Abstract
Background
Sepsis is a life-threatening complication of infection. The incidence of sepsis is thought to be on the increase, but estimates making use of administrative data in the United States may be affected by administrative bias.
Methods
We studied the population-based incidence of sepsis in the Waikato region of New Zealand from 2007 to 2012 using International Classification of Diseases, Tenth Revision, Australian Modification, which lacks a specific code for sepsis.
Results
Between 2007 and 2012, 1643 patients met coding criteria for sepsis in our hospitals. Sixty-three percent of patients were 65 or over, 17% of cases were admitted to an intensive care unit, and the in-hospital and 1-year mortality with sepsis was 19% and 38%, respectively. Age-standardized rate ratios (ASRRs) demonstrated that sepsis was associated with male sex (ASRR 1.4; 95% confidence interval [CI], 1.23–1.59), Maori ethnicity (ASRR 3.22 compared with non-Maori; 95% CI, 2.85–3.65), study year (ASRR 1.62 comparing 2012 with 2008; 95% CI, 1.18–2.24), and socioeconomic deprivation (ASRR 1.72 comparing the highest with the lowest quintile of socioeconomic deprivation; 95% CI, 1.5–1.97). Multiorgan failure was present in approximately 20% of cases in all age groups. Intensive care unit admission rate fell from 30% amongst 25- to 34-year-olds to less than 10% amongst those aged 75 and over.
Conclusions
In a 9% sample of the New Zealand population, the incidence of sepsis increased by 62% over a 5-year period. Maori, elderly, and disadvantaged populations were most affected.
Keywords: epidemiology, ICD-10, sepsis
Sepsis is a life-threatening illness caused by a dysregulated host response to infection [1]. Definitions have evolved over the last 3 decades, but typical findings in a patient with an infectious illness include evidence of organ dysfunction, tissue hypoperfusion, and circulatory failure [1, 2]. It is generally accepted that the incidence of sepsis is rising, but this assertion is based almost entirely on studies of temporal trends in the United States [3]. These have made use of coding datasets to suggest that this increase may have been as much as 13% annually from 2004 to 2009 [4]. Although the light that these headline figures shines on a major public health problem is clearly welcome, it has been asserted that coding artifact and improved documentation may be driving these observations. Rhee et al [5, 6] showed stable or falling incidence of hospitalization with bacteremia and objective clinical markers of organ failure at 2 US hospitals. This was accompanied by an increase in the incidence of sepsis detected using International Classification of Diseases, Nineth Revision (ICD-9) codes and a fall in the threshold for coding organ failure. Gohil et al [7] showed that the introduction of specific sepsis codes to the ICD and changes to the diagnosis-related group reimbursement system (in 2002 and 2007, respectively) were independently associated with increases in sepsis diagnosis in California’s statewide Mandatory Hospital Discharge Set, an administrative bias described as “up-capture”. The true extent of changes in sepsis incidence are therefore unknown internationally and difficult to assess in the United States. The assertion that sepsis incidence may even be stable rather than increasing could damage high-profile efforts to improve recognition and treatment [8].
New Zealand has a single-payer public heatlhcare system that collects administrative data using the International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Australian Modification (ICD-10-AM). Coding practices in New Zealand may be less prone to the administrative and clinical bias reported in the United States. The ICD-10-AM does not have a coding series for the sepsis syndrome, and data are universally extracted by trained administrators under robust systems of quality control to allow submission to a National Minimum Data Set. This is in contrast to the United States where clinicians are often involved in submitting coded data linked to reimbursement [9]. We conducted an investigation to determine the incidence of sepsis in the Waikato region of New Zealand, to describe the demographic and clinical features of the condition and (given ethnic disparities in sepsis reported elsewhere) to compare incidence specifically between Maori and non-Maori populations.
METHODS
Ethical approval for this study was obtained through the Ministry of Health Northern B ethics committee.
Population and Data Sources
The Waikato District Health Board (DHB) provides comprehensive, publically funded healthcare to a population of 403368 people, representing 9.5% of the New Zealand population at the 2013 national census. A total of 20.7% of the population are Maori. A 600-bed, tertiary-level hospital operates in the regional center, and 4 other regional and community hospitals have emergency and in-patient departments. No acute in-patient care is provided in private hospitals.
We used an administrative database containing demographic and coded data to identify patients admitted to our facilities with sepsis. The Waikato DHB clinical coding service makes use of ICD-10-AM and the Australian Classification of Healthcare Interventions systems (New Zealand Ministry of Health). Coding is regularly audited and is in adherence with the Australian Coding Standards and New Zealand Conventions. Coded data are submitted to the New Zealand National Minimum Dataset (NMDS), which has been used extensively for population health research [10]. Population denominators and patient survival were determined using census, and mortality data were distributed by Statistics New Zealand and the New Zealand Health Information Office, respectively.
Case Definition and Data Collection
Patients admitted with sepsis admitted between the July 1, 2007 and the June 30, 2012 were identified. A case of sepsis was defined as a primary diagnosis of infection accompanied by 1 or more secondary codes indicating organ failure. Primary and secondary ICD-10-AM codes were based on the ICD-9 algorithm published by Angus et al [11], translated to ICD-10-AM by Sundararajan et al [12]. Excluded from study were elective admissions and non-overnight admissions that did not end in death. For estimates of sepsis incidence, we excluded patients not domiciled in the region, and, because the outcomes of sepsis are time and treatment dependent, we excluded sepsis cases from analysis if the patient had been admitted and discharged with the same primary diagnosis in the preceding 30 days. For all cases, we recorded age, sex, date of death (up to July 2013), domicile, ethnicity, New Zealand residency status, admitting hospital, length of hospital stay, and need for intensive care unit (ICU) admission. The Charlson comorbidity index was calculated using additional diagnosis codes according to the method of Quan et al [13]. Multiorgan failure was defined by the presence of >1 organ failure code.
Ethnicity and Socioeconomic Status
The NMDS allows for the recording of multiple ethnicities per patient. We classified ethnicity using a total response system for “European/Other”, “Maori”, “Pacific”, and “Asian”, whereby patients recording multiple ethnicities were counted in each of those groups [10]. A further comparison (previously used by Baker et al [10]) was made between patients identifying as Maori and those belonging exclusively to the “European/Other” group, which was non-Maori, non-Pacific, and non-Asian. Patient domiciles were assigned to a New Zealand Index for Socioeconomic Deprivation (NZDep). This is a method for determining socioeconomic status that has been extensively used in health outcomes research and is validated elsewhere [14]. Scores are assigned based on national deciles of socioeconomic status with 1 indicating least deprived and 10 most deprived.
Statistical Analysis
Data were analyzed using STATA 13 (StatCorp, College Station, TX) and Microsoft Excel (Microsoft Corporation, Seattle, WA) supported by an add-in provided by Public Health England (http://www.wmpho.org.uk/tools/). Crude annual incidence of sepsis was calculated using Waikato regional mid-year population estimates provided by Statistics New Zealand. Age-standardized rate ratios and confidence intervals (CIs) were calculated to allow comparisons based on age and ethnic group. Five-year age, gender, and ethnicity-specific incidence was calculated using 2013 Waikato census data. Annualized and age-standardized incidence rates were calculated using Waikato mid-year population estimates. In each case, the 2013 New Zealand census population was taken as the direct standard.
RESULTS
Clinical Characteristics of Infection and Sepsis
Over the 5-year study period, there were 209 730 acute overnight admissions to Waikato DHB facilities. Of these 8% (16624) were assigned a primary diagnosis of infection. Our definition of sepsis was met for 1701 (10.2%) of these cases. After excluding nonresidents and 30-day readmissions, we identified 1643 individual sepsis episodes, the general characteristics of which are shown in Table 1. The median length of stay (LOS) for sepsis was 6 days (range 0–203; interquartile range [IQR], 3–11) with in-hospital and 1-year mortality of 18.7% (308 of 1643) and 37.7% (620 of 1643), respectively. A total of 16.9% (278 of 1643) of sepsis episodes led to an ICU admission with a median ICU LOS of 1.75 days (range 0.25–49.75 days; IQR, 0.75–4). The respective in-hospital and 1-year mortality of sepsis for patients admitted to ICU was 33.8% (94 of 278) and 42.4% (118 of 278).
Table 1.
Basic Descriptive Characteristics for Cases Admitted With Severe Sepsis: Number, Proportions, and Specific Rates (per 100000 Population), July 2007 to June 2012, Waikato DHB Resident Population (Total = 1643)
| Variable | N | % | Specific Rate/100000 | 95% CI |
|---|---|---|---|---|
| Female | 779 | 47.4 | 450.5 | 419.4–483.3 |
| Male | 864 | 52.6 | 519.8 | 466.9–534.1 |
| Ethnicity | ||||
| European | 1161 | 70.7 | 467.8 | 441.3–495.5 |
| Maori | 418 | 25.4 | 617.9 | 560.1–680.1 |
| Pacific | 36 | 2.2 | 489.3 | 342.7–677.3 |
| Asian | 28 | 1.7 | 175.4 | 116.6–253.5 |
| Non-Maori | 1255 | 74.6 | 430.0 | 406.3–454.8 |
| Maori | 418 | 25.4 | 571.7 | 518.2–629.3 |
| Age | ||||
| 0–14 | 32 | 3.2 | 50.2 | 37.5–65.8 |
| 15–24 | 36 | 2.2 | 84.5 | 59.2–117.0 |
| 25–64 | 137 | 31.7 | 346.1 | 317.0–377.1 |
| 65+ | 384 | 62.9 | 2437.9 | 2291.5–2591.1 |
| Median age (years) (IQR) | 38 (13–67), Range 0–102 | |||
| Mortality | ||||
| In-hospital mortality | 308 | 18.7 | ||
| One-year mortality | 620 | 37.7 | ||
| ICU | ||||
| Admit to ICU | 279 | 17.0 | ||
| Median days in ICU (IQR) | 1.75 (0.75–4), Range 0.25–49.75 | |||
| NZ Dep Quintiles | ||||
| 1–2 | 152 | 9.3 | 329.4 | 279.1–386.2 |
| 3–4 | 102 | 6.2 | 322.9 | 262.7–392.7 |
| 5–6 | 295 | 18.0 | 366.5 | 325.8–410.8 |
| 7–8 | 494 | 30.2 | 465.5 | 425.4–508.5 |
| 9–10 | 595 | 36.3 | 631.3 | 581.6–684.2 |
| Year (July to June) | ||||
| 2007/2008 | 66.9 | 58.6–75.9 | ||
| 2008/2009 | 74.0 | 65.4–83.4 | ||
| 2009/2010 | 86.7 | 77.4–96.8 | ||
| 2010/2011 | 104.0 | 93.8–114.9 | ||
| 2011/2012 | 115.3 | 104.6–126.7 | ||
Abbreviations: CI, confidence interval; Dep, deprivation; DHB, District Health Board; ICU, intensive care unit; IQR, interquartile range; NZ, New Zealand.
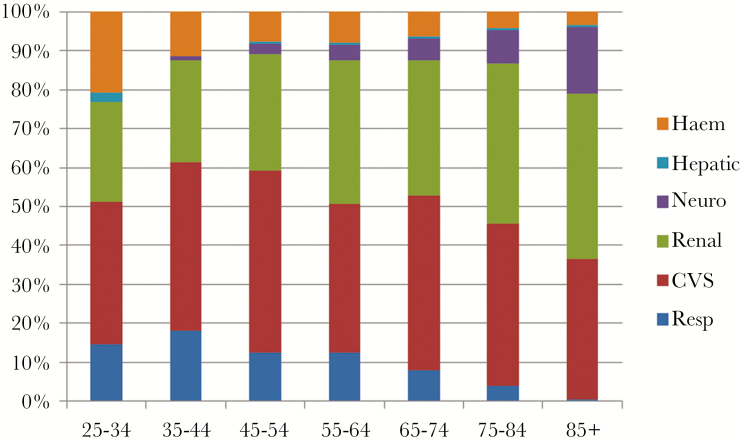
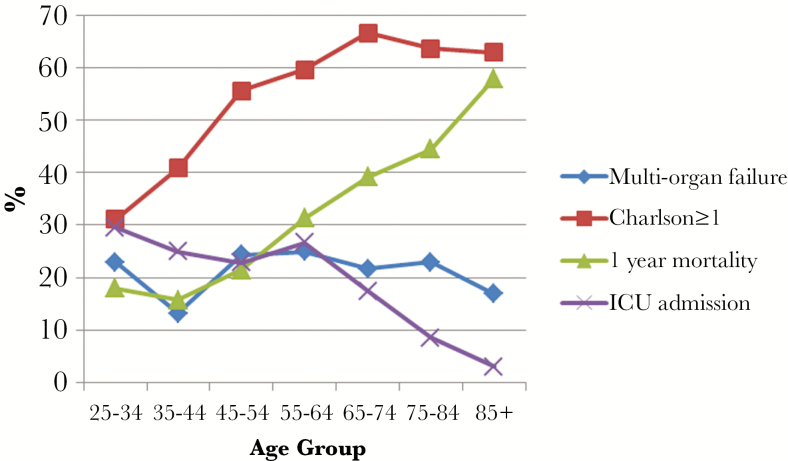
Infections of the bloodstream, heart, lower respiratory tract, and nervous system were classified as severe in >10% of cases so identified. The total organ failures taken to indicate sepsis in each age group are shown in Figure 1. With increasing age, the proportion of sepsis cases identified by respiratory and hematologic dysfunction fell whereas rates of neurologic, cardiovascular, and renal dysfunction increased. Proportionate multiorgan failure, comorbidity, ICU admission, and 1-year mortality are shown by age group in Figure 2. The proportion of patients admitted to ICU fell steadily with increasing age, from 30% of 25- to 34-year-olds to 3% amongst those 85 or older, despite the proportion of patients with multiorgan failure remaining constant.
Figure 1.
Organ failures identifying sepsis by age group. Data are shown as proportions of all recorded organ failures in all episodes (n = 1643). Abbreviations: CVS, cardiovascular system; Haem, hematologic; Neuro, neurologic; Resp, respiratory.
Figure 2.
Proportions by age-group; multiorgan failure, comorbidity (Charlson score ≥1) 1-year mortality, and intensive care unit (ICU) admission.
Epidemiology of Sepsis
Sepsis was a disease of older patients, with approximately two thirds (63%) aged 65 or over (Table 1). Table 2 shows 5-year, age-standardized incidence and age-standardized rate ratios of sepsis by age, gender, and ethnicity. From 2007 to 2012, the age-standardized incidence of sepsis rose from 65.5 to 106.7 per 100000 population (rate ratio 1.62; 95% CI, 1.18–2.24) with two thirds (66.6%) of patients whose NZDep score could be assigned residing in the 2 highest quintiles of deprivation. Corresponding to this, the incidence of sepsis was highest in the most deprived NZDep quintile (age-standardized rate ratio 1.72 [95% CI, 1.5–1.97] compared with the least deprived quintile). Compared with non-Maori, Maori were more than 3 times as likely to suffer an episode of sepsis (age-standardized rate ratio 3.22; 95% CI, 2.85–3.65). This corresponded to an increased risk of in-hospital death with sepsis amongst Maori in all age groups. By age 0–19, 20–59, and ≥60, the annual rates (per 100000 population) of in-hospital death with sepsis were 0.77, 4.23, and 91 in the general population and 1.87, 12.58, and 188 amongst Maori.
Table 2.
Five-Year Age Standardized Rates and Rate Ratios
| Variable | ASR | 95% CI | ASRR | 95% CI |
|---|---|---|---|---|
| Gender | ||||
| Female | 419.9 | 390.5–449.2 | 1.00 | — |
| Male | 586.2 | 547.3–625.2 | 1.40 | 1.23–1.59 |
| NZ Dep Quintiles | ||||
| 1 | 341.5 | 286.9–396.1 | 1.00 | — |
| 2 | 364.5 | 292.8–436.1 | 1.07 | 0.92–1.24 |
| 3 | 364.2 | 322.8–405.6 | 1.07 | 0.92–1.24 |
| 4 | 376.5 | 342.7–410.3 | 1.11 | 0.95–1.28 |
| 5 | 578.8 | 532.2–625.4 | 1.72 | 1.50–1.97 |
| Ethnicity | ||||
| Non-Maori | 341.5 | 322.3–361.5 | 1.00 | — |
| Maori | 1100.5 | 966.8–1244.2 | 3.22 | 2.85–3.65 |
| Study Year | ||||
| 2007–08 | 65.5 | 57.5–74.4 | 1.00 | — |
| 2008–09 | 71.8 | 63.4–80.9 | 1.09 | 0.77–1.55 |
| 2009–10 | 83.0 | 74.1–92.6 | 1.26 | 0.90–1.76 |
| 2010–11 | 98.3 | 88.7–108.7 | 1.48 | 1.08–2.06 |
| 2011–12 | 106.7 | 96.8–117.3 | 1.62 | 1.18–2.24 |
Abbreviations: ASR, age-standardized rate; ASRR, ASR ratio; CI, confidence interval; Dep, deprivation; NZ, New Zealand.
DISCUSSION
To our knowledge, this is the only non-US study in the last decade that reports multiyear, population-based incidence of sepsis [3]. Our findings parallel those of others from resource-rich countries by showing a significant increase in the burden of sepsis borne disproportionately by aged, ethnic minority, and socioeconomically deprived populations.
New Zealand is an ideal country in which to study the epidemiology of infection. All acute in-patient care is provided by networks of publically funded hospitals; all hospital discharges are coded using robust systems of quality control; detailed census-based population data are available; and extensive research has validated the local use of ICD-10-AM and the NZDep score in relation to the population burden of disease and a range of clinical and nonclinical outcomes [10, 14, 15]. The clinical coding rules in use during this study did not include specific codes for sepsis, and we are not aware of any changes in the prevailing mechanism of healthcare funding that might have changed coding practices specifically to optimize hospital reimbursement. Against this background, the age-standardized incidence of sepsis rose by 63% over the 5-year period studied.
The validity of administrative methods for recording sepsis has now been extensively studied, and efforts are ongoing to optimize available schema [16, 17]. Although sensitivity varies dramatically depending on method, reported specificities ranges from 78% to 100% compared with objective clinical data [16]. We chose a translation of the ICD-9-Clinical Modification algorithm published by Angus et al [11] in 2001 and since used extensively to produce national and international comparisons of sepsis incidence. The original Angus et al [11] criteria were validated against a prospective sample of in-patient admissions meeting clinical criteria for sepsis [11. Wang et al [18] recently reported the sensitivity and specificity of the Angus et al [11] method to be 43% and 86%, respectively, in a contemporary cohort of patients with community-acquired sepsis in the United States. Although the sensitivity of the ICD-10-AM translation that we used is unknown, its specificity is likely to be high, and reports of poor sensitivity imply that we have significantly underreported on true cases of disease.
It has been suggested that the increase in incidence and falling mortality of sepsis seen in some studies can be explained by administrative “up-capture” and falling thresholds for defining organ failure [6]. We are confident that we have reported on a population of critically ill patients. The observed in-hospital and 1-year mortality for sepsis was 19% and 38%, respectively. In a system that carefully rations bed-days and ICU resources, the median length of hospital stay was 6 days, and 17% of our sepsis cases required ICU admission [19]. The in-hospital mortality of the cohort as a whole (19%) was the same as that reported for 2012 in a large prospective study of sepsis in Australian and New Zealand ICUs [20]. Of the 17% of patients admitted to ICU, the in-hospital mortality was 34%. All patients in our cohort had an infectious disease as a primary diagnosis. This strategy would have excluded cases in which infection was coded as a complicating rather than primary illness. Our inclusion criteria were designed to detect community-onset sepsis cases. Nosocomial sepsis accounts for approximately 10% of total cases and carries a higher mortality than community-onset disease [21]. Therefore, we are likely to have underreported nosocomial cases of sepsis and consequently the true sepsis-associated mortality in our population.
Sepsis as the primary cause of ICU admission in Australia and New Zealand rose from 7.2% to 11.1% between 1997 and 2012 [20]. By reporting population-based data, we provide support for the argument that this reflects an increase in sepsis in the populations that these units serve, something that was by no means clear based on ICU observations alone. The decision to admit to an ICU depends on such parameters as bed availability, the need for intubation, local precedent, and the skill mix of non-ICU wards. Varying proportions of sepsis cases are managed outside the ICU; therefore, the ICU incidence of sepsis represents the treated rather than the true incidence of disease [22]. The sequelae of sepsis exert major burdens of physical and cognitive impairment on survivors for years after the event, independently of ICU admission [23]. That 83% of the episodes we report in this study were managed in general wards suggests that in New Zealand, sepsis is a common condition that is not primarily managed in the ICU, an important consideration when implementing interventions to improve sepsis outcomes.
It is relevant to consider why the incidence of sepsis would be on the increase while disproportionately affecting Maori and those of lower socioeconomic status. Life-course studies provide strong evidence that experience of ill health, risk behaviors, and social stress contribute to morbid events later in life [24, 25]. Chronic medical conditions, lower education and income, alcohol and tobacco use, elevated baseline high-sensitivity C-reactive protein, household overcrowding, and obesity are all linked to incident infectious disease [26–29], whereas lower socioeconomic status and (in the United States) lack of insurance contribute to mortality [30, 31]. Baker et al [10] used the need for hospital admission to define “serious” infection in a landmark study, making use of the New Zealand National Minimum Data Set. Between 2 time periods, 1989–1993 and 2004–2008, there was a 51.3% relative increase in admission for infection-related illness against a 16.3% relative increase in total hospital admissions. In keeping with our findings, the rate of hospitalization for infection was more than double (1) amongst Maori compared with non-Maori and (2) amongst the most socioeconomically deprived compared with the least. It is intriguing that hospital admission for infection has been rising in New Zealand since the early 1990s, a time of widening socioeconomic disparity and growing obesity, for example, amongst Maori and non-Maori alike [10, 32]. Exposures to risks of environment (ie, household overcrowding, social dislocation and socioeconomic deprivation), host (ie, smoking, diabetes, obesity, chronic disease, malnutrition), and organism (ie, colonization with Staphylococcus aureus, Streptococcus pneumoniae and other pathogens) are concentrated amongst deprived and marginalized populations and, we postulate, are likely to account for higher rates of sepsis. It would follow that the individual, societal, and financial burdens of sepsis will not be attenuated until entrenched health and socioeconomic disparities are addressed.
CONCLUSIONS
We acknowledge that our study has several limitations. An apparent increase in sepsis incidence could still be explained by a trend toward more complete capture of organ failure by clinical coding staff. However, we have pointed to the quality-control efforts in place to prevent this, and we have referenced published studies that would have been vulnerable to the same bias if present. We were unable to validate the coding definition of sepsis against a prospective sample of patients meeting confirmatory clinical criteria. This may have been valuable, for example, to explain the low proportion of cases amongst children less than 1 year old. In the report by Sundararajan et al [12], 8% of patients admitted with sepsis to hospitals in the state of Victoria from 1999 to 2003 were less than 1 year of age. In our study, using the same coding algorithm, only 0.7% of cases were in this age group. It is possible that natural or epidemic variation in the incidence of important infectious illnesses contributed to the reported incidence of sepsis. Finally, the findings in one region of New Zealand may not extrapolate to all others or indeed to any other jurisdiction. Therefore, ongoing research is needed to describe the incidence of sepsis at a national and international level. Existing methods seem to be sufficient to document significant disparities in sepsis incidence based on age, socioeconomic status, and ethnicity. Given the paucity of data describing sepsis morbidity and mortality, their use should be prioritized to generate accurate comparisons between different healthcare settings and populations [33].
Acknowledgments
We acknowledge the support of colleagues at Waikato District Health Board.
Financial support. This work was supported by a summer studentship awarded by the Waikato Clinical School, Faculty of Medicine and Health Sciences, University of Auckland.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003; 29:530–8. [DOI] [PubMed] [Google Scholar]
- 3. Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016; 193:259–72. [DOI] [PubMed] [Google Scholar]
- 4. Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013; 41:1167–74. [DOI] [PubMed] [Google Scholar]
- 5. Rhee C, Murphy MV, Li L, et al. Comparison of trends in sepsis incidence and coding using administrative claims versus objective clinical data. Clin Infect Dis 2015; 60:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rhee C, Murphy MV, Li L, et al. Improving documentation and coding for acute organ dysfunction biases estimates of changing sepsis severity and burden: a retrospective study. Crit Care 2015; 19:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gohil SK, Cao C, Phelan M, et al. Impact of policies on the rise in sepsis incidence, 2000–2010. Clin Infect Dis 2016; 62:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rhee C, Gohil S, Klompas M. Regulatory mandates for sepsis care–reasons for caution. N Engl J Med 2014; 370:1673–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beck DE, Margolin DA. Physician coding and reimbursement. Ochsner J 2007; 7:8–15. [PMC free article] [PubMed] [Google Scholar]
- 10. Baker MG, Barnard LT, Kvalsvig A, et al. Increasing incidence of serious infectious diseases and inequalities in New Zealand: a national epidemiological study. Lancet 2012; 379:1112–9. [DOI] [PubMed] [Google Scholar]
- 11. Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29:1303–10. [DOI] [PubMed] [Google Scholar]
- 12. Sundararajan V, Macisaac CM, Presneill JJ, et al. Epidemiology of sepsis in Victoria, Australia. Crit Care Med 2005; 33:71–80. [DOI] [PubMed] [Google Scholar]
- 13. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 14. Salmond C, Crampton P, Atkinson J.NZDep2006 Index of Deprivation 2007; Wellington: University of Otago, 5541:1–61. [Google Scholar]
- 15. Pearson AL, Apparicio P, Riva M. Cumulative disadvantage? Exploring relationships between neighbourhood deprivation trends (1991 to 2006) and mortality in New Zealand. Int J Health Geogr 2013; 12:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jolley RJ, Sawka KJ, Yergens DW, et al. Validity of administrative data in recording sepsis: a systematic review. Crit Care 2015; 19:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jolley RJ, Quan H, Jetté N, et al. Validation and optimisation of an ICD-10-coded case definition for sepsis using administrative health data. BMJ Open 2015; 5:e009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang HE, Addis DR, Donnelly JP, et al. Discharge diagnoses versus medical record review in the identification of community-acquired sepsis. Crit Care 2015; 19:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Streat: Cost containment: the Pacific. New Zealand. Available at: https://scholar.google.com/scholar_lookup?title=Cost+containment%3A+the+Pacific.+New+Zealand&author=S.+Streat&author=J.+A.+Judson&publication_year=1994. Accessed 6 November 2016.
- 20. Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically Ill patients in Australia and New Zealand, 2000–2012. JAMA 2014; 311:1308–16. [DOI] [PubMed] [Google Scholar]
- 21. Tsertsvadze A, Royle P, Seedat F, et al. Community-onset sepsis and its public health burden: a systematic review. Syst Rev 2016; 5:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Linde-Zwirble WT, Angus DC. Severe sepsis epidemiology: sampling, selection, and society. Crit Care 2004; 8:222–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc 2012; 60:1070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poulton R, Caspi A, Milne BJ, et al. Association between children’s experience of socioeconomic disadvantage and adult health: a life-course study. Lancet 2002; 360:1640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Danese A. Adverse childhood experiences and adult risk factors for age-related disease. Arch Pediatr Adolesc Med 2009; 163:1135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang HE, Shapiro NI, Griffin R, et al. Chronic medical conditions and risk of sepsis. PLoS One 2012; 7:e48307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang HE, Shapiro NI, Safford MM, et al. High-sensitivity C-reactive protein and risk of sepsis. PLoS One 2013; 8:e69232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang J, Howden-Chapman P.Infectious Diseases Attributable to Household Crowding in New Zealand: A Systematic Review and Burden of Disease Estimate. 2013. Wellington: University of Otago. Available at: http://www.healthyhousing.org.nz/wp-content/uploads/2010/01/HH-Crowding-ID-Burden-25-May-2013.pdf. Accessed 12 July 2017. [Google Scholar]
- 29. Wang HE, Griffin R, Judd S, et al. Obesity and risk of sepsis: a population-based cohort study. Obesity 2013; doi:10.1002/oby.20468. [DOI] [PMC free article] [PubMed]
- 30. Kumar G, Taneja A, Majumdar T, et al. The association of lacking insurance with outcomes of severe sepsis: retrospective analysis of an administrative database. Crit Care Med 2014; 42:583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koch K, Nørgaard M, Schønheyder HC, et al. Effect of socioeconomic status on mortality after bacteremia in working-age patients. A Danish population-based cohort study. PLoS One 2013; 8:e70082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384:766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016; 193:259–72. [DOI] [PubMed] [Google Scholar]
- 34. Sands KE, Bates DW, Lanken PN, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA 1997; 278:234–40. [PubMed] [Google Scholar]