Abstract
Aristolochic acid (AA) is considered to be a causative agent for progressive interstitial renal fibrosis, leading to AA nephropathy. Lysophosphatidic acid (LPA) is a mediator in the onset of renal fibrosis. In this study, we analyzed the molecular species of LPA and its precursor lysophospholipids in kidney tissue from rats exposed to AA. Daily intraperitoneal injections of AA for 35 days to rats gave rise to fibrosis in kidney, decreased the kidney levels of LPA, lysophosphatidylserine and lysophosphatidylinositol. In rat renal cell lines (NRK52E and NRK49F), AA-induced cytotoxicity was potentiated by Ki16425, LPA1,3 receptor antagonist. The level of mRNA encording α-smooth muscle actin was significantly increased by AA-treatment only in NRK52E cells, while the mRNA level of collagen III was decreased in both NRK52E and NRK49F cells. These results suggest that endogenous LPA in rat kidney prevents AA-induced renal fibrosis.
Abbreviations: 18S, ribosomal protein S18; AA, aristolochic acid; α-SMA, α-smooth muscle actin; LC–MS/MS, liquid chromatography–tandem mass spectrometry; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; LPG, lysophosphatidylglycerol; LPI, lysophosphatidylinositol; LPS, lysophosphatidylserine; lysoPLD, lysophospholipase D; LPL, lysophospholipid; PLA1, phospholipase A1; PLA2, phospholipase A2; HE, hematoxylin/eosin; AZ, azan Mallory
Keywords: Lysophospholipid, Aristolochic acid, Lysophosphatidic acid, Chronic kidney disease, Fibrosis, Nephrotoxicity
1. Introduction
The physiological and pathophysiological roles of lysophospholipids (LPLs), especially lysophosphatidic acid (LPA), have been studied extensively in mammals. LPA is now recognized as not only an intermediate in synthesis of glycerophospholipids but also an important lipid mediator [1], [2], [3]. Recent rapid progress in liquid chromatography–tandem mass spectrometry (LC–MS/MS) with high selectivity and sensitivity has contributed greatly to the progress of biochemical, physiological and pathological studies of LPLs [4]. In a unilateral ureteral obstruction model in rats, we found higher production of LPA from lysophosphatidylcholine (LPC) in the swollen renal pelvis in the ligated kidney than in bladder urine [5]. We reported prevention by LPA of Cd2+-induced death of cultured rat renal proximal epithelial cells (NRK52E) and interstitial fibroblast cells (NRK49F), suggesting that endogenously generated LPA protects rats from the renal toxicity of Cd2+ [6]. However, there is no information on renal tissue level of LPA in rats that were subjected to renal injury.
Aristolochic acid (AA) is an alkaloid extracted from mainly seeds of Aristolochia clematitis and roots of Aristolochia fangchi and Aristolochia manshuriensis. Aristolochia species are used for treatment for arthritis, gout and rheumatism [7]. AA was shown to act as an analgesic, diuretic and anti-inflammatory agent [8]. However, AA is now considered a causative agent for progressive interstitial renal fibrosis and urothelial carcinoma development in the upper urinary tract. These pathological lesions developed in seemingly unrelated events. The first event occurred in the 1950s in rural villages in the drainage basin of the Danube river [9] and the second occurred in Belgium in young women who had taken slimming pills including Chinese herbs [10]. Since these events, the uses of AA-containing medicinal substances and supplements have been banned in many countries. On the other hand, AA is known to have an inhibitory effect on phospholipase A2 (PLA2) and to prevent inflammatory diseases, such as Clostridium difficile-induced diarrhea-related events [11]. Therefore, we postulated that chronic AA-induced nephropathy leads to altered levels of LPA and its related LPLs in animal body tissues. In this study, we examined whether AA-nephropathy is accompanied by altered levels of LPLs in whole kidney tissue of rats.
2. Materials and methods
2.1. Materials
AA, a mixture of AAI and AAII, was purchased from Across Organics (Geel, Belgium). Ki16425, an LPA1, 3 receptor antagonist, was obtained from Cayman Chemical (Ann Arbor, MI, USA). Sepasol RNA I super and polyethylene glycol #400 were obtained from Nakarai tesque (Kyoto, Japan). 1-Heptadecanoyl (17:0) LPC, 1-heptadecenoyl (17:1) lysophosphatidylinositol (LPI), 17:0 lysophosphatidylglycerol (LPG), 17:0 lysophosphatidylethanolamine (LPE), and 17:0 lysophosphatidylserine (LPS) were purchased from Avanti Polar Lipids (Alabaster, AL, USA). 17:0 LPA was prepared from 17:0 LPC using phospholipase D from Streptomyces chromofuscus as described previously [12]. Egg yolk phosphatidylcholine (PC), 1-palmitoyl LPC, and 1-palmitoyl LPA as standards for TLC were obtained from Funakoshi Co. (Tokyo, Japan). Alzet™ osmotic pumps were purchased from Durect (Cupertino, CA, USA). Chow (MF) purchased from Oriental Yeast (Tokyo, Japan) had the following components: powders of wheat, defatted soybean, alfalfa, defatted rice, defatted bovine milk, soybean oil, corn, white fish meal, and beer yeast.
2.2. Animal experiments
Male Wistar/ST rats (4–5 weeks old) were purchased from Japan SLC (Shizuoka, Japan). The breeding room was kept at 20–25 °C with a light–dark cycle of 12 h each. Rats were allowed free access to a chow and water throughout the experimental period. All rats were handled in accordance with the principles and guidelines of the Experimental Animal Committee of Kyushu University of Health and Welfare. The rats were divided into two groups of 6 animals each in three separate experiments and acclimatized for 1 week. One week later, AA was dissolved in 100% polyethylene glycol #400 at a final concentration of 10 mg/ml and was subcutaneously (with and without Alzet™) or intraperioneally injected at 10 mg/kg body weight daily for 35 days. The sham rats were given the vehicle solution (1 ml/kg).
2.3. Histological analysis
After AA treatment for 35 days, kidneys were quickly removed. The kidney slices were fixed with 10% formaldehyde and then embedded in paraffin. Degrees of renal tissue injury were evaluated by microscopic observation after staining with hematoxylin/eosin (HE) and azan Mallory (AZ).
2.4. Lipid extraction from kidney tissue
The frozen kidney (0.24 ± 0.11 g for 6 rats) was placed on ice and then de-frosted tissues were homogenized in a glass homogenizer containing 4 ml of a mixture of chloroform–methanol–water at a final proportion of 1:2:0.8 (v/v), followed by centrifugation at 1100 × g for 10 min at 4 °C after standing on ice for 2 h. The precipitates were mixed with 0.5 ml chloroform, 1 ml methanol and 1 ml distilled water, and the mixture was centrifuged at 1100 × g for 10 min at 4 °C. The supernatants were combined, and the pH was adjusted to 9–10 with 20% NH4OH. After mixing the supernatant with 1.5 ml chloroform and 1.5 ml distilled water, the mixture was centrifuged at 1100 × g for 10 min at 4 °C. The resultant upper phase was withdrawn and mixed with 3 ml of a mixture of chloroform–methanol (17:3), followed by centrifugation. The lower phases were combined for LC–MS/MS of LPC. The pH of the remaining upper phase was adjusted to 2–3 with 5 M HCl, and polar acidic LPLs were extracted twice with 3 ml of chloroform–methanol mixture (17:3). The lower phases were combined for LC–MS/MS of LPA, LPG, LPI and LPS.
Before the lipid extraction, 10 nmol 17:0 LPC, 0.5 nmol 17:0 LPS, 0.5 nmol 17:0 LPG, 0.2 nmol 17:1 LPI and 0.5 nmol 17:0 LPA were added as internal standards for corrections of efficiencies of lipid extraction and electrospray ionization. The first lipid extract was dried under a stream of nitrogen gas, and were dissolved in 0.5 ml of a mixture of methanol/water (95:5, v/v) containing 5 mM formic acid for LC–MS/MS. The second lipid extract containing acidic polar lipids such as LPA, LPS, LPG and LPI were dissolved in 0.1 ml of a mixture of methanol/water (95:5, v/v) containing 5 mM formic acid for LC–MS/MS.
2.5. LC–MS/MS of LPLs
LC–MS/MS was performed on a quadrupole-linear iontrap hybrid MS, 4000 QTRAP™ (Applied Biosystems/MDS Sciex; Concord, ON, Canada), with an Agilent 1100 LC system combined with an autosampler (HTS PAL, CTC Analytics, Zwingen, Switzerland), as previously described [13]. Separation of LPCs in the neutral lipid fractions by LC was achieved using an Agilent ZORBAX Eclipse XDB-C18 column (50 mm × 1 mm; 3.5-μm particle size silica). The composition of the mobile phase was methanol/water (4:1, v/v) containing 5 mM ammonium formate, which was pumped at a flow rate of 0.1 ml/min for isocratic elution. Separation of LPA, LPI, LPG and LPS in the acidic LPL fraction by LC was performed with a Tosoh TSK-ODS-100Z column (150 mm × 2 mm; 5-μm particle size silica) developed with methanol/water (19:1, v/v) containing 5 mM ammonium formate at a flow rate of 0.22 ml/min in isocratic elution mode. At regular intervals, 5 μl aliquots of test solutions were applied to the mass spectrometer for analysis. LPL were analyzed by multiple reaction monitoring (MRM) in positive ion mode for LPC or in negative ion mode for LPA, LPS, LPG and LPI, as previously described [13]. In the positive ion MRM, Q1 and Q3 were set for the protonated molecular ion and [phosphorylcholine]+ at m/z 184 for LPC. In the negative ion MRM, Q3 was set to [cyclic glycerol phosphate]− at m/z 153 for LPA, LPG and LPS, [inositolphosphate–H2O]− at m/z 241 for LPI in combination with the deprotonated molecular ion as Q1. The amounts of the different molecular species of LPC were calculated from the ratios of their areas of positive ions to those of internal standards 17:0 LPC. Similarly, the amounts of molecular species of LPA, LPI, LPG and LPS were calculated from the ratios of their peak areas of negative ions to those of internal standards 17:0 LPA, 17:1 LPI, 17:0 LPG, and 17:0 LPS, respectively. Each measurement included a standard curve with serial dilutions.
2.6. mRNA quantification
Total RNAs were extracted using Sepasol RNA I Super G according to the manufacturer's manual. A Turbo DNA-free kit (Ambion, Austin, TX, USA) was used to remove contaminating DNAs from the RNAs obtained. The total RNA level was quantified by determination of ultraviolet absorbance at 260 nm, and its purity was verified by measuring the absorbance ratio at 260 and 280 nm. cDNA was prepared using ReverTra Ace qPCR RT kit (Toyobo, Osaka, Japan). Real time RT-PCR with 50 ng cDNA and 400 nM of each sense and antisense primers in a final volume of 20 μl using the Thunderbird Sybr qPCR Mix (Toyobo) were performed in triplicate using a Step one plus (Applied Biosystems, Tokyo, Japan) under PCR cycling conditions according to manufacturer's instructions. Analysis of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) for kidney homogenates or ribosomal protein S18 (18S) RNA for renal cell pellets was performed in parallel as a housekeeping RNA control to normalize gene expression. The primers used are shown in Supplementary Table 1.
Supplementary Table 1 related to this article can be found, in the online version, at doi:10.1016/j.toxrep.2015.02.012.
Primer sequences used for real time RT-PCR.
2.7. Cell culture and AA treatment
NRK52 and NRK49F cells were provided by Riken Bioresource Center (Ibaragi, Japan) and Health Science Research Resources Bank (Osaka, Japan), respectively. The cells were maintained in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% (v/v) fetal calf serum (FCS) and penicillin/streptomycin and incubated at 37 °C in humidified atmosphere of 5% CO2 and 95% air. The cells were seeded in a culture dish with 85 mm in diameter or 96-well culture plate at a density of 2 × 105 cells/ml for 48 h in DMEM with 10% FCS. Cells were washed with phosphate-buffered saline twice and were treated with AA and/or Ki16425 for up to 65 h in DMEM without FCS. Cell viability was determined by Cell Titer 96 Aqueous one solution cell proliferation assay (Promega, Fitchburg, WI, USA).
2.8. Statistical analysis
All data are expressed as means ± S.E. Comparisons were analyzed by Student's unpaired t-test, and multiple comparisons were carried out by using one-way ANOVA with Tukey's post hoc test.
3. Results
3.1. Effects of AA-treatment on renal injury
Rats received daily subcutaneous injections of AA, but not vehicle alone, for 35 days were found to form scab. The subcutaneous tissue was infiltrated with polymorphonuclear leukocytes and macrophages (Supplementary Fig. 1A and B). Under our experimental conditions, this AA-injection route did not affect the renal function of rats. Next, we inserted an Alzet™ osmotic pump into the subcutaneous tissue according to manufacturer's instructions to continuously infuse AA for 4 weeks. However, this injection technique also did not induce significant histological and biological changes in renal tissues (data not shown). We finally selected intraperitoneal injection of AA for further experiments because we confirmed previous reports that this route evoked chronic AA-nephropathy in mice [14], [15].
Supplementary Fig. 1 related to this article can be found, in the online version, at doi:10.1016/j.toxrep.2015.02.012.
Formation of scabs in rats receiving subcutaneously injected AA for 35 days. Rats received a subcutaneous injection of AA (10 mg/kg) daily for 35 days. (A) Photomicrographs of subcutaneous tissue of rat treated with AA (scale bar = 200 μm; HE stain). (B) Filtration of many polymorphonuclear leukocytes (shown by an elliptical dashed line) and macrophages (shown by a rectangular dashed line) (scale bar = 100 μm; HE stain).
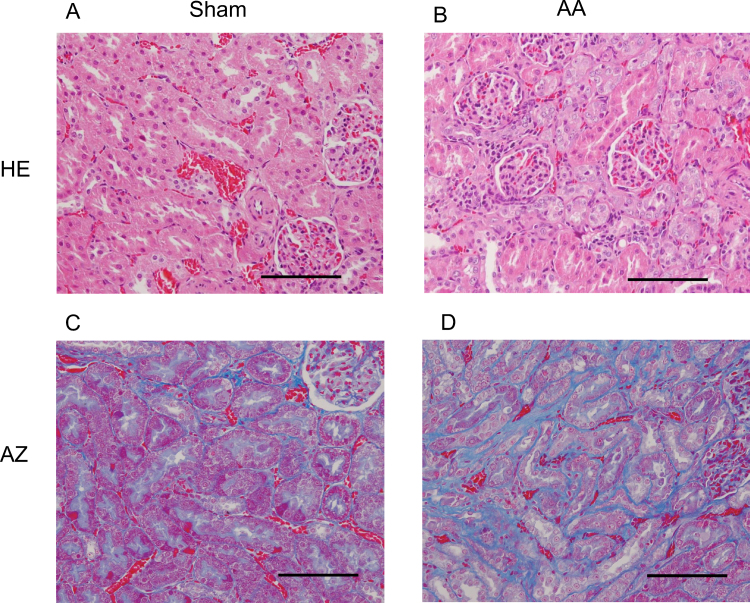
As shown in Table 1, the plasma urea–nitrogen and creatinine values in rats treated with AA for 35 day were significantly higher than those of corresponding sham rats. After exposure of rats to AA for 35 days, HE staining of isolated rat kidney revealed apoptosis of epithelial cells, focal vacuolar changes, and thickening of tubular basement membrane in the renal tubular region (Fig. 1B). On the rat without AA-treatment, no abnormalities were observed in described above area (Fig. 1A). In the interstitial region, slight lymphocytic infiltration was detected in 2 of 6 rats exposed to AA. In contrast, no remarkable glomerular alteration was observed in rats treated with and without AA. We performed AZ staining to examine whether fibrosis proceeded in the interstitial area. After an exposure of 35 days with AA, we found prominent collagen fibers in interstitial areas around the renal tubules of rats (Fig. 1D).
Table 1.
Renal function in AA-exposed rats.
| Sham | AA | |
|---|---|---|
| Urea nitrogen (mmol/l) | 5.79 ± 0.27 | 8.40 ± 0.64* |
| Creatinine (μmol/l) | 22.71 ± 1.28 | 28.76 ± 0.88* |
Data are the mean ± S.E. for 4–6 rats/group.
p < 0.05 compared to the sham.
Fig. 1.
Photomicrographs of renal cortices of rats. Rats were exposed to AA (10 mg/kg/day) or vehicle by daily intraperitoneal injection for 35 days. The kidneys were excised and stained with HE (A and B) or AZ (C and D). Data are representatives of each group with 4 or 6 test and sham rats. Scale bar = 100 μm.
3.2. Effect of AA on mRNA levels of collagens and α-SMA
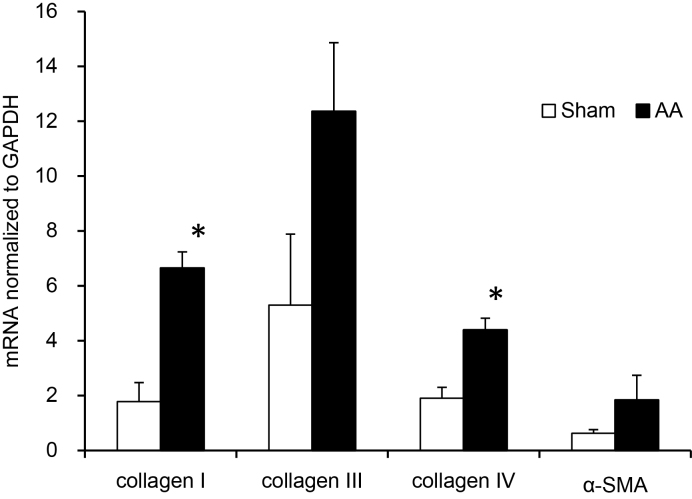
The levels of mRNA encoding collagen I and collagen IV in the kidney of AA-exposed rats were significantly higher than those of the sham rats (Fig. 2). There was a tendency of higher mean mRNA levels of collagen III and α-SMA in the kidney of AA-exposed rats, but the mean values did not reach to significant difference compared to the sham rats (collagen III; p = 0.06, α-SMA; p = 0.09).
Fig. 2.
Effects of AA on mRNA expression of collagen I, collagen III, collagen IV and α-SMA. RNAs were extracted from kidneys of rat that received AA (10 mg/kg/day) for 35 days. mRNAs encoding collagen I, collagen III, collagen IV and α-SMA were quantified by real-time RT-PCR. Results are means ± S.E. (n = 6, 4). *p < 0.05 compared to the sham.
3.3. Effects of AA on kidney levels of LPLs
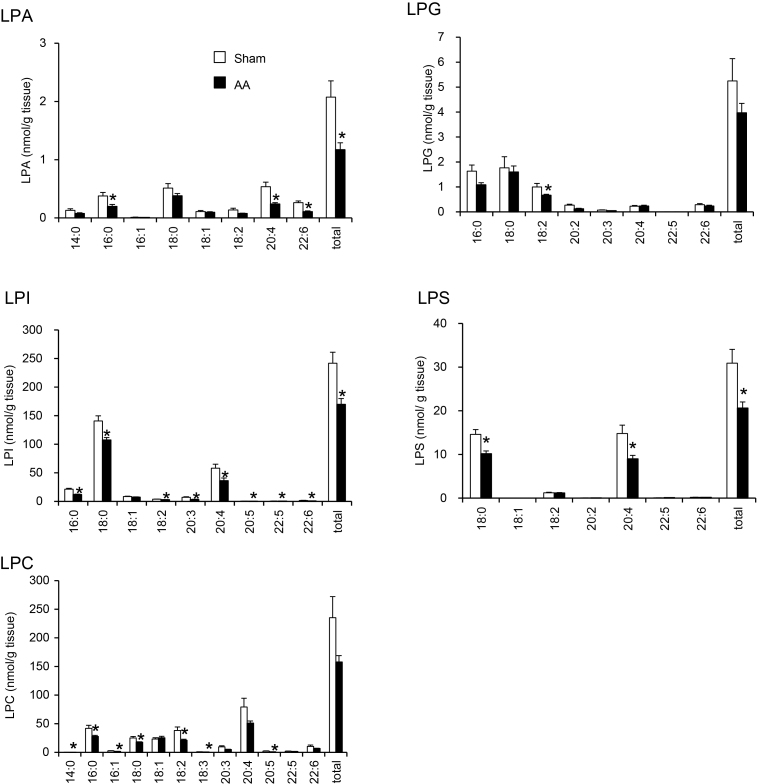
The AA exposure to rats significantly lowered renal tissue levels of total LPA, LPI and LPS compared to the corresponding sham rats (Fig. 3). The markedly lowered molecular species levels in the kidney were LPA (16:0, 20:4 and 22:6), LPS (18:0 and 20:4), LPG (18:2), LPI (16:0, 18:0, 18:2, 20:3, 20:4, 20:5, 22:5 and 22:6) and LPC (14:0, 16:0, 16:1, 18:0, 18:2, 18:3 and 20:5).
Fig. 3.
Effects of AA on total amount and molecular species of five LPL in kidney tissue of rats. Rats were treated with AA (10 mg/kg/day) for 35 days. Extracted lipids were analyzed by LC–MS/MS. *p < 0.05 compared to the sham.
3.4. Effect of Ki16425 on AA-induced cytotoxicity
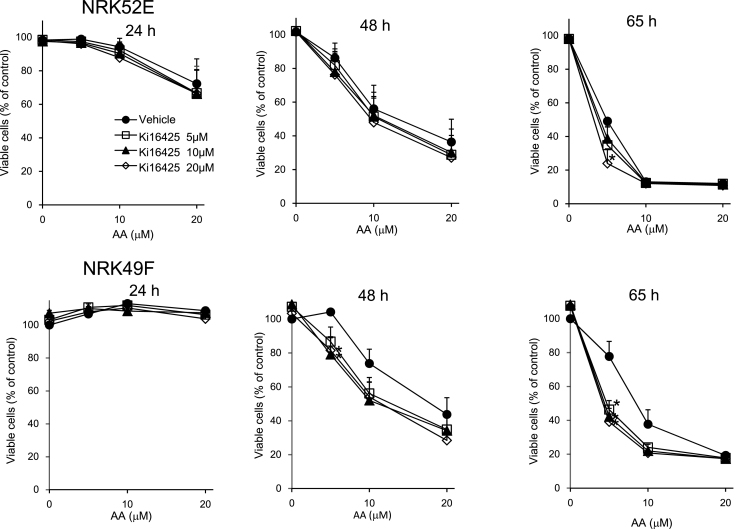
We examined whether endogenous LPA is involved in the regulation of viability of NRK52E cells and NRK49F cells treated with and without AA for various culture times. The populations of the survival cells were reduced by AA-treatment in a concentration- and time-dependent manner. The viability of AA-exposed cells treated with Ki16425 was not different from that of the AA-exposed cells treated the vehicle, except for decreased viabilities observed with Ki16425 (20 μM for 65 h) in NRK52E cells (Fig. 4). The number of the survival cells after treatment of 5 μM AA was significantly decreased by co-incubation with Ki16425 for 48 and 65 h in NRK49F cells.
Fig. 4.
Effects of AA and Ki16425 on cell viability. FCS-starved NRK52E and NRK49F cells were incubated with various concentrations (5, 10, 20 μM) of AA and Ki16425 for 24, 48 and 65 h. Cell viability was determined as described in Section 2. Results are means ± S.E. (n = 3). *p < 0.05 compared to the cells treated with vehicle.
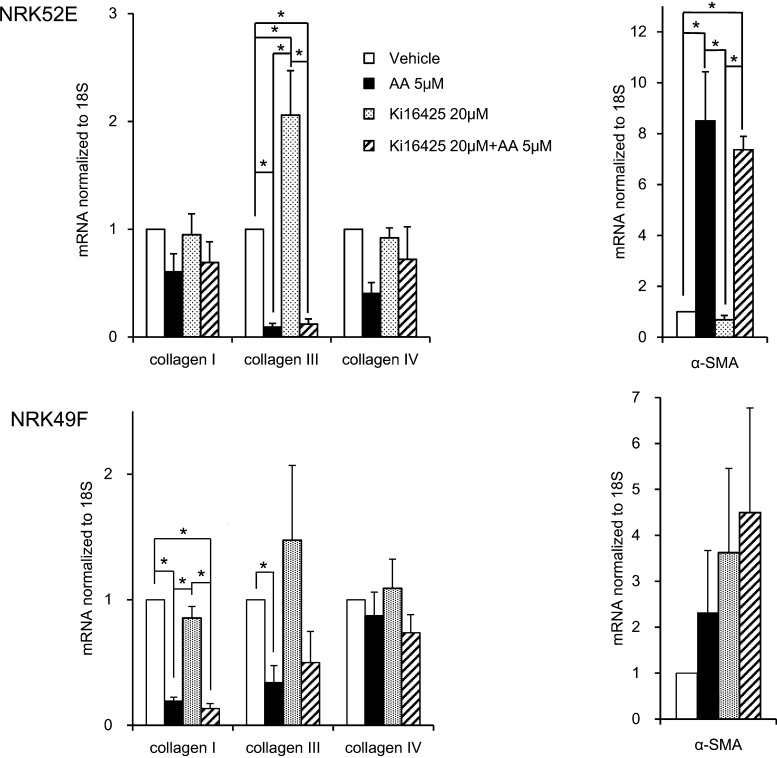
3.5. Effects of AA and Ki16425 on mRNA expression of collagens and α-SMA in NRK52E and NRK49F cells
To obtain information on possibility whether endogenous LPA affects AA-induced renal fibrosis in rats, we measured the mRNA expression of collagens and α-SMA in cultured rat kidney-derived cells (Fig. 5). Based on the preliminary experiments with AA (5 or 10 μM) for 24, 48 or 65 h, we selected the data obtained with AA 5 μM for 65 h. The level of mRNA encoding α-SMA in NRK52E cells treated with AA at 5 μM were about 8.5-fold higher compared to that in NRK52E cells treated with vehicle alone, and co-incubation of Ki16425 with AA resulted in no significant reduction of AA-increased mRNA level (7.4-fold increase compared to the Ki16425 alone) in NRK52E cells. Contrary to our expectation, the expression of collagen III in NRK52E cell was significantly lower in rats treated with AA 5 μM compared to the vehicle alone. Treatment of Ki16425 alone resulted in about 2-fold increase in mRNA expression of collagen III, but caused no effect on AA-induced decrease in mRNA expression of collagen III.
Fig. 5.
Effects of AA on mRNA expressions of collagen I, collagen III, collagen IV and α-SMA in rat kidney-derived cell lines. RNAs were extracted from NRK52E or NRK49F cells treated with 5 μM AA and/or 20 μM Ki16425 for 65 h. mRNAs encoding collagen I, collagen III, collagen IV and α-SMA were quantified by real-time RT-PCR. Results are means ± S.E. (NRK52E; n = 6, NRK49F; n = 4). *p < 0.05 compared to the cells treated with vehicle.
In NRK49F cells, the expressions of collagens I and collagen III after AA-treatment were significantly lower than those after treatment with the vehicle. There was no significant effect of Ki16425 on AA-induced reduction of mRNA expressions of collagen III. The level of mRNA encoding α-SMA in AA-treated rats was not significantly different from other groups.
4. Discussion
In order to elucidate the pathophysiology of AA nephropathy in humans, AA has been administered to various experimental animals. Diverse AA administration procedures were reported: oral for 3 days [8], 7 days [16] and 1 month [17], gavage for 3 days [18], intragastric injection for 12 weeks [19] and subcutaneous injection for 35 days [20]. Among these procedures, we first tested the daily subcutaneous injection at a dose of 10 mg/kg body weight for 35 days in rats, because this protocol was developed renal fibrosis within a relatively short time and its dose was verified. However, the procedure produced no renal lesion, but led to formation of scabs. We assumed that formation of the scabs was enhanced by repeated needle-punctures in narrow area, resulting in the insufficient movement of AA in bloodstream. In rats exposed daily to AA by intraperitoneal injection for up to 35 days, several indices of renal injury in the rats were higher than those of sham rats: plasma levels of urea–nitrogen and creatinine, fibrosis in interstitial areas observed by histological observation, and significantly higher mRNA levels of collagens I and IV, but not collagen III and α-SMA, in kidney tissue by real time RT-PCR were significantly higher in AA-treated rats than in sham rats. Similarly, Huang et al. [14] showed that intraperitoneal injection of AA into mice increases collagens I, III and IV but not α-SMA in tubular interstitium and fibrosis in the region by Masson's trichrome stain, indicating a worsening of renal function.
Total LPA, LPS and LPI levels in kidney tissue from AA-exposed rats were significantly lower than those of the sham rats. PLA2 may be suppressed by AA-treatment, leading to a decrease in levels of saturated molecular species of LPC, LPS and LPI. In addition, phospholipase A1 (PLA1) may be also suppressed in the kidney by AA treatment, resulting in reduced unsaturated molecular species of LPA, LPC, LPG, LPS, and LPI. It should be mentioned that the enzymes for LPI formation were suggested to be cytosolic calcium-dependent phospholipase A2α and PLA1 [21], [22], [23]. AA-treatment probably lowers the kidney levels of various LPLs that express differently homeostatic actions of the kidney integrity and functions. Gao et al. [24] showed that intraperitoneal injection of LPA under ischemia was able to protect against the kidney injury due to ischemia/reperfusion.
Previously, LPA/LPA1 axis was proposed to be participated in renal fibrosis in mice with unilateral ureter obstruction [25]. Therefore, using rat kidney-derived cells (NRKE52 and NRK49F) treated with Ki16425, an LPA1,3 antagonist, we examined whether endogenous production of LPA is involved in AA-induced cell dysfunctions such as reduced cell viability and stimulated renal fibrosis. In the latter case, mRNA expressions of collagens (I, III and IV) and α-SMA were selected as marker proteins for extracellular matrix production and epithelial–mesenchymal transitions, respectively. We showed that AA-induced cytotoxicity in NRK52E and NRK49F cells was significantly augmented by treatment with Ki16425, but that treatment with Ki16425 alone induced no cytotoxicity in the cells. This result suggests that endogenous LPA protectively acts as an anti-apoptotic factor against AA-induced cytotoxicity to the renal cells via LPA1,3 receptors.
We found that AA-treatment did not increase the mRNA levels of collagens, but significantly increased that of α-SMA in NRK52E. In NRK 52E cells, AA was reported to potentiate the mRNA levels of collagens I and III [26] and the protein level of α-SMA [27]. Although we have varied the concentration and time of AA-exposure, we detected no increased mRNA level of collagens under our experimental conditions. Inconsistency of real time RT-PCR results with whole kidney and those with NRK52E cells are unclear at the present time. AA may indirectly augment the mRNA expression for collagens through secretion of endogenous collagen expression-stimulating substances from renal cells. Another possible factor is its strong cytotoxicity of AA toward NRK52E and NRK49F cells, that leads to reduced mRNA expression of collagen III and collagen I, but not collagen IV.
Co-incubation of Ki16425 with AA resulted in no additive or reductive effect in mRNA expressions of collagen IV and α-SMA induced by AA alone, indicating no involvement of endogenous LPA in the AA-induced inhibition of their mRNA expressions. Interestingly, however, endogenous LPA in NRK52E cells was suggested to significantly inhibit mRNA expression of collagen III in the absence of AA, whereas it did not affect AA-induced expression of collagen III mRNA, possibly due to very high inhibitory potency of AA.
In summary, our results suggest that endogenous LPA in rat kidney exerts a weak, but significant protective effect on the AA-induced renal fibrosis.
Transparency document
References
- 1.Grzelczyk A., Gendaszewska-Darmach E. Novel bioactive glycerol-based lysophospholipids: new data – new insight into their function. Biochimie. 2013;95:667–679. doi: 10.1016/j.biochi.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Makide K., Kitamura H., Sato Y., Okutani M., Aoki J. Emerging lysophospholipid mediators, lysophosphatidylserine, lysophosphatidylthreonine, lysophosphatidylethanolamine and lysophosphatidylglycerol. Prostaglandins Other Lipid Mediat. 2009;89:135–139. doi: 10.1016/j.prostaglandins.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Tokumura A. Metabolic pathways and physiological and pathological significances of lysolipid phosphate mediators. J. Cell. Biochem. 2004;92:869–881. doi: 10.1002/jcb.20147. [DOI] [PubMed] [Google Scholar]
- 4.Murph M., Tanaka T., Pang J., Felix E., Liu S., Trost R., Godwin A.K., Newman R., Mills G. Liquid chromatography mass spectrometry for quantifying plasma lysophospholipids: potential biomarkers for cancer diagnosis. Methods Enzymol. 2007;433:1–25. doi: 10.1016/S0076-6879(07)33001-2. [DOI] [PubMed] [Google Scholar]
- 5.Tsutsumi T., Adachi M., Nikawadori M., Morishige J., Tokumura A. Presence of bioactive lysophosphatidic acid in renal effluent of rats with unilateral ureteral obstruction. Life Sci. 2011;89:195–203. doi: 10.1016/j.lfs.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Tsutsumi T., Ishihara A., Yamamoto A., Asaji H., Yamakawa S., Tokumura A. The potential protective role of lysophospholipid mediators in nephrotoxicity induced by chronically exposed cadmium. Food Chem. Toxicol. 2014;65:52–62. doi: 10.1016/j.fct.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Arlt V.M., Stiborova M., vom Brocke J., Simoes M.L., Lord G.M., Nortier J.L., Hollstein M., Phillips D.H., Schmeiser H.H. Aristolochic acid mutagenesis: molecular clues to the aetiology of Balkan endemic nephropathy-associated urothelial cancer. Carcinogenesis. 2007;28:2253–2261. doi: 10.1093/carcin/bgm082. [DOI] [PubMed] [Google Scholar]
- 8.Yeh Y.H., Lee Y.T., Hsieh H.S., Hwang D.F. Short-term toxicity of aristolochic acid, aristolochic acid-I and aristolochic acid-II in rats. Food Chem. Toxicol. 2008;46:1157–1163. doi: 10.1016/j.fct.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Tatu C.A., Orem W.H., Finkelman R.B., Feder G.L. The etiology of Balkan endemic nephropathy: still more questions than answers. Environ. Health Perspect. 1998;106:689–700. doi: 10.1289/ehp.106-1533478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanherweghem J.L., Depierreux M., Tielemans C., Abramowicz D., Dratwa M., Jadoul M., Richard C., Vandervelde D., Verbeelen D., Vanhaelen-Fastre R. Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including Chinese herbs. Lancet. 1993;341:387–391. doi: 10.1016/0140-6736(93)92984-2. [DOI] [PubMed] [Google Scholar]
- 11.Lima A.A., Nascimento N.R., Fang G.D., Yotseff P., Toyama M.H., Guerrant R.L., Fonteles M.C. Role of phospholipase A2 and tyrosine kinase in Clostridium difficile toxin A-induced disruption of epithelial integrity, histologic inflammatory damage and intestinal secretion. J. Appl. Toxicol. 2008;28:849–857. doi: 10.1002/jat.1348. [DOI] [PubMed] [Google Scholar]
- 12.Tokumura A., Iimori M., Nishioka Y., Kitahara M., Sakashita M., Tanaka S. Lysophosphatidic acids induce proliferation of cultured vascular smooth muscle cells from rat aorta. Am. J. Physiol. 1994;267:C204–C210. doi: 10.1152/ajpcell.1994.267.1.C204. [DOI] [PubMed] [Google Scholar]
- 13.Tokumura A., Carbone L.D., Yoshioka Y., Morishige J., Kikuchi M., Postlethwaite A., Watsky M.A. Elevated serum levels of arachidonoyl-lysophosphatidic acid and sphingosine 1-phosphate in systemic sclerosis. Int. J. Med. Sci. 2009;6:168–176. doi: 10.7150/ijms.6.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang L., Scarpellini A., Funck M., Verderio E.A., Johnson T.S. Development of a chronic kidney disease model in C57BL/6 mice with relevance to human pathology. Nephron Extra. 2013;3:12–29. doi: 10.1159/000346180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou L., Fu P., Huang X.R., Liu F., Chung A.C., Lai K.N., Lan H.Y. Mechanism of chronic aristolochic acid nephropathy: role of Smad3. Am. J. Physiol. Renal Physiol. 2010;298:F1006–F1017. doi: 10.1152/ajprenal.00675.2009. [DOI] [PubMed] [Google Scholar]
- 16.Lou Y., Li J., Lu Y., Wang X., Jiao R., Wang S., Kong L. Aristolochic acid-induced destruction of organic ion transporters and fatty acid metabolic disorder in the kidney of rats. Toxicol. Lett. 2011;201:72–79. doi: 10.1016/j.toxlet.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Wu H.Z., Guo L., Mak Y.F., Liu N., Poon W.T., Chan Y.W., Cai Z. Proteomics investigation on aristolochic acid nephropathy: a case study on rat kidney tissues. Anal. Bioanal. Chem. 2011;399:3431–3439. doi: 10.1007/s00216-010-4463-4. [DOI] [PubMed] [Google Scholar]
- 18.Cui M., Liu Z.H., Qiu Q., Li H., Li L.S. Tumour induction in rats following exposure to short-term high dose aristolochic acid I. Mutagenesis. 2005;20:45–49. doi: 10.1093/mutage/gei007. [DOI] [PubMed] [Google Scholar]
- 19.Li W., Jiang H., Feng J.M. Isogenic mesenchymal stem cells transplantation improves a rat model of chronic aristolochic acid nephropathy via upregulation of hepatic growth factor and downregulation of transforming growth factor beta1. Mol. Cell. Biochem. 2012;368:137–145. doi: 10.1007/s11010-012-1352-5. [DOI] [PubMed] [Google Scholar]
- 20.Debelle F.D., Nortier J.L., De Prez E.G., Garbar C.H., Vienne A.R., Salmon I.J., Deschodt-Lanckman M.M., Vanherweghem J.L. Aristolochic acids induce chronic renal failure with interstitial fibrosis in salt-depleted rats. J. Am. Soc. Nephrol. 2002;13:431–436. doi: 10.1681/ASN.V132431. [DOI] [PubMed] [Google Scholar]
- 21.Ghomashchi F., Naika G.S., Bollinger J.G., Aloulou A., Lehr M., Leslie C.C., Gelb M.H. Interfacial kinetic and binding properties of mammalian group IVB phospholipase A2 (cPLA2beta) and comparison with the other cPLA2 isoforms. J. Biol. Chem. 2010;285:36100–36111. doi: 10.1074/jbc.M110.165647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pineiro R., Falasca M. Lysophosphatidylinositol signalling: new wine from an old bottle. Biochim. Biophys. Acta. 2012;1821:694–705. doi: 10.1016/j.bbalip.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita A., Kumazawa T., Koga H., Suzuki N., Oka S., Sugiura T. Generation of lysophosphatidylinositol by DDHD domain containing 1 (DDHD1): Possible involvement of phospholipase D/phosphatidic acid in the activation of DDHD1. Biochim. Biophys. Acta. 2010;1801:711–720. doi: 10.1016/j.bbalip.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Gao J., Zhang D., Yang X., Zhang Y., Li P., Su X. Lysophosphatidic acid and lovastatin might protect kidney in renal I/R injury by downregulating MCP-1 in rat. Ren. Fail. 2011;33:805–810. doi: 10.3109/0886022X.2011.601829. [DOI] [PubMed] [Google Scholar]
- 25.Pradère J.P., Gonzalez J., Klein J., Valet P., Grès S., Salant D., Bascands J.L., Saulnier-Blache J.S., Schanstra J.P. Lysophosphatidic acid and renal fibrosis. Biochim. Biophys. Acta. 2008;1781:582–587. doi: 10.1016/j.bbalip.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bai Y., Lu H., Hu L., Hong D., Ding L., Chen B. Effect of Sedum sarmentosum BUNGE extract on aristolochic acid-induced renal tubular epithelial cell injury. J. Pharmacol. Sci. 2014;124:445–456. doi: 10.1254/jphs.13216fp. [DOI] [PubMed] [Google Scholar]
- 27.Liu M., Yang X., Fan J., Zhang R., Wu J., Zeng Y., Nie J., Yu X. Altered tight junctions and fence function in NRK-52E cells induced by aristolochic acid. Hum. Exp. Toxicol. 2012;31:32–41. doi: 10.1177/0960327111407645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences used for real time RT-PCR.
Formation of scabs in rats receiving subcutaneously injected AA for 35 days. Rats received a subcutaneous injection of AA (10 mg/kg) daily for 35 days. (A) Photomicrographs of subcutaneous tissue of rat treated with AA (scale bar = 200 μm; HE stain). (B) Filtration of many polymorphonuclear leukocytes (shown by an elliptical dashed line) and macrophages (shown by a rectangular dashed line) (scale bar = 100 μm; HE stain).