal | 347 (23.2) | 175 (29.0) |
| Overweight | 473 (31.6) | 189 (31.3) |
| Obese | 644 (43.0) | 229 (37.9) |
| Outpatient at diagnosis | 590 (39.4) | 220 (36.4) |
| Prior Hypertension | 1346 (89.9) | 557 (92.1) |
| Prior Coronary artery disease | 860 (57.4) | 353 (58.4) |
| Prior Arrhythmia | 1048 (70.0) | 452 (74.7) |
| Prior Hyperlipidemia | 899 (60.0) | 339 (56.0) |
| Prior Stroke | 237 (15.8) | 112 (18.5) |
| Prior Arthritis | 629 (42.0) | 303 (50.1) |
| Prior Asthma | 148 (9.9) | 69 (11.4) |
| Prior Cancer | 512 (34.2) | 252 (41.7) |
| Prior Chronic kidney disease | 382 (25.5) | 164 (27.1) |
| Prior COPD | 401 (26.8) | 179 (29.6) |
| Prior Dementia | 143 (9.6) | 79 (13.1) |
| Prior Depression | 333 (22.2) | 146 (24.1) |
| Prior Diabetes | 588 (39.3) | 229 (37.9) |
| Prior Osteoporosis | 243 (16.2) | 135 (22.3) |
| Prior Schizophrenia | 46 (3.1) | 25 (4.1) |
| Prior Substance abuse disorder | 71 (4.7) | 29 (4.8) |
| Ejection fraction | ||
| Unknown, n | 257 | 105 |
| Reduced | 594 (47.9) | 202 (40.4) |
| Preserved | 647 (52.1) | 298 (59.6) |
Data are presented as N (%) unless indicated otherwise.
COPD=chronic obstructive pulmonary disease, eGFR=estimated glomerular filtration rate
During a median (min, max) follow-up of 3.6 (0-13.0) years, 605 (40.4%) patients were admitted to a SNF (mean [SD] age 81.3 [9.3] years, 35% male; 60% preserved EF; Table 1). The proportion of SNF admissions did not change over the study period (P=.34). Among patients admitted to a SNF, a total of 966 SNF admissions occurred with a mean (SD) length of stay per admission of 144.0 (366.9) days. Of the patients admitted to a SNF, 225 (37%) had 2 or more admissions; the maximum number of admissions was 12. Among all patients, those with preserved EF had more SNF admissions compared to those with reduced EF (Figure 2, P<.001).
Figure 2.
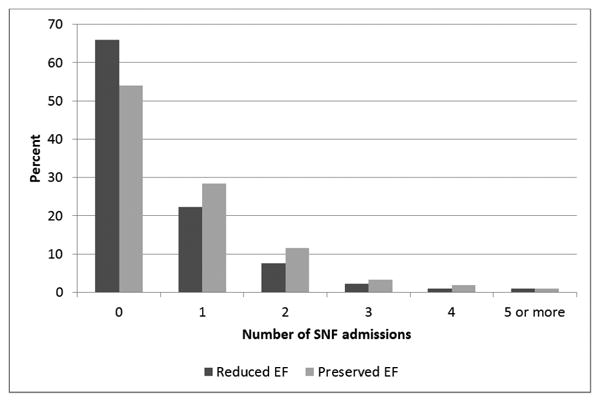
Number of skilled nursing facility admissions per person after HF diagnosis according to ejection fraction.
The overall age- and sex- adjusted rate of days per person-year in a SNF was 19.2. After adjustment for age, no difference in rate of days per person-year in a SNF was observed for women compared to men (21.9 and 16.7 days per person-year, respectively; P=.11). After adjustment for sex, patients aged 75 or older had a higher rate compared to those younger (48.6 vs. 7.2; P<.001). After adjustment for age and sex, the rate of days per person-year in a SNF for patients with preserved EF vs. reduced EF did not differ (20.2 vs. 19.8; P=.92).
SNF and Risk of Hospitalizations
Over a median (min, max) follow-up of 3.6 (0-13.0) years, 688 people died and 5158 hospitalizations occurred among 1219 people. After adjustment for age and sex, residing in a SNF was associated with a 70% increased risk of hospitalization compared to not residing in a SNF (adjusted HR, 95% CI: 1.69, 1.45-1.97). Further adjustment for EF and comorbidities only marginally attenuated these results; residing in a SNF was associated with a 50% increased risk of hospitalization compared to not residing in a SNF (adjusted HR, 95% CI: 1.52, 1.31-1.76).
Predictors of Hospitalization among Patients Admitted to a SNF
Among the 605 patients admitted to a SNF, 518 hospitalizations occurred during the SNF stays. After adjustment for sex, older age was associated with a decreased risk of hospitalization among those in a SNF (HR, 95% CI: 0.96, 0.95-0.98 for a 1 year increase in age, Table 2). After adjustment for age and sex, arrhythmia, asthma, chronic kidney disease and COPD were all associated with a higher risk of hospitalization while being underweight and having dementia were associated with a reduced risk of hospitalization (Table 2).
Table 2. Age- and Sex-Adjusted Predictors of Hospitalization among Heart Failure Patients in a Skilled Nursing Facility.
| HR (95% CI) | P | |
|---|---|---|
| Male | 1.15 (0.87-1.54) | .33 |
| Age at HF | 0.96 (0.95-0.98) | <.001 |
| Marrieda | 1.04 (0.79-1.37) | .77 |
| Normal weight | 1.00 (ref) | .01 |
| Underweight | 0.23 (0.08-0.69) | |
| Overweight | 0.88 (0.62-1.24) | |
| Obese | 1.18 (0.85-1.62) | |
| Hypertensiona | 2.24 (0.77-6.47) | .14 |
| CADa | 1.26 (0.94-1.69) | .13 |
| Arrhythmiaa | 2.22 (1.61-3.06) | <.001 |
| Hyperlipidemiaa | 1.13 (0.85-1.51) | .41 |
| Strokea | 1.21 (0.94-1.57) | .14 |
| Arthritisa | 0.98 (0.74-1.30) | .91 |
| Asthmaa | 1.77 (1.24-2.53) | .002 |
| Cancera | 1.24 (0.93-1.67) | .15 |
| Chronic kidney diseasea | 1.66 (1.30-2.12) | <.001 |
| COPDa | 1.37 (1.03-1.82) | .03 |
| Dementiaa | 0.74 (0.57-0.96) | .03 |
| Depressiona | 0.80 (0.62-1.04) | .10 |
| Diabetesa | 1.27 (0.97-1.66) | .09 |
| Osteoporosisa | 0.80 (0.60-1.08) | .14 |
| Schizophreniaa | 0.91 (0.62-1.36) | .65 |
| Substance abusea | 1.12 (0.75-1.67) | .59 |
| Reduced EF | 0.90 (0.65-1.26) | .55 |
Treated as time-dependent covariates
CAD=coronary artery disease, COPD=chronic obstructive pulmonary disease, EF=ejection fraction, HF=heart failure
We then examined ADLs among patients in SNF with at least one complete assessment; 582 patients had a total of 3980 assessments. Extensive assistance/total dependence was highly prevalent for numerous ADLs, with the exception of eating (Table 3). In 2679 (67.3%) assessments, patients required assistance with five or six ADLs. Limitations in the ADLs were associated with an increased risk of hospitalization (Table 3) including assistance or extensive assistance/total dependence in dressing, toileting, getting in and out of bed and locomotion.
Table 3. Age- and Sex-Adjusted Activities of Daily Livinga as Predictors of Hospitalization among Heart Failure Patients in a Skilled Nursing Facility.
| N | HR (95% CI) | P | |
|---|---|---|---|
| Eating | .80 | ||
| Independent/supervision | 3159 | 1.00 (ref) | |
| Limited assistance | 365 | 1.13 (0.79-1.63) | |
| Extensive assistance/Total dependence | 465 | 1.04 (0.66-1.65) | |
| Dressing | <.001 | ||
| Independent/supervision | 559 | 1.00 (ref) | |
| Limited assistance | 597 | 2.19 (1.46-3.27) | |
| Extensive assistance/Total dependence | 2824 | 1.79 (1.28-2.51) | |
| Bathing | .28 | ||
| Independent/supervision | 140 | 1.00 (ref) | |
| Limited assistance | 258 | 1.63 (0.74-3.62) | |
| Extensive assistance/Total dependence | 3582 | 1.79 (0.87-3.67) | |
| Toileting | .02 | ||
| Independent/supervision | 702 | 1.00 (ref) | |
| Limited assistance | 691 | 1.57 (1.07-2.28) | |
| Extensive assistance/Total dependence | 2587 | 1.53 (1.11-2.10) | |
| In/Out bed | .04 | ||
| Independent/supervision | 1014 | 1.00 (ref) | |
| Limited assistance | 651 | 1.40 (1.02-1.93) | |
| Extensive assistance/Total dependence | 2315 | 1.40 (1.05-1.85) | |
| Locomotion on unitb | <.001 | ||
| Independent/supervision | 1018 | 1.00 (ref) | |
| Limited assistance | 585 | 1.54 (1.12-2.12) | |
| Extensive assistance/Total dependence | 2377 | 1.73 (1.32-2.26) | |
| Number of ADLs requiring assistancec | .001 | ||
| 0-1 | 430 | 1.00 (ref) | |
| 2-4 | 871 | 1.59 (1.05-2.41) | |
| 5-6 | 2679 | 2.12 (1.41-3.19) |
Treated as time dependent covariates.
Walking or self-sufficient wheeling.
includes patients requiring limited or extensive assistance or total dependence.
ADL=activity of daily living
We further examined if the number of ADLs for which patients required limited or extensive assistance or total dependence (further referred to as ADLs requiring assistance) was associated with risk of hospitalization. Indeed, an increasing number of ADLs requiring assistance was associated with an increased risk of hospitalization in a dose-response manner (Table 3). Patients with 2-4 ADLs requiring assistance had a 1.6 fold increased risk of hospitalization compared to those with 0-1 ADLs requiring assistance (HR, 95% CI: 1.59, 1.05-2.41, Table 3). Patients who required assistance with 5-6 ADLs had more than a two-fold increased risk of hospitalization compared to patients requiring assistance with 0-1 ADLs (HR, 95% CI: 2.12, 1.41-3.19).
After adjustment for all of the individual predictors, needing assistance with 2-4 or 5-6 ADLs was independently and strongly associated with an increased risk of hospitalization (HR, 95% CI: 1.84, 1.25-2.71; HR, 95% CI: 2.30, 1.55-3.40, respectively; Figure 3). Additional clinical predictors of increased risk of hospitalization included arrhythmia, asthma and chronic kidney disease. Conversely, increasing age, being underweight and having depression or osteoporosis were associated with a decreased risk of hospitalization among patients referred to a SNF (Figure 3).
Figure 3.
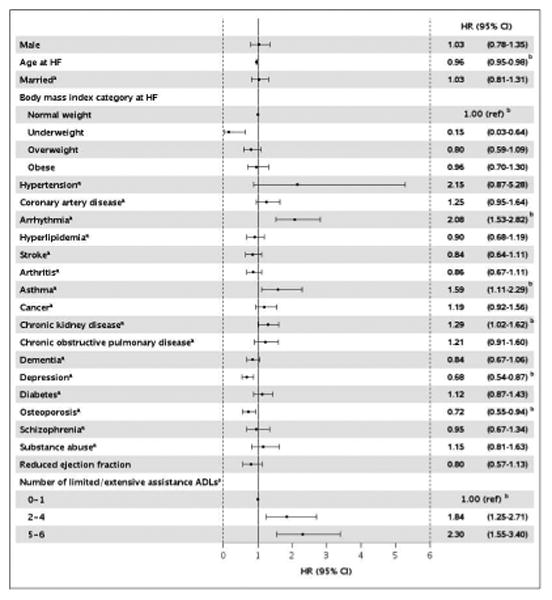
Fully-adjusted predictors of hospitalizations among heart failure patients in a skilled nursing facility.
aTreated as time dependent covariates.
bP<.05
ADL=activity of daily living
Discussion
Assembling the aforementioned comprehensive community dataset in a population-based HF incidence cohort enabled characterizing the complete experience of patients living with HF from diagnosis to end of life. We reported that after the first diagnosis of HF, admission to a SNF is frequent, that once admitted to a SNF, the duration of the stay is prolonged (on average 144 days) and that almost 40% of the patients who have been in a SNF will return to a SNF. Patients in a SNF had an increased risk of hospitalization compared to those who did not. Among the patients in a SNF, many non-cardiovascular characteristics, including ADLs, were predictors of hospitalization.
SNF Utilization in HF
Our data pertaining to a comprehensive community cohort of patients with incident (first-ever) HF document that 40% of HF patients are admitted to a SNF at some point after HF diagnosis. Data on this subject are sparse. The study presented herein utilizes longitudinal data on SNF admissions among both in- and outpatient HF patients. Because previous studies relied on administrative data and voluntary registries, only included incident and prevalent hospitalized HF cases, and characterized SNF use at hospital discharge only, the overall utilization of SNF among HF patients was underestimated. Indeed, a study of Medicare fee-for-service hospitalizations for HF found that the utilization of SNF had increased from 13% in 1993 to 20% in 2006.23 In the Get with the Guidelines–HF (GWTG-HF) program, a voluntary registry of the American Heart Association, approximately 24% of patients hospitalized with HF were discharged to a SNF in 2005 and 2006.6 The characteristics associated with admission to a SNF in the GWTG-HF study were longer hospital stay, older age, female sex, hypotension, higher EF, no ischemic heart disease and a variety of comorbidities.6 Our study brings new knowledge to this matter by reporting that the use of SNF in HF patients in the community is more than double what had been previously reported. Indeed, linking clinical data to SNF data including MDS assessments, we reported on all SNF admissions over the course of the entire follow-up and identified that SNF admissions are very frequent in this population as almost half of patients diagnosed with HF are admitted at least once to a SNF during their follow-up. Among those admitted to a SNF, 37% are admitted to a SNF more than once. The proportion of persons admitted to a SNF remained constant over a decade and the length of their stay was prolonged (on average 144 days). A higher proportion of patients with reduced EF had 0 SNF admissions compared to patients with preserved EF. Conversely, a higher proportion of patients with preserved EF had 1, 2, 3, or 4 SNF admissions compared to those with reduced EF. However, a higher proportion of patients with preserved EF are women and older compared to patients with reduced EF. Consequently, we looked at the rate of days per person year in a SNF and after adjusting for age and sex, the rate was similar between those with reduced and preserved EF.
SNF and Hospitalizations
In our cohort, HF patients in a SNF had a 50% increased risk of hospitalization compared to those not in a SNF, independently of comorbidity burden. Prior reports were discrepant as a hospital-level Medicare claims study found no association between SNF rates and 30-day hospital readmission rates24 while data from the GWTG-HF program indicated that HF patients referred to SNF had an increased risk of death and rehospitalization compared to those discharged home.6 Our data clearly document an increased risk of hospitalization among patients referred to SNF, even after adjusting for factors contributing to SNF use.
As was recently emphasized, “research is critically needed to understand and ameliorate the multiple factors that contribute to increased mortality and rehospitalizations among patients with HF discharged to SNFs”.7 Since claims data have limitations that hinder adequate adjustment and voluntary registries may suffer from selection bias, our population-based study addresses this gap in knowledge by reporting on a population of optimal clinical relevance with detailed in- and outpatient clinical data linked with CMS MDS data including ADLs, thus capturing the complete experience of patients with rigorously validated incident HF diagnoses. Our linked dataset enabled us to uncover new insights into the predictors of hospitalization among patients with HF admitted to a SNF. Indeed, many non-cardiovascular factors, including the number of ADLs requiring assistance, were associated with an increased risk of hospitalization.
Clinical Implications
These results suggest that intensive transitional care focusing on improving physical function for patients with HF admitted to SNFs could help reduce hospitalizations. Recent evidence suggesting that physical, nutritional and cognitive interventions were effective in reversing frailty in community-dwelling older persons25 resonate with this point. The healthcare environment and characteristics of persons using SNF are distinct from those of community-dwelling adults; thus, it is important to study the SNF population. Interventions targeted at HF patients in SNFs could lead to different care guidelines and quality measures for patients with HF admitted to SNFs as opposed to patients with HF living independently.6,7 Indeed, data suggest that providing HF-specific care education to SNF staff may help increase their knowledge and confidence in caring for HF patients.26 Furthermore, a SNF HF quality initiative program led to better HF care and enhanced teamwork among the SNF staff in addition to improving staff satisfaction.27
Limitations and Strengths
Although most nursing homes in the Olmsted County area are SNFs, we could not determine with certainty if a patient was placed into skilled nursing care or intermediate care. Furthermore, we cannot rule out unmeasured confounding. Our study has many notable strengths, including the geographically-defined community setting, rigorous validation of HF cases, and our ability to merge medical record data with MDS data, allowing complete ascertainment of in- and outpatient clinical data as well as SNF utilization after an incident HF diagnosis.
Conclusion
In this community cohort of patients with incident HF, 40% were admitted to a SNF sometime after their diagnosis. Patients in a SNF had a 50% increased risk of hospitalization compared to those not in a SNF. Characteristics associated with hospitalization among the SNF users were mostly non-cardiovascular, including ADLs. This work provides new understanding in the use of SNFs in HF and its impact on hospitalization, which is necessary to plan effective interventions.
Acknowledgments
We thank Ellen Koepsell, RN and Deborah S. Strain for their study support.
Funding Sources: This work was supported by grants from the National Institute on Aging (R21 AG045228 and R01 AG034676) and National Heart Lung and Blood Institute (HL120859). The funding sources played no role in the design, conduct, or reporting of this study.
Dr. Chamberlain is a Co-Investigator of the Rochester Epidemiology Project, funded by a grant from the National Institute on Aging. Dr. Boyd reports grants from NIA during the conduct of the study, and royalty for a chapter on Multimorbidity from UptoDate, outside of the submitted work. Dr. St. Sauver reports grants from National Institute on Aging during the conduct of the study. Dr. Roger reports grants from National Institutes on Aging, grants from National Heart, Lung, Blood, Institute, during the conduct of the study.
Abbreviations
- ADLs
activities of daily living
- CAD
coronary artery disease
- COPD
chronic obstructive pulmonary disease
- HF
heart failure
- MDS
minimum data set
- REP
Rochester Epidemiology Project
- SNF
skilled nursing facility
Footnotes
Disclosures: All other authors have no disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sheila M. Manemann, Department of Health Sciences Research, Mayo Clinic, Rochester, MN.
Alanna M. Chamberlain, Department of Health Sciences Research, Mayo Clinic, Rochester, MN.
Cynthia M. Boyd, Division of Geriatric Medicine and Gerontology, Johns Hopkins University, Baltimore, MD.
Susan A. Weston, Department of Health Sciences Research, Mayo Clinic, Rochester, MN.
Jill Killian, Department of Health Sciences Research, Mayo Clinic, Rochester, MN.
Cynthia L. Leibson, Department of Health Sciences Research, Mayo Clinic, Rochester, MN.
Andrea Cheville, Division of Physical Medicine and Rehabilitation.
Jennifer St. Sauver, Department of Health Sciences Research, Mayo Clinic, Rochester, MN.
Shannon M. Dunlay, Department of Health Sciences Research, Mayo Clinic, Rochester, MN; Division of Cardiovascular Diseases in the Department of Internal Medicine, Mayo Clinic, Rochester, MN.
Ruoxiang Jiang, Department of Health Sciences Research, Mayo Clinic, Rochester, MN.
Véronique L. Roger, Department of Health Sciences Research, Mayo Clinic, Rochester, MN.
References
- 1.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the american heart association. Circ Heart Fail. 2013;6(3):606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jurgens CY, Goodlin S, Dolansky M, et al. Heart failure management in skilled nursing facilities: a scientific statement from the American Heart Association and the Heart Failure Society of America. Circ Heart Fail. 2015;8(3):655–687. doi: 10.1161/HHF.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 5.Mor V, Intrator O, Feng Z, Grabowski DC. The revolving door of rehospitalization from skilled nursing facilities. Health Affairs. 2010;29(1):57–64. doi: 10.1377/hlthaff.2009.0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen LA, Hernandez AF, Peterson ED, et al. Discharge to a skilled nursing facility and subsequent clinical outcomes among older patients hospitalized for heart failure. Circ Heart Fail. 2011;4(3):293–300. doi: 10.1161/CIRCHEARTFAILURE.110.959171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pressler SJ. Heart failure patients in skilled nursing facilities: evidence needed. Circ Heart Fail. 2011;4(3):241–243. doi: 10.1161/CIRCHEARTFAILURE.111.962258. [DOI] [PubMed] [Google Scholar]
- 8.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 11.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 12.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22(4 Suppl A):6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 13.Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis. 2013:10E66. doi: 10.5888/pcd10.120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services. Multiple chronic conditions - a strategic framework: optimum health and quality of life for individuals with multiple chronic conditions. Washington, DC: Dec, 2010. [Google Scholar]
- 15.Chamberlain AM, St Sauver JL, Gerber Y, et al. Multimorbidity in heart failure: a community perspective. Am J Med. 2015;128(1):38–45. doi: 10.1016/j.amjmed.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocca WA, Boyd CM, Grossardt BR, et al. Prevalence of multimorbidity in a geographically defined American population: patterns by age, sex, and race/ethnicity. Mayo Clin Proc. 2014;89(10):1336–1349. doi: 10.1016/j.mayocp.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296(18):2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 20.Nursing Home Quality Initiative. [May 25, 2016];CMS.gov Centers for Medicare & Medicaid Services. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/index.html.
- 21.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 22.White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28(15):1982–1998. doi: 10.1002/sim.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. JAMA. 2010;303(21):2141–2147. doi: 10.1001/jama.2010.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Ross JS, Carlson MD, et al. Skilled nursing facility referral and hospital readmission rates after heart failure or myocardial infarction. Am J Med. 2012;125(1):100. doi: 10.1016/j.amjmed.2011.06.011. e101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng TP, Feng L, Nyunt MS, et al. Nutritional, Physical, Cognitive, and Combination Interventions and Frailty Reversal Among Older Adults: A Randomized Controlled Trial. Am J Med. 2015;128(11):1225–1236. doi: 10.1016/j.amjmed.2015.06.017. e1221. [DOI] [PubMed] [Google Scholar]
- 26.Boxer RS, Dolansky MA, Frantz MA, Prosser R, Hitch JA, Pina IL. The Bridge Project: improving heart failure care in skilled nursing facilities. J Am Med Dir Assoc. 2012;13(1):83. doi: 10.1016/j.jamda.2011.01.005. e81-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nazir A, Dennis ME, Unroe KT. Implementation of a heart failure quality initiative in a skilled nursing facility: lessons learned. J Gerontol Nurs. 2015;41(5):26–33. doi: 10.3928/00989134-20141216-01. [DOI] [PubMed] [Google Scholar]


