ABSTRACT
Nocardioides sp. strain PD653 was the first identified aerobic bacterium capable of mineralizing hexachlorobenzene (HCB). In this study, strain PD653-B2, which was unexpectedly isolated from a subculture of strain PD653, was found to lack the ability to transform HCB or pentachloronitrobenzene into pentachlorophenol. Comparative genome analysis of the two strains revealed that genetic rearrangement had occurred in strain PD653-B2, with a genomic region present in strain PD653 being deleted. In silico analysis allowed three open reading frames within this region to be identified as candidate genes involved in HCB dechlorination. Assays using recombinant Escherichia coli cells revealed that an operon is responsible for both oxidative HCB dechlorination and pentachloronitrobenzene denitration. The metabolite pentachlorophenol was detected in the cultures produced in the E. coli assays. Significantly less HCB-degrading activity occurred in assays under oxygen-limited conditions ([O2] < 0.5 mg liter−1) than under aerobic assays, suggesting that monooxygenase is involved in the reaction. In this operon, hcbA1 was found to encode a monooxygenase involved in HCB dechlorination. This monooxygenase may form a complex with the flavin reductase encoded by hcbA3, increasing the HCB-degrading activity of PD653.
IMPORTANCE The organochlorine fungicide HCB is widely distributed in the environment. Bioremediation can effectively remove HCB from contaminated sites, but HCB-degrading microorganisms have been isolated in few studies and the genes involved in HCB degradation have not been identified. In this study, possible genes involved in the initial step of the mineralization of HCB by Nocardioides sp. strain PD653 were identified. The results improve our understanding of the protein families involved in the dechlorination of HCB to give pentachlorophenol.
KEYWORDS: HCB, Nocardioides sp. strain PD653, aerobic dechlorination, monooxygenase
INTRODUCTION
A wide range of halogenated organic compounds have been used in different applications. Hexachlorobenzene (C6Cl6; HCB) is an organochlorine fungicide that has been used worldwide since the 1940s (1). The use of HCB was discontinued in many countries in the 1970s because of its toxicity and environmental persistence, and it was classed as a persistent organic pollutant at the Stockholm Convention in 2001. However, HCB remains a widely distributed environmental contaminant.
Bioremediation is a microorganism-based approach to remediating contaminated sites. Bioremediation is expected to be an effective way of removing pollutants, including persistent organic pollutants, from contaminated environments. However, heavily halogenated organic compounds are less biodegradable than are many other organic compounds. Microorganisms capable of degrading HCB have been isolated and identified in few studies.
There have been many insights into the bacterium-driven reductive dehalogenation of HCB. Dehalococcoides sp. strain CBDB1 is one of the most extensively studied anaerobic bacteria capable of dechlorinating HCB (2, 3). Strain CBDB1 uses polychlorinated benzenes as growth-supporting electron acceptors, and dechlorinates HCB through organohalide respiration; the end products have been found to be 1,3,5-trichlorobenzene, 1,3-dichlorobenzene, and 1,4-dichlorobenzene (4). This strain also dechlorinates pentachlorobenzene (4), 1,2,3-trichlorobenzene, 1,2,4-trichlorobenzene, all of the tetrachlorobenzene isomers (5), and polychlorinated phenols (6). The chlorobenzene reductive dehalogenase gene cbrA was recently identified (7). Strain CBDB1 has been studied biochemically, and the complete genome has been sequenced and 32 reductive dehalogenase homologs identified (8). Sequencing Dehalococcoides genomes will provide additional insights into polychlorinated benzene biodegradation under anaerobic conditions.
The biodegradation of HCB under strictly aerobic conditions has been described in few publications. To the best of our knowledge, only a genetically engineered mutant of CYP101 (P450cam) has been found to aerobically dehalogenate HCB to give pentachlorophenol (PCP) (9). The reaction is catalyzed by a two-component monooxygenase formed by a putidaredoxin reductase, PdR, that transfers an electron from the NADH cofactor to terminal oxygenase CYP101. Analysis of the three-dimensional structure of the mutant demonstrated the importance of the L244A mutation, which improves the catalytic site by making space for pentachlorobenzene and HCB chlorine atoms (10). An engineered Sphingobium chlorophenolicum ATCC 32723 with a gene cassette (camA+ camB+ camC) encoding the F87W/Y96F/L244A/V247L cytochrome P-450cam variant has been found to degrade HCB without toxic intermediates accumulating (11).
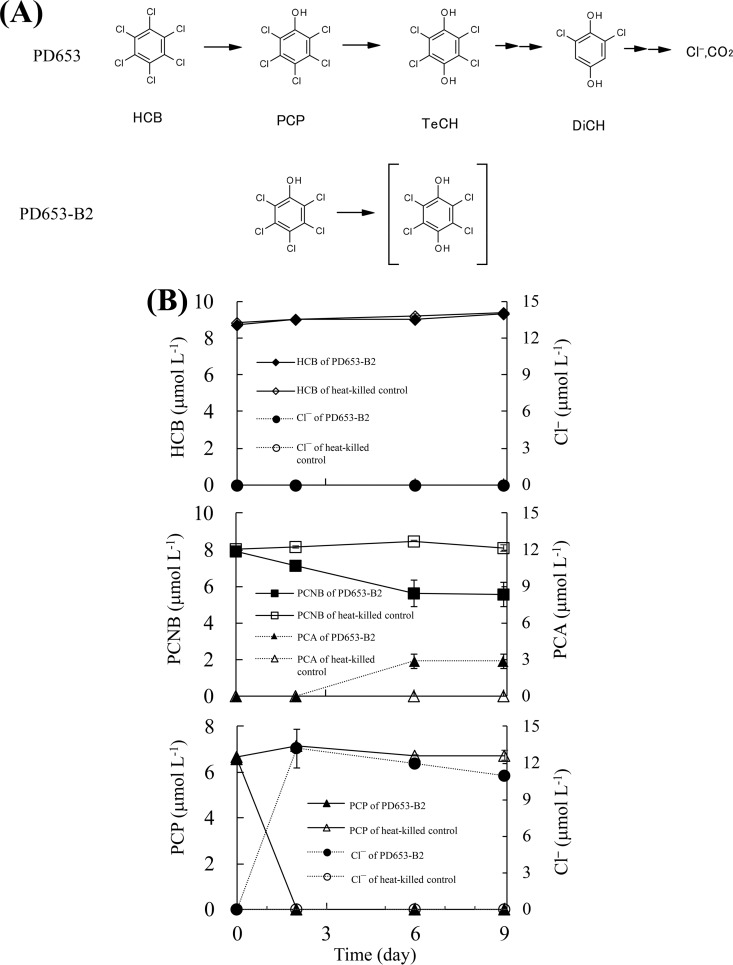
The metabolism of HCB by naturally occurring bacteria under aerobic conditions has been studied. Liu et al. found that the genera Azospirillum and Alcaligenes were dominant members of a HCB-using community isolated from contaminated soil (12). Nocardioides sp. strain PD653 was the first bacterium capable of mineralizing HCB under aerobic conditions that was identified (13). This strain was isolated from an upland soil contaminated with pentachloronitrobenzene (PCNB), and it was found to degrade both PCNB and HCB. The proposed HCB metabolic pathway in strain PD653 is shown in Fig. 1A. Strain PD653 mineralizes HCB via PCP. The intermediate metabolites tetrachlorohydroquinone (TeCH) and 2,6-dichlorohydroquinone (DiCH) have also been detected. Various bacteria degrade PCP (14–18). The genes encoding enzymes involved in PCP degradation by Sphingobium chlorophenolicum ATCC 39723 have been elucidated in detail (19). However, the gene encoding the dehalogenase involved in the first step of aerobic HCB dechlorination remains unidentified.
FIG 1.
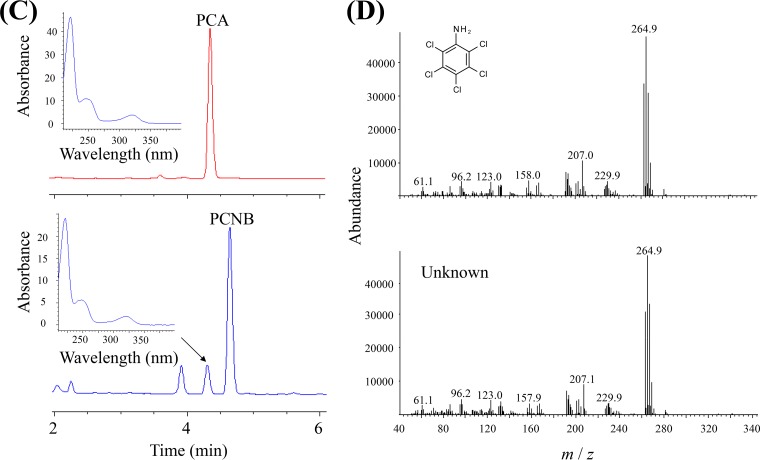
(A) Proposed pathway through which hexachlorobenzene and pentachlorophenol are degraded by Nocardioides sp. strains PD653 and PD653-B2. (B) Determination of catabolic ability of strain PD653-B2. Chloroaromatic compound degradation and Cl− generation were analyzed. Cl− and NO2− generated during the degradation of pentachloronitrobenzene (PCNB) are not shown. Each concentration shown is the mean (n = 3) with the standard deviation. (C) Detection and identification of PCNB-derived metabolites produced by strain PD653-B2. (Top) Authentic pentachloroaniline (PCA) standard. (Bottom) Solution of strain PD653-B2 on day 9. The UV spectrum of PCA and the metabolite indicated with an arrow are also shown. (D) Spectrum of PCA acquired by gas chromatography mass spectrometry. (Top) Authentic PCA standard. (Bottom) The unknown metabolite of pentachloronitrobenzene on day 9.
In this study, strain PD653-B2, which was found to have lost the ability to transform HCB into PCP, was isolated and characterized. Comparing the PD653 and PD653-B2 draft genomes allowed a genomic region that had been deleted in the derivative strain to be identified. This region was found to contain genes specific to the native PD653 strain. Heterologous expression in Escherichia coli revealed that the genes encode HCB-oxidative dehalogenase components.
RESULTS
Ability of strain PD653-B2 to catabolize HCB and PCP.
We isolated Nocardioides sp. strain PD653-B2 (GenBank accession number LC196157) from preculture medium when subcultures of strain PD653 (GenBank accession number DQ673618) were prepared. Strain PD653-B2 was unable to transform HCB into PCP.
The catabolic ability of strain PD653-B2 was determined by inoculating the strain into mineral salts medium (MM) containing HCB, PCNB, or PCP.
We found no HCB degradation or chloride ion accumulation. In contrast, 30.4% of the PCNB (initial concentration of 8 μmol liter−1) was degraded. No liberation of nitrite or chloride ions was found (data not shown), but an unidentified peak was found when the culture solution was analyzed by high-performance liquid chromatography (HPLC) on days 6 and 9. The retention time and mass spectrum of the unidentified peak were consistent with the compound being pentachloroaniline (PCA) (Fig. 1D). All of the PCP disappeared when the PD653-B2 strain was cultivated in a solution containing PCP at a concentration of 6.4 μmol liter−1 for 9 days, and the chloride concentration reached 10.9 μmol liter−1, suggesting approximately two chlorine atoms were released per PCP molecule through dechlorination (Fig. 1B).
Comparative genome analysis revealed a region conserved only in strain PD653.
We hypothesized that the derivative strain PD653-B2 lacked the ability to degrade HCB and PCNB because of a genetic rearrangement leading to the genomic region harboring the genes coding for enzymes involved in HCB and PCNB metabolism being deleted. This hypothesis was tested by fully sequencing the native and derivative strain genomes and comparing the results to identify regions that had been deleted from the derivative strain genome.
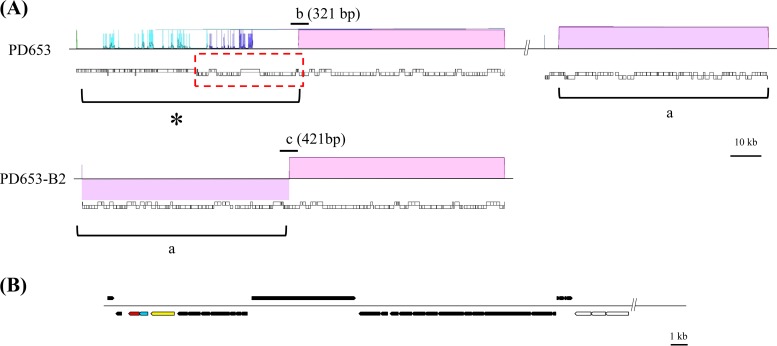
The estimated genome sizes (assembled sequence size) of the native PD653 strain and the derivative PD653-B2 strain were 5.08 Mb (87 contigs) and 4.99 Mb (81 contigs), respectively. Both genomes had G+C contents of 70.9%. The assembled strain PD653 and PD653-B2 data were subjected to DDBJ MiGAP, and 5,087 and 4,968 coding sequences, respectively, were identified. The draft genomes were compared using the Mauve tool (20), and a region of interest from positions 1 to 71,874 of contig 22 (GenBank accession number BDJG01000022) was found in which a deletion (indicated by an asterisk in Fig. 2A) consisting of 96 coding sequences had occurred in strain PD653-B2. The raw reads of strain PD653-B2 were mapped directly to contig 22 in strain PD653 to determine whether the region of interest was completely deleted from strain PD653-B2. The raw reads were completely assembled to strain PD653 contig 22, suggesting that the region of interest may not have been deleted but remained partially present in strain PD653-B2 (data not shown). This indicated that misassembly may have occurred, so we analyzed the sequences marked “b” and “c” in Fig. 2A. This confirmed that the sequences were consistent with those obtained from MiSeq, indicating that the genome structure shown in Fig. 2A was appropriate.
FIG 2.
Comparative analysis of the Nocardioides sp. strain PD653 and PD653-B2 genomes. (A) The region present only in strain PD653 (lower column) is indicated with an asterisk (*). The translocated segments are marked “a.” The regions amplified to confirm that misassembly had occurred are marked “b” and “c” (not to scale). (B) Structure of the locus marked with a red dotted line in panel A. The arrows indicate the sizes, locations, and directions of open reading frame (ORF) transcription. The genes located in the region marked with an asterisk (*) are marked with black arrows, and the genes conserved in both strains are marked with open arrows. The ORF1, ORF2, and ORF3 candidate genes are marked with yellow, azure, and magenta arrows, respectively.
In the region of interest, the annotations and structures indicated that the open reading frames (ORFs) ORF1, ORF2, and ORF3 were produced by the genes that most probably encode enzymes involved in the initial HCB metabolism step (Fig. 2B and Table 1). ORF1 was predicted to code coenzyme F420-dependent N5,N10-methylenetetrahydromethanopterin reductase and related flavin-dependent oxidoreductases (encoded by PD653_2189). ORF2 was predicted to code a flavoprotein (encoded by PD653_2188), and ORF3 was predicted to code a conserved protein/domain typically associated with flavoprotein oxygenases in the DIM6/NTAB family (encoded by PD653_2187). Multicomponent enzyme systems are often involved in aromatic compound hydroxylation, so we assumed that ORF1, ORF2, and ORF3 belong to a multicomponent monooxygenase system and selected them as candidate genes. An approximately 60-kb segment of the strain PD653-B2 genome, assigned as contig 64 in strain PD653 (marked “a” in Fig. 2A) (GenBank accession number BDJG01000064), was translocated to the region containing the candidate genes in strain PD653, suggesting that genome rearrangement had occurred in strain PD653-B2.
TABLE 1.
List of CDSs identified in the region present only in strain PD653a
| Locus tag | Gene length (bp) | Protein length (aa)b | Closest related protein | Bit score | E-value | Identity (%) | Accession no. | Organism |
|---|---|---|---|---|---|---|---|---|
| PD653_2118 | 483 | 161 | RusA family crossover junction endodeoxyribonuclease | 129 | 2.0e-35 | 51 | WP_050670494 | Luteipulveratus halotolerans |
| PD653_2119 | 771 | 257 | RusA family crossover junction endodeoxyribonuclease | 106 | 5.0e-24 | 38 | WP_064943947 | Mycobacterium sp. 852013-50091_SCH5140682 |
| PD653_2120 | 312 | 104 | Hypothetical protein TPY_2741 | 112 | 4.0e-29 | 53 | AEJ40901 | Sulfobacillus acidophilus TPY |
| PD653_2121 | 762 | 254 | Site-specific DNA methyltransferase | 325 | 9.0e-110 | 63 | WP_015297969 | Mycobacterium sp. JS623 |
| PD653_2122 | 300 | 100 | Lsr2 family protein | 48.5 | 5.0e-05 | 31 | WP_029146266 | Microbacterium luticocti |
| PD653_2123 | 363 | 121 | Hypothetical protein | 63.9 | 4.0e-11 | 41 | WP_028474062 | Nocardioides alkalitolerans |
| PD653_2124 | 585 | 195 | Adenine methyltransferase | 244 | 9.0e-80 | 71 | WP_074404865 | Mycobacterium fortuitum |
| PD653_2125 | 894 | 298 | Hypothetical protein | 205 | 1.0e-62 | 66 | WP_038679418 | Pimelobacter simplex |
| PD653_2126 | 1,680 | 560 | DnaB-like helicase N-terminal domain-containing protein | 672 | 0.0 | 67 | SFI85990 | Nocardioides psychrotolerans |
| PD653_2127 | 678 | 226 | Hypothetical protein SAMN05216561_11433 | 273 | 3.0e-90 | 64 | SFI86065 | Nocardioides psychrotolerans |
| PD653_2128 | 456 | 152 | DUF4326 domain-containing protein | 109 | 3.0e-28 | 47 | WP_067955981 | Mycobacterium sp. NAZ190054 |
| PD653_2129 | 309 | 103 | Protein of unknown function | 100 | 9.0e-24 | 59 | SDT36843 | Jiangella sp. DSM 45060 |
| PD653_2130 | 270 | 90 | GTPase ObgE | 35.4 | 6.1e+00 | 73 | WP_075711905 | Eubacterium sp. 68-3-10 |
| PD653_2131 | 306 | 102 | Hypothetical protein | 120 | 9.0e-34 | 61 | WP_055962014 | Aeromicrobium sp. Leaf291 |
| PD653_2132 | 204 | 68 | Alkaline phosphatase | 35 | 3.7e+00 | 50 | WP_011847724 | Shewanella baltica |
| PD653_2133 | 366 | 122 | Hypothetical protein SAMN05216561_11438 | 39.3 | 2.1e-01 | 39 | SFI86241 | Nocardioides psychrotolerans |
| PD653_2134 | 294 | 98 | Hypothetical protein | 45.1 | 5.0e-04 | 35 | WP_018793587 | Salinispora arenicola |
| PD653_2135 | 273 | 91 | Bifunctional (p)ppGpp synthetase/guanosine-3′,5′-bis(diphosphate) 3′-pyrophosphohydrolase | 38.1 | 6.6e-01 | 32 | WP_059580539 | Burkholderia vietnamiensis |
| PD653_2136 | 186 | 62 | Hypothetical protein SAMN05216561_11439 | 60.8 | 3.0e-10 | 57 | SFI86273 | Nocardioides psychrotolerans |
| PD653_2137 | 201 | 67 | ||||||
| PD653_2138 | 201 | 67 | Hypothetical protein TREMEDRAFT_64958 | 36.6 | 1.1e+00 | 43 | XP_007007165 | Tremella mesenterica DSM 1558 |
| PD653_2139 | 1,455 | 485 | Hypothetical protein SAMN05216561_11441 | 522 | 2.0e-180 | 57 | SFI86340 | Nocardioides psychrotolerans |
| PD653_2140 | 297 | 99 | Hypothetical protein | 66.2 | 3.0e-12 | 42 | WP_038676231 | Pimelobacter simplex |
| PD653_2141 | 156 | 52 | 3-Dehydroquinate synthase | 33.1 | 8.4e+00 | 60 | WP_036384679 | Muricauda sp. MAR_2010_75 |
| PD653_2142 | 1,482 | 494 | Hypothetical protein | 566 | 0.0 | 67 | WP_018585440 | Salinispora arenicola |
| PD653_2143 | 1,554 | 518 | Hypothetical protein SAMN05216561_11447 | 701 | 0.0 | 70 | SFI86582 | Nocardioides psychrotolerans |
| PD653_2144 | 198 | 66 | DUF2283 domain-containing protein | 56.2 | 1.0e-08 | 54 | WP_051681722 | Cellulomonas sp. HZM |
| PD653_2145 | 717 | 239 | HNH endonuclease | 114 | 4.0e-28 | 38 | WP_060921055 | Microbacterium paraoxydans |
| PD653_2146 | 531 | 177 | Hypothetical protein AHiyo6_03890 | 80.9 | 3.0e-15 | 61 | GAP53824 | Arthrobacter sp. Hiyo6 |
| PD653_2147 | 306 | 102 | ||||||
| PD653_2148 | 189 | 63 | Hypothetical protein F443_18763 | 32.7 | 6.4e+00 | 48 | ETI34804 | Phytophthora parasitica P1569 |
| PD653_2149 | 201 | 67 | Hypothetical protein | 55.1 | 1.0e-08 | 42 | WP_038679443 | Pimelobacter simplex |
| PD653_2150 | 1,068 | 356 | Phage Mu protein F-like protein | 477 | 2.0e-166 | 69 | SFI86619 | Nocardioides psychrotolerans |
| PD653_2151 | 1,254 | 418 | Hypothetical protein SAMN05216561_11449 | 468 | 9.0e-161 | 63 | SFI86659 | Nocardioides psychrotolerans |
| PD653_2152 | 408 | 136 | Uncharacterized conserved protein | 142 | 2.0e-41 | 59 | SFI86700 | Nocardioides psychrotolerans |
| PD653_2153 | 1,152 | 384 | Hypothetical protein SAMN05216561_11451 | 619 | 0.0 | 77 | SFI86736 | Nocardioides psychrotolerans |
| PD653_2154 | 420 | 140 | ||||||
| PD653_2155 | 333 | 111 | Hypothetical protein SAMN05216561_11456 | 105 | 4.0e-27 | 58 | SFI86932 | Nocardioides psychrotolerans |
| PD653_2156 | 435 | 145 | Hypothetical protein SAMN05216561_11453 | 87.8 | 2.0e-19 | 47 | SFI86816 | Nocardioides psychrotolerans |
| PD653_2157 | 372 | 124 | Phage protein, HK97 gp10 family | 141 | 5.0e-41 | 58 | SFI43596 | Nocardioides psychrotolerans |
| PD653_2158 | 495 | 165 | Hypothetical protein SAMN05216561_11455 | 159 | 3.0e-47 | 56 | SFI86892 | Nocardioides psychrotolerans |
| PD653_2159 | 231 | 77 | Hypothetical protein | 51.6 | 1.0e-06 | 49 | WP_038679463 | Pimelobacter simplex |
| PD653_2160 | 513 | 171 | Hypothetical protein SAMN05216561_11458 | 233 | 3.0e-76 | 66 | SFI87011 | Nocardioides psychrotolerans |
| PD653_2161 | 636 | 212 | Hypothetical protein SAMN05216561_11459 | 171 | 2.0e-50 | 47 | SFI87042 | Nocardioides psychrotolerans |
| PD653_2162 | 180 | 60 | Hypothetical protein SAMN05216561_11460 | 68.6 | 2.0e-13 | 58 | SFI87082 | Nocardioides psychrotolerans |
| PD653_2163 | 3,390 | 1,130 | Phage tail tape measure protein, TP901 family, core region | 415 | 2.0e-122 | 48 | SDD40725 | Auraticoccus monumenti |
| PD653_2164 | 1,812 | 604 | Hypothetical protein | 87 | 2.0e-14 | 24 | WP_038676804 | Pimelobacter simplex |
| PD653_2165 | 1,014 | 338 | Hypothetical protein | 107 | 1.0e-22 | 29 | WP_038676802 | Pimelobacter simplex |
| PD653_2166 | 315 | 105 | ||||||
| PD653_2167 | 321 | 107 | ||||||
| PD653_2168 | 201 | 67 | ||||||
| PD653_2169 | 342 | 114 | ||||||
| PD653_2170 | 363 | 121 | Hypothetical protein | 97.4 | 3.0e-24 | 67 | WP_056903920 | Nocardioides sp. Leaf307 |
| PD653_2171 | 732 | 244 | Hypothetical protein | 80.1 | 3.0e-14 | 31 | WP_052337072 | Nocardioides alkalitolerans |
| PD653_2172 | 678 | 226 | Hypothetical protein | 77.4 | 2.0e-13 | 31 | WP_027768815 | Streptomyces sp. CNQ865 |
| PD653_2173 | 222 | 74 | Hypothetical protein | 78.2 | 1.0e-17 | 53 | WP_026923285 | Glycomyces arizonensis |
| PD653_2174 | 192 | 64 | Hypothetical protein | 76.3 | 4.0e-17 | 60 | WP_067428397 | Nocardioides jensenii |
| PD653_2175 | 2,709 | 903 | Polysaccharide deacetylase | 103 | 2.0e-19 | 63 | ONH30622 | Frankia sp. M16386 |
| PD653_2176 | 402 | 134 | Hypothetical protein AUJ48_03015 | 108 | 7.0e-26 | 46 | OIN95531 | Deltaproteobacteria bacterium CG1_02_45_11 |
| PD653_2177 | 189 | 63 | Hypothetical protein | 58.5 | 2.0e-08 | 52 | WP_018018316 | Corynebacterium capitovis |
| PD653_2178 | 180 | 60 | Multispecies: hypothetical protein | 63.2 | 4.0e-12 | 50 | WP_056689176 | Nocardioides |
| PD653_2179 | 924 | 308 | Hypothetical protein | 342 | 1.0e-114 | 61 | WP_041545914 | Nocardioides sp. JS614 |
| PD653_2180 | 174 | 58 | Aryl-alcohol oxidase | 35.4 | 1.9e+00 | 34 | KDQ59695 | Jaapia argillacea MUCL 33604 |
| PD653_2181 | 1,068 | 356 | DUF4192 domain-containing protein | 413 | 2.0e-141 | 59 | WP_056689300 | Nocardioides sp. Root140 |
| PD653_2182 | 681 | 227 | Hypothetical protein | 317 | 6.0e-107 | 70 | WP_011751463 | Nocardioides sp. JS614 |
| PD653_2183 | 504 | 168 | Hypothetical protein | 129 | 4.0e-35 | 52 | WP_043806996 | Paenarthrobacter aurescens |
| PD653_2184 | 1,128 | 376 | Hypothetical protein SAMN05428985_101622 | 179 | 2.0e-49 | 40 | SDJ82508 | Nocardioides sp. YR527 |
| PD653_2185 | 369 | 123 | Hypothetical protein | 74.3 | 3.0e-15 | 45 | WP_056689157 | Nocardioides sp. Root140 |
| PD653_2186 | 336 | 112 | Hypothetical protein SAMN04487968_110141 | 35.8 | 7.3e+00 | 62 | SFC74443 | Nocardioides terrae |
| PD653_2187 | 621 | 207 | Flavin reductase | 212 | 4.0e-67 | 64 | WP_071047937 | Frankia sp. BMG5.36 |
| PD653_2188 | 507 | 169 | FMN reductase | 212 | 5.0e-68 | 68 | WP_029112870 | Mycobacterium sp. URHB0044 |
| PD653_2189 | 1,356 | 452 | LLM class flavin-dependent oxidoreductase | 538 | 0.0 | 58 | WP_061291802 | Azotobacter vinelandii |
| PD653_2190 | 585 | 195 | Multispecies: thermonuclease | 252 | 9.0e-83 | 70 | WP_011776883 | Micrococcaceae |
| PD653_2191 | 750 | 250 | Hypothetical protein SAMN05216467_2870 | 209 | 1.0e-64 | 55 | SFK31985 | Cellulomonas sp. KH9 |
| PD653_2192 | 531 | 177 | Hypothetical protein WILDE_87 | 60.5 | 3.0e-08 | 39 | ALY10869 | Arthrobacter phage Wilde |
| PD653_2193 | 1,149 | 383 | Hypothetical protein | 258 | 3.0e-81 | 70 | WP_011751639 | Nocardioides sp. JS614 |
| PD653_2194 | 351 | 117 | Hypothetical protein | 38.9 | 3.8e-01 | 49 | WP_041545943 | Nocardioides sp. JS614 |
| PD653_2195 | 324 | 108 | Hypothetical protein | 35.4 | 5.7e+00 | 24 | WP_006292151 | Parascardovia denticolens |
| PD653_2196 | 378 | 126 | Hypothetical protein ARZXY2_4076 | 142 | 3.0e-41 | 82 | AOY73576 | Arthrobacter sp. ZXY-2 |
| PD653_2197 | 6,069 | 2,023 | DNA primase | 2,971 | 0.0e+00 | 76 | WP_057294205 | Nocardioides sp. Soil796 |
| PD653_2198 | 1,233 | 411 | DNA polymerase V | 439 | 1.0e-149 | 59 | SDL33667 | Nocardioides sp. YR527 |
| PD653_2199 | 414 | 138 | DNA polymerase V | 97.1 | 4.0e-23 | 45 | KXS38722 | Halomonadaceae bacterium T82-2 |
| PD653_2200 | 483 | 161 | Hypothetical protein | 268 | 2.0e-90 | 83 | WP_011751413 | Nocardioides sp. JS614 |
| PD653_2201 | 756 | 252 | Hypothetical protein | 290 | 3.0e-96 | 59 | WP_057294213 | Nocardioides sp. Soil796 |
| PD653_2202 | 756 | 252 | Chromosomal replication initiator DnaA | 300 | 3.0e-100 | 67 | WP_056689293 | Nocardioides sp. Root140 |
| PD653_2203 | 723 | 241 | Hypothetical protein | 363 | 2.0e-125 | 77 | WP_011751416 | Nocardioides sp. JS614 |
| PD653_2204 | 1,491 | 497 | Membrane protein | 867 | 0.0e+00 | 87 | WP_011776835 | Paenarthrobacter aurescens |
| PD653_2205 | 465 | 155 | Hypothetical protein | 191 | 2.0e-59 | 63 | WP_067431475 | Nocardioides jensenii |
| PD653_2206 | 708 | 236 | Hypothetical protein | 223 | 2.0e-70 | 62 | WP_056689283 | Nocardioides sp. Root140 |
| PD653_2207 | 2,739 | 913 | Transglycosylase | 1,288 | 0.0 | 72 | WP_067431470 | Nocardioides jensenii |
| PD653_2208 | 483 | 161 | ATP-binding protein | 291 | 1.0e-94 | 90 | WP_067431563 | Nocardioides jensenii |
| PD653_2209 | 822 | 274 | Hypothetical protein | 256 | 4.0e-81 | 58 | WP_076180369 | Mycobacterium fortuitum |
| PD653_2210 | 207 | 69 | ||||||
| PD653_2211 | 153 | 51 | Hypothetical protein SAMN05421872_102369 | 44.7 | 6.0e-05 | 49 | SDC47105 | Nocardioides lianchengensis |
| PD653_2212 | 297 | 99 | Hypothetical protein SAMN05421872_101566 | 58.9 | 3.0e-09 | 38 | SDC21565 | Nocardioides lianchengensis |
| PD653_2213 | 297 | 99 | DUF3263 domain-containing protein | 127 | 3.0e-36 | 70 | WP_062101763 | Cellulomonas sp. B6 |
Coding sequences (CDSs) that exhibited no similarity to proteins in the public databases are blank.
aa, amino acids.
Analysis of the genes presumed to be involved in the lower pathway revealed that an ORF annotated as 4-hydroxyphenylacetate 3-hydroxylase (encoded by PD653_1114 in PD653 and PD653B2_0297 in PD653-B2) was similar to TftD, chlorophenol 4-monooxygenase in Burkholderia cepacia strain AC1100 (590 bit; 54% identity) (21). The tftC gene encoding flavin reductase was not found close to this ORF, but the putative flavin reductase gene was found upstream of the ORF (PD653_1112 in PD653 and PD653B2_0299 in PD653-B2). A gene cluster containing an ORF encoding a protein homologous to 2,6-dichlorophenol hydroxylase (TfdB; 55% identify) in Ralstonia eutropha strain JMP 134 was found (encoded by PD653_3537 in PD653 and PD653B2_3864 in PD653-B2) (22). An ORF encoding a protein homologous to maleylacetate reductase (TfdF; 40% identity) was also found adjacent to the putative tfdB gene (23). The pcp genes responsible for PCP degradation were not found in the draft genome of either strain.
Nucleotide and amino acid sequence analyses of the hcb genes.
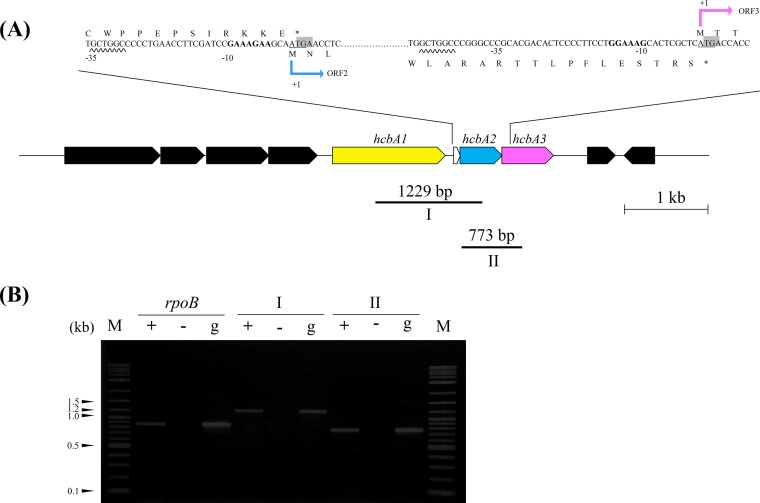
A short ORF (called ORF2a) was found in the flanking region of 177 nucleotides between ORF1 and ORF2. The ORF2a termination codon overlapped with the downstream ORF2 start codon (Fig. 3A). Similarly, the ORF2 stop codon overlapped with the ORF3 start codon. The putative ribosome-binding sites GAAAGAA and GGAAAG were found 10 nucleotides upstream of ORF2 and 15 nucleotides upstream of ORF3, respectively. The GCTGGC hexamer, which was similar to the hexamer in Streptomyces glaucescens (24, 25), was 35 nucleotides upstream of ORF2 and 48 nucleotides upstream of ORF3, suggesting it is a promoter.
FIG 3.
(A) Schematic representation of the candidate gene flanking region for Nocardioides sp. strain PD653 with putative ribosome-binding sites. The initiation codons (ATG or GTG) are underlined. The termination codons (TGA) are shaded. The translation start sites (+1) are shown. The putative promoter regions are underlined with wavy lines. The putative ribosome-binding sites are marked with bold letters. The candidate genes ORF1, ORF2, ORF3, and small ORF2a are marked with yellow (hcbA1), azure (hcbA2), magenta (hcbA3), and open arrows, respectively. The regions amplified for reverse transcription-PCR (RT-PCR) analysis are indicated below. The primer binding site and target amplified region of rpoB are not shown. (B) Polycistronic transcription of hcbA genes. Intergenic regions I and II were analyzed by RT-PCR. No-RT and genomic DNA, used as negative and positive controls, respectively, are presented in lanes − and +, respectively. cDNA from strain PD653 cells exposed to hexachlorobenzene for 3 h was used as the template.
The amino acid sequence deduced for ORF1 was similar to the sequence in Ese, the monooxygenase enzyme involved in endosulfan and endosulfan sulfate metabolism, in Arthrobacter sp. KW (432 bits; 49% identity) (26). Ese belongs to the two-component flavin-dependent monooxygenase family. The distribution of secondary structural elements was predicted from the deduced ORF1 amino acid sequence, and it was similar to other known flavin-dependent monooxygenases with conformations that have been determined (Fig. 4). The deduced ORF2 amino acid sequence was similar to the sequence in EmoB, an NADH:flavin mononucleotide oxidoreductase in Mesorhizobium sp. BNC1 involved in the two-component enzyme system (77 bits; 37% identity) (27). The deduced ORF3 amino acid sequence contained a flavin reductase-like domain (smart00903). The secondary structure of ORF3 was predicted in a similar way to the secondary structure of ORF1 and had a strongly conserved distribution of known flavin reductase secondary structural elements except for strand β4 (see Fig. S1 in the supplemental material).
FIG 4.
Comparison of the deduced amino acid sequences of ORF1 and other flavin-dependent monooxygenases. Secondary structural elements are highlighted in green for β-sheets and red for α-helices. ORF1, hexachlorobenzene oxidative dehalogenase from Nocardioides sp. PD653; LadA, long-chain alkane monooxygenase from Geobacillus thermodenitrificans NG80-2 (gi 165761309) (32, 33); SsuD, alkanesulfonate monooxygenase from Escherichia coli (gi 2507139) (34); LuxA, alkanal monooxygenase from Vibrio harveyi (gi 126509) (35); Adf, F420-dependent secondary alcohol dehydrogenase from Methanobacterium thermophilus (gi 47168785) (36); kMer, coenzyme F420-dependent methylenetetrahydromethanopterin reductase from Methanobacterium kandleri (gi 126509) (37); tMer, coenzyme F420-dependent methylenetetrahydromethanopterin reductase from Methanobacterium thermoautotrophicum (gi 13787050) (37). Identical residues are marked with asterisks, and conserved residues are marked with dots. Residues involved in coenzyme binding are labeled with bold letters.
Degradation of HCB and PCNB by recombinant E. coli cells.
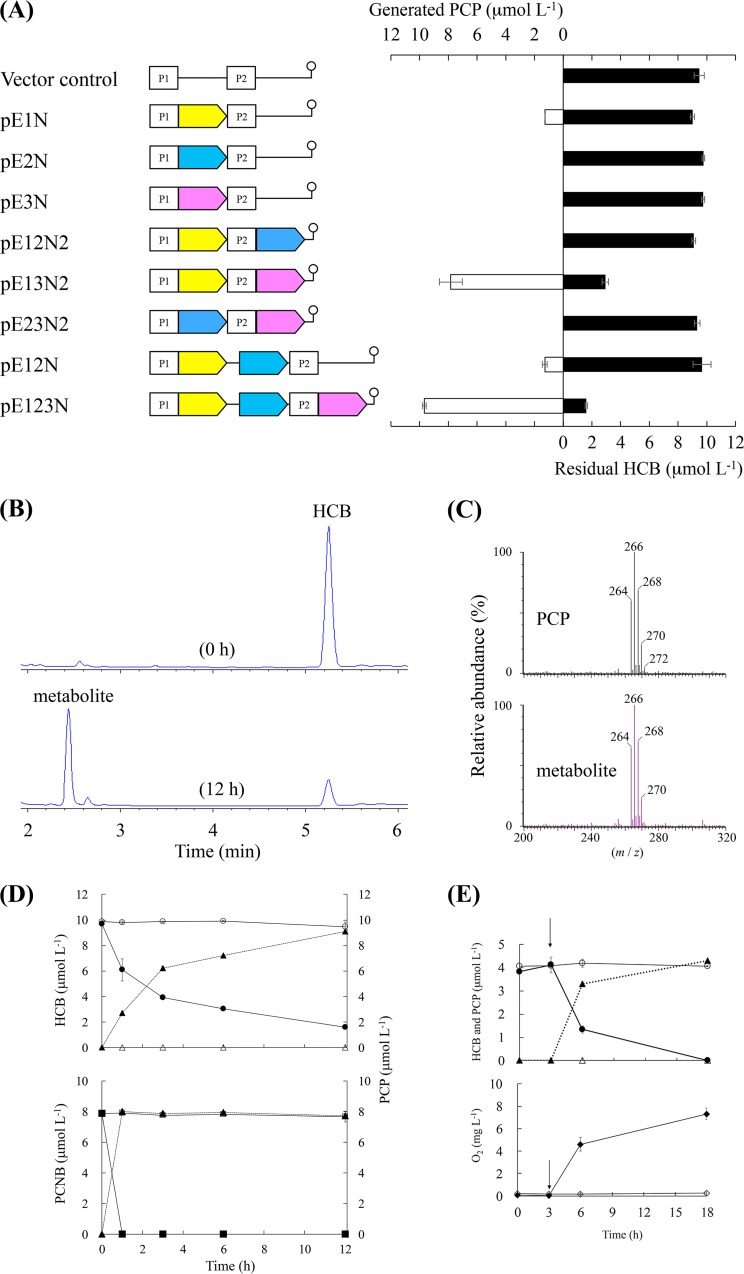
We determined whether the products of ORF1, ORF2, and ORF3 contributed to HCB and PCNB degradation by determining the abilities of recombinant E. coli BL21(DE3) cells harboring each construct to degrade HCB when incubated for 12 h in a medium containing HCB at 10 μmol liter−1 (Fig. 5A, solid bars). The degradation activities of E. coli cells containing plasmids separately carrying ORF1, ORF2, and ORF3 were first compared. E. coli BL21(DE3)/pE1N (ORF1 at the first multicloning site [MCS1]) degraded 3.9% of the HCB, and E. coli cells harboring pE2N (ORF2 at MCS1) or pE3N (ORF3 at MCS1) did not degrade HCB at all. The HCB-degrading activities of the two ORFs in the coexpression system were then compared. E. coli BL21(DE3)/pE13N2 (ORF1 at MCS1 and ORF3 at the second multicloning site [MCS2]) degraded 68.7% of the HCB, but E. coli BL21(DE3)/pE12N2 (ORF1 at MCS1 and ORF2 at MCS2), pE12N (ORF1 and ORF2 at MCS1), and pE23N2 (ORF2 at MCS1 and ORF3 at MCS2) did not degrade HCB. HCB was degraded most effectively (83.0%) by E. coli BL21(DE3)/pE123N (ORF1 and ORF2 at MCS1 and ORF3 at MCS2), which coexpressed all three ORFs. An unknown peak that indicated HCB degradation activity was found when the E. coli BL21(DE3)/pE123N culture fluid was analyzed by HPLC (Fig. 5B). The peak had the same retention time as PCP (data not shown) and was identified as PCP because the mass spectrum was identical to the mass spectrum of the PCP standard (Fig. 5C). As HCB was degraded, PCP was stoichiometrically accumulated (Fig. 5D), suggesting that HCB was dehalogenated to give PCP as shown in Fig. 5A (open bars). Recombinant E. coli cells harboring pE123N at a dry weight cell concentration of 0.8 g liter−1 degraded 6.9 μmol liter−1 HCB within 6 h, giving a degradation rate of 1.44 nmol mg−1 h−1 on a dry cell weight basis.
FIG 5.
Transformation of hexachlorobenzene (HCB) by recombinant Escherichia coli cells. (A) Comparison of dechlorination activity for E. coli cells transformed using expression vectors. Solid and open bars indicate residual HCB and pentachlorophenol (PCP) generated in the culture, respectively. Each error bar indicates the standard error for triplicate samples. The genetic elements of all of the expression vectors are shown; the candidate genes ORF1, ORF2, and ORF3 are marked with yellow, azure, and magenta arrows, respectively. The stem-loop symbol indicates the position of the terminator. P1 and P2 denote T7 promoters 1 and 2, respectively. The scheme is not to scale. (B) High-performance liquid chromatography chromatograms for sample solutions at 0 and 12 h. (C) PCP mass spectrum. (Top) Authentic PCP standard. (Bottom) The metabolite of HCB. (D) Time courses for HCB degradation (■) and PCP generation (▲). The HCB concentrations (□) and PCP concentrations (△) in the vector control cultures are also shown. Each concentration shown is the mean (n = 3) with the standard deviation. (D) Time courses for HCB degradation (●), pentachloronitrobenzene (PCNB) degradation (■), and PCP generation (▲). The HCB concentrations (○), PCNB concentrations (□), and PCP concentrations (△) in the vector control cultures are also shown. Each concentration shown is the mean (n = 3) with the standard deviation. (E) Effect of oxygen on HCB dechlorination by recombinant E. coli cells. (Top) HCB degradation (○) and PCP generation (△) under oxygen-limited conditions. The HCB concentrations (●) and PCP concentrations (▲) were determined in the replicate samples in which the N2 atmosphere had been replaced with ambient air at the times indicated by arrows. Closed diamonds indicate the oxygen concentrations in the replicate samples to which oxygen had been reintroduced, and the open diamonds indicate the oxygen concentrations in the controls.
The dechlorination of HCB was strongly affected by the presence of oxygen (Fig. 5E). HCB was not degraded and PCP was not generated under oxygen-limited conditions, but HCB degradation and PCP generation resumed when oxygen was reintroduced by replacing the N2 atmosphere with ambient air. When the oxygen concentration was increased, 4 μmol liter−1 HCB was transformed into PCP in 3 h, suggesting that ORF1 encodes a monooxygenase. We hypothesized that ORF1, ORF2, and ORF3 are genes that are involved in the initial HCB dechlorination step, so we called them hcbA1, hcbA2, and hcbA3, respectively.
We determined whether the hcbA genes are involved in PCNB degradation by investigating the PCNB degradation and metabolite generation time courses using E. coli BL21(DE3)/pE123N. As shown in Fig. 5D, the recombinant cells degraded 8 μmol liter−1 PCNB within 6 h. PCP was found to be a metabolite and was accumulated stoichiometrically.
Operon structures of hcbA1, hcbA2, and hcbA3.
We determined whether the three identified genes were transcribed polycistronically by analyzing the intergenic regions hcbA1-hcbA2 (region I) and hcbA2-hcbA3 (region II) by reverse transcription-PCR (RT-PCR) using as a template total RNA extracted from strain PD653 cells grown in a medium containing HCB. Both intergenic regions were successfully amplified, suggesting that all three genes were transcribed as a single mRNA molecule (Fig. 3B).
DISCUSSION
The PD653-B2 and PD653 strains had different abilities to dechlorinate HCB and denitrate PCNB (28). Interestingly, an approximately equal molar amount of PCA was generated when PCNB was degraded by PD653-B2, so we hypothesized that there was an alternative degradation pathway. The results suggest that the gene(s) involved in both HCB dechlorination and PCNB denitration was deleted when PD653-B2 was derived from PD653. Comparative genome analysis indicated that the approximately 60-kb region marked with an asterisk in Fig. 2A was present in PD653 but not in PD653-B2. Direct read mapping results led us to suspect that misassembly may have occurred in this region, but the sequence accuracy obtained from MiSeq was supported by PCR amplification and sequence analysis. As a result, the region of interest (marked with an asterisk in Fig. 2A) may have been translocated and less likely to be sequenced by MiSeq because of mutations in strain PD653-B2.
In the region of interest, almost half of the coding sequence (42/96) had similar sequences to genes in the Nocardioides genus (Table 1), suggesting that the region is not unique to strain PD653. Efforts were made to search the 60-kb region for inverted repeat sequences recognized by transposase and for genes encoding transposase typical of insertion sequence elements known to be involved in genetic rearrangements (29), but none were detected (data not shown). There were, therefore, no clues to allow us to gain an understanding of how the genomic rearrangement occurred. Comparative genome analysis did not allow us to identify hcbA genes in the chromosome or plasmid of the native PD653 strain.
Recombinant E. coli cells biotransformed HCB and PCNB into PCP. It was found in a previous study that genetically engineered Sphingobium chlorophenolicum ATCC 32723 harboring a gene cassette (camA+ camB+ camC) degraded HCB at a rate of 0.67 nmol mg−1 h−1 on a dry cell weight basis (11). In our assays, E. coli BL21(DE3)/pE123N (hcbA1 and hcbA2 at ORF1 and hcbA3 at ORF2) degraded HCB at more than twice that rate. The deduced amino acid sequence comparisons revealed that HcbA1, HcbA2, and HcbA3 showed no significant homology to the proteins encoded by the gene cassette (camA+ camB+ camC) (9). Considering that hcbA genes were deleted from PD653-B2, we concluded that these genes may play important roles in the initial steps of the metabolism of both HCB and PCNB. However, it is not clear if only these genes are responsible for HCB and PCNB metabolism by PD653, so further studies of hcbA genes using knockout and complementation techniques are necessary.
The hcbA gene, which has characteristics associated with flavoproteins, was identified in Nocardioides sp. strain PD653. Flavoproteins are involved in various biological processes, such as the degradation of natural and anthropogenic compounds and the biosynthesis of hormones, vitamins, and antibiotics (30). Our results suggest the possibility that hcbA1 encodes a monooxygenase responsible for transforming both HCB and PCNB into PCP. This was supported by the results obtained under oxygen-limited conditions (Fig. 5E). We propose, according to Huijbersa et al. (31), to classify HcbA1 as part of the group C flavin monooxygenases because it contains a conserved luciferase-like domain (IPR011251). This domain is found in different bacterial luciferase family proteins, including LadA produced by Geobacillus thermodenitrificans MG80-2 (32, 33), SsuD produced by E. coli (34), LuxA produced by Vibrio harveyi (35), and Adf (36), kMer, and tMer produced by methanogenic archaea (37), and consists of eight α-helices and eight parallel β-strands, forming a characteristic three-dimensional structure called the TIM barrel fold. Despite the low overall sequence identity, there are structural similarities between bacterial luciferases and nonfluorescent flavoproteins, which make up clearly related families with somewhat different folds (32–38). The predicted HcbA1 secondary structure was similar to the secondary structures of TIM barrel enzymes, suggesting that they have similar three-dimensional structures (Fig. 4).
The hcbA3 gene encodes a putative reductase component associated with HcbA1. We identified a conserved domain typical of the flavin reductase component belonging to the two-component flavin-diffusible monooxygenase (TC-FDM) family, including TftC (39), PheA2 (40), and HpaC (41), from the deduced HcbA3 amino acid sequence. These enzymes catalyze the NAD(P)H-dependent reduction of flavins and supply reduced flavins to a monooxygenase (21, 39–43). Taking the comparison of secondary structural elements into account, we hypothesized that HcbA3 can fold in a similar way to TC-FDM and acts as a flavin reductase. This hypothesis was supported by the HCB degradation activity being higher when hcbA1 and hcbA3 were coexpressed by recombinant E. coli than when only hcbA1 was expressed by recombinant E. coli. Therefore, the hcbA1 and hcbA3 genes in PD653 may encode a flavin-dependent two-component oxygenase that catalyzes the dehalogenation of HCB to PCP.
The hcbA3 and hcbA2 genes, which slightly overlap (by three nucleotides) (Fig. 3A) upstream of hcbA3, were indicative of translational coupling (44–47). Genetic organizations resembling this structure have been found in several Gram-positive bacterial gene clusters involved in the degradation of long-chain n-alkanes (48), thiocarbamate (49), vinyl chloride, and ethene (50). We could not fully determine the functional role of hcbA2, but we did find that it is part of the hcb operon and is transcribed polycistronically; therefore we hypothesized that it is part of the three-component enzyme system that catalyzes the oxidative dehalogenation of HCB in strain PD653 cells. Further studies of protein-protein interactions and the biochemical properties of the hcb gene products should be performed to test this hypothesis.
In conclusion, we describe the characterization of the hcb operon in Nocardioides sp. strain PD653 that transforms HCB and PCNB into PCP under aerobic conditions. This strain has previously been found to completely mineralize HCB under aerobic conditions (13). We did not identify genes similar to known PCP-degrading genes in the draft PD653 genome, but ongoing work is aimed at locating genes that may encode PCP-degrading enzymes.
MATERIALS AND METHODS
Materials.
PCA was purchased from TCI Tokyo Casei (Tokyo, Japan). PCNB and PCP were purchased from Wako Pure Chemical Industries (Osaka, Japan). HCB was purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). Difco R2A agar medium was purchased from Becton Dickinson and Company (Franklin Lakes, NJ, USA). MM was prepared as previously described (51) and then autoclaved and supplemented with 50 μg liter−1 p-aminobenzoic acid. The preculture medium for strains PD653 and PD653-B2 contained 1 g glucose, 1 g Bacto tryptone, 0.3 g (NH4)2SO4, 1.2 g Na2HPO4·12H2O, and 0.5 g liter−1 KH2PO4. The preculture medium was autoclaved and then supplemented with 10 ml of a solution of trace elements (52) and 50 μg liter−1 p-aminobenzoic acid.
Analytical methods.
Chloride ion concentrations were measured by ion chromatography (761 Compact IC; Metrohm, Herisau, Switzerland). An IC SI-90 4E column (Shodex, Tokyo, Japan) was used, and the mobile phase, 1.8 mM Na2CO3 and 1.7 mM NaHCO3, was used at a flow rate of 1.0 ml min−1 at 40°C. The concentrations of all of the chloroaromatic compounds were monitored by HPLC (Hewlett-Packard series 1100; Hewlett-Packard, Waldbronn, Germany) equipped with a UV detector (set at 220 nm). A Poroshell 120 EC-C18 column (150 mm long, 4.6-mm inner diameter; Agilent Technologies, Tokyo, Japan) was used, and the temperature was 40°C. The mobile phase was a mixture of acetonitrile and 0.1% phosphoric acid in water, and the pump was set to run in isocratic mode with a flow rate of 1.0 ml min−1. The mobile-phase composition was 90:10 acetonitrile/aqueous phosphoric acid for HCB analysis and 87:13 acetonitrile/aqueous phosphoric acid for PCNB, PCP, and PCA analysis. PCP was analyzed using an Acquity ultraperformance liquid chromatography (UPLC) system (Waters, Milford, MA, USA) equipped with a Micromass Quattro micro API tandem quadrupole system (Waters). Mass spectra of the compounds were acquired using a Z-spray source in electrospray ionization mode and total ion current mode. The electrospray ionization conditions used to analyze PCP were a capillary voltage of 1.2 kV, a cone voltage of 17.46 V, a source temperature of 100°C, a desolvation temperature of 350°C, a cone gas flow rate of 50 liters h−1, and a desolvation gas flow rate of 600 liters h−1. PCP was detected in negative ion mode using an m/z range of 150 to 300. The ultraperformance liquid chromatography and electrospray ionization mass spectrometry systems were controlled using MassLynx 4.1 software (Waters). Separation was performed using an Acquity UPLC BEH C18 column (1.7-mm particle size, 2.1-mm inner diameter, 100 mm long; Waters) at 40°C. Linear gradient elution was used, using two solvents. Solvent A was 0.1% (vol/vol) acetic acid in water, and solvent B was 0.1% (vol/vol) acetic acid in acetonitrile. The flow rate was 200 μl min–1, and the gradient profile was 10% solvent B for 2 min, linear change to 90% solvent B over 12 min, and 90% solvent B for 4 min. The identity of PCA was confirmed by analyzing samples using an HP6890 series gas chromatograph coupled to an HP 5973 mass selective detector. The gas chromatograph was fitted with an HT8-PCB capillary column (60 m long, 0.25-mm inner diameter; Kanto Kagaku, Tokyo, Japan). The oven temperature program started at 120°C, increased to 210°C at 20°C min−1, increased to 290°C at 10°C min−1, and then increased to 320°C at 25°C min−1. The inlet temperature was 250°C.
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 2. Strain PD653-B2 (GenBank accession number LC196157) was isolated unexpectedly from the preculture medium during the subculturing of strain PD653 (GenBank accession number DQ673618), and it was found to lack the ability to transform HCB into PCP. E. coli cells were grown in Luria-Bertani (LB) medium. E. coli DH5α (Toyobo, Osaka, Japan) and BL21(DE3) (Novagen, Madison, WI, USA) were used as host strains for the plasmids pGEM-T Easy (Promega, Madison, WI, USA), pETDuet-1 (Novagen), and their derivatives. The transformants harboring the plasmids were cultured in LB medium supplemented with 100 μg ml−1 ampicillin.
TABLE 2.
Bacterial strains and plasmids used in the study
| Strain or plasmid | Relevant characteristicsa | Reference or origin |
|---|---|---|
| Strain | ||
| Nocardioides sp. PD653 | HCB+, PCP+ | 13 |
| Nocardioides sp. PD653-B2 | HCB−, PCP+ | This study |
| E. coli DH5α | F−, l−, f80dlacZΔM15, Δ(lacZYA-argF)U169, deoR, recA1, endA1, hsdR17(rK−, mK+), pho A, supE44, thi-1, gyrA96, relA1 | Toyobo |
| E. coli BL21(DE3) | F− ompT hsdSB(rB− mB−) gal dcm λ(DE3); T7 RNA polymerase gene under the control of the lacUV promoter | Novagen |
| Plasmid | ||
| pGEM-T Easy | Apr lacZ, pMB1-derived replicon, TA cloning vector | Promega |
| pGEM-T Easy::2177-2179 | Apr, pGEM-T Easy with 2.9-kb PCR-amplified DNA fragment containing ORF1, ORF2, and ORF3 of PD653 | This study |
| pETDuet-1 | Apr pBR322-derived ColE1 replication, T7 promoter, two MCS, expression vector | Novagen |
| pE123N | Apr, pE12N with 0.7-kb PCR-amplified DNA fragment containing ORF3 in MCS2 | This study |
| pE12N | Apr, pETDuet-1with PCR-amplified DNA fragment containing ORF1 and ORF2 in MCS1 | This study |
| pE12N2 | Apr, pE1N with PCR-amplified DNA fragment containing ORF2 in MCS2 | This study |
| pE23N2 | Apr, pE2N with PCR-amplified DNA fragment containing ORF3 in MCS2 | This study |
| pE13N2 | Apr, pE1N with PCR-amplified DNA fragment of ORF3 in MCS2 | This study |
| pE1N | Apr, pETDuet-1 with PCR-amplified DNA fragment of ORF1 in MCS1 | This study |
| pE2N | Apr, pETDuet-1 with PCR-amplified DNA fragment of ORF2 in MCS1 | This study |
| pE3N | Apr, pETDuet-1 with PCR-amplified DNA fragment of ORF3 in MCS1 | This study |
HCB+, able to degrade hexachlorobenzene (HCB); HCB−, unable to degrade HCB; PCP+, able to degrade pentachlorophenol (PCP); PCP−, unable to degrade PCP; Apr, ampicillin resistant.
DNA isolation.
Total DNA from strains PD653 and PD653-B2 grown on R2A medium at 30°C was purified using a DNeasy Tissue kit (Qiagen, Valencia, CA, USA). Plasmid DNA from E. coli transformants was extracted using a Wizard miniprep system (Promega).
Characterization of strain PD653-B2.
The catabolic abilities of strain PD653-B2 were determined by performing biodegradation tests. Strain PD653-B2 cells were grown in a preculture medium until the optical density at 600 nm (OD600) reached ∼1.2. Cells were harvested at 3,000 × g at 4°C for 10 min, and then the cell pellets were washed with MM and then resuspended in MM. A 1-ml aliquot of the cell suspension (OD = 1.0) was added to each of a series of 50-ml glass-stoppered Erlenmeyer flasks, each containing 9 ml of MM supplemented with one of the chloroaromatic compounds tested. The initial PCP, HCB, and PCNB concentrations were 6.5, 9, and 8 μmol liter−1, respectively. The flasks were shaken at 180 rpm at 30°C for 9 days. Triplicate flasks were withdrawn at selected time points, and a 600-μl aliquot of the fluid in each flask was analyzed by ion chromatography. The remaining fluid was mixed with 18.8 ml of acetonitrile, and the mixture was centrifuged at 19,000 × g for 10 min. The chloroaromatic compound concentrations in the supernatant were determined by HPLC with a photodiode array detector. The metabolite of PCNB that was produced was identified by gas chromatography mass spectrometry.
Draft genome sequences and analysis.
Samples of genomic DNA from strains PD653 and PD653-B2 were fragmented to approximately 500 bp using a Covaris S2-A system (Covaris, Woburn, MA, USA). A library for sequencing was prepared using a NEBNext DNA library prep master mix set for the Illumina platform (New England BioLabs, Ipswich, MA, USA), and the library samples were paired-end sequenced (2 × 300 bp) using a MiSeq sequencer and a MiSeq version 3 reagent kit (Illumina KK, Tokyo, Japan). The total read bases for strains PD653 and PD653-B2 were 2.51 and 1.90 Gb, respectively. Raw reads were trimmed and assembled de novo using CLC Genomics Workbench version 7.5.1 (Qiagen). The trimming parameters were as follows: ambiguous limit, 2; quality limit, 0.001; 10 5′-terminal nucleotides; and 40 3′-terminal nucleotides. The de novo assembly parameters were as follows: update contigs, yes; bubble size, 600; minimum contig length, 1,000; automatic word size, 51; perform scaffolding, yes; auto-detect paired distances, yes; mismatch cost, 2; insertion cost, 3; deletion cost, 3; length fraction, 0.5; and similarity fraction, 0.8. The assembled contigs of strains PD653 and PD653-B2 were annotated using the DDBJ Microbial Genome Annotation Pipeline (http://www.migap.org/index.php/en) and edited manually for entry into nucleotide sequence databases (DDBJ/ENBL/GenBank).
No complete genome was available for the reference sequence with the strain PD653 draft genome, so the distances between the contigs and the arrangement order were not clear. The strain PD653 scaffold was therefore built using a rule of filling gaps with 100 Ns between contigs, as described on the NCBI website (https://www.ncbi.nlm.nih.gov/assembly/agp/AGP_Specification/). Comparative genome analysis was performed by merging and orienting the strain PD653-B2 contigs using ABACAS (53) guided by the strain PD653 genome sequence as the reference genome. The genes involved in HCB metabolism were identified by aligning the draft strain PD653-B2 genome with the draft strain PD653 genome using Mauve (20). Strain PD653-specific sequences were identified from the aligned sequences. The candidate genes were selected according to their protein-coding sequence annotations.
The sequences in the regions indicated in Fig. 2A were analyzed to determine whether misassembly had occurred in strain PD653 contig 22 and strain PD653-B2 contig 26. The nucleotide sequences of the primers used in this experiment are shown in Table 3. Region b was amplified by PCR using primer set wt_MS22_F and wt_delta_MS_R. Region c was amplified by PCR using primer set delta_MS26_F and wt_delta_MS_R. The PCR amplicons were cloned into the pGEM-T Easy vector (Promega) and sequenced.
TABLE 3.
PCR primers used in the study
| Primer | 5′ to 3′a |
|---|---|
| Specific primer used for amplification of candidate genes involved in dechlorination of HCB | |
| orf1_F | TCAGGACAACACCGACGTCT |
| orf3_R | ACCTCCTGTGGTGGAGCGGA |
| orf1-NcoI_mcs1_F | AAACCATGGGGCGGGATACCCTTGTACTC |
| orf1-NcoI_mcs1_R | AAACCATGGTCAGGAGAAGATGCCCCG |
| orf2-NcoI_mcs1_F | AAACCATGGGGAACCTCGTCACCGTCATC |
| orf2-NcoI_mcs1_R | AAACCATGGTCATGAGCGAGTGCTTTCCAG |
| orf2-NdeI_mcs2_F | AAACATATGAACCTCGTCACCGTCATCGGC |
| orf2_mcs2_R | GTCATGAGCGAGTGCTTT |
| orf3-BspHI_mcs1_F | AAATCATGACCACCTCCGCACCGATC |
| orf3-BspHI_mcs1_R | AAATCATGATCAGGCGGTGGTGAGGCG |
| orf3-NdeI_mcs2_F | AAACATATGACCACCTCCGCACCGATC |
| RT-PCR primer | |
| hcbA1_q_F | ACCCATCGAAGATCCACAAG |
| hcbA2_q_ F | TGAACCTCGTCACCGTCATC |
| hcbA2_q_R | AACTGGTCGAGGAAGAGCTTG |
| hcbA3_q_R | TCAAGGGAGACTGAGGTAAAGG |
| rpoB_q_F | AGATCTCCGAACCACTCGAA |
| rpoB_q_R | TGTTGATCTTGTAGCGACCG |
| Primer used for amplification to confirm misassembly | |
| wt_MS22_F | CGCTACTACCAGGTCCTCAA |
| delta_MS26_F | TACTTCACCTGGTTGAGGGC |
| wt_delta_MS_R | AGGAGGTCTTCATGATGGTG |
Specified restriction sites are underlined.
The putative genes involved in the lower chloroaromatic compound pathway were analyzed according to the University of Minnesota Biocatalysis/Biodegradation Database (http://eawag-bbd.ethz.ch/pcp/pcp_map.html) (54).
Comparison of the deduced amino acid sequences in the genes.
The amino acid sequences that were identified were compared with the amino acid sequences of other bacterial genes using the BLAST algorithm (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Amino acid sequence alignment analysis was performed using the ClustalW program on the DDBJ website (http://clustalw.ddbj.nig.ac.jp/index.php?lang=ja). The secondary structures of HcbA1 and HcbA3 were predicted using Jpred4 (http://www.compbio.dundee.ac.uk/jpred/) (55).
Construction of expression plasmids.
Primers were synthesized to amplify the candidate gene ORFs (Table 3). The region including ORF1, ORF2, and ORF3 was PCR amplified from strain PD653 genomic DNA using the primer set orf1_F and orf3_R. The PCR product was ligated into the pGEM-T Easy vector and then used as a PCR template in the experiments described below. An expression vector for putative HCB-oxidative dehalogenase was constructed by amplifying ORF1 by PCR using primer set orf1-NcoI_mcs1_F and orf1-NcoI_mcs1_R. A PCR-amplified fragment containing ORF1 was digested and ligated into the NcoI site in the first multicloning site (MCS1) of pETDuet-1, and the resulting plasmid was designated pE1N. The pE2N plasmid, including ORF2, was constructed in a similar manner using primers orf2-NcoI_mcs1_F and orf2-NcoI_mcs1_R. Plasmid pE3N was formed by amplifying the fragment containing ORF3 by PCR using primer set orf3-BspHI_mcs1_F and orf3-BspHI_mcs1_R. The PCR-amplified fragment containing ORF3 was digested with BspHI and ligated into the NcoI site of pETDuet-1. Plasmid pE12N was formed by amplifying the fragment containing ORF1 and ORF2 by PCR using primers orf1-NcoI_mcs1_F and orf2-NcoI_mcs1_R. The PCR-amplified fragment containing ORF1 and ORF2 was digested and ligated into the NcoI site of pETDuet-1. Plasmid pE12N2, containing ORF1 at MCS1 and ORF2 at the second multicloning site (MCS2) of pETDuet-1, was formed by amplifying ORF2 by PCR using primers orf2-NdeI_mcs2_F and orf2_mcs2_R. The PCR-amplified fragment containing ORF2 was digested with NdeI and cloned into the NdeI-EcoRV site (MCS2) of pE1N. Plasmids pE123N (containing ORF1 and ORF2 at MCS1 and ORF3 at MCS2), pE13N2 (containing ORF1 at MCS1 and ORF3 at MCS2), and pE23N2 (containing ORF2 at MCS1 and ORF3 at MCS2) were formed by amplifying ORF3 by PCR using primers orf3-NdeI_mcs2_F and orf3_R and then digesting and ligating the fragment into the NdeI-EcoRV sites of pE12N, pE1N, and pE2N, respectively. The recombinant plasmids were sequenced and transferred into E. coli BL21(DE3). E. coli cells harboring the expression vectors were inoculated into LB medium containing 100 μg ml−1 ampicillin and shaken overnight at 210 rpm and 37°C. The overnight culture was inoculated into fresh LB medium containing 100 μg ml−1 ampicillin and 0.25 mM isopropyl-β-thiogalactoside. The initial OD600 was adjusted to 0.6, and gene expression was induced in a culture kept at 37°C and shaken at 210 rpm for 4 h. The induced cells were then harvested by centrifuging the mixture at 1,800 × g for 10 min and washed with phosphate-buffered saline.
Degradation of HCB and PCNB by the recombinant E. coli cells.
Washed recombinant E. coli cells were suspended in 1 ml of MM and inoculated into 9 ml of MM containing 10 μmol liter−1 HCB or 8 μmol liter−1 PCNB in a glass-stoppered Erlenmeyer flask. The initial OD600 was 1.8 (a dry weight cell concentration of 0.8 g liter−1). The flask cultures were kept at 37°C and shaken at 210 rpm for 12 h. Triplicate flasks were withdrawn at selected time points, and 20 ml of acetonitrile was added to each, giving a sample solution of 30 ml. Each sample solution was centrifuged at 19,000 × g for 10 min, and the HCB and PCP concentrations in the supernatant were analyzed by HPLC with a photodiode array detector.
The effect of oxygen on HCB degradation activity was investigated using 40-ml aliquots of an isopropyl β-d-1-thiogalactopyranoside-induced cell culture washed with phosphate-buffered saline. The subsequent procedures were performed in a glove box (UN-650F; UNICO, Ibaraki, Japan) with a N2 atmosphere. Washed cells were suspended in 1 ml of MM that had been purged with N2 (99.9%) for 20 min to remove dissolved oxygen. The cells were then inoculated into 9 ml of MM containing 4 μmol liter−1 HCB in a glass-stoppered Erlenmeyer flask. The initial OD600 was 9.0. The oxygen concentration was measured independently using a dissolved oxygen meter (SG6–SevenGo pro dissolved oxygen; Mettler-Toledo, Greifensee, Switzerland). Each culture was kept at room temperature and constantly stirred using a magnetic stirrer, and then after 3 h, oxygen was reintroduced by replacing the N2 atmosphere with ambient air. Triplicate flasks were withdrawn at specified time points. Control cultures were kept under oxygen-limited conditions ([O2] < 0.5 mg liter−1) throughout the experiment. The samples were prepared and analyzed as described above.
RT-PCR.
A single colony of strain PD653 was inoculated into the preculture medium and cultured to the mid-exponential phase (OD600 = 1.0) at 30°C on a shaker at 180 rpm for 4 days. An aliquot of the culture was inoculated into fresh preculture medium in a glass-stoppered Erlenmeyer flask to give an OD600 of 0.1, and then the culture was cultivated. Strain PD653 was grown in the medium, and HCB was added during the exponential phase (OD600 = 0.3 to 0.4). Cells were harvested after 3 h of incubation and stored at −80°C until use. Total RNA was extracted according to the manufacturer's instructions with some modifications to the initial steps. Briefly, cells were resuspended in 200 μl Tris-EDTA buffer containing 5 mg ml−1 lysozyme, the cells were incubated at 37°C for 5 min, and then 500 μl Sepasol-RNA I (Nacalai Tesque, Kyoto, Japan) was added. The sample was placed in a 1.5-ml screw-cap tube containing glass beads (50 mg of beads 100 μm in diameter and 30 mg of beads 1 mm in diameter). The mixture was disrupted using a bead beater at 5,000 rpm for 5 min, and then 500 μl of Sepasol-RNA I was added. DNA traces were removed using total RNA and recombinant DNase (TaKaRa, Tokyo, Japan). The RNA quality was checked by performing 1% native agarose gel electrophoresis. The RNA concentration was determined using a NanoDrop ND-1000 system (Thermo Fisher, Lafayette, CO, USA). cDNA was synthesized from 1 μg total RNA by reverse transcription using a ReverTra Ace quantitative PCR (qPCR) RT kit (Toyobo) following the manufacturer's recommendations. Reverse transcription reaction 8.0 was performed at 37°C for 15 min, 50°C for 15 min, and 98°C for 5 min. The cDNA product was diluted by a factor of eight and then used as the template for RT-PCR analyses using the primers shown in Table 3. The primers used in the RT-PCR process were hcbA1_q_F and hcbA2_q_R for intergenic region I and hcbA2_q_F and hcbA3_q_R for intergenic region II. rpoB_q_R for rpoB, a housekeeping gene encoding the RNA polymerase β-subunit, was used as a positive control in the RT-PCR experiments. The PCR conditions were one cycle at 1.5 min, 30 cycles at 98°C for 15 s, 62.5°C for 30 s, 68°C for 30 s, and finally one cycle at 68°C for 30 s. The PCR products were separated by electrophoresis using 1.5% (wt/vol) agarose gel.
Accession number(s).
The strain PD653 and PD653-B2 genome sequences have been deposited in DDBJ/EMBL/GenBank under accession numbers BDJG01000001 to BDJG01000087 and BDJE01000001 to BDJE01000081, respectively.
Strain PD653-B2 was submitted to the Japanese National Agriculture and Food Research Organization GeneBank project (https://www.gene.affrc.go.jp/about_en.php) under MAFF number 304153.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Kuramata for help with the experimental design and M. Devers-Lamrani for scientific discussions. We thank Gareth Thomas from Edanz Group for editing a draft of the manuscript.
This work was supported by a grant from the MEXT-Supported Program for the Strategic Research Foundation at Private Universities 2013 to 2017 (grant S1311017) and the Cooperative Research Programme, Trade and Agriculture (TAD/PROG) OECD/2010.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00824-17.
REFERENCES
- 1.Barber JL, Sweetman AJ, van Wijk D, Jones KC. 2005. Hexachlorobenzene in the global environment: emissions, levels, distribution, trends and processes. Sci Total Environ 349:1–44. doi: 10.1016/j.scitotenv.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Adrian L, Szewzyk U, Wecke J, Görisch H. 2000. Bacterial dehalorespiration with chlorinated benzenes. Nature 408:580–583. doi: 10.1038/35046063. [DOI] [PubMed] [Google Scholar]
- 3.Leys D, Adrian L, Smidt H. 2013. Organohalide respiration: microbes breathing chlorinated molecules. Philos Trans R Soc B Biol Sci 368:20120316. doi: 10.1098/rstb.2012.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jayachandran G, Görisch H, Adrian L. 2003. Dehalorespiration with hexachlorobenzene and pentachlorobenzene by Dehalococcoides sp. strain CBDB1. Arch Microbiol 180:411–416. doi: 10.1007/s00203-003-0607-7. [DOI] [PubMed] [Google Scholar]
- 5.Hölscher T, Görisch H, Adrian L. 2003. Reductive dehalogenation of chlorobenzene congeners in cell extracts of Dehalococcoides sp. strain CBDB1. Appl Environ Microbiol 69:2999–3001. doi: 10.1128/AEM.69.5.2999-3001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adrian L, Hansen SK, Fung JM, Görisch H, Zinder SH. 2007. Growth of Dehalococcoides strains with chlorophenols as electron acceptors. Environ Sci Technol 41:2318–2323. doi: 10.1021/es062076m. [DOI] [PubMed] [Google Scholar]
- 7.Adrian L, Rahnenführer J, Gobom J, Hölscher T. 2007. Identification of a chlorobenzene reductive dehalogenase in Dehalococcoides sp. strain CBDB1. Appl Environ Microbiol 73:7717–7724. doi: 10.1128/AEM.01649-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kube M, Beck A, Zinder SH, Kuhl H, Reinhardt R, Adrian L. 2005. Genome sequence of the chlorinated compound-respiring bacterium Dehalococcoides species strain CBDB1. Nat Biotechnol 23:1269–1273. doi: 10.1038/nbt1131. [DOI] [PubMed] [Google Scholar]
- 9.Jones JP, O'Hare EJ, Wong LL. 2001. Oxidation of polychlorinated benzenes by genetically engineered CYP101 (cytochrome P450cam). Eur J Biochem 268:1460–1467. doi: 10.1046/j.1432-1327.2001.02018.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Christopher A, Jones JP, Bell SG, Guo Q, Xu F, Rao Z, Wong LL. 2002. Crystal structure of the F87W/Y96F/V247L mutant of cytochrome P-450cam with 1,3,5-trichlorobenzene bound and further protein engineering for the oxidation of pentachlorobenzene and hexachlorobenzene. J Biol Chem 277:37519–37526. doi: 10.1074/jbc.M203762200. [DOI] [PubMed] [Google Scholar]
- 11.Yan DZ, Liu H, Zhou NY. 2006. Conversion of Sphingobium chlorophenolicum ATCC 39723 to a hexachlorobenzene degrader by metabolic engineering. Appl Environ Microbiol 72:2283–2286. doi: 10.1128/AEM.72.3.2283-2286.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu T, Chen ZL, Shen YF. 2009. Aerobic biodegradation of hexachlorobenzene by an acclimated microbial community. Int J Environ Pollut 37:235–244. doi: 10.1504/IJEP.2009.025127. [DOI] [Google Scholar]
- 13.Takagi K, Iwasaki A, Kamei I, Satsuma K, Yoshioka Y, Harada N. 2009. Aerobic mineralization of hexachlorobenzene by newly isolated pentachloronitrobenzene-degrading Nocardioides sp. strain PD653. Appl Environ Microbiol 75:4452–4458. doi: 10.1128/AEM.02329-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apajalahti JHA, Salkinoja-Salonen MS. 1986. Degradation of polychlorinated phenols by Rhodococcus chlorophenolicus. Appl Microbiol Biotechnol 25:62–67. doi: 10.1007/BF00252514. [DOI] [Google Scholar]
- 15.Karn SK, Chakrabarti SK, Reddy MS. 2011. Degradation of pentachlorophenol by Kocuria sp. CL2 isolated from secondary sludge of pulp and paper mill. Biodegradation 22:63–69. doi: 10.1007/s10532-010-9376-6. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Chandra R, Patel DK, Rai V. 2007. Isolation and characterization of novel Serratia marcescens (AY927692) for pentachlorophenol degradation from pulp and paper mill waste. World J Microbiol Biotechnol 23:1747–1754. doi: 10.1007/s11274-007-9424-5. [DOI] [PubMed] [Google Scholar]
- 17.Sharma A, Thakur IS, Dureja P. 2009. Enrichment, isolation and characterization of pentachlorophenol degrading bacterium Acinetobacter sp. ISTPCP-3 from effluent discharge site. Biodegradation 20:643–650. [DOI] [PubMed] [Google Scholar]
- 18.Lee SG, Yoon BD, Park YH, Oh HM. 1998. Isolation of a novel pentachlorophenol-degrading bacterium, Pseudomonas sp. Bu34. J Appl Microbiol 85:1–8. doi: 10.1046/j.1365-2672.1998.00456.x. [DOI] [PubMed] [Google Scholar]
- 19.Cai M, Xun L. 2002. Organization and regulation of pentachlorophenol-degrading genes in Sphingobium chlorophenolicum ATCC 39723. J Bacteriol 184:4672–4680. doi: 10.1128/JB.184.17.4672-4680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darling AC, Mau B, Blattner FR, Perna NT. 2004. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res 14:1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gisi MR, Xun L. 2003. Characterization of chlorophenol 4-monooxygenase (TftD) and NADH:flavin adenine dinucleotide oxidoreductase (TftC) of Burkholderia cepacia AC1100. J Bacteriol 185:2786–2792. doi: 10.1128/JB.185.9.2786-2792.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perkins EJ, Gordon MP, Caceres O, Lurquin PF. 1990. Organization and sequence analysis of the 2,4-dichlorophenol hydroxylase and dichlorocatechol oxidative operons of plasmid pJP4. J Bacteriol 172:2351–2359. doi: 10.1128/jb.172.5.2351-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seibert V, Stadler-Fritzsche K, Schlömann M. 1993. Purification and characterization of maleylacetate reductase from Alcaligenes eutrophus JMP134(pJP4). J Bacteriol 175:6745–6754. doi: 10.1128/jb.175.21.6745-6754.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strohl WR. 1992. Compilation and analysis of DNA sequences associated with apparent streptomycete promoters. Nucleic Acids Res 20:961–974. doi: 10.1093/nar/20.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vögtli M, Hütter R. 1987. Characterization of the hydroxystreptomycin phosphotransferase gene (sph) of Streptomyces glaucescens: nucleotide sequence and promoter analysis. Mol Gen Genet 208:195–203. doi: 10.1007/BF00330442. [DOI] [PubMed] [Google Scholar]
- 26.Weir KM, Sutherland TD, Horne I, Russell RJ, Oakeshott JG. 2006. A single monooxygenase, ese, is involved in the metabolism of the organochlorides endosulfan and endosulfate in an Arthrobacter sp. Appl Environ Microbiol 72:3524–3530. doi: 10.1128/AEM.72.5.3524-3530.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bohuslavek J, Payne JW, Liu Y, Bolton H, Xun L. 2001. Cloning, sequencing, and characterization of a gene cluster involved in EDTA degradation from the bacterium BNC1. Appl Environ Microbiol 67:688–695. doi: 10.1128/AEM.67.2.688-695.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takagi K, Yoshioka Y, Iwasaki A, Kamei I, Harada N. 2007. Metabolic pathways of hexachlorobenzene (HCB), quintozene (PCNB) and pentachlorophenol (PCP) by a newly isolated strain Nocardioides sp. PD653 under aerobic conditions. Organohalogen Compd 69:2576–2579. [Google Scholar]
- 29.Siguier P, Filée J, Chandler M. 2006. Insertion sequences in prokaryotic genomes. Curr Opin Microbiol 9:526–531. doi: 10.1016/j.mib.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Ghisla S, Massey V. 1989. Mechanisms of flavoprotein-catalyzed reactions. Eur J Biochem 181:1–17. doi: 10.1111/j.1432-1033.1989.tb14688.x. [DOI] [PubMed] [Google Scholar]
- 31.Huijbersa MME, Montersinoa S, Westphala AH, Tischlera D, van Berkela WJH. 2014. Flavin dependent monooxygenases. Arch Biochem Biophys 544:2–17. doi: 10.1016/j.abb.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Feng L, Wang W, Cheng J, Ren Y, Zhao G, Gao C, Tang Y, Liu X, Han W, Peng X, Liu R, Wang L. 2007. Genome and proteome of long-chain alkane degrading Geobacillus thermodenitrificans NG80-2 isolated from a deep-subsurface oil reservoir. Proc Natl Acad Sci U S A 104:5602–5607. doi: 10.1073/pnas.0609650104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L, Liu X, Yang W, Xu F, Wang W, Feng L, Bartlam M, Wang L, Rao Z. 2008. Crystal structure of long-chain alkane monooxygenase (LadA) in complex with coenzyme FMN: unveiling the long-chain alkane hydroxylase. J Mol Biol 376:453–465. doi: 10.1016/j.jmb.2007.11.069. [DOI] [PubMed] [Google Scholar]
- 34.Eichhorn E, Davey CA, Sargent DF, Leisinger T, Richmond TJ. 2002. Crystal structure of Escherichia coli alkanesulfonate monooxygenase SsuD. J Mol Biol 324:457–468. doi: 10.1016/S0022-2836(02)01069-0. [DOI] [PubMed] [Google Scholar]
- 35.Fisher AJ, Thompson TB, Thoden JB, Baldwin TO, Rayment I. 1996. The 1.5-Å resolution crystal structure of bacterial luciferase in low salt conditions. J Biol Chem 271:21956–21968. doi: 10.1074/jbc.271.36.21956. [DOI] [PubMed] [Google Scholar]
- 36.Aufhammer SW, Warkentin E, Berk H, Shima S, Thauer RK, Ermler U. 2004. Coenzyme binding in F420-dependent secondary alcohol dehydrogenase, a member of the bacterial luciferase family. Structure 12:361–370. doi: 10.1016/j.str.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Shima S, Warkentin E, Grabarse W, Sordel M, Wicke M, Thauer RK, Ermler U. 2000. Structure of coenzyme F420 dependent methylenetetrahydromethanopterin reductase from two methanogenic archaea. J Mol Biol 300:935–950. doi: 10.1006/jmbi.2000.3909. [DOI] [PubMed] [Google Scholar]
- 38.Moore SA, James MNG. 1994. Common structural features of the LuxF protein and the subunits of bacterial luciferase: evidence for a (βα)8 fold in luciferase. Protein Sci 3:1914–1926. doi: 10.1002/pro.5560031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xun L. 1996. Purification and characterization of chlorophenol 4-monooxygenase from Burkholderia cepacia AC1100. J Bacteriol 178:2645–2649. doi: 10.1128/jb.178.9.2645-2649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duffner FM, Mueller R. 1998. A novel phenol hydroxylase and catechol 2,3-dioxygenase from the thermophilic Bacillus thermoleovorans strain A2: nucleotide sequence and analysis of the genes. FEMS Microbiol Lett 161:37–45. doi: 10.1111/j.1574-6968.1998.tb12926.x. [DOI] [PubMed] [Google Scholar]
- 41.Prieto MA, Perez-Aranda A, Garcia JL. 1993. Characterization of an Escherichia coli aromatic hydroxylase with a broad substrate range. J Bacteriol 175:2162–2167. doi: 10.1128/jb.175.7.2162-2167.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirchner U, Westphal AH, Müller R, van Berkel WJH. 2003. Phenol hydroxylase from Bacillus thermoglucosidasius A7, a two-protein component monooxygenase with a dual role for FAD. J Biol Chem 278:47545–47553. doi: 10.1074/jbc.M307397200. [DOI] [PubMed] [Google Scholar]
- 43.Galán B, Díaz E, Prieto MA, García JL. 2000. Functional analysis of the small component of the 4-hydroxyphenylacetate 3-monooxygenase of Escherichia coli W: a prototype of a new flavin:NAD(P)H reductase subfamily. J Bacteriol 182:627–636. doi: 10.1128/JB.182.3.627-636.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spanjaard RA, van Duin J. 1989. Translational reinitiation in the presence and absence of a Shine and Dalgarno sequence. Nucleic Acids Res 17:5501–5507. doi: 10.1093/nar/17.14.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sprengel R, Reiss B, Schaller H. 1985. Translationally coupled initiation of protein synthesis in Bacillus subtilis. Nucleic Acids Res 13:893–909. doi: 10.1093/nar/13.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adhin MR, van Duin J. 1990. Scanning model for translational reinitiation in eubacteria. J Mol Biol 213:811–818. doi: 10.1016/S0022-2836(05)80265-7. [DOI] [PubMed] [Google Scholar]
- 47.Yoo JH, RajBhandary UR. 2008. Requirements for translation re-initiation in Escherichia coli: roles of initiator tRNA and initiation factors IF2 and IF3. Mol Microbiol 67:1012–1026. doi: 10.1111/j.1365-2958.2008.06104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whyte LG, Smits THM, Labbé D, Witholt B, Greer CW, van Beilen JB. 2002. Gene cloning and characterization of multiple alkane hydroxylase systems in Rhodococcus strains Q15 and NRRL B-16531. Appl Environ Microbiol 68:5933–5942. doi: 10.1128/AEM.68.12.5933-5942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagy I, Schoofs G, Compernolle F, Proost P, Vanderleyden J, De Mot R. 1995. Degradation of the thiocarbamate herbicide EPTC (S-ethyl dipropylcarbamothioate) and biosafening by Rhodococcus sp. strain NI86/21 involve an inducible cytochrome P-450 system and aldehyde dehydrogenase. J Bacteriol 177:676–687. doi: 10.1128/jb.177.3.676-687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mattes TE, Coleman NV, Spain JC, Gossett JM. 2005. Physiological and molecular genetic analyses of vinyl chloride and ethene biodegradation in Nocardioides sp. strain JS614. Arch Microbiol 183:95–106. doi: 10.1007/s00203-004-0749-2. [DOI] [PubMed] [Google Scholar]
- 51.Yamazaki K, Fujii K, Iwasaki A, Takagi K, Satsuma K, Harada N, Uchimura T. 2008. Different substrate specificities of two triazine hydrolases (TrzNs) from Nocardioides species. FEMS Microbiol Lett 286:171–177. doi: 10.1111/j.1574-6968.2008.01271.x. [DOI] [PubMed] [Google Scholar]
- 52.Yanze-Kontchou C, Gschwind N. 1994. Mineralization of the herbicide atrazine as a carbon source by a Pseudomonas strain. Appl Environ Microbiol 60:4297–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Assefa S, Keane TM, Otto TD, Newbold C, Berriman M. 2009. ABACAS: algorithm-based automatic contiguation of assembled sequences. Bioinformatics 25:1968–1969. doi: 10.1093/bioinformatics/btp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ellis LBM, Wackett LP. 2012. Use of the University of Minnesota Biocatalysis/Biodegradation Database for study of microbial degradation. Microb Inform Exp 2:1. doi: 10.1186/2042-5783-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drozdetskiy A, Cole C, Procter J, Barton GJ. 2015. JPred4: a protein secondary structure prediction server. Nucleic Acids Res 43:W389–W394. doi: 10.1093/nar/gkv332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.