The objective of this study was to identify independent prognostic factors for survival in a real‐world population of patients with EGFR mutation‐positive advanced non‐small cell lung cancer undergoing treatment with gefitinib. The study also evaluates whether brain metastasis or intracranial progression is a poor prognostic predictor for gefitinib therapy.
Keywords: Non‐small cell lung cancer, Gefitinib, Prognostic factors, Chronic hepatitis C, Intracranial progression
Abstract
Background.
This study aimed to identify independent prognostic factors for overall survival (OS) of patients with advanced non‐small cell lung cancer (NSCLC) harboring an activating epidermal growth factor receptor (EGFR) mutation and receiving gefitinib as first‐line treatment in real‐world practice.
Materials and Methods.
We enrolled 226 patients from June 2011 to May 2013. During this period, gefitinib was the only EGFR‐tyrosine kinase inhibitor reimbursed by the Bureau of National Health Insurance of Taiwan.
Results.
The median progression‐free survival and median OS were 11.9 months (95% confidence interval [CI]: 9.7–14.2) and 26.9 months (21.2–32.5), respectively. The Cox proportional hazards regression model revealed that postoperative recurrence, performance status (Eastern Cooperative Oncology Grade [ECOG] ≥2), smoking index (≥20 pack‐years), liver metastasis at initial diagnosis, and chronic hepatitis C virus (HCV) infection were independent prognostic factors for OS (hazard ratio [95% CI] 0.3 [0.11–0.83], p = .02; 2.69 [1.60–4.51], p < .001; 1.92 [1.24–2.97], p = .003; 2.26 [1.34–3.82], p = .002; 3.38 [1.85–7.78], p < .001, respectively). However, brain metastasis (BM) at initial diagnosis or intracranial progression during gefitinib treatment had no impact on OS (1.266 [0.83–1.93], p = .275 and 0.75 [0.48–1.19], p = .211, respectively).
Conclusion.
HCV infection, performance status (ECOG ≥2), newly diagnosed advanced NSCLC without prior operation, and liver metastasis predicted poor OS in EGFR mutation‐positive advanced NSCLC patients treated with first‐line gefitinib; however, neither BM at initial diagnosis nor intracranial progression during gefitinib treatment had an impact on OS.
Implications for Practice.
The finding that chronic hepatitis C virus (HCV) infection might predict poor overall survival (OS) in epidermal growth factor receptor mutation‐positive advanced non‐small cell lung cancer (NSCLC) patients treated with first‐line gefitinib may raise awareness of benefit from anti‐HCV treatment in this patient population. Brain metastasis in the initial diagnosis or intracranial progression during gefitinib treatment is not a prognostic factor for OS. This study, which enrolled a real‐world population of NSCLC patients, including sicker patients who were not eligible for a clinical trial, may have impact on guiding usual clinical practice.
摘要
背景. 本研究使用携带有激活的表皮生长因子受体(EGFR)突变基因且在真实世界接受吉非替尼一线治疗的晚期非小细胞肺癌(NSCLC)患者确定总生存期(OS)的独立预后因素。
材料和方法. 本研究从2011年6月至2013年5月共招募226例患者。这期间, 吉非替尼是唯一一种可由台湾中央健康保险局报销的EGFR‐酪氨酸激酶抑制剂。
结果. 中位无进展生存期和中位OS分别为11.9 [95%置信区间(CI):9.7‐14.2] 和26.9(21.2‐32.5)个月。Cox 比例风险回归模型显示术后复发、体力状态[东部肿瘤协作组评分(ECOG)≥2分]、吸烟指数(≥20包年)、首次诊断时肝转移和慢性丙型肝炎病毒(HCV)感染为OS的独立预后因素[风险比(95% CI)分别为0.3(0.11‐0.83), p = 0.02;2.69(1.60‐4.51), p<0.001;1.92(1.24‐2.97), p = 0.003;2.26(1.34‐3.82), p = 0.002;3.38 (1.85‐7.78), p<0.001]。但首次诊断时为脑转移(BM)或吉非替尼治疗期间颅内进展不影响OS [风险比(95% CI)分别为1.266(0.83‐1.93), p = 0.275;0.75(0.48‐1.19), p = 0.211]。
结论. 在接受吉非替尼一线治疗的EGFR突变阳性的晚期NSCLC患者中, HCV感染、体力状态(ECOG ≥ 2分)、既往未接受过手术的首次诊断晚期NSCLC和肝转移预测OS不佳, 但首次诊断时BM和吉非替尼治疗期间颅内进展均不影响OS。
对临床实践的提示:慢性HCV感染可能预测接受吉非替尼一线治疗的EGFR突变阳性的晚期NSCLC患者OS不佳, 这将提高该人群通过抗HCV治疗获益的意识。首次诊断时为BM或吉非替尼治疗期间颅内进展不是OS的预后因素。本研究招募真实世界的NSCLC患者人群, 包括不符合临床试验要求的病情较重患者, 对指导常规临床实践可能会有一定影响。
Introduction
Of the common cancer types, lung cancer, although not the most common cancer, causes the most cancer‐related deaths worldwide (1.59 million deaths in 2012) [1]. In 2004, investigators identified patients with non‐small cell lung cancer (NSCLC) who harbored specific mutations in the epidermal growth factor receptor (EGFR) gene and found that these EGFR mutations predicted a response to the tyrosine kinase inhibitor (TKI) gefitinib [2]. After the IPASS study, gefitinib became first‐line treatment for patients with advanced NSCLC harboring an activated EGFR mutation [3], [4]. In addition to gefitinib, both erlotinib and afatinib showed promising effects in the treatment of advanced lung cancer with an activated EGFR mutation [5], [6], [7]. In one study, the penetration rate of gefitinib in cerebrospinal fluid (CSF) was approximately half that of erlotinib (1.13 ± 0.36% and 2.77 ± 0.45%, respectively) [8]. Therefore, some physicians may think gefitinib is less effective in treating patients with brain metastasis (BM).
The National Health Insurance of Taiwan has reimbursed gefitinib for first‐line treatment of advanced NSCLC with EGFR mutations since June 2011 but did not reimburse erlotinib and afatinib for the same indication until November 2013 and May 2014, respectively. Therefore, from June 2011 to November 2013, gefitinib was almost the only choice for first‐line therapy for patients with advanced NSCLC harboring an activated EGFR mutation in Taiwan, regardless of the mutation type, performance status, or a consideration of side effects. Some prognostic factors have been proposed to predict survival of patients harboring activated EGFR mutations, including Eastern Cooperative Oncology Group (ECOG) performance status, metastatic site, smoking status, and age [9], [10], [11], [12]. However, in these prior reports, whether they were prospective clinical trials or retrospective studies, selection of the patient population may have been confounded by disease severity or the physician's preference. Therefore, we wanted to identify independent prognostic factors for survival in a real‐world population of patients with EGFR mutation‐positive advanced NSCLC using gefitinib. We also wanted to evaluate if BM or intracranial progression is a poor prognostic predictor for gefitinib therapy.
Materials and methods
Patients and Populations
In this retrospective study, all patients aged 18 years or older with a diagnosis of NSCLC from June 1, 2011, to May 31, 2013, at National Taiwan University Hospital (NTUH) were identified. Other eligibility criteria included the following: EGFR‐activating mutation (either exon 19 deletion, L858R in exon 21, or other uncommon mutations), stage IIIB/IV newly diagnosed NSCLC or postoperative recurrence after curative surgery with or without adjuvant therapy, and receiving gefitinib as first‐line therapy. Exclusion criteria included the following: initial treatment at another hospital, treatment less than 3 months due to adverse events, lost to follow‐up within less than 3 months, enrollment in another clinical trial, history of other malignancies, sarcomatoid type cancer, and EGFR exon 20 insertion. Baseline clinical characteristics, including age at diagnosis, sex, EGFR mutation type, newly diagnosed or postoperative recurrence, ECOG performance status at the beginning of gefitinib treatment, smoking status, tumor histology, and viral hepatitis profile, were recorded by retrospective chart review. This investigation was approved by the NTUH Research Ethics Committee.
Treatment
Patients took gefitinib 250 mg/day orally until disease progression, at which time they received subsequent anticancer therapy or continued gefitinib, depending on their physician's discretion
Assessment
Tumor response to treatment was assessed using computed tomography (CT) every 3 months and was evaluated based on Response Evaluation Criteria In Solid Tumors version 1.1 [13]. The primary outcome of this study was overall survival (OS), defined as the duration between the start of gefitinib treatment and the time of all‐cause death. We contacted the Taiwan Death Registry to check the survival status of patients who were lost to follow‐up during the study period. If the patient had died, we recorded the date of death and calculated the OS of the patient. The secondary outcome was progression‐free survival (PFS), defined as the duration between the start of gefitinib treatment and the time of disease progression.
Statistical Analysis
Patient OS and PFS were assessed using the Kaplan‐Meier method. The log‐rank test was used for comparison of survival curves of different characteristics. Prognostic factors for OS and PFS were analyzed by univariate and multivariate analysis. Variables with p ≤ .1 were added to the final multivariate Cox regression model. The hazard ratio (HR) and 95% confidence interval (CI) were calculated for all variables in the regression model. All tests were two‐sided. Statistical significance was set at p < .05. Statistical analysis was conducted using SPSS 17.0 for Windows (SPSS, Inc., Chicago, IL, http://www.spss.com.hk/corpinfo/index.htm). The data cut‐off date was September 30, 2015.
Results
Patient Characteristics
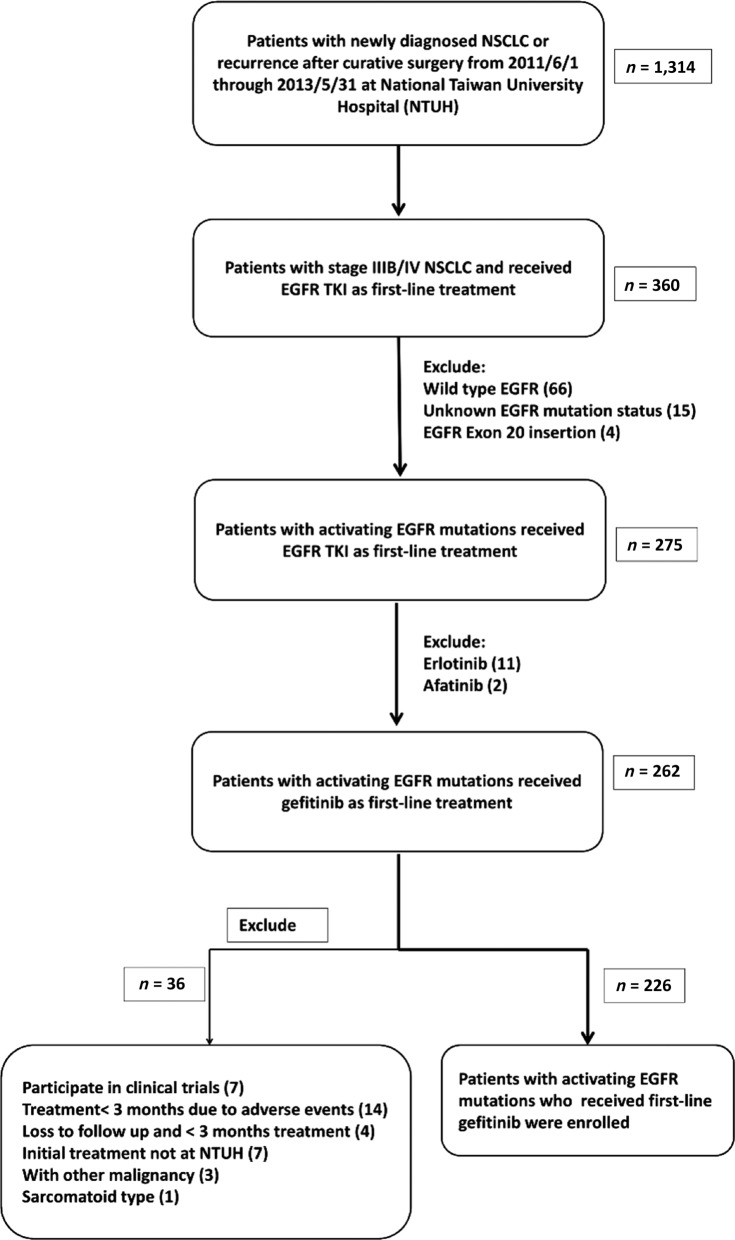
From June 1, 2011, to May 31, 2013, 1,341 patients were diagnosed as having lung cancer or postoperative recurrence at NTUH. Of these patients, 275 had stage IIIB or IV lung cancer and harbored an EGFR mutation; they received EGFR‐TKI as first‐line therapy, including 13 who participated in clinical trials for erlotinib or afatinib (11 and 2, respectively). After excluding those who met the exclusion criteria, 226 patients were enrolled in the study (Fig. 1). The median follow‐up time for these patients was 37.8 months (95% CI 35.6–40.0). The patients were predominantly younger than 70 years old (<70 years old, 61.5%), female (65%), with an ECOG performance status <2 (88.5%), clinical stage IV (97.8%), histology of adenocarcinoma (99.1%), and common EGFR mutation (95.1%, 46% with exon 19 deletion and 49.1% with L858R mutation). The median age at diagnosis was 64.7 years. Patients with an exon 19 deletion were younger than those with L858R mutation (median, 61.7 years old vs. 67.9 years old, p = .028, independent t test). Most patients (92%) were newly diagnosed as having advanced lung cancer without prior treatment or operation (Table 1).
Figure 1.
Patient selection and exclusion criteria.
Abbreviations: EGFR, epidermal growth factor receptor; NSCLC, non‐small cell lung cancer; NTUH, National Taiwan University Hospital; TKI, tyrosine kinase inhibitor.
Table 1. Baseline characteristics.
| Characteristic | n (%) |
|---|---|
| Age (28–88 years) | |
| ≥70 years old | 87 (38.5%) |
| <70 years old | 139 (61.5%) |
| Gender | |
| Female | 147 (65%) |
| Male | 79 (35%) |
| Smoking history | |
| <20 pack‐years | 190 (84.1%) |
| ≥20 pack‐years | 36 (15.9%) |
| Performance status | |
| ECOG <2 | 200 (88.5%) |
| ECOG ≥2 | 26 (11.5%) |
| Stage | |
| IIIB | 5 (2.2%) |
| IV | 221 (97.8%) |
| EGFR mutation status | |
| Exon 19 deletion | 104 (46%) |
| L858R | 111 (49.1%) |
| Uncommon mutationa | 7 (3.1%) |
| Complexb | 4 (1.8%) |
| HBV status | |
| HBV (+) | 36 (15.9%) |
| HBV (−) | 145 (64.2%) |
| Unknown HBV status | 45 (19.9%) |
| HCV status | |
| HCV (+) | 12 (5.3%) |
| HCV (−) | 171 (75.6%) |
| Unknown HCV status | 43 (19.1%) |
| Disease status | |
| Postoperative recurrence | 18 (8.0%) |
| Advanced NSCLC | 208 (92.0%) |
| Pathology | |
| Adenocarcinoma | 224 (99.1%) |
| Adenosquamous cell carcinoma | 1 (0.45%) |
| NOS | 1 (0.45%) |
| M1a | 75 (33.2%) |
| Brain metastasis | 63 (27.9%) |
| Bone metastasis | 111 (49.1%) |
| Liver metastasis | 24 (10.6%) |
Mutation other than del‐19 or L858R, for example, L861Q, T790M, G719X, V769M.
Two or more distinct mutations co‐occurring in the same tumor, for example, G719X + S768I.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; HBV, hepatitis B virus; HCV, hepatitis C virus; NOS, not otherwise specified; NSCLC, non‐small cell lung cancer.
OS
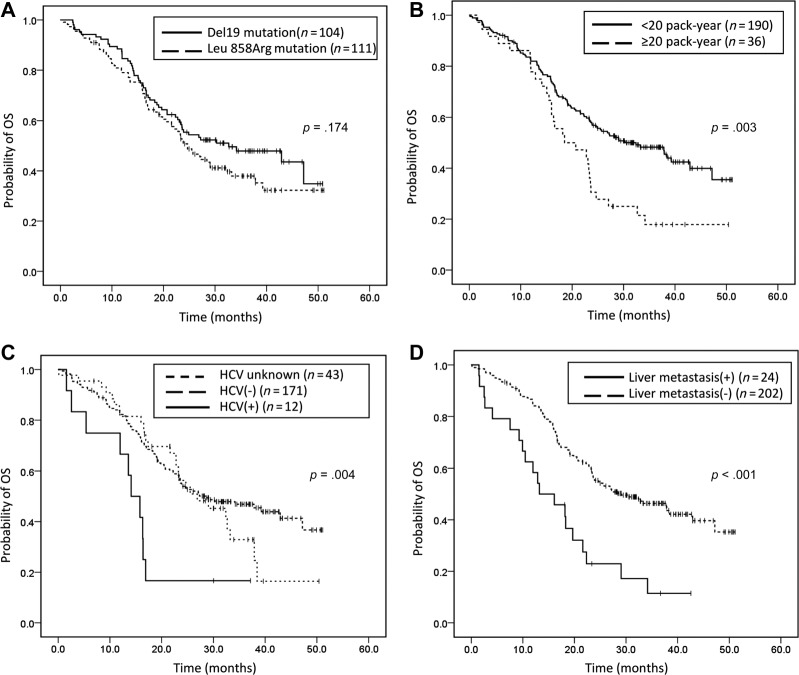
The median OS was 26.9 months (95% CI 21.2–32.5). Significantly shorter OS was noted in patients with an ECOG performance status ≥2 (median: 14 months, 95% CI 8.0–20.0), advanced lung cancer without prior operation (median: 24.7 months 95% CI 21.0–28.3), chronic hepatitis C virus (HCV) infection (median: 14.1 months, 95% C 10.3–17.9), and a smoking index ≥20 pack‐years (median: 18.5, 95% CI 9.5–27.6), and in those patients who had brain or liver metastasis at initial diagnosis (Table 2; Fig. 2; supplemental online Fig. 1). In multivariate analyses using multiple Cox proportional hazards models, we observed that performance status (ECOG ≥2; HR 2.69, 95% CI 1.6–4.51, p < .001), smoking index (>20 pack‐years; HR1.92, 95% CI 1.24–2.97, p = .003), liver metastasis at initial diagnosis (HR 2.26, 95% CI 1.34–3.82, p = .002), and chronic HCV infection (HR 3.8, 95% CI 1.85–7.78, p < .001) were independent prognostic factors for OS, while BM at initial diagnosis was not (HR 1.27, 95% CI 0.83–1.93, p = .275; Table 3). Among the 187 patients with disease progression, 40 experienced intracranial progression and 147 experienced extracranial progression during gefitinib treatment. There was no statistical difference in OS between the two groups (median OS in patients with intracranial progression and with extracranial progression: 23.6 months and 23.3 months, respectively, p = .222; Fig. 3).
Table 2. PFS and OS according to baseline characteristics.
| Variables | Number | Median PFS (months) | p valuea | Median OS (months) | p valuea |
|---|---|---|---|---|---|
| All | 226 | 11.9 (10.4–13.3) | NA | 26.9 (21.2–32.5) | NA |
| Age | |||||
| ≥70 y/o | 87 | 12.1 (8.8–15.4) | .261 | 23.4 (15.5–31.3) | .24 |
| <70 y/o | 139 | 11.4 (9.9–12.9) | 28.4 (20.3–36.6) | ||
| Gender | |||||
| Female | 147 | 11.9 (9.7–14.1) | .211 | 28.4 (17.5–39.3) | .315 |
| Male | 79 | 11.6 (10.6–12.6) | 23.4 (17.6–29.2) | ||
| Smoking history | |||||
| <20 pack‐years | 190 | 12.1 (10.4–13.8) | .362 | 32.4 (23.7–41.1) | .003 |
| ≥20 pack‐years | 36 | 10.6 (8.7–12.5) | 18.5 (9.5–27.6) | ||
| Performance status | |||||
| ECOG <2 | 200 | 12.1 (10.3–13.9) | .001 | 29.0 (21.9–36.2) | <.001 |
| ECOG ≥2 | 26 | 9.6 (8.4–10.7) | 14.0 (8.0–20.0) | ||
| Stage | |||||
| IIIB | 5 | 9.6 (9.5–9.6) | .285 | 39.3 (7.9–70.6) | .704 |
| IV | 221 | 11.9 (10.4–13.3) | 25.7 (19.9–31.6) | ||
| EGFR mutation status | |||||
| Exon 19 deletion | 104 | 11.9 (10.1–13.7) | <.001 | 32.7 (16.8–48.6) | .136 |
| L858R | 111 | 11.9 (9.7–14.2) | 24.7 (20.8–28.5) | ||
| Uncommon mutation | 7 | 8.8 (4.3–13.4) | 38.4 (0–78.5) | ||
| Complex | 4 | 2.7 (0–7.8) | 12.9 (0.4–25.4) | ||
| HBV status | |||||
| HBV (+) | 36 | 10.3 (5.7–14.8) | .227 | 25.5 (NA) | .656 |
| HBV (−) | 145 | 11.8 (10.2–13.4) | 23.8 (19.9–27.8) | ||
| Unknown HBV status | 45 | 11.9 (9.7–14.1) | 32.7 (24.8–40.5) | ||
| HCV status | |||||
| HCV (+) | 12 | 5.6 (4.1–7.1) | .39 | 14.1 (10.3–17.9) | .004 |
| HCV (−) | 171 | 11.9 (10.2–13.5) | 28.4 (17.9–39.0) | ||
| Unknown HCV status | 43 | 11.9 (9.8–14.0) | 25.5 (17.7–33.3) | ||
| Disease status | |||||
| Post‐op recurrence | 18 | 17.3 (1.6–33.0) | .12 | NR | .018 |
| Advanced NSCLC | 208 | 11.4 (10.0–12.8) | 24.7 (21.0–28.3) | ||
| Pathology | |||||
| Adenocarcinoma | 224 | 11.9 (10.4–13.3) | NA | 26.9 (20.8–32.9) | NA |
| Adenosquamous cell carcinoma | 1 | 3.3 | 33.4 | ||
| NOS | 1 | 6.1 | 19.1 | ||
| M1a | 75 | 14.9 (11.0–18.3) | .001b | 33.2 (19.3–47.1) | .08b |
| Brain metastasis | 63 | 9.9 (8.3–11.6) | .02c | 20.7 (16.4–25.0) | .042c |
| Bone metastasis | 111 | 10.3 (8.8–11.9) | .001d | 23.6 (19.9–27.2) | .1d |
| Liver metastasis | 24 | 8.9 (5.9–11.8) | <.001e | 13.2 (5.9–20.5) | <.001e |
Log‐rank test.
Versus extra‐pulmonary metastasis.
Versus without brain metastasis.
Versus without bone metastasis.
Versus without liver metastasis.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; HBV, hepatitis B virus; HCV, hepatitis C virus; NA, not applicable; NOS, not otherwise specified; NR, not reached; OS, overall survival; PFS, progression‐free survival.
Figure 2.
Kaplan‐Meier survival curves of overall survival in (A): patients with exon 19 deletion and L858R mutation (p = .174, log‐rank test). (B): Patients with a smoking index ≥20 pack‐years and a smoking index <20 pack‐years (p = .003, log‐rank test). (C): Patients with chronic hepatitis C, without chronic hepatitis C, or an unknown hepatitis C virus status (p = .004, log‐rank test). (D): Patients with or without liver metastasis (p < .001, log‐rank test).
Abbreviation: OS, overall survival.
Table 3. Overall survival: univariate and multivariate analysis.
| Univariate analysis | Multivariate analysis‐reduced | |||||
|---|---|---|---|---|---|---|
| Variable | Hazard ratio | 95% CI | p value | Hazard ratio | 95% CI | p value |
| Age (≥70 y/o) | 1.24 | 0.87–1.77 | .241 | |||
| Gender (male) | 1.2 | 0.84–1.72 | .315 | |||
| Smoke (≥20 pack‐years) | 1.88 | 1.24–2.84 | .003 | 1.92 | 1.24–2.97 | .003 |
| ECOG ≥2 | 2.35 | 1.44–3.84 | .001 | 2.69 | 1.60–4.51 | <.001 |
| Exon 19 deletiona | 0.78 | 0.54–1.12 | .175 | |||
| HBV | 0.89 | 0.54–1.46 | .643 | |||
| HCV | 2.89 | 1.49–5.60 | .002 | 3.80 | 1.85–7.78 | <.001 |
| Postoperative recurrence | 0.32 | 0.12–0.87 | .025 | 0.30 | 0.11–0.83 | .02 |
| M1a | 0.71 | 0.48–1.04 | .082 | 1.02 | 0.65–1.60 | .93 |
| Brain metastasis | 1.47 | 1.01–2.14 | .043 | 1.266 | 0.83–1.93 | .275 |
| Bone metastasis | 1.34 | 0.94–1.91 | .101 | |||
| Liver metastasis | 2.62 | 1.62–4.25 | <.001 | 2.26 | 1.34–3.82 | .002 |
Compared to L858R.
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus.
Figure 3.
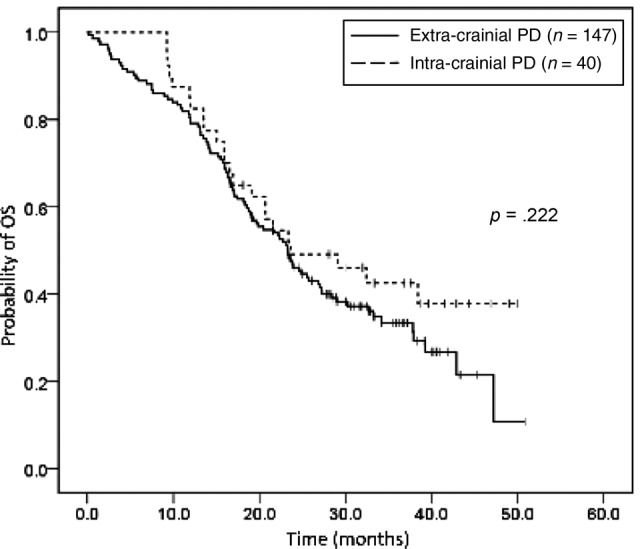
Kaplan‐Meier survival curves of overall survival in patients with intracranial progression and extracranial progression (p = .222, log‐rank test).
Abbreviation: OS, overall survival; PD, progressive disease.
PFS
The median PFS was 11.9 months (95% CI 10.4–13.3). Of the 226 patients, 187 suffered from progressive lung cancer. Shorter PFS was noted in patients with an ECOG performance status ≥2 (median: 9.6 months, 95% CI 8.4–10.7), a complex or uncommon EGFR mutation (median: 2.7 months and 8.8 months, respectively) and extrapulmonary metastasis at initial diagnosis. There was no significant PFS difference relative to age or smoking index. No extrapulmonary involvement at initial diagnosis of advanced lung cancer significantly predicted longer PFS (Table 2; supplemental online Fig. 1, supplemental online Fig. 2).
Patients with chronic HCV
Of the 226 patients, 183 underwent an anti‐HCV antibody test and 12 were diagnosed with chronic HCV infection. Ten patients with chronic HCV infection died during follow‐up, all due to cancer‐related causes (supplemental online Table 2). Among 12 patients with HCV infection, six patients had received second‐line chemotherapy. During gefitinib treatment, 11 patients developed abnormal hepatic function, among whom 9 patients had grade 1 hepatotoxicity and 2 patients had grade 2 hepatotoxicity. The severity of hepatotoxicity in patients with or without HCV infection and with unknown HCV status was summarized in supplemental online Table 2. Less hepatotoxicity developed in patients with unknown HCV status during gefitinib treatment. For patients who received anti‐HCV antibody examination, there is no significant correlation between the severity of hepatotoxicity and chronic hepatitis C infection (p = .533, Pearson's chi‐square test). Also, there is no significant difference in OS between different grade of hepatotoxicity (p = .677, log‐rank test; supplemental online Fig. 1).
Patients with BM
Among 63 patients with BM at diagnosis, 26 patients received gefitinib without first brain local treatments, and local treatments were performed to 37 patients, including whole brain radiotherapy to 28 patients, stereotactic radiosurgery to 1 patient, and brain tumor resection to 8 patients (7 patients received postoperation whole brain radiotherapy). There is no significant difference in OS between patients with BM who received brain local treatments and those who did not (21.6 months and 20.7 months, respectively, p = .517)
Postprogression Treatment
In all, 153 of the 187 patients who suffered from progressive lung cancer received subsequent treatment (supplemental online Table 2). Among these patients, chemotherapy and EGFR‐TKIs accounted for a similar proportion of treatment (68.6% and 69.1%, respectively). Platinum doublet‐chemotherapy and erlotinib were the most common chemotherapy and EGFR TKI regimens, respectively. Sixty‐eight patients with progressive disease after failure of chemotherapy were re‐treated with EGFR TKIs. In addition to chemotherapy and EGFR TKIs, 10 patients (5.3%) received combined therapy with bevacizumab. Postprogression treatment for patients with chronic HCV infection is summarized in supplemental online Table 2.
Discussion
In this study, we found that ECOG performance status, smoking index, hepatic metastasis at initial diagnosis, disease status (newly diagnosed or postoperative recurrence), and chronic HCV infection were independent prognostic factors for OS in patients harboring an EGFR mutation and receiving gefitinib as first‐line treatment in real‐world practice. This is the first time that chronic hepatitis C infection has been proposed as a poor prognostic factor. We also found that BM at initial diagnosis or intracranial progression during gefitinib treatment did not predict poor OS.
A retrospective study found that patients with exon 19 deletion tended to be younger and had more locally advanced lymphadenopathy [14]. Our study also revealed the presence of exon 19 deletion in younger patients. Previous studies have found that exon 19 deletion predicted better OS than L858R mutation in NSCLC patients receiving EGFR‐TKI treatment, especially afatinib [15], [16]. This was not observed in our study, however. Median OS of patients with exon 19 deletion and L858R mutation in our study was 32.7 months and 24.7 months, respectively (95% CI 16.8–48.6 and 20.8–28.5, respectively, p = .174). Another study with post‐hoc analysis of the NEJ002 study, but without a direct comparison of the exon 19 deletion and L858R mutation, revealed no OS differences between patients receiving gefitinib and carboplatin‐paclitaxel, irrespective of exon 19 deletion (29.3 months vs. 29.7 months, p = .53) or L858R mutation (28.4 months vs. 25.1 months, p = .45) [17].
Smoking status as a prognostic predictor has been reviewed previously. A retrospective study found that a smoking index of >30 pack‐years was an independent negative predictor for both PFS and OS [11]. Our study, however, found that a smoking index of >20 pack‐years was a negative predictor for OS but not for PFS. We divided patients into never‐smokers and ever‐smokers and still found no significant difference in PFS between these two groups (median: 11.9 months vs. 11.3 months, respectively, p = .252, log‐rank test). This may imply that the most important predictor is activating EGFR mutations. In addition, a better response to post‐progression treatment in light smokers (smoking index <20 pack‐years) determined longer OS. Further study is needed to evaluate the underlying mechanism of why patients with a smoking index of >20 pack‐years responded poorly to postprogression treatment.
Liver metastasis has been reported to be a poor prognostic factor in other malignancies [18]. A retrospective study found that liver metastasis predicted a poor prognosis in patients with an activated EGFR mutation receiving gefitinib as first‐line treatment [9]. In addition, liver metastasis in patients with NSCLC was recently reported to predict a poor response to erlotinib as second‐ and third‐line therapy [19]. Some studies have proposed hypotheses to explain the impact of liver metastasis. Because the liver is an important component of the immune system, cancer cells that metastasize to the liver must hamper immune surveillance mechanisms and compromise tumor immunity [20], [21]. These cancer cells exhibit a more invasive feature and therefore result in a poor prognosis.
A study evaluated gefitinib treatment in patients with postoperative recurrent NSCLC harboring EGFR mutations and in patients with stage IV disease [22]. Similar to our study, this study found that patients with postoperative recurrence had better OS than those with newly diagnosed advanced lung cancer. The reason for this difference, although unclear, may be related to tumor burden [23]. At NTUH, patients with lung cancer after complete resection receive regular chest and brain CT follow‐up every 3–6 months for at least 2–5 years, and then annually, according to NCCN guidelines. Therefore, recurrent lung cancer is usually detected when the tumor is relatively small or when it involves fewer extrapulmonary organs. However, this early detection of recurrent lung cancer may result in lead‐time bias.
The most interesting finding of our study was that chronic HCV infection predicted poor OS. Chronic HCV infection is often associated with primary liver cancer [24]. Several studies have reported HCV infection is associated with a higher incidence of cancers other than liver cancer [25]. In addition to an increased incidence of non‐liver cancer, some studies showed that HCV infection increased non‐liver‐related natural death [26]. The increased presence of comorbidity, alcohol abuse, injected drug use, and a poor socioeconomic status among HCV‐infected patients has been proposed to explain this finding [26]. In our study, the prevalence of HCV infection in lung cancer was higher than the estimated prevalence rate in Taiwan (6.6% vs. 4.4%) [27]. An increased incidence of lung cancer in patients with chronic hepatitis C has also been reported [28]. The mechanism by which chronic HCV infection affects OS is still unclear. HCV infection is now considered to be a systemic disease, with a lot of extrahepatic manifestations such as autoimmune or lymphoproliferative disorders [29]. Some studies have indicated that the severity of extrahepatic disorders may correlate with the serum HCV viral load, and that eradication of the HCV viral load significantly reduced the rate of extrahepatic deaths [30], [31]. Further studies are needed to clarify the impact of chronic HCV infection on lung cancer survival. Although chronic HCV infection predicted a poor prognosis, this negative effect was not seen among patients with chronic hepatitis B (HR 0.89, 95% CI 0.54–1.46, p = .643). One study using National Health Insurance Research Database of Taiwan revealed that hepatitis B virus (HBV) infection might increase risk of mortality in extrahepatic cancers, such as non‐Hodgkin lymphoma, gallbladder, and extrahepatic bile duct cancers among women with surface antigen of the hepatitis B virus prenatal screening [32]. However, in agreement with our study, they did not find association of HBV infection and lung cancer mortality. In Taiwan, anti‐HBV treatment usually reserves for patients with liver cirrhosis, decompensated liver disease, or active hepatitis caused by HBV infection, or patients who are HBV carriers and receive chemotherapy. Nevertheless, anti‐HBV treatment is not recommended in patients receiving EGFR TKI treatment. Among our patients with HBV infection (n = 36), 13 patients received anti‐HBV treatment (9 patients received second‐line chemotherapy and 4 patients received EGFR TKIs alone). There is no significant OS difference between patients with anti‐HBV treatment or not (p = .479, log‐rank test). However, due to the small number of patients with HBV infection, further study may be conducted to clarify whether anti‐HBV treatment is a prognostic factor in this patient population.
The prognosis of NSCLC patients with BM is variable [33]. Some case reports and case series recently reported promising effects of EGFR TKIs alone on patients harboring activated EGFR mutations [34], [35], [36]. In our study, no significant difference in OS was noted between patients with BM who received brain local treatments and those who did not. However, selection bias existed because brain local treatments in our hospital are usually reserved for patients with neurologic symptoms. Therefore, a prospective, randomized controlled study is still needed to confirm the efficacy of EGFR TKIs alone for such patients.
Recently, gefitinib was compared to other TKIs as first‐line treatment in prospective clinical trials in patients with NSCLC harboring activated EGFR mutations (the CTONG 0901 study, comparing erlotinib with gefitinib; the LUX‐Lung 7 study) [37], [38]. Lux‐Lung 7 directly compared the gefitinib and afatinib in treating patients with advanced NSCLC and harboring common EGFR mutations (exon 19 deletion or Leu858Arg). In summary, patients treating with afatinib have significant longer PFS than those with gefitinib (median 11.0 months [95% CI 10.6–12.9] with afatinib vs. 10.9 months [9.1–11.5] with gefitinib; HR 0.73 [95% CI 0.57–0.95], p = 0.017). However, in subgroup analysis, patients with BM showed no significant difference in PFS between gefitinib and afatinib treatment groups. Furthermore, regarding OS, there was no statistical significance between these two groups [39]. In the CTONG 0901 study, erlotinib was not superior to gefitinib in PFS or OS. However, due to the better penetration rate of erlotinib in CSF compared to gefitinib [40], some physicians may prefer erlotinib for treatment of patients with BM. Therefore, gefitinib may be considered less effective in treating BM than extra‐BM. In our study, although univariate analysis showed BM predicted shorter OS, this finding could not be seen in multivariate analysis (HR 1.27, 95% CI 0.83–1.93, p = .275). Liver metastasis was the only site of metastasis that predicted poor prognosis. In addition, OS of 187 patients with progressive disease during gefitinib treatment was similar between those with intracranial progression and those with extracranial progression (median OS: 26.6 months and 26.3 months, respectively, p = .222). Although we could not conclude whether gefitinib is active in patients with brain metastases from our data, we suggested that BM is not a negative predictor in this patient population treating with gefitinib.
Almost all patients with advanced metastatic lung cancer harboring activated EGFR mutation during the study period received gefitinib as first‐line therapy. This was due to the policy of the Bureau of National Health Insurance in Taiwan at that time to reimburse only gefitinib for first‐line treatment of advanced NSCLC with EGFR mutation. Therefore, our study gave us real‐world data about the efficacy of gefitinib in treating advanced NSCLC with an EGFR mutation, regardless of EGFR mutation type, performance status of the patient, metastatic sites, or physician's preference. There are still limitations in this study. First, lead‐time bias may have resulted in prolonged survival for patients with recurrent lung cancer after operation due to frequent CT follow‐up. Second, the number of patients with HCV is small (12 patients), and there were 43 patients (19%) with unknown HCV status. However, among patients with unknown HCV status, all of their hepatic enzymes showed within normal limit before gefitinib treatment. According to prior studies [41], [42], around 30% of patients with chronic hepatitis C have normal alanine transaminase levels, and the prevalence of HCV infection in Taiwan is only 4.4%. Taking above two points into consideration, patients with unknown HCV status may not change the result that HCV possibly predicted poor prognosis. Third, our study enrolled a real‐world population including patients with poor performance status (i.e., ECOG ≥2), which could have biased this study towards a sicker population of patients who were not eligible for a clinical trial.
Conclusion
Gefitinib is an effective treatment in patients with advanced NSCLC harboring EGFR mutation. Even with a lower penetration rate to CSF, BM in the initial diagnosis or intracranial failure during gefitinib treatment is not a negative prognostic factor for OS. Chronic HCV infection might predict poor OS in EGFR mutation‐positive advanced NSCLC patients treated with first‐line gefitinib. However, the underlying mechanism needs further study to clarify.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Author Contributions
Conception/design: James C Yang, Chong‐Jen Yu, Wei‐Yu Liao, Jin‐Yuan Shih
Provision of study material or patients: Jin‐Shing Chen, Zhong‐Zhe Lin, Chia‐Chi Lin, James C Yang, Chong‐Jen Yu, Wei‐Yu Liao, Jin‐Yuan Shih, Zong‐Han Yao, Chao‐Chi Ho, Kuan Yu Chen, Jin‐Yuan Shih
Collection and/or assembly of data: Jin‐Shing Chen, Zhong‐Zhe Lin, Chia‐Chi Lin, James C Yang, Chong‐Jen Yu, Wei‐Yu Liao, Jin‐Yuan Shih, Zong‐Han Yao, Chao‐Chi Ho, Kuan Yu Chen, Jin‐Yuan Shih
Data analysis and interpretation: James C Yang, Chong‐Jen Yu, Wei‐Yu Liao, Jin‐Yuan Shih, Zong‐Han Yao
Manuscript writing: Wei‐Yu Liao, Jin‐Yuan Shih, Zong‐Han Yao
Final approval of manuscript: Wei‐Yu Liao
Disclosures
Kuan Yu Chen: AstraZeneca, Roche, Eli Lilly, Boehringer Ingelheim, Merck Sharp & Dohme, Sanofi (H); Jin‐Yuan Shih: AstraZeneca, Roche, Pfizer, Boehringer Ingelheim, Norvatis, Eli Lilly (H); James C Yang: Boehringer Ingelheim, Eli Lilly, Roche/Genentech/Chugai, Astellas, Merck Sharp & Dohme, Pfizer, Novartis, Yuhan, Bristol‐Myers Squibb, Ono, Merrimack (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Stewart BW, Wild CP, eds. World cancer report. World Health Organization, 2014.
- 2. Lynch TJ, Bell DW, Sordella R et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2004;350:2129–2139. [DOI] [PubMed] [Google Scholar]
- 3. Mok TS, Wu Y‐L, Thongprasert S et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–957. [DOI] [PubMed] [Google Scholar]
- 4. Fukuoka M, Wu YL, Thongprasert S et al. Biomarker analyses and final overall survival results from a phase III, randomized, open‐label, first‐line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non‐small‐cell lung cancer in asia (IPASS). J Clin Oncol 2011;29:2866–2874. [DOI] [PubMed] [Google Scholar]
- 5. Wu YL, Zhou C, Hu CP et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐Lung 6): An open‐label, randomised phase 3 trial. Lancet Oncol 2014;15:213–222. [DOI] [PubMed] [Google Scholar]
- 6. Sequist LV, Yang JC, Yamamoto N et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–3334. [DOI] [PubMed] [Google Scholar]
- 7. Rosell R, Carcereny E, Gervais R et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012;13:239–246. [DOI] [PubMed] [Google Scholar]
- 8. Togashi Y, Masago K, Masuda S et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non‐small cell lung cancer. Cancer Chemother Pharmacol 2012;70:399–405. [DOI] [PubMed] [Google Scholar]
- 9. Wu KL, Tsai MJ, Yang CJ et al. Liver metastasis predicts poorer prognosis in stage IV lung adenocarcinoma patients receiving first‐line gefitinib. Lung Cancer 2015;88:187–194. [DOI] [PubMed] [Google Scholar]
- 10. Liu M, Zhou C, Zheng J. Cigarette smoking impairs the response of EGFR‐TKIs therapy in lung adenocarcinoma patients by promoting EGFR signaling and epithelial‐mesenchymal transition. Am J Transl Res 2015;7:2026–2035. [PMC free article] [PubMed] [Google Scholar]
- 11. Kim MH, Kim HR, Cho BC et al. Impact of cigarette smoking on response to epidermal growth factor receptor (EGFR)‐tyrosine kinase inhibitors in lung adenocarcinoma with activating EGFR mutations. Lung Cancer 2014;84:196–202. [DOI] [PubMed] [Google Scholar]
- 12. Mitchell P, Mok T, Barraclough H et al. Smoking history as a predictive factor of treatment response in advanced non‐small‐cell lung cancer: A systematic review. Clin Lung Cancer 2012;13:239–251. [DOI] [PubMed] [Google Scholar]
- 13. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, He D, Fang W et al. The difference of clinical characteristics between patients with exon 19 deletion and those with L858R mutation in nonsmall cell lung cancer. Medicine (Baltimore) 2015;94:e1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Riely GJ, Pao W, Pham D et al. Clinical course of patients with non‐small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res 2006;12:839–844. [DOI] [PubMed] [Google Scholar]
- 16. Yang JC, Wu YL, Schuler M et al. Afatinib versus cisplatin‐based chemotherapy for EGFR mutation‐positive lung adenocarcinoma (LUX‐Lung 3 and LUX‐Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141–151. [DOI] [PubMed] [Google Scholar]
- 17. Watanabe S, Inoue A, Nukiwa T et al. Comparison of gefitinib versus chemotherapy in patients with non‐small cell lung cancer with exon 19 deletion. Anticancer Res 2015;35:6957–6961. [PubMed] [Google Scholar]
- 18. McKay RR, Kroeger N, Xie W et al. Impact of bone and liver metastases on patients with renal cell carcinoma treated with targeted therapy. Eur Urol 2014;65:577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He Y, Wang Y, Boyle T et al. Hepatic metastases is associated with poor efficacy of erlotinib as 2nd/3rd line therapy in patients with lung adenocarcinoma. Med Sci Monit 2016;22:276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol 2013;14:996–1006. [DOI] [PubMed] [Google Scholar]
- 21. Langley RR, Fidler IJ. The seed and soil hypothesis revisited–The role of tumor‐stroma interactions in metastasis to different organs. Int J Cancer 2011;128:2527–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ko R, Kenmotsu H, Hisamatsu Y et al. The effect of gefitinib in patients with postoperative recurrent non‐small cell lung cancer harboring mutations of the epidermal growth factor receptor. Int J Clin Oncol 2015; 20:668–673. [DOI] [PubMed] [Google Scholar]
- 23. Park JH, Kim TM, Keam B et al. Tumor burden is predictive of survival in patients with non‐small‐cell lung cancer and with activating epidermal growth factor receptor mutations who receive gefitinib. Clin Lung Cancer 2013;14:383–389. [DOI] [PubMed] [Google Scholar]
- 24. Bruix J, Barrera JM, Calvet X et al. Prevalence of antibodies to hepatitis C virus in spanish patients with hepatocellular carcinoma and hepatic cirrhosis. Lancet 1989;2:1004–1006. [DOI] [PubMed] [Google Scholar]
- 25. Fiorino S, Bacchi‐Reggiani L, de Biase D et al. Possible association between hepatitis C virus and malignancies different from hepatocellular carcinoma: A systematic review. World J Gastroenterol 2015;21:12896–12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Omland LH, Jepsen P, Krarup H et al. Increased mortality among persons infected with hepatitis C virus. Clin Gastroenterol Hepatol 2011;9:71–78. [DOI] [PubMed] [Google Scholar]
- 27. Sievert W, Altraif I, Razavi HA et al. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int 2011;31(suppl 2):61–80. [DOI] [PubMed] [Google Scholar]
- 28. Allison RD, Tong X, Moorman AC et al. Increased incidence of cancer and cancer‐related mortality among persons with chronic hepatitis C infection, 2006‐2010. J Hepatol 2015;63:822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cacoub P, Comarmond C, Domont F et al. Extrahepatic manifestations of chronic hepatitis C virus infection. Ther Adv Infect Dis 2016;3:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Backus LI, Boothroyd DB, Phillips BR et al. A sustained virologic response reduces risk of all‐cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol 2011;9:509–516.e1. [DOI] [PubMed] [Google Scholar]
- 31. Nahon P, Bourcier V, Layese R et al. Eradication of hepatitis C virus infection in patients with cirrhosis reduces risk of liver and non‐liver complications. Gastroenterology 2017;152:142–156.e2. [DOI] [PubMed] [Google Scholar]
- 32. Fwu CW, Chien YC, Nelson KE et al. Mortality after chronic hepatitis B virus infection: A linkage study involving 2 million parous women from Taiwan. J Infect Dis 2010;201:1016–1023. [DOI] [PubMed] [Google Scholar]
- 33. Sperduto PW, Kased N, Roberge D et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis‐specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Park SJ, Kim HT, Lee DH et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non‐small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer 2012;77:556–560. [DOI] [PubMed] [Google Scholar]
- 35. Jamal‐Hanjani M, Spicer J. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of epidermal growth factor receptor‐mutant non‐small cell lung cancer metastatic to the brain. Clin Cancer Res 2012;18:938–944. [DOI] [PubMed] [Google Scholar]
- 36. Wu YL, Zhou C, Cheng Y et al. Erlotinib as second‐line treatment in patients with advanced non‐small‐cell lung cancer and asymptomatic brain metastases: A phase II study (CTONG‐0803). Ann Oncol 2013;24:993–999. [DOI] [PubMed] [Google Scholar]
- 37. Park K, Tan EH, O'Byrne K et al. Afatinib versus gefitinib as first‐line treatment of patients with EGFR mutation‐positive non‐small‐cell lung cancer (LUX‐Lung 7): A phase 2b, open‐label, randomised controlled trial. Lancet Oncol 2016;17:577–589. [DOI] [PubMed] [Google Scholar]
- 38. Yang JJ, Zhou Q, Yan HH, et al. A phase III randomised controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with egfr mutations. British journal of cancer 2017;116:568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paz‐Ares L, Tan E, Zhang L et al. Afatinib (A) vs gefitinib (G) in patients (pts) with EGFR mutation‐positive (EGFRm+) non‐small‐cell lung cancer (NSCLC): Overall survival (OS) data from the phase IIb trial LUX‐Lung 7 (LL7). Abstract #LBA43 presented at: ESMO 2016. Congress; Denmark.
- 40. Deng Y, Feng W, Wu J et al. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non‐small‐cell lung cancer. Mol Clin Oncol 2014;2:116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sanai FM, Benmousa A, Al‐Hussaini H et al. Is serum alanine transaminase level a reliable marker of histological disease in chronic hepatitis C infection? Liver Int 2008;28:1011–1018. [DOI] [PubMed] [Google Scholar]
- 42. Bacon BR. Treatment of patients with hepatitis C and normal serum aminotransferase levels. Hepatology 2002;36(5 suppl 1):S179–S184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.