Abstract
The targeting of vaccine antigens to antigen presenting cells (APC), such as dendritic cells (DCs), is a promising strategy for boosting vaccine immunogenicity and, in turn, protective and/or therapeutic efficacy. However, in vivo systems are needed to evaluate the potential of this approach for testing human vaccines. To this end, we examined human CD8+ T–cell expansion to novel DC–targeting vaccines in vitro and in vivo in humanized mice. Vaccines incorporating the influenza matrix protein-1 (FluM1) antigen fused to human specific antibodies targeting different DC receptors, including DEC-205, DCIR, Dectin-1, and CD40, elicited human CD8+ T-cell responses, as defined by the magnitude of specific CD8+ T-cells to the targeted antigen. In vitro we observed differences in response to the different vaccines, particularly between the weakly immunogenic DEC-205-targeted and more strongly immunogenic CD40-targeted vaccines, consistent with previous studies. However, in humanized mice adoptively transferred (AT) with mature human T cells (HM-T), vaccines that performed weakly in vitro (i.e., DEC-205, DCIR, and Dectin-1) gave stronger responses in vivo, some resembling those of the strongly immunogenic CD40-targeted vaccine. These results demonstrate the utility of the humanized mouse model as a platform for studies of human vaccines.
Keywords: Human Dendritic Cells, Cytotoxic T cells, Humanized mice, Dendritic cell targeted vaccines
INTRODUCTION
There is much interest in targeting vaccine antigens to DCs to boost vaccine immunogenicity. Seminal studies from the Nussenzweig and Steinman laboratories demonstrated the potential of monoclonal antibody (mAb)–based vaccines to cell-surface DC receptors (DC targeting vaccines) [1,2]. In these studies, a mAb-ovalbumin fusion protein specific for DEC-205, a C-type lectin receptor abundant on DCs, was 400 times more effective at eliciting ovalbumin-specific mouse CD8+ T-cell responses in vivo than non-targeted ovalbumin. Also, targeting antigens to dectin-1 or DCIR, both C-type lectins expressed by DCs, results in antigen specific CD8+ T cell responses in vitro [3,4]. Recent in vitro comparisons of DC targeting vaccines revealed differences in cross presentation [5] and identified CD40, a co-stimulatory molecule, as an optimal DC receptor target for cross presentation [6,7]. The superiority of CD40 was attributed to antigen accrual in minimally degradative compartments that, presumably, preserves antigen structure long enough for access to cross presentation machinery [6]. With the realization that early endosomal antigen targeting extends cross presentation to multiple human DC subsets (i.e., CD1c+ and CD141+ conventional (c)DCs), it was proposed that targeting multiple cross presenting subsets would maximize vaccine immunogenicity [7]. However, which DC receptors are best for antigen cross presentation in vivo remains unknown.
Humanized mice can be used to study the human immune system [8,9]. Human T cells develop endogenously in models like the NOD/SCID/IL-2γ−/− (NSG) mouse [9]. However, T cells in NSG are restricted to mouse MHC molecules, precluding the use of human specific vaccines [9]. Using HLA-A201 transgenic NSG mice in vaccine studies is complicated by a lack of human MHC Class II expression and subsequent lack of human MHC Class II restricted CD4+ T helper cells [9]. To circumvent these issues, we chose to use a model where mature T cells from human donors are AT into humanized NOD/SCID-β2microglobulin-deficient mice transplanted with syngeneic human CD34+ hematopoietic progenitor cells (HPCs) (T cell adoptive transfer humanized mouse model or “HM-T”). With HM-T, mice are reconstituted with all human DC subsets and B cells a few weeks following HPC transplant [10–12]. Subsequent AT of T cells permits analysis of memory responses to immunological stimuli. Whereas influenza challenge studies are not possible in HM-T because of the lack of human MHC molecule expression in mouse lung tissues, HM-T vaccinated with live attenuated influenza vaccine (LAIV) or with subunit vaccine (TIV) expand CD8+ T cells specific to FluM1 and nonstructural protein-1 in blood, spleen, and lungs [11].
Here, we tested whether HM-T can be used for assessing DC targeting vaccines. Herein, we find that FluM1-specific human CD8+ T-cell expansion using low doses of vaccine required DC antigen targeting. Importantly, results from vaccine comparisons in vivo differed from results generated in vitro. Thus, HM-T can be used to compare human-specific DC targeting vaccines.
MATERIALS AND METHODS
DC targeting vaccines
Human specific targeting IgG4 monoclonal antibody or corresponding IgG4 “isotype” control antibody fusion protein, which is not specific toward DC receptors, with heavy chain-fused FluM1 peptide (GILGFVFTL), here known as “FluM1” (i.e., “anti-CD40-FluM1 [13], anti-DCIR-FluM1 [4], anti-Dectin-1-FluM1 [3], anti-DEC-205-FluM1 [13], control “isotypeFluM1” vaccine [13]), were produced by the Baylor Institute for Immunology Research Biotechnology Core. The core antibody IgG4 sequences have been described (GenBank accession numbers KM660791 with at Kabat Q3K substitution sequence change, KM660792, JX002666, JX002667, KM246789, KM246790) or are referenced in [13]. [13] describes the process of transferring the variable regions of the human specific anti-DEC-205 MG38 clone (from Ralph Steinman) onto a human IgG4 framework. The control “isotype” IgG4 sequence is described in [13] along with procedures for appending antigen sequence to the H chain C-terminus and methods of vaccine production and quality assurance such as LPS determination (<0.01 ng LPS/mg protein). The FluM1 peptide sequence appended to the H chain C-termini has two copies of the CD8+ T-cell peptide (in bold) in the context of natural FluM1 sequences (underlined) [14]. ASQTPTNTISVTPTNNSTPTNNSNPKPNPASDTTEPATPTTPVTTPTTTLLPILSPLTKGILGFVFTLTVPSERK GILGFVFTLTRKNGSGETSPTSTPADSSTITPTATPTATPTIKGAS.
DC purification from humanized mice and human blood
Humanized mice
Sublethally irradiated NOD-SCID B2m−/− mice were transplanted with 3×106 CD34+ HPCs isolated from G-CSF-mobilized blood of HLA-A*0201+ healthy donors. All protocols were approved by the Institutional Review Board (IRB, 097-053 and 099-076) and the Institutional Animal Care and Use Committee (A01-005) at Baylor Research Institute. At 4–6 weeks post-transplant, humanized mice (without AT) were either mock inoculated with PBS or given 10 μg of CD40 fusion protein (Figure 4 specifically) and harvested. Harvested spleens were digested for 10 minutes with DNase and collagenase. Following purification with Ficoll-Paque Plus density-gradient centrifugation (Stemcell Technologies, Vancouver, BC, Canada), cells were stained on ice with fluorochrome-conjugated specific antibodies. DCs were then sorted with FACSAria (BD) using Diva software (BD) (supplementary figure 2).
Human blood
PBMCs were isolated from leukapheresis products using Ficoll-Paque Plus density-gradient centrifugation. DCs were enriched using human Pan-DC Pre-Enrichment Kit (StemCell Technologies) before FACS-sorting or use in co-culture.
Human PBMC assay for CD8+ T-cell responses
Human PBMC (1×106/ml) were cultured with IL-2 (100U/ml) for eight days with DC targeting vaccines. Following culture, cells were analyzed for FluM1-specific CD8+ T cell responses as in [11].
Autologous DC- CD8+ T-cell co-culture
CD8+ T cells were isolated from PBMCs using human T-cell enrichment kits (StemCell Technologies). CD8+ T cells were co-cultured with FACS sorted DC subsets or purified pan DCs (StemCell Technologies), IL-2 (10U/ml, added at day 0 and fresh every second day), IL-7 (10U/ml, day 0 only) for eight days in completed RPMI with 10% AB serum.
CD8+ T-cell responses in vivo
Sublethally irradiated NOD/SCID-β2microglobulin-deficient mice were transplanted with human CD34+ HPCs isolated from the PBMCs of granulocyte colony-stimulating factor mobilized donors. FLT3L or IFNα were not used in HM-T. ~4 weeks following injection of CD34+ HPC, total T cells (autologous to CD34+ HPCs) were isolated from PBMCs using human T-cell enrichment kits (StemCell Technologies). The day preceding vaccination, mice were AT i.v. with 2×107 total T cells in PBS. The day after adoptive transfer, mice were vaccinated i.v. with DC targeting vaccine. 75μl of blood was obtained via retro-orbital bleeding at indicated days. CD8+ T cells were stained in blood with specific antibodies and FluM1-specific MHC Class I tetramers. Following staining, blood was lysed with FACSlyse (BD) according to instructions and analyzed by FACS. Spleens and lungs were digested as described above, except that lungs were digested for 40 minutes. A total of ten different donors were used to create cohorts of HM-T for experiments.
RESULTS
CD8+ T-cell responses to CD40 targeted antigen in vitro
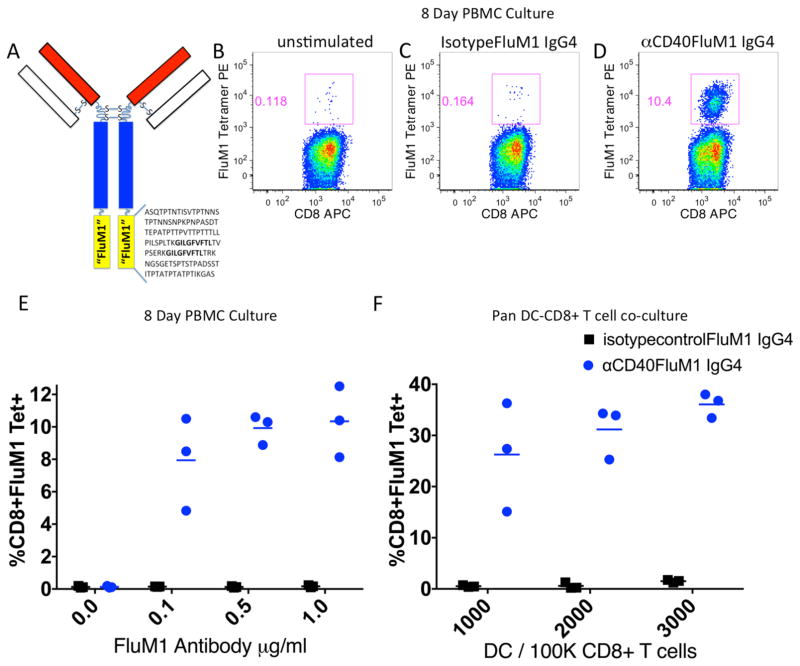
We first sought to determine whether a DC-targeted fusion protein vaccine encompassing canonical FluM1 epitope (GILGFVFTL) would elicit FluM1-specific CD8+ T-cell responses in vitro. To this end, human PBMCs were cultured with titrated amounts of vaccine composed of an antibody to CD40 fused to FluM1 peptide (Figure 1A), hereafter referred to as CD40-targeted vaccine, or a non-targeted control “isotypeFluM1” vaccine (containing an identical FluM1 sequence). After eight days of culture, flow cytometry was used to determine the expansion of antigen-specific CD8+ T cells as a percentage of CD3+CD8+ T cells bound with a HLA-A*0201-FluM1 peptide tetramer. The specificity of this tetramer is demonstrated in supplemental figure 1. As demonstrated in Figure 1B–D, CD40-targeted vaccine elicited expansion of FluM1-tetramer+ CD8+ T cells. Vaccine titration experiments showed a near-maximum response at 0.1 micrograms/ml of vaccine (Figure 1E). Next, we tested our fusion vaccine in co-cultures of CD8+ T cells and titrated numbers of autologous DCs. Again, only the CD40-targeted vaccine elicited expansion of FluM1-tetramer+ CD8+ T cells (Figure 1F). Thus, when targeted to CD40, the FluM1 antigen incorporated into a fusion protein vaccine drives antigen-specific CD8+ T-cell expansion in vitro.
Figure 1. Human FluM1-specific CD8+ T-cell responses in vitro.
(A) Cartoon depicting a targeting fusion protein with FluM1 linker sequence. (B–E) PBMCs were co-cultured for eight days with IL-2 (100U/ml) alone (B), isotype control-FluM1 IgG4 (1μg/ml) (C), or αCD40-FluM1 IgG4 (1μg/ml) (D). After culture, FluM1-specific CD8+ T cells were enumerated by FluM1-specific MHC class I tetramer staining and FACS. (E) Graphical representation of the experiments depicted in (B–D). Each dot or square represents a replicate. (F) Titrated numbers of negatively selected and purified human blood DCs were co-cultured for eight days with 100,000 negatively selected autologous CD8+ T cells in the presence of IL-7 (10U/ml, day 0 only) and IL-2 (10U/ml, added at days zero, two, four and six), αCD40-FluM1 IgG4, or isotype control-FluM1 IgG4 (both at 1μg/ml). Each dot or square represents a replicate. Experiments portrayed in B–E and F utilized different donors. Data in B–E and F is representative of three experiments using different human donors.
Cross presentation of CD40-targeted antigen in vitro
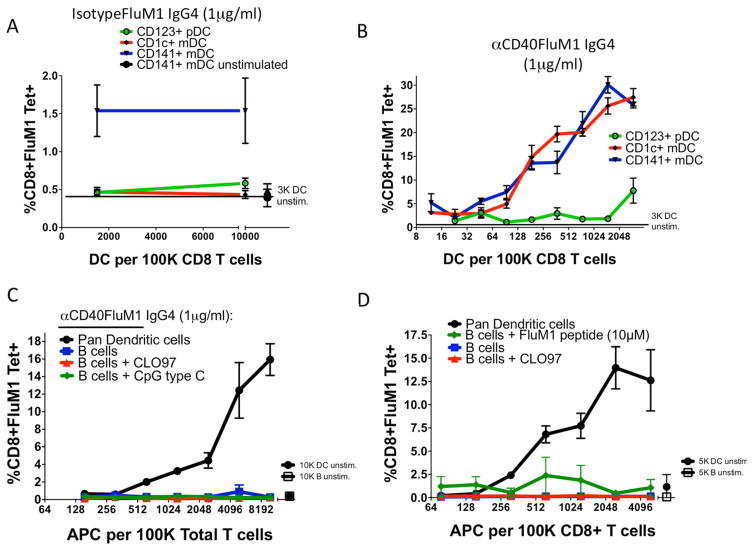
To determine which APCs cross-present antigens from CD40-targeted vaccine, FACS-purified human CD45+CD4+CD14−CD11c+CD141+ or CD1c+ cDC or human CD45+CD4+CD14−CD11c−CD123+ plasmacytoid (p)DC (supplementary Figure 2) were cultured at titrated doses with autologous CD8+ T cells. CD8+ T-cell expansion was measured as in Figure 1. Consistent with previous results [15,16], the non-targeted control “isotypeFluM1” vaccine induced FluM1-specific CD8+ T-cell expansion only in co-cultures with CD141+ DCs (Figure 2A). In contrast, the CD40-targeted vaccine prompted equivalent expansion in co-cultures with CD141+ or CD1c+ cDC subsets, and a ~32 fold weaker response from pDCs (Figure 2B).
Figure 2. Antigen presentation by human DC subsets after antigen targeting in vitro.
Titrated numbers of FACS-purified human blood DC subsets were cultured with either (A) non-targeted isotype control-FluM1 IgG4 (1μg/ml) or (B) αCD40-FluM1 IgG4 (1μg/ml). Lines indicate FluM1-specific CD8+ T-cell responses generated by CD141+ DCs (blue), CD1c+ DCs (red), and CD123+ plasmacytoid (p)DCs (green). Data is representative of three different experiments using different human donors. (C–D) Two separate experiments from the same donor in which negatively selected purified DCs or B cells are co-cultured with total T cells (C) or CD8+ T cells alone (D). Black lines indicate FluM1-specific CD8+ T-cell responses in co-cultures containing human blood DCs and αCD40-FluM1 IgG4 (1μg/ml), blue lines indicate human B cells and αCD40-FluM1 IgG4 (1μg/ml), red lines indicate B cells stimulated with CLO97 (1μg/ml) and αCD40-FluM1 IgG4 (1μg/ml), and green lines indicate in (C) B cells stimulated with CpG C (1μg/ml) and αCD40-FluM1 IgG4 (1μg/ml) or in (D) B cells and 10μM FluM1 peptide.
We asked whether human B cells could cross-present CD40-targeted antigen using the same co-culture approach. Human DCs and B cells were exposed to vaccine and cultured with autologous CD8+ T cells (Figure 2D) or autologous CD4+ and CD8+ T cells (Figure 2C). In contrast to DCs, B cells were unable to expand FluM1-tetramer+ CD8+ T cells following exposure to CD40-targeted vaccine (Figure 2C). Addition of CD4+ T cells (Figure 2C) and/or TLR ligands to cultures did not enable B cells to elicit a CD8+ T cell response. These results suggest that the B cell contribution to cross presentation is minor.
CD8+ T-cell responses and CD40 targeting in vivo
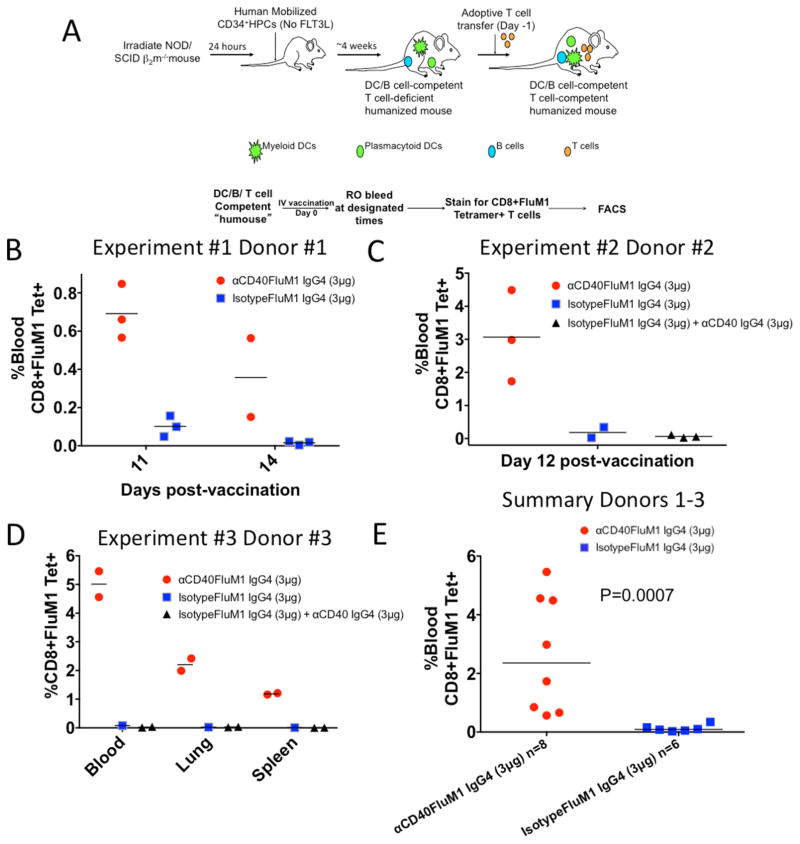
To test DC-targeting vaccines in vivo, we performed studies with HM-T (Figure 3A). HM-T were vaccinated with CD40-targeted vaccine or non-targeted control “isotypeFluM1” vaccine (Figure 3B). CD40-targeted vaccination led to higher blood FluM1-specific CD8+ T-cell responses in vivo (percentage of blood FluM1-specific CD8+ T cells, day 11: CD40 fusion protein, 0.69%±0.14%; non-targeted control fusion protein, 0.1%±0.06%). At day 14, mice immunized with CD40-targeted vaccine still displayed elevated numbers of circulating FluM1-specific CD8+ T cells (Day 14: CD40 fusion protein, 0.15% and 0.56%) compared to mice receiving the non-targeted control (0.01%±0.01%)
Figure 3. Human FluM1-specific CD8+ T-cell responses in vivo.
(A) Cartoon demonstrating a typical humanized mouse experiment. (B) Graphical representation of an experiment in which HM-T were vaccinated IV with either 3 μg αCD40-FluM1 IgG4 or 3 μg isotype control-FluM1 IgG4. The percentage of blood FluM1–specific CD8+ T cells was determined at days 11 and 14 post-vaccination. Each dot or square represents a single mouse. One αCD40-FluM1 IgG4 vaccinated mouse died between days 11 and 14. (C) A separate experiment in which groups of HM-T were vaccinated with 3 μg αCD40-FluM1 IgG4, 3 μg isotype control-FluM1 IgG4, or 3 μg isotype control-FluM1 IgG4 and 3 μg αCD40 IgG4. At day 12 post-vaccination, blood was analyzed to determine the percentage of FluM1-specific CD8+ T cells in each vaccination group. Each dot, square or triangle represents a single mouse. (D) Repeat of the experiment depicted in (B) except the lung and spleen compartments were also analyzed for FluM1-specific CD8+ T cells. (E) A summary of data from B–D comparing αCD40-FluM1 IgG4 and isotype control-FluM1 IgG4 at days 11 and 12 post-vaccination using a two-tailed non-parametric Mann-Whitney test. P<0.05 is a statistically significant difference. Red circles indicate mice vaccinated with 3 μg αCD40-FluM1 IgG4, blue squares are mice vaccinated with 3 μg isotype control-FluM1 IgG4, and black triangles are mice vaccinated with 3 μg isotype control-FluM1 IgG4 and 3 μg αCD40. Each experiment in B–D utilized a different human donor. FluM1-specific CD8+ T cells were enumerated by FluM1-specific MHC class I tetramer staining and FACS.
To determine if the targeting of FluM1 antigen is necessary for CD8+ T cell expansion, HM-T were vaccinated with CD40-targeted vaccine, control “isotypeFluM1” vaccine alone, or control “isotypeFluM1” vaccine plus CD40-specific monoclonal antibody with no FluM1. At day 12 post-vaccination, CD40-targeted vaccine–immunized mice developed elevated responses in the blood compared to mice receiving control “isotypeFluM1” vaccine alone or with naked anti-CD40 antibody (anti-CD40-FluM1, 3.08%±1.39%; control “isotypeFluM1” vaccine, 0.34% and 0.025%; control “isotypeFluM1” vaccine and CD40-specific antibody, 0.07%±0.04) (Figure 3C). In a separate experiment, only mice receiving the CD40-targeted vaccine displayed FluM1-specific CD8+ T-cell responses in blood and other tissues (Figure 3D). When blood FluM1-specific CD8+ T-cell responses from Figures 3B–3D were compared, the CD40-FluM1 vaccinated mice displayed significantly higher FluM1-specific CD8+ T cell responses (P=0.0007, Figure 3E). Similar to other results [6,13,14], our CD40-targeted vaccine did not induce co-stimulatory molecule upregulation by human DCs in vitro (data not shown). Thus, at low vaccine doses, antigen targeting is necessary for inducing FluM1-specific CD8+ T-cell responses to fusion protein in HM-T.
Cross presentation by DC subsets in HM-T
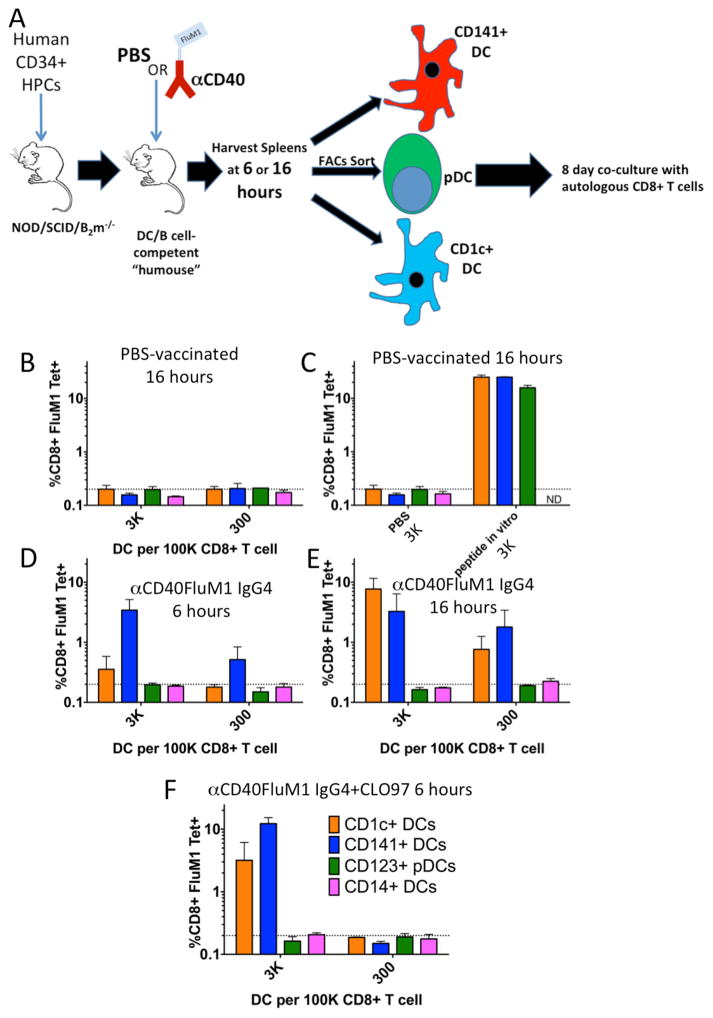
To understand human DC subset cross presentation in vivo, we compared DC subsets targeted with antigens in HM-T. Mice vaccinated with PBS or CD40-targeted vaccine were rested for six or 16 hours, and then spleen DC subsets were isolated (Figure 4A, Supplemental Figure 2A–E). CD1c+ cDCs and pDCs had purities greater than 95%, while CD141+ cDCs were 86.5% pure (Supplemental Figure 2B–E). Titrated numbers of each subset were co-cultured as in Figure 1F. We observed no CD8+ T-cell expansion with PBS treatment, though all DC subsets induced responses when pulsed with FluM1 peptide ex vivo (Figure 4B, C). Vaccination with CD40-targeted vaccine led to expansion of FluM1-specific CD8+ T cells co-cultured with either CD141+ or CD1c+ DCs after 16 hours of antigen targeting in vivo (Figure 4E). CD141+ DCs induced responses with just six hours of in vivo antigen targeting (Figure 4D).
Figure 4. Antigen presentation by human DC subsets after antigen targeting in vivo.
(A) Cartoon illustrating the humanized mouse antigen cross presentation assay. (B–F) Graphical representations of FluM1-specific CD8+ T-cell responses generated by purified human DCs (Supplemental Figure 2) isolated from groups of HM-T vaccinated with (B) PBS for 16 hours, (C) purified human DC from PBS vaccinated mice pulsed with 1μM FluM1 epitope peptide for 1 hour in vitro prior to co-culture (D) αCD40-FluM1 IgG4 for six hours (10μg/mouse IV), (E) αCD40-FluM1 IgG4 for 16 hours (10μg/mouse IV), or (F) αCD40-FluM1 IgG4 (10μg/mouse IV) and CLO97 (20μg/mouse IV, mixed with αCD40-FluM1 IgG4) for six hours. DC-CD8+ T-cell co-cultures were performed as in Figure 1F. Orange bars indicate responses mediated by CD1c+ DCs, blue bars by CD141+ DCs, green bars by CD123+ pDCs, and pink bars by CD14+ DC. Data is representative of three experiments using two different human donors.
CD1c+ DCs and pDCs express less CD40 than CD141+ DCs (Supplemental Figure 2F–G). Adjuvants like the TLR7/8 agonist CLO97 enhance CD40 expression (Supplemental Figure 2F–G). When CLO97 was mixed with the CD40-targeted vaccine, CD1c+ DCs gained the ability to expand FluM1-tetramer+ CD8+ T cells after six hours of antigen targeting in vivo (Figure 4F). Thus, both CD141+ and CD1c+ DC subsets can cross-present CD40-targeted antigen in vivo, albeit with different kinetics.
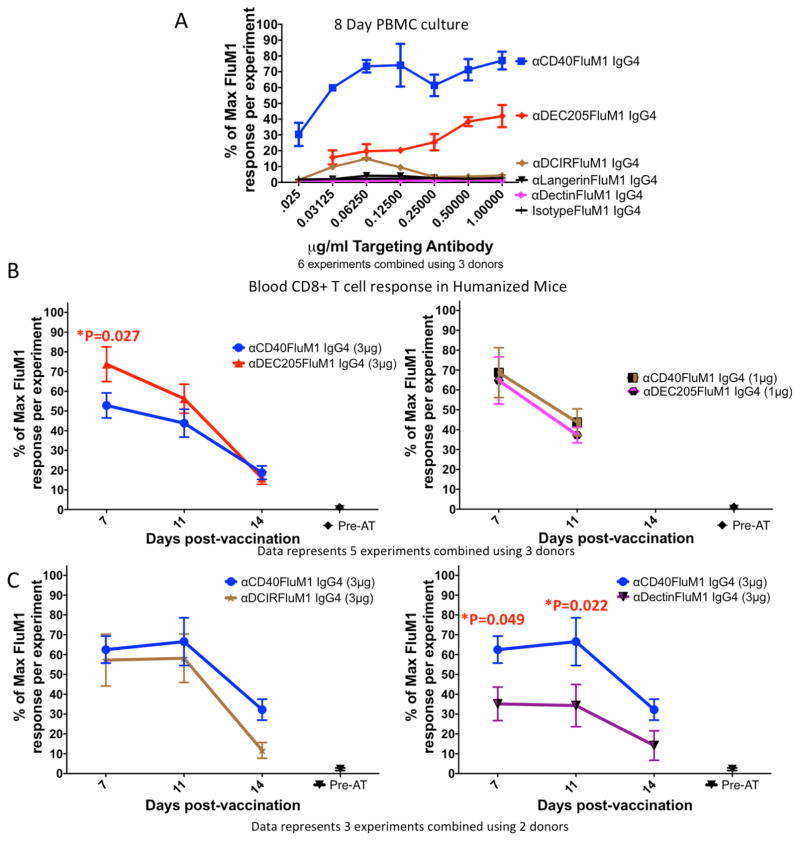
Comparing DC-targeting vaccines in vitro and in vivo
The cross presentation potential of human DC subsets purified from the blood is different from lymphoid organ DCs [17]. Thus, the humanized mouse model could offer a novel way to assess human CD8+ T-cell vaccines. To this end, HM-T received DC targeting vaccines to DCIR, Dectin-1, DEC-205, or CD40 and blood FluM1–specific CD8+ T-cell expansion was measured as in Figure 3. The frequency of FluM1-specific CD8+ T cells present at the time of AT was used as a baseline (Figure 5, see “Pre-AT”) because FluM1-specific CD8+ T cell expansion in in HM-T requires vaccination (Figure 3 and [11]).
Figure 5. Comparisons of different antigen-targeting vaccines in vitro and in vivo.
(A) FluM1-specific CD8+ T-cell responses in human PBMC cultures performed as in Figures 1B–E. FluM1-specific CD8+ T-cell responses mediated by αCD40-FluM1 IgG4 are in blue, αDEC205-FluM1 IgG4 in red, αDCIR-FluM1 in magenta, and αDCIR-FluM1 IgG4 in brown. Black lines indicate responses by FluM1 containing antibodies directed against Langerin or a non-targeted isotype control FluM1 antibody. All antibodies were titrated 1:2 starting at a concentration of 1μg/ml. Error bars indicate replicates across six experiments using three separate human donors. To normalize data across multiple experiments and donors, every data point was configured as a percentage of the highest response in the corresponding experiment according to the following formula: % of maximum FluM1 response per experiment = (response of replicate “X”/replicate with max response in experiment)*100. (B–C) Mice were vaccinated IV and FluM1-specific CD8+ T-cell responses measured from blood as in Figure 3. Responses were then normalized and compiled as in Figure 5A. (B) Mice were vaccinated with either 3 or 1μg doses of αDEC205-FluM1 IgG4 or αCD40-FluM1 IgG4. (B) represents normalized data in graphical form (mean and SEM) of five experiments using a total of 30 HM-T mice (15 vaccinated with αCD40-FluM1 IgG4, 15 with αDEC205-FluM1 IgG4) constructed from three donors. (C) Represents normalized data (as in 5B) from three separate experiments using two different donors (20 HM-T mice total, 8 vaccinated with αCD40-FluM1 IgG4, 6 with αDCIR-FluM1 IgG4, and 6 with αDectin-FluM1 IgG4). Mice were vaccinated with 3μg αDCIR-FluM1 IgG4, 3μg αDectin-FluM1 IgG4, or 3μg αCD40-FluM1 IgG4. For experiments in this figure, a single dot triangle, hexagon, square or star represents one vaccinated humanized mouse. “Pre-AT” means the frequency of FluM1-specific CD8+ T cells from human donors at the time of adoptive transfer one day before vaccination. To account for the repeated measures of the longitudinal studies presented in Figure 5B and 5C, a Linear Mixed Model analysis was performed. Vaccine-induced responses induced at different time points were compared using PROC MIXED in SAS, using REPEATED statement. As fixed effects, we included treatment (vaccines) and day, and treatment-day interaction. A covariance structure of SP (POW) was used to account for the fact that responses from the same mice are more correlated for adjacent time points and correlation decreases with increasing distance between time points. P<0.05 is a statistically significant difference.
Similar to in vitro comparisons (Figure 5A), both the CD40 and DEC-205 vaccines expanded FluM1-specific CD8+ T cells in vivo at two low doses (1 and 3 μg/mouse; 167 fold over pre-AT levels at maximum response) (Figure 5B). However, unlike the in vitro comparison, the DEC-205 vaccine induced a significantly higher response at day 7 (3μg/mouse dose) (right panel, P=0.027). There were no significant differences between the extent and durability of responses induced by both vaccines at the higher dose on days 11 and 14 and at all time points measured using the lower dose (left panel). Thus, in contrast to in vitro findings (Figure 5A and [6,7]), the DEC-205-targeted vaccine was at least as potent as the CD40-targeted vaccine at promoting FluM1-specific CD8+ T cell expansion in vivo.
To further determine whether vaccine-induced FluM1-specific CD8+ T cell expansion differed in HM-T versus in vitro, we evaluated the DCIR- and Dectin-1-targeted vaccines in vivo. In contrast to the very weak response noted in vitro (Figure 5A), the DCIR vaccine-induced responses which did not differ significantly from those induced by the CD40-targeted vaccine (Figure 5C, left panel). Thus, immunization with the DCIR-targeted vaccine resulted in much more substantial responses in vivo than in vitro. Lastly, we tested the Dectin-1-targeted vaccine that did not elicit CD8+ T-cell responses in vitro (Figure 5A). Although the responses to Dectin-1-targeted vaccine were significantly lower compared to the CD40-targeted vaccine at days 7 and 11 post-vaccination (Figure 5C, right panel, P=0.049 and P=0.022, respectively), they were 49-fold higher than pre-AT levels at the maximum response. Thus, the humanized mouse model revealed responses to vaccines that were undetectable in vitro.
DISCUSSION
Here we show that HM-T can be used to study DC targeting vaccines. Expansion of influenza-specific CD8+ T cells upon TIV vaccination in HM-T depends on the human myeloid compartment [22]. Here, cDC in HM-T are the main APCs cross presenting CD40-targeting vaccines. Furthermore, in regards to the use of low vaccine doses, human CD8+ T-cell expansion requires antigen targeting in HM-T.
Human T cells can develop endogenously in some models, like the NOD/SCID/IL-2γ−/− (NSG) mouse [9]. However, these T cells are restricted to mouse MHC molecules limiting the use of human MHC-specific epitope vaccines and human MHC class I-specific tetramers [9]. The use of HLA-A2.1 transgenic NSG mice in immunological studies is complicated by a lack of human MHC Class II expression in the mouse thymus and subsequent lack of human MHC Class II restriction by CD4+ T helper cells [9]. To circumvent these issues, we have chosen to use a humanized mouse model where mature peripheral blood human T cells from human donors are adoptively transferred into NOD/SCID-β2microglobulin-deficient mice transplanted with human CD34+ hematopoietic progenitor cells (HPCs). In addition, the lack of stable mouse MHC class I in periphery of HM-T could possibly lessen graft versus host disease as compared to NOD/SCID mice [9].
Our CD40-targeted vaccine does not enhance DC-co-stimulatory molecule expression [6,7,13]. Vaccination with CD40-specific antibody and non-targeted control “isotypeFluM1” vaccine was unable to induce FluM1-specific CD8+ T cell expansion in HM-T (Fig. 3). These results suggest that targeting of antigen to an APC, rather than the activation status of the APC, is of primary importance for inducing antigen specific CD8+ T cell expansion.
In HM-T, HPCs develop into human DCs that home to tissues [10] where, most likely, they interact with AT T cells. For instance, when LAIV is delivered intranasally, both the lung and lung-draining lymph nodes of HM-T contain human DCs that expand influenza-specific CD8+ T cells [11]. Upon injection, DC-targeting vaccines likely distribute via the bloodstream to tissues, such as the spleen, where DCs reside. Upon binding to DC-expressed receptors, the vaccine-derived antigens are internalized and processed [6,7] and presented to T cells. Surprisingly, we found that some vaccine-induced responses were more potent in vivo than their in vitro results would predict. These results suggest dynamics inherent to the HM-T model that are either absent or unimportant to the outcomes of in vitro experimental systems.
One such dynamic may be that a greater number and a larger variety of cell types can cross present vaccines in vivo. For example, we used blood DC in vitro whereas the HM-T model contains both lymphoid and tissue-resident DCs, as well as those from the blood [10,11]. Correspondingly, our results revealed differences in vaccine-induced responses depending on the receptor targeted. CD40-targeted vaccine-induced responses were detectable in vitro and in vivo, as would be expected given the broad expression of CD40 amongst DC subsets. In contrast, CD8+ T-cell responses to DEC-205-, Dectin-1- and DCIR-targeted vaccines were more readily detectable in vivo.
In contrast to the fusion proteins used here in vitro (Figure 5A), DCs treated with non-covalent conjugate vaccines targeting DCIR or Dectin-1 yield robust memory FluM1-specific CD8+ T-cell expansion in vitro [3,4]. These contrasting results suggest that antigen incorporated into a fusion protein is more difficult to cross-present. As noted above, the APCs able to cross-present fusion proteins may not be present in blood. Nonetheless, because CD8+ T-cell expansion was noted after just one immunization with our DCIR or Dectin-1 fusion proteins, both given without adjuvants, we conclude that the HM-T is a sensitive platform for vaccine testing.
The DEC-205 vaccine elicited higher responses in vivo than in vitro (Figure 5), yet the CD40-targeted vaccine may still be superior [6,7]. Future experiments testing variables like dosage, addition of adjuvants, and type of vaccine antigen used (self/cancer antigens versus viral antigens, priming versus recall antigens) are required for broadening the scope of our findings.
Whereas target receptor expression by cell type is not predictive of cross presentation ability, the breadth of target receptor expression across cell types is likely to determine the potency of a targeting vaccine in vivo. For instance, CD40’s wide expression pattern may dilute the CD40-targeted vaccine. Hypothetically, some CD40-targeted vaccine will bind cells, like CD40+ B cells, unable to cross-present vaccine antigen (Figure 2). Despite its cross presentation disadvantage [6,7], DEC-205 targeted antigen may be more restricted to cells able to cross-present in vivo. Thus, targeting receptors with expression limited primarily to cells capable of cross presentation may maximize vaccine potency in vivo.
CONCLUSION
The HM-T model revealed responses to DC targeting vaccines not appreciated in vitro. Thus, this model may provide additional insights in the study of human CD8+ T cell vaccines.
Supplementary Material
Acknowledgments
The authors would like to thank members of the Baylor Institute for Immunology Research flow cytometry core and tissue procurement core for excellent reagents and assistance, Zhiqing Wang for reagents, and Dr. Gerard Zurawski (BIIR) and Anna Lisa Lucido (JAX-GM) for help editing the manuscript.
Supported by Roche Collaborative Research Grant and Baylor Research Institute.
Abbreviations
- DCs
dendritic cells
- c
conventional
- p
plasmacytoid
- HPCs
CD34+ hematopoietic stem cells
- FluM1
influenza M1 protein
- FACS
fluorescent activated cell sorting
- PBMCs
peripheral blood mononuclear cells
- mAb
monoclonal antibody
- APCs
antigen presenting cells
- AT
adoptive transfer
Footnotes
AUTHOR CONTRIBUTIONS
JPG, KP, and JB designed experiments and wrote the manuscript. JPG, PA, FM, and CY performed experiments. SZ, PK, XL, ZW and AF prepared reagents. AA performed statistical analyses.
Conflict of interest: The authors have no conflicting financial interests, except that G.Z. and S.Z are named inventors of patents relating to DC targeting that are held by Baylor Research Institute.
References
- 1.Bonifaz LC, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient Targeting of Protein Antigen to the Dendritic Cell Receptor DEC-205 in the Steady State Leads to Antigen Presentation on Major Histocompatibility Complex Class I Products and Peripheral CD8+ T Cell Tolerance. Journal of Experimental Medicine. 2002;196:1627–38. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–79. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ni L, Gayet I, Zurawski SM, Duluc D, Flamar AL, Li XH, et al. Concomitant Activation and Antigen Uptake via Human Dectin-1 Results in Potent Antigen-Specific CD8+ T Cell Responses. The Journal of Immunology. 2010;185:3504–13. doi: 10.4049/jimmunol.1000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klechevsky E, Flamar AL, Cao Y, Blanck JP, Liu M, O’Bar A, et al. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2010;116:1685–97. doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–69. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee B, Smed-Sorensen A, Cohn L, Chalouni C, Vandlen R, Lee BC, et al. Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells. Blood. 2012;120:2011–20. doi: 10.1182/blood-2012-01-402370. [DOI] [PubMed] [Google Scholar]
- 7.Cohn L, Chatterjee B, Esselborn F, Smed-Sorensen A, Nakamura N, Chalouni C, et al. Antigen delivery to early endosomes eliminates the superiority of human blood BDCA3+ dendritic cells at cross presentation. Journal of Experimental Medicine. 2013;210:1049–63. doi: 10.1002/eji.201242477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, et al. Development and function of human innate immune cells in a humanized mouse model. Nat Biotech. 2014;32:364–72. doi: 10.1038/nbt.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12:786–98. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palucka AK, Gatlin J, Blanck JP, Melkus MW, Clayton S, Ueno H, et al. Human dendritic cell subsets in NOD/SCID mice engrafted with CD34+ hematopoietic progenitors. Blood. 2003;102:3302–10. doi: 10.1182/blood-2003-02-0384. [DOI] [PubMed] [Google Scholar]
- 11.Yu CI, Gallegos M, Marches F, Zurawski G, Ramilo O, Garcia-Sastre A, et al. Broad influenza-specific CD8+ T-cell responses in humanized mice vaccinated with influenza virus vaccines. Blood. 2008;112:3671–8. doi: 10.1182/blood-2008-05-157016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu CI, Becker C, Wang Y, Marches F, Helft J, Leboeuf M, et al. Human CD1c+ dendritic cells drive the differentiation of CD103+ CD8+ mucosal effector T cells via the cytokine TGF-β. Immunity. 2013;38:818–30. doi: 10.1016/j.immuni.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flamar AL, Zurawski SM, Scholz F, Gayet I, Ni L, Li XH, et al. Noncovalent Assembly of Anti-Dendritic Cell Antibodies and Antigens for Evoking Immune Responses In Vitro and In Vivo. The Journal of Immunology. 2012;189:2645–55. doi: 10.4049/jimmunol.1102390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flamar A-L, Xue Y, Zurawski SM, Montes M, King B, Sloan L, et al. Targeting concatenated HIV antigens to human CD40 expands a broad repertoire of multifunctional CD4+ and CD8+ T cells. Aids. 2013;27:2041–51. doi: 10.1097/QAD.0b013e3283624305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8 + dendritic cells. Journal of Experimental Medicine. 2010;207:1261–71. doi: 10.1006/cimm.1995.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre CA, Ventre E, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8 + dendritic cells. Journal of Experimental Medicine. 2010;207:1283–92. doi: 10.1093/intimm/dxm119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segura E, Durand M, Amigorena S. Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells. Journal of Experimental Medicine. 2013;210:1035–47. doi: 10.1084/jem.20121103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.