Summary
The Conserved Oligomeric Golgi (COG) complex is a key evolutionally conserved multisubunit protein machinery that regulates tethering and fusion of intra-Golgi transport vesicles. The Golgi apparatus specifically promotes sorting and complex glycosylation of glycoconjugates. Without proper glycosylation and processing, proteins and lipids will be mislocalized and/or have impaired function. The Golgi glycosylation machinery is kept in homeostasis by a careful balance of anterograde and retrograde trafficking to ensure proper localization of the glycosylation enzymes and their substrates. This balance, like other steps of membrane trafficking, is maintained by vesicle trafficking machinery that includes COPI vesicular coat proteins, SNAREs, Rabs, and both coiled-coil and multi-subunit vesicular tethers. COG complex interacts with other membrane trafficking components and is essential for proper localization of Golgi glycosylation machinery. Here we describe using CRISPR-mediated gene editing coupled with a phenotype-based selection strategy directly linked to the COG complex’s role in glycosylation homeostasis to obtain COG complex subunit knock-outs (KOs). This has resulted in clonal KOs for each COG subunit in HEK293T cells and gives the ability to further probe the role of the COG complex in Golgi homeostasis.
Keywords: CRISPR, COG, Conserved oligomeric Golgi complex, fluorescently tagged lectins, FAC sorting, glycosylation defects, Knock-outs, SubAB toxin, Cholera toxin
1. Introduction
The COG complex is a bi-lobed protein complex that has eight subunits (lobe A: 1–4 lobe B: 5–8) and functions in retrograde trafficking of vesicles (containing glycosylation enzymes among other cargo) at the Golgi apparatus (1–6). Its conservation across a variety of organisms (yeast, worms, plants, and humans to name a few)(7–9, 1, 10, 11) hints at its crucial role in the cell, and indeed a loss of function of the COG complex results in a genetic disorder in the family of Congenital Disorders of Glycosylation (CDG)(12). These COG associated disorders fall under type II which is characterized by the improper processing of glycosylated proteins, presumably due to lack of proper glycosylation enzyme homeostasis. Patients with these disorders exhibit a wide variety of symptoms due to the many proteins that are dependent upon COG for proper location and function. Some of these include; issues with clotting, elevated liver enzymes, mental and growth retardation, seizures, and-- in the most severe cases-- lethality within the first year of life (13, 14). Additionally pathogens, such as Chlamydia and HIV, are known to depend on COG function for their trafficking and survival in host cells (15–17). Though it is clear that the COG complex is important for Golgi homeostasis, its specific mechanism of action is still being elucidated.
One of the main drawbacks of COG complex studies has been a lack of immortal human cell lines that are deficient in each COG subunit to provide a clean background for functional assays. The current work-around for this is to use RNAi approaches to significantly reduce protein levels (18–20), or to re-localize over expressed COG protein to a non-physiological location using a mitochondrial targeting tag (3).
The following method alleviates this issue by creating immortal human KO cell lines deficient for individual COG complex subunits using CRISPR technology paired with a COG deficiency specific sorting methodology we developed for this project. The phenotypic differences between the KOs and their parental cell lines are more numerous and far more drastic than seen in the past knock-down and re-localization assays used to characterize the COG complex thus far.
CRISPR-Cas9 gene editing has made a great impact in many fields since its discovery as a gene editing platform in 2012 (21). The CRISPR-Cas9 system allows for quick gene KO or modification in a highly specific manner by using a single guide RNA sequence, or sgRNA, of 17 to 20 base pairs to target bacterial endonuclease Cas9 to create a double strand break. This sgRNA can be any sequence as long as it lies adjacent to an NGG protospacer motif (or PAM) sequence. The double strand endonuclease action causes a homozygous KO <1% to 99% of the time depending on transfection conditions.
Because of the variable efficiency, CRISPR-Cas9 treated cells must be screened to determine that the gene of interest has been homozygously knocked-out.
In this chapter we detail a gene-knock out and selection method based on the arising glycosylation defects resulting from COG complex malfunction that have previously been shown (22).
We also include a section that highlights two examples of phenotypic analysis that were done with the resulting KO cells.
2. Materials
All buffers should be prepared using ultrapure water (18 MΩ water) except for electrophoresis (running) and electroblot buffers. All reagents are analytical grade.
2.1 Transfection of cells with CRISPR constructs
E.coli NEB 10-beta Competent E. coli for transformation and obtaining plasmids (see Note 1).
SOC (Super Optimal Broth) media
LB (Luria-Bertrani) broth: add 20 g of LB Broth to 1 L of water and autoclave for 30 min.
Kanamycin antibiotic (1000x stock: 150 mg kanamycin sulfate in 5 ml dd H2O)
Kanamycin (30 mg/L) LB agar plates.
Plasmids containing Cas9 endonuclease and gRNA targeting the first exon of the COG 7 subunit (Catalog # HCP222287-CG01-3-B-a, HCP222287-CG01-3-B-b, HCP222287-CG01-3-B-c) (Gencopoeia, Rockville, MD).
Control plasmid containing GFP (see Note 2)
QIAprep Spin Miniprep Kit.
HEK293T cells (CRL-3216 ATCC, Mananas, VA) grown to ~70% confluency on 6-well plates
Lipofectamine 2000 Transfection Reagent
Dulbecco’s Phosphate Buffered Saline (DPBS 1x) without calcium and magnesium
Transfection media: Opti-MEM I Reduced Serum Media buffered with HEPES and sodium bicarbonate and supplemented with hypoxanthine, thymidine, sodium bicarbonate and supplemented with hypoxanthine, thymidine, sodium pyruvate, L-glutamine, trace elements, and growth factors.
Growth media: dilute 50 mL of heat-inactivated Fetal Bovine Serum (FBS) in 450 mL of DMEM/F-12 50/50 medium supplemented with 2.5 mM L-glutamine , 15 mM HEPES. Sterile filtered.
2.2 Lectin staining, FAC sorting, and colony expanding
12 mm round glass coverslips (#1.5, 0.17 mm thickness),
Glass slides: frosted microscope slides (precleaned).
Galanthus nivalus lectin (GNL) (20 μg/mL, Vector laboratories; Burlingame, CA) labeled with an Alexa-647 protein labeling kit (Life Technologies) according to the manufacturer protocol.
0.1% bovine serum albumin (BSA) in PBS, 0.2 μm sterile filtered
Collagen solution: 50 μg/mL Collagen, Bovine, Type I in 0.01 N HCl
Paraformaldehyde 16% stock solution
ProlongR Gold antifade reagent
Cell Sorting Media: PBS, 25 mM Hepes pH= 7.0, 2% FBS (heat inactivated) 1mM EDTA.0.2 μm sterile filtered
Mammalian cell Culture Plates: 10 cm, 12-well, 24-well, and 96-well
1.5 mL microcentrifuge tubes
Filtered Cap 5ml- 12x75mm polystyrene round bottom tubes
. Gibco 100x Antibiotic/Antimycotic
Widefield or confocal fluorescence microscope.
2.3 Lysate and DNA preparation for validation
2% SDS in water
Heat blocks set to 95°C for lysates and 63°C and 98°C for DNA
1.5 mL microcentrifuge tubes
Quick Extract DNA extraction solution
2.4 Sodium Dodecyl Sulfate- Polyacrylamide Gel Electrophoresis and Western Blot Components
9% SDS-PAGE mini-gels
Vertical mini-gel electrophoresis unit
SDS PAGE running buffer: 25 mM Tris, 0.192 M glycine, 0.1% SDS
0.2 μm Whatman Protran Nitrocellulose Blotting Membranes
Blotting paper
Pierce G2 Fast Blotter and 1-Step Transfer Buffer (Thermo Scientific, Rockford, IL)
Odyssey blocking buffer (LI-COR Biosciences, Pittsburgh,NE)
Secondary antibody incubation solution: PBS containing 5% non-fat dry milk.
-
Antibodies:
Primary: rabbit anti-COG7 antibody were developed using purified His6-COG7 expressed in bacteria (2) and mouse monoclonal β-actin (Sigma #030M4788)
Secondary: LI-COR Donkey anti-Mouse 680 (#926-68072), LI-COR Goat anti-Rabbit 800 (#925-32211) (LI-COR Biosciences, Lincoln England)
LI-COR Odyssey imaging system.
6x Laemmli sample buffer with 5% 2-mercaptoethanol
Ponceau S stain: 0.2 g Ponceau S , 5 mL of Acetic acid ( 100 %), 200 mL dd H2O
A Western Incubation Box
Dulbecco’s Phosphate Buffered Saline (DPBS 1x) without calcium and magnesium
Fat free evaporated milk
2.5 Polymerase Chain Reaction (PCR) and Purification for sequencing
-
Primers used to amplify and targeting region of COG 7 gene:
COG 7 Forward Primer: AGAGGAGGAAAAACAACACCCAA
COG 7 Reverse Primer: AGTTACCCGTCCTGGCGTTT
DMSO
dNTPs
10x exTaq buffer
exTaq polymerase
0.2 mL PCR tubes
Thermocycler
Zymoclean gel DNA recovery kit
2.6 Phenotypic analysis by SubAB trafficking
6-Well plates (TPP) with cells between 50–80%
SubAB toxin (22)
Growth media (see Section 2.1. part 13)
95°C 2%SDS
-
Antibodies:
Primary: goat polyclonal anti-GRP78 (Santa Cruz Biotechnology, Santa Cruz, CA, SC-1051) and mouse monoclonal β-actin (Sigma #030M4788)
Secondary: Donkey anti-Mouse 680, Donkey anti-Goat 800 (LI-COR Biosciences)
Gradient gels 4–15% (commercially available)
All materials listed in 2.4 for SDS-PAGE and Western blotting.
2.6 Phenotypic analysis by Cholera Toxin binding and flow cytometry
6-Well plates with cells (COG KO and WT HEK293 cells as a control) with confluency between 50–80%
Cholera toxin lectin (B subunit only) (CTxB) (20 μg/mL) was labeled with an Alexa-647 protein labeling kit from Life Technologies.
Growth Media (see Section 2.1. part 13)
0.1%BSA in PBS. 0.2 μm sterile filtered
1.5 mL microcentrifuge tubes
Filtered Cap 5 mL- 12x75mm polystyrene round bottom tubes
1 μM 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI)
BD Fortessa Flow Cytometer (or equivalent) detecting at 647 nm.
3. Methods
3.1 Plasmid transformation and preparation
Day One
-
1
Add 1 μl of COG 7 targeting CRISPR plasmid to the tube containing competent E.coli cells; let incubate for 30 min. on ice.
-
2
Heat-shock the cells: Place tube with cells in a 42°C water bath for 40 sec.
-
3
Put tube back on ice for one minute for recovery.
-
5
Add 300 μl SOC media to cells.
-
6
Incubate in 37°C water bath for one hour.
-
7
Spin down for 1 min (6,000 x g).
-
8
Remove 200 μl and resuspend pellet in remaining liquid.
-
9
Take suspension and spread on a LB Agar plate (with Kanamycin, encoded for by the CRISPR plasmid).
-
10
Place plate in incubator at 37°C overnight.
Day Two
-
11
Pick up a single colony with sterile plastic loop and place it in 5 mL of LB broth (with Kanamycin, encoded for by the CRISPR plasmid).
-
12
Let bacteria incubate in a shaker set at 200 rpm and 37°C overnight.
-
13
Overnight culture was then used to obtain DNA using the QIAprep Spin Miniprep Kit and following the manufacturer’s instructions.
3.2 Transfection
Plate HEK293T cells on a 6 well plate so that they will be at 50–70% confluency the next day, approximately 1.5x106 cells/well.
The following day transfect the cells using mini prepped DNA and following the Lipofectamine 2000 standard protocol (see Note 3).
Remove OptiMem containing Lipofectamine 2000 and DNA the next morning and replace with regular culture media (see Note 4).
3.3 Galanthus nivalis lectin labeling
Lectin staining (see Note 5) is performed with both fixed and unfixed cells as in (23, 24) with some modifications. Solutions are stored at 4°C until use.
-
1
5–7 days after transfection, remove the 6-well plate containing cells transfected with CRISPR plasmids from the incubator. Resuspend the cells by gently pipetting up and down and take an aliquot of cells (see Note 6 and 7) to plate in a 24-well plate on collagen coated coverslips (see Note 8) (Keep a large portion on the 6 well plate for cell sorting).
-
2
24–48 hours after plating cells prepare to do lectin labelling and immunofluroscence.
-
3
Wash cells grown on coverslips 3 times with PBS (see Note 9).
-
4
Fix each coverslip for 15 min with 1% PFA in PBS (made from 16% stock solution).
-
5
After fixation, incubate cells with 1% BSA in PBS for 10 minutes to block nonspecific binding. Repeat.
-
6
Incubate cells with Galanthus Nivalus lectin (GNL) labeled with AlexaFluor 647 diluted 1:500 in 1% BSA in PBS for 30 min. (Keep cells in the dark during incubation)
-
7
Wash cells 5 times with DPBS and then fix again with 4% PFA for 15 min.
-
8
Wash cells 5 times with PBS.
-
9
Wash with 0.1% Triton-X 100 in PBS for 1 minute for further permeablization.
-
9
Dunk coverslips in PBS containing DAPI 10 times then dunk coverslips in water 10 times before mounting on glass microscope slides using ProlongR Gold antifade reagent.
-
10
Coverslips are then cured in the dark overnight at room temperature.
-
11
Examine cells and obtain images with fluorescence microscope. Our images were taken on a Zeiss LSM510 confocal microscope with a 63x oil immersion objective.
3.4 FAC Sorting
After ensuring that a population of your cells are GNL positive via immunofluorescence, remove the remaining CRISPR transfected cells from the 6-well plate via resuspension in regular culture media by gentle pipetting up and down with 1 ml pipette, then place in 1.5 mL microcentrifuge tubes and spin down at 600 x g for 3 minutes.
Remove the media carefully and resuspend cells in ice cold 0.1% filtered BSA in PBS solution.
Spin down cells again then resuspend in ice cold 0.1% BSA solution containing GNL labeled with AlexaFluor 647 at a 1:1000 dilution, and place on ice in the dark for 30 minutes.
Spin cells down (600 x g for 3 min), wash three times with 0.1% BSA solution, resuspend in ice cold cell sorting media (sterile filtered), and pipet through filtered Cap 5mL- 12x75mm polystyrene round bottom tubes to remove clumps. Place tubes on ice.
Cells need to be sorted with a cell sorting capable flow cytometer that can properly register 647 nm. We use the FACSAria (BD Biosciences) and run wild type GNL-647 stained and unstained cells as a control each time.
The FACSAria can sort one lectin positive cell per well in a 96 well plate. 100 μL of culture medium containing 1x Gibco Antibiotic/Antimycotic is added into each well prior to sorting (see Note 10 and 11).
3.5 Expanding Colonies and Lectin testing
10 days after sorting, the 96 well plates were screened for growing patches of cells (see Note 12).
Mark wells which only have one patch of cells growing as they are assumed to be from one common cell.
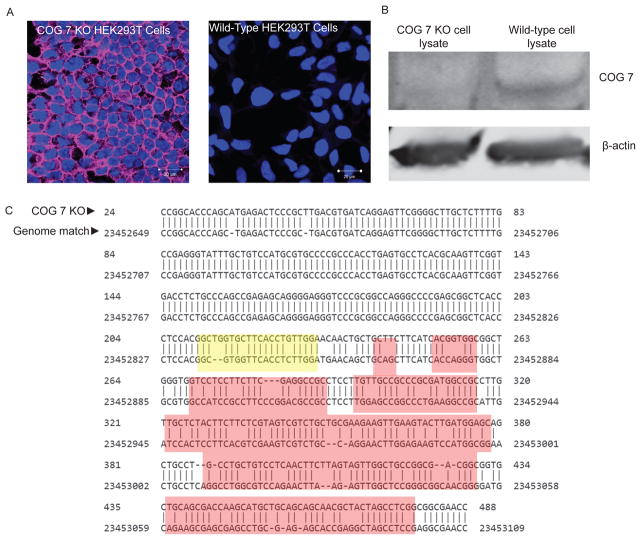
15 days after sorting split wells that have colonies growing and grow up in 24 well plates (see Note 13) with collagen coated coverslips for lectin staining analysis as detailed above (Figure 1a).
Fig 1.
Analysis of COG 7 KO: A) Lectin staining of WT HEK293 and COG 7 KO HEK293 cells with Galanthus nivalis lectin-Alexa 647 (GNL). Cells were mounted in DAPI containing media to visual the nuclei (blue). GNL is false colored pink. Images were takes with a 63x immersion lens on a confocal microscope. Settings were calibrated using stained control cells and kept the same throughout.
B) Western immunoblot for WT HEK and COG 7 KO cells: delta 7 cell line G3 blotted next to WT HEK293 lysate as a control.
C) Sequencing and BLAST of COG gene in KO cells: PCR products were obtained by amplifying the gene of interest from chromosomal DNA. These products were then cleaned and sent for sequencing. Products appeared to be of the expected size by gel (531 bp). Once the sequence was obtained this was BLASTed against the human genome. The expected cut site based on the guide RNA is highlighted in yellow and resultant frameshift mutations can be seen in red. WT HEK293 cells were also sequenced and BLASTed as a control. Frameshift mutations were not seen in control samples.
3.6 Validation using SDS-PAGE and Western Blot
After colonies are confirmed to be positive for GNL binding, colonies were expanded onto one well of a 6 well plate and grown to ~90% confluency.
Resuspend cells in 1 mL of regular culture medium and take a 100 μL aliquot for cell counting for chromosomal DNA prep (see Note 14). Include a wild type cell sample as well as a control.
After 100,000 cells are removed for chromosomal DNA prep (See 3.7 for more details), move the remaining cells to a 1.5 mL microcentrifuge tube.
Spin cells down at 600 x g for 3 min.
While cells are spinning down, heat 2% SDS to 95°C.
Remove the supernatant from the cells and resuspend in PBS. Spin down again.
Remove supernatant again and add 250 μL of 95°C 2% SDS to cells then resuspend and heat at 95°C for 5 additional min.
Add 50 μL of 6x sample buffer to cells and vortex.
Heat solution for 3 additional minutes at 95°C then remove from heat (see Note 15).
Store lysate at −20°C until needed.
Load 10 μL of lysate per well in a 9% SDS-PAGE gel and run at 180V until the dye front reaches the bottom of the gel. Turn off the machine.
Soak 2 thick sheets of filter paper and the nitrocellulose membrane in 1-Step Transfer Buffer.
Remove the gel from the chamber and pry open glass. Place gel in ddH2O
Place one piece of filter paper soaked in 1-Step Transfer Buffer on the anode side of the Pierce G2 Fast Blotter cassette. Then place soaked nitrocellulose membrane on top of this.
Next, carefully place gel on nitrocellulose membrane and roll gel with clean roller to remove air bubbles.
Place second soaked sheet of filter paper on top of the gel and carefully roll again to remove any residual air bubbles.
Place cathode portion on top and press to secure the cassette. Place cassette into Fast Blotter machine.
Select Mixed Range MW protocol and press Start.
After transfer stain the membrane with Ponceau S stain for 5 min to ensure the transfer went well and also to assess over-all protein levels in each lysate.
Trim down membrane and place in a blotting box with PBS. Rinse membrane in PBS (3x for 4 minutes each) at room temperature while rocking on a table rocker.
Block the membrane for 20 minutes using Odyssey blocking buffer while rocking at room temperature.
Add primary antibodies and incubate overnight, on a table rocker at 4°C.
Wash the membrane 3x for 4 minutes each with PBS while rocking.
Add the secondary antibody solution diluted in PBS containing 5% milk. Incubate in the dark at room temperature while rocking for 40 minutes.
Repeat washing step (Step 23).
Scan blot on LI-COR Odyessy imaging system (Figure 1b).
3.7 Validation using PCR and sequencing
After colonies were confirmed to be positive for GNL binding, each colony was expanded onto one well of a 6 well plate and grown to ~90%.
Resuspend cells from each colony in 1mL of regular culture medium take a 100 μL aliquot for cell counting. Include a wild type cell sample as well as a control.
After determining number of viable cells per μL, remove 100,000 cells and place in a microcentrifuge tube.
Spin down cells at 600 x g for 3 min, remove supernatant.
Add 0.5 mL of Quick Extract DNA Solution from Epicenter, using the recommended protocol for cells (see Note 16).
Store at −20°C until needed.
-
For chromosomal PCR use the following for 50 μL reactions:
2 μL of 10 mM each of Cog 7 forward and reverse primer
2–3 μL of the chromosomal DNA
2.5 μL of DMSO
4 μL of dNTPs
5 μL of 5x exTaq buffer
2 μL of exTaq polymerase
*Do two reactions for each colony.
-
Place tubes in a thermocycler and use the following run settings (see Note 17):
95°C, 1:00 minute
95°C, 0:10
57°C, 0:10
72°C, 0:30
Repeat 2–4, 35x
6 72°C, 0:30
4°C, ∞
Add loading dye to each reaction then load on a 1% agarose gel
Purify product of correct size using the Zymoclean gel DNA recovery kit and following the manufacturer’s instructions (see Note 18).
After eluting, take 2 μL of gel purified product and dilute in water with loading dye. Run on a gel to ensure a product of the correct size was obtained.
Sequence samples. (We then send samples for sequencing at the UAMS core sequencing facility.)
Run the resulting sequences against the human genome using NCBI BLAST to look for mismatches, deletions and insertions near CRISPR cut site (Figure 1c).
Optional further verification (see Note 19).
3.8 Phenotypic analysis using SubAB trafficking
Analysis of Subtilase cytotoxin (SubAB) trafficking was performed as in (22) with some modifications. In brief, cells are incubated with the toxin for varying lengths of time. Lysates are taken after each time point, then analyzed for GRP78 cleavage via western blot. The amount of time it takes for SubAB to cleave its target (GRP78) (determined by western blot) is an indicator for the efficiency of retrograde trafficking from the plasma membrane through the Golgi and to the ER. We have shown previously that SubAB retrograde trafficking is delayed in COG complex deficient cells (22).
Grow each KO clone on 6-wells of a 6-well plate. Also grow wild-type HEK293T cells.
Each well will be a different time point. Label the wells: 0 min, 20 min, 40 min, 60 min, 120 min, 180 min. Remove media from each well and replace with 500 μL of fresh media. Place back at 37°C.
Make a 6x stock of SubAB toxin in culture medium. At each time point 100 μL will be added to the appropriate well to get a final working concentration of 20 μg per mL (See Note 20).
Add 100 μL of 6x SubAB stock to the wells labeled 180 min. Your experiment will end 3 h from this time point. Place back at 37°C.
Continue adding toxin to the wells at the appropriate time points with relation to the end time. Gently rock the plate back and forth to mix the toxin with the media. Place back at 37°C after each addition of toxin until the next time point is reached.
After 3 h from the initial toxin addition, remove media containing SubAB from all wells and place this into a sealed tube and throw into a biohazard bag. Replace with 1 mL of PBS.
Resuspend cells by gently pipetting up and down. Place cell suspension into a microcentrifuge tube. Each time point of each sample goes into a separate tube.
Spin cells down at 600 x g for 3 min.
Remove PBS and lyse samples with 250 μL of 95°C 2% SDS (See Note 21). Vortex, then heat samples for 5 min at 95°C.
Follow Steps 8–26 of section 3.6 for running the gel and blotting (Figure 2a).
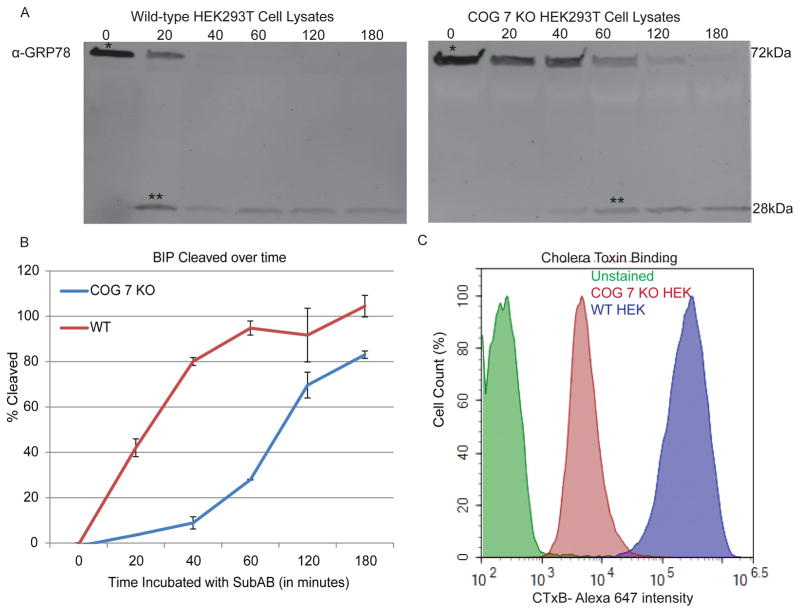
Fig 2.
Phenotypic changes in COG 7 KOs: A) SubAB toxin trafficking and activity is affected in COG 7 KO cells: Cells were incubated at the indicated times (in minutes) with SubAB toxin. Cells were collected and lysates were made as described in section 3.8. * indicates full length GRP78 and ** indicates cleaved GRP78, mediated by SubAB after it has reached the ER. 50% cleavage can be seen in 20 min in control cells, but it takes 60–120 min for the same amount of cleavage in COG 7 KO cells B) Quantification of GRP78 cleavage over time. Two separate COG 7 KOs were ran. C) Cholera Toxin binding is affected in COG 7 KO cells: Cells were incubated with CTx-647 for 30 minutes on ice flow cytometry was performed to analyze CTx binding as described in Section 3.9. COG 7 KO cells have a lesser binding capacity than WT cells, possibly due to mis-glycosylated GM1, the target of CTx binding.
3.9 Phenotypic analysis using Cholera Toxin
Cholera Toxin binds to GM1 ganglioside residues. These glycolipids have a terminal sialic acid. Sialic acid residues are added in the Trans Golgi, so only cells with properly functioning Trans Golgi glycosylation machinery will have these modifications. COG complex deficient cells demonstrate altered sialylation of plasma membrane glycoconjugates(23). This assay is a complement to the GNL screening (high GNL binding and low Cholera Toxin binding in COG KO cells).
Grow each KO clone (and wild-type HEK293T cells as a control) to ~90% confluency on a 6-well plate. Have one well of a 6-well plate for each cell clone.
Remove media and resuspend cells in 1 mL of PBS. Place in 1.5 ml microcentrifuge tubes and spin down at 600 x g for 3 min.
Remove the PBS carefully and resuspend cells in ice cold 0.1% filtered BSA in PBS solution.
Spin down cells again at 600 x g for 3 min, then resuspend in ice cold 0.1% BSA solution containing Cholera Toxin (See Note 22) labeled with AlexaFluor 647 at a 1:1000 dilution and place tubes on ice in the dark for 30 min.
After this, spin cells down again at 600g for 3 minutes then resuspended in 500 μL of ice cold 0.1% BSA solution DAPI (1:10,000) and placed into a 5 mL- 12x75mm polystyrene round bottom tube. Pipet up and down to remove clumps. Place tubes on ice. (See Note 23)
Cells need to be analyzed with a flow cytometer that ca that can properly register Alexa647. We use the BD Fortessa at the UAMS Flow Cytometry Core and run wild type HEK293T CTx-647 stained and unstained cells as a control.
Gating is done to flow data based on size/shape of each event and viability based on DAPI staining (see Note 24 and 25).
Acknowledgments
We would like to thank the Digital Microscopy, Flow Cytometry, and DNA sequencing Core Facilities at UAMS for their help in this project. This work was supported, in part, by the NIH grants GM083144 and U54 GM105814.
Footnotes
Small quantities of DNA are sent from Genecopia. It is best to transform competent cells to get higher quantities of DNA for transfection of target cell line.
The mCherry tag on the Genecopia plasmid is very dim in HEK293T cells, so it is best to use a small amount (1/10) EGFP DNA to track transfection efficiency.
CRISPR-Cas9 is dose dependent, but so are its potential off target effects. At lower efficiencies, such as the 1% efficiency we observed, off target effects are rarely seen.
OptiMem with Lipofectamine 2000 plus DNA can be changed anytime between 4–18 hours with HEK293T cells. Little transfection related cell death occurs.
Lectins can target different glyco-conjugates on the cell surface. GNL-Alexa647 was chosen to make COG knockouts because it has been shown to give a more robust signal when COG function is impaired (23, 25). GNL binds high-mannose residues, which would normally be at low levels on the cell surface, since these residues are further processed in the medial and Trans Golgi.
Glycosylation defects can be seen beginning at 2 days, but we wait until transient expression of Cas9 (as assessed by the flurophore mCherry or co-transfected EGFP) has diminished to assess glycosylation defects.
A confluency of ~50–80% is also the best to analyze the plasma membrane glycoproteins and lipids when doing lectin staining.
When plating cells on coverslips for lectin staining and immunofluorescence we have found that cells best adhere using collagen coating. This is also the best surface to plate the cells on for live-cell imaging.
Though cells plated on collagen adhere better, HEK293T cells are only semi-adherent so caution needs to be taken when staining or changing media. Additionally it is best for cells to be ~50–80% confluent when analyzing so they adhere better and do not come off in a sheet.
Some cell lines do not tolerate single cell sorting well. For this reason you may want to sort additional cells into a 3–6 cm dish to have a population enriched for glycosylation defects as a backup.
Cell sorting appears to be a step that can allow for contamination on occasion. For this reason we recommend sorting cells into wells containing media with 1% antibiotic/antimycotics.
Cells generally grow on the edge of wells, for this reason it is difficult to see colonies before 10 days.
Because HEK293T cells are semi-adherent we passage the cells from 96-well to 24-well for analysis simply by pipetting up and down to resuspend. We used a 1000 μL tip for this instead of a 200 μL pipet tip to cause less shearing forces on the cells.
Because Chromosomal DNA does not require many cells, and it and whole cell lysates are both needed for validation, we usually do these steps in tandem.
When creating cell lysates for western blot analysis the lysates are often quite viscous due to chromosomal DNA. To prevent this you can use a higher volume of 2%SDS, sonicate the lysate briefly, or boil the lysate for an additional minute or two.
When preparing chromosomal DNA from the cells for PCR and sequencing be sure to vortex well between each step.
For PCR we have found that 3–4 μL of chromosomal DNA and a few extra cycles in the thermocycler gives the best product yields.
After PCR we have found that gel purified PCR produce gives better sequencing results that just running the product through a PCR clean up kit.
As an additional verification step and to ensure that defects in KOs are from target specific mutations, we transiently transfect functioning COG protein back into the cells to ensure that it can correct any defects found.
SubAB is a potent toxin so caution should be used while performing this assay. Dispose of toxin containing media in a sealed tube and place in a biohazard bag.
An additional PBS wash maybe desired to remove BSA contaminates before lysing.
We use the B subunit of cholera toxin, since this is a binding assay only. This enables the toxin to keep its lectin abilities, but is safer to work with.
We use DAPI as a viability dye because live cells take up the dye much slower than dead cells. Cells brightly stained for DAPI can be excluded from flow data by gating. Many other dyes, including Propidium Iodide, are also available for this purpose.
We try to make sure that there are at least 10,000 events after gating to analyze for CTx binding. For this reason it is helpful to gate when collecting data to be sure enough events have been obtained.
Here we used the NovoExpress software from ACEA Biosciences. FlowJo or other flow analysis software can also be used.
References
- 1.Ungar D, Oka T, Brittle EE, Vasile E, Lupashin VV, Chatterton JE, et al. Characterization of a mammalian Golgi-localized protein complex, COG, that is required for normal Golgi morphology and function. J Cell Biol. 2002;157:405–15. doi: 10.1083/jcb.200202016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shestakova A, Zolov S, Lupashin V. COG complex-mediated recycling of Golgi glycosyltransferases is essential for normal protein glycosylation. Traffic. 2006;7:191–204. doi: 10.1111/j.1600-0854.2005.00376.x. [DOI] [PubMed] [Google Scholar]
- 3.Willett R, Kudlyk T, Pokrovskaya I, Schonherr R, Ungar D, Duden R, et al. COG complexes form spatial landmarks for distinct SNARE complexes. Nat Commun. 2013;4:1553. doi: 10.1038/ncomms2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ungar D, Oka T, Vasile E, Krieger M, Hughson FM. Subunit architecture of the conserved oligomeric Golgi complex. J Biol Chem. 2005;280:32729–35. doi: 10.1074/jbc.M504590200. [DOI] [PubMed] [Google Scholar]
- 5.Fotso P, Koryakina Y, Pavliv O, Tsiomenko AB, Lupashin VV. Cog1p plays a central role in the organization of the yeast conserved oligomeric Golgi complex. J Biol Chem. 2005;280:27613–23. doi: 10.1074/jbc.M504597200. [DOI] [PubMed] [Google Scholar]
- 6.Willett R, Ungar D, Lupashin V. The Golgi puppet master: COG complex at center stage of membrane trafficking interactions. Histochem Cell Biol. 2013;140:271–83. doi: 10.1007/s00418-013-1117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whyte JRC, Munro S. The SeC34/35 golgi transport complex is related to the exocyst, defining a family of complexes involved in multiple steps of membrane traffic. Developmental Cell. 2001;1:527–37. doi: 10.1016/s1534-5807(01)00063-6. [DOI] [PubMed] [Google Scholar]
- 8.Suvorova ES, Duden R, Lupashin VV. The Sec34/Sec35p complex, a Ypt1p effector required for retrograde intra-Golgi trafficking, interacts with Golgi SNAREs and COPI vesicle coat proteins. J Cell Biol. 2002;157:631–43. doi: 10.1083/jcb.200111081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suvorova ES, Kurten RC, Lupashin VV. Identification of a human orthologue of Sec34p as a component of the cis-Golgi vesicle tethering machinery. J Biol Chem. 2001;276:22810–8. doi: 10.1074/jbc.M011624200. [DOI] [PubMed] [Google Scholar]
- 10.Kubota Y, Sano M, Goda S, Suzuki N, Nishiwaki K. The conserved oligomeric Golgi complex acts in organ morphogenesis via glycosylation of an ADAM protease in C. elegans. Development. 2006;133:263–73. doi: 10.1242/dev.02195. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa T, Machida C, Yoshioka Y, Ueda T, Nakano A, Machida Y. EMBRYO YELLOW gene, encoding a subunit of the conserved oligomeric Golgi complex, is required for appropriate cell expansion and meristem organization in Arabidopsis thaliana. Genes Cells. 2008;13:521–35. doi: 10.1111/j.1365-2443.2008.01186.x. [DOI] [PubMed] [Google Scholar]
- 12.Foulquier F. COG defects, birth and rise! Biochim Biophys Acta. 2009;1792:896–902. doi: 10.1016/j.bbadis.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Zeevaert R, Foulquier F, Jaeken J, Matthijs G. Deficiencies in subunits of the Conserved Oligomeric Golgi (COG) complex define a novel group of Congenital Disorders of Glycosylation. Mol Genet Metab. 2008;93:15–21. doi: 10.1016/j.ymgme.2007.08.118. [DOI] [PubMed] [Google Scholar]
- 14.Wu X, Steet RA, Bohorov O, Bakker J, Newell J, Krieger M, et al. Mutation of the COG complex subunit gene COG7 causes a lethal congenital disorder. Nat Med. 2004;10:518–23. doi: 10.1038/nm1041. [DOI] [PubMed] [Google Scholar]
- 15.Pokrovskaya ID, Szwedo JW, Goodwin A, Lupashina TV, Nagarajan UM, Lupashin VV. Chlamydia trachomatis hijacks intra-Golgi COG complex-dependent vesicle trafficking pathway. Cell Microbiol. 2012;14:656–68. doi: 10.1111/j.1462-5822.2012.01747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S, Dominska-Ngowe M, Dykxhoorn DM. Target silencing of components of the conserved oligomeric Golgi complex impairs HIV-1 replication. Virus Res. 2014;192:92–102. doi: 10.1016/j.virusres.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu J, Davoli T, Perriera JM, Chin CR, Gaiha GD, John SP, et al. Comprehensive identification of host modulators of HIV-1 replication using multiple orthologous RNAi reagents. Cell Rep. 2014;9:752–66. doi: 10.1016/j.celrep.2014.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zolov SN, Lupashin VV. Cog3p depletion blocks vesicle-mediated Golgi retrograde trafficking in HeLa cells. J Cell Biol. 2005;168:747–59. doi: 10.1083/jcb.200412003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kudlyk T, Willett R, Pokrovskaya ID, Lupashin V. COG6 interacts with a subset of the Golgi SNAREs and is important for the Golgi complex integrity. Traffic. 2013;14:194–204. doi: 10.1111/tra.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laufman O, Freeze HH, Hong W, Lev S. Deficiency of the Cog8 subunit in normal and CDG-derived cells impairs the assembly of the COG and Golgi SNARE complexes. Traffic. 2013;14:1065–77. doi: 10.1111/tra.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–21. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith RD, Willett R, Kudlyk T, Pokrovskaya I, Paton AW, Paton JC, et al. The COG complex, Rab6 and COPI define a novel Golgi retrograde trafficking pathway that is exploited by SubAB toxin. Traffic. 2009;10:1502–17. doi: 10.1111/j.1600-0854.2009.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pokrovskaya ID, Willett R, Smith RD, Morelle W, Kudlyk T, Lupashin VV. Conserved oligomeric Golgi complex specifically regulates the maintenance of Golgi glycosylation machinery. Glycobiology. 2011;21:1554–69. doi: 10.1093/glycob/cwr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willett RA, Pokrovskaya ID, Lupashin VV. Fluorescent microscopy as a tool to elucidate dysfunction and mislocalization of Golgi glycosyltransferases in COG complex depleted mammalian cells. Methods Mol Biol. 2013;1022:61–72. doi: 10.1007/978-1-62703-465-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ha JY, Pokrovskaya ID, Climer LK, Shimamura GR, Kudlyk T, Jeffrey PD, et al. Cog5-Cog7 crystal structure reveals interactions essential for the function of a multisubunit tethering complex. Proc Natl Acad Sci USA. 2014;111:15762–7. doi: 10.1073/pnas.1414829111. [DOI] [PMC free article] [PubMed] [Google Scholar]