Abstract
Cytoplasmic incompatibility (CI) is a conditional sterility in numerous arthropods that is caused by inherited, intracellular bacteria such as Wolbachia. Matings between males carrying CI-inducing Wolbachia and uninfected females, or between males and females infected with different Wolbachia strains, result in progeny that die during very early embryogenesis. Multiple studies in diploid (Drosophila) and haplodiploid (Nasonia) insects have shown that CI-Wolbachia cause a failure of the paternally derived chromatin from resolving into distinct chromosomes. This leads to the formation of chromatin bridges and other mitotic defects as early as the first mitotic division, and to early mitotic arrest. It is currently unknown if CI-inducing symbionts other than Wolbachia affect similar cellular processes. Here, we investigated CI caused by an unrelated bacterium, Cardinium, which naturally infects a parasitic wasp, Encarsia suzannae. CI crosses in this host–symbiont system resulted in early mitotic defects including asynchrony of paternal and maternal chromosome sets as they enter mitosis, chromatin bridges and improper chromosome segregation that spanned across multiple mitotic divisions, triggering embryonic death through accumulated aneuploidy. We highlight small differences with CI-Wolbachia, which could be due to the underlying CI mechanism or host-specific effects. Our results suggest a convergence of CI-related cellular phenotypes between these two unrelated symbionts.
Keywords: bacterial endosymbionts, embryonic death, mitotic defects, parasitoid, reproductive manipulators
1. Background
Numerous arthropod species, particularly insects, carry vertically transmitted, intracellular bacterial symbionts that manipulate reproductive developmental processes of their hosts in ways that enhance symbiont transmission. By far the most common symbiont-induced reproductive manipulation is cytoplasmic incompatibility (CI). This form of sterility, best characterized in Wolbachia (α-Proteobacteria), results when infected males mate with uninfected females (unidirectional CI) or when mates harbour different strains of Wolbachia (bidirectional CI) [1]. The current understanding is that male chromosomes are marked during male gametogenesis to cause a lethal paternal effect (as Wolbachia are not present in the sperm). Paternal chromosomes are rescued in the egg if Wolbachia of a similar type are present in the egg cytoplasm (reviewed by [2,3]). The understanding of the molecular mechanism of Wolbachia-induced CI has been recently improved [4,5], supporting the modification–rescue framework, with two CI-inducing genes that appear to work together causing modification that can be rescued by CI-Wolbachia-infected host females.
Initial cytological studies of CI performed in Wolbachia-infected Nasonia vitripennis (jewel wasp) embryos revealed a tangled paternal chromatin mass next to the female chromosomes during metaphase of the first mitosis [6]. This abnormal mitotic behaviour in Nasonia was subsequently confirmed [7] and attributed to improper condensation of the paternal chromatin. Subsequently, mitotic defects were observed in the cleavage products of Wolbachia-infected Drosophila simulans embryos, such as the formation of a chromatin bridge during the first cleavage division (CD) and smaller, condensed chromatin bodies, presumably breakdown products resulting from defective subsequent cleavage divisions [8]. Later work confirmed the improper condensation of paternal chromosomes, causing some fragments to become incorporated into daughter nuclei (DN) and extensive chromosome bridging during anaphase in the first and later cleavage divisions [9–12].
The mitotic defects caused by Wolbachia-induced CI affect development in an organism-dependent manner. In diploid species, CI leads to early embryonic developmental arrest. By contrast, in haplodiploid organisms including Nasonia, CI results in either haploid male embryos (male development) or embryonic lethality (female mortality [13,14]). In N. vitripennis, defects in the male-derived pronucleus (PN) are so severe that the paternal chromosomes do not segregate, or segregate to one daughter nucleus and embryonic development proceeds from the remaining normal haploid nucleus at the end of the first mitotic division [15]. Embryonic lethality in Drosophila and Nasonia results from interference of the defective paternal chromatin with mitotic progression of the viable maternal chromosomes, with consequent aneuploidy in all cleavage nuclei [12,15]. The fact that CI-embryos from Drosophila and Nasonia exhibit very similar mitotic defects suggests that Wolbachia may be disrupting the same host targets that are conserved between these two organisms.
The entirety of what is known regarding the cellular basis of CI stems from studies on Wolbachia–host systems. Interestingly, Cardinium, an unrelated bacterial symbiont in the Bacteroidetes that appears to infect 9% of arthropods [16], also causes CI [17–19]. Cardinium strains, like Wolbachia strains, can elicit a range of effects on host development in addition to CI, including parthenogenetic female development [20,21] and feminization of genetic male embryos [22,23]. The sequencing of the genome (cEper1) of a CI-inducing Cardinium strain infecting the parasitic wasp Encarsia pergandiella, now known as Encarsia suzannae [24], found little homology and no evidence of lateral gene transfer between these two symbionts [25], despite records of co-infections in other species [18,26,27]. This lack of homology between Wolbachia and Cardinium genomes indicates CI has arisen independently in these two bacterial lineages [25]. A compelling question, then, is how similar are the underlying cellular and molecular mechanisms of CI-induced by these bacteria? In E. suzannae infected by CI-causing Cardinium, CI-affected embryos do not hatch into larvae, but the cytological mechanism and specific developmental window of Cardinium-induced mortality has remained unknown [17].
Here, we performed the first detailed cytological analysis of CI-Cardinium-affected embryos in Encarsia. Our work suggests that Cardinium-induced CI results from mitotic defects occurring during the first and subsequent mitotic divisions. There is a large degree of overlap in the specific cytological phenotypes of CI between Cardinium and Wolbachia (e.g. mitotic asynchrony, chromatin bridging), with some differences (e.g. absence of mortality at the first mitotic division in Cardinium), pointing to a possible convergence of Wolbachia and Cardinium on the same targeted pathway(s) in their respective hosts.
2. Material and methods
(a). Insect cultures
We used laboratory cultures of Cardinium-infected and uninfected E. suzannae. The infected line is fixed for Cardinium and was originally collected from its natural whitefly host Bemisia tabaci in the Rio Grande Valley in Texas in 2003. An uninfected line was obtained by curing adult wasps of a subpopulation of the infected line with 50 mg ml−1 rifampicin in honey for three generations. Both cultures were maintained on B. tabaci reared on cowpea plants (Vigna unguiculata) in a climatic chamber at 27°C, 60–70% relative humidity and 16 L : 8 D photoperiod. Encarsia suzannae has an unusual biology; only diploid female eggs are laid in whiteflies (male eggs are laid in parasitoid pupae developing within the whitefly [28]). All eggs examined in this study were dissected from whiteflies and were therefore diploid, incipient females.
(b). Egg collection
Three types of matings were set up in glass vials: the CI cross between infected males and cured females of E. suzannae, and two control crosses, cured males × cured females and infected males × infected females. Cytological events of embryogenesis were studied from the eggs taken from small oviposition arenas prepared by laying a leaf disc hosting third and fourth instar nymphs of whiteflies into a Petri dish (50 mm diameter) containing a moist filter paper disc. Several mated E. suzannae females were allowed to oviposit under observation for about 30 min, parasitized whiteflies were marked and the time of each oviposition recorded on a map of the whiteflies in the dish. After removing the parasitoids, the wasp eggs were left to develop by incubating arenas at room temperature (25 ± 2°C) until shortly before the chosen fixation time. Each hydropic egg, measuring 70–80 µm at oviposition [29,30], was extracted from whitefly nymphs by dissection under a binocular microscope and immediately fixed. Varying the incubation time yielded embryos that ranged in age from 15 min to 96 h post-oviposition, although we focused, in particular, on the first 24 h of embryo development.
(c). Cytological technique
Eggs were dissected from nymphs on a microscope slide in 50 µl of tris-buffered saline (TBS), and, in order to remove most of the whitefly debris, subsequently transferred to 50 µl of 3.7% formaldehyde in TBST (TBS plus Tween 20) for fixation, using a glass Pasteur pipette that had been pulled to a narrow capillary end. The slide with the drop of fixative was then placed in a humid chamber for 1 h, followed by three washes in TBST at 5 min intervals with fluid removed by either a slightly moist twisted Kimwipe (Kimtech Science) or the modified pipette. After the third wash, eggs were stained with 1 µg ml−1 DAPI (4′,6-diamidino-2-phenylindole) (Molecular Probes) in TBST for 5 min and washed three more times in TBST as above. Each stained egg was mounted on a Superfrost Plus slide (Fisher Scientific) or on a slide previously rubbed with siliconized paper (Bausch and Lomb). The mounting medium was a solution of 80% glycerol, 20% TBST with 2% n-propyl-gallate (Sigma), and the coverslip was sealed with clear nail polish. Slides were then stored at 4°C in the dark for at least one day to allow sufficient stain penetration.
(d). Confocal microscopy
Meiosis and early embryonic development of DAPI-stained eggs were imaged by optically sectioning embryos using a laser scanning confocal inverted microscope, either a Zeiss 510 LSCM or Nikon C1si. DAPI fluorescence was generated with a 405 nm excitation beam, using either a 40× dry objective or a 63× immersion oil objective at 1 to 2× zoom.
(e). Data analysis
Stacked images and three-dimensional videos of the different optical sections were obtained from multifocal Z-stack imaging using the microscopes' native software: Zeiss's LSM Image Browser and Nikon's EZ-C1. Images were then edited and assembled using GIMP. Cytological events occurring during embryo development were compared between CI crosses and cured and infected compatible crosses.
3. Results
(a). Normal development of compatible embryos
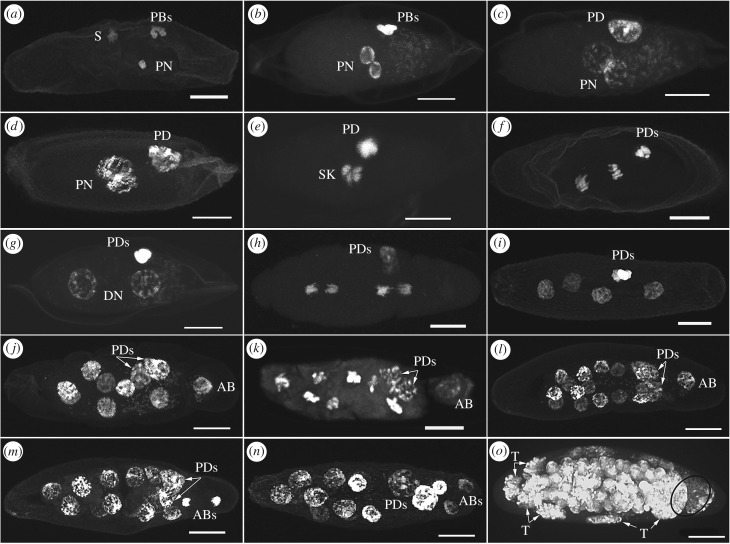
We observed embryogenesis in 102 eggs from the control crosses (infected female × infected male and cured female × cured male), most in stages before the end of the fourth CD. We found no difference in meiosis and early embryonic development between the two control crosses. By 50–70 min after egg deposition (AED), the second division of meiosis produced four haploid nuclei. One of these products became the haploid female PN that moved towards the interior of the egg's cytoplasm, while the other three products formed haploid polar bodies (PB), remaining at a position near the plasma membrane (figure 1a,b). Simultaneously, the sperm nucleus changed from a needle-like shape to become more ovoid and decondensed, and became the paternal PN. By 75–90 min AED, the male PN had migrated towards the female PN in the middle of the egg. By 01.30–01.50 h AED, the two PN in prophase were physically apposed (figure 1c,d), forming the synkaryon (figure 1e), while the PB coalesced into a single polar body derivative (PD) (figure 1c). At the end of metaphase through early anaphase of the first CD, four groups of chromosomes, two from each PN, were visible (figure 1e). The first CD was completed by 02.15–02.30 h AED (figure 1f,g), ultimately resulting in two DN and two PDs arising from the division of the first one. The second CD was completed by 03.00–03.30 h AED, resulting in four DN, while the two PDs did not undergo a further division (figure 1h,i). Within 04.00–04.30 h AED, the third CD resulted in eight DN, while the two PDs remained apparently inactive (figure 1j). The DN at the pole close to the PDs then lost synchrony with the other DN, so that at the end of the fourth CD (05.00–05.30 h AED) there were 14 DN and one apical blastomere (AB), a mitotic product that loses synchrony with the other DN and does not contribute to the formation of the multinucleated embryo (blastula) [30]. The AB completed a division 01.00–01.30 h later, forming two ABs, while the other 14 DN approached the prophase of the fifth CD. At the same time, the two PDs underwent polyploidization, each of them giving rise to a giant nucleus (figure 1k–n). Later observations of infected embryos at 24 and 48 h AED showed a blastula surrounded by big extra-embryonic nuclei stretched against the chorion (figure 1o). These giant nuclei, forming an extra-embryonic membrane, are known as teratocytes and have been found in the yolkless (hydropic) eggs of many families of Hymenoptera [30]. The normal development of compatible embryos that we observed in E. suzannae closely matched the embryonic development of the sibling species Encarsia gennaroi (E. pergandiella in [30]), to which the reader is referred for a detailed description of embryonic development in Encarsia, and the normal development of the sibling species Encarsia marthae (E. pergandiella in [31]).
Figure 1.
Normal embryo development of E. suzannae in compatible crosses. (b,c,g,j,l,m,n,o) Eggs of infected females mated with infected males, containing the bacterial endosymbiont Cardinium (bright small dots). (a,d,e,f,h,i,k) Eggs of cured females mated with cured males. (a) Fertilized egg at the end of meiosis, containing the female pronucleus (PN) and three polar bodies (PBs) and the peripheral sperm nucleus (S). (b) Female and male pronuclei approaching at interphase, three PBs. (c,d) Apposition of female and male pronuclei at prophase of the first cleavage division (CD), one polar body derivative (PD) is occurring. (e) First CD: synkaryon (SK) at the end of metaphase–early anaphase with four groups of chromosomes, a pair from each pronucleus. (f) First CD, end of anaphase and two PDs. (g) First two daughter nuclei (DN) and two PDs. (h) Anaphase of second CD and two PDs. (i) At the end of second CD, there are four DN. (j) End of third CD: eight DN of which one migrating to the pole close to the two PDs forms the apical blastomere (AB). (k) Fourth CD: seven DN in metaphase while the asynchronous AB is still in interphase, the two PDs polyploidize. (l) End of fourth CD: 14 DN, 1 AB in prophase and two PDs. (m) End of fourth CD: 14 DN, the AB at the end of anaphase, two PDs. (n) End of fourth CD: 14 DN, two ABs and two PDs. (o) Embryo after 24 h surrounded by stretched polyploid nuclei (teratocytes: T) forming the extra-embryonic membrane and with a high density of Cardinium bacteria near the right (posterior) pole in the black circle.
(b). Abnormal development of cytoplasmic incompatibility embryos
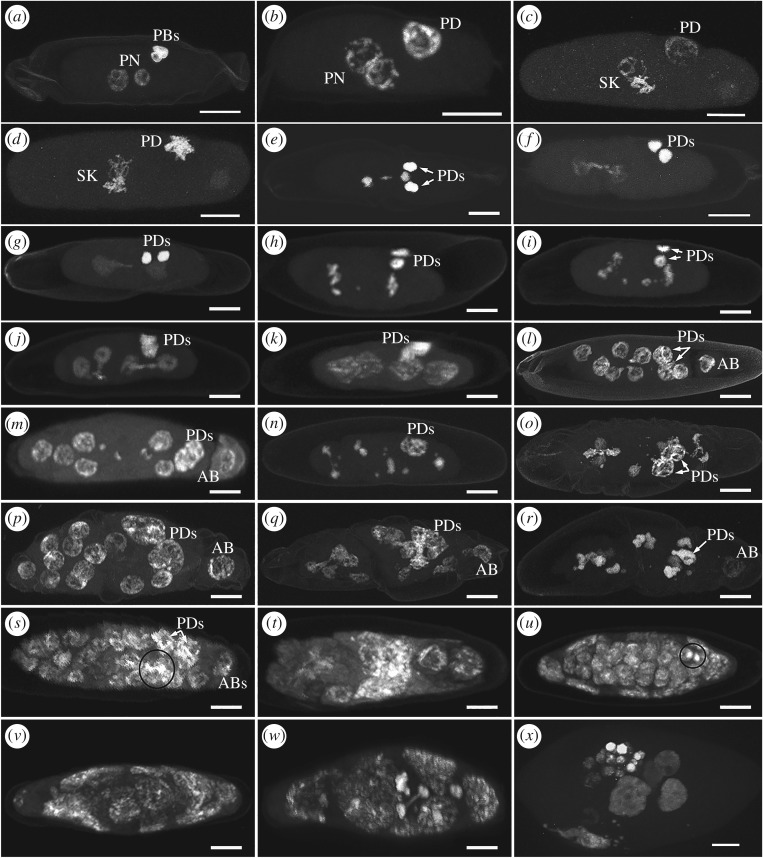
We observed 112 eggs from the incompatible cross (cured female × infected male), representative of each stage of embryonic development until the end of the fifth CD (7–9 h AED) and of later embryonic development occurring at 16–96 h AED. As about 70% of progeny die in the CI cross [32], we expected a minority of eggs to exhibit normal embryogenesis. For all eggs examined, events leading to the apposition of female and male PN (figure 2a) did not differ between control and CI-embryos. Like eggs from control crosses, meiosis was completed by 60–75 min, and in the same time frame, the sperm underwent apparently normal modifications. At pronuclear apposition (90–120 min), the two PN seemed to be at the same condensation level during prophase as in the control embryos (figure 2b). Aberrant mitotic events were observed immediately following synkaryon formation (02.00–02.10 h). During prometaphase and metaphase (02.10–02.40 h AED) chromosomes of one PN appeared less condensed than the other (figure 2c,d) and at the end of the first CD (02.40–03.00 h) one set of chromosomes lagged behind the two DN (figure 2e), often along with the formation of a chromatin bridge between the two DN (figure 2f). Occasionally along with, or instead of, bridge formation, we observed misshapen DN at the end of mitosis (figure 2g), probably a result of bridge formation. Chromatin bridges and misshapen nuclei are both considered products of improper chromosome segregation [15]. Overall, of the 28 eggs observed during the first CD, only two did not show any mitotic defects.
Figure 2.
Abnormal embryo development of E. suzannae in CI crosses. (a) Fertilized egg containing the male pronucleus (PN) approaching the female PN and three polar bodies (PBs). (b) Apposition of female and male PN showing synchronous prophase, one polar body derivative (PD). (c,d) At prometaphase (c) and metaphase (d) one PN is less condensed than the other one, and the PD is dividing. (e) At the end of telophase, one set of chromosomes lags between the segregating maternal chromosomes and two PDs occur. (f) Irregularly segregating chromosomes form a bridge between the first two daughter nuclei (DN). (g) Misshapen first two DN and two PDs much more condensed. (h) Slightly aberrant second cleavage division (CD): the two DN are in metaphase and only one chromosome lags between them. (i) Highly aberrant second CD: many chromosomes do not segregate correctly lagging between the DN or are scattered in the egg cytoplasm. (j,k) Highly aberrant second CD: the two pairs of sister DN are connected by a chromatin bridge (j) and the resulting four DN are misshapen (k). (l,m) Third CD results in the normal number of CN (eight) but misshapen (l) or unsegregated chromosomes lag in the egg cytoplasm (m), the apical blastomere (AB) is differentiated. (n,o) Third CD aborted: unsegregated chromosomes lag in the egg cytoplasm and nuclei are connected by chromatin bridges; four nuclei connected in (o). (p) Normal embryo development at the end of the fourth CD: 14 DN, one AB and two PDs. (q) Irregular fourth CD producing 14 DN, misshapen or connected by chromatin bridges. (r) Aborted fourth CD: highly condensed and some less condensed groups of chromosomes, and unsegregated chromosomes scattered in the egg cytoplasm. (s) The end of the fifth CD: highly condensed chromatin bodies (inside the black circle) co-occur with nuclei of regular shape. (t) At the end of the fifth CD, embryonic development arrested, only few nuclei are clearly recognizable and largely diffuse chromatin is visible. (u–w) At 16–20 h from oviposition a percentage of the embryos appear normally developed and surrounded by the polyploid teratocytes forming the extra-embryonic membrane, nevertheless bearing the products of defected mitosis like few small condensed chromatin bodies, here inside the black circle (u); in most of the embryos, development has stopped, showing only few nuclei (v) or fragmented nuclei originating many condensed chromatin bodies (w) together with diffuse chromatin, while peripheral teratocytes are still clearly visible. (x) Embryo in which development has arrested, observed at 72 h after egg deposition: within the hydropic egg, a few small nuclei and large chromatin masses occur.
During the second CD, which was completed at 03.15–03.40 h AED, we observed more mitotic defects, including improper segregation of one set of chromosomes, chromatin bridges and/or small, condensed chromatin bodies (figure 2h–k). Abnormal segregation and asynchrony of one chromosome set continued in the third CD (04.20–05.30 h AED). At this stage, there would normally be eight DN and two PDs, but we sometimes observed fewer than eight DN, as some nuclei may have stopped dividing. In addition, we recorded many small, condensed chromatin bodies resulting from irregular divisions, chromatin bridges sometimes connecting several pairs of sister nuclei, and misshapen nuclei with a distinct peduncle (figure 2l–o). However, the severity of the aberration was variable, with instances of normal numbers of DN and only slight defects, like some misshapen nuclei or a few unsegregated chromosomes scattered in the egg cytoplasm (figure 2l,m). In the fourth CD (05.30–07.00 h AED), only 10% of observed embryos reached the 17–18 nuclei stage (14 DN + 1–2 AB + 2PDs) seen in the compatible control embryos without apparent mitotic defects (figure 2p). The remaining 90% included either embryos with the normal number of nuclei and small mitotic defects or embryos with severe aberrations. In general, the defects observed consisted of different condensation levels among nuclei, abnormal segregation of chromosomes, irregularly shaped nuclei, small condensed chromatin bodies or chromatin bridges (figure 2q,r). Similarly, at the fifth CD (07.00–09.00 h AED), we found aborted embryos and a few developed embryos with slight defects (figure 2s–t). In eggs fixed at 16–20 h AED, all embryos were defective, with 42% showing normal development (multinucleated blastula surrounded by big stretched extra-embryonic nuclei) associated with slight mitotic defects consisting of one to four very dense chromatin bodies of much smaller size than the blastula nuclei (figure 2u). In the remaining 58% of embryos, the deleterious effects of the accumulation of mitotic defects in previous divisions resulted in aberrant embryos that did not complete normal development (figure 2v,w). Even in these later embryos, we could sometimes observe chromatin bridges (figure 2w). Eggs imaged from 48 to 96 h from oviposition did not complete embryogenesis (figure 2x), and in only one case did we observe a first instar larva at 96 h (they normally hatch within 48 h). The frequencies of anomalies at different developmental stages remained fairly constant throughout development, suggesting that all mitotic defects follow from what happened at the first mitosis, following pronuclear apposition, but not necessarily as a result of chromatin bridging, which was evident only in 28% of defective embryos (table 1).
Table 1.
Frequency of mitotic defects (md), in general, and of chromatin bridging (cb), in particular, over the first five mitotic divisions and subsequent divisions up to 96 h from oviposition in incompatible embryos. n, number of embryos observed; AED, after egg deposition.
| mitotic division | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st |
2nd |
3rd |
4th |
5th |
greater than 5th (16–96 h AED) |
||||||||||||
| n | md | cb | n | md | cb | n | md | cb | n | md | cb | n | md | cb | n | md | cb |
| 28 | 26 | 8 | 8 | 7 | 3 | 15 | 13 | 3 | 11 | 9 | 3 | 9 | 9 | 2 | 29 | 28 | 5 |
Therefore, Cardinium causes widespread mitotic defects that are nearly identical to those caused by Wolbachia, but unlike Wolbachia, the defects span a much wider range of cleavage divisions, leading not to early (i.e. at first CD) but to late embryonic death, probably through the accumulation of mitotic defects in the first several divisions.
4. Discussion
Our results showed a remarkable resemblance of cellular phenotypes caused by CI-Cardinium (Bacteroidetes) to the distantly related Wolbachia (α-Proteobacteria). Hallmarks of Wolbachia-induced CI in Drosophila and Nasonia parasitic wasp species are the asynchrony of the paternal and maternal chromosome sets as they enter into mitosis, and abnormal chromosome segregation, usually revealed by chromatin bridging, which leads to the formation of aneuploid nuclei during early embryonic development [2,3]. Here, we showed that Cardinium also causes such phenotypes, although the prevalence of chromatin bridges seems to be somewhat lower than in Wolbachia-mediated CI. Owing to the lack of chromosomal differences between the sexes in Hymenoptera, our current experimental approach does not allow us to distinguish between the paternal and maternal chromosome sets. Genetic studies in CI-Wolbachia-influenced N. vitripennis embryos have shown that it is the paternal genome that is excluded from the developing embryo, as haploid males resulting from CI-embryos express maternal but not paternal markers [6,7]. Molecular markers of paternal chromatin confirmed Wolbachia CI-induced defects are limited to paternal chromosomes in Drosophila [33]. It is highly likely that the paternal chromatin is the one affected also in CI-Cardinium-affected embryos in Encarsia, as the hypercondensation of one of the two nuclei is very consistent with Wolbachia-induced CI in all Drosophila and Nasonia species examined thus far [11,15]. In E. suzannae, the expression of Cardinum-induced CI matches the phenotypic expression of Wolbachia-induced CI, that is, sterility when an uninfected female crosses with an infected male, and fertility recovery when an infected female mates with an infected male [16]. In Drosophila and Nasonia systems, this effect is driven by Wolbachia products that mark male chromosomes during gametogenesis, causing mitotic abnormalities and arrest during early embryogenesis and rescue of paternal chromosomes in the egg infected by Wolbachia of a similar type [4,5]. Similarly, it is highly unlikely that in the unidirectional CI of our system the modification mechanism targets the female nucleus and spares the male nucleus, making our hypothesis that the paternal set is the one affected the most plausible at this time. However, this hypothesis needs to be tested in future experiments.
The first mitosis in insects is unique, because the female and male pronuclei do not fuse upon apposition but stay on two separate regions of the metaphase plate, and enter independently into anaphase [12,34]. It is thus essential that the female and male pronuclei enter and exit mitosis synchronously to successfully produce DN that contain both maternal and paternal chromosomes. Instead, in Encarsia embryos affected by CI-Cardinium, chromosome condensation of the two pronuclei is defective, just as it is in both Drosophila and Nasonia infected by Wolbachia [12,35]. Although similar phenotypes could have different genetic bases, the striking similarity of pronuclear asynchrony and abnormal chromosome segregation caused by both symbionts suggests that, despite their phylogenetic distance and evidence of independent evolution of CI [25], Wolbachia and Cardinium affect mitotic progression in similar ways during very early embryo development, possibly aiming at conserved molecular targets in their hosts.
Despite the similar cellular phenotypes between these two symbiont–host systems, we also observed several differences. Wolbachia-induced incompatible crosses in Drosophila cause the arrest of almost 50% of embryos during very early mitosis, with the remaining part arrested at the syncytial blastoderm stage or before hatching [11]. In haplodiploids, the most common phenotype of Wolbachia-induced CI is the arrest of embryo development as early as the first mitotic division in N. longicornis and N. giraulti, while in N. vitripennis more variants are observed due to the possible expression of both types of CI (male development and female mortality) [15]. However, in Encarsia, most of the Cardinium-induced CI-embryos do not arrest during the first mitotic division. In fact, the mitotic anomalies we observed during the first mitosis did not prevent embryos from progressing in their development. We also did not observe other phenomena seen in Wolbachia CI in Nasonia: embryos with two morphologically normal nuclei and one highly condensed genome that does not segregate, or embryos where one chromosome set segregates to only one daughter nucleus, which in N. vitripennis results in normal development of haploid males [15]. The absence of such embryos provides cytological evidence that E. suzannae does not have the male development type of CI, confirming the evidence of crossing experiments [17]. The lack of abnormal embryos that will develop as males signals a difference from the Wolbachia–Nasonia system, where the two species with predominantly female mortality type of CI (N. longicornis and N. giraulti) also have a few such ‘male development’ embryos [15]. However, like the Cardinium–Encarsia system, 30% of the N. giraulti CI-embryos were found to reach the blastoderm stage with evidence of aneuploid cells [14].
Recently, the understanding of the molecular mechanism of Wolbachia-induced CI has been substantially improved [4,5]. Homologous genes to the ‘CI genes’ identified in Wolbachia are absent in Cardinium, and the conserved domains of these genes across taxa are still not characterized [5]. Given that the molecular mechanism of CI-Cardinium is probably different, the observed phenotypic differences could be due to functional disparity in how these two symbionts cause CI. However, the differences seen could also be attributed to symbiont density or host-species effects. Defective embryos that develop as males in Nasonia are thought to be associated with the highest levels of Wolbachia modification and potentially density-dependent, with higher titres of the symbiont resulting in complete condensation and exclusion of the paternal set in embryogenesis [15]. The observed differences in cytological events between Wolbachia- and Cardinium-induced CI could also be influenced by interactions of the symbiont with particular host species, as is well known for Wolbachia CI in different hosts [36].
CI in E. suzannae, as well as in other systems with CI-Cardinium, is often incomplete, with egg hatching reduced but not eliminated [17,18,26]. We speculate that the disparity in the overall frequency of embryos with mitotic defects (mild or severe) observed here (90%) and the known lethality of CI in E. suzannae (73%) [32] may result from the likelihood that a small portion of embryos can survive mild mitotic defects. In such embryos, the affected nuclei may be those not contributing to the blastula formation. It has been demonstrated that Drosophila embryos can undergo substantial mitotic defects and still survive to adulthood [37,38].
The prevailing model for Wolbachia-induced CI is that Wolbachia modify sperm chromatin during spermatogenesis and these modifications render the sperm unable to successfully participate in embryonic development. It has been hypothesized that the fate of the paternal genome during the first mitosis of the zygote is determined by the extent of Wolbachia modification present in the sperm [2,39]. Thus, sperm nuclei that have been strongly altered by Wolbachia may produce chromosomes that will not segregate, resulting in embryos that will develop as haploid males, whereas sperm nuclei that have been only moderately modified may produce chromosomes that mis-segregate, resulting in detrimental aneuploidy. It would be interesting to know if this model also holds for Cardinium. If so, the sperm nuclei mis-segregate, allowing the embryo to survive for a time while ensuring it ultimately dies from the accumulated insults of incomplete segregation.
Acknowledgments
We thank Patty Jansma and Brooke Massani for training on and assistance with the confocal microscopes.
Data accessibility
All data necessary to replicate the experiments have been provided. The best images of cytological events have been selected. However, additional images are available upon request to the corresponding author.
Authors' contributions
M.S.H. conceived and coordinated the study; M.Ge., M.Gi., S.E.K., M.S.H., P.M.F. participated in the design of the study; M.Ge., S.E.K., M.R.D. carried out the experiments; M.Ge., M.Gi. analysed the data; P.M.F., M.S.H. helped with interpretation of the data; M.Ge., M.Gi., M.S.H. drafted the manuscript; P.M.F. revised it critically for important intellectual content. All authors commented on drafts of the manuscript and gave final approval for publication.
Competing interests
We declare no conflicting or competing interests.
Funding
This research was supported by the National Science Foundation grant IOS-1256905 to M.S.H. and by a Marie Curie International Outgoing Fellowship within the 7th European Community Framework Programme, Grant Agreement Number PIOF-GA-2012-327425 to M.Ge. and M.Gi.
References
- 1.Werren JH. 1997. Biology of Wolbachia . Annu. Rev. Entomol. 42, 587–609. ( 10.1146/annurev.ento.42.1.587) [DOI] [PubMed] [Google Scholar]
- 2.Tram U, Ferree PM, Sullivan W. 2003. Identification of Wolbachia–host interacting factors through cytological analysis. Microb. Infect. 5, 999–1011. ( 10.1016/S1286-4579(03)00192-8) [DOI] [PubMed] [Google Scholar]
- 3.Serbus L, Casper-Lindley C, Landmann F, Sullivan W. 2008. The genetics and cell biology of Wolbachia–host interactions. Annu. Rev. Genet. 42, 683–707. ( 10.1146/annurev.genet.41.110306.130354) [DOI] [PubMed] [Google Scholar]
- 4.Beckmann JF, Ronau JA, Hochstrasser M. 2017. A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat. Microbiol. 2, 17007 ( 10.1038/nmicrobiol.2017.7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LePage DP, et al. 2017. Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature 543, 243–247. ( 10.1038/nature21391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan SL, Saul GB. 1968. Post-fertilization effect of incompatibility factors in Mormoniella. Mol. Gen. Genet. 103, 29–36. ( 10.1007/BF00271154) [DOI] [PubMed] [Google Scholar]
- 7.Breeuwer JA, Werren JH. 1990. Microorganisms associated with chromosome destruction and reproductive isolation between two insect species. Nature 346, 558–560. ( 10.1038/346558a0) [DOI] [PubMed] [Google Scholar]
- 8.O'Neill SL, Karr TL. 1990. Bidirectional incompatibility between conspecific populations of Drosophila simulans. Nature 348, 178–180. ( 10.1038/348178a0) [DOI] [PubMed] [Google Scholar]
- 9.Reed KM, Werren JH. 1995. Induction of paternal genome loss by the paternal-sex-ratio chromosome and cytoplasmic incompatibility bacteria (Wolbachia): a comparative study of early embryonic events. Mol. Reprod. Dev. 40, 408–418. ( 10.1002/mrd.1080400404) [DOI] [PubMed] [Google Scholar]
- 10.Lassy CW, Karr TL. 1996. Cytological analysis of fertilization and early embryonic development in incompatible crosses of Drosophila simulans. Mech. Dev. 57, 47–58. ( 10.1016/0925-4773(96)00527-8) [DOI] [PubMed] [Google Scholar]
- 11.Callaini G, Riparbelli MG, Giordano R, Dallai R. 1996. Mitotic defects associated with cytoplasmic incompatibility in Drosophila simulans. J. Invertebr. Pathol. 67, 55–64. ( 10.1006/jipa.1996.0009) [DOI] [Google Scholar]
- 12.Callaini G, Dallai R, Riparbelli MG. 1997. Wolbachia-induced delay of paternal chromatin condensation does not prevent maternal chromosomes from entering anaphase in incompatible crosses of Drosophila simulans. J. Cell Sci. 110, 271–280. [DOI] [PubMed] [Google Scholar]
- 13.Vavre F, Fleury F, Varaldi J, Fouillet P, Boulétreau M. 2000. Evidence for female mortality in Wolbachia-mediated cytoplasmic incompatibility in haplodiploid insects: epidemiologic and evolutionary consequences. Evolution 54, 191–200. [DOI] [PubMed] [Google Scholar]
- 14.Vavre F, Dedeine F, Quillon M, Fouillet P, Fleury F, Boulétreau M. 2001. Within-species diversity of Wolbachia-induced cytoplasmic incompatibility in haplodiploid insects. Evolution 55, 1710–1714. ( 10.1111/j.0014-3820.2001.tb00691.x) [DOI] [PubMed] [Google Scholar]
- 15.Tram U, Fredrick K, Werren JH, Sullivan W. 2006. Paternal chromosome segregation during the first mitotic division determines Wolbachia-induced cytoplasmic incompatibility phenotype. J. Cell Sci. 119, 3655–3663. ( 10.1242/jcs.03095) [DOI] [PubMed] [Google Scholar]
- 16.Russell JA, Funaro CF, Giraldo YM, Goldman-Huertas B, Suh D, Kronauer DJC, Moreau CS, Pierce NE. 2012. A veritable menagerie of heritable bacteria from ants, butterflies, and beyond: broad molecular surveys and a systematic review. PLoS ONE 7, e51027 ( 10.1371/journal.pone.0051027.s014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter MS, Perlman SJ, Kelly SE. 2003. A bacterial symbiont in the Bacteroidetes induces cytoplasmic incompatibility in the parasitoid wasp Encarsia pergandiella. Proc. R. Soc. Lond. B 270, 2185–2190. ( 10.1098/rspb.2003.2475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotoh T, Noda H, Ito S. 2007. Cardinium symbionts cause cytoplasmic incompatibility in spider mites. Heredity 98, 13–20. ( 10.1038/sj.hdy.6800881) [DOI] [PubMed] [Google Scholar]
- 19.Gebiola M, White JA, Cass BN. 2016. Cryptic diversity, reproductive isolation and cytoplasmic incompatibility in a classic biological control success story. Biol. J. Linn. Soc. 117, 217–230. ( 10.1111/bij.12648) [DOI] [Google Scholar]
- 20.Zchori-Fein E, Gottlieb Y, Kelly SE, Brown JK, Wilson JM, Karr TL, Hunter MS. 2001. A newly discovered bacterium associated with parthenogenesis and a change in host selection behavior in parasitoid wasps. Proc. Natl Acad. Sci. USA 98, 12 555–12 560. ( 10.1073/pnas.221467498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zchori-Fein E, Perlman S, Kelly SE, Katzir N, Hunter MS. 2004. Characterization of a ‘Bacteroidetes’ symbiont in Encarsia wasps (Hymenoptera: Aphelinidae): proposal of ‘Candidatus Cardinium hertigii’. Int. J. Syst. Evol. Microbiol. 54, 961–968. ( 10.1099/ijs.0.02957-0) [DOI] [PubMed] [Google Scholar]
- 22.Weeks AR, Marec F, Breeuwer JAJ. 2001. A mite species that consists entirely of haploid females. Science 292, 2479–2482. ( 10.1126/science.1060411) [DOI] [PubMed] [Google Scholar]
- 23.Giorgini M, Monti M, Caprio E, Stouthamer R, Hunter M. 2009. Feminization and the collapse of haplodiploidy in an asexual parasitoid wasp harboring the bacterial symbiont Cardinium. Heredity 102, 365–371. ( 10.1038/hdy.2008.135) [DOI] [PubMed] [Google Scholar]
- 24.Gebiola M, Monti MM, Johnson RC, Woolley JB, Hunter MS, Giorgini M, Pedata PA. 2016. A revision of the Encarsia pergandiella species complex (Hymenoptera: Aphelinidae) shows cryptic diversity in parasitoids of whitefly pests. Syst. Entomol. 42, 31–59. ( 10.1111/syen.12187) [DOI] [Google Scholar]
- 25.Penz T, Schmitz-Esser S, Kelly SE, Cass BN, Müller A, Woyke T, Malfatti SA, Hunter MS, Horn M. 2012. Comparative genomics suggests an independent origin of cytoplasmic incompatibility in Cardinium hertigii. PLoS Genet. 8, e1003012 ( 10.1371/journal.pgen.1003012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ros VID, Breeuwer JAJ. 2009. The effects of, and interactions between, Cardinium and Wolbachia in the doubly infected spider mite Bryobia sarothamni . Heredity 102, 413–422. ( 10.1038/hdy.2009.4) [DOI] [PubMed] [Google Scholar]
- 27.White JA, Kelly SE, Perlman SJ, Hunter MS. 2009. Cytoplasmic incompatibility in the parasitic wasp Encarsia inaron: disentangling the roles of Cardinium and Wolbachia symbionts. Heredity 102, 483–489. ( 10.1038/hdy.2009.5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter MS, Woolley JB. 2001. Evolution and behavioral ecology of heteronomous aphelinid parasitoids. Annu. Rev. Entomol. 46, 251–290. ( 10.1146/annurev.ento.46.1.251) [DOI] [PubMed] [Google Scholar]
- 29.Collier T, Kelly SE, Hunter MS. 2002. Egg size, intrinsic competition, and lethal interference in the parasitoids Encarsia pergandiella and Encarsia formosa. Biol. Control 23, 254–261. ( 10.1006/bcon.2001.1007) [DOI] [Google Scholar]
- 30.Mancini D, Garonna AP, Pedata PA. 2013. A new embryonic pattern in parasitic wasps: divergence in early development may not be associated with lifestyle. Evol. Dev. 15, 418–425. ( 10.1111/ede.12051) [DOI] [PubMed] [Google Scholar]
- 31.Hunter MS, Nur U, Werren JH. 1993. Origin of males by genome loss in an autoparasitoid wasp. Heredity 70, 162–171. ( 10.1038/hdy.1993.25) [DOI] [Google Scholar]
- 32.Gebiola M, Kelly SE, Hammerstein P, Giorgini M, Hunter MS. 2016. ‘Darwin's corollary’ and cytoplasmic incompatibility induced by Cardinium may contribute to speciation in Encarsia wasps (Hymenoptera: Aphelinidae). Evolution 70, 2447–2458. ( 10.1111/evo.13037) [DOI] [PubMed] [Google Scholar]
- 33.Landmann F, Orsi GA, Loppin B, Sullivan W. 2009. Wolbachia-mediated cytoplasmic incompatibility is associated with impaired histone deposition in the male pronucleus. PLoS Pathog. 5, e1000343 ( 10.1371/journal.ppat.1000343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawamura N. 2001. Fertilization and the first cleavage mitosis in insects. Dev. Growth Differ. 43, 343–349. ( 10.1046/j.1440-169x.2001.00584.x) [DOI] [PubMed] [Google Scholar]
- 35.Tram U, Sullivan W. 2002. Role of delayed nuclear envelope breakdown and mitosis in Wolbachia-induced cytoplasmic incompatibility. Science 296, 1124–1126. ( 10.1126/science.1070536) [DOI] [PubMed] [Google Scholar]
- 36.Bordenstein SR, Uy JJ, Werren JH. 2003. Host genotype determines cytoplasmic incompatibility type in the haplodiploid genus Nasonia. Genetics 164, 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferree PM, Barbash DA. 2009. Species-specific heterochromatin prevents mitotic chromosome segregation to cause hybrid lethality in Drosophila. PLoS Biol. 7, e1000234 ( 10.1371/journal.pbio.1000234.s007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferree PM, Gomez K, Rominger P, Howard D, Kornfeld H, Barbash DA. 2014. Heterochromatin position effects on circularized sex chromosomes cause filicidal embryonic lethality in Drosophila melanogaster. Genetics 196, 1001–1005. ( 10.1534/genetics.113.161075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breeuwer J, Werren J. 1993. Cytoplasmic incompatibility and bacterial density in Nasonia vitripennis. Genetics 135, 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data necessary to replicate the experiments have been provided. The best images of cytological events have been selected. However, additional images are available upon request to the corresponding author.