Abstract
Background
Pain present for at least 3 months after a surgical procedure is considered chronic postsurgical pain (CPSP) and affects 10–50 per cent of patients. Interventions for CPSP may focus on the underlying condition that indicated surgery, the aetiology of new‐onset pain or be multifactorial in recognition of the diverse causes of this pain. The aim of this systematic review was to identify RCTs of interventions for the management of CPSP, and synthesize data across treatment type to estimate their effectiveness and safety.
Methods
MEDLINE, Embase, PsycINFO, CINAHL and the Cochrane Library were searched from inception to March 2016. Trials of pain interventions received by patients at 3 months or more after surgery were included. Risk of bias was assessed using the Cochrane risk‐of‐bias tool.
Results
Some 66 trials with data from 3149 participants were included. Most trials included patients with chronic pain after spinal surgery (25 trials) or phantom limb pain (21 trials). Interventions were predominantly pharmacological, including antiepileptics, capsaicin, epidural steroid injections, local anaesthetic, neurotoxins, N‐methyl‐d‐aspartate receptor antagonists and opioids. Other interventions included acupuncture, exercise, postamputation limb liner, spinal cord stimulation, further surgery, laser therapy, magnetic stimulation, mindfulness‐based stress reduction, mirror therapy and sensory discrimination training. Opportunities for meta‐analysis were limited by heterogeneity. For all interventions, there was insufficient evidence to draw conclusions on effectiveness.
Conclusion
There is a need for more evidence about interventions for CPSP. High‐quality trials of multimodal interventions matched to pain characteristics are needed to provide robust evidence to guide management of CPSP.
Short abstract
Flimsy evidence base
Introduction
Pain present for at least 3 months after a surgical procedure is described as chronic postsurgical pain (CPSP)1. CPSP affects between 10 and 50 per cent of patients after common operations such as mastectomy, cardiac surgery, hysterectomy, hernia repair, joint replacement, back surgery and also more minor procedures2, 3, 4, 5, 6, 7, 8. In a European survey9 of surgical patients, the prevalence of moderate to severe CPSP at 12 months after operation was 11·8 per cent. Chronic pain is associated with poor general health, disability, depression9, 10, 11, 12 and social withdrawal, and increases the risk of further co‐morbidities13. CPSP has been defined previously as pain that develops after surgery5, and a proposed update to the definition includes the possibility that CPSP is pain that increases in intensity after surgery14. This update allows for the possibility that pain among patients who undergo surgery to relieve pain is also, appropriately, included in the definition.
Risk factors for CPSP may be genetic, psychosocial, or related to preoperative or acute postoperative pain severity2, 15. However, certain surgical procedure‐related factors are key for the development of chronic pain16. Surgical procedures lasting longer than 3 h may increase the risk of postoperative pain5. A major surgical factor in the development of chronic pain is nerve injury, and patients undergoing thoracic, breast and hernia surgery are at particular risk of neuropathic pain8. Inflammation resulting from intraoperative tissue injury can contribute to central sensitization and further pain2. Inadequate preventive analgesia may also contribute17.
Knowledge of determinants and predictors of CPSP can guide the development of interventions and help target care. Possible forms of management for CPSP may focus on the underlying condition that needed surgery, on the aetiology of the pain, or be multifactorial in recognition of the diverse causes of postoperative pain. Although some forms of management may have limited applicability outside of the specific condition for which they were intended, others may be transferrable, regardless of the surgical procedure.
There are systematic reviews of pharmacological and other interventions for the management of chronic pain, defined generally, or specific to the presumed mechanisms (such as neuropathic pain17 and cancer pain18). A number of Cochrane reviews17, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 have included studies evaluating interventions for CPSP, although this was not the primary focus of these reviews. It is rare for any review to focus specifically on chronic pain in the postoperative context. Exceptions include reviews that have focused on interventions for chronic pain after particular surgical procedures, including phantom limb pain after amputation30 and knee replacement31. The aim of the present review was to identify RCTs of interventions for the management of CPSP and to synthesize data across treatment type to provide an estimate of their effectiveness and safety. In keeping with recommended practice, a systematic review is a key step toward the development of future trials to evaluate interventions for CPSP32.
Methods
The protocol was registered in the international prospective register of systematic reviews (PROSPERO; http://www.crd.york.ac.uk/PROSPERO/) on 15 January 2015 (registration number 15957). The review was conducted in accordance with PRISMA guidelines33.
Eligibility criteria
Published articles describing RCTs involving any intervention that aimed to provide management of CPSP were included. Eligible studies reflected PICO criteria34: patients aged 18 years or more and at least 90 per cent of study participants reporting CPSP; interventions for pain received by patients at a minimum of 3 months after surgery; comparison arm of placebo, usual care or an alternative pain management intervention; and outcomes were pain reported using any data collection tool(s).
Information sources and searches
MEDLINE, Embase, PsycINFO, CINAHL and the Cochrane Library were searched from inception to 23 March 2016. The search strategies were modified for different bibliographic databases (Appendix S1, supporting information). No language restrictions were applied. Reference lists were checked and registers inspected; grey literature (literature not formally published as journal articles) was sought in OpenGrey (http://www.greynet.org/opengreyrepository.html), a database of grey literature, on 30 March 2016. A minimum sample size was not specified in the protocol to ensure inclusion of all treatments of potential interest to clinicians and researchers working in a range of surgical specialties.
Published conference abstracts were followed up to obtain any full publications, but otherwise excluded. After completion of data extraction, relevant systematic reviews were identified from the Cochrane Database of Systematic Reviews, and included studies were reviewed to identify any studies missed in the initial searches because eligibility was not apparent from the title and abstract.
Study selection
All records identified in the search were imported into EndNote X7 (Thomson Reuters, New York, New York, USA). Abstracts or full‐text articles were screened to remove obviously irrelevant reports. Reasons for excluding studies were recorded as free text in EndNote X7. This was performed by one author who was overinclusive if eligibility was not clear. A sample of 10 per cent was double screened by a second author, which identified one eligible study that had been missed. The final selection of studies was then performed in duplicate by two authors. When there was insufficient information to determine eligibility, study author e‐mail addresses were obtained and supplementary information was requested.
Data collection
Data from included studies were extracted using standard forms by one author and checked by a second author. Study setting, participant demographics, methodology, recruitment, duration, treatment characteristics, length of follow‐up, outcomes, tools used to measure outcomes, and information for the risk‐of‐bias assessment were recorded. Authors of studies were contacted where necessary for clarification and to provide missing or incomplete data.
Outcomes
In accordance with GRADE guidelines35 and Cochrane guidance, the total number of outcomes planned to be included in this review was limited to seven (2 primary and 5 secondary). The primary clinical effectiveness outcome was pain intensity and the primary harm outcome was serious adverse events. These reflect recommendations from the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT)36 and the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS)37. Studies were required to report the primary outcome of pain intensity to be eligible for inclusion in the review. The first secondary outcome was the presence or absence of neuropathic pain, which is particularly relevant to chronic pain after a surgical intervention8. The other four secondary outcomes reflected the IMMPACT core outcome domains for chronic pain clinical trials: physical functioning, emotional functioning, participants' ratings of global improvement and satisfaction with treatment, and participant disposition36. No limits were placed on the tools used to measure these outcomes.
Risk of bias in individual studies
Risk of bias was assessed using the Cochrane risk‐of‐bias tool38. Two authors assessed the risk of bias independently across the six domains of the tool for each study. Results are reported through graphical representation of bias judgements grouped by intervention.
Statistical analysis
In the protocol, meta‐analyses were planned using RevMan 5 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) with general guidance from the Cochrane Handbook38. For dichotomous (binary) data, the odds ratio with 95 per cent c.i. would be used. For continuous data, if outcomes were measured identically across studies, an overall mean difference and 95 per cent c.i. would be calculated. If continuous outcomes were measured differently across studies, overall standardized mean differences and 95 per cent c.i. would be calculated. For data from crossover trials, the generic inverse‐variance method in RevMan 5 would be used.
Opportunities for meta‐analysis were limited by heterogeneity between studies. Even when multiple studies for a particular intervention were identified, variation in modes of administration, comparator groups and/or format of outcome data precluded pooling. Thus, the majority of results are reported narratively, with results of meta‐analyses described only for gabapentin and capsaicin. Planned subgroup analysis of pharmacotherapy, physical/self‐management and multidisciplinary interventions was not possible owing to clinical and methodological heterogeneity. Results for pain outcome at final follow‐up within individual studies are presented as reported by investigators (Tables S1 and S2, supporting information).
Results
Included trials
Searches identified 17 029 articles, of which 660 were considered potentially relevant after initial screening. Author e‐mail addresses were traced for 57 of 78 studies that contained insufficient information to determine eligibility, and further data were requested. Replies were received for 16 studies, and only one was eligible for inclusion. The remaining articles were assumed to be ineligible as the abstract or full text made no reference to patients having CPSP. After evaluation of full‐text articles, 66 trials39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104 with data from 3149 participants were included (Fig. 1).
Figure 1.
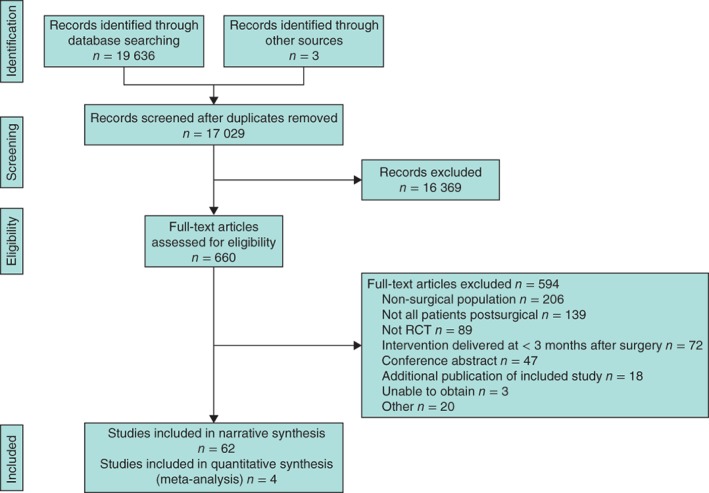
Flow diagram showing selection of articles for review
An overall summary of trial characteristics is provided in Table 1 and characteristics of individual trials are shown in Tables S1 and S2 (supporting information). Table 2 summarizes studies according to the index surgery and intervention. Individual components of risk‐of‐bias assessment are provided in Appendix S2 (supporting information).
Table 1.
Overall summary of trial characteristics
| Trial design | Parallel design (41), crossover study (25) |
| No. of arms | Two arms (53), three arms (12), five arms (1) |
| Countries | USA (21), Germany (8), Denmark (7), Iran (3), China (2), Egypt (2), Finland (2), France (2), Italy (2), Korea (2), Sweden (2), Turkey (2), Belgium (1), Canada (1), Israel (1), Mozambique (1), The Netherlands (1), Norway (1), Serbia (1), Spain (1), Switzerland (1), UK (1), international multisite (1) |
| Surgery types | Spinal surgery (25), amputation (21), breast cancer surgery (8), inguinal hernia repair (3), neck dissection for cancer (2), knee replacement (1), sternotomy (1), abdominal surgery (1), shoulder surgery (1), various surgical procedures (3) |
| Interventions Pharmacological | Antidepressants as analgesics (4), antiepileptics (8), capsaicin (3), epidural steroid injections and associated interventions (11), local anaesthetic (11), neurotoxins (3), N‐methyl‐d‐aspartate receptor antagonist (7), opioids (6), calcitonin (1), naloxone as an adjuvant to morphine (1) |
| Physical, surgical, | |
| psychological and | |
| other pain | |
| management | Acupuncture/dry needling (2), exercise (4), limb cover/liner for patients who had undergone amputation (2), spinal cord stimulation (5), further surgery (2), laser therapy (1), magnetic stimulation (1), mindfulness‐based stress reduction (1), mirror therapy for amputation (1), sensory discrimination training (1), joint manipulation (1), combined package of hot packs, ultrasound treatment and transcutaneous electrical nerve stimulation (1) |
| Comparator interventions | Active treatment (31), placebo or sham (31), usual care (2), waiting list (1), no treatment (1) |
Values in parentheses are number of studies.
Table 2.
Summary of included studies according to the index surgery and intervention
| Amputation | Spinal surgery | Breast cancer | Abdominal surgery | Hernia repair | Knee replacement | Neck dissection | Sternotomy | Shoulder surgery | Mixed | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pharmacological interventions | ||||||||||
| Antidepressants | 239, 40 | 241, 42 | ||||||||
| Antiepileptics | 243, 44 | 247, 48 | 245, 49 | 150 | 146 | |||||
| Capsaicin | 152 | 151 | 153 | |||||||
| Epidural injection and | 1154, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64 | |||||||||
| associated interventions | ||||||||||
| Local anaesthetic | 470, 71, 72, 73 | 267, 68 | 245, 74 | 265, 66 | 169 | |||||
| Neurotoxins | 177 | 175 | 176 | |||||||
| NMDA receptor antagonist | 678, 79, 80, 81, 82, 83 | 169 | ||||||||
| Opioids | 440, 70, 71, 84 | 159 | 185 | |||||||
| Other | 178 | 186 | ||||||||
| Physical, surgical, psychological | ||||||||||
| and other interventions | ||||||||||
| Acupuncture/dry needling | 188 | 187 | ||||||||
| Exercise | 191 | 389, 90, 92 | ||||||||
| Limb cover/liner | 293, 94 | |||||||||
| Spinal cord stimulation | 595, 96, 97, 98, 99 | |||||||||
| Surgery | 290, 95 | |||||||||
| Other | 2102, 104 | 392, 100, 103 | 1101 | |||||||
Trial design
Trials were generally small, ranging in size from three to 250 participants (median 38). The study with three participants was a pilot trial, but a total of 18 trials recruited fewer than 20 participants. Sample size calculations were reported in 34 of 66 studies; of these, 13 failed to recruit or retain sufficient numbers of participants to meet their calculation. Authors did not always state dates of recruitment; publication dates ranged from 1989 to 2016. There has been an increase over time in the number of published trials in this field, from ten trials published before 2001 to 23 published between 2011 and 2015.
Interventions
The primary method of reporting results was grouped according to treatment type. The majority of studies evaluated pharmacological interventions, and so studies were grouped as primarily pharmacological, or as primarily physical, surgical, psychological and other (Table 1).
Outcome measurements
A visual analogue scale (VAS) was used to assess pain intensity in 37 of 66 studies (56 per cent). Validated pain‐specific tools were used infrequently; the most common was the McGill Pain Questionnaire, which was used in eight studies. Only one trial referenced IMMPACT criteria36 for patient outcomes. In terms of presentation of the primary outcome (pain intensity) as the percentage of patients reporting 30 or 50 per cent improvement, a format preferred by both IMMPACT105 and the Cochrane PaPaS Group106, only a minority of authors (6 of 31 trials published after 2008) were compliant. Serious adverse events and the secondary outcomes in this review were reported inconsistently and there was no opportunity to summarize these outcomes; therefore, only pain outcomes are presented in Tables S1 and S2 (supporting information).
Pharmacological interventions
Antidepressants
Four trials, including data from 177 participants, evaluated the effect of antidepressants on chronic pain after amputation39, 40 or breast surgery41, 42. Risk of bias was evident in two studies owing to incomplete outcome data40, 42, and a change to the definition of responder and potential funder bias40. Amitriptyline was evaluated in three trials39, 40, 42, with some issues suggesting risk of bias, and venlafaxine in one trial41 with a low risk of bias. There was no evidence that a 4–6‐week course of antidepressants reduced pain intensity compared with placebo, except in one trial42 involving 20 patients which found that patients reported lower breast scar pain intensity after 4 weeks of 100 mg/day amitriptyline compared with placebo. However, this trial also found evidence that amitriptyline resulted in more adverse events than placebo.
Antiepileptics
Eight trials including data from 338 participants evaluated the effects of antiepileptic medications on CPSP. The largest number of studies for any one technology was for gabapentin (6 studies, 293 participants with pain after amputation43, 44, breast cancer surgery45, sternotomy46 and spinal surgery47, 48). Four trials were at risk of bias owing to issues relating to blinding48, blinding and randomization45, incomplete outcome data44, and blinding and single‐authored article47. Meta‐analysis using the generic inverse‐variance method was possible for the primary outcome for one subgroup only (gabapentin versus placebo for 6 weeks) involving two crossover trials with 43 patients43, 44. This demonstrated a within‐person mean difference in pain intensity measured on a VAS and numerical rating scale score of –1·12 (95 per cent c.i. –1·89 to –0·36; I 2 = 53 per cent), favouring gabapentin (Fig. S1, supporting information). Similar results are reported in a Cochrane review30 and no studies additional to those included in the previous review were identified. The two trials that compared gabapentin with non‐steroidal anti‐inflammatory drugs (NSAIDs) found that gabapentin provided more effective pain relief after 1 month46 and 6 months48 of treatment. An 8‐week course of gabapentin was found to be superior to a stellate ganglion block using bupivacaine in a trial involving 60 patients after mastectomy45. The addition of 1 month of oral gabapentin to standard epidural corticosteroids was found to result in lower pain at 6 months compared with epidural corticosteroids alone after spinal surgery47.
One trial49 with a low risk of bias found no differences in pain relief between levetiracetam and placebo over 4 weeks of treatment. Pain relief after taking pregabalin for 7 weeks for chronic pain after abdominal surgery was compared with placebo in a study of 13 patients50; results favouring the treatment group must be interpreted with caution as the trial was terminated early by the industry sponsor.
Capsaicin
Three trials including data from 174 participants evaluated capsaicin for relief of chronic pain after inguinal hernia repair51, mastectomy52, and diverse procedures for cancer53. All studies were at risk of bias because of issues relating to blinding of a preparation with a burning sensation and erythema. One study52 was also at risk of bias owing to selective reporting. The trial51 assessing a single 60‐min application of a capsaicin patch (8 per cent) found no evidence of pain relief compared with placebo after 3 months. Two trials52, 53 of low‐dose (0·075 per cent) capsaicin topical cream applied four times daily for 6–8 weeks reported some evidence of reduced pain intensity compared with placebo. Meta‐analysis suggested a modest positive effect of capsaicin topical cream on the proportion of patients reporting pain improvement (odds ratio 2·64, 95 per cent c.i. 1·02 to 6·86; I 2 = 0 per cent) (Fig. S2, supporting information), although caution is warranted owing to risk of bias and, as a previous Cochrane review107 advised, the total number of events was too few to be reliable. In both trials, a commonly reported side‐effect was local skin reaction.
Epidural injections and associated interventions
Eleven trials including data from 886 participants evaluated epidural injections and associated interventions after spinal surgery54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64. Risk of bias was evident in ten studies relating to allocation concealment54, 55, 56, 64, blinding54, 58, 61, incomplete outcome data54, 57, 61, 63, selective reporting56, 57, 59, 62 and single‐authored article63. Two trials55, 56 comparing epidural injections with, and without steroids found no difference in pain relief between groups. The addition of steroids to 3‐monthly morphine epidural injections was not found to influence pain intensity after 6 months59. A three‐arm trial63 involving 206 patients evaluated epidural injections of 1 mg indomethacin, 2 mg indomethacin and 80 mg methylprednisolone, and found that all treatments resulted in a similar pain reduction. Two trials evaluating epidural injections of steroids (prednisolone acetate) versus saline alone produced contrasting results: one57 noted no benefit at 120 days and the other58 reported a reduction in pain at 18 months after multiple epidural injections. The addition of hyaluronidase to an epidural steroid injection was found to lead to lower pain intensity at 4 weeks62 and 12 months60, and a combination of hyaluronidase and triamcinolone provided more effective pain relief for 12 weeks than either agent alone61. Two trials reported that adding percutaneous adhesiolysis to an epidural injection led to better pain relief at 6 months64 and 12 months54 after treatment.
Local anaesthetics
Eleven trials including data from 324 participants assessed the effectiveness of local anaesthetics in providing pain relief after inguinal hernia repair65, 66, spinal surgery67, 68, 69, amputation70, 71, 72, 73 and breast cancer surgery45, 74. Risk of bias was evident in three studies, and concerned incomplete outcome data73, random sequence generation and blinding45, and early trial termination65. Interventions assessed included lidocaine block65, repeated epidural nerve blocks68, stellate ganglion block45, 74, bupivacaine72, intravenous lidocaine67, 69, 70, ropivacaine73, oral mexiletine71 and lidocaine patch66.
Five trials evaluated local anaesthetic nerve blocks. No difference in pain intensity was found after ultrasound‐guided lidocaine nerve block compared with placebo block65 or after repeated epidural sympathetic nerve block compared with saline blocks68. A trial45 of stellate ganglion blocks for pain after breast surgery found that they were inferior to gabapentin; another trial74 noted that pain relief at 8 weeks was improved with ultrasound guidance compared with unguided blocks. One trial72 found that injections of bupivacaine into contralateral painful muscle sites that mirror phantom limb pains were more effective at providing pain relief than placebo saline injections.
Five trials evaluated systemic administration of intravenous local anaesthetic or oral mexiletine. Two trials67, 69 found that intravenous lidocaine did not reduce pain intensity compared with saline, and one70 reported that it reduced stump pain, but not phantom limb pain. A pilot trial73 of three patients reported that ropivacaine reduced phantom limb pain after 12 weeks, although no statistical tests were performed on this small patient sample. An 8‐week course of oral mexiletine was found to have no effect on pain intensity compared with placebo in a trial with 60 patients71.
A single trial66 of 21 patients evaluated lidocaine patches (5 per cent); applied for 2 weeks, they were found to produce similar results to placebo patches.
Neurotoxins
Three trials including data from 91 participants evaluated botulinum toxin A injections for chronic pain after knee replacement75, neck dissection76 and lower limb amputation77. One study77 had evidence of bias relating to incomplete outcome data. In patients with knee replacement treated with botulinum toxin A, pain intensity was reduced compared with that in the placebo group after 2 and 7 months, with no increase in adverse events75. In a dose‐finding study76 involving patients with chronic pain after neck dissection, a lower dose of botulinum A toxin was associated with reduced pain intensity. There was little evidence that botulinum A was more effective than lidocaine/Depo‐Medrol® (Pharmacia & Upjohn, New York, New York, USA) injection after 6 months in a pilot trial of patients with phantom limb pain77.
N‐methyl‐d‐aspartate receptor antagonists
Seven trials, including data from 122 participants, evaluated N‐methyl‐d‐aspartate (NMDA) receptor antagonists. One study78 had evidence of risk of bias relating to blinding and incomplete outcome data. No differences in pain relief after 3–5 weeks of memantine compared with placebo were found in four trials79, 80, 81, 82 involving patients with pain after amputation. Three studies, involving 11–20 patients each, evaluated ketamine alone or in conjunction with calcitonin with placebo for patients with phantom limb pain78, 83 or pain after diverse procedures69. All trials provided evidence that the intervention reduced pain intensity, although follow‐up was short (80 min to 48 h).
Opioids
Six trials including data from 297 participants evaluated opioids for chronic pain after amputation40, 70, 71, 84, breast surgery85 and spinal surgery59. Opioids evaluated included tramadol40, oral morphine71, 84, 85, morphine infusion70 and epidural morphine59. In three trials, risk of bias was noted relating to blinding84, incomplete outcome data40 and selective reporting59. Compared with placebo, oral morphine was found to provide better pain relief at 4–6 weeks70, 71, 84, although a common side‐effect was constipation. Trials evaluating 4 weeks of tramadol compared with placebo40, 6 weeks of morphine compared with gabapentin with, or without NSAIDs85, and 3‐monthly injections of epidural morphine and steroids compared with steroids alone59, found no differences in pain intensity between treatment groups.
Other pharmacological interventions
One trial78, with risk of bias relating to blinding and incomplete outcome data, evaluated intravenous calcitonin for phantom limb pain in 20 patients, and found no effect up to 48 h after infusion compared with saline. Another trial86, with no clear evidence of risk of bias, evaluated low doses of oral/or intravenous naloxone as a supplement in 12 patients whose severe CPSP was already managed by continuous intrathecal morphine administration. No evidence of an effect on pain relief was found after two 3‐week sessions on differing doses of the drug across a 9‐week period
Physical, surgical, psychological and other interventions
Acupuncture/dry needling
No evidence of differences in pain relief was found in a trial of 20 patients comparing dry needling and physiotherapy with physiotherapy alone for pain after shoulder surgery87. Another trial88 involving 70 patients with chronic pain after neck dissection reported that acupuncture resulted in better pain relief than usual care after 42 days. Neither participants nor assessors were blinded and this may have introduced bias.
Exercise
Four trials89, 90, 91, 92 involving 323 participants evaluated exercise interventions, often as a component of a broader package of care. Two studies were at risk of bias owing to lack of blinding90, 92. No evidence of differences in pain relief was found in trials comparing 3 months of exercise with, and without hyperextension exercises after lumbar surgery89, and 3 weeks of exercise combined with a cognitive intervention compared with lumbar fusion after disc herniation surgery90. A 4‐week training programme of progressive muscle relaxation, mental imagery and phantom exercises was found to be more effective at relieving phantom limb pain than a general exercise programme91. A trial92 of treatment of pain after laminectomy found that 8 weeks of low‐tech exercises (McKenzie‐type and spinal stabilization training exercise) or high‐tech exercises (cardiovascular, isotonic and isokinetic exercises) resulted in a reduction in pain‐related disability compared with no treatment.
Limb cover/lining
One trial93 with 57 patients reported that non‐invasive limb covering for 12 weeks compared with sham limb covering did not reduce phantom limb pain. Another trial94 involving 30 patients, which was at risk of bias owing to incomplete outcome data, found evidence that a stump liner worn by amputees for 2 weeks reduced pain compared with a placebo liner.
Spinal cord stimulation
Five trials including 260 participants assessed the impact of spinal cord stimulation (SCS) on chronic pain after spinal surgery. Two studies95, 96 had a risk of bias related to blinding, and another97 owing to blinding and commercial interests. Two trials found that patients who received SCS for 6 months reported better pain relief than those who had conventional management (100 patients)97 or reoperation (60)95. Subcutaneous stimulation as adjunct therapy to SCS was noted to provide better relief of back pain, but not leg pain, compared with sham treatment in a trial of 20 participants96. Burst SCS was found to be more effective at providing pain relief after 1 week than tonic or placebo SCS98. In a trial involving 15 patients99, there was no difference in pain after 2 weeks on stimulation with 1000‐ versus 500‐Hz bursts.
Surgery
No evidence of differences in pain relief was found when lumbar fusion was compared with exercise for chronic pain after disc herniation surgery in a trial of 60 participants90. In a trial95 involving 60 patients with failed back surgery syndrome, less pain relief after 6 months was reported by patients who had reoperation than was reported by patients who had SCS.
Other interventions
No evidence of differences in pain relief were found in a trial of cutaneous magnetic stimulation for 24 h compared with sham treatment after spinal surgery in 17 patients100. Four weeks of laser therapy compared with placebo laser therapy was found to reduce mastectomy pain at 12 weeks in a trial of 61 participants101. An unblinded trial that included ten patients102, which was at risk of bias, found that 2 weeks of sensory discrimination training led to a reduction in phantom limb pain at 3 months compared with comprehensive psychophysiological assessment. One trial103 with 40 patients, reported that 8 weeks of mindfulness‐based stress reduction following spinal surgery led to better pain relief after 12 weeks compared with that in the waiting list control group. However, the trial was at risk of bias because of lack of blinding and incomplete outcome data. In a three‐arm trial104 that included 22 patients with phantom limb pain, mirror therapy was found to reduce pain intensity after 4 weeks compared with sham mirror therapy and mental visualization. The study was at risk of bias owing to lack of blinding. A trial92 involving patients with pain after laminectomy found that an 8‐week course of joint manipulation did not reduce pain‐related disability compared with no treatment. The same trial also found no difference between a combined package of hot packs, ultrasound treatment and transcutaneous electrical nerve stimulation, and no treatment.
Discussion
The best evidence to guide the implementation of effective interventions comes from their evaluation in high‐quality randomized trials, and ultimately in systematic reviews and meta‐analyses. Given the prevalence and impact of CPSP, it is imperative to establish robust methods for its management. This systematic review aimed to provide a comprehensive evaluation of the evidence base for the management of CPSP. Although some of the interventions identified were procedure‐specific, others had wider applicability to other types of CPSP. However, owing to heterogeneity in the interventions and trial design, pooling of data in meta‐analysis was rarely possible or warranted. Of the 66 included trials, most evaluated pharmacological interventions. For all interventions, there was insufficient evidence to draw conclusions on effectiveness or harm.
There are few systematic reviews in the field of CPSP, with existing reviews focusing on predictors108, characteristics8 and prevention109, 110, 111, 112. The previous reviews that have evaluated treatments have been procedure‐specific, focusing on chronic pain after total knee replacement31 and phantom limb pain30. This contrasts with other areas of pain research in which numerous systematic reviews21, 22, 113, 114, 115, 116 of treatments have been published. Typically, the focus of a review is on a defined condition (such as fibromyalgia, back pain) or a presumed mechanism of chronic pain (for example neuropathic pain). Patients with CPSP are, of course, embedded within broader trials investigating chronic pain, but it has not previously been possible to identify these patients.
This review highlighted some difficulties with conducting a broad systematic review of CPSP. First, there was heterogeneity in the definition of CPSP within research studies; some trials included only patients with neuropathic pain and there was variability across studies in key eligibility criteria, such as duration and severity of pain. Second, one‐third of the studies included in the review evaluated interventions for phantom limb pain. Although previous reviews of CPSP have also included amputation109, 111, the commonality in the aetiology of phantom limb pain and other forms of CPSP could be questioned. However, phantom limb pain was included as the aim of this review was to provide a broad overview of interventions for chronic pain in the surgical context. The identification of interventions that show effectiveness in one well studied surgical model could provide directions for the evaluation of interventions for CPSP in other surgical areas.
It is important to acknowledge the limitations of this review when interpreting the results. Searches yielded a large volume of literature and therefore initial eligibility screening was performed in duplicate for only 10 per cent of the studies; this may have increased the risk of eligible studies being discarded117. However, the final selection of studies was undertaken by two reviewers in accordance with guidance from the Cochrane Handbook38. Given the hidden nature of patients with CPSP included within other trials, relevant studies were often difficult to identify from titles and abstracts, and required investigation of the full text to establish whether or not patients were likely to have CPSP. Although the search terms identified a large volume of literature, search of relevant Cochrane reviews identified three other relevant studies that were not identified in the initial searches. This highlights the difficulty of conducting such a systematic review owing to limited reference to the patient sample by conventional means – there is no medical subject heading (MeSH) for CPSP, so indexers and even study authors did not necessarily use CPSP as a descriptor or keyword. Limits were not placed on the tools used to assess secondary outcomes and the resulting heterogeneity precluded their inclusion in analysis. Adverse events were found to be poorly and inconsistently reported. This has previously been described as a common issue in chronic pain trials30, and poor reporting precluded conclusions about intervention safety in this review. Opportunities for meta‐analysis were limited because of variability in the identified interventions, and conclusions are predominantly based on narrative synthesis. However, this review has produced a comprehensive overview of the evidence for management of CPSP, and the findings have a number of methodological and clinical implications.
Only three trials included patients with CPSP after various surgical procedures; the remainder focused on one surgery type. Of these, the majority of trials were conducted to evaluate treatments for phantom limb pain and failed back surgery syndrome, which is likely to reflect the historical recognition of these pain conditions118, 119. Although an encouraging temporal increase in the number of trials conducted was identified, the paucity of research into the management of CPSP, particularly after operations other than amputation and spinal surgery, highlights the need for further research. The majority of trials in this review evaluated pharmacological interventions, reflecting the commonplace role of these therapies in the management of chronic pain. There was insufficient evidence to evaluate the effectiveness of any treatment modality in reducing CPSP. It has previously been proposed that commonly prescribed pharmacological treatments are insufficient to treat chronic non‐cancer pain when used in isolation120. Given the complex and multifactorial nature of CPSP, an individualized and multimodal model of care may be required, as recommended more widely for chronic non‐cancer pain120.
Similar to a previous review of interventions for phantom limb pain30, the present analysis identified the need for more methodological rigour in the reporting and conduct of randomized trials in this field. This need is highlighted by the unclear or high risk of bias rating assigned to many aspects of the included trials. Frequently encountered issues included lack of transparency, as shown by lack of preregistration of trials or publication of trial protocols, failure to report conduct/results according to CONSORT standards121, 122, 123, and limited and variable assessment of pain and adverse events. IMMPACT recommendations36 suggest the use of a comprehensive approach to pain assessment in clinical trials addressing chronic pain. Many of the trials included in this review were conducted before publication of the IMMPACT recommendations in 2003. However, the trials conducted and published after the IMMPACT guidance generally limited their outcome assessment to pain intensity. Inconsistent reporting of the secondary outcomes of interest precluded their analysis, highlighting the need for standardization of outcomes assessment. For many included trials, sample sizes were small and duration of follow‐up was short, limiting the conclusions that can be drawn about the therapeutic benefit of interventions in the context of chronic pain.
For many included trials, threats to both internal and external validity existed. Reports of trials did not always include a sample size calculation. The inclusion criteria did not specify an a priori sample size, owing to the heterogeneity of the definition of CPSP and the range of potential interventions. Such a broad approach allowed this review to meet the intended aim of comprehensiveness, and to identify and present all trials within this complex and evolving field, including those in which events led to early trial termination or lower recruitment than planned. In keeping with recommendations in the Cochrane risk‐of‐bias tool, sample size was not considered to present a risk of bias per se 124, although small studies do not improve the precision of estimates. The relatively small sample sizes in some of the studies that met the inclusion criteria, as well as the high risk of bias among many of the largest trials, impacted on both results and generalizability.
In addition to issues of bias in trial conduct and reporting, the authors were initially keen to report on the quality of the evidence, potentially using GRADE. However, this was not possible because of the inability to estimate effects of treatments: all findings would have been downgraded for quality owing to the absence of evidence for synthesis. However, as this field develops and more trials emerge, it would be expected that new reviews will report effect estimates and examine the quality of the evidence.
This review highlights the need for more evidence about interventions for CPSP, and a focus not on the presumed pathological mechanism or location of pain, but on the relationship of pain to surgery. Many patients experience CPSP and it is imperative that evidence‐based interventions are offered to these individuals to improve postoperative outcome. Trials to date have focused on pharmacological interventions, and no trials have been conducted to evaluate multimodal interventions matched to pain characteristics for the management of CPSP. Given the complexity of pain that extends or emerges after surgery, individualized interventions should be developed and evaluated. High‐quality trials of these interventions are needed to provide a robust evidence base to guide the management of CPSP.
Supporting information.
Additional supporting information may be found online in the supporting information tab for this article:
Appendix S1 Search terms (Word document)
Appendix S2 Risk of bias by intervention type (Word document)
Table S1 Characteristics of included studies evaluating pharmacological interventions (Word document)
Table S2 Characteristics of included studies evaluating physical, surgical, psychological and other interventions (Word document)
Fig. S1 Forest plot showing trials of gabapentin versus placebo for treatment of chronic phantom limb pain (Word document)
Fig. S2 Forest plot for trials of low‐dose capsaicin versus placebo for treatment of chronic postsurgical pain after cancer surgery (Word document)
Supporting information
Appendix S1 Search terms (Word document)
Appendix S2 Risk of bias by intervention type (Word document)
Table S1 Characteristics of included studies evaluating pharmacological interventions (Word document)
Table S2 Characteristics of included studies evaluating physical, surgical, psychological and other interventions (Word document)
Fig. S1 Forest plot showing trials of gabapentin versus placebo for treatment of chronic phantom limb pain (Word document)
Fig. S2 Forest plot for trials of low‐dose capsaicin versus placebo for treatment of chronic postsurgical pain after cancer surgery (Word document)
Acknowledgements
This article summarizes independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research programme (RP‐PG‐0613‐20001). The views expressed in this article are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health.
Disclosure: The authors declare no conflict of interest.
References
- 1. International Association for the Study of Pain. Classification of chronic pain . Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl 1986; 3: S1–S226. [PubMed] [Google Scholar]
- 2. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006; 367: 1618–1625. [DOI] [PubMed] [Google Scholar]
- 3. Brandsborg B, Nikolajsen L, Hansen CT, Kehlet H, Jensen TS. Risk factors for chronic pain after hysterectomy: a nationwide questionnaire and database study. Anesthesiology 2007; 106: 1003–1012. [DOI] [PubMed] [Google Scholar]
- 4. Thomson S. Failed back surgery syndrome – definition, epidemiology and demographics. Br J Pain 2013; 7: 56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Macrae WA. Chronic post‐surgical pain: 10 years on. Br J Anaesth 2008; 101: 77–86. [DOI] [PubMed] [Google Scholar]
- 6. Beswick AD, Wylde V, Gooberman‐Hill R, Blom A, Dieppe P. What proportion of patients report long‐term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012; 2: e000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoofwijk DM, Fiddelers AA, Peters ML, Stessel B, Kessels AG, Joosten EA et al Prevalence and predictive factors of chronic postsurgical pain and poor global recovery 1 year after outpatient surgery. Clin J Pain 2015; 31: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 8. Haroutiunian S, Nikolajsen L, Finnerup NB, Jensen TS. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain 2013; 154: 95–102. [DOI] [PubMed] [Google Scholar]
- 9. Fletcher D, Stamer UM, Pogatzki‐Zahn E, Zaslansky R, Tanase NV, Perruchoud C et al Chronic postsurgical pain in Europe: an observational study. Eur J Anaesthesiol 2015; 32: 725–734. [DOI] [PubMed] [Google Scholar]
- 10. Smith BH, Elliott AM, Chambers WA, Smith WC, Hannaford PC, Penny K. The impact of chronic pain in the community. Fam Pract 2001; 18: 292–299. [DOI] [PubMed] [Google Scholar]
- 11. Leadley RM, Armstrong N, Reid KJ, Allen A, Misso KV, Kleijnen J. Healthy aging in relation to chronic pain and quality of life in Europe. Pain Pract 2014; 14: 547–558. [DOI] [PubMed] [Google Scholar]
- 12. Magni G, Caldieron C, Rigatti‐Luchini S, Merskey H. Chronic musculoskeletal pain and depressive symptoms in the general population. An analysis of the 1st National Health and Nutrition Examination Survey data. Pain 1990; 43: 299–307. [DOI] [PubMed] [Google Scholar]
- 13. Hawton A, Green C, Dickens AP, Richards SH, Taylor RS, Edwards R et al The impact of social isolation on the health status and health‐related quality of life of older people. Qual Life Res 2011; 20: 57–67. [DOI] [PubMed] [Google Scholar]
- 14. Werner MU, Kongsgaard UE. I. Defining persistent post‐surgical pain: is an update required? Br J Anaesth 2014; 113: 1–4. [DOI] [PubMed] [Google Scholar]
- 15. Hinrichs‐Rocker A, Schulz K, Jarvinen I, Lefering R, Simanski C, Neugebauer EA. Psychosocial predictors and correlates for chronic post‐surgical pain (CPSP) – a systematic review. Eur J Pain 2009; 13: 719–730. [DOI] [PubMed] [Google Scholar]
- 16. Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology 2000; 93: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 17. Chaparro LE, Wiffen PJ, Moore RA, Gilron I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev 2012; (7)CD008943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmidt‐Hansen M, Bennett MI, Arnold S, Bromham N, Hilgart JS. Oxycodone for cancer‐related pain. Cochrane Database Syst Rev 2015; (2)CD003870. [DOI] [PubMed] [Google Scholar]
- 19. Wiffen PJ, Derry S, Moore RA, Lunn MPT. Levetiracetam for neuropathic pain in adults. Cochrane Database Syst Rev 2014; (7)CD010943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oosterhuis T, Costa LOP, Maher CG, De Vet HCW, Van Tulder MW, Ostelo RWJG. Rehabilitation after lumbar disc surgery – an updated Cochrane review. Physiotherapy (United Kingdom) 2015; 101: eS1158–eS1159. [Google Scholar]
- 21. Moore RA, Wiffen PJ, Derry S, Toelle T, Rice AS. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev 2014; (4)CD007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McNicol ED, Midbari A, Eisenberg E. Opioids for neuropathic pain. Cochrane Database Syst Rev 2013; (8)CD006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hayden JA, van Tulder MW, Malmivaara A, Koes BW. Exercise therapy for treatment of non‐specific low back pain. Cochrane Database Syst Rev 2005; (3)CD000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Challapalli V, Tremont‐Lukats IW, McNicol ED, Lau J, Carr DB. Systemic administration of local anesthetic agents to relieve neuropathic pain. Cochrane Database Syst Rev 2005; (4)CD003345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Derry S, Moore RA. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2012; (9)CD010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Derry S, Wiffen PJ, Moore RA, Quinlan J. Topical lidocaine for neuropathic pain in adults. Cochrane Database Syst Rev 2014; (7)CD010958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gallagher HC, Gallagher RM, Butler M, Buggy DJ, Henman MC. Venlafaxine for neuropathic pain in adults. Cochrane Database Syst Rev 2015; (8)CD011091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Staal JB, de Bie R, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low‐back pain. Cochrane Database Syst Rev 2008; (3)CD001824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rubinstein SM, van Middelkoop M, Assendelft WJ, de Boer MR, van Tulder MW. Spinal manipulative therapy for chronic low‐back pain. Cochrane Database Syst Rev 2011; (2)CD008112. [DOI] [PubMed] [Google Scholar]
- 30. Alviar MJ, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev 2016; (10)CD006380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beswick AD, Wylde V, Gooberman‐Hill R. Interventions for the prediction and management of chronic postsurgical pain after total knee replacement: systematic review of randomised controlled trials. BMJ Open 2015; 5: e007387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosenthal R, Bucher HC, Dwan K. The use of systematic reviews when designing and reporting surgical trials. Ann Surg 2017; 265: e35–e36. [DOI] [PubMed] [Google Scholar]
- 33. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well‐built clinical question: a key to evidence‐based decisions. ACP J Club 1995; 123: A12–A13. [PubMed] [Google Scholar]
- 35. Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R et al GRADE guidelines: 12. Preparing summary of findings tables – binary outcomes. J Clin Epidemiol 2013; 66: 158–172. [DOI] [PubMed] [Google Scholar]
- 36. Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB et al Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain 2003; 106: 337–345. [DOI] [PubMed] [Google Scholar]
- 37. Cochrane Pain, Palliative and Supportive Care Review Group . PaPaS Author and Referee Guidance: Supplementary Information with Evidence About Pain Studies and Their Outcomes Over the Past Two Decades; 2011. http://papas.cochrane.org/sites/papas.cochrane.org/files/uploads/PaPaSAuthorandRefereeGuidance.pdf [accessed 19 May 2017].
- 38. Higgins J, Green SE. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration ; 2011. www.cochrane-handbook.org [accessed 19 May 2017].
- 39. Robinson LR, Czerniecki JM, Ehde DM, Edwards WT, Judish DA, Goldberg ML et al Trial of amitriptyline for relief of pain in amputees: results of a randomized controlled study. Arch Phys Med Rehabil 2004; 85: 1–6. [DOI] [PubMed] [Google Scholar]
- 40. Wilder‐Smith CH, Hill LT, Laurent S. Postamputation pain and sensory changes in treatment‐naive patients: characteristics and responses to treatment with tramadol, amitriptyline, and placebo. Anesthesiology 2005; 103: 619–628. [DOI] [PubMed] [Google Scholar]
- 41. Tasmuth T, Härtel B, Kalso E. Venlafaxine in neuropathic pain following treatment of breast cancer. Eur J Pain 2002; 6: 17–24. [DOI] [PubMed] [Google Scholar]
- 42. Kalso E, Tasmuth T, Neuvonen Pertti J. Amitriptyline effectively relieves neuropathic pain following treatment of breast cancer. Pain 1995; 64: 293–302. [DOI] [PubMed] [Google Scholar]
- 43. Smith DG, Ehde DM, Hanley MA, Campbell KM, Jensen MP, Hoffman AJ et al Efficacy of gabapentin in treating chronic phantom limb and residual limb pain. J Rehabil Res Dev 2005; 42: 645–654. [DOI] [PubMed] [Google Scholar]
- 44. Bone M, Critchley P, Buggy DJ. Gabapentin in postamputation phantom limb pain: a randomized, double‐blind, placebo‐controlled, cross‐over study. Reg Anesth Pain Med 2002; 27: 481–486. [DOI] [PubMed] [Google Scholar]
- 45. Hoseinzade H, Mahmoodpoor A, Agamohammadi D, Sanaie S. Comparing the effect of stellate ganglion block and gabapentin on the post mastectomy pain syndrome. Rawal Med J 2008; 33: 21–24. [Google Scholar]
- 46. Biyik I, Gülcüler M, Karabiga M, Ergene O, Tayyar N. Efficacy of gabapentin versus diclofenac in the treatment of chest pain and paresthesia in patients with sternotomy. Anadolu Kardiyol Derg 2009; 9: 390–396. [PubMed] [Google Scholar]
- 47. Zencirci B. Analgesic efficacy of oral gabapentin added to standard epidural corticosteroids in patients with failed back surgery. Clin Pharmacol 2010; 2: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Khosravi MB, Azemati S, Sahmeddini MA. Gabapentin versus naproxen in the management of failed back surgery syndrome; a randomized controlled trial. Acta Anaesthesiol Belg 2014; 65: 31–37. [PubMed] [Google Scholar]
- 49. Vilholm OJ, Cold S, Rasmussen L, Sindrup SH. Effect of levetiracetam on the postmastectomy pain syndrome. Eur J Neurol 2008; 15: 851–857. [DOI] [PubMed] [Google Scholar]
- 50. Silverman A, Samuels Q, Gikas H, Nawras A. Pregabalin for the treatment of abdominal adhesion pain: a randomized, double‐blind, placebo‐controlled trial. Am J Ther 2012; 19: 419–428. [DOI] [PubMed] [Google Scholar]
- 51. Bischoff JM, Ringsted TK, Petersen M, Sommer C, Uçeyler N, Werner MU. A capsaicin (8%) patch in the treatment of severe persistent inguinal postherniorrhaphy pain: a randomized, double‐blind, placebo‐controlled trial. PLoS ONE 2014; 9: e109144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Watson CPN, Evans RJ. The postmastectomy pain syndrome and topical capsaicin: a randomized trial. Pain 1992; 51: 375–379. [DOI] [PubMed] [Google Scholar]
- 53. Ellison N, Loprinzi CL, Kugler J, Hatfield AK, Miser A, Sloan JA et al Phase III placebo‐controlled trial of capsaicin cream in the management of surgical neuropathic pain in cancer patients. J Clin Oncol 1997; 15: 2974–2980. [DOI] [PubMed] [Google Scholar]
- 54. Manchikanti L, Singh V, Cash KA, Pampati V, Datta S. A comparative effectiveness evaluation of percutaneous adhesiolysis and epidural steroid injections in managing lumbar post surgery syndrome: a randomized, equivalence controlled trial. Pain Physician 2009; 12: E355–E368. [PubMed] [Google Scholar]
- 55. Manchikanti L, Malla Y, Cash KA, McManus CD, Pampati V. Fluoroscopic cervical interlaminar epidural injections in managing chronic pain of cervical postsurgery syndrome: preliminary results of a randomized, double‐blind, active control trial. Pain Physician 2012; 15: 13–25. [PubMed] [Google Scholar]
- 56. Manchikanti L, Singh V, Cash KA, Pampati V, Datta S. Fluoroscopic caudal epidural injections in managing post lumbar surgery syndrome: two‐year results of a randomized, double‐blind, active‐control trial. Int J Med Sci 2012; 9: 582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Meadeb J, Rozenberg S, Duquesnoy B, Kuntz JL, Le Loët X, Sebert JL et al Forceful sacrococcygeal injections in the treatment of postdiscectomy sciatica. A controlled study versus glucocorticoid injections. Joint Bone Spine 2001; 68: 43–49. [DOI] [PubMed] [Google Scholar]
- 58. Revel M, Auleley GR, Alaoui S, Nguyen M, Duruoz T, Eck‐Michaud S et al Forceful epidural injections for the treatment of lumbosciatic pain with post‐operative lumbar spinal fibrosis. Rev Rhum Engl Ed 1996; 63: 270–277. [PubMed] [Google Scholar]
- 59. Rocco AG, Frank E, Kaul AF, Lipson SJ, Gallo JP. Epidural steroids, epidural morphine and epidural steroids combined with morphine in the treatment of post‐laminectomy syndrome. Pain 1989; 36: 297–303. [DOI] [PubMed] [Google Scholar]
- 60. Yousef AA, EL‐Deen AS, Al‐Deeb AE. The role of adding hyaluronidase to fluoroscopically guided caudal steroid and hypertonic saline injection in patients with failed back surgery syndrome: a prospective, double‐blinded, randomized study. Pain Pract 2010; 10: 548–553. [DOI] [PubMed] [Google Scholar]
- 61. Kim SB, Lee KW, Lee JH, Kim MA, An BW. The effect of hyaluronidase in interlaminar lumbar epidural injection for failed back surgery syndrome. Ann Rehabil Med 2012; 36: 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rahimzadeh P, Sharma V, Imani F, Faiz HR, Ghodraty MR, Nikzad‐Jamnani AR et al Adjuvant hyaluronidase to epidural steroid improves the quality of analgesia in failed back surgery syndrome: a prospective randomized clinical trial. Pain Physician 2014; 17: E75–E82. [PubMed] [Google Scholar]
- 63. Aldrete JA. Epidural injections of indomethacin for postlaminectomy syndrome: a preliminary report. Anesth Analg 2003; 96: 463–468. [DOI] [PubMed] [Google Scholar]
- 64. Chun‐jing H, Hao‐xiong N, jia‐xiang N. The application of percutaneous lysis of epidural adhesions in patients with failed back surgery syndrome. Acta Cir Bras 2012; 27: 357–362. [DOI] [PubMed] [Google Scholar]
- 65. Bischoff JM, Koscielniak‐Nielsen ZJ, Kehlet H, Werner MU. Ultrasound‐guided ilioinguinal/iliohypogastric nerve blocks for persistent inguinal postherniorrhaphy pain: a randomized, double‐blind, placebo‐controlled, crossover trial. Anesth Analg 2012; 114: 1323–1329. [DOI] [PubMed] [Google Scholar]
- 66. Bischoff JM, Petersen M, Uçeyler N, Sommer C, Kehlet H, Werner MU. Lidocaine patch (5%) in treatment of persistent inguinal postherniorrhaphy pain: a randomized, double‐blind, placebo‐controlled, crossover trial. Anesthesiology 2013; 119: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 67. Park CH, Jung SH, Han CG. Effect of intravenous lidocaine on the neuropathic pain of failed back surgery syndrome. Korean J Pain 2012; 25: 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fredman B, Zohar E, Ben Nun M, Iraqi R, Jedeikin R, Gepstein R. The effect of repeated epidural sympathetic nerve block on ‘failed back surgery syndrome’ associated chronic low back pain. J Clin Anesth 1999; 11: 46–51. [DOI] [PubMed] [Google Scholar]
- 69. Kvarnström A, Karlsten R, Quiding H, Emanuelsson BM, Gordh T. The effectiveness of intravenous ketamine and lidocaine on peripheral neuropathic pain. Acta Anaesthesiol Scand 2003; 47: 868–877. [DOI] [PubMed] [Google Scholar]
- 70. Wu CL, Tella P, Staats PS, Vaslav R, Kazim DA, Wesselmann U et al Analgesic effects of intravenous lidocaine and morphine on postamputation pain: a randomized double‐blind, active placebo‐controlled, crossover trial. Anesthesiology 2002; 96: 841–848. [DOI] [PubMed] [Google Scholar]
- 71. Wu CL, Agarwal S, Tella PK, Klick B, Clark MR, Haythornthwaite JA et al Morphine versus mexiletine for treatment of postamputation pain: a randomized, placebo‐controlled, crossover trial. Anesthesiology 2008; 109: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Casale R, Ceccherelli F, Labeeb AA, Biella GE. Phantom limb pain relief by contralateral myofascial injection with local anaesthetic in a placebo‐controlled study: preliminary results. J Rehabil Med 2009; 41: 418–422. [DOI] [PubMed] [Google Scholar]
- 73. Ilfeld BM, Moeller‐Bertram T, Hanling SR, Tokarz K, Mariano ER, Loland VJ et al Treating intractable phantom limb pain with ambulatory continuous peripheral nerve blocks: a pilot study. Pain Med 2013; 14: 935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu CJ, Cai HF, Liu DC, Liu YQ, Sun ZG, Li N et al [Efficacy of ultrasound‐stellate ganglion block in breast cancer with postoperative neuropathic pain.] Chin J Contemp Neurol Neurosurg 2013; 13: 872–875. [Google Scholar]
- 75. Singh JA, Mahowald ML, Noorbaloochi S. Intraarticular botulinum toxin A for refractory painful total knee arthroplasty: a randomized controlled trial. J Rheumatol 2010; 37: 2377–2386 [Erratum in: J Rheumatol 2011; 38: 1534]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wittekindt C, Liu WC, Preuss SF, Guntinas‐Lichius O. Botulinum toxin A for neuropathic pain after neck dissection: a dose‐finding study. Laryngoscope 2006; 116: 1168–1171. [DOI] [PubMed] [Google Scholar]
- 77. Wu H, Sultana R, Taylor KB, Szabo A. A prospective randomized double‐blinded pilot study to examine the effect of botulinum toxin type A injection versus Lidocaine/Depomedrol injection on residual and phantom limb pain: initial report. Clin J Pain 2012; 28: 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Eichenberger U, Neff F, Sveticic G, Björgo S, Petersen‐Felix S, Arendt‐Nielsen L et al Chronic phantom limb pain: the effects of calcitonin, ketamine, and their combination on pain and sensory thresholds. Anesth Analg 2008; 106: 1265–1273. [DOI] [PubMed] [Google Scholar]
- 79. Maier C, Dertwinkel R, Mansourian N, Hosbach I, Schwenkreis P, Senne I et al Efficacy of the NMDA‐receptor antagonist memantine in patients with chronic phantom limb pain – results of a randomized double‐blinded, placebo‐controlled trial. Pain 2003; 103: 277–283. [DOI] [PubMed] [Google Scholar]
- 80. Wiech K, Kiefer RT, Töpfner S, Preissl H, Braun C, Unertl K et al A placebo‐controlled randomized crossover trial of the N‐methyl‐D‐aspartic acid receptor antagonist, memantine, in patients with chronic phantom limb pain. Anesth Analg 2004; 98: 408–413, table of contents. [DOI] [PubMed] [Google Scholar]
- 81. Schwenkreis P, Maier C, Pleger B, Mansourian N, Dertwinkel R, Malin JP et al NMDA‐mediated mechanisms in cortical excitability changes after limb amputation. Acta Neurol Scand 2003; 108: 179–184. [DOI] [PubMed] [Google Scholar]
- 82. Nikolajsen L, Gottrup H, Kristensen AG, Jensen TS. Memantine (a N‐methyl‐d‐aspartate receptor antagonist) in the treatment of neuropathic pain after amputation or surgery: a randomized, double‐blinded, cross‐over study. Anesth Analg 2000; 91: 960–966. [DOI] [PubMed] [Google Scholar]
- 83. Nikolajsen L, Hansen CL, Nielsen J, Keller J, Arendt‐Nielsen L, Jensen TS. The effect of ketamine on phantom pain: a central neuropathic disorder maintained by peripheral input. Pain 1996; 67: 69–77. [DOI] [PubMed] [Google Scholar]
- 84. Huse E, Larbig W, Flor H, Birbaumer N. The effect of opioids on phantom limb pain and cortical reorganization. Pain 2001; 90: 47–55. [DOI] [PubMed] [Google Scholar]
- 85. Patarica‐Huber E, Boskov N, Pjevic M. Multimodal approach to therapy‐related neuropathic pain in breast cancer. J BUON 2011; 16: 40–45. [PubMed] [Google Scholar]
- 86. Block L, Lundborg C, Bjersing J, Dahm P, Hansson E, Biber B. Ultralow dose of naloxone as an adjuvant to intrathecal morphine infusion improves perceived quality of sleep but fails to alter persistent pain: a randomized, double‐blind, controlled study. Clin J Pain 2015; 31: 968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Arias‐Buría JL, Valero‐Alcaide R, Cleland JA, Salom‐Moreno J, Ortega‐Santiago R, Atín‐Arratibel MA et al Inclusion of trigger point dry needling in a multimodal physical therapy program for postoperative shoulder pain: a randomized clinical trial. J Manipulative Physiol Ther 2015; 38: 179–187. [DOI] [PubMed] [Google Scholar]
- 88. Pfister DG, Cassileth BR, Deng GE, Yeung KS, Lee JS, Garrity D et al Acupuncture for pain and dysfunction after neck dissection: results of a randomized controlled trial. J Clin Oncol 2010; 28: 2565–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Manniche C, Asmussen K, Lauritsen B, Vinterberg H, Karbo H, Abildstrup S et al Intensive dynamic back exercises with or without hyperextension in chronic back pain after surgery for lumbar disc protrusion. A clinical trial. Spine (Phila Pa 1976) 1993; 18: 560–567. [DOI] [PubMed] [Google Scholar]
- 90. Brox JI, Reikerås O, Nygaard Ø, Sørensen R, Indahl A, Holm I et al Lumbar instrumented fusion compared with cognitive intervention and exercises in patients with chronic back pain after previous surgery for disc herniation: a prospective randomized controlled study. Pain 2006; 122: 145–155. [DOI] [PubMed] [Google Scholar]
- 91. Brunelli S, Morone G, Iosa M, Ciotti C, De Giorgi R, Foti C et al Efficacy of progressive muscle relaxation, mental imagery, and phantom exercise training on phantom limb: a randomized controlled trial. Arch Phys Med Rehabil 2015; 96: 181–187. [DOI] [PubMed] [Google Scholar]
- 92. Timm KE. A randomized‐control study of active and passive treatments for chronic low back pain following L5 laminectomy. J Orthop Sports Phys Ther 1994; 20: 276–286. [DOI] [PubMed] [Google Scholar]
- 93. Hsiao AF, York R, Hsiao I, Hansen E, Hays RD, Ives J et al A randomized controlled study to evaluate the efficacy of noninvasive limb cover for chronic phantom limb pain among veteran amputees. Arch Phys Med Rehabil 2012; 93: 617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kern U, Altkemper B, Kohl M. Management of phantom pain with a textile, electromagnetically‐acting stump liner: a randomized, double‐blind, crossover study. J Pain Symptom Manage 2006; 32: 352–360. [DOI] [PubMed] [Google Scholar]
- 95. North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery 2005; 56: 98–106. [DOI] [PubMed] [Google Scholar]
- 96. Van Gorp EJ, Teernstra OPM, Gültuna I, Hamm‐Faber T, Bürger K, Schapendonk R et al Subcutaneous stimulation as ADD‐ON therapy to spinal cord stimulation is effective in treating low back pain in patients with failed back surgery syndrome: a multicenter randomized controlled trial. Neuromodulation 2016; 19: 171–178. [DOI] [PubMed] [Google Scholar]
- 97. Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J et al Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007; 132: 179–188. [DOI] [PubMed] [Google Scholar]
- 98. Schu S, Slotty PJ, Bara G, Knop M, Edgar D, Vesper J. A prospective, randomised, double‐blind, placebo‐controlled study to examine the effectiveness of burst spinal cord stimulation patterns for the treatment of failed back surgery syndrome. Neuromodulation 2014; 17: 443–450. [DOI] [PubMed] [Google Scholar]
- 99. Van Havenbergh T, Vancamp T, Van Looy P, Vanneste S, De Ridder D. Spinal cord stimulation for the treatment of chronic back pain patients: 500‐Hz vs. 1000‐Hz burst stimulation. Neuromodulation 2015; 18: 9–12. [DOI] [PubMed] [Google Scholar]
- 100. Weintraub MI, Steinberg RB, Cole S. The role of cutaneous magnetic stimulation in failed back syndrome. Semin Integr Med 2005; 3: 101–103. [Google Scholar]
- 101. Ebid AA, El‐Sodany AM. Long‐term effect of pulsed high‐intensity laser therapy in the treatment of post‐mastectomy pain syndrome: a double blind, placebo‐control, randomized study. Lasers Med Sci 2015; 30: 1747–1755. [DOI] [PubMed] [Google Scholar]
- 102. Flor H, Denke C, Schaefer M, Grüsser S. Effect of sensory discrimination training on cortical reorganisation and phantom limb pain. Lancet 2001; 357: 1763–1764. [DOI] [PubMed] [Google Scholar]
- 103. Esmer G, Blum J, Rulf J, Pier J. Mindfulness‐based stress reduction for failed back surgery syndrome: a randomized controlled trial. J Am Osteopath Assoc 2010; 110: 646–652. [PubMed] [Google Scholar]
- 104. Chan BL, Witt R, Charrow AP, Magee A, Howard R, Pasquina PF et al Mirror therapy for phantom limb pain. N Engl J Med 2007; 357: 2206–2207. [DOI] [PubMed] [Google Scholar]
- 105. Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT et al Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008; 9: 105–121. [DOI] [PubMed] [Google Scholar]
- 106. Moore A, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S et al ‘Evidence’ in chronic pain – establishing best practice in the reporting of systematic reviews. Pain 2010; 150: 386–389. [DOI] [PubMed] [Google Scholar]
- 107. Derry S, Lloyd R, Moore RA, McQuay HJ. Topical capsaicin for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2009; (4)CD007393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Masselin‐Dubois A, Attal N, Fletcher D, Jayr C, Albi A, Fermanian J et al Are psychological predictors of chronic postsurgical pain dependent on the surgical model? A comparison of total knee arthroplasty and breast surgery for cancer. J Pain 2013; 14: 854–864. [DOI] [PubMed] [Google Scholar]
- 109. Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev 2013; (7)CD008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Humble SR, Dalton AJ, Li L. A systematic review of therapeutic interventions to reduce acute and chronic post‐surgical pain after amputation, thoracotomy or mastectomy. Eur J Pain 2015; 19: 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. McNicol ED, Schumann R, Haroutounian S. A systematic review and meta‐analysis of ketamine for the prevention of persistent post‐surgical pain. Acta Anaesthesiol Scand 2014; 58: 1199–1213. [DOI] [PubMed] [Google Scholar]
- 112. Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta‐analysis. Anesth Analg 2012; 115: 428–442. [DOI] [PubMed] [Google Scholar]
- 113. Moore RA, Chi C‐C, Wiffen PJ, Derry S, Rice ASC. Oral nonsteroidal anti‐inflammatory drugs for neuropathic pain. Cochrane Database Syst Rev 2015; (10)CD010902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev 2015; (7)CD008242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH et al Pharmacotherapy for neuropathic pain in adults: a systematic review and meta‐analysis. Lancet Neurol 2015; 14: 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wiffen PJ, Derry S, Moore RA, Stannard C, Aldington D, Cole P et al Buprenorphine for neuropathic pain in adults. Cochrane Database Syst Rev 2015; (9)CD011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Edwards P, Clarke M, DiGuiseppi C, Pratap S, Roberts I, Wentz R. Identification of randomized controlled trials in systematic reviews: accuracy and reliability of screening records. Stat Med 2002; 21: 1635–1640. [DOI] [PubMed] [Google Scholar]
- 118. Pool JL. Posterior cordotomy for relief of phantom limb pain. Ann Surg 1946; 124: 386–391. [PubMed] [Google Scholar]
- 119. Blume H, Richardson R, Rojas C. Epidural nerve stimulation of the lower spinal cord and cauda equina for the relief of intractable pain in failed low back surgery. Appl Neurophysiol 1982; 45: 456–460. [DOI] [PubMed] [Google Scholar]
- 120. Turk DC, Wilson HD, Cahana A. Treatment of chronic non‐cancer pain. Lancet 2011; 377: 2226–2235. [DOI] [PubMed] [Google Scholar]
- 121. Schulz KF, Altman DG, Moher D; CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Moher D, Schulz KF, Altman DG; CONSORT Group . The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. Ann Intern Med 2001; 134: 657–662. [DOI] [PubMed] [Google Scholar]
- 123. Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I et al Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996; 276: 637–639. [DOI] [PubMed] [Google Scholar]
- 124. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD et al; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Search terms (Word document)
Appendix S2 Risk of bias by intervention type (Word document)
Table S1 Characteristics of included studies evaluating pharmacological interventions (Word document)
Table S2 Characteristics of included studies evaluating physical, surgical, psychological and other interventions (Word document)
Fig. S1 Forest plot showing trials of gabapentin versus placebo for treatment of chronic phantom limb pain (Word document)
Fig. S2 Forest plot for trials of low‐dose capsaicin versus placebo for treatment of chronic postsurgical pain after cancer surgery (Word document)


