Abstract
Obesity is a prognostic risk factor in the progression of prostate cancer (PCa), however, the molecular mechanisms involved are unclear. In this study, we provide preclinical proof of concept for the role of a pro-inflammatory CXCL12-CXCR4/CXCR7 signaling axis in an obesity-driven mouse model of HiMyc prostate cancer. Analysis of the stromal vascular fraction (SVF) from periprostatic white adipose tissue (ppWAT) from obese HiMyc mice at 6 months of age revealed a dramatic increase in mRNAs encoding various chemokines, cytokines, growth factors and angiogenesis mediators, with CXCL12 among the most significantly upregulated genes. Immunofluorescence staining of ventral prostate tissue from obese HiMyc mice revealed high levels of CXCL12 in the stromal compartment as well as high staining for CXCR4 and CXCR7 in the epithelial compartment of tumors. PCa cell lines derived from HiMyc tumors (HMVP2 and derivative cell lines) displayed increased protein expression of both CXCR4 and CXCR7 compared to protein lysates from a non-tumorigenic prostate epithelial cell line (NMVP cells). CXCL12 treatment stimulated migration and invasion of HMVP2 cells but not NMVP cells. These effects of CXCL12 on HMVP2 cells were inhibited by the CXCR4 antagonist AMD3100 as well as knockdown of either CXCR4 or CXCR7. CXCL12 treatment also produced rapid activation of STAT3, NFkB, and MAPK signaling in HMVP2 cells which was again attenuated by either AMD3100 or knockdown of CXCR4 or CXCR7. Collectively, these data suggest that CXCL12 secreted by stromal cells activates invasiveness of PCa cells and may play a role in driving tumor progression in obesity. Targeting the CXCL12-CXCR4/CXCR7 axis could lead to novel approaches for offsetting the effects of obesity on PCa progression.
Keywords: CXCL12, CXCR4, CXCR7, Prostate cancer, Obesity, WAT, HiMyc
Introduction
Data associating obesity and PCa risk have been inconclusive [1, 2], however, a number of studies have shown increased risk of biochemical failure and metastasis, as well as poorer survival among obese PCa patients with androgen-dependent tumors, especially those who experienced rapid weight gain [3]. Men with low-volume PCa have a lower BMI, less body fat, and a smaller waist-to-hip ratio than men with high-volume PCa, which agrees with other reported findings [4, 5]. Fat is the most energy dense dietary component and has been the focus of most PCa dietary epidemiologic investigations. High consumption of energy and fat, especially saturated fat [6, 7] is associated with advanced stage PCa and mortality [6, 8]. As the prevalence of obesity increases [9], a clearer understanding of the underlying mechanism(s) and its link with cancer aggressiveness are urgently needed to develop new strategies for reducing PCa related morbidity and mortality.
Abdominal obesity, resulting from overgrowth of visceral white adipose tissue (WAT), has been specifically linked with progression of PCa [10, 11]. The state of chronic inflammation ensuing as a result of pathological WAT overgrowth has been proposed to play a role in linking obesity and cancer [12, 13]. WAT is a potent endocrine organ [14], comprised of mature adipocytes, together with other cell types known specifically as the stromal vascular fraction (SVF). The SVF consists of mesenchymal adipocyte progenitors termed adipose stromal cells (ASCs), monocytes, macrophages, and other infiltrating leukocytes [13, 15]. These cells contribute to the production of adipokines, among which IGF-1, IL-6, TNFα, leptin, adiponectin and steroid hormones are implicated in cancer [16, 17]. Specifically, periprostatic WAT (ppWAT), which is expanded in obesity, is believed to play a significant role in promoting PCa progression [18, 19].
Recent studies have suggested an important role of chemokines in PCa progression [20, 21]. Chemokines are small chemotactic cytokines involved in a variety of developmental processes in addition to their function in recruitment and activation of leukocytes and directed migration of invasive cancer cells [22–24]. One of the chemokines promoting cancer progression is CXCL12, also known as stromal-derived factor-1 (SDF-1). CXCL12 is a potent chemoattractant for hematopoietic cells [25] and activates signaling events through two different receptors, CXCR4 and CXCR7 [24]. Binding of CXCL12 to CXCR4 induces trimeric G protein signaling leading to activation of the Src, PI3K/AKT, ERK, and JNK pathways, contributing to protease production and cellular migration and invasion [22, 26]. CXCR4 has been shown to be a key receptor in mediating the metastasis of multiple types of tumors [27]. The role of CXCL12-CXCR4 axis in cancer cell migration, invasion and metastasis has been demonstrated [28–31]. AMD3100, a CXCR4 inhibitor, sensitizes PCa to chemotherapy [32], however the main source of CXCL12 and its contribution to obesity-associated cancer progression has not been investigated.
We previously reported the effect of dietary energy balance manipulation on cancer development and progression in HiMyc mice [33], a spontaneous genetic PCa model. The HiMyc mouse tumors share molecular and histopathologic features with human prostate tumors [34]. c-Myc is overexpressed in many cancers including PCa [35, 36]. The MYC locus (8q24) is amplified in patients [37], which correlates with high histological grade scores and a worse prognosis [38]. Upregulation of nuclear c-MYC protein expression is a highly prevalent and early change in human tumors, which suggests that it drives cancer initiation [39]. In the HiMyc model, over-expression of c-Myc in the prostate is directed via the ARR2Pb probasin promoter [34]. Prostatic epithelial expression of c-Myc in the dorsolateral prostate (DLP), VP, and anterior prostate (AP) lobes results in complete penetrance of PIN as early as 2 to 4 weeks of age, which progressed to locally invasive adenocarcinomas within 6 to 12 months of age [34]. While the HiMyc model is not metastatic, it is advantageous for studying the obesity-cancer relationship because there is sufficient time to establish DIO and study disease progression.
We recently reported the effect of dietary energy balance manipulation across the spectrum of CR to DIO on PCa development and progression in HiMyc mice [33]. Male HiMyc mice were placed on three dietary regimens, 30% CR, a modified AIN76A with 10kcal% fat, and a DIO regimen (60kcal% fat), resulting in cohorts of lean, overweight and obese animals. All diet groups had an approximately similar incidence of hyperplasia and low grade PIN in the VP at 3 and 6 months of age. However, 30% CR significantly reduced the incidence of in situ adenocarcinoma at 3 months compared to the DIO group and at 6 months compared to both the overweight control and DIO groups. The DIO regimen significantly increased the incidence of adenocarcinoma with aggressive stromal invasion, as compared to the overweight group (96% versus 65% respectively, p=0.02) at the 6-month time point. Additionally, at both 3 and 6 months, only in situ carcinomas were observed in mice maintained on the 30% CR diet. Expression of both growth factor and inflammatory genes was significantly increased in prostate tissue obtained from obese HiMyc mice compared to mice on both the control and CR diets [33].
Here, we provide evidence that CXCL12 signaling through CXCR4 and CXCR7 mediates obesity-induced PCa progression in HiMyc mice [33]. We show that DIO leads to increased expression of CXCL12 from the SVF of ppWAT and demonstrate that CXCL12 is expressed by ASCs. CXCL12 selectively stimulated migration and invasion of murine PCa cells derived from HiMyc mice (HMVP2 cells) [40] compared to non-tumorigenic prostate epithelial cells derived from FVB/N mice. CXCL12 also induced several important oncogenic signaling pathways including STAT3, NFκB and MAPK in PCa cells. The migration, invasion and activation of signaling events were abrogated by inhibition (either pharmacologically or genetically) of CXCR4 or CXCR7 indicating that CXCL12 functions through these receptors in mouse PCa cells. Collectively, these data demonstrate an important role of ASC-derived CXCL12, in obesity-driven PCa progression.
Materials and Methods
Cell culture
The murine prostate cell lines NMVP, HMVP1, HMVP2, HMVP2A1 and HMVP2A2 were generated in our laboratory from the mouse ventral prostate in 2012 as described previously [40] and we periodically check for the expression of prostate epithelial cell markers. No futher authentication was performed. NMVP cells were cultured in DMEM/F12 (Lonza) medium containing 10% fetal bovine serum (FBS), bovine pituitary extract (28 mg/ml) (Hammond Cell Tech), epidermal growth factor (10 ng/ml) (Sigma-Aldrich), insulin (8 mg/ml) (Sigma-Aldrich) and transferrin (5 mg/ml) (Sigma-Aldrich). All other cell lines were cultured in RPMI-1640 (Life Technologies) medium containing 10% FBS (Life Technologies). Cell lines were regularly (every two months) evaluated for mycoplasma by PCR with commercially available PCR mycoplasma detection kit (Applied Biological Materials Inc.). All experiments were performed within one month after thawing the cells. All cells were maintained in 95% air and 5% CO2 at 37°C.
Isolation of SVF
HiMyc mice [34] were obtained from the NIH MMRRC on an FVB/N genetic background and bred in-house for the current experiments. Diets were purchased from Research Diets Inc. For all the studies, mice were housed in suspended polycarbonate cages on autoclaved hardwood bedding at room temperatures of 20°C–22°C, relative humidity of 60–70%, and 14/10-hour light/dark cycle. All animal studies have been conducted in accordance with an approved Institutional (The Univerity of Texas at Austin) Animal Care and Use Committee (IACUC) protocol. Mice were placed on AIN76A semipurified diet at 4 to 5 weeks of age for a 1-week equilibration period and then randomized into the following dietary groups for the duration of the study: a) 30% calorie restriction (CR) diet, b) control diet (continued on the modified AIN76A diet, 10 kcal% fat), fed ad libitum; and c) diet induced obesity [41] diet (60 kcal% fat), fed ad libitum as previously described [33]. Groups of mice were terminated by CO2 asphyxiation at 6 months age and ppWAT were collected and immersed in DMEM (Life Technologies) media. The pooled (n=5) fat tissues were minced into fine pieces and resuspended in equal volume of digestion solution containing 0.5 mg/mL collagenase type 1(Sigma-Aldrich) and 25 u/mL dispase (BD) in phosphate buffer saline (PBS). The resulting mixture was incubated on water bath at 37°C for 1h with through shaking every 10 minutes. Then the samples were centrifuged at 300g for 5 min and the supernatant including the upper white layer (adipocytes) were aspirated and the cell pellet were resuspended in DMEM containing 5% FBS and 2mM EDTA (Sigma-Aldrich) and filtered through 100 μm nylon cell strainer followed by rinsing the filter with additional DMEM solution. The cell suspensions were centrifuged at 300g for 10 min, and the pellet were resuspended in PBS with 2mM EDTA. Finally, the cell suspensions were centrifuged at 300g for 10 min to isolate the SVF.
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from the SVF by using Qiagen RNeasy mini kit (Qiagen) according to the manufacturer’s protocol and subjected to reverse-transcription as previously described [33]. mRNA levels of genes were quantitatively determined by qRT-PCR using the Viia7 Real Time PCR System (Applied Biosystems) with SYBR Green Master Mix (Qiagen). The relative abundance of the mRNA was normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA for quantitative evaluation.
Immunofluorescence (IF) and Immunocytochemical (ICC) analyses
IF analyses were performed on formalin fixed, paraffin-embedded prostate tissue sections and ICC analyses were performed on cultured HMVP2 cells as described previously [40, 42]. Briefly, tissue sections were deparaffinized with serial incubation and washing in xylene, 100-70% ethanol and water followed by antigen unmasking with citrate buffer. The samples were then blocked for 1h at room temperature and incubated with primary antibodies [Perilipin (9349) from Cell Signaling, CD3 (550275) from BD Pharmingen, RM0029-11H3 (ab56297), CXCR7 (ab72100) from Abcam, CXCL12 (sc-28876) from Santa Cruz, CXCR4 (MAB21651) from R&D Systems and αSMA (C6198) from Sigma] overnight at 4°C. Proteins were detected with fluorochrome-conjugated secondary antibodies and visualized using an Olympus BX60 fluorescence microscope.
Migration Assay
The migration of cells was measured by widely used wound healing or scratch assay [43]. In brief, cells were seeded in 12 well plate, allowed to grow overnight followed by starvation. Scratch was made in the monolayer confluent cells with sterile 200 μL pipette tips and the cells were treated with CXCL12 either alone or in combination with AMD3100 (Abcam) for 18h. The images were acquired at 0 and 18 hours time point and the migration was calculated from the distance of the closure of the scratch before and the after the treatment.
Transwell Invasion Assay
The invasion of cells was measured by matrigel transwell invasion chamber (EMD Millipore) assay [44]. Cells were seeded into the upper chamber and the inserts were placed in 24 well plate containing CXCL12, AMD3100 or their combination. 18h after incubation in tissue culture incubator, the cells were fixed with 10% formalin solution (Fisher Scientific), permeabilized with methanol and stained with Giemsa stain (Sigma-Aldrich). After washing with water, the non-migrated cells were scraped from the upper chamber with cotton swab, and migrated cells remaining on the bottom surface were counted with an inverted microscope.
Knockdown of CXCR4 and CXCR7
Knockdown of CXCR4 and CXCR7 in HMVP2 cells were performed with pGFP-C-shLenti vector from Origene according to the manufacturer’s instructions. Mouse CXCR4 or CXCR7 29mer shRNA constructs (TL500383 and TL515747) with 4 different sequences was used for both CXCR4 or CXCR7 knockdown and scrambled negative control non-effective shRNA cassette in pGFP-C-shLenti plasmid (TR30021) was used as shRNA control. Cells were infected with lentivirus for 2 days and stable CXCR4 or CXCR7 knockdown cells were generated by puromycin selection. The knockdown efficiency was measured by quantitative real-time PCR (qPCR) and Western blotting.
Western blotting
The following antibodies were used for Western blotting: STAT3 (9139), pSTAT3Tyr705 (9145), JNK (9252), pJNKThr183/185 (9251), AKT (9272), pAKTSer473 (4060), ERK1/2 (9102), pERK1/2Thr202/Tyr204 (4370), pNFκBp65Ser536 (3033) from cell Signaling; CXCR4 (ab124824), CXCR7 (ab72100) from Abcam; NFκBp65 (sc-372), β-actin (A5316) from Sigma-Aldrich. After plating cells were serum starved overnight and treated with CXCL12 for indicated time periods. In some experiments cells were pretreated with AMD3100 2h before the CXCL12 treatment. After incubation, cells were lysed in RIPA buffer. Proteins were separated by SDS-PAGE gel and transferred to nitrocellulose membranes. Levels of phosphorylated and total proteins were measured by Western blot as described previously [33].
Statistical analyses
Statistical analyses were performed using two-tailed Student’s t-test or one-way ANOVA followed by Bonferroni’s multiple comparison tests as applicable. Statistical significance was set at p<0.05.
Results
Recruitment of adipocytes and inflammatory cells to the ventral prostate (VP) of obese HiMyc mice
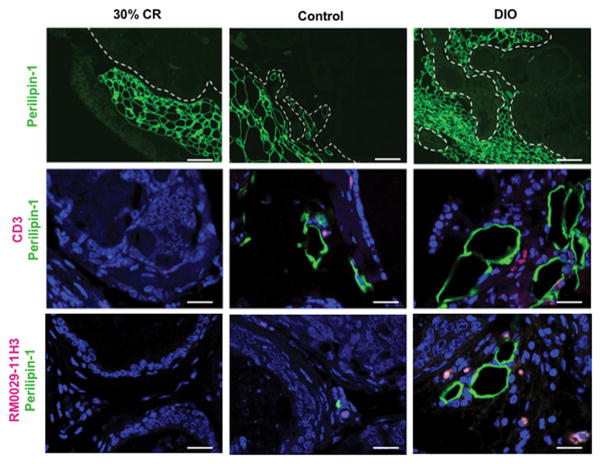
HiMyc mice were placed on the three dietary regimens: 30% CR (lean), ad libitum 10 kcal% diet (control) and ad libitum 60 kcal% diet (DIO) [41]. At 6 months of age, VP tissues were subjected to IF analyses. As shown in Fig. 1 (Top panels), staining for perilipin-1, a lipid droplet coat protein, revealed that adipocytes were restricted to ppWAT at the periphery of the VP of lean HiMyc mice. In contrast, adipocytes could be seen infiltrating the VP gland of control HiMyc mice. Notably, in DIO mice, many of the glands in the VP were completely surrounded by adipocytes. We also stained the VP tissues from mice on the three dietary regimens with a CD3 antibody. This revealed infiltration of T-lymphocytes (Fig. 1, middle panels) in the VP of control diet mice, which was even more pronounced in the DIO mice. Similarly, infiltration of macrophages (detected with antibody RM0029-11H3) was dramatically higher in the VP gland of DIO mice compared to the other two diet groups (Fig. 1, bottom panels). Inflammatory cells were observed in close proximity of the infiltrating adipocytes in the VP of the DIO diet group. Collectively, these results suggest that adipocytes and inflammatory cells recruited into the tumor stroma in obesity might contribute to accelerated PCa progression.
Figure 1.
Recruitment and infiltration of adipocytes and inflammatory cells in the ventral prostate of HiMyc mice as a function of dietary energy balance manipulation. Immunofluorescence analysis of representative prostate tissue sections from HiMyc mice fed on 30 %CR, control or DIO diets with antibodies against perilipin-1, CD3 (T-lymphocytes) and RM0029-11H3 (macrophages) with red and green fluorophore conjugated secondary antibodies. Representative images of tissue sections from 5 mice per group are shown. All tissue sections from each diet group gave similar results. Scale bar 200 μm (top row), 25 μm (middle and bottom rows).
Obesity-induced gene expression changes in the SVF of ppWAT from HiMyc mice
To identify factors secreted by obesity-recruited stroma, we isolated the SVF from the ppWAT of control and obese mice. mRNA was analyzed by qRT-PCR for expression of selected genes previously implicated in the obesity-cancer link [33, 45]. As shown in Supplemental Fig. 1A, we observed highly significant upregulation of mRNA levels for IGF-1, IL-6, CCL5, IKKα, cyclinD1, cMyc, NFκB1 and RelA (NFκBp65) in SVF from the obese mice. mRNAs for a number of other genes [TGFβ, PDGFα, PDGFβ, HIF-1α, VEGF (A, B, C), IL-1α, IL-1β, IL-7, IL-17, IL-23, IL-23R and TNFα] were also significantly upregulated in the SVF from from ppWAT of obese HiMyc mice compared to mice on the control diet (Supplemental Fig. 1B–C). These data indicate that ASCs and possibly other SVF cells present in ppWAT secrete growth factor and inflammatory mediators that may play an important role in driving PCa progression in obesity.
CXCL12 and its receptors are elevated in VP of obese HiMyc mice
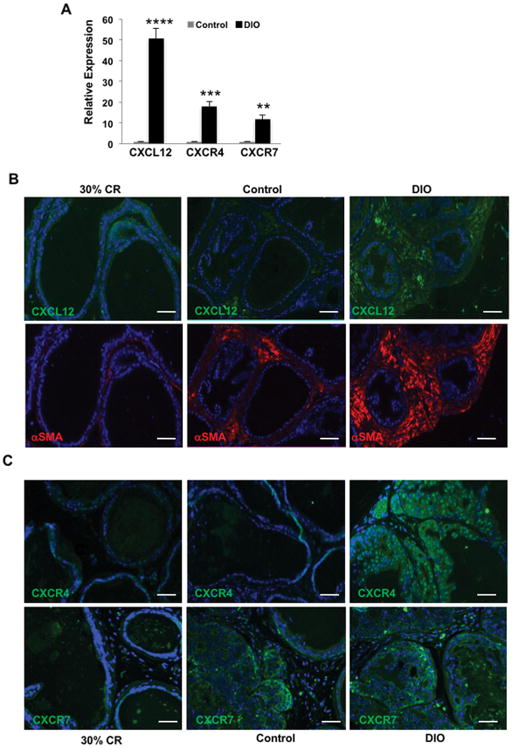
Analysis of mRNA levels demonstrated that CXCL12 expression was highly upregulated in the SVF of ppWAT from obese HiMyc mice (Fig. 2A). Expression of CXCL12 receptors, CXCR4 and CXCR7, was also increased. It has been reported that ASCs secrete large amounts of CXCL12 [46], however CXCL12 expression in ppWAT has not been investigated. In order to determine the cellular location of CXCL12 and its receptors in the VP of HiMyc mice, tissue sections from mice on the three different diets were analyzed. As shown in Fig. 2B (top panel), we detected a higher IF signal for CXCL12 protein in the VP stroma of obese HiMyc mice compared to CR and control diet mice. α-SMA, a myofibroblast marker whose expression is activated in obesity-associated cancer progression [47] was expressed in CXCL12-positive stroma (Fig. 2B, bottom panels). Expression of CXCR4 and CXCR7, two receptors for CXCL12, was detected primarily in the tumor epithelial compartment in the VP of HiMyc mice (Fig. 2C) and was highest in the VP of obese mice.
Figure 2.
A, Increased mRNA expression of CXCL12, CXCR4 and CXCR7 in the SVF of obese HiMyc mice. mRNA was isolated from the SVF of adipose tissue surrounding the ventral prostate of HiMyc mice at 6 months of age. Fold change in gene expression in obese mice fed the DIO (60kcal% fat) diet relative to mice fed the control (10kcal% fat) diet is shown. Quantitation of fold change of mRNA is presented as mean ± SEM of three independent experiments. Significantly different, *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001 (Student’s t-test). B,C, Immunofluorescence analysis of representative prostate tissue sections from age-matched HiMyc mice fed on CR, Control or DIO diet with antibodies against CXCL12, α-SMA, CXCR4 and CXCR7 with red and green fluorophore-conjugated secondary antibodies. Shown are representative images from one mouse out of a group of 5 mice. All tissue sections from each diet group gave similar results. Scale bar 100 μm.
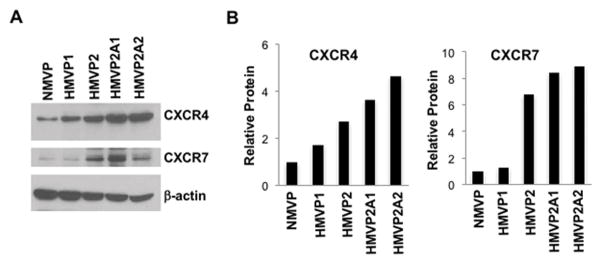
We also compared the expression of CXCL12 and and its receptors in cultured immortalized mouse ASCs and HMVP2 cells derived from HiMyc tumors [40]. As shown in Supplemental Fig. 1D, the mRNA expression of CXCL12 was significantly higher in the mASCs compared to the HMVP2 cells. In contrast, CXCR4 and CXCR7 mRNA expression was higher in HMVP2 cells than in ASCs. Interestingly, CXCR4 and CXCR7 proteins were expressed in HMVP2 cells as well as in two other more tumorigenic cell lines (HMVP2A1 and HMVP2A2 derived from in vivo/in vitro passage of HMVP2 cells [40]) to a greater extent than NMVP (non-tumorigenic) and HMVP1 (weakly tumorigenic) cells (Fig. 3A). Quantification revealed that HMVP2 cells had ~2.5 and ~6 fold higher expression of CXCR4 and CXCR7, respectively, compared to NMVP cells and the two additional PCa cell lines had even higher expression of these receptors (Fig. 3B). Protein expression of CXCR4 and CXCR7 in the more aggressive cancer cells was confirmed by IF (data not shown). These results demonstrate that CXCR4 and CXCR7 expression in PCa epithelium is associated with obesity and cancer aggressiveness.
Figure 3.
Increased CXCR4 and CXCR7 protein expression in cancer cell lines. A, Protein lysates prepared from cultured cells were subjected to Western blotting. B, Relative protein levels were quantitated by densitometry; β-actin was used to control for protein loading. Representative blots of two separate experiments with similar results are shown. Note increased expression in PCa cells compared to normal mouse (NMVP) prostate epithelial cells.
CXCL12 selectively stimulates migration and invasion of HMVP2 PCa cells
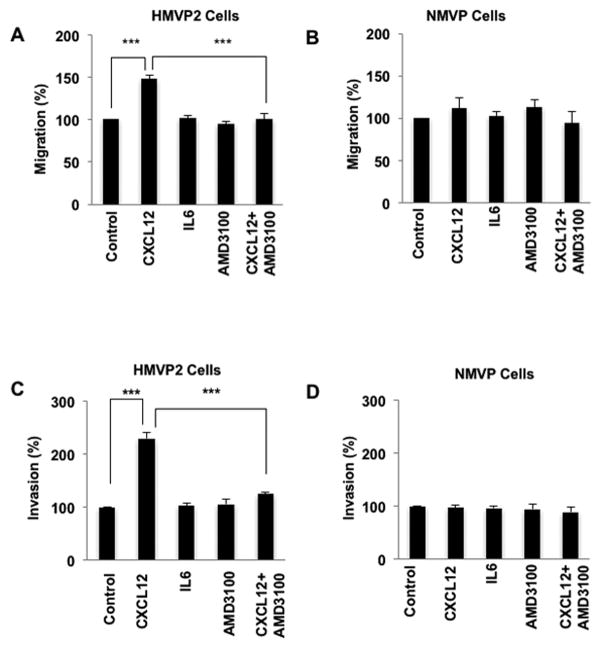
CXCL12 is implicated in chemotaxis of various cell types (reviewed in[24]). Therefore, we examined the effect of CXCL12 on migration and invasion of HMVP2 cells compared to NMVP cells that have lower CXCR4 and CXCR7 protein expression. In these experiments, we also compared the effect of IL-6, which was also found to be highly upregulated in the SVF from ppWAT of obese HiMyc mice (Supplemental Fig. 1A). CXCL12 had no significant effects on proliferation of any of the cell lines (data not shown). However, CXCL12 stimulated migration of HMVP2 cells (Fig. 4A and Supplemental Fig. 2A) but not NMVP cells (Fig. 4B and Supplemental Fig. 2B). In contrast, IL-6 treatment had little or no stimulatory effect on the migration of either HMVP2 or NMVP cells. To further investigate the role of CXCL12 and its receptor, CXCR4, on migration, we pretreated the cells with the CXCR4 inhibitor, AMD3100 [32], and then stimulated with CXCL12. As shown in Fig. 4, AMD3100 significantly inhibited the CXCL12-induced migration of HMVP2 cells.
Figure 4.
CXCL12 induces migration and invasion of HMVP2 cells. A, B. cells in 12 well plates scratched with 200 μl pipette tips and treated with vehicle control, CXCL12 (100 ng/mL), IL-6 (10 ng/mL), AMD3100 (40 ug/mL) or CXCL12 + AMD3100 at 0 and 18 h post-treatment. Quantitation of migration levels are presented as mean ± SEM of three independent experiments. ***P<0.001; (One Way ANOVA). C, D. NMVP and HMVP2 cells were seeded in Matrigel in the upper transwell chambers and then placed into 24 well plates with medium that contained the vehicle (control), CXCL12 (100 ng/mL), IL-6 (10 ng/mL), AMD3100 (40 ug/ml) or CXCL12 + AMD3100. After 18h, the invading cells in the lower chamber were fixed, stained and visualized by light microscopy. Quantitation of invading cells presented as mean ± SEM of three independent experiments. ***P<0.001; (One Way ANOVA)
As shown in Fig. 4C and Supplemental Fig. 3A, CXCL12 also significantly stimulated invasion of HMVP2 cells but had little effect on the invasion of NMVP cells (Fig. 4D and Supplemental Fig. 3B). Again, IL-6 had little or no effect on the invasive properties of HMVP2 cells in this assay. Pretreatment with AMD3100 inhibited CXCL12-induced invasion of HMVP2 cells (Fig. 4). Collectively, these results indicate that CXCL12 stimulates migration and invasion of HMVP2 cells through its receptor, CXCR4. In addition, the data demonstrate a selectivity for the tumor cells compared to non tumorigenic cells based on the level of expression of CXCR4.
Silencing of CXCR4 or CXCR7 attenuates CXCL12 induced migration and invasion
In order to investigate whether CXCL12-induced migration and invasion of HMVP2 cells is mediated through both CXCR4 and CXCR7, we performed migration and invasion assays with CXCR4 and CXCR7 knockdown cells. We successfully knocked down CXCR4 and CXCR7 with two different targeting sequences, as confirmed by both qRT-PCR (Supplemental Fig. 4A) and Western blotting (Supplemental Fig. 4B and C). We performed migration and invasion analysis with CXCR4 and CXCR7-silenced cells in parallel with HMVP2 cells transfected with control non-targeted scrambled shRNA. As shown in Fig. 5 (panels A and Supplemental Fig. 5A), treatment with CXCL12 induced significantly less migration in both CXCR4 shRNA expressing cells compared to the cells with control shRNA. CXCR4 knockdown also decreased the invasiveness of HMVP2 cells, compared the control shRNA-expressing cells, following CXCL12 treatment (Fig. 5, panel B and Supplemental Fig. 5B). Similarly, CXCR7 silencing also decreased the CXCL12-dependent migration (Fig. 5C and Supplemental Fig. 5C) and invasion (Fig. 5D and Supplemental Fig. 5D) of HMVP2 cells. These results further confirm that CXCL12 induces migration and invasion of HMVP2 cells by signaling thorugh both of its receptors CXCR4 and CXCR7.
Figure 5.
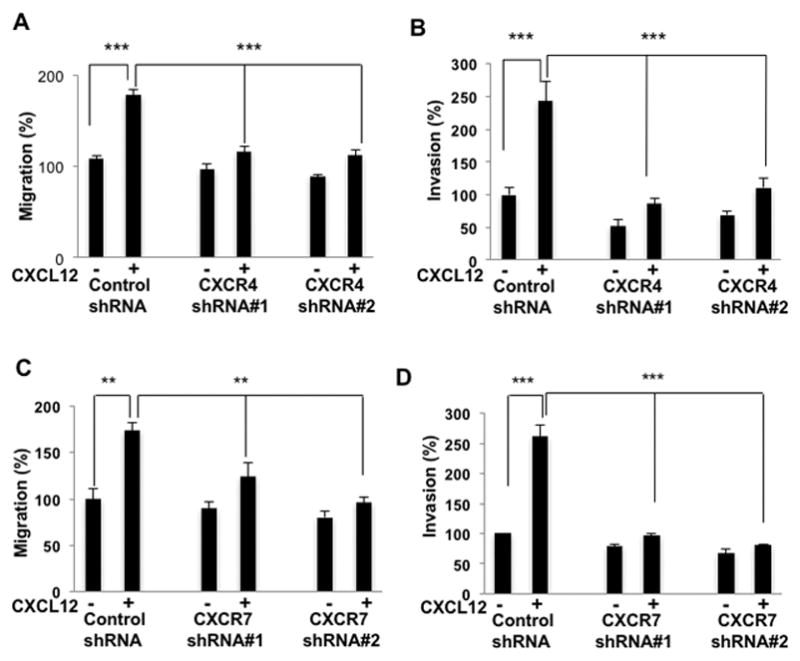
CXCR4 and CXCR7 knockdown decreases migration and invasion in HMVP2 cells. Cells (A, transfected with control or CXCR4 shRNA; C, transfected with control or CXCR7 shRNA) in 12 well plates scratched with 200 μl pipette tips and treated with vehicle control or CXCL12 (100 ng/mL). Quantitation of migration levels were presented as mean ± SEM of three independent experiments. ***P<0.001; (Student’s t-test). Cells (B, transfected with control or CXCR4 shRNA; D, transfected with control or CXCR7 shRNA) were seeded in Matrigel in the upper transwell chambers and then placed into 24 well plates with medium that contained the vehicle (control) or CXCL12 (100 ng/mL). After 18h, the invading cells in the lower chamber were fixed, stained and visualized by light microscopy. Quantitation of invading cells were presented as mean ± SEM of three independent experiments. ***P<0.001; (Student’s t-test)
CXCL12 induces cancer-associated signaling pathways in HMVP2 cells
Previous reports indicate that binding of CXCL12 to its receptor(s) activates various cell signaling pathways such as PI3K/AKT, ERK and JNK pathways [24, 27]. As shown in Figure 6, CXCL12 treatment of HMVP2 cells led to activation of signaling pathways, including AKT, STAT3, NFκB and MAPKs (both JNK and ERK), as assessed by the phosphorylation status of these proteins over a 30 min time course. We also examined the effect of CXCL12 on these signaling pathways in NMVP cells. Treatment of cells with CXCL12 led to a rapid (15 min) activation of multiple signaling pathways in HMVP2 cells but not NMVP cells (Figure 6, panels A and B). This is consistent with lower CXCR4 and CXCR7 expression level in NMVP and supports the hypothesis that the activation of these signaling events is mediated through CXCR4 and CXCR7. The involvement of CXCR4 was further confirmed in HMVP2 cells pretreated with AMD3100 which led to significantly decreased activation of STAT3, NFκB, ERK and JNK signaling (see Supplemental Fig. 6).
Figure 6.
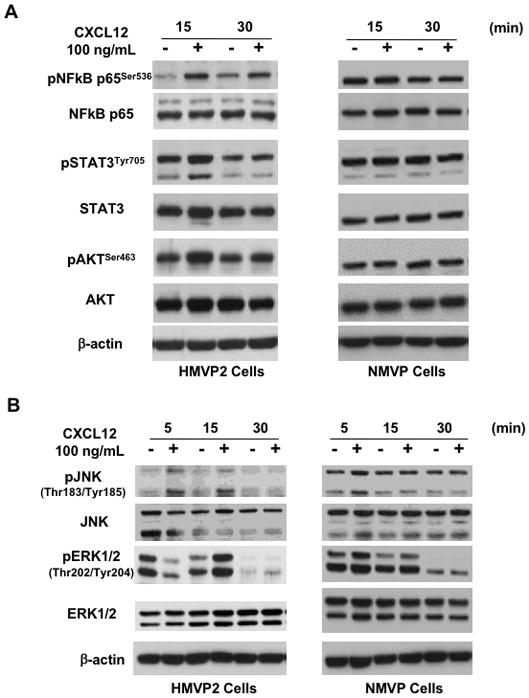
Activation of NFκB, STAT3 and MAPK signaling by CXCL12 in HMVP2 cells. A,B. Protein lysates were prepared from cultured NMVP and HMVP2 cells treated with CXCL12 (100 ng/mL) for 5 to 30 min and subjected to Western blot analysis using antibodies for the indicated phospho and total proteins. Relative protein levels were quantitated by densitometry; β-actin was used to control for protein loading. Representative blots of three separate experiments with similar results are shown.
Finally, we used CXCR4 and CXCR7 knockdown HMVP2 cells to measure CXCL12-induced signaling. As shown in Supplemental Fig. 7, treatment with CXCL12 led to activation of STAT3, NFκB and JNK in control shRNA expressing cells similar to the parental HMVP2 cells. However, cells with CXCR4 knockdown showed lower activation of these signaling pathways upon treatment with CXCL12. Similarly, knockdown of CXCR7 also led to decreased activation of these signaling pathways upon stimulation with CXCL12 (Supplemental Fig. 8).
Discussion
Obesity is a risk factor for higher grade and more aggressive PCa and a number of mechanisms have been proposed to account for this observation [48]. Recent attention has turned to ppWAT as a source of locally produced factors that may drive tumor progression [49]. Furthermore, evidence from our laboratories has revealed that ASCs, the perivascular mesenchymal stem cells, serve as adipocyte progenitors in the WAT and represent a cell population that may be of particular importance to the obesity-cancer link [50]. In mouse models, ASCs have been shown to infiltrate tumors and promote cancer progression by increasing vascularization, and stimulating malignant cell survival and proliferation [51–53]. Our recent report provides evidence that in obese PCa patients, ASCs are guided to tumors by chemokines, CXCL1 and CXCL8 [20]. Here, by using both in vivo and in vitro models, we show that the SVF of ppWAT is a rich source of growth factors, cytokines and chemokines that are produced at high levels in obese mice. The role of CXCL12-CXCR4 axis in progression of various cancers including PCa is well documented [28–31]. However, the cell source of CXCL12 has remained unclear and whether this chemokine gradient contributes to caner-promoting effects of obesity has remained a subject of debate.
Here, we provide evidence that ASC-derived CXCL12 signaling via CXCR4 and CXCR7 in malignant epithelial cells contributes to PCa progression in obese HiMyc mice. As we recently reported, the DIO regimen significantly increased whereas 30% CR significantly reduced the incidence and progression rate of locally invasive adenocarcinoma in HiMyc mice [33] As shown in Fig. 1, increased adiposity in HiMyc mice led to infiltration of adipocytes and inflammatory cells (T-lymphocyte and macrophages) into the stromal compartment of the prostate tumors that develop in the VP of these mice. In previous studies, we also showed that there was a dramatic increase in both macrophages and T-cells in the stromal compartment of these tumors consistent with the current results [33]. Analysis of the SVF from ppWAT of obese HiMyc mice revealed dramatic upregulation (mRNA) of various chemokines (including CXCL12 and its receptors CXCR4 and CXCR7), cytokines, growth factors and other signaling molecules known to be involved in cancer and cancer progression. Furthermore, immunofluorescence staining confirmed high levels of CXCL12 protein in the stromal compartment of the VP of obese HiMyc mice and high protein expression of CXCR4 and CXCR7 in the epithelial compartment of the tumors. Thus, obesity dramatically affected the tumor microenvironment supporting the hypothesis that these changes contributed to the observed effects on tumor progression [33].
CXCL12 and its receptors have been described in a variety of solid tumors and tumor cells including breast, lung, prostate, and pancreatic cancers (reviewed in ref. [24]), are strongly linked to PCa bone metastasis and have been shown to be markers for poor prognosis [54, 55]. By using cultured mouse ASCs, we found that these cells do express high mRNA levels of CXCL12 relative to HMVP2 PCa tumor cells indicating that the dramatically increased expression of CXCL12 in the SVF of obese HiMyc mice might be contributed by the ASCs that are present. It is also possible that other cells in the ppWAT, including macrophages and neutrophils [56] may contribute to the increase in CXCL12, as well as a number of other molecules that were found to be upregulated, and this remains to be fully explored. Nevertheless, we believe that the ASCs are an important source of CXCL12 and other chemokines, especially in the obese state that can drive tumor progression and represent a potential target for therapeutic interventions. The current results support the hypotheses that upregulation of CXCL12 in either in the ppWAT, the tumor stromal compartment or both as well as upregulation of CXCR4 and CXCR7 in the prostate cancer epithelial cells contribute to the effects of obesity on PCa progression in HiMyc mice. These data also suggest the possibility that differences in levels of CXCR4 and CXCR7 in subpopulations of tumor cells may provide selectivity in response to CXCL12 in driving tumor progression. HMVP2 cells represent a tumor initiating cell line with stem/progenitor properties as previously described [40]. As shown in Fig. 3, these cells expressed relatively high levels of CXCR4 and CXCR7 compared to NMVP and HMVP1 cells. In further support of this hypothesis, Dubrovska et al reported that in human PCa cell lines (DU145 and PC3), CXCR4 expression was elevated in the CD44+/CD133+ subpopulation of cells and that these cells were considerably more tumorigenic [57] and recent evidence suggests that CXCR7 is involved in maintaining the stem like properties of breast, glioblastoma and lung cancer cells [58–60].
Using HMVP2 PCa cells, we found that CXCL12 significantly simulated their migration and invasion but did not produce similar effects on normal (immortalized) prostate epithelial cells (NMVP cells) derived from the VP of wild-type (FVB/N) mice. IL-6, a cytokine whose mRNA was also found to be highly upregulated in the SVF of ppWAT from obese HiMyc mice did not induce migration or invasion of HMVP2 cells. These effects of CXCL12 correlated with the levels of CXCR4 and CXCR7 protein detected in the cells and with selective activation of several key signaling pathways such as NFκB, STAT3 and MAPKs. Furthermore, blocking CXCR4 or CXCR7 either genetically or pharmacologically abrogated the effect of CXCL12 on migration, invasion and signal transduction. Collectively, these data further support the hypothesis that differences in levels of CXCR4 and CXCR7 in subpopulations of tumor cells may provide selectivity in response to CXCL12 in driving tumor progression.
The results presented here demonstrate that obesity induced PCa progression in HiMyc mice is mediated, at least in part, via the CXCL12-CXCR4/CXCR7 signaling axis. While these data confirm the importance of CXCL12 signaling, other factors are likely involved in obesity-driven PCa progression. Indeed, a number of other molecules were significantly upregulated in the SVF of ppWAT (Supplemental Fig. 1). Previously, other chemokines including CCL5, CCL7, CXCL1, CXCL8, CXCL16 and their receptors CCR3, CXCR1, CXCR2, and CXCR6, have been implicated in PCa progression [20, 21, 23]. In a recent report, Laurent et al. showed that periprostatic adipocytes secrete higher CCL7 in obesity and that this chemokine stimulates the invasion of CCR3-expressing tumor cells [21]. This suggests that in obesity CXCL12 may work together with other chemokines and adipokines to promote PCa aggressiveness. Finally, recent studies indicate that NF-κB activation in mesenchymal progenitors (such as ASCs) ‘licenses’ them to execute their immunosuppressive properties, which could contribute to tumor immune evasion and increased progression [61, 62]. Therefore, other mechanisms may also contribute to enhanced tumor progression in obesity such as altered immune surveillance.
In conclusion, the current data suggest that targeting the CXCL12-CXCR4/CXCR7 signaling axis may be a potential strategy for offsetting the effects of obesity on PCa progression. We demonstrate that ASCs are an important source of CXCL12 in the ppWAT as well as the tumor stroma. Several recent reports [51, 53, 63] identify ASCs as a potential target for therapeutic interventions that could simultaneously inactivate numerous signaling processes induced by these cells in the tumor microenvironment. Future studies will explore the possibility of using these approaches in combination with conventional cancer therapies.
Supplementary Material
Acknowledgments
Grant Support: This study was supported by NIH grant R01 CA196259 (to J. DiGiovanni and M. G. Kolonin) and Start-up funds (J. DiGiovanni) from the University of Texas at Austin.
References
- 1.Nomura AM. Body size and prostate cancer. Epidemiol Rev. 2001;23:126–31. doi: 10.1093/oxfordjournals.epirev.a000777. [DOI] [PubMed] [Google Scholar]
- 2.Porter MP, Stanford JL. Obesity and the risk of prostate cancer. Prostate. 2005;62:316–21. doi: 10.1002/pros.20121. [DOI] [PubMed] [Google Scholar]
- 3.Flavin R, Zadra G, Loda M. Metabolic alterations and targeted therapies in prostate cancer. J Pathol. 2011;223:283–94. doi: 10.1002/path.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63:800–9. doi: 10.1016/j.eururo.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spitz MR, Strom SS, Yamamura Y, Troncoso P, Babaian RJ, Scardino PT, et al. Epidemiologic determinants of clinically relevant prostate cancer. Int J Cancer. 2000;89:259–64. doi: 10.1002/1097-0215(20000520)89:3<259::aid-ijc8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 6.Graham S, Haughey B, Marshall J, Priore R, Byers T, Rzepka T, et al. Diet in the epidemiology of carcinoma of the prostate gland. J Natl Cancer Inst. 1983;70:687–92. [PubMed] [Google Scholar]
- 7.Strom SS, Yamamura Y, Forman MR, Pettaway CA, Barrera SL, DiGiovanni J. Saturated fat intake predicts biochemical failure after prostatectomy. Int J Cancer. 2008;122:2581–5. doi: 10.1002/ijc.23414. [DOI] [PubMed] [Google Scholar]
- 8.Kondo Y, Homma Y, Aso Y, Kakizoe T. Promotional effect of two-generation exposure to a high-fat diet on prostate carcinogenesis in ACI/Seg rats. Cancer Res. 1994;54:6129–32. [PubMed] [Google Scholar]
- 9.Eheman C, Henley SJ, Ballard-Barbash R, Jacobs EJ, Schymura MJ, Noone AM, et al. Annual Report to the Nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118:2338–66. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Nunzio C, Albisinni S, Freedland SJ, Miano L, Cindolo L, Finazzi Agro E, et al. Abdominal obesity as risk factor for prostate cancer diagnosis and high grade disease: a prospective multicenter Italian cohort study. Urol Oncol. 2013;31:997–1002. doi: 10.1016/j.urolonc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 11.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–78. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 12.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park J, Euhus DM, Scherer PE. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocr Rev. 2011;32:550–70. doi: 10.1210/er.2010-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–55. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 15.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886–95. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 17.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–16. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 18.Ribeiro RJ, Monteiro CP, Cunha VF, Azevedo AS, Oliveira MJ, Monteiro R, et al. Tumor cell-educated periprostatic adipose tissue acquires an aggressive cancer-promoting secretory profile. Cell Physiol Biochem. 2012;29:233–40. doi: 10.1159/000337604. [DOI] [PubMed] [Google Scholar]
- 19.van Roermund JG, Hinnen KA, Tolman CJ, Bol GH, Witjes JA, Bosch JL, et al. Periprostatic fat correlates with tumour aggressiveness in prostate cancer patients. BJU Int. 2011;107:1775–9. doi: 10.1111/j.1464-410X.2010.09811.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang T, Tseng C, Zhang Y, Sirin O, Corn PG, Li-Ning-Tapia EM, et al. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nat Commun. 2016;7:11674. doi: 10.1038/ncomms11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laurent V, Guerard A, Mazerolles C, Le Gonidec S, Toulet A, Nieto L, et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat Commun. 2016;7:10230. doi: 10.1038/ncomms10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, et al. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010;29:709–22. doi: 10.1007/s10555-010-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 24.Hattermann K, Mentlein R. An infernal trio: the chemokine CXCL12 and its receptors CXCR4 and CXCR7 in tumor biology. Ann Anat. 2013;195:103–10. doi: 10.1016/j.aanat.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–20. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin Q, Jin P, Liu X, Wei H, Lin X, Chi C, et al. SDF-1alpha inhibits hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells through PI3K/Akt and ERK1/2 signaling pathways. Mol Biol Rep. 2011;38:9–16. doi: 10.1007/s11033-010-0071-9. [DOI] [PubMed] [Google Scholar]
- 27.Domanska UM, Kruizinga RC, Nagengast WB, Timmer-Bosscha H, Huls G, de Vries EG, et al. A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur J Cancer. 2013;49:219–30. doi: 10.1016/j.ejca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Sakao K, Vyas AR, Chinni SR, Amjad AI, Parikh R, Singh SV. CXCR4 is a novel target of cancer chemopreventative isothiocyanates in prostate cancer cells. Cancer Prev Res (Phila) 2015;8:365–74. doi: 10.1158/1940-6207.CAPR-14-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh S, Singh UP, Grizzle WE, Lillard JW., Jr CXCL12-CXCR4 interactions modulate prostate cancer cell migration, metalloproteinase expression and invasion. Lab Invest. 2004;84:1666–76. doi: 10.1038/labinvest.3700181. [DOI] [PubMed] [Google Scholar]
- 30.Conley-LaComb MK, Saliganan A, Kandagatla P, Chen YQ, Cher ML, Chinni SR. PTEN loss mediated Akt activation promotes prostate tumor growth and metastasis via CXCL12/CXCR4 signaling. Mol Cancer. 2013;12:85. doi: 10.1186/1476-4598-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dirat B, Ader I, Golzio M, Massa F, Mettouchi A, Laurent K, et al. Inhibition of the GTPase Rac1 mediates the antimigratory effects of metformin in prostate cancer cells. Mol Cancer Ther. 2015;14:586–96. doi: 10.1158/1535-7163.MCT-14-0102. [DOI] [PubMed] [Google Scholar]
- 32.Domanska UM, Timmer-Bosscha H, Nagengast WB, Oude Munnink TH, Kruizinga RC, Ananias HJ, et al. CXCR4 inhibition with AMD3100 sensitizes prostate cancer to docetaxel chemotherapy. Neoplasia. 2012;14:709–18. doi: 10.1593/neo.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blando J, Moore T, Hursting S, Jiang G, Saha A, Beltran L, et al. Dietary energy balance modulates prostate cancer progression in Hi-Myc mice. Cancer Prev Res (Phila) 2011;4:2002–14. doi: 10.1158/1940-6207.CAPR-11-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–38. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 35.Nesbit CE, Tersak JM, Prochownik EV. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18:3004–16. doi: 10.1038/sj.onc.1202746. [DOI] [PubMed] [Google Scholar]
- 36.Hawksworth D, Ravindranath L, Chen Y, Furusato B, Sesterhenn IA, McLeod DG, et al. Overexpression of C-MYC oncogene in prostate cancer predicts biochemical recurrence. Prostate Cancer Prostatic Dis. 2010;13:311–5. doi: 10.1038/pcan.2010.31. [DOI] [PubMed] [Google Scholar]
- 37.Jenkins RB, Qian J, Lieber MM, Bostwick DG. Detection of c-myc oncogene amplification and chromosomal anomalies in metastatic prostatic carcinoma by fluorescence in situ hybridization. Cancer Res. 1997;57:524–31. [PubMed] [Google Scholar]
- 38.Sato K, Qian J, Slezak JM, Lieber MM, Bostwick DG, Bergstralh EJ, et al. Clinical significance of alterations of chromosome 8 in high-grade, advanced, nonmetastatic prostate carcinoma. J Natl Cancer Inst. 1999;91:1574–80. doi: 10.1093/jnci/91.18.1574. [DOI] [PubMed] [Google Scholar]
- 39.Gurel B, Iwata T, Koh CM, Jenkins RB, Lan F, Van Dang C, et al. Nuclear MYC protein overexpression is an early alteration in human prostate carcinogenesis. Mod Pathol. 2008;21:1156–67. doi: 10.1038/modpathol.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saha A, Blando J, Fernandez I, Kiguchi K, DiGiovanni J. Linneg Sca-1high CD49fhigh prostate cancer cells derived from the Hi-Myc mouse model are tumor-initiating cells with basal-epithelial characteristics and differentiation potential in vitro and in vivo. Oncotarget. 2016;7:25194–207. doi: 10.18632/oncotarget.7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leri A, Liu Y, Claudio PP, Kajstura J, Wang X, Wang S, et al. Insulin-like growth factor-1 induces Mdm2 and down-regulates p53, attenuating the myocyte renin-angiotensin system and stretch-mediated apoptosis. Am J Pathol. 1999;154:567–80. doi: 10.1016/S0002-9440(10)65302-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saha A, Blando J, Tremmel L, DiGiovanni J. Effect of Metformin, Rapamycin and Their Combination on Growth and Progression of Prostate Tumors in HiMyc Mice. Cancer Prev Res (Phila) 2015 doi: 10.1158/1940-6207.CAPR-15-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–33. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 44.Valster A, Tran NL, Nakada M, Berens ME, Chan AY, Symons M. Cell migration and invasion assays. Methods. 2005;37:208–15. doi: 10.1016/j.ymeth.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Sirin O, Kolonin MG. Treatment of obesity as a potential complementary approach to cancer therapy. Drug Discov Today. 2013;18:567–73. doi: 10.1016/j.drudis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Nakao N, Nakayama T, Yahata T, Muguruma Y, Saito S, Miyata Y, et al. Adipose tissue-derived mesenchymal stem cells facilitate hematopoiesis in vitro and in vivo: advantages over bone marrow-derived mesenchymal stem cells. Am J Pathol. 2010;177:547–54. doi: 10.2353/ajpath.2010.091042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun. 2013;4:1795. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freedland SJ, Banez LL, Sun LL, Fitzsimons NJ, Moul JW. Obese men have higher-grade and larger tumors: an analysis of the duke prostate center database. Prostate Cancer Prostatic Dis. 2009;12:259–63. doi: 10.1038/pcan.2009.11. [DOI] [PubMed] [Google Scholar]
- 49.Finley DS, Calvert VS, Inokuchi J, Lau A, Narula N, Petricoin EF, et al. Periprostatic adipose tissue as a modulator of prostate cancer aggressiveness. J Urol. 2009;182:1621–7. doi: 10.1016/j.juro.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Daquinag A, Traktuev DO, Amaya-Manzanares F, Simmons PJ, March KL, et al. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;69:5259–66. doi: 10.1158/0008-5472.CAN-08-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daquinag AC, Tseng C, Zhang Y, Amaya-Manzanares F, Florez F, Dadbin A, et al. Targeted Proapoptotic Peptides Depleting Adipose Stromal Cells Inhibit Tumor Growth. Mol Ther. 2016;24:34–40. doi: 10.1038/mt.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Daquinag AC, Amaya-Manzanares F, Sirin O, Tseng C, Kolonin MG. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 2012;72:5198–208. doi: 10.1158/0008-5472.CAN-12-0294. [DOI] [PubMed] [Google Scholar]
- 53.Klopp AH, Zhang Y, Solley T, Amaya-Manzanares F, Marini F, Andreeff M, et al. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin Cancer Res. 2012;18:771–82. doi: 10.1158/1078-0432.CCR-11-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akashi T, Koizumi K, Tsuneyama K, Saiki I, Takano Y, Fuse H. Chemokine receptor CXCR4 expression and prognosis in patients with metastatic prostate cancer. Cancer Sci. 2008;99:539–42. doi: 10.1111/j.1349-7006.2007.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mochizuki H, Matsubara A, Teishima J, Mutaguchi K, Yasumoto H, Dahiya R, et al. Interaction of ligand-receptor system between stromal-cell-derived factor-1 and CXC chemokine receptor 4 in human prostate cancer: a possible predictor of metastasis. Biochem Biophys Res Commun. 2004;320:656–63. doi: 10.1016/j.bbrc.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 56.Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239–54. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 57.Dubrovska A, Elliott J, Salamone RJ, Telegeev GD, Stakhovsky AE, Schepotin IB, et al. CXCR4 expression in prostate cancer progenitor cells. PLoS One. 2012;7:e31226. doi: 10.1371/journal.pone.0031226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang X, Li X, Li Z, Liu Y, Yao L, Song S, et al. Downregulation of CXCR7 inhibits proliferative capacity and stem cell-like properties in breast cancer stem cells. Tumour Biol. 2016;37:13425–33. doi: 10.1007/s13277-016-5180-1. [DOI] [PubMed] [Google Scholar]
- 59.Wu YC, Tang SJ, Sun GH, Sun KH. CXCR7 mediates TGFbeta1-promoted EMT and tumor-initiating features in lung cancer. Oncogene. 2016;35:2123–32. doi: 10.1038/onc.2015.274. [DOI] [PubMed] [Google Scholar]
- 60.Walters MJ, Ebsworth K, Berahovich RD, Penfold ME, Liu SC, Al Omran R, et al. Inhibition of CXCR7 extends survival following irradiation of brain tumours in mice and rats. Br J Cancer. 2014;110:1179–88. doi: 10.1038/bjc.2013.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol. 2013;91:19–26. doi: 10.1038/icb.2012.56. [DOI] [PubMed] [Google Scholar]
- 62.Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood. 2011;118:330–8. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nowicka A, Marini FC, Solley TN, Elizondo PB, Zhang Y, Sharp HJ, et al. Human omental-derived adipose stem cells increase ovarian cancer proliferation, migration, and chemoresistance. PLoS One. 2013;8:e81859. doi: 10.1371/journal.pone.0081859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.