Abstract
Mesenchymal stem/progenitor cells and induced pluripotent stem cells have become viable cell sources for prospective cell-based cartilage engineering and tissue repair. The development and function of stem cells are influenced by the tissue microenvironment. Specifically, the local tissue microenvironment can dictate how stem cells integrate into the existing tissue matrix and how successfully they can restore function to the damaged area in question. This review focuses on the microenvironmental features of articular cartilage and how they influence stem cell–based cartilage tissue repair. Also discussed are current tissue-engineering strategies used in combination with cell-based therapies, all of which are designed to mimic the natural properties of cartilage tissue in order to achieve a better healing response.
Keywords: cartilage repair, stem cells, progenitor, chondroprogenitor, microenvironment, articular cartilage
Introduction
Articular cartilage is aneural and lacks vasculature—ideal for minimizing the occurrence of inflammation and preventing the sensation of pain during joint loading. However, these conditions are not conducive to tissue repair because of the lack of a systemic blood supply. As such, articular cartilage heals poorly and there is much focus on finding effective methodologies to restore its structure and function following cartilage injury. Given its complex architecture, articular cartilage is challenging to reengineer. Current cell-based cartilage repair strategies utilize both mature and progenitor/stem cells. For instance, autologous chondrocyte implantation (ACI) implants presumably healthy mature chondrocytes from non-load-bearing regions of a joint into a cartilage defect.1,2 On the other hand, for smaller cartilage defects, microfracture surgery is performed to drill through the subchondral bone in order to create a fibrin clot that facilitates the migration of bone marrow–derived mesenchymal stem cells (BM-MSCs) into the cartilage to promote healing.3 Both methods are conventionally used for cartilage repair but have been reported to result in the formation of fibrous or hypertrophic repair tissues,4–6 which can deteriorate over time because of poor mechanical properties in comparison to hyaline cartilage. Indeed, while there is certainly room to improve the cell sources currently being considered for cartilage repair strategies, the aforementioned findings strongly suggest that the local tissue microenvironment ultimately plays the most crucial role in regulating the development and function of these cells. The questions arise as to what happens to cells that are implanted into a highly inflammatory or osteogenic microenvironment, and whether they will develop into fibrocartilage or hypertrophic cartilage, respectively. As such, it is important to recognize that regulating the tissue microenvironment may be another point of control that can be used to dictate the cartilage repair response.
The functional simplicity and biological complexity of articular cartilage
Development
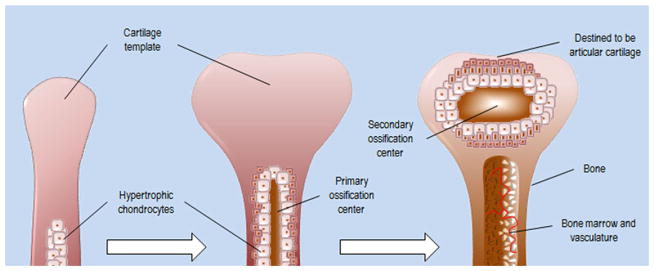
Articular cartilage is a product of long bone formation, which occurs through the highly regulated process of endochondral ossification (Fig. 1). During early embryonic limb development, cells of the mesenchymal lineage condense to form the limb bud. These mesenchymal chondrocyte precursors will highly express SOX-5/SOX-6/SOX-9 and undergo chondrogenesis-producing types II/IX/XI collagen and aggrecan, all of which are components of the cartilage extracellular matrix (ECM).7–9 These cells proliferate longitudinally from proximal to distal end, as they are coordinated by Indian hedgehog (IHH) and Wnt signaling to form a cartilage template that will eventually be replaced by bone.10,11 Articular cartilage represents the remains of the cartilage template from which the fully formed long bone developed.
Figure 1.
Endochondral ossification and articular cartilage. During the process of endochondral ossification, chondrocytes of the cartilage template, from which long bones will form, become hypertrophic and are replaced by osteoblasts. Articular cartilage tissue represents the remnants of the cartilage template from which long bones developed.
Function and organization
The anatomical function of articular cartilage is to bare load and minimize friction during joint movement, which is mostly reflected in its structural heterogeneity, organization, and cellularity. Compared to many other tissues, it has a high water content and consists mainly of an extracellular matrix that is produced by a sparse population of chondrocytes12—two features that contribute to the compressive resistance or characteristic “bounce” exhibited by this tissue. Although outwardly appearing as a relatively simple tissue, the architecture of articular cartilage is, in fact, elegantly complex. Articular cartilage of the knee and hip are approximately 1.5–2.55 mm and 1.35–2 mm thick, respectively.13 Articular cartilage is also compartmentalized into several zones (superficial zone, middle zone, deep zone, and calcified cartilage),14 and there is a distinct heterogeneity in both ECM and cellular organization between the zones. As such, each can be thought to have a different microenvironment and cellular organization that is best suited for its anatomical function.
For instance, the superficial zone makes up 10–20% of the full articular cartilage thickness in healthy adults and consists of an ECM that is organized in parallel to the articular surface to capitalize on the tensile strength of its network of collagen fibrils.12,15 This is important for preventing the tissue from tearing during physical stress. The articular surface has a higher cellularity than the rest of the cartilage and houses cells that produce a lubricating proteoglycan, known as lubricin (PRG4),16,17 which minimizes surface friction generated by diarthrodial joint movement. In clear contrast to the superficial zone, the middle zone, which makes up 40–60% of the full cartilage thickness, shows a columnar organization of cells and collagen fibrils of larger diameter.18 While the superficial zone mostly consists of some proteoglycans among collagen types II/IX, the middle zone has a relatively larger proteoglycan content, which helps with water retention. The large area and composition of the middle zone is well adapted to its primary function of providing compressive resistance during joint loading. Furthermore, the deep zone represents approximately 30% of the entire cartilage thickness and has essentially the same function as the middle zone but provides even more compressive resistance, as it has the densest ECM of the aforementioned zones.19 Deep zone chondrocytes are large, hypertrophic, and sparsely distributed. This zone has the largest collagen fibrils in articular cartilage tissue, organized longitudinally, and completely perpendicular to the articular surface to maximize compressive resistance against mechanical stress. Unlike in the other zones, the deep zone chondrocytes produce type X collagen, which is mostly found in the calcified cartilage below the tidemark. The calcified cartilage is a transitory sector of tissue that separates cartilage from the subchondral bone tissue.
Local stem/progenitor cells
In the last two decades, important discoveries have been made with respect to mesenchymal progenitor cells that can be isolated from adult mesenchymal tissues. The existence of these progenitor cells was first proposed by Hayes et al. on the basis of their observation that cartilage exhibits appositional growth during development.20 Mesenchymal progenitor cells have been found in many areas of the joint, including cartilage, synovial lining, bone marrow, infrapatellar fat pad, meniscus, and even tendons,21–29 and it has been proposed that they may be quiescent remnant cells from the developing limb bud mesenchyme.30 Cartilage-derived mesenchymal progenitors make up a small percentage of all cells in the cartilage tissue31 and reside side-by-side with the more abundant chondrocytes throughout cartilage; however, they are most abundantly found in the superficial zone, specifically in the articular surface.
Cartilage-derived mesenchymal progenitor cells differ from chondrocytes in many ways. For example, they have been reported to proliferate faster in vitro than mature articular chondrocytes and exhibit several common mesenchymal progenitor cell surface markers, including CD49e, CD90, CD105, CD166, and Notch1.22,23,32,33 Specific subsets of progenitors found on the articular surface are also capable of homing/migrating to areas of tissue that have been impacted or damaged.34 Most importantly, these cells are multipotent and have the capacity to differentiate in a chondrogenic microenvironment and form cartilage-like tissue.23 The presence of these cells opens up new possibilities for cartilage tissue repair and, in the process, calls attention to the potential importance of the stem cell niche in the cartilage healing response.
Changes to stem cell niche
In injury
Sustained injury as well as chronic diseases, such as osteoarthritis (OA), can result in substantial changes to the architecture of articular cartilage tissue. As expected, these changes can significantly affect the stem cell niche, which is best described as the local tissue microenvironment of resident stem/progenitor cells. For instance, a direct cartilage injury that occurs at the articular surface extending into the neighboring middle zone will not only compromise its primary function of resisting compressive forces, but will also result in an acute inflammatory response that will begin changing the local tissue microenvironment. This inflammatory response to articular cartilage injury primarily involves the production of interleukins, in particular interleukin-1 (IL-1), IL-6, IL-17, and IL-18, as well as tumor necrosis factor α (TNF-α).14,35,36 The production of these cytokines is not exclusive to joint cartilage tissue; indeed, much of it comes from the neighboring joint synovium, which, unlike cartilage, is vascularized and allows circulating immune cells, such as macrophages, to enter the joint and further heighten the immune response. In addition to the acute immune response brought on by cartilage injury, it would also result in the immediate disruption and chronic breakdown of the pericellular matrix, leading to the release of collagen, hyularonan, aggrecan, and fibronectin cleavage fragments.14 Such neoepitopes have previously been shown to perpetuate cartilage breakdown by promoting the production of collagenases, aggrecanases, reactive oxygen species (i.e., H2O2, hydroxyl radicals), and nitric oxide (NO).37,38
In osteoarthritis
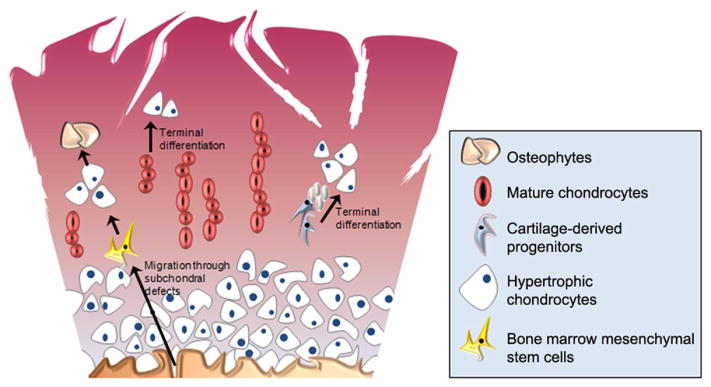
Many of the same conditions persist in OA cartilage. In addition to the active breakdown of the ECM by the matrix metalloproteinase (MMP) family of collagenases and ADAMTS family of aggrecanases, COX-2 and prostaglandins are actively produced by OA chondrocytes.36 Additionally, due to chronic inflammation and tissue wear-and-tear, higher grades of OA present with complete erosion of the articular surface. Furthermore, in contrast to a cartilage injury, OA is more defined by its osteogenic features. Chondrocyte hypertrophy,39 osteophyte formation,40 and changes to the subchondral bone and bone marrow41 are hallmarks of OA, many of which influence the immediate tissue microenvironment of OA cartilage. The subchondral bone undergoes striking changes during OA pathogenesis. The bone begins to thicken and bone marrow edema can be clearly observed. The subchondral bone also becomes more permeable, and bone morphogenetic proteins (BMPs) and members of the transforming growth factor β (TGF-β) super family produced by osteoblasts potentially leak into cartilage, favoring terminal differentiation of chondrocytes and osteophyte formation.42 Overall, such events promote a microenvironment that favors chondrocyte hypertrophy and osteogenesis (Fig. 2). Hypertrophic chondrocytes exhibit elevated RUNX2 expression resulting in the production of type X collagen, which accumulates at, or just above, the tidemark and becomes incorporated into the OA cartilage ECM. Increased integration of type X collagen into the cartilage ECM not only alters the pericellular microenvironment of local cell populations, but also alters the mechanical properties of the tissue itself by facilitating mineralization.43,44
Figure 2.
Osteoarthritis cartilage microenvironment promotes cellular hypertrophy and terminal differentiation. Cartilage tissue homeostasis is thrown off balance during osteoarthritis, as the combined effects of inflammatory factors and degradative enzymes erode cartilage tissue, resulting in matrix remodeling and changes to the subchondral bone. These events affect the cartilage stem cell niche, potentially altering the differentiation of local and migratory progenitor cell populations and ultimately leading to cellular hypertrophy and/or heterotopic bone formation.
During OA, there are also a greater number of mesenchymal progenitors that are present in the articular cartilage.21,32 It is speculated that this increase in progenitor cell number could be due to the activation and proliferation of once dormant progenitor cells in the tissue as a last-resort attempt to recover from tissue erosion. It has also been speculated that the increasing permeability to the subchondral bone may allow the migration of bone marrow stromal cells to penetrate into the cartilage. In both scenarios, the inflammatory and hypertrophic microenvironment of OA cartilage may decrease the likelihood that these cells will undergo chondrogenesis properly to replenish the damaged/eroded cartilage tissue. In such a case, the increasing presence of progenitor cells, which are entrained by the microenvironment of OA cartilage, may not be sufficient to restore tissue homeostasis.
Repairing damaged cartilage
Considerations for stem cell–based cartilage repair
Despite its aforementioned limitations, the use of autologous chondrocytes for articular joint cartilage repair is clinically practiced. However, in patients who have OA or are in the early stages of OA development, the utility of using autologous chondrocytes for cartilage repair is not the best option since these cells may already exhibit pathogenic features that limit their ability to repopulate the defect and produce new and healthy cartilage tissue. In such situations, mesenchymal progenitor cells with chondrogenic potential may represent a desirable alternative cell type for use in autologous cell implantation.
Although chondrogenic progenitor cells are regarded as being biologically primed for chondrogenesis, they are not entirely lineage restricted, and, as such, they exhibit enough plasticity to differentiate along other mesenchymal cell lineages, namely, osteogenic and adipogenic lineages.23,45 The local tissue stem cell niche is a critical feature that can dictate which path to maturation these cells will ultimately take. Similarly, when seeking to utilize stem cells in cell-based therapy for cartilage repair/regeneration, it is essential to consider the effects of the local tissue microenvironment into which these stem cells are being introduced. From a tissue-engineering perspective, there are two important factors to consider in this regard. First, a viable source of stem/progenitor cells that offer a biological repertoire that complements the desired path of differentiation is required. Second, one must consider how to provide the best stem cell niche for these cells to mature. There is currently a great need to develop regulatory strategies that can be used in conjunction with stem cells to promote the most desirable repair response. Such strategies include anything that will favorably alter the local stem cell niche, including the use of recombinant growth factors, implementation of artificial/biological scaffolds, and even the use of small interfering RNAs (siRNAs) to attenuate further damage.
Cell sources
Mesenchymal progenitor cells
Mesenchymal progenitors are proposed to be a good fit for cartilage repair since they are self-renewing and exhibit high proliferative capability, in comparison to mature articular chondrocytes. Progenitor cell sources under consideration for use in cartilage defect repair include the bone marrow, joint fat pad, periostium, synovium, and even non-weight-bearing regions of articular cartilage. BM-MSCs are the most studied source of these cells that are currently clinically used for cartilage repair and have been found to be a good cell source for progenitor cell–based cartilage repair because of their relative abundance in the body and their ability to be easily induced into differentiating down the chondrogenic lineage in culture by simply treating with growth factors, such as TGF-β1. Indeed, there are many comparative studies where BM-MSCs are used as the gold standard to which lesser known progenitor cell sources are held. For example, BM-MSCs have been compared to adipose tissue–derived progenitor cells of the infrapatellar fat pad, and, remarkably, the cells from the infrapatellar fat pad of OA patients retain the same chondrogenic properties as those in nondiseased tissue.46 Although adipose tissue is relatively expendable in the joint, making it a convenient source of cells for cell-based therapeutic approaches, there is also evidence suggesting that adipose-derived progenitors generally have reduced chondrogenic differentiation potential, relative to MSCs.47,48
Another suitable progenitor cell source that has been investigated is the periosteum,49,50 which, like bone marrow, is considered to be a built-in reservoir of progenitor cells and mediates bone growth and fracture healing. These cells can nevertheless be induced into chondrogenesis under the correct culture condition and used in cartilage-tissue repair strategies.
Interestingly, the joint synovium contains progenitor cells that are reported to have even higher chondrogenic potential than those derived from adipose tissues, periosteum, and bone marrow.51 It has been previously postulated that the increased chondrogenic potential of synovium-derived progenitors may be attributed to their close proximity to the articular cartilage stem cell niche.52 This is likely true considering that articular cartilage–derived progenitors exhibit high chondrogenic potential.
A recent study by Jiang et al. demonstrated that mature chondrocytes can be induced into a progenitor cell–like state via low-glucose 2D-culture conditions that promote expression of the early MSC marker, CD146.53 While these cells are similar to MSCs, they exhibited higher chondrogenic potential than MSCs. The authors used these cells to successfully repair the cartilage defects in 15 patients, strongly suggesting that the cartilage-derived progenitor cells from committed mature chondrocytes have future potential use for cartilage regenerative therapies.
OA cartilage was once considered an unlikely source of chondrogenic progenitor cells given the pathological characteristics of this tissue. However, it is now known that OA cartilage consists of a larger-than-normal pool of progenitor cells,22 some of which are promising potential cell sources for tissue repair. It has been demonstrated that articular cartilage from late-stage OA patients contains a population of chondrogenic progenitors, in which inhibition of hypertrophic transcription factor RUNX2 results in elevated expression of the master chondrogenesis regulator SOX-9.54
Induced pluripotent stem cells
Induced pluripotent stem cells (iPSCs) have become a potentially viable cell source for cartilage tissue repair. These cells are mature adult cells, typically from the skin, which have been genetically reprogrammed into a pluripotent stem cell state by transducing them with factors present in embryonic stem cells (ESCs)55 and can be induced into differentiating along all three germ-line lineages. They can also be modified to become committed to a specific germline.56 It has been confirmed that human ESCs can be induced into differentiating along the chondrogenic lineage using in vitro culture conditions that mimic cartilage microenvironmental conditions and endogenous growth factors.57,58 However, the remarkable plasticity of iPSCs is simultaneously an advantage and a disadvantage when considering these cells as a viable cell source for tissue repair. Yamashita et al. recently demonstrated that suspension culture conditions can be used to generate chondrogenic cells from human iPSCs.59 While some studies have reported that ESCs express little in the way of off-target cell markers,57 other studies have reported that the use of iPSCs in cartilage defect repair can result in instances of immature teratoma formation as part of the repair tissue when the transplanted cells are not completely homologous with the genome of the host.60,61 In this regard, in comparison to mesenchymal progenitors isolated from mature tissue sources, iPSCs must be regulated more tightly to ensure that they differentiate in a desirable fashion.
Creating the best stem cell niche for cartilage repair
Given the important role of stem cells in maintaining cartilage homeostasis and the great potential of stem cells in cartilage repair, researchers have developed the use of natural extracellular matrix proteins, scaffolding, and combined growth factors for stem cell attachment, growth, and regulation of cell differentiation. In the following sections, we discuss how each of these factors combines with stem cells in improving cartilage regeneration. Table 1 lists these factors and their desirable qualities.
Table 1.
Components with current or prospective use in cartilage tissue repair
| Stem cell type | Desirable qualities |
|---|---|
| Bone marrow mesenchymal stem cells | Relatively abundant |
| Cartilage-derived mesenchymal progenitors | High colony-forming efficiency |
| Induced pluripotent stem cells | Most abundant |
|
| |
| Extracellular matrices | Desirable qualities |
|
| |
| Decellularized extracellular matrix (ECM) | Acts like natural scaffolding |
| Promotes cell attachment | |
| Helps maintain stem cell niche | |
| Individual matrix proteins | Depending on which protein is used, they can promote cell attachment, proliferation, and chondrogenesis |
|
| |
| Scaffolds | Desirable qualities |
|
| |
| Synthetic biodegradable polymers | Helps maintain tissue infrastructure |
| Biodegradable to allow for replacement by natural ECM over time | |
| Hydrogels | Flexible/malleable |
| Low cytotoxicity | |
| High water content, similar to that in natural cartilage tissue | |
| Biomimetic materials | Mimics natural molecules and structures found in cartilage tissue |
|
| |
| Growth factors | Desirable qualities |
|
| |
| Transforming growth factor β 1 | Promotes cell proliferation, chondrogenesis, and ECM synthesis |
| Insulin-like growth factor 1 | Promotes cell proliferation and ECM synthesis |
| Bone morphogenetic factor | Stimulates proteoglycan synthesis |
Extracellular matrices
Decellularized ECMs (dECMs) are used for cartilage repair because they have little to no cytotoxicity62 and contain many of the natural structural components that modulate cell attachment, growth, and differentiation.63 The dECM can be used as a scaffold that closely mimics the natural tissue matrix in which cells can reside and function. The successful use of the dECM for promoting cartilage repair embodies the importance of preserving the local tissue microenvironment for improving cellular function, as demonstrated by a recent study using the dECM in combination with adipose-derived stem cells to achieve a superior cartilage healing response, resulting in the production of repair tissue that closely resembles articular cartilage in molecular and biomechanical properties. 64 Using a canine model, a study by Yang et al. also confirmed that decellularized osteochondral scaffolds can successfully induce primary canine BM-MSCs to produce repair tissue with a stiffness (70.77% of normal cartilage) and glycosaminoglycan content (74.95% of normal cartilage) comparable to that of native cartilage.65
The idea behind using the dECM in this capacity is to preserve as much as possible the natural architecture of the tissue while “evicting” the resident cells in order to repopulate with cells that are more suitable to facilitate tissue repair. There are mainly two categories of the dECM: tissue-derived and cell-derived matrices. To obtain a dECM that retains its natural structural architecture, native cells are removed by physical and chemical means, such as exposure to freeze/thaw cycles and treatment with Triton X-100.64 Tissue-derived matrices can be further divided into allografts and xenografts. Allograft dECMs are obtained from a donor that is the same species as the host, whereas xenograft dECMs are obtained from a donor of a different species. Additionally, Cheng et al. have demonstrated that the cartilage tissue matrix can be mechanically homogenized and lyophilized to produce scaffolds that promote chondrogenic differentiation of adipose-derived stem cells.66 Although the natural tissue architecture is not well preserved when using a lyophilized ECM to build a scaffold, this study makes it clear that the presence of the cartilage ECM alone can be sufficient to induce chondrogenesis.
Alternatively to using whole dECMs, individual ECM proteins can be used to accomplish many of the same functions. The best example of this is the use of collagen as the active component of sponges to promote adhesion and chondrogenesis of cells.67 Collagen is biocompatible and its degradation products have low cytotoxicity. Type I collagen sponges have been used to deliver growth factors, such as insulin and fibroblast growth factor (FGF), which not only increases proteoglycan content, but also results in a repair response that produces new tissue that more closely resembles hyaline cartilage instead of fibrocartilage.67 Likewise, the cartilage matrix protein matrilin-3 is shown to stimulate chondrogenesis of progenitor cells,68,69 suggesting that it too carries potential for future development into a biologically active component of engineered matrices. Matrilin-3 is highly expressed during development by proliferating chondrocytes in the growth plate, and ATDC5 chondroprogenitor cell proliferation and differentiation in the presence of matrilin-3 demonstrate that specific matrix molecules can be used to regulate cell behavior.69 While it is clear that matrilin-3 is not highly expressed in articular cartilage, there is evidence to suggest that its presence provides a chondrogenic microenvironment for progenitor cells, which promotes their differentiation along the chondrocyte lineage.68,69 Matrilin-3 also has anti-inflammatory properties that inhibit several downstream targets of the IL-1β pathway;70 a BMP-2 pathway antagonist has been shown to ultimately repress collagen X expression and chondrocyte hypertrophy.71 All of these features contribute to future consideration of matrilins in cartilage tissue repair strategies, as they currently hold promise for helping to provide an optimal microenvironment that promotes chondrogenesis, while simultaneously inhibiting chondrocyte hypertrophy and terminal differentiation.
Synthetic scaffolding
As discussed above, cartilage is a connective tissue with a dense ECM, low cell density, and no vasculature. The first challenge in cartilage regeneration is creating an environment suitable for stem cell homing and growth. In this manner, cell growth scaffolding is critical. Synthetic scaffolding materials used for cartilage tissue repair and engineering are designed to be low in cytotoxicity and often emulate various features of the natural cartilage microenvironment in order to facilitate a better healing response from chondrocytes and progenitor/stem cells.
One of the most commonly used scaffolding is biodegradable polymers, which have advantages in the precise control of chemical composition, crystallinity, molecular weight, molecular weight distribution, as well as easily fabricated microstructure and macrostructure (including porosity).72,73 However, since all polymers are covalently linked, biodegradation may be an issue. Some high–molecular weight polymers present a high cytotoxicity.74 Therefore, polymers with biodegradable linkage or components are the favored scaffolding materials. For instance, polylactide-co-glycolide (PLGA) is a widely used, U.S. Food and Drug Administration (FDA)-approved polymer for stem cell adhesion and growth in cartilage tissue engineering. PLGA was originally fabricated for biodegradable sutures in surgeries. Since then, its potential in many applications, such as medical devices, drug delivery, and tissue engineering, has attracted much attention. PLGA is the co-polymer of PGA (polyglycolide) and PLA (polylactide).73 Its ester bonds can degrade via hydrolysis, and, subsequently, the long polymer chains gradually break into small–molecular weight pieces in the physiological environment, which are eventually cleared by circulation (the degradation mechanism is shown in Fig. 3). The hydrolysis of polymers is closely related to the molecular weight and degree of polymerization. Many factors can increase the degradation rate of a polymer, such as high water content, large surface area, small particle size, lower molecular weight, and higher surface area. The regeneration of cartilage is a slow process because of its avascular nature. Thus, the degradation rate of the polymers for cartilage repair should occur at an appropriate rate—neither too fast nor too slow. The optimal rate would complement and mimic as much as possible the natural low rate of turnover that is characteristic of cartilage ECM. Numerous studies have made an effort to control the degradation rate of the polymer scaffolding.75–77 However, one of the complications associated with many hydrolysis polymers (e.g., PLGA) relates to the acidic degradation byproducts, which can lower the local tissue pH and result in cell and tissue necrosis.78
Figure 3.
Mechanisms of the hydrolysis of ester bonds during the degradation of polymers.
In addition to synthetic polymers, hydrogels extracted from natural materials are very popular scaffolding for cartilage repair. Hydrogels contain polymeric networks of branched molecules and are colloidal gels when water is the dispersion medium. Owing to their high water content, hydrogels present a degree of flexibility that is very similar to natural soft tissues, especially the polysaccharide family hydrogels, such as alginate and agarose, which have a similar chemical formula as glycosaminoglycans (one of the major components of natural cartilage ECM). This close similarity to glycosaminoglycans may account for the success that has been attributed to the use of alginate and agarose in cartilage defect repair applications. In addition, hydrogels exhibit high biocompatibility with cartilage stem cells, low cytotoxicity,79–81 and do not undergo hydrolysis through ester bonds. Although they usually take longer to degrade than hydrolysis polymers (e.g., PLGA), they are suitable for cartilage, a slow regeneration tissue.82–84 More importantly, they do not produce acidic by-products during degradation, which greatly increase the potential for clinical applications. Both synthetic polymers and natural hydrogels can benefit from the recent development of nanotechnology to improve the surface and structure, as well as to lower inflammatory responses.84 They can also improve select protein adsorption capability, which is important for mediating cell adhesion and function.85 Furthermore, nanofiber hydrogels can mimic the collagen orientation and alignment of cartilage; the biomimetic architecture can promote chondrogenic differentiation of mesenchymal stem cells, improving cartilage regeneration.86,87
Most recently, newer developments in cell scaffolding involve bioinspired materials, which can be derived and engineered from either DNA/ RNA or protein/peptides and usually mimic the chemistry, structure, and assembly of natural biomolecules, presenting excellent biocompatibility and biodegradability. For example, rosette nanotubes are a class of biomimetic self-assembled supramolecular structures, for which the basic building blocks are derived from guanine and cytosine DNA base pairs with the linkage of a lysine side chain.88 Their units undergo a hierarchical process to form a six-membered supermacrocycle by the formation of 18 hydrogen bonds under physiological conditions; the rosettes then form a stable stack, with an inner channel that is 11 Å in diameter, on the basis of electrostatic force, base-stacking interactions, and hydrophobic effects. It was reported that such nanotubes have a similar size and morphology to collagen, enhanced protein absorption, and stem cell and chondrocyte functions.89 For these reasons, rosette nanotubes have future potential for cartilage tissue engineering and repair. Interestingly, the structure of these bioinspired materials contains a hydrophobic core (aromatic rings) and a hydrophilic surface (lysine side chains), allowing their self-assembly to incorporate a variety of hydrophobic drugs into a stable water medium in order to improve cell differentiation and functions.90,91 Other examples of bioinspired materials are those that are peptide based, including nanostructured biomaterials that are genetically selected and/or design peptides with specific binding to functional solids. They tailor their binding and assembly characteristics, develop bifunctional peptide/protein genetic constructs with both material binding and biological activity, and use these constructs as molecular synthesizers, erectors, and assemblers.92 For example, a self-assembling peptide RADA16 (Ac-RADARADARADARADA-COHN2) was engineered to form a 3D scaffolding for cell growth.93 When it was exposed to physiological salt conditions, the peptide formed a hydrogel due to the formation of ionic bonds and a hydrophobic interaction. The authors stated that the nanofiber structure resembles natural collagen, with a fiber diameter of 5–10 nm, and enables cell proliferation more than any other synthetic scaffold, such as PLGA.
In short, scaffolding suitable for cartilage regeneration should be biocompatible and biodegradable and should support stem cell adhesion and growth. Some materials may also promote differentiation, but a well-mediated chondrogenesis process can only be achieved by adding growth factors. One shortcoming of synthetic scaffolding, such as PLGA, is that—unlike natural materials, such as collagens, that are better at mimicking features of the surrounding cartilage matrix, including biodegradability—its breakdown products can be relatively more cytotoxic to cells that they are designed to harbor.
Growth factors
Growth factors, another critical component in regenerative medicine, can regulate stem cell differentiation to control which cells they become and how fast they differentiate. Specifically for cartilage regeneration, TGF-β, insulin-like growth factor-1 (IGF-1), and BMPs are the most commonly used growth factors.
IGF-1 is a small protein containing only 70 amino acids, but it plays an important role in regulating DNA synthesis in multiple cell types, including chondrocytes. It accounts for most of the chondrocyte stimulation that is induced by serum94 and has a positive effect on chondrocyte proliferation and proteoglycan and type II collagen synthesis.95,96
TGF-β, a 25-kDa homodimeric protein, has three homologous isoforms (TGF-β1, TGF-β2, and TGF-β3), for which sequences have been identified in all mammalian species.97 TGF-β plays an important role in cell proliferation and differentiation, including chondrogenesis, bone formation, angiogenesis, neuroprotection, and wound repair.98–100 Especially for cartilage, TGF-β controls the production of extracellular matrices by stimulating the synthesis of collagens, fibronectin, and proteoglycans,101,102 exerting positive effects on cartilage regeneration. However, TGF-β can also induce undesired side effects, such as inflammatory responses and osteophyte formation in articular cartilage defects, if present in the knee joint for too long.103
BMPs, consisting of 30 members, are a subfamily of the TGF super family and play an important role in mediating chondrocyte and osteoblast growth and differentiation. The most widely studied BMPs are BMP-2, BMP-4, and BMP-7, which can promote chondrogenesis and stimulate proteoglycan synthesis.104 They can also induce chondrogenic differentiation of mesenchymal stem cells.105 Although BMPs are used for cartilage repair, they have also been found to stimulate osteoblast growth and osteogeneic differentiation. In addition, they appear to be less potent than TGF-β1 in promoting proteoglycan synthesis in the joint.106
There are two obstacles in using these growth factors in regenerative medicine: (1) the short half-life in vivo—for example, TGF-β has a 30-min half-life107 in the body, and IGF-1 in the body has an even shorter half-life (10–12 min);108 and (2) the high cost in producing and maintaining the full-length recombinant protein. Growth factors, especially TGF-β and BMP-2, have a hundred amino acids and complex 3D structures, and are easy to deactivate during shipping and storage. In addition, physicians have to administer an extremely high dose of the growth factors to achieve desired clinical outcomes, making growth factor therapy very expensive. Nowadays, scientists apply different approaches to identify the bioactive area of growth factors and use shorter peptides to achieve similar functions as the full-length growth factors.109,110 Moreover, many of the short peptides have been modified onto the cell growth scaffolding, greatly enhancing their half-life, delivery, and efficacy.111–113 The use of growth factors in combination with scaffolds/dECM is an excellent strategy to try to mimic the structural and bioactive conditions of native articular cartilage. Although this strategy is not perfect in that not every element of native healthy cartilage is represented in the resulting synthetic microenvironment, it has helped to enhance the healing response and produce repair tissue that is comparable to hyaline cartilage.
Conclusions
Understanding the stem cell niche is critical in cell-based tissue repair strategies because it determines the developmental fate of stem cells. As such, recent advances in stem cell–based cartilage repair strategies have come from paying close attention to the role of the tissue microenvironment in the healing process. Biocompatible scaffolding can facilitate stem cell attachment and growth, while growth factors promote chondrogenesis and enhance cellular functions. Further advancement in this field will depend on the ability to find new and innovative approaches to the combined use of stem cells, growth factors, and biomimetic scaffolds in order to emulate the native cartilage architecture, its biological composition, and its mechanical properties.
Acknowledgments
This study is supported by NIH P20GM104937.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 2.Peterson L, Vasiliadis HS, Brittberg M, et al. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38:1117–1124. doi: 10.1177/0363546509357915. [DOI] [PubMed] [Google Scholar]
- 3.Williams RJ, 3rd, Harnly HW. Microfracture: indications, technique, and results. Instr Course Lect. 2007;56:419–428. [PubMed] [Google Scholar]
- 4.Henderson I, Gui J, Lavigne P. Autologous chondrocyte implantation: natural history of postimplantation periosteal hypertrophy and effects of repair-site debridement on outcome. Arthroscopy. 2006;22:1318–1324.e1. doi: 10.1016/j.arthro.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 5.Kreuz PC, Steinwachs M, Erggelet C, et al. Classification of graft hypertrophy after autologous chondrocyte implantation of full-thickness chondral defects in the knee. Osteoarthritis Cartilage. 2007;15:1339–1347. doi: 10.1016/j.joca.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14:1119–1125. doi: 10.1016/j.joca.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Lefebvre V, Li P, de Crombrugghe B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 1998;17:5718–5733. doi: 10.1093/emboj/17.19.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akiyama H, Chaboissier MC, Martin JF, et al. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandell LJ, Nalin AM, Reife RA. Alternative splice form of type II procollagen mRNA (IIA) is predominant in skeletal precursors and non-cartilaginous tissues during early mouse development. Dev Dyn. 1994;199:129–140. doi: 10.1002/aja.1001990206. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi T, Chung UI, Schipani E, et al. PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development. 2002;129:2977–2986. doi: 10.1242/dev.129.12.2977. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Topol L, Lee H, et al. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003;130:1003–1015. doi: 10.1242/dev.00324. [DOI] [PubMed] [Google Scholar]
- 12.Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1:461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shepherd DE, Seedhom BB. Thickness of human articular cartilage in joints of the lower limb. Ann Rheum Dis. 1999;58:27–34. doi: 10.1136/ard.58.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayasuriya CT, Chen Q. Cartilage extracellular matrix integrity and OA. In: Rothschild BM, editor. Principles of Osteoarthritis—Its Definition, Character, Derivation and Modality-Related Recognition. Rijeka: InTechOpen; 2012. p. 337. [Google Scholar]
- 15.Bellucci G, Seedhom BB. Mechanical behaviour of articular cartilage under tensile cyclic load. Rheumatology (Oxford) 2001;40:1337–1345. doi: 10.1093/rheumatology/40.12.1337. [DOI] [PubMed] [Google Scholar]
- 16.Schumacher BL, Block JA, Schmid TM, et al. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys. 1994;311:144–152. doi: 10.1006/abbi.1994.1219. [DOI] [PubMed] [Google Scholar]
- 17.Jay GD, Britt DE, Cha CJ. Lubricin is a product of megakaryocyte stimulating factor gene expression by human synovial fibroblasts. J Rheumatol. 2000;27:594–600. [PubMed] [Google Scholar]
- 18.Minns RJ, Steven FS. The collagen fibril organization in human articular cartilage. J Anat. 1977;123:437–457. [PMC free article] [PubMed] [Google Scholar]
- 19.Scuderi GR, Tria AJ. The Knee: A Comprehensive Review. Hackensack: World Scientific; 2010. [Google Scholar]
- 20.Hayes AJ, MacPherson S, Morrison H, et al. The development of articular cartilage: evidence for an appositional growth mechanism. Anat Embryol (Berl) 2001;203:469–479. doi: 10.1007/s004290100178. [DOI] [PubMed] [Google Scholar]
- 21.Fickert S, Fiedler J, Brenner RE. Identification of subpopulations with characteristics of mesenchymal progenitor cells from human osteoarthritic cartilage using triple staining for cell surface markers. Arthritis Res Ther. 2004;6:R422–R432. doi: 10.1186/ar1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grogan SP, Miyaki S, Asahara H, et al. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11:R85. doi: 10.1186/ar2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams R, I, Khan M, Richardson K, et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS One. 2010;5:e13246. doi: 10.1371/journal.pone.0013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Bari C, Dell’Accio F, Tylzanowski P, et al. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura K, Solchaga LA, Caplan AI, et al. Chondroprogenitor cells of synovial tissue. Arthritis Rheum. 1999;42:2631–2637. doi: 10.1002/1529-0131(199912)42:12<2631::AID-ANR18>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 26.De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multilineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 27.Wickham MQ, Erickson GR, Gimble JM, et al. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res. 2003;412:196–212. doi: 10.1097/01.blo.0000072467.53786.ca. [DOI] [PubMed] [Google Scholar]
- 28.Muhammad H, Schminke B, Bode C, et al. Human migratory meniscus progenitor cells are controlled via the TGF-β pathway. Stem Cell Reports. 2014;3:789–803. doi: 10.1016/j.stemcr.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 30.Koelling S, Miosge N. Stem cell therapy for cartilage regeneration in osteoarthritis. Expert Opin Biol Ther. 2009;9:1399–1405. doi: 10.1517/14712590903246370. [DOI] [PubMed] [Google Scholar]
- 31.Pretzel D, Linss S, Rochler S, et al. Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res Ther. 2011;13:R64. doi: 10.1186/ar3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alsalameh S, Amin R, Gemba T, et al. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50:1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 33.Khan IM, Bishop JC, Gilbert S, et al. Clonal chondroprogenitors maintain telomerase activity and Sox9 expression during extended monolayer culture and retain chondrogenic potential. Osteoarthritis Cartilage. 2009;17:518–528. doi: 10.1016/j.joca.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Seol D, McCabe DJ, Choe H, et al. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum. 2012;64:3626–3637. doi: 10.1002/art.34613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lotz M. Cytokines in cartilage injury and repair. Clin Orthop Relat Res. 2001;391(Suppl):S108–S115. doi: 10.1097/00003086-200110001-00011. [DOI] [PubMed] [Google Scholar]
- 36.Martel-Pelletier J, Boileau C, Pelletier JP, et al. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol. 2008;22:351–384. doi: 10.1016/j.berh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11:747–755. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- 38.Scher JU, Pillinger MH, Abramson SB. Nitric oxide synthases and osteoarthritis. Curr Rheumatol Rep. 2007;9:9–15. doi: 10.1007/s11926-007-0016-z. [DOI] [PubMed] [Google Scholar]
- 39.van der Kraan PM, van den Berg WB. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage. 2012;20:223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Felson DT, Gale DR, Elon Gale M, et al. Osteophytes and progression of knee osteoarthritis. Rheumatology (Oxford) 2005;44:100–104. doi: 10.1093/rheumatology/keh411. [DOI] [PubMed] [Google Scholar]
- 41.Tanamas SK, Wluka AE, Pelletier JP, et al. Bone marrow lesions in people with knee osteoarthritis predict progression of disease and joint replacement: a longitudinal study. Rheumatology (Oxford) 2010;49:2413–2419. doi: 10.1093/rheumatology/keq286. [DOI] [PubMed] [Google Scholar]
- 42.Zhen G, Wen C, Jia X, et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19:704–712. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen G. The role of type X collagen in facilitating and regulating endochondral ossification of articular cartilage. Orthod Craniofac Res. 2005;8:11–17. doi: 10.1111/j.1601-6343.2004.00308.x. [DOI] [PubMed] [Google Scholar]
- 44.Arias JL, Nakamura O, Fernandez MS, et al. Role of type X collagen on experimental mineralization of eggshell membranes. Connect Tissue Res. 1997;36:21–33. doi: 10.3109/03008209709160211. [DOI] [PubMed] [Google Scholar]
- 45.McCarthy HE, Bara JJ, Brakspear K, et al. The comparison of equine articular cartilage progenitor cells and bone marrow-derived stromal cells as potential cell sources for cartilage repair in the horse. Vet J. 2012;192:345–351. doi: 10.1016/j.tvjl.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Buckley CT, Almeida HV, et al. Infrapatellar fat pad-derived stem cells maintain their chondrogenic capacity in disease and can be used to engineer cartilaginous grafts of clinically relevant dimensions. Tissue Eng Part A. 2014;20:3050–3062. doi: 10.1089/ten.tea.2014.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang JI, Kazmi N, Durbhakula MM, et al. Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: a patient-matched comparison. J Orthop Res. 2005;23:1383–1389. doi: 10.1016/j.orthres.2005.03.008.1100230621. [DOI] [PubMed] [Google Scholar]
- 48.Diekman BO, Rowland CR, Lennon DP, et al. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage-derived matrix. Tissue Eng Part A. 2010;16:523–533. doi: 10.1089/ten.tea.2009.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Bari C, Dell’Accio F, Vanlauwe J, et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54:1209–1221. doi: 10.1002/art.21753. [DOI] [PubMed] [Google Scholar]
- 50.De Bari C, Dell’Accio F, Luyten FP. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001;44:85–95. doi: 10.1002/1529-0131(200101)44:1<85::AID-ANR12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 51.Yoshimura H, Muneta T, Nimura A, et al. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 52.O’Sullivan J, D’Arcy S, Barry FP, et al. Mesenchymal chondroprogenitor cell origin and therapeutic potential. Stem Cell Res Ther. 2011;2:8. doi: 10.1186/scrt49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang Y, Cai Y, Zhang W, et al. Human cartilage-derived progenitor cells from committed chondrocytes for efficient cartilage repair and regeneration. Stem Cells Transl Med. 2016;5:733–744. doi: 10.5966/sctm.2015-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koelling S, Kruegel J, Irmer M, et al. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell. 2009;4:324–335. doi: 10.1016/j.stem.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 56.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 57.Oldershaw RA, Baxter MA, Lowe ET, et al. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol. 2010;28:1187–1194. doi: 10.1038/nbt.1683. [DOI] [PubMed] [Google Scholar]
- 58.Craft AM, Rockel JS, Nartiss Y, et al. Generation of articular chondrocytes from human pluripotent stem cells. Nat Biotechnol. 2015;33:638–645. doi: 10.1038/nbt.3210. [DOI] [PubMed] [Google Scholar]
- 59.Yamashita A, Morioka M, Yahara Y, et al. Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem Cell Reports. 2015;4:404–418. doi: 10.1016/j.stemcr.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uto S, Nishizawa S, Takasawa Y, et al. Bone and cartilage repair by transplantation of induced pluripotent stem cells in murine joint defect model. Biomed Res. 2013;34:281–288. doi: 10.2220/biomedres.34.281. [DOI] [PubMed] [Google Scholar]
- 61.Gutierrez-Aranda I, Ramos-Mejia V, Bueno C, et al. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells. 2010;28:1568–1570. doi: 10.1002/stem.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Q, Peng J, Guo Q, et al. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials. 2008;29:2378–2387. doi: 10.1016/j.biomaterials.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 63.Cheng CW, Solorio LD, Alsberg E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol Adv. 2014;32:462–484. doi: 10.1016/j.biotechadv.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang H, Peng J, Lu S, et al. In vivo cartilage repair using adipose-derived stem cell-loaded decellularized cartilage ECM scaffolds. J Tissue Eng Regen Med. 2014;8:442–453. doi: 10.1002/term.1538. [DOI] [PubMed] [Google Scholar]
- 65.Yang Q, Peng J, Lu SB, et al. Evaluation of an extracellular matrix-derived acellular biphasic scaffold/cell construct in the repair of a large articular high-load-bearing osteochondral defect in a canine model. Chin Med J (Engl) 2011;124:3930–3938. [PubMed] [Google Scholar]
- 66.Cheng NC, Estes BT, Awad HA, et al. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng Part A. 2009;15:231–241. doi: 10.1089/ten.tea.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ben-Yishay A, Grande DA, Schwartz RE, et al. Repair of articular cartilage defects with collagen-chondrocyte allografts. Tissue Eng. 1995;1:119–133. doi: 10.1089/ten.1995.1.119. [DOI] [PubMed] [Google Scholar]
- 68.Pei M, Luo J, Chen Q. Enhancing and maintaining chondrogenesis of synovial fibroblasts by cartilage extracellular matrix protein matrilins. Osteoarthritis Cartilage. 2008;16:1110–1117. doi: 10.1016/j.joca.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jayasuriya CT, Zhou FH, Pei M, et al. Matrilin-3 chondrodysplasia mutations cause attenuated chondrogenesis, premature hypertrophy and aberrant response to TGF-β in chondroprogenitor cells. Int J Mol Sci. 2014;15:14555–14573. doi: 10.3390/ijms150814555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jayasuriya CT, Goldring MB, Terek R, et al. Matrilin-3 induction of IL-1 receptor antagonist is required for upregulating collagen II and aggrecan and down-regulating ADAMTS-5 gene expression. Arthritis Res Ther. 2012;14:R197. doi: 10.1186/ar4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang X, Trehan SK, Guan Y, et al. Matrilin-3 inhibits chondrocyte hypertrophy as a bone morphogenetic protein-2 antagonist. J Biol Chem. 2014;289:34768–34779. doi: 10.1074/jbc.M114.583104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boccaccini AR, Maquet V. Bioresorbable and bioactive polymer/Bioglass® composites with tailored pore structure for tissue engineering applications. Compos Sci Technol. 2003;63:2417–2429. [Google Scholar]
- 73.Thomson RC, Mikos AG, Beahm E, et al. Guided tissue fabrication from periosteum using preformed biodegradable polymer scaffolds. Biomaterials. 1999;20:2007–2018. doi: 10.1016/s0142-9612(99)00103-9. [DOI] [PubMed] [Google Scholar]
- 74.Fischer D, Li Y, Ahlemeyer B, et al. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24:1121–1131. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 75.Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices. 2011;8:607–626. doi: 10.1586/erd.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martin JR, Gupta MK, Page JM, et al. A porous tissue engineering scaffold selectively degraded by cell-generated reactive oxygen species. Biomaterials. 2014;35:3766–3776. doi: 10.1016/j.biomaterials.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liao J, Qu Y, Chu B, et al. Biodegradable CSMA/ PECA/graphene porous hybrid scaffold for cartilage tissue engineering. Sci Rep. 5:9879. doi: 10.1038/srep09879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu H, Slamovich EB, Webster TJ. Less harmful acidic degradation of poly(lacticco-glycolic acid) bone tissue engineering scaffolds through titania nanoparticle addition. Int J Nanomed. 2006;1:541–545. doi: 10.2147/nano.2006.1.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Almeida HV, Eswaramoorthy R, Cunniffe GM, et al. Fibrin hydrogels functionalized with cartilage extracellular matrix and incorporating freshly isolated stromal cells as an injectable for cartilage regeneration. Acta Biomater. 2016;36:55–62. doi: 10.1016/j.actbio.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 80.Dua R, Comella K, Butler R, et al. Integration of stem cell to chondrocyte-derived cartilage matrix in healthy and osteoarthritic states in the presence of hydroxyapatite nanoparticles. PLoS One. 2016;11:e0149121. doi: 10.1371/journal.pone.0149121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reppel L, Schiavi J, Charif N, et al. Chondrogenic induction of mesenchymal stromal/stem cells from Wharton’s jelly embedded in alginate hydrogel and without added growth factor: an alternative stem cell source for cartilage tissue engineering. Stem Cell Res Ther. 2015;6:260. doi: 10.1186/s13287-015-0263-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eslaminejad MB, Mirzadeh H, Mohamadi Y, et al. Bone differentiation of marrow-derived mesenchymal stem cells using beta-tricalcium phosphate–alginate–gelatin hybrid scaffolds. J Tissue Eng Regen Med. 2007;1:417–424. doi: 10.1002/term.49. [DOI] [PubMed] [Google Scholar]
- 83.Kurth T, Hedbom E, Shintani N, et al. Chondrogenic potential of human synovial mesenchymal stem cells in alginate. Osteoarthritis Cartilage. 2007;15:1178–1189. doi: 10.1016/j.joca.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 84.Chung TW, Yang J, Akaike T, et al. Preparation of alginate/galactosylated chitosan scaffold for hepatocyte attachment. Biomaterials. 2002;23:2827–2834. doi: 10.1016/s0142-9612(01)00399-4. [DOI] [PubMed] [Google Scholar]
- 85.Thomas CH, McFarland CD, Jenkins ML, et al. The role of vitronectin in the attachment and spatial distribution of bone-derived cells on materials with patterned surface chemistry. J Biomed Mater Res. 1997;37:81–93. doi: 10.1002/(sici)1097-4636(199710)37:1<81::aid-jbm10>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 86.Moon S, Ryu B, Choi J, et al. The morphology and mechanical properties of sodium alginate based electrospun poly(ethylene oxide) nanofibers. Polym Eng Sci. 2009;49:52–59. [Google Scholar]
- 87.Wise JK, Yarin AL, Megaridis CM, et al. Chondrogenic differentiation of human mesenchymal stem cells on oriented nanofibrous scaffolds: engineering the superficial zone of articular cartilage. Tissue Eng Part A. 2009;15:913–921. doi: 10.1089/ten.tea.2008.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang L, Chen Y, Rodriguez J, et al. Biomimetic helical rosette nanotubes and nanocrystalline hydroxyapatite coatings on titanium for improving orthopedic implants. Int J Nanomed. 2008;3:323–333. doi: 10.2147/ijn.s2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen Y, Bilgen B, Pareta RA, et al. Self-assembled rosette nanotube/hydrogel composites for cartilage tissue engineering. Tissue Eng Part C Methods. 2010;16:1233–1243. doi: 10.1089/ten.TEC.2009.0400. [DOI] [PubMed] [Google Scholar]
- 90.Song S, Chen Y, Yan Z, et al. Self-assembled rosette nanotubes for incorporating hydrophobic drugs in physiological environments. Int J Nanomed. 2011;6:101–107. doi: 10.2147/IJN.S11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen Y, Song S, Yan Z, et al. Self-assembled rosette nanotubes encapsulate and slowly release dexamethasone. Int J Nanomed. 2011;6:1035–1044. doi: 10.2147/IJN.S18755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sarikaya M, Tamerler C, Jen AK, et al. Molecular biomimetics: nanotechnology through biology. Nat Mater. 2003;2:577–585. doi: 10.1038/nmat964. [DOI] [PubMed] [Google Scholar]
- 93.Gelain F, Bottai D, Vescovi A, et al. Designer self-assembling peptide nanofiber scaffolds for adult mouse neural stem cell 3-dimensional cultures. PLoS One. 2006;1:e119. doi: 10.1371/journal.pone.0000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Giudice LC, de Zegher F, Gargosky SE, et al. Insulin-like growth factors and their binding proteins in the term and preterm human fetus and neonate with normal and extremes of intrauterine growth. J Clin Endocrinol Metab. 1995;80:1548–1555. doi: 10.1210/jcem.80.5.7538146. [DOI] [PubMed] [Google Scholar]
- 95.Darling EM, Athanasiou KA. Growth factor impact on articular cartilage subpopulations. Cell Tissue Res. 2005;322:463–473. doi: 10.1007/s00441-005-0020-4. [DOI] [PubMed] [Google Scholar]
- 96.Morales TI. The role and content of endogenous insulin-like growth factor-binding proteins in bovine articular cartilage. Arch Biochem Biophys. 1997;343:164–172. doi: 10.1006/abbi.1997.0166. [DOI] [PubMed] [Google Scholar]
- 97.Chin D, Boyle GM, Parsons PG, et al. What is transforming growth factor-beta (TGF-β)? Br J Plast Surg. 2004;57:215–221. doi: 10.1016/j.bjps.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 98.Sherris DA, Murakami CS, Larrabee WF, Jr, et al. Mandibular reconstruction with transforming growth factor-beta 1. Laryngoscope. 1998;108:368–372. doi: 10.1097/00005537-199803000-00011. [DOI] [PubMed] [Google Scholar]
- 99.Dinbergs ID, Brown L, Edelman ER. Cellular response to transforming growth factor-beta 1 and basic fibroblast growth factor depends on release kinetics and extracellular matrix interactions. J Biol Chem. 1996;271:29822–29829. doi: 10.1074/jbc.271.47.29822. [DOI] [PubMed] [Google Scholar]
- 100.Wakefield LM, Winokur TS, Hollands RS, et al. Recombinant latent transforming growth factor beta 1 has a longer plasma half-life in rats than active transforming growth factor beta 1, and a different tissue distribution. J Clin Invest. 1990;86:1976–1984. doi: 10.1172/JCI114932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ignotz RA, Endo T, Massague J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987;262:6443–6446. [PubMed] [Google Scholar]
- 102.Chen JK, Hoshi H, McKeehan WL. Transforming growth factor type beta specifically stimulates synthesis of proteoglycan in human adult arterial smooth muscle cells. Proc Natl Acad Sci USA. 1987;84:5287–5291. doi: 10.1073/pnas.84.15.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Beuningen HM, Glansbeek HL, van der Kraan PM, et al. Osteoarthritis-like changes in the murine knee joint resulting from intra-articular transforming growth factor-beta injections. Osteoarthritis Cartilage. 2000;8:25–33. doi: 10.1053/joca.1999.0267. [DOI] [PubMed] [Google Scholar]
- 104.Luyten FP, Chen P, Paralkar V, et al. Recombinant bone morphogenetic protein-4, transforming growth factor-beta 1, and activin A enhance the cartilage phenotype of articular chondrocytes in vitro. Exp Cell Res. 1994;210:224–229. doi: 10.1006/excr.1994.1033. [DOI] [PubMed] [Google Scholar]
- 105.Noel D, Gazit D, Bouquet C, et al. Short-term BMP-2 expression is sufficient for in vivo osteochondral differentiation of mesenchymal stem cells. Stem Cells. 2004;22:74–85. doi: 10.1634/stemcells.22-1-74. [DOI] [PubMed] [Google Scholar]
- 106.van Beuningen HM, Glansbeek HL, van der Kraan PM, et al. Differential effects of local application of BMP-2 or TGF-beta 1 on both articular cartilage composition and osteophyte formation. Osteoarthritis Cartilage. 1998;6:306–317. doi: 10.1053/joca.1998.0129. [DOI] [PubMed] [Google Scholar]
- 107.Nimni ME. Polypeptide growth factors: targeted delivery systems. Biomaterials. 1997;18:1201–1225. doi: 10.1016/s0142-9612(97)00050-1. [DOI] [PubMed] [Google Scholar]
- 108.Guler HP, Zapf J, Schmid C, et al. Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinol (Copenh) 1989;121:753–758. doi: 10.1530/acta.0.1210753. [DOI] [PubMed] [Google Scholar]
- 109.Saito A, Suzuki Y, Ogata S, et al. Activation of osteoprogenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope. Biochim Biophys Acta. 2003;1651:60–67. doi: 10.1016/s1570-9639(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 110.Chen Y, Webster TJ. Increased osteoblast functions in the presence of BMP-7 short peptides for nanostructured biomaterial applications. J Biomed Mater Res A. 2009;91:296–304. doi: 10.1002/jbm.a.32246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mercado AE, Yang X, He X, et al. Effect of grafting BMP2-derived peptide to nanoparticles on osteogenic and vasculogenic expression of stromal cells. J Tissue Eng Regen Med. 2014;8:15–28. doi: 10.1002/term.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Meng Q, Man Z, Dai L, et al. A composite scaffold of MSC affinity peptide-modified demineralized bone matrix particles and chitosan hydrogel for cartilage regeneration. Sci Rep. 2015;5:17802. doi: 10.1038/srep17802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim TG, Park TG. Biomimicking extracellular matrix: cell adhesive RGD peptide modified electrospun poly(D,L-lactic-co-glycolic acid) nanofiber mesh. Tissue Eng. 2006;12:221–233. doi: 10.1089/ten.2006.12.221. [DOI] [PubMed] [Google Scholar]