ABSTRACT
The immune receptor NKG2D is predominantly expressed on NK cells and T cell subsets and confers anti-tumor activity. According to the current paradigm, immune surveillance is counteracted by soluble ligands shed into the microenvironment, which down-regulate NKG2D receptor expression. Here, we analyzed the clinical significance of the soluble NKG2D ligands sMICA and sULBP2 in the malignancy-associated ascites of ovarian cancer. We show that high levels of sMICA and sULBP2 in ascites were associated with a poor prognosis. Ascites inhibited the activation of normal NK cells, which, in contrast to the prevailing notion, was not associated with decreased NKG2D expression. Of note, an inverse correlation of soluble NKG2D ligands with effector memory T cells and a direct correlation with pro-tumorigenic CD163+CD206+ macrophages was observed. Thus, the role of soluble NKG2D ligands within the ovarian cancer microenvironment is more complex than anticipated and does not exclusively function via NKG2D downregulation.
Keywords: Ascites; immunosurveillance, NKG2D, ovarian cancer
Introduction
Natural killer (NK) cells represent an important element of the innate immune surveillance. These innate lymphocytes contribute to the first line of defense against pathogens and are mainly responsible for the elimination of virus-infected cells and tumor cells. NK cells recognize their target cells via an antigen-independent set of receptors and kill them through cytotoxic granules containing perforins and granzymes, by expression of death ligands such as tumor necrosis factors α (TNFα), TNF-related apoptosis-inducing ligand (TRAIL), or CD95L (Fas ligand).4
Moreover, NK cells secrete cyto- and chemokines such as TNFα, interferon-γ (IFN-γ) and MIP-1α, MIP1-β and RANTES, which activate immune cells including dendritic cells and macrophages.7 Given the high cytotoxic potential of NK cells, these cells need to be tightly regulated. Their activation is fine-tuned by the balance of activating and inhibitory receptors. Normal healthy cells are protected from the NK cell attack due to the expression of self-defining major histocompatibility complex (MHC) class I molecules, which bind to inhibitory receptors on NK cells. Tumor cells may lose expression of MHC class I molecules to evade T cells but in turn allow target cell killing by NK cells. In addition, NK cells are selectively activated by stressed or malignant cells, upon upregulation of activating ligands for NK cells, which overcome the inhibitory signaling delivered by MHC class I molecules.19,20
One of the major activating receptors involved in recognition and killing of transformed cells is the natural-killer group 2, member D (NKG2D) receptor.3 In humans, NKG2D is abundantly expressed on cytotoxic NK cells and CD8+ T cells and is also detectable on CD4+ T cell subsets, γδ T cells, NKT cells, and regulatory T cells (Tregs). Activating ligands for NKG2D (MICA/B, ULBP1–6) are barely expressed on healthy cells but induced upon malignant transformation on cancer cells. Thus, the inducible expression of NKG2D-ligands represents a danger signal, which alerts the immune system to the dangerous cell. Engagement of NKG2D on NK cells results in direct target cell killing and release of pro-inflammatory cytokines, while NKG2D expressed on the subsets of T cells provides co-stimulation.21,28,42
Immune surveillance via NKG2D and the corresponding ligands appears to be particularly effective in the early stages of tumor growth. Later, tumors develop strategies to circumvent immune detection via NKG2D. Immune escape during tumor progression results from the release of soluble ligands from the tumor cell surface into the environment by shedding.5,26 These soluble molecules (sNKG2D-L) passively block NKG2D and induce receptor internalization to down-regulate NKG2D on the surface of NK cells and thereby impair NK cell function.1,2,3,10,13,15,29,39 Serum levels of sNKG2D-L correlate with a poor prognosis and are used as prognostic markers in some tumor entities.37,38,45 Further, cytokines such as TGFβ and MIF (macrophage migration-inhibitory factor) down-regulate NKG2D expression on effector cells.16,32 Thus, an immunosuppressive environment characterized by high levels of sNKG2D-L, MIF and TGFβ can promote immune evasion by downregulation of NKG2D on NK and T effector cells. Finally, NKG2D ligand expression can be switched off by tumor cells during tumor progression, e.g., via mRNA degradation by microRNAs.41
Ovarian carcinoma has a dire prognosis and is the most lethal of all gynecological cancers.6 It is characterized by a unique tumor microenvironment, including the peritoneal fluid, which occurs as a malignancy-associated effusion at more advanced stages.23,46 This ascites contains a plethora of tumor-promoting and immunosuppressive cytokines, growth factors, lipids, extracellular vesicles, tumor cell spheroids and large numbers of immune cells, which form a malicious alliance to support tumor progression and immune evasion12,46).
Not much is known about the relevance of NKG2D and its ligands in ovarian cancer. Immunohistology revealed a higher expression of surface MICA/B and ULBP2 in ovarian cancer cells compared with normal ovary, and this was surprisingly associated with a poor prognosis.25 These findings, which are in contrast with previous data showing that high NKG2D ligand expression on tumor cells is associated with a good prognosis (e.g., colorectal cancer31 were also confirmed by an independent study.30 Using tissue microarrays, it was shown that the high expression of several surface NKG2D ligands was inversely correlated with disease survival. In vitro data using ovarian cancer cells lines suggest that NKG2D activity is regulated by sNKG2D-L and MIF, a leaderless cytokine that contributes to NKG2D downregulation.16,17,32 However, data on the expression level of sNKG2D-L in the ascites fluid of patients as well as its correlation with the NKG2D receptor expression are not available.
Given the immune suppressive microenvironment in ovarian cancer and the malignancy-associated ascites we sought to investigate a possible role for MIF and sNKG2D-L in immune evasion in ovarian cancer.
Results and discussion
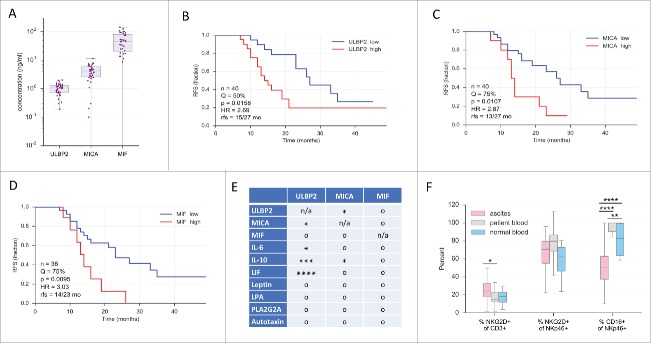
It is well established that tumor cells release soluble ligands for NKG2D to inhibit anti-tumor immune responses via blocking and downregulation of the receptor. To elucidate the clinical relevance of sNKG2D-L, we analyzed the levels of soluble NKG2D ligands (MICA, ULBP2) and MIF in the ascites and measured NKG2D expression on tumor associated lymphocytes in human ovarian carcinoma ascites. MIF, sMICA and sULBP2 (n = 36–40, Fig. 1A) were detectable in ascites fluid by ELISA and ascites levels were comparable to the serum levels published for samples from patients with other tumors.5,22,34 To identify potential associations of these ascites mediators with relapse-free survival (RFS) we performed Kaplan-Meier analyses. Ascites samples split at different thresholds (“high” and “low”) and longrank p values were determined. As shown in Fig. 1 B-D, this analysis yielded significant associations with RFS for sULBP2, sMICA and MIF. In each case, the hazard ratio (HR) was approximately 3 and the difference in median RFS 13-15 versus 23-27 months. As controls we determined the CD68+ and CD163+ fractions of CD14+ tumor-associated macrophages (TAMs) in the same samples and obtained the expected results,36 i.e., a significant association of RFS with CD163+ cells but not with CD68+ cells (Fig. S4).
Figure 1.
Clinical significance of sULBP2, sMICA and MIF and in ovarian cancer ascites. (A) Concentrations of sULBP2, sMICA and MIF in ovarian cancer ascites (sample size: n = 36–40) as measured by ELISA. Boxes: upper and lower quartile with median; bars: 95% CI. (B-D) Kaplan-Meier plots of the data in panel 1A using data sets split at the median (Q = 50%) or the upper quartile (Q = 75%) as indicated. n: total sample size; p: logrank test p value; HR: hazard ratio; rfs: median RFS of “high” vs. “low” groups. (E) Probabilities of independent prognostic power of sULBP2, sMICA and MIF. For each variable, the best Kaplan-Maier split by log-rank test (panel B) was determined to discretize samples into 2 groups. Hypergeometric testing was used to determine significant overlaps between groups. *, ***, ****: p < 0.05, p < 0.001: p < 0.0001; o: not significant; n/a: not applicable. Significance indicates lack of independence. (F) Percentage of positive NKG2D and CD16 cells of the indicated lymphocyte subsets in ovarian cancer ascites, patient blood or blood from donors with non-malignant conditions (designated as “normal blood)” measured using flow cytometry. Statistical significance between samples was determined by unpaired t-test and is indicated by asterisks if p < 0.05. Gating strategy see Fig. S6.
Next, we assessed whether sULBP2, sMICA and MIF might represent independent prognostic factors. Since multivariate regression analysis is appropriate only for large sample sizes, we addressed this issue by testing distribution probabilities. We included in this analysis several other mediators of ovarian cancer ascites previously reported to be associated with a poor RFS, i.e., IL-6, IL-10, leukemia inhibitory factor (LIF), leptin, lysophosphatidic acid (LPA), phospholipase PLA2G2A and the lysophospholipase autotaxin, all determined by ELISA.35,36 For each mediator, samples were split into 2 groups by a “best-fit” approach (according to logrank test) and the overlapping fraction of samples was determined. Hypergeometric testing (Fig. 1E) revealed statistically significant results for sULBP2 vs. sMICA, IL-6, IL-10 and LIF, and for sMICA vs. sULBP2 and IL-10, suggesting that these molecules are not independent prognostic factors of RFS. In contrast, no significant overlaps were observed for MIF, pointing to an independent prognostic power.
To evaluate whether elevated MIF and sNKG2D ligands are associated with a downregulation of NKG2D on ascites-associated NK and/or T cells, NKG2D expression was analyzed by flow cytometry. Surprisingly, CD3-expressing cells (T and NKT cells) from ascites had a higher NKG2D surface-expression relative to patient-derived peripheral T cells (Fig. 1F). NKG2D expression on patient-derived peripheral T cells was not altered in comparison to donors with non-malignant conditions (myomatosis uteri or ovarian cysts), subsequently referred to as “normal.” In line with these observations, there was no significant difference in the percentage of NKG2D-expressing NKp46-positive NK cells isolated from ascites, patient blood or normal blood (MFI values see Suppl. Table S1). Most strikingly, a highly significant downregulation of CD16 on NK cells from ascites was observed, whereas CD16-expression on peripheral cells was very similar between patients and normal donors (Fig. 1F, see Table S1 for MFI values). These data indicate that high levels of MIF and sNKG2D-L do not diminish NKG2D expression on lymphocytes in ascites to evade immune surveillance. However, the strong clinical associations of sNKG2D-L and MIF implicate that these factors contribute to ovarian cancer progression through hitherto unidentified mechanisms.
To investigate the impact of sNKG2D-L containing ascites on NK cells from healthy donors, purified NK cells, either resting (IL2 – 10 U/ml primed) or activated (IL2 – 200 U/ml + IL15 – 10 ng/ml primed), were incubated with ascites samples from 4 different ovarian cancer patients and NK cell receptor expression and degranulation were tested. While there was a slight reduction in the surface expression of NKG2D on resting NK cells (Fig. S1), this was not observed with activated NK cells (Fig. 2A). Strikingly, CD16 surface expression was strongly reduced on NK cells in response to ascites irrespective of the NK cell activation status (Figs. 2B, and S1).
Figure 2.
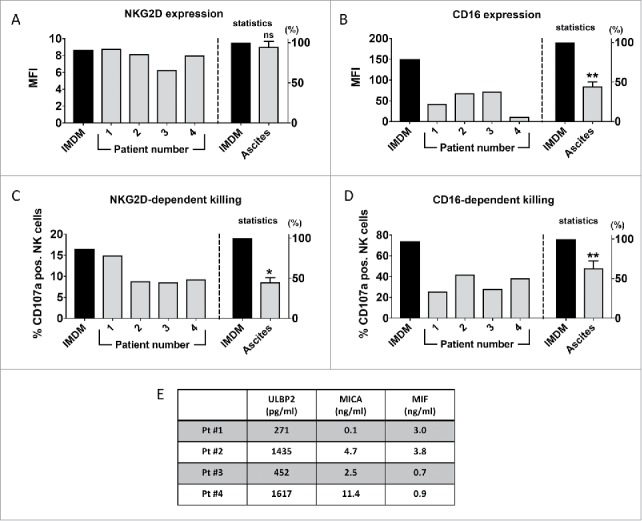
Ovarian cancer ascites diminishes CD16-surface expression and inhibits NKG2D- and CD16-dependent target cell killing. IL2 and IL15 primed NK cells from healthy donors were incubated with ovarian cancer ascites (4 different patients) diluted 1:4 in IMDM medium or with pure IMDM medium (control) for 18 hours. The surface expression of NKG2D (A) and CD16 (B) receptors were analyzed by flow cytometry. In both cases, receptor expression was normalized to the control (IMDM) group and the normalized values from all 4 patients were combined for the statistics. Following an 18-hour incubation with ovarian ascites or IMDM, NK cells were co-cultured with Drosophila Schneider-2 (S2) cells stably expressing ULBP1 (S2-ULBP1 (C)) or coated with IgG (S2-Fc (D)) to assess NK cell degranulation in response to NKG2D or CD16 stimulus, respectively. Degranulation was detected by flow cytometric analysis of CD107a. In both cases, CD107a positive NK cells were normalized to the control (IMDM) group and the normalized values from all 4 patients were combined for the statistics. Error bars indicate standard error of the mean (SEM) and statistical analysis by paired t-test. Statistical significance is indicated by asterisks if p < 0.05). Gating strategy see Fig. S6. (E) Amount of soluble NKG2D ligands and MIF in ovarian ascites samples used in A-D as measured by ELISA.
Next, we analyzed whether ascites inhibits NKG2D- and CD16-dependent NK cell degranulation. To this end, Drosophila Schneider 2 (S2) cells, either transfected with an expression vector for ULBP1 ligand or coated with rabbit IgG, were used. Drosophila S2 ULBP1-transfectants are used as a tool to monitor NKG2D-activity. These cells do not express any NK cell ligands, which allows to determine the NKG2D-dependent target cell killing without the potential interference from other stimuli.27 Analysis of all 4 patients showed that ovarian ascites significantly inhibited NK cell degranulation in response to NKG2D stimulation (S2-ULBP1 target cells) and the reduction was in the range of 35-40% (Fig. 2C). Of note, sample 4 with about 6-fold higher sULBP2 and 100-fold higher sMICA levels than sample 1 (Fig. 2E) was not significantly more effective than other samples at inhibiting NKG2D-dependent killing. These observations were confirmed with another set of ascites samples (n = 5) using 2 independent NK cell donors (Fig. S2). We conclude that the inhibition of NKD2D-dependent killing was independent of MIF, sMICA and sULBP2 concentrations in the respective ascites samples. Moreover, the inhibition of NK cell degranulation did not correlate with a reduction of NKG2D expression on activated NK cells. In contrast, the ascites-induced downregulation of CD16 correlated with a significant and robust decrease of CD16-dependent NK cell degranulation (Fig. 2D). Taken together, this suggests that soluble ascites factors potentially including sNKG2D-L and MIF cooperate with other factors to inhibit CD16- and NKG2D-mediated target cell killing, and that this is obviously independent of the NKG2D surface expression. Recently it was shown that extracellular vesicles purified from ascites fluid could down-regulate NKG2D, but not DNAM-1 expression on healthy T and NK cells.18 Such a mechanism is obviously not relevant in the patient cohort analyzed here, since NKG2D expression is unchanged or even increased on ascites derived lymphocytes.
We also investigated whether the ascites-mediated interference with functions of other immune cells might be associated with MIF or sNKG2D-L levels. To this end, we analyzed the impact of ascites on (i) the pro-inflammatory response by monocyte-derived macrophages, monitored by the LPS/IFNγ-induce expression of IL12B, and (ii) the activation of normal peripheral T cells by CD3/CD28 ligation, assayed by the induction of IFNγ and TNF, as described previously.11 An ascites-dependent inhibition of these immune cell functions was observed, however, we were unable to detect any link of inhibitory effects and the concentrations of MIF or sNKG2D-L (Fig. S3).
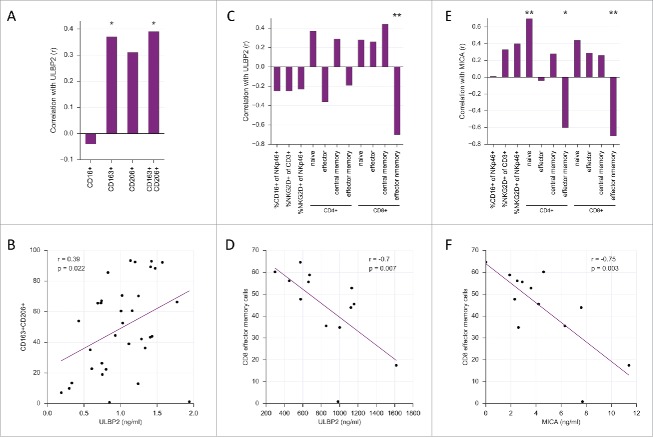
A hallmark of ovarian cancer ascites is the presence of numerous types of immune cells, partly in large numbers, which contribute to disease progression, drug resistance and an adverse survival (recent review).46 Given the inverse correlation of MIF and sNKG2D-L with RFS we analyzed potential correlations of these factors with clinically relevant immune cells. A clear correlation was observed for sULBP2 levels and CD14+ cells expressing CD163 or CD163 and CD206 (r = 0.37 and r = 0.39, respectively; Fig. 3A and B), previously identified as a pro-tumorigenic TAM subset in ovarian cancer ascites that is associated with a short RFS.35,36 In contrast, no significant correlations were found of TAM subsets with sMICA or MIF concentrations (Fig. S4).
Figure 3.
Correlations of sULBP2 and sMICA levels with the accumulation of immune cell subsets in ovarian cancer ascites. (A) Correlation of sULBP2 with CD16-expressing cells and TAM subsets from ascites fluid analyzed by low cytometry. Spearman correlation was used because the data were not normally distributed. (B) Scatter plot of the correlation of sULBP2 with CD163+CD206+ TAMs. (C, E) Correlation of sULBP2 and sMICA with NK cells, CD4+ and CD8+ T cell subsets. Naive T cells: CD45RA+CCR7+; central memory: CD45RA−CCR7+; effector: CD45RA+CCR7−; effector memory: CD45RA−CCR7− cells. (D, F) Scatter plot of the correlation of sULBP2 and sMICA with CD8+ effector memory cells. Gating strategy see Fig. S6, comparison of lymphocytes in OC ascites and periphery of normal/OC patients see Fig. S7.
The NKG2D/NKG2D-L axis is involved in the regulation of macrophages. Thus, it is tempting to speculate that a causal connection of ULBP2 and the TAM phenotype exists. Recently, it was shown that sMICA triggers the expansion of mouse myeloid-derived suppressor cells (MDSCs) and shifts macrophages to an anti-inflammatory phenotype via NKG2D-mediated STAT3 activation.47 However, the relevance of this pathway in humans is unclear, since we were unable to detect NKG2D on macrophages isolated from human ascites (Fig. S5). Moreover, it is possible that indirect effects exist such an interference of sNKG2D-L with the crosstalk of NKG2D-L on macrophages and NKG2D on T and NK cells. Based on the surprising observation that the murine NKG2D ligand MULT-1 stimulates anti-tumor immunity in its soluble form it was postulated that chronic interaction of NKG2D-L expressed on macrophages with NKG2D on NK cells desensitizes NK cells, which could be blocked by soluble NKG2D.8,33
Human macrophages upregulate NKG2D-L in response to TLR-4 stimulation, which renders them more susceptible to NK cell-mediated killing.9 This was regarded as a mechanism to dampen the immune response, although the NKG2D/NKG2D-L crosstalk was also reported to activate NK cell anti-tumor responses while sparing macrophages from killing due to the expression of the inhibitory ligand Qa-1b.48 The role of the reciprocal NKG2D/NKG2D-L interaction in modulating macrophage functions therefore remains to be investigated both in the tumor context and in physiologic conditions.
A particularly striking correlation was observed for both sULBP2 and sMICA with T cell subtypes (Fig. 3C–F). The profiling of ascites T cell subsets based on CD45RA and CCR7 expression revealed an inverse correlation of both ligands with CD8+ effector memory cells (sULBP2, r = −0.63, Fig. 3C and D; sMICA, r = −0.75, Fig. 3E and F). This was accompanied by a significant positive correlation (r > 0.75) of sMICA with naive CD8+ cells (Fig. 3C), consistent with an inverse correlation between naïve and effector memory cells (r = 0.77). A similar trend was seen with CD4+ cells, albeit with a slightly lower statistical significance (Fig. 3C and E). In contrast, there was no significant correlation of any T cell subset with MIF (Fig. S4).
We show that soluble ligands for NKG2D correlate with an immunosuppressive microenvironment and with a poor prognosis, as summarized by the scheme in Fig. 4. Currently, human sNKG2D-L are described to downregulate NKG2D on effector cells as part of the immune escape strategy (Fig. 4A), yet this is fundamentally different in ovarian cancer ascites. NKG2D expression was not diminished on NK cells and was even elevated on T cells isolated from ascites. On the other hand, high levels of sNKG2D-L correlated with increased numbers of pro-tumorigenic TAMs and a decreased accumulation or differentiation of memory effector T cells (Fig. 4B and C). In line with an immunosuppressive tumor micromilieu, we also identified an sNKG2D-L independent impact on NK cell activity (Fig. 4D). Ascites fluid induced a strong downregulation of CD16 and inhibited CD16-dependent target cell killing in vitro (Fig. 2B and D), which was also reflected by a reduced CD16 expression on ascites NK cells compared with NK cells from peripheral blood (Fig. 1B). Of note, CD16 expression of peripheral NK cells isolated from normal donors and patients was similar.
Figure 4.
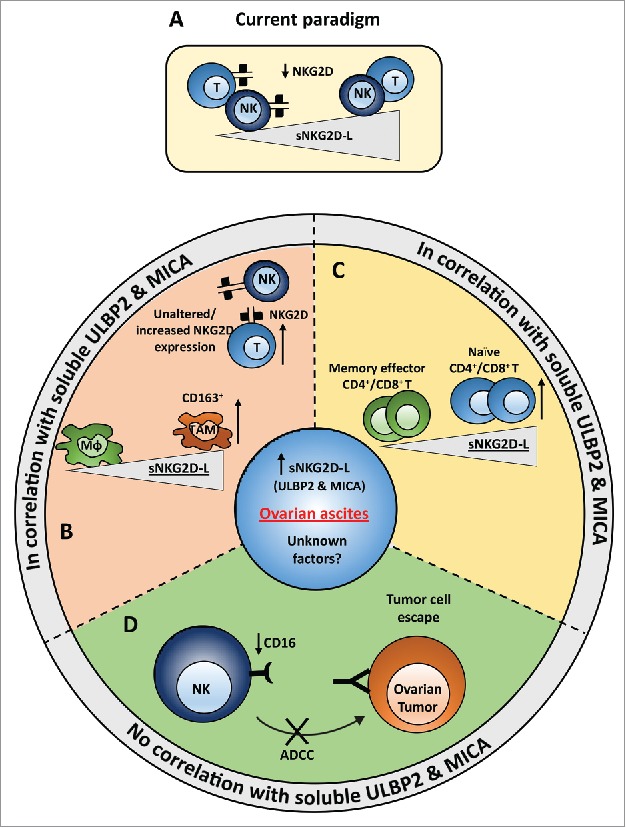
Model of immune regulatory effects of ovarian cancer ascites that are either associated or independent of sNKG2D-L. (A) Current paradigm. Presence of soluble NKG2D ligands is known to down-regulate NKG2D receptor on the surface of NK and CD8+ T cells (in humans). (B,C) In contrast to the current understanding, no significant difference or rather slight but significant increase in the surface expression of NKG2D on NK and T cells, respectively, was seen in ovarian cancer ascites. Instead, a correlation of soluble sULBP2 and sMICA ligands with the number of tumor associated macrophages (B) and naïve T cells (C) was observed. The number of memory effector T cells correlated inversely with the level of soluble ULBP2 and MICA. (D) Strong reduction of CD16 expression was observed on NK cells from ascites samples compared with NK cells from peripheral blood of ovarian cancer patients and healthy donors. Further, ex vivo incubation of healthy donor NK cells with ovarian cancer ascites strongly reduced the surface expression of CD16 on NK cells and inhibited the NK cell-mediated cytotoxicity. This effect was most likely due to yet unknown factors present in ascites and there was no correlation with sULBP2 and sMICA levels.
In summary, ovarian cancer ascites contains elevated levels of MIF and sNKG2D-L, which correlated inversely with the relapse free survival of the patients. Cell-free ascites inhibited CD16 and NKG2D-dependent NK cell cytotoxicity. However, in contrast to the current paradigm, this effect was independent of NKG2D downregulation on the surface of T and NK cells. The NKG2D/NKG2D-L interactions play complex roles in both the activation and regulation of immune responses and the current view of their role in immune escape seems to be oversimplified. Understanding the biology of NK cells of the ovarian cancer ascites will most likely identify novel and more complex mechanisms of sNKG2D-L activity. It is conceivable that ovarian cancer NK cells do not only lack cytolytic functions, but also promote tumor growth, e.g., though the release of IL-8 and interferon-inducible protein-10, as it has been shown for decidual NK cells.14,24
Finally, a comprehensive analysis of the NKG2D/NKG2D-L system may be of high therapeutic relevance. Initial preclinical mouse experiments showed promising anti-tumor activity of adoptively transferred T cells bearing a chimeric NKG2D-based antigen receptor (CAR) to retarget T cells to NKG2D-L expressing target cells.40 Unexpectedly, significant clinical toxicity resulting in morbidity and mortality were observed in follow up studies, which were dependent on the genetic background of the host strain.43 This underlines that NKG2D ligands and NKG2D are probably useful targets for immunotherapy to combat ovarian cancer provided a deeper understanding of their regulation and function is available.
Material and methods
Patient samples
Ascites and peripheral blood were collected from patients with high grade ovarian carcinoma undergoing primary surgery at the University Hospital in Marburg according to the protocols approved by the ethics committee of Marburg University and in compliance with the Helsinki Declaration. Peripheral blood was equally obtained from patients with benign gynecological disease, e.g., myomatosis uteri or ovarian cysts, and further referred to as normal controls.
Mononuclear cells were isolated from ascites and peripheral blood by Lymphocyte Separation Medium 1077 (PromoCell) density gradient centrifugation and cryopreserved or used freshly for immunophenotyping. Cell-free ascites and plasma was preserved for analysis of NKG2D ligands and cytokines as well as functional NK cell assays.
The collection and the analysis of human material were approved by the local ethics committee of the Philipps University Marburg under reference number 205/10. Donors provided written consent in accordance with the Declaration of Helsinki.
Immunophenotyping
Peripheral lymphocytes (OC patients: n = 27; normal: n = 10) and ascites-associated lymphocyte subsets (n = 43) were characterized using the following antibodies for surface expression by flow cytometry (FACSCanto BD Bioscience): anti-human CD19 FITC, CD45RA FITC, CD8 APC (all Miltenyi Biotec), CD16 PE-Cy7 and CD335 (NKp46) eFluor450 (both eBiosciences), CD4 PE-Cy7 (Southern Biotech), CD3 APC (Biolegend), CCR7 PE (BD Biosciences), and NKG2D FITC (abcam). Corresponding isotype-matched controls were purchased from Miltenyi Biotec, BD Biosciences and eBioscience. TAMs in ascites were analyzed for surface expression of CD14, CD163, CD206 and intracellular expression of CD68 by flow cytometry, as described previously.36
Elisa
Ascites levels of sULBP2, MICA and MIF were determined using the commercially available R&D duosets for ULBP2, MICA and MIF except that the antibody BUM01 was used as a capture antibody for ULBP2 (kindly provided by Alexander Steinle). Ascites levels of IL-6, IL-10, LIF, leptin, LPA, PLA2G2A and autotaxin were determined by commercial ELISAs, as described previously.36,35
Cytotoxicity assays
Schneider-2 cells expressing ULBP1 or coated with rabbit polyclonal serum were used as target cells to activate NKG2D or CD16a receptor on NK cells, respectively. Purified 2.5 × 104 (resting or IL-2/IL-15 primed) NK cells were incubated with 2.5 × 104 Schneider-2 cells. After 2h co-incubation 2 μM monensin and PE-labeled anti-human CD107a mAB (both BioLegend) was added. Cells were incubated for additional 4h and then samples were stained with BV421™–labeled anti-human CD56 antibody to discriminate CD56-positive NK cells from CD56-negative Schneider-2 cells. NK cell degranulation was assessed by flow cytometric analysis as described previously.44
Statistical analyses
Comparative data were statistically analyzed by Student's t-test (2-sided, equal variance or as indicated in the figure legends). Box pots depicting medians (line), upper and lower quantiles (box) and confidence intervals (bars) were constructed using the Seaborn boxplot function. Normal distribution and correlations were analyzed using the scipy.stats functions normaltest and spearmanr. Associations between mediator concentrations and relapse-free survival (logrank test), HR and median survival times were analyzed using the Python Lifelines KaplanMeierFitter and CoxPHFitter functions.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgements
We are grateful to A. Wortmann for providing the data in Figure S3B and to F. Finkernagel for advice with statistical analyses. This work was supported by grants from the DFG (MU601/17-1 to RM, RE1590/1-1 to SR, PO1408/7-1 and PO1408/13-1 to EPvS) and by the Wilhelm-Sander-Stiftung (grant 62082555 to EPvS and 2016.123.1 to SR and UW.
References
- 1.Ashiru O, Boutet P, Fernandez-Messina L, Aguera-Gonzalez S, Skepper JN, Vales-Gomez M, Reyburn HT. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res 2010; 70:481-9; PMID:20068167; https://doi.org/ 10.1158/0008-5472.CAN-09-1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashiru O, Lopez-Cobo S, Fernandez-Messina L, Pontes-Quero S, Pandolfi R, Reyburn HT, Vales-Gomez M. A GPI anchor explains the unique biological features of the common NKG2D-ligand allele MICA*008. Biochem J 2013; 454:295-302; PMID:23772752; https://doi.org/ 10.1042/BJ20130194 [DOI] [PubMed] [Google Scholar]
- 3.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999; 285:727-9; PMID:10426993; https://doi.org/ 10.1126/science.285.5428.727 [DOI] [PubMed] [Google Scholar]
- 4.Campbell KS, Hasegawa J. Natural killer cell biology: An update and future directions. J Allergy Clin Immunol 2013; 132:536-44; PMID:23906377; https://doi.org/ 10.1016/j.jaci.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chitadze G, Bhat J, Lettau M, Janssen O, Kabelitz D. Generation of soluble NKG2D ligands: Proteolytic cleavage, exosome secretion and functional implications. Scand J Immunol 2013; 78:120-9; PMID:23679194; https://doi.org/ 10.1111/sji.12072 [DOI] [PubMed] [Google Scholar]
- 6.Colombo N, Peiretti M, Parma G, Lapresa M, Mancari R, Carinelli S, Sessa C, Castiglione M, Group EGW. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010; 21(Suppl 5):v23-30; PMID:20555088; https://doi.org/ 10.1093/annonc/mdq244 [DOI] [PubMed] [Google Scholar]
- 7.De Sanctis JB, Blanca I, Bianco NE. Secretion of cytokines by natural killer cells primed with interleukin-2 and stimulated with different lipoproteins. Immunology 1997; 90:526-33; PMID:9176105; https://doi.org/ 10.1046/j.1365-2567.1997.00174.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng W, Gowen BG, Zhang L, Wang L, Lau S, Iannello A, Xu J, Rovis TL, Xiong N, Raulet DH. Antitumor immunity. A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science 2015; 348:136-9; PMID:25745066; https://doi.org/ 10.1126/science.1258867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eissmann P, Evans JH, Mehrabi M, Rose EL, Nedvetzki S, Davis DM. Multiple mechanisms downstream of TLR-4 stimulation allow expression of NKG2D ligands to facilitate macrophage/NK cell crosstalk. J Immunol 2010; 184:6901-9; PMID:20488792; https://doi.org/ 10.4049/jimmunol.0903985 [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Messina L, Ashiru O, Boutet P, Aguera-Gonzalez S, Skepper JN, Reyburn HT, Vales-Gomez M. Differential mechanisms of shedding of the glycosylphosphatidylinositol (GPI)-anchored NKG2D ligands. J Biol Chem 2010; 285:8543-51; PMID:20080967; https://doi.org/ 10.1074/jbc.M109.045906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finkernagel F, Reinartz S, Lieber S, Adhikary T, Wortmann A, Hoffmann N, Bieringer T, Nist A, Stiewe T, Jansen JM, et al.. The transcriptional signature of human ovarian carcinoma macrophages is associated with extracellular matrix reorganization. Oncotarget 2016; 7:75339-52; PMID:27659538; https://doi.org/ 10.18632/oncotarget.12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giuntoli RL 2nd, Webb TJ, Zoso A, Rogers O, Diaz-Montes TP, Bristow RE, Oelke M. Ovarian cancer-associated ascites demonstrates altered immune environment: Implications for antitumor immunity. Anticancer Res 2009; 29:2875-84; PMID:19661290. [PubMed] [Google Scholar]
- 13.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 2002; 419:734-8; PMID:12384702; https://doi.org/ 10.1038/nature01112 [DOI] [PubMed] [Google Scholar]
- 14.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, et al.. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 2006; 12:1065-74; PMID:16892062; https://doi.org/ 10.1038/nm1452 [DOI] [PubMed] [Google Scholar]
- 15.Jinushi M, Takehara T, Tatsumi T, Hiramatsu N, Sakamori R, Yamaguchi S, Hayashi N. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. J Hepatol 2005; 43:1013-20; PMID:16168521; https://doi.org/ 10.1016/j.jhep.2005.05.026 [DOI] [PubMed] [Google Scholar]
- 16.Krockenberger M, Dombrowski Y, Weidler C, Ossadnik M, Honig A, Hausler S, Voigt H, Becker JC, Leng L, Steinle A, et al.. Macrophage migration inhibitory factor contributes to the immune escape of ovarian cancer by down-regulating NKG2D. J Immunol 2008; 180:7338-48; PMID:18490733; https://doi.org/ 10.4049/jimmunol.180.11.7338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krockenberger M, Kranke P, Hausler S, Engel JB, Horn E, Nurnberger K, Wischhusen J, Dietl J, Honig A. Macrophage migration-inhibitory factor levels in serum of patients with ovarian cancer correlates with poor prognosis. Anticancer Res 2012; 32:5233-8; PMID:23225421. [PubMed] [Google Scholar]
- 18.Labani-Motlagh A, Israelsson P, Ottander U, Lundin E, Nagaev I, Nagaeva O, Dehlin E, Baranov V, Mincheva-Nilsson L. Differential expression of ligands for NKG2D and DNAM-1 receptors by epithelial ovarian cancer-derived exosomes and its influence on NK cell cytotoxicity. Tumour Biol 2016; 37(4):5455-66; PMID:26563374; https://doi.org/ 10.1007/s13277-015-4313-2 [DOI] [PubMed] [Google Scholar]
- 19.Lanier LL. Follow the leader: NK cell receptors for classical and nonclassical MHC class I. Cell 1998; 92:705-7; PMID:9529246; https://doi.org/ 10.1016/S0092-8674(00)81398-7 [DOI] [PubMed] [Google Scholar]
- 20.Lanier LL. NK cell receptors. Annu Rev Immunol 1998; 16:359-93; https://doi.org/ 10.1146/annurev.immunol.16.1.359 [DOI] [PubMed] [Google Scholar]
- 21.Lanier LL. NKG2D receptor and its ligands in host defense. Cancer Immunol Res 2015; 3:575-82; PMID:26041808; https://doi.org/ 10.1158/2326-6066.CIR-15-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Bert N, Gasser S. Advances in NKG2D ligand recognition and responses by NK cells. Immunol Cell Biol 2014; 92:230-6; PMID:24445601; https://doi.org/ 10.1038/icb.2013.111 [DOI] [PubMed] [Google Scholar]
- 23.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol 2010; 177:1053-64; PMID:20651229; https://doi.org/ 10.2353/ajpath.2010.100105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levi I, Amsalem H, Nissan A, Darash-Yahana M, Peretz T, Mandelboim O, Rachmilewitz J. Characterization of tumor infiltrating natural killer cell subset. Oncotarget 2015; 6:13835-43; PMID:26079948; https://doi.org/ 10.18632/oncotarget.3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li K, Mandai M, Hamanishi J, Matsumura N, Suzuki A, Yagi H, Yamaguchi K, Baba T, Fujii S, Konishi I. Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in ovarian cancer: High expression of ULBP2 is an indicator of poor prognosis. Cancer Immunol Immunother 2009; 58:641-52; PMID:18791713; https://doi.org/ 10.1007/s00262-008-0585-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Soto A, Huergo-Zapico L, Acebes-Huerta A, Villa-Alvarez M, Gonzalez S. NKG2D signaling in cancer immunosurveillance. Int J Cancer 2015; 136:1741-50; PMID:24615398; https://doi.org/ 10.1002/ijc.28775 [DOI] [PubMed] [Google Scholar]
- 27.March ME, Gross CC, Long EO. Use of transfected Drosophila S2 cells to study NK cell activation. Methods Mol Biol 2010; 612:67-88; PMID:20033635; https://doi.org/ 10.1007/978-1-60761-362-6_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcus A, Gowen BG, Thompson TW, Iannello A, Ardolino M, Deng W, Wang L, Shifrin N, Raulet DH. Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol 2014; 122:91-128; PMID:24507156; https://doi.org/ 10.1016/B978-0-12-800267-4.00003-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marten A, von Lilienfeld-Toal M, Buchler MW, Schmidt J. Soluble MIC is elevated in the serum of patients with pancreatic carcinoma diminishing gammadelta T cell cytotoxicity. Int J Cancer 2006; 119:2359-65; PMID:16929491; https://doi.org/ 10.1002/ijc.22186 [DOI] [PubMed] [Google Scholar]
- 30.McGilvray RW, Eagle RA, Rolland P, Jafferji I, Trowsdale J, Durrant LG. ULBP2 and RAET1E NKG2D ligands are independent predictors of poor prognosis in ovarian cancer patients. Int J Cancer 2010; 127:1412-20; PMID:20054857; https://doi.org/ 10.1002/ijc.25156 [DOI] [PubMed] [Google Scholar]
- 31.McGilvray RW, Eagle RA, Watson NF, Al-Attar A, Ball G, Jafferji I, Trowsdale J, Durrant LG. NKG2D ligand expression in human colorectal cancer reveals associations with prognosis and evidence for immunoediting. Clin Cancer Res 2009; 15:6993-7002; PMID:19861434; https://doi.org/ 10.1158/1078-0432.CCR-09-0991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mittelbronn M, Platten M, Zeiner P, Dombrowski Y, Frank B, Zachskorn C, Harter PN, Weller M, Wischhusen J. Macrophage migration inhibitory factor (MIF) expression in human malignant gliomas contributes to immune escape and tumour progression. Acta Neuropathol 2011; 122:353-65; PMID:21773885; https://doi.org/ 10.1007/s00401-011-0858-3 [DOI] [PubMed] [Google Scholar]
- 33.Pardoll DM. Distinct mechanisms of tumor resistance to NK killing: Of mice and men. Immunity 2015; 42:605-6; PMID:25902479; https://doi.org/ 10.1016/j.immuni.2015.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinart N, Nguyen PH, Boucas J, Rosen N, Kvasnicka HM, Heukamp L, Rudolph C, Ristovska V, Velmans T, Mueller C, et al.. Delayed development of chronic lymphocytic leukemia in the absence of macrophage migration inhibitory factor. Blood 2013; 121:812-21; PMID:23118218; https://doi.org/ 10.1182/blood-2012-05-431452 [DOI] [PubMed] [Google Scholar]
- 35.Reinartz S, Finkernagel F, Adhikary T, Rohnalter V, Schumann T, Schober Y, Nockher WA, Nist A, Stiewe T, Jansen JM, et al.. A transcriptome-based global map of signaling pathways in the ovarian cancer microenvironment associated with clinical outcome. Genome Biol 2016; 17:108; PMID:27215396; https://doi.org/ 10.1186/s13059-016-0956-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reinartz S, Schumann T, Finkernagel F, Wortmann A, Jansen JM, Meissner W, Krause M, Schworer AM, Wagner U, Muller-Brusselbach S, et al.. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: Correlation of CD163 expression, cytokine levels and early relapse. Int J Cancer 2014; 134:32-42; PMID:23784932; https://doi.org/ 10.1002/ijc.28335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salih HR, Goehlsdorf D, Steinle A. Release of MICB molecules by tumor cells: Mechanism and soluble MICB in sera of cancer patients. Hum Immunol 2006; 67:188-95; PMID:16698441; https://doi.org/ 10.1016/j.humimm.2006.02.008 [DOI] [PubMed] [Google Scholar]
- 38.Salih HR, Rammensee HG, Steinle A. Cutting edge: Down-regulation of MICA on human tumors by proteolytic shedding. J Immunol 2002; 169:4098-102; PMID:12370336; https://doi.org/ 10.4049/jimmunol.169.8.4098 [DOI] [PubMed] [Google Scholar]
- 39.Song H, Kim J, Cosman D, Choi I. Soluble ULBP suppresses natural killer cell activity via down-regulating NKG2D expression. Cell Immunol 2006; 239:22-30; PMID:16630603; https://doi.org/ 10.1016/j.cellimm.2006.03.002 [DOI] [PubMed] [Google Scholar]
- 40.Spear P, Barber A, Sentman CL. Collaboration of chimeric antigen receptor (CAR)-expressing T cells and host T cells for optimal elimination of established ovarian tumors. Oncoimmunology 2013; 2:e23564; PMID:23734311; https://doi.org/ 10.4161/onci.23564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stern-Ginossar N, Gur C, Biton M, Horwitz E, Elboim M, Stanietsky N, Mandelboim M, Mandelboim O. Human microRNAs regulate stress-induced immune responses mediated by the receptor NKG2D. Nat Immunol 2008; 9:1065-73; PMID:18677316; https://doi.org/ 10.1038/ni.1642 [DOI] [PubMed] [Google Scholar]
- 42.Ullrich E, Koch J, Cerwenka A, Steinle A. New prospects on the NKG2D/NKG2DL system for oncology. Oncoimmunology 2013; 2:e26097; PMID:24353908; https://doi.org/ 10.4161/onci.26097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.VanSeggelen H, Hammill JA, Dvorkin-Gheva A, Tantalo DG, Kwiecien JM, Denisova GF, Rabinovich B, Wan Y, Bramson JL. T cells engineered with chimeric antigen receptors targeting NKG2D ligands display lethal toxicity in mice. Mol Ther 2015; 23:1600-10; PMID:26122933; https://doi.org/ 10.1038/mt.2015.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vyas M, Schneider AC, Shatnyeva O, Reiners KS, Tawadros S, Kloess S, Kohl U, Hallek M, Hansen HP, Pogge von Strandmann E. Mono- and dual-targeting triplebodies activate natural killer cells and have anti-tumor activity in vitro and in vivo against chronic lymphocytic leukemia. Oncoimmunology 2016; 5:e1211220; PMID:27757305; https://doi.org/ 10.1080/2162402X.2016.1211220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene 2008; 27:5932-43; PMID:18836474; https://doi.org/ 10.1038/onc.2008.267 [DOI] [PubMed] [Google Scholar]
- 46.Worzfeld T, Pogge von Strandmann E, Huber M, Adhikary T, Wagner U, Reinartz S, Muller R. The unique molecular and cellular microenvironment of ovarian cancer. Front Oncol 2017; 7:24; PMID:28275576; https://doi.org/ 10.3389/fonc.2017.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao G, Wang X, Sheng J, Lu S, Yu X, Wu JD. Soluble NKG2D ligand promotes MDSC expansion and skews macrophage to the alternatively activated phenotype. J Hematol Oncol 2015; 8:13; PMID:25887583; https://doi.org/ 10.1186/s13045-015-0110-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Z, Zhang C, Zhang J, Tian Z. Macrophages help NK cells to attack tumor cells by stimulatory NKG2D ligand but protect themselves from NK killing by inhibitory ligand Qa-1. PLoS One 2012; 7:e36928; PMID:22629344; https://doi.org/ 10.1371/journal.pone.0036928 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.