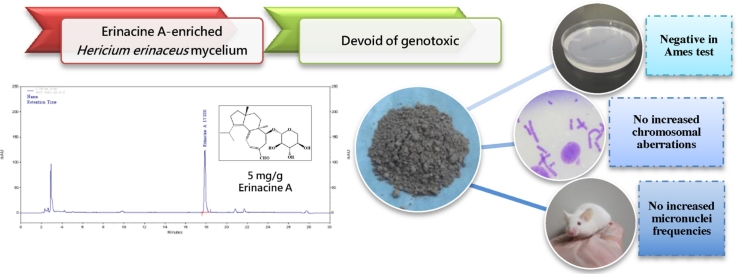
Graphical abstract
Keywords: Erinacine A, Hericium erinaceus, Genotoxicity, Safety assessment
Chemical compounds studied in this article: Dimethyl sulfoxide (PubChem CID: 679), 4-Nitroquinoline N-oxide (PubChem CID: 5955), Sodium azide (PubChem CID: 33557), Mitomycin C (PubChem CID: 5746), 9-Aminoacridine (PubChem CID: 7019), 2-Aminoanthracene (PubChem CID: 11937), benzo[a]pyrene (PubChem CID: 2336), 2-Aminofluorene (PubChem CID: 1539), Thiazolyl blue tetrazolium bromide (PubChem CID: 64965), Demecolcine (PubChem CID: 220401), Potassium chloride (PubChem CID: 4873), Methanol (PubChem CID: 887), Glacial acetic acid (PubChem CID: 176), Cyclophosphamide (PubChem CID: 2907)
Highlights
-
•
EAHE mycelium was standardized to have 5 mg/g erinacine A and was characterized by NMR spectroscopy, LC-MSMS, and HPLC.
-
•
No adverse or test article-related differences were observed during the study.
-
•
EAHE mycelium is non-genotoxic in a three standard battery of tests.
Abstract
Hericium erinaceus (H. erinaceus) has a long history of usage in traditional Chinese medicine for the treatment of gastric disorders. Recently, it has become a well-established candidate in causing positive brain and nerve health-related activities by inducing nerve growth factor (NGF) from its bioactive ingredient, erinacine A. This active compound, which exists only in fermented mycelium but not in its fruiting body, increases NGF levels in astroglial cells in vitro as well as catecholamine and NGF levels in vivo. With increasing recognition of erinacine A in H. erinaceus (EAHE) mycelium improving neurodegenerative diseases, numerous products are being marketed based on these functional claims. To our knowledge, there have been no reports on the mutagenicity of EAHE prior to this paper. Hence, the present study was undertaken to determine the mutagenicity and genotoxicity effects of EAHE mycelium conducted in three standard battery of tests (reverse mutation, chromosomal aberration, and micronuclei tests) according to the latest guidelines in order to meet all international regulatory requirements and provide information on the safety of this new and promising natural remedy. Our results have indicated that EAHE mycelium did not significantly increase the number of revertant colonies in the bacterial reverse mutation test nor induce higher frequency of aberrations in the chromosome aberration test. Moreover, no statistically significant EAHE mycelium-related increase was observed in the incidence of reticulocytes per 1000 red blood cells and micronucleated reticulocytes per 1000 reticulocytes. In conclusion, the three standard battery of tests suggested that EAHE mycelium was devoid of mutagenicity and genotoxicity in the tested doses and experimental conditions.
1. Introduction
Senile dementia, such as Alzheimer's disease, is a serious global public health crisis as there is no effective therapy for it currently. Neurohealth, is thus a major concern of the predicted Silver Tsunami – the growing wave of people who will reach the age of 65 over the next two decades and may be affected by geriatric cognitive disorders – which will greatly impact society in the next 40 years as the number of dependent older people is estimated to increase three-fold, from 101 million in 2010 to 277 million in 2050 [1]. It has been shown that dysregulation of nerve growth factor (NGF) signaling is linked to early stages of neurological diseases [2], [3]. The absence of NGF has shown to cause an Alzheimer-like symptom in the brains of 15 to 17 months old anti-NGF transgenic mice [4], but such symptoms could be ameliorated by the intranasal administration of NGF in transgenic anti-NGF mice (AD11 mice) that have a progressive neurodegenerative phenotype resembling Alzheimer's disease [5]. Although there is a widespread interest in NGF as a potential therapeutic agent, the high molecular weight of NGF makes it unable to cross the blood–brain barrier. Alternatively, there are new approaches being developed which focus on low-molecular weight compounds that can cross the brain–blood and promote NGF biosynthesis [6].
Hericium erinaceus (H. erinaceus), a culinary and medicinal mushroom, has been extensively studied for its neurohealth properties. It has a long history of usage in traditional Chinese medicine for the treatment of digestive diseases [7], but recently, it has also been reported to exhibit other various pharmacological activities, including anti-cancer, anti-microbial, anti-diabetic, anti-hypertensive, anti-fat deposit, antioxidant, gastro-protective, neuro-protective, immuno-modulating, and wound-healing properties [8], [9], [10], [11], [12], [13], [14], [15], [16], [17]. Among these health-beneficial roles, its role in protecting and stimulating nerve cells, however, is the most sought after characteristic out of all the other edible mushrooms with medicinal value. Extracts of H. erinaceus, such as hericenones C–H [18], [19] and erinacines A–I [20], [21], [22], [23], have all shown to induce the expression of NGF in cultured rodent astrocytes. Erinacine A, the main representative of the compounds in H. erinaceus extracts, has demonstrated epinephrine-stronger NGF-inducing activities in vitro and in vivo [24]. Furthermore, such NGF stimulating effects have been augmented by the increased availability of the active compound (up to 8 mg/kg body weight) [24], and it has also been achieved via oral administration [25]. Hence, there is potential in developing H. erinaceus enriched with erinacine A (EAHE) as an ingredient in medicinal foods or products to help in the reduction or even the prevention of age-related neurodegenerative diseases.
To our knowledge, there have been no reports on the mutagenicity of H. erinaceus prior to this paper. Furthermore, mushroom mycelium has an identity distinct from mushrooms, which are categorized into two specific classes of compounds: hericenones and erinacines, where they can only be extracted from the fruit body and the cultured mycelium, respectively. Our previous 28-day sub-chronic toxicity test in Sprague-Dawley rats showed no evidence of systemic toxicity attributable to EAHE administration [26]. It was estimated that NOAEL (no adverse effect level) of EAHE mycelium is greater than 3 g/kg of body weight/day, which is 171.4 times the recommended daily intake for humans (1.05 g/60 kg of body weight/day). Additional research on EAHE, including an assessment of its mutagenic and carcinogenic potential, however, should be included to further support the safety of its consumption. Evaluation of the genotoxic properties is important in the context of European Union and international legislations aiming to protect human health. For an adequate assessment of the genotoxic potential, three endpoints need to be considered: gene mutations, structural chromosome aberrations, and numerical chromosome aberrations. Hence, the present study was undertaken to determine the mutagenicity and genotoxicity effects of EAHE mycelium conducted in three standard battery of tests (reverse mutation, chromosomal aberration, and micronuclei tests) according to the latest guidelines in order to meet all international regulatory requirements and provide information on the safety of this new and promising natural remedy.
2. Materials and methods
2.1. Preparation, identification, and quantification of EAHE
H. erinaceus (BCRC 35669) purchased from Bioresources Collection and Research Center (BCRC) in Food Industry Research and Development Institute (Hsinchu, Taiwan) were maintained and cultivated according to the method described earlier [27]. The proximate composition analysis, which includes ash, total proteins, lipids, and carbohydrates of the freeze-dried mycelia, was determined according to official Association of Official Analytical Chemists (AOAC) methods [28]. The 5 mg/g active erinacine A content in the mycelia was quantified and qualified via high performance liquid chromatography (HPLC) according to our previous published protocol [27]. In brief, a COSMOSIL 5C18-AR-II (250 × 4.6 mm; particle size 5 μm; Nacalai USA, Inc.) column with a column temperature of 40 °C was employed, and a solvent system consisting of methanol (A) and 2.0% acetic acid in water (B) was used for HPLC elution. Retention time of erinacine A detected by UV at 340 nm was approximately ∼17 min at a flow rate of 1.0 mL/min.
2.2. Animals and husbandry
7-week old male ICR mice used for the in vivo erythrocyte micronucleus test were procured from BioLASCO Taiwan Co., Ltd and were utilized after a week of acclimatization and quarantine. ICR mice were housed in groups of five in polypropylene cages with absorbent hardwood bedding (Beta Chip; Northeastern Products Corp, USA). The animals were maintained in a well-ventilated room (10–15 air changes/h) under an ambient temperature of 22 ± 3 °C and 55 ± 15% relative humidity on a 12:12 h light regime, and they were provided with standard rodent diet (MF-18; Oriental Yeast Co., Ltd, Tokyo, Japan) as well as purified water ad libitum. All husbandry conditions were conformed to the guidelines set forth by the National Institutes of Health (NIH) for the care and use of laboratory animals, and were carried out under Good Laboratory Practice (21 CFR Part 58). These protocols were approved by the Institutional Animal Care and Use Committee (IACUC 101-11e).
2.3. Bacterial reverse mutation test (Ames test)
This test was performed in accordance to the Organization for Economic Cooperation and Development (OECD) Guideline for the testing of chemicals no. 471 [29] with the bacterial reverse mutation test. EAHE at doses of 0.3125, 0.625, 1.25, 2.5, and 5 mg/plate were tested for gene mutation using Salmonella typhimurium strains TA98, TA100, TA102, TA1535, and TA1537 (MolTox Inc., USA), both in the presence (0.5 ml S9 mix) and absence (0.5 ml of 0.2 M phosphate buffer, pH 7.4) of metabolic activation. The S9 mix of aroclor 1254-induced rat liver homogenate was freshly prepared before each test as described by the previous study [30]. Dimethyl sulfoxide was used as the diluent for EAHE and as the negative control. Positive controls used were 4-nitroquinoline-N-oxide, sodium azide, mitomycin C, 9-aminoacridine, 2-aminoanthracene, benzo[a]pyrene, and 2-aminofluorene (Sigma-Aldrich, MO, USA). In brief, 100 μl of bacterial culture was exposed to different doses of EAHE in both the presence and absence of metabolic activation before adding it to 2 ml of molten top agar containing 0.5 mM of histidine/biotin. The mixture was subsequently poured on the surface of minimal glucose agar plates which were then incubated at 37 °C for 48 h prior to revertant colonies counting. All testing groups were set up in triplicates. A positive result was determined by the dose dependent increase and the two-fold increase in revertant numbers over the negative control.
2.4. In vitro chromosome aberration test
This test was conducted in Chinese Hamster Ovary (CHO-K1) cells according to the OECD Guideline for the testing of chemicals no. 473 [31] with the in vitro mammalian chromosome aberration test. As the amount of precipitation recorded for EAHE was at 5 mg/ml, dose levels of 2.5, 1.25, and 0.625 mg/ml were selected and exposed to the CHO-K1 cells (BCRC 60006) in the presence and absence of a metabolic activation system derived from rat liver S9 mix [30]. The cells were maintained in Ham's/F-12 medium at 5% CO2 and 37 °C. Two independent experiments were performed in duplicate. In the temporary treatment, CHO-K1 cells were exposed to EAHE for 3 h followed by a recovery period of 17 h, with and without metabolic activation. In the continuous treatment, cells were incubated for 20 h in the absence of metabolic activation. At the end of the treatment, parallel experiments were conducted where cells were either determined by MTT assay for cell growth inhibition or prepared for chromosome observation. In brief, cells were treated with 0.1 μg/ml demecolcine solution (Sigma-Aldrich, MO, USA) for 4 h prior to harvesting. Cell pellets of each treatment group were resuspended in 0.075 M KCl solution and were fixed using methanol/glacial acetic acid at a ratio of 3:1 v/v. After fixation, cells were applied to a glass slide, stained with Diff Quik (Sysmex Corporation, Kobe, Japan), mounted with Neo-Mount Anhydrous Mounting Medium, and then microscopically evaluated (at least 100 well-spread metaphases/dish). 80 μg/ml of cyclophosphamide (Sigma-Aldrich, MO, USA) with metabolic activation and 6 μg/ml of mytomycin C without metabolic activation were used as positive controls. EAHE is considered to damage chromosomes in CHO-K1 cells when the frequency of aberrant cells is >3% with a dose dependent increase. Abnormal cells were determined by the observation of chromosome gap (G), chromosome break (B), chromosome dicentric (D), chromosome ring (R), chromatid gap (g), chromatid break (b), and chromatid exchange (e).
2.5. In vivo erythrocyte micronucleus test
This test was carried out using the OECD Guideline for the testing of chemicals #474 [32] with the mammalian erythrocyte micronucleus test. EAHE at dose levels of 1.25, 2.5, and 5 g/kg BW (20 ml/kg by gavage) were evaluated for its potential to induce micronuclei in the peripheral blood lymphocytes of male ICR mice. The doses were selected according to the results of the single-dose acute study and were given once for the study. Each experimental group (low, mid, and high dose) contained five male mice. These mice received the vehicle (distilled water) by gavage and were administrated with 80 μg/ml cyclophosphamide (10 ml/kg) by intraperitoneal injection as negative and positive controls, respectively. All experimental animals were observed once a day for signs of toxicity, mortality, and morbidity until the completion of the treatment. The body weight of each animal was recorded at the initiation of the treatment and prior to bone marrow sampling. Peripheral blood samples (3 to 4 μl) from tail vein were collected at 48 h and 72 h after dosing and then applied to acridine orange-coated slides for 3 to 4 h at room temperature. The smear samples were microscopically examined with a fluorescent microscope (Zeiss, Oberkochen, Germany) for the number of reticulocytes (orange-red signal), and reticulocytes that contained at least one positive micronucleus (yellow-green signal). The results were expressed as the frequency of reticulocytes per 1000 red blood cells and micronucleated reticulocytes per 1000 reticulocytes.
2.6. Statistical analysis
All values presented throughout this manuscript were expressed as mean ± standard error of mean (SEM). Mean differences between the control and the treatment groups were analyzed by one-way ANOVA followed by Duncan's test. Aberrant cells from each concentration were compared to the negative control values using Chi-square analyses. A value of p < 0.05 was considered to be statistically significant.
3. Results and discussion
A resurgence of interest in natural products is spreading across many parts of the world currently as they are thought to be new alternative medicines for conventional therapies with fewer side effects. In East Asian countries and more recently in the United States, intensive research has increasingly demonstrated the potential beneficial health properties of compounds extracted from mushrooms for the prevention and management of cancer and other life-debilitating diseases. For such reasons, the genotoxicity of culinary–medicinal mushrooms that are used by the general population should be warranted in order to identify the ingredients that pose mutagenic and carcinogenic risks. To our knowledge, there have been no reports on the mutagenicity of H. erinaceus prior to this paper. Therefore, this is the first report undertaken to evaluate the genotoxicity of a standardized H. erinaceus mycelium enriched with 5 mg/g erinacine A by using the Ames test, the chromosomal aberration test, and the erythrocyte micronucleus test.
The Ames test is widely used as an initial screening method to determine the mutagenic potential of newly discovered products since there is a high correlation between the positive responses in test mutagenicity and carcinogenicity [33], [34]. The proximate analysis and HPLC analysis of EAHE mycelium are shown in Table S1 and Fig. S1, respectively. These results are in line with those previously published [27]. Our initial test revealed no pronounced toxicity on test strains at concentrations as high as 50 mg/ml (5 mg/plate) (data not shown); therefore, we set this concentration as the upper limit of the tested dose range and performed the Ames test with serial dilutions. The results of in vitro bacterial genetic mutation assay are summarized in Table 1. In all bacterial strains exposed to different doses of EAHE mycelium (5, 2.5, 1.25, 0.625, and 0.3125 mg/plate) with the presence and absence of S9-Mix metabolic activators, the revertant colonies showed no dose dependency and were similar to those of negative control. These findings suggest that EAHE mycelium displayed no mutagenicity, especially in S. typhimurium strains TA98, TA100, TA102, TA1535, and TA1597.
Table 1.
Ames Test of EAHE mycelium using Salmonella typhimurium strains TA98, TA100, TA102, TA1535, and TA1537.
| Dose (mg/plate) | Number of revertants/plate (without S9 activation) |
||||
|---|---|---|---|---|---|
| TA98 | TA100 | TA102 | TA1535 | TA1537 | |
| Negative controla | 29 ± 3 | 148 ± 5 | 233 ± 9 | 20 ± 2 | 13 ± 2 |
| Positive controlb | 304 ± 8* | 447 ± 19* | 561 ± 15* | 80 ± 10* | 48 ± 5* |
| 5 | 30 ± 5 | 149 ± 4 | 204 ± 13 | 26 ± 2 | 16 ± 1 |
| 2.5 | 32 ± 2 | 140 ± 13 | 216 ± 10 | 19 ± 6 | 15 ± 4 |
| 1.25 | 27 ± 3 | 144 ± 6 | 192 ± 15 | 27 ± 1 | 14 ± 3 |
| 0.625 | 30 ± 6 | 146 ± 6 | 207 ± 10 | 23 ± 4 | 14 ± 1 |
| 0.3125 | 25 ± 9 | 148 ± 12 | 225 ± 12 | 19 ± 4 | 13 ± 2 |
| Number of revertants/plate (with S9 activation) |
|||||
| TA98 |
TA100 |
TA102 |
TA1535 |
TA1537 |
|
| Negative controla | 20 ± 1 | 160 ± 16 | 226 ± 13 | 17 ± 1 | 9 ± 2 |
| Positive controlc | 192 ± 17* | 1103 ± 15* | 1108 ± 15* | 937 ± 8* | 1089 ± 11* |
| 5 | 26 ± 7 | 160 ± 2 | 211 ± 17 | 21 ± 3 | 11 ± 4 |
| 2.5 | 25 ± 2 | 169 ± 8 | 234 ± 19 | 22 ± 2 | 12 ± 2 |
| 1.25 | 26 ± 9 | 160 ± 4 | 228 ± 13 | 17 ± 3 | 11 ± 4 |
| 0.625 | 34 ± 6 | 155 ± 15 | 238 ± 12 | 19 ± 6 | 12 ± 2 |
| 0.3125 | 25 ± 5 | 164 ± 10 | 242 ± 13 | 15 ± 1 | 8 ± 3 |
Data were expressed as mean ± SD (n = 3).
Two-fold or more increase in revertant numbers over the negative control.
Dimethyl sulfoxide.
TA98: 4-nitroquinoline-N-oxide, 0.5 μg/plate; TA100: sodium azide, 0.4 μg/plate; TA102: mitomycin C, 0.5 μg/plate; TA1535: sodium azide, 0.4 μg/plate; TA1537: 9-aminoacridine, 50 μg/plate.
TA98: benzo[a] pyrene, 4 μg/plate; TA100: 2-aminofluorene, 4 μg/plate; TA102: benzo[a] pyrene, 4 μg/plate; TA1535: 2-aminoanthracene, 4 μg/plate; TA1537: 9-aminoacridine, 50 μg/plate.
Since the Ames test cannot detect chromosome aberrations induced by chemicals [35], it has been recommended to use in vitro tests as a minimum requirement for mutagenicity testing [36]. According to the guidelines OECD 473 [31], the highest dose used in the in vitro chromosome aberration test should be a concentration above the limit of solubility to avoid false positive results. As the amount of precipitation recorded for EAHE was at 5 mg/ml, dose levels of 2.5, 1.25, and 0.625 mg/ml were selected and exposed to the CHO-K1 cells (BCRC 60006) in the presence and absence of a metabolic activation system derived from rat liver S9 mix [30]. The cell proliferation of CHO-K1 in the 3 h treatment with the presence and absence of S9 activation was inhibited by <7.7% and <21.4%, respectively. Incubation of these cells under the same condition for 20 h in the absence of S9 activation resulted in <34.3% decreases of cell growth (data not shown). As the highest concentration showed a significant reduction in the degree of confluency, such doses were then used for chromosome observation. The validity of the tests was observed in the incidence of cells having aberrant chromosomes, which was 0% to 1% and 6.5% to 12.5% in negative groups and positive groups, respectively (Table 2).
Table 2.
Chromosome aberration test of EAHE mycelium using CHO-K1 cells.
| Dose | S9-Mixture | G | B | D | R | g | b | e | AFd |
|---|---|---|---|---|---|---|---|---|---|
| Short-term treatment (3 h) | |||||||||
| Negative controla | − | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/200 |
| Positive controlb | − | 0 | 0 | 0 | 1 | 1 | 5 | 18 | 25/200* |
| 2.5 | − | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 4/200 |
| 1.25 | − | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/200 |
| 0.625 | − | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1/200 |
| Negative controla | + | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2/200 |
| Positive controlc | + | 0 | 1 | 2 | 0 | 0 | 8 | 2 | 13/200* |
| 2.5 | + | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1/200 |
| 1.25 | + | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1/200 |
| 0.625 | + | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1/200 |
| Long-term treatment (20 h) | |||||||||
| Negative controla | − | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/200 |
| Positive controlb | − | 0 | 0 | 2 | 0 | 2 | 5 | 11 | 20/200* |
| 2.5 | − | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/200 |
| 1.25 | − | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/200 |
| 0.625 | − | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/200 |
G: chromosome gap; B: chromosome break; D: dicentric; R: ring; g: chromatid gap; b: chromatid break; e: chromatid exchange.
Statistically significant (p < 0.05, Chi-square test) when compared to the control group.
Ham's/F-12 Culture medium.
6 μM mitomycin C.
80 μM cyclophosphamide.
Aberration frequency: Number of cells with chromosome aberration in 200 metaphase cells (n/200).
Neither 3 h nor 20 h EAHE mycelium treatments induced higher frequency of aberrations that were significantly different from negative controls (p > 0.05, Chi-square test) (Table 2). In summary, these data indicate that exposure to EAHE mycelium does not result in genetic damage in cultured mammalian cells under the test conditions.
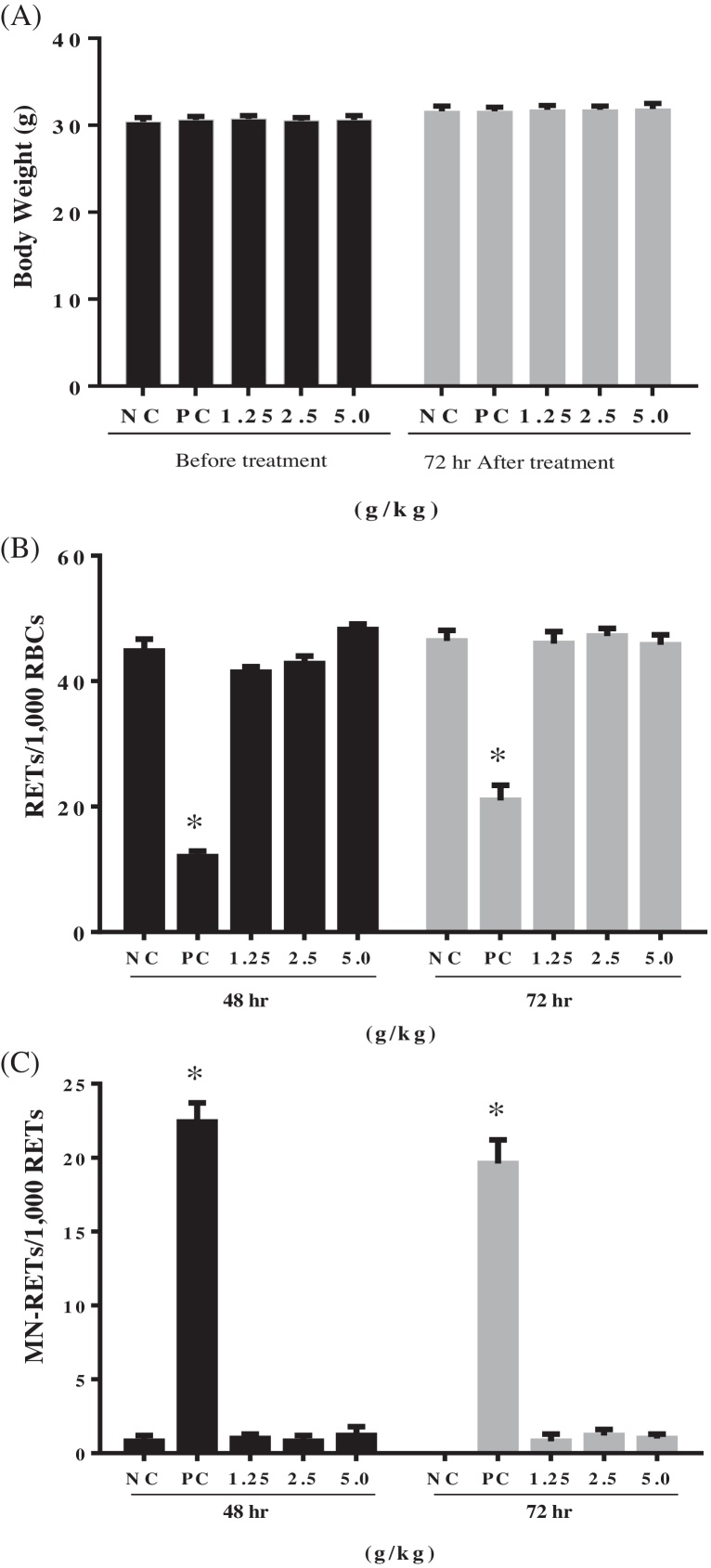
The uptake of EAHE mycelium that resulted in chromosomal damage was further investigated by using the in vivo erythrocyte micronucleus test in ICR mice since the in vivo assay takes into account whole animal processes, such as absorption, distribution, metabolism, and excretion. Our earlier study on acute oral toxicity indicated that acute oral LD50 of EAHE mycelium was greater than 5 g/kg (data not shown). Hence, doses of 1.25 g/kg (low dose), 2.5 g/kg (mid dose) and 5 g/kg (high dose) were selected for this study, whereas distilled water and cyclophosphamide were served as negative and positive controls, respectively. During the 72-h post-treatment, EAHE at all tested doses (1.25, 2.5, and 5 g/kg) did not induce any symptoms of toxicity, morbidity, or mortality in all mice (n = 25). Regarding body weight, no significant difference was observed between the control and treated groups (p > 0.05, one-way ANOVA followed by Duncan's test) (Fig. 1a). The rate of reticulocytes occurrence per 1000 red blood cells (RBC) was 44.8 ± 1.9% and 46.4 ± 1.7% in negative control groups at 48-h and 72-h post-treatment, respectively (Fig. 1b). Results showed that cyclophosphamide effectively induced myelosuppression [37] by significantly decreasing the rates in the positive control groups (p < 0.05, one-way ANOVA followed by Duncan's test) after dosing, but no significant differences were noted in all EAHE testing groups when compared to the negative control (p > 0.05, one-way ANOVA followed by Duncan's test) (Fig. 1b).
Fig. 1.
Effects of EAHE on the (A) body weight, incidence of (B) reticulocytes (RETs), and (C) micronucleated reticulocytes (MN-RETs) in ICR mice (n = 25). Distilled water and cyclophosphamide (0.05 g/kg) were served as negative (NC) and positive controls (PC), respectively. Data were expressed as mean ± SEM. * Statistically significant (p < 0.05, one-way ANOVA followed by Duncan's test) when compared to the control group.
On the other hand, the incidence of micronucleated reticulocytes in the peripheral blood per 1000 reticulocytes was 0.8 ± 0.4% and 0.0 ± 0.0% in negative control groups at 48-hr and 72-hr post-treatment, respectively (Fig. 1c). The positive control group had a mean frequency of 22.4 ± 1.3% and 19.6 ± 1.6% at 48-h and 72-h post-treatment, respectively, which were statistically significant increases compared to the negative control groups (p < 0.05, one-way ANOVA followed by Duncan's test). At 48-h post-treatment, the EAHE treated groups had 1.0 ± 0.3%, 0.8 ± 0.4%, and 1.2 ± 0.6% micronucleated reticulocytes per 1000 reticulocytes at low, mid, and high test dose levels, respectively (Fig. 1c). Similar trends were also observed after 72-h treatment. These values were not statistically significant, and hence did not demonstrate any signs of toxicity with the administration of EAHE mycelium in the mouse erythrocyte micronucleus assay. The results of the in vivo assay were thus consistent with those of the in vitro mutagenicity test, which strongly suggest that consumption of standardized EAHE does not pose genotoxic hazards to individuals.
4. Conclusion
This pioneering work showed that through use of the three-core test system for genetic damage, 5 mg/g erinacine A-enriched H. erinaceus mycelium was devoid of its genotoxic effects under our experimental conditions. Our findings support that the risk of EAHE having genotoxic activity is low. Additional research on EAHE, including a randomized controlled clinical trial, may be included in future studies to further support the safety of its consumption.
Transparency document
Acknowledgement
The authors thank Hsin-Yun Yang for editing the manuscript.
Footnotes
Available online 15 November 2014
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.11.009.
Contributor Information
I-Chen Li, Email: ichen.li@grapeking.com.tw.
Yen-Lien Chen, Email: yanlan1208@gmail.com.
Wan-Ping Chen, Email: wp.chen@grapeking.com.tw.
Li-Ya Lee, Email: ly.lee@grapeking.com.tw.
Yueh-Ting Tsai, Email: r91445202@gmail.com.
Chin-Chu Chen, Email: gkbioeng@grapeking.com.tw.
Chin-Shuh Chen, Email: cschen@mail.nchu.edu.tw.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Schulte-Herbruggen O., Braun A., Rochlitzer S., Jockers-Scherubl M.C., Hellweg R. Neurotrophic factors—a tool for therapeutic strategies in neurological neuropsychiatric and neuroimmunological diseases? Curr. Med. Chem. 2007;14(22):2318–2329. doi: 10.2174/092986707781745578. [DOI] [PubMed] [Google Scholar]
- 2.Schulte-Herbruggen O., Jockers-Scherubl M.C., Hellweg R. Neurotrophins: from pathophysiology to treatment in Alzheimer's disease. Curr. Alzheimer Res. 2008;5(1):38–44. doi: 10.2174/156720508783884620. [DOI] [PubMed] [Google Scholar]
- 3.Capsoni S., Ugolini G., Comparini A., Ruberti F., Berardi N., Cattaneo A. Alzheimer-like neurodegeneration in aged antinerve growth factor transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 2000;97(12):6826–6831. doi: 10.1073/pnas.97.12.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capsoni S., Giannotta S., Cattaneo A. Nerve growth factor and galantamine ameliorate early signs of neurodegeneration in anti-nerve growth factor mice. Proc. Natl. Acad. Sci. U.S.A. 2002;99(19):12432–12437. doi: 10.1073/pnas.192442999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J., Lacoske M.H., Theodorakis E.A. Neurotrophic natural products: chemistry and biology. Angew. Chem. Int. Ed. Engl. 2014;53(4):956–987. doi: 10.1002/anie.201302268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li G., Yu K., Li F., Xu K., Li J., He S., Cao S., Tan G. Anticancer potential of Hericium erinaceus extracts against human gastrointestinal cancers. J. Ethnopharmacol. 2014;153(2):521–530. doi: 10.1016/j.jep.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Abdulla M.A., Fard A.A., Sabaratnam V., Wong K.H., Kuppusamy U.R., Abdullah N., Ismail S. Potential activity of aqueous extract of culinary-medicinal Lion's Mane mushroom, Hericium erinaceus (Bull.: Fr.) Pers. (Aphyllophoromycetideae) in accelerating wound healing in rats. Int. J. Med. Mushrooms. 2011;13(1):33–39. doi: 10.1615/intjmedmushr.v13.i1.50. [DOI] [PubMed] [Google Scholar]
- 8.Hiwatashi K., Kosaka Y., Suzuki N., Hata K., Mukaiyama T., Sakamoto K., Shirakawa H., Komai M. Yamabushitake mushroom (Hericium erinaceus) improved lipid metabolism in mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 2010;74(7):1447–1451. doi: 10.1271/bbb.100130. [DOI] [PubMed] [Google Scholar]
- 9.Kim S.P., Kang M.Y., Kim J.H., Nam S.H., Friedman M. Composition and mechanism of antitumor effects of Hericium erinaceus mushroom extracts in tumor-bearing mice. J. Agric. Food Chem. 2011;59(18):9861–9869. doi: 10.1021/jf201944n. [DOI] [PubMed] [Google Scholar]
- 10.Kim S.P., Moon E., Nam S.H., Friedman M. Hericium erinaceus mushroom extracts protect infected mice against Salmonella Typhimurium-Induced liver damage and mortality by stimulation of innate immune cells. J. Agric. Food Chem. 2012;60(22):5590–5596. doi: 10.1021/jf300897w. [DOI] [PubMed] [Google Scholar]
- 11.Kim S.P., Nam S.H., Friedman M. Correction to Hericium erinaceus (Lion's Mane) mushroom extracts inhibit metastasis of cancer cells to the lung in CT-26 colon cancer-transplanted mice. J. Agric. Food Chem. 2014;62(2):528. doi: 10.1021/jf400916c. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y.O., Lee S.W., Oh C.H., Rhee Y.H. Hericium erinaceus suppresses LPS-induced pro-inflammation gene activation in RAW264.7 macrophages. Immunopharmacol. Immunotoxicol. 2012;34(3):504–512. doi: 10.3109/08923973.2011.633527. [DOI] [PubMed] [Google Scholar]
- 13.Shang X., Tan Q., Liu R., Yu K., Li P., Zhao G.P. In vitro anti-Helicobacter pylori effects of medicinal mushroom extracts, with special emphasis on the Lion's Mane mushroom, Hericium erinaceus (higher Basidiomycetes) Int. J. Med. Mushrooms. 2013;15(2):165–174. doi: 10.1615/intjmedmushr.v15.i2.50. [DOI] [PubMed] [Google Scholar]
- 14.Wong J.-Y., Abdulla M.A., Raman J., Phan C.-W., Kuppusamy U.R., Golbabapour S., Sabaratnam V. Gastroprotective effects of Lion's Mane mushroom Hericium erinaceus (Bull.: Fr.) Pers. (Aphyllophoromycetideae) extract against ethanol-induced ulcer in rats. Evid. Based Complement. Alternat. Med. 2013;2013:9. doi: 10.1155/2013/492976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong K.H., Naidu M., David P., Abdulla M.A., Abdullah N., Kuppusamy U.R., Sabaratnam V. Peripheral nerve regeneration following crush injury to rat peroneal nerve by aqueous extract of medicinal mushroom Hericium erinaceus (Bull.: Fr) Pers. (Aphyllophoromycetideae) Evid. Based Complement. Alternat. Med. 2011;2011:10. doi: 10.1093/ecam/neq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z., Lv G., Pan H., Pandey A., He W., Fan L. Antioxidant and hepatoprotective potential of endo-polysaccharides from Hericium erinaceus grown on tofu whey. Int. J. Biol. Macromol. 2012;51(5):1140–1146. doi: 10.1016/j.ijbiomac.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Kawagishi H., Ando M., Sakamoto H., Yoshida S., Ojima F., Ishiguro Y., Ukai N., Furukawa S. Hericenones C, D and E, stimulators of nerve growth factor (NGF)-synthesis, from the mushroom Hericium erinaceum. Tetrahedron Lett. 1991;32(35):4561–4564. [Google Scholar]
- 18.Kawagishi H., Ando M., Shinba K., Sakamoto H., Yoshida S., Ojima F., Ishiguro Y., Ukai N., Furukawa S. Chromans, hericenones F, G and H from the mushroom Hericium erinaceum. Phytochemistry. 1992;32(1):175–178. [Google Scholar]
- 19.Kawagishi H., Shimada A., Hosokawa S., Mori H., Sakamoto H., Ishiguro Y., Sakemi S., Bordner J., Kojima N., Furukawa S. Erinacines E, F, and G, stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1996;37(41):7399–7402. [Google Scholar]
- 20.Kawagishi H., Shimada A., Shirai R., Okamoto K., Ojima F., Sakamoto H., Ishiguro Y., Furukawa S. Erinacines A, B and C, strong stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1994;35(10):1569–1572. [Google Scholar]
- 21.Kawagishi H., Simada A., Shizuki K., Ojima F., Mori H., Okamoto K., Sakamoto H., Furukawa S. Erinacine D, a stimulator of NGF-synthesis, from the mycelia of Hericium erinaceum. Heterocycl. Commun. 1996;2(1):51. [Google Scholar]
- 22.Lee E.W., Shizuki K., Hosokawa S., Suzuki M., Suganuma H., Inakuma T., Li J., Ohnishi-Kameyama M., Nagata T., Furukawa S., Kawagish H. Two novel diterpenoids, erinacines H and I from the mycelia of Hericium erinaceum. Biosci. Biotechnol. Biochem. 2000;64(11):2402–2405. doi: 10.1271/bbb.64.2402. [DOI] [PubMed] [Google Scholar]
- 23.Shimbo M., Kawagishi H., Yokogoshi H. Erinacine A increases catecholamine and nerve growth factor content in the central nervous system of rats. Nutr. Res. 2005;25(6):617–623. [Google Scholar]
- 24.Mori K., Inatomi S., Ouchi K., Azumi Y., Tuchida T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: a double-blind placebo-controlled clinical trial. Phytother. Res. 2009;23(3):367–372. doi: 10.1002/ptr.2634. [DOI] [PubMed] [Google Scholar]
- 25.Prince M., Prina M., Guerchet M. Alzheimer's Disease International; 2013. World Alzheimer Report 2013. [Google Scholar]
- 26.Li I.C., Chen Y.L., Lee L.Y., Chen W.P., Tsai Y.T., Chen C.C., Chen C.S. Evaluation of the toxicological safety of erinacine A-enriched Hericium erinaceus in a 28-day oral feeding study in Sprague-Dawley rats. Food Chem. Toxicol. 2014;70(0):61–67. doi: 10.1016/j.fct.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 27.International A. AOAC International; Gaithersburg, MD: 1995. Official Methods of Analysis of AOAC International. [Google Scholar]
- 28.OECD . OECD Publishing; 1997. Test No. 471: Bacterial Reverse Mutation Test. [Google Scholar]
- 29.Maron D.M., Ames B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983;113(3–4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 30.OECD . OECD Publishing; 1997. Test No. 473: In vitro Mammalian Chromosome Aberration Test. [Google Scholar]
- 31.OECD . OECD Publishing; 1997. Test No. 474: Mammalian Erythrocyte Micronucleus Test. [Google Scholar]
- 32.McCann J., Choi E., Yamasaki E., Ames B.N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proc. Natl. Acad. Sci. U.S.A. 1975;72(12):5135–5139. doi: 10.1073/pnas.72.12.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortelmans K., Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res. 2000;455(1–2):29–60. doi: 10.1016/s0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 34.Ohuchida A., Yoshida R. Mutagenicity test of cefodizime sodium. J. Toxicol. Sci. 1988;13(1):245–256. doi: 10.2131/jts.13.supplementi_245. [DOI] [PubMed] [Google Scholar]
- 35.Ishidate M., Jr. A proposed battery of tests for the initial evaluation of the mutagenic potential of medicinal and industrial chemicals. Mutat. Res. 1988;205(1–4):397–407. doi: 10.1016/0165-1218(88)90030-4. [DOI] [PubMed] [Google Scholar]
- 36.Daniel D., Crawford J. Myelotoxicity from chemotherapy. Semin. Oncol. 2006;33(1):74–85. doi: 10.1053/j.seminoncol.2005.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.