Abstract
The amyloid precursor protein (APP) has long been appreciated for its role in Alzheimer’s disease (AD) pathology. However, less is known about the physiologic function of APP outside of AD. Particularly, whether and how APP may regulate functions of cell surface receptors, including GPCRs, remains largely unclear. In this study, we identified a novel direct interaction between APP and the α2A-adrenergic receptor (α2AAR) that occurs at the intracellular domains of both proteins. The APP interaction with α2AAR is promoted by agonist stimulation and competes with arrestin 3 binding to the receptor. Consequently, the presence of APP attenuates α2AAR internalization and desensitization, which are arrestin-dependent processes. Furthermore, in neuroblastoma neuro-2A cells and primary superior cervical ganglion neurons, where APP is highly expressed, the lack of APP leads to a dramatic increase in plasma membrane recruitment of endogenous arrestin 3 following α2AAR activation. Concomitantly, agonist-induced internalization of α2AAR is significantly enhanced in these neuronal cells. Our study provided the first evidence that APP fine tunes GPCR signaling and trafficking. Given the important role of α2AAR in controlling norepinephrine release and response, this novel regulation of α2AAR by APP may have an impact on modulation of noradrenergic activity and sympathetic tone.—Zhang, F., Gannon, M., Chen, Y., Zhou, L., Jiao, K., Wang, Q. The amyloid precursor protein modulates α2A-adrenergic receptor endocytosis and signaling through disrupting arrestin 3 recruitment.
Keywords: internalization, desensitization, interactions, cell surface receptor
As the source of amyloid-β peptides, the amyloid precursor protein (APP) has been well appreciated for its function in AD. Outside of the disease, APP plays an important role in neuronal development, regulating neurite outgrowth, and synaptogenesis (1, 2). APP-deficient mice show several neurologic phenotypes that could be related to synaptogenesis, including decreased grip strength (3), alterations in spine density, and diminished performance on spatial memory tasks (4, 5). In mammals, the APP family also includes 2 APP-like proteins, APP-like protein (APLP)-1 and -2, which share partially overlapping biologic activities with APP (6). Despite significant progress being made in understanding the physiologic significance of APP after several decades of research on the protein, the mechanisms behind its functions are still unclear, and our understanding of the diverse activities of APP remains incomplete.
APP has been shown to interact with several transmembrane and cytosolic proteins (7, 8). Although more efforts have been made to understand how such interactions regulate APP trafficking and processing, less is known about the role of APP in regulating the functions of its partners. A previous finding on regulation of the NGF/TrkA signaling (9) by APP suggests that interacting with other cell surface receptors and regulating their downstream signaling may represent an important means for APP to elicit its physiologic functions. Despite GPCRs being the largest family of cell surface receptors, whether and how APP may regulate GPCR functions has not been investigated.
The α2A-adrenergic receptor (α2AAR) is a prototypical GPCR, and mediates a variety of critical physiologic/pharmacological responses, which include lowering blood pressure, evoking sedation, reducing pain perception, and decreasing epileptogenesis and anxiety (10–12). Positioned at both the presynaptic noradrenergic terminal and the postsynaptic compartments of targeting neurons, the α2AAR controls both noradrenergic input to the cerebrum and the resulting response in this brain region (13). In addition, α2AAR serves as the primary autoreceptor in sympathetic neurons, controlling norepinephrine (NE) release and sympathetic tone (14, 15). Like many GPCRs, the non-G-protein–interacting partners of α2AAR modulate multiple aspects of the receptor function both in vitro and in vivo (16–20). Among these partners, a universal GPCR regulator, arrestin 3, binds to α2AAR after receptor activation and mediates agonist-induced endocytosis and desensitization of α2AAR (17, 18, 21). As a result, arrestin 3 determines the response sensitivity of α2AAR in multiple pharmacological settings in vivo (18, 20).
In this study, we discovered a novel direct interaction between APP and the α2AAR through the intracellular portions of each protein. We hypothesized that APP binding to α2AAR has functional consequences on receptor trafficking and signaling. Using both gain- and loss-of-function approaches, we demonstrated that the presence of APP antagonizes arrestin-dependent endocytosis and desensitization of α2AAR. Consistent with these observations, we discovered that the interaction of APP with α2AAR competes with the interaction of arrestin and α2AAR. Furthermore, we extended our studies to primary superior cervical ganglion (SCG) neurons, where the α2AAR is the major autoreceptor and demonstrated the APP antagonism of arrestin function in this native setting.
MATERIALS AND METHODS
Antibodies and chemicals
Antibodies (Abs) for GAPDH and APP (22C11) were purchased from EMD Millipore (Billerica, MA, USA); APP rabbit mAb (Y188) from Abcam (Cambridge, United Kingdom); HA.11 Ab for detecting HA-tagged α2AAR from Covance (Princeton, NJ, USA); Abs for phospho-ERK1/2 (Thr202/Tyr204), ERK, and the green fluorescent protein (GFP) mAb from Cell Signaling Technology (Danvers, MA, USA); Flag M2 Ab from Sigma-Aldrich (St. Louis, MO, USA); secondary Abs used for immunostaining (Alexa Fluor 488- and 594-conjugated) from Thermo Fisher Scientific (Waltham, MA, USA); secondary Abs used for Western blot with the Li-Cor Odyssey Imaging System (IRDye 680 and 800; Li-Cor Biosciences, Lincoln, NE, USA); Lipofectamine 2000 from Thermo Fisher Scientific; NE, clonidine, guanfacine, UK14304, yohimbine, propranolol, and prazosin from Sigma-Aldrich; and [35S]Methionine from GE Healthcare (Little Chalfont, United Kingdom).
Cell culture
Neuro-2A (N2a) cells were cultured in 1:1 DMEM/Opti-MEM mix (Thermo Fisher Scientific) supplemented with 5% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin. HEK293 cells were cultured in DMEM with 10% fetal bovine serum plus 100 U/ml penicillin, and 100 µg/ml streptomycin (Thermo Fisher Scientific).
N2a-HA-α2AAR-APP-CRISPR cells are a stable N2a cell line expressing HA-α2AAR with APP knocked out by the CRISPR/Cas9 system. This cell line was generated according to a published protocol (22). Two target genomic DNA sequence primers were designed and annealed (forward: 5′-CACCACTGCAGATCACAAACGTGG-3′ and reverse: 5′-AAACCCACGTTTGTGATCTGCAGT-3′). Using the BbsI restriction enzyme, the primers were introduced to the pSpCas9(BB)-2A-GFP plasmid. An N2a-HA-α2AAR stable cell line (23) was transfected with the resulting plasmid using Lipofectamine 2000 and selected for GFP expression by fluorescence-activated cell sorting. Single clones were allowed to grow and screened for loss of expression of APP. N2a-HA-α2AAR cells transfected with the empty pSpCas9(BB)-2A-GFP vector were established as a control cell line. APP knockout (KO) in the N2a-HA-α2AAR-APP-CRISPR line was confirmed using both Western blot and immunostaining, and continued expression of APP in the N2a-HA-α2AAR-control-CRISPR was confirmed in the same way.
For small interfering RNA (siRNA) treatment, siRNAs targeting mouse APP were purchased from Integrated DNA Technologies (Coralville, IA, USA) (TriFecta Kit DsiRNA Duplex), Duplex sequences: 5′-ACUAGUGCAUGAAUAGAUUCUCUCC-3′ and 3′-UUUGAUCACGUACUUAUCUAAGAGAGG-5′; 5′-GGAGAUUCAAGAUGAAGUCGAUGAG-3′ and 3′-CUCCUCUAAGUUCUACUUCAGCUACUC-5′ and were transfected into cells using Lipofectamine 2000. A negative control siRNA, also from Integrated DNA Technologies (NC1 Control Duplex) was transfected into cells to use as the control.
Animals and drug treatment
Mice were housed in the Association for Assessment and Accreditation of Laboratory Animal Care–accredited Animal Resources Program facility at the University of Alabama at Birmingham, in accordance with procedures of the Animal Welfare Act and the 1989 amendments to the Act, and all studies followed protocols approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
The generation of α2AAR-knock-in mice has been described in Lu et al. (24). This line has been backcrossed for more than 10 generations to a pure C57BL/6 background. The mice (6 mo old) were treated though intraperitoneal injection with saline, clonidine, guanfacine, or UK14304 (1 mg/kg) 1 h before euthanizing for the experiments. Postnatal d 5–7 pups were used to culture SCG neurons.
Primary culture of SCG neurons
SCG neurons were cultured from HA-α2AAR-knock-in (24) mouse pups at postnatal d 5–7, as described elsewhere (24–26), with slight modifications. SCGs were dissected and placed into HBSS (Thermo Fisher Scientific) with 25 mM glucose and 20 mM HEPES (pH 7.3). The neurons were enzymatically digested with 3 mg/ml collagenase (Sigma-Aldrich) and 1 mg/ml trypsin (Sigma-Aldrich). Using trituration with a fire-polished siliconized Pasteur pipette, we dissociated the neurons by trituration. A preplating step was then performed to reduce non-neuronal cell types in the final culture. The neurons were then plated onto coverslips treated with poly-d-lysine (Sigma-Aldrich) and laminin (Thermo Fisher Scientific). Growth medium was L-15 base medium (Thermo Fisher Scientific) supplemented with 10% Nu-serum (Corning, Corning, NY, USA), 30% glucose, 2% GlutaMax (Thermo Fisher Scientific), 1% insulin/transferrin/selenium supplement (Thermo Fisher Scientific), 25 ng/ml nerve growth factor (Thermo Fisher Scientific), and 24 nM NaHCO3. Medium was changed on days in vitro (DIV) 1, 4, and 6. On DIV 1 and 4, 10 µM 5-fluoro-2′-deoxyuridine (Sigma-Aldrich) was added to control nonneuronal cell growth, and on DIV 4 and 6, 1 µM yohimbine (α2AAR antagonist) was added to preserve cell surface α2AARs. All experiments were performed on DIV 8.
Immunofluorescence staining
To examine colocalization between α2AAR and APP on the plasma membrane, live cells were first incubated with a hemagglutinin (HA; rat anti-HA.11) and APP (mouse 22C11) Ab to label cell surface HA-α2AAR and APP, respectively. Cells were then treated with vehicle or clonidine (1 μM) for 5 min. After stimulation, the cells were fixed and then incubated with Alexa Fluor 488–conjugated anti-mouse and Alexa Fluor 594–conjugated anti-rat secondary Abs. Images were obtained using an LSM 710 confocal microscope (Zeiss, Oberkochen, Germany), with a ×63 oil magnification. Colocalization was estimated with Pearson’s correlation coefficient in ImageJ software (27).
For the arrestin recruitment staining, N2a cells or SCG neurons were treated with NE (10 μM) in the presence of prazosin (1 μM) and propranolol (1 μM) for various times. Cell were then fixed, permeabilized, and incubated with rabbit arrestin 3 Ab (kindly provided by the J. Benovic laboratory at Thomas Jefferson University, Philadelphia PA, USA) and mouse APP Ab (22C11) followed by Alexa Fluor 488-conjugated anti-rabbit and Alexa Fluor 594-conjugated anti-mouse secondary Ab. Images were obtained with a U-TBI90 confocal microscope (Olympus Corporation, Shinjuku, Tokyo, Japan) at ×63 oil magnification. The percentage of recruitment was calculated with ImageJ to determine both surface and internal fluorescence activity, and the relative amount of fluorescence activity on the membrane compared to the total fluorescence activity was calculated.
α2AAR internalization assays
Immunofluorescent staining
To assess α2AAR internalization, a published prelabeling method was used (24, 25, 28). In brief, the HA-tagged α2AARs on the surface of live cells were labeled with a HA Ab (HA.11) before treatment. After labeling of the receptor, the cells were stimulated with 10 μM NE (plus 1 µM prazosin and 1 µM propranolol to selectively activate the α2AAR) for various time points. After fixation and permeabilization, the cells were incubated with a rabbit APP Ab (Y188) followed by anti-mouse Alexa Fluor 488- and anti-rabbit Alexa Fluor 594 conjugated secondary Abs. Images were obtained with an LSM 710 confocal microscope (Zeiss), with ×63 oil magnification. Quantification of internalization was performed with MetaMorph software (Molecular Devices, Sunnyvale, CA, USA), to calculate both surface and internal fluorescence activity, and a ratio of internal fluorescence over total fluorescence was determined to give an arbitrary unit of internalization.
Intact cell surface ELISA
Internalization of α2AAR was examined by intact cell surface ELISA, according to our procedure (21, 28). In brief, HEK293 cells coexpressing HA-α2AAR, with or without APP, were plated onto 96-well culture plates at 1 × 104 cells/well. Cells were stimulated acutely with NE for the indicated time, followed by fixation. Samples were then subjected to blocking, primary Ab (HA.11), and secondary Ab (horseradish peroxidase–conjugated anti-mouse) and then incubated with o-phenylenediamine substrate (Thermo Fisher Scientific). Cell surface receptor density was determined by measuring absorbance at 490 nm.
In vitro glutathione S-transferase pull-down assay
cDNA encoding the C terminus of APP (APP-C; aa 639–695) was cloned into the pGEX4T vector. Preparation of glutathione S-transferase (GST) and GST-fused APP-C was performed as previously described (16, 29). [35S]-labeled, in vitro translated, α2AAR third intracellular loop (3iloop) was prepared with the TNT rabbit reticulocyte lysate kit (Promega, Madison, WI, USA) (16). Pull-down assays with 2 μg of GST or GST-fused APP-C in each sample were performed according to procedures described elsewhere (16, 29). In each assay, the same SDS-PAGE gel for autoradiography was stained with Coomassie Blue to confirm that a comparable amount of GST or GST-fusion protein was loaded in each lane.
Coimmunoprecipitation to detect APP-α2AAR interaction
Coimmunoprecipitation (co-IP) assays were performed according to published procedures (28, 29). In brief, after stimulation, HEK or N2a cells were lysed on ice in immunoprecipitation (IP) buffer [10 mM Tris (pH = 7.4), 10% glycerol, 5 mM EGTA, 5 mM EDTA, 0.3% NP-40 and protease inhibitors], after stimulation with the appropriate ligands or vehicle. After 20 min of centrifugation at 14,800 rpm at 4°C, the supernatant was subjected to IP. For anti-HA IP, cell lysates were incubated with rat Anti-HA Affinity Matrix (Roche, Basel, Switzerland) overnight at 4°C. For anti-APP IP, cell lysates were incubated with anti-APP-C Ab (1:100 dilution; Sigma-Aldrich), followed by incubation with protein A/G beads. HA-α2AAR and the full-length APP in the IP complex or total lysates were detected by Western blot with mouse HA.11 and rabbit anti-APP (Y188; Abcam) Ab, respectively. Western blots were quantified with the Odyssey Imaging System (Li-Cor) according to the manufacturer’s instruction.
To examine α2AAR interaction with APPΔC, the HindIII–ApaI fragment coding aa 1–649 of APP was amplified by PCR, using (forward) 5′-CCCAAGCTTATGCTGCCCGGTTTGGCACTGCTCCTG-3′ and (reverse) 5′-CCAGGGCCCCTACTTGTCGTCGTCGTCCTTGTAATCTGCTGCCTTCAGCATCACCAAGGTGATG-3′, and cloned into the pCMV-tag2 vector (Agilent Technologies, La Jolla, CA, USA) to generate the FLAG-APPΔC construct.
Quantification of ERK1/2 activation
To test ERK1/2 activation, both HEK293 and N2a-HA-α2AAR-APP-CRISPR or control cells with the empty vector were used. HEK293 cells were transfected with either the empty vector or APP. Cells were stimulated by 10 μM NE (plus 1 μM prazosin and 1 μM propranolol) for various time points. Cell lysates were collected and run in a Western blot, using Abs for p-ERK and t-ERK. Western blots were quantified using the Odyssey Imaging System (Li-Cor) according to the manufacturer’s instructions. Activation of ERK1/2 was calculated by dividing p-ERK1/2 by t-ERK1/2. To calculate the relative change of the ERK1/2 activation, the percentage of activation remaining at 10 min was determined by dividing the amount of activated ERK at 10 min by the amount of activated ERK at the peak activation, or 5 min.
Statistical analyses
Statistical analyses were performed with Prism software (GraphPad, La Jolla, CA, USA). All values are presented as means ± sem. To determine difference between 2 groups with 1 variable, an unpaired Student’s t test was performed, with P < 0.05 considered significant. Intact cell surface ELISA was analyzed by 2-way ANOVA followed by Sidak’s multiple comparisons test.
RESULTS
APP interacts with the α2AAR in an α2AAR agonist-regulated manner
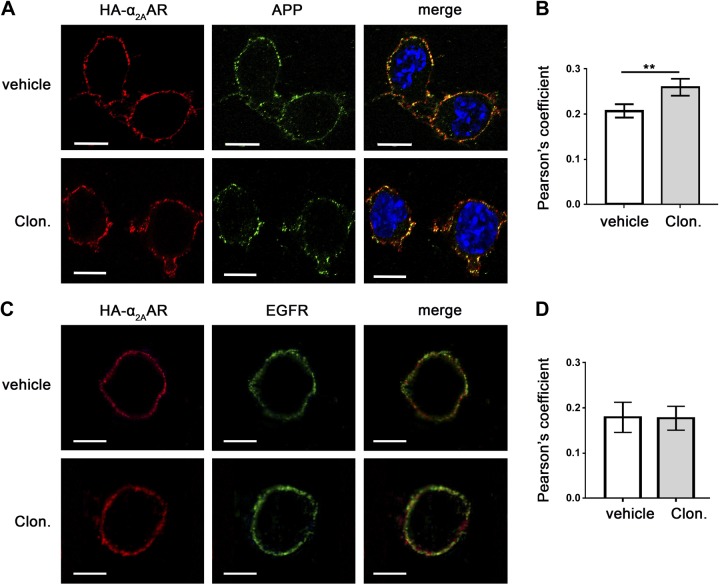
Both APP and α2AAR are transmembrane proteins. We used an intact-cell Ab-prelabeling method to specifically detect α2AAR and APP localized on the plasma membrane. A significant amount of APP (endogenously expressed) and HA-tagged α2AAR (exogenously transfected) were colocalized in the same compartment on the cell surface of N2a cells (Fig. 1A). This colocalization was further enhanced with stimulation of the receptor with an α2AAR agonist, clonidine, as revealed by a significant increase in Pearson’s correlation coefficient in clonidine-treated cells compared to vehicle-treated cells (Fig. 1B). Although colocalization of α2AAR with another cell surface protein, epidermal growth factor receptor, was detected (Fig. 1C), clonidine stimulation did not change the degree of the colocalization between these two proteins (Fig. 1D).
Figure 1.
Agonist stimulation enhances APP and α2AAR colocalization on the plasma membrane. A, B) N2a cells stably expressing HA-α2AAR were first labeled with primary Abs against HA (rat) and APP (22C11, mouse) and then treated with vehicle or 1 µM clonidine for 5 min. After treatment, cells were fixed and stained with fluorescence-conjugated secondary Abs. A) Representative images showing localization of HA-α2AAR and endogenous APP in cells. B) Quantification of colocalization between APP and α2AAR, using Pearson’s coefficient. Data are means ± sem (n = 11–23 cells per condition). **P < 0.01 (Student’s t test). C, D) N2a cells stably expressing the HA-α2AAR were first labeled with primary Abs against HA and then stimulated. Cells were fixed, permeabilized, and incubated with rabbit anti–epidermal growth factor receptor Ab, followed by fluorescence-conjugated secondary Abs. C) Representative images showing localization of HA-α2AAR and endogenous APP in cells. D) Quantification of colocalization between APP and α2AAR, using Pearson’s coefficient. Data are means ± sem (n = 12–14 per condition). Scale bars, 10 μm.
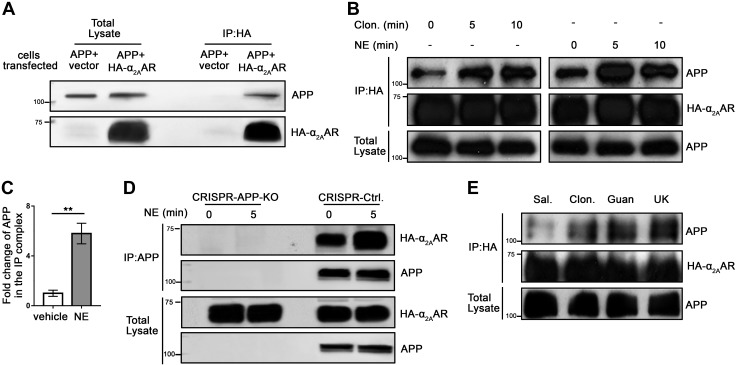
Since APP and α2AAR appear to be located in the same compartment on the plasma membrane, we next determined whether these two proteins actually interact with each other by co-IP assays. In HEK293 cells coexpressing APP and HA-α2AAR, but not in cells expressing APP alone, APP was coimmunoisolated with HA-α2AAR by an anti-HA Ab (Fig. 2A and Supplemental Fig. 1A). Furthermore, when α2AAR was stimulated with an agonist, NE (in the presence of propranolol and prazosin to selectively activate α2AAR) or clonidine, the level of APP coimmunoprecipitated with HA-α2AAR was markedly increased (Fig. 2B). The quantification shows that 5 min of NE stimulation led to a nearly 5-fold increase in the level of APP in the IP complex over the control (Fig. 2C). We then tested the interaction between APP (endogenously expressed) and HA-α2AAR (exogenously transfected) in N2a cells by co-IP with an APP Ab. To control the specificity of the IP assays, we generated an N2a cell line with the App gene inactivated (APP-KO) using the CRISPR/Cas9 technique (Fig. 2D). In control N2a cells (generated in parallel with the empty CRISPR vector), but not in APP-KO cells, HA-α2AAR was coimmunoisolated with APP by an anti-APP Ab, and the amount of α2AAR in the immunoprecipitation complex was further increased by NE stimulation (Supplemental Fig. 1B). Together, these results indicate that α2AAR and APP form a complex in cells and activation of α2AAR enhances the formation of this complex.
Figure 2.
α2AAR agonists promote complex formation between APP and α2AAR in cells and in the mouse brain. A–C) HEK293 cells coexpressing APP with or without HA-α2AAR were treated with 10 µM NE (plus 1 µM prazosin and 1 µM propranolol), clonidine (1 μM), or vehicle. Cell lysates were then subjected to co-IP assays with anti-HA affinity matrix. A, B) Representative Western blots. C) Quantification of the amount of APP coimmunoprecipitated with HA-α2AAR in cells treated with NE for 5 min. Data (means ± sem) are expressed as fold change compared with vehicle control (defined as 1.0) (n = 8 in each group). **P < 0.01, NE vs. control (Student’s t test). D) Control or APP-KO N2a cells expressing HA-α2AAR were stimulated with vehicle or 10 µM NE (plus 1 µM prazosin and 1 µM propranolol) for 5 min. Cell lysates were subjected to co-IP assays using an anti-APP Ab. Representative Western blots are shown. E) Adult HA-α2AAR-knock-in mice were injected (i.p. with saline or 1 mg/kg of clonidine, guanfacine, or UK14304). At 1 h after injection, cortices were dissected and homogenized. Cortical lysates were subjected to IP assays using anti-HA matrix. Representative Western blots are shown.
We further examined the interaction between endogenous APP and α2AAR in vivo in the brain by exploiting an HA-α2AAR-knock-in mouse line that we had generated (24). Mice were injected with saline or one of 3 different α2 agonists: clonidine, guanfacine, and UK14304 (1 mg/kg, i.p.). One hour after injection, the cortices were collected, lysed, and subjected to co-IP assays using an HA Ab. Stimulation with any one of the 3 agonists markedly increased the amount of APP in the IP complex with α2AAR (Fig. 2E). These data clearly demonstrate endogenous interaction between APP and α2AR in the mouse brain, which is promoted by α2AR agonist treatment.
APP and α2AAR directly interact through their intracellular domains
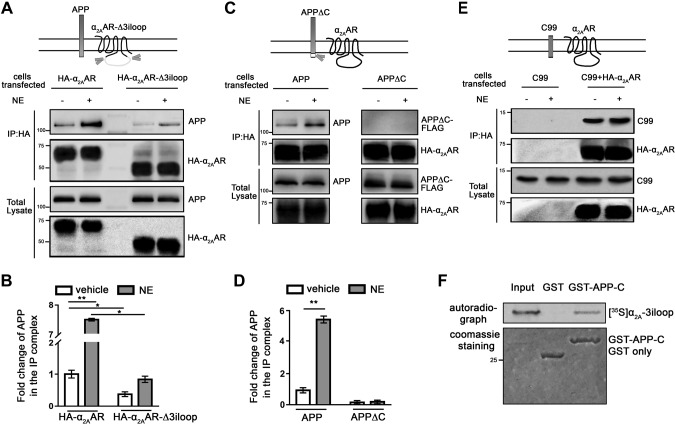
We have reported that the 3iloop of α2AAR can interact with multiple regulatory proteins (16–18). We therefore sought to test whether this region is also involved in the interaction with APP. We performed co-IP assays with HEK293 cells coexpressing APP and HA-tagged mutant α2AAR with the 3iloop deleted (HA-α2AAR-Δ3iloop) (30). The removal of the 3iloop significantly decreased the level of APP coimmunoprecipitated with α2AAR (Fig. 3A, B), indicating that this portion of the receptor is essential for its interaction with APP. This result also suggests that the interaction between APP and α2AAR likely occurs intracellularly. In this case, the intracellular domain of APP would be necessary for its interaction with α2AAR. To test this idea, we examined the interaction between HA-α2AAR and Flag-tagged mutant APP, in which the C-terminal intracellular domain was deleted. Although we readily detected the full-length APP in the IP complex with α2AAR, we failed to detect the APPΔC-α2AAR interaction in parallel experiments using the same experimental conditions (Fig. 3C, D), suggesting the requirement of APP-C for interaction with α2AAR. Furthermore, when coexpressed with HA-α2AAR, the C99 fragment of APP, which contains the transmembrane and intracellular domains of APP, also formed a complex with the receptor (Fig. 3E). Complex formation between α2AAR and C99 seemed not to be affected by agonist stimulation of the receptor.
Figure 3.
APP and α2AAR interact through their intracellular domains. A, B) Deletion of the α2AAR 3iloop significantly reduced its ability to interact with APP. HEK293 cells coexpressing APP with HA-α2AAR or HA-α2AAR-Δ3iloop were treated with 10 µM NE (plus 1 µM prazosin and 1 µM propranolol) or vehicle for 5 min. Cell lysates were subjected to IP assays with an HA Ab. A) Representative Western blots. B) Quantification of the amount of APP in the IP complex. Data (means ± sem) are expressed as fold change vs. vehicle control (defined as 1.0) (n = 3 in each group). *P < 0.05; **P < 0.01 (Student’s t test). C, D) Removal of the APP-C abolished its interaction with the α2AAR. HEK293 cells coexpressing HA-α2AAR with APP or APPΔC were treated with 10 µM NE (plus 1 µM prazosin and 1 µM propranolol) or vehicle for 5 min. Cell lysates were subjected to IP assays. C) Representative Western blots. D) Quantification of the amount of APP and APPΔC in the IP complex. Data (means ± sem) are expressed as fold change vs. vehicle control (defined as 1.0) (n = 3 in each group). **P < 0.01 (Student’s t test). E) The C99 fragment of APP formed a complex with HA-α2AAR. HEK293 cells coexpressing C99, with or without HA-α2AAR were stimulated with vehicle or 10 µM NE (plus 1 µM prazosin and 1 µM propranolol), and cell lysates were subjected to IP assays. Representative Western blots are shown. F) In vitro GST pull-down assay showing direct interaction between the α2AAR 3iloop and APP C-terminal domain. Purified GST or GST-fused APP-C was incubated with [35S]-labeled, in vitro translated α2AAR-3iloop. Input probe represents one-tenth of the total input in each reaction.
We next examined whether the α2AAR 3iloop and C-terminal intracellular domain of APP directly interact with each other by in vitro GST pull-down assays. [35S]-labeled, in vitro translated α2AAR-3iloop was successfully pulled down with purified GST-fused APP-C but not with GST alone (Fig. 3F). Taken together, these results reveal a direct interaction between APP and α2AAR that requires the intracellular domains of these 2 proteins.
APP reduces agonist-dependent endocytosis of α2AAR
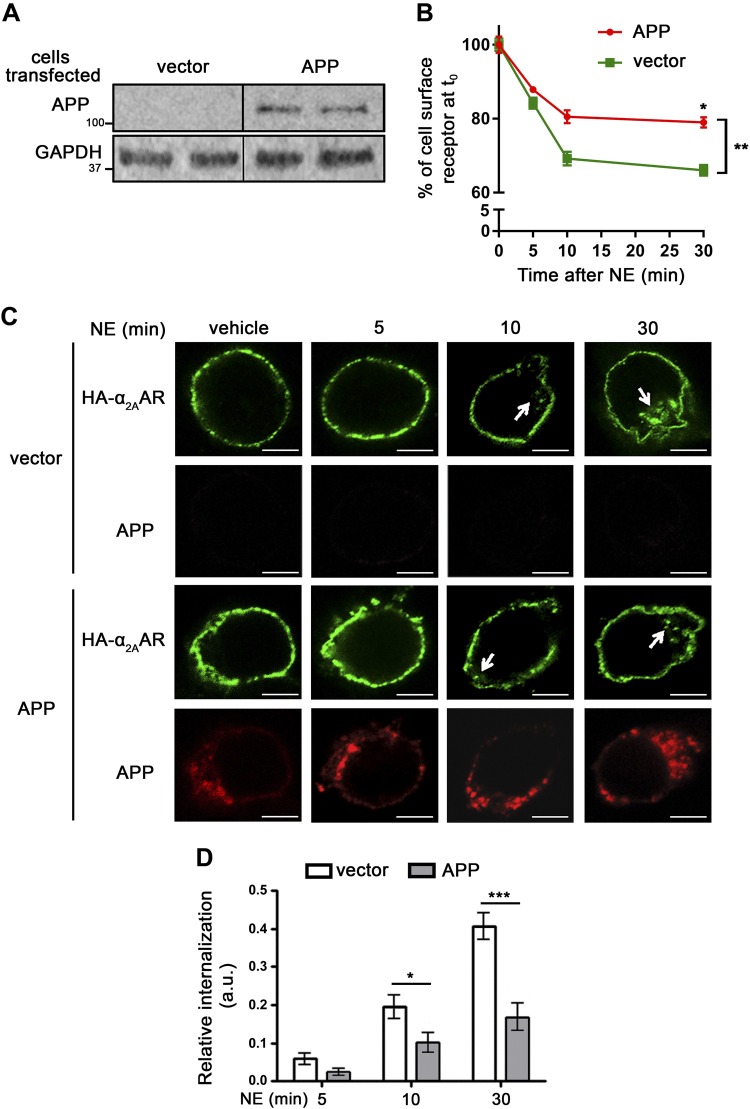
Having discovered this novel α2AAR–APP interacting pair, we next sought to determine the impact of this interaction on receptor trafficking and signaling. We first examined agonist-induced endocytosis of the receptor using a receptor prelabeling method that we have described in several publications (24, 25, 28). We used 2 different types of cell lines, HEK293 and N2a, for these studies, taking advantage of the different expression levels of endogenous APP in these cell lines. In HEK293 cells, the endogenous APP level is very low and undetectable by Western blot (Fig. 4A). In these cells, a significant portion of α2AAR internalized after treatment with NE (in the presence of prazosin and propranolol), as detected using 2 independent methods, intact cell surface ELISA (Fig. 4B) and Ab-prelabeling immunofluorescence (Fig. 4C). The expression of exogenous APP in HEK293 cells significantly reduced the amount of α2AAR that was internalized after NE treatment for both 10 and 30 min when compared to that in vehicle-treated control cells in both intact cell surface ELISA (Fig. 4B) and Ab-prelabeling immunofluorescence assays (Fig. 4C, D).
Figure 4.
Overexpression of APP in HEK293 cells reduces α2AAR internalization. HEK293 cells were cotransfected with cDNA vectors encoding HA-α2AAR, together with APP or empty vector. A) Western blot image showing expression of exogenous APP in HEK293 cells, which have a very low level of endogenous APP. B) Internalization of cell surface HA-α2AAR was assessed by intact cell surface ELISA. Internalization is indicated by the decrease in the percentage of surface receptor (with t = 0 set as 100%). Data are means ± sem (n = 4 per condition). *P < 0.05 by Sidak’s multiple comparisons test; **P < 0.01 by 2-way ANOVA. C, D) Internalization of HA-α2AAR was examined by an Ab-prelabeling method. C) Representative images showing internalization of cell surface HA-α2AAR after 10 µM NE treatment (plus 1 µM prazosin and 1 µM propranolol). Arrows: perinuclear punctate staining, which is indicative of endocytosed receptors. Scale bars, 5 μm. D) Quantitation of α2AAR endocytosis. Data are means ± sem (n = 17–23 cells per condition). *P < 0.05, ***P < 0.001 (Student’s t test).
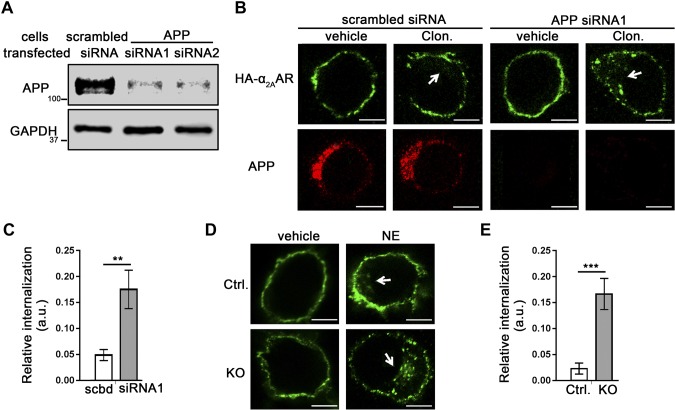
Contrary to HEK293 cells, N2a cells have a relatively high level of endogenous APP (Figs. 2D and 5A). We used siRNAs against APP to knock down its expression in these cells (Fig. 5A) and examined α2AAR endocytosis in response to the agonist clonidine. In N2a cells transfected with siRNA1, α2AAR internalization in response to 30 min of clonidine stimulation was significantly increased compared with that in cells treated with scrambled siRNA (Fig. 5B, C). Similar results were obtained with siRNA2 (data not shown). We further examined α2AAR internalization in APP-KO N2a cells, in which NE-induced α2AAR internalization was significantly enhanced compared to that in control cells that were generated in parallel with the empty CRISPR vector (Fig. 5D, E). Taken together, our experiments using both gain- and loss-of-function approaches strongly suggest that APP reduces agonist-dependent endocytosis of α2AAR and stabilizes the receptor on the cell surface.
Figure 5.
Loss of endogenous APP in N2a cells increases α2AAR internalization. A–C) N2a cells stably expressing HA-α2AAR were transfected with APP siRNAs or a scrambled siRNA. Internalization of surface HA-α2AAR was examined by an Ab-prelabeling method. A) Western blot image showing expression of the knockdown efficiency of scrambled or APP siRNAs. B) Representative images showing internalization of cell surface HA-α2AAR after 1 µM clonidine treatment for 30 min. Arrows: perinuclear punctate staining, which is indicative of endocytosed receptors. C) Quantitation of α2AAR endocytosis. Data are means ± sem (n = 11–13 cells per condition). D, E) α2AAR internalization was examined in APP-KO and control N2a cells. D) Representative images showing internalization of cell surface HA-α2AAR after 30 min 10 µM NE treatment (plus 1 µM prazosin and 1 µM propranolol). Arrows: perinuclear punctate staining, indicative of endocytosed receptors. E) Quantitation of α2AAR endocytosis. Data are means ± sem (n = 14–22 cells per condition). **P < 0.01, ***P < 0.001 (Student’s t test). Scale bars, 10 μm.
APP enhances α2AAR signaling intensity and duration
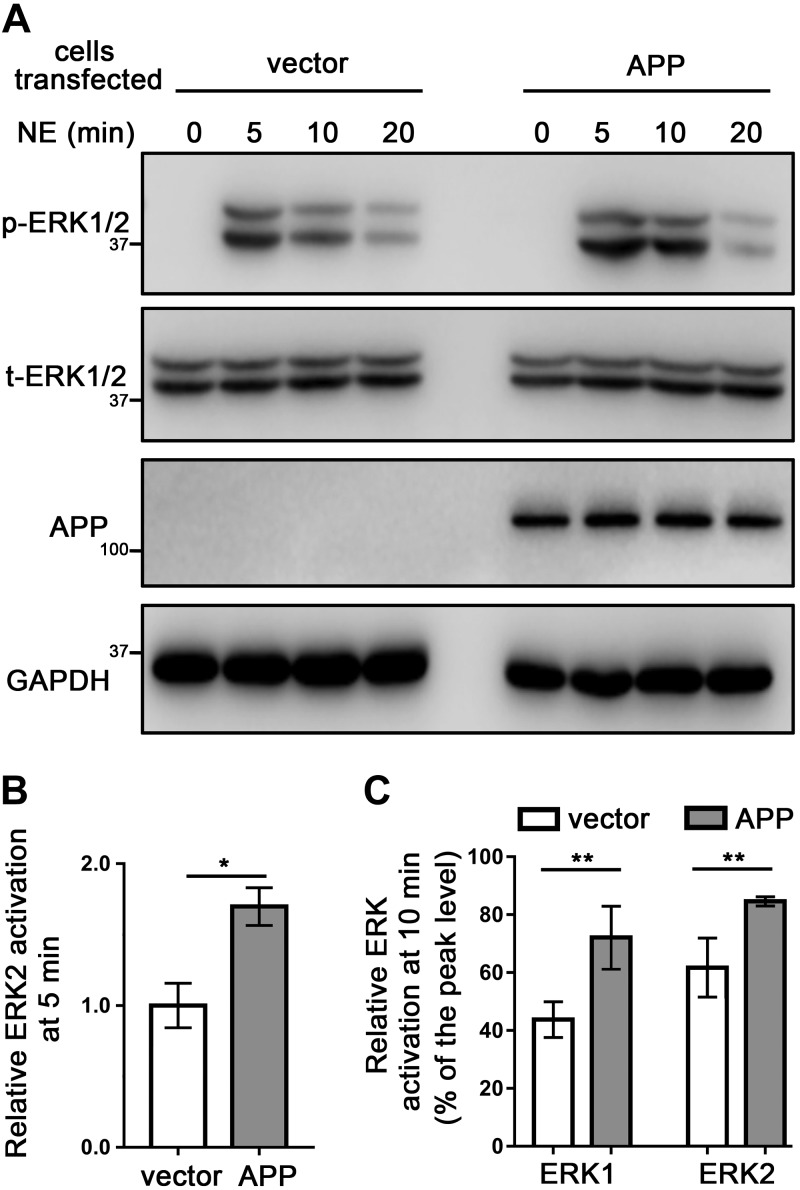
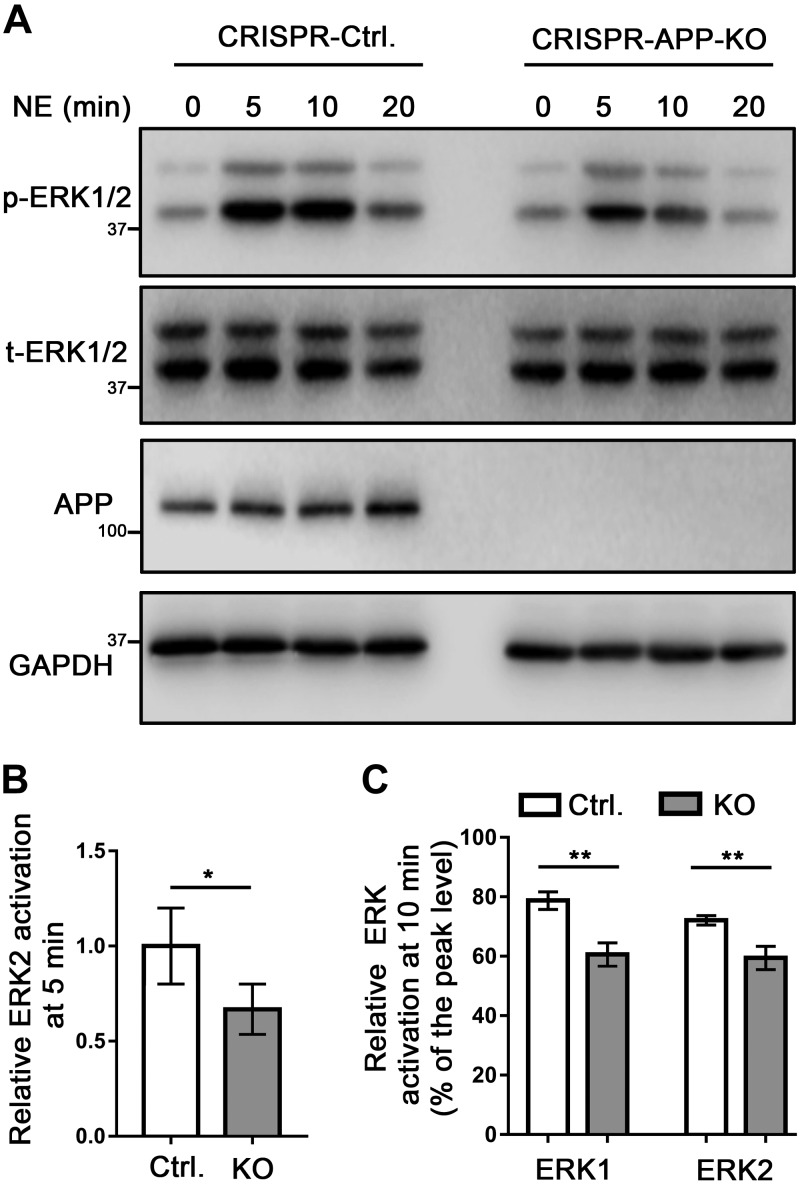
We next addressed how APP may affect α2AAR signaling by measuring α2AAR-mediated ERK1/2 activation, which is a G-protein-dependent process (18, 31). In HEK293 cells expressing exogenous APP, 5 min treatment of NE (in the presence of prazosin and propranolol) was able to induce a significantly higher level of ERK1/2 activation (reflected by p-ERK/t-ERK ratio) compared to that in control cells (Fig. 6A, B). Moreover, the decrease in ERK1/2 signals from the 5-min (peak) to 10-min time point (indicating signal desensitization) in APP-overexpressing cells was reduced compared with that in control cells (Fig. 6A, C). On the other hand, α2AAR-mediated ERK1/2 activation after 5 min NE treatment was significantly reduced in APP-KO N2a cells compared with that in control cells transfected with the empty vector (Fig. 7A, B). In addition, loss of APP led to a stronger reduction of ERK1/2 signals from the 5- to 10-min time point, compared with that in control cells transfected with the empty vector (Fig. 7A, C). Taken together, our data suggest that the presence of APP both strengthens and prolongs α2AAR signaling.
Figure 6.
Overexpression of APP increases the strength and duration of α2AAR-mediated ERK signaling. HEK293 cells were cotransfected with cDNA vectors encoding HA-α2AAR together with APP or empty vector. A) Representative Western blots showing p- and t-ERK in HEK293 cells, with or without APP overexpression. B) Quantification of ERK2 activation with 10 µM NE (plus 1 µM prazosin and 1 µM propranolol) treatment for 5 min. Data (means ± sem) are expressed as fold change vs. control cells expressing empty vector (defined as 1.0). C) Quantification of the relative change in ERK1/2 activation at 10 vs. 5 min of NE treatment. *P < 0.05, **P < 0.01 (Student’s t test; n = 8 per group).
Figure 7.
KO of endogenous APP in N2a cells decreases the strength and duration of α2AAR-mediated ERK signaling. A) Representative Western blots showing p-ERK and t-ERK in control and APP-KO N2a cells. B) Quantification of ERK2 activation with 10 µM NE (plus 1 µM prazosin and 1 µM propranolol) treatment for 5 min. Data (means ± sem) are expressed as fold change vs. that in WT control cells (defined as 1.0). C) Quantification of the relative change in ERK1/2 activation at 10 vs. 5 min of NE treatment. n = 5 in each group. *P < 0.05, **P < 0.01 (Student’s t test).
APP interaction with the α2AAR competes with arrestin binding to the receptor
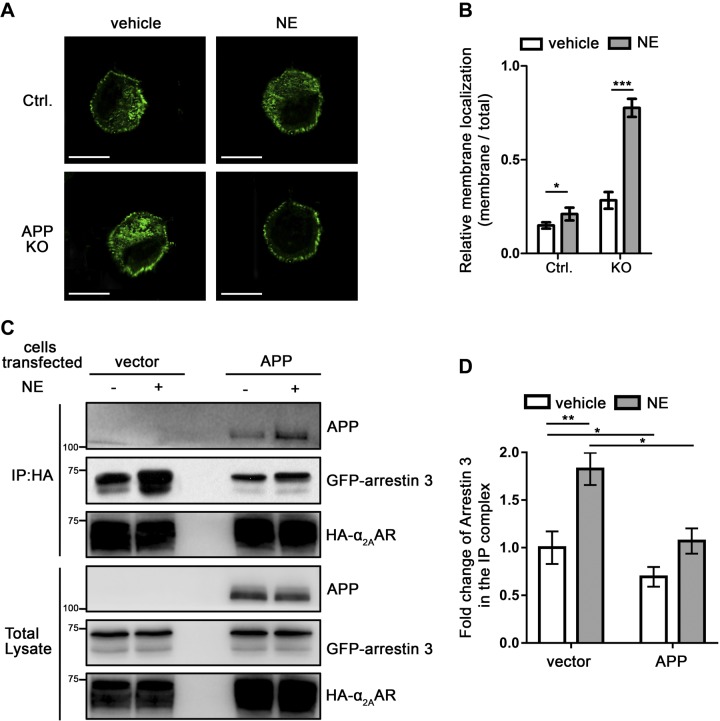
Because APP affects endocytosis and desensitization of α2AAR, which are arrestin-dependent processes (18, 21, 32), we speculate that the APP-α2AAR interaction may alter the α2AAR interaction with arrestin 3. We first examined arrestin 3 translocation after α2AAR stimulation by NE in N2a cells. In control cells transfected with the empty vector, 10 min NE treatment (in the presence of prazosin and propranolol) induced a slight but significant translocation of the endogenous arrestin 3 from cytoplasm to the plasma membrane (Fig. 8A, B). However, in APP-KO cells, almost all visible arrestin 3 was translocated to the plasma membrane after α2AAR activation by NE (Fig. 8A, B). These data suggest that the presence of APP blocks arrestin 3 recruitment to the α2AAR on the plasma membrane.
Figure 8.
APP reduces the interaction between α2AAR and arrestin in cells. A, B) APP-KO and control N2a cells were simulated with 10 µM NE (plus 1 µM prazosin and 1 µM propranolol) for 10 min. Endogenous arrestin 3 and APP were examined. A) Representative image of control and APP-KO N2a cells showing endogenous arrestin. Scale bars, 15 μm. B) Quantification of arrestin membrane recruitment. Data are means ± sem. (n = 16 cells per condition). C, D) HEK293 cells coexpressing HA-α2AAR and GFP-arrestin 3, together, with or without exogenous APP, were treated with 10 µM NE (plus 1 µM prazosin and 1 µM propranolol) for 5 min. Cell lysates were subjected to co-IP assays. C) Representative Western blots are shown. D) Quantification of the amount of arrestin 3 coimmunoprecipitated with HA-α2AAR. Data (means ± sem) are expressed as fold change vs. vehicle control without APP expression (defined as 1.0) (n = 5 in each group). *P < 0.05, **P < 0.01, ***P < 0.001 (Student’s t test).
We next examined the α2AAR-arrestin 3 interaction in HEK293 cells, with or without APP overexpression by co-IP assays. In control cells, we were able to readily coimmunoisolate GFP-tagged arrestin 3 with HA-α2AAR using an HA Ab, and the amount of arrestin 3 in the IP complex was enhanced by 5 min of NE stimulation (Fig. 8C, D). However, in APP-overexpressing cells, the level of arrestin 3 immunoprecipitated with α2AAR was significantly reduced compared with that in controls, both with and without NE stimulation (Fig. 8C, D). These data suggest that APP competes with arrestin 3 for binding to α2AAR.
Loss of APP promotes arrestin recruitment to plasma membrane and enhances α2AAR endocytosis in primary neurons
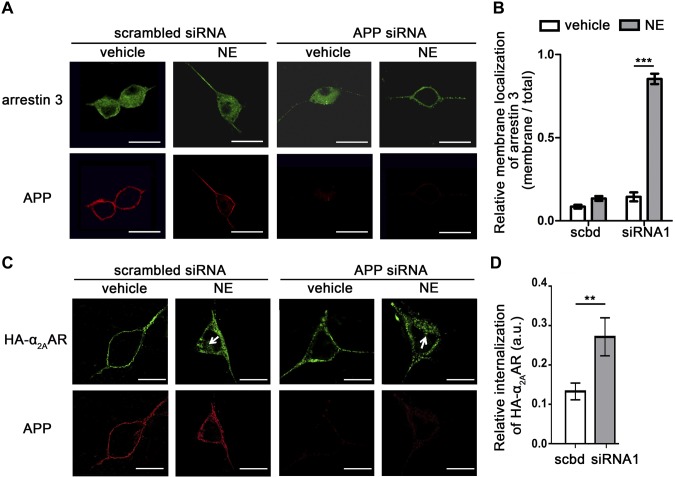
The above studies demonstrate regulation of α2AAR by APP in heterologous cells. We next sought to determine whether such regulation exists in native SCG neurons, which have a relatively high level of expression of both APP and α2AAR. siRNA was used to knock down endogenous APP. We first examined translocation of endogenous arrestin 3 after α2AAR stimulation. In control neurons transfected with scrambled siRNA, 10 min NE treatment slightly enriched arrestin 3 at the plasma membrane. However, in neurons with APP knocked down, almost all detectable arrestin 3 was localized onto the plasma membrane after NE treatment (Fig. 9A, B). These data suggest that APP blocks arrestin 3 recruitment to α2AAR in native neurons.
Figure 9.
Loss of APP promotes α2AAR-induced arrestin recruitment to the membrane and α2AAR internalization in primary SCG neurons. Primary SCG neurons derived from HA-α2AAR-knock-in mice were transfected with APP siRNA or scrambled siRNA and treated with 10 µM NE (plus 1 µM prazosin and 1 µM propranolol) for 10 min. A) Representative images showing endogenous arrestin 3 and APP. Scale bars, 25 μm. B) Quantification of arrestin membrane recruitment. Data are means ± sem. n = 12 cell per condition. C) Representative images showing internalization of cell surface HA-α2AAR after 10 µM NE (plus 1 µM prazosin and 1 µM propranolol) treatment for 10 min. Arrows: perinuclear punctate staining, which is indicative of endocytosed receptors. Scale bars, 15 μm. D) Quantitation of α2AAR endocytosis. Data are means ± sem (n = 18 cells per condition). **P < 0.01, ***P < 0.001 (Student’s t test).
Taking advantage of our HA-α2AAR-knock-in mouse line (24), we examined agonist-dependent endocytosis of endogenous α2AAR in SCG neurons, with or without APP knockdown. After 10 min of NE stimulation (plus prazosin and propranolol), internalization of endogenous α2AAR was significantly increased in neurons treated with the APP siRNA, compared that in neurons treated with the scrambled siRNA (Fig. 9C, D). These data suggest that, as in the heterologous cells tested, APP stabilizes α2AAR to the cell surface in native neurons.
DISCUSSION
In the present study, we identified a novel function of APP in regulating trafficking and signaling of a GPCR, namely α2AAR. APP directly interacts with the 3iloop of α2AAR through its C-terminal intracellular domain (Fig. 3). The APP-α2AAR interaction occurs in an agonist-dependent fashion in both cultured cells and the mouse brain (Figs. 1 and 2), and competes with arrestin 3 binding to the receptor (Figs. 8 and 9). Consequently, the presence of APP blocks arrestin translocation to the plasma membrane (Figs. 8 and 9) and attenuates arrestin-mediated internalization (Figs. 4, 5, and 9) and desensitization (Figs. 6 and 7) of α2AAR. Our study provided the first evidence demonstrating that APP stabilizes a GPCR on the cell surface and potentiates its signaling. Furthermore, the APP antagonism of arrestin translocation and α2AAR endocytosis exists in primary SCG neurons (Fig. 9), demonstrating the physiologic relevance of APP-α2AAR interaction.
It has now been well established that non-G-protein-interacting partners play a crucial role in fine tuning GPCR signaling and functions (17, 33–35). The nonvisual arrestins 2 and 3, have been appreciated as universal regulators of GPCRs (36, 37). Agonist-induced arrestin translocation from cytosol to the plasma membrane represents a hallmark of GPCR activation and desensitization of G-protein-dependent signaling (38–40). Like many GPCRs, agonist stimulation of α2AAR leads to receptor phosphorylation by GRK2/3 and subsequent binding of arrestin 3 (17). In WT N2a cells and SCG neurons, α2AAR activation was able to induce recruitment of only a small portion of arrestin 3 to the plasma membrane (Figs. 8 and 9). However, when APP expression is disrupted, by CRISPR KO or siRNA knockdown, almost all arrestin 3 was translocated to plasma membrane after α2AAR stimulation (Figs. 8 and 9). Concomitantly, agonist-induced α2AAR endocytosis was significantly increased (Figs. 4, 5 and 9). Our experiments thus revealed a novel role of APP in antagonizing arrestin-mediated regulation of α2AAR in neuronal cells. We have reported that a dendritic spine-enriched scaffolding protein, spinophilin, also competes with arrestin 3 for interaction with α2AAR (16, 18). Spinophilin is mainly localized in the postsynaptic compartment (41, 42), whereas APP has been shown to localize in both dendrites and axons (43, 44) and can regulate localization and activity of presynaptic membrane proteins such as the high-affinity choline transporter (45). It is plausible that spinophilin and APP play a dominant role in different neuronal compartments in impeding arrestin-mediated regulation of α2AAR.
Regulation of other cell surface receptor-initiated signaling may represent an important mechanism underlying APP-elicited physiologic functions. APP has been shown to interact with the NGF receptor, TrkA (46), and this interaction is critical for TrkA activation in response to NGF (9). To the best of our knowledge, how APP could regulate GPCRs has not been investigated. APP has been reported to interact with 2 other GPCRs, the orphan GPCR, GPR3, and the prostaglandin E receptor 2 subtype EP2 (PTGER2), in HEK cells (47). However, whether APP interactions with these receptors are direct or indirect and what the functional consequences of such interactions are on receptor trafficking or signaling remain to be addressed. In our current study, we showed by multiple lines of evidence that a direct APP-α2AAR interaction occurs intracellularly in an agonist-promoted manner (Figs. 1–3). Different from the case of GPR3, in which the complex formation between APP and GPR3 can be enhanced by the presence of arrestin 3 (47), α2AAR interactions with APP and arrestin 3 seem to be mutually exclusive (Figs. 8 and 9). Such a result is not surprising, given that both APP (Fig. 3) and arrestin 3 (16) directly bind to the 3iloop of α2AAR. Because arrestin-dependent mechanisms are essential for α2AAR internalization and desensitization (17), by competing for arrestin 3 binding to the receptor (Figs. 8 and 9), APP stabilizes α2AAR on the cell surface (Figs. 4, 5, and 9) and potentiates its signaling (Figs. 6 and 7).
We have demonstrated that stimulation of α2AAR promotes amyloidogenic processing of APP through activation of Gi/o proteins (23). The direct interaction between APP and α2AAR would bring APP to the proximity of α2AAR downstream signaling to modulate its processing route. Meanwhile, such an interaction potentiates α2AAR signaling, as suggested by the current study, which may further facilitate amyloidogenic processing of APP. The signaling molecules that mediate α2AAR-promoted APP processing and potential effects of APP-α2AAR interaction on activation of these signaling molecules are currently under investigation.
As the primary autoreceptor of the noradrenergic neurons, α2AAR plays a key role in controlling the brain noradrenergic activity and sympathetic outflow (48). In addition, α2AAR activation in sympathetic neurons inhibits high-frequency stimulus-induced NE release and reduces sympathetic tone (48, 49). Pharmacological stimulation of α2AAR lowers blood pressure and represents an effective strategy for long-term control of hypertension (50, 51). Through enhancing α2AAR surface retention and signaling, APP could influence noradrenergic activity and sympathetic tone. Indeed, sympathetic alterations have been indicated in APP-deficient mice (52). Future studies are necessary to understand the mechanistic contribution of impaired α2AAR signaling in this process. Nonetheless, our current study using multiple complementary approaches and native neurons provides new and valuable insights into the physiologic functions of APP, and may have implications for understanding sympathetic regulation.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Jeffrey Benovic (Thomas Jefferson University, Philadelphia PA, USA) for generously providing the anti-arrestin 3 Ab. This work was supported by U.S. National Institutes of Health, National Institute of Mental Health Grant MH081917 (to Q.W.). The authors declare no conflicts of interest.
Glossary
- 3iloop
third intracellular loop
- α2AAR
α2A-adrenergic receptor
- Ab
antibody
- AD
Alzheimer’s disease
- APLP1
APP-like protein 1
- APLP2
APP-like protein 2
- APP
amyloid precursor protein
- APP-C
C terminus of APP
- co-IP
coimmunoprecipitation
- DIV
days in vitro
- GFP
green fluorescent protein
- GST
glutathione S-transferase
- HA
hemagglutinin
- IP
immunoprecipitation
- KO
knockout
- N2a
neuro-2A
- NE
norepinephrine
- siRNA
small interfering RNA
- SCG
superior cervical ganglion
- t-ERK
total ERK
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
K. Jiao and Q. Wang designed research; F. Zhang, M. Gannon, and Y. Chen performed experiments; F. Zhang, M. Gannon, Y. Chen, K. Jiao, and Q. Wang analyzed data; L. Zhou provided analytic tools; and M. Gannon, F. Zhang, and Q. Wang wrote the manuscript.
REFERENCES
- 1.Nicolas M., Hassan B. A. (2014) Amyloid precursor protein and neural development. Development 141, 2543–2548 [DOI] [PubMed] [Google Scholar]
- 2.Van der Kant R., Goldstein L. S. (2015) Cellular functions of the amyloid precursor protein from development to dementia. Dev. Cell 32, 502–515; Correction 33, 240 [DOI] [PubMed] [Google Scholar]
- 3.Zheng H., Jiang M., Trumbauer M. E., Sirinathsinghji D. J., Hopkins R., Smith D. W., Heavens R. P., Dawson G. R., Boyce S., Conner M. W., Stevens K. A., Slunt H. H., Sisoda S. S., Chen H. Y., Van der Ploeg L. H. (1995) beta-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell 81, 525–531 [DOI] [PubMed] [Google Scholar]
- 4.Steinbach J. P., Müller U., Leist M., Li Z. W., Nicotera P., Aguzzi A. (1998) Hypersensitivity to seizures in beta-amyloid precursor protein deficient mice. Cell Death Differ. 5, 858–866 [DOI] [PubMed] [Google Scholar]
- 5.Dawson G. R., Seabrook G. R., Zheng H., Smith D. W., Graham S., O’Dowd G., Bowery B. J., Boyce S., Trumbauer M. E., Chen H. Y., Van der Ploeg L. H., Sirinathsinghji D. J. (1999) Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience 90, 1–13 [DOI] [PubMed] [Google Scholar]
- 6.Klevanski M., Saar M., Baumkötter F., Weyer S. W., Kins S., Müller U. C. (2014) Differential role of APP and APLPs for neuromuscular synaptic morphology and function. Mol. Cell. Neurosci. 61, 201–210 [DOI] [PubMed] [Google Scholar]
- 7.Russo C., Venezia V., Repetto E., Nizzari M., Violani E., Carlo P., Schettini G. (2005) The amyloid precursor protein and its network of interacting proteins: physiological and pathological implications. Brain Res. Brain Res. Rev. 48, 257–264 [DOI] [PubMed] [Google Scholar]
- 8.Deyts C., Thinakaran G., Parent A. T. (2016) APP receptor? to be or not to be. Trends Pharmacol. Sci. 37, 390–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matrone C., Barbagallo A. P., La Rosa L. R., Florenzano F., Ciotti M. T., Mercanti D., Chao M. V., Calissano P., D’Adamio L. (2011) APP is phosphorylated by TrkA and regulates NGF/TrkA signaling. J. Neurosci. 31, 11756–11761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hein L. (2006) Adrenoceptors and signal transduction in neurons. Cell Tissue Res. 326, 541–551 [DOI] [PubMed] [Google Scholar]
- 11.Gyires K., Zádori Z. S., Török T., Mátyus P. (2009) α(2)-Adrenoceptor subtypes-mediated physiological, pharmacological actions. Neurochem. Int. 55, 447–453 [DOI] [PubMed] [Google Scholar]
- 12.Massé F., Hascoët M., Dailly E., Bourin M. (2006) Effect of noradrenergic system on the anxiolytic-like effect of DOI (5-HT2A/2C agonists) in the four-plate test. Psychopharmacology (Berl.) 183, 471–481 [DOI] [PubMed] [Google Scholar]
- 13.Gilsbach R., Hein L. (2012) Are the pharmacology and physiology of α2 adrenoceptors determined by α2-heteroreceptors and autoreceptors respectively? Br. J. Pharmacol. 165, 90–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlicker E., Göthert M. (1998) Interactions between the presynaptic alpha2-autoreceptor and presynaptic inhibitory heteroreceptors on noradrenergic neurones. Brain Res. Bull. 47, 129–132 [DOI] [PubMed] [Google Scholar]
- 15.Starke K., Göthert M., Kilbinger H. (1989) Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol. Rev. 69, 864–989 [DOI] [PubMed] [Google Scholar]
- 16.Wang Q., Limbird L. E. (2002) Regulated interactions of the alpha 2A adrenergic receptor with spinophilin, 14-3-3zeta, and arrestin 3. J. Biol. Chem. 277, 50589–50596 [DOI] [PubMed] [Google Scholar]
- 17.Wang Q., Limbird L. E. (2007) Regulation of alpha2AR trafficking and signaling by interacting proteins. Biochem. Pharmacol. 73, 1135–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q., Zhao J., Brady A. E., Feng J., Allen P. B., Lefkowitz R. J., Greengard P., Limbird L. E. (2004) Spinophilin blocks arrestin actions in vitro and in vivo at G protein-coupled receptors. Science 304, 1940–1944 [DOI] [PubMed] [Google Scholar]
- 19.Lu R., Chen Y., Cottingham C., Peng N., Jiao K., Limbird L. E., Wyss J. M., Wang Q. (2010) Enhanced hypotensive, bradycardic, and hypnotic responses to alpha2-adrenergic agonists in spinophilin-null mice are accompanied by increased G protein coupling to the alpha2A-adrenergic receptor. Mol. Pharmacol. 78, 279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cottingham C., Li X., Wang Q. (2012) Noradrenergic antidepressant responses to desipramine in vivo are reciprocally regulated by arrestin3 and spinophilin. Neuropharmacology 62, 2354–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cottingham C., Chen Y., Jiao K., Wang Q. (2011) The antidepressant desipramine is an arrestin-biased ligand at the α(2A)-adrenergic receptor driving receptor down-regulation in vitro and in vivo. J. Biol. Chem. 286, 36063–36075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ran F. A., Hsu P. D., Wright J., Agarwala V., Scott D. A., Zhang F. (2013) Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y., Peng Y., Che P., Gannon M., Liu Y., Li L., Bu G., van Groen T., Jiao K., Wang Q. (2014) α(2A) adrenergic receptor promotes amyloidogenesis through disrupting APP-SorLA interaction. Proc. Natl. Acad. Sci. USA 111, 17296–17301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu R., Li Y., Zhang Y., Chen Y., Shields A. D., Winder D. G., Angelotti T., Jiao K., Limbird L. E., Zhou Y., Wang Q. (2009) Epitope-tagged receptor knock-in mice reveal that differential desensitization of alpha2-adrenergic responses is because of ligand-selective internalization. J. Biol. Chem. 284, 13233–13243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cottingham C., Lu R., Jiao K., Wang Q. (2013) Cross-talk from β-adrenergic receptors modulates α2A-adrenergic receptor endocytosis in sympathetic neurons via protein kinase A and spinophilin. J. Biol. Chem. 288, 29193–29205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brum P. C., Hurt C. M., Shcherbakova O. G., Kobilka B., Angelotti T. (2006) Differential targeting and function of alpha2A and alpha2C adrenergic receptor subtypes in cultured sympathetic neurons. Neuropharmacology 51, 397–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zinchuk V., Zinchuk O., Okada T. (2007) Quantitative colocalization analysis of multicolor confocal immunofluorescence microscopy images: pushing pixels to explore biological phenomena. Acta Histochem. Cytochem. 40, 101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J., Chen Y., Lu R., Cottingham C., Jiao K., Wang Q. (2008) Protein kinase A phosphorylation of spinophilin modulates its interaction with the alpha 2A-adrenergic receptor (AR) and alters temporal properties of alpha 2AAR internalization. J. Biol. Chem. 283, 14516–14523 [DOI] [PubMed] [Google Scholar]
- 29.Chen Y., Liu Y., Cottingham C., McMahon L., Jiao K., Greengard P., Wang Q. (2012) Neurabin scaffolding of adenosine receptor and RGS4 regulates anti-seizure effect of endogenous adenosine. J. Neurosci. 32, 2683–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards S. W., Limbird L. E. (1999) Role for the third intracellular loop in cell surface stabilization of the alpha2A-adrenergic receptor. J. Biol. Chem. 274, 16331–16336 [DOI] [PubMed] [Google Scholar]
- 31.Wang Q., Lu R., Zhao J., Limbird L. E. (2006) Arrestin serves as a molecular switch, linking endogenous alpha2-adrenergic receptor to SRC-dependent, but not SRC-independent, ERK activation. J. Biol. Chem. 281, 25948–25955 [DOI] [PubMed] [Google Scholar]
- 32.DeGraff J. L., Gagnon A. W., Benovic J. L., Orsini M. J. (1999) Role of arrestins in endocytosis and signaling of alpha2-adrenergic receptor subtypes. J. Biol. Chem. 274, 11253–11259 [DOI] [PubMed] [Google Scholar]
- 33.Bockaert J., Perroy J., Bécamel C., Marin P., Fagni L. (2010) GPCR interacting proteins (GIPs) in the nervous system: roles in physiology and pathologies. Annu. Rev. Pharmacol. Toxicol. 50, 89–109 [DOI] [PubMed] [Google Scholar]
- 34.Bockaert J., Fagni L., Dumuis A., Marin P. (2004) GPCR interacting proteins (GIP). Pharmacol. Ther. 103, 203–221 [DOI] [PubMed] [Google Scholar]
- 35.Ritter S. L., Hall R. A. (2009) Fine-tuning of GPCR activity by receptor-interacting proteins. Nat. Rev. Mol. Cell Biol. 10, 819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lohse M. J., Hoffmann C. (2014) Arrestin interactions with G protein-coupled receptors. Handb. Exp. Pharmacol. 219, 15–56 [DOI] [PubMed] [Google Scholar]
- 37.Lefkowitz R. J. (2013) Arrestins come of age: a personal historical perspective. Prog. Mol. Biol. Transl. Sci. 118, 3–18 [DOI] [PubMed] [Google Scholar]
- 38.Barak L. S., Ferguson S. S., Zhang J., Caron M. G. (1997) A beta-arrestin/green fluorescent protein biosensor for detecting G protein-coupled receptor activation. J. Biol. Chem. 272, 27497–27500 [DOI] [PubMed] [Google Scholar]
- 39.Ma L., Pei G. (2007) Beta-arrestin signaling and regulation of transcription. J. Cell Sci. 120, 213–218 [DOI] [PubMed] [Google Scholar]
- 40.Claing A., Laporte S. A., Caron M. G., Lefkowitz R. J. (2002) Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog. Neurobiol. 66, 61–79 [DOI] [PubMed] [Google Scholar]
- 41.Muly E. C., Smith Y., Allen P., Greengard P. (2004) Subcellular distribution of spinophilin immunolabeling in primate prefrontal cortex: localization to and within dendritic spines. J. Comp. Neurol. 469, 185–197 [DOI] [PubMed] [Google Scholar]
- 42.Feng J., Yan Z., Ferreira A., Tomizawa K., Liauw J. A., Zhuo M., Allen P. B., Ouimet C. C., Greengard P. (2000) Spinophilin regulates the formation and function of dendritic spines. Proc. Natl. Acad. Sci. USA 97, 9287–9292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allinquant B., Moya K. L., Bouillot C., Prochiantz A. (1994) Amyloid precursor protein in cortical neurons: coexistence of two pools differentially distributed in axons and dendrites and association with cytoskeleton. J. Neurosci. 14, 6842–6854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schubert W., Prior R., Weidemann A., Dircksen H., Multhaup G., Masters C. L., Beyreuther K. (1991) Localization of Alzheimer beta A4 amyloid precursor protein at central and peripheral synaptic sites. Brain Res. 563, 184–194 [DOI] [PubMed] [Google Scholar]
- 45.Wang B., Yang L., Wang Z., Zheng H. (2007) Amyloid precursor protein mediates presynaptic localization and activity of the high-affinity choline transporter. Proc. Natl. Acad. Sci. USA 104, 14140–14145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q., Descamps O., Hart M. J., Poksay K. S., Spilman P., Kane D. J., Gorostiza O., John V., Bredesen D. E. (2014) Paradoxical effect of TrkA inhibition in Alzheimer’s disease models. J. Alzheimers Dis. 40, 605–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson C. D., Sheng M. (2013) Gpr3 stimulates Aβ production via interactions with APP and β-arrestin2. PLoS One 8, e74680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hein L., Altman J. D., Kobilka B. K. (1999) Two functionally distinct alpha2-adrenergic receptors regulate sympathetic neurotransmission. Nature 402, 181–184 [DOI] [PubMed] [Google Scholar]
- 49.Trendelenburg A. U., Nörenberg W., Hein L., Meyer A., Starke K. (2001) Alpha2-adrenoceptor-mediated inhibition of cultured sympathetic neurons: changes in alpha2A/D-adrenoceptor-deficient mice. Naunyn Schmiedebergs Arch. Pharmacol. 363, 110–119 [DOI] [PubMed] [Google Scholar]
- 50.MacMillan L. B., Hein L., Smith M. S., Piascik M. T., Limbird L. E. (1996) Central hypotensive effects of the alpha2a-adrenergic receptor subtype. Science 273, 801–803 [DOI] [PubMed] [Google Scholar]
- 51.Giovannitti J. A. Jr., Thoms S. M., Crawford J. J. (2015) Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth. Prog. 62, 31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duce J. A., Ayton S., Miller A. A., Tsatsanis A., Lam L. Q., Leone L., Corbin J. E., Butzkueven H., Kilpatrick T. J., Rogers J. T., Barnham K. J., Finkelstein D. I., Bush A. I. (2013) Amine oxidase activity of β-amyloid precursor protein modulates systemic and local catecholamine levels. Mol. Psychiatry 18, 245–254 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.