Abstract
Amino acids are essential building blocks of life, and fluorinated derivatives have gained interest in chemistry and medicine. Modern mass spectrometry has enabled the study of oligo‐ and polypeptides as isolated entities in the gas phase, but predominantly as singly or even multiply charged species. While laser desorption of neutral peptides into adiabatically expanding supersonic noble gas jets is possible, UV–VIS spectroscopy, electric or magnetic deflectometry as well as quantum interferometry would profit from the possibility to prepare thermally slow molecular beams. This has typically been precluded by the fragility of the peptide bond and the fact that a peptide would rather ‘fry’, i.e. denature and fragment than ‘fly’. Here, we explore how tailored perfluoroalkyl functionalization can reduce the intermolecular binding and thus increase the volatility of peptides and compare it to previously explored methylation, acylation and amidation of peptides. We show that this strategy is essential and enables the formation of thermal beams of intact neutral tripeptides, whereas only fragments were observed for an extensively fluoroalkyl‐decorated nonapeptide. © 2017 The Authors. Journal of Mass Spectrometry Published by John Wiley & Sons Ltd.
Keywords: fluorination, molecular beams, peptides, thermal evaporation, vuv ionization
Introduction
Since the early days of Otto Stern1 and Immanuel Estermann,2 neutral molecular beams have played a key role in fundamental studies of physics and physical chemistry.3, 4, 5 Experiments with isolated molecules in the gas phase have laid the ground for high‐precision spectroscopy,6, 7 molecule and cluster deflectometry8, 9, 10 and for an improved understanding of chemical reactions with quantum state control.11, 12 Modern molecular beam experiments have allowed setting new bounds on the electric dipole moment of the electron13, 14 and enabled the observation of quantum interference with clusters and molecules,15, 16 even with masses exceeding 10′000 amu.17 Modern research in molecular beam methods has recently focused on obtaining improved control over the motional and internal states of polyatomic molecules using selectors,18, 19 electrical,20, 21, 22, 23, 24 magnetic25 and mechanical26 decelerators as well as laser cooling of dimers and trimers.27, 28, 29 Polyatomic particles were even successfully trapped at mK temperatures.22, 26, 30, 31 Complementary to that, there has been a growing effort to prepare neutral beams of large molecules. Our present contribution addresses the question how to bring complex biomolecular building blocks into the gas phase.32 D. Gross and G. Grodsky reported on the sublimation and decomposition of unmodified amino acids and certain dipeptides in 1955.33, 34, 35, 36, 37, 38 Methylation and acylation of peptides have already been investigated in the late 60s and early 70s in combination with electron impact mass spectrometry (EI‐MS) as a means for increased volatility in sequence analysis of unknown proteins.39, 40, 41 Here, we aim at preparing neutral continuous beams of peptides at low velocity as required for spectroscopy, deflectometry and quantum interferometry or even nanostructuring using soft‐landing of individual biomolecules on surfaces. Even though one may argue that biomolecules are most naturally studied in an aqueous environment, it is meaningful to start from isolated species to which one may later add an increasing number of water molecules to compare their physical data with quantum chemical models.42, 43 Additionally, gas phase studies of biomolecules enable the elucidation of intrinsic folding preferences without interference of solvents or other molecules.44, 45, 46, 47
While some biomolecules or biomolecular moieties – such as nucleobases and some vitamins – can be readily sublimated or evaporated48 oligopeptides, proteins and oligonucleotides will rather fragment than fly when heated. To suppress fragmentation, one may reduce the heating time or add collisional cooling, once the molecules are airborne. Both ideas have been earlier explored for biomolecules injected into supersonic noble gas jets.43, 49, 50 This way, neutral intact macromolecules can be volatilized, but at the expense of being 300–1000 m/s fast, depending on the gas type and temperature. In contrast, velocities down to several 10 m/s have been achieved in buffer gas cooled sources51 for molecules up to stilbene or using laser‐induced acoustic desorption even for molecules beyond 10′000 amu.52, 53 However, the generation of thermally slow neutral beams of oligopeptides, which we take here as examples of relevant but fragile biomolecules, poses a considerable challenge.
Fluorination has gained increasing attention in medicinal chemistry over the last 50 years.54, 55 Around 20% of all pharmaceuticals contain at least one carbon–fluorine bond. Fluorine modification of single amino acids, peptides and proteins substantially alters their properties and provides new opportunities for peptide and protein design.56, 57 The strong electron‐withdrawing effect of fluorine lowers the pk a value of proximal protons, affects hydrogen bonding and – despite the high polarization of the individual carbon fluorine bond – perfluoroalkyls exhibit a low overall dipole moment due to their inherent geometry. The very low polarizability of perfluoroalkyls results in very weak intermolecular dispersion forces and consequently low boiling points compared to hydrocarbons of similar mass.58
In our present work, we focus on the question how to derivatize biomolecular structures such that their sublimation enthalpy is reduced and their thermal stability increased. Perfluoroalkyl functionalization of individual molecules is expected to reduce the binding to neighbouring particles and surfaces because earlier studies have shown that it enhances the volatility of large organic compounds.17, 59, 60, 61 Here, we apply this strategy for the first time to oligopeptides and study its influence on their volatility.
Flying rather than frying the peptide
The volatility of the first model system, namely the tripeptide alanine–tryptophan–alanine (AWA) was compared in various modifications, i.e. in its native, methylated or perfluoroalkyl modified form including acylation and amidation of the termini, respectively, as shown in Fig. 1.
Figure 1.
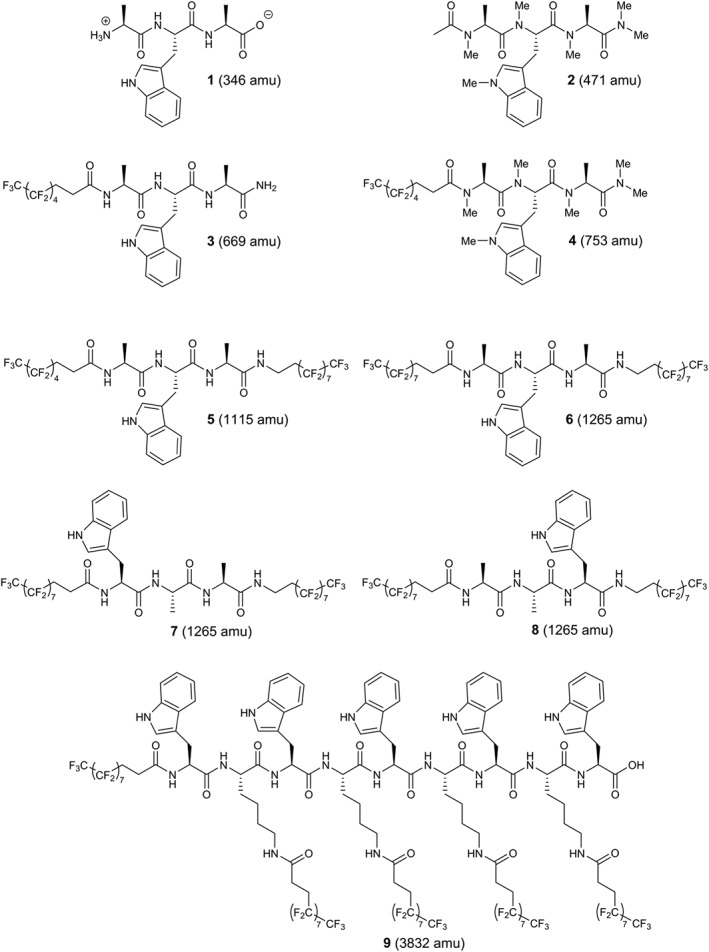
Gallery of peptides 1–9 with increasing molecular weight employed in this study. Tripeptides 1–8 resulted from variation of the Ala‐Trp‐Ala motif: charges and hydrogen bond donors present in parent tripeptide 1 were removed by acylation, methylation and amidation in derivative 2; one perfluoroalkyl chain was introduced at the N‐terminus by acylation and the C‐terminus amidated in derivative 3; 4 was obtained by methylation of 3; fluorinated alkyl chains of different or equal length were introduced at the N‐ and C‐terminus by acylation and amidation, respectively, in derivative 5 and 6; 7 and 8 are sequence isomers of 6; high Trp and fluoroalkyl content realized by alternating Trp and Lys followed by acylation of the lysine side chains and the N‐terminus as exemplified in peptide 9.
All peptides were volatilized in a resistively heated oven whose temperature was monitored on its outside and inside with an absolute uncertainty of ±5 K. The sublimated or evaporated molecules passed a differential pumping stage before they entered the probe chamber, where they were ionized (see Fig. 2). For selected compounds, electron impact ionization (E impact = 70 eV) in quadrupole mass spectrometry (EI‐QMS) was compared with vacuum‐ultraviolet (VUV) photoionization (λ = 157 nm, τ = 10 ns) in time‐of‐flight mass spectrometry (TOF‐MS) to distinguish ionization induced from thermal decomposition processes.
Figure 2.
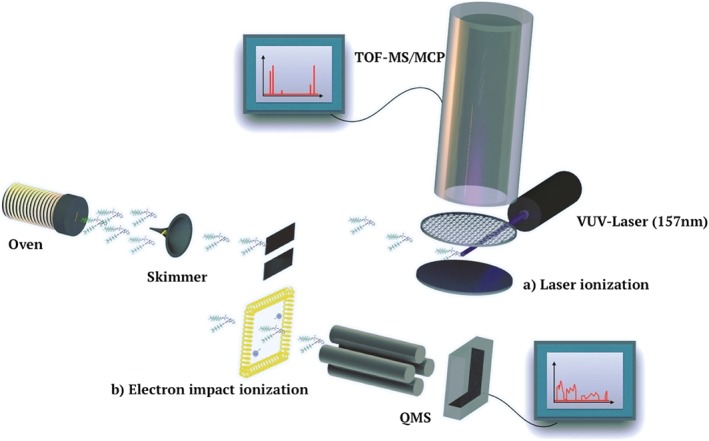
Experimental scheme for the volatilization/ionization tests. The peptides were heated in a ceramic cell with an aperture of 3 × 0.05 mm2. The molecular beam reached the mass spectrometer through two differential pumping stages, separated by one skimmer and one slit of 3 mm as the relevant dimension. Under heat load, the pressure in the three chambers was 1 × 10−5, 3 × 10−6 and 1.5 × 10−7 mbar, respectively. Pulsed photoionization of the molecular beam was combined with time‐of‐flight mass spectrometry (panel (a)). Alternatively, continuous electron impact ionization (b) was combined with a quadrupole mass spectrometer. Because both spectrometers were optimized for high transmission, their mass resolution is limited to about 2% with a calibration uncertainty of 5% across the entire mass range.
Results
We started by comparing the native tripeptide 1 with its methylated derivative 2 where internal charges were removed by acetylation and amidation of the termini (Fig. 3). Upon evaporation of 1 at varying source temperatures up to T = 595 K, only molecular fragments were detected, both in EI‐QMS and VUV‐TOF‐MS. Three major fragments were observed that were tentatively assigned to the loss of the C‐terminal alanine (H2N‐CH(CH3)‐COOH) possibly resulting from thermal diketopiperazine formation (C14H15N3O2, 257 amu)62, 63 as well as two common tryptophan fragments64 which were observed in all following mass spectra: the indole cation (C8H6N+, 116 amu) and the skatole cation (C9H8N+, 130 amu).
Figure 3.
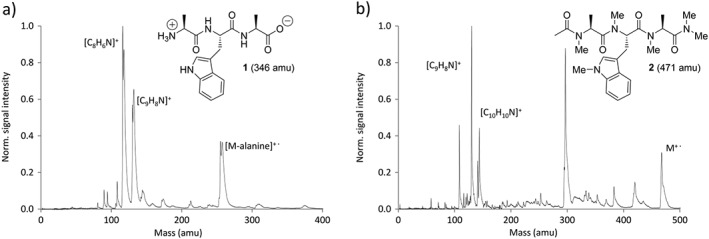
Panel (a) shows the mass spectrum of the native tripeptide Ala‐Trp‐Ala 1 after evaporation at 595 K and VUV postionization with a pulse intensity of I ion = 2.9(3)MW/cm2. The native biomolecule (346 amu) falls apart under these conditions, and the following main fragments are observed: the indole cation (C8H6N+, 116 amu), the skatole cation (C9H8N+,130 amu) and a signal that is tentatively assigned to a cationic Ala‐Trp diketopiperazine fragment (C14H15N3O2 +, 257 amu). The spectrum was calibrated to the indole cation (C8H6N+, 116 amu). (b) Under similar experimental conditions, but at lower temperature (T = 525 K), the mass spectrum of the methylated tripeptide 2 displays the intact parent ion at 471 amu. (b). Fragments include the N‐methyl indole cation (C9H8N+,130 amu) and the N‐methylated skatole cation (C10H10N+, 144 amu) as well as several unidentified species. The spectrum was calibrated to the N‐methyl indole cation (C9H8N+,130 amu).
As reported in the literature, the removal of internal charges and hydrogen bond donors – through acylation of the N‐terminus, amidation of the C‐terminus and methylation of all nitrogen atoms – reduces the intermolecular binding and increases the volatility of the peptides.39 Indeed, evaporation of 2 permitted the observation of intact molecular ions (m = 471 amu), already at 525 K (see Fig. 3(b)).
Earlier studies with stable organic molecules showed that their volatility and stability can be enhanced by functionalization with perfluoroalkyl chains.65 The high electronegativity of fluorine reduces the polarizability‐to‐mass ratio in the compound and redistributes electron density slightly to the outside of the neutral molecule. Pictorially speaking, this functionalization aims at ‘wrapping’ the peptide in a protective fluorinated shell. Even though the particle is technically not encapsulated, the attachment of the chains is assumed to be beneficial.
This hypothesis was verified for derivative 3 of the thermolabile tripeptide AWA. Upon heating to 548 K and exposure to VUV‐TOF mass spectrometry with laser settings equal to those used for the native peptide 1, a substantial parent peak was observed (Fig. 4(a)) corroborating our design hypothesis. It was also noted that VUV photoionization is softer than electron impact ionization (Fig. 4(b)).66 This was apparent in the substantially reduced number of fragments and the higher parent‐to‐fragment ratio shown in Fig. 4(a).
Figure 4.
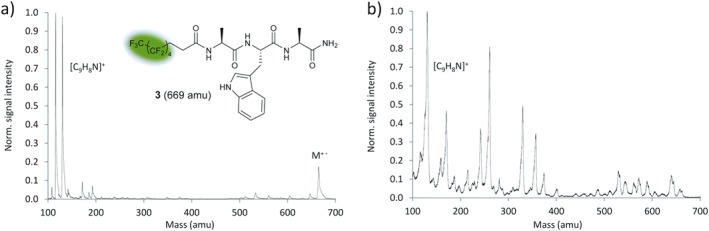
VUV‐TOF versus EI‐QMS. (a) VUV‐TOF mass spectrum of a thermal perfluoroalkyl functionalized peptide beam (3), recorded at T = 548 K. A strong parent peak (m = 669 amu) is observed and accompanied by the indole cation (C8H6N+, 116 amu) and skatole cation (C9H8N+, 130 amu). The spectrum was calibrated to the indole cation (C8H6N+, 116 amu). (b) In contrast, the EI‐QMS spectrum at 70‐eV electron energy yields a pronounced fragment spectrum under otherwise identical boundary conditions. The spectrum was calibrated to the skatole cation (C9H8N+, 130 amu). The green highlight indicates the fluoroalkyl‐tag. [Colour figure can be viewed at wileyonlinelibrary.com]
A dense mass spectrum was observed upon photoionization of a thermal beam of the methylated derivative 4, which carries one fluoroalkyl chain introduced by acylation of the N‐terminus (Fig. 5(a)). Intact parent molecules were detected over the entire temperature range from 467 to 585 K. Based on this positive trend, additional perfluoroalkylation by amidation of the C‐terminus was investigated in the absence of N‐methylation. The mass spectrum of the peptide derivative 5 is shown in Fig. 5(b). The parent peak (m = 1115 amu) appears at 548 K, reaches its maximum at 586 K and now clearly dominates the spectrum. A similar but less pronounced effect is observed with the non‐N‐methylated peptide 3, which carries only one fluoroalkyl chain (Fig. 4(a)). This corroborates the hypothesis that perfluoroalkylation enhances the volatility of the peptides and stabilizes them against thermal and photo‐induced dissociation whereas N‐methylation seems to promote fragmentation.
Figure 5.
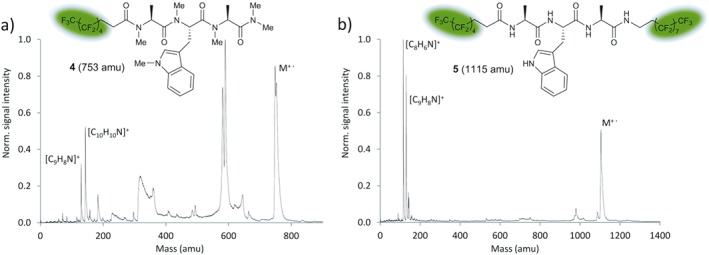
(a) VUV‐TOF mass spectrum of a thermal beam of perfluoroalkyl functionalized and methylated tripeptide 4 at T = 552 K and I ion = 2.9(3) MW/cm2. We observe the methylated skatole cation (C10H10N+, 144 amu) and the N‐methyl indole cation (C9H8N+, 130 amu) as well as unidentified fragments. The spectrum was calibrated to the N‐methyl indole cation (C9H8N+,130 amu). (b) A second perfluoroalkyl chain adds to the molecular mass but results in a relatively clean mass spectrum of the non N‐methylated peptide 5 (compare also Fig. 4(a)). The spectrum was calibrated to the indole cation (C8H6N+, 116 amu). [Colour figure can be viewed at wileyonlinelibrary.com]
To elucidate whether peptide stability and detectability are sequence dependent, the three perfluoroalkylated compounds 6, 7 and 8 were synthesized, which differ only in the order of the three amino acid residues. Their VUV‐TOF mass spectra are shown in Fig. 6(a–c). The strongest parent signal is found in the temperature range of 575–585 K and for the highest photoionization intensity Iion = 2.9(3)MW/cm2. In all three cases, we find a dominant parent peak (1265 amu) and a simple mass pattern with the indole and skatole cation (C8H6N+, 116 amu and C9H8N+, 130 amu, respectively) as prominent fragments (Fig. 6(a–c)). Interestingly, the Trp‐Ala‐Ala sequence isomer 6 led to additional unidentified fragments over 600 amu compared to the other two sequence permutations 7 and 8 (Fig. 6(a)).
Figure 6.
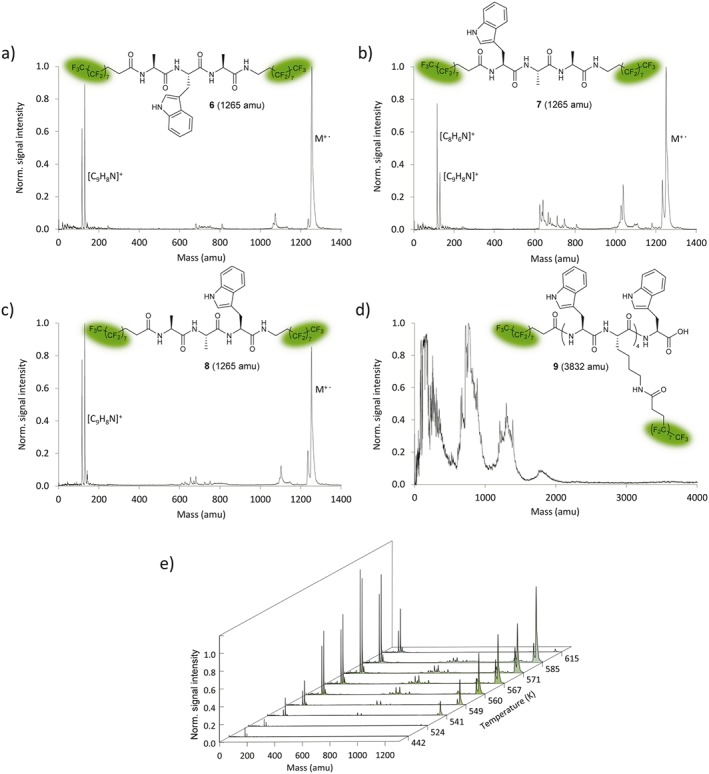
(a–c) Sequence isomers of tripeptide derivative with perfluoroalkyl decorated termini. Mass spectra for 6, 7 and 8 are very similar, although a higher proportion of fragments is observed for 7. Spectra were calibrated to the indole cation (C8H6N+, 116 amu). The indole cation is not indicated in (a) and (c) for improved clarity. (d) No intact ion was detected for highly perfluoroalkyl decorated Trp‐Lys oligomer 9 after thermal evaporation and photoionization. (e) Variation of the oven temperature leads to an increase of both the parent signal and the fragments of compound 8. Significant thermal decomposition is observed at 615 K. [Colour figure can be viewed at wileyonlinelibrary.com]
To probe the thermal contribution to fragmentation, we have studied the mass spectrum of 8 as a function of the source temperature (see Fig. 6(e)). Both the parent peak and its fragments rise in a constant relation with increasing temperature, up to 615 K, where the parent molecule finally disintegrates. This suggests that the molecules remain stable up to this temperature and that the fragments are predominantly due to the ionization process.
Because perfluoroalkyl functionalization substantially increases the molecular volatility,65 it is intriguing to test the mass limit of this method. The ideal model analyte would (i) have a high fluoroalkyl content and (ii) include a high tryptophan content for efficient photo‐ionization. Compound 9 fulfils these requirements and was heated in the same setup (Fig. 2), under high vacuum. The resulting VUV‐TOF‐mass spectrum (Fig. 6(d)) contains no intact parent molecule.
In order to test for the presence of an intact parent fraction in the neutral molecular beam, we have collected the evaporated material on a glass slide next to the oven. This sample was post‐analysed in a commercial MALDI instrument and did not show any intact parent peak, suggesting that the functionalized nonapeptide did not reach the glass slide as an intact entity in sufficient quantities.
Discussion
By comparing the mass spectra of all tailored peptides depicted in Fig. 1 and assuming similar photoionization efficiency for compounds 1–8, it can be seen that compounds 6, 7 and 8 produce the most intense thermal beams and VUV‐TOF mass signals with a small proportion of fragments, even though they are 3.6 times more massive than the free AWA tripeptide alone. This observation can be attributed to reduced intermolecular interactions resulting from the removal of internal charges and the low polarizability of the perfluoroalkyl chains. The chain length of the perfluoroalkyl decoration, although not investigated in detail, seemed to play a minor but significant role with a more pronounced parent ion peak for compound 6 (C‐ and N‐terminal C8F17) in comparison to compound 5, which carries a shorter perfluoroalkyl chain (C5F11) at the N‐terminus (Figs 5(b) and 6(a)). For compound 4 which has only one short perfluoroalkyl chain (C5F11), the relative intensity of the indole and skatole fragment ions versus the parental ion is significantly increased (Fig. 4(a)) although little additional fragments were observed. Interestingly, sequence isomers with identical decoration of perfluoroalkyl groups exhibited different degrees of fragmentation with the Trp‐Ala‐Ala sequence isomer being the most fragile in the series.
N‐methylation appears to promote fragmentation in combination with fluoroalkyl chains or without (Figs 3(b) and 5(a)). In contrast, already a single perfluoroalkyl chain with native N―H bonds in the peptide delivers significantly cleaner spectra than N‐methylated compounds.
The parent–fragment ratio does also depend on the ionization mechanism. Many mass spectrometry experiments use electron impact ionization with energies of 70 eV for optimum sensitivity. However, the excess of electron energy can open a variety of fragmentation channels.67 In contrast, VUV photoionization at 157 nm (7.90 eV) was confirmed as a soft ionization technique,66 whenever the peptide chain contained one or more tryptophan units.
Even though derivatization changes the geometry and chemical response of the molecule, we argue that our method can serve many practical purposes. In recent years, substantial research effort has been dedicated to evaluate, understand and explore the effects and benefits of fluorination in biochemistry, medicine and pharmacology.54, 55, 56, 57, 68 Studying fluoroalkyl derivatized molecules in the gas phase with an increasing number of hydration layers shall soon allow to systematically study the change of their electro‐optical properties in quantum interferometry experiments.43 Furthermore, it allows the preservation of native N―H functionality and thereby investigation of intramolecular interactions which might well reflect those of native peptides in the absence of solvent or other molecules.44, 45
Methods
Synthesis and characterization of peptide constructs are detailed in the Supporting Information. Figures 3, 4, 5 and 6(a–d) were generated with Microsoft Excel 2010 using the ‘Scatter with Smooth Lines’ function. Figure 6e was prepared with Origin 9.2.214.
Author contribution statement
J.S. and U.S. contributed equally to the work. J.S. synthesized and characterized the molecular compounds, U.S. and S.P designed and conducted the volatilization experiment with subsequent photoionization, J.C. designed and conducted the volatilization experiment with subsequent electron‐impact ionization, U.S., S.P. and J.C. analysed the data. V.K., M.A. and M. M. conceived and supervised the experiments. U.S., J.S., V.K. and M.A. wrote the paper, with all authors reviewing it.
Additional information
There are no additional accession codes; the authors declare to have no competing financial interests.
Supporting information
Data S1. Ala‐Trp‐Ala 1. Top: 1H‐NMR (DMSO‐d6, 500 MHz, 293 K), Bottom: UV–Vis‐trace 190–500 nm of UPLC chromatogram (Method 1).
S2. Ac‐Ala‐Trp‐Ala‐NH2. Top: 1H‐NMR (DMSO‐d6, 500 MHz, 293 K), Bottom: UV–Vis‐trace 190–500 nm of UPLC chromatogram (Method 1).
S3. (2H,2H,3H,3H‐perfluorooctanoyl)‐Ala‐Trp‐Ala. Top: 1H‐NMR (DMSO‐d6, 500 MHz, 293 K), Bottom: UV–Vis‐trace 190–500 nm of UPLC chromatogram (Method 2).
S4. (2H,2H,3H,3H‐perfluoroundecanoyl)‐Ala‐Trp‐Ala. Top: 1H‐NMR (DMSO‐d6, 500 MHz, 293 K), Bottom: UV–Vistrace 190–500 nm of UPLC chromatogram (Method 2).
S5. (2H,2H,3H,3H‐perfluoroundecanoyl)‐Trp‐Ala‐Ala: Top: 1H‐NMR (DMSO‐d6, 500 MHz, 293 K), Bottom: UV‐Vistrace 190–500 nm of UPLC chromatogram (Method 2).
S6. (2H,2H,3H,3H‐perfluoroundecanoyl)‐Ala‐Ala‐Trp: Top: 1H‐NMR (DMSO‐d6, 500 MHz, 293 K), Bottom: UV‐Vistrace 190–500 nm of UPLC chromatogram (Method 2).
S7. Ac‐Nα‐Me‐Ala‐Nα‐Me‐Trp(Me)‐Nα‐Me‐Ala‐N(Me)2 2. Top: 1H–NMR (CDCl3, 500 MHz, 293 K), Bottom: UV–Vis‐trace 190–500 nm of UPLC chromatogram (Method 2).
S8. (2H,2H,3H,3H‐perfluorooctanoyl)‐Ala‐Trp‐Ala‐NH2. Top: 1H‐NMR (DMSO‐d6, 500 MHz, 293 K), Bottom: UV–Vistrace 190–500 nm of UPLC chromatogram (Method 2).
S9. (2H,2H,3H,3H‐perfluorooctanoyl)‐Nα‐Me‐Ala‐Nα‐Me‐Trp(Me)‐Nα‐Me‐Ala‐N(Me)2. Top: 1H‐NMR (CDCl3, 500 MHz, 293 K), Bottom: UV–Vis‐trace 190–500 nm of UPLC chromatogram (Method 2).
S10. (2H,2H,3H,3H‐perfluorooctanoyl)‐Ala‐Trp‐Ala‐(2H,2H,3H,3H‐perfluorodecylamid). Top: 1H‐NMR (DMSO‐d6, 500 MHz, 293 K), Bottom: UV–Vis‐trace 190–500 nm of UPLC chromatogram (Method 2).
S11. (2H,2H,3H,3H‐perfluoroundecanoyl)‐Ala‐Trp‐Ala‐(2H,2H,3H,3H‐perfluorodecylamid) 6. Top: 1H‐NMR (DMF‐d7, 600 MHz, 333 K), Bottom: UV–Vis‐trace 190–500 nm of UPLC chromatogram (Method 2).
S12. (2H,2H,3H,3H‐perfluoroundecanoyl)‐Trp‐Ala‐Ala‐(2H,2H,3H,3H‐perfluorodecylamid) 7. Top: 1H‐NMR (DMF‐d7, 600 MHz, 333 K), Bottom: UV–Vis‐trace 190–500 nm of UPLC chromatogram (Method 2).
S13. (2H,2H,3H,3H‐perfluoroundecanoyl) ‐Ala‐Ala‐Trp‐(2H,2H,3H,3H‐perfluorodecylamid) 8. Top: 1H‐NMR (DMF‐d7, 600 MHz, 333 K), Bottom: UV–Vis‐trace 190–500 nm (UPLC, Method 2).
S14. 2H,2H,3H,3H‐perfluorodecanoic acid NHS ester. 1H‐NMR (CDCl3, 500 MHz, 293 K).
S15. Trp‐Lys‐Trp‐Lys‐Trp‐Lys‐Trp‐Lys‐Trp. Top: 1H‐NMR (DMSO‐d6, 500 MHz, 293 K), Bottom: UV‐Vis‐trace 190–500 nm of UPLC chromatogram (Method 1). The chromatogram shows multiple peaks with identical absorption and MS‐spectra which are possibly caused by interconverting conformers.
S16. Compound 9. Top: 1H‐NMR (HFIP‐d2+ 20 mol% H2O), presat (water and OH‐HFIP signal suppression, 600 MHz, 293 K), Bottom: 1H‐NMR (HFIP‐d2, 600 MHz, 293 K).
Acknowledgments
We thank the FP7 Ideas: European Research Council for support under grant agreement no 320694, the Austrian Science Fund (W1210‐25) and the Swiss Nanoscience Institute at the University of Basel, project P1403. We thank Jan Pac for support in early measurements and the Mass Spectrometry Center of the University of Vienna for MALDI‐MS. We thank PD Dr. Daniel Häussinger, University of Basel, for the NMR spectra of compound 9. JPC gratefully acknowledges a Vienna Quantum Fellowship.
Schätti, J. , Sezer, U. , Pedalino, S. , Cotter, J. P. , Arndt, M. , Mayor, M. , and Köhler, V. (2017) Tailoring the volatility and stability of oligopeptides. J. Mass Spectrom., 52: 550–556. doi: 10.1002/jms.3959.
Contributor Information
M. Arndt, Email: markus.arndt@univie.ac.at.
V. Köhler, Email: valentin.koehler@unibas.ch.
References
- 1. Stern O.. Eine direkte Messung der thermischen Molekularstrahlgeschwindigkeit. Z. Phys. 1920, 2, 49. [Google Scholar]
- 2. Estermann I., Stern O.. Diffraction of molecular beams. Z. Phys. 1930, 61, 95. [Google Scholar]
- 3. van de Meerakker S. Y. T., Bethlem H. L., Meijer G.. Taming molecular beams. Nat. Phys. 2008, 4, 595. [Google Scholar]
- 4. Henini M.. Molecular Beam Epitaxy: From Research to Mass Production. Elsevier Science: Oxford, 2012. [Google Scholar]
- 5. Scoles G., Bassi D., Buck U., Laine D. C.. Atomic and Molecular Beam Methods. 1 Oxford University Press: New York, 1988. [Google Scholar]
- 6. Bordé C. J., Avrillier S., Van Lerberghe A., Salomon C., Bassi D., Scoles G.. Observation of optical Ramsey fringes in the 10 μm spectral region using a supersonic beam of SF6. J. Phys. Colloq 1981, 42, C8‐15‐C18‐19. [Google Scholar]
- 7. Eibenberger S., Doyle J., Patterson D.. Enantiomer‐specific state transfer of chiral molecules. arXiv:1608.04691v1 [physics.chem‐ph] 15 Aug 2016 2016. [DOI] [PubMed]
- 8. Antoine R., Dugourd P., Rayane D., Benichou E., Broyer M., Chandezon F., Guet C.. Direct measurement of the electric polarizability of isolated C60 molecules. J. Chem. Phys. 1999, 110, 9771. [Google Scholar]
- 9. Antoine R., Compagnon I., Rayane D., Broyer M., Dugourd P., Breaux G., Hagemeister F. C., Pippen D., Hudgins R. R., Jarrold M. F.. Electric dipole moments and conformations of isolated peptides. Eur. Phys. J. D 2002, 20, 583. [Google Scholar]
- 10. de Heer W. A., Kresin V. V.. In Handbook of Nanophysics, Clusters and Fullerenes. (Sattler K. D. Ed.). CRC Press: Boca Raton, 2010. 10.11. [Google Scholar]
- 11. Kirste M., Wang X., Schewe H. C., Meijer G., Liu K., van der Avoird A., Janssen L. M. C., Gubbels K. B., Groenenboom G. C., van de Meerakker S. Y. T.. Quantum‐state resolved bimolecular collisions of velocity‐controlled OH with NO radicals. Science 2012, 338, 1060. [DOI] [PubMed] [Google Scholar]
- 12. Willitsch S., Bell M. T., Gingell A. D., Procter S. R., Softley T. P.. Cold reactive collisions between laser‐cooled ions and velocity‐selected neutral molecules. Phys. Rev. Lett. 2008, 100, 043203. [DOI] [PubMed] [Google Scholar]
- 13. Hudson J. J., Sauer B. E., Tarbutt M. R., Hinds E. A.. Measurement of the electron electric dipole moment using YbF molecules. Phys. Rev. Lett. 2002, 89, 023003. [DOI] [PubMed] [Google Scholar]
- 14. Baron J., Campbell W. C., DeMille D., Doyle J. M., Gabrielse G., Gurevich Y. V., Hess P. W., Hutzler N. R., Kirilov E., Kozyryev I., O'Leary B. R., Panda C. D., Parsons M. F., Petrik E. S., Spaun B., Vutha A. C., West A. D.. Order of magnitude smaller limit on the electric dipole moment of the electron. Science 2014, 343, 269. [DOI] [PubMed] [Google Scholar]
- 15. Schöllkopf W., Toennies J. P.. Nondestructive mass selection of small Van der Waals clusters. Science 1994, 266, 1345. [DOI] [PubMed] [Google Scholar]
- 16. Haslinger P., Dörre N., Geyer P., Rodewald J., Nimmrichter S., Arndt M.. A universal matter‐wave interferometer with optical ionization gratings in the time domain. Nat. Phys. 2013, 9, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eibenberger S., Gerlich S., Arndt M., Mayor M., Tüxen J.. Matter‐wave interference of particles selected from a molecular library with masses exceeding 10 000 amu. Phys. Chem. Chem. Phys. 2013, 15, 14696. [DOI] [PubMed] [Google Scholar]
- 18. Rieger T., Junglen T., Rangwala S. A., Pinkse P. W. H., Rempe G.. Continuous loading of an electrostatic trap for polar molecules. Phys. Rev. Lett. 2005, 95, 173001. [DOI] [PubMed] [Google Scholar]
- 19. Junglen T., Rieger T., Rangwala S. A., Pinkse P. W. H., Rempe G.. Slow ammonia molecules in an electrostatic quadrupole guide. Eur. Phys. J. D 2004, 31, 365. [Google Scholar]
- 20. van de Meerakker S. Y. T., Bethlem H. L., Vanhaecke N., Meijer G.. Manipulation and control of molecular beams. Chem. Rev. 2012, 112, 4828. [DOI] [PubMed] [Google Scholar]
- 21. Küpper J., Filsinger F., Meijer G.. Manipulating the motion of large neutral molecules. Faraday Discuss. 2009, 142, 155. [DOI] [PubMed] [Google Scholar]
- 22. Heiner C. E., Carty D., Meijer G., Bethlem H. L.. A molecular synchrotron. Nat. Phys. 2007, 3, 115. [Google Scholar]
- 23. Bethlem H. L., Crompvoets F. M. H., Jongma R. T., van de Meerakker S. Y. T., Meijer G.. Deceleration and trapping of ammonia using time‐varying electric fields. Phys. Rev. A 2002, 65, 053416. [Google Scholar]
- 24. Bethlem H. L., Berden G., Meijer G.. Decelerating neutral dipolar molecules. Phys. Rev. Lett. 1999, 83, 1558. [Google Scholar]
- 25. Narevicius E., Parthey C. G., Libson A., Riedel M. F., Even U., Raizen M. G.. Towards magnetic slowing of atoms and molecules. New J. Phys. 2007, 9. [Google Scholar]
- 26. Chervenkov S., Wu X., Bayerl J., Rohlfes A., Gantner T., Zeppenfeld M.. Continuous centrifuge decelerator for polar molecules. Phys. Rev. Lett. 2014, 112, 013001. [DOI] [PubMed] [Google Scholar]
- 27. Shuman E. S., Barry J. F., DeMille D.. Laser cooling of a diatomic molecule. Nature 2010, 467, 820. [DOI] [PubMed] [Google Scholar]
- 28. Barry J., Yale (New Haven), 2013.
- 29. Zhelyazkova V., Cournol A., Wall T. E., Matsushima A., Hudson J. J., Hinds E. A., Tarbutt M. R., Sauer B. E.. Laser cooling and slowing of CaF molecules. Phys. Rev. A 2014, 89, 053416. [Google Scholar]
- 30. Englert B., Mielenz M., Sommer C., Bayerl J., Motsch M., Pinkse P., Rempe G., Zeppenfeld M.. Storage and adiabatic cooling of polar molecules in a microstructured trap. Phys. Rev. Lett. 2011, 107. [DOI] [PubMed] [Google Scholar]
- 31. Zeppenfeld M., Englert B. G. U., Glockner R., Prehn A., Mielenz M., Sommer C., van Buuren L. D., Motsch M., Rempe G.. Sisyphus cooling of electrically trapped polyatomic molecules. Nature 2012, 491, 570. [DOI] [PubMed] [Google Scholar]
- 32. Schermann J.‐P.. Spectroscopy and Modeling of Biomolecular Building Blocks. Elsevier, 2007. [Google Scholar]
- 33. Gross D., Grodsky G.. The sublimation of amino acids and peptides. J. Am. Chem. Soc. 1955, 77, 1678. [Google Scholar]
- 34. Badelin V. G., Tyunina E. Y., Krasnov A. V., Tyunina V. V., Giricheva N. I., Girichev A. V.. Mass spectrometry study of the sublimation of aliphatic dipeptides. Russ. J. Phys. Chem. A 2012, 86, 457. [Google Scholar]
- 35. Tyunina V. V., Krasnov A. V., Tyunina E. Y., Badelin V. G., Girichev G. V.. Enthalpy of sublimation of natural aromatic amino acids determined by Knudsen's effusion mass spectrometric method. J. Chem. Thermodyn. 2014, 74, 221. [Google Scholar]
- 36. Tyunina V. V., Krasnov A. V., Badelin V. G., Girichev G. V.. Enthalpy of sublimation of hydroxyl‐containing amino acids: Knudsen's effusion mass spectrometric study. J. Chem. Thermodyn. 2016, 98, 62. [Google Scholar]
- 37. de Kruif C. G., Voogd J., Offringa J. C. A.. Enthalpies of sublimation and vapour pressures of 14 amino acids and peptides. J. Chem. Thermodyn. 1979, 11, 651. [Google Scholar]
- 38. Glavin D. P., Bada J. L.. Isolation of amino acids from natural samples using sublimation. Anal. Chem. 1998, 70, 3119. [DOI] [PubMed] [Google Scholar]
- 39. Das B. C., Gero S. D., Lederer E.. N‐methylation of N‐acyl oligopeptides. Biochem. Biophys. Res. Commun. 1967, 29, 211. [DOI] [PubMed] [Google Scholar]
- 40. Morris H. R., Williams D. H., Ambler R. P.. Determination of the sequences of protein‐derived peptides and peptide mixtures by mass spectrometry. Biochem. J. 1971, 125, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roepstorff P., Spear R. K., Brunfeldt K.. Mass spectrometric sequence determination of permethylated peptide mixtures. FEBS Lett. 1971, 15, 237. [DOI] [PubMed] [Google Scholar]
- 42. de Vries M. S., Hobza P.. Gas‐phase spectroscopy of biomolecular building blocks. Annu. Rev. Phys. Chem. 2007, 58, 585. [DOI] [PubMed] [Google Scholar]
- 43. Geyer P., Sezer U., Rodewald J., Mairhofer L., Dörre N., Haslinger P., Eibenberger S., Brand C., Arndt M.. Perspectives for quantum interference with biomolecules and biomolecular clusters. Phys. Scripta 2016, 91, 063007. [Google Scholar]
- 44. Walsh P. S., Blodgett K. N., McBurney C., Gellman S. H., Zwier T. S.. Inherent conformational preferences of Ac‐Gln‐Gln‐NHBn: sidechain hydrogen bonding supports a β‐turn in the gas phase. Angew. Chem. Int. Ed. 2016, 55, 14618. [DOI] [PubMed] [Google Scholar]
- 45. Fricke H., Schaefer G., Schrader T., Gerhards M.. Secondary structure binding motifs of the jet cooled tetrapeptide model Ac‐Leu‐Val‐Tyr(Me)‐NHMe. Phys. Chem. Chem. Phys. 2007, 9, 4592. [DOI] [PubMed] [Google Scholar]
- 46. Fricke H., Funk A., Schrader T., Gerhards M.. Investigation of secondary structure elements by IR/UV double resonance spectroscopy: analysis of an isolated beta‐sheet model system. J. Am. Chem. Soc. 2008, 130, 4692. [DOI] [PubMed] [Google Scholar]
- 47. Jarrold M. F.. Peptides and proteins in the vapor phase. Annu. Rev. Phys. Chem. 2000, 51, 179. [DOI] [PubMed] [Google Scholar]
- 48. Grégoire G.. In Nucleic Acids in the Gas Phase. (Gabelica V. Ed.). Springer: Heidelberg, 2014. 21. [Google Scholar]
- 49. Dey M., Moritz F., Atkinson G. H., Grotemeyer J., Schlag E.. Molecular beams of polyenes: retinals and beta‐carotene. J. Chem. Phys. 1991, 95, 4584. [Google Scholar]
- 50. Meijer G., de Vries M. S., Hunziker H. E., Wendt H. R.. Laser desorption jet‐cooling of organic molecules cooling characteristics and detection sensitivity. Appl. Phys. B Lasers Opt. 1990, 51, 395. [Google Scholar]
- 51. Piskorski J., Patterson D., Eibenberger S., Doyle J. M.. Cooling, spectroscopy and non‐sticking of trans‐stilbene and Nile Red. Chem Phys Chem 2014, 15, 3800. [DOI] [PubMed] [Google Scholar]
- 52. Sezer U., Wörner L., Horak J., Felix L., Tüxen J., Götz C., Vaziri A., Mayor M., Arndt M.. Laser‐induced acoustic desorption of natural and functionalized biochromophores. Anal. Chem. 2015, 87, 5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zinovev A. V., Veryovkin I. V., Moore J. F., Pellin M. J.. Laser‐driven acoustic desorption of organic molecules from back‐irradiated solid foils. Anal. Chem. 2007, 79, 8232. [DOI] [PubMed] [Google Scholar]
- 54. Zhou Y., Wang J., Gu Z., Wang S., Zhu W., Acena J. L., Soloshonok V. A., Izawa K., Liu H.. Next generation of fluorine‐containing pharmaceuticals, compounds currently in Phase II–III clinical trials of major pharmaceutical companies: new structural trends and therapeutic areas. Chem. Rev. 2016, 116, 422. [DOI] [PubMed] [Google Scholar]
- 55. Müller K., Faeh C., Diederich F.. Fluorine in pharmaceuticals: looking beyond intuition. Science 2007, 317, 1881. [DOI] [PubMed] [Google Scholar]
- 56. Marsh E. N. G.. Fluorinated proteins: from design and synthesis to structure and stability. Acc. Chem. Res. 2014, 47, 2878. [DOI] [PubMed] [Google Scholar]
- 57. Tang Y., Ghirlanda G., Petka W. A., Nakajima T., DeGrado W. F., Tirrell D. A.. Fluorinated coiled‐coil proteins prepared in vivo display enhanced thermal and chemical stability. Angew. Chem. Int. Ed. 2001, 40, 1494. [DOI] [PubMed] [Google Scholar]
- 58. Kirsch P.. Modern Fluoroorganic Chemistry. Wiley‐VCH Verlag GmbH & Co. KGaA: Weinheim, 2013. 1. [Google Scholar]
- 59. Felix L., Sezer U., Arndt M., Mayor M.. Synthesis of highly fluoroalkyl‐functionalized oligoporphyrin systems. Eur. J. Org. Chem. 2014, 6884. [Google Scholar]
- 60. Tüxen J., PhD. thesis, University of Basel (Basel), 2012.
- 61. O'Hagan D.. Understanding organofluorine chemistry. An introduction to the C―F bond. Chem. Soc. Rev. 2008, 37, 308. [DOI] [PubMed] [Google Scholar]
- 62. Lichtenstein N., Hestrin S., Dimant E., Brzoza H.. Behavior of peptides when heated in β‐naphthol. J. Am. Chem. Soc. 1938, 60, 560. [Google Scholar]
- 63. Drabik E., Jeziorna A., Bienias U., Trzeciak‐Karlikowska K., Pawlak T., Paluch P., Potrzebowski M. J.. Study of the mechanism of thermal chemical processes in the crystals of YAF tripeptides by means of mass spectrometry and solid state NMR. J. Phys. Chem. B 2013, 117, 13481. [DOI] [PubMed] [Google Scholar]
- 64. Piuzzi F., Dimicoli I., Mons M., Tardivel B., Zhao Q.. A simple laser vaporization source for thermally fragile molecules coupled to a supersonic expansion: application to the spectroscopy of tryptophan. Chem. Phys. Lett. 2000, 320, 282. [Google Scholar]
- 65. Sezer U., Schmid P., Felix L., Mayor M., Arndt M.. Stability of high‐mass molecular libraries: the role of the oligoporphyrin core. J. Mass Spectrom. 2015, 50, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hanley L., Zimmermann R.. Light and molecular ions: the emergence of vacuum UV single‐photon ionization in MS. Anal. Chem. 2009, 81, 4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vékey K.. Internal energy effects in mass spectrometry. J. Mass Spectrom. 1996, 31, 445. [Google Scholar]
- 68. O'Hagan D., Schaffrath C., Cobb S. L., Hamilton J. T., Murphy C. D.. Biochemistry: biosynthesis of an organofluorine molecule. Nature 2002, 416, 279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Ala‐Trp‐Ala 1. Top: 1H‐NMR (DMSO‐d6, 500 MHz, 293 K), Bottom: UV–Vis‐trace 190–500 nm of UPLC chromatogram (Method 1).
S2. Ac‐Ala‐Trp‐Ala‐NH2. Top: 1H‐NMR (DMSO‐d6, 500 MHz, 293 K), Bottom: UV–Vis‐trace 190–500 nm of UPLC chromatogram (Method 1).
S3. (2H,2H,3H,3H‐perfluorooctanoyl)‐Ala‐Trp‐Ala. Top: 1H‐NMR (DMSO‐d6, 500 MHz, 293 K), Bottom: UV–Vis‐trace 190–500 nm of UPLC chromatogram (Method 2).
S4. (2H,2H,3H,3H‐perfluoroundecanoyl)‐Ala‐Trp‐Ala. Top: 1H‐NMR (DMSO‐d6, 500 MHz, 293 K), Bottom: UV–Vistrace 190–500 nm of UPLC chromatogram (Method 2).
S5. (2H,2H,3H,3H‐perfluoroundecanoyl)‐Trp‐Ala‐Ala: Top: 1H‐NMR (DMSO‐d6, 500 MHz, 293 K), Bottom: UV‐Vistrace 190–500 nm of UPLC chromatogram (Method 2).
S6. (2H,2H,3H,3H‐perfluoroundecanoyl)‐Ala‐Ala‐Trp: Top: 1H‐NMR (DMSO‐d6, 500 MHz, 293 K), Bottom: UV‐Vistrace 190–500 nm of UPLC chromatogram (Method 2).
S7. Ac‐Nα‐Me‐Ala‐Nα‐Me‐Trp(Me)‐Nα‐Me‐Ala‐N(Me)2 2. Top: 1H–NMR (CDCl3, 500 MHz, 293 K), Bottom: UV–Vis‐trace 190–500 nm of UPLC chromatogram (Method 2).
S8. (2H,2H,3H,3H‐perfluorooctanoyl)‐Ala‐Trp‐Ala‐NH2. Top: 1H‐NMR (DMSO‐d6, 500 MHz, 293 K), Bottom: UV–Vistrace 190–500 nm of UPLC chromatogram (Method 2).
S9. (2H,2H,3H,3H‐perfluorooctanoyl)‐Nα‐Me‐Ala‐Nα‐Me‐Trp(Me)‐Nα‐Me‐Ala‐N(Me)2. Top: 1H‐NMR (CDCl3, 500 MHz, 293 K), Bottom: UV–Vis‐trace 190–500 nm of UPLC chromatogram (Method 2).
S10. (2H,2H,3H,3H‐perfluorooctanoyl)‐Ala‐Trp‐Ala‐(2H,2H,3H,3H‐perfluorodecylamid). Top: 1H‐NMR (DMSO‐d6, 500 MHz, 293 K), Bottom: UV–Vis‐trace 190–500 nm of UPLC chromatogram (Method 2).
S11. (2H,2H,3H,3H‐perfluoroundecanoyl)‐Ala‐Trp‐Ala‐(2H,2H,3H,3H‐perfluorodecylamid) 6. Top: 1H‐NMR (DMF‐d7, 600 MHz, 333 K), Bottom: UV–Vis‐trace 190–500 nm of UPLC chromatogram (Method 2).
S12. (2H,2H,3H,3H‐perfluoroundecanoyl)‐Trp‐Ala‐Ala‐(2H,2H,3H,3H‐perfluorodecylamid) 7. Top: 1H‐NMR (DMF‐d7, 600 MHz, 333 K), Bottom: UV–Vis‐trace 190–500 nm of UPLC chromatogram (Method 2).
S13. (2H,2H,3H,3H‐perfluoroundecanoyl) ‐Ala‐Ala‐Trp‐(2H,2H,3H,3H‐perfluorodecylamid) 8. Top: 1H‐NMR (DMF‐d7, 600 MHz, 333 K), Bottom: UV–Vis‐trace 190–500 nm (UPLC, Method 2).
S14. 2H,2H,3H,3H‐perfluorodecanoic acid NHS ester. 1H‐NMR (CDCl3, 500 MHz, 293 K).
S15. Trp‐Lys‐Trp‐Lys‐Trp‐Lys‐Trp‐Lys‐Trp. Top: 1H‐NMR (DMSO‐d6, 500 MHz, 293 K), Bottom: UV‐Vis‐trace 190–500 nm of UPLC chromatogram (Method 1). The chromatogram shows multiple peaks with identical absorption and MS‐spectra which are possibly caused by interconverting conformers.
S16. Compound 9. Top: 1H‐NMR (HFIP‐d2+ 20 mol% H2O), presat (water and OH‐HFIP signal suppression, 600 MHz, 293 K), Bottom: 1H‐NMR (HFIP‐d2, 600 MHz, 293 K).


