Abstract
Extracellular ATP plays important roles in coordinating the activities of astrocytes and neurons, and aberrant signalling is associated with neurodegenerative diseases. In rodents, ATP stimulates opening of Ca2+‐permeable channels formed by P2X receptor subunits in the plasma membrane. It is widely assumed, but not verified, that P2X receptors also evoke Ca2+ signals in human astrocytes. Here, we directly assess this hypothesis. We showed that cultured foetal cortical human astrocytes express mRNA for several P2X receptor subunits (P2X4, P2X5, P2X6) and G protein‐coupled P2Y receptors (P2Y1, P2Y2, P2Y6, P2Y11). In these astrocytes, ATP stimulated Ca2+ release from intracellular stores through IP 3 receptors and store‐operated Ca2+ entry. These responses were entirely mediated by P2Y1 and P2Y2 receptors. Agonists of P2X receptors did not evoke Ca2+ signals, and nor did ATP when Ca2+ release from intracellular stores and store‐operated Ca2+ entry were inhibited. We conclude that ATP‐evoked Ca2+ signals in cultured human foetal astrocytes are entirely mediated by P2Y1 and P2Y2 receptors, with no contribution from P2X receptors.
Keywords: P2X receptor, P2Y1 receptor, P2Y2 receptor, phospholipase C, purinoceptor, store‐operated Ca2+ entry
Abbreviations used
- [Ca2+]i
intracellular free Ca2+ concentration
- 2‐APB
2‐aminoethoxydiphenyl borate
- ER
endoplasmic reticulum
- GFAP
glial fibrillary acidic protein
- HBS
HEPES‐buffered saline
- IP3
inositol 1,4,5‐trisphosphate
- pEC50
−log of the half‐maximally effective drug concentration
- PLC
phospholipase C
- SOCE
store‐operated Ca2+ entry
Astrocytes comprise a diverse population of glial cells that express glial fibrillary acidic protein, synthesise and store glycogen granules and are linked to each other by gap junctions (Haydon 2001; Verkhratsky and Butt 2013). Astrocytes are abundant throughout the brain and spinal cord, where their roles include directing migration of neurons during development; release of extracellular matrix molecules and growth factors; secretion of neurotransmitters, including ATP and glutamate; regulation of the neuronal environmental; providing neurons with nutrients; and inactivation and recycling of neurotransmitters (Haydon 2001).
Astrocytes respond to many neurotransmitters, but ATP and glutamate are the most prominent, and both can evoke Ca2+ signals that trigger further release of ATP or glutamate. This interplay allows reciprocal interactions between astrocytes and neurons, and it contributes, alongside diffusion of inositol 1,4,5‐trisphosphate (IP3) through gap junctions, to regenerative propagation of ATP‐evoked Ca2+ signals between astrocytes (Haydon 2001). ATP signalling in astrocytes thereby contributes to diverse physiological and pathophysiological processes, including gliotransmitter release, cytokine expression, nociception, regulation of synaptic strength, astrogliosis, ischaemia‐induced injury and Alzheimer's disease (Franke et al. 2001; Duan et al. 2003; Pascual et al. 2005; Lammer et al. 2006; Delekate et al. 2014).
Responses to ATP are mediated by two families of P2 receptors. P2X receptors are ligand‐gated cation channels, which are permeable to Na+, K+ and Ca2+ (Burnstock and Kennedy 2011). The seven P2X receptor subtypes (P2X1‐7) form homo‐ or hetero‐trimeric channels. ATP is the major endogenous agonist for all P2X receptors (Soto et al. 1996; Nicke et al. 1998). P2Y receptors are G protein‐coupled receptors. Five of the eight P2Y receptor subtypes (P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11) stimulate phospholipase C (PLC) through Gq, and the others inhibit adenylyl cyclase through Gi (P2Y12, P2Y13 and P2Y14) (Alexander et al. 2011). Endogenous ligands of P2Y receptors include ADP, ATP, UDP (uridine 5′‐diphosphate), UTP (uridine 5′‐triphosphate) and UDP‐glucose (Jacobson and Muller 2016).
Despite acceptance of the importance of ATP‐evoked Ca2+ signals in astrocytes, the evidence derives almost entirely from rodents, where mRNA for most P2 receptors has been detected, and both P2X and P2Y receptors have been implicated in Ca2+ signalling (Fumagalli et al. 2003; Verkhratsky et al. 2009). However, there is a widespread assumption that most ATP‐evoked Ca2+ signals in rodent astrocytes are mediated by P2X1/5 and P2X7 receptors (Lalo et al. 2008, 2011). The evidence implicating P2X7 receptors is controversial and derives largely from analyses of reactive astroctyes (Sim et al. 2004; Verkhratsky et al. 2009; Oliveira et al. 2011), where morphology and function are changed by the inflammatory mediators that are inevitably released during preparation of brain slices (Takano et al. 2014; Ben Haim et al. 2015). Furthermore, in humans, the P2X5 subunit is truncated and retained within the endoplasmic reticulum (ER) (Kotnis et al. 2010). In cultured rodent astrocytes, P2Y1 and P2Y2 receptors, and to a lesser extent P2Y4 receptors, can also initiate ATP‐evoked Ca2+ signals (Verkhratsky et al. 2009). Hence, even in rodent astrocytes, the identities of the receptors that mediate ATP‐evoked Ca2+ signals are unresolved (Fumagalli et al. 2003; Verkhratsky et al. 2009).
In human astrocytes, the receptors that mediate ATP‐evoked Ca2+ signals are unknown. There has been no complete or quantitative analysis of mRNA expression levels for P2 receptors, although in cultures of human astrocytes mRNAs for P2Y1, P2Y2, P2Y4, P2X4, P2X5 and P2X7 receptors were detected (John et al. 2001; Narcisse et al. 2005; Hashioka et al. 2014). The only P2 receptor protein shown to be expressed is the P2X7 subunit, but in healthy astrocytes it was exclusively expressed on intracellular membranes, and in brain sections it was detected only in diseased tissue (Narcisse et al. 2005). In the only analyses of Ca2+ signals, 2‐MeS‐ATP and UTP evoked Ca2+ signals in cultured human astrocytes, but the receptor pharmacology was not further defined (John et al. 1999). In another study, an agonist of P2X receptors (BzATP), which also stimulates P2Y11 receptors (Communi et al. 1999), evoked a convincing Ca2+ signal only in reactive astrocytes (Narcisse et al. 2005). Hence, the common but unverified, assumption that ATP‐evoked Ca2+ signals in healthy human astrocytes are largely mediated by P2X receptors requires further investigation (Burnstock 2008; Illes et al. 2012).
In this study, we define the receptors responsible for ATP‐evoked Ca2+ signals in human astrocytes. We used cultures of foetal cortical human astrocytes to quantify mRNA expression for all P2 receptors, and we identified the P2 receptors through which ATP evokes Ca2+ signals. There are limitations to the use of cultured cells, but for human brain tissue, it provides the only practicable means of directly measuring cytosolic Ca2+ signals. Furthermore, it avoids the persistent astrogliosis caused by the traumatic injury and hypoxia inherent in preparing brain slices, which has been shown to affect expression of P2 receptors (Narcisse et al. 2005; Takano et al. 2014). Our results show that cultured human foetal astrocytes express mRNA for several P2X and P2Y receptors, but the Ca2+ signals evoked by ATP are entirely mediated by P2Y1 and P2Y2 receptors.
Materials and methods
Materials
Fura‐2 AM was from Invitrogen (Paisley, UK). Fluo‐8 AM was from Stratech Scientific (Suffolk, UK). MRS2365 ((N)‐methanocarba‐2‐methylthio‐adenosine‐5′‐diphosphate), MRS2179 (2′‐deoxy‐N 6‐methyladenosine 3′,5′‐bisphosphate), U73122 (1‐[6‐[[(17β)‐3‐methoxyestra‐1,3,5(10)‐trien‐17‐yl]amino]hexyl]‐1H‐pyrrole‐2,5‐dione), U73343 (1‐[6‐[[(17β)‐3‐methoxyestra‐1,3,5(10)‐trien‐17‐yl]amino]hexyl]‐2,5‐pyrrolidinedione), SKF96365 (1‐[2‐(4‐methoxyphenyl)‐2‐[3‐(4‐methoxyphenyl)propoxy]ethyl‐1H‐imidazole hydrochloride) and BTP‐2 (N‐[4‐[3,5‐bis(trifluoromethyl)‐1H‐pyrazol‐1‐yl]phenyl]‐4‐methyl‐1,2,3‐thiadiazole‐5‐carboxamide) were from Tocris (Bristol, UK). Fibronectin was from Merck Millipore (Watford, UK). Bovine serum albumin was from Europa Bioproducts Ltd (Cambridge, UK). 2′‐amino‐UTP (2′‐amino‐2′‐deoxyuridine‐5′‐triphosphate) and 2′‐thio‐UTP (2′‐thio‐2′‐deoxyuridine‐5′‐triphosphate) were from Trilink Biotechnologies (San Diego, CA, USA). Thapsigargin was from Bio Techne (Abingdon, UK). qPCR primers and related reagents were from Qiagen (Crawley, West Sussex, UK). All other reagents, including 2‐aminoethoxydiphenyl borate (2‐APB), ADP, ATP, probenecid, UDP and UTP were from Sigma‐Aldrich (Gillingham, UK). The properties of the drugs used are summarized in Table S1.
Cell culture
Human astrocytes isolated from foetal cortex were supplied as frozen cells that had not been passaged (catalogue number CC‐2565, Lonza, Slough, UK). The cells were confirmed, by Lonza, to be free of infection with HIV‐1 and hepatitis B and C, and we confirmed that they were free of mycoplasma. Astrocytes were grown at 37°C in humidified air containing 5% CO2, using astrocyte growth medium (Lonza) supplemented with 3% foetal bovine serum. Astrocyte growth medium includes human epidermal growth factor, insulin, ascorbic acid, gentamycin and l‐glutamine. Cells were passaged using trypsin, according to the supplier's instructions, when they reached 70–80% confluence. Cells were used for up to four passages after receipt, during which they maintained an astrocyte‐like morphology and expressed glial fibrillary acidic protein, assessed by qPCR and immunostaining.
Quantitative PCR
For quantitative PCR (qPCR), confluent cultures of astrocytes in 24‐well plates were lysed (200 μL cell processing buffer/well), mRNA was then isolated from the lysate (4 μL) and cDNA was synthesized using Fastlane cell cDNA kit (Qiagen,Crawley, UK). The cDNA was diluted fivefold with RNAase‐free water. Incubations for qPCR included Rotor‐Gene SYBR™ Green PCR master mix (10 μL), cDNA (5 μL), Quantitect primer assay (2 μL, Table S2) and RNAase‐free water (3 μL). In negative controls, the primers were omitted during qPCR or the reverse‐transcriptase was omitted during cDNA synthesis. A Rotor‐Gene 6000 thermocycler (Qiagen) was used for qPCR with a denaturation step (95°C, 5 min), 40 amplification cycles (5 s at 95°C, 10 s at 60°C) and then a melting curve (70–95°C). Expression of mRNA relative to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was calculated from: Relative expression , where E is the amplification efficiency, calculated as 10m, where m is the average increase in fluorescence for four cycles after the cycle threshold CT for the indicated PCR product. The effectiveness of all primer pairs was verified using BioBank generic pooled cDNA (Primerdesign Ltd, Chandler's Ford UK). All primers included in this study amplified a single product from the BioBank pooled cDNA (Figure S1). The melting temperatures of all products amplified from cDNA from astrocytes were identical to the respective BioBank controls. Results are reported as means from cDNA samples independently obtained from three different cell cultures.
Measurements of [Ca2+]i in populations of astrocytes
Confluent cultures of astrocytes grown in fibronectin‐coated 96‐well plates (Greiner Bio‐One, Stonehouse, UK) were incubated at 20°C with fluo‐8 AM (4 μM) in HEPES‐buffered saline (HBS) containing probenecid (2.5 mM) (Di Virgilio et al. 1990). HBS had the following composition: 135 mM NaCl, 5.9 mM KCl, 1.2 mM MgCl2, 1.5 mM CaCl2, 11.5 mM glucose and 11.6 mM HEPES, pH 7.3. After 60 min, the cells were washed with HBS, incubated for 90 min in HBS containing probenecid (2.5 mM) to allow de‐esterification of the indicator, washed and then used immediately for experiments. All experiments were performed at 20°C, to avoid extrusion and intracellular compartmentalization of fluo‐8, in HBS without probenecid. Where indicated, Ca2+‐free HBS containing BAPTA (1,2‐bis(o‐aminophenoxy)ethane‐N,N,N′,N′‐tetraacetic acid, final concentration 2.5 mM) was added immediately before stimulation to reduce the free [Ca2+] of HBS to < 100 nM.
Fluorescence (excitation at 490 nm, emission at 520 nm) was recorded at 1.44‐s intervals using a FlexStation III fluorescence plate‐reader (MDS Analytical Technologies, Wokingham, UK), which allows automated fluid additions during the recording (Tovey et al. 2006). Fluorescence (F) was calibrated to intracellular free Ca2+ concentration ([Ca2+]i) from: , where KD is the equilibrium dissociation constant of fluo‐8 for Ca2+ (389 nM), Fmin and Fmax are the minimal and maximal fluorescence values determined after addition of Triton X‐100 (0.2%) in Ca2+‐free HBS with BAPTA (10 mM, Fmin) or ionomycin (10 μM) in normal HBS (Fmax).
Measurement of [Ca2+]i in single astrocytes
Almost confluent cultures of astrocytes grown on fibronectin‐coated eight‐well imaging slides (Thistle Scientific Ltd, Glasgow, UK) were loaded with fura‐2 by incubation with fura‐2 AM (2 μM) in HBS containing 2.5 mM probenecid (45 min, 20°C). After a further 45 min in the same medium without fura‐2 AM, the cells were used for experiments at 20°C in HBS without probenecid. Imaging was performed using an Olympus IX71 inverted fluorescence microscope with alternating excitation (340 nm and 380 nm) provided by a Xe‐arc lamp at 1‐s intervals. Emission was recorded at 510 nm using a Luca EMCCD camera (Andor Technology, Belfast, UK) and MetaFluor software (Molecular Devices, Sunnyvale, CA, USA). Background‐corrected ratios of F340/F380 fluorescence were used to determine whether ligands evoked increases in [Ca2+]i.
Statistical analysis
For each experiment, the concentration‐response relationship was fitted to a logistic equation (GraphPad Prism 5, La Jolla, CA, USA) from which the maximal amplitude and pEC50 values (−log of the half‐maximally effective drug concentration) were determined. All analyses, except when otherwise stated, show pooled results from cells from two donors (Lonza lot numbers: 0000289765 and 0000402839). Key results from these and a third donor (0000514417) are shown individually in Figure S2. Results are presented as means ± SEM of values from at least three independent experiments. Sample sizes (n) refer to independent experiments.
Results
ATP evokes Ca2+ release and Ca2+ entry in cultured human foetal astrocytes
ATP evoked a concentration‐dependent increase in [Ca2+]i (pEC50 = 5.94 ± 0.03, n = 8) in populations of cultured foetal human cortical astrocytes. Similar results and with similar sensitivities to ATP, but with Ca2+ signals of different amplitude, were observed in cells from three different donors (Figure S2A). Removal of extracellular Ca2+ affected neither the peak amplitude of the increase in [Ca2+]i nor its sensitivity to ATP (pEC50 = 5.64 ± 0.08, n = 5), but the sustained phase of the response was abolished (Fig. 1a and b). These results establish that release of Ca2+ from intracellular stores and Ca2+ entry across the plasma membrane contribute to the ATP‐evoked Ca2+ signals. U73122, an inhibitor of phospholipase C (Bleasdale et al. 1990), but not its inactive analogue (U73343), caused a concentration‐dependent inhibition of the ATP‐evoked Ca2+ signals (Fig. 1c). 2‐APB, an antagonist at IP3Rs (Saleem et al. 2014), also inhibited ATP‐evoked Ca2+ signals (Fig. 1d). Neither 2‐APB nor U73122 completely blocked the response to ATP, but the range of useable concentrations is limited by off‐target effects of the inhibitors (Grierson and Meldolesi 1995; Mogami et al. 1997; Peppiatt et al. 2003). These results demonstrate that ATP‐evoked Ca2+ signals are at least substantially dependent on stimulation of PLC and IP3‐evoked release of Ca2+ from intracellular stores.
Figure 1.
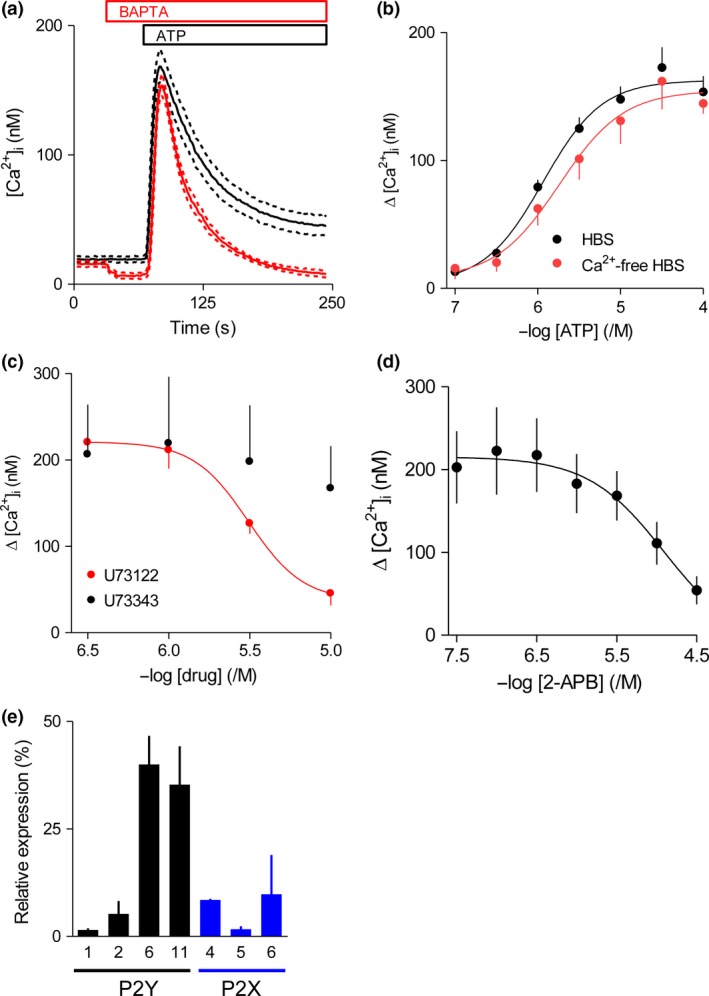
ATP stimulates Ca2+ release from intracellular stores and Ca2+ entry in cultured human foetal astrocytes. (a) Populations of fluo‐8‐loaded astrocytes were stimulated with ATP (100 μM) in HEPES‐buffered saline (HBS) (black; n = 8) or Ca2+‐free HBS (red; n = 5, the addition of BAPTA, final concentration 2.5 mM, to chelate extracellular Ca2+ is shown). Results show [Ca2+]i as means (solid lines) ± SEM (dashed lines). (b) Summary results (means ± SEM) show effects of the indicated concentrations of ATP on the peak increase in [Ca2+]i (Δ[Ca2+]i) in the presence (n = 8) or absence (n = 5) of extracellular Ca2+. (c) Effects of pre‐treatment (5 min) with the indicated concentrations of U73122 or U73343 in HBS on Δ[Ca2+]i evoked by ATP (100 μM). (d) Similar analysis of the effects of pre‐treatment (5 min) with 2‐2‐aminoethoxydiphenyl borate (APB) in HBS. Results (c and d) show means ± SEM, n = 3. For clarity, only a single error bar is shown in (b) and (c). (e) Expression of mRNA for P2 receptors was measured by qPCR relative to mRNA for glyceraldehyde 3‐phosphate dehydrogenase (GAPDH). Results (means ± SEM from three independent samples, each measured in duplicate) are expressed as percentages of all P2 receptor mRNA. There was no detectable expression of mRNA for the remaining P2Y (4, 12–14) or P2X (1‐3, 7) receptor subtypes, although the primers used were all shown to be effective (Figure S1).
We used qPCR with primers demonstrated to selectively amplify mRNA encoding each of the human P2Y and P2X receptors (Table S2) to quantify expression of these mRNAs in cultured foetal human cortical astrocytes. The results confirmed expression of mRNA for four of the eight subtypes of P2Y receptors (P2Y1, P2Y2, P2Y6 and P2Y11) and three of the seven subunits of P2X receptors (P2X4, P2X5 and P2X6) (Fig. 1e). There was no detectable expression of mRNA for the remaining P2Y or P2X receptors, despite the proven effectiveness of the primers used (Figure S1).
P2X receptors do not evoke Ca2+ signals
Since some P2Y receptors, but no P2X receptors, can stimulate PLC (Burnstock and Kennedy 2011), our results so far suggest a major (and perhaps exclusive) role for P2Y receptors in initiating ATP‐evoked Ca2+ signals in human astrocytes. This contrasts with the prominent role ascribed to P2X receptors in rodent astrocytes. We therefore assessed whether P2X receptors contribute to the Ca2+ signals evoked by ATP in human astrocytes.
We detected mRNA for P2X4, P2X5 and P2X6 receptor subunits in human astrocytes (Fig. 1e). P2X4 and P2X5, but not P2X6, subunits can form functional homo‐trimers (Torres et al. 1999). However, P2X6 subunits can form hetero‐trimers with P2X4 or P2X5 subunits, and the P2X4/6 structure has been shown to be functional (Le et al. 1998; Torres et al. 1999). Since our cultured astrocytes express mRNA for only three P2X receptor subunits, agonists that might otherwise inadequately distinguish between P2 receptors could be used to activate the candidate receptors (Table S1). Hence, astrocytes were stimulated in HBS with either α,β‐meATP, an agonist of human P2X4 and heteromeric P2X4/6 receptors (Le et al. 1998; Jones et al. 2000), or BzATP, an agonist of human P2X5 receptors (Bo et al. 2003). Neither α,β‐meATP nor BzATP, at concentrations more than sufficient to stimulate these P2X receptors (Le et al. 1998; Jones et al. 2000; Bo et al. 2003), evoked an increase in [Ca2+]i (Fig. 2a and b). In these experiments, the peak increases in [Ca2+]i (Δ[Ca2+]i) evoked by α,β‐meATP and BzATP were 5 ± 0 nM and 5 ± 3 nM respectively (n = 3); the parallel measurements of ATP‐evoked Δ[Ca2+]i were 132 ± 26 nM and 150 ± 16 nM. We avoided higher concentrations of α,β‐meATP (100 μM) and BzATP (300 μM) because they evoked Ca2+ signals in Ca2+‐free HBS (not shown).
Figure 2.
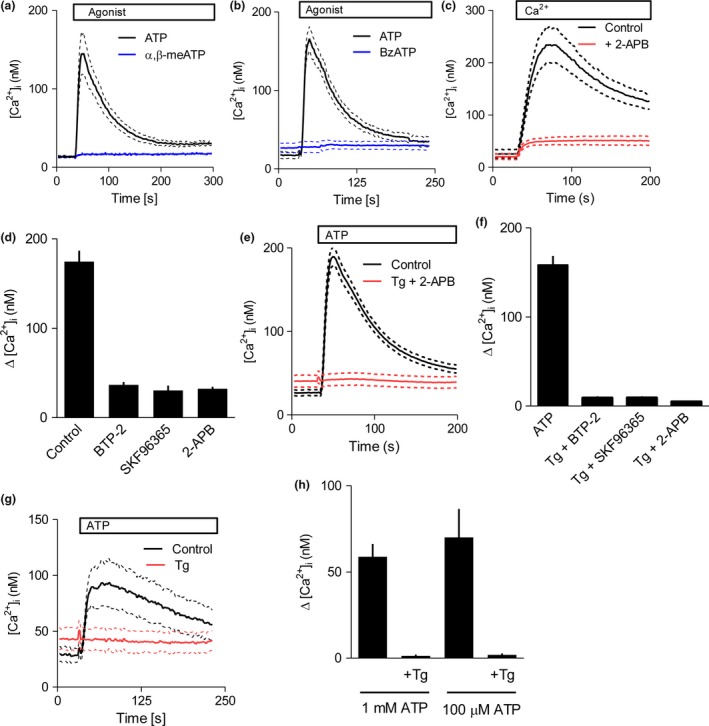
P2X receptors do not contribute to ATP‐evoked Ca2+ signals. (a and b) Populations of fluo‐8‐loaded astrocytes in HEPES‐buffered saline (HBS) were stimulated with ATP (100 μM, black), α,β‐meATP (30 μM, a; blue) or BzATP (100 μM, b; blue) as indicated. Results show [Ca2+]i as means (solid lines) ± SEM (dashed lines; n = 3). (c) Cells were incubated with thapsigargin (5 μM, 15 min) in Ca2+‐free HBS alone or with 2‐2‐aminoethoxydiphenyl borate (APB) (100 μM). Traces (in the same format as a, n ≥ 4) show [Ca2+]i after restoration of extracellular Ca2+ (2 mM). (d) Summary results (means ± SEM, n ≥ 4) show Δ[Ca2+]i evoked by restoration of extracellular Ca2+ to thapsigargin‐treated cells after pre‐treatment (15 min) with 2‐APB (100 μM), SKF96365 (10 μM) or BTP‐2 (10 μM). (e) Astrocytes in HBS were stimulated with ATP (100 μM) alone or after pre‐treatment with thapsigargin (5 μM, 15 min) to deplete intracellular Ca2+ stores and 2‐APB (100 μM, 15 min) to inhibit store‐operated Ca2+ entry (SOCE). Traces are in the same format as a; n ≥ 6. (f) Summary results (means ± SEM, n ≥ 4) show Δ[Ca2+]i evoked by ATP alone or after pre‐treatment with thapsigargin and the inhibitors shown (same concentrations as in d). (g) Ca2+ signals evoked by ATP in HBS (100 μM) alone or after pre‐treatment with thapsigargin (Tg, 5 μM, 15 min). Traces are in the same format as a; n = 3. (h) Summary results (means ± SEM, n = 3) show Δ[Ca2+]i evoked by ATP alone (100 or 1 mM) or after pre‐treatment with thapsigargin.
We next attempted to eliminate the Ca2+ signals evoked by P2Y receptors to unmask any possible contribution from P2X receptors. This required inhibition of both the Ca2+ release and Ca2+ entry components of the response evoked by P2Y receptors (Fig. 1a and b). Thapsigargin, which inhibits Ca2+ pumps in the ER, is commonly used to deplete the ER of Ca2+ and to thereby stimulate store‐operated Ca2+ entry (SOCE) (Parekh and Putney 2005). We confirmed that thapsigargin stimulated SOCE in human astrocytes (Fig. 2c). Pre‐treatment of astrocytes with three structurally unrelated inhibitors of SOCE, BTP‐2 (10 μM), SKF96365 (10 μM) and 2‐APB (100 μM) (Bootman et al. 2002; Liou et al. 2005; Ohga et al. 2008) almost abolished the SOCE evoked by thapsigargin (Fig. 2d). Although 2‐APB inhibits both IP3R and SOCE, its effects on thapsigargin‐evoked Ca2+ entry are probably due to it inhibiting formation of the STIM1 puncta that stimulate SOCE (DeHaven et al. 2008).
In astrocytes pre‐treated with thapsigargin to deplete intracellular Ca2+ stores and so prevent IP3‐evoked Ca2+ release, and with BTP‐2, SKF96365 or 2‐APB present to inhibit SOCE, a normally maximally effective concentration of ATP (100 μM) had no significant effect on [Ca2+]i (Fig. 2e and f). Similar results were observed in cells from all three donors (Figure S2B). These results confirm that the Ca2+ entry evoked by ATP is likely mediated by SOCE, and that there is no additional response to ATP mediated by P2X receptors.
To exclude any possible off‐target effects of the SOCE inhibitors on P2X receptors, we compared the effects of ATP in HBS on astrocytes with and without prior thapsigargin treatment. This experiment is practicable because the amplitude of the Ca2+ signal evoked by SOCE decays relatively quickly in the continued presence of extracellular Ca2+ (Fig. 2c), such that the small residual SOCE‐mediated Ca2+ signal detected after 15 min would not obscure a response to ATP. Under these conditions, addition of ATP (100 μM or 1 mM) to thapsigargin‐treated cells in normal HBS had no significant effect on [Ca2+]i (Fig. 2g and h). The lack of response to such high concentrations of ATP excludes a role for P2X receptors, including P2X7 receptors which have low affinity for ATP (Surprenant et al. 1996). These results demonstrate that P2X receptors make no detectable contribution to the Ca2+ signals evoked by ATP in cultured human cortical astrocytes, despite evidence that the cells express mRNA for three P2X receptor subunits (Fig. 1e).
An increase in [Ca2+]i has been reported to stimulate translocation of P2X4 receptors from intracellular membranes to the plasma membrane (Qureshi et al. 2007; Vacca et al. 2009). We therefore considered whether release of Ca2+ from intracellular stores might stimulate a similar translocation of P2X receptors in human astrocytes and thereby allow ATP to sequentially activate P2Y and then P2X receptors. However, when astrocytes were first stimulated with ADP to activate P2Y (but not P2X) receptors, there was the expected increase in [Ca2+]i, but subsequent addition of α,β‐meATP to stimulate P2X receptors (30 μM after 5 min) evoked no further increase in [Ca2+]i (Figure S3).
Collectively, these results demonstrate that the Ca2+ signals evoked by ATP in cultured human cortical astrocytes are entirely mediated by P2Y receptors with no detectable contribution from P2X receptors.
P2Y1 and P2Y2 receptors mediate ATP‐evoked Ca2+ signals
All four of the P2Y receptor subtypes for which mRNA was detected in human astrocytes (P2Y1, P2Y2, P2Y6 and P2Y11) are coupled to Gq/11 and can thereby stimulate PLC. We used ligands that distinguish between the subtypes for which mRNA was detected to resolve the contributions of different P2Y receptors to the ATP‐evoked Ca2+ signals (Table S1).
ADP is an agonist of P2Y1, but not of P2Y2 or P2Y11 receptors. ADP caused a concentration‐dependent increase in [Ca2+]i (pEC50 = 6.00 ± 0.11, n = 3) (Fig. 3a). Since ADP might also activate P2Y6 receptors (Communi et al. 1996), we also used MRS2365, a selective agonist of P2Y1 receptors (Table S1). MRS2365 evoked a concentration‐dependent increase in [Ca2+]i (pEC50 = 6.20 ± 0.19, n = 5) and the maximal amplitude of the response was similar to that evoked by ADP (Fig. 3b). UDP is a potent agonist of P2Y6 receptors, but not of P2Y1, P2Y2 or P2Y4 receptors (Table S1). UDP had no effect on [Ca2+]i (Figure S4A). Hence, P2Y1 receptors, but not P2Y6 receptors, contribute to the Ca2+ signals evoked by ATP.
Figure 3.
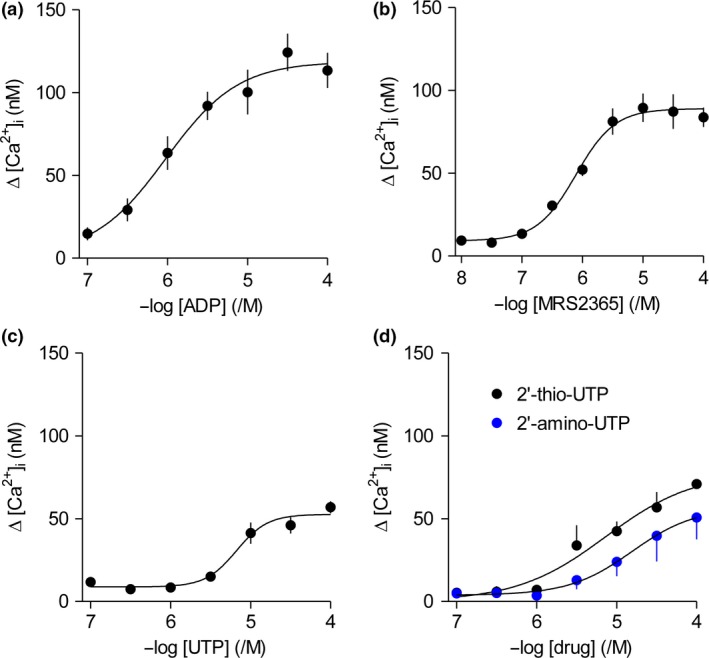
ATP‐evoked Ca2+ signals are mediated by P2Y1 and P2Y2 receptors. (a–d) Concentration‐dependent effects of the indicated agonists on Δ[Ca2+]i were determined in normal HEPES‐buffered saline (HBS): ADP (P2Y1 and P2Y11) (a, n = 5), MRS2365 (P2Y1) (b, n = 5), UTP (P2Y2 and P2Y6) (c, n = 5), 2’‐thio‐UTP and 2’‐amino‐UTP (P2Y2) (d, n = 3).
UTP is a potent agonist of P2Y2 and P2Y11 receptors, but not of P2Y1 receptors (White et al. 2003). UTP caused a concentration‐dependent increase in [Ca2+]i (pEC50 = 4.86 ± 0.18, n = 5) (Fig. 3c). The effect of UTP on [Ca2+]i was mimicked by two additional agonists of P2Y2 receptors: 2′‐amino‐UTP (pEC50 = 4.57 ± 0.22, n = 3) and 2′‐thio‐UTP (pEC50 = 4.87 ± 0.47, n = 3) (Fig. 3d), but not by the related analogue, 2′‐azido UTP, which is a selective agonist of P2Y4 receptors (Jacobson et al. 2006) (Figure S5). NF546, a selective agonist of P2Y11 receptors, had no effect on [Ca2+]i (Figure S4B). Hence, P2Y2 receptors also contribute to the ATP‐evoked Ca2+ signals. MRS2179 is a selective antagonist of P2Y1 receptors, and as expected, it caused a rightward shift of the concentration‐response relationship for the Ca2+ signals evoked by MRS2365, a selective agonist of P2Y1 receptors. In the presence of MRS2179 (5 μM), the ΔpEC50 value for MRS2365 was 0.64 ± 0.26 (where ΔpEC50 = ; mean ± SEM, n = 6) (Figure S6A). In keeping with the ability of ATP to evoke Ca2+ signals through both P2Y1 and P2Y2 receptors, MRS2179 (5 μM) caused a smaller shift in the ΔpEC50 value for the Ca2+ signals evoked by ATP (0.47 ± 011, n = 7) (Figure S6B). The shifts in pEC50 values caused by MRS2179 were statistically significant (unpaired Student's t‐test, p = 0.003 and 0.048 for ATP and MRS2365 respectively). We conclude that P2Y1 and P2Y2 receptors, but not P2Y6 or P2Y11 receptors, evoke Ca2+ signals in cultured foetal human astrocytes.
To determine whether activation of P2Y1 receptors (with ADP) and of P2Y2 receptors (with UTP) are entirely responsible for the Ca2+ signals evoked by ATP, we compared the maximal amplitudes of the responses evoked by the three stimuli in parallel measurements. The Δ[Ca2+]i evoked by ATP, ADP and UTP were 142 ± 5 nM, 88 ± 11 nM and 51 ± 13 nM (n = 3) respectively. Hence, the sum of the responses to ADP and UTP (139 ± 25 nM) was not significantly different from the response evoked by ATP (142 ± 5 nM). These results confirm that ATP evokes Ca2+ signals through P2Y1 and P2Y2 receptors, but they do not resolve whether the two receptors are expressed in different cells or whether both contribute to the responses in individual cells. We therefore examined the responses of single fura‐2‐loaded cells to ATP, UTP and ADP.
In these single‐cell analyses, 87 ± 6% of cells responded to ATP (101 cells, 4 independent fields), 65 ± 5% responded to ADP (502 cells, 13 fields) and 41 ± 6% responded to UTP (386 cells, 11 fields), suggesting that at least 22% of ATP‐responsive cells express both P2Y1 and P2Y2 receptors. Analyses of responses to sequential stimulation with ADP and UTP revealed that 59 ± 8% of the cells that responded to ADP then responded to UTP (204 cells, 7 fields), while 92 ± 6% of cells that responded to UTP responded to a subsequent challenge with ADP (152 cells, 5 fields), suggesting that about half of the cells responded to both stimuli. These results demonstrate that most cells respond to ATP and that many express both P2Y1 and P2Y2 receptors. We considered whether autocrine release of ATP might contribute to the sustained phase of the Ca2+ signal evoked by selective activation of P2Y receptors. This seems unlikely, since in cell populations the relative amplitudes of the initial and sustained phases were similar for cells stimulated with ATP to activate all P2Y receptors or with ADP to activate only P2Y1 receptors (Fig. 1a and S3). Furthermore, in our single‐cell analyses, none of the cells that failed to respond initially to selective activation of P2Y1 receptors (176 cells) or P2Y2 receptors (228 cells) responded during the next 3–6 min with a detectable increase in [Ca2+]i.
Discussion
We provide the first complete quantitative analysis of mRNA expression for P2 receptors in cultured foetal human cortical astrocytes, and a comprehensive pharmacological characterization of ATP‐evoked Ca2+ signals. We showed that mRNAs for four P2Y receptors (P2Y6 ~ P2Y11 > P2Y2 > P2Y1) and three P2X receptor subunits (P2X6 > P2X4 > P2X5) are expressed. There was no detectable mRNA for any of the remaining P2 receptors (Fig. 1e). The expression pattern is broadly consistent with previous studies of cultured human astrocytes from both adult (Hashioka et al. 2014) and foetal tissue (John et al. 2001; Narcisse et al. 2005), which examined mRNA for only seven of the fifteen P2 receptors, and detected mRNA for P2Y1, P2Y2, P2Y4, P2X4, P2X5 and P2X7 receptors. The notable differences are the absence of mRNA for P2Y4 and P2X7 receptors in our analyses, with the latter perhaps explained by the presence of fewer reactive astrocytes in our analysis (Narcisse et al. 2005). Neither we nor others have verified the relationship between mRNA and protein expression in human astrocytes because the P2 receptor‐selective antibodies generally lack specificity (Sim et al. 2004; Takano et al. 2014).
In keeping with many analyses of rodent astrocytes, ATP evoked an increase in [Ca2+]i in both confluent populations of human cultured foetal astrocytes and sub‐confluent single cells (Verkhratsky et al. 2009). In human astrocytes, the initial response to ATP was because of Ca2+ release from intracellular stores through IP3 receptors (Fig. 1a–d), but the sustained response required Ca2+ entry across the plasma membrane. The Ca2+ entry had pharmacological properties typical of SOCE (Fig. 2e and f). In most cells, receptors that stimulate PLC usually activate SOCE (Parekh and Putney 2005), and in rodent astrocytes P2Y receptors have been shown to evoke Ca2+ entry by stimulating PLC (Fumagalli et al. 2003), but SOCE evoked by P2Y receptors has not, to the best of our knowledge, been previously reported for human astrocytes. These results are not consistent with the prominent role ascribed to P2X receptors in rodent astrocytes. Since mRNAs for three P2X receptor subunits were expressed in human astrocytes, we looked more closely to determine whether there was any underlying contribution from P2X receptors to ATP‐evoked Ca2+ signals. ATP analogues that would be expected to stimulate human P2X receptors assembled from P2X4, P2X5 or P2X6 subunits (α,β‐meATP and BzATP) did not increase [Ca2+]i (Fig. 2a and b). Furthermore, under conditions where responses from IP3 receptors and SOCE were inhibited, there was no response to ATP (Fig. 2e and f). We confirmed that this lack of effect of ATP was not due to off‐target effects of the inhibitors used to block SOCE (Fig. 2g and h). Hence, whether assessed using ATP analogues selective for P2X receptors or ATP itself, there is no evidence that P2X receptors evoke Ca2+ signals in cultured human foetal astrocytes. Finally, we considered whether the IP3‐evoked Ca2+ signal might stimulate translocation of intracellular P2X4 receptors to the plasma membrane (Qureshi et al. 2007; Vacca et al. 2009), but we found no evidence to suggest that Ca2+ release and SOCE unmasked a response to P2X receptors (Figure S3).
The only published argument suggesting a role for P2X receptors in Ca2+ signalling in normal human astrocytes derives from their expression of mRNA for some P2X receptor subunits (John et al. 2001; Narcisse et al. 2005; Hashioka et al. 2014). Our results demonstrate that although cultured foetal cortical human astrocytes express mRNA for some P2X receptor subunits (Fig. 1e), P2X receptors do not contribute to the Ca2+ signals evoked by ATP (Fig. 2). Instead, we have shown that two of the four P2Y receptor subtypes for which mRNA was detected, P2Y1 and P2Y2 receptors, are entirely responsible for ATP‐evoked Ca2+ signals (Fig. 3 and Figure S2). Our conclusion is consistent with a previous report in which two non‐selective analogues, UTP and 2‐MeS‐ATP, which would together activate P2Y1 and P2Y2 receptors, evoked Ca2+ signals in human astrocytes (John et al. 1999).
Our analyses of mRNA for P2X receptors were not predictive for expression of functional plasma membrane receptors. Others have also noted expression of mRNA for P2 receptors for which there was no corresponding functional response (Fumagalli et al. 2003). For P2X5 subunits, a likely explanation is that the human protein is truncated and retained in the ER, where it may also trap other P2X subunits with which it can oligomerize (P2X4 and P2X6) (Torres et al. 1999; Kotnis et al. 2010). For P2Y receptors too, the most abundant mRNAs (for P2Y6 and P2Y11) were not associated with expression of functional P2Y receptors. In rodents too, there is no functional response to P2Y6 receptors, although their mRNA is expressed (Fumagalli et al. 2003). These observations are relevant because mRNA expression in astrocytes has often been used to infer the likely identity of the receptors that mediate ATP‐evoked Ca2+ signals (Verkhratsky et al. 2009).
We conclude that in cultured foetal cortical human astrocytes, ATP evokes Ca2+ signals that are entirely mediated by P2Y1 and P2Y2 receptors, each of which stimulates PLC and thereby IP3‐evoked Ca2+ release and SOCE. Many astrocytes express both of these receptors, but some express only one or the other. We have not further explored this heterogeneity. Although mRNA for P2X receptor subunits is expressed, P2X receptors do not contribute to ATP‐evoked Ca2+ signals.
Supporting information
Figure S1. Melting curves for qPCR analyses of the expression of purinoreceptor subtypes.
Figure S2. ATP evokes Ca2+ signals through P2Y receptors in astrocytes from three donors.
Figure S3. Stimulation of P2Y receptors does not cause translocation of functional P2X receptors to the plasma membrane.
Figure S4. Neither P2Y6 nor P2Y11 receptors evoke Ca2+ signals in cultured human foetal astrocytes.
Figure S5. 2′‐azido‐UTP does not evoke Ca2+ signals.
Figure S6. Effects of MRS2179, a selective antagonist of P2Y1 receptors, on the Ca2+ signals evoked by ATP and MRS2365.
Table S1. Properties of the drugs used.
Table S2. Primers used for qPCR analyses.
Table S3. Ca2+ signals evoked by P2Y‐selective agonists in cultured human foetal astrocytes.
Acknowledgements and conflict of interest disclosure
The authors have no conflict of interest related to this research. This work was supported by the Wellcome Trust (101844) and a European Union Horizon 2020 Marie Sklodowska‐Curie Individual European Fellowship to M.S.M. (658386).
All experiments were conducted in compliance with local regulations of the University of Cambridge.
References
- Alexander S. P., Mathie A. and Peters J. A. (2011) Guide to receptors and channels (GRAC), 5th edition. Br. J. Pharmacol. 164(Suppl 1), S1–S324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Haim L., Carrillo‐de Sauvage M. A., Ceyzeriat K. and Escartin C. (2015) Elusive roles for reactive astrocytes in neurodegenerative diseases. Front. Cell. Neurosci. 9, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleasdale J. E., Thakur N. R., Gremban R. S., Bundy G. L., Fitzpatrick F. A., Smith R. J. and Bunting S. (1990) Selective inhibition of receptor‐coupled phospholipase C‐dependent processes in human platelets and polymorphonuclear neutrophils. J. Pharmacol. Exp. Ther. 255, 756–768. [PubMed] [Google Scholar]
- Bo X., Jiang L. H., Wilson H. L., Kim M., Burnstock G., Surprenant A. and North R. A. (2003) Pharmacological and biophysical properties of the human P2X5 receptor. Mol. Pharmacol. 63, 1407–1416. [DOI] [PubMed] [Google Scholar]
- Bootman M. D., Collins T. J., Mackenzie L., Roderick H. L., Berridge M. J. and Peppiatt C. M. (2002) 2‐aminoethoxydiphenyl borate (2‐APB) is a reliable blocker of store‐operated Ca2+ entry but an inconsistent inhibitor of InsP3‐induced Ca2+ release. FASEB J. 16, 1145–1150. [DOI] [PubMed] [Google Scholar]
- Burnstock G. (2008) Purinergic signalling and disorders of the central nervous system. Nat. Rev. Drug Discov. 7, 575–590. [DOI] [PubMed] [Google Scholar]
- Burnstock G. and Kennedy C. (2011) P2X receptors in health and disease. Adv. Pharmacol. 61, 333–372. [DOI] [PubMed] [Google Scholar]
- Communi D., Parmentier M. and Boeynaems J. M. (1996) Cloning, functional expression and tissue distribution of the human P2Y6 receptor. Biochem. Biophys. Res. Commun. 222, 303–308. [DOI] [PubMed] [Google Scholar]
- Communi D., Robaye B. and Boeynaems J. M. (1999) Pharmacological characterization of the human P2Y11 receptor. Br. J. Pharmacol. 128, 1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHaven W. I., Smyth J. T., Boyles R. R., Bird G. S. and Putney J. W., Jr (2008) Complex actions of 2‐aminoethyldiphenyl borate on store‐operated calcium entry. J. Biol. Chem. 283, 19265–19273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delekate A., Fuchtemeier M., Schumacher T., Ulbrich C., Foddis M. and Petzold G. C. (2014) Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer's disease mouse model. Nat. Commun. 5, 5422. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Steinberg T. H. and Silverstein S. C. (1990) Inhibition of Fura‐2 sequestration and secretion with organic anion transport inhibitors. Cell Calcium 11, 57–62. [DOI] [PubMed] [Google Scholar]
- Duan S., Anderson C. M., Keung E. C., Chen Y. and Swanson R. A. (2003) P2X7 receptor‐mediated release of excitatory amino acids from astrocytes. J. Neurosci. 23, 1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke H., Krugel U., Schmidt R., Grosche J., Reichenbach A. and Illes P. (2001) P2 receptor‐types involved in astrogliosis in vivo. Br. J. Pharmacol. 134, 1180–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli M., Brambilla R., D'Ambrosi N., Volonte C., Matteoli M., Verderio C. and Abbracchio M. P. (2003) Nucleotide‐mediated calcium signaling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia 43, 218–230. [DOI] [PubMed] [Google Scholar]
- Grierson J. P. and Meldolesi J. (1995) Shear stress‐induced [Ca2+]i transients and oscillations in mouse fibroblasts are mediated by endogenously released ATP. J. Biol. Chem. 270, 4451–4456. [DOI] [PubMed] [Google Scholar]
- Hashioka S., Wang Y. F., Little J. P., Choi H. B., Klegeris A., McGeer P. L. and McLarnon J. G. (2014) Purinergic responses of calcium‐dependent signaling pathways in cultured adult human astrocytes. BMC Neurosci. 15, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon P. G. (2001) GLIA: listening and talking to the synapse. Nat. Rev. Neurosci. 2, 185–193. [DOI] [PubMed] [Google Scholar]
- Illes P., Verkhratsky A., Burnstock G. and Franke H. (2012) P2X receptors and their roles in astroglia in the central and peripheral nervous system. Neuroscientist 18, 422–438. [DOI] [PubMed] [Google Scholar]
- Jacobson K. A. and Muller C. E. (2016) Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacol. 104, 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K. A., Costanzi S., Ivanov A. A., Tchilibon S., Besada P., Gao Z. G., Maddileti S. and Harden T. K. (2006) Structure activity and molecular modeling analyses of ribose‐ and base‐modified uridine 5’‐triphosphate analogues at the human P2Y2 and P2Y4 receptors. Biochem. Pharmacol. 71, 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John G. R., Scemes E., Suadicani S. O., Liu J. S., Charles P. C., Lee S. C., Spray D. C. and Brosnan C. F. (1999) IL‐1β differentially regulates calcium wave propagation between primary human fetal astrocytes via pathways involving P2 receptors and gap junction channels. Proc. Natl Acad. Sci. USA 96, 11613–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John G. R., Simpson J. E., Woodroofe M. N., Lee S. C. and Brosnan C. F. (2001) Extracellular nucleotides differentially regulate interleukin‐1β signaling in primary human astrocytes: implications for inflammatory gene expression. J. Neurosci. 21, 4134–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. A., Chessell I. P., Simon J., Barnard E. A., Miller K. J., Michel A. D. and Humphrey P. P. (2000) Functional characterization of the P2X4 receptor orthologues. Br. J. Pharmacol. 129, 388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotnis S., Bingham B., Vasilyev D. V. et al (2010) Genetic and functional analysis of human P2X5 reveals a distinct pattern of exon 10 polymorphism with predominant expression of the nonfunctional receptor isoform. Mol. Pharmacol. 77, 953–960. [DOI] [PubMed] [Google Scholar]
- Lalo U., Pankratov Y., Wichert S. P., Rossner M. J., North R. A., Kirchhoff F. and Verkhratsky A. (2008) P2X1 and P2X5 subunits form the functional P2X receptor in mouse cortical astrocytes. J. Neurosci. 28, 5473–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U., Verkhratsky A. and Pankratov Y. (2011) Ionotropic ATP receptors in neuronal‐glial communication. Sem. Cell Dev. Biol. 22, 220–228. [DOI] [PubMed] [Google Scholar]
- Lammer A., Gunther A., Beck A., Krugel U., Kittner H., Schneider D., Illes P. and Franke H. (2006) Neuroprotective effects of the P2 receptor antagonist PPADS on focal cerebral ischaemia‐induced injury in rats. Eur. J. Neurosci. 23, 2824–2828. [DOI] [PubMed] [Google Scholar]
- Le K. T., Babinski K. and Seguela P. (1998) Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. J. Neurosci. 18, 7152–7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J., Kim M. L., Heo W. D., Jones J. T., Myers J. W., Ferrell J. E., Jr and Meyer T. (2005) STIM is a Ca2+ sensor essential for Ca2+‐store‐depletion‐triggered Ca2+ influx. Curr. Biol. 15, 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogami H., Lloyd Mills C. and Gallacher D. V. (1997) Phospholipase C inhibitor, U73122, releases intracellular Ca2+, potentiates Ins(1,4,5)P3‐mediated Ca2+ release and directly activates ion channels in mouse pancreatic acinar cells. Biochem. J. 324, 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narcisse L., Scemes E., Zhao Y., Lee S. C. and Brosnan C. F. (2005) The cytokine IL‐1β transiently enhances P2X7 receptor expression and function in human astrocytes. Glia 49, 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicke A., Baumert H. G., Rettinger J., Eichele A., Lambrecht G., Mutschler E. and Schmalzing G. (1998) P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand‐gated ion channels. EMBO J. 17, 3016–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohga K., Takezawa R., Arakida Y., Shimizu Y. and Ishikawa J. (2008) Characterization of YM‐58483/BTP2, a novel store‐operated Ca2+ entry blocker, on T cell‐mediated immune responses in vivo. Int. Immunopharmacol. 8, 1787–1792. [DOI] [PubMed] [Google Scholar]
- Oliveira J. F., Riedel T., Leichsenring A., Heine C., Franke H., Krugel U., Norenberg W. and Illes P. (2011) Rodent cortical astroglia express in situ functional P2X7 receptors sensing pathologically high ATP concentrations. Cereb. Cortex 21, 806–820. [DOI] [PubMed] [Google Scholar]
- Parekh A. B. and Putney J. W. (2005) Store‐operated calcium channels. Physiol. Rev. 85, 757–810. [DOI] [PubMed] [Google Scholar]
- Pascual O., Casper K. B., Kubera C. et al (2005) Astrocytic purinergic signaling coordinates synaptic networks. Science 310, 113–116. [DOI] [PubMed] [Google Scholar]
- Peppiatt C. M., Collins T. J., Mackenzie L., Conway S. J., Holmes A. B., Bootman M. D., Berridge M. J., Seo J. T. and Roderick H. L. (2003) 2‐Aminoethoxydiphenyl borate (2‐APB) antagonises inositol 1,4,5‐trisphosphate‐induced calcium release, inhibits calcium pumps and has a use‐dependent and slowly reversible action on store‐operated calcium entry channels. Cell Calcium 34, 97–108. [DOI] [PubMed] [Google Scholar]
- Qureshi O. S., Paramasivam A., Yu J. C. and Murrell‐Lagnado R. D. (2007) Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J. Cell Sci. 120, 3838–3849. [DOI] [PubMed] [Google Scholar]
- Saleem H., Tovey S. C., Molinski T. F. and Taylor C. W. (2014) Interactions of antagonists with subtypes of inositol 1,4,5‐trisphosphate (IP3) receptor. Br. J. Pharmacol. 171, 3298–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim J. A., Young M. T., Sung H. Y., North R. A. and Surprenant A. (2004) Reanalysis of P2X7 receptor expression in rodent brain. J. Neurosci. 24, 6307–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F., Garcia‐Guzman M., Karschin C. and Stuhmer W. (1996) Cloning and tissue distribution of a novel P2X receptor from rat brain. Biochem. Biophys. Res. Commun. 223, 456–460. [DOI] [PubMed] [Google Scholar]
- Surprenant A., Rassendren F., Kawashima E., North R. A. and Buell G. (1996) The cytolytic P2X receptor for extracellular ATP identified as a P2Z receptor (P2X7). Science 272, 735–738. [DOI] [PubMed] [Google Scholar]
- Takano T., He W., Han X. et al (2014) Rapid manifestation of reactive astrogliosis in acute hippocampal brain slices. Glia 62, 78–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres G. E., Egan T. M. and Voigt M. M. (1999) Hetero‐oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners. J. Biol. Chem. 274, 6653–6659. [DOI] [PubMed] [Google Scholar]
- Tovey S. C., Sun Y. and Taylor C. W. (2006) Rapid functional assays of intracellular Ca2+ channels. Nat. Prot. 1, 259–263. [DOI] [PubMed] [Google Scholar]
- Vacca F., Giustizieri M., Ciotti M. T., Mercuri N. B. and Volonte C. (2009) Rapid constitutive and ligand‐activated endocytic trafficking of P2X receptor. J. Neurochem. 109, 1031–1041. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. and Butt A. (2013) Glial Physiology and Pathophysiology. Wiley‐Blackwell, Oxford. [Google Scholar]
- Verkhratsky A., Krishtal O. A. and Burnstock G. (2009) Purinoceptors on neuroglia. Mol. Neurobiol. 39, 190–208. [DOI] [PubMed] [Google Scholar]
- White P. J., Webb T. E. and Boarder M. R. (2003) Characterization of a Ca2+ response to both UTP and ATP at human P2Y11 receptors: evidence for agonist‐specific signaling. Mol. Pharmacol. 63, 1356–1363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Melting curves for qPCR analyses of the expression of purinoreceptor subtypes.
Figure S2. ATP evokes Ca2+ signals through P2Y receptors in astrocytes from three donors.
Figure S3. Stimulation of P2Y receptors does not cause translocation of functional P2X receptors to the plasma membrane.
Figure S4. Neither P2Y6 nor P2Y11 receptors evoke Ca2+ signals in cultured human foetal astrocytes.
Figure S5. 2′‐azido‐UTP does not evoke Ca2+ signals.
Figure S6. Effects of MRS2179, a selective antagonist of P2Y1 receptors, on the Ca2+ signals evoked by ATP and MRS2365.
Table S1. Properties of the drugs used.
Table S2. Primers used for qPCR analyses.
Table S3. Ca2+ signals evoked by P2Y‐selective agonists in cultured human foetal astrocytes.


