Abstract
Central retinal vein occlusion (CRVO) can cause vision loss. The pathogenesis of CRVO involves a thrombus formation leading to increased retinal capillary pressure, increased vascular permeability, and possibly retinal neovascularization. Vision loss due to CRVO is commonly caused by macular edema. Multiple treatment modalities have been used to treat macular edema. Currently, the most common therapy used is intravitreal inhibition of vascular endothelial growth factor (VEGF). The three most widely used agents are aflibercept, bevacizumab, and ranibizumab and they are effective at blocking VEGF. In addition, intraocular steroids can be used to treat macular edema. This review will briefly cover the treatment options and discuss in greater detail the efficacy and safety of aflibercept.
Keywords: Aflibercept, antivascular endothelial growth factor, central retinal vein occlusion
Background
Central retinal vein occlusion (CRVO) is a common cause of sight-threatening retinal disease.[1] The pathogenesis of CRVO is believed to involve vascular endothelial damage and compression of the retinal vein, leading to thrombus formation. This leads to increased retinal capillary pressure, which causes transudation into the extracellular space and macular edema.[2,3] CRVO may be further divided into ischemic or nonischemic subtypes, with the ischemic subtype having worse visual outcomes.[2,3] In a recent natural history cohort study, the baseline visual acuity was 20/100 or better for 78% of patients with nonischemic CRVO, while only 1% of patients with ischemic CRVO achieved 20/100 or better. Final visual acuity was 20/100 or better in 83% of patients with nonischemic CRVO versus only 12% of patients with ischemic CRVO.[4] Visual loss from CRVO is most commonly caused by macular edema but may also be caused by macular ischemia, neovascular glaucoma, retinal neovascularization, or a combination of these complications.[2,3] Imaging from a patient with CRVO is shown in Figure 1.
Figure 1.
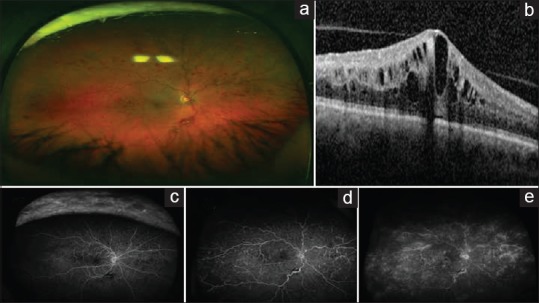
Retinal imaging of central retinal vein occlusion. (a) The ultra-wide fundus photograph shows numerous intraretinal hemorrhages and vascular tortuosity consistent with central retinal vein occlusion. (b) The ocular coherence tomography scan shows intraretinal edema with thickening in the central macula. (c-e) The fluorescein angiogram progresses with time from left to right. The fluorescein angiogram shows leakage to the macula, perivascular leakage in the periphery, and shunt vessels due to old central retinal vein occlusion
Globally, CRVO has a large impact with an estimated 2.5 million affected people.[5,6] Prevalence rates are similar across the world, ranging between 0.3% and 2.1% in data collected from international epidemiological studies.[5,7,8,9,10,11,12] CRVO prevalence rates do not significantly vary with regard to race or gender. Increasing age is the biggest risk factor for developing CRVO, which is likely due to increased arteriosclerosis and other systemic and ocular risk factors.[5,6] Other risk factors for developing CRVO include hypertension, diabetes mellitus, atherosclerosis, high cholesterol, thrombophilia, glaucoma, and other inflammatory and autoimmune conditions.[13]
An extensive laboratory workup for the cause of the CRVO is usually not recommended because the majority of testing will not reveal a systemic coagulopathy. However, in younger patients, especially those with bilateral CRVO disease, a thrombophilic workup is recommended.[14] A workup including blood pressure measurement for hypertension screening, intraocular pressure (IOP) measurement to screen for glaucoma, complete blood count with glucose to evaluate if the patient is a diabetic, and a lipid panel to see if the patient is hyperlipidemic is recommended in younger patients under 56-year-old with CRVO. Further work up with tests for thrombophilias such as homocysteinemia, anticardiolipin antibodies, antiphospholipid antibodies, activated protein C resistance, antithrombin III activity, and proteins S and C can be investigated if the initial workup is negative on a case by case basis.[14] Additional testing may be performed to detect other systemic disorders associated with CRVO including but not limited to cardiovascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, lupus, and blood dyscrasias.
Antivascular Endothelial Growth Factor Agents in the Treatment of Central Retinal Vein Occlusion
Vascular endothelial growth factor (VEGF) is a cytokine produced by hypoxic cells to stimulate vascular permeability and proliferation by binding to endothelial cell receptors. This increased vascular permeability leads to the development of macular edema in CRVO. The development of macular edema may cause vision loss. Anti-VEGF agents such as pegaptanib, bevacizumab, ranibizumab, and aflibercept work by binding to VEGF to inhibit endothelial receptor binding. Figure 2 demonstrates the effectiveness of anti-VEGF agents in decreasing macular edema.
Figure 2.
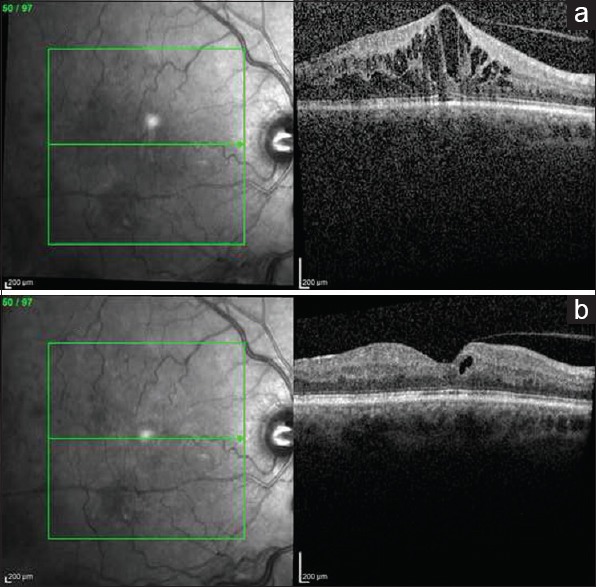
Ocular coherence tomography changes with antivascular endothelial growth factor treatment. (a) Central retinal thickness before antivascular endothelial growth factor treatment. (b) Central retinal thickness after treatment with aflibercept demonstrating significantly less macular edema
The CRUISE study was a double-masked, randomized, controlled trial (RCT) evaluating the efficacy of ranibizumab injections in treating CRVO compared to sham injections.[15] Participants were randomized to one of three groups to receive monthly sham intraocular injections or intraocular ranibizumab injections of 0.3 mg or 0.5 mg. Significant statistical difference was observed between the ranibizumab groups and the sham group for those achieving 15-letter improvement in BCVA at 7 days, 1 month, and 6 months. At 6 months, 46.2% and 47.7% of participants in the 0.3 mg and 0.5 mg ranibizumab groups, respectively, had achieved 15-letter improvement in BCVA compared to only 16.9% of participants in the sham group.[15]
A recent meta-analysis of six RCTs described the positive outcomes associated with anti-VEGF injections compared to sham injections in patients with macular edema secondary to CRVO [Table 1].[3] These trials include the GALILEO and COPERNICUS trials for aflibercept, CRUISE and ROCC trials for ranibizumab, the Epstein study for bevacizumab, and the Wroblewski study for pegaptanib sodium.[16,17,18,19,20] This meta-analysis showed that patients receiving anti-VEGF treatment were 2.71 times more likely to gain 15 letters or more of visual acuity after 6 months compared to patients who were treated with sham injections.[3] The likelihood of gaining 15 letters of visual acuity at 6 months did not show a statistically significant difference among the different anti-VEGF agents. However, aflibercept had the highest rate of 15-letter improvement in visual acuity. Nearly sixty percent (60.2%) of those receiving aflibercept injections gained 15 letters.[16] Visual acuity was largely maintained at 12 months using anti-VEGF dosing as needed in the applicable studies.[3] Similarly, those who received anti-VEGF agents had a mean visual acuity 15.23 letters higher than those receiving sham injections at 6 months.[3] These anti-VEGF agents not only improved visual acuity but were also protective in deterioration of visual acuity. The same meta-analysis revealed that receiving intravitreal anti-VEGF treatment was associated with an 80% lower risk of losing 15 letters of visual acuity at 6 months.[3] Anti-VEGF agents also demonstrated the ability to decrease macular edema. Anti-VEGF agents caused an average reduction of 267.4 μm in central retinal thickness (CRT) compared to participants who received sham injections.[3] The positive outcomes from using anti-VEGF agents in the treatment of CRVO comes with no increased risk of IOP elevation or cataract progression. Anti-VEGF therapy was associated with decreased risk of developing iris or retinal neovascularization and neovascular glaucoma compared to sham groups at 6 months. Endophthalmitis, rhegmatogenous retinal detachment, and retinal artery occlusion, which are all known complications of intravitreal injections, occurred at extremely low frequency in all the studies included in the meta-analysis.[16,17,18,19,20] No systemic adverse events were identified in these studies at 6 months. Anti-VEGF therapy for CRVO macular edema is effective at improving and maintaining visual acuity with an excellent safety profile.
Table 1.
Comparison of antivascular endothelial growth factor agents in the treatment of central retinal vein occlusiona
| Study | Treatment groups | BCVA gain of ≥15 letters | BCVA loss of ≥15 letters | Mean BCVA change (letters) | Mean change in CRT (µm) |
|---|---|---|---|---|---|
| GALILEO 2013 | Aflibercept 2.0 mg (n=106) | 60.2% (62/103) | Not reported | +18.0 | −448.6 |
| Sham (n=71) | 22.1% (15/68) | Not reported | +3.3 | −169.3 | |
| Copernicus 2012 | Aflibercept 2.0 mg (n=114) | 56.1% (64/114) | 1.8% (2/114) | +17.3 | −457.2 |
| Sham (n=74) | 12.3% (9/73) | 27.4% (20/73) | −4.0 | −144.8 | |
| CRUISE 2010 | Ranibizumab 0.3 mg (n=132) | 46.2% (61/132) | 3.8% (5/132) | +12.7 | −433.7 |
| Ranibizumab 0.5 mg (n=130) | 47.7% (62/130) | 1.5% (2/130) | +14.9 | −452.3 | |
| Sham (n=130) | 16.9% (22/130) | 15.4% (20/130) | +0.8 | −167.0 | |
| ROCC 2010 | Ranibizumab 0.5 mg (n=16) | 53.3% (8/15) | 13.3% (2/15) | +12.0 | −304.0 |
| Sham (n=16) | 14.3% (2/14) | 4/14 (28.6%) | −1.0 | −151.0 | |
| Epstein 2012 | Bevacizumab 1.25 mg (n=30) | 60.0% (18/30) | 6.7% (2/30) | +14.1 | −426.0 |
| Sham (n=30) | 20.0% (6/30) | 23.3% (7/30) | −2.0 | −102.0 | |
| Wroblewski 2009 | Pegaptanib sodium 0.3 mg (n=33) | 36.4% (12/33) | 9.1% (3/33) | +7.1 | −243.0 |
| Pegaptanib sodium 1.0 mg (n=33) | 39.4% (13/33) | 6.1% (2/33) | +9.9 | −179.0 | |
| Sham (n=32) | 28.1% (9/32) | 31.2% (10/32) | −3.2 | −148.0 |
aData reported at 6-month follow-up visit for each study. VEGF = Vascular endothelial growth factor, CRVO = Central retinal vein occlusion, BCVA = Best-corrected visual acuity, CRT = Central retinal thickness
Aflibercept in the Treatment of Central Retinal Vein Occlusion
Aflibercept is a receptor fusion protein that includes the second domain of human VEGF receptor 1 and the third domain of VEGF receptor 2. These are fused to the Fc domain of human immunoglobulin G1.[21] Aflibercept has been designed to have a greater binding affinity for VEGF than bevacizumab and ranibizumab and has shown this ability in vitro.[22,23] Aflibercept has been associated with significant visual improvement for patients with CRVO macular edema and the Food and Drug Administration has been approved for this indication since September 2012. Two of the most important RCTs evaluating the use of aflibercept for the treatment of CRVO macular edema were the COPERNICUS and GALILEO studies.
In the COPERNICUS trial, patients with CRVO-related macular edema with a BCVA between 20/40 and 20/320 (Early Treatment Diabetic Retinopathy Study [ETDRS] 73–24 letters) and a CRT of ≥250 μm were randomized into two groups. The first group received a 2.0 mg intravitreal injection of aflibercept every 4 weeks for 24 weeks, while the second group to receive a sham injection at the same time intervals.[24] From weeks 24 to 52, all the patients were evaluated monthly and received an intravitreal aflibercept injection if they had a >50 μm CRT increase from their lowest previous measurement, persistent diffuse edema ≥250 μm, new or persistent cystic retinal changes or subretinal fluid, or a loss of ≥5 letters in BCVA. All the patients were evaluated at a minimum interval of 3 months from weeks 52 to 100 to assess the need for intravitreal aflibercept injections based on the same criteria.[24]
The GALILEO trial enrolled patients with CRVO-related macular edema with the same visual acuity and CRT requirements in the COPERNICUS trial. Patients were randomized to the treatment group, which received a 2.0 mg intravitreal injection of aflibercept every 4 weeks for 20 weeks, or the sham group, which received a sham injection at the same interval.[16] From weeks 24 to 48, patients in the aflibercept group received another 2.0 mg injection of aflibercept if they met the same criteria in the COPERNICUS trial, while the sham group participants continued to receive sham injections. From weeks 52 to 68, all patients were evaluated every 8 weeks and received an intravitreal aflibercept injection if they met the specified criteria.[16]
After 6 months, 56.1% of patients who received aflibercept injections had a 15-letter increase in visual acuity versus 12.3% receiving sham injections in the COPERNICUS study.[24] About 60.2% of patients receiving aflibercept injections had at 15-letter improvement compared to 22.1% of participants receiving sham injections in the GALILEO study.[16] Combined, 58.1% of participants in these studies had a 15-letter increase in visual acuity after receiving aflibercept injections, and participants receiving aflibercept were 3.37 times more likely to have a 15-letter increase in visual acuity compared to those who receive sham injections.[3]
After the 6-month primary endpoint, the GALILEO and COPERNICUS trials continued to show promising results for treatment of CRVO macular edema with aflibercept. At week 52, 55.3% of participants in the original aflibercept group had gained ≥15 letters of visual acuity, and 49.1% of the participants in the aflibercept had achieved this increase in visual acuity at 100 weeks while adhering to study protocol.[17] Similarly, 60.2% of participants in the aflibercept group of the GALILEO study had gained ≥15 letters of visual acuity compared to 32.4% in the original sham group at week 52. By 76 weeks, 57.3% of the aflibercept group participants had achieved this increase in visual acuity compared to 29.4% of participants in the sham group.[16]
The findings of the COPERNICUS and GALILEO trials suggest that patients with CRVO-related macular edema may benefit from early aflibercept injections following the initial event. Visual outcomes were best in the groups that initially received aflibercept injections rather than the groups that initially received sham injections and then received aflibercept as needed many weeks later. Intravitreal aflibercept was generally well tolerated, with the most common adverse events being those typically associated with intravitreal injections or events related to the progression of underlying CRVO. When the intervals between treatments were increased in both trials, decreased visual and anatomic gains were observed. This suggests that the treatment interval may be extended after the initiation of treatment, but the monitoring and treatment intervals should be chosen carefully based on clinician discretion.[16] No significant difference in the rate of adverse events was noted between the aflibercept groups and sham groups for both trials.[16,17] The results of the COPERNICUS and GALILEO trials suggest that early, regular intravitreal aflibercept injections are effective at improving visual and anatomic outcomes in patients with CRVO-related macular edema.
Recently, the results from the The Study of Comparative Treatments for Retinal Vein Occlusion 2 trial (SCORE 2) trial demonstrated that intravitreal bevacizumab was non-inferior to intravitreal aflibercept with respect to visual acuity outcomes in patients who had macular edema from CRVO or hemiretinal vein occlusion. Patients were randomized 1:1 to receive either intravitreal aflibercept (2 mg) or intravitreal bevacizumab (1.25 mg) every 4 weeks for 6 months. In the SCORE2 trial, mean visual acuity letter score improved over 6 months from 50.3 to 69.3 in patients treated with bevacizumab and from 50.4 to 69.3 in patients treated with aflibercept (P = 0.001 for non-inferiority). In addition, there were no significant differences in adverse events between the two treatment groups.
A Possible Role for Switching Antivascular Endothelial Growth Factor Agents
There is limited data on how to treat eyes with persistent macular edema and a suboptimal response to a particular anti-VEGF agent. Currently, retina specialists do not have a consensus definition of a suboptimal responder. Multiple retrospective studies have indicated that aflibercept may be an effective agent in treating CRVO refractory to other anti-VEGF agents. Eadie et al. described a review of six patients who received a minimum of 10 monthly intravitreal ranibizumab or bevacizumab injections and were transitioned to intravitreal aflibercept every 4–6 weeks for refractory macular edema.[25] All of the patients had improvement in their macular edema with a mean decrease in central optical coherence tomography thickness of 316 μm at 1 month and 290 μm at 3 months. Three of the six patients had lasting modest VA improvement, while four of the six had subjective visual improvement.[25] Similarly, Lehmann-Clarke et al. reported a retrospective series of six patients with CRVO who were switched to aflibercept for persistent macular edema after a minimum of 6 monthly ranibizumab injections. These patients had a mean improvement of 9.2 ETDRS letters and a mean decreased central macular thickness of 248.0 μm after switching to monthly intravitreal aflibercept.[26]
In a retrospective study by Pfau et al., 13 patients had 48 months of persistent macular edema due to CRVO despite treatment with ranibizumab and/or bevacizumab. These eyes were switched to treatment with aflibercept.[27] After 1 year of aflibercept treatment, the mean injection interval increased by 0.51 months, and the relapse-free interval increased by 3.02 weeks. The mean ETDRS score increased from 66.15 to 76.54 letters, and the mean CRT decreased by 195.84 μm.[27] Another retrospective study was performed by Papakostas et al. who evaluated the efficacy of aflibercept injections in 42 eyes from 42 patients with CRVO-related macular edema refractory to treatment with ranibizumab and/or bevacizumab. Median visual acuity before aflibercept treatment was 20/126. One month after switching to aflibercept, acuity was 20/89, and acuity was 20/100 at the end of the follow-up period, which averaged 14 months.[28] Median CRT before aflibercept was 536 μm, 293.5 μm at 1 month after the first aflibercept injection, and 279 μm at the end of the follow-up period. These results are limited by their retrospective nature and possible recall bias. It is possible that aflibercept may improve visual acuity and anatomy of patients with CRVO-related macular edema that is not responsive to intravitreal injections of bevacizumab and/or ranibizumab. In addition, some clinicians suggest adding intravitreal steroids in eyes with a suboptimal response to aflibercept, ranibizumab, or bevacizumab.[29,30] Prospective studies are necessary to determine how to best manage eyes with recalcitrant macular edema secondary to CRVO who have had a suboptimal response to anti-VEGF agents.
Other Treatment Options for Central Retinal Vein Occlusion
Laser-induced chorioretinal venous anastomosis
Moderate efficacy was demonstrated in a small, randomized RCT comparing laser-induced chorioretinal venous anastomosis to sham treatment in adults with nonischemic CRVO macular edema.[31] Efficacy was highest in the 76% of patients who developed a functional anastomosis. However, 9% of patients required vitrectomy for macular traction or nonresolving vitreous hemorrhage, and 18% in the treatment arm developed neovascularization at the treatment site.[31] Currently, this type of laser procedure is not commonly used because there are more effective pharmacologic agents available.
Surgically induced chorioretinal venous anastomosis
A non-RCT comparing surgically induced chorioretinal venous anastomosis to controls for treatment of patients with ischemic CRVO demonstrated improvement of VA in 80% of patients undergoing surgery compared to 28% of the controls after 8 months.[32] The mean improvement of VA at 8 months was 1.5 logMAR units more in the surgical group than the controls, and all the patients had at least one successful shunt formation. Despite these positive results, three of the ten surgical patients developed significant side effects, including cataract formation, retinal detachment, and vitreous cavity hemorrhage.[32]
Radial optic neurotomy
RON involves a radial incision through the cribriform plate, scleral rim, and adjacent sclera to relax the scleral outlet and was first proposed by Opremcak et al.[33] Its efficacy has been demonstrated in multiple studies. A recent meta-analysis evaluated 200 patients from five studies and found there was no improvement in VA at 6 months for those receiving RON compared to controls, but those receiving RON had significantly improved VA at 12 months compared to controls. Those who had received RON had a pooled risk ratio of 2.27 for improvement of ≥3 lines of logMAR scale after 12 months when compared to controls.[34] RON also demonstrated the ability to decrease the risk of neovascular glaucoma and had similar rates of retinal detachment and vitreous hemorrhage compared to control groups.[34]
Corticosteroids
Intravitreal corticosteroids are a treatment option for CRVO. The “Standard Care versus Corticosteroid for Retinal Vein Occlusion” trial was a randomized clinical trial which treated individuals with nonischemic CRVO macular edema with 1 mg of 4 mg of intravitreal triamcinolone injections every 4 months compared to observation alone. At 12 months, those eyes treated with either dose of triamcinolone were five times more likely to have a 15-letter gain in visual acuity compared to the control group.[35] About 26.5% of participants who received 1 mg triamcinolone injections achieved a 15-letter improvement in best-corrected visual acuity (BCVA) at 12 months, while 25.6% of participants in the 4 mg injection group achieved this improvement. Only 6.8% of those in the observation group had a 15-letter improvement in BCVA at 12 months.[35] However, the participants who received triamcinolone injections were also more likely to require IOP-lowering treatment and have new development or progression of cataract.[35] In the 1 mg injection group, 16.3% of individuals had ≥10 mm Hg elevation in IOP and 5.4% had ≥35 mm Hg elevation in IOP. In the 4 mg injection group, 26.4% of participants had ≥10 mm Hg IOP elevation and 8.8% had ≥35 mm Hg IOP elevation. Control group participants were less likely to develop elevated IOP with only 2.3% developing ≥10 mm Hg elevation in IOP and 1.1% developing ≥35 mm Hg IOP elevation.[35]
The GENEVA trial compared a single dexamethasone implant injection at a dose of 0.35 mg or 0.7 mg to a sham implant for participants with nonischemic BRVO or CRVO macular edema. Participants receiving the dexamethasone implant had increased visual acuity at 30 and 60 days, but not at 90 or 180 days.[36] At day 180, 41% of participants in the 0.7 mg implant group achieved 15-letter improvement in BCVA compared to 40% and 23% in the 0.35 mg implant and sham implant groups, respectively.[36] There was also a higher incidence of ocular hypertension (4% vs. 0.7%, P < 0.002), requirement for IOP-lowering medication (24% in the steroid group), and procedural treatment for elevated IOP among the patients who received dexamethasone implants. These participants also had increased anterior chamber activity compared to the sham group.[36] In an additional continuation study for the dexamethasone implant, patients who received a 0.7 mg dexamethasone implant had increased visual acuity at 60 days but no evidence of sustained visual acuity gain at 12 months.[37] Participants who received the dexamethasone implant also had increased progression of cataracts (29.8% in the retreated 0.7 mg group), increased incidence of elevated IOP (32.8% of patients in the 0.7 mg group), and more individuals requiring IOP-lowering medications and procedures than those who received a sham implant.[37] A recent retrospective study has also demonstrated that dexamethasone intravitreal implants are not only effective as a first-line agent for CRVO-related macular edema but also for refractory cases as well.[38] Corticosteroid treatments have demonstrated the ability to improve visual acuity in the short-term and have proven, especially useful in pseudophakic patients or patient who do not experience significant IOP elevation with local steroid use. They are a useful option for the treatment of macular edema due to retinal vein occlusion.
Conclusion
Intravitreal VEGF blockade is a standard treatment for macular edema due to CRVO. Intravitreal aflibercept is an effective and safe treatment option. Level 1 medical evidence has demonstrated that aflibercept can improve vision and reduce retinal thickening in eyes with macular edema and CRVO.
Financial support and sponsorship
Dr. Do is a consultant and receives research funding from Allergan, Genentech, and Regeneron.
Dr. Nguyen serves on the Scientific Advisory Board for AbbVie, Genentech, Regeneron, and Santen.
Conflicts of interest
The authors have no any conflicts of interest to declare.
References
- 1.Rogers SL, McIntosh RL, Lim L, Mitchell P, Cheung N, Kowalski JW, et al. Natural history of branch retinal vein occlusion: An evidence-based systematic review. Ophthalmology. 2010;117:1094–101.e5. doi: 10.1016/j.ophtha.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 2.Mitry D, Bunce C, Charteris D. Anti-vascular endothelial growth factor for macular oedema secondary to branch retinal vein occlusion. Cochrane Database Syst Rev. 2013;1:CD009510. doi: 10.1002/14651858.CD009510.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Braithwaite T, Nanji AA, Lindsley K, Greenberg PB. Anti-vascular endothelial growth factor for macular oedema secondary to central retinal vein occlusion. Cochrane Database Syst Rev. 2014;5:CD007325. doi: 10.1002/14651858.CD007325.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011;118:119–33.e1-2. doi: 10.1016/j.ophtha.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, et al. The prevalence of retinal vein occlusion: Pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117:313–9.e1. doi: 10.1016/j.ophtha.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laouri M, Chen E, Looman M, Gallagher M. The burden of disease of retinal vein occlusion: Review of the literature. Eye (Lond) 2011;25:981–8. doi: 10.1038/eye.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol. 1996;114:1243–7. doi: 10.1001/archopht.1996.01100140443012. [DOI] [PubMed] [Google Scholar]
- 8.Liu W, Xu L, Jonas JB. Vein occlusion in Chinese subjects. Ophthalmology. 2007;114:1795–6. doi: 10.1016/j.ophtha.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda M, Kiyohara Y, Arakawa S, Hata Y, Yonemoto K, Doi Y, et al. Prevalence and systemic risk factors for retinal vein occlusion in a general Japanese population: The Hisayama study. Invest Ophthalmol Vis Sci. 2010;51:3205–9. doi: 10.1167/iovs.09-4453. [DOI] [PubMed] [Google Scholar]
- 10.Lim LL, Cheung N, Wang JJ, Islam FM, Mitchell P, Saw SM, et al. Prevalence and risk factors of retinal vein occlusion in an Asian population. Br J Ophthalmol. 2008;92:1316–9. doi: 10.1136/bjo.2008.140640. [DOI] [PubMed] [Google Scholar]
- 11.Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: The Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–41. [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung N, Klein R, Wang JJ, Cotch MF, Islam AF, Klein BE, et al. Traditional and novel cardiovascular risk factors for retinal vein occlusion: The multiethnic study of atherosclerosis. Invest Ophthalmol Vis Sci. 2008;49:4297–302. doi: 10.1167/iovs.08-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolar P. Risk factors for central and branch retinal vein occlusion: A meta-analysis of published clinical data. J Ophthalmol 2014. 2014:724780. doi: 10.1155/2014/724780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lahey JM, Tunç M, Kearney J, Modlinski B, Koo H, Johnson RN, et al. Laboratory evaluation of hypercoagulable states in patients with central retinal vein occlusion who are less than 56 years of age. Ophthalmology. 2002;109:126–31. doi: 10.1016/s0161-6420(01)00842-9. [DOI] [PubMed] [Google Scholar]
- 15.Brown DM, Campochiaro PA, Singh RP, Li Z, Gray S, Saroj N, et al. Ranibizumab for macular edema following central retinal vein occlusion: Six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124–33.e1. doi: 10.1016/j.ophtha.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Holz FG, Roider J, Ogura Y, Korobelnik JF, Simader C, Groetzbach G, et al. VEGF trap-eye for macular oedema secondary to central retinal vein occlusion: 6-month results of the phase III GALILEO study. Br J Ophthalmol. 2013;97:278–84. doi: 10.1136/bjophthalmol-2012-301504. [DOI] [PubMed] [Google Scholar]
- 17.Heier JS, Clark WL, Boyer DS, Brown DM, Vitti R, Berliner AJ, et al. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: Two-year results from the COPERNICUS study. Ophthalmology. 2014;121:1414–20.e1. doi: 10.1016/j.ophtha.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 18.Epstein DL, Algvere PV, von Wendt G, Seregard S, Kvanta A. Benefit from bevacizumab for macular edema in central retinal vein occlusion: Twelve-month results of a prospective, randomized study. Ophthalmology. 2012;119:2587–91. doi: 10.1016/j.ophtha.2012.06.037. [DOI] [PubMed] [Google Scholar]
- 19.Wroblewski JJ, Wells JA, 3rd, Adamis AP, Buggage RR, Cunningham ET, Jr, Goldbaum M, et al. Pegaptanib sodium for macular edema secondary to central retinal vein occlusion. Arch Ophthalmol. 2009;127:374–80. doi: 10.1001/archophthalmol.2009.14. [DOI] [PubMed] [Google Scholar]
- 20.Kinge B, Stordahl PB, Forsaa V, Fossen K, Haugstad M, Helgesen OH, et al. Efficacy of ranibizumab in patients with macular edema secondary to central retinal vein occlusion: Results from the sham-controlled ROCC study. Am J Ophthalmol. 2010;150:310–4. doi: 10.1016/j.ajo.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 21.Economides AN, Carpenter LR, Rudge JS, Wong V, Koehler-Stec EM, Hartnett C, et al. Cytokine traps: Multi-component, high-affinity blockers of cytokine action. Nat Med. 2003;9:47–52. doi: 10.1038/nm811. [DOI] [PubMed] [Google Scholar]
- 22.Stewart MW, Grippon S, Kirkpatrick P. Aflibercept. Nat Rev Drug Discov. 2012;11:269–70. doi: 10.1038/nrd3700. [DOI] [PubMed] [Google Scholar]
- 23.Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171–85. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyer D, Heier J, Brown DM, Clark WL, Vitti R, Berliner AJ, et al. Vascular endothelial growth factor trap-eye for macular edema secondary to central retinal vein occlusion: Six-month results of the phase 3 COPERNICUS study. Ophthalmology. 2012;119:1024–32. doi: 10.1016/j.ophtha.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 25.Eadie JA, Ip MS, Kulkarni AD. Response to aflibercept as secondary therapy in patients with persistent retinal edema due to central retinal vein occlusion initially treated with bevacizumab or ranibizumab. Retina. 2014;34:2439–43. doi: 10.1097/IAE.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann-Clarke L, Dirani A, Mantel I, Ambresin A. The effect of switching ranibizumab to aflibercept in refractory cases of macular edema secondary to ischemic central vein occlusion. Klin Monbl Augenheilkd. 2015;232:552–5. doi: 10.1055/s-0035-1545783. [DOI] [PubMed] [Google Scholar]
- 27.Pfau M, Fassnacht-Riederle H, Becker MD, Graf N, Michels S. Clinical outcome after switching therapy from ranibizumab and/or bevacizumab to aflibercept in central retinal vein occlusion. Ophthalmic Res. 2015;54:150–6. doi: 10.1159/000439223. [DOI] [PubMed] [Google Scholar]
- 28.Papakostas TD, Lim L, van Zyl T, Miller JB, Modjtahedi BS, Andreoli CM, et al. Intravitreal aflibercept for macular oedema secondary to central retinal vein occlusion in patients with prior treatment with bevacizumab or ranibizumab. Eye (Lond) 2016;30:79–84. doi: 10.1038/eye.2015.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manousaridis K, Peter S, Mennel S. Outcome of intravitreal dexamethasone implant for the treatment of ranibizumab-resistant macular edema secondary to retinal vein occlusion. Int Ophthalmol. 2017;37:47–53. doi: 10.1007/s10792-016-0226-3. [DOI] [PubMed] [Google Scholar]
- 30.Ozkok A, Saleh OA, Sigford DK, Heroman JW, Schaal S. THE OMAR STUDY: Comparison of ozurdex and triamcinolone acetonide for refractory cystoid macular edema in retinal vein occlusion. Retina. 2015;35:1393–400. doi: 10.1097/IAE.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 31.McAllister IL, Gillies ME, Smithies LA, Rochtchina E, Harper CA, Daniell MD, et al. The central retinal vein bypass study: A trial of laser-induced chorioretinal venous anastomosis for central retinal vein occlusion. Ophthalmology. 2010;117:954–65. doi: 10.1016/j.ophtha.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 32.Mirshahi A, Roohipoor R, Lashay A, Mohammadi SF, Mansouri MR. Surgical induction of chorioretinal venous anastomosis in ischaemic central retinal vein occlusion: A non-randomised controlled clinical trial. Br J Ophthalmol. 2005;89:64–9. doi: 10.1136/bjo.2004.045278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Opremcak EM, Bruce RA, Lomeo MD, Ridenour CD, Letson AD, Rehmar AJ. Radial optic neurotomy for central retinal vein occlusion: A retrospective pilot study of 11 consecutive cases. Retina. 2001;21:408–15. doi: 10.1097/00006982-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Chen ZN, Shao Y, Li XR. Radial optic neurotomy in treating central retinal vein occlusion: A Meta-analysis. Int J Ophthalmol. 2016;9:898–903. doi: 10.18240/ijo.2016.06.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott IU, Van Veldhuisen PC, Ip MS, Blodi BA, Oden NL, Awh CC, et al. Effect of bevacizumab vs. aflibercept on visual acuity 15 among patients with macular edema due to central retinal vein 16 occlusion: The SCORE2 Randomized Clinical Trial. J Am Med Assoc. 2017 doi: 10.1001/jama.2017.4568. [Doi: 10.1001/jama.2017.4568] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haller JA, Bandello F, Belfort R, Jr, Blumenkranz MS, Gillies M, Heier J, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134–46.e3. doi: 10.1016/j.ophtha.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 37.Haller JA, Bandello F, Belfort R, Jr, Blumenkranz MS, Gillies M, Heier J, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118:2453–60. doi: 10.1016/j.ophtha.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Sheu SJ, Wu TT, Horng YH. Efficacy and safety of dexamethasone intravitreal implant for treatement of refractory macular edema secondary to retinal vein occlusion in Taiwan. J Ocul Pharmacol Ther. 2015;31:461–7. doi: 10.1089/jop.2014.0119. [DOI] [PubMed] [Google Scholar]


