Abstract
Cisplatin (CP) is one of the most active cytotoxic drugs. However, it has several side effects that are associated with increased oxidative stress. Aged garlic extract (AGE) is a natural product containing different compounds with antioxidant activity. The present study aimed to evaluate the effect of AGE on CP-induced hepatotoxicity. Four equally male rat groups: control, AGE-treated (250 mg/kg once for 21 days), CP-treated (7.5 mg/kg, once intraperitoneal), combined AGE and CP-treated were used. Blood samples were collected to investigate blood picture and serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin (TSB) and albumin. The liver of each rat was excised, cleaned, weighed, rinsed in ice-cold saline and homogenized for assessment malondialdehyde (MDA) level, catalase (CAT) and superoxide dismutase (SOD) activities and level of reduced glutathione (GSH). Histological examination was also carried out. AGE-pretreated rats revealed significant reduction in serum levels of AST, ALT & TSB and increase serum albumin level induced by CP administration. Furthermore, AGE significantly ameliorated CP-induced increase in MDA level and decrease in GSH level, CAT and SOD activities in liver tissue homogenates. Additionally, histopathological and blood picture examinations revealed markedly ameliorated CP-induced toxicity on blood cells parameters and liver structure. Our results prove that AGE has antioxidant and protective effects against CP-induced oxidative stress and changes in parameters of blood cells and liver structure in rats. Thus, it could be used as a dietary supplementation to reduce toxic side effects of anticancer drugs.
Keywords: Cisplatin, Aged garlic extract, Liver, Oxidative stress, Histopathology, Rat
1. Introduction
Cisplatin (CP), a potent antineoplastic drug, is widely used in the treatment of different solid-organ tumors. However, its clinical use is limited due to its toxic side effects including nephrotoxicity, neurotoxicity, ototoxicity and hepatotoxicity [1]. The toxicity of CP seems to be dose-dependent due to the cumulative effect of the drug [2], where the accumulation of CP produces obvious necrotic changes within the tissues of the affected organs. The generation of reactive oxygen species (ROS) and nitrogen species (NS) is one of the possible mechanisms responsible for CP toxicity through their oxidative stress injury and suppression of the antioxidant defense system [3]. To ameliorate the toxic effect of CP without inhibiting its antitumor effects, different experimental studies were carried out using a combination of CP with various radicular scavengers, enzyme inhibitors, sulfur-containing antioxidants and natural foods with antioxidant properties [1], [4], [5].
Natural and herbal products have been used in traditional medicine to treat a variety of diseases including malignancies [6]. The anticancer activities of the extract from a number of herbal plants have been demonstrated. A number of previous studies concluded that herbal medicine might have anticancer effect by enhancing the immune system, including cell differentiation, inhibiting telomerase activities and inducing apoptosis of cancer cells [7].
Garlic (Allium sativum) is a worldwide traditional food and dietary supplement. Nowadays, many garlic preparations are used in the medical field including fresh garlic extract, garlic oil, aged garlic extract (AGE) and a number of organosulfur compounds. AGE is an odorless garlic preparation produced by prolonged extraction of fresh garlic at room temperature for at least 20 months [8].
AGE contains many important water-soluble organo-sulfur compounds with potent antioxidant and free radical scavenging activities [8], [9]. So far, AGE has been demonstrated to possess several physiological activities in experimental animals, including vasodilative and hypotensive activities, the induction of decrease in serum cholesterol levels, antimicrobial, antiallergic, anti-inflammatory, immunomodulatory, and antioxidant properties [8], [9]. Recently, AGE has received particular attention because of studies that have reported that it is a highly efficient antioxidant and has free radical scavenging capacity [9], [10].
The present study aimed to investigate the possible protective effect of AGE on some hematological parameters, as well as on the activity of antioxidant enzymes and lipid peroxidation in liver of rats treated with CP.
2. Materials and methods
2.1. Chemicals
CP was obtained in the form of commercial Egyptian Unistin Vial (Egyptian International medical Company (EIMC) United Pharmaceuticals, Cairo, Egypt). AGE (kyolic) was obtained from Wakunaga of America (Mission Viejo, CA). It was prepared by soaking sliced raw garlic (Allium sativum) in 15–20% aqueous ethanol for at least 10 months at room temperature. The extract was then filtered and concentrated under reduced pressure at low temperature. The content of water-soluble compounds was relatively high while that of oil-soluble compounds was low. The AGE used in this study contained 28.6% extracted solids (286 mg/ml), and S-allyl cysteine, the most abundant water-soluble compound in AGE, was present at 1.47 mg/ml.
2.2. Animals
Twenty-four adult male Wister albino rats (12–14 weeks of age) were obtained from the animal house, Faculty of Medicine, Zagazig University, Zagazig, Egypt. The rats were kept under appropriate conditions of temperature (25 ± 2 °C), humidity (60–70%) and light (12 h dark/light cycle), free access of a commercial balanced diet and tap water ad libitum.
2.3. Experimental design
After one week of acclimatization, the animals were randomly divided into four equal groups in separate plastic cages, six rats each. Two groups (I and II) were used as control and received normal saline 0.5 ml i.p. and distilled water P.O. (group I) and AGE, 250 mg/kg orally (group II) for 21 days. Groups (III and IV) received single i.p. dose of CP (7.5 mg/kg) on day 16th, after successive administration of distilled water (0.5 ml, orally, group III) or AGE (250 mg/kg orally, group IV). The rats of each group were weighed on 1st, 4th, 7th, 10th, 13th, 16th, 19th and 22nd days.
2.4. Samples collection
On day 22th, (6 days after CP injection), the rats were anesthetized by ether inhalation. Blood samples were collected through a direct intracardiac puncture from each rat. Two blood samples from each rat were collected one sample was collected on EDTA (heparinized tubes) for determination of hematological parameters and the other was left to clot at 37 °C and centrifuged at 3000 rpm for 15 min. The serum (supernatant) was collected and stored at −20 °C for biochemical analysis.
Tissue samples: A vertical midline thoracic and abdominal incision was done to explore their viscera. Liver of each animal was excised, cleaned from their surrounding fat and connective tissue, washed with normal saline, blotted with filter paper, weighed and rinsed in ice-cold saline. Half of each liver was homogenized for biochemical analysis and the other half was processed for histological examination.
2.5. Hematological studies
The heparinized blood samples were analyzed for the number of red blood cells (RBCs), white blood cells (WBCs), and platelets, hemoglobin concentration (Hb%), hematocrit value (HTC), packed cells volume (PCV), mean corpuscle volume (MCV), mean corpuscle hemoglobin (MCH), mean corpuscle hemoglobin concentration (MCHC) and the differential count of polymorphs and lymphocytes according to standard methods using an Animal Blood Counter-ABC vet (Horiba ABX, France).
2.6. Biochemical assays
Liver biomarkers assessment: the levels of aspartate transaminase (AST) and alanine transaminase (ALT) enzymes were estimated in the sera of the blood samples using commercial kits (Roche Diagnostics, GmbH, D-68298, Mannheim, Germany) according to Reitman and Frankel [11]. Also, serum albumin was determined using commercial kit supplied by Diamond, RA50, Ireland and total serum bilirubin (TSB) was assayed according to the method of Schmidt and Eisenburg [12] as well.
Lipid peroxidation and antioxidants assessment: Half of each rat's liver was minced and homogenized in ice-cold 10% trichloroacetic acid phosphate buffer saline (0.05 M, pH 7.4). The homogenates were centrifuged at 15.000 × g for 15 min at 4 °C. The supernatants were collected for the measurement of catalase (CAT), superoxide dismutase (SOD), activities and the levels of reduced glutathione (GSH), and malondialdehyde (MDA). The antioxidant markers GSH, SOD, and CAT were measured by a colorimetric method using commercial kits (Biodiagnostic, Co., Cairo, Egypt) according to the manufacturer procedures. GSH was determined spectrophotometrically according to the method of Carlberg and Mannervik [13]. The method was based on the formation of a stable yellow colored compound in a reaction between Ellman's reagent (5,5-dithiobis(-2-nitrobenzoic acid)) and GSH to give a colored compound that has a characteristic absorption at 412 nm. SOD activity in kidney tissues was determined based on the ability of the enzyme to inhibit nitroblue tetrazolium (NBT) reduction by superoxide. The results were expressed as U/mg protein [14]. CAT activity was estimated by measuring the decomposition of hydrogen peroxide at 240 nm, according to the method of Aebi [15] and was expressed as U/mg protein. One unit of activity is equal to the moles of H2O2 degraded/min/mg protein. The malondialdehyde (MDA), (the marker of lipid peroxidation) was determined in homogenate by estimating level of thiobarbituric acid reactive substances (TBARS) measured as malondialdehyde (MDA), according to the method of Mihara and Uchiyama [16], or using commercial kits (Biodiagnostic, Cairo, Egypt). The results were expressed as nmol/g protein for MDA.
2.7. Histological studies
Specimens from each liver were fixed in 10% neutral-buffered formalin solution for 48 h, dehydrated in ascending grades of ethyl alcohol, cleared in xylol and embedded in paraffin blocks. Serial sections (3–5 μm) were cut using microtome (Leica RM 2125, Leica Biosystems Nussloch GmbH, Germany). The sections were washed in a water bath and left in the oven for dewaxing. Thereafter, the sections were stained with hematoxylin and eosin for general histological features determination, Periodic acid Schiff (PAS) stain to demonstrate mucopolysaccharides as PAS positive materials and Masson's trichrome stain for connective tissue staining [17]. The stained tissue-slides were mounted with DPX (Di-N-Butyle Phthalate Xylene) and covered with cover slips. All slides were examined by a light microscope (Olympus BH-2, Olympus, Tokyo, Japan).
2.8. Statistical analyses
Results were expressed as mean ± SEM. Comparison of means was done by the Student's t-test (One way ANOVA) and the Mann–Whitney U test. Values of P < 0.05 were considered statistically significant. Statistical evaluation was conducted with SPSS version 16.0 (SPSS Inc., Chicago, IL, USA).
The study was performed after the approval of the Medical Ethical Committee of the Faculty of Medicine, Zagazig University, Zagazig, Egypt and followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals.
3. Results
3.1. Hematological findings
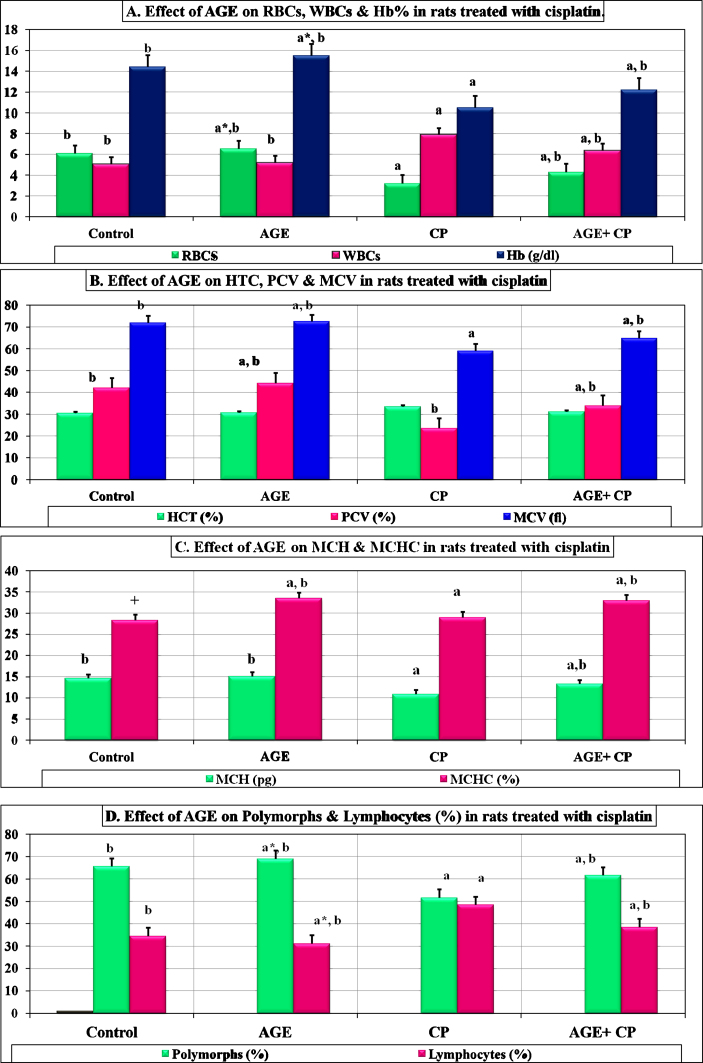
AGE pre-treated rats exhibited significant (P < 0.01) improvement in most of the hematological parameters and red blood cells indices compared to CP-treated rats (Fig. 1A–D). These observations were confirmed by increased RBCs and platelets counts, Hb%, PCV, MCV, MCH, and MCHC values. However, a significant (P < 0.01) reduction in RBCs and platelets counts as well as a significant (P < 0.01) increase in WBCs and lymphocytes counts and HTV value were recorded in CP-treated rats compared to control rats.
Fig. 1.
Effect of aged garlic extract on hematological indices in rats treated with cisplatin. Data are represented as mean ± SEM (n = 6). a: significant difference vs. control at P < 0.01. b: significant difference vs. cisplatin-treated group at P < 0.01. a*: P < 0.05. b*: P < 0.05. +: P = 0.594.
3.2. Liver biomarkers of rats
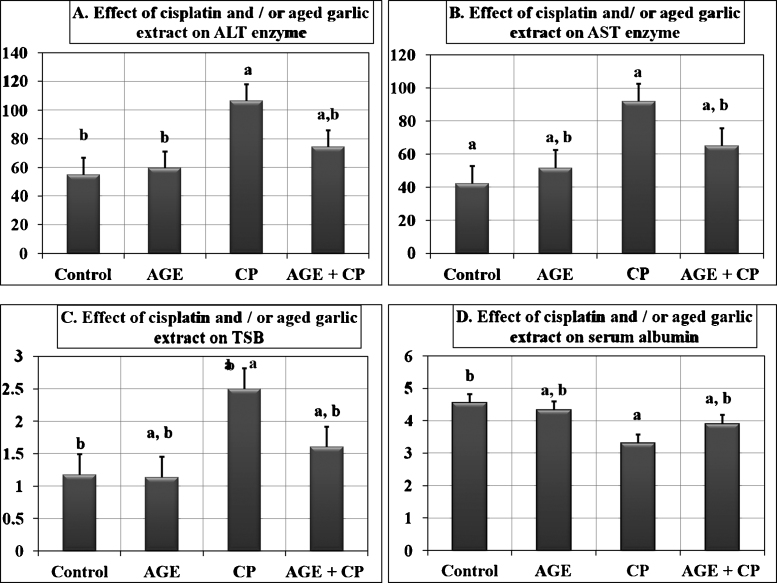
A significant (P < 0.01) elevation in serum AST, ALT and TSB levels was observed in CP-treated rats compared to those of the other three groups. However, the serum levels of these biomarkers revealed significant (P < 0.01) decrease in AGE+ CP-treated rats compared to CP-treated group. In addition, a significant (P < 0.01) decrease in the serum albumin level was observed in CP-treated rats as compared to control rats, meanwhile in AGE+ CP-treated rats, a significant (P < 0.05) increase in serum albumin level was reported compared to CP-treated group (Fig. 2).
Fig. 2.
Effect of aged garlic extract on liver biomarkers in rats treated with cisplatin. Data are expressed as mean ± SEM (n = 6). P < 0.001 compared to control (a) or cisplatin-treated (b) rats. a*: P < 0.05; a**: P < 0.005. TSB: total serum bilirubin; AST: Aspartate amino transferase enzyme; ALT: Alanine amino transferase enzyme.
3.3. Antioxidants and lipid peroxidation
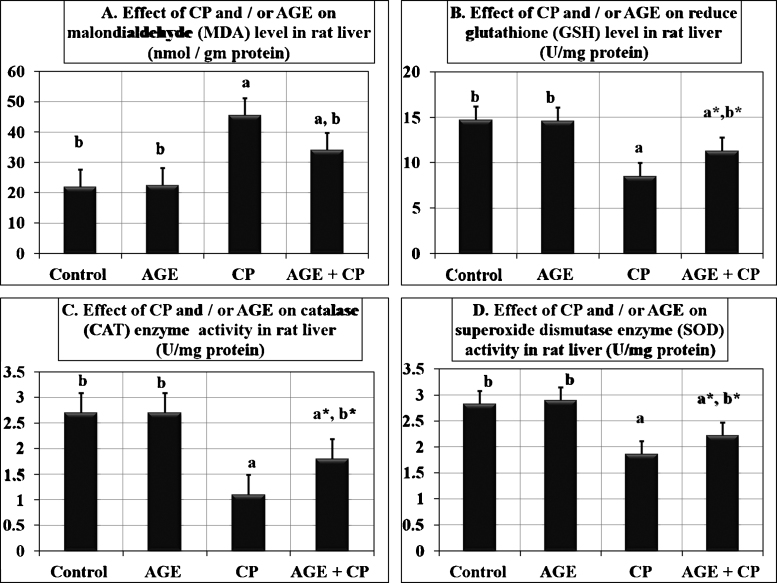
CP induced significant (P < 0.001) reduction in SOD, and CAT activities and GSH level compared to control group. However, CP pre-treated with AGE group showed significant increase of CAT (P = 0.0138), and SOD (P = 0.014) activities and GSH (P = 0.0018) level compared to CP-treated group. MDA level significantly (P < 0.0001) increased in CP-treated rats relative to other groups indicating the enhancement of lipid peroxidation. Meanwhile, MDA level in both CP-treated and combined AGE+ CP-treated groups revealed significant (P < 0.0001) difference compared to control group (Fig. 3).
Fig. 3.
Effect of aged garlic extract on hepatic antioxidant enzymes and lipid peroxidation in rats treated with cisplatin. Data are expressed as mean ± SEM (n = 6). a: significant difference vs. control group at P < 0.001. b: significant difference vs. Cisplatin-treated group at P < 0.001. a*: P < 0.05. b*: P < 0.05.
3.4. Histopathological examination
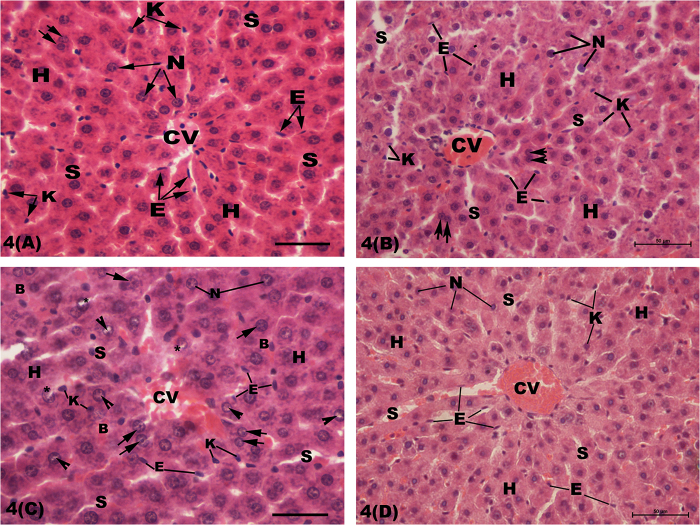
Normal architecture of radiating cords of hepatocytes from the central vein with blood sinusoids lined by flat endothelial and large kupffer cells in-between was observed in the centrilobular zone of the hepatic lobule in control rats (Fig. 4A), while mild congestion of central vein was seen in AGE-treated rats (Fig. 4B). However, the parenchymal structure of centrilobular zone of hepatic lobule in CP-treated rats exhibited disorganized centrilobular hepatic cords, dilated and congested central vein and blood sinusoids, cellular necrosis, nuclear pyknosis with margination of its heterochromatin and destructed nuclear membrane (Fig. 4C). On the other hand, normal hepatic parenchymal architecture with mild dilatation and congestion of the central vein and blood sinusoids was observed in combined AGE+ CP-treated rats (Fig. 4D).
Fig. 4.
Light micrographs of the centrilobular zone of rats’ livers showing; (A): normal hepatic cords (H) radiating around the central vein (CV) with blood sinusoids (S) in-between. The hepatocytes exhibit central rounded nuclei (N). Few hepatocytes have two nuclei (double arrows). The sinusoids are lined by numerous flat endothelial (E) and few large kupffer (K) cells. (B): Congested central vein (CV) is seen in aged garlic extract-treated rats. (C): In cisplatin-treated rats, disorganized hepatic cords (H), centrilobular cellular degeneration with dilated and congested central vein (CV) and blood sinusoids (S) are observed. The nuclei (N) of some hepatocytes reveal peripheral condensation of heterochromatin (arrow head) and discontinuous nuclear envelope (*) with few binucleated cells (double arrows). (D): Mild dilatation and congestion central vein (CV) is observed in the hepatic parenchyma of combined aged garlic extract and cisplatin-treated rats. B: blood cells. H & E stain. Bar = 50 μm.
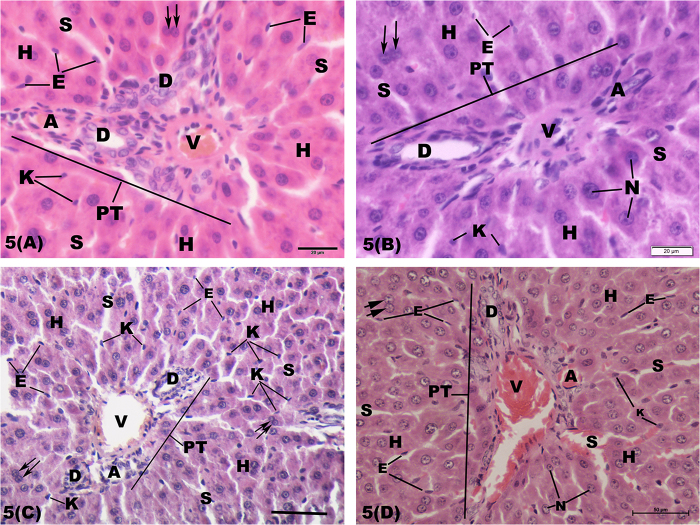
Normal portal tracts surrounded by condensed cords of small-sized hepatocytes with irregular blood sinusoids in-between was seen in the periportal area of liver parenchyma in control rats. Each portal tract consisted of bile ductule, hepatic arteriole and portal venule (Fig. 5A). However, there were wide portal tracts with dilated and congested portal venule, proliferation and dilatation of bile ductules in the periportal area of CP-treated rats (Fig. 5C). On the opposite side, normal hepatic architecture with dilated congested portal venules was seen in the periportal area in both AGE-treated and combined AGE+ CP-treated rats (Fig. 5B and D).
Fig. 5.
Light micrographs of the periportal zone of hepatic lobule of the rats showing (A): normal portal tract (PT) consisting of bile ductule (D), hepatic arteriole (A) and portal venule (V) in control rats. The tracts are surrounded by a network of hepatic cords (H) with blood sinusoids (S) in-between. (B): In aged garlic extract-treated rats; congested portal venules (V) are seen within the portal tract. (C): In cisplatin-treated rats; wide portal tract (PT) with dilated congested portal venule (V), hyperplastic bile ductule (D) are seen within the periportal area. (D): In combined aged garlic extract and cisplatin-treated rats; normal portal tract with congested portal venule (V) is observed in periportal area. E: endothelial cells; K: kupffer cells; N: nucleus. H & E stain. Bar = 20, 50 μm.
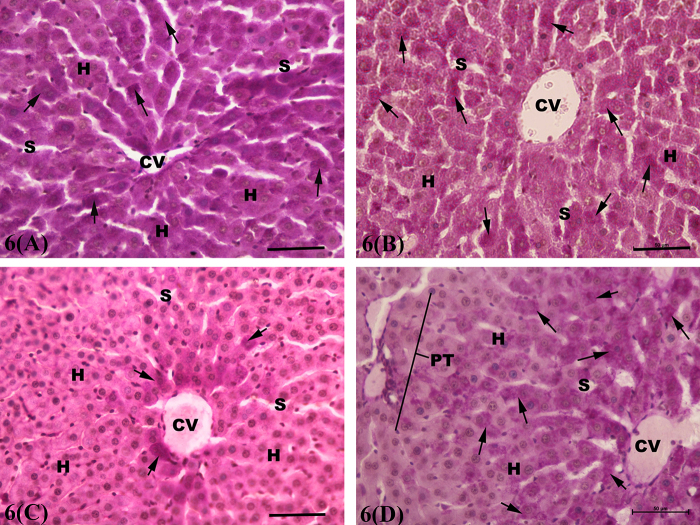
In PAS-stained sections, strong positive reaction was observed in the cytoplasm of hepatocytes all over the hepatic lobule in both control (Fig. 6A) and AGE-treated rats (Fig. 6B). However, scare positive PAS reaction was seen in few centrilobular hepatocytes in CP-treated rats (Fig. 6C). Conversely, in combined AGE+ CP-treated rats, an excessive glycogen deposition was observed in the centrilobular hepatic parenchymal cells as indicated by presence of strong positive PAS reaction within their cytoplasm (Fig. 6D).
Fig. 6.
Light micrographs of the PAS-stained sections of rat liver showing strong positive (arrow) PAS reaction within the cytoplasm of the parenchymal hepatocytes (H) throughout the hepatic lobule in control (Fig. 6A) and aged garlic extract-treated (Fig. 6B) groups. Scare positive PAS-reaction (arrow) is seen within the cytoplasm of few centrilobular hepatocytes in cisplatin-treated rats (Fig. 6C). Strong positive PAS reaction (arrow) is observed in the cytoplasm of centrilobular hepatocytes in combined aged garlic extract + cisplatin-treated rats. CV: central vein; S: blood sinusoid; H: hepatocytes; PT: portal tract. PAS Stain. Bar = 50 μm.
4. Discussion
CP is one of the most cytotoxic agents that is widely used to treat a variety of cancers but it is associated with toxic side effects on different body organs. The oxidative stress through formation of free radicals is one of the mechanisms of cisplatin-induced toxicity [3]. The free radical scavengers, that prevent the formation and/or scavenge the reactive hydroxyl free radicals, can provide protection against cisplatin-induced toxicity [18]. Different natural products and dietary compounds have been recently investigated and evaluated as potential protective antioxidant agents against cisplatin-induced hepatotoxicity [1], [3], [5]. AGE, natural dietary substance, has been previously investigated to ameliorate the toxic side effects of different substances through its antioxidant, radical-scavengering and antiperoxidative activities [8], [9]. The present study aimed to investigate the possible protective effect of AGE against CP-induced disturbance in hematological indices, oxidative stress and histopathological changes in rat liver.
Hematopoietic system is one of the most sensitive systems to evaluate the hazards effects of poisons and drugs in humans and animals [19]. In consistency, the present study indicated that a single dose of CP induced significant adverse effect in hematological parameters in rats six days after treatment and the pretreatment with AGE had successfully ameliorated the hematological disturbances induced by CP. The toxic effect of CP on blood parameters was demonstrated by the significant reduction in RBCs and platelets counts with subsequent decline in the values of Hb%, MCV, MCH, MCHC and PCV. Indeed, an increase in HTC, WBCs and lymphocytes was recorded in CP-treated rats as well. The previous results suggested that there was an etiological relationship between anemia and CP treatment. Such relationship could be explained through different mechanisms including destruction of bone marrow cells or increase osmotic fragility of RBCs. Thus, CP intoxication might lead to anemia as a result of either suppression the activity of hematopoietic tissues, impaired erythropoiesis, and accelerated RBCs destruction because of the altered RBCs membrane permeability, increased RBCs mechanical fragility, and/or defective Fe metabolism [20]. In agree with our results, Hassan et al. [21] reported that, the disturbances in RBCs could reflect an imbalance between its production and loss. The authors added that, the nephrotoxic effect of CP showed a negative effect on erythropoiesis that resulted in low production and count of RBCs.
Moreover, Marković et al. [22] stated that aside from the reduction in RBC number, chronic application of CP induced a reduction in the number of platelets and an increase in the number WBCs in the blood of rats. The decrease in platelets count might be due to CP inhibiting bone marrow activity or could be due to decreased production or increased consumption of platelets or due to the increased platelets aggregation [23]. In support of the above, Olas et al. [24] showed that CP causes oxidative stress in human platelets and lymphocytes, which might reflect on their life expectancy, the induction of apoptosis, and thereby ultimately reduce the number of these cells in the blood. However, the increase in the WBCs number could be the consequence of infection and inflammation during CP treatment and CP metabolism in the experimental rats. While, elevation of HTC value in CP-treated rats could be related to loss of the body fluid and hemoconcentration that resulted from the toxic effect of CP on the function of rat kidney [22].
However, the animals pretreated with AGE revealed significant modulation in most of the hematological indices changed in CP-treated rats. AGE was found to have beneficial effects against CP-induced suppression in most of hematological parameters and RBCs indices as it increased number of RBCs and Hb concentration about to normal levels. Also, AGE induced great improvement in the reduction of MCV, PCV, MCH, MCHC levels in CP-treated animals. Garlic-induced increase in erythrocytes count might be linked either to an increase in erythropoiesis or to the ability of garlic in decreasing membrane rigidity inherent to its cholesterol lowering effect [25]. These results indicated that AGE had preventive and protective effects against CP-induced hematological changes. AGE has been shown to exert antioxidant properties in plasma and erythrocytes of elderly subjects [26]. AGE exhibited profound antianemic, antifatigue, lipid-lowering activity and transaminases lowering when compared with i.p. route of treatment. Garlic high dose oral treatment exhibited profound antioxidant activity in red blood cells and plasma [25].
Previously, AGE has been shown to significantly improve erythrocyte deformability through stabilization of erythrocyte membranes in non-sickle RBC. This phenomenon was attributed to the antioxidant activities of AGE [9]. The ameliorative effect of AGE could be due to reduction of lipid peroxidation level in cell membrane with subsequent prevention of free radicals induced damage through its antioxidant activity achieved by its active compounds [8].
Evidence of CP-induced liver injury via a mechanism of oxidative stress caused by increased MDA (a potential lipid peroxidation biomarker), reduced GSH levels and decreased activity of CAT, SOD and GPx were demonstrated by various studies [1], [4], [26], [27]. Such oxidative stress was mediated through the generation of ROS that induced disturbance of membrane permeability and severe cell damage [3], [4]. In the present study, CP-induced higher MDA level, while decreasing SOD, CAT activities and GSH level in the homogenate of rat liver tissue. Increase MDA level enhanced the lipid peroxidation and increased ROS production with subsequent disturbance of membrane function and integrity [28]. Furthermore, inhibition of antioxidant CAT, SOD, and GSH enzymes was implicated in the pathogenesis of CP-induced hepatotoxicity [1], [27]. Our results agreed with the previous studies which revealed the involvement of oxidative stress and lipid peroxidation in the mechanisms of CP-induced hepatotoxicity [3], [27].
The natural products-derived antioxidants were previously used to protect against CP-induced oxidative stress in hepatic tissues in several studies [1], [21], [27]. This study was the first report to evaluate the preventive effect of AGE against CP-induced oxidative stress on hepatic tissues in rats. The water-soluble organo-sulfur compounds of AGE exhibited potent antioxidant and free radical scavenging activities [8], [9]. In the present study, pretreatment with AGE induced significant increase in hepatic CAT, SOD and GPx activities accompanied with significant decrease in hepatic MDA level in CP-treated rats. The protective effect of AGE might be mediated by its highly bioavailable and significant antioxidant compounds including S-allyl cysteine, S-allyl mercaptocyteine, allicin, and selenium that exhibited potent antioxidant activity [8]. The water-soluble S-allyl cysteine reduced the extent of lipid peroxidation and significantly enhanced antioxidant activities in vitro and in vivo [29]. Thus, AGE could ameliorate the lipid peroxidation and oxidative damages of rat liver tissues induced by CP through its antioxidant compounds. In accordance with present study, significant increase in cardiac and hepatic CAT, SOD and GPx activities accompanied with significant decrease in MDA level were reported in animals treated with AGE [10], [29], [30]. Garlic pretreatment increased the activity of SOD and CAT and it scavenges superoxide radicals and reduced myocardial damage caused by free radicals [26], [30]. Also, garlic extracts increased SOD and CAT activities in vascular cultured cells. S-allyl cysteine sulfoxide (alliin), a bioactive compound of garlic, prevented the reduction of hepatic SOD and CAT activities in diabetic rats [30].
AST and ALT are the most sensitive biomarker enzymes used in evaluation of the function and integrity of liver cells. Both enzymes are present mainly in the cytoplasm of hepatocytes [31]. In the present study, the rats injected with CP showed elevation of serum levels of ALT, AST, TSB and reduction of serum level of albumin as compared with saline control group. As the elevation in the serum activity of ALT, a liver cytoplasmic enzyme, indicates a necrotic lesions in the liver cells while the decrease in serum albumin level indicates that there was an impairment in both synthetic and execratory activities of liver cells [32]. In agree with our results, Abdelmeguid et al. [1] and Adaramoye et al. [3] reported that there was an increase in the levels of liver biomarkers in CP-treated rats.
Oral administration of AGE prior to and after CP significantly reduced its toxic effect on serum levels of AST & ALT enzymes compared to untreated rats. In agree with results of the present study, administration of AGE caused a significant reduction in the serum levels of AST & ALT in rats treated with cadmium [33]; lead [34] and doxorubicin [10]. The reduction of the liver enzymes in AGE pre-treated rats may be due to its antioxidant effect that reduces the free radical-induced oxidative damage in the liver, thereby stabilizing the membrane permeability and reducing the leakage of enzymes into the blood [18]. Similarly, reduction in serum levels of AST & ALT enzymes was reported with administration of other herbal plants such as silymarin [5] and ginseng extract [35] in cisplatin-induced hepatotoxicity.
In agree with our results, Abdelmeguid et al. [5] reported that, the biochemical findings in CP-treated rats were confirmed by the histopathological changes in the liver, where centrilobular necrotic changes, apoptotic nuclear changes, dilated congested central vein and blood sinusoids and wide portal tracts were observed. Congestion of veins and blood sinusoids within the hepatic parenchyma might be in part due to the direct irritant effect of cisplatin on the wall of blood vessels or secondary to the fibrotic changes in periportal areas affecting the intra-biliary system with subsequent obstruction of the Hering duct and dual blood supply of the liver [36].
In the current study, the oral intake of AGE before and after CP injection significantly improved most of the histopathological findings of CP-induced hepatotoxicity. Similar results were reported by Iseri et al. [4], Kart et al. [3], Abdelmeguid et al. [5] and El-Sharaky et al. [32] who demonstrated histopathological changes in liver parenchyma in CP-treated rats and their improvement by various natural antioxidant agents such as caffeic acid phenyl ester, silymarin, pomegranate seed extract and ginger extract. Due to its ability to reduce free radical-induced oxidative damage in the liver and to scavenge the hydroxyl and peroxyl radicals, AGE has been shown to improve the histopathological changes of the damaged liver cells [37]. Moreover, AGE or garlic powder were used to protect the liver against different other toxic agents including lead [34]; cadmium [33]. In addition, Alkreathy et al. [10] reported that AGE ameliorated the toxic effect of doxorubicin on the heart muscle in rats. Thus, administration of AGE improved most of hematological, biochemical and histopathological changes induced by CP through its antioxidant and free radical scavenging activities.
5. Conclusion
The present study revealed that administration of CP-induced hepatotoxicity in rats through the oxidative stress and lipid peroxidation. Such hepatotoxic effect of CP was indicated by alterations of liver biomarkers, lipid peroxidation biomarker (MDA), antioxidant enzymes including CAT, SOD, GPx and GSH and histological parameters. Also, different changes of hematological parameters were observed in CP-treated rats as well. However, the pre-treatment of AGE provided a beneficial role in CP-induced such previous changes through its antioxidant and free radical scavenging activities. Thus, AGE may be considered a useful dietary supplementary compound to patients treated with antineoplastic drugs including CP. This provides a cheap protective strategy in the management of acute hepatotoxicity or CP-induced liver damage. However further study is needed to explore the effect of AGE on the antineoplastic activity of CP.
Funding
The research received no specific grant from any funding agency in the public, community, or non-for profit sectors.
Conflict of interest
The author declares that there are no conflicts of interest. The research received no specific grant from any funding agency in the public, community, or non-for profit sectors.
Transparency document
Acknowledgments
The author thanks Prof. Abdel Moneim Osman and Prof. Sherif Hassan for revision of this manuscript.
Footnotes
Available online 16 September 2014
References
- 1.Mansour H.H., Hafez H.F., Fahmy N.M. Silymarin modulates Cisplatin 10 mg kg−1 induced oxidative stress and hepatotoxicity in rats. J. Biochem. Mol. Biol. 2006;39:656–661. doi: 10.5483/bmbrep.2006.39.6.656. [DOI] [PubMed] [Google Scholar]
- 2.Sastry J., Kellie S.J. Severe neurotoxicity, ototoxicity and nephrotoxicity following high-dose cisplatin and amifostine. Pediatr. Hematol. Oncol. 2005;22:441–445. doi: 10.1080/08880010590964381. [DOI] [PubMed] [Google Scholar]
- 3.Kart A., Yilmaz C., Musa K., Hasan O. Caffeic acid phenethylester (CAPE) ameliorates cisplatin-induced hepatotoxicity in rabbit. Exp. Toxicol. Pathol. 2010;62:45–52. doi: 10.1016/j.etp.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 4.Iseri S., Ercan F., Gedik N., Yuksel M., Alican I. Simvastatin attenuates cisplatin-induced kidney and liver damage in rats. Toxicology. 2007;230(2–3):256–264. doi: 10.1016/j.tox.2006.11.073. [DOI] [PubMed] [Google Scholar]
- 5.Abdelmeguid N.E., Chmaisse H.N., Abou Zeinab N.S. Silymarin ameliorates cisplatin-induced hepatotoxicity in rats: histopathological and ultrastructural studies. Pak. J. Biol. Sci. 2010;13:463–479. doi: 10.3923/pjbs.2010.463.479. [DOI] [PubMed] [Google Scholar]
- 6.Yin X., Zhou J., Jie C., Xing D., Zhang Y. Anticancer activity and mechanism of Scutellaria barbata extract on human lung cancer cell line. Life Sci. 2004;75:2233–2244. doi: 10.1016/j.lfs.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Gill C.L., Boyed A., McDermott E., McConn M., Servili M., Selvaggini R. Potential anticancer effects of virgin olive oil phenols on colorectal carcinogenesis models in vitro. Int. J. Cancer. 2005;117(1):1–7. doi: 10.1002/ijc.21083. [DOI] [PubMed] [Google Scholar]
- 8.Capasso A. Antioxidant action and therapeutic efficacy of Allium sativum L. Molecules. 2013;18(January (1)):690–700. doi: 10.3390/molecules18010690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ana L.C., Santana R.A., Silva-Islas C.A., Chanez-Cardenas M.E., Santamarıa A., Maldonado P.D. The antioxidant mechanisms underlying the aged garlic extract- and s-allylcysteine-induced protection. Oxid. Med. Cell. Longev. 2012:16. doi: 10.1155/2012/907162. Article ID 907162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alkreathy H., Zoheir A.D., Nessar A., Mark S., Soad S.A., Osman A.M. Aged garlic extract protects against doxorubicin-induced cardiotoxicity in rats. Food Chem. Toxicol. 2010;48:951–956. doi: 10.1016/j.fct.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Reitman S., Frankel A.S. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. Am. J. Clin. Pathol. 1957;28:53–60. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt M., Eisenburg J. Serum bilirubin determination in newborn infants. A new micro method for the determination of serum of plasma bilirubin in newborn infants. Fortschr. Med. 1975;93:1461–1466. [PubMed] [Google Scholar]
- 13.Carlberg I., Mannervik B. Glutathione reductase. In: Meister A., editor. Methods in Enzymology. Academic Press; New York: 1985. pp. 484–490. [DOI] [PubMed] [Google Scholar]
- 14.Kakkar P.S., Das B., Viswanathan P.N.A. Modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 15.Aebi H. Catalase. In: Bergmeyer H.U., editor. vol. 3. Academic Press; New York: 1983. pp. 276–286. (Methods in Enzymatic Analysis). [Google Scholar]
- 16.Mihara M., Uchiyama M. Determination of malondialdehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978;86(1):271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 17.Bancroft J.D., Gamble M. 5th ed. Churchill Livingstone Pub.; Edinburgh/New York/London/Philadelphia: 2002. Theory and Practice of Histological Techniques; pp. 125–138. 172–175, 184–193, 593–620. [Google Scholar]
- 18.Pradeep K., Mohan C.V.R., Gobianand K., Karthikeyan S. Silymarin modulates the oxidant–antioxidant imbalance during diethylnitrosamine induced oxidative stress in rats. Eur. J. Pharmacol. 2007;560:110–116. doi: 10.1016/j.ejphar.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Lijuv B., Jeena K., Kuttan R. Acute and subchronic toxicity as well as mutagenic evaluation of essential oil from turmeric Curcuma longa L. Food Chem. Toxicol. 2013;53:52–61. doi: 10.1016/j.fct.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 20.Yuan G., Dai S., Yin Z., Lu H., Jia R., Song X., Shu Y., Zhao X. Toxicological assessment of combined lead and cadmium: acute and sub-chronic toxicity study in rats. Food Chem. Toxicol. 2014;65:260–268. doi: 10.1016/j.fct.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 21.Hassan I., Chibber S., Naseem I. Ameliorative effect of riboflavin on the cisplatin induced nephrotoxicity and hepatotoxicity under photoillumination. Food Chem. Toxicol. 2010;48(8–9):2052–2058. doi: 10.1016/j.fct.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Markovic S.D., Žizic J.B., Djacic D.S., Obradovic A.D., Ćurcic M.G., Cvetkovic D.M., Đordevic N.Z., Ognjanovic B.A., Štajn A.Š. Alteration of oxidative stress parameters in red blood cells of rats after chronic in vivo treatment with cisplatin and selenium. Arch. Biol. Sci., Belgrade. 2011;63(4):991–999. [Google Scholar]
- 23.Sirage H.M. Biochemical and hematological studies for the protective effect of Oyster Mushroom (Pleurotus ostreatus) against glycerol-induced acute renal failure in rats. J. Biol. Sci. 2009;9(7):746–752. [Google Scholar]
- 24.Olas B., Wachowicz B., Majsterek I., Blasiak J. Resveratrol may reduce oxidative stress induced by platinum compounds in human plasma, blood platelets and lymphocytes. Anticancer Drugs. 2005;16:659–665. doi: 10.1097/00001813-200507000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Hamlaoui-Gasmi S., Mokni M., Limam N., Limam F., Amri M., Aouani E., Marzouki L. Effect of garlic's mode of administration on erythrocytes and plasma parameters in Wistar rat. Afr. J. Biotechnol. 2012;11(33):8259–8263. [Google Scholar]
- 26.Avci A., Atli T., Erguder I.B., Varli M., Devrim E., Aras S., Durak I. Effects of garlic consumption on plasma and erythrocyte antioxidant parameters in elderly subjects. Gerontology. 2008;54:173–176. doi: 10.1159/000130426. [DOI] [PubMed] [Google Scholar]
- 27.Palipoch S., Chuchard P., Phanit K., Prasit S. Hepatoprotective effect of curcumin and alpha-tocopherol against cisplatin-induced oxidative stress. Complement. Altern. Med. 2014;14:111–1118. doi: 10.1186/1472-6882-14-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estuo N. Lipid peroxidation: physiological levels and dual biological effects. Free Radic. Biol. Med. 2009;47(5):469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 29.Almaca G. Antioxidant effects of sulfur containing amino acids. Yonsei Med. J. 2004;45(5):776–788. doi: 10.3349/ymj.2004.45.5.776. [DOI] [PubMed] [Google Scholar]
- 30.Gorinstein S., Leontowicz H., Leontowicz M., Drzewiecki J., Najman K., Katrich E., Barasch D., Yamamoto K., Trakhtenberg S. Raw and boiled garlic enhances plasma antioxidant activity and improves plasma lipid metabolism in cholesterol-fed rats. Life Sci. 2006;78:655–663. doi: 10.1016/j.lfs.2005.05.069. [DOI] [PubMed] [Google Scholar]
- 31.Adaramoye O.A., Osaimoje D.O., Akinsanya A.M., Nneji C.M., Fafunso M.A., Ademowo O.G. Changes in antioxidant status and biochemical indices after acute administration of artemether, artemether-lumefantrine and halofantrine in rats. J. Compil. Basic Clin. Pharmacol. Toxicol. 2008;102:412–418. doi: 10.1111/j.1742-7843.2008.00211.x. [DOI] [PubMed] [Google Scholar]
- 32.El-Sharaky A.S., Newairy A.A., Kamel M.A., Eweda S.M. Protective effect of ginger extract against bromobenzene-induced hepatotoxicity in male rats. Food Chem. Toxicol. 2009;47:1584–1590. doi: 10.1016/j.fct.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Padalko V., Kozlova E., Leonova I. Protective efficacy of garlic on cadmium induced oxidative stress in young and adult rats. Oxid. Antioxid. Med. Sci. 2012;1(2):101–109. [Google Scholar]
- 34.Sharma A., Sharma V., Kansal L. Amelioration of lead-induced hepatotoxicity by Allium sativum extracts in Swiss albino mice. Libyan J. Med. 2010;5:4621. doi: 10.4176/091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdel-Wahhab K.G., Mahmoud M.S., Mona E.K., Mohamed A.A. Protective effect of a natural extract against cisplatin induced hepatotoxicity. J. Appl. Res. 2013;9(4):3129–3140. [Google Scholar]
- 36.Shona S., Abdel-Wakeel E., Sherif M., Tarek Zaki., Abdel-Galil I. Effect of cisplatinum on the liver of the adult albino rat and the possible protective role of vitamin E (Histological and Ultrastructural Study) Anat. Physiol. 2012;2(3):1–5. [Google Scholar]
- 37.Shaarawy M.S., Amany A.T., Saad M.E., Zakaria Y.A., Abeer B., Maha S.M., Emad K., Khalid M. Protective effects of garlic and silymarin on NDE-induced rats hepatotoxicity. Int. J. Biol. Sci. 2009;5:549–557. doi: 10.7150/ijbs.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.