Abstract
Aquporins are intrinsic membrane proteins that function as water channel to transport water and/or mineral nutrients across biological membranes. In this study, we aimed to clarify whether water structure can be changed by the presence of ceramics and whether such a change can be determined by aquaporin. First, we confirmed that ceramics could transform tap water into active tap water by increasing water permeability through aquaporin. We also found that this change in water permeability by treatment with ceramics occurred in distilled water. The distilled water was determined to exhibit the same aquaporin permeability as the original tap water. Our data indicate that the aquaporin permeability of water can be changed by severe physical shocks, such as slapping and sonication, which is consistent with the implication that the aquaporin permeability is closely related to the structure of the water. In this study, using aquaporins, we first reported that the treatment of water with ceramics can affect the structure of water, and the water can retain the structure for a given period under certain condition
Keywords: Aquaporin, Water permeability, Ceramics, Water structure
Highlights
-
•
The ceramics can change the structure of distilled water.
-
•
The aquaporin permeability is closely related to the structure of water.
-
•
Aquaporin permeability of the water is increased with vigorous slapping.
-
•
We first reported that ceramics can affect the structure of water which can be determined by aquaporin.
1. Introduction
Water is a critical component in all living cells. Drinking water greatly affects human health [1]. Increasingly, science is providing evidence linking the human health with water quality [2]. For example, certain mineral waters could enhance immune activity in humans and anti-cancer immunity in mice by increasing the activity of natural killer cells [3]. Because natural mineral water contains various solutes, it is believed that mineral water is likely to be good for human health. Conversely, water treated with special ceramics and stone is anecdotally reported to be good for human health and good for the growth for animals and plants, even when it is confirmed that minerals do not elute into the water from the ceramics or stone [4]. If this finding is observed, it is considered that the water molecules themselves have changed. The structure of water has not been sufficiently demonstrated scientifically to date. Furthermore, the consideration that the water structure affects human health or the growth of animals and plants has not been scientifically accepted to date. In the present study, we measured water permeability of ceramic-treated water through human aquaporins and attempt to clarify whether the structure of water is changed by exposure to ceramics. Aquaporin was first discovered as water channel in human blood cells [5]. Aquaporins are present in the plasma membrane of the cells and have a narrow hole of approximately 3 Å which contributes directly to water entrance [6], [7] and exit to maintain cellular water balance [8], [9]. We have already demonstrated that aquaporin permeability differs depending on the type of water [10]. The factors affecting the gating behavior may include phosphorylation, pH, Ca2+, pressure, solute gradients, temperature and nutritional conditions [11]. If these factors can be excluded completely, the difference in aquaporin water permeability depends on the nature of the water molecule itself.
2. Materials and methods
2.1. Water samples and treatments
Distilled water was prepared from the tap water of Hita city. The ceramics, referred to as “Tadanoumi ceramics®”, were produced by Cosmic Co. Ltd., Hiroshima, Mihara, 723-0015, Japan. This solid ceramic was made by melting and mixing the iron and clay. We use another sample consisting of the clay only and yet another consisting of the iron and clay without melting. Fifty milliliters of tap water and distilled water were treated with 4 g of ceramic for 24 h at room temperature. As the controls, the water samples were treated with microwaves (Toshiba ER-501S) for 3–5 min at high power and treated with sonication (Branson 2510) for 3–5 min at normal power at room temperature. Degassing was carried out for 3 h using a vacuum pump. Heating at 100 °C was performed in a boiled water bath for 10 min. The flower Begonia was used for the long shelf life test: the stem of the flower was cut and placed in a glass bottle containing tap water or ceramic-treated tap water.
2.2. In vitro transcription of aquaporin genes, microinjection of Xenopus oocytes and measurement of water permeability
The human aquaporin genes (AQP1, AQP2, AQP3 and AQP5) inserted in the pXβG-ev1 vector were furnished by Dr. Ishibashi, K., Dr. Yasui, M., and Dr. Sasaki, S. The capped complementary RNA (cRNA) was synthesized using T3 RNA polymerase of the mMESSAG EmMACHINE High Yield Capped RNA Transcription Kit (Cat no.: AM1348, Ambion, USA) after linearization of the aquaporin pXβG-ev1 constructs [10]. The synthesized RNA samples were purified, and the concentrations were measured. Oocytes (1.0–1.2 mm in diameter) of stage V–VI were incubated in 0.1% collagenase (Clostridium histolytium, Type II, Sigma) solution [100 mM NaCl, 2 mM KCl, 1 mM MgCl2, and 5 mM Hepes-Tris (pH 7.5)] at 20 °C for 2 h. The exfoliated oocytes were washed with Modified Barth's Saline (MBS) medium [88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 0.4 mM Ca(NO3)2, 0.4 mM CaCl2, 0.8 mM MgSO4, 100 µg ml−1 Na-penicillin, and 100 µg ml−1 Streptomycin, 15 mM Tris–HCl (pH 7.4)]. 50 nl of cRNA (10–50 ng) or 50 nl water was injected into oocytes using a Nanoject injector (Narishige, Tokyo, Japan). The injected oocytes were cultured in 1× MBS (200 m Osmin) prepared by Milli-Q water for 48 h at 20 °C. For determination of water permeability, the injected and cultured oocytes were transferred into water (0 m Osmout), and the oocyte swelling velocity was measured with a digital camera (Shimazu, Kyoto, Japan) and calculated with Motic Images Plus 21S software (Shimazu, Kyoto, Japan) [10]. Osmotic water permeability (Pf) was determined from the initial slope of the time course of V/V0 (d(V/V0)/dt), initial oocyte volume (V0=9×10−4 cm3), initial oocyte surface area (S=0.045 cm2), and molar volume of water (Vw=18 cm3 mol−1) [7]:
2.3. Proliferation test of cultured cells
Normal skin cells (YK-cell) were originally isolated from human skin. Dulbecco's Modified Eagle Medium (DMEM) was purchased from Wako Co. Ltd. (Osaka Japan). DMEM medium containing 10% fetal bovine serum was used as control medium. The ceramic-treated medium was created by treating the control medium with Tadanoumi ceramics® (approximately 1 g for 50 ml medium) for 24 h at room temperature. The medium was used after the filter was sterilized. Human skin cells were grown in the control medium. Full grown cells were trypsinized and used at a concentration of 1.0×105 cells/ml. The cells cultured in control or ceramic-treated medium for 48 h were treated with Cell counting kit-8. The number of viable cells is determined by measuring the absorbance at 450 nm of water soluble formazan, which is produced by intracellular dehydrogenase.
3. Results
3.1. Effect of the ceramic-treated water on shelf life of begonia
The Begonia flowers were grown in the tap water or the ceramic-treated tap water, and their flower growing status was compared (Supplemental figure). The Begonia flowers in tap water withered after 7 days, but the Begonia flowers in the ceramic-treated water maintained their original shape after 7 days.
3.2. Influence of the ceramic-treated water on the growth of culture cells
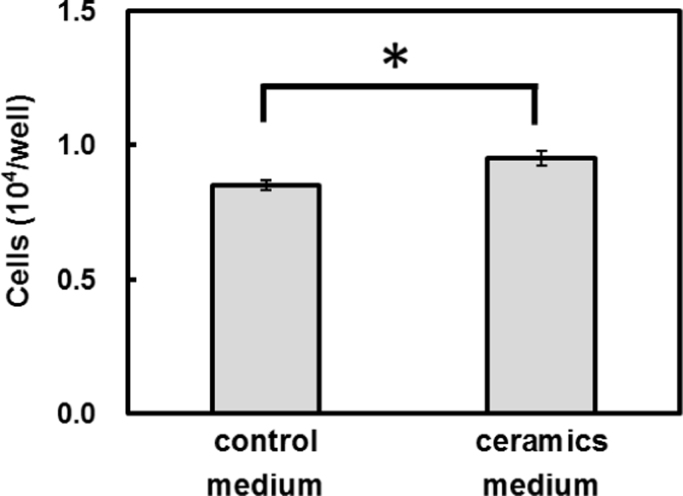
Because the ceramic-treated water has different physical and chemical features, these differences may affect culture cell activity. In this study, we investigated the effect of the ceramic-treated water on the human skin culture cell activity.The results of human skin cell activity assay show that proliferation of the cells culture in the ceramic-treated culture medium was 12% higher than the cells culture in the control medium (Fig. 1), suggesting that the use of ceramic-treated water in the medium promoted the proliferation of cells.
Fig. 1.
Proliferation of the human skin cells in the ceramic-treated culture medium (*P<0.05).
3.3. Effect of ceramics on water permeability of aquaporins
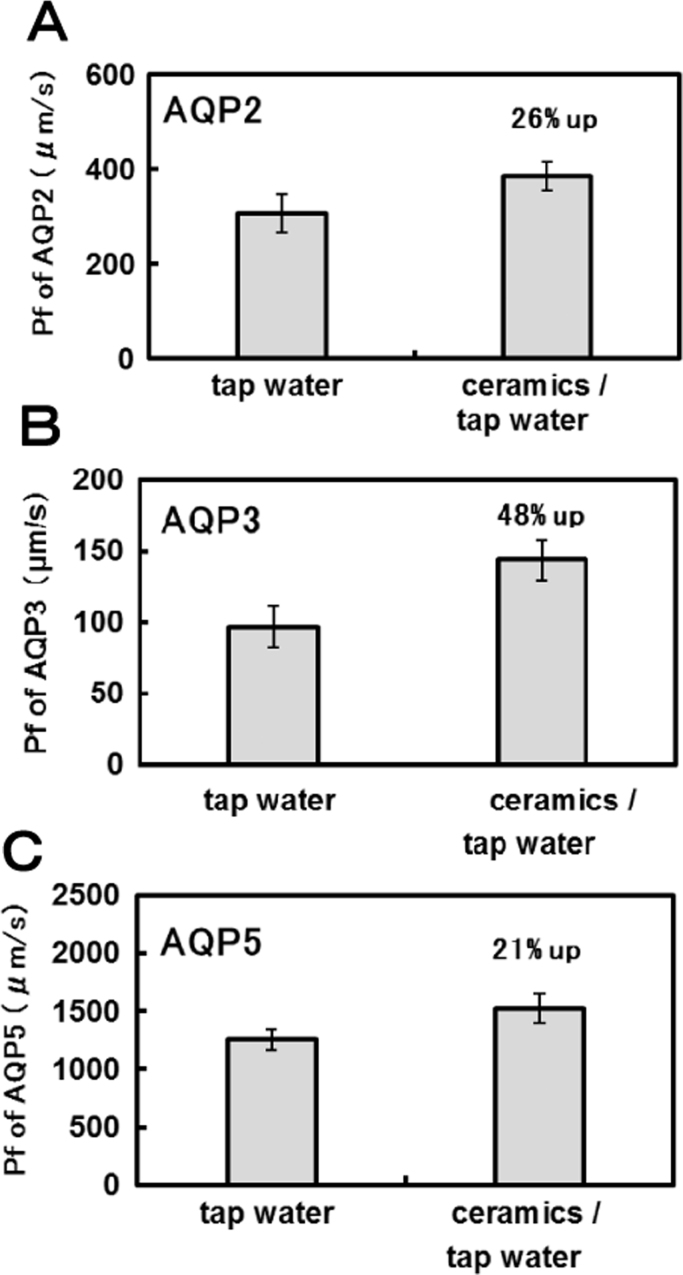
If the nature of water is changed by ceramics, we hypothesize that the modified water molecules can change their permeability through aquaporins. To elucidate how ceramic-treated water affects aquaporin permeability, oocytes expressing human aquaporins (AQP2, AQP3 and AQP5) were transferred directly to the untreated and ceramic-treated tap water. The expansion rates of oocytes expressing different aquaporin in untreated and ceramic-treated tap water samples were recorded, and the water permeability (Pf) was calculated. As shown in Fig. 2, AQP2, AQP3 and AQP5 showed significantly higher permeability in the ceramic-treated tap water than the untreated tap water. The Pf values of the ceramic-treated water through AQP2 increased by 26% compared with the tap water (Pf was 305 μm/s and 386 μm/s for the untreated and ceramic-treated tap water, respectively). Similar to AQP2, the Pf value of ceramics-treated water through AQP3 increased by 26% (Pf was 97 μm/s and 144 μm/s for the untreated and ceramic-treated tap water, respectively), and AQP5 increased by 21% (Pf was 1258 μm/s and 1523 μm/s for the untreated and ceramic-treated tap water, respectively). Statistically, the ceramic-treated water had significantly higher permeability compared to the tap water for AQP2, AQP3 and AQP5 (p<0.05).
Fig. 2.
Aquaporin permeability of the tap water treated with the ceramics. A: AQP2, B: AQP3, C:AQP5.
3.4. Distilled water treated with the ceramics increases its permeability through aquaporins
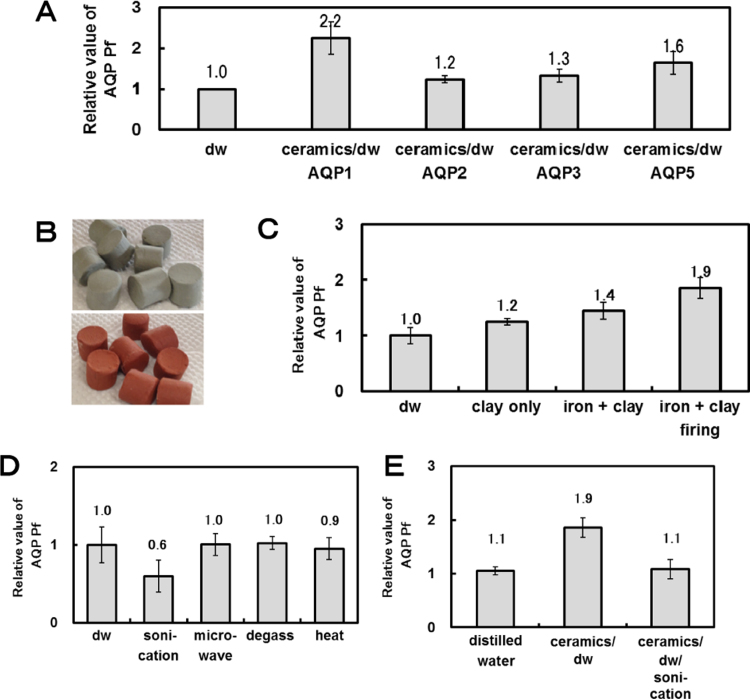
The ceramic-treated water increases the storage stability of the flower and promotes the growth of cultured cells. Such effects are a result of a change in the water within the ceramics. The changed nature of the water can be detected by aquaporin. We also demonstrate aquaporin permeability of distilled water treated with the ceramics because tap water contains many various minerals. We used four types of aquaporin: AQP1, AQP2, AQP3 and AQP5. The results shown in Fig. 3A suggested that AQP1, AQP2, AQP3 and AQP5 showed significantly higher water permeability in the distilled water treated with ceramics compared with the untreated distilled water. The value of Pf in the distilled water treated with ceramics increased by a factor of 2.2 for AQP1, of 1.2 for AQP2, of 1.3 for AQP3, and of 1.6 for AQP5 compared with untreated distilled water (Pf was 1074 μm/s for AQP1, 351 μm/s for AQP2, 467 μm/s for AQP3 and 1714 μm/s for AQP5. From these results, it is speculated that the nature of the water molecules is changed by the ceramics because the distilled water did not contain any minerals that affect the water structure.
Fig. 3.
Aquaporin permeability of the distilled water (dw) treated with the ceramics. A: Relative value of AQP1, 2, 3 and 5 permeability in ceramic treated dw (ceramics/dw). B: The ceramics (bottom) and clay (top). C: Relative value of AQP1 permeability in dw treated with clay only, iron sand and clay, and Tadanoumi ceramics® (iron sand plus clay was fired). D: Relative values of AQP1 permeability in the dw treated with sonicator, microwave, degassing, and heating. E: Relative values of AQP1 permeability in the dw and the dw treated with Tadanoumi ceramics® and the dw treated with Tadanoumi ceramics® and later sonicated.
3.5. How the ceramics affect the water permeability of aquaporin
The ceramics are a registered commodity that are manufactured by Cosmic Co. Ltd. under the name “Tadanoumi ceramics®. Samples of the clay only and of a simple mixture of the iron sands and the clay were prepared and dried. Tadanoumi ceramics® were made by melting the mixture of the iron sands and the clay. Photographs of the clay and the Tadanoumi ceramics® are shown in Fig. 3B. These samples were immersed in distilled water for 24 h. The results of water permeability measurements in these water samples for AQP1 are shown in Fig. 3C. Water permeability of AQP1 was greatest for water treated with the Tadanoumi ceramics® followed by the iron sand and clay, and lowest for the clay. The relative value of Pf for the distilled water treated by the clay, the iron sand and clay, and Tadanoumi ceramics® was 1.2, 1.4, and 1.9 times that of untreated distilled water, respectively (Pf was 660, 766, and 984 μm/s). The iron sand and clay mixture was found to have a higher activity in aquaporin water permeability after melting.
3.6. Aquaporin permeability with water samples from different locations
We studied whether aquaporin permeability is different in tap water compared to water distilled from tap water. Water permeability of AQP1 in the distilled water was the same as in the original tap water (Data not shown). The distilled water showed different values of water permeability (Pf) depending on the original tap water (Data not shown). The aquaporin permeability of tap water underlying the production of distilled water differed by locality. For example, tap water from Tokyo and Osaka had different aquaporin permeability (the Pf of Tokyo 1, Tokyo2, Osaka1 and Osaka2 was 156, 719, 511 and 723 μm/s, respectively). The aquaporin permeability of the distilled water is presumed to be affected by the location and procedure by which the original water was produced. These results suggest that aquaporin water permeability is a property of the water molecules themselves and not of minerals contained in the water.
3.7. Decrease in aquaporin permeability of the water treated with sonication
If aquaporin permeability is a property of water molecules themselves, it is considered that when physical force is applied to the water, aquaporin permeability of the water will be changed. As shown in Fig. 3D, the water permeability of AQP1 in distilled water was reduced by 40% by sonication; however, it was not changed by microwave treatment, degassing action or heating. Similar results even when using water treated with the ceramics, were obtained, as shown in Fig. 3E. Aquaporin permeability of distilled water treated with the ceramics increased by a factor of 1.9 compared to untreated distilled water. (Pf was 529 μm/s for distilled water and 984 μm/s for the ceramic-treated distilled water.) The increase in aquaporin permeability resulting from ceramic treatment was reduced after treatment with a sonicator (574 μm/s for the sonicated ceramic-treated distilled water).
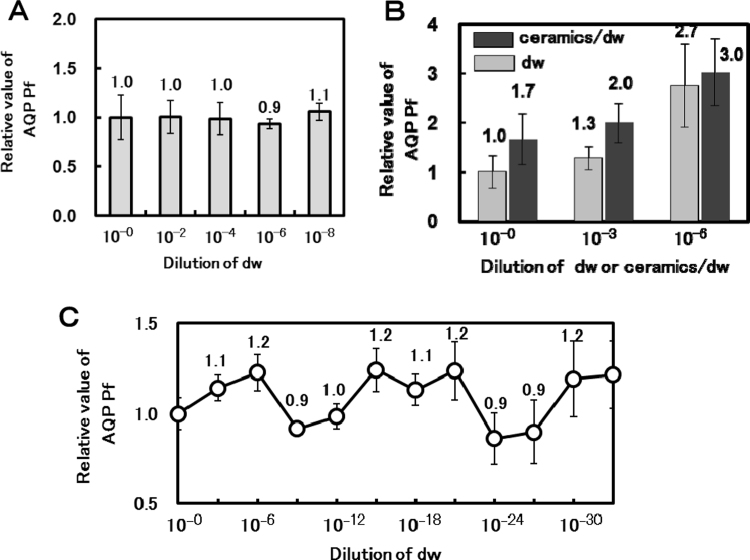
3.8. Aquaporin permeability of the water is changed by dilution
A change in the nature of the water following treatment with ceramics was detected by aquaporin. This change in nature is a change in the water molecule itself, not the properties of the minerals, because the nature of distilled water is changed by the treatment. This nature can be retained for more than one week; however, it is reversed by sonication. We also determined whether this nature is reversed by dilution. First, 10 μl distilled water was taken from the first tube containing the distilled water, it was later added into the next tube containing 10 ml distilled water and gently mixed. The first water sample was diluted by 10−2. These operations were repeated four times. The water permeability AQP1 in the diluted distilled water is shown in Fig. 4A. Aquaporin permeability of the distilled water diluted by 10−8 was same as the distilled water in the first tube. Next, the distilled water was mixed vigorously by slapping the tube on a desk at the stage of each dilution. As shown in Fig. 4B, aquaporin permeability of the vigorously slapped distilled water was increased by a factor of 2.7 compared to the original distilled water at 10−6 dilution. Similarly, the ceramic-treated distilled water increased by a factor of 3.0. The aquaporin permeability of the distilled water with a higher dilution and slapping is shown in Fig. 4C. The aquaporin permeability of the distilled water repeatedly shifted up and down in each step of dilution and slapping. In this experiment, Pf rose at 10−6, 10−18 and 10−30, and Pf decreased at 10−12 and 10−24. The up and down phenomenon of aquaporin permeability of the diluted water and the ceramic-treated distilled water is not specific to AQP1, and the experiments using AQP2 also observed this effect (data not shown).
Fig. 4.
Change of aquaporin permeability of the dw by dilution and slapping. A: Relative value of AQP1 permeability in dw diluted with the same dw. B: Change in the relative value of AQP1 permeability in the dw or Tadanoumi ceramics® treated dw by dilution and slapping operation. C: Change of relative value of AQP1 permeability in dw that is highly diluted and slapped.
4. Discussion
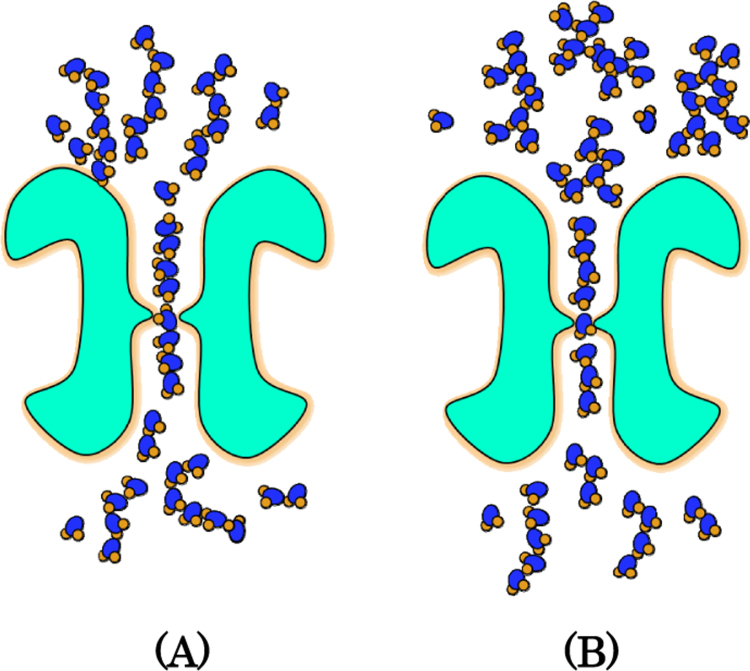
In a previous paper, we reported that aquaporins of humans and plants demonstrated different water permeability in different types of water [10]. In our present study, we demonstrated increased water permeability of the ceramic-treated distilled water through aquaporin. This observation suggests that the water itself is changed by the ceramics because we observed an increased Pf value in the distilled water treated by the ceramics. Distilled water was prepared from tap water. We should understand that the distilled water exhibits different water permeability, depending on the origin of the tap water. The water permeability of distilled water does not change after treatment by heating or microwaves, but it was decreased by sonication. From these results, the water is presumed to have a certain structure. The aquaporin permeability of the distilled water did not change when the water was gently diluted with the same distilled water. However, the permeability was changed by the slapping operation, and the value of permeability varied greatly in each dilution step, indicating that the unique aquaporin permeability of the water is changed by severe physical shocks, such as slapping and sonication. From these results, it is presumed that the water has a certain structure and that this structure is changed by physical force. Aquaporin has a hole capable of allowing water molecules to pass through only one molecule at a time. Whether it is easy to pass through the aquaporin is closely related to the structure of the water. When the water passes through the pores of aquaporin, single-file water structure with 4–8 water molecules has been estimated by the simulation [12], [13]. We are thinking from the results of high aquaporin permeability of the water treated with the ceramic that the water treated with ceramics is easy to form single-file water structure. It is considered that electrostatic state by contact with the ceramic contributes to form it. This image is shown in Fig. 5. Our data showed that the water molecules can memorize the structure under certain condition. Benveniste insisted that the water is able to memorize the structure and function of a substance, such as an antibody [14]. The author showed experimentally that the highly diluted antibody solution, diluted to 10−30, retains the reactivity. In this case, antibody solution was slapped in each dilution steps. This approach is a technique to create “Remedy” according to homeopathic theory. This author's hypothesis was denied because the reproducibility of the experiments was not obtained publicly. In this report, we showed that water structure can be detected by aquaporin. This technology will be useful for studying the challenges facing water memory.
Fig. 5.
Speculation of the structure of the water passing through the aquaporin. A: The water structure which is easy to make single-file water. B: The water structure which is difficult to create a single file water.
Acknowledgments
We are thankful to Dr. Sasaki, S. at Tokyou Medical and Dental University, Dr. Yasui, M. at Keio University and Dr. Ishibashi, K. at Meiji Pharmaceutical University for providing human aquaporins in pXβG-ev1.
Footnotes
Transparency document associated with this article can be found in the online version at doi:10.1016/j.bbrep.2016.01.002.
Contributor Information
Tadao Kozumi, Email: tadaokozumi@yahoo.co.jp.
Yoshichika Kitagawa, Email: kitagawa@kitagawainst.com.
Appendix A. Supplementary material
Supplementary material Supplementary Fig 1. Growth of Begonia in the ceramic-treated tap water. Left: ceramic-treated tap water, Right: tap water.
Supplementary material
References
- 1.Petraccia L., Liberati G., Giuseppe Masciullo S., Grassi M., Fraioli A. Water, mineral waters and health. Clin. Nutr. 2006;25:377–385. doi: 10.1016/j.clnu.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Agre P., Kozono D. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett. 2003;555:72–78. doi: 10.1016/s0014-5793(03)01083-4. [DOI] [PubMed] [Google Scholar]
- 3.Wada J., Hou T., Fujii S. Increasing of Immune activity by Kirishima City Makizonochō Seki-Daira mineral water (brand name: Seki-Daira mineral water)-enhancing immune activity in humans and anti-cancer immunity in mice. Food Style. 2008;21:48–52. [Google Scholar]
- 4.Niwa Y., Kawahira K., Matsumoto K., Matsumoto K. Effect of far infrared ray emitting products and stones on human leukocyte functions, lipid peroxidation, the growth of tumor on mice, drug-induced hepatilis rat, and clinical course and serum lipid peroxide levels of rheumatoid arthritis patients. J. Inflamm. 1996;16:425–436. [Google Scholar]
- 5.Preston G.M., Carroll T.P., Guggino W.B., Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 6.Murata K., Mitsuoka K., Hirai T., Walz T., Agre P., Heymann J.B., Engel A., Fujiyoshi Y. Structural determinants of water permeation through aquaporin-1. Nature. 2000;407:599–605. doi: 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- 7.Kozono D., Ding X., Iwasaki I., Meng X., Kamagata Y., Agre P., Kitagawa Y. Functional expression and characterization of an archaeal aquaporin, AqpM from Methanothermobacter marburgensis. J. Biol. Chem. 2003;278:10649–10656. doi: 10.1074/jbc.M212418200. [DOI] [PubMed] [Google Scholar]
- 8.Verkman A.S. Mammalian aquaporins: diverse physiological roles and potential clinical significance. Mol. Med. 2008;10:e13. doi: 10.1017/S1462399408000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maurel C., Verdoucq L., Luu D.-T., Santoni V. Plant aquaporins: membrane channels with multiple integrated functions. Plant Biol. 2008;59:595–624. doi: 10.1146/annurev.arplant.59.032607.092734. [DOI] [PubMed] [Google Scholar]
- 10.Kitagawa Y., Liu C., Ding X. The influence of natural mineral water on aquaporin water permeability and human natural killer cell activity. Biochem. Biophys. Res. Commun. 2010;409:40–45. doi: 10.1016/j.bbrc.2011.04.102. [DOI] [PubMed] [Google Scholar]
- 11.Yukutake Y., Yasui M. Regulation of water permeability through aquaporin-4. Neuroscience. 2010;168:885–891. doi: 10.1016/j.neuroscience.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Cui Y., Bastien D.A. Water transport in human aquaporin-4: molecular dynamics (MD) simulations. Biochem. Biophys. Res. Commun. 2011;412:654–659. doi: 10.1016/j.bbrc.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horner A., Zocher F., Preiner J., Ollinger N., Siligan C., Akimov S.A., Pohl P. The mobility of single-file water molecules is governed by the number of H-bonds they may form with channel-lining residues. Sci. Adv. 2015;1:e1400083. doi: 10.1126/sciadv.1400083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davenas E., Beauvais F., Amara J., Oberbaum M., Robinzon B., MiadonnaiI A., Tedeschi A., Pomeranz B., Fortner P., Belon P., Sainte-laudy J., Poitevin B., Benveniste J. Human basophil degranulation triggered by very dilute antiserum against IgE. Nature. 1988;333:816–818. doi: 10.1038/333816a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material Supplementary Fig 1. Growth of Begonia in the ceramic-treated tap water. Left: ceramic-treated tap water, Right: tap water.
Supplementary material