Abstract
The burden of disability, premature death, escalating health care costs and lost economic productivity due to obesity and its associated complications including hypertension, stroke, cardiovascular disease and type 2 diabetes is staggering [1,2]. A better understanding of metabolic homeostatic pathways will provide us with insights into the biological mechanisms of obesity and how to fundamentally address this epidemic [3–6]. In mammals, energy balance is maintained via a homeostatic system involving both peripheral and central melanocortin systems; changes in body weight reflect an unbalance of the energetic state [7–9]. Although the primary cause of obesity is unknown, there is significant effort to understand the role of the central melanocortin pathway in the brain as it has been shown that deficiency of proopiomelanocortin (POMC) [10,11] and melanocortin 4 receptors (MC4R) [12–15] in both rodents and humans results in severe hyperphagia and obesity [16–23]. In this review, we will summarize how the central melanocortin pathway helps regulate body mass and adiposity within a ‘healthy’ range through the ‘nutrient sensing’ network [24–28]. This article is part of a Special Issue entitled: Melanocortin Receptors - edited by Ya-Xiong Tao.
Keywords: Central melanocortin pathway, Melanocortin 4 receptors, Metabolism, Proopiomelanocortin neurons, Agouti related peptide neurons
1. Introduction
The central melanocortin pathway consists of neurons that release endogenous melanocortin ligands (ACTH, α-, β-, γ-melanocyte-stimulating hormone (MSH)), five receptors (MC1R, MC2R, MC3R, MC4R and MC5R) [29] and the endogenous melanocortin antagonist/inverse agonist agouti and agouti related peptide (AgRP) [30]. The melanocortin ligands (ACTH and α-, β-, γ-MSH) are derived from POMC, which are produced in POMC neurons [31]. In the central nervous system, POMC neurons are located in the arcuate nucleus of the hypothalamus (ARC) and the nucleus of the solitary tract (NTS) of the brain stem, both of which are involved in the regulation of energy balance [32,33]. In response to caloric sufficiency, POMC neurons are activated resulting in decreased food intake, increased energy expenditure and weight loss [34–37]. In contrast, AgRP is the antagonist/inverse agonist for MC3R and MC4R [38]. AgRP is released by AgRP neurons, coexpressed with Neuropeptide Y (NPY) [39,40]. AgRP neurons are stimulated by the orexigenic hormone-ghrelin, but inhibited by anorexigenic hormones such as serotonin or leptin [38,41–46].
ACTH and α-, β-, γ-MSH are the agonists of the central melanocortin system and bind to five different G-protein-coupled melanocortin receptors (MC1R, MC2R, MC3R, MC4R and MC5R) [30]. Of these five identified melanocortin receptors only MC3R and MC4R are expressed in the CNS and are linked to the regulation of energy balance [47]. Neural MC4R has been shown to regulate satiety signals and modulate glucose and lipid metabolism in the periphery [24]. Central administration of MC4R agonists such as α-MSH decreases food intake and increases energy expenditure resulting in weight loss. Central administration of MC4R antagonists such as AgRP have the opposite effect to α–MSH; they increase food intake, decrease energy expenditure, alter metabolism to promote deposition of adipose mass and suppress systemic thermogenesis resulting in weight gain [8,48–50]. MC4R mutations are the most common cause of monogenic obesity in humans [51]. Similar to human mutation, MC4R knockout mice exhibit hyperphagia, hyperglycemia, hyperleptinemia, and hyperinsulinemia [13]. In contrast, the role of MC3R in the regulation of energy homeostasis is more subtle. MC3R knockout mice only slightly increased adiposity and an accelerated diet-induced obesity. Nonetheless, MC3R expression is important for maintenance of glucose rhythms and lipid metabolism [47].
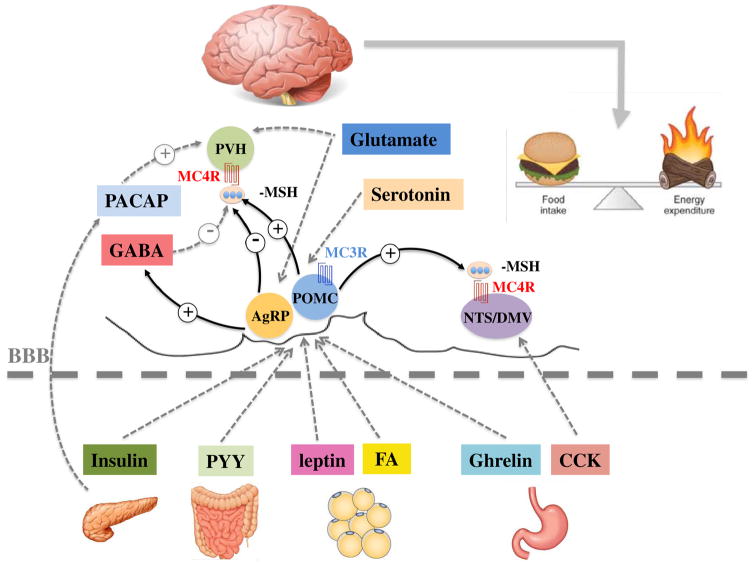
As mentioned previously, AgRP and POMC in the hypothalamic arcuate nucleus are the two upstream neurons in the central melanocortin pathway. These two upstream neurons integrate and distribute the central or peripheral information from hormonal and neural signals including fatty acids (FA), cholecystokinin (CCK), peptide YY (PYY), leptin, insulin, ghrelin, pituitary adenylate cyclase-activating peptide (PACAP), serotonin, GABA and glutamate (Fig. 1 and Table 1). We now summarize the current evidence of the roles of these signals within the ‘nutrient sensing’ central melanocortin pathway in maintaining body mass and adiposity within a healthy range.
Fig. 1.
Participation of the central melanocortin system in metabolic regulation and energy homeostasis.
Table 1.
Overview of the effects of administration of neuropeptides on energy balance in control and genetic or pharmacological blockade of MC4-Rs/MC3-Rs.
| Group of experiments | Body weight | Energy intake | Energy expenditure | HGP | POMC neuron | AgRP neuron | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Leptin | Control | ↓ | ↓ | ↑ | ↑/= | ↑ | ↓ | [33,52–57] |
| SHU9119 | = | = | ↓ | ↑ | ↑ | |||
| Insulin | Wild type | ↓ | ↓ | ↓ | ↑ | ↓ | [53,58–62] | |
| MC4−/− | ↓ | |||||||
| FA | Control | ↓ | ↓ | ↓ | ↑ | ↓ | [63,64] | |
| SHU9119 | ↑ | ↑ | ||||||
| CCK | Wild type | ↓/= | ↓ | [65,66] | ||||
| MC4−/− | = | |||||||
| Chrenlin | Wild type | ↑ | ↑ | ↓ | ↓ | ↑ | [67–70] | |
| MC4−/− | ↓ | |||||||
| PYY | Wild type | ↓ | ↓ | ↑ | ↓ | [71–76] | ||
| MC4−/− | ↓ | |||||||
| PACAP | Control | ↓ | ↑ | ↑ | ↑ | [77–80] | ||
| SHU9119 | ↓ | ↓/= | ||||||
| Serotonin | Wild type | ↓ | ↓ | = | ↑ | ↓ | [42,81,82] | |
| MC4−/− | = | = | ||||||
| GABA | Wild type | ↑ | ↑ | [83] | ||||
| MC4−/− | ||||||||
| Glutamate | Wild type | ↓ | ↓ | ↑ | [84–88] | |||
| MC4−/− | ↓ | ↓ | ↓ |
↑, increased compared to control group; ↓, decreased compared to control group; =, normal compared to control group. Empty fields, no data available. SHU9119, an MC3-R/MC4-R antagonist.
2. Hormonal signals
2.1. Leptin
Leptin is released from peripheral adipose tissue and has an important role on the regulation of energy homeostasis [89,90]. Leptin binds to the leptin receptor (Ob-Rb) in the ARC [91–95] and stimulates the cellular activity of POMC neurons while inhibiting the cellular activity of NPY/AgRP neurons [33]. Leptin or leptin receptor -deficient rodents and humans are obese due to hyperphagia and reduced energy expenditure [91,96]. The administration of leptin into leptin-deficient mice (ob/ob) can totally rescue hyperphagia and limit obesity, while chronic infusions of leptin have been shown to completely deplete visible adipose tissue [97]. Furthermore, ICV administration of leptin into obese mice increases energy expenditure and reduces food intake [52,53]. ICV administration of non-selective MC4R antagonist SHU9119 inhibited the anorexigenic effects of leptin on obese mice [54,55]. Also, ICV administration of leptin could not rescue hyperphagia in obese mice deficient of MC4R (MC4R−/−) [98,99]. Leptin also plays another crucial role in the regulation of glucose homeostasis by decreasing the synthesis/release of AgRP [56,100–102]. Intriguingly, central administration of leptin resulted in decreased glucose production only if the central melanocortin pathway is prevented by SHU9119 [57]. These results suggest that the central melanocortin pathway is the downstream target of leptin in the regulation of body weight, energy balance and glucose homeostasis.
2.2. Insulin
Insulin, a peptide hormone secreted by the pancreatic β-cells, plays a key role in regulating plasma glucose levels in the periphery. The level of blood glucose and the level of adiposity influence insulin secretions in the short and long term, respectively [103,104]. Insulin in the central nervous system is associated with suppression of food intake and body weight gain. Central administration of insulin will bind to insulin receptors (IR) and mimic a state of energy surplus to inhibit food intake and decrease body weight [53,58]. IR are widely expressed in the CNS while the hypothalamus contains the highest expression of IR [105–107]. IR have also been found to be expressed on NPY/AgRP neurons and POMC neurons [108]. Electrophysiological recordings revealed that insulin hyperpolarized NPY/AgRP neurons and depolarized POMC neurons via activation of KATP channels [59,109–112]. Mice lacking IR in the CNS showed mild and sex-specific obesity, hyperleptinemia, and insulin resistance [113]. Deletion of IR alone in AgRP neurons found that insulin action on AgRP neurons was required to suppression of hepatic glucose production [114]. However, deletion of IR alone from POMC neurons failed to influence energy or glucose homeostasis [60,114]. Nonetheless, the melanocortin pathway did have an effect on insulin action, specifically POMC neurons in ARC projected to and acted on distinct MC4R expressing neuronal populations in the intermediolateral nucleus (IML) and dorsal motor nucleus of the vagus (DMV) to decrease insulin secretion or to increase insulin sensitivity separately [115–117]. Additionally, ICV administration of melanocortin agonist (α-MSH) in the third cerebral ventricle of rats improved insulin sensitivity independent of food intake [61]. Furthermore, it has been shown that MC4R KO mice have impaired insulin tolerance, suggesting the role for MC4R in the regulation of insulin action [62]. One potential mechanism is that the activation of MC4R potentiates insulin-stimulated mTOR signaling via the AMPK pathway [61], inhibits c-Jun N-terminal kinase activity and promotes insulin signaling [118]. These findings suggest the strong interaction between the melanocortin pathway and insulin signaling pathway.
2.3. Fatty acids
Fatty acids (FA) act as a sensor of nutrient availability in the brain to control energy homeostasis [119]. In the hypothalamus, FA detect signals from peripheral tissues to regulate food intake, insulin secretion and hepatic glucose production (HGP) [120,121]. Intracerebroventricular (ICV) infusion of monounsaturated acid oleic acid (OA) or polyunsaturated O-3 docosahexaenoic acid (DHA) into rodents has been shown to increase POMC mRNA expression and decrease NPY mRNA expression, subsequently resulting in release of α-MSH that leads to reduced food intake, body weight and glucose production. The anorexigenic effect of OA is completely abolished if the MC4R antagonist SHU9119 is centrally administered at the same time [63,64,122]. However, a similar result, even after 48 h following centrally administered SHU9119, was not apparent on serum glucose levels – OA continued to have an inhibitory effect on serum glucose production [63,64,122]. Fatty acid synthase (FAS) has been found to co-localize with orexigenic NPY in ARC neurons [123]. The inhibition of FAS C75 activity can significantly decrease food intake and body weight [124,125]. Moreover, this anorexigenic effect is mediated by influencing NPY production in the melanocortin pathway [126]. In conclusion, POMC neurons and AgRP neurons within the hypothalamus indicator the variations in plasma FA levels and their metabolites to regulate energy balance and glucose homoeostasis.
2.4. Cholecystokinin
Cholecystokinin (CCK) is a gut released peptide synthesized by both the gastrointestinal system and the central nervous system [127]. Both peripheral and central administration of CCK lead to the reduction of food intake [65,128,129]. First, following a meal, CCK activates CCKNTS neurons, a subset of nucleus tractus solitaries (NTS) neurons that respond to CCK, to inhibit food intake and reduce meal size [130–135]. Both chemogenetic and optogenetic experiments have shown that this satiating function is mediated by a CCKNTS → PVH pathway. Second, electrophysiological recordings revealed that approximately 23% of PVH MC4R-expressing neurons were excited by CCK-8, indicating that CCK activated the appetite-controlling PVH MC4R-expressing neurons [136]. Third, SHU9119 blocked CCK-induced inhibition of feeding in rats. IP injection of CCK-8 significantly reduced food intake in wild type mice but not in MC4R KO mice [66]. Finally, fourth ventricular administration of MC4R agonist melanotan II (MTII) stimulated phosphorylation of ERK1/2 in NTS while fourth ventricular administration of MC4R antagonist SHU9119 in freely feeding rats restrained the IP injection of CCK-induced phosphorylation of ERK1/2 in the NTS and prevented the reduction of food intake by CCK [137–139]. These results suggest that activation of MC4R is required for CCK-induced suppression of feeding.
2.5. Ghrelin
Ghrelin is an orexigenic peptide and is primarily synthesized in the stomach. Ghrelin binds to growth hormone secretagogue receptor (GHSR) which is mainly located in the medial part of the hypothalamic arcuate nucleus and potently stimulates growth hormone secretion [140–144]. Ghrelin affects energy balance through its involvement in regulation of feeding behavior, glucose and lipid metabolism [67,145–147]. Mice lacking ghrelin receptors are hypophagic and lean when fed a high-fat diet. In addition, these GHSR-null mice exhibit increased locomotor activity and improved glucose homeostasis [148,149]. Ghrelin stimulates food intake and increases fat mass [69,150,151] partly by activating GHSR in the NPY/AgRP neurons within ARC [152,153]. In the ARC, 94% of NPY/AgRP neurons contain GHSR mRNA [154]. Electrophysiological recordings reveal that ghrelin depolarized NPY/AgRP neurons and simultaneously hyperpolarize POMC neurons [155]. Central and peripheral administration of ghrelin increase hypothalamic NPY and AGRP mRNA expression and induce c-fos in NPY/AgRP neurons [69,152]. Therefore, the orexigenic effects of ghrelin are thought to depend on NPY/AgRP neuron release of NPY/AgRP and their subsequent release of GABA inhibiting POMC neurons [70]. However, it is shown that ghrelin cannot stimulate food intake in MC4R null mice, suggesting that the orexigenic effects of ghrelin are partly mediated by the central melanocortin pathway [68]. ICV administration of unacylated ghrelin increases MC4R expression and decreases MC3R expression [156]. In humans, the postprandial suppression of total ghrelin is attenuated in patients with MC4R deficiency compared to lean controls [157], suggesting that the regulation of postprandial ghrelin suppression in humans may involve central melanocortin signaling. These results suggest a critical role of central melanocortin signaling on mediating the orexigenic effects of ghrelin.
2.6. Peptide YY
The peptide YY (PYY) is an anorexigenic gut hormone expressed predominantly in the intestinal L-cells. PYY is implicated in the regulation of energy balance and glucose homeostasis. In response to a meal, PYY is co-secreted with glucagon like peptide 1 [158,159]. PYY knockout mice increases food intake and develop obesity [71,72]. In contrast, PYY overexpressed transgenic mice reduces food intake and are protected against diet-induced obesity [160].
PYY has two main forms, PYY1–36 and PYY3–36. PYY3–36 represents approximately half of the total postprandial circulating PYY in humans [161]. In humans, peripheral administration of PYY3–36 reduces food intake [73,74,162–164]. In diet-induced obese rodent models, peripheral administration of PYY3–36 reduce food intake, decreasing body weight and improving insulin sensitivity [165–167]. PYY3–36 has a high binding affinity to the NPY receptor Y2 subtype [168,169]. Y2R is shown to be the receptor responsible for mediating the anorectic effect of PYY3–36 since PYY3–36 is not able to reduce food intake in Y2R-null mice [73]. Peripheral administration of PYY3–36 increases POMC mRNA expression and decreases NPY mRNA expression in the ARC [73,75,76] suggesting that the anorexigenic effects of PYY3–36 in mice might be mediated by the central melanocortin pathway. Surprisingly, a subsequent study show that MC4R is not essential for the anorexigenic role of PYY3–36 [74] since PYY3–36 is equally effective in inducing satiety in wild type and MC4R deficient mice. So, further studies are needed to clarify the interactions between PYY and the melanocortin pathway.
2.7. Pituitary adenylate cyclase-activating peptide
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a peptide originally isolated from the bovine hypothalamus [170]. PAC-AP belongs to the vasoactive intestinal polypeptide/secretin/glucagon family of neuropeptides and is expressed throughout the central nervous system and peripheral tissues including the hypothalamus and pancreas [171]. PACAP and its receptors (PAC1R) abundantly express in the hypothalamus [172,173] and are implicated in the regulation of energy balance in rodents [77,174,175]. Genetic ablation of PACAP in mice is associated with impaired lipid metabolism, carbohydrate intake and brown adipose tissue thermogenesis [78,176,177] while genetic ablation of PAC1R in mice results in impaired insulin response to glucose and reduces glucose tolerance [178]. In addition, ICV administration of PACAP bound to PAC1R increases the expression of POMC mRNA and MC4R mRNA, decreasing food intake and increasing energy expenditure [79]. This effect is attenuated in the genetic ablation of PACAP or PAC1R in mice [174,175]. This effect of PACAP on food consumption is also attenuated by a pretreatment with MC3-R/MC4-R antagonist SHU9119 [79,80]. Thus, these results suggest that PACAP affects energy balance through the melanocortin-dependent pathway.
3. Neural signals
3.1. Serotonin
Serotonin (or 5-hydroxytryptamine, 5-HT) is a multifunctional monoamine neurotransmitter secreted from both peripheral tissues and the brain [179,180]. Serotonin bound to 5-HT2C or 5-HT1B receptors have been shown to inhibit food intake and promote weight loss [181,182]. Pharmacological agents that increase 5-HT activity in the CNS can induce this anorexigenic action of serotonin [183,184]. Global deficiency of 5-HT2C or 5-HT1B receptors in mice resulted in hyperphagia, obesity, impaired glucose homeostasis, and showed attenuated responses to anorexigenic 5-HT drugs [185–188]. Recent studies have shown that the melanocortin pathway is an important downstream mediator of serotonin’s negative action on energy balance. For example, serotonergic terminals made synaptic contacts with arcuate nucleus of the hypothalamus POMC and AgRP neurons [42,189], indicating that the serotonin system was anatomically positioned to influence melanocortin neuron activity [190]. The functional importance of the melanocortin pathway in serotonin’s effects on energy balance has been assessed using pharmacological or genetic inactivation of MC4R. Serotonin or 5-HT2C/5-HT1B receptor agonist mCPP induced MC4R activation by activation of 5-HT2C receptors on POMC neurons and inhibition of 5-HT1B receptors on AgRP neurons [81,82]. Also 5-HT2C receptor-specific agonist D-Fen’s anorectic effects were attenuated in rats and agouti mice pretreated with MC3R/MC4R abbreviation SHU9119 [81,82,190]. Interestingly, mice lacking MC4Rs were not responsive to 5-HT2C receptor agonist-induced hypophagia [188]. Reexpression of MC4Rs only in single-minded homolog 1 neurons in the hypothalamic paraventricular nucleus and in the amygdala was sufficient to restore the hypophagic property of 5-HT2C receptor agonist [188]. These findings demonstrate that an intact central melanocortin pathway through MC4R is necessary for the anorexigenic action of serotonin [82].
3.2. GABA
γ-Aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the brain and acts via two different types of membrane GABA receptors: ionotropic (GABAA) and metabotropic (GABAB) receptors. Pharmacological studies suggest that both central GABAA and GABAB receptor signaling exert prominent influences on feeding and body weight in various brain regions [191–196]. Bilateral lateral hypothalamus injection of GABAA receptor antagonist picrotoxin acutely evokes feeding while injection of GABAA receptor agonist muscimol acutely suppresses feeding and decreases body weight [197,198]. In addition, peripheral administration of the GABAB agonist baclofen significantly reduces food intake and body weight in both diabetic (db/db) and diet-induced obese mice by decreasing NPY expression and increasing POMC expression in ARC [199]. GABA is released by AgRP neurons co-localized with AgRP/NPY–immuno-positive axon terminals which are innervated with local POMC neurons (~70% of POMC neurons expressed GABAB receptors) in ARC [200–203], suggesting that GABA has a direct inhibitory effect on POMC neurons to rapidly affect the activity of downstream neurons [204]. Additionally, a study of POMC-specific GABAB receptor-deficient mice shows that GABAB signaling in POMC neurons protects against obesity and increases insulin sensitivity on the high-fat diet induced mice [205]. Finally, it is shown that GABA released from AgRP neurons bind to GABAA receptor neurons on the lateral PBN of the hindbrain to regulate appetite and body weight [83,206–208]. Future study using genetic mouse model is necessary to understand the role of GABA on central melanocortin pathway.
3.3. Glutamate
Glutamate is the major excitatory neurotransmitter in the brain [209,210] and plays a role in regulating body weight, food intake and metabolism [84–86,211–213]. The metabotropic glutamate mGluR5 receptor agonist (R,S)-2-chloro-5-hydroxyphenylglycine (CHPG) has been shown to stimulate food intake [214] while the antagonists of glutamatergic NMDA and mGluR5 receptors have been shown to decrease food consumption in a baboon model of binge-eating disorder [215]. Selective disruption of glutamate release in leptin receptor-expressing neurons was found to lead to development of mild obesity due to reduced energy expenditure, suggesting that glutamate release mediates leptin action on energy expenditure [87]. Glutamate increases hypothalamic expression of NPY, POMC and cocaine- and amphetamine-regulated transcript (CART) while reducing AgRP expression [216]. Also, a significant number of vesicular glutamate transporter 2 (VGluT2)-immunoreactive terminals have been observed on NPY neurons and POMC neurons, suggesting that glutamatergic fibers are located in the ARC [217,218]. These results suggest that glutamate may affect feeding behavior through the melanocortin-dependent pathway. Indeed, selective disruption of glutamate release from paraventricular nucleus (PVH) neurons led to hyperphagia, reduced energy expenditure and rapid development of obesity [88]. Furthermore, it has been shown that conditionally restored MC4R expression only on Sim1 neurons in the background of MC4R-null mice completely reversed the obese phenotype by reversing hyperphagia. Thus, these results demonstrate that MC4R-expressing glutamatergic neurons in PVH of the hypothalamus are both necessary and sufficient for MC4R control of feeding.
4. Conclusion
Recent advances in the molecular biology and the neuroscience of the melanocortin system using genetic mutations and pharmacological compounds have greatly extended our knowledge of its role in the regulation of energy balance [219,220]. We have provided an overview of the current understanding of the neural systems and the involvement of melanocortins in metabolic homeostasis and the development of obesity. The effect of leptin, CCK, fatty acids, ghrelin and serotonin on energy balance is dependent on melanocortin system. In contrast, the effect of PYY, PACAP and glutamate on energy balance is independent on melanocortin system. We have also raised important questions that will need to be addressed so that we can further understand how the central melanocortin pathway regulates both energy intake and energy expenditure. The development of neuron specific mouse models, CRISPR technologies, optogenetics, chemogenetics, anterograde/retrograde mapping techniques and sing cell sequencing has allowed characterization of neuronal or humoral inputs that are important for body weight regulation [221]. Development of primate models will be also necessary to verify the find finds from rodents. These future studies will provide important insights to human diseases.
Acknowledgments
This work was supported by grants to T.Y. (China Scholarship Council 201406280111), X.K. (US National Institutes of Health Grant 5K99DK106550), K.W.W. (R01 DK100699) and T.L. (AHA 14SDG20370016).
Footnotes
This article is part of a Special Issue entitled: Melanocortin Receptors - edited by Ya-Xiong Tao.
Transparency document
The http://dx.doi.org/10.1016/j.bbadis.2017.05.007 associated with this article can be found, in the online version.
References
- 1.Gautron L, Elmquist JK, Williams KW. Neural control of energy balance: translating circuits to therapies. Cell. 2015;161:133–145. doi: 10.1016/j.cell.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xi L, Chow CM, Kong X. Role of tissue and systemic hypoxia in obesity and type 2 diabetes. J Diabetes Res. 2016;2016:1527852. doi: 10.1155/2016/1527852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez-Dominguez JR, Bai Z, Xu D, Yuan B, Lo KA, Yoon MJ, Lim YC, Knoll M, Slavov N, Chen S, Chen P, Lodish HF, Sun L. De novo reconstruction of adipose tissue transcriptomes reveals long non-coding RNA regulators of brown adipocyte development. Cell Metab. 2015;21:764–776. doi: 10.1016/j.cmet.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kong X, Banks A, Liu T, Kazak L, Rao RR, Cohen P, Wang X, Yu S, Lo JC, Tseng YH, Cypess AM, Xue R, Kleiner S, Kang S, Spiegelman BM, Rosen ED. IRF4 is a key thermogenic transcriptional partner of PGC-1alpha. Cell. 2014;158:69–83. doi: 10.1016/j.cell.2014.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, Accili D. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell. 2012;150:620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y, Gao Y, Tao C, Shao M, Zhao S, Huang W, Yao T, Johnson JA, Liu T, Cypess AM, Gupta O, Holland WL, Gupta RK, Spray DC, Tanowitz HB, Cao L, Lynes MD, Tseng YH, Elmquist JK, Williams KW, Lin HV, Scherer PE. Connexin 43 mediates white adipose tissue beiging by facilitating the propagation of sympathetic neuronal signals. Cell Metab. 2016;24:420–433. doi: 10.1016/j.cmet.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sohn JW, Elmquist JK, Williams KW. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 2013;36:504–512. doi: 10.1016/j.tins.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krashes MJ, Lowell BB, Garfield AS. Melanocortin-4 receptor-regulated energy homeostasis. Nat Neurosci. 2016;19:206–219. doi: 10.1038/nn.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu T, Elmquist JK, Williams KW. Mrap2: an accessory protein linked to obesity. Cell Metab. 2013;18:309–311. doi: 10.1016/j.cmet.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barsh G. From Agouti to Pomc—100 years of fat blonde mice. Nat Med. 1999;5:984–985. doi: 10.1038/12415. [DOI] [PubMed] [Google Scholar]
- 11.Millington GW. The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr Metab. 2007;4:18. doi: 10.1186/1743-7075-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 13.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 14.Berglund ED, Liu T, Kong X, Sohn JW, Vong L, Deng Z, Lee CE, Lee S, Williams KW, Olson DP, Scherer PE, Lowell BB, Elmquist JK. Melanocortin 4 receptors in autonomic neurons regulate thermogenesis and glycemia. Nat Neurosci. 2014;17:911–913. doi: 10.1038/nn.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31:506–543. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 17.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 18.Greenfield JR. Melanocortin signalling and the regulation of blood pressure in human obesity. J Neuroendocrinol. 2011;23:186–193. doi: 10.1111/j.1365-2826.2010.02088.x. [DOI] [PubMed] [Google Scholar]
- 19.Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension. 2006;48:58–64. doi: 10.1161/01.HYP.0000227966.36744.d9. [DOI] [PubMed] [Google Scholar]
- 20.Ni XP, Butler AA, Cone RD, Humphreys MH. Central receptors mediating the cardiovascular actions of melanocyte stimulating hormones. J Hypertens. 2006;24:2239–2246. doi: 10.1097/01.hjh.0000249702.49854.fa. [DOI] [PubMed] [Google Scholar]
- 21.Sayk F, Heutling D, Dodt C, Iwen KA, Wellhoner JP, Scherag S, Hinney A, Hebebrand J, Lehnert H. Sympathetic function in human carriers of melanocortin-4 receptor gene mutations. J Clin Endocrinol Metab. 2010;95:1998–2002. doi: 10.1210/jc.2009-2297. [DOI] [PubMed] [Google Scholar]
- 22.Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, Balthasar N, Williams KW, Elmquist JK. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell. 2013;152:612–619. doi: 10.1016/j.cell.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao YX, Segaloff DL. Follicle stimulating hormone receptor mutations and reproductive disorders. Prog Mol Biol Transl Sci. 2009;89:115–131. doi: 10.1016/S1877-1173(09)89005-4. [DOI] [PubMed] [Google Scholar]
- 24.Girardet C, Begriche K, Ptitsyn A, Koza RA, Butler AA. Unravelling the mysterious roles of melanocortin-3 receptors in metabolic homeostasis and obesity using mouse genetics. Int J Obes Suppl. 2014;4:S37–S44. doi: 10.1038/ijosup.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garfield AS, Lam DD, Marston OJ, Przydzial MJ, Heisler LK. Role of central melanocortin pathways in energy homeostasis. Trends Endocrinol Metab. 2009;20:203–215. doi: 10.1016/j.tem.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Adan RA, Tiesjema B, Hillebrand JJ, la Fleur SE, Kas MJ, de Krom M. The MC4 receptor and control of appetite. Br J Pharmacol. 2006;149:815–827. doi: 10.1038/sj.bjp.0706929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu H, Inoue K, Mori M. The leptin-dependent and -independent melanocortin signaling system: regulation of feeding and energy expenditure. J Endocrinol. 2007;193:1–9. doi: 10.1677/JOE-06-0144. [DOI] [PubMed] [Google Scholar]
- 28.Williams KW, Liu T, Kong X, Fukuda M, Deng Y, Berglund ED, Deng Z, Gao Y, Liu T, Sohn JW, Jia L, Fujikawa T, Kohno D, Scott MM, Lee S, Lee CE, Sun K, Chang Y, Scherer PE, Elmquist JK. Xbp1s in Pomc neurons connects ER stress with energy balance and glucose homeostasis. Cell Metab. 2014;20:471–482. doi: 10.1016/j.cmet.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 30.Gantz I, Fong TM. The melanocortin system. Am J Physiol Endocrinol Metab. 2003;284:E468–E474. doi: 10.1152/ajpendo.00434.2002. [DOI] [PubMed] [Google Scholar]
- 31.Pritchard LE, Turnbull AV, White A. Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity. J Endocrinol. 2002;172:411–421. doi: 10.1677/joe.0.1720411. [DOI] [PubMed] [Google Scholar]
- 32.Wikberg JE. Melanocortin receptors: perspectives for novel drugs. Eur J Pharmacol. 1999;375:295–310. doi: 10.1016/s0014-2999(99)00298-8. [DOI] [PubMed] [Google Scholar]
- 33.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 34.Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P, Luo M. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci. 2013;33:3624–3632. doi: 10.1523/JNEUROSCI.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao T, Deng Z, Gao Y, Sun J, Kong X, Huang Y, He Z, Xu Y, Chang Y, Yu KJ, Findley BG, Berglund ED, Wang RT, Guo H, Chen H, Li X, Kaufman RJ, Yan J, Liu T, Williams KW. Ire1alpha in Pomc neurons is required for thermogenesis and glycemia. Diabetes. 2017;66:663–673. doi: 10.2337/db16-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 39.He Y, Shu G, Yang Y, Xu P, Xia Y, Wang C, Saito K, Hinton A, Jr, Yan X, Liu C, Wu Q, Tong Q, Xu Y. A small potassium current in AgRP/NPY neurons regulates feeding behavior and energy metabolism. Cell Rep. 2016;17:1807–1818. doi: 10.1016/j.celrep.2016.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang D, He X, Zhao Z, Feng Q, Lin R, Sun Y, Ding T, Xu F, Luo M, Zhan C. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front Neuroanat. 2015;9:40. doi: 10.3389/fnana.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bewick GA, Gardiner JV, Dhillo WS, Kent AS, White NE, Webster Z, Ghatei MA, Bloom SR. Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. FASEB J. 2005;19:1680–1682. doi: 10.1096/fj.04-3434fje. [DOI] [PubMed] [Google Scholar]
- 42.Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, Lee CE, Aschkenasi CJ, Zhang CY, Yu J, Boss O, Mountjoy KG, Clifton PG, Lowell BB, Friedman JM, Horvath T, Butler AA, Elmquist JK, Cowley MA. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Yang D, Liu T, Williams KW. Motivation to Eat-AgRP neurons and homeostatic need. Cell Metab. 2015;22:62–63. doi: 10.1016/j.cmet.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Lin YC, Kuo TW, Knight ZA. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 2015;160:829–841. doi: 10.1016/j.cell.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steculorum SM, Ruud J, Karakasilioti I, Backes H, Engstrom Ruud L, Timper K, Hess ME, Tsaousidou E, Mauer J, Vogt MC, Paeger L, Bremser S, Klein AC, Morgan DA, Frommolt P, Brinkkotter PT, Hammerschmidt P, Benzing T, Rahmouni K, Wunderlich FT, Kloppenburg P, Bruning JC. AgRP neurons control systemic insulin sensitivity via myostatin expression in brown adipose tissue. Cell. 2016;165:125–138. doi: 10.1016/j.cell.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Begriche K, Girardet C, McDonald P, Butler AA. Melanocortin-3 receptors and metabolic homeostasis. Prog Mol Biol Transl Sci. 2013;114:109–146. doi: 10.1016/B978-0-12-386933-3.00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, Smith DM, Yagaloff K, Ghatei MA, Bloom SR. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- 49.Ruan HB, Dietrich MO, Liu ZW, Zimmer MR, Li MD, Singh JP, Zhang K, Yin R, Wu J, Horvath TL, Yang X. O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell. 2014;159:306–317. doi: 10.1016/j.cell.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi YC, Lau J, Lin Z, Zhang H, Zhai L, Sperk G, Heilbronn R, Mietzsch M, Weger S, Huang XF, Enriquez RF, Baldock PA, Zhang L, Sainsbury A, Herzog H, Lin S. Arcuate NPY controls sympathetic output and BAT function via a relay of tyrosine hydroxylase neurons in the PVN. Cell Metab. 2013;17:236–248. doi: 10.1016/j.cmet.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Girardet C, Butler AA. Neural melanocortin receptors in obesity and related metabolic disorders. Biochim Biophys Acta. 2014;1842:482–494. doi: 10.1016/j.bbadis.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci U S A. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown LM, Clegg DJ, Benoit SC, Woods SC. Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behav. 2006;89:687–691. doi: 10.1016/j.physbeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 54.Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G, Baskin DG, Schwartz MW. Melanocortin receptors in leptin effects. Nature. 1997;390:349. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- 55.Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, Burn P, Palmiter RD. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet. 1999;21:119–122. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 56.Goncalves GH, Li W, Garcia AV, Figueiredo MS, Bjorbaek C. Hypothalamic agouti-related peptide neurons and the central melanocortin system are crucial mediators of leptin’s antidiabetic actions. Cell Rep. 2014;7:1093–1103. doi: 10.1016/j.celrep.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gutierrez-Juarez R, Obici S, Rossetti L. Melanocortin-independent effects of leptin on hepatic glucose fluxes. J Biol Chem. 2004;279:49704–49715. doi: 10.1074/jbc.M408665200. [DOI] [PubMed] [Google Scholar]
- 58.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 59.Plum L, Belgardt BF, Bruning JC. Central insulin action in energy and glucose homeostasis. J Clin Invest. 2006;116:1761–1766. doi: 10.1172/JCI29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu J, Zhang C, Borgquist A, Nestor CC, Smith AW, Bosch MA, Ku S, Wagner EJ, Ronnekleiv OK, Kelly MJ. Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metab. 2014;19:682–693. doi: 10.1016/j.cmet.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L. Central melanocortin receptors regulate insulin action. J Clin Invest. 2001;108:1079–1085. doi: 10.1172/JCI12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fan W, Dinulescu DM, Butler AA, Zhou J, Marks DL, Cone RD. The central melanocortin system can directly regulate serum insulin levels. Endocrinology. 2000;141:3072–3079. doi: 10.1210/endo.141.9.7665. [DOI] [PubMed] [Google Scholar]
- 63.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 2002;51:271–275. doi: 10.2337/diabetes.51.2.271. [DOI] [PubMed] [Google Scholar]
- 64.Schwinkendorf DR, Tsatsos NG, Gosnell BA, Mashek DG. Effects of central administration of distinct fatty acids on hypothalamic neuropeptide expression and energy metabolism. Int J Obes. 2011;35:336–344. doi: 10.1038/ijo.2010.159. [DOI] [PubMed] [Google Scholar]
- 65.Schick RR, Stevens CW, Yaksh TL, Go VL. Chronic intraventricular administration of cholecystokinin octapeptide (CCK-8) suppresses feeding in rats. Brain Res. 1988;448:294–298. doi: 10.1016/0006-8993(88)91266-8. [DOI] [PubMed] [Google Scholar]
- 66.Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci. 2004;7:335–336. doi: 10.1038/nn1214. [DOI] [PubMed] [Google Scholar]
- 67.Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 68.Shaw AM, Irani BG, Moore MC, Haskell-Luevano C, Millard WJ. Ghrelin-induced food intake and growth hormone secretion are altered in melanocortin 3 and 4 receptor knockout mice. Peptides. 2005;26:1720–1727. doi: 10.1016/j.peptides.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 69.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 70.Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM, Ye Z, Nargund RP, Smith RG, Van der Ploeg LH, Howard AD, MacNeil DJ, Qian S. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 71.Shi YC, Hammerle CM, Lee IC, Turner N, Nguyen AD, Riepler SJ, Lin S, Sainsbury A, Herzog H, Zhang L. Adult-onset PYY overexpression in mice reduces food intake and increases lipogenic capacity. Neuropeptides. 2012;46:173–182. doi: 10.1016/j.npep.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 72.Boey D, Lin S, Karl T, Baldock P, Lee N, Enriquez R, Couzens M, Slack K, Dallmann R, Sainsbury A, Herzog H. Peptide YY ablation in mice leads to the development of hyperinsulinaemia and obesity. Diabetologia. 2006;49:1360–1370. doi: 10.1007/s00125-006-0237-0. [DOI] [PubMed] [Google Scholar]
- 73.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 74.Halatchev IG, Ellacott KL, Fan W, Cone RD. Peptide YY3-36 inhibits food intake in mice through a melanocortin-4 receptor-independent mechanism. Endocrinology. 2004;145:2585–2590. doi: 10.1210/en.2003-1754. [DOI] [PubMed] [Google Scholar]
- 75.Challis BG, Pinnock SB, Coll AP, Carter RN, Dickson SL, O’Rahilly S. Acute effects of PYY3-36 on food intake and hypothalamic neuropeptide expression in the mouse. Biochem Biophys Res Commun. 2003;311:915–919. doi: 10.1016/j.bbrc.2003.10.089. [DOI] [PubMed] [Google Scholar]
- 76.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 77.Mizuno Y, Kondo K, Terashima Y, Arima H, Murase T, Oiso Y. Anorectic effect of pituitary adenylate cyclase activating polypeptide (PACAP) in rats: lack of evidence for involvement of hypothalamic neuropeptide gene expression. J Neuroendocrinol. 1998;10:611–616. doi: 10.1046/j.1365-2826.1998.00244.x. [DOI] [PubMed] [Google Scholar]
- 78.Nakata M, Kohno D, Shintani N, Nemoto Y, Hashimoto H, Baba A, Yada T. PACAP deficient mice display reduced carbohydrate intake and PACAP activates NPY-containing neurons in the rat hypothalamic arcuate nucleus. Neurosci Lett. 2004;370:252–256. doi: 10.1016/j.neulet.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 79.Mounien L, Do Rego JC, Bizet P, Boutelet I, Gourcerol G, Fournier A, Brabet P, Costentin J, Vaudry H, Jegou S. Pituitary adenylate cyclase-activating polypeptide inhibits food intake in mice through activation of the hypothalamic melanocortin system. Neuropsychopharmacology. 2009;34:424–435. doi: 10.1038/npp.2008.73. [DOI] [PubMed] [Google Scholar]
- 80.Tanida M, Shintani N, Hashimoto H. The melanocortin system is involved in regulating autonomic nerve activity through central pituitary adenylate cyclase-activating polypeptide. Neurosci Res. 2011;70:55–61. doi: 10.1016/j.neures.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 81.Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Marcus JN, Holstege H, Lee CE, Cone RD, Elmquist JK. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- 82.Lam DD, Przydzial MJ, Ridley SH, Yeo GS, Rochford JJ, O’Rahilly S, Heisler LK. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology. 2008;149:1323–1328. doi: 10.1210/en.2007-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nagata M, Suzuki W, Iizuka S, Tabuchi M, Maruyama H, Takeda S, Aburada M, Miyamoto K. Type 2 diabetes mellitus in obese mouse model induced by monosodium glutamate. Exp Anim. 2006;55:109–115. doi: 10.1538/expanim.55.109. [DOI] [PubMed] [Google Scholar]
- 85.Hermanussen M, Garcia AP, Sunder M, Voigt M, Salazar V, Tresguerres JA. Obesity, voracity, and short stature: the impact of glutamate on the regulation of appetite. Eur J Clin Nutr. 2006;60:25–31. doi: 10.1038/sj.ejcn.1602263. [DOI] [PubMed] [Google Scholar]
- 86.Guyenet SJ, Matsen ME, Morton GJ, Kaiyala KJ, Schwartz MW. Rapid glutamate release in the mediobasal hypothalamus accompanies feeding and is exaggerated by an obesogenic food. Mol Metab. 2013;2:116–122. doi: 10.1016/j.molmet.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu Y, Kim ER, Zhao R, Myers MG, Jr, Munzberg H, Tong Q. Glutamate release mediates leptin action on energy expenditure. Mol Metab. 2013;2:109–115. doi: 10.1016/j.molmet.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu Y, Wu Z, Sun H, Zhu Y, Kim ER, Lowell BB, Arenkiel BR, Xu Y, Tong Q. Glutamate mediates the function of melanocortin receptor 4 on Sim1 neurons in body weight regulation. Cell Metab. 2013;18:860–870. doi: 10.1016/j.cmet.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Allison MB, Myers MG., Jr 20 years of leptin: connecting leptin signaling to biological function. J Endocrinol. 2014;223:T25–T35. doi: 10.1530/JOE-14-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Friedman J. The long road to leptin. J Clin Invest. 2016;126:4727–4734. doi: 10.1172/JCI91578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 92.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 93.Xu Y, Chang JT, Myers MG, Jr, Xu Y, Tong Q. Euglycemia restoration by central leptin in type 1 diabetes requires STAT3 signaling but not fast-acting neurotransmitter release. Diabetes. 2016;65:1040–1049. doi: 10.2337/db15-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu J, Lee J, Salazar Hernandez MA, Mazitschek R, Ozcan U. Treatment of obesity with celastrol. Cell. 2015;161:999–1011. doi: 10.1016/j.cell.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun J, Gao Y, Yao T, Huang Y, He Z, Kong X, Yu KJ, Wang RT, Guo H, Yan J, Chang Y, Chen H, Scherer PE, Liu T, Williams KW. Adiponectin potentiates the acute effects of leptin in arcuate Pomc neurons. Mol Metab. 2016;5:882–891. doi: 10.1016/j.molmet.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 97.Friedman JM. Leptin at 14 y of age: an ongoing story. Am J Clin Nutr. 2009;89:973S–979S. doi: 10.3945/ajcn.2008.26788B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gonzalez-Jimenez E, Aguilar Cordero MJ, Padilla Lopez CA, Garcia Garcia I. Monogenic human obesity: role of the leptin-melanocortin system in the regulation of food intake and body weight in humans. An Sist Sanit Navar. 2012;35:285–293. doi: 10.4321/s1137-66272012000200010. [DOI] [PubMed] [Google Scholar]
- 99.Catli G, Abaci A, Anik A, Bober E. Low serum nesfatin-1 levels may be a contributing factor for monogenic obesity due to prohormone convertase 1 deficiency. Med Hypotheses. 2013;81:172–174. doi: 10.1016/j.mehy.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 100.do Carmo JM, Tallam LS, Roberts JV, Brandon EL, Biglane J, da Silva AA, Hall JE. Impact of obesity on renal structure and function in the presence and absence of hypertension: evidence from melanocortin-4 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2009;297:R803–R812. doi: 10.1152/ajpregu.00187.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marino JS, Xu Y, Hill JW. Central insulin and leptin-mediated autonomic control of glucose homeostasis. Trends Endocrinol Metab. 2011;22:275–285. doi: 10.1016/j.tem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Varela L, Horvath TL. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012;13:1079–1086. doi: 10.1038/embor.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bannon AW, Seda J, Carmouche M, Francis JM, Norman MH, Karbon B, McCaleb ML. Behavioral characterization of neuropeptide Y knockout mice. Brain Res. 2000;868:79–87. doi: 10.1016/s0006-8993(00)02285-x. [DOI] [PubMed] [Google Scholar]
- 105.Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- 106.Corp ES, Woods SC, Porte D, Jr, Dorsa DM, Figlewicz DP, Baskin DG. Localization of 125I-insulin binding sites in the rat hypothalamus by quantitative autoradiography. Neurosci Lett. 1986;70:17–22. doi: 10.1016/0304-3940(86)90430-1. [DOI] [PubMed] [Google Scholar]
- 107.Werther GA, Hogg A, Oldfield BJ, McKinley MJ, Figdor R, Allen AM, Mendelsohn FA. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology. 1987;121:1562–1570. doi: 10.1210/endo-121-4-1562. [DOI] [PubMed] [Google Scholar]
- 108.Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci Off J Soc Neurosci. 2002;22:9048–9052. doi: 10.1523/JNEUROSCI.22-20-09048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwartz MW, Marks JL, Sipols AJ, Baskin DG, Woods SC, Kahn SE, Porte D., Jr Central insulin administration reduces neuropeptide Y mRNA expression in the arcuate nucleus of food-deprived lean (Fa/Fa) but not obese (fa/fa) Zucker rats. Endocrinology. 1991;128:2645–2647. doi: 10.1210/endo-128-5-2645. [DOI] [PubMed] [Google Scholar]
- 110.Schwartz MW, Sipols AJ, Marks JL, Sanacora G, White JD, Scheurink A, Kahn SE, Baskin DG, Woods SC, Figlewicz DP, et al. Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology. 1992;130:3608–3616. doi: 10.1210/endo.130.6.1597158. [DOI] [PubMed] [Google Scholar]
- 111.Sipols AJ, Baskin DG, Schwartz MW. Effect of intracerebroventricular insulin infusion on diabetic hyperphagia and hypothalamic neuropeptide gene expression. Diabetes. 1995;44:147–151. doi: 10.2337/diab.44.2.147. [DOI] [PubMed] [Google Scholar]
- 112.Choudhury AI, Heffron H, Smith MA, Al-Qassab H, Xu AW, Selman C, Simmgen M, Clements M, Claret M, Maccoll G, Bedford DC, Hisadome K, Diakonov I, Moosajee V, Bell JD, Speakman JR, Batterham RL, Barsh GS, Ashford ML, Withers DJ. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J Clin Invest. 2005;115:940–950. doi: 10.1172/JCI24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 114.Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Bruning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 115.Coppari R, Bjorbaek C. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat Rev Drug Discov. 2012;11:692–708. doi: 10.1038/nrd3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu Y, Elmquist JK, Fukuda M. Central nervous control of energy and glucose balance: focus on the central melanocortin system. Ann N Y Acad Sci. 2011;1243:1–14. doi: 10.1111/j.1749-6632.2011.06248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev. 2011;91:389–411. doi: 10.1152/physrev.00007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chai B, Li JY, Zhang W, Wang H, Mulholland MW. Melanocortin-4 receptor activation inhibits c-Jun N-terminal kinase activity and promotes insulin signaling. Peptides. 2009;30:1098–1104. doi: 10.1016/j.peptides.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moulle VS, Picard A, Le Foll C, Levin BE, Magnan C. Lipid sensing in the brain and regulation of energy balance. Diabetes Metab. 2014;40:29–33. doi: 10.1016/j.diabet.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 120.Oomura Y, Nakamura T, Sugimori M, Yamada Y. Effect of free fatty acid on the rat lateral hypothalamic neurons. Physiol Behav. 1975;14:483–486. doi: 10.1016/0031-9384(75)90015-3. [DOI] [PubMed] [Google Scholar]
- 121.Migrenne S, Magnan C, Cruciani-Guglielmacci C. Fatty acid sensing and nervous control of energy homeostasis. Diabetes Metab. 2007;33:177–182. doi: 10.1016/j.diabet.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 122.Auestad N, Korsak RA, Morrow JW, Edmond J. Fatty acid oxidation and ketogenesis by astrocytes in primary culture. J Neurochem. 1991;56:1376–1386. doi: 10.1111/j.1471-4159.1991.tb11435.x. [DOI] [PubMed] [Google Scholar]
- 123.Kim EK, Miller I, Landree LE, Borisy-Rudin FF, Brown P, Tihan T, Townsend CA, Witters LA, Moran TH, Kuhajda FP, Ronnett GV. Expression of FAS within hypothalamic neurons: a model for decreased food intake after C75 treatment. Am J Physiol Endocrinol Metab. 2002;283:E867–E879. doi: 10.1152/ajpendo.00178.2002. [DOI] [PubMed] [Google Scholar]
- 124.Makimura H, Mizuno TM, Yang XJ, Silverstein J, Beasley J, Mobbs CV. Cerulenin mimics effects of leptin on metabolic rate, food intake, and body weight independent of the melanocortin system, but unlike leptin, cerulenin fails to block neuroendocrine effects of fasting. Diabetes. 2001;50:733–739. doi: 10.2337/diabetes.50.4.733. [DOI] [PubMed] [Google Scholar]
- 125.Gao S, Lane MD. Effect of the anorectic fatty acid synthase inhibitor C75 on neuronal activity in the hypothalamus and brainstem. Proc Natl Acad Sci U S A. 2003;100:5628–5633. doi: 10.1073/pnas.1031698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lopez-Buesa P, Burgos C, Galve A, Varona L. Joint analysis of additive, dominant and first-order epistatic effects of four genes (IGF2, MC4R PRKAG3 and LEPR) with known effects on fat content and fat distribution in pigs. Anim Genet. 2014;45:133–137. doi: 10.1111/age.12091. [DOI] [PubMed] [Google Scholar]
- 127.Little TJ, Horowitz M, Feinle-Bisset C. Role of cholecystokinin in appetite control and body weight regulation. Obesity Rev. 2005;6:297–306. doi: 10.1111/j.1467-789X.2005.00212.x. [DOI] [PubMed] [Google Scholar]
- 128.Moran TH, Bi S. Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos Trans R Soc Lond Ser B Biol Sci. 2006;361:1211–1218. doi: 10.1098/rstb.2006.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Montgomery IA, Irwin N, Flatt PR. Beneficial effects of (pGlu-Gln)-CCK-8 on energy intake and metabolism in high fat fed mice are associated with alterations of hypothalamic gene expression. Horm Metab Res. 2013;45:471–473. doi: 10.1055/s-0032-1331767. [DOI] [PubMed] [Google Scholar]
- 130.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16:296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baskin DG, Kim F, Gelling RW, Russell BJ, Schwartz MW, Morton GJ, Simhan HN, Moralejo DH, Blevins JE. A new oxytocin-saporin cytotoxin for lesioning oxytocin-receptive neurons in the rat hindbrain. Endocrinology. 2010;151:4207–4213. doi: 10.1210/en.2010-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rinaman L. Hindbrain noradrenergic lesions attenuate anorexia and alter central cFos expression in rats after gastric viscerosensory stimulation. J Neurosci Off J Soc Neurosci. 2003;23:10084–10092. doi: 10.1523/JNEUROSCI.23-31-10084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McMinn JE, Sindelar DK, Havel PJ, Schwartz MW. Leptin deficiency induced by fasting impairs the satiety response to cholecystokinin. Endocrinology. 2000;141:4442–4448. doi: 10.1210/endo.141.12.7815. [DOI] [PubMed] [Google Scholar]
- 134.Gibbs J, Falasco JD, McHugh PR. Cholecystokinin-decreased food intake in rhesus monkeys. Am J Phys. 1976;230:15–18. doi: 10.1152/ajplegacy.1976.230.1.15. [DOI] [PubMed] [Google Scholar]
- 135.Blumberg S, Schroeder M, Haba D, Malkesman O, Torregrossa AM, Weller A, Smith GP. Effects of CCK-8 on independent ingestion and central c-Fos-like immunoreactivity in rats on postnatal days 10 and 11. Peptides. 2006;27:2820–2828. doi: 10.1016/j.peptides.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 136.D’Agostino G, Lyons DJ, Cristiano C, Burke LK, Madara JC, Campbell JN, Garcia AP, Land BB, Lowell BB, Dileone RJ, Heisler LK. Appetite controlled by a cholecystokinin nucleus of the solitary tract to hypothalamus neurocircuit. elife. 2016;5 doi: 10.7554/eLife.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Campos CA, Wright JS, Czaja K, Ritter RC. CCK-induced reduction of food intake and hindbrain MAPK signaling are mediated by NMDA receptor activation. Endocrinology. 2012;153:2633–2646. doi: 10.1210/en.2012-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sutton GM, Duos B, Patterson LM, Berthoud HR. Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology. 2005;146:3739–3747. doi: 10.1210/en.2005-0562. [DOI] [PubMed] [Google Scholar]
- 139.Blevins JE, Morton GJ, Williams DL, Caldwell DW, Bastian LS, Wisse BE, Schwartz MW, Baskin DG. Forebrain melanocortin signaling enhances the hindbrain satiety response to CCK-8, American journal of physiology. Regul Integr Comp Physiol. 2009;296:R476–R484. doi: 10.1152/ajpregu.90544.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 141.Hassouna R, Zizzari P, Tolle V. The ghrelin/obestatin balance in the physiological and pathological control of growth hormone secretion, body composition and food intake. J Neuroendocrinol. 2010;22:793–804. doi: 10.1111/j.1365-2826.2010.02019.x. [DOI] [PubMed] [Google Scholar]
- 142.Horvath TL, Diano S, Sotonyi P, Heiman M, Tschop M. Minireview: ghrelin and the regulation of energy balance—a hypothalamic perspective. Endocrinology. 2001;142:4163–4169. doi: 10.1210/endo.142.10.8490. [DOI] [PubMed] [Google Scholar]
- 143.Perello M, Scott MM, Sakata I, Lee CE, Chuang JC, Osborne-Lawrence S, Rovinsky SA, Elmquist JK, Zigman JM. Functional implications of limited leptin receptor and ghrelin receptor coexpression in the brain. J Comp Neurol. 2012;520:281–294. doi: 10.1002/cne.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang Q, Liu C, Uchida A, Chuang JC, Walker A, Liu T, Osborne-Lawrence S, Mason BL, Mosher C, Berglund ED, Elmquist JK, Zigman JM. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol Metab. 2014;3:64–72. doi: 10.1016/j.molmet.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Toshinai K, Yamaguchi H, Sun Y, Smith RG, Yamanaka A, Sakurai T, Date Y, Mondal MS, Shimbara T, Kawagoe T, Murakami N, Miyazato M, Kangawa K, Nakazato M. Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology. 2006;147:2306–2314. doi: 10.1210/en.2005-1357. [DOI] [PubMed] [Google Scholar]
- 146.Sakata I, Sakai T. Ghrelin cells in the gastrointestinal tract. Int J Pept. 2010;2010 doi: 10.1155/2010/945056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li Z, Xu G, Qin Y, Zhang C, Tang H, Yin Y, Xiang X, Li Y, Zhao J, Mulholland M, Zhang W. Ghrelin promotes hepatic lipogenesis by activation of mTOR-PPARgamma signaling pathway. Proc Natl Acad Sci U S A. 2014;111:13163–13168. doi: 10.1073/pnas.1411571111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee JH, Lin L, Xu P, Saito K, Wei Q, Meadows AG, Bongmba OY, Pradhan G, Zheng H, Xu Y, Sun Y. Neuronal deletion of ghrelin receptor almost completely prevents diet-induced obesity. Diabetes. 2016;65:2169–2178. doi: 10.2337/db15-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 151.Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 152.Hewson AK, Dickson SL. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J Neuroendocrinol. 2000;12:1047–1049. doi: 10.1046/j.1365-2826.2000.00584.x. [DOI] [PubMed] [Google Scholar]
- 153.Dickson SL, Luckman SM. Induction of c-fos messenger ribonucleic acid in neuropeptide Y, growth hormone (GH)-releasing factor neurons in the rat arcuate nucleus following systemic injection of the GH secretagogue, GH-releasing peptide-6. Endocrinology. 1997;138:771–777. doi: 10.1210/endo.138.2.4907. [DOI] [PubMed] [Google Scholar]
- 154.Willesen MG, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–316. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- 155.Renquist BJ, Murphy JG, Larson EA, Olsen D, Klein RF, Ellacott KL, Cone RD. Melanocortin-3 receptor regulates the normal fasting response. Proc Natl Acad Sci U S A. 2012;109:E1489–E1498. doi: 10.1073/pnas.1201994109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stevanovic DM, Grefhorst A, Themmen AP, Popovic V, Holstege J, Haasdijk E, Trajkovic V, van der Lely AJ, Delhanty PJ. Unacylated ghrelin suppresses ghrelin-induced neuronal activity in the hypothalamus and brainstem of male rats [corrected] PLoS One. 2014;9:e98180. doi: 10.1371/journal.pone.0098180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.van der Klaauw AA, Keogh JM, Henning E, Blackwood A, Haqq AM, Purnell JQ, Farooqi IS. Postprandial total ghrelin suppression is modulated by melanocortin signaling in humans. J Clin Endocrinol Metab. 2013;98:E288–E292. doi: 10.1210/jc.2012-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- 159.Pedersen-Bjergaard U, Host U, Kelbaek H, Schifter S, Rehfeld JF, Faber J, Christensen NJ. Influence of meal composition on postprandial peripheral plasma concentrations of vasoactive peptides in man. Scand J Clin Lab Invest. 1996;56:497–503. doi: 10.3109/00365519609088805. [DOI] [PubMed] [Google Scholar]
- 160.Boey D, Lin S, Enriquez RF, Lee NJ, Slack K, Couzens M, Baldock PA, Herzog H, Sainsbury A. PYY transgenic mice are protected against diet-induced and genetic obesity. Neuropeptides. 2008;42:19–30. doi: 10.1016/j.npep.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 161.Grandt D, Schimiczek M, Beglinger C, Layer P, Goebell H, Eysselein VE, Reeve JR., Jr Two molecular forms of peptide YY (PYY) are abundant in human blood: characterization of a radioimmunoassay recognizing PYY 1-36 and PYY 3-36. Regul Pept. 1994;51:151–159. doi: 10.1016/0167-0115(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 162.Moran TH, Smedh U, Kinzig KP, Scott KA, Knipp S, Ladenheim EE. Peptide YY(3-36) inhibits gastric emptying and produces acute reductions in food intake in rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 2005;288:R384–R388. doi: 10.1152/ajpregu.00535.2004. [DOI] [PubMed] [Google Scholar]
- 163.Koegler FH, Enriori PJ, Billes SK, Takahashi DL, Martin MS, Clark RL, Evans AE, Grove KL, Cameron JL, Cowley MA. Peptide YY(3-36) inhibits morning, but not evening, food intake and decreases body weight in rhesus macaques. Diabetes. 2005;54:3198–3204. doi: 10.2337/diabetes.54.11.3198. [DOI] [PubMed] [Google Scholar]
- 164.Sloth B, Davidsen L, Holst JJ, Flint A, Astrup A. Effect of subcutaneous injections of PYY1-36 and PYY3-36 on appetite, ad libitum energy intake, and plasma free fatty acid concentration in obese males. Am J Physiol Endocrinol Metab. 2007;293:E604–E609. doi: 10.1152/ajpendo.00153.2007. [DOI] [PubMed] [Google Scholar]
- 165.Chelikani PK, Haver AC, Reeve JR, Jr, Keire DA, Reidelberger RD. Daily,intermittent intravenous infusion of peptide YY(3-36) reduces daily food intake and adiposity in rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R298–R305. doi: 10.1152/ajpregu.00674.2005. [DOI] [PubMed] [Google Scholar]
- 166.Vrang N, Madsen AN, Tang-Christensen M, Hansen G, Larsen PJ. PYY(3-36) reduces food intake and body weight and improves insulin sensitivity in rodent models of diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2006;291:R367–R375. doi: 10.1152/ajpregu.00726.2005. [DOI] [PubMed] [Google Scholar]
- 167.Chelikani PK, Haver AC, Reidelberger RD. Intermittent intraperitoneal infusion of peptide YY(3-36) reduces daily food intake and adiposity in obese rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R39–R46. doi: 10.1152/ajpregu.00164.2007. [DOI] [PubMed] [Google Scholar]
- 168.Keire DA, Bowers CW, Solomon TE, Reeve JR., Jr Structure and receptor binding of PYY analogs. Peptides. 2002;23:305–321. doi: 10.1016/s0196-9781(01)00602-7. [DOI] [PubMed] [Google Scholar]
- 169.Karra E, Chandarana K, Batterham RL. The role of peptide YY in appetite regulation and obesity. J Physiol. 2009;587:19–25. doi: 10.1113/jphysiol.2008.164269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K, Fujino M, Minamino N, Arimura A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38) Biochem Biophys Res Commun. 1990;170:643–648. doi: 10.1016/0006-291x(90)92140-u. [DOI] [PubMed] [Google Scholar]
- 171.Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 172.Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129:2787–2789. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- 173.Hannibal J, Mikkelsen JD, Clausen H, Holst JJ, Wulff BS, Fahrenkrug J. Gene expression of pituitary adenylate cyclase activating polypeptide (PACAP) in the rat hypothalamus. Regul Pept. 1995;55:133–148. doi: 10.1016/0167-0115(94)00099-j. [DOI] [PubMed] [Google Scholar]
- 174.Morley JE, Horowitz M, Morley PM, Flood JF. Pituitary adenylate cyclase activating polypeptide (PACAP) reduces food intake in mice. Peptides. 1992;13:1133–1135. doi: 10.1016/0196-9781(92)90019-y. [DOI] [PubMed] [Google Scholar]
- 175.Chance WT, Thompson H, Thomas I, Fischer JE. Anorectic and neurochemical effects of pituitary adenylate cyclase activating polypeptide in rats. Peptides. 1995;16:1511–1516. doi: 10.1016/0196-9781(95)02048-9. [DOI] [PubMed] [Google Scholar]
- 176.Gray SL, Cummings KJ, Jirik FR, Sherwood NM. Targeted disruption of the pituitary adenylate cyclase-activating polypeptide gene results in early postnatal death associated with dysfunction of lipid and carbohydrate metabolism. Mol Endocrinol. 2001;15:1739–1747. doi: 10.1210/mend.15.10.0705. [DOI] [PubMed] [Google Scholar]
- 177.Gray SL, Yamaguchi N, Vencova P, Sherwood NM. Temperature-sensitive phenotype in mice lacking pituitary adenylate cyclase-activating polypeptide. Endocrinology. 2002;143:3946–3954. doi: 10.1210/en.2002-220401. [DOI] [PubMed] [Google Scholar]
- 178.Jamen F, Persson K, Bertrand G, Rodriguez-Henche N, Puech R, Bockaert J, Ahren B, Brabet P. PAC1 receptor-deficient mice display impaired insulinotropic response to glucose and reduced glucose tolerance. J Clin Invest. 2000;105:1307–1315. doi: 10.1172/JCI9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lam DD, Garfield AS, Marston OJ, Shaw J, Heisler LK. Brain serotonin system in the coordination of food intake and body weight. Pharmacol Biochem Behav. 2010;97:84–91. doi: 10.1016/j.pbb.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 180.Gao Y, Yao T, Deng Z, Sohn JW, Sun J, Huang Y, Kong X, Yu KJ, Wang RT, Chen H, Guo H, Yan J, Cunningham KA, Chang Y, Liu T, Williams KW. TrpC5 mediates acute leptin and serotonin effects via Pomc neurons. Cell Rep. 2017;18:583–592. doi: 10.1016/j.celrep.2016.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron. 2011;71:488–497. doi: 10.1016/j.neuron.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Berglund ED, Liu C, Sohn JW, Liu T, Kim MH, Lee CE, Vianna CR, Williams KW, Xu Y, Elmquist JK. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J Clin Invest. 2013;123:5061–5070. doi: 10.1172/JCI70338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Heisler LK, Tecott LH. Knockout corner: neurobehavioural consequences of a serotonin 5-HT(2C) receptor gene mutation. Int J Neuropsychopharmacol. 1999;2:67–69. doi: 10.1017/S1461145799001327. [DOI] [PubMed] [Google Scholar]
- 184.Simansky KJ. Serotonergic control of the organization of feeding and satiety. Behav Brain Res. 1996;73:37–42. doi: 10.1016/0166-4328(96)00066-6. [DOI] [PubMed] [Google Scholar]
- 185.Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- 186.Bouwknecht JA, van der Gugten J, Hijzen TH, Maes RA, Hen R, Olivier B. Male and female 5-HT(1B) receptor knockout mice have higher body weights than wildtypes. Physiol Behav. 2001;74:507–516. doi: 10.1016/s0031-9384(01)00589-3. [DOI] [PubMed] [Google Scholar]
- 187.Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, Elmquist JK. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60:582–589. doi: 10.1016/j.neuron.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xu Y, Jones JE, Lauzon DA, Anderson JG, Balthasar N, Heisler LK, Zinn AR, Lowell BB, Elmquist JK. A serotonin and melanocortin circuit mediates D-fenfluramine anorexia. J Neurosci. 2010;30:14630–14634. doi: 10.1523/JNEUROSCI.5412-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kiss J, Leranth C, Halasz B. Serotoninergic endings on VIP-neurons in the suprachiasmatic nucleus and on ACTH-neurons in the arcuate nucleus of the rat hypothalamus. A combination of high resolution autoradiography and electron microscopic immunocytochemistry. Neurosci Lett. 1984;44:119–124. doi: 10.1016/0304-3940(84)90068-5. [DOI] [PubMed] [Google Scholar]
- 190.Marston OJ, Garfield AS, Heisler LK. Role of central serotonin and melanocortin systems in the control of energy balance. Eur J Pharmacol. 2011;660:70–79. doi: 10.1016/j.ejphar.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 191.Cooper SJ. Palatability-dependent appetite and benzodiazepines: new directions from the pharmacology of GABA(A) receptor subtypes. Appetite. 2005;44:133–150. doi: 10.1016/j.appet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 192.Duke AN, Platt DM, Cook JM, Huang S, Yin W, Mattingly BA, Rowlett JK. Enhanced sucrose pellet consumption induced by benzodiazepine-type drugs in squirrel monkeys: role of GABAA receptor subtypes. Psychopharmacology. 2006;187:321–330. doi: 10.1007/s00213-006-0431-2. [DOI] [PubMed] [Google Scholar]
- 193.Martire M, Barrese V, D’Amico M, Iannotti FA, Pizzarelli R, Samengo I, Viggiano D, Ruth P, Cherubini E, Taglialatela M. Pre-synaptic BK channels selectively control glutamate versus GABA release from cortical and hippocampal nerve terminals. J Neurochem. 2010;115:411–422. doi: 10.1111/j.1471-4159.2010.06938.x. [DOI] [PubMed] [Google Scholar]
- 194.Pecina S, Berridge KC. Brainstem mediates diazepam enhancement of palatability and feeding: microinjections into fourth ventricle versus lateral ventricle. Brain Res. 1996;727:22–30. doi: 10.1016/0006-8993(96)00325-3. [DOI] [PubMed] [Google Scholar]
- 195.Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci Off J Soc Neurosci. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kim ER, Wu Z, Sun H, Xu Y, Mangieri LR, Xu Y, Tong Q. Hypothalamic non-AgRP,non-POMC GABAergic neurons are required for postweaning feeding and NPY hyperphagia. J Neurosci. 2015;35:10440–10450. doi: 10.1523/JNEUROSCI.1110-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kelly J, Alheid GF, Newberg A, Grossman SP. GABA stimulation and blockade in the hypothalamus and midbrain: effects on feeding and locomotor activity. Pharmacol Biochem Behav. 1977;7:537–541. doi: 10.1016/0091-3057(77)90250-7. [DOI] [PubMed] [Google Scholar]
- 198.Turenius CI, Htut MM, Prodon DA, Ebersole PL, Ngo PT, Lara RN, Wilczynski JL, Stanley BG. GABA(A) receptors in the lateral hypothalamus as mediators of satiety and body weight regulation. Brain Res. 2009;1262:16–24. doi: 10.1016/j.brainres.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 199.Sato I, Arima H, Ozaki N, Ozaki N, Watanabe M, Goto M, Shimizu H, Hayashi M, Banno R, Nagasaki H, Oiso Y. Peripherally administered baclofen reduced food intake and body weight in db/db as well as diet-induced obese mice. FEBS Lett. 2007;581:4857–4864. doi: 10.1016/j.febslet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 200.Ovesjo ML, Gamstedt M, Collin M, Meister B. GABAergic nature of hypothalamic leptin target neurones in the ventromedial arcuate nucleus. J Neuroendocrinol. 2001;13:505–516. doi: 10.1046/j.1365-2826.2001.00662.x. [DOI] [PubMed] [Google Scholar]
- 201.Hentges ST, Nishiyama M, Overstreet LS, Stenzel-Poore M, Williams JT, Low MJ. GABA release from proopiomelanocortin neurons. J Neurosci. 2004;24:1578–1583. doi: 10.1523/JNEUROSCI.3952-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Meister B. Neurotransmitters in key neurons of the hypothalamus that regulate feeding behavior and body weight. Physiol Behav. 2007;92:263–271. doi: 10.1016/j.physbeh.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 203.Sinchak K, Dewing P, Ponce L, Gomez L, Christensen A, Berger M, Micevych P. Modulation of the arcuate nucleus-medial preoptic nucleus lordosis regulating circuit: a role for GABAB receptors. Horm Behav. 2013;64:136–143. doi: 10.1016/j.yhbeh.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dicken MS, Tooker RE, Hentges ST. Regulation of GABA and glutamate release from proopiomelanocortin neuron terminals in intact hypothalamic networks. J Neurosci Off J Soc Neurosci. 2012;32:4042–4048. doi: 10.1523/JNEUROSCI.6032-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ito Y, Banno R, Shibata M, Adachi K, Hagimoto S, Hagiwara D, Ozawa Y, Goto M, Suga H, Sugimura Y, Bettler B, Oiso Y, Arima H. GABA type B receptor signaling in proopiomelanocortin neurons protects against obesity, insulin resistance, and hypothalamic inflammation in male mice on a high-fat diet. J Neurosci Off J Soc Neurosci. 2013;33:17166–17173. doi: 10.1523/JNEUROSCI.0897-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Horvath TL, Garcia-Segura LM, Naftolin F. Control of gonadotropin feedback: the possible role of estrogen-induced hypothalamic synaptic plasticity. Gynecol Endocrinol. 1997;11:139–143. doi: 10.3109/09513599709152525. [DOI] [PubMed] [Google Scholar]
- 207.Pu S, Jain MR, Horvath TL, Diano S, Kalra PS, Kalra SP. Interactions between neuropeptide Y and gamma-aminobutyric acid in stimulation of feeding: a morphological and pharmacological analysis. Endocrinology. 1999;140:933–940. doi: 10.1210/endo.140.2.6495. [DOI] [PubMed] [Google Scholar]
- 208.Wu Q, Howell MP, Cowley MA, Palmiter RD. Starvation after AgRP neuron ablation is independent of melanocortin signaling. Proc Natl Acad Sci U S A. 2008;105:2687–2692. doi: 10.1073/pnas.0712062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci Off J Soc Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Collin M, Backberg M, Ovesjo ML, Fisone G, Edwards RH, Fujiyama F, Meister B. Plasma membrane and vesicular glutamate transporter mRNAs/proteins in hypothalamic neurons that regulate body weight. Eur J Neurosci. 2003;18:1265–1278. doi: 10.1046/j.1460-9568.2003.02840.x. [DOI] [PubMed] [Google Scholar]
- 211.Ren X, Ferreira JG, Yeckel CW, Kondoh T, de Araujo IE. Effects of ad libitum ingestion of monosodium glutamate on weight gain in C57BL6/J mice. Digestion. 2011;83(Suppl 1):32–36. doi: 10.1159/000323405. [DOI] [PubMed] [Google Scholar]
- 212.Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, Ding JB, Yang Z, Sabatini BL, Lowell BB. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73:511–522. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Liu T, Wang Q, Berglund ED, Tong Q. Action of neurotransmitter: a key to unlock the AgRP neuron feeding circuit. Front Neurosci. 2012;6:200. doi: 10.3389/fnins.2012.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ploj K, Albery-Larsdotter S, Arlbrandt S, Kjaer MB, Skantze PM, Storlien LH. The metabotropic glutamate mGluR5 receptor agonist CHPG stimulates food intake. Neuroreport. 2010;21:704–708. doi: 10.1097/WNR.0b013e32833b4fe7. [DOI] [PubMed] [Google Scholar]
- 215.Bisaga A, Danysz W, Foltin RW. Antagonism of glutamatergic NMDA and mGluR5 receptors decreases consumption of food in baboon model of binge-eating disorder. Eur Neuropsychopharmacol. 2008;18:794–802. doi: 10.1016/j.euroneuro.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Razolli DS, Solon C, Roman EA, Ignacio-Souza LM, Velloso LA. Hypothalamic action of glutamate leads to body mass reduction through a mechanism partially dependent on JAK2. J Cell Biochem. 2012;113:1182–1189. doi: 10.1002/jcb.23445. [DOI] [PubMed] [Google Scholar]
- 217.Kiss J, Csaba Z, Csaki A, Halasz B. Glutamatergic innervation of neuropeptide Y and pro-opiomelanocortin-containing neurons in the hypothalamic arcuate nucleus of the rat. Eur J Neurosci. 2005;21:2111–2119. doi: 10.1111/j.1460-9568.2005.04012.x. [DOI] [PubMed] [Google Scholar]
- 218.Eyigor O, Centers A, Jennes L. Distribution of ionotropic glutamate receptor subunit mRNAs in the rat hypothalamus. J Comp Neurol. 2001;434:101–124. doi: 10.1002/cne.1167. [DOI] [PubMed] [Google Scholar]
- 219.Yang YK, Harmon CM. Recent developments in our understanding of melanocortin system in the regulation of food intake. Obes Rev. 2003;4:239–248. doi: 10.1046/j.1467-789x.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- 220.Williams KW, Scott MM, Elmquist JK. Modulation of the central melanocortin system by leptin, insulin, and serotonin: co-ordinated actions in a dispersed neuronal network. Eur J Pharmacol. 2011;660:2–12. doi: 10.1016/j.ejphar.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sternson SM, Atasoy D, Betley JN, Henry FE, Xu S. An emerging technology framework for the neurobiology of appetite. Cell Metab. 2016;23:234–253. doi: 10.1016/j.cmet.2015.12.002. [DOI] [PubMed] [Google Scholar]