Abstract
Age-related cataracts are closely associated with lens chronological aging, oxidation, calcium imbalance, hydration and crystallin modifications. Accumulating evidence indicates that misfolded proteins are generated in the endoplasmic reticulum (ER) by most cataractogenic stresses. To eliminate misfolded proteins from cells before they can induce senescence, the cells activate a clean-up machinery called the ER stress/unfolded protein response (UPR). The UPR also activates the nuclear factor- erythroid-2-related factor 2 (Nrf2), a central transcriptional factor for cytoprotection against stress. Nrf2 activates nearly 600 cytoprotective target genes. However, if ER stress reaches critically high levels, the UPR activates destructive outputs to trigger programmed cell death. The UPR activates mobilization of ER-Ca2+ to the cytoplasm and results in activation of Ca2+-dependent proteases to cleave various enzymes and proteins which cause the loss of normal lens function. The UPR also enhances the overproduction of reactive oxygen species (ROS), which damage lens constituents and induce failure of the Nrf2 dependent cytoprotection. Kelch-like ECH-associated protein 1 (Keap1) is an oxygen sensor protein and regulates the levels of Nrf2 by the proteasomal degradation. A significant loss of DNA methylation in diabetic cataracts was found in the Keap1 promoter, which overexpresses the Keap1 protein. Overexpressed Keap1 significantly decreases the levels of Nrf2. Lower levels of Nrf2 induces loss of the redox balance toward to oxidative stress thereby leading to failure of lens cytoprotection. Here, this review summarizes the overall view of ER stress, increases in Ca2+ levels, protein cleavage, and loss of the well-established stress protection in somatic lens cells.
Keywords: ER stress, Unfolded protein response, Nrf2, Keap1, DNA methylation, Age-related cataracts
1. Introduction
Protein misfolding and aggregation is considered as a key factor for various conformational diseases including cataract, a leading cause of blindness worldwide. Age-related cataracts (ARCs) have been characterized as conformational diseases as posttranslational modifications of lens proteins cause their conformational changes that lead to destabilization and eventual aggregation (Crabbe, 1998; Harding, 2002). It is well-documented that almost all cataract lenses have similar characters such as oxidation of lens constituents, loss of antioxidants, increased levels of cytosolic Ca2+, activation of proteolysis, imbalances of ions, hydration and crystallin degradation and aggregation. The risk of ARCs significantly increases with age as many stressors play critical roles in the cataract formation. Importantly, it has also been reported that the involvement of endoplasmic reticulum (ER) stress-mediated unfolded protein response (UPR) in human lens epithelial cells in the context of ARCs formation (Elanchezhian et al., 2012a; Elanchezhian et al., 2012b; Ikesugi et al., 2006a; Ikesugi et al., 2006b; Mulhern, 2012; Mulhern et al., 2006; Mulhern et al., 2007; Shinohara et al., 2006).
This review mainly discusses, 1) how environmental stressors induce misfolded protein conformation via ER stress; 2) the general UPR pathways; 3) how UPR drives Ca2+ release from ER to initiate a sequence of measures that culminates in the loss of various membrane-associated enzymes such as sarco/endoplasmic reticulum Ca2+-ATPases (SERCA) and plasma membrane Ca2+-ATPases (PMCA) and other proteins including transcriptional factors; 4) the link between reactive oxygen species (ROS) and ER stress 5) the importance of “Nrf2 dependent stress protection mechanism in the lens”, a major stress protection pathway during UPR; 6) the role of Keap1 promoter DNA demethylation in the suppression of Nrf2 dependent stress protection; 7) the fact that ER stress is also induced in immature lens fiber cells. These discussions further define how different cataract types can be determined in human age-related cataracts.
2. Cataractogenic stress and the ER stress/UPR
Cells are well equipped to protect themselves against various stresses. Environmental stresses and mutant proteins are known to induce cataracts. Among the numerous environmental stresses, certain ones appear to induce misfolded protein conformation. Membrane, luminal or secretory proteins are synthesized in the rough ER and transported into the highly oxidized ER lumen. Stress appears to modify these proteins in the ER to generate misfolded proteins. Misfolded protein conformation in the ER initiates processes of cataract formation and full blown; chronic UPR further amplifies crystallin and protein degradation, modification, and aggregation in the downstream cascade.
2.1. Environmental stress
Cataracts can result from metabolic, nutritional or environmental insults, or they may be secondary to other ocular or systemic diseases such as retinal degenerative diseases, uveitis, glaucoma or diabetes (Harding, 1991). By far the most crucial risk factor is age; the prevalence of ARCs escalates from 4% at ages 52–64 to 50% at ages 75–85. The prevalence is expected to nearly double by the year 2020 due to an increased aging population. ARCs constitute a vast majority of all cataracts and represent a major public health problem worldwide.
Most ARCs are thought to be caused by environmental stresses in combination with ecliptic genetic inheritance. Considering the fact that severe bilateral cataracts can be found in 40-year-old individuals, but a 90-year-old can have clear lenses, pathogenic processes, not age, are responsible for the formation of ARCs. Significant increases in the incidence of ARCs with age suggest cumulative processes or age-associated processes might play a vital role in ARCs. In the past several decades, large numbers of cataractogenic stresses have been characterized. Reviews of various aspects of ARCs have been published with lists of some of them in the last several years; posttranslational modification (Zhang and Lu, 2011), oxidation (Zoric et al., 2010), a model of normal and pathological aging (Michael and Bron, 2011), cataract-map (Beebe et al., 2010), redox regulation in the lens (Lou, 2003), oxidative damage (Bassnett and McNulty, 2003; Baynes and Thorpe, 1999; Brennan and Kantorow, 2009; Lou, 2003; Truscott and Augusteyn, 1977), alpha-crystallin on lens cell function (Andley, 2007, 2009), nutritional antioxidant (McGinty and Truscott, 2006), and presbyopia (Chiu and Taylor, 2007). A literature search indicates that most of the cataractogenic stresses in Table 1 induce ER stress in various tissues.
Among the many environmental stressors, very few are known to induce cataractogenic stress, but all environmental stressor-driven cataractogenic stress can cause ER stress that culminates in calcium imbalance, protein degradation, oxidative insults, perturbations of redox status, and loss of antioxidant defense mechanisms. Interestingly, the majority of cataractogenic stressors generate an accumulation of misfolded protein conformations in the ER, which is an essential requirement for activation of ER stress. Next, this review describes the literature related to the ER stressor that generates misfolded protein conformation in the ER lumen.
2.2. Cataractogenic stressor induces misfolded protein conformation
Here, the review describes how these stressors generate misfolded protein conformation in the lens epithelial cells thereby leading to cataract formation. Secretory, luminal and membrane proteins are primarily synthesized in the rough ER; ER lumen has developed a chemical environment which is more highly oxidized than the cytoplasm. The ER is equipped with a variety of molecular chaperones such as Ig binding protein (BiP), heat shock proteins and folding enzymes that assist the folding process, while at the same time exerting tight quality control measures. One of the common post-translational modifications of ER-synthesized proteins is disulfide bridge formation, catalyzed by the family of protein disulfide isomerases. This covalent modification provides unique structural advantages. However, the benefits that disulfide bonds impart to these proteins come at a high cost to the cell. Recent reports have demonstrated the mechanism by which the cell can deal with or even exploit the side reactions of disulfide bond formation to maintain homeostasis of the ER and its folding machinery (Feige and Hendershot, 2011).
The key question in cataractogenic ER stress is “does the cataractogenic stressor alter protein conformation in the ER?” Literature searches show that chronic alcoholics generate acetaldehyde, a highly reactive metabolite of ethanol, that forms covalent acetaldehyde-protein adducts with several proteins via lysine, cysteine, histidine and tyrosine residues (Stevens et al., 1981). Similarly, malondialdehyde-adducts or methylglyoxal adducts in diabetic patients react with arginine residues in proteins (Suji et al., 2008). Higher levels of urea induce a ‘‘direct interaction mechanism’’ with the protein backbone as well as side chains whereby urea has a stronger dispersion interaction with that protein than water thereby changing the protein conformation (Hua et al., 2008). Either extreme high or low-temperature changes protein conformation in mammalian cells (Caldwell, 1989). Dehydration or osmolality changes also affect protein conformation, and for several proteins, these conformational changes are at least partially irreversible (Prestrelski et al., 1993). Excess levels of molecular oxygen or other oxidative insults induce conformational changes by cysteine oxidation (Murray and Van Eyk, 2012). Similarly, ionizing radiation also changes protein conformation (Chapelier et al., 2001). The receptors of steroid hormones bind to ligands to induce significant conformational changes in the nucleus of the cell, which is central to steroid receptor activation (Allan et al., 1992). Reports have shown that steroid hormone induces ER stress in islet cells (Yusta et al., 2006; Yusta and Drucker, 2004) and prostate cancer cells (Yemelyanov et al., 2012). These results suggest that certain newly synthesized steroid receptors in the ER lumen bind excess levels of steroid hormone, activating ER stress and leading to cataract by small vacuole formation in the nucleus of the embryonic chick lens (Nishigori, 2006). Some drugs such as valproic acid (Cheema et al., 2007; Mandula et al., 2006), 5-Aza-2'-deoxycytidine (5-Aza) (Christman, 2002), thapsigargin (Davidson and Varhol, 1995), phenothiazine and tunicamycin (Lane et al., 1987), naphthoquinone, a toxic metabolite of naphthalene (Jali et al., 2014), bind to the proteins and disturb protein conformation. The well-known ER stressors, calcimycin (ionophore A23187) also induces a Ca2+-dependent conformational change in SERCA (Champeil et al., 1986; Ohmiya and Kanazawa, 1991). Photosensitized paraquat has also reported to inducing protein structural alterations and free radical-mediated fragmentation of serum albumin (Jaiswal et al., 2002). Thapsigargin is a high-affinity inhibitor of SERCA and binds to SERCA and changes conformation as well as inhibits SERCA activity (Davidson and Varhol, 1995). Tunicamycin is an inhibitor of N-glycosylation thereby inhibiting the proper protein folding and induces the so-called UPR (Elbein, 1987). The deficiency of vitamins and amino acids are relatively slow to alter the protein conformation since they have some pooled materials in the cells. Tryptophan deficiency induces deficiency mutants and alters protein conformation (Kolvenbach et al., 1993), and vitamin deficiency results in the loss of cofactors of many enzymes and disturbs protein conformation. Homocysteine binds to cysteine by disulfide binding (Gonzalez et al., 2004), whereas sodium-selenite binds to cysteine by disulfide-selenite formation (Protein-S-Se-S-Protein). Soluble forms of mercury, copper, and lead also bind multiple cysteine-containing proteins such as papain (Guo et al., 2011), and change protein conformation. Environmental toxins such as paraquat (Dhakshinamoorthy and Jaiswal, 2002) and bisphenol A (Hiroi et al., 2006) also bind to proteins and interferes with functional roles as well as altering protein conformation. Thus, higher levels of cataractogenic stressors in the ER bind to newly synthesized membrane, luminal and secretory proteins. These bindings disturb protein conformation in the highly oxidized ER lumen, resulting in activation of ER stress. This is an important concept to elucidate cataractogenic stressors. It has been speculated that most of the stress damage in the lens may be recovered throughout life since most stresses are low enough to generate the protective ER stress. Those lower levels of stress may be beneficial to enhance stress protection, which is called hormesis (Calabrese and Baldwin, 2003). However, continued activation of the ER stress indicates the inability to re-establish homeostasis. Chronic ER stress can be generated by either prolonged time with weak levels of stresses or severe stresses in short time, but the both ways provide similar cellular damage (Papa, 2012). Lens-specific cataract formation might be induced by a predominant interaction between lens specific protein(s) and the ER stressor(s). It has been demonstrated that human lens epithelial cells exposed to a certain oxygen environment activated a protective UPR while chronic hypoxic conditions induced ROS production and apoptotic UPR resulting in lens epithelial cell death and lens oxidation (Elanchezhian et al., 2012b; Zheng et al., 2015). Thus, cataract is one of the protein misfolding diseases, and misfolded protein conformation in the ER initiates pathogenic processes and results in misfolded crystallin aggregations. Such diversified cataractogenic stressors have a common principle to share a common pathway for cataract formation.
2.3. Mutant protein also induces ER stress/UPR
More than 30% of proteins are synthesized in the rough ER (Ghaemmaghami et al., 2003). Alterations in protein trafficking occur mainly in the ER, the central site for folding, post-translational modifications, and transport of these proteins. An imbalance between a load of misfolded proteins and the folding capacity of the ER leads to ER stress. Also, missense mutations may result in protein misfolding and disruption of protein trafficking. Many transgenic animals having mutant or chimeric genes also develop a cataract in the lens as well as dysfunctions in the other tissues (Firtina et al., 2009; Graw, 1999). Accumulating evidence also suggests that ER stress contributes to the development and progression of cystic fibrosis, α1-antitrypsin deficiency, retinitis pigmentosa, and Alzheimer disease (Ogen-Shtern et al., 2016). This review focuses only on the relationship between environmental stresses-induced ER stress and cataracts. Gene mutant or inherited cataracts are beyond the scope of this review.
3. Activation of the ER stress/UPR
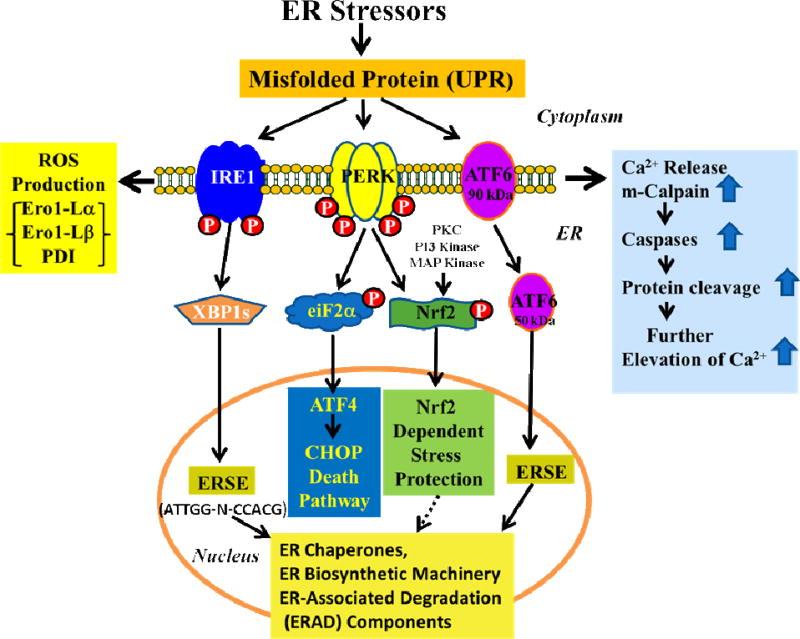
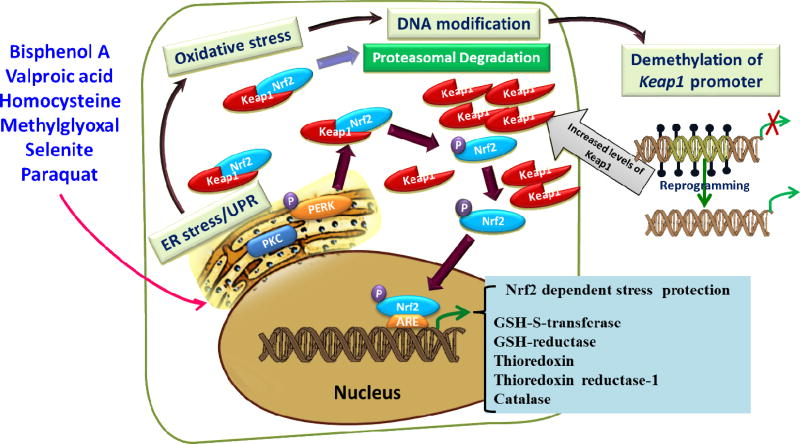
Cells induce a cascade of reactions, called the UPR, to cope with any perturbation by misfolded protein conformations (Kaufman et al., 2002; Schroder and Kaufman, 2005; Shen et al., 2004; Zhang et al., 2015; Zhang et al., 2005). Generally, the UPR responses are comprised of (1) translational attenuation to prevent further production of misfolded proteins (Banks et al., 1999) including degradation of mRNA (Narasimhan et al., 2012; Singh et al., 2013; Yang et al., 2011); (2) transcriptional induction of ER resident proteins to further assist protein folding (Gething and Sambrook, 1992); (3) induction of ER-associated degradation (ERAD) machinery to reduce the burden on ER folding capacity (Yoshida et al., 2000a); and (4) enlargement of the ER to deal with the massive load of unfolded proteins (Selye, 1985). The successful execution of compensatory responses to stress by these mechanisms restores normal cell function and ensures survival. Alternatively, irreversibly stressed cells would undergo senescence or apoptosis in response to chronic UPR (Haeri and Knox, 2012). There are three intertwined pathways that comprise the UPR (Walter and Ron, 2011). Each pathway is initiated by an integral ER membrane protein such as the protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK), the ER resident, inositol-requiring kinase 1 (IRE1), and activating transcription factor 6 (ATF6) that serves as a sensor for unfolded proteins in the ER lumen (Fig. 1).
Fig. 1.
Schematic diagram for molecular mechanisms of activation of ER stress. Accumulation of misfolded protein triggers the phosphorylation of PERK and IRE1 and cleavage of ATF6 to activate the UPR. This rapidly reduces the misfolded protein load through reversible translational attenuation and transcriptional induction of ER resident proteins to further assist protein folding and through induction of chaperones, ER biosynthetic machinery, and ER-associated degradation (ERAD) components. Chronic ER stress induces the apoptotic UPR which generates excessive levels of ROS, through the involvement of Ero1-Lα and-Lβ and PDI. Furthermore, the UPR releases Ca2+ from the ER to activate m-calpain and caspases. P-PERK phosphorylates eIF2α which further activates ATF4 and the CHOP-death pathway. P-PERK, PKC, PI3 kinase and MAP kinase phosphorylate Nrf2; the P-Nrf2 dissociates from Keap1 and translocates into the nucleus to bind to the antioxidant response element (ARE), and this activates the transcription of more than 200 stress/antioxidant enzymatic genes such as glutathione, glutathione reductase, thioredoxin, thioredoxin-S-transferase, and catalase (see Fig. 2). These stress/antioxidant enzymes regenerate reduced glutathione, and the resultant reduced glutathione eliminates ROS so the cell can survive and recover from the stress. Chronic UPR activates the death pathway and results in senescence or death.
PERK
Essentially all ER stressors that induce the UPR’s transcriptional components can also transiently attenuate translation via phosphorylation of the eukaryotic translation initiation factor 2α (eIF2α), which is mediated by the PERK signaling pathway (Harding et al., 1999; Shi et al., 1998). PERK, an ER stress sensor, is a type I transmembrane protein kinase receptor which is activated by ER stress with the release of BiP from its luminal domain. The release of BiP from PERK results in its oligomerization with subsequent autophosphorylation of its cytosolic domain (Banks et al., 1999; Yoshida et al., 2000b). P-PERK receptors then reduce the activity of ribosomal initiating factor (eIF2α) by phosphorylation of its α-subunit, which inhibits 80S ribosome assembly, and consequently attenuates protein synthesis/translation (Banks et al., 1999). In contrast, phosphorylation of eIF2α promotes the translation of ATF4 upon ER stress (Zhang et al., 2005).
Similarly, P-PERK, (Cullinan and Diehl, 2004; Cullinan et al., 2003) protein kinase C (PKC) (Bloom and Jaiswal, 2003; Chiu et al., 2002), PI3 kinase (Kang et al., 2002) and/or MAP kinase (Zipper and Mulcahy, 2000) phosphorylates the nuclear factor-erythroid-2-related factor 2 (Nrf2) (Fig. 1). Phosphorylation of Nrf2 disrupts its association with the Kelch-like ECH-associated protein 1 (Keap1) and the P-Nrf2 is then translocated to the nucleus where it binds to the antioxidant response element (ARE) in the promoter (Desagher et al., 2005) to activate 600 target genes, including 20 for antioxidant associated enzymes such as glutathione (GSH)-reductase, GSH-S-transferase, thioredoxin, thioredoxin reductase, and many more antioxidant defense genes (Aldana et al., 2003; Datta et al., 2017; Kwak et al., 2003a; Thimmulappa et al., 2002). This system is called as Nrf2 dependent stress protection (Fig. 1). Human lens epithelial cells exposed to hypoxic/hypoglycemic conditions (Elanchezhian et al., 2012b), sodium selenite (Palsamy et al., 2014b), high or low glucose (Ikesugi et al., 2006a; Ikesugi et al., 2006b), homocysteine (Elanchezhian et al., 2012a), tunicamycin (Ikesugi et al., 2006b), thapsigargin, streptozotocin-induced diabetes and methylglyoxal (Palsamy et al., 2014a), and galactose (Mulhern et al., 2006) activate PERK and P-PERK and apparently activate ER stress. Of the three membrane signaling receptors, PERK is most strongly activated by the above ER stressors in human lens epithelial cells.
IRE1
Two isoforms (IRE1α and 1β) are found in mammalian ER membranes (Tirasophon et al., 1998) (Ding et al., 2011). In unstressed cells, the IRE1 protein kinase is maintained in an inactive monomeric form through association with BiP (Bertolotti et al., 2000). Once liberated from BiP, IRE1 is free to dimerize, trans-autophosphorylate and thereby activate its RNase activity (Tirasophon et al., 1998). IRE1 is required for splicing of the X-box transcription factor-1, XBP-1 (Yoshida et al., 2001) (Fig. 1). The spliced form of XBP1 is a potent transcriptional activator and plays a key role in the transcriptional induction of a broad range of UPR target genes, including several ER-resident chaperones (Lee et al., 2003a; Yoshida et al., 2001). The UPR-dependent transcriptional program in mammalian cells does not rely solely on IRE1 signaling. Human lens epithelial cells exposed to hypoxic/hypoglycemic conditions (Elanchezhian et al., 2012b), high or low glucose (Ikesugi et al., 2006b), galactose (Mulhern et al., 2006), homocysteine (Elanchezhian et al., 2012a), tunicamycin (Ikesugi et al., 2006b), thapsigargin, streptozotocin-induced diabetes and methylglyoxal (Palsamy et al., 2014b), sodium selenite (Palsamy et al., 2014b), and valproic acid (Palsamy et al., 2014c), activated IRE1 suggesting that the ER stress is activated by cataractogenic stressors.
ATF6
Two isoforms (90 kDa ATF6α and 110 kDa ATF6β, also known as CREB-RP) are maintained at the ER membrane by their binding to BiP (Haze et al., 1999; Thuerauf et al., 2007). Upon BiP release, both isoforms transit to the Golgi compartment where they are cleaved by site-specific proteases, S1P and S2P to generate ATF6 (50–60 kDa) protein (Abraham et al., 1999; Haze et al., 1999). The cleaved product then transits to the nucleus and promotes the transcription of many UPR target genes, including BiP and XBP-1 (Li et al., 2000; Okada et al., 2002). The overlap in targets between IRE1 and ATF6-dependent signaling demonstrates the convergence of the two pathways and the ability of the UPR to amplify its signaling potential. Transcriptional activation of ER-resident proteins is mediated by ATF6 and IRE1 receptors (Yoshida et al., 1998). One of the clearest demonstrations of ATF6 activation by ER stressor was observed in human lens epithelial cells treated with 10 µM sodium selenite (Palsamy et al., 2014b). Also, human lens epithelial cells treated with methylglyoxal upregulated both the ATF4 and the phosphorylation of IRE1 (Palsamy et al., 2014a). Furthermore, the fact that BiP and XBP-1 are also increased in these cells further confirmed the finding that ER stress is induced by methylglyoxal (Palsamy et al., 2014a). Consistent with these observations, human lens epithelial cells treated with homocysteine (Elanchezhian et al., 2012a), non-physiologically high and low glucose (Ikesugi et al., 2006b), galactose (Mulhern et al., 2006), hypoxic/hypoglycemic conditions (Elanchezhian et al., 2012b), valproic acid (Palsamy et al., 2014c), thapsigargin (Palsamy et al., 2014a), tunicamycin (Ikesugi et al., 2006b), sodium selenite (Palsamy et al., 2014b) and calcium ionophore-A23187 (Ikesugi et al., 2006b) are also upregulated the UPR pathway.
4. ER stress/UPR initiates a Ca2+ imbalance by releasing ER-Ca2+
Cortical cataracts are the main type of cataract that exhibits elevated levels of Ca2+ ranging from 0.1 to 64 mM. However, nuclear cataracts possess normal levels of calcium and sodium (Baruch et al., 2001; Duncan and Jacob, 1984; Harding, 1991; Marcantonio et al., 1986). Ca2+ levels in human aqueous humor are approximately 1 mM, and there is a large, 10,000-fold-inwardly directed gradient across the plasma membrane. An increased Ca2+ level in the ER compartment generates a large Ca2+ gradient across the ER membrane. ER stressors such as methylglyoxal are also found to induce an ER-Ca2+ release spike for 2–39 seconds (Palsamy et al., 2014a), and valproic acid does the same for 2–183 seconds (Palsamy et al., 2014c) whereas homocysteine-induced ER-Ca2+ release and uptake occur several times within a few minutes. Sodium selenite provokes ER-Ca2+ release and induces severe chronic UPR (Palsamy et al., 2014b). SERCA, a family of proteins responsible for transporting Ca2+ from the cytosol into the lumen of the ER, is a major regulator of ER stress (Park et al., 2010). Repeated treatment with selenite gradually reduces the spontaneous ER-Ca2+ release, suggesting either a loss of ATP from the ER or decrease the rate of SERCA pumps. Reports also showed that SERCA was gradually decreased within 12 h and it was further degraded by 24 h in human lens epithelial cells exposed with sodium selenite (Palsamy et al., 2014b). These results suggest that ER stress initiates a spontaneous ER-Ca2+ release, and inhibition of SERCA can prevent the refilling of Ca2+ to the ER stores causing a measured release of Ca2+ into the cytosol through leak channels (Hofer et al., 1996). The basal Ca2+ leak occurs via the translocon, a transmembrane aqueous pore located in the ER (Giunti et al., 2007; Ong et al., 2007) that is responsible for the transport of newly synthesized secretory proteins into the ER lumen (Swanton and Bulleid, 2003). Gating of this translocon is found to be facilitated by BiP and Ca2+-Calmodulin (Erdmann et al., 2011; Schauble et al., 2012). A research report by Paredes et al., suggests that Ca2+ may be both a cause and a consequence of ER protein misfolding and it appears that ER Ca2+ leak is a significant co-factor for the initiation of the UPR (Paredes et al., 2013).
The ER-Ca2+ store is entirely depleted by chronic UPR in lens cells, for example by exposure to thapsigargin, a widely used SERCA inhibitor and a well-known ER stressor (Foldi et al., 2013; Treiman et al., 1998). Cells treated with thapsigargin exhibit a marked decrease in protein synthesis. Net leakage of proteins was associated more strongly with an increase in ER-Ca2+ than with an increase in sodium (Abraham et al., 1999; Duncan and Jacob, 1984; Duncan and Wormstone, 1999; Shearer et al., 1997). Because ER-Ca2+ release under ER stress takes a matter of seconds, it has been speculated that ER-Ca2+ release is sufficient to activate ERAD components or m-calpain and induce SERCA degradation for slowing down Ca2+ uptake from the cytoplasm to ER (Palsamy et al., 2014a). The loss of SERCA generates higher Ca2+ levels in the cytoplasm for prolonged times. ERAD components and m-calpain further damage the PMCA, which might induce Ca2+ influx from aqueous humor. Since human lens cells normally possess very low Ca2+ permeability, depletion of intracellular stores by thapsigargin initiates a Ca2+ entry pathway. As an outcome, this phenomenon potentially provides a signal transduction mechanism with minimal risk of Ca2+ overload to the lens, whereas over-activation of the store-operated channel is a possible way of increasing Ca2+ in the lens (Park et al., 2010). Similar to this, studies have also reported that methylglyoxal (Palsamy et al., 2014a), homocysteine (Elanchezhian et al., 2012b), valproic acid (Palsamy et al., 2014c), sodium selenite (Palsamy et al., 2014b), and tunicamycin, calcium ionophore-A23187 (Ikesugi et al., 2006b) induce ER-Ca2+ verload and increasing ER-Ca2+ levels in the lens cytoplasm. Higher levels of Ca2+ activate ERAD components and m-calpain and several proteases including caspases, resulting in degradation of many important enzymes including SERCA (Palsamy et al., 2014b), and more importantly PMCA in the lens. This observation further suggested that sodium selenite induces a significant elevation of Ca2+ in the rat lens (Hightower et al., 1987). Lenses from rats injected with sodium selenite showed a 50% decrease in PMCA activity (Wang et al., 1993). Ca2+ homeostasis is, therefore, recognized as having a fundamental importance in lens pathophysiology. Theoretically, increased lens Ca2+ could be due to inhibition of outwardly directed PMCA pumps. The only path for Ca2+ to leave from the intact lens is by transporting it against its electrochemical gradient into fiber cells, and then passaging it by electrodiffusion from cell to cell through gap junctions to surface cells, where PMCA pump activity and Na+/Ca2+ exchange can transport it back to the aqueous or vitreous humors (Gao et al., 2004). The Na+/Ca2+ exchanger and PMCA actively remove Ca2+ from the lens epithelial cells, whereas SERCA sequesters Ca2+ in the ER, which contains the largest intracellular store of Ca2+ (mM range) and then the Ca2+ store is replenished by a high affinity, low capacity SERCA uptake system (Berridge, 1993; Pozzan et al., 1994).
5. Increased Ca2+ activates m-calpain, caspases, cysteine proteases and ERAD components
The accumulation of unfolded proteins within the ER, while promoting the UPR, also elicits ERAD activation (Jarosch et al., 2003). Highlighted by an increase in ubiquitin-mediated proteolysis, this response encourages the removal of misfolded proteins from the ER lumen (Jarosch et al., 2003) and ERAD activation prevents promotion of the chronic UPR (Haynes et al., 2004). Further, recent reports demonstrated that Nrf2 is one of the important transcription factors in the UPR, and can directly promote the transcription of several cytoprotective target genes encoding proteasome subunits, thereby promoting proteasome assembly, during oxidative stress conditions (Kwak et al., 2003a; Kwak et al., 2003b). This suggests that PERK-dependent Nrf2 activation may contribute to protein degradation, during the UPR. Thus, mild UPR promotes cell survival, but chronic UPR leads toward self-destruction. One can expect that severity and duration of ER stress must determine this delicate balance.
An important consequence of ER-Ca2+ mobilization in lenses is activation of cysteine proteases such as m-calpain which is a major calcium-activated non-lysosomal, cysteine protease in human (Wang et al., 1992), cows (David et al., 1989), rodent (David and Shearer, 1986; Morishima et al., 2002; Nakagawa et al., 2000; Shearer et al., 1997), rabbits (Shearer et al., 1997), and guinea pigs (Shearer et al., 1997). Ca2+ influx mediated calpain activation results in caspase-3-mediated apoptosis in the retina degeneration model (Sharma and Rohrer, 2004). The ER-Ca2+ elevation also activates multiple caspases including caspase-12, caspase-9, and caspase-3 in the mouse (Nakagawa et al., 2000). The human caspase-12 gene has frame-shifts and a premature stop codon and therefore identifies with human caspase-4, which has sequence homology with mouse sequences (Hitomi et al., 2004a; Hitomi et al., 2004b). In general, caspase-mediated apoptosis begins with signaling from an initiator caspase-12 (mouse) or caspase-4 (human), which, subsequently promotes the release of cytochrome c and SMAC/Diablo from the mitochondria as well as the activation of a series of effector caspases, such as caspase-3, that cleave many other proteins. While many caspases are localized in the cytoplasm, in human cells caspase-4 is found in the ER membrane (Hitomi et al., 2004a; Yukioka et al., 2008). UPR induction results in the cleavage of procaspase-4, followed by the proto-typical activation of downstream effector caspases, although the exact mechanism remains uncertain (Hitomi et al., 2004a; Kahns et al., 2003; Kalai et al., 2003; Yukioka et al., 2008). Further increasing Ca2+ activates the Ca2+-dependent m-calpain and proteases and results in crystallin degradation, denaturation, and aggregation and are considered to cause cataract in rodents (Marcantonio, 1996; Shearer et al., 1997). Also, the loss of cytoskeletal proteins was also reported from lenses of selenite-treated rats (David and Shearer, 1984). Sodium selenite accelerated the loss of the cytoskeletal proteins and unidentified nuclear proteins of 49, 60 and 90 kDa (Matsushima et al., 1997). Interestingly, calcium electrode studies, carried out on human lenses, have shown that in those with localized cataracts, the free calcium only rises in the opaque areas, whereas surrounding clear regions have near-normal free calcium levels (Duncan and Jacob, 1984). In a nutshell as a key regulator of cell survival Ca2+ can induce ER stress-mediated apoptosis regulated at both the early and late apoptotic stages in response to various conditions (Bahar et al., 2016).
6. Protein folding and assembly generate ROS
In aerobic respiratory tissues, 1) ROS are usually created by leakage of superoxide from the mitochondrial respiratory chain due to perturbation of mitochondrial structure and function. 2) ROS are also generated from subsequent reduction of molecular oxygen. Molecular oxygen usually accepts a total of four electrons, one at a time, and the equivalent quantity of protons to produce two molecules of water. During this process, different oxygen radicals are continuously formed as intermediate products, including superoxide and peroxide. 3) Also, ROS are generated inside ER by hyperactivity of the so-called oxidative protein folding.
Since the lens is localized in an hypoxic environment (Elanchezhian et al., 2012b; Hockwin, 1971; McNulty et al., 2004; Neelam et al., 2013; Siegfried et al., 2010), the low levels of the enzymes associated with the aerobic oxidation of glucose restrict metabolism mainly to anaerobic glycolysis (Kinoshita, 1965). Even in the epithelium, anaerobic glycolysis appears to be the principal source of bioenergy, nearly 50% of the ATP in the lens epithelial cells is generated by oxidative metabolism (Elanchezhian et al., 2012b; Kinoshita, 1965; Winkler and Riley, 1991). The anaerobic respiration does not generate ROS, therefore certain ROS must be produced from other sources such as the chronic UPR, and the oxidative protein folding might play a significant role in ROS production in the lens.
The ER lumen is a highly oxidized environment and is favorable for disulfide formation (Hwang et al., 1992). Disulfide formation generates ROS as a byproduct of thiol/disulfide exchange reactions (Zhang and Kaufman, 2004). This response involves ER oxidoreductin 1 (Ero1), protein disulfide isomerase (PDI) and ER client proteins (Higa and Chevet; Pagani et al., 2000). There are two Ero1 isoforms in humans, Ero1α, and Ero1β (Cabibbo et al., 2000; Pagani et al., 2000) and both proteins facilitate oxidative folding (Mezghrani et al., 2001). Ero1 is a flavoenzyme, which may interact directly with molecular oxygen, producing ROS in the form of hydrogen peroxide and thereby contributes to the burden of oxidative stress. Protein disulfide bond formation by PDIs is dependent on the redox status in the ER, which is in part maintained by glutathione. Glutathione is synthesized in the cytosol and presented as the main redox buffer in the ER lumen. It has been shown that oxidized glutathione is essential to provide oxidizing equivalents for disulfide bond formation and glutathione-mediated oxidation of PDI is the major pathway of thiol-disulfide exchange (Cai et al., 2000; Hwang et al., 1992). However, Ero1 (Cabibbo et al., 2000) is now thought as the primary electron acceptor for disulfide bond formation. In oxidized conditions, Ero1 oxidizes the active cysteinyl thiol groups in PDI. PDI subsequently oxidizes the client proteins to create disulfide bonds. Ero1 transfers electrons from PDI to molecular oxygen and produces ROS. The reduced form of PDI breaks and rearranges disulfides in the nascent proteins. Also, reduced glutathione may be required for the disulfide isomerization by reducing the disulfides in PDI to the thiol (Csala et al., 2010). Recent reports have also revealed that PDI is the major substrate of Ero1α (Hwang et al., 1992) and the interaction between PDI and Ero1α is mediated through binding between the hydrophobic pocket in the b′ domain of PDI and the protruding β-hairpin of Ero1α (Masui et al., 2011).
To deal with ROS formed during protein folding in ER stress, the PERK pathway induces the Nrf2 dependent stress/antioxidant cytoprotection pathway (Fig. 1) which in turn activates the transcription of glutathione-S-transferase, NAD(P)H:quinone oxidoreductase-1, γ-glutamate cysteine ligase, and heme oxygenase-1 to protect the cells from oxidative stress (Itoh et al., 1997). Intriguingly, the ROS production associated enzymes, PDI, and Ero1-Lβ are constant in human lens epithelial cells treated with methylglyoxal and other ER stressors suggesting that ER stress-mediated ROS production must be constant. However, the continued ROS production diminishes the Nrf2 dependent antioxidant protection. On the other hand, human lens epithelial cells treated with sodium selenite produce significantly higher levels of ROS (Palsamy et al., 2014b). Similarly, human lens epithelial cells treated with homocysteine (Elanchezhian et al., 2012a), non-physiologically high and low levels of glucose (Ikesugi et al., 2006b), hypoxia with hypoglycemia (Elanchezhian et al., 2012b), valproic acid (Palsamy et al., 2014c), thapsigargin, tunicamycin, and calcium ionophore-A23187 (Ikesugi et al., 2006b) produce significant levels of ROS in human lens epithelial cells. Also, the levels of PDI and Ero1-Lβ are consistently elevated in human lens epithelial cells treated with each of these ER stressors.
7. Nrf2 is a central transcriptional activator for cytoprotective target genes
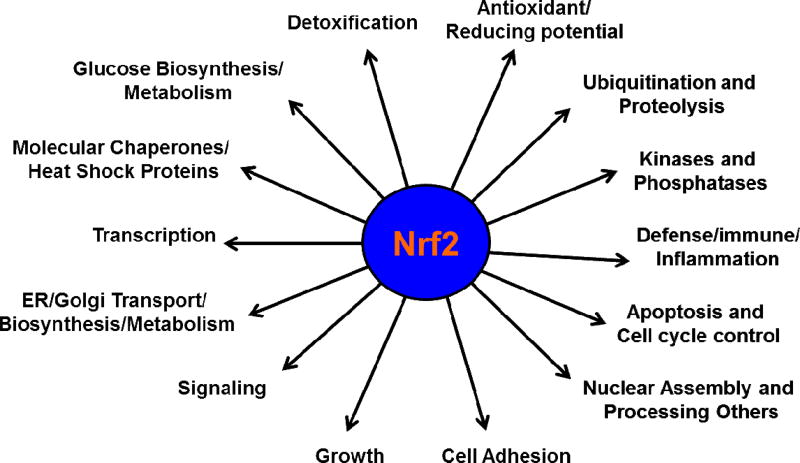
When cells are challenged by ER stressors, they must quickly augment their antioxidant capacity to counteract increased ROS production and maintain homeostasis. Nrf2 is a central transcription factor that functions as the key controller of the redox homeostatic gene regulatory network (Fig. 1). Under ER stresses, the Nrf2 signaling pathway is activated to enhance the expression of a multitude of cytoprotective enzymes that restore redox homeostasis. Keap1, a negative regulatory protein for Nrf2, is fastened to cytosolic actin and interacts with Nrf2 by acting as an adaptor protein. This interaction is a comparatively quick incident, with Nrf2 exhibiting a short half-life of 13–21 minutes (Hong et al., 2005; Katoh et al., 2005; Kobayashi and Yamamoto, 2006). Such a rapid turnover maintains a low, basal level of Nrf2, under unstressed conditions. An accumulation of phosphorylated-Nrf2 (P-Nrf2) in the nucleus that is associated with its transcriptional partner, Maf protein, forms a heterodimer that binds to ARE core sequence (GTGACNNNGC) on the DNA of target cytoprotective genes (Alam et al., 1999; Itoh et al., 1997; Itoh et al., 1999; Kobayashi et al., 1999; Motohashi and Yamamoto, 2004; Taguchi et al., 2011). Nrf2 protein is known to regulate nearly 200–600 cytoprotective genes (Enomoto et al., 2001; Kwak et al., 2003a; Lee et al., 2003b; Nguyen et al., 2000; Thimmulappa et al., 2002; Venugopal and Jaiswal, 1996; Wild et al., 1999), and are mainly associated with the expression of antioxidant enzymes, cellular transporters (Hayashi et al., 2003; Sasaki et al., 2002), enzymes that exclude the entry of xenobiotic metabolites and toxic compounds (Hayashi et al., 2003), detoxification enzymes, several components of the proteasome as well as those involved in protein folding and degradation (Kwak et al., 2003c). Also, Nrf2 promotes the expression of genes implicated in cell growth, cell adhesion, protein folding, cell signaling, cell-cycle control, survival, and glucose metabolism (Kwak et al., 2003c; Lee et al., 2003b). Nrf2 also promotes the expression of molecular chaperones/heat shock proteins and wound healing response proteins (Braun et al., 2002). (Fig. 2)
Fig. 2.
The Nrf2 target genes. The transcriptional factor, Nrf2 binds to the ARE site in the promoter of cytoprotective genes, and activates (>2.0 fold) or suppresses (<1/2 fold) more than 200–600 target genes in non-lens tissues.
8. Loss of DNA methylation in the Keap1 promoter
Extensive studies of protein, lipid and DNA modifications by oxidative insults have been investigated, and multiple review papers have been published in the past (Bassnett and McNulty, 2003; Cvekl and Duncan, 2007; Kowluru et al., 2015; Lou, 2003; Sobrin and Seddon, 2014; Truscott and Augusteyn, 1977; Zoric et al., 2010). Oxidative insults are also well known to induce DNA modifications, and DNA methylation is a most common epigenetic modification in somatic cells during aging that limits the activity of gene regulatory elements, including cell type-specific gene promoters and enhancers. Further, the patterns of DNA methylation are non-random, well-regulated, and tissue-specific (Eckhardt et al., 2006). Cell type-specific DNA methylation patterns are established during mammalian development and maintained in adult somatic cells (Crider et al., 2012). In essence, DNA methylation is a biochemical process that occurs predominantly at CpG dinucleotides where DNA methyltransferases (Dnmts) transfer the methyl group to cytosine nucleotides, generating 5-methylcytosine (5mC) which is found almost entirely within CpG dinucleotides (about 70–80%) present in DNA of mammalian somatic cells. Interestingly, CpG DNA methylation is highly prevalent in repetitive sequences but rare in CpG islands within housekeeping promoters. A detailed literature search supports the concept that DNA methylation of a gene functions to stabilize or lock in the silent state of certain genes (Razin and Riggs, 1980). However, DNA methylation patterns are perturbed in human diseases, such as imprinting disorders, degenerative diseases, and cancer (Jaenisch and Bird, 2003; Kagey et al., 2010). Also, studies with in vitro and animal models have shown that loss of genomic DNA methylation is associated with cellular senescence and aging of organisms (Oakes et al., 2003; Wilson and Jones, 1983). In humans, loss of genomic DNA methylation, measured in blood or cancer DNA samples (Guz et al., 2008; Hsiung et al., 2007; Moore et al., 2008) has been found in a variety of age-related diseases (Fraga et al., 2005; Fraga and Esteller, 2007), but little information is available on changes in promoter DNA methylation patterns during normal lens aging and ARCs (Palsamy et al., 2012). The importance of an epigenetic event is that it represents a mechanism by which the gene function is selectively altered in response to environmental and aging stresses. The Nrf2 and Keap1 genes have 60, and 90 CpG dinucleotides in their promoters, respectively, and methylated DNA sequence analyses showed that the CpG dinucleotides in the Keap1 gene are indeed epigenetically modified but not in the Nrf2 gene (Palsamy et al., 2012). Thus, age-dependent environmental stresses appear to lead to epigenetic DNA modifications, which cause aberrant gene expression . Specifically, loss of DNA methylation in the promoter of Keap1 gene decreases Nrf2-dependent antioxidant protection and results in a redox imbalance altered towards lens oxidation (Elanchezhian et al., 2012a; Elanchezhian et al., 2012b; Palsamy et al., 2012) (Palsamy et al., 2014a; Palsamy et al., 2014b, c). To be precise, there is a significant loss of methylcytosine in diabetic cataractous lenses (possibly more than 90%), and it appears to be very rapid. This notion is substantiated by human lens epithelial cell culture experiments with ER stressors such as methylglyoxal or sodium selenite (Palsamy et al., 2014a; Palsamy et al., 2014b). These findings indicate that diabetic cataracts are not induced by aging per se, but rather by age-associated diabetic stresses. In contrast, it has been shown that loss of DNA methylation in the Keap1 promoter in clear lenses is at a rate of 1.25% per year. Interestingly, diabetic lenses with cortical, nuclear, posterior subcapsular, and mixed type cataracts demonstrated significant loss of DNA methylation in the Keap1 promoter (Palsamy et al., 2014a). These data are of huge importance as they suggest that most of the population, with appropriate preventive care, would not develop a cataract in their natural lifetime. Characterization of dysregulation in DNA methylation during aging is at a very early stage and even understudied research area. This signifies the importance of studying the epigenetic DNA remodeling during age-related variations, including ARCs. In contrast, DNA is methylated with age in repetitive DNA segments such as Alu repeats, where Alu elements exhibit a striking tissue-specific pattern of methylation (Xie et al., 2009). Further studies are required to explore how some genes are methylated, while other genes are demethylated.
9. Regulation of DNA methylation
Although Ca2+ dependent proteolysis through ERAD might be responsible for the decreased levels of Nrf2, reports have also shown that loss of DNA methylation in the Keap1 gene also plays a major role in suppression of Nrf2 and loss of Nrf2-dependent cytoprotection. There are two known DNA demethylation pathways; one is the passive demethylation pathway in the germinative zone of replicating lens epithelial cells, and the other is the active demethylation pathway in the remaining non-replicating lens epithelial cells. Epigenetic modifications of DNA can last for a lifetime, and some of them might be imprinted (Kagey et al., 2010), a fact which can be used as a new tool to look at the history of and the correlations between the various stresses that are encountered throughout life and their pathogenic consequences. Since age-dependent cataractogenic stresses reprogram a broad range of genes by the epigenetic loss of promoter DNA methylation, this research study may predict the no-return pathway for ARCs formation and senescence.
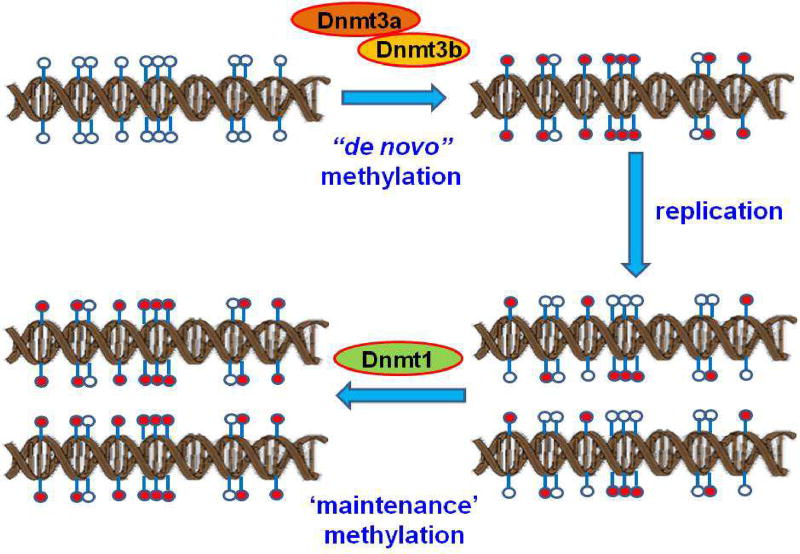
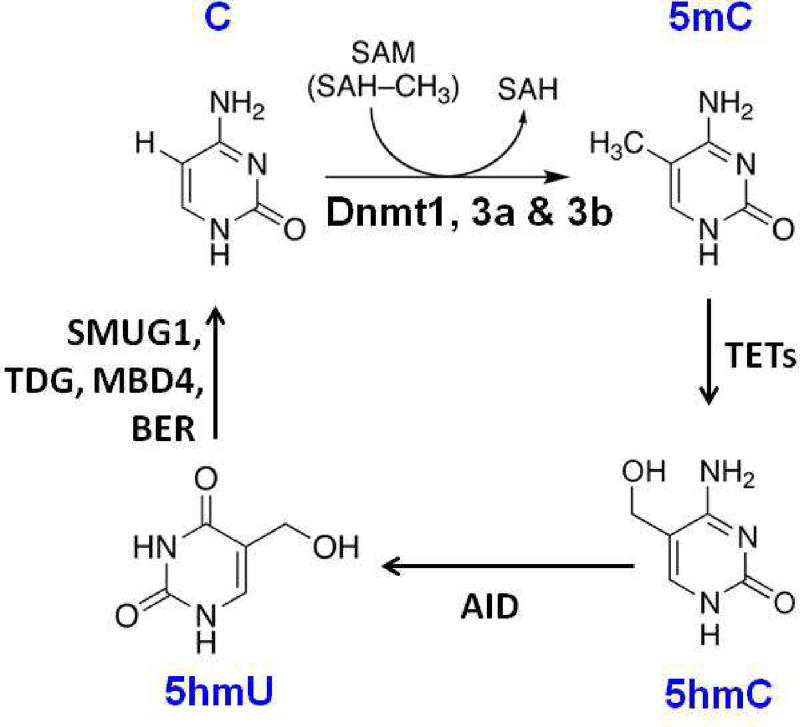
It is thought that DNA methylation patterns are established during embryonic development and are then faithfully inherited in the cells by a 'maintenance' mechanism in somatic cells. Failure of such 'maintenance' mechanism leads to DNA demethylation, which can result from passive demethylation via Dnmt1, Dnmt3a and Dnmt3b following DNA replication (Fig. 3), or from an active, replication-independent process of enzymatic removal of the 5mC by ten-eleven translocation 1 (TET1), activation-induced cytidine deaminase (AID), and thymine-DNA glycosylase (TDG). This suggests that promoter DNA methylation dynamics play an important part in human disease development and aging (Fig. 4).
Fig. 3.
Passive DNA demethylation pathway. Passive DNA demethylation is caused by a reduction in activity or absence of Dnmts. Dnmt3a and 3b are responsible for de novo DNA methylation of parent DNA. When methylated DNA is replicated, the daughter strands of DNA are unmethylated. This hemimethylated DNA is recognized by the maintenance DNA methyltransferase1 (Dnmt1) and restores parental DNA methylation patterns through successive rounds of cell division. If Dnmt1 is inhibited or absent when the cell divides, the newly synthesized strand of DNA will not be methylated, and successive rounds of cell division will result in passive demethylation. In this figure, unmethylated CpGs are shown by empty circles, and methylated CpGs are indicated by red circles.
Fig. 4.
Active DNA demethylation pathway. Active DNA demethylation takes place through the enzymatic replacement of 5mC with cytosine via the base-excision DNA repair pathway (BER) pathway. Cytosine is methylated by Dnmt1, 3a, and 3b and the resulting 5mC is oxidized to 5-hydroxymethylcytosine (5hmC) by TET1 proteins. 5hmC is then deaminated by AID into 5-hydroxy uracil (5hmU). Finally, 5hmU can be excised by TDG and repaired by the BER pathway with unmethylated cytosines. Active DNA demethylation pathway is induced in the non-replicating cells.
It has been hypothesized that ER stress-mediated chronic UPR further degrades/inactivates Dnmt1, Dnmt3a and Dnmt3b and lower levels of Dnmts no longer methylate the hemimethylated DNA through ‘maintenance methylation’ and result in loss of DNA methylation (Palsamy et al., 2012; Palsamy et al., 2014a). In contrast, chronic UPR could stimulate active DNA demethylation, which can be a major demethylation pathway in the rest of lens epithelial cells of diabetic cataractous lenses since there is no apparent DNA replication in those cells throughout life (Palsamy et al., 2012; Palsamy et al., 2014a).
In addition to promoter DNA demethylation of the Keap1 promoter in ARCs formation, there are reports demonstrated the involvement of promoter DNA hypermethylation in the context of ARCs formation. For instance, promoter DNA hypermethylation of CRYAA has been characterized in ARCs, thereby correlating the increased DNA methylation with decreased gene as well as protein expression (Zhou et al., 2012). Interestingly, treatment with zebularine, an inhibitor of DNMTs, was reported to increase the CRYAA expression levels in the lens epithelial cells (Zhou et al., 2012). Also, the lens epithelial-derived growth factor, LEDGF is expressed by lens epithelial cells, upregulates heat shock proteins and β-crystallins, and responds to environmental stress. LEDGF is an oncogene, and its expression is regulated by a promoter CpG island methylation (Bhargavan et al., 2013; Shinohara et al., 2002). Histone modification is also known to regulate the LEDGF response to UV damage, one of the environmental exposures that lead to ARCs formation (Bhargavan et al., 2013). DNA damage is an additional consequence of oxidative stress, and there are several reports have demonstrated the association between DNA repair genes with ARCs formation. More specifically, the promoter of the DNA repair genes, such as O6-methylguanine DNA methyltransferase (MGMT), and 8-oxoguanine DNA glycosylase 1 (OGG1) have been shown to hypermethylated in the cataractous tissues (Li et al., 2014; Wang et al., 2015). However, to understand the major mechanisms and function of DNA methylation in ARCs formation needs further research in this field.
10. ER stressor alters levels of DNA methylation in the Keap1 promoter
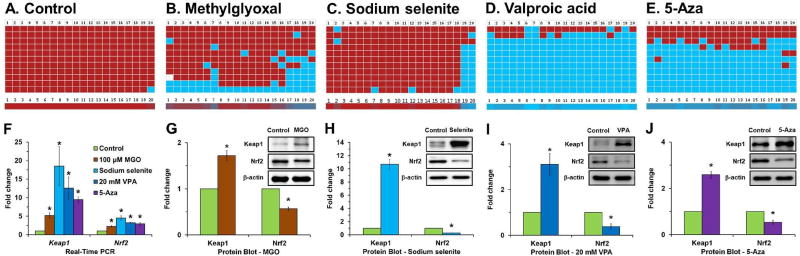
Diabetes is a well-documented cataract risk factor that accelerates early cataract formation. DNA methylation in the Keap1 gene is significantly decreased in diabetic cataractous lenses (Palsamy et al., 2012), and it is unknown what induces this loss in the lenses of these patients. It is speculated that loss of DNA modification enzymes (Dnmts and TET1) in the active and passive DNA demethylation pathways might be responsible for the loss of DNA methylation. mRNA and protein expressions studies confirmed that methylglyoxal, valproic acid, 5-Aza and sodium selenite treatment significantly activates the transcription of Nrf2 and Keap1 genes, while significantly suppressing Nrf2 and increasing Keap1 protein (Palsamy et al., 2014a; Palsamy et al., 2014b, c). Furthermore, DNA methyltransferases, Dnmt1, Dnmt3a and Dnmt3b and the active DNA demethylation enzymes TET1, AID, and TDG are over-expressed in human lens epithelial cells treated with methylglyoxal, valproic acid, and sodium selenite (Palsamy et al., 2014a; Palsamy et al., 2014b, c). Bisulfite-converted DNA sequencing results revealed a significant loss of DNA demethylation in the Keap1 promoter with notable increases in Keap1 mRNA and protein in human lens epithelial cells treated with methylglyoxal, sodium selenite, and valproic acid, but not in the Nrf2 promoter (Palsamy et al., 2012; Palsamy et al., 2014a; Palsamy et al., 2014b, c). Similarly, all other ER stressors listed in Table 1 might induce the loss of DNA methylation as shown (Fig. 5).
Fig. 5.
Loss of Keap1 promoter DNA methylation in human lens epithelial cells treated with methylglyoxal, sodium selenite, valproic acid (VPA) and 5-Aza-2'-deoxycytidine (5- Aza). Control human lens epithelial cells do not show any loss of DNA methylation of the Keap1 promoter (A). However, significant loss of DNA methylation of the Keap1 promoter is found in human lens epithelial cells treated with either 100 µM methylglyoxal for 1 day (B), 1 µM sodium selenite for 1 day (C), 20 mM of VPA for 5 days (D) and/or 10 µM 5-Aza for 7 days (E). Ten individual clones of the bisulfite-converted DNA sequences of human lens epithelial cells were analyzed for DNA methylation in fragment-1 (contains 20 CpG dinucleotides) of the Keap1 promoter using BISMA software with default filtering threshold settings (http://biochem.jacobs-university.de/BDPC/BISMA/). Each row of red squares represents methylated CpG dinucleotides. Blue squares represent unmethylated CpG dinucleotides, and white squares represent an undetermined CpG status. The color gradient bar shows that the red region contains more methylated CpG dinucleotides, and the blue region contains more unmethylated CpG dinucleotides. Fold changes in the mRNA expression levels of Keap1 and Nrf2 in control human lens epithelial cells and treated with methylglyoxal, sodium selenite, and 5-Aza (F). The corresponding fold variations in the protein levels of Keap1 and Nrf2 in human lens epithelial cells treated with methylglyoxal (G), sodium selenite (H), VPA (I) and 5-Aza (J).
These studies have established that treatment of human lens epithelial cells with ER stressor diminishes DNA methylation in the Keap1 promoter, similar to what occurs in human diabetic cataractous lenses. This finding indicates that loss of DNA methylation in the CpG islands of the Keap1 promoter in lenses of diabetic patients activates expression of the Keap1 protein, which then increases Nrf2 proteasomal degradation. As an outcome, decreased Nrf2 activity further represses the transcription of many cytoprotective target genes and alters the redox balance towards lens protein oxidation and blocks cytoprotection against cataractogenic stresses. Thus, the failure of antioxidant protection due to demethylation of CpG islands in the Keap1 promoter is linked to diabetic cataracts and possibly ARCs.
11. ER stressor activates proteasomal degradation of Nrf2 in lens epithelial cells
The mRNA levels of Nrf2/Keap1 in human lens epithelial cells are increased significantly, but the levels of Nrf2 protein is decreased significantly in human lens epithelial cells treated with ER stressor, especially cataractogenic stressors (Elanchezhian et al., 2012a; Palsamy et al., 2014a; Palsamy et al., 2014b, c). This discrepancy might be due to proteasomal degradation or ERAD, and this was confirmed in human lens epithelial cells that were pretreated with MG-132, an inhibitor of proteasome protease, followed by methylglyoxal treatment for another 24 h. The protein profiles of Nrf2 and Keap1 revealed that these ER stressors such as methylglyoxal treatment augmented significant proteasomal degradation of Nrf2, but not of Keap1 protein. However, when MG-132 pretreated human lens epithelial cells were then cultured with methylglyoxal revealed a substantial increase in the levels of Nrf2 and Keap1 protein compared to that of human lens epithelial cells treated with methylglyoxal alone (Palsamy et al., 2014a). These results further confirm that ER stressors augment the degradation of Nrf2 and Keap1 proteins through activation of proteasomal degradation. Also, the passive DNA demethylation enzymes, Dnmts, also decreased in human lens epithelial cells treated with ER stressor, but human lens epithelial cells pretreated with MG-132 augment degradation of Dnmts through activation of proteasomal degradation.
12. Lower levels of Nrf2 suppress expression of Nrf2-target cytoprotective genes
Since levels of Nrf2 decrease in human lens epithelial cells treated with ER stressor, levels of mRNA expression profiles of Nrf2-target cytoprotective genes such as glutamate-cysteine ligase catalytic subunit, glutamate-cysteine ligase modifier subunit, quinone reductase, thioredoxin reductase 1 and sulfiredoxin-1 must be significantly decreased. These Nrf2-target genes in human lens epithelial cells treated with methylglyoxal also decrease because Nrf2 protein is suppressed by higher levels of Keap1 protein leading to repression of the availability of Nrf2 for this transcription of Nrf2-target genes (Enomoto et al., 2001; Kwak et al., 2003a; Lee and Ye, 2004; Nair et al., 2007; Palsamy et al., 2014a; Palsamy et al., 2014b, c). Glutathione reductase and catalase levels also decrease in human lens epithelial cells treated with ER stressors such as methylglyoxal, sodium selenite and valproic acid (Palsamy et al., 2014a; Palsamy et al., 2014b, c). These findings strongly suggest that Nrf2-target cytoprotective genes may be suppressed upon exposure to either ER stressors or cataractogenic stressors in lens epithelial cells. Further, cultured lens epithelial cells of Nrf2 knockout (Nrf2−/−) mice treated with varying concentrations of methylglyoxal showed enhanced cell death, compared to cultured lens epithelial cells of wild-type mice. These results demonstrate the importance of retaining normal levels of Nrf2 and Nrf2 target cytoprotective genes for the survival of the lens under stressed conditions (Palsamy et al., 2014a).
13. UPR activation in immature lens fiber cells
Induction of ER stress in lens epithelial cells is well documented, but it is still obscure in the case of immature lens fiber cells. As lens opacity develops in these fiber cell layers, it is crucial to identify whether ER stress is induced in immature lens fiber cells and generates Nrf2-deficiency in these cells. Immature lens fiber cells contain ER, mitochondria and intact DNA and mRNAs (Bassnett and Sikic, 2017). Crystallin mRNAs are present for at least 1 month from the time of initiation of lens fiber differentiation in the chicken lens (Shinohara and Piatigorsky, 1976). Proliferating lens epithelial cells in the lens germinative zone remain as the most sensitive to ER stress (Elanchezhian et al., 2012b), and immature lens fiber cells appear to overexpress ROS in suckling rat lenses (Palsamy et al., 2014b). Firtina et al. reported that the ER-resident chaperones, BiP and PDI, were expressed at high levels in the newly forming fiber cells of mouse embryonic lens (Firtina and Duncan, 2011). These fiber cells also expressed the UPR- associated molecules; XBP-1, ATF6, P-PERK, and ATF4 during embryogenesis. Moreover, spliced XBP-1, cleaved ATF6, and P-eIF2α were detected in embryonic mouse lenses suggesting that the UPR pathway is active in this tissue (Firtina and Duncan, 2011). Also, the expression of Ero-1β, an ER stress-mediated ROS-producing enzyme, was found in the cortical lens fiber cells of an ARC lens from a 57-year-old individual, but the rest of UPR proteins, such as Nrf2, BiP, and Keap1, were not detected (Elanchezhian et al., 2012b). Lens epithelial cells and immature lens fiber cells are partially liquefied in selenite injected 14-day suckling rat lenses (Palsamy et al., 2014b; Shearer et al., 1997) suggesting significant induction of UPR induced damage in immature lens fiber cells. Also, detection of string-like DNA staining with 2',7'- dichlorodihydrofluorescein diacetate (H2-DCFH) in the lens fiber cells suggests that ER stressors-induced increased the permeability of the plasma membrane and binding of H2-DCFH to DNA, resulting in detection of DCF fluorescence on DNA (Palsamy et al., 2014b).
Based on these observations, this review summarizes the possibility that lens fiber cells that form under ER stress might develop cataract due to a deficiency in Nrf2 dependent cytoprotection in the multi-layer of the cells. Interestingly, the localization of these cells determines the type of cataract to develop. An unsettled report that nuclear cataract patients have significantly higher mortality than those with other types of cataracts (Wang et al., 2014) suggests that nuclear cataract patients may have mild levels of ER stress in the lens throughout life.
14. Prevention of ARCs
It can be expected that almost all the ER stressors listed in Table 1 generate a misfolded protein conformation which triggers an activation of ER stress. Loss of DNA methylation in somatic cells is considered irreversible, as is aging, and ARCs, but these are preventable. Avoiding ER stressors by changing one’s lifestyle, improving systemic diseases, preventing nutritional deficiencies, and avoiding toxic drugs, environmental toxins, and irradiations may prevent ARCs formation. Those are the best strategies to protect the lens from initiation of chronic UPR. Many reports indicate that intake of excess levels of antioxidants may help to prevent cataract formation. It is also important to block the loss of DNA methylation in somatic lens cells in order to maintain the preprogrammed methylated DNA status for a prolonged period of time. Huang et al. recently reported that the stable knockdown of Tet1 results in a decrease of Keap1 mRNA when compared to control cells (Huang et al., 2014). From their report, one can speculate that if Tet1 is suppressed, the Keap1 gene retains its methylation status and Keap1 expression is also suppressed. The bisulfite genomic DNA sequencing of the human Keap1 gene suggests that if promoter DNA demethylation is avoided, ARCs can be preventable up to ages over 100. Also, although the Keap1−/− mouse dies postnatally, the lung-specific knockdown of Keap1 significantly enhances antioxidant protection (Blake et al., 2010; Wakabayashi et al., 2003) suggesting that Keap1 suppression can be a potential therapeutic approach for the prevention of ARCs. These strategies protect the lens from cataract formation, though they are also beneficial for longevity.
15. Future directions
Oxidative stress is associated with DNA hypomethylation in the Keap1 gene in the cataractous lens, but DNA hypermethylation is associated with colorectal (Hanada et al., 2012) and lung cancers (Muscarella et al., 2011). Interestingly, the lens is the only organ in the human body which does not develop cancer (Bhat, 2001). A better understanding of the DNA methylation status in the Keap1 promoter might reveal links between cellular malignancy and senescence.
The DNA methylation status of the Keap1 promoter suggests that aging directly affects ARC formation in term of the loss of Nrf2 dependent cytoprotection. Loss of DNA methylation in the Keap1 promoter is nearly 0% at age 17, but at the ages 60 and 75, it is 40% and 50%, respectively (Palsamy et al., 2012; Palsamy et al., 2014a). A 40–50% loss of DNA methylation stress in elderly populations can increase to an average of 90% loss with cataractogenic stress in those who develop ARCs (Palsamy et al., 2012; Palsamy et al., 2014a). Thus, the incidence of ARCs shows significant increases with loss of DNA methylation. Also, loss of DNA methylation is known to occur in multiple genes, such as hypoxia-inducible factor-1 (HIF-1) (Ke and Costa, 2006). HIF-1 is the first gene involved in homeostatic processes in the lens and plays an integral role in the response of the lens to low oxygen concentrations or hypoxia. HIF-1 activates transcription of numerous genes including vascular endothelial growth factor (VEGF), glucose transporter-1 (GLUT-1), and genes involved in glucose metabolism (Willam et al., 2002). HIF-1 interacts with the von Hippel-Lindau (pVHL) ubiquitin E3 ligase complex (Masson et al., 2001; Srinivas et al., 1999). In normoxic conditions, hydroxylation at two proline residues and acetylation at a lysine residue in the oxygen-dependent degradation domain of HIF-1 triggers its connotation with pVHL E3 ligase complex, leading to HIF-1 degradation via the ubiquitin-proteasome pathway. Methylation of pVHL regulates the levels of HIF-1 in patients with metastatic clear-cell renal cell carcinoma (Choueiri et al., 2013). One can speculate that it is not HIF-1, but instead the pVHL promoter (HIF-1/pVHL), which can significantly lose DNA methylation and reprogram the oxygen response pathway in the lens. Extensive analysis of the DNA methylation status of these genes might yield a significant contribution. Also, nuclear factor-κB (NF-κB) activation is induced by a wide variety of agents including stress, cigarette smoke, other carcinogens, bacteria, inflammatory stimuli, cytokines, free radicals, tumor promoters, and endotoxins. Upon activation, NF-κB regulates the expression of almost 400 different genes, which include angiogenic factors, cell cycle regulatory molecules, cytokines, enzymes, and viral proteins. NF-κB is sequestered in the cytoplasm in an inactive form through its association with one of the several inhibitory molecules, including IκB-α, which is the most abundant form; NF-κB in NF-κB/IκB-α complexes are ubiquitinated and undergo proteasomal degradation (Oeckinghaus and Ghosh, 2009).
Interestingly, Nrf2, HIF-1, and NF-κB are central transcriptional activators and Keap1, pVHL and IκB-α are regulatory proteins which induce proteasomal degradation of these transcriptional factors under stress conditions. One can expect a loss of DNA methylation in the Keap1, pVHL, and IκB-α promoters, which can ultimately result in an overexpression of these proteins, resulting in decreased levels of transcriptional activators and loss of cytoprotection. Elucidating the epigenetic mechanisms in the whole genome is an exciting challenge that is expected to lead to a clear understanding of the development of human senescence and malignancy through ARCs research.
16. Summary and conclusions
Most cataractogenic stress generates misfolded protein conformation of the membrane luminal and secretory proteins in the highly oxidized ER lumen. The conformational change of the protein is one of the most important and essential triggers to generate a cascade of cytoprotective and self-destructive chain reactions, the so-called ER stress/UPR. The protective ER stress eliminates misfolded proteins from the cell by ERAD pathway. Chronic UPR releases ER-Ca2+ to activate m-calpain and proteolysis enzymes. It also produces aggravated ROS, that are sufficient to oxidize cellular constituents, leading thereby to cellular apoptosis. Induction of apoptosis in lens epithelial cells have been reported in certain types of cataracts (Li et al., 1995), but not in nuclear cataracts (Konofsky et al., 1987). Cortical cataracts typically begin in a small region of the lens periphery and can spread around the lens circumference. Chronic and strong UPR might produce severe ROS thereby inducing apoptosis in lens epithelial cells. On the contrary, the weak UPR under mild ER stress conditions could be simply overcome by the lens epithelial cellular defense system. The ROS generated by these cells might cause little pathology (Papa, 2012). However, though ER is not present in the lens nucleus, it is speculated that nuclear cataracts, which do not require apoptosis in lens epithelial cells, can be induced by non-lethal exposures of ER stressors for extended times or recurrent times (Ikesugi et al., 2006b; Palsamy et al., 2014b). There are several experimental nuclear cataract models describing the involvement of UPR in the disease pathology, and none of them provided the detailed mechanism. Thus, one can suggest that various ER stressors with different potential and exposure durations may affect the development of the different types of cataracts (Berthoud et al., 2016; Lyu et al., 2016; Mulhern et al., 2006; Palsamy et al., 2014b). Recently, it has been demonstrated the activation of the UPR through discrete pathways in the lens of age- related, high myopia-related and congenital cataracts (Yang et al., 2015). Specifically, all three UPR pathways are activated in age-related and high myopia-related cataractous lenses; however, only the IRE1/XBP-1and PERK/eIF2α/ATF4 pathways are activated in the congenital cataract lenses (Yang et al., 2015). Overall, the functional consequences of differential UPR activation in the context of the mechanism(s) of development of various cataracts are largely unknown but can be a topic of future research.
In addition, the expression of the Keap1 gene is upregulated by the loss of DNA methylation. The demethylation is induced by the decreased level of Dnmts and increasing levels of TET1, AID, and TDG, which further induce the loss of DNA methylation in the Keap1 promoter. The demethylated promoter of Keap1 results in overexpression of the Keap1 gene and produces higher levels of Keap1 protein. Higher levels of Keap1 induce the degradation of Nrf2 by ubiquitin-mediated proteasomal degradation and ERAD. As a result, Nrf2 dependent stress protection declines and the redox balance is altered towards lens oxidation leading to cataract formation. Thus, misfolded protein conformation initiates the production of misfolded crystallin aggregation (Fig. 6).
Fig. 6.
A schematic diagram showing the involvement of various cataractogenic stressors mediating ER stress mediated Nrf2 dependent antioxidant system failure via Keap1 promoter DNA demethylation.
Acknowledgments
We are grateful to Prof. Robert C. Augusteyn and Dr. Sangeetha Nagarajan for their immense help in proof reading. This research was supported by a grant from the National Eye Institute to Prof.Shinohara (EY018172).
List of abbreviations
- 5-Aza
5-aza-2'-deoxycytidine
- 5mC
5-methylcytosine
- AID
activation-induced cytidine deaminase
- ARCs
age-related cataracts
- ARE
antioxidant response element
- ATF6
activating transcription factor 6
- BiP
Ig binding protein
- Dnmts
DNA methyltransferases
- eIF2α
eukaryotic translation initiation factor 2α
- ER
endoplasmic reticulum
- ERAD
ER-associated degradation
- Ero1
ER oxidoreductin 1
- GSH
glutathione
- H2DCFDA
2',7'-dichlorodihydrofluorescein diacetate
- HIF-1
hypoxia-inducible factor-1
- IRE1
inositol-requiring kinase 1
- Keap1
Kelch-like ECH-associated protein 1
- NF-κB
nuclear factor-κB
- Nrf2
nuclear factor-erythroid-2-related factor 2
- PDI
protein disulfide isomerase
- PERK
protein kinase R (PKR)-like endoplasmic reticulum kinase
- PKC
protein kinase C
- PMCA
plasma membrane Ca2+-ATPases
- pVHL
von Hippel-Lindau
- ROS
reactive oxygen species
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPases
- TDG
thymine-DNA glycosylase
- TET1
ten-eleven translocation 1
- UPR
unfolded protein response
- XBP-1
X-box transcription factor-1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham MA, Korula A, Jayakrishnan K, John GT, Thomas PP, Jacob CK. Prognostic factors in diffuse proliferative lupus nephritis. J Assoc Physicians India. 1999;47:862–865. [PubMed] [Google Scholar]
- Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- Aldana JP, Marcovich R, Singhal P, Reddy K, Morgenstern N, El-Hakim A, Smith AD, Lee BR. Immune response to laparoscopic bowel injury. J Endourol. 2003;17:317–322. doi: 10.1089/089277903322145503. [DOI] [PubMed] [Google Scholar]
- Allan GF, Leng X, Tsai SY, Weigel NL, Edwards DP, Tsai MJ, O'Malley BW. Hormone and antihormone induce distinct conformational changes which are central to steroid receptor activation. J Biol Chem. 1992;267:19513–19520. [PubMed] [Google Scholar]
- Alves MS, Reis PA, Dadalto SP, Faria JA, Fontes EP, Fietto LG. A novel transcription factor, ERD15 (Early Responsive to Dehydration 15), connects endoplasmic reticulum stress with an osmotic stress-induced cell death signal. J Biol Chem. 2011;286:20020–20030. doi: 10.1074/jbc.M111.233494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andley UP. Crystallins in the eye: Function and pathology. Prog Retin Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Andley UP. Effects of alpha-crystallin on lens cell function and cataract pathology. Curr Mol Med. 2009;9:887–892. doi: 10.2174/156652409789105598. [DOI] [PubMed] [Google Scholar]
- Archer AG. Cataract formation in anorexia nervosa. Br Med J (Clin Res Ed) 1981;282:274. doi: 10.1136/bmj.282.6260.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi J, Kamo H, Baba R, Doi Y, Yamashita A, Murakami D, Hanada A, Hirano T. Bisphenol A induces endoplasmic reticulum stress-associated apoptosis in mouse non-parenchymal hepatocytes. Life Sci. 2010;87:431–438. doi: 10.1016/j.lfs.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Athanasiadis I, Konstantinidis A, Kyprianou I, Robinson R, Moschou V, Kouzi-Koliakos K. Rapidly progressing bilateral cataracts in a patient with beta thalassemia and pellagra. J Cataract Refract Surg. 2007;33:1659–1661. doi: 10.1016/j.jcrs.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Azuma M, Fukiage C, David LL, Shearer TR. Activation of calpain in lens: a review and proposed mechanism. Exp Eye Res. 1997;64:529–538. doi: 10.1006/exer.1996.0234. [DOI] [PubMed] [Google Scholar]
- Bahar E, Kim H, Yoon H. ER Stress-Mediated Signaling: Action Potential and Ca(2+) as Key Players. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17091558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks V, Hagelstein S, Thomas N, Bale S, Harding KG. Comparing hydrocolloid dressings in management of exuding wounds. Br J Nurs. 1999;8:640–646. doi: 10.12968/bjon.1999.8.10.6600. [DOI] [PubMed] [Google Scholar]
- Barnes NA, Stephenson SJ, Tooze RM, Doody GM. Amino acid deprivation links BLIMP-1 to the immunomodulatory enzyme indoleamine 2,3-dioxygenase. J Immunol. 2009;183:5768–5777. doi: 10.4049/jimmunol.0803480. [DOI] [PubMed] [Google Scholar]
- Baruch A, Greenbaum D, Levy ET, Nielsen PA, Gilula NB, Kumar NM, Bogyo M. Defining a link between gap junction communication, proteolysis, and cataract formation. J Biol Chem. 2001;276:28999–29006. doi: 10.1074/jbc.M103628200. [DOI] [PubMed] [Google Scholar]
- Bassnett S, McNulty R. The effect of elevated intraocular oxygen on organelle degradation in the embryonic chicken lens. J Exp Biol. 2003;206:4353–4361. doi: 10.1242/jeb.00670. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Sikic H. The lens growth process. Prog Retin Eye Res. 2017 doi: 10.1016/j.preteyeres.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- Beebe DC, Holekamp NM, Shui YB. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res. 2010;44:155–165. doi: 10.1159/000316481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berthoud VM, Minogue PJ, Lambert PA, Snabb JI, Beyer EC. The Cataract-linked Mutant Connexin50D47A Causes Endoplasmic Reticulum Stress in Mouse Lenses. J Biol Chem. 2016;291:17569–17578. doi: 10.1074/jbc.M115.707950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Bhargavan B, Chhunchha B, Fatma N, Kubo E, Kumar A, Singh DP. Epigenetic repression of LEDGF during UVB exposure by recruitment of SUV39H1 and HDAC1 to the Sp1-responsive elements within LEDGF promoter CpG island. Epigenetics. 2013;8:268–280. doi: 10.4161/epi.23861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat SP. The ocular lens epithelium. Biosci Rep. 2001;21:537–563. doi: 10.1023/a:1017952128502. [DOI] [PubMed] [Google Scholar]
- Bhuyan KC, Bhuyan DK. Molecular mechanism of cataractogenesis: III. Toxic metabolites of oxygen as initiators of lipid peroxidation and cataract. Curr Eye Res. 1984;3:67–81. doi: 10.3109/02713688408997188. [DOI] [PubMed] [Google Scholar]
- Bhuyan KC, Bhuyan DK, Podos SM. Lipid peroxidation in cataract of the human. Life Sci. 1986;38:1463–1471. doi: 10.1016/0024-3205(86)90559-x. [DOI] [PubMed] [Google Scholar]
- Bhuyan KC, Bhuyan DK, Podos SM. Free radical enhancer xenobiotic is an inducer of cataract in rabbit. Free Radic Res Commun. 1991;12–13(Pt 2):609–620. doi: 10.3109/10715769109145837. [DOI] [PubMed] [Google Scholar]
- Blake DJ, Singh A, Kombairaju P, Malhotra D, Mariani TJ, Tuder RM, Gabrielson E, Biswal S. Deletion of Keap1 in the lung attenuates acute cigarette smoke-induced oxidative stress and inflammation. Am J Respir Cell Mol Biol. 2010;42:524–536. doi: 10.1165/rcmb.2009-0054OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau EB. Congenital cataracts and maternal vitamin D deficiency. Lancet. 1996;347:626. doi: 10.1016/s0140-6736(96)91331-8. [DOI] [PubMed] [Google Scholar]
- Bloom DA, Jaiswal AK. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J Biol Chem. 2003;278:44675–44682. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- Bonashevskaia TI. Experimental cataracts induced by alpha-2,4-dinitrophenol. Vestn Oftalmol. 1970;3:73–75. [PubMed] [Google Scholar]
- Braun S, Hanselmann C, Gassmann MG, auf dem Keller U, Born-Berclaz C, Chan K, Kan YW, Werner S. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Molecular and cellular biology. 2002;22:5492–5505. doi: 10.1128/MCB.22.15.5492-5505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan LA, Kantorow M. Mitochondrial function and redox control in the aging eye: role of MsrA and other repair systems in cataract and macular degenerations. Exp Eye Res. 2009;88:195–203. doi: 10.1016/j.exer.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabibbo A, Pagani M, Fabbri M, Rocchi M, Farmery MR, Bulleid NJ, Sitia R. ERO1-L, a human protein that favors disulfide bond formation in the endoplasmic reticulum. J Biol Chem. 2000;275:4827–4833. doi: 10.1074/jbc.275.7.4827. [DOI] [PubMed] [Google Scholar]
- Cai J, Nelson KC, Wu M, Sternberg P, Jr, Jones DP. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: the dose-response revolution. Annu Rev Pharmacol Toxicol. 2003;43:175–197. doi: 10.1146/annurev.pharmtox.43.100901.140223. [DOI] [PubMed] [Google Scholar]
- Caldwell CR. Temperature-Induced Protein Conformational Changes in Barley Root Plasma Membrane-Enriched Microsomes: III. Effect of Temperature and Cations on Protein Sulfhydryl Reactivity. Plant Physiol. 1989;91:1339–1344. doi: 10.1104/pp.91.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champeil P, Le Maire M, Moller JV, Riollet S, Guillain F, Green NM. Does intrinsic fluorescence reflect conformational changes in the Ca2+-ATPase of sarcoplasmic reticulum? FEBS Lett. 1986;206:93–98. doi: 10.1016/0014-5793(86)81347-3. [DOI] [PubMed] [Google Scholar]
- Chang D, Zhang X, Rong S, Sha Q, Liu P, Han T, Pan H. Serum antioxidative enzymes levels and oxidative stress products in age-related cataract patients. Oxid Med Cell Longev. 2013;2013:587826. doi: 10.1155/2013/587826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapelier A, Desmadril M, Houee-Levin C. Gamma radiation effects on alpha-lactalbumin: structural modifications. Can J Physiol Pharmacol. 2001;79:154–157. [PubMed] [Google Scholar]
- Cheema MA, Taboada P, Barbosa S, Castro E, Siddiq M, Mosquera V. Energetics and conformational changes upon complexation of a phenothiazine drug with human serum albumin. Biomacromolecules. 2007;8:2576–2585. doi: 10.1021/bm070354j. [DOI] [PubMed] [Google Scholar]
- Chen KJ, Pan WH, Shaw NS, Huang RF, Lin BF. Association between dietary folate-rich food intake and folate status of elderly Taiwanese. Asia Pac J Clin Nutr. 2005;14:244–249. [PubMed] [Google Scholar]
- Chen YW, Yang YT, Hung DZ, Su CC, Chen KL. Paraquat induces lung alveolar epithelial cell apoptosis via Nrf-2-regulated mitochondrial dysfunction and ER stress. Arch Toxicol. 2012;86:1547–1558. doi: 10.1007/s00204-012-0873-8. [DOI] [PubMed] [Google Scholar]
- Cheung N, Wong TY. Obesity and eye diseases. Surv Ophthalmol. 2007;52:180–195. doi: 10.1016/j.survophthal.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CC, Kang YL, Yang TH, Huang CH, Fang K. Ectopic expression of herpes simplex virus-thymidine kinase gene in human non-small cell lung cancer cells conferred caspase-activated apoptosis sensitized by ganciclovir. Int J Cancer. 2002;102:328–333. doi: 10.1002/ijc.10701. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Taylor A. Nutritional antioxidants and age-related cataract and maculopathy. Experimental eye research. 2007;84:229–245. doi: 10.1016/j.exer.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Chiu HW, Fang WH, Chen YL, Wu MD, Yuan GF, Ho SY, Wang YJ. Monascuspiloin enhances the radiation sensitivity of human prostate cancer cells by stimulating endoplasmic reticulum stress and inducing autophagy. PLoS One. 2012;7:e40462. doi: 10.1371/journal.pone.0040462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choueiri TK, Fay AP, Gagnon R, Lin Y, Bahamon B, Brown V, Rosenberg JE, Hutson TE, Baker-Neblett KL, Carpenter C, Liu Y, Pandite L, Signoretti S. The role of aberrant VHL/HIF pathway elements in predicting clinical outcome to pazopanib therapy in patients with metastatic clear-cell renal cell carcinoma. Clin Cancer Res. 2013;19:5218–5226. doi: 10.1158/1078-0432.CCR-13-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman JK. 5-Azacytidine and 5-aza-2'-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene. 2002;21:5483–5495. doi: 10.1038/sj.onc.1205699. [DOI] [PubMed] [Google Scholar]
- Clayton RM, Cuthbert J, Phillips CI, Bartholomew RS, Stokoe NL, Ffytche T, Reid JM, Duffy J, Seth J, Alexander M. Analysis of individual cataract patients and their lenses: a progress report. Exp Eye Res. 1980;31:553–566. doi: 10.1016/s0014-4835(80)80014-5. [DOI] [PubMed] [Google Scholar]
- Crabbe MJ. Cataract as a conformational disease--the Maillard reaction, alpha-crystallin and chemotherapy. Cell Mol Biol (Noisy-le-grand) 1998;44:1047–1050. [PubMed] [Google Scholar]
- Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate's role. Adv Nutr. 2012;3:21–38. doi: 10.3945/an.111.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csala M, Margittai E, Banhegyi G. Redox control of endoplasmic reticulum function. Antioxid Redox Signal. 2010;13:77–108. doi: 10.1089/ars.2009.2529. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushnir VN, Slepova OS, Cruglova TB, Dumbrava VA, Cushnir RI, Zaitseva NS, Vovk EM. The role of HBV-infection in development of cataracts in children and adults. Oftalmologia. 1997;41:318–322. [PubMed] [Google Scholar]
- Cvekl A, Duncan MK. Genetic and epigenetic mechanisms of gene regulation during lens development. Prog Retin Eye Res. 2007;26:555–597. doi: 10.1016/j.preteyeres.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Cano M, Ebrahimi K, Wang L, Handa JT. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res 2017 [Google Scholar]
- David LL, Shearer TR. Calcium-activated proteolysis in the lens nucleus during selenite cataractogenesis. Investigative ophthalmology & visual science. 1984;25:1275–1283. [PubMed] [Google Scholar]
- David LL, Shearer TR. Purification of calpain II from rat lens and determination of endogenous substrates. Experimental eye research. 1986;42:227–238. doi: 10.1016/0014-4835(86)90057-6. [DOI] [PubMed] [Google Scholar]
- David LL, Varnum MD, Lampi KJ, Shearer TR. Calpain II in human lens. Investigative ophthalmology & visual science. 1989;30:269–275. [PubMed] [Google Scholar]
- Davidson GA, Varhol RJ. Kinetics of thapsigargin-Ca(2+)-ATPase (sarcoplasmic reticulum) interaction reveals a two-step binding mechanism and picomolar inhibition. The Journal of biological chemistry. 1995;270:11731–11734. doi: 10.1074/jbc.270.20.11731. [DOI] [PubMed] [Google Scholar]
- De Filippi L, Fournier M, Cameroni E, Linder P, De Virgilio C, Foti M, Deloche O. Membrane stress is coupled to a rapid translational control of gene expression in chlorpromazine-treated cells. Curr Genet. 2007;52:171–185. doi: 10.1007/s00294-007-0151-0. [DOI] [PubMed] [Google Scholar]
- De Miguel C, Pollock JS. Does endoplasmic reticulum stress mediate endothelin-1-induced renal inflammation? Am J Physiol Regul Integr Comp Physiol. 2013;305:R107–109. doi: 10.1152/ajpregu.00184.2013. [DOI] [PubMed] [Google Scholar]
- Derham BK, Harding JJ. Effects of modifications of alpha-crystallin on its chaperone and other properties. Biochem J. 2002;364:711–717. doi: 10.1042/BJ20011512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desagher S, Severac D, Lipkin A, Bernis C, Ritchie W, Le Digarcher A, Journot L. Genes regulated in neurons undergoing transcription-dependent apoptosis belong to signaling pathways rather than the apoptotic machinery. The Journal of biological chemistry. 2005;280:5693–5702. doi: 10.1074/jbc.M408971200. [DOI] [PubMed] [Google Scholar]
- Dhakshinamoorthy S, Jaiswal AK. c-Maf negatively regulates ARE-mediated detoxifying enzyme genes expression and anti-oxidant induction. Oncogene. 2002;21:5301–5312. doi: 10.1038/sj.onc.1205642. [DOI] [PubMed] [Google Scholar]
- Ding Y, Lin M, Liu H, Zhang W, Wang L, Li Y. Outcomes of post-cataract surgery endophthalmitis referred to a tertiary center from local hospitals in the south of China. Infection. 2011;39:451–460. doi: 10.1007/s15010-011-0138-0. [DOI] [PubMed] [Google Scholar]
- Duncan G, Jacob TJ. Calcium and the physiology of cataract. Ciba Found Symp. 1984;106:132–152. doi: 10.1002/9780470720875.ch8. [DOI] [PubMed] [Google Scholar]
- Duncan G, Wormstone IM. Calcium cell signalling and cataract: role of the endoplasmic reticulum. Eye (Lond) 1999;13(Pt 3b):480–483. doi: 10.1038/eye.1999.125. [DOI] [PubMed] [Google Scholar]
- Duncan G, Wormstone IM, Liu CS, Marcantonio JM, Davies PD. Thapsigargin-coated intraocular lenses inhibit human lens cell growth. Nat Med. 1997;3:1026–1028. doi: 10.1038/nm0997-1026. [DOI] [PubMed] [Google Scholar]
- Eckhardt F, Lewin J, Cortese R, Rakyan VK, Attwood J, Burger M, Burton J, Cox TV, Davies R, Down TA, Haefliger C, Horton R, Howe K, Jackson DK, Kunde J, Koenig C, Liddle J, Niblett D, Otto T, Pettett R, Seemann S, Thompson C, West T, Rogers J, Olek A, Berlin K, Beck S. DNA methylation profiling of human chromosomes 6, 20 and 22. Nat Genet. 2006;38:1378–1385. doi: 10.1038/ng1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elanchezhian R, Palsamy P, Madson CJ, Lynch DW, Shinohara T. Age-related cataracts: Homocysteine coupled endoplasmic reticulum stress and suppression of Nrf2-dependent antioxidant protection. Chem Biol Interact. 2012a;200:1–10. doi: 10.1016/j.cbi.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elanchezhian R, Palsamy P, Madson CJ, Mulhern ML, Lynch DW, Troia AM, Usukura J, Shinohara T. Low glucose under hypoxic conditions induces unfolded protein response and produces reactive oxygen species in lens epithelial cells. Cell Death Dis. 2012b;3:e301. doi: 10.1038/cddis.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein AD. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O'Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- Erdmann F, Schauble N, Lang S, Jung M, Honigmann A, Ahmad M, Dudek J, Benedix J, Harsman A, Kopp A, Helms V, Cavalie A, Wagner R, Zimmermann R. Interaction of calmodulin with Sec61alpha limits Ca2+ leakage from the endoplasmic reticulum. EMBO J. 2011;30:17–31. doi: 10.1038/emboj.2010.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang DL, Wan Y, Shen W, Cao J, Sun ZX, Yu HH, Zhang Q, Cheng WH, Chen J, Ning B. Endoplasmic reticulum stress leads to lipid accumulation through upregulation of SREBP-1c in normal hepatic and hepatoma cells. Mol Cell Biochem. 2013;381:127–137. doi: 10.1007/s11010-013-1694-7. [DOI] [PubMed] [Google Scholar]
- Fatma N, Singh P, Chhunchha B, Kubo E, Shinohara T, Bhargavan B, Singh DP. Deficiency of Prdx6 in lens epithelial cells induces ER stress response-mediated impaired homeostasis and apoptosis. Am J Physiol Cell Physiol. 2011;301:C954–967. doi: 10.1152/ajpcell.00061.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige MJ, Hendershot LM. Disulfide bonds in ER protein folding and homeostasis. Curr Opin Cell Biol. 2011;23:167–175. doi: 10.1016/j.ceb.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrini W, Aubert V, Balmer A, Munier FL, Abouzeid H. Anterior uveitis and cataract after rubella vaccination: a case report of a 12-month-old girl. Pediatrics. 2013;132:e1035–1038. doi: 10.1542/peds.2012-2930. [DOI] [PubMed] [Google Scholar]
- Firtina Z, Danysh BP, Bai X, Gould DB, Kobayashi T, Duncan MK. Abnormal expression of collagen IV in lens activates unfolded protein response resulting in cataract. The Journal of biological chemistry. 2009;284:35872–35884. doi: 10.1074/jbc.M109.060384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firtina Z, Duncan MK. Unfolded Protein Response (UPR) is activated during normal lens development. Gene Expr Patterns. 2011;11:135–143. doi: 10.1016/j.gep.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flach AJ, Dolan BJ. Progression of amiodarone induced cataracts. Doc Ophthalmol. 1993;83:323–329. doi: 10.1007/BF01204334. [DOI] [PubMed] [Google Scholar]
- Flach AJ, Peterson JS, Dolan BJ. Anterior subcapsular cataracts: a review of potential etiologies. Ann Ophthalmol. 1985;17:78–80. [PubMed] [Google Scholar]
- Foldi I, Toth AM, Szabo Z, Mozes E, Berkecz R, Datki ZL, Penke B, Janaky T. Proteome-wide study of endoplasmic reticulum stress induced by thapsigargin in N2a neuroblastoma cells. Neurochem Int. 2013;62:58–69. doi: 10.1016/j.neuint.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Foster A, Gilbert C, Rahi J. Epidemiology of cataract in childhood: a global perspective. J Cataract Refract Surg. 1997;23(Suppl 1):601–604. doi: 10.1016/s0886-3350(97)80040-5. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suner D, Cigudosa JC, Urioste M, Benitez J, Boix-Chornet M, Sanchez-Aguilera A, Ling C, Carlsson E, Poulsen P, Vaag A, Stephan Z, Spector TD, Wu YZ, Plass C, Esteller M. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga MF, Esteller M. Epigenetics and aging: the targets and the marks. Trends Genet. 2007;23:413–418. doi: 10.1016/j.tig.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Frederikse PH, Farnsworth P, Zigler JS., Jr Thiamine deficiency in vivo produces fiber cell degeneration in mouse lenses. Biochem Biophys Res Commun. 1999;258:703–707. doi: 10.1006/bbrc.1999.0560. [DOI] [PubMed] [Google Scholar]
- Friend B. Special delivery. Nurs Times. 1990;86:18. [PubMed] [Google Scholar]
- Gao J, Sun X, Martinez-Wittinghan FJ, Gong X, White TW, Mathias RT. Connections between connexins, calcium, and cataracts in the lens. J Gen Physiol. 2004;124:289–300. doi: 10.1085/jgp.200409121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe E, Suissa S, LeLorier J. Exposure to allopurinol and the risk of cataract extraction in elderly patients. Arch Ophthalmol. 1998;116:1652–1656. doi: 10.1001/archopht.116.12.1652. [DOI] [PubMed] [Google Scholar]
- Geng T, Hu W, Broadwater MH, Snider JM, Bielawski J, Russo SB, Schwacke JH, Ross J, Cowart LA. Fatty acids differentially regulate insulin resistance through endoplasm reticulum stress-mediated induction of tribbles homologue 3: a potential link between dietary fat composition and the pathophysiological outcomes of obesity. Diabetologia. 2013 doi: 10.1007/s00125-013-2973-2. [DOI] [PubMed] [Google Scholar]
- Gerkowicz M, Kosior-Jarecka E, Koziol-Montewka M. Role of nitric oxide in ophthalmic diseases. Klin Oczna. 2005;107:533–536. [PubMed] [Google Scholar]
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O'Shea EK, Weissman JS. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- Ghemrawi R, Pooya S, Lorentz S, Gauchotte G, Arnold C, Gueant JL, Battaglia-Hsu SF. Decreased vitamin B12 availability induces ER stress through impaired SIRT1-deacetylation of HSF1. Cell Death Dis. 2013;4:e553. doi: 10.1038/cddis.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum MP, Kotas ME, Erion DM, Kursawe R, Chatterjee P, Nead KT, Muise ES, Hsiao JJ, Frederick DW, Yonemitsu S, Banks AS, Qiang L, Bhanot S, Olefsky JM, Sears DD, Caprio S, Shulman GI. SirT1 regulates adipose tissue inflammation. Diabetes. 2011;60:3235–3245. doi: 10.2337/db11-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunti R, Gamberucci A, Fulceri R, Banhegyi G, Benedetti A. Both translocon and a cation channel are involved in the passive Ca2+ leak from the endoplasmic reticulum: a mechanistic study on rat liver microsomes. Arch Biochem Biophys. 2007;462:115–121. doi: 10.1016/j.abb.2007.03.039. [DOI] [PubMed] [Google Scholar]
- Gonzalez B, Pajares MA, Martinez-Ripoll M, Blundell TL, Sanz-Aparicio J. Crystal structure of rat liver betaine homocysteine s-methyltransferase reveals new oligomerization features and conformational changes upon substrate binding. J Mol Biol. 2004;338:771–782. doi: 10.1016/j.jmb.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Graw J. Cataract mutations and lens development. Prog Retin Eye Res. 1999;18:235–267. doi: 10.1016/s1350-9462(98)00018-4. [DOI] [PubMed] [Google Scholar]
- Guan L, Han B, Li Z, Hua F, Huang F, Wei W, Yang Y, Xu C. Sodium selenite induces apoptosis by ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction in human acute promyelocytic leukemia NB4 cells. Apoptosis. 2009;14:218–225. doi: 10.1007/s10495-008-0295-5. [DOI] [PubMed] [Google Scholar]
- Gueant JL, Elakoum R, Ziegler O, Coelho D, Feigerlova E, Daval JL, Gueant-Rodriguez RM. Nutritional models of foetal programming and nutrigenomic and epigenomic dysregulations of fatty acid metabolism in the liver and heart. Pflugers Arch. 2013 doi: 10.1007/s00424-013-1339-4. [DOI] [PubMed] [Google Scholar]
- Guo Y, Wang Z, Qu W, Shao H, Jiang X. Colorimetric detection of mercury, lead and copper ions simultaneously using protein-functionalized gold nanoparticles. Biosens Bioelectron. 2011;26:4064–4069. doi: 10.1016/j.bios.2011.03.033. [DOI] [PubMed] [Google Scholar]
- Guz J, Foksinski M, Siomek A, Gackowski D, Rozalski R, Dziaman T, Szpila A, Olinski R. The relationship between 8-oxo-7,8-dihydro-2'-deoxyguanosine level and extent of cytosine methylation in leukocytes DNA of healthy subjects and in patients with colon adenomas and carcinomas. Mutat Res. 2008;640:170–173. doi: 10.1016/j.mrfmmm.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Haas MJ, Raheja P, Jaimungal S, Sheikh-Ali M, Mooradian AD. Estrogen-dependent inhibition of dextrose-induced endoplasmic reticulum stress and superoxide generation in endothelial cells. Free Radic Biol Med. 2012;52:2161–2167. doi: 10.1016/j.freeradbiomed.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Haeri M, Knox BE. Endoplasmic Reticulum Stress and Unfolded Protein Response Pathways: Potential for Treating Age-related Retinal Degeneration. J Ophthalmic Vis Res. 2012:45–59. [PMC free article] [PubMed] [Google Scholar]
- Hammer GP, Scheidemann-Wesp U, Samkange-Zeeb F, Wicke H, Neriishi K, Blettner M. Occupational exposure to low doses of ionizing radiation and cataract development: a systematic literature review and perspectives on future studies. Radiat Environ Biophys. 2013;52:303–319. doi: 10.1007/s00411-013-0477-6. [DOI] [PubMed] [Google Scholar]
- Han F, Yan S, Shi Y. Single-prolonged stress induces endoplasmic reticulum-dependent apoptosis in the hippocampus in a rat model of post-traumatic stress disorder. PLoS One. 2013;8:e69340. doi: 10.1371/journal.pone.0069340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada N, Takahata T, Zhou Q, Ye X, Sun R, Itoh J, Ishiguro A, Kijima H, Mimura J, Itoh K, Fukuda S, Saijo Y. Methylation of the KEAP1 gene promoter region in human colorectal cancer. BMC Cancer. 2012;12:66. doi: 10.1186/1471-2407-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Harding J. Cataract : biochemistry, epidemiology, and pharmacology. 1. Chapman and Hall; London;New York: 1991. [Google Scholar]
- Harding JJ. Viewing molecular mechanisms of ageing through a lens. Ageing Res Rev. 2002;1:465–479. doi: 10.1016/s1568-1637(02)00012-0. [DOI] [PubMed] [Google Scholar]
- Harding JJ, Rixon KC. Carbamylation of lens proteins: a possible factor in cataractogenesis in some tropical countries. Exp Eye Res. 1980;31:567–571. doi: 10.1016/s0014-4835(80)80015-7. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Suzuki H, Itoh K, Yamamoto M, Sugiyama Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochemical and biophysical research communications. 2003;310:824–829. doi: 10.1016/j.bbrc.2003.09.086. [DOI] [PubMed] [Google Scholar]
- Haynes CM, Titus EA, Cooper AA. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell. 2004;15:767–776. doi: 10.1016/j.molcel.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa A, Chevet E. Redox signaling loops in the unfolded protein response. Cell Signal. 2012;24:1548–1555. doi: 10.1016/j.cellsig.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Hightower KR, David LL, Shearer TR. Regional distribution of free calcium in selenite cataract: relation to calpain II. Investigative ophthalmology & visual science. 1987;28:1702–1706. [PubMed] [Google Scholar]
- Hiroi T, Okada K, Imaoka S, Osada M, Funae Y. Bisphenol A binds to protein disulfide isomerase and inhibits its enzymatic and hormone-binding activities. Endocrinology. 2006;147:2773–2780. doi: 10.1210/en.2005-1235. [DOI] [PubMed] [Google Scholar]
- Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, Tsujimoto Y, Tohyama M. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol. 2004a;165:347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi J, Katayama T, Taniguchi M, Honda A, Imaizumi K, Tohyama M. Apoptosis induced by endoplasmic reticulum stress depends on activation of caspase-3 via caspase-12. Neurosci Lett. 2004b;357:127–130. doi: 10.1016/j.neulet.2003.12.080. [DOI] [PubMed] [Google Scholar]
- Hockwin O. Age changes of lens metabolism. Altern Entwickl Aging Dev. 1971;1:95–129. [PubMed] [Google Scholar]
- Hofer AM, Curci S, Machen TE, Schulz I. ATP regulates calcium leak from agonist-sensitive internal calcium stores. FASEB J. 1996;10:302–308. doi: 10.1096/fasebj.10.2.8641563. [DOI] [PubMed] [Google Scholar]
- Hong F, Sekhar KR, Freeman ML, Liebler DC. Specific patterns of electrophile adduction trigger Keap1 ubiquitination and Nrf2 activation. The Journal of biological chemistry. 2005;280:31768–31775. doi: 10.1074/jbc.M503346200. [DOI] [PubMed] [Google Scholar]
- Horke S, Witte I, Wilgenbus P, Altenhofer S, Kruger M, Li H, Forstermann U. Protective effect of paraoxonase-2 against endoplasmic reticulum stress-induced apoptosis is lost upon disturbance of calcium homoeostasis. Biochem J. 2008;416:395–405. doi: 10.1042/BJ20080775. [DOI] [PubMed] [Google Scholar]
- Hough TA, Bogani D, Cheeseman MT, Favor J, Nesbit MA, Thakker RV, Lyon MF. Activating calcium-sensing receptor mutation in the mouse is associated with cataracts and ectopic calcification. Proc Natl Acad Sci U S A. 2004;101:13566–13571. doi: 10.1073/pnas.0405516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung DT, Marsit CJ, Houseman EA, Eddy K, Furniss CS, McClean MD, Kelsey KT. Global DNA methylation level in whole blood as a biomarker in head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2007;16:108–114. doi: 10.1158/1055-9965.EPI-06-0636. [DOI] [PubMed] [Google Scholar]
- Hua L, Zhou R, Thirumalai D, Berne BJ. Urea denaturation by stronger dispersion interactions with proteins than water implies a 2-stage unfolding. Proc Natl Acad Sci U S A. 2008;105:16928–16933. doi: 10.1073/pnas.0808427105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Chavez L, Chang X, Wang X, Pastor WA, Kang J, Zepeda-Martinez JA, Pape UJ, Jacobsen SE, Peters B, Rao A. Distinct roles of the methylcytosine oxidases Tet1 and Tet2 in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 2014;111:1361–1366. doi: 10.1073/pnas.1322921111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- Ikesugi K, Mulhern ML, Madson CJ, Hosoya K, Terasaki T, Kador PF, Shinohara T. Induction of endoplasmic reticulum stress in retinal pericytes by glucose deprivation. Curr Eye Res. 2006a;31:947–953. doi: 10.1080/02713680600966785. [DOI] [PubMed] [Google Scholar]
- Ikesugi K, Yamamoto R, Mulhern ML, Shinohara T. Role of the unfolded protein response (UPR) in cataract formation. Exp Eye Res. 2006b;83:508–516. doi: 10.1016/j.exer.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Inada ET, Watanabe KP, Tanaka SG, Sakakisbara LA. Ocular toxicity caused by chloroquine: case report. Arq Bras Oftalmol. 2005;68:407–409. doi: 10.1590/s0004-27492005000300027. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaanus SD. Drug-related cataract. Optom Clin. 1991;1:143–157. [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jain K, Suryakumar G, Prasad R, Singh SN, Ganju L. Myocardial ER chaperone activation and protein degradation occurs due to synergistic, not individual, cold and hypoxic stress. Biochimie. 2013;95:1897–1908. doi: 10.1016/j.biochi.2013.06.018. [DOI] [PubMed] [Google Scholar]
- Jaiswal R, Khan MA, Musarrat J. Photosensitized paraquat-induced structural alterations and free radical mediated fragmentation of serum albumin. J Photochem Photobiol B. 2002;67:163–170. doi: 10.1016/s1011-1344(02)00321-4. [DOI] [PubMed] [Google Scholar]
- Jali BR, Kuang Y, Neamati N, Baruah JB. Selective binding of naphthoquinone derivatives to serum albumin proteins and their effects on cytotoxicity. Chemico-biological interactions. 2014 doi: 10.1016/j.cbi.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Jancevski M, Foster CS. Cataracts and uveitis. Discov Med. 2010;9:51–54. [PubMed] [Google Scholar]
- Jarosch E, Lenk U, Sommer T. Endoplasmic reticulum-associated protein degradation. Int Rev Cytol. 2003;223:39–81. doi: 10.1016/s0074-7696(05)23002-4. [DOI] [PubMed] [Google Scholar]
- Ji C. Mechanisms of alcohol-induced endoplasmic reticulum stress and organ injuries. Biochem Res Int. 2012;2012:216450. doi: 10.1155/2012/216450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagey JD, Kapoor-Vazirani P, McCabe MT, Powell DR, Vertino PM. Long-term stability of demethylation after transient exposure to 5-aza-2'-deoxycytidine correlates with sustained RNA polymerase II occupancy. Mol Cancer Res. 2010;8:1048–1059. doi: 10.1158/1541-7786.MCR-10-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahns S, Kalai M, Jakobsen LD, Clark BF, Vandenabeele P, Jensen PH. Caspase-1 and caspase-8 cleave and inactivate cellular parkin. The Journal of biological chemistry. 2003;278:23376–23380. doi: 10.1074/jbc.M300495200. [DOI] [PubMed] [Google Scholar]
- Kalai M, Lamkanfi M, Denecker G, Boogmans M, Lippens S, Meeus A, Declercq W, Vandenabeele P. Regulation of the expression and processing of caspase-12. J Cell Biol. 2003;162:457–467. doi: 10.1083/jcb.200303157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KW, Lee SJ, Park JW, Kim SG. Phosphatidylinositol 3-kinase regulates nuclear translocation of NF-E2-related factor 2 through actin rearrangement in response to oxidative stress. Mol Pharmacol. 2002;62:1001–1010. doi: 10.1124/mol.62.5.1001. [DOI] [PubMed] [Google Scholar]
- Kaphalia L, Calhoun WJ. Alcoholic lung injury: Metabolic, biochemical and immunological aspects. Toxicol Lett. 2013;222:171–179. doi: 10.1016/j.toxlet.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh Y, Iida K, Kang MI, Kobayashi A, Mizukami M, Tong KI, McMahon M, Hayes JD, Itoh K, Yamamoto M. Evolutionary conserved N-terminal domain of Nrf2 is essential for the Keap1-mediated degradation of the protein by proteasome. Arch Biochem Biophys. 2005;433:342–350. doi: 10.1016/j.abb.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ, Scheuner D, Schroder M, Shen X, Lee K, Liu CY, Arnold SM. The unfolded protein response in nutrient sensing and differentiation. Nat Rev Mol Cell Biol. 2002;3:411–421. doi: 10.1038/nrm829. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Azuma N. Morphological studies on cataract and small lens formation in neonatal rats treated with monosodium-L-glutamate. Ophthalmic Res. 1992;24:289–297. doi: 10.1159/000267181. [DOI] [PubMed] [Google Scholar]
- Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- Kenche H, Baty CJ, Vedagiri K, Shapiro SD, Blumental-Perry A. Cigarette smoking affects oxidative protein folding in endoplasmic reticulum by modifying protein disulfide isomerase. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:965–977. doi: 10.1096/fj.12-216234. [DOI] [PubMed] [Google Scholar]
- Kim HN, Guo Z, Zhu W, Yoon J, Tian H. Recent progress on polymer-based fluorescent and colorimetric chemosensors. Chem Soc Rev. 2011a;40:79–93. doi: 10.1039/c0cs00058b. [DOI] [PubMed] [Google Scholar]
- Kim I, Shu CW, Xu W, Shiau CW, Grant D, Vasile S, Cosford ND, Reed JC. Chemical biology investigation of cell death pathways activated by endoplasmic reticulum stress reveals cytoprotective modulators of ASK1. The Journal of biological chemistry. 2009;284:1593–1603. doi: 10.1074/jbc.M807308200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IY, Kang YJ, Yoon MJ, Kim EH, Kim SU, Kwon TK, Kim IA, Choi KS. Amiodarone sensitizes human glioma cells but not astrocytes to TRAIL-induced apoptosis via CHOP-mediated DR5 upregulation. Neuro Oncol. 2011b;13:267–279. doi: 10.1093/neuonc/noq195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita JH. Pathways of Glucose Metabolism in the Lens. Invest Ophthalmol. 1965;4:619–628. [PubMed] [Google Scholar]
- Kobayashi A, Ito E, Toki T, Kogame K, Takahashi S, Igarashi K, Hayashi N, Yamamoto M. Molecular cloning and functional characterization of a new Cap'n' collar family transcription factor Nrf3. J Biol Chem. 1999;274:6443–6452. doi: 10.1074/jbc.274.10.6443. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Kolvenbach CG, Elliott S, Sachdev R, Arakawa T, Narhi LO. Characterization of two fluorescent tryptophans in recombinant human granulocyte-colony stimulating factor: comparison of native sequence protein and tryptophan-deficient mutants. J Protein Chem. 1993;12:229–236. doi: 10.1007/BF01026045. [DOI] [PubMed] [Google Scholar]
- Konofsky K, Naumann GO, Guggenmoos-Holzmann I. Cell density and sex chromatin in lens epithelium of human cataracts. Quantitative studies in flat preparation. Ophthalmology. 1987;94:875–880. doi: 10.1016/s0161-6420(87)33543-2. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Kowluru A, Mishra M, Kumar B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 2015;48:40–61. doi: 10.1016/j.preteyeres.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuckel CL, Lubit BW, Lambooy PK, Farnsworth PN. Methylisocyanate and actin polymerization: the in vitro effects of carbamylation. Biochim Biophys Acta. 1993;1162:143–148. doi: 10.1016/0167-4838(93)90140-m. [DOI] [PubMed] [Google Scholar]
- Kushnir VN, Slepova OS, Zaitseva NS, Titarenko ZD, Dumbrava VA, Vovk EM. Viral hepatitis B as a factor in the etiology of cataracts in adults and children. Vestn Oftalmol. 1996;112:46–50. [PubMed] [Google Scholar]
- Kwak MK, Kensler TW, Casero RA., Jr Induction of phase 2 enzymes by serum oxidized polyamines through activation of Nrf2: effect of the polyamine metabolite acrolein. Biochemical and biophysical research communications. 2003a;305:662–670. doi: 10.1016/s0006-291x(03)00834-9. [DOI] [PubMed] [Google Scholar]
- Kwak MK, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Molecular and cellular biology. 2003b;23:8786–8794. doi: 10.1128/MCB.23.23.8786-8794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem. 2003c;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- Lane MD, Slieker LJ, Olson TS, Martensen TM. Post-translational acquisition of ligand binding-and tyrosine kinase-domain function by the epidermal growth factor and insulin receptors. J Recept Res. 1987;7:321–354. doi: 10.3109/10799898709054992. [DOI] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003a;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003b;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- Lee JN, Ye J. Proteolytic activation of sterol regulatory element-binding protein induced by cellular stress through depletion of Insig-1. J Biol Chem. 2004;279:45257–45265. doi: 10.1074/jbc.M408235200. [DOI] [PubMed] [Google Scholar]
- Li F, Wang Y, Zhang G, Zhou J, Yang L, Guan H. Expression and methylation of DNA repair genes in lens epithelium cells of age-related cataract. Mutat Res. 2014;766–767:31–36. doi: 10.1016/j.mrfmmm.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Li M, Baumeister P, Roy B, Phan T, Foti D, Luo S, Lee AS. ATF6 as a transcription activator of the endoplasmic reticulum stress element: thapsigargin stress-induced changes and synergistic interactions with NF-Y and YY1. Mol Cell Biol. 2000;20:5096–5106. doi: 10.1128/mcb.20.14.5096-5106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Liu Z, Guo J, Chen J, Yang P, Tian J, Sun J, Zong Y, Qu S. Cholesterol overloading leads to hepatic L02 cell damage through activation of the unfolded protein response. Int J Mol Med. 2009;24:459–464. doi: 10.3892/ijmm_00000253. [DOI] [PubMed] [Google Scholar]
- Li S, Li J, Shen C, Zhang X, Sun S, Cho M, Sun C, Song Z. Tert-butylhydroquinone (tBHQ) protects hepatocytes against lipotoxicity via inducing autophagy independently of Nrf2 activation. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbalip.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Kuszak JR, Dunn K, Wang RR, Ma W, Wang GM, Spector A, Leib M, Cotliar AM, Weiss M, et al. Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. J Cell Biol. 1995;130:169–181. doi: 10.1083/jcb.130.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien YC, Kung HN, Lu KS, Jeng CJ, Chau YP. Involvement of endoplasmic reticulum stress and activation of MAP kinases in beta-lapachone-induced human prostate cancer cell apoptosis. Histol Histopathol. 2008;23:1299–1308. doi: 10.14670/HH-23.1299. [DOI] [PubMed] [Google Scholar]
- Lopez JJ, Redondo PC, Salido GM, Pariente JA, Rosado JA. N,N,N',N'-tetrakis(2-pyridylmethyl)ethylenediamine induces apoptosis through the activation of caspases-3 and -8 in human platelets. A role for endoplasmic reticulum stress. J Thromb Haemost. 2009;7:992–999. doi: 10.1111/j.1538-7836.2009.03431.x. [DOI] [PubMed] [Google Scholar]
- Lou MF. Redox regulation in the lens. Prog Retin Eye Res. 2003;22:657–682. doi: 10.1016/s1350-9462(03)00050-8. [DOI] [PubMed] [Google Scholar]
- Lyu L, Whitcomb EA, Jiang S, Chang ML, Gu Y, Duncan MK, Cvekl A, Wang WL, Limi S, Reneker LW, Shang F, Du L, Taylor A. Unfolded-protein response-associated stabilization of p27(Cdkn1b) interferes with lens fiber cell denucleation, leading to cataract. FASEB J. 2016;30:1087–1095. doi: 10.1096/fj.15-278036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalakshmi B, Therese KL, Devipriya U, Pushpalatha V, Margarita S, Madhavan HN. Infectious aetiology of congenital cataract based on TORCHES screening in a tertiary eye hospital in Chennai, Tamil Nadu, India. Indian J Med Res. 2010;131:559–564. [PubMed] [Google Scholar]
- Mandula H, Parepally JM, Feng R, Smith QR. Role of site-specific binding to plasma albumin in drug availability to brain. J Pharmacol Exp Ther. 2006;317:667–675. doi: 10.1124/jpet.105.097402. [DOI] [PubMed] [Google Scholar]
- Manthey KC, Chew YC, Zempleni J. Riboflavin deficiency impairs oxidative folding and secretion of apolipoprotein B-100 in HepG2 cells, triggering stress response systems. J Nutr. 2005;135:978–982. doi: 10.1093/jn/135.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantonio JM. Calcium-induced disruption of the lens cytoskeleton. Ophthalmic Res. 1996;28(Suppl 1):48–50. doi: 10.1159/000267943. [DOI] [PubMed] [Google Scholar]
- Marcantonio JM, Duncan G, Rink H. Calcium-induced opacification and loss of protein in the organ-cultured bovine lens. Exp Eye Res. 1986;42:617–630. doi: 10.1016/0014-4835(86)90051-5. [DOI] [PubMed] [Google Scholar]
- Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. The EMBO journal. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui S, Vavassori S, Fagioli C, Sitia R, Inaba K. Molecular bases of cyclic and specific disulfide interchange between human ERO1alpha protein and protein-disulfide isomerase (PDI) J Biol Chem. 2011;286:16261–16271. doi: 10.1074/jbc.M111.231357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima H, David LL, Hiraoka T, Clark JI. Loss of cytoskeletal proteins and lens cell opacification in the selenite cataract model. Experimental eye research. 1997;64:387–395. doi: 10.1006/exer.1996.0220. [DOI] [PubMed] [Google Scholar]
- McGinty SJ, Truscott RJ. Presbyopia: the first stage of nuclear cataract? Ophthalmic research. 2006;38:137–148. doi: 10.1159/000090645. [DOI] [PubMed] [Google Scholar]
- McNulty R, Wang H, Mathias RT, Ortwerth BJ, Truscott RJ, Bassnett S. Regulation of tissue oxygen levels in the mammalian lens. J Physiol. 2004;559:883–898. doi: 10.1113/jphysiol.2004.068619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meo SA, Al-Khlaiwi T. Health hazards of welding fumes. Saudi Med J. 2003;24:1176–1182. [PubMed] [Google Scholar]
- Mezghrani A, Fassio A, Benham A, Simmen T, Braakman I, Sitia R. Manipulation of oxidative protein folding and PDI redox state in mammalian cells. Embo J. 2001;20:6288–6296. doi: 10.1093/emboj/20.22.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael R, Bron AJ. The ageing lens and cataract: a model of normal and pathological ageing. Philos Trans R Soc Lond B Biol Sci. 2011;366:1278–1292. doi: 10.1098/rstb.2010.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoia C, Ronchi A, Pigatto PD, Guzzi G. Influence of selenium and mercury on age-related cataracts in the Brazilian Amazon. Environ Health Perspect. 2011;119:A159. doi: 10.1289/ehp.1003242. author reply A159-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyokawa-Gorin K, Takahashi K, Handa K, Kitahara A, Sumitani Y, Katsuta H, Tanaka T, Nishida S, Yoshimoto K, Ohno H, Ishida H. Induction of mitochondrial uncoupling enhances VEGF(1)(2)(0) but reduces MCP-1 release in mature 3T3-L1 adipocytes: possible regulatory mechanism through endogenous ER stress and AMPK-related pathways. Biochem Biophys Res Commun. 2012;419:200–205. doi: 10.1016/j.bbrc.2012.01.145. [DOI] [PubMed] [Google Scholar]
- Moore LE, Pfeiffer RM, Poscablo C, Real FX, Kogevinas M, Silverman D, Garcia-Closas R, Chanock S, Tardon A, Serra C, Carrato A, Dosemeci M, Garcia-Closas M, Esteller M, Fraga M, Rothman N, Malats N. Genomic DNA hypomethylation as a biomarker for bladder cancer susceptibility in the Spanish Bladder Cancer Study: a case-control study. Lancet Oncol. 2008;9:359–366. doi: 10.1016/S1470-2045(08)70038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morck C, Olsen L, Kurth C, Persson A, Storm NJ, Svensson E, Jansson JO, Hellqvist M, Enejder A, Faergeman NJ, Pilon M. Statins inhibit protein lipidation and induce the unfolded protein response in the non-sterol producing nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2009;106:18285–18290. doi: 10.1073/pnas.0907117106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002;277:34287–34294. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Mulhern ML, Madson CJ, Danford A, Ikesugi K, Kador PF, Shinohara T. The unfolded protein response in lens epithelial cells from galactosemic rat lenses. Invest Ophthalmol Vis Sci. 2006;47:3951–3959. doi: 10.1167/iovs.06-0193. [DOI] [PubMed] [Google Scholar]
- Mulhern ML, Madson CJ, Kador PF, Randazzo J, Shinohara T. Cellular osmolytes reduce lens epithelial cell death and alleviate cataract formation in galactosemic rats. Mol Vis. 2007;13:1397–1405. [PubMed] [Google Scholar]
- Mulhern ML, Madson CJ, Troia A, Elanchezhian R, Palsamy P, Shinohara T. Endoplasmic reticulum stress associated retinal photoreceptor cell death in the transgenic mutant rhodopsin S334ter-3 rats. Biomedicine and Aging Pathology. 2012;2:143–150. [Google Scholar]
- Murray CI, Van Eyk JE. Chasing cysteine oxidative modifications: proteomic tools for characterizing cysteine redox status. Circ Cardiovasc Genet. 2012;5:591. doi: 10.1161/CIRCGENETICS.111.961425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscarella LA, Parrella P, D'Alessandro V, la Torre A, Barbano R, Fontana A, Tancredi A, Guarnieri V, Balsamo T, Coco M, Copetti M, Pellegrini F, De Bonis P, Bisceglia M, Scaramuzzi G, Maiello E, Valori VM, Merla G, Vendemiale G, Fazio VM. Frequent epigenetics inactivation of KEAP1 gene in non-small cell lung cancer. Epigenetics. 2011;6:710–719. doi: 10.4161/epi.6.6.15773. [DOI] [PubMed] [Google Scholar]
- Nair S, Xu C, Shen G, Hebbar V, Gopalakrishnan A, Hu R, Jain MR, Liew C, Chan JY, Kong AN. Toxicogenomics of endoplasmic reticulum stress inducer tunicamycin in the small intestine and liver of Nrf2 knockout and C57BL/6J mice. Toxicol Lett. 2007;168:21–39. doi: 10.1016/j.toxlet.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Narasimhan M, Patel D, Vedpathak D, Rathinam M, Henderson G, Mahimainathan L. Identification of novel microRNAs in post-transcriptional control of Nrf2 expression and redox homeostasis in neuronal, SH-SY5Y cells. PLoS One. 2012;7:e51111. doi: 10.1371/journal.pone.0051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelam S, Brooks MM, Cammarata PR. Lenticular cytoprotection. Part 1: the role of hypoxia inducible factors-1alpha and-2alpha and vascular endothelial growth factor in lens epithelial cell survival in hypoxia. Molecular vision. 2013;19:1–15. [PMC free article] [PubMed] [Google Scholar]
- Nemet AY, Vinker S, Levartovsky S, Kaiserman I. Is cataract associated with cardiovascular morbidity? Eye (Lond) 2010;24:1352–1358. doi: 10.1038/eye.2010.34. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Huang HC, Pickett CB. Transcriptional regulation of the antioxidant response element. Activation by Nrf2 and repression by MafK. The Journal of biological chemistry. 2000;275:15466–15473. doi: 10.1074/jbc.M000361200. [DOI] [PubMed] [Google Scholar]
- Nishigori H. Steroid (glucocorticoid)-induced cataract. Yakugaku Zasshi. 2006;126:869–884. doi: 10.1248/yakushi.126.869. [DOI] [PubMed] [Google Scholar]
- Nozaki S, Sledge GW, Jr, Nakshatri H. Repression of GADD153/CHOP by NF-kappaB: a possible cellular defense against endoplasmic reticulum stress-induced cell death. Oncogene. 2001;20:2178–2185. doi: 10.1038/sj.onc.1204292. [DOI] [PubMed] [Google Scholar]
- Oakes CC, Smiraglia DJ, Plass C, Trasler JM, Robaire B. Aging results in hypermethylation of ribosomal DNA in sperm and liver of male rats. Proc Natl Acad Sci U S A. 2003;100:1775–1780. doi: 10.1073/pnas.0437971100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogen-Shtern N, Ben David T, Lederkremer GZ. Protein aggregation and ER stress. Brain Res. 2016;1648:658–666. doi: 10.1016/j.brainres.2016.03.044. [DOI] [PubMed] [Google Scholar]
- Ohmiya H, Kanazawa T. Inhibition by A23187 of conformational changes involved in the Ca(2+)-induced activation of sarcoplasmic reticulum Ca(2+)-ATPase. J Biochem. 1991;109:751–757. doi: 10.1093/oxfordjournals.jbchem.a123452. [DOI] [PubMed] [Google Scholar]
- Okada T, Yoshida H, Akazawa R, Negishi M, Mori K. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J. 2002;366:585–594. doi: 10.1042/BJ20020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira RA, Azevedo-Ximenes E, Luzzati R, Garcia RC. The hydroxy-naphthoquinone lapachol arrests mycobacterial growth and immunomodulates host macrophages. Int Immunopharmacol. 2010;10:1463–1473. doi: 10.1016/j.intimp.2010.08.023. [DOI] [PubMed] [Google Scholar]
- Ong HL, Liu X, Sharma A, Hegde RS, Ambudkar IS. Intracellular Ca(2+) release via the ER translocon activates store-operated calcium entry. Pflugers Arch. 2007;453:797–808. doi: 10.1007/s00424-006-0163-5. [DOI] [PubMed] [Google Scholar]
- Osada N, Kosuge Y, Ishige K, Ito Y. Characterization of neuronal and astroglial responses to ER stress in the hippocampal CA1 area in mice following transient forebrain ischemia. Neurochem Int. 2010;57:1–7. doi: 10.1016/j.neuint.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Pagani M, Fabbri M, Benedetti C, Fassio A, Pilati S, Bulleid NJ, Cabibbo A, Sitia R. Endoplasmic reticulum oxidoreductin 1-lbeta (ERO1-Lbeta), a human gene induced in the course of the unfolded protein response. J Biol Chem. 2000;275:23685–23692. doi: 10.1074/jbc.M003061200. [DOI] [PubMed] [Google Scholar]
- Palsamy P, Ayaki M, Elanchezhian R, Shinohara T. Promoter demethylation of Keap1 gene in human diabetic cataractous lenses. Biochem Biophys Res Commun. 2012;423:542–548. doi: 10.1016/j.bbrc.2012.05.164. [DOI] [PubMed] [Google Scholar]
- Palsamy P, Bidasee KR, Ayaki M, Augusteyn RC, Chan JY, Shinohara T. Methylglyoxal induces endoplasmic reticulum stress and DNA demethylation in the Keap1 promoter of human lens epithelial cells and age-related cataracts. Free Radic Biol Med. 2014a;72:134–148. doi: 10.1016/j.freeradbiomed.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsamy P, Bidasee KR, Shinohara T. Selenite cataracts: activation of endoplasmic reticulum stress and loss of Nrf2/Keap1-dependent stress protection. Biochim Biophys Acta. 2014b;1842:1794–1805. doi: 10.1016/j.bbadis.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsamy P, Bidasee KR, Shinohara T. Valproic acid suppresses Nrf2/Keap1 dependent antioxidant protection through induction of endoplasmic reticulum stress and Keap1 promoter DNA demethylation in human lens epithelial cells. Exp Eye Res. 2014c;121:26–34. doi: 10.1016/j.exer.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa FR. Endoplasmic reticulum stress, pancreatic beta-cell degeneration, and diabetes. Cold Spring Harb Perspect Med. 2012;2:a007666. doi: 10.1101/cshperspect.a007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes RM, Bollo M, Holstein D, Lechleiter JD. Luminal Ca2+ depletion during the unfolded protein response in Xenopus oocytes: cause and consequence. Cell Calcium. 2013;53:286–296. doi: 10.1016/j.ceca.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Zhou Y, Lee J, Ozcan U. Sarco(endo)plasmic reticulum Ca2+-ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity. Proc Natl Acad Sci U S A. 2010;107:19320–19325. doi: 10.1073/pnas.1012044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim C, O'Dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, Ogier-Denis E. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penas C, Verdu E, Asensio-Pinilla E, Guzman-Lenis MS, Herrando-Grabulosa M, Navarro X, Casas C. Valproate reduces CHOP levels and preserves oligodendrocytes and axons after spinal cord injury. Neuroscience. 2011;178:33–44. doi: 10.1016/j.neuroscience.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Pestka JJ. Deoxynivalenol-induced proinflammatory gene expression: mechanisms and pathological sequelae. Toxins (Basel) 2010;2:1300–1317. doi: 10.3390/toxins2061300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrasek J, Iracheta-Vellve A, Csak T, Satishchandran A, Kodys K, Kurt-Jones EA, Fitzgerald KA, Szabo G. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1308331110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrou M, Hanna PE, Cribb AE. Cisplatin, gentamicin, and p-aminophenol induce markers of endoplasmic reticulum stress in the rat kidneys. Toxicological sciences : an official journal of the Society of Toxicology. 2007;99:346–353. doi: 10.1093/toxsci/kfm152. [DOI] [PubMed] [Google Scholar]
- Popov CS, Yantchev I, Popova MP, Vultcheva GM. Effect of different factors modifying the activity of some enzyme systems of the endoplasmic reticulum on the sensitivity of cell organelles against the damaging action of chemical agents. II. Studies with chlorpromazine, 2,4-dinitrophenol, phenobarbital and DDT. Br J Exp Pathol. 1979;60:85–95. [PMC free article] [PubMed] [Google Scholar]
- Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Prchal JT, Conrad ME, Skalka HW. Association of presenile cataracts with heterozygosity for galactosaemic states and with riboflavin deficiency. Lancet. 1978;1:12–13. doi: 10.1016/s0140-6736(78)90359-8. [DOI] [PubMed] [Google Scholar]
- Prestrelski SJ, Tedeschi N, Arakawa T, Carpenter JF. Dehydration-induced conformational transitions in proteins and their inhibition by stabilizers. Biophys J. 1993;65:661–671. doi: 10.1016/S0006-3495(93)81120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokofyeva E, Wegener A, Zrenner E. Cataract prevalence and prevention in Europe: a literature review. Acta Ophthalmol. 2013;91:395–405. doi: 10.1111/j.1755-3768.2012.02444.x. [DOI] [PubMed] [Google Scholar]
- Pyati UJ, Gjini E, Carbonneau S, Lee JS, Guo F, Jette CA, Kelsell DP, Look AT. p63 mediates an apoptotic response to pharmacological and disease-related ER stress in the developing epidermis. Dev Cell. 2011;21:492–505. doi: 10.1016/j.devcel.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racape M, Duong Van Huyen JP, Danger R, Giral M, Bleicher F, Foucher Y, Pallier A, Pilet P, Tafelmeyer P, Ashton-Chess J, Dugast E, Pettre S, Charreau B, Soulillou JP, Brouard S. The involvement of SMILE/TMTC3 in endoplasmic reticulum stress response. PLoS One. 2011;6:e19321. doi: 10.1371/journal.pone.0019321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju TN, Kanth VR, Reddy PU. Influence of kynurenines in pathogenesis of cataract formation in tryptophan-deficient regimen in Wistar rats. Indian J Exp Biol. 2007;45:543–548. [PubMed] [Google Scholar]
- Razin A, Riggs AD. DNA methylation and gene function. Science. 1980;210:604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Richa S, Yazbek JC. Ocular adverse effects of common psychotropic agents: a review. CNS Drugs. 2010;24:501–526. doi: 10.2165/11533180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Roba AA, Zondervan M, Patel D. Trachoma visual impairment in a SAFE area in Ethiopia. Trop Doct. 2010;40:157–159. doi: 10.1258/td.2010.090390. [DOI] [PubMed] [Google Scholar]
- Rosin A. The long-term consequences of exposure to lead. Isr Med Assoc J. 2009;11:689–694. [PubMed] [Google Scholar]
- Salih MA, Bender DA, McCreanor GM. Lethal familial pellagra-like skin lesion associated with neurologic and developmental impairment and the development of cataracts. Pediatrics. 1985;76:787–793. [PubMed] [Google Scholar]
- Santi-Rocca J, Smith S, Weber C, Pineda E, Hon CC, Saavedra E, Olivos-Garcia A, Rousseau S, Dillies MA, Coppee JY, Guillen N. Endoplasmic reticulum stress-sensing mechanism is activated in Entamoeba histolytica upon treatment with nitric oxide. PLoS One. 2012;7:e31777. doi: 10.1371/journal.pone.0031777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Hatta S, Wada K, Ueda N, Yoshimura T, Endo T, Sakata M, Tanaka T, Haga M. Effects of extract of Ginkgo biloba leaves and its constituents on carcinogen-metabolizing enzyme activities and glutathione levels in mouse liver. Life Sci. 2002;70:1657–1667. doi: 10.1016/s0024-3205(01)01557-0. [DOI] [PubMed] [Google Scholar]
- Sato M, Suzuki S. Endoplasmic reticulum stress and metallothionein. Yakugaku Zasshi. 2007;127:703–708. doi: 10.1248/yakushi.127.703. [DOI] [PubMed] [Google Scholar]
- Schauble N, Lang S, Jung M, Cappel S, Schorr S, Ulucan O, Linxweiler J, Dudek J, Blum R, Helms V, Paton AW, Paton JC, Cavalie A, Zimmermann R. BiP-mediated closing of the Sec61 channel limits Ca2+ leakage from the ER. EMBO J. 2012;31:3282–3296. doi: 10.1038/emboj.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schocket SS, Esterson J, Bradford B, Michaelis M, Richards RD. Induction of cataracts in mice by exposure to oxygen. Isr J Med Sci. 1972;8:1596–1601. [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Selye H. The nature of stress. Basal Facts. 1985;7:3–11. [PubMed] [Google Scholar]
- Sen SK, Pukazhvanthen P, Abraham R. Plasma Homocysteine, Folate and Vitamin B(12) levels in senile cataract. Indian J Clin Biochem. 2008;23:255–257. doi: 10.1007/s12291-008-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer RN, Hetherington J., Jr Anticholinesterase drugs and cataracts. Am J Ophthalmol. 1966;62:613–618. doi: 10.1016/0002-9394(66)92181-7. [DOI] [PubMed] [Google Scholar]
- Shah M, Shah S, Upadhyay P, Agrawal R. Controversies in traumatic cataract classification and management: a review. Can J Ophthalmol. 2013;48:251–258. doi: 10.1016/j.jcjo.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Rohrer B. Calcium-induced calpain mediates apoptosis via caspase-3 in a mouse photoreceptor cell line. J Biol Chem. 2004;279:35564–35572. doi: 10.1074/jbc.M401037200. [DOI] [PubMed] [Google Scholar]
- Shearer TR, Ma H, Fukiage C, Azuma M. Selenite nuclear cataract: review of the model. Mol Vis. 1997;3:8. [PubMed] [Google Scholar]
- Shen X, Zhang K, Kaufman RJ. The unfolded protein response--a stress signaling pathway of the endoplasmic reticulum. J Chem Neuroanat. 2004;28:79–92. doi: 10.1016/j.jchemneu.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18:7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Kaji T. Cellular defense mechanisms against lead toxicity in the vascular system. Biol Pharm Bull. 2012;35:1885–1891. doi: 10.1248/bpb.b212018. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Ikesugi K, Mulhern ML. Cataracts: role of the unfolded protein response. Med Hypotheses. 2006;66:365–370. doi: 10.1016/j.mehy.2005.08.044. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Piatigorsky J. Quantitation of delta-crystallin messenger RNA during lens induction in chick embryos. Proc Natl Acad Sci U S A. 1976;73:2808–2812. doi: 10.1073/pnas.73.8.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Singh DP, Fatma N. LEDGF, a survival factor, activates stress-related genes. Prog Retin Eye Res. 2002;21:341–358. doi: 10.1016/s1350-9462(02)00007-1. [DOI] [PubMed] [Google Scholar]
- Siegfried CJ, Shui YB, Holekamp NM, Bai F, Beebe DC. Oxygen distribution in the human eye: relevance to the etiology of open-angle glaucoma after vitrectomy. Invest Ophthalmol Vis Sci. 2010;51:5731–5738. doi: 10.1167/iovs.10-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Ronghe AM, Chatterjee A, Bhat NK, Bhat HK. MicroRNA-93 regulates NRF2 expression and is associated with breast carcinogenesis. Carcinogenesis. 2013;34:1165–1172. doi: 10.1093/carcin/bgt026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalka HW, Prchal JT. Cataracts and riboflavin deficiency. Am J Clin Nutr. 1981a;34:861–863. doi: 10.1093/ajcn/34.5.861. [DOI] [PubMed] [Google Scholar]
- Skalka HW, Prchal JT. Riboflavin deficiency and cataract formation. Metab Pediatr Ophthalmol. 1981b;5:17–20. [PubMed] [Google Scholar]
- Sobrin L, Seddon JM. Nature and nurture-genes and environment-predict onset and progression of macular degeneration. Prog Retin Eye Res. 2014;40:1–15. doi: 10.1016/j.preteyeres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas V, Zhang LP, Zhu XH, Caro J. Characterization of an oxygen/redox-dependent degradation domain of hypoxia-inducible factor alpha (HIF-alpha) proteins. Biochemical and biophysical research communications. 1999;260:557–561. doi: 10.1006/bbrc.1999.0878. [DOI] [PubMed] [Google Scholar]
- Stevens VJ, Fantl WJ, Newman CB, Sims RV, Cerami A, Peterson CM. Acetaldehyde adducts with hemoglobin. J Clin Invest. 1981;67:361–369. doi: 10.1172/JCI110043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suji G, Khedkar SA, Singh SK, Kishore N, Coutinho EC, Bhor VM, Sivakami S. Binding of lipoic acid induces conformational change and appearance of a new binding site in methylglyoxal modified serum albumin. Protein J. 2008;27:205–214. doi: 10.1007/s10930-008-9126-3. [DOI] [PubMed] [Google Scholar]
- Swanton E, Bulleid NJ. Protein folding and translocation across the endoplasmic reticulum membrane. Mol Membr Biol. 2003;20:99–104. doi: 10.1080/0968768031000069241. [DOI] [PubMed] [Google Scholar]
- Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16:123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- Tan SX, Teo M, Lam YT, Dawes IW, Perrone GG. Cu, Zn superoxide dismutase and NADP(H) homeostasis are required for tolerance of endoplasmic reticulum stress in Saccharomyces cerevisiae. Mol Biol Cell. 2009;20:1493–1508. doi: 10.1091/mbc.E08-07-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Benedek GB. Measurement of the Velocity of Blood Flow (in vivo) Using a Fiber Optic Catheter and Optical Mixing Spectroscopy. Appl Opt. 1975a;14:189–196. doi: 10.1364/AO.14.000189. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Benedek GB. Observation of protein diffusivity in intact human and bovine lenses with application to cataract. Invest Ophthalmol. 1975b;14:449–456. [PubMed] [Google Scholar]
- Tardito S, Bassanetti I, Bignardi C, Elviri L, Tegoni M, Mucchino C, Bussolati O, Franchi-Gazzola R, Marchio L. Copper binding agents acting as copper ionophores lead to caspase inhibition and paraptotic cell death in human cancer cells. J Am Chem Soc. 2011;133:6235–6242. doi: 10.1021/ja109413c. [DOI] [PubMed] [Google Scholar]
- Thiagarajan R, Manikandan R. Antioxidants and cataract. Free Radic Res. 2013;47:337–345. doi: 10.3109/10715762.2013.777155. [DOI] [PubMed] [Google Scholar]
- Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- Thuerauf DJ, Marcinko M, Belmont PJ, Glembotski CC. Effects of the isoform-specific characteristics of ATF6 alpha and ATF6 beta on endoplasmic reticulum stress response gene expression and cell viability. J Biol Chem. 2007;282:22865–22878. doi: 10.1074/jbc.M701213200. [DOI] [PubMed] [Google Scholar]
- Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998;12:1812–1824. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiman M, Caspersen C, Christensen SB. A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca(2+)-ATPases. Trends Pharmacol Sci. 1998;19:131–135. doi: 10.1016/s0165-6147(98)01184-5. [DOI] [PubMed] [Google Scholar]
- Truscott RJ, Augusteyn RC. Oxidative changes in human lens proteins during senile nuclear cataract formation. Biochim Biophys Acta. 1977;492:43–52. doi: 10.1016/0005-2795(77)90212-4. [DOI] [PubMed] [Google Scholar]
- Validandi V, Reddy VS, Srinivas PN, Mueller NH, Bhagyalaxmi SG, Padma T, Petrash JM, Reddy GB. Temperature-dependent structural and functional properties of a mutant (F71L) alphaA-crystallin: molecular basis for early onset of age-related cataract. FEBS Lett. 2011;585:3884–3889. doi: 10.1016/j.febslet.2011.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brule J, Degueldre F, Galand A. Drug-induced cataracts. Rev Med Liege. 1998;53:766–769. [PubMed] [Google Scholar]
- Van Summeren A, Renes J, Lizarraga D, Bouwman FG, Noben JP, van Delft JH, Kleinjans JC, Mariman EC. Screening for drug-induced hepatotoxicity in primary mouse hepatocytes using acetaminophen, amiodarone, and cyclosporin a as model compounds: an omics-guided approach. Omics. 2013;17:71–83. doi: 10.1089/omi.2012.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanz AL, Lunsdorf H, Adnan A, Nimtz M, Gurramkonda C, Khanna N, Rinas U. Physiological response of Pichia pastoris GS115 to methanol-induced high level production of the Hepatitis B surface antigen: catabolic adaptation, stress responses, and autophagic processes. Microb Cell Fact. 2012;11:103. doi: 10.1186/1475-2859-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadarajan S, Bampton ET, Smalley JL, Tanaka K, Caves RE, Butterworth M, Wei J, Pellecchia M, Mitcheson J, Gant TW, Dinsdale D, Cohen GM. A novel cellular stress response characterised by a rapid reorganisation of membranes of the endoplasmic reticulum. Cell Death Differ. 2012;19:1896–1907. doi: 10.1038/cdd.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrensen GF, van Marle J, Jonges R, Voorhout W, Breipohl W, Wegener AR. Tryptophan deficiency arrests chromatin breakdown in secondary lens fibers of rats. Exp Eye Res. 2004;78:661–672. doi: 10.1016/j.exer.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, Harada T, Engel JD, Yamamoto M. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li F, Zhang G, Kang L, Qin B, Guan H. Altered DNA Methylation and Expression Profiles of 8-Oxoguanine DNA Glycosylase 1 in Lens Tissue from Age-related Cataract Patients. Curr Eye Res. 2015;40:815–821. doi: 10.3109/02713683.2014.957778. [DOI] [PubMed] [Google Scholar]
- Wang YX, Zhang JS, You QS, Xu L, Jonas JB. Ocular diseases and 10-year mortality: The Beijing Eye Study 2001/2011. Acta Ophthalmol 2014 [Google Scholar]
- Wang Z, Bunce GE, Hess JL. Selenite and Ca2+ homeostasis in the rat lens: effect on Ca-ATPase and passive Ca2+ transport. Current eye research. 1993;12:213–218. doi: 10.3109/02713689308999466. [DOI] [PubMed] [Google Scholar]
- Wang Z, Hess JL, Bunce GE. Calcium efflux in rat lens: Na/Ca-exchange related to cataract induced by selenite. Current eye research. 1992;11:625–632. doi: 10.3109/02713689209000735. [DOI] [PubMed] [Google Scholar]
- Weng S, Sprague JE, Oh J, Riek AE, Chin K, Garcia M, Bernal-Mizrachi C. Vitamin D deficiency induces high blood pressure and accelerates atherosclerosis in mice. PLoS One. 2013;8:e54625. doi: 10.1371/journal.pone.0054625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild AC, Moinova HR, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. The Journal of biological chemistry. 1999;274:33627–33636. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- Willam C, Masson N, Tian YM, Mahmood SA, Wilson MI, Bicknell R, Eckardt KU, Maxwell PH, Ratcliffe PJ, Pugh CW. Peptide blockade of HIFalpha degradation modulates cellular metabolism and angiogenesis. Proc Natl Acad Sci U S A. 2002;99:10423–10428. doi: 10.1073/pnas.162119399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VL, Jones PA. DNA methylation decreases in aging but not in immortal cells. Science. 1983;220:1055–1057. doi: 10.1126/science.6844925. [DOI] [PubMed] [Google Scholar]
- Winkler BS, Riley MV. Relative contributions of epithelial cells and fibers to rabbit lens ATP content and glycolysis. Invest Ophthalmol Vis Sci. 1991;32:2593–2598. [PubMed] [Google Scholar]
- Xie H, Wang M, Bonaldo Mde F, Smith C, Rajaram V, Goldman S, Tomita T, Soares MB. High-throughput sequence-based epigenomic analysis of Alu repeats in human cerebellum. Nucleic Acids Res. 2009;37:4331–4340. doi: 10.1093/nar/gkp393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Liu M, Zhu X, Yang L, Miao Y, Shi D, Yin H. Effective Components of Panax quinquefolius and Corydalis tuber Protect Myocardium through Attenuating Oxidative Stress and Endoplasmic Reticulum Stress. Evid Based Complement Alternat Med. 2013;2013:482318. doi: 10.1155/2013/482318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalon M, Goldberg EP, Osborn D, Stacholy J, Sheets JW. Polycarbonate intraocular lenses. J Cataract Refract Surg. 1988;14:393–395. doi: 10.1016/s0886-3350(88)80145-7. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhou S, Gu J, Wang Y, Guo M, Liu Y. Differences in Unfolded Protein Response Pathway Activation in the Lenses of Three Types of Cataracts. PLoS One. 2015;10:e0130705. doi: 10.1371/journal.pone.0130705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Yao Y, Eades G, Zhang Y, Zhou Q. MiR-28 regulates Nrf2 expression through a Keap1-independent mechanism. Breast Cancer Res Treat. 2011;129:983–991. doi: 10.1007/s10549-011-1604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemelyanov A, Bhalla P, Yang X, Ugolkov A, Iwadate K, Karseladze A, Budunova I. Differential targeting of androgen and glucocorticoid receptors induces ER stress and apoptosis in prostate cancer cells: a novel therapeutic modality. Cell Cycle. 2012;11:395–406. doi: 10.4161/cc.11.2.18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem. 1998;273:33741–33749. doi: 10.1074/jbc.273.50.33741. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol. 2000a;20:6755–6767. doi: 10.1128/mcb.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Fujisawa A, Tsuzaki Y, Saitoh S. Identification and expression of a Mycoplasma gallisepticum surface antigen recognized by a monoclonal antibody capable of inhibiting both growth and metabolism. Infect Immun. 2000b;68:3186–3192. doi: 10.1128/iai.68.6.3186-3192.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Yu B, Wang Y, Jiang J, Liu L, Zhao H, Qi W, Zheng Q. Involvement of endoplasmic reticulum stress in isoliquiritigenin-induced SKOV-3 cell apoptosis. Recent Pat Anticancer Drug Discov. 2013;8:191–199. [PubMed] [Google Scholar]
- Yukioka F, Matsuzaki S, Kawamoto K, Koyama Y, Hitomi J, Katayama T, Tohyama M. Presenilin-1 mutation activates the signaling pathway of caspase-4 in endoplasmic reticulum stress-induced apoptosis. Neurochem Int. 2008;52:683–687. doi: 10.1016/j.neuint.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, Pipeleers D, Ling Z, Drucker DJ. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006;4:391–406. doi: 10.1016/j.cmet.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Yusta B, Drucker DJ. GLP-2 peptide(glucagon-like peptide-2) controls energetic homeostasis by actions on proliferation and cytoprotection in the gastrointestinal epithelium. Journ Annu Diabetol Hotel Dieu. 2004:127–137. [PubMed] [Google Scholar]
- Yusuf IH, Sandford V, Hildebrand GD. Congenital cataract in a child with pyridoxine-dependent epilepsy. J Aapos. 2013;17:315–317. doi: 10.1016/j.jaapos.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Zhang K. Integration of ER stress, oxidative stress and the inflammatory response in health and disease. Int J Clin Exp Med. 2010;3:33–40. [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem. 2004;279:25935–25938. doi: 10.1074/jbc.R400008200. [DOI] [PubMed] [Google Scholar]
- Zhang P, Xing K, Randazzo J, Blessing K, Lou MF, Kador PF. Osmotic stress, not aldose reductase activity, directly induces growth factors and MAPK signaling changes during sugar cataract formation. Exp Eye Res. 2012;101:36–43. doi: 10.1016/j.exer.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SX, Ma JH, Bhatta M, Fliesler SJ, Wang JJ. The unfolded protein response in retinal vascular diseases: implications and therapeutic potential beyond protein folding. Prog Retin Eye Res. 2015;45:111–131. doi: 10.1016/j.preteyeres.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Smith DL, Meriin AB, Engemann S, Russel DE, Roark M, Washington SL, Maxwell MM, Marsh JL, Thompson LM, Wanker EE, Young AB, Housman DE, Bates GP, Sherman MY, Kazantsev AG. A potent small molecule inhibits polyglutamine aggregation in Huntington's disease neurons and suppresses neurodegeneration in vivo. Proc Natl Acad Sci U S A. 2005;102:892–897. doi: 10.1073/pnas.0408936102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Lu Y. Protein post-translational modification and age-related cataract. Zhonghua Yan Ke Za Zhi. 2011;47:656–659. [PubMed] [Google Scholar]
- Zhang Z, Tong N, Gong Y, Qiu Q, Yin L, Lv X, Wu X. Valproate protects the retina from endoplasmic reticulum stress-induced apoptosis after ischemia-reperfusion injury. Neurosci Lett. 2011;504:88–92. doi: 10.1016/j.neulet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yang XY, Cohen DM. Urea-associated oxidative stress and Gadd153/CHOP induction. Am J Physiol. 1999;276:F786–793. doi: 10.1152/ajprenal.1999.276.5.F786. [DOI] [PubMed] [Google Scholar]
- Zheng XY, Xu J, Chen XI, Li W, Wang TY. Attenuation of oxygen fluctuation-induced endoplasmic reticulum stress in human lens epithelial cells. Exp Ther Med. 2015;10:1883–1887. doi: 10.3892/etm.2015.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Luo Y, Liu X, Fan L, Lu Y. Down-regulation and CpG island hypermethylation of CRYAA in age-related nuclear cataract. FASEB J. 2012;26:4897–4902. doi: 10.1096/fj.12-213702. [DOI] [PubMed] [Google Scholar]
- Zimmerman TJ, Wheeler TM. Miotics: side effects and ways to avoid them. Ophthalmology. 1982;89:76–80. doi: 10.1016/s0161-6420(82)34866-6. [DOI] [PubMed] [Google Scholar]
- Zipper LM, Mulcahy RT. Inhibition of ERK and p38 MAP kinases inhibits binding of Nrf2 and induction of GCS genes. Biochem Biophys Res Commun. 2000;278:484–492. doi: 10.1006/bbrc.2000.3830. [DOI] [PubMed] [Google Scholar]
- Zoric L, Colak E, Canadanovic V, Kosanovic-Jakovic N, Kisic B. Oxidation stress role in age-related cataractogenesis. Med Pregl. 2010;63:522–526. doi: 10.2298/mpns1008522z. [DOI] [PubMed] [Google Scholar]