Appendiceal mucinous neoplasms are rare and challenging to manage. This review summarizes the current literature and controversies in classification, clinical presentation, molecular alterations, and treatment outcomes in this heterogeneous group of tumors and provides guidelines for treatment.
Keywords: Appendix carcinoma, Abdominal surgery, Review
Abstract
Objective.
Appendiceal mucinous neoplasms (AMNs) are a rare and heterogeneous disease for which clinical management is challenging. We aim to review the literature regarding modalities of treatment to guide the management of AMNs.
Methods and Review Criteria.
We conducted a PubMed search in February 2016 for English‐language publications, using the terms “appendiceal,” “appendix,” “carcinoma,” “cancer,” “mucinous,” “treatment,” “genes,” “target,” “genomic,” and terms listed in the articles' subheadings. Published reports and abstracts from the American Society of Clinical Oncology meetings were also searched.
Results.
In this review, we summarize current data and controversies in AMN classification, clinical presentation, molecular alterations, treatment outcomes with regard to cytoreductive surgery, hyperthermic intraperitoneal chemotherapy (HIPEC), and the role of systemic chemotherapy.
Conclusion.
Appendiceal mucinous neoplasms are a heterogeneous group of tumors with a rising incidence. Treatment is based on stage and histology. Low‐grade tumors are treated surgically with resection of the primary site in early stage disease, or peritoneal debulking and HIPEC in patients with advanced stage disease. Treatment of high‐grade tumors requires further prospective trials, and options include debulking surgery and HIPEC with or without preoperative chemotherapy. Trials evaluating novel therapies based on the molecular profiling of AMN tumors are needed to evaluate therapeutic options in patients who are not surgical candidates.
Implications for Practice.
This review provides a reference to guide gastroenterologists, pathologists, surgeons, and oncologists in the management of appendiceal mucinous neoplasms (AMNs), a rare and heterogeneous disease with no consensus on histologic classification or guidelines for treatment algorithms. This review summarizes all AMN classifications and proposes a treatment algorithm based on stage and histology of disease.
Introduction
Appendiceal mucinous neoplasms (AMNs) are rare tumors accounting for less than 1% of all cancers. Appendiceal mucinous neoplasms include a heterogeneous group of diseases with varying malignant potential as reflected by different classification systems. Early stage AMNs are usually incidentally diagnosed at resection for suspicion of appendicitis. Advanced stage disease presents with abdominal distension related to the accumulation of mucin in the peritoneal space. Due to the lack of well‐controlled randomized trials, several management algorithms have been proposed. This article reviews the different classification systems and the currently accepted therapeutic management for this rare and complex disease.
Background
Malignant tumors of the appendix include mucinous epithelial neoplasms, neuroendocrine (typical carcinoid) tumors, goblet/ex‐goblet cell or composite carcinoid, lymphomas, adenocarcinomas, and lymphoid or mesenchymal sarcomas. Histologically, 65% of appendiceal tumors are of neuroendocrine origin, while adenocarcinomas (mucinous, signet ring or non‐mucinous) constitute approximately 20% of these tumors [1], [2]. Appendiceal mucinous neoplasms are present in 0.2%–0.3% of appendectomy specimens [2]. The treatment of AMN is not well defined, with controversies regarding the extent of surgery and the role of chemotherapy, including early postoperative intraperitoneal chemotherapy (EPIC) and hyperthermic intraperitoneal chemotherapy (HIPEC).
Early classification systems considered AMN a benign disease, with different terminologies including appendiceal mucocele [3], cystadenoma, and cystadenocarcinoma [4]. Criteria for simple mucoceles (benign adenomas) were established and distinguished from those of malignant histologies. Carr et al. [5] described mucinous neoplasm with uncertain malignant potential with the characteristics of low‐grade tumors with acellular mucin in or beyond the appendiceal wall. Appropriate categorization and nomenclature of these neoplasms remained problematic, with various pathologic grading systems [5], [6], [7], [8], [9]. Pseudomyxoma peritonei (PMP) [3] is the diffuse collections of gelatinous material in the abdomen and pelvis, and mucinous implants on the peritoneal surfaces. Follow‐up studies revealed that the majority of PMP arise from the appendix and represent local spread into the peritoneal cavity. Therefore, it has been recommended that the term PMP be limited to describing the clinical entity of mucinous ascites and not be used as a histologic diagnosis [10].
The clinical course of AMN appears to be determined by the stage at diagnosis, as well as the histological features reflecting cellular differentiation [2], [6], [7], [8], [11], [12]. Mucinous ascites indicates an advanced stage portending worse disease outcomes [2]. Histologic differentiation has gained vital importance, as well‐differentiated mucin‐producing appendiceal tumors clearly have a better prognosis than poorly differentiated tumors. The treatment of AMN is largely based on stage and histology. This review addresses controversies in classification staging and provides therapeutic guidance for AMN.
Methods and Review Criteria
We conducted a PubMed search in February 2016 for English‐language publications, using the terms “appendiceal,” “appendix,” “carcinoma,” “cancer,” “mucinous,” “treatment,” “genes,” “target,” “genomic,” and terms listed in the articles' subheadings. Published reports and abstracts from the American Society of Clinical Oncology meetings were also searched. No exclusion criteria were used. Articles were selected on the basis of relevance and additional papers were identified from their reference lists. Articles published in high‐impact journals were reviewed thoroughly and selected to contribute to the information summarized in this article.
Epidemiology and Characteristics
Appendiceal mucinous neoplasms account for 0.4%–1% of all gastrointestinal malignancies in the U.S., roughly translating to 1,500 new cases annually [1], [13], [14], [15], [16]. In sharp contrast to colorectal cancer (CRC), AMNs are usually a relatively indolent disease and rarely develop metastases outside the peritoneal cavity. The age‐adjusted incidence of AMN is estimated at 0.12 cases per 1 million individuals per year [11], [15]. The incidence of AMN in the U.S. has increased over time, from 0.6 cases per million persons in 1973 [1] to 2.8 cases per million persons in 2011, with an annual percentage increase of 3.1%. In addition, age at diagnosis has decreased over the same time period [17]. A similar pattern of increase in incidence and decrease in age at diagnosis was observed in a large registry study from the Netherlands spanning 1980–2010 [18]. The trends over time in incidence, demographics, type of surgery, and survival of patients with AMN were analyzed using the Surveillance, Epidemiology, and End Results Program database [17]. Women account for 50%–55% of the appendiceal tumor population [9], [17], and the majority (70%–74%) of patients are white. No significant change in race or gender has been observed over time. Peritoneal involvement is the presenting stage for the majority (53.2%) of patients with AMN (localized [26.3%] and regional [20.5%] stages). There seems to be a trend toward a decrease in regional disease stage over time, while distant stage disease is increasing in incidence [17].
Clinical Presentation
Patients with appendiceal tumors can present with nonspecific clinical manifestations delaying diagnosis [19], [20]. An acute appendicitis‐like presentation with right lower quadrant pain secondary to distention of the appendix by mucin is the most common clinical presentation in early stage disease [21]. Appendicitis or perforation of the appendix can be present, specifically if the tumor obstructs the appendiceal orifice. Carr et al. [5] reported that 32% of patients with appendiceal neoplasms received a preoperative diagnosis of acute appendicitis, while 23% were incidentally diagnosed.
Advanced stage disease presents with increasing abdominal girth due to the accumulation of mucinous ascites in the peritoneum. Other clinical presentations for this stage include chronic abdominal pain, weight loss, anemia, infertility, and new‐onset umbilical or inguinal hernias [20], [22]. Even though patients commonly have mucinous peritoneal, serosal, and omental implants, intestinal obstruction is an uncommon initial presentation [12], [23].
Histologic Classification
The classification of appendiceal adenocarcinomas has been controversial, with conflicting results. Well‐recognized categories include mucinous adenocarcinomas (previously called mucinous cystadenocarcinomas or malignant mucoceles), which are the main subject of this article, and ordinary colonic‐type adenocarcinomas (non‐mucinous), which are virtually identical to those in the colorectal region [24]. A third category, appendiceal crypt cell adenocarcinoma (also called adenocarcinoma ex‐goblet‐cell‐carcinoid) is becoming better characterized [25] and may also have a mucinous component. Most non‐mucinous carcinomas are colonic type adenocarcinoma, but occasionally adenocarcinomas that closely resemble pancreatobiliary adenocarcinomas also arise in the appendix, although they have not been properly documented in the literature.
Most primary appendiceal adenocarcinomas are of AMN subtype, in which mucin is involved in more than 50% of the lesion. Appendiceal mucinous neoplasms most commonly arise from low‐grade appendiceal mucinous neoplasms (LAMN), which are adenomatous changes in the appendiceal mucosa. Less frequently, AMN may arise from a polyp‐forming adenoma that is identical to those seen in the colon, and/or serrated adenoma [25], [26]. Appendiceal mucinous neoplasms with signet ring cell features (in which signet ring‐shaped cells are seen floating within the mucin nodules) or poorly differentiated histology are believed to be more aggressive and have a worse prognosis [27]. Most appendiceal adenocarcinomas that were previously designated as “signet ring carcinoma” are either goblet cell patterns in appendiceal crypt cell adenocarcinoma (“adenocarcinoma‐ex‐GCC (goblet cell carcinoid)”), which is now regarded as a distinct tumor type, or mucinous adenocarcinomas with signet ring cells within the mucin. True primary signet ring cell carcinoma (poorly cohesive cell carcinoma), which is characterized by individual cells or cord‐like infiltration without any mucinous component or crypt cell adenocarcinoma components, is virtually non‐existent [1]. Published literature suggests a disparity between mucinous and colonic‐type (non‐mucinous) appendiceal adenocarcinoma with respect to disease course and prognosis [5], [28], [29]. Figure 1 shows a gross specimen of the appendix in which mucin is confined to the appendix without perforation. Figures 2, 3, 4, 5, 6 show different pathologies that range from LAMN to high‐grade AMN and appendiceal crypt cell adenocarcinoma.
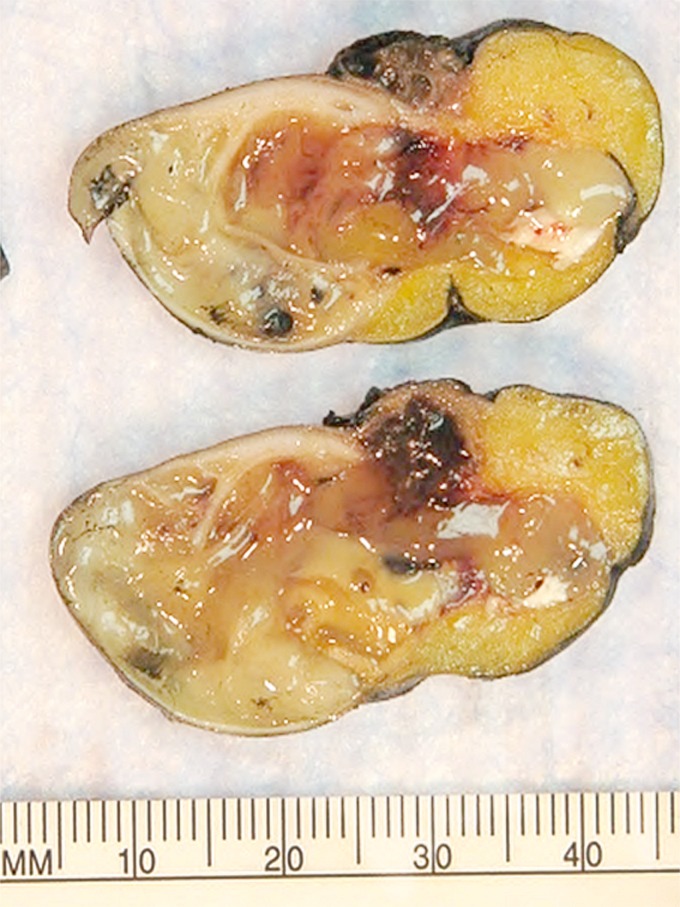
Figure 1.
Gross specimen. The appendix lumen is filled with gelatinous mucinous material characteristic of low‐grade appendiceal mucinous neoplasms LAMN (LAMN; so‐called “mucocele” formation). In this case, it shows protrusion into the mesoappendix in a diverticular fashion, which is not uncommon in LAMNs.
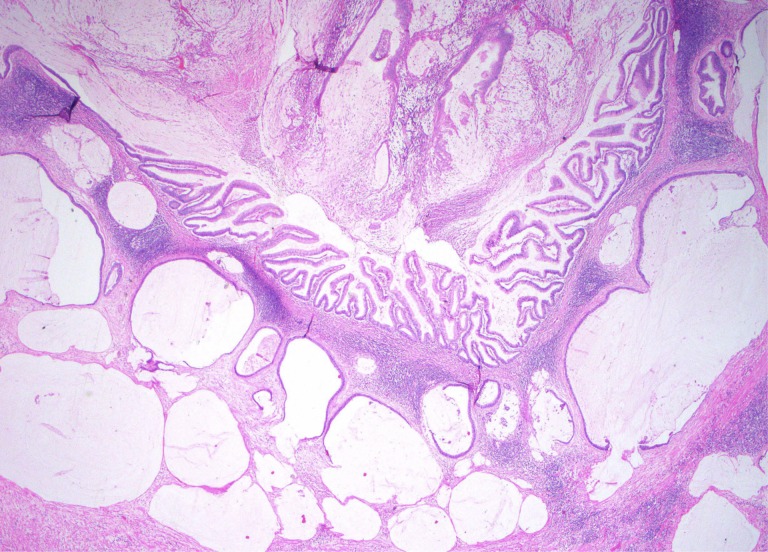
Figure 2.
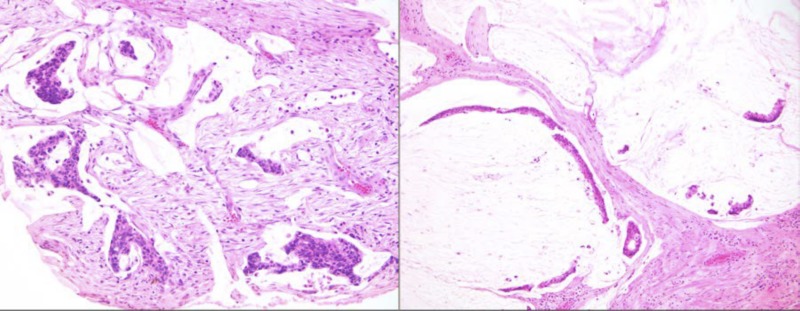
Low‐grade appendiceal mucinous neoplasms LAMN with invasive low‐grade mucinous adenocarcinoma component. The well‐formed and well‐preserved epithelium with papillary villous elements (upper middle) represents the mucosal adenomatous component. The mucin lakes, which are represented as pale nodules of white to slightly blue nodules with scant epithelial cells, represent invasive mucinous adenocarcinoma. Based on the degree of mucin and the relatively mild cytologic atypia, this qualifies as a “low‐ grade” mucinous adenocarcinoma.
Figure 3.
Invasive intestinal type adenocarcinoma, not low‐grade appendiceal mucinous neoplasmsLAMN. Some invasive adenocarcinomas of the appendix are conventional intestinal (colonic) type, as illustrated in this example.
Figure 4.
Low‐grade appendiceal mucinous neoplasms LAMN with paucicellullar mucinous spread to the peritoneal surfaces (low‐grade mucinous adenocarcinoma of the so‐called disseminated peritoneal adenomucinosis type). Some of the mucin nodules also contain detached signet‐ring like cells, but this does not qualify as signet ring (poorly cohesive) cell adenocarcinoma because there was no individual cell or cord‐like non‐mucinous infiltration into the stroma by the carcinoma cells.
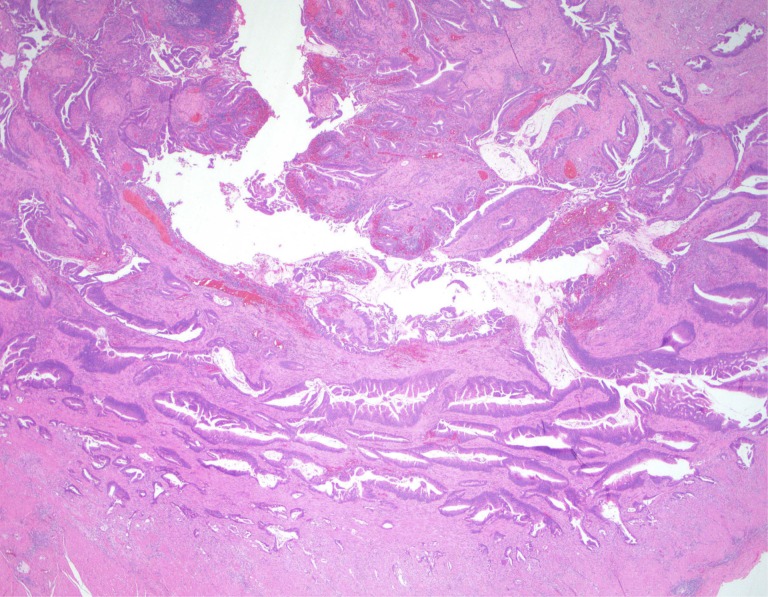
Figure 5.
Invasive mucinous adenocarcinoma of high grade involving the peritoneal surfaces (“peritoneal mucinous carcinomatosis”) which arose in a low‐grade appendiceal mucinous neoplasms LAMN (not shown here).
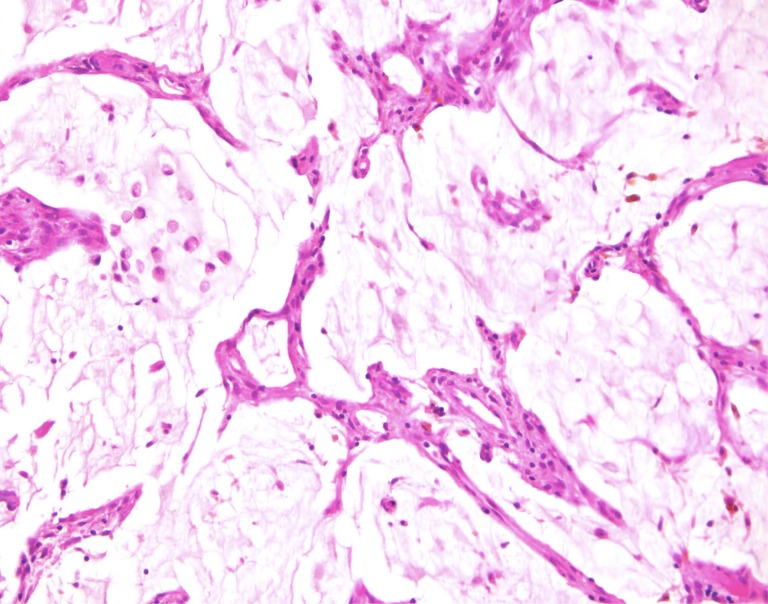
Figure 6.
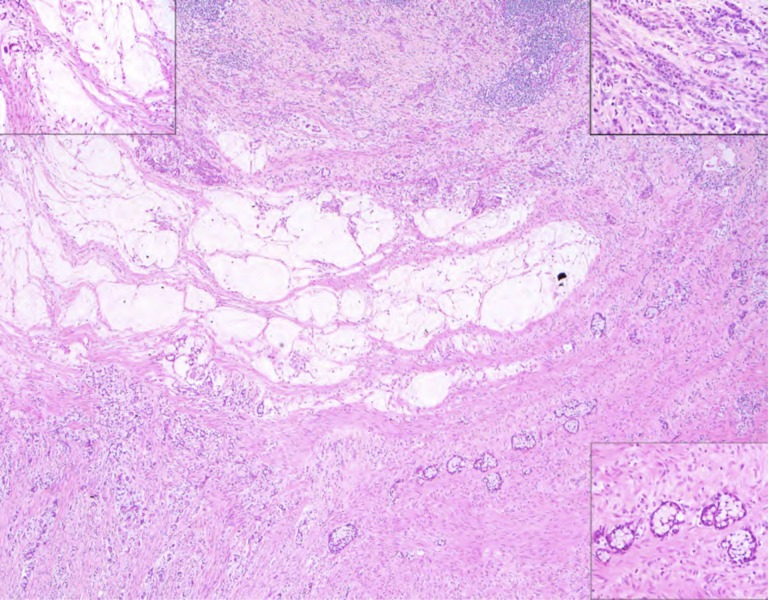
This is an example of an appendiceal crypt cell adenocarcinoma (so‐called adenocarcinoma ex‐goblet‐cell carcinoid), which can also show mucinous areas, as illustrated in the middle of the photograph and the upper left inset. The other components of this tumor, however, which are detailed in the insets in upper right and lower right, show features more characteristic of appendiceal crypt cell adenocarcinoma. In the upper right, there is a poorly cohesive (signet ring) cell pattern, and the lower right shows the classical goblet‐cell carcinoid pattern. Such cases should be distinguished from low‐grade appendiceal mucinous neoplasms LAMN or related appendiceal mucinous adenocarcinoma, as which they are commonly misclassified.
Different classification systems for AMN have been used and are mainly based on cellularity and differentiation. Ronnett et al. [6], [30] initially classified AMN into three major variants: disseminated peritoneal adenomucinosis (DPAM), peritoneal mucinous carcinomatosis (PMCA), and PMCA of indeterminate or discordant features (PMCA I/D). This classification predicted survival differences between the three groups. Patients with DPAM have an indolent clinical course without distant metastatic spread (extra‐peritoneal). Peritoneal mucinous carcinomatosis histology has a higher cellular‐to‐mucin ratio and possesses higher metastatic potential to lymph nodes and extra‐peritoneal organs, projecting worse outcomes [30], [31]. With advanced disease (peritoneal involvement), these classifications become more challenging without a known primary (after a peritoneal biopsy). The diagnosis is then limited to a peritoneal biopsy suggesting AMN, but cannot be confirmed without further debulking surgery, if this is possible.
More recently, the same authors [6], [19], [30] acknowledged that the intermediate category behaves more like the PMCA group. Thus, Ronnett's classification model [6] was subsequently revised and simplified into low‐ and high‐grade carcinoma [7], in which any mucinous epithelium beyond the muscularis mucosa is indisputable evidence of an invasive appendiceal malignancy [5], [21], [32]. Bradley et al. [21] further classified DPAM and PMCA I/D into three variants: (a) well‐differentiated mucinous adenocarcinoma, grade 1 of 3 (DPAM), (b) mucinous adenocarcinoma, grade 2 of 3 (PMCA‐I type), and (c) high‐grade mucinous adenocarcinoma, grade 3 of 3 (PMCA type). The Bradley classification system found the intermediate classification tracked with the low‐grade histology outcomes. A major limitation of these earlier classification systems is that the stage was only limited to disease involving the peritoneal cavity. Misdraji et al. [8] developed a classification system that was broadly concordant with the report by Ronnett but also included classification for localized appendiceal disease [6], [33]. In this classification, the term LAMN was introduced to replace the term mucocele. Low‐grade appendiceal mucinous neoplasms were further subclassified based on the degree of mucin and differentiation. The Ronnett classification is the most endorsed internationally.
Immunohistochemistry and Molecular Alterations
The diagnosis of AMN depends largely on the presence of mucin on pathologic examination. Appendiceal mucinous neoplasms stain diffusely positive for CK20 (100%) and are often negative for CK7 (71%). Furthermore, AMNs are usually positive for MUC5AC (86%) and DPC4 (100%). Of note, both CRC and appendiceal tumors share the same pattern of CK positivity: a patchy CK7 and a diffuse CK20 positivity [34], [35]. It was therefore postulated that AMNs follow the same pathogenic pathway as CRCs, most often arising from adenomatous polyps and following a well‐established genetic progression in an adenoma–carcinoma sequence [36], [37]. These steps include (a) point mutations in the KRAS proto‐oncogene and mutations and/or deletions in the TP53 gene on chromosome 17p, (b) truncating mutations or deletions in the adenomatous polyposis coli (APC) gene on chromosome 5q, and (c) mutations in the beta‐catenin gene. An alternative pathway of carcinogenesis involves microsatellite instability (MSI) caused by mutations in nucleotide mismatch repair genes, including hMSH2, hMLH1, PMS1, PMS2, and GTBP [36], [37], [38]. In a study of 30 appendiceal adenocarcinomas evaluated for the presence of MSI, TP53 overexpression, and KRAS mutations, 23 mucinous subtypes were included [25]. All AMNs had microsatellite stable genotype. Szych et al. [39] described random allelic shift in AMNs [38], but AMNs seem to possess detectable levels of MSI, reported at 6% in another study of 149 patients [40]. However, it should be noted that, due to variable definitions, some of this data remains controversial and requires further verification.
Many recent studies have employed molecular profiling as a potential adjunct predictor of outcomes. The few available genetic studies on AMNs support clonality and the presence of specific molecular signatures [41]. Szych et al. found that of 16 samples of AMN with peritoneal involvement [39], 100% possessed KRAS mutations in the corresponding appendiceal adenomas, while of 16 specimens from patients with localized disease (no peritoneal involvement), 11 had KRAS mutation variants in the adenomas adjacent to the cancer. This observation may suggest that KRAS mutations represent an early event in the tumorigenesis of AMNs, but are insufficient to drive peritoneal spread. Levine et al. [42] studied low grade appendiceal cancer gene expression as a biomarker predictive of efficacy in patients who underwent complete debulking surgery and HIPEC. Gene expression profiles were analyzed for the transcriptome of appendiceal cancers utilizing 139 gene‐cassette (including actionable genes) and dichotomizing the profiles into low‐ and high‐expression cancers. The authors concluded that high gene expression predicted poor survival outcomes even when adjusting for grade, PCI score, performance status, and age. This study was based on previous findings demonstrating the difference in genomic signatures between appendiceal cancers and CRCs. Kabbani et al. also evaluated and compared genetic alterations in appendiceal carcinomas [25]. In this study, KRAS mutations were detected in 11 of 20 (55%) tumors; 8 of 16 (50%) were mucinous and 3 of 4 (75%) were non‐mucinous (colonic‐type) carcinomas. However, TP53 overexpression is present in only 1 of 30 (3%) AMNs. Raghav et al. reported the largest molecular study [40] of 149 patients in a single institution. COX‐2 expression, KRAS, PI3K, and BRAF mutations were seen in 61%, 55%, 17%, and 4% of patients, respectively. Additionally, KRAS mutation was strongly associated with well or moderately differentiated histologies. Finally, a comprehensive genomic profiling study of 11 AMN samples was recently reported by Goldstein et al. [41], using second‐generation sequencing including base substitutions, short insertions, deletions, copy number alterations, and fusions/rearrangements. Base substitutions in KRAS were detected in 73% and TP53 mutations in 64% of cases (MYC [36%], SMAD4 [27%], and APC [27%]). The clinical application of targeted therapies in this setting appears to be a promising strategy. Given the rarity of the disease and its heterogeneous nature, and the fact that no prospective trials have been designed, AMN molecular targets are targets. These studies are retrospective series with limited sample size and do not take into account the heterogeneous nature of the disease. In addition, there are few published studies on the genetic alterations within various subgroups of AMNs.
The clinical application of targeted therapies in this setting appears to be a promising strategy. Given the rarity of the disease and its heterogeneous nature, and the fact that no prospective trials have been designed, AMN molecular targets are considered as similar to colon cancer targets.
Treatment of Appendiceal Neoplasms
Table 1 summarizes the staging of AMNs according to the American Joint Committee on Cancer Cancer Staging Manual, Seventh Edition (2010) [43].
Table 1. TNM stage classification.
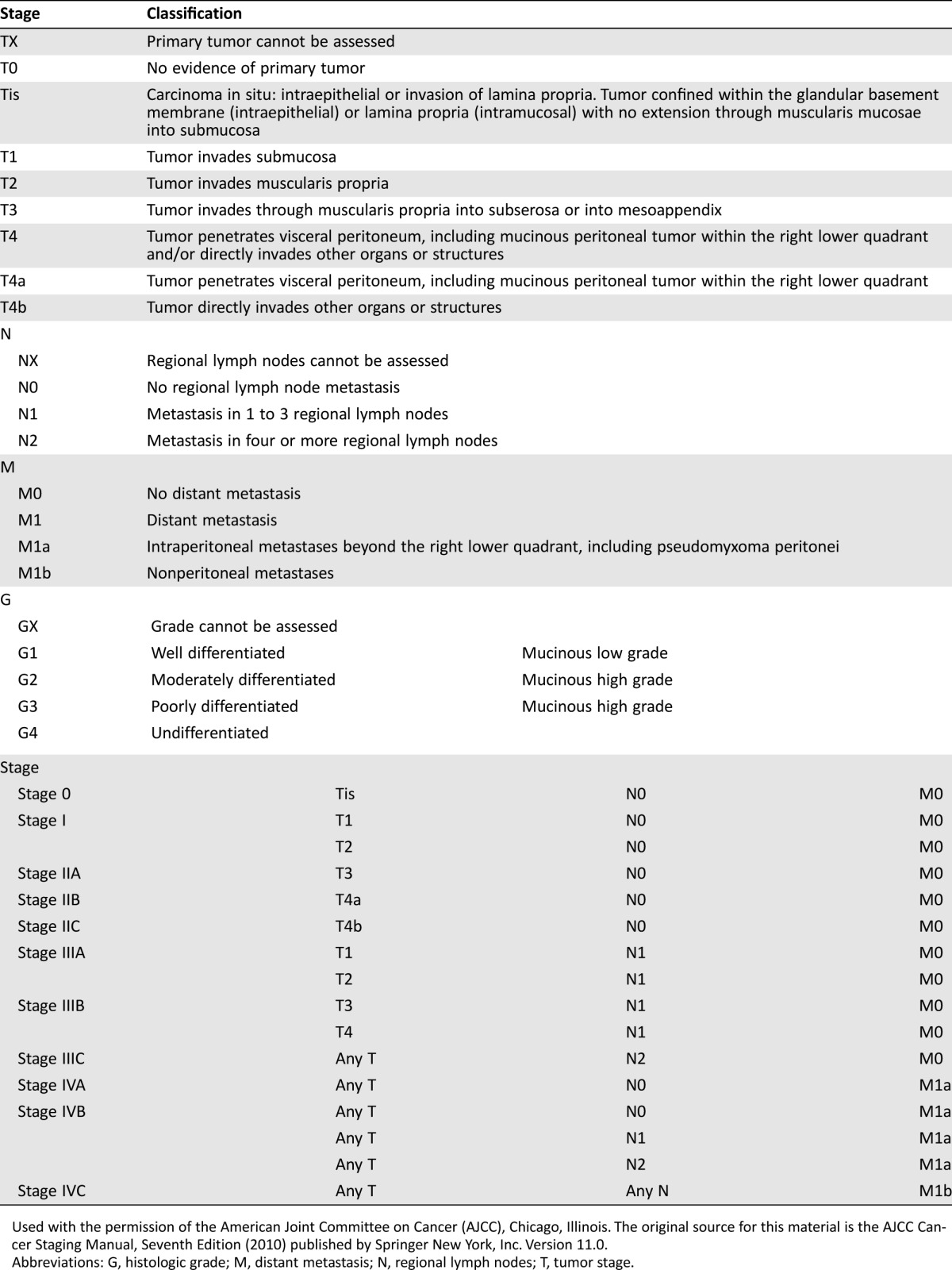
Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original source for this material is the AJCC Cancer Staging Manual, Seventh Edition (2010) published by Springer New York, Inc. Version 11.0.
Abbreviations: G, histologic grade; M, distant metastasis; N, regional lymph nodes; T, tumor stage.
Localized AMNs
Because the incidence of nodal spread of well‐differentiated localized appendiceal tumors is less than 2%, most of the published surgical literature suggests that simple appendectomy is sufficient for tumors exhibiting only local disease [44]. Right hemicolectomy should be considered to clear the tumor margin in case of involvement after appendectomy, and it should be considered for tumors involving the peri‐appendiceal area, tumor size of 2 cm or larger, high grade histology, or tumor that invades through the muscularis propria [45]. Similar criteria for right hemicolectomy were proposed by Pahlavan et al. [46] and include the following: (a) the degree of cellular undifferentiation, (b) increased mitotic activity, (c) involvement of the base of the appendix, (d) lymph node metastasis, or (e) tumor size greater than 2 cm. We suggest that any of the risk factors mentioned here put patients at risk of higher rates of local recurrences, thus supporting right hemicolectomy. In addition, patients presenting with perforation, high grade, or poorly differentiated histology should be considered for hemicolectomy.
Surgical management of low‐grade AMN with peritoneal mucin spillage is controversial. Published literature suggests a significant prognostic difference between acellular and cellular mucin [5], [8], [46], [47]. Accordingly, the use of cytoreductive surgery (CRS) for relatively early lesions with localized cellular mucin spillage is advocated based on population‐based [46] and pathology‐based [7], [12] series, showing high likelihood of progression to extensive intra‐abdominal disease if treated by appendectomy or right hemicolectomy alone. Three‐, 5‐, 7‐, and 10‐year overall survival (OS) rates for low‐grade AMN with extra‐appendiceal spread are 100%, 86%, 60%, and 45%, respectively.
The role of adjuvant chemotherapy for resected localized AMN has not been evaluated in prospective randomized trials. Fluorouracil (5‐FU)‐based adjuvant chemotherapy is usually recommended in specific high‐risk situations such as poorly differentiated tumors (signet ring histology) with evidence of lymph node involvement or perforation. Adjuvant chemotherapy is not recommended for low‐grade well‐differentiated mucinous tumors and should only be considered in specific situations where the cancer shows invasive features such as lymphovascular or lymph node involvement or has mixed‐type histology.
Treatment of AMN with Peritoneal Metastasis
Evolution of Treatment.
Treatment of peritoneal metastasis of appendiceal origin is based on retrospective case series. Historically, treatment consisted of repeated drainage of the mucinous ascites, and evolved to serial debulking surgeries. In the 1980s, Spratt et al. demonstrated that the addition of intraperitoneal chemotherapy to traditional debulking surgery improved disease control duration [48]. In the 1990s, Sugarbaker [49] introduced the concept of a one‐stage aggressive cytoreductive debulking procedure via several macroscopic peritonectomies, followed by intraoperative HIPEC infusion [50].
HIPEC.
The aim of HIPEC is to deliver a regionally high intraperitoneal dose of heated chemotherapy with minimal systemic effects. Hyperthermic intraperitoneal chemotherapy is usually reserved for patients who have complete CRS with minimal or no residual disease. A high dose of heated (to around 107°F or 40°C–42°C) chemotherapeutic agents is perfused intraoperatively throughout the abdomen to eradicate any residual microscopic cancer cells. Hyperthermia has been proven to be cytotoxic at the cellular and tissue levels and results in the production of heat shock proteins [51], [52], [53], altered thermoregulation of malignant cells, and massive cellular destruction upon prolonged exposure. Animal studies suggest that hyperthermia might potentially enhance the cytotoxicity of chemotherapeutic agents [53]. In the setting of AMN, the most commonly used agents are mitomycin C at 10–12.5 mg/m2 [53], [54], oxaliplatin at 460 mg/m2, cisplatin, and 5‐FU, as single agents or in various combinations [54].
Evaluation of Complete Cytoreduction and HIPEC.
The completeness of cytoreduction is a significant predictor of survival in AMN with peritoneal spread. Complete resection (CCR)0 is defined as no evidence of visible disease; that is, no malignant deposits; CCR1 is defined as <2.5 mm of disease that could not be resected; CCR2 is defined as disease of 2.5–5 mm; and CCR3 is defined as ≥5 mm of visible disease left after surgery. Sugarbaker et al. developed the peritoneal cancer index (PCI), an intraoperative score assessing the extent of cancer in 13 abdomino‐pelvic regions. Peritoneal cancer index scores range from 1–39 and have been shown to be an accurate predictor of achieving CCR and of survival [55]. Determining PCI using imaging pre‐operatively helps to guide decisions regarding surgery. A PCI greater than 20 is a clear indicator of low probability of achieving a CCR [56].
Combining CRS and HIPEC [57] resulted in 5‐year survival rates of 86% for DPAM and 50% for the more aggressive PMCA, only in patients who achieved CCR. The 5‐year survival rate drops to 20% with incomplete cytoreduction. Gough et al. [58] reported a 10‐year survival rate of 32% among patients with low‐grade AMNs following debulking surgery. Histopathology and completeness of cytoreduction highly correlate with favorable outcomes [54], [57], [59], [60]. While no randomized trials have been conducted in appendiceal cancers, the expert consensus panel discussion at the Fifth International Workshop on Peritoneal Surface Malignancy in Milan, Italy (December 2006) [61], acknowledged the survival benefit of complete cytoreduction in AMNs compared to historical controls. Following these guidelines becomes challenging in the setting of high‐grade histologies.
Chua et al. [54] retrospectively evaluated data from 2,298 patients with AMN with peritoneal involvement who were treated with CRS with or without HIPEC in 16 specialized centers. The treatment‐related mortality was 2% and major operative complications occurred in 24% of patients. The median OS was 16.3 years and the median progression‐free survival (PFS) was 8.2 years, with 10‐ and 15‐year survival rates of 63% and 59%, respectively. Incomplete CRS was again identified as an independent predictor of poor outcomes. This difference remained significant after multivariate analysis, providing evidence that optimal CRS is the strongest predictor of long‐term disease outcomes in peritoneal metastasis from AMN. Hyperthermic intraperitoneal chemotherapy was also found to be associated with significantly improved PFS but not OS benefits on multivariate analysis. A high PCI score was identified as a significant prognostic variable.
Shaib et al. [60] evaluated the role of HIPEC in peritoneal metastasis from AMN in a retrospective analysis of three tertiary care centers: Emory, Ohio State, and Wayne State Universities. One of the three centers does not use HIPEC as a treatment modality in managing patients with AMN. This analysis evaluated the survival of 163 patients, with DPAM in 60 patients (36.8%), PMCA in 88 patients (53.9%), and PMCA I/D in 15 patients (9.2%). Complete resection was achieved in 76 patients (44.7%) while HIPEC was performed in 79 patients (48.4%). The median OS was 55 months for all patients in the series, 77 months for patients who received HIPEC, and 25 months for patients who did not receive HIPEC. Complete resection predicted better survival. Because HIPEC utilization in this study was driven by the treatment center rather than the degree of surgical debulking, this analysis suggests that the benefit of HIPEC is not driven by the selection of patients.
Role of Systemic Chemotherapy
Preoperative Chemotherapy.
The role of preoperative chemotherapy in patients with AMN with peritoneal metastasis has been evaluated in retrospective studies. Use of systemic chemotherapy prior to CRS predicted worse OS and PFS [54]. Similarly, in the study by Shaib et al. [60], systemic chemotherapy was associated with worse OS, with a median of 32 months compared with 82 months. In another retrospective study, Baratti et al. [62] analyzed prognostic factors among 104 patients who had received CRS, 25% of whom had previously received systemic chemotherapy. Patients receiving preoperative chemotherapy had a worse PFS and OS by multivariate analysis. In a prospective neoadjuvant chemotherapy study [63], 34 patients with appendiceal adenocarcinomas received 5‐fluorouracil and oxaliplatin (FOLFOX). Stable disease was detected on computed tomography imaging in 65% of patients. Surprisingly, 50% of patients were intraoperatively found to have disease progression, with only 29% having actual response to chemotherapy. These discrepant results underscore the limitations of current imaging modalities and standard response criteria to evaluate disease status with peritoneal involvement [64]. Bijelic et al. [65] reported a neoadjuvant approach with systemic 5‐FU‐based chemotherapy in patients with PMCA that engendered complete or near‐complete pathologic response, translating to more limited surgical resections. These promising results may be due to selection of a more aggressive histology and exclusion of low‐grade DPAM.
A retrospective trial [40] evaluated the use of non‐cytotoxic targeted agents in the preoperative setting. Immunohistochemistry for COX‐2 expression and KRAS mutational status were used to select patients for celecoxib and epidermal growth factor receptor (EGFR) inhibitors (cetuximab or panitumumab). Unfortunately, this personalized approach had no impact on OS (p = .84 and .83, respectively). These studies indicate that patients with resectable peritoneal metastasis from low‐grade AMN should not receive preoperative chemotherapy. Selection bias, poor efficacy of chemotherapy, side effects of chemotherapy, and delay of surgical intervention may explain the negative impact of chemotherapy results.
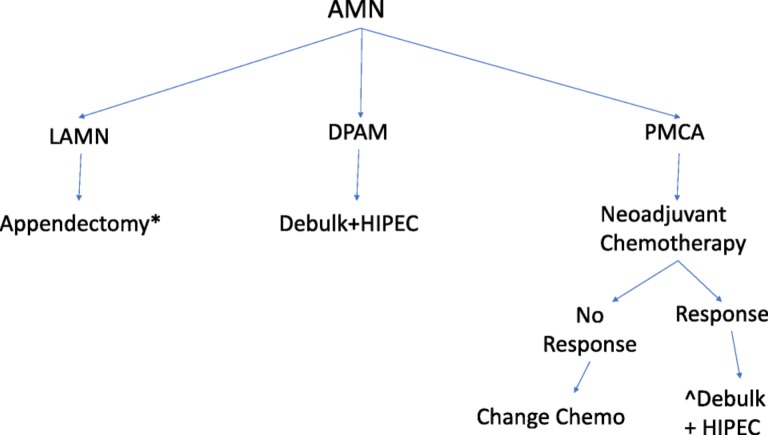
In summary, retrospective reports confirm that histopathology, pre‐operative systemic chemotherapy, degree of CRS, and use of HIPEC are significant independent predictors of survival outcomes. Optimal CRS and HIPEC treatment without preoperative systemic chemotherapy remains the standard of care in the management of peritoneal metastasis of low‐grade AMN. High‐grade appendiceal adenocarcinomas should be considered for systemic preoperative chemotherapy; if there is response, surgical resection and HIPEC treatment should be considered. Figure 7 is a proposed treatment approach for each histologic subtype and stage.
Optimal CRS and HIPEC treatment without preoperative systemic chemotherapy remains the standard of care in the management of peritoneal metastasis of low‐grade AMN. High‐grade appendiceal adenocarcinomas should be considered for systemic preoperative chemotherapy and if there is response, surgical resection and HIPEC treatment should be considered.
Figure 7.
Treatment recommendations for appendiceal mucinous neoplasms.
*Right hemicolectomy if tumor invades base of appendix, has high mitotic rate, size >2 cm, margin positive.
^Adjuvant chemotherapy if R1 or R2 surgery, or if lymph node positive.
Abbreviations: AMN, appendiceal mucinous neoplasms; DPAM, disseminated peritoneal adenomucinosis; HIPEC, hyperthermic intraperitoneal chemotherapy; LAMN,: lLow‐grade appendiceal mucinous neoplasms; DPAM: disseminated peritoneal adenomucinosis, PMCA:, peritoneal mucinous carcinomatosis; PMCA I/D:, PMCAperiotoneal mucinous carcinomatosis of indeterminate or discordant features. The classification is dependent on the grade of differentiation rather than the anatomic invasion of the disease.
Palliative Systemic Chemotherapy.
The role and optimal type of systemic chemotherapy in the management of AMN have not been established in prospective trials. Chemotherapy is usually reserved for patients with recurrent disease for which surgical resection is no longer an option. In general, high‐grade mucinous adenocarcinomas of the appendix behave in a similar fashion to mucinous colon tumors, and one would argue the same for adenocarcinomas. Low‐grade mucinous cancers have a lower chance of response to chemotherapy. Due to the paucity of literature regarding chemotherapy treatments and the poor treatment response in relation to the grade of disease, we focus in this section on the systemic treatment approach.
Fluorouracil‐based regimens are commonly used in the treatment of AMN. The combination of capecitabine and mitomycin C was evaluated in 39 patients enrolled on single arm phase II trial. The regimen was based on the observed activity of these agents used in HIPEC [67]. The overall response rate was 38%. A reduction in tumor marker levels by 50% or greater was achieved in 20% of patients. Survival at 1‐ and 2‐years was 84% and 61%, respectively. This study was, however, criticized for not using standard response evaluation criteria such as Response Evaluation Criteria In Solid Tumors (RECIST), which has established limitations in evaluating peritoneal disease.
Combination chemotherapy [68] with FOLFOX‐4 (5‐FU, leucovorin, oxaliplatin) was evaluated in a retrospective study of 20 patients with peritoneal metastasis from AMN. A partial response rate was seen in 20% of cases, while median PFS and OS were 8 and 26 months, respectively. Overman et al. criticized this study [9] for the absence of a clear reference grading system, imprecise definitions of high‐ and low‐grade tumors, and the failure of RECIST criteria to quantify disease response.
The role of biologic targeted therapy such as EGFR inhibitors and anti‐angiogenic agents has not been formally studied [66]. Shapiro et al. [69] tested the effect of more modern agents, including targeted agents, (irinotecan, platinum, capecitabine, gefitinib, bevacizumab, cetuximab) on response rates and PFS in patients who were non‐surgical or poor candidates for CRS or HIPEC. The use of anti‐angiogenic agents in particular was supported by a report on vascular endothelial growth factor (VEGF) expression portending poor prognosis of OS in AMN [70]. The 5‐FU‐based regimens prolonged disease control to a median of 7.6 months in patients who were not CRS candidates. Moderate to poorly differentiated tumors, as well as signet‐ring histology, were found to be negative predictors of OS in patients with peritoneal metastasis from AMN treated with chemotherapy. Lieu et al. [71] collected data on 78 patients with metastatic disease who received chemotherapy. Radiographic response was seen in 44% of cases. The median PFS was 6.9 months, and median OS was 1.7 years, the latter being markedly shorter than reported for patients with PMCA [62]. Response to chemotherapy predicted improved PFS, and CCR predicted improved OS.
Conclusion
Appendiceal mucinous neoplasms are a heterogeneous group of tumors with a rising incidence. Treatment is based on stage and histology. Low‐grade tumors are treated surgically with resection of the primary site in early stage disease, or peritoneal debulking and HIPEC in patients with advanced stage disease. Treatment of high‐grade tumors requires further prospective trials, and options include debulking surgery and HIPEC, with or without preoperative chemotherapy. Trials evaluating novel therapies based on molecular profiling of AMN tumors are needed to evaluate therapeutic options in patients who are not surgical candidates.
Author Contributions
Conception/design: Walid L. Shaib, Bassel F. El‐Rayes
Collection and/or assembly of data: Walid L. Shaib, Rita Assi, Volkan Adsay
Manuscript writing: Walid L. Shaib, Ali Shamseddine, Olantunji B. Alese, Charles III Staley, Bahar Memis, Tonios Bekaii‐Saab
Final approval of manuscript: Volkan Adsay, Tonios Bekaii‐Saab, Bassel F. El‐Rayes
Disclosures
The authors indicated no financial relationships.
References
- 1. McCusker ME, Cote TR, Clegg LX et al. Primary malignant neoplasms of the appendix: A population‐based study from the surveillance, epidemiology and end‐results program, 1973–1998. Cancer 2002;94:3307–3312. [DOI] [PubMed] [Google Scholar]
- 2. Smeenk RM, van Velthuysen ML, Verwaal VJ et al. Appendiceal neoplasms and pseudomyxoma peritonei: A population based study. Eur J Surg Oncol 2008;34:196–201. [DOI] [PubMed] [Google Scholar]
- 3. Rokitansky K, Swaine WE, Sir Sieveking EH, Moore CH, Day GE. A Manual of Pathological Anatomy. Vol. 2 Philadelphia, PA: Blanchard and Lea, 1855;24:100–118. [Google Scholar]
- 4. Elting AW. IX. Primary carcinoma of the vermiform appendix, with a report of three cases. Ann Surg 1903;37:549–574. [PMC free article] [PubMed] [Google Scholar]
- 5. Carr NJ, McCarthy WF, Sobin LH. Epithelial noncarcinoid tumors and tumor‐like lesions of the appendix. A clinicopathologic study of 184 patients with a multivariate analysis of prognostic factors. Cancer 1995;75:757–768. [DOI] [PubMed] [Google Scholar]
- 6. Ronnett BM, Zahn CM, Kurman RJ et al. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol 1995;19:1390–1408. [DOI] [PubMed] [Google Scholar]
- 7. Pai RK, Beck AH, Norton JA et al. Appendiceal mucinous neoplasms: Clinicopathologic study of 116 cases with analysis of factors predicting recurrence. Am J Surg Pathol 2009;33:1425–1439. [DOI] [PubMed] [Google Scholar]
- 8. Misdraji J, Yantiss RK, Graeme‐Cook FM et al. Appendiceal mucinous neoplasms: A clinicopathologic analysis of 107 cases. Am J Surg Pathol 2003;27:1089–1103. [DOI] [PubMed] [Google Scholar]
- 9. Overman MJ, Fournier K, Hu CY et al. Improving the AJCC/TNM staging for adenocarcinomas of the appendix: The prognostic impact of histological grade. Ann Surg 2013;257:1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carr NJ, Cecil TD, Mohamed F et al. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: The results of the Peritoneal Surface Oncology Group International (PSOGI) modified delphi process. Am J Surg Pathol 2016;40:14–26. [DOI] [PubMed] [Google Scholar]
- 11. Gupta S, Parsa V, Adsay V et al. Clinicopathological analysis of primary epithelial appendiceal neoplasms. Med Oncol 2010;27:1073–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Young RH. Pseudomyxoma peritonei and selected other aspects of the spread of appendiceal neoplasms. Semin Diagn Pathol 2004;21:134–150. [DOI] [PubMed] [Google Scholar]
- 13. Collins DC. 71,000 human appendix specimens. A final report, summarizing forty years' study. Am J Proctol 1963;14:265–281. [PubMed] [Google Scholar]
- 14. Connor SJ, Hanna GB, Frizelle FA. Appendiceal tumors: Retrospective clinicopathologic analysis of appendiceal tumors from 7,970 appendectomies. Dis Colon Rectum 1998;41:75–80. [DOI] [PubMed] [Google Scholar]
- 15. Jemal A, Siegel R, Ward E et al. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225–249. [DOI] [PubMed] [Google Scholar]
- 16. Ozakyol AH, Saricam T, Kabukcuoglu S et al. Primary appendiceal adenocarcinoma. Am J Clin Oncol 1999;22:458–459. [DOI] [PubMed] [Google Scholar]
- 17. Shaib WL, Goodman M, Chen Z et al. Incidence and survival of appendiceal mucinous neoplasms: A SEER analysis. Am J Clin Oncol 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18. van den Heuvel MG, Lemmens VE, Verhoeven RH et al. The incidence of mucinous appendiceal malignancies: A population‐based study. Int J Colorectal Dis 2013;28:1307–1310. [DOI] [PubMed] [Google Scholar]
- 19. Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol 2006;7:69–76. [DOI] [PubMed] [Google Scholar]
- 20. Ito H, Osteen RT, Bleday R et al. Appendiceal adenocarcinoma: Long‐term outcomes after surgical therapy. Dis Colon Rectum 2004;47:474–480. [DOI] [PubMed] [Google Scholar]
- 21. Bradley RF, Stewart JH 4th, Russell GB et al. Pseudomyxoma peritonei of appendiceal origin: A clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol 2006;30:551–559. [DOI] [PubMed] [Google Scholar]
- 22. Garg PK, Prasad D, Aggarwal S et al. Acute intestinal obstruction: An unusual complication of mucocele of appendix. Eur Rev Med Pharmacol Sci 2011;15:99–102. [PubMed] [Google Scholar]
- 23. Rutledge RH, Alexander JW. Primary appendiceal malignancies: Rare but important. Surgery 1992;111:244–250. [PubMed] [Google Scholar]
- 24. Reid MD, Basturk O, Shaib WL et al. Adenocarcinoma ex‐goblet cell carcinoid (appendiceal‐type crypt cell adenocarcinoma) is a morphologically distinct entity with highly aggressive behavior and frequent association with peritoneal/intra‐abdominal dissemination: An analysis of 77 cases. Mod Pathol 2016;29:1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kabbani W, Houlihan PS, Luthra R et al. Mucinous and nonmucinous appendiceal adenocarcinomas: Different clinicopathological features but similar genetic alterations. Mod Pathol 2002;15:599–605. [DOI] [PubMed] [Google Scholar]
- 26. Muller D. Appendiceal neoplasm resulting from a malignant transformation of a villous adenoma [in German]. Chirurg 1980;51:609–610. [PubMed] [Google Scholar]
- 27. Stewart JH 4th, Shen P, Russell GB et al. Appendiceal neoplasms with peritoneal dissemination: Outcomes after cytoreductive surgery and intraperitoneal hyperthermic chemotherapy. Ann Surg Oncol 2006;13:624–634. [DOI] [PubMed] [Google Scholar]
- 28. Turaga KK, Pappas SG, Gamblin T. Importance of histologic subtype in the staging of appendiceal tumors. Ann Surg Oncol 2012;19:1379–1385. [DOI] [PubMed] [Google Scholar]
- 29. Nitecki SS, Wolff BG, Schlinkert R et al. The natural history of surgically treated primary adenocarcinoma of the appendix. Ann Surg 1994;219:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ronnett BM, Yan H, Kurman RJ et al. Patients with pseudomyxoma peritonei associated with disseminated peritoneal adenomucinosis have a significantly more favorable prognosis than patients with peritoneal mucinous carcinomatosis. Cancer 2001;92:85–91. [DOI] [PubMed] [Google Scholar]
- 31. Esquivel J, Sugarbaker PH. Clinical presentation of the pseudomyxoma peritonei syndrome. Br J Surg 2000;87:1414–1418. [DOI] [PubMed] [Google Scholar]
- 32. Higa E, Rosai J, Pizzimbono CA et al. Mucosal hyperplasia, mucinous cystadenoma, and mucinous cystadenocarcinoma of the appendix. A re‐evaluation of appendiceal “mucocele”. Cancer 1973;32:1525–1541. [DOI] [PubMed] [Google Scholar]
- 33. Misdraji J. Appendiceal mucinous neoplasms: Controversial issues. Arch Pathol Lab Med 2010;134:864–870. [DOI] [PubMed] [Google Scholar]
- 34. Ji H, Isacson C, Seidman JD et al. Cytokeratins 7 and 20, Dpc4, and MUC5AC in the distinction of metastatic mucinous carcinomas in the ovary from primary ovarian mucinous tumors: Dpc4 assists in identifying metastatic pancreatic carcinomas. Int J Gynecol Pathol 2002;21:391–400. [DOI] [PubMed] [Google Scholar]
- 35. Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell 1996;87:159–170. [DOI] [PubMed] [Google Scholar]
- 36. Perucho M. Microsatellite instability: The mutator that mutates the other mutator. Nat Med 1996;2:630–631. [DOI] [PubMed] [Google Scholar]
- 37. Marra G, Boland CR. Hereditary nonpolyposis colorectal cancer: The syndrome, the genes, and historical perspectives. J Natl Cancer Inst 1995;87:1114–1125. [DOI] [PubMed] [Google Scholar]
- 38. Chung DC. The genetic basis of colorectal cancer: Insights into critical pathways of tumorigenesis. Gastroenterology 2000;119:854–865. [DOI] [PubMed] [Google Scholar]
- 39. Szych C, Staebler A, Connolly DC et al. Molecular genetic evidence supporting the clonality and appendiceal origin of pseudomyxoma peritonei in women. Am J Pathol 1999;154:1849–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Raghav KP, Shetty AV, Kazmi SM et al. Impact of molecular alterations and targeted therapy in appendiceal adenocarcinomas. The Oncologist 2013;18:1270–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goldstein D EJ, Wang K, et al. Comprehensive genomic profiling of cancer of the appendix to reveal new routes to targeted therapies. J Clin Oncol 2015;33(suppl 3):608a. [Google Scholar]
- 42. Levine EA, Votanopoulos KI, Qasem SA, Philip J, Cummins KA, Chou JW, Ruiz J, D'Agostino R, Shen P, Miller LD. Prognostic molecular subtypes of low‐grade cancer of the appendix. J Am Coll Surg 2016;222:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original and primary source for this information is the AJCC Cancer Staging Manual, Seventh Edition (2010) published by Springer Science Business Media.
- 44. Yantiss RK, Shia J, Klimstra DS et al. Prognostic significance of localized extra‐appendiceal mucin deposition in appendiceal mucinous neoplasms. Am J Surg Pathol 2009;33:248–255. [DOI] [PubMed] [Google Scholar]
- 45. Gonzalez‐Moreno S, Sugarbaker PH. Right hemicolectomy does not confer a survival advantage in patients with mucinous carcinoma of the appendix and peritoneal seeding. Br J Surg 2004;91:304–311. [DOI] [PubMed] [Google Scholar]
- 46. Pahlavan PS, Kanthan R. Goblet cell carcinoid of the appendix. World J Surg Oncol 2005;3:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Young RH, Gilks CB, Scully RE. Mucinous tumors of the appendix associated with mucinous tumors of the ovary and pseudomyxoma peritonei. A clinicopathological analysis of 22 cases supporting an origin in the appendix. Am J Surg Pathol 1991;15:415–429. [DOI] [PubMed] [Google Scholar]
- 48. Spratt JS, Adcock RA, Muskovin M et al. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res 1980;40:256–260. [PubMed] [Google Scholar]
- 49. Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jacquet P, Stephens AD, Averbach AM et al. Analysis of morbidity and mortality in 60 patients with peritoneal carcinomatosis treated by cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy. Cancer 1996;77:2622–2629. [DOI] [PubMed] [Google Scholar]
- 51. Gerweck LE. Hyperthermia in cancer therapy: The biological basis and unresolved questions. Cancer Res 1985;45:3408–3414. [PubMed] [Google Scholar]
- 52. Lindquist S. The heat‐shock response. Annu Rev Biochem 1986;55:1151–1191. [DOI] [PubMed] [Google Scholar]
- 53. Oleson JR, Calderwood SK, Coughlin CT et al. Biological and clinical aspects of hyperthermia in cancer therapy. Am J Clin Oncol 1988;11:368–380. [DOI] [PubMed] [Google Scholar]
- 54. Chua TC, Moran BJ, Sugarbaker PH et al. Early‐ and long‐term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 2012;30:2449–2456. [DOI] [PubMed] [Google Scholar]
- 55. Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359–374. [DOI] [PubMed] [Google Scholar]
- 56. Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg 1995;221:124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol 1999;6:727–731. [DOI] [PubMed] [Google Scholar]
- 58. Gough DB, Donohue JH, Schutt AJ et al. Pseudomyxoma peritonei. Long‐term patient survival with an aggressive regional approach. Ann Surg 1994;219:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dayal S, Taflampas P, Riss S et al. Complete cytoreduction for pseudomyxoma peritonei is optimal but maximal tumor debulking may be beneficial in patients in whom complete tumor removal cannot be achieved. Dis Colon Rectum 2013;56:1366–1372. [DOI] [PubMed] [Google Scholar]
- 60. Shaib WL, Martin LK, Choi M et al. Hyperthermic intraperitoneal chemotherapy following cytoreductive surgery improves outcome in patients with primary appendiceal mucinous adenocarcinoma: A pooled analysis from three tertiary care centers. The Oncologist 2015;20:907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Baratti D, Kusamura S, Deraco M. The Fifth International Workshop on Peritoneal Surface Malignancy (Milan, Italy, December 4–6, 2006): Methodology of disease‐specific consensus. J Surg Oncol 2008;98:258–262. [DOI] [PubMed] [Google Scholar]
- 62. Baratti D, Kusamura S, Nonaka D et al. Pseudomyxoma peritonei: Clinical pathological and biological prognostic factors in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol 2008;15:526–534. [DOI] [PubMed] [Google Scholar]
- 63. Sugarbaker PH, Bijelic L, Chang D et al. Neoadjuvant FOLFOX chemotherapy in 34 consecutive patients with mucinous peritoneal carcinomatosis of appendiceal origin. J Surg Oncol 2010;102:576–581. [DOI] [PubMed] [Google Scholar]
- 64. Esquivel J, Chua TC, Stojadinovic A et al. Accuracy and clinical relevance of computed tomography scan interpretation of peritoneal cancer index in colorectal cancer peritoneal carcinomatosis: A multi‐institutional study. J Surg Oncol 2010;102:565–570. [DOI] [PubMed] [Google Scholar]
- 65. Bijelic L, Kumar AS, Stuart OA et al. Systemic chemotherapy prior to cytoreductive surgery and HIPEC for carcinomatosis from appendix cancer: Impact on perioperative outcomes and short‐term survival. Gastroenterol Res Pract 2012;2012:163284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Farquharson AL, Pranesh N, Witham G et al. A phase II study evaluating the use of concurrent mitomycin C and capecitabine in patients with advanced unresectable pseudomyxoma peritonei. Br J Cancer 2008;99:591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Livingston EH, Woodward WA, Sarosi GA et al. Disconnect between incidence of nonperforated and perforated appendicitis: Implications for pathophysiology and management. Ann Surg 2007;245:886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pietrantonio F, Maggi C, Fanetti G et al. FOLFOX‐4 chemotherapy for patients with unresectable or relapsed peritoneal pseudomyxoma. The Oncologist 2014;19:845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shapiro JF, Chase JL, Wolff RA et al. Modern systemic chemotherapy in surgically unresectable neoplasms of appendiceal origin: A single‐institution experience. Cancer 2010;116:316–322. [DOI] [PubMed] [Google Scholar]
- 70. Logan‐Collins JM, Lowy AM, Robinson‐Smith TM et al. VEGF expression predicts survival in patients with peritoneal surface metastases from mucinous adenocarcinoma of the appendix and colon. Ann Surg Oncol 2008;15:738–744. [DOI] [PubMed] [Google Scholar]
- 71. Lieu CH, Lambert LA, Wolff RA et al. Systemic chemotherapy and surgical cytoreduction for poorly differentiated and signet ring cell adenocarcinomas of the appendix. Ann Oncol 2012;23:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]