Abstract
The vertebrate retina develops in close proximity to the forebrain and neural crest-derived cartilages of the face and jaw. Coloboma, a congenital eye malformation, is associated with aberrant forebrain development (holoprosencephaly) and with craniofacial defects (frontonasal dysplasia) in humans, suggesting a critical role for cross-lineage interactions during retinal morphogenesis. ZIC2, a zinc-finger transcription factor, is linked to human holoprosencephaly. We have previously used morpholino assays to show zebrafish zic2 functions in the developing forebrain, retina and craniofacial cartilage. We now report that zebrafish with genetic lesions in zebrafish zic2 orthologs, zic2a and zic2b, develop with retinal coloboma and craniofacial anomalies. We demonstrate a requirement for zic2 in restricting pax2a expression and show evidence that zic2 function limits Hh signaling. RNA-seq transcriptome analysis identified an early requirement for zic2 in periocular neural crest as an activator of alx1, a transcription factor with essential roles in craniofacial and ocular morphogenesis in human and zebrafish. Collectively, these data establish zic2 mutant zebrafish as a powerful new genetic model for in-depth dissection of cell interactions and genetic controls during craniofacial complex development.
Keywords: zic2, alx1, Hedgehog signaling, coloboma, zebrafish
INTRODUCTION
The key portion of the vertebrate eye, the neural retina, begins its development as an integral part of the forebrain primordium. It evaginates to form bilateral optic vesicles that connect to the forebrain via the optic stalks (OS). Optic vesicles then fold into cup-like structures that briefly remain open at the site adjacent to OS, termed the choroid fissure (Bazin-Lopez et al., 2015; Gestri et al., 2012; Kwan, 2014; Schmitt and Dowling, 1994). The edges of the choroid fissure come together and fuse during normal development; failure of this process results in uveal coloboma, estimated to occur once in every 5,000 births (Williamson and FitzPatrick, 2014). Coloboma is a significant cause of congenital blindness, found in 3–11% of blind children (Onwochei et al., 2000). Despite its prevalence and debilitating consequences, the underlying molecular defects that cause coloboma are not well understood.
The choroid fissure forms in a complex environment that includes the adjacent forebrain and neural crest (NC)-derived mesenchymal cells on their way to becoming skeletal and vascular elements of the face and jaw. In zebrafish, NC cells that migrate in the anterior streams around the optic vesicle form the neurocranium (ethmoid plate and the trabeculae) (Schilling et al., 1999; Wada et al., 2005). The retina and OS signal to the anterior NC, directing it to its destinations (Eberhart et al., 2008; Kish et al., 2011; Swartz et al., 2011). There is emerging evidence for a reciprocal interaction, whereby NC cells signal back to the eye and brain (reviewed in (Bazin-Lopez et al., 2015; Gestri et al., 2012; Le Douarin et al., 2007). In humans, significant comorbidity has been reported between frontonasal dysplasia and coloboma (Wu et al., 2007), suggesting that NC plays a specific role in choroid fissure morphogenesis. The importance of this mechanism has only recently come to light and needs robust genetic models to be fully understood.
Dysregulation in several signaling pathways has been implicated in coloboma, with Hedgehog (Hh) signaling arguably the best characterized (Williamson and FitzPatrick, 2014). Disruption of Hh signaling also causes forebrain anomalies called holoprosencephaly (HPE)(Roessler et al., 1996; Roessler and Muenke, 2010), and facial dysmorphologies that range from hypertelorism (increased distance between eye orbits) attributed to increased Hh signaling to orofacial clefting caused by Hh reduction (Brugmann et al., 2010; Gongal et al., 2011; Young et al., 2010). To understand how Hh signaling controls these processes, it is necessary to examine the downstream effectors of Hh signaling in each developmental context.
zic2, a member of the conserved Zic family of zinc-finger transcription factors, is one such effector. zic2 plays a key role in brain morphogenesis, as indicated by the high incidence of zic2 mutations in human patients with HPE (Brown et al., 2001; Brown et al., 1998; Ribeiro et al., 2012; Roessler et al., 2009; Solomon et al., 2010). Extensive studies in mouse and Xenopus have demonstrated essential roles for zic2 in early neural development, namely, neural crest specification and neural tube closure (Elms et al., 2004; Elms et al., 2003; Houtmeyers et al., 2016; Nagai et al., 2000; Nagai et al., 1997; Nakata et al., 1997, 1998; Nyholm et al., 2009; Teslaa et al., 2013; Warr et al., 2008; Ybot-Gonzalez et al., 2007). Zic2 is also required for specification of embryonic stem cells, where it functions as an enhancer-binding cofactor in concert with the Mbd3-NuRD chromatin remodeling complex (Luo et al., 2015). Later in development, zic2 function is required for correct migration of cortical neurons (Murillo et al., 2015), for cerebellar granular neuron differentiation (Frank et al., 2015) and as a key determinant of ipsilateral vs contralateral projection in retinal ganglion cells (Escalante et al., 2013; Garcia-Frigola et al., 2008; Herrera et al., 2003),. In hypomorphic zic2 mice, defective retinal morphogenesis has been reported, but not characterized (Herrera et al., 2003). The underlying mechanism of its functions during development have been elusive until recently, when zic2 was found to inhibit canonical Wnt signaling (Fujimi et al., 2012; Pourebrahim et al., 2011), and to control forebrain morphogenesis via a direct interaction with Smad2/3 in the Nodal signal transduction pathway (Houtmeyers et al., 2016). To fully understand the mechanism of zic2 functions in the context of the developing embryo, it is essential that we dissect these functions further, and in more than one model organism.
Here we report that zebrafish with genetic lesions in zebrafish zic2 orthologs, zic2a and zic2b, develop with profound retinal and craniofacial anomalies, similar to those observed after transient depletion of zic2 by antisense morpholino oligos (Sanek et al., 2009; Teslaa et al., 2013). We show that zic2 function is required for the correct morphogenesis of the OS and for juxtaposing the edges of the fissure to allow its subsequent closure. We demonstrate a requirement for zic2 in restricting pax2a expression at the OS/ventral retina border, and show evidence of increased Hh signaling in the absence of zic2 function. Using RNA-seq-based transcriptome analysis, we confirm an early requirement for zic2 function in NC-derived pharyngeal and periocular neural crest, and identify a novel role for zic2 as a transcriptional activator of Alx1, a paired homeobox transcription factor with key functions in craniofacial and ocular morphogenesis in human and zebrafish embryos (Dee et al., 2013; Uz et al., 2010). Collectively, these data establish zic2 mutant zebrafish as a powerful new genetic model for in-depth dissection of the complex inter-lineage cell interactions and genetic controls during craniofacial complex development.
RESULTS
Zebrafish zic2 orthologs function redundantly during retinal and craniofacial morphogenesis
Zebrafish zic2 orthologs zic2a and zic2b, the only members of the Zic gene family duplicated in teleosts, reside on chromosomes 9 and 1, respectively. To build a genetic model of zic2-linked HPE in zebrafish, we set out to establish lines mutant at both loci. Toward this end, we obtained a mutagenic gene-trap insertion in the first coding exon of zic2a, zic2agbt133, isolated in a screen by Clark et al. (Clark et al., 2011) (Fig. S1). zic2agbt133 homozygous embryos develop normally for the first 2 weeks (data not shown), likely due to functional redundancy with its ortholog, zic2b. We used targeted mutagenesis with TALEN and CRISPR/Cas9 to generate frame-shift alleles at two distinct sites in the first exon of the zic2b locus (see Materials and Methods for details). Three mutant alleles were isolated that contained a 27-nt insertion (zic2b1127) or a 16-nt insertion (zic2b1116) at the CRISPR target site, and a 4-nt deletion (zic2bt104) at the TALEN target site (Fig. S1). zic2b homozygous mutants developed normally and were viable as adults despite the predicted absence of full-length zic2b protein; in contrast, ~5% of the embryos derived from a double heterozygous (zic2a+/−; zic2b+/−) incross exhibited retinal coloboma by 48 hpf (Fig. 1A–F; Table S1). The expected proportion of zic2a;zic2b homozygous mutants (zic2 mutants) is 6.25%. Affected embryos frequently presented with mild coloboma, defined here as a relatively small gap present in only one of the two retinae (Fig. 1B, G). Unexpectedly, a subset of embryos with coloboma exhibited periocular hemorrhage and edema, indicative of vascular deficits (Fig. 1C, F).
Figure 1. Zebrafish Zic2 is required during retinal morphogenesis.
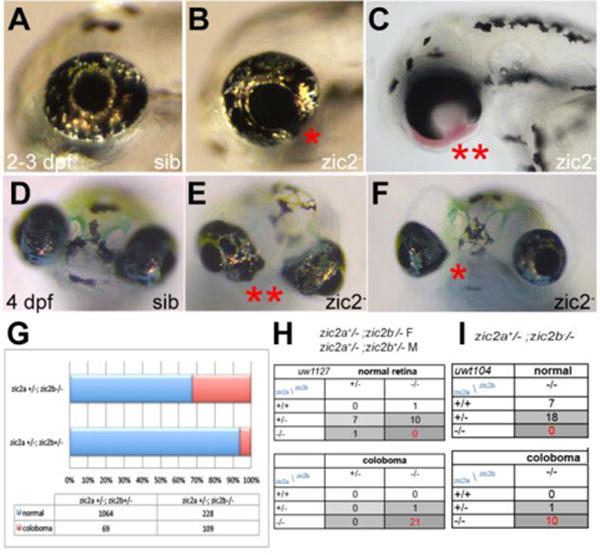
A: normal retinal morphology. B: retina exhibiting mild coloboma (*). C: retina exhibiting severe coloboma with periocular hemorrhaging (**). D: normal retinal morphology. E: bilateral coloboma in a severely affected embryo (**). F: mild, unilateral coloboma in an affected embryo. G: Penetrance and expressivity of coloboma is increased in progeny that lack maternal zic2b, derived from zic2agbt133/+; zic2bt104/zic2bt104 parents, compared to those from double heterozygous (zic2agbt133/+; zic2bt104/+) parents (see Table S1 for details). H, I: Both CRISPR- and TALEN-induced mutant alleles of zic2b are tightly associated with coloboma in MZ-zic2 embryos. Embryos in A–C are at 2–3 dpf, shown in lateral views, anterior to the left. Embryos in D–F are at 4 dpf, shown in anterior views, dorsal at the top.
We next asked if the maternal function of zic2 played a role during retinal development by assessing embryonic phenotypes in progeny from a cross between zic2a+/−; zic2b−/− parents, 25% of which are predicted to be zic2 mutants. 25% of these embryos exhibited coloboma by 2 dpf (Table S1); coloboma was primarily severe, i.e. bilateral with large ventral gaps (Fig. 1C, E, G), consistent with a requirement for maternal zic2b during retinal morphogenesis. To confirm that zic2 mutations were responsible for abnormal retinal morphogenesis, we genotyped representative embryos with and without coloboma individually (Fig. S2). This analysis showed that the majority of embryos with coloboma were zic2 mutants (going forward, we will refer to zic2 mutants derived from zic2b-/-mothers as MZ-zic2 mutants). Coloboma was also occasionally observed in maternally depleted embryos with one wildtype allele of zic2a (Fig 1H, I).
By 5 dpf, all embryos with coloboma exhibited profoundly hypoplastic craniofacial cartilages, both in the neurocranium and pharyngeal arches, and severe periocular and cranial edema (Fig. 2A, B; Table S2). Similar defects were observed in embryos produced by heterozygous parents and those from zic2a+/−; zic2b−/− parents (data not shown). Genotypic analysis revealed that this phenotype was restricted to zic2 mutants and embryos with one wildtype allele of zic2a (Fig. 2C,D). Collectively, these data clearly demonstrate a requirement for zygotic zic2 function in the developing retina and craniofacial cartilages, and an early contribution of maternal zic2b function to retinal morphogenesis.
Figure 2. Zebrafish Zic2 is required for craniofacial cartilage development.
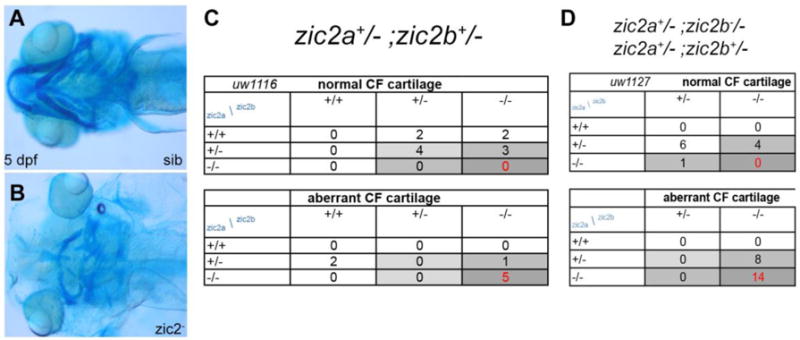
A: normal neurocranium and branchial arches. B: hypoplastic, disorganized craniofacial cartilages in a zic2 mutant. C: Craniofacial defects are enriched in zic2 mutants derived from double heterozygous parents. D: In embryos that lack maternal zic2b, craniofacial defects are observed in zic2 mutants and in embryos with one wildtype copy of zic2a. Cartilage was visualized by staining with Alcian Blue. Embryos at 5 dpf are shown in ventral views, anterior to the left.
zic2 restricts expression of pax2a in the optic stalk
We next asked if ventral retinal patterning and/or morphology were disrupted in zic2 mutants at 24 hpf, prior to the first appearance of overt coloboma. The choroid fissure and the OS are marked by patterned expression of several homeobox transcription factors, including pax2a (Macdonald et al., 1995; Mui et al., 2005; Take-uchi et al., 2003). We examined pax2a expression in zic2 embryos using whole mount in situ hybridization (WISH). Embryos derived from zic2a+/−;zic2b+/− parents exhibited normal expression of pax2a overall with the exception of the OS domain, which was mispatterned in 12% of the embryos (Fig. 3A, B). Post-hoc genotyping confirmed that all the embryos with mispatterned pax2a were homozygous for zic2b (Fig. 3C). All embryos with mispatterned pax2a exhibited aberrant ventral retina (Fig. 3D–F), i.e. were also zic2agbt133 homozygous. We next applied confocal microscopy to examine distribution of the Pax2a epitope in 24 hpf MZ-zic2 mutants. Normal retina expressed Pax2a in the restricted portion of the retina adjacent to the choroid fissure (Fig. 3G). In MZ-zic2 mutants, retinal edges of the choroid fissure expressed Pax2a, but were separated by a large gap. The OS were abnormally wide and contained aberrantly high pax2a signal (Fig. 3H). We also noted an intense concentration of F-actin at the choroid fissure in normal siblings and an absence thereof in mutant retina, consistent with aberrant morphogenesis. These observations are consistent with our previous finding that pa2a is ectopically expressed in embryos transiently depleted of zic2a (Sanek et al., 2009). When examined in ventral cross-sections, MZ-zic2 mutants exhibited aberrant expansion of Pax2a both in the ventral retina and in the pre-optic diencephalon contiguous with the OS (Fig. 3I, J). Pax2a-expressing diencephalon appeared dysmorphic, with thinner walls and larger lumen than the equivalent structure in the unaffected siblings (Fig. 3K, L). Collectively, these observations indicate an early requirement for zic2 function during morphogenesis of both the OS/choroid fissure boundary and the adjacent diencephalon.
Figure 3. Pax2a expression is aberrant in MZ-zic2 mutants at 1 dpf.
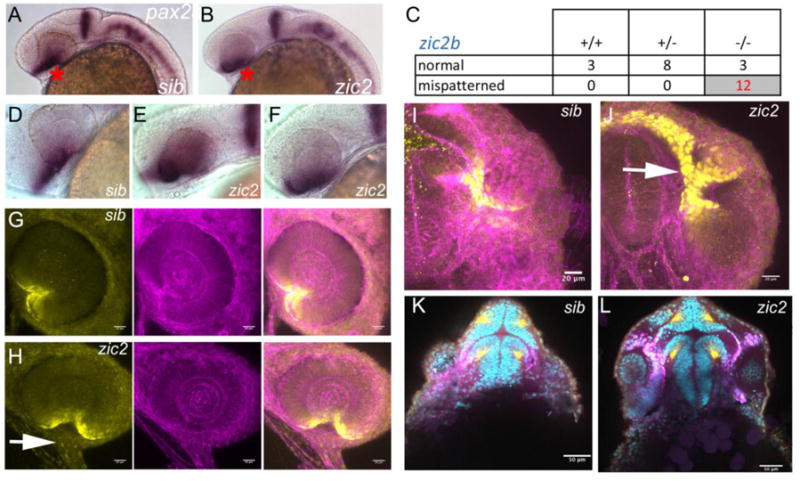
pax2a expression at 1 dpf was visualized in embryos derived from zic2agbt133/+; zic2buw1116/+ parents using WISH (A–F) or in progeny of zic2agbt133/+; zic2buwt104 parents using immunohistochemistry (G–L). A: normal pax2a expression in the ventral retina (*). B: mispatterned pax2a expression (*) was observed in 12 out of 103 embryos (12%, 2 expts.). C: Only zic2b homozygous embryos exhibit pax2a mispatterning. zic2a genotype was not tested because PCR genotyping was not robust after WISH. D–F: Embryos with mispatterned pax2a expression also exhibit coloboma, indicative of homozygosity for zic2agbt133. G, H: confocal stacks through representative retina of normal (G) and zic2 mutant (H) retina. I, J: confocal stacks through the ventral aspects of a normal (I) and zic2 mutant (J) diencephalon and retina. Arrowheads in H, J point to the aberrant optic stalk. In G–J, yellow = Pax2a, magenta = F-actin cytoskeleton visualized by phalloidin. K, L: single confocal sections through representative normal (K) and zic2 mutant (L) embryos, imaged ventrally at the level of choroid fissure. magenta = Pax2a; yellow = acetylated tubulin; cyan = nuclei visualized by DAPI. Embryos are shown in lateral views, anterior to the left (A–F) or anterior to the right (G, H); in ventral views with anterior at the top (I–L).
Hedgehog growth factors secreted from the ventral diencephalic midline pattern the OS and retina, partitioning it into three domains: the OS, ventral retina and dorsal retina (Ekker et al., 1995; Lee et al., 2008a; Lupo et al., 2005; Schimmenti et al., 2003; Varga et al., 2001; Wang et al., 2015). Since pax2a requires Hh signaling for its expression, pax2a expansion in zic2 mutants may indicate aberrant levels of Hh or an aberrant downstream transcriptional response. Zebrafish that lack the functional Hh receptor blowout/ptc1 due to mutations show increased levels Hh signaling and develop with incompletely penetrant coloboma; this defect is efficiently rescued by exposure to low levels of cyclopamine, a small molecule that inhibits Hh signaling (Lee et al., 2008a). Cyclopamine treatment also rescues coloboma caused by knockdown of sox4 or sox11, transcription factors that function as inhibitory modulators of Hh signaling during retinal morphogenesis (Pillai-Kastoori et al., 2014; Wen et al., 2015). We reasoned that, if Hh signaling is expanded in zic2 mutants, inhibition of Hh signaling should reduce the penetrance and/or expressivity of coloboma. To test this prediction, we exposed progeny from zic2a+/−; zic2b−/− incrosses to low concentration of cyclopamine that was sufficient to rescue coloboma in blowout/ptc1 mutants (Lee et al., 2008a). When progeny from zic2a+/−; zic2bt104 parents were exposed to cyclopamine during gastrulation and somitogenesis, they developed with significantly milder coloboma than their vehicle-treated siblings (Fisher Exact test, p <0.02, Fig. 4A–E, Fig. S3). The overall morphology of the embryos was not affected at this cyclopamine concentration (3–4.5 μM). To test specificity of the rescue, we asked if cyclopamine rescue is allele-independent. Progeny from zic2a+/−; zic2buw1116 parents exhibited significant alleviation of coloboma phenotype after cyclopamine treatment (Fisher Exact test, p <0.04; Fig. 4E, Fig. S3). In contrast, cyclopamine did not significantly affect coloboma penetrance or expressivity in zygotic zic2 mutants (Fig. 4F, Fig. S3). These findings are consistent with the notion that Hh signaling is de-repressed in the absence of functional zic2 and that this de-repression contributes to retinal dysmorphology in zic2 mutants.
Figure 4. Cyclopamine treatment reduces frequency and severity of coloboma in zic2 mutants.
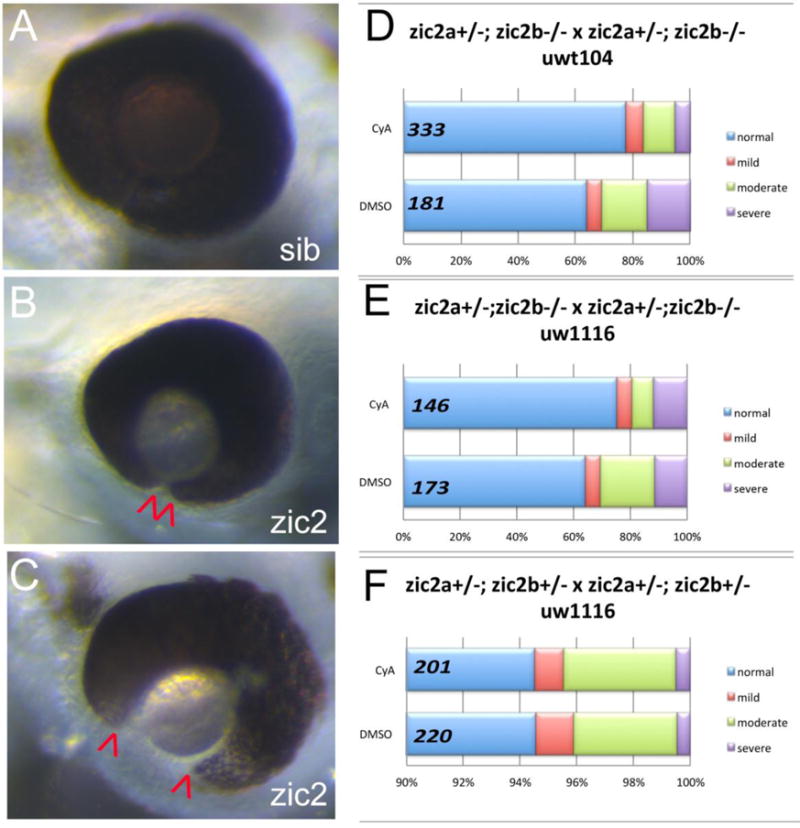
A: normal retinal morphology; B: retina with mild coloboma; C: retina with moderate coloboma. D: Embryos were derived from zic2agbt133/+; zic2b t104 parental crosses and exposed to 3 or 4.5 μM cyclopamine (CyA) from 3–5 hpf until 24–26 hpf. In CyA-treated groups (3 expts; Fig. S3A), the proportion of embryos with coloboma was reduced significantly compared to vehicle-treated control siblings (Fisher’s Exact test, P < 0.001). Proportion of severely affected embryos among all embryos with coloboma was also decreased in CyA-treated siblings (Fisher Exact test, p<0.02). E: Embryos were derived from zic2agbt133/+; zic2b uw1116 parents, and treated starting at 3 hpf with 4.5 uM Cya. CyA-treated groups exhibited reduction in coloboma penetrance (Fisher’s Exact test p<0.04) compared to vehicle-treated control siblings (2 expts; Fig. S3B). F: Embryos were derived from zic2agbt133/+; zic2b uw1116/+ parents, and treated as in D with 4.5 uM CyA or vehicle starting at 3 hpf. Proportion of embryos with coloboma was not affected by exposure to cyclopamine (2 expts). Embryos with unilateral mild coloboma were scored as “mild”; embryos with bilateral mild coloboma were scored as “moderate”, and embryos with bilateral moderate coloboma were scored as “severe”.
Zic2 function is required for neural crest-derived craniofacial lineage formation
We next set out to identify downstream effectors of zic2, i.e. genes whose transcription levels depend on zic2 function, using RNA-seq transcriptomic analysis (Fig. 5A). Embryos derived from zic2a+/−; zic2b+/− parents were sorted by coloboma at 25–28 hpf, when coloboma first manifests in zic2 mutants. RNA was isolated from individual embryos without pooling to increase the statistical power of analysis and subjected to high-throughput sequencing (see Materials and Methods for details). RNA-seq was performed on 10 samples in total, 5 normal and 5 with coloboma. One of the wild type samples was determined to be a mutant and excluded from further analysis. This approach identified a large set of 362 genes differentially expressed in zic2 mutants (199 increased and 169 reduced) (Table S3). We used the online bioinformatics tool (Huang da et al., 2009) to sort the responsive gene list into categories based on tissue enrichment in zebrafish (ZFIN_ANATOMY). This analysis identified myotome/somite, craniofacial elements, and heart (enrichment scores 2.78, 2.24 and 1.71, respectively). Myotome/somite markers and heart markers were found primarily in the upregulated set, consistent with expression of zic2b in zebrafish somites (Toyama et al., 2004) and with the demonstrated function for mammalian Zic2 during myogenesis (Inoue et al., 2007; Pan et al., 2011). In contrast, craniofacial markers appeared among transcripts depleted in zic2 mutants. (Fig. 5A). This set included chondrogenic neural crest markers dlx1a, dlx2a, dlx4a and barx1, and xanthophore lineage markers aox5, gch2 and cax1 (references in Table S4). We have previously shown both lineages to be strongly dependent on zic2 function in morpholino assays (Teslaa et al., 2013). A small number of retinal markers were also reduced in zic2 mutants, namely, vax1 and vax2, Hh-regulated OS/ventral retina markers (Take-uchi et al., 2003), and atoh7, expressed in retinal ganglion cells (Masai et al., 2000). Notably, pax2a transcript levels were unchanged in zic2 mutants, perhaps because the mispatterning we have documented in Fig. 3 affects a small portion of its expression domain.
Figure 5. RNA sequencing transcriptome analysis identifies a set of Zic2-dependent targets.
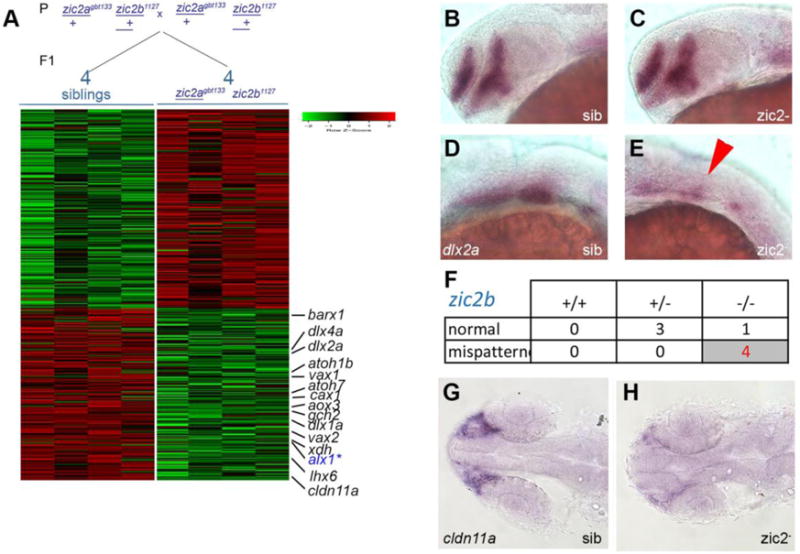
A: Embryos derived from zic2agbt133/+; zic2b1127/+ parents were sorted by presence or absence of coloboma into zic2 mutants and sibling groups, respectively. RNA extracted from individual embryos (4 wildtype and 5 with coloboma) was used to prepare cDNA libraries for illumina high-throughput sequencing. Genes with assigned value of False-Discovery Rate below 0.05 were preliminarily selected. The heat-map color represents relative expression levels of differentially expressed genes; 4 out of 5 coloboma-representing libraries are shown to maintain visual balance with the 4 normal sibling samples. B, C: Representative sibling and zic2 mutant embryos derived from zic2agbt133/+;zic2buw1116/+ parents show normal dlx2a expression by WISH in the telencephalon and diencephalon. D: normal dlx2a expression in branchial arch primordia. E: depleted branchial arch dlx2a expression (arrowhead) was observed in 7 out of 127 (6%, 3 expts.) of embryos from this cross. F: Only zic2b− homozygotes exhibited dlx2a reduction in branchial arch primordia. G: normal cldn11a expression adjacent to the optic stalk of embryo with normal retinal morphology. H: depleted cldn11a expression in zic2 mutant with coloboma. Embryos in B – E are shown in lateral views, anterior to the left. Embryos in G and H are shown in dorsal views, anterior to the left.
To validate these results, we used WISH on zic2 mutants and siblings. Dlx2a was specifically reduced in pharyngeal arch primordia at 24 hpf, but not in the tel- or diencephalon, in 6% of progeny from zic2a+/−;zic2b+/− parents (Fig. 5B–E), and this reduction was restricted to zic2b−/− embryos (Fig. 5F). Reduced expression of atoh7 and vax2 was also confirmed by WISH (Fig. S4).
Cldn11a, a tight junction component enriched in myelinating oligodendrocytes (Bronstein et al., 1997; Morita et al., 1999) was the most strongly depleted gene identified by RNA-seq (Fig. 5A). In zebrafish, cldn11a expression has been reported in vascular endothelium (Cannon et al., 2013). In contrast, we found cldn11a expression to be restricted to a small group of cells anterior to the retina, in close proximity to the choroid fissure, at 24 hpf (Fig. 5G). Consistent with RNA-seq results, cldn11a expression was nearly abolished in zic2 mutants assayed by WISH (Fig. 5H). Notably, cldn11 transcription requires zic2 function in the differentiating mammalian cerebellar ganglion cells (Frank et al., 2015).
Zic2 controls transcription of alx1 in the periocular neural crest
One of the candidate targets of zic2 identified by RNA-seq, Alx1, is expressed in chondrogenic neural crest and functions during retinal and craniofacial cartilage morphogenesis. Homozygous mutations in alx1 are associated with profound frontonasal dysplasia and microophthalmia in humans (Bertola et al., 2013; Uz et al., 2010), and zebrafish alx1 morphants develop with hypoplastic craniofacial cartilages and coloboma (Dee et al., 2013). WISH analysis corroborated depletion of alx1 transcript in zic2 mutants (Fig. 6A,B). We examined expression of alx1 during normal development to verify its restriction to neural crest. At 16 hpf, alx1 was expressed in the frontonasal neural crest, which forms the facial skeleton (Couly et al., 2002; Langenberg et al., 2008) (Fig. 6C, D). alx1 was expressed in the periocular region at 24 and 36 hpf (Fig. 6E, F) and in the ethmoid plate anlagen at 48 hpf (Fig. 6F). alx1 was also expressed in the prospective swim bladder, but this domain did not appear to be affected in zic2 mutants (not shown).
Figure 6. Alx1 is a novel target of zic2 in the periocular neural crest.
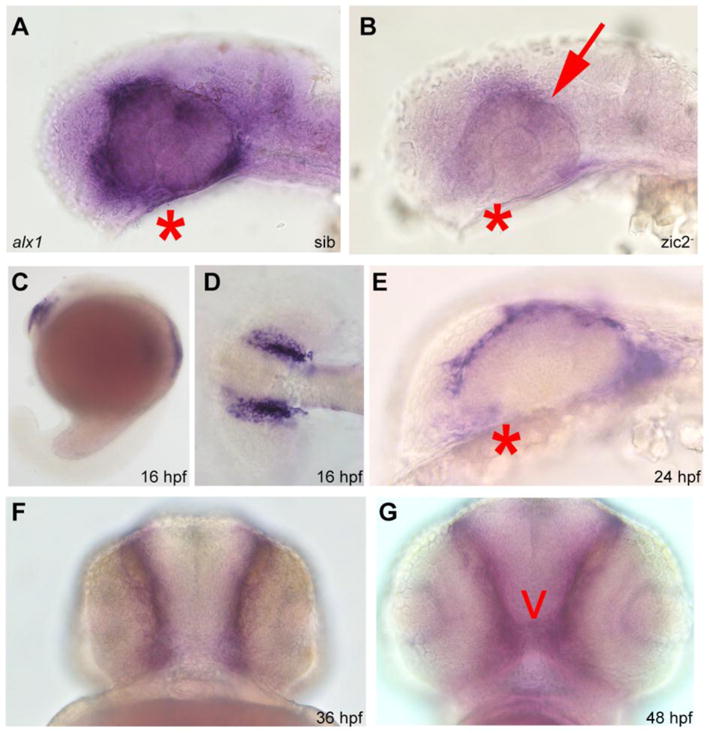
Embryos derived from zic2agbt133/+; zic2bt104/zic2bt104 parents were stained for alx1 expression by WISH. A: normal expression in periocular mesenchyme of sibling embryo. B: depleted expression in zic2 (arrow) in mutant embryo (39 out of 112 total, 2 expts). C–G: wild type embryos stained for alx1 expression by WISH. C–D: alx1 is expressed in frontonasal neural crest at 16 hpf. E, F: alx1 is expressed in periocular mesenchyme (*) at 24 hpf and 36 hpf. G: alx1 is expressed in the ethmoid plate (arrowhead) at 48 hpf. Embryos in A, B, C, and E are shown in lateral views, anterior to the left. Embryo in D is shown dorsally, anterior to the left. Embryos in F and G are shown in anterior views, ventral at the top.
To ask if alx1 depletion was indicative of a broader periocular mesenchyme deficit, we examined expression of crestin, an early general marker of neural crest (Langenberg et al., 2008) in MZ-zic2 mutants and siblings. We found the anterior stream of crestin-positive neural crest and its pharyngeal domain strongly reduced in zic2 mutants (Fig. 7A–E). In contrast, crestin-expressing cells in the trunk appeared unaffected (Fig. 7C, F).
Figure 7. Frontonasal and pharyngeal neural crest is depleted in MZ-zic2 mutants.
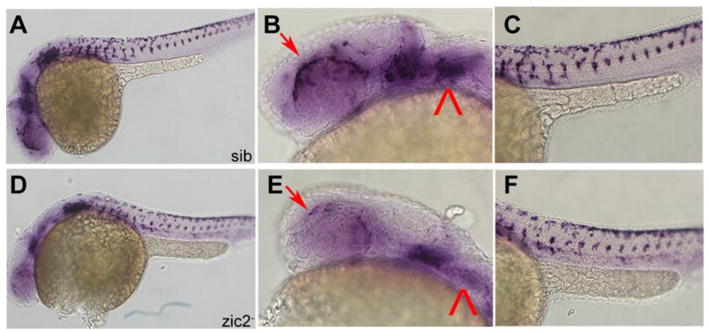
A–C: normal crestin expression in frontonasal and pharyngeal neural crest. D–F: depleted crestin expression in 14 of 55 embryos from a zic2agbt133/+; zic2bt104/zic2bt104 incross. Arrows point to periocular neural crest. Arrowhead points to pharyngeal arch expression. Embryos are shown in lateral views, anterior to the left.
Since crestin is not expressed in the ventral portion of periocular neural crest, we examined this NC population directly by high-resolution confocal imaging. MZ-zic2 mutants and their siblings were fixed at 24 hpf and stained for F-actin and DNA to visualize cell outlines and nuclei. In normal siblings, cells with mesenchymal appearance were observed in the periocular space adjacent to the choroid fissure; these were enriched in the proximal (closest to the OS) half of the optic cup (Fig. 8A–C). In contrast, the ventral periocular space was largely devoid of cells along the entire proximodistal axis of the mutant optic cup (Fig. 8D–F). Taken together, these data argue that zic2 plays a critical early role during periocular neural crest formation.
Figure 8. Ventral periocular neural crest is depleted in MZ-zic2 mutants.
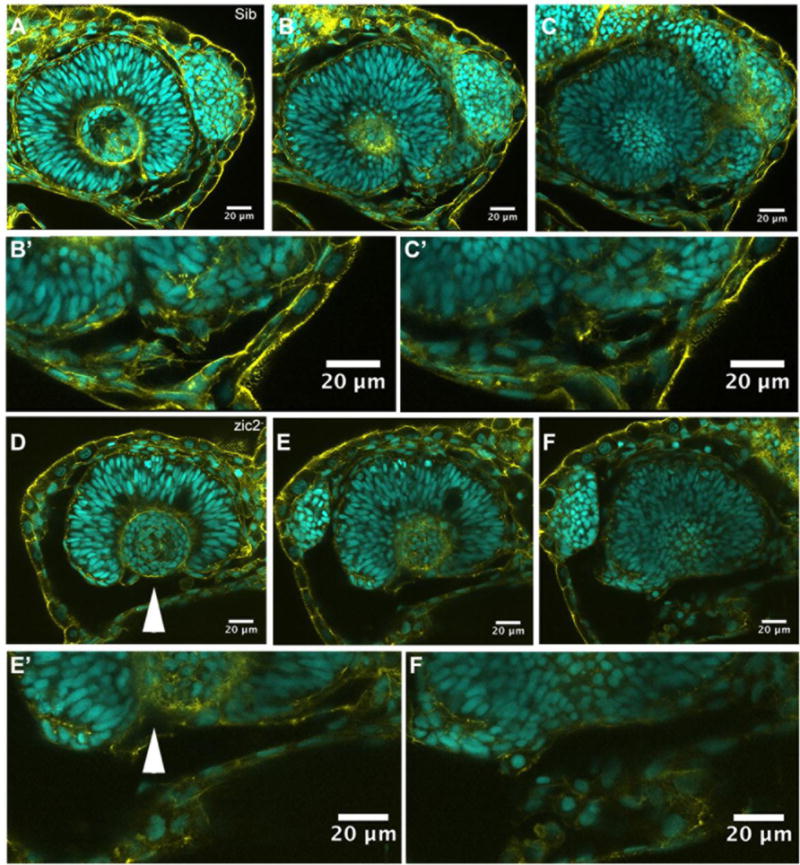
Single confocal sections through optic cups of embryos derived from zic2agbt133/+; zic2b1116/zic2b1116 parents. A–C′: embryo with normal retinal morphology. D–F′: embryo with coloboma. Embryos were imaged in lateral mounts. Cyan = nuclei visualized by DAPI; yellow=F-actin cytoskeleton visualized by phalloidin. Arrowheads point to aberrant gap in the ventral retina (coloboma). Embryos are shown in lateral views, anterior to the right (A–C) or anterior to the left (E–G). B′, C′, E′, F′ are enlarged from B,C,E and F, respectively.
DISCUSSION
The data presented here establish the first genetic model of zic-linked birth defects in zebrafish and extends our understanding of how zic2 coordinates development of the craniofacial complex comprised of brain, retina and craniofacial cartilages. We show that zebrafish zic2a and zic2b function redundantly to promote Hh-dependent retinal morphogenesis, and demonstrate a requirement for both zygotic and maternal zic2 in controlling morphogenesis of the optic stalk and retina, and to restrict pax2a expression in this region. This study confirms a key early role for zic2 in neural crest formation and identifies a homeobox transcription factor Alx1 as a novel effector of zic2 function in the periocular neural crest.
Where does zic2 function to control eye morphogenesis?
Our transcriptome analysis identified an early requirement for zic2 function in a number of neural crest lineages, particularly in the cartilage precursors of the branchial arches and in periocular neural crest. Zebrafish zic2a and zic2b are predominantly expressed in the presumptive neural crest (Grinblat and Sive, 2001; Nyholm et al., 2007; Teslaa et al., 2013; Toyama et al., 2004); hence it is tempting to speculate that zic2 controls ventral retinal morphogenesis indirectly, via a primary function in neural crest. Alx1 is an attractive candidate effector of Zic2, since alx gene family members function in periocular neural crest to regulate retinal morphogenesis in human, mouse and zebrafish (Bertola et al., 2013; Dee et al., 2013; Lakhwani et al., 2010; Qu et al., 1999; Uz et al., 2010; Zhao et al., 1996).
Non-cell-autonomous roles for periocular neural crest in choroid fissure morphogenesis have been demonstrated in mouse mutants (Evans and Gage, 2005; Matt et al., 2008) and in zebrafish morpholino knockdowns (Lupo et al., 2011; McMahon et al., 2009). There is yet much to learn about the mechanisms of these cell interaction, e.g. which specific neural crest lineages are important, when and how they interact with the retina, and which genes direct these interactions. Addressing these questions in robust mutant-based models will be essential going forward, yet such models are currently few and far between. The zic2 mutant zebrafish is an important step toward filling this gap.
Genetic removal of maternal zic2b enhances penetrance and expressivity of retinal coloboma in zic2 mutant embryos (Fig.1), suggesting a potential requirement for zebrafish zic2 function during gastrulation. Zic2a and 2b are expressed in the gastrula mesoderm (Drummond et al., 2013; Grinblat and Sive, 2001; Toyama et al., 2004), including the prechordal plate, which induces formation of the hypothalamus in the ventral diencephalon (Mathieu et al., 2002; Pera and Kessel, 1997; Rubenstein et al., 1998). The hypothalamus subsequently becomes an important source of Shh and is required for regionalization of the anterior diencephalon and optic fissure (Shimamura and Rubenstein, 1997; Zhao et al., 2012). It is therefore feasible that zic2 functions in the prechordal plate during gastrulation to promote ventral diencephalic/hypothalamic specification and the establishment of the hypothalamic signaling center. This hypothesis is supported by the partial rescue of coloboma by Hh inhibition in MZ-zic2 mutants. Notably, mouse zic2 promotes formation of the embryonic organizer during gastrulation (Barratt et al., 2014; Houtmeyers et al., 2016; Warr et al., 2008).
zic2a, but not zic2b, is also expressed in a restricted domain in the distal OS (Nyholm et al., 2007; Sanek et al., 2009; Toyama et al., 2004), where it may function to promote choroid fissure formation cell-autonomously. However, zic2b function should then be largely dispensable for normal retinal morphogenesis; this prediction is not born out by our results, which instead point toward a strict requirement for zic2b and a somewhat relaxed requirement for zic2a function during retinal morphogenesis (Fig. 1).
zic2a and zic2b are also expressed in the retina itself, as is zic2 in higher vertebrates (Brown et al., 2003; Nagai et al., 1997; Toyama et al., 2004). While this expression begins relatively late, ~ 24 hpf, it may contribute to retinal morphogenesis cell-autonomously. The timing of the zic2 mutant deficits described here, which manifest by 24 hpf, argues against a cell-autonomous function of zic2 in the retina. However, the accumulated evidence in mouse models warrants a detailed examination of retinal function of zebrafish zic2 (Garcia-Frigola et al., 2008; Herrera et al., 2003; Lee et al., 2008b). zic2 mutants in combination with powerful methodologies available in zebrafish, e.g. transplant assays, tissue-specific transgenesis and high-resolution live imaging, provide a robust platform for testing these hypotheses efficiently in future studies.
What is the molecular mechanism of zic2 function in the developing retina?
Misregulation of PAX2 has been causally linked to coloboma in humans (reviewed in (Gregory-Evans et al., 2004) and in chick (Sehgal et al., 2008). Regardless of the cell type where zic2 exerts its primary function, it is likely that misregulation of pax2a in zic2 mutants demonstrated here and in zic2 morphants (Sanek et al., 2009) contributes to their retinal anomalies; for this reason, it will be important to ask if this mechanism is conserved in mouse models.
Our demonstration that cyclopamine exposure ameliorates zic2-linked coloboma supports, albeit indirectly, the idea that Hh signaling upregulation is responsible for retinal defects in zic2 mutants. pax2a, a target of Hh signaling, is mispatterned, but is not reduced overall in zic2 mutants. Likewise, upregulation of Hh signaling is not detectable at the level of whole-transcriptome gene expression of known direct targets of Hh at the diencephalic midline, ptc1 and nkx2.2 (Bergeron et al., 2008). These genes are also expressed normally in zic2 mutants when assayed by WISH (data not shown).
An alternate hypothesis, consistent with the apparent de-repression of Hh signaling in zic2 mutants posits that Zic2 regulates transcription of Hh pathway components in a small portion of the embryo, such that would not be detected by our whole-embryo transcriptome approach. For example, it is plausible that a sub-lineage of the periocular neural crest modulates Hh signaling by producing secreted Hh inhibitors or creating physical barriers for Hh diffusion. It is also possible that zic2 restricts pax2a transcription via a parallel, Hh-independent mechanism. If this were the case, cyclopamine acting through Hh signaling may counteract pax2a expansion in zic2 mutants, thereby alleviating severity of coloboma observed in MZ-zic2 mutants. Our data are also consistent with the possibility that zic2 controls cell movements of pax2a-expressing cells rather than pax2a transcription. While the apparent increase in the number of pax2a-positive cells in the mutant diencephalon (Fig. 3G–I) argues against this hypothesis, additional studies are needed to test these hypotheses.
We have likely missed important targets of zic2 in our whole-embryo transcriptome analysis. In other contexts, zic2 has been shown to directly modulate Nodal signaling and canonical Wnt signaling (Fujimi et al., 2012; Houtmeyers et al., 2016; Murgan et al., 2015; Pourebrahim et al., 2011) and may function in this capacity in the developing zebrafish. Exciting recent data identify Zic2 as a key co-factor for chromatin remodeling in embryonic stem cells (Luo et al., 2015), and may function in this capacity in the developing embryos. Nonetheless, the broad-stroke approach taken here has correctly identified a number of cell lineages that depend on zic2 function, among them periocular neural crest, which is necessary for the formation and subsequent closure of the choroid fissure and whose migration is guided by the optic vesicle and by the optic stalks (Eberhart et al., 2008; Langenberg et al., 2008). Despite its limitations, this approach has led us to identify a strong candidate effector of zic2 function in retinal and craniofacial development, alx1. Going forward, RNA-seq to MZ-zic2 embryos at earlier stages of development will allow identification of a more complete and focused set of proximal zic2 targets and effectors.
How does this work inform our understanding of mammalian HPE and related disorders?
Loss-of-function alleles of ZIC2 are found in 10% of patients with the HPE (Brown et al., 2005; Solomon et al., 2010). zic2-linked HPE is unusual for two reasons. First, its penetrance in human patients is the highest of the common HPE-linked genes, 87%; by comparison, penetrance of HPE in patients with Shh mutations is only 36% (Solomon et al., 2012). Second, in contrast to Shh-linked HPE, facial structures of zic2-linked HPE patients are largely normal, although their cerebral morphology ranges from microform to severe alobar (Solomon et al., 2010). This suggests that the developing human forebrain is very sensitive to reduction in ZIC2 levels during human embryogenesis (in contrast, duplication of the ZIC2-containing region does not disrupt human development (Jobanputra et al., 2012).
Mouse and zebrafish embryos are less sensitive to zic2 depletion, since they develop normally when heterozygous for loss-of-function alleles of zic2. Coloboma is the most obvious defect in zebrafish that lack zic2, but diencephalic deficits are also present, as indicated by dysmorphic preoptic diencephalon (Fig. 3), narrowing of forebrain midline in the MZ-zic2 mutants (Fig. 1) and reduction of ventral diencephalic marker expression in zic2 mutants (e.g. lhx6 and nkx2.1; Fig. S3). In contrast, homozygous zic2 mouse mutants develop with prominent forebrain defects (Elms et al., 2003; Nagai et al., 2000). Gongal et al (Gongal et al., 2011) have proposed that HPE and coloboma represent mild and severe aspects of the same phenotypic spectrum; by this token, it is likely that the overt differences between mouse and zebrafish mutant phenotypes reflect quantitative rather than qualitative differences in brain primordium architecture in teleosts vs mammals. This argument further emphasizes the need for in-depth analysis of zic2 functions in more than one model organism.
It is important to note that zic2 double mutant phenotypes largely, but not completely, replicate the phenotypes observed after morpholino-mediated knockdown of zic2a and zic2b individually. The biggest difference between the assays is observed in the anterior diencephalon, which forms normally in MZ-zic2 mutants (Fig. 3), but is disrupted in zic2a morphants (Sanek and Grinblat, 2008; Sanek et al., 2009; Teslaa et al., 2013). This difference may indicate genetic compensation by other members of the zebrafish Zic family that function during brain morphogenesis (Elsen et al., 2008; Maurus and Harris, 2009; Winata et al., 2013) and retinal morphogenesis (Maurus and Harris, 2009). More generally, this study demonstrates the ability of closely related orthologs to compensate for each other’s functions when disrupted chromosomally, but not via transient knockdown. These data will contribute to the collective efforts to understand the mechanisms that underlie the well-documented differences in outcomes of gene disruption through transient knockdown and chromosomal lesions in target genes (Kok et al., 2015; Rossi et al., 2015).
Collectively, our data identify a novel role for zic2 in frontonasal/periocular neural crest development and establish a new animal model of inherited coloboma with frontonasal dysplasia. These data suggest that ZIC2 mutations may contribute to human conditions other than HPE, e.g. frontonasal dysplasia. Human hereditary coloboma frequently presents unilaterally, an indication that modifiers (genetic or environmental) are important contributors to choroid fissure formation. We find that coloboma in zygotic zic2 mutants is incompletely penetrant and predominantly unilateral, making this model ideally suited for modifier screens to identify molecular pathways that interact with zic2. This model will also facilitate in-depth analysis of other key roles for zic2, e.g. their functions in post-mitotic neurons such as cerebellar granule neurons (Frank et al., 2015) and Cajal-Retzius cells (Escalante et al., 2013; Murillo et al., 2015) and its potential link to schizophrenia (Hatayama et al., 2011).
MATERIALS AND METHODS
Zebrafish strains and embryo manipulation
Adult zebrafish were maintained according to established methods (Westerfield, 1993). All experimental protocols using zebrafish were approved by the University of Wisconsin Animal Care and Use Committee, and carried out in accordance with the institutional animal care protocols. Embryos were obtained from natural matings and staged according to (Kimmel et al., 1995). The following mutant strains of zebrafish were used: zic2a GBT133 insertional mutant (Clark et al., 2011); zic2b UWt104 mutant, generated by TALEN mutagenesis in the course of the study; zic2bUW1127 and zic2bUW1116 mutants, generated by CRISPR/Cas9 mutagenesis in the course of this study. Double zic2 mutants were obtained by crossing zic2a GBT133/+ zebrafish to each of the three zic2 mutant allele carriers; F1s were selected by RFP fluorescence to identify zic2aGBT133 carriers, which express RFP (Clark et al., 2011) and raised to adulthood. Adult zic2b+/− zebrafish were identified by PCR genotyping of genomic DNA extracted from tail clips.
Cyclopamine treatments were carried out as follows: Cyclopamine (AdipoGen) was diluted in DMSO and added to E3 to final concentrations of 3uM or 4.5uM; final DMSO concentration was adjusted to 0.5%. Embryos were placed in E3 with cyclopamine or 0.5%DMSO for vehicle-only control. Treatments were started at 3hpf (before maternal-zygotic transition), 5 hpf or 6hpf. Embryos were moved to fresh E3 at 24 hpf and allowed to develop until 3–4 dpf, when they were assayed for retinal morphology.
Engineered nuclease mutagenesis and high-resolution melt analysis (HRMA)
Zic2b TALEN was designed and synthesized by the Mutation Generation and Detection Core (MGD) Facility, University of Utah to the following target left and right sites in exon 1, respectively: 5′-TCCTCTTGCGCAGCCGAGG-3′ and 5′-GGGGTGTTGTCCACTGGCCG-3.
Design of zic2b CRISPR site 5′-GGTGGAGTTAAAAGTGGAGC-3′ in exon1, mutagenesis and founder identifications were carried out as previously described ((Sedykh et al., 2016), with the following HRMA primers pairs: TALEN site - 5′-TGGACAACACCCCATCTTCA-3′ and 5′-GGATGTTTGGAGAGCCGTGAT-3; CRISPR site - 5′-TATTCTGCGGCCGCTCTT3′ and 5′-GGAGTCGAATCCCCAAATC-3′.
Sequencing and PCR genotyping of zic2 mutant alleles
To determine zic2a genotype, DNA was extracted from individual embryos or adult fish and subjected to PCR with the following primers: gbt forward 5′-CCCCGTAATGCAGAAGAAGA-3′, gbt reverse 5′-GTCCAGCTTGATGTCGGTCT-3′, wt forward and wt reverse 5′-ATTCATGGAGCCGTACTGGTTGTG-3′ and 5′-TGTTACTGGACGCAGGGCATCAGTT-3′. (see Supp. Fig. 2 for details).
Zic2b PCR fragments identified as mutant by HRMA were subcloned via TA cloning into pGEMT-Easy (Promega) and sequenced to characterize the mutations. Subsequently, PCR followed by Metaphor gel electrophoresis was used to efficiently genotype individual embryos and adult fish, HRMA primer sequence above were used for CRISPR allele genotyping. TALEN allele genotyping used 5′-GGACAACACCCCATCTTC-3′ and 5′-CGGGGAAAAGTAGGTGAC-3′ (Supp. Fig. 2).
RNA-seq transcriptome analysis
Embryos derived from a zic2aGBT133/+; zic2bUW1127/+ incross were sorted by presence or absence of coloboma. RNA was prepared from each individual embryo using the RNAEasy kit (Qiagen) according to (de Jong et al., 2010). cDNA libraries were prepared using the TruSeq stranded mRNA library preparation protocol with poly-A selection and sequenced on the Illumina HiSeq2500. Gene-level read counts were estimated from the raw sequencing data using RSEM v1.2.18 (Li and Dewey, 2011) and Bowtie v1.1.1 (Langmead et al., 2009). The gene set used consisted of all genes classified as protein coding or lincRNA within the Ensembl v77 annotation of the Zv9 assembly of the zebrafish genome. RSEM was run with option “–forward-prob 0” to take into account that the RNA-seq libraries were strand-specific. A matrix of gene-level counts from all libraries was compiled and analyzed for differential gene expression using the R statistical language and environment (R core team, 2014). Specifically, the count matrix was first pre-normalized using the median normalization routine from the EBSeq v1.5.4 package (Leng et al., 2013). The normalized dataset was then filtered to exclude genes that did not show coverage of at least 10 counts in at least 1 library across the entire dataset. The edgeR v3.14.0 package, (Robinson et al., 2010) with internal normalization switched off, was subsequently applied to call for differential expression (DE). Genes with assigned value of False Discovery Rate (FDR) below 0.05 by edgeR were preliminarily selected. Since a) low-expressed genes tend to be artificially enriched in the list of genes called DE by statistical algorithms and b) DE genes expressed at higher levels have more biological relevance and follow-up potential, we applied an additional filter to the edgeR output by retaining genes that have expression exceeding a certain quantile (0.2) of genome-wide distribution of expression values in at least 60% of libraries representing the strain with a larger mean expression of that gene.
Immunohistochemistry, in situ hybridization and Alcian Blue staining
Embryos were fixed in 4% paraformaldehyde (PFA) in PBS, or in 4% PFA/0.25% glutaraldehyde, 5mM EGTA, 0.2% TritonX-100, 1xPBS for optimal phalloidin staining. After PFA/glutaraldehyde fix, embryos were treated with 100mM sodium borohydride to reduce auto-fluorescence. Primary antibodies were detected fluorescently with Alexa-labeled goat anti-mouse or goat anti-rabbit secondary antibodies. Embryos were mounted in VectaShield and imaged on an Olympus IX81 inverted confocal microscope with the Fluoview 1000 confocal package, using a 60x water immersion objective (NA 1.10), a 60x oil immersion objective (NA 1.35) or a 20x objective (NA 0.75).
|
| ||
| Antibody/stain reagent | Source | Dilution |
|
| ||
| Rabbit anti-pax2a | GeneTex, cat# GTX128127 | 1:500 |
| Mouse anti-acetylated tubulin | Sigma, T6793 | 1:400 |
| Goat Anti-Mouse Alexa 488 | Invitrogen, cat#A-11001 | 1:500 |
| Goat Anti-Rabbit Alexa 568 | Invitrogen, cat#A-11011 | 1:500 |
| phalloidin Alexa 488 or 568 | Molecular Probes, cat#A12379; A12380 | 1:100 |
| DAPI | Molecular Probes, cat#D21490 | 1:5000 |
|
| ||
In situ hybridization was carried out as previously described (Gillhouse et al., 2004), using the following probes: pax2a (Hoyle et al., 2004); dlx2a (Akimenko et al., 1994); crestin (Luo et al., 2001); vax2 (Gross and Dowling, 2005); atoh7 (Masai et al., 2000). cldn11a was synthesized as a gBlocks® Gene Fragments (IDT) and TA-cloned into pGEMT-Easy (Promega). Full length alx1 cDNA was amplified from total mRNA of 24 hpf embryos by PCR with primers 5′-TTGAGACGAGGCCAGAGGAC-3′ and 5′-CCTGGCTCTGTGAATAATTACAAG-3′ primers using OneTaq One-Step RT-PCR kit (NEB), and TA-cloned into pGEMT-Easy (Promega). After WISH, embryos were mounted in 100% glycerol and imaged on Axioskop2 Plus (Zeiss) compound or Leica MZ FLIII stereo microscopes equipped with Leica DFC310 FX camera and LAS v4.0 software. For cartilage staining, zebrafish larvae were fixed at 5–6 dpf in 4% paraformaldehyde and stained with Alcian Blue according to (Kimmel et al., 1998). Samples were flat-mounted in glycerol for imaging as described above.
Supplementary Material
Highlights.
Zic2 controls choroid fissure morphogenesis in zebrafish.
Zic2 is required for periocular mesenchyme formation.
Zic2 activates transcription of alx1, a transcription factor with essential functions during craniofacial and retinal development.
Acknowledgments
We are grateful to Abby Keller for establishing technical expertise in CRISPR mutagenesis, Kelsey Baubie and Lizzie Roehl for fish husbandry. We thank Steve Ekker for providing the zic2agbt133 mutant zebrafish and Kristen Kwan for the gift of pax2a antibody. We also wish to thank David Grunwald for advice and support, the University of Utah Mutation Generation and Detection Core for TALEN design, and the University of Wisconsin Biotechnology Center DNA Sequencing Facility for providing sequencing facilities and services.
FUNDING
This work was supported by grants from the National Institutes of Health (EY022098-01) and American Heart Association (11GRNT7770002) to Y.G, and the Vilas Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA AVAILABILITY
Original data in support of this publication is available upon request. The RNA-seq data files from this study have been deposited at the National Center for Biotechnology Information Gene Expression Omnibus (NCBI GEO) database (accession number GSE99382).
COMPETING INTERESTS
No competing interests declared.
AUTHOR CONTRIBUTIONS
The study was designed by YG. IS, BY and LR carried out the bulk of the experiments. IS, BY and YG analyzed the data. OM and CD carried out bioinformatic analysis of RNAseq data. YG, IS and BY wrote the manuscript. All authors approved the manuscript prior to submission.
References
- Akimenko MA, Ekker M, Wegner J, Lin W, Westerfield M. Combinatorial expression of three zebrafish genes related to distal-less: part of a homeobox gene code for the head. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:3475–3486. doi: 10.1523/JNEUROSCI.14-06-03475.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt KS, Glanville-Jones HC, Arkell RM. The Zic2 gene directs the formation and function of node cilia to control cardiac situs. Genesis. 2014;52:626–635. doi: 10.1002/dvg.22767. [DOI] [PubMed] [Google Scholar]
- Bazin-Lopez N, Valdivia LE, Wilson SW, Gestri G. Watching eyes take shape. Current opinion in genetics & development. 2015;32:73–79. doi: 10.1016/j.gde.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron SA, Milla LA, Villegas R, Shen MC, Burgess SM, Allende ML, Karlstrom RO, Palma V. Expression profiling identifies novel Hh/Gli-regulated genes in developing zebrafish embryos. Genomics. 2008;91:165–177. doi: 10.1016/j.ygeno.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertola DR, Rodrigues MG, Quaio CR, Kim CA, Passos-Bueno MR. Vertical transmission of a frontonasal phenotype caused by a novel ALX4 mutation. American journal of medical genetics Part A. 2013;161A:600–604. doi: 10.1002/ajmg.a.35762. [DOI] [PubMed] [Google Scholar]
- Bronstein JM, Micevych PE, Chen K. Oligodendrocyte-specific protein (OSP) is a major component of CNS myelin. Journal of neuroscience research. 1997;50:713–720. doi: 10.1002/(SICI)1097-4547(19971201)50:5<713::AID-JNR8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Brown L, Paraso M, Arkell R, Brown S. In vitro analysis of partial loss-of-function ZIC2 mutations in holoprosencephaly: alanine tract expansion modulates DNA binding and transactivation. Human molecular genetics. 2005;14:411–420. doi: 10.1093/hmg/ddi037. [DOI] [PubMed] [Google Scholar]
- Brown LY, Kottmann AH, Brown S. Immunolocalization of Zic2 expression in the developing mouse forebrain. Gene expression patterns : GEP. 2003;3:361–367. doi: 10.1016/s1567-133x(03)00043-7. [DOI] [PubMed] [Google Scholar]
- Brown LY, Odent S, David V, Blayau M, Dubourg C, Apacik C, Delgado MA, Hall BD, Reynolds JF, Sommer A, Wieczorek D, Brown SA, Muenke M. Holoprosencephaly due to mutations in ZIC2: alanine tract expansion mutations may be caused by parental somatic recombination. Human molecular genetics. 2001;10:791–796. doi: 10.1093/hmg/10.8.791. [DOI] [PubMed] [Google Scholar]
- Brown SA, Warburton D, Brown LY, Yu CY, Roeder ER, Stengel-Rutkowski S, Hennekam RC, Muenke M. Holoprosencephaly due to mutations in ZIC2, a homologue of Drosophila odd-paired. Nature genetics. 1998;20:180–183. doi: 10.1038/2484. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Allen NC, James AW, Mekonnen Z, Madan E, Helms JA. A primary cilia-dependent etiology for midline facial disorders. Human molecular genetics. 2010;19:1577–1592. doi: 10.1093/hmg/ddq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JE, Place ES, Eve AM, Bradshaw CR, Sesay A, Morrell NW, Smith JC. Global analysis of the haematopoietic and endothelial transcriptome during zebrafish development. Mechanisms of development. 2013;130:122–131. doi: 10.1016/j.mod.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KJ, Balciunas D, Pogoda HM, Ding Y, Westcot SE, Bedell VM, Greenwood TM, Urban MD, Skuster KJ, Petzold AM, Ni J, Nielsen AL, Patowary A, Scaria V, Sivasubbu S, Xu X, Hammerschmidt M, Ekker SC. In vivo protein trapping produces a functional expression codex of the vertebrate proteome. Nature methods. 2011;8:506–515. doi: 10.1038/nmeth.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couly G, Creuzet S, Bennaceur S, Vincent C, Le Douarin NM. Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development. 2002;129:1061–1073. doi: 10.1242/dev.129.4.1061. [DOI] [PubMed] [Google Scholar]
- de Jong M, Rauwerda H, Bruning O, Verkooijen J, Spaink HP, Breit TM. RNA isolation method for single embryo transcriptome analysis in zebrafish. BMC research notes. 2010;3:73. doi: 10.1186/1756-0500-3-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dee CT, Szymoniuk CR, Mills PE, Takahashi T. Defective neural crest migration revealed by a Zebrafish model of Alx1-related frontonasal dysplasia. Human molecular genetics. 2013;22:239–251. doi: 10.1093/hmg/dds423. [DOI] [PubMed] [Google Scholar]
- Drummond DL, Cheng CS, Selland LG, Hocking JC, Prichard LB, Waskiewicz AJ. The role of Zic transcription factors in regulating hindbrain retinoic acid signaling. BMC developmental biology. 2013;13:31. doi: 10.1186/1471-213X-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart JK, He X, Swartz ME, Yan YL, Song H, Boling TC, Kunerth AK, Walker MB, Kimmel CB, Postlethwait JH. MicroRNA Mirn140 modulates Pdgf signaling during palatogenesis. Nature genetics. 2008;40:290–298. doi: 10.1038/ng.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekker SC, Ungar AR, Greenstein P, von Kessler DP, Porter JA, Moon RT, Beachy PA. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Current biology : CB. 1995;5:944–955. doi: 10.1016/s0960-9822(95)00185-0. [DOI] [PubMed] [Google Scholar]
- Elms P, Scurry A, Davies J, Willoughby C, Hacker T, Bogani D, Arkell R. Overlapping and distinct expression domains of Zic2 and Zic3 during mouse gastrulation. Gene expression patterns : GEP. 2004;4:505–511. doi: 10.1016/j.modgep.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Elms P, Siggers P, Napper D, Greenfield A, Arkell R. Zic2 is required for neural crest formation and hindbrain patterning during mouse development. Developmental biology. 2003;264:391–406. doi: 10.1016/j.ydbio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Elsen GE, Choi LY, Millen KJ, Grinblat Y, Prince VE. Zic1 and Zic4 regulate zebrafish roof plate specification and hindbrain ventricle morphogenesis. Developmental biology. 2008;314:376–392. doi: 10.1016/j.ydbio.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante A, Murillo B, Morenilla-Palao C, Klar A, Herrera E. Zic2-dependent axon midline avoidance controls the formation of major ipsilateral tracts in the CNS. Neuron. 2013;80:1392–1406. doi: 10.1016/j.neuron.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Evans AL, Gage PJ. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Human molecular genetics. 2005;14:3347–3359. doi: 10.1093/hmg/ddi365. [DOI] [PubMed] [Google Scholar]
- Frank CL, Liu F, Wijayatunge R, Song L, Biegler MT, Yang MG, Vockley CM, Safi A, Gersbach CA, Crawford GE, West AE. Regulation of chromatin accessibility and Zic binding at enhancers in the developing cerebellum. Nature neuroscience. 2015;18:647–656. doi: 10.1038/nn.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimi TJ, Hatayama M, Aruga J. Xenopus Zic3 controls notochord and organizer development through suppression of the Wnt/beta-catenin signaling pathway. Developmental biology. 2012;361:220–231. doi: 10.1016/j.ydbio.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Garcia-Frigola C, Carreres MI, Vegar C, Mason C, Herrera E. Zic2 promotes axonal divergence at the optic chiasm midline by EphB1-dependent and - independent mechanisms. Development. 2008;135:1833–1841. doi: 10.1242/dev.020693. [DOI] [PubMed] [Google Scholar]
- Gestri G, Link BA, Neuhauss SC. The visual system of zebrafish and its use to model human ocular diseases. Developmental neurobiology. 2012;72:302–327. doi: 10.1002/dneu.20919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillhouse M, Wagner Nyholm M, Hikasa H, Sokol SY, Grinblat Y. Two Frodo/Dapper homologs are expressed in the developing brain and mesoderm of zebrafish. Developmental dynamics : an official publication of the American Association of Anatomists. 2004;230:403–409. doi: 10.1002/dvdy.20060. [DOI] [PubMed] [Google Scholar]
- Gongal PA, French CR, Waskiewicz AJ. Aberrant forebrain signaling during early development underlies the generation of holoprosencephaly and coloboma. Biochimica et biophysica acta. 2011;1812:390–401. doi: 10.1016/j.bbadis.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Gregory-Evans CY, Williams MJ, Halford S, Gregory-Evans K. Ocular coloboma: a reassessment in the age of molecular neuroscience. J Med Genet. 2004;41:881–891. doi: 10.1136/jmg.2004.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinblat Y, Sive H. zic Gene expression marks anteroposterior pattern in the presumptive neurectoderm of the zebrafish gastrula. Developmental dynamics : an official publication of the American Association of Anatomists. 2001;222:688–693. doi: 10.1002/dvdy.1221. [DOI] [PubMed] [Google Scholar]
- Gross JM, Dowling JE. Tbx2b is essential for neuronal differentiation along the dorsal/ventral axis of the zebrafish retina. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4371–4376. doi: 10.1073/pnas.0501061102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatayama M, Ishiguro A, Iwayama Y, Takashima N, Sakoori K, Toyota T, Nozaki Y, Odaka YS, Yamada K, Yoshikawa T, Aruga J. Zic2 hypomorphic mutant mice as a schizophrenia model and ZIC2 mutations identified in schizophrenia patients. Scientific reports. 2011;1:16. doi: 10.1038/srep00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera E, Brown L, Aruga J, Rachel RA, Dolen G, Mikoshiba K, Brown S, Mason CA. Zic2 patterns binocular vision by specifying the uncrossed retinal projection. Cell. 2003;114:545–557. doi: 10.1016/s0092-8674(03)00684-6. [DOI] [PubMed] [Google Scholar]
- Houtmeyers R, Tchouate Gainkam O, Glanville-Jones HA, Van den Bosch B, Chappell A, Barratt KS, Souopgui J, Tejpar S, Arkell RM. Zic2 mutation causes holoprosencephaly via disruption of NODAL signalling. Human molecular genetics. 2016;25:3946–3959. doi: 10.1093/hmg/ddw235. [DOI] [PubMed] [Google Scholar]
- Hoyle J, Tang YP, Wiellette EL, Wardle FC, Sive H. nlz gene family is required for hindbrain patterning in the zebrafish. Developmental dynamics : an official publication of the American Association of Anatomists. 2004;229:835–846. doi: 10.1002/dvdy.20001. [DOI] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Inoue T, Ota M, Mikoshiba K, Aruga J. Zic2 and Zic3 synergistically control neurulation and segmentation of paraxial mesoderm in mouse embryo. Developmental biology. 2007;306:669–684. doi: 10.1016/j.ydbio.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Jobanputra V, Burke A, Kwame AY, Shanmugham A, Shirazi M, Brown S, Warburton PE, Levy B, Warburton D. Duplication of the ZIC2 gene is not associated with holoprosencephaly. American journal of medical genetics Part A. 2012;158A:103–108. doi: 10.1002/ajmg.a.34375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Developmental dynamics : an official publication of the American Association of Anatomists. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Miller CT, Kruze G, Ullmann B, BreMiller RA, Larison KD, Snyder HC. The shaping of pharyngeal cartilages during early development of the zebrafish. Developmental biology. 1998;203:245–263. doi: 10.1006/dbio.1998.9016. [DOI] [PubMed] [Google Scholar]
- Kish PE, Bohnsack BL, Gallina D, Kasprick DS, Kahana A. The eye as an organizer of craniofacial development. Genesis. 2011;49:222–230. doi: 10.1002/dvg.20716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok FO, Shin M, Ni CW, Gupta A, Grosse AS, van Impel A, Kirchmaier BC, Peterson-Maduro J, Kourkoulis G, Male I, DeSantis DF, Sheppard-Tindell S, Ebarasi L, Betsholtz C, Schulte-Merker S, Wolfe SA, Lawson ND. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Developmental cell. 2015;32:97–108. doi: 10.1016/j.devcel.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KM. Coming into focus: the role of extracellular matrix in vertebrate optic cup morphogenesis. Developmental dynamics : an official publication of the American Association of Anatomists. 2014;243:1242–1248. doi: 10.1002/dvdy.24162. [DOI] [PubMed] [Google Scholar]
- Lakhwani S, Garcia-Sanz P, Vallejo M. Alx3-deficient mice exhibit folic acid-resistant craniofacial midline and neural tube closure defects. Developmental biology. 2010;344:869–880. doi: 10.1016/j.ydbio.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Langenberg T, Kahana A, Wszalek JA, Halloran MC. The eye organizes neural crest cell migration. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237:1645–1652. doi: 10.1002/dvdy.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome biology. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin NM, Brito JM, Creuzet S. Role of the neural crest in face and brain development. Brain research reviews. 2007;55:237–247. doi: 10.1016/j.brainresrev.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Lee J, Willer JR, Willer GB, Smith K, Gregg RG, Gross JM. Zebrafish blowout provides genetic evidence for Patched1-mediated negative regulation of Hedgehog signaling within the proximal optic vesicle of the vertebrate eye. Developmental biology. 2008a;319:10–22. doi: 10.1016/j.ydbio.2008.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Petros TJ, Mason CA. Zic2 regulates retinal ganglion cell axon avoidance of ephrinB2 through inducing expression of the guidance receptor EphB1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008b;28:5910–5919. doi: 10.1523/JNEUROSCI.0632-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng N, Dawson JA, Thomson JA, Ruotti V, Rissman AI, Smits BM, Haag JD, Gould MN, Stewart RM, Kendziorski C. EBSeq: an empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics. 2013;29:1035–1043. doi: 10.1093/bioinformatics/btt087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R, An M, Arduini BL, Henion PD. Specific pan-neural crest expression of zebrafish Crestin throughout embryonic development. Developmental dynamics : an official publication of the American Association of Anatomists. 2001;220:169–174. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1097>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Luo Z, Gao X, Lin C, Smith ER, Marshall SA, Swanson SK, Florens L, Washburn MP, Shilatifard A. Zic2 is an enhancer-binding factor required for embryonic stem cell specification. Molecular cell. 2015;57:685–694. doi: 10.1016/j.molcel.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo G, Gestri G, O’Brien M, Denton RM, Chandraratna RA, Ley SV, Harris WA, Wilson SW. Retinoic acid receptor signaling regulates choroid fissure closure through independent mechanisms in the ventral optic cup and periocular mesenchyme. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8698–8703. doi: 10.1073/pnas.1103802108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo G, Liu Y, Qiu R, Chandraratna RA, Barsacchi G, He RQ, Harris WA. Dorsoventral patterning of the Xenopus eye: a collaboration of Retinoid, Hedgehog and FGF receptor signaling. Development. 2005;132:1737–1748. doi: 10.1242/dev.01726. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Barth KA, Xu Q, Holder N, Mikkola I, Wilson SW. Midline signalling is required for Pax gene regulation and patterning of the eyes. Development. 1995;121:3267–3278. doi: 10.1242/dev.121.10.3267. [DOI] [PubMed] [Google Scholar]
- Masai I, Stemple DL, Okamoto H, Wilson SW. Midline signals regulate retinal neurogenesis in zebrafish. Neuron. 2000;27:251–263. doi: 10.1016/s0896-6273(00)00034-9. [DOI] [PubMed] [Google Scholar]
- Mathieu J, Barth A, Rosa FM, Wilson SW, Peyrieras N. Distinct and cooperative roles for Nodal and Hedgehog signals during hypothalamic development. Development. 2002;129:3055–3065. doi: 10.1242/dev.129.13.3055. [DOI] [PubMed] [Google Scholar]
- Matt N, Ghyselinck NB, Pellerin I, Dupe V. Impairing retinoic acid signalling in the neural crest cells is sufficient to alter entire eye morphogenesis. Developmental biology. 2008;320:140–148. doi: 10.1016/j.ydbio.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Maurus D, Harris WA. Zic-associated holoprosencephaly: zebrafish Zic1 controls midline formation and forebrain patterning by regulating Nodal, Hedgehog, and retinoic acid signaling. Genes & development. 2009;23:1461–1473. doi: 10.1101/gad.517009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon C, Gestri G, Wilson SW, Link BA. Lmx1b is essential for survival of periocular mesenchymal cells and influences Fgf-mediated retinal patterning in zebrafish. Developmental biology. 2009;332:287–298. doi: 10.1016/j.ydbio.2009.05.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Fujimoto K, Furuse M, Tsukita S. Claudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in testis. The Journal of cell biology. 1999;145:579–588. doi: 10.1083/jcb.145.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mui SH, Kim JW, Lemke G, Bertuzzi S. Vax genes ventralize the embryonic eye. Genes Dev. 2005;19:1249–1259. doi: 10.1101/gad.1276605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgan S, Kari W, Rothbacher U, Iche-Torres M, Melenec P, Hobert O, Bertrand V. Atypical Transcriptional Activation by TCF via a Zic Transcription Factor in C. elegans Neuronal Precursors. Developmental cell. 2015;33:737–745. doi: 10.1016/j.devcel.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo B, Ruiz-Reig N, Herrera M, Fairen A, Herrera E. Zic2 Controls the Migration of Specific Neuronal Populations in the Developing Forebrain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:11266–11280. doi: 10.1523/JNEUROSCI.0779-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Aruga J, Minowa O, Sugimoto T, Ohno Y, Noda T, Mikoshiba K. Zic2 regulates the kinetics of neurulation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1618–1623. doi: 10.1073/pnas.97.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Aruga J, Takada S, Gunther T, Sporle R, Schughart K, Mikoshiba K. The expression of the mouse Zic1, Zic2, and Zic3 gene suggests an essential role for Zic genes in body pattern formation. Developmental biology. 1997;182:299–313. doi: 10.1006/dbio.1996.8449. [DOI] [PubMed] [Google Scholar]
- Nakata K, Nagai T, Aruga J, Mikoshiba K. Xenopus Zic3, a primary regulator both in neural and neural crest development. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:11980–11985. doi: 10.1073/pnas.94.22.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, Nagai T, Aruga J, Mikoshiba K. Xenopus Zic family and its role in neural and neural crest development. Mechanisms of development. 1998;75:43–51. doi: 10.1016/s0925-4773(98)00073-2. [DOI] [PubMed] [Google Scholar]
- Nyholm MK, Abdelilah-Seyfried S, Grinblat Y. A novel genetic mechanism regulates dorsolateral hinge-point formation during zebrafish cranial neurulation. Journal of cell science. 2009;122:2137–2148. doi: 10.1242/jcs.043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm MK, Wu SF, Dorsky RI, Grinblat Y. The zebrafish zic2a-zic5 gene pair acts downstream of canonical Wnt signaling to control cell proliferation in the developing tectum. Development. 2007;134:735–746. doi: 10.1242/dev.02756. [DOI] [PubMed] [Google Scholar]
- Onwochei BC, Simon JW, Bateman JB, Couture KC, Mir E. Ocular colobomata. Survey of ophthalmology. 2000;45:175–194. doi: 10.1016/s0039-6257(00)00151-x. [DOI] [PubMed] [Google Scholar]
- Pan H, Gustafsson MK, Aruga J, Tiedken JJ, Chen JC, Emerson CP., Jr A role for Zic1 and Zic2 in Myf5 regulation and somite myogenesis. Developmental biology. 2011;351:120–127. doi: 10.1016/j.ydbio.2010.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera EM, Kessel M. Patterning of the chick forebrain anlage by the prechordal plate. Development. 1997;124:4153–4162. doi: 10.1242/dev.124.20.4153. [DOI] [PubMed] [Google Scholar]
- Pillai-Kastoori L, Wen W, Wilson SG, Strachan E, Lo-Castro A, Fichera M, Musumeci SA, Lehmann OJ, Morris AC. Sox11 is required to maintain proper levels of Hedgehog signaling during vertebrate ocular morphogenesis. PLoS genetics. 2014;10:e1004491. doi: 10.1371/journal.pgen.1004491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourebrahim R, Houtmeyers R, Ghogomu S, Janssens S, Thelie A, Tran HT, Langenberg T, Vleminckx K, Bellefroid E, Cassiman JJ, Tejpar S. Transcription factor Zic2 inhibits Wnt/beta-catenin protein signaling. The Journal of biological chemistry. 2011;286:37732–37740. doi: 10.1074/jbc.M111.242826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu S, Tucker SC, Zhao Q, deCrombrugghe B, Wisdom R. Physical and genetic interactions between Alx4 and Cart1. Development. 1999;126:359–369. doi: 10.1242/dev.126.2.359. [DOI] [PubMed] [Google Scholar]
- Ribeiro LA, Roessler E, Hu P, Pineda-Alvarez DE, Zhou N, Jones M, Chandrasekharappa S, Richieri-Costa A, Muenke M. Comparison of mutation findings in ZIC2 between microform and classical holoprosencephaly in a Brazilian cohort. Birth defects research Part A, Clinical and molecular teratology. 2012;94:912–917. doi: 10.1002/bdra.23047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC, Muenke M. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nature genetics. 1996;14:357–360. doi: 10.1038/ng1196-357. [DOI] [PubMed] [Google Scholar]
- Roessler E, Lacbawan F, Dubourg C, Paulussen A, Herbergs J, Hehr U, Bendavid C, Zhou N, Ouspenskaia M, Bale S, Odent S, David V, Muenke M. The full spectrum of holoprosencephaly-associated mutations within the ZIC2 gene in humans predicts loss-of-function as the predominant disease mechanism. Human mutation. 2009;30:E541–554. doi: 10.1002/humu.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler E, Muenke M. The molecular genetics of holoprosencephaly. American journal of medical genetics Part C, Seminars in medical genetics. 2010;154C:52–61. doi: 10.1002/ajmg.c.30236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Kontarakis Z, Gerri C, Nolte H, Holper S, Kruger M, Stainier DY. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature. 2015;524:230–233. doi: 10.1038/nature14580. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Shimamura K, Martinez S, Puelles L. Regionalization of the prosencephalic neural plate. Annual review of neuroscience. 1998;21:445–477. doi: 10.1146/annurev.neuro.21.1.445. [DOI] [PubMed] [Google Scholar]
- Sanek NA, Grinblat Y. A novel role for zebrafish zic2a during forebrain development. Developmental biology. 2008;317:325–335. doi: 10.1016/j.ydbio.2008.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanek NA, Taylor AA, Nyholm MK, Grinblat Y. Zebrafish zic2a patterns the forebrain through modulation of Hedgehog-activated gene expression. Development. 2009;136:3791–3800. doi: 10.1242/dev.037820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling TF, Concordet JP, Ingham PW. Regulation of left-right asymmetries in the zebrafish by Shh and BMP4. Developmental biology. 1999;210:277–287. doi: 10.1006/dbio.1999.9214. [DOI] [PubMed] [Google Scholar]
- Schimmenti LA, de la Cruz J, Lewis RA, Karkera JD, Manligas GS, Roessler E, Muenke M. Novel mutation in sonic hedgehog in non-syndromic colobomatous microphthalmia. American journal of medical genetics Part A. 2003;116A:215–221. doi: 10.1002/ajmg.a.10884. [DOI] [PubMed] [Google Scholar]
- Schmitt EA, Dowling JE. Early eye morphogenesis in the zebrafish, Brachydanio rerio. The Journal of comparative neurology. 1994;344:532–542. doi: 10.1002/cne.903440404. [DOI] [PubMed] [Google Scholar]
- Sedykh I, TeSlaa JJ, Tatarsky RL, Keller AN, Toops KA, Lakkaraju A, Nyholm MK, Wolman MA, Grinblat Y. Novel roles for the radial spoke head protein 9 in neural and neurosensory cilia. Scientific reports. 2016;6:34437. doi: 10.1038/srep34437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal R, Karcavich R, Carlson S, Belecky-Adams TL. Ectopic Pax2 expression in chick ventral optic cup phenocopies loss of Pax2 expression. Dev Biol. 2008;319:23–33. doi: 10.1016/j.ydbio.2008.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- Solomon BD, Bear KA, Wyllie A, Keaton AA, Dubourg C, David V, Mercier S, Odent S, Hehr U, Paulussen A, Clegg NJ, Delgado MR, Bale SJ, Lacbawan F, Ardinger HH, Aylsworth AS, Bhengu NL, Braddock S, Brookhyser K, Burton B, Gaspar H, Grix A, Horovitz D, Kanetzke E, Kayserili H, Lev D, Nikkel SM, Norton M, Roberts R, Saal H, Schaefer GB, Schneider A, Smith EK, Sowry E, Spence MA, Shalev SA, Steiner CE, Thompson EM, Winder TL, Balog JZ, Hadley DW, Zhou N, Pineda-Alvarez DE, Roessler E, Muenke M. Genotypic and phenotypic analysis of 396 individuals with mutations in Sonic Hedgehog. Journal of medical genetics. 2012;49:473–479. doi: 10.1136/jmedgenet-2012-101008. [DOI] [PubMed] [Google Scholar]
- Solomon BD, Lacbawan F, Mercier S, Clegg NJ, Delgado MR, Rosenbaum K, Dubourg C, David V, Olney AH, Wehner LE, Hehr U, Bale S, Paulussen A, Smeets HJ, Hardisty E, Tylki-Szymanska A, Pronicka E, Clemens M, McPherson E, Hennekam RC, Hahn J, Stashinko E, Levey E, Wieczorek D, Roeder E, Schell-Apacik CC, Booth CW, Thomas RL, Kenwrick S, Cummings DA, Bous SM, Keaton A, Balog JZ, Hadley D, Zhou N, Long R, Velez JI, Pineda-Alvarez DE, Odent S, Roessler E, Muenke M. Mutations in ZIC2 in human holoprosencephaly: description of a novel ZIC2 specific phenotype and comprehensive analysis of 157 individuals. Journal of medical genetics. 2010;47:513–524. doi: 10.1136/jmg.2009.073049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz ME, Sheehan-Rooney K, Dixon MJ, Eberhart JK. Examination of a palatogenic gene program in zebrafish. Developmental dynamics : an official publication of the American Association of Anatomists. 2011;240:2204–2220. doi: 10.1002/dvdy.22713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Take-uchi M, Clarke JD, Wilson SW. Hedgehog signalling maintains the optic stalk-retinal interface through the regulation of Vax gene activity. Development. 2003;130:955–968. doi: 10.1242/dev.00305. [DOI] [PubMed] [Google Scholar]
- Teslaa JJ, Keller AN, Nyholm MK, Grinblat Y. Zebrafish Zic2a and Zic2b regulate neural crest and craniofacial development. Developmental biology. 2013;380:73–86. doi: 10.1016/j.ydbio.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama R, Gomez DM, Mana MD, Dawid IB. Sequence relationships and expression patterns of zebrafish zic2 and zic5 genes. Gene expression patterns : GEP. 2004;4:345–350. doi: 10.1016/j.modgep.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Uz E, Alanay Y, Aktas D, Vargel I, Gucer S, Tuncbilek G, von Eggeling F, Yilmaz E, Deren O, Posorski N, Ozdag H, Liehr T, Balci S, Alikasifoglu M, Wollnik B, Akarsu NA. Disruption of ALX1 causes extreme microphthalmia and severe facial clefting: expanding the spectrum of autosomal-recessive ALX-related frontonasal dysplasia. American journal of human genetics. 2010;86:789–796. doi: 10.1016/j.ajhg.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga ZM, Amores A, Lewis KE, Yan YL, Postlethwait JH, Eisen JS, Westerfield M. Zebrafish smoothened functions in ventral neural tube specification and axon tract formation. Development. 2001;128:3497–3509. doi: 10.1242/dev.128.18.3497. [DOI] [PubMed] [Google Scholar]
- Wada N, Javidan Y, Nelson S, Carney TJ, Kelsh RN, Schilling TF. Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull. Development. 2005;132:3977–3988. doi: 10.1242/dev.01943. [DOI] [PubMed] [Google Scholar]
- Wang X, Lupo G, He R, Barsacchi G, Harris WA, Liu Y. Dorsoventral patterning of the Xenopus eye involves differential temporal changes in the response of optic stalk and retinal progenitors to Hh signalling. Neural development. 2015;10:7. doi: 10.1186/s13064-015-0035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr N, Powles-Glover N, Chappell A, Robson J, Norris D, Arkell RM. Zic2-associated holoprosencephaly is caused by a transient defect in the organizer region during gastrulation. Human molecular genetics. 2008;17:2986–2996. doi: 10.1093/hmg/ddn197. [DOI] [PubMed] [Google Scholar]
- Wen W, Pillai-Kastoori L, Wilson SG, Morris AC. Sox4 regulates choroid fissure closure by limiting Hedgehog signaling during ocular morphogenesis. Developmental biology. 2015;399:139–153. doi: 10.1016/j.ydbio.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book : a guide for the laboratory use of zebrafish (Brachydanio rerio) M Westerfield; Eugene, OR: 1993. [Google Scholar]
- Williamson KA, FitzPatrick DR. The genetic architecture of microphthalmia, anophthalmia and coloboma. European journal of medical genetics. 2014;57:369–380. doi: 10.1016/j.ejmg.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Winata CL, Kondrychyn I, Kumar V, Srinivasan KG, Orlov Y, Ravishankar A, Prabhakar S, Stanton LW, Korzh V, Mathavan S. Genome wide analysis reveals Zic3 interaction with distal regulatory elements of stage specific developmental genes in zebrafish. PLoS genetics. 2013;9:e1003852. doi: 10.1371/journal.pgen.1003852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu E, Vargevik K, Slavotinek AM. Subtypes of frontonasal dysplasia are useful in determining clinical prognosis. American journal of medical genetics Part A. 2007;143A:3069–3078. doi: 10.1002/ajmg.a.31963. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Gaston-Massuet C, Girdler G, Klingensmith J, Arkell R, Greene ND, Copp AJ. Neural plate morphogenesis during mouse neurulation is regulated by antagonism of Bmp signalling. Development. 2007;134:3203–3211. doi: 10.1242/dev.008177. [DOI] [PubMed] [Google Scholar]
- Young NM, Chong HJ, Hu D, Hallgrimsson B, Marcucio RS. Quantitative analyses link modulation of sonic hedgehog signaling to continuous variation in facial growth and shape. Development. 2010;137:3405–3409. doi: 10.1242/dev.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Zevallos SE, Rizzoti K, Jeong Y, Lovell-Badge R, Epstein DJ. Disruption of SoxB1-dependent Sonic hedgehog expression in the hypothalamus causes septo-optic dysplasia. Developmental cell. 2012;22:585–596. doi: 10.1016/j.devcel.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Behringer RR, de Crombrugghe B. Prenatal folic acid treatment suppresses acrania and meroanencephaly in mice mutant for the Cart1 homeobox gene. Nature genetics. 1996;13:275–283. doi: 10.1038/ng0796-275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


