Abstract
Background
Methamphetamine (METH) use is a risk factor for the transmission of HIV. Each is associated with neurocognitive impairment and subsequent problems in everyday functioning, yet additive effects of HIV and METH are not consistently observed. This study used the UCSD Performance-Based Skills Assessment (UPSA-2) to assess whether METH use disorder and HIV together resulted in poorer functional outcome than either condition alone.
Method
Participants in the Translational Methamphetamine AIDS Research Center (TMARC) cohort were stratified based upon HIV infection and METH use disorder: HIV−/METH− (n=49), HIV−/METH+ (n=48), HIV+/METH− (n= 37), and HIV+/METH+ (n=38). They were administered the UPSA-2 which measures abilities in six domains of everyday functioning. Main effects and interactions of HIV and METH were examined, as were relationships between UPSA-2 scores and disease characteristics.
Results
Significant HIV-by-METH interactions were observed for the UPSA-2 total score and Comprehension/Planning and Financial subscales such that METH was associated with lower scores in HIV- participants but not HIV+ participants. METH was associated with lower scores on the Communications subscale. All three risk groups had lower scores than HIV−/METH−participants. Recency and frequency of METH use were associated with lower scores. Lower Medication Management scores were related to lower nadir CD4 counts.
Conclusions
METH use disorder and HIV each impair functional performance, but there is no additive effect when the two conditions occur together. The neurocognitive sequelae of combined HIV infection and METH use are complex and warrant further study, as do the potential effects of compensatory strategies and other factors.
Keywords: HIV, methamphetamine, UPSA, everyday functioning, cognition
1. Introduction
Methamphetamine (METH) dependence commonly accompanies HIV infection, typically because of behaviors during drug use that increase the risk of viral transmission (Buchacz et al., 2005; Cohen, 2012). While each of these conditions by themselves often have negative effects on the individual’s cognition and functional behaviors, there is also evidence that the combined effect of HIV infection and heavy METH use may have additive effects, e.g., resulting in worse neuronal injury (Chana et al., 2006), compounded damage to frontostriatal circuits (Chang et al., 2005), and more profound neuropsychological impairment (Rippeth et al., 2004) than either condition alone.
Neuropsychological deficits, particularly those that are frontally-mediated, are thought to substantially impact everyday functional ability, i.e., the ability to engage in vital tasks of daily living (Cattie et al., 2012; Twamley et al., 2002). For people living with HIV, an added demand is adherence to an antiretroviral therapy (ART) regimen. Although HIV and METH dependence have each been associated with worse performance on tasks of everyday functioning (Blackstone et al., 2012; Henry et al., 2010; Iudicello et al., 2014; Morgan et al., 2014; Vance et al., 2011), the combined effects of HIV infection and heavy drug use on the ability to carry out tasks of daily living have not been widely studied [but see (Sadek et al., 2007)] but are important to understand given the high co-occurrence of these two conditions and the potential adverse implications for medication adherence and other important functional behaviors. Parsing the relationships among HIV illness, METH use characteristics, and everyday functioning may help inform treatment decisions. For example ART seems to reduce the severity of HIV-associated neurocognitive disorders (Cysique and Brew, 2009), however some antiretroviral medications do appear to have neurotoxic effects (Meeker et al., 2014).
Furthermore, there are limitations in the operationalization of everyday functioning in previous investigations. Many studies of everyday functional ability, including those conducted in HIV and substance dependence (Blackstone et al., 2013; Sadek et al., 2007) have used self-report measures that ask the individual to rate how well they perform activities of daily living. We (Henry et al., 2010) and others (Elliott et al., 2015; Karagiozis et al., 1998; Patterson et al., 2001a) have proposed that reliance on self-report is problematic especially during the study of conditions with known cognitive impairment. Performance-based functional measures, such as the UCSD Performance Based Skills Assessment (UPSA-2), are useful in that they divide everyday function into specific components and have high reliability and validity (Patterson et al., 2001a; Patterson et al., 2001b), e.g., comprehension and planning abilities, financial skills, knowledge in use of transportation and managing the household, and the extent to which individuals can internalize and plan to take a complex daily regimen of medications.
The objective of the current study was to assess everyday functional ability in four groups of individuals stratified based upon their HIV status and history of METH dependence, using the UPSA-2 to assess the individual and interactive effects of HIV and METH dependence on specific and essential domains of everyday functioning. We hypothesized that participants with both HIV infection and a history of METH dependence would have lower scores on the UPSA-2 than subjects with either condition alone and non-infected non-METH dependent comparison subjects. A secondary aim was to assess to what extent performance on the UPSA-2 measures would correlate with HIV illness features (e.g., CD4+ T-cell counts, viral load, ART use), and METH use characteristics (e.g., length of abstinence, quantity of use) within our HIV+ and METH-dependent participants, respectively.
2. Materials and Methods
2.1 Participants
This investigation was a component of the Translational Methamphetamine AIDS Research Center (TMARC), which is a multi-project center grant focused on translational approaches to understanding the combined effects of HIV and methamphetamine dependence on brain structure and function. The UCSD Human Research Protections Program approved the study. All human participants in the TMARC cohort were included. Participants were stratified by HIV and METH dependence status into one of four groups: HIV−/METH− (n=49), HIV−/METH+ (n=48), HIV+/METH− (n= 37), and HIV+/METH+ (n=38). HIV infection was determined by Enzyme-linked immunosorbent assay (ELISA) and a confirmatory Western blot. The presence and history of METH use disorders were determined via the DSM-IV criteria for METH abuse and dependence as assessed by the Composite International Diagnostic Interview Version 2.1 [CIDI; (World Health Organization, 1997)]. Participants who met criteria for both lifetime METH dependence and METH abuse or dependence within the past 18 months were included in the two METH+ groups.
Potential participants were excluded if they reported histories of psychosis (e.g., schizophrenia) or significant medical (e.g., hepatitis C infection) or neurological (e.g., head injury with loss of consciousness for more than 30 minutes, seizure disorders, stroke, multiple sclerosis) conditions known to affect cognitive functions. To minimize the confounding effects of acute drug use (intoxication and withdrawal), participants who tested positive for METH or any other illicit drugs (aside from cannabis) were also excluded.
2.2. Measures
Functional ability was assessed with the UPSA-2 (Patterson and Goldman, 2005). Participants were asked to perform tasks in 6 separate domains considered necessary for successful and independent functioning in the community: 1) Comprehension and Planning, 2) Financial ability, 3) Communication skills, 4) Transportation, 5) Household skills, and 6) Medication management as described below.
In the first domain, comprehension and planning skills were assessed by having participants read a fictional article about the opening of a theme park and then described the activities at the park and planned a trip to the recreational facility. Financial ability was evaluated by a counting change task and having the subjects identify important aspects of a utility bill. Communication skills were assessed through role-playing tasks that asked participants to use telephone to schedule a medical appointment. The Transportation domain involved reading and interpreting a generic bus route and planning the use of a public bus system. Household skills were assessed by requiring preparation of a shopping list for a specific cooking task based on food items presented in a mock pantry. Finally, for the Medication Management domain, participants were required to organize a medication routine where they were asked to role-play how they would take a number of different medications over the course of a single day.
Administration of the UPSA-2 requires about 30 minutes. Each of the UPSA-2 domains generates a raw score that is converted to a domain score ranging from 0 to 20 points. The 6 domain scores are summed to create a total UPSA-2 score up to a maximum of 120 points, with higher scores indicating better performance.
For the purpose of examining the UPSA-2 measures in relation to HIV disease characteristics and METH use patterns, participant characterization data were drawn from the TMARC parent study. Selected HIV disease-related variables included current and nadir CD4+ T-cell counts, plasma viral loads, and self-reported duration of HIV infection. Self-reported METH use characteristics were collected using a comprehensive timeline-followback procedure and included age of first use of METH, days since last use of METH, total days of METH use, and total quantity of METH used.
2.3 Statistical Analyses
UPSA-2 total and subscale scores were examined for outliers, normality of distribution, and homogeneity of variance. Outliers were determined as values greater than 3 standard deviations from the grand mean (Stevens, 1992). In order to preserve the relative value of these data points and retain power, outliers were truncated to within 3 standard deviations of the mean, per published methods (Minassian et al., 2015a; Minassian et al., 2015b). A total of ten outliers were truncated (1 HIV−/METH−, 3 HIV−/METH+, 4 HIV+/METH−, 2 HIV+/METH+).
Main effects of HIV, METH, and their interaction on UPSA-2 performance were examined for the Total Score and subscale scores using Univariate Analysis of Variance (ANOVA). Three between-subjects factors were entered into the models, as well as their interaction: METH status (METH+ vs. METH−), HIV status (HIV+ vs. HIV−), and education level (12 years or less vs. more than 12 years). Years of education was selected a priori as an additional between-subjects factor given the difference in education among the groups, the significant relationship between education and UPSA scores (see Results), and previous reports of a significant relationship between education and UPSA scores (Leifker et al., 2010). Planned contrasts were conducted on the four groups. When other demographics were associated with the subscales, those factors were included in the analysis to account for their effects (i.e., gender in the Communication subscale; see Results).
Pearson’s correlation coefficients were used to assess the relationship among UPSA-2 scores and HIV disease characteristics as well as METH use features. An independent samples t-test was used to assess difference in UPSA-2 scores between participants with and without a detectable plasma viral load. The HIV viral load measure and METH use measures were significantly positively skewed; log transformations of those variables were thus entered into the analyses. All analyses were performed using SPSS 20. Significance values were set at p < .05, and effect sizes were calculated using partial Eta-squared (ηp2) and Cohen’s d.
3. Results
3.1 Demographics and Illness Features
See Table 1 for demographic information and HIV and METH features. The groups did not differ by age or ethnicity, but a greater proportion of the HIV−/METH− group were women. The HIV−/METH+ group had significantly fewer years of education and lower scores on the Wide Range Achievement Test (WRAT-4) than the other three groups. Consistent with previous literature (Bing et al., 2001; Bousman et al., 2009; Zweben et al., 2004), all three risk groups had higher scores on a depression scale, the Beck Depression Inventory (BDI-II), than the HIV−/METH− group. Global Deficit Scores (GDS), a gross index of neurocognitive deficits (Carey et al., 2004), were lower in the HIV−/METH− group but not significantly different among the three risk groups. Participants in the METH groups had a higher prevalence of lifetime substance use disorder than the METH-negative groups. Ten participants (6 HIV−/METH+, 3 HIV+/METH−, 1 HIV+/METH+) had a positive urine toxicology for cannabis; these participants did not have significantly different UPSA-2 scores than the remainder of the cohort.
Table 1.
Demographic and Illness Features in HIV and METH participants
| HIV−/METH− (n=46) | HIV−/METH+ (n=46) | HIV+/METH− (n=37) | HIV+/METH+ (n=38) | Group difference | |
|---|---|---|---|---|---|
| Age (years) | 38.4 (14.0) [18–68] | 39.4 (10.8) [21–64] | 41.5 (11.9) [23–66] | 41.6 (9.1) [24–64] | ns |
| Gender | 13 F, 33 M | 8 F, 38 M | 5 F, 32 M | 1 F, 37 M | Fisher’s Exact =10.8, p =0.01 |
| Education (years) | 13.9 (2.0) [8–20] | 12.6 (2.0) [9–18] | 14.3 (2.2) [9–18] | 13.7 (2.4) [8–20] | HIV−/METH+ < other groups, p<.0.02 |
| WRAT-4 Score | 103.3 (12.2) [82–134] | 96.7 (10.6) [83–126] | 103.5 (11.6) [85–134] | 105.4 (12.6) [86–131] | HIV−/METH+ < other groups, p < 0.01 |
| Ethnicity (n) | 0 Asian 3 Afr-Am. 13 Hispanic 28 Cauc. 2 Other | 0 Asian 11 Afr-Am. 8 Hispanic 25 Cauc. 2 Other | 2 Asian 7 Afr-Am. 7 Hispanic 20 Cauc. 1 Other | 0 Asian 7 Afr-Am. 4 Hispanic 26 Cauc. 1 Other | ns |
| Global Deficit Score | 0.28 (0.29) [0–1.05] | 0.43 (0.42) [0–1.72] | 0.47 (0.62) [0–3.39] | 0.46 (0.40) [0–1.65] | HIV−/METH− < HIV+ groups, p < 0.06 |
| BDI-II total score | 3.2 (4.4) [0–17] | 17.0 (11.3) [0–46] | 13.1 (11.9) [0–40] | 13.0 (12.5) [0–47] | HIV−/METH− < other groups, p < 0.001 |
| Current CD4+ T-cell count | – | – | 518.0 (237.4) [81–1061] | 546.0 (266.7) [82–1185] | ns |
| Lifetime Substance Use Disorder a (n) | 15 | 37 | 12 | 21 | METH+ groups > METH− groups, p <0.001 |
| Current Alcohol Abuse (n) | 1 | 0 | 1 | 0 | ns |
| Current Cannabis Use (n) | 0 | 1 | 1 | 1 | ns |
| Nadir CD4+ T- cell count | – | – | 279.7 (213.5) [3–763] | 248.7 (248.7) [4–707] | ns |
| Duration of HIV infection (months) | – | – | 97.9 (102.8) [1.1–313.1] | 121.6 (96.5) [1.1–340.4] | ns |
| Plasma viral load b | – | – | 2.5 (1.3) [1.6–5.5] | 2.6 (1.4) [0.9–6.0] | ns |
| Detectable plasma viral load (n) | – | – | 14 | 16 | ns |
| ART use (n) | – | – | 26 | 26 | ns |
| Months on current regimen | 21.8 (24.0) [0.8–93.9] | 33.2 (33.4] [0.5–123.8] | ns | ||
| Months of exposure to all ART | – | – | 55.2 (75.5) [0–269.0] | 49.0 (51.1) [0–207.8] | ns |
| Detectable plasma viral load while on ART (n) | – | – | 4 | 4 | ns |
| Participants with AIDS (n) | – | – | 16 | 19 | ns |
| Days since last METH use | – | 103.5 (126.2) [4.0–456.5] | – | 134.2 (144.1) [3.0–547.9] | ns |
| Total Days used METH | – | 2325.8 (2372.4) [86–10588] | – | 2233.8 (2494.3) [59–9771] | ns |
| Total Quantity METH (grams) | – | 3594.8 (6777.5) [27.8–40184.9] | – | 1889.6 (4470.8) [12.4–26525.55] | ns |
Note: values are means (standard deviations) unless otherwise specified. Ranges [min-max] for continuous variables appear in brackets. WRAT-4= Wide Range Achievement Test,- 4th Edition. BDI-II= Beck Depression Inventory. METH=methamphetamine. ART=antiretroviral therapy.
Presence of lifetime substance use disorder for alcohol, cocaine, and/or opioids.
Plasma viral load is reported in log base 10 copies per ML. ns= p > 0.05 unless otherwise noted.
Better-educated participants performed better on the Financial and Communication subscales (p<.05), with a trend toward Medication Management (p=.06) and Comprehension (p=.09) subscales (significant Pearson r range 0.21–0.26). Thus, education level (12 years or less vs. more than 12 years) was included as a between-subjects factor in the analyses. Higher WRAT-4 scores were positively correlated with higher scores on the Medication Management, Financial, Communication, Transportation, and Household Management scales (p<.05; significant Pearson r range=0.21–0.30). Given that WRAT-4 performance is an index of premorbid verbal intelligence, which in turn is highly dependent on education level, including education in the analyses was intended to account for the group differences in premorbid intelligence (HIV−/METH+ < all other groups) as reflected by both education and WRAT-4 scores. Men and women did not differ on most UPSA-2 measures although women performed better than men on the Communication subscale. The analysis involving Communication was thus repeated with gender as a between-subjects factor in a univariate ANOVA to account for its influence. The two HIV+ groups did not differ on any HIV illness features, and the two METH+ groups did not significantly differ on self-reported METH use history and characteristics.
Approximately 70% of the HIV+ subjects reported current use of ART medications (Table 1); this proportion did not differ in the METH− vs. METH+ groups nor did the groups significantly differ on duration of ART treatment. All participants were taking a nucleoside reverse transcriptase inhibitor (e.g., emtricitabine); 56% were also on a protease inhibitor (e.g., ritanovir); 33% were on a non-nucleoside reverse transcriptase inhibitor (e.g., efavirenz); 15% were on an integrase inhibitor (e.g., raltegravir); and 6% were on an entry inhibitor (e.g., mariviroc). A small proportion (17%) of participants on ART had a detectable plasma viral load; these participants were equally distributed in the two METH groups.
3.2 HIV and METH effects on UPSA-2
The ANOVA on UPSA-2 total scores indicated a statistically significant HIV-by-METH interaction with a medium effect size [F(1,159)=7.48, p=0.007, ηp2=0.05] such that METH dependence was associated with lower scores in the HIV-negative participants but not in the HIV-positive participants (Figure 1). There was a statistically significant main effect of METH [F(1,159)=4.35, p=0.04, ηp2=0.03] (Figure 1) but not a main effect of HIV [F(1,159)=1.71, ns, ηp2=0.01]. There was no main effect of education nor any interactions with METH and HIV and education. The ANOVA on each of the subscales indicated a statistically significant HIV-by-METH interaction for Comprehension/Planning [F(1,159)=9.20, p=0.003, ηp2=0.06)] and Financial [F(1,159)=4.86, p=0.03, ηp =0.03)] subscales (Figure 2). There was a significant main effect of HIV for the Comprehension/Planning [F(1,159)=4.85, p=0.03, ηp2=0.03] and Financial [F(1,159)=4.01, p=0.05, ηp2=0.03] subscales such that HIV-positive participants had lower scores than HIV-negative participants. There was a significant main effect of METH for the Communication subscale [F(1,159)=6.22, p=0.01, ηp2 =0.04] such that METH-positive participants had lower scores than METH-negative participants (Figure 2). There was a significant main effect of education on the Financial subscale [F(1,159)=6.28, p=0.01, ηp2 =0.04] such that participants with less education had lower scores, but there were no interactions of education with HIV or METH. There were no other main effects of education or interactions with education on the other subscales. When gender was included as a between-subjects factor for the Communication subscale, there were no interactions between gender and HIV, gender and METH, nor interactions among gender, education, HIV, and METH. There was no significant interaction or main effects on the Medication Management, Transportation or Household Skills subscales.
Figure 1.
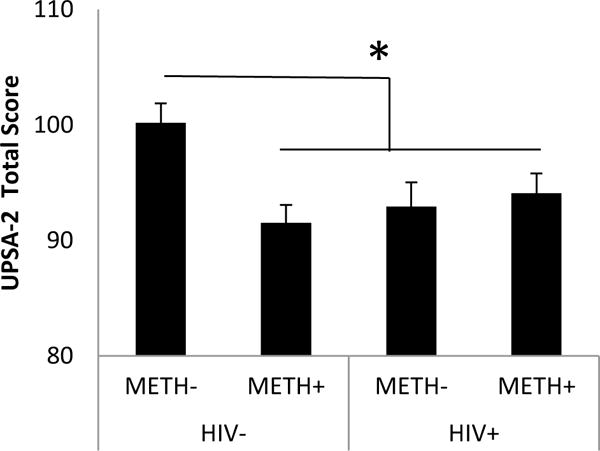
Mean (+/− 1 standard error) UPSA-2 Total scores in HIV and METH participants. * p < .05. Data are based on marginal means from analyses of variance which include education level as a between-subjects factor.
Figure 2.
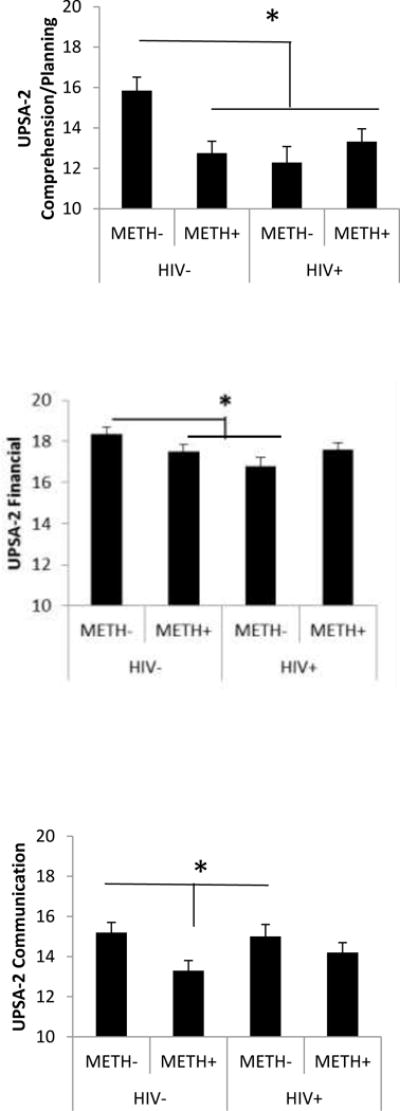
Mean (+/− 1 standard error) UPSA-2 subscale scores in HIV and METH participants * p < .05. Data are based on marginal means from analyses of variance which include education level as a between-subjects factor.
Planned contrasts of the four groups on the UPSA-2 subscale scores indicated that HIV−/METH− subjects had significantly higher (better) scores compared to each of the three of the other groups on the Comprehension/Planning subscale (p<0.007). On the Financial subscale, HIV–METH− subjects had significantly higher scores than the HIV+/METH− subjects (p=0.007) and on the Communication subscale, they had significantly higher scores than the HIV−/METH+ subjects (p=0.007). Also on the Communication subscale, HIV+/METH− subjects had higher scores than HIV−/METH+ subjects (p=0.04). HIV−/METH− subjects had a trend towards higher scores on the Medication Management subscale compared to HIV+/METH− subjects (p=0.07). For the Transportation subscale, HIV−/METH− subjects had higher scores than the HIV−/METH+ subjects (p=0.04). Table 2 displays mean UPSA-2 scores and effect sizes (difference from HIV−/METH−) for the groups.
Table 2.
Means, standard deviations, and Cohen’s d effect sizes (relative to HIV–METH−) for UPSA-2 scores in HIV and METH participants.
| HIV−/METH− (n=46) | HIV−/METH+ (n=46) | HIV+/METH− (n=37) | HIV+/METH+ (n=38) | |
|---|---|---|---|---|
| Total Score | 101.2 (7.5) | 91.2 (14.6) d=0.9 | 92.9 (9.9) d=0.8 | 94.6 (10.6) d=0.6 |
| Medication Management | 18.4 (1.6) | 17.4 (2.3) d=0.5 | 17.5 (2.1) d=0.5 | 17.4 (1.9) d=0.5 |
| Comprehension/Planning | 15.8 (3.3) | 12.5 (4.1) d=0.8 | 12.3(4.4) d=0.9 | 13.3(3.5) d=0.6 |
| Financial | 18.6 (1.8) | 17.4 (2.3) d=0.5 | 17.3 (2.1) d=0.6 | 17.7 (2.2) d=0.4 |
| Communication | 15.7 (2.8) | 13.1 (3.5) d=0.8 | 14.3 (3.0) d=0.4 | 14.4 (3.2) d=0.4 |
| Transportation | 15.9 (2.5) | 14.5 (3.4) d=0.5 | 15.5 (2.5) d=0.2 | 15.6 (2.7) d=0.1 |
| Household Skills | 16.9 (3.1) | 16.6 (3.0) d=0.1 | 16.2 (3.8) d=0.2 | 16.5 (3.7) d=0.1 |
Post-hoc analyses stratifying for ART use did not substantially alter the above findings, e.g., the HIV+ participants had lower UPSA-2 total scores than HIV−/METH− subjects regardless of ART use. The HIV–by-METH interaction was statistically significant in the analysis that included only HIV+ participants taking ART [F(1,144)=8.89, p=0.003, ηp2=0.06] and in that which included only HIV+ participants not taking ART [F(1,115)=6.56, p=0.012, ηp2=0.06].
3.3 Relationship between HIV illness features and METH use characteristics with UPSA-2
Because of equivalent impairments in UPSA-2 scores among the three risk groups and to preserve power, analyses were conducted on collapsed groups, i.e., HIV+/METH− and HIV+/METH+ were collapsed for analyses relating the UPSA-2 to HIV disease features, and HIV−/METH+ and HIV+/METH+ were collapsed for analyses relating the UPSA-2 to METH use characteristics. Participants with a detectable viral load had higher Medication Management scores (mean score=18.1) than those without a detectable viral load (mean score=17.0); [t(73)=2.5, p=0.02, Cohen’s d=0.59]. Correlations with plasma viral load were conducted only in HIV+ participants with a detectable plasma viral load (n=30); eight of those participants were taking ART medications while the remainder were not. Among the HIV+ participants, there was a significant positive correlation between nadir CD4+ T-cell count and Medication Management, and a significant positive correlation between plasma viral load and Medication Management (Table 3). Among METH+ participants, lower UPSA scores were related to younger age of first METH use, more recent METH use, higher days of METH use, and higher quantity of METH used (Table 4).
Table 3.
Pearson’s correlation coefficients for UPSA-2 scores and HIV disease characteristics in all HIV+ participants (n=75).
| Total Score | Med. Management | Comprehension/Planning | Financial | Communication | Transportation | Household Skills | |
|---|---|---|---|---|---|---|---|
| Current CD4+ T-cell Count | .08 | .21 | .02 | −.15 | −.08 | .12 | .10 |
| Nadir CD4+ T-cell Count | .07 | .36** | .02 | −.17 | −.14 | .02 | .10 |
| Plasma viral loada | .27 | .40* | .31 | −.15 | .24 | −.04 | .05 |
| Duration of HIV (months) | .02 | −.15 | .04 | .03 | .19 | −.02 | −.06 |
p <.01.
p < .05
Plasma viral load is reported in log base 10 copies per ML. Correlations with plasma viral load were conducted only on participants with a detectable plasma viral load (n=30).
Table 4.
Pearson R correlation coefficients for UPSA-2 scores and METH use characteristics, in all METH+ participants (n=84).
| Total Score | Med. Management | Comprehension/Planning | Financial | Communication | Transportation | Household Skills | |
|---|---|---|---|---|---|---|---|
| Age of first METH use (yrs) | .20 | .17 | .07 | .10 | .27* | .10 | .03 |
| Days since last METH usea | .22* | .20 | .14 | .12 | .16 | .06 | .16 |
| Total days used METHa | −.29** | −.24** | −.21 | −.30** | −.25* | −.17 | −.10 |
| Total Quantity METH (grams)a | −.20 | −.22* | −.12 | −.18 | −.26* | −.18 | .03 |
p < .05,
p <.01
variables were log-transformed.
4. Discussion
People with METH dependence evidence difficulty with tasks of daily living (Henry et al., 2010; Morgan et al., 2014; Rendell et al., 2009), as do those with HIV infection (Blackstone et al., 2012; Cattie et al., 2012), and in combination these risk factors result in an additive effect on self-reported everyday functioning (Blackstone et al., 2013). However, the combined effect of METH dependence and HIV has not previously been investigated with the use of performance-based functional measures that are likely a better reflection of an individual’s actual real-world functioning than self-report. We expected that there would be an additive deleterious effect of METH and HIV given previous findings of worse neurocognitive impairment in dually-affected participants. Contrary to our hypotheses, people with both HIV infection and METH dependence showed impairment on a performance-based measure, the UPSA-2, which was equivalent to that evidenced by individuals with either condition alone. It is important to note that the three risk groups performed comparably and it is unclear why having two risk factors did not result in disproportionately worse performance relative to those with single risk factors. The lower level of education in the HIV−/METH+ group does not account for the findings, nor does use of ART. The number of individuals with a detectable viral load despite ART use, which may reflect suboptimal adherence to ART (Wood et al., 2003) but may also indicate non-responsivity to ART, was not different in the two HIV groups. We were likely underpowered to detect effects of ART non-adherence and differential effects of specific ART regimens.
Although Blackstone and colleagues did observe additivity of HIV and METH on a composite measure of everyday functioning that was largely comprised of self-reported information (and there are cohort differences between theirs and the present study; see below), the present findings are consistent with an earlier report of a lack of additive effect of METH and HIV on self-reported measures of functioning (Sadek et al., 2007). Our group and others have previously observed an additive effect of METH and HIV on neurological and neurocognitive functions (Chana et al., 2006; Chang et al., 2005; Rippeth et al., 2004), but the current results are consistent with findings based on other neurobehavioral and neuroimaging markers. For example, additive effects of HIV and METH were not observed for behaviors associated with frontal systems dysfunction, including sensation-seeking, impulsivity/disinhibition, and apathy; moreover, a similar pattern of results was observed for impulsivity/disinhibition such that an HIV-by-METH interaction revealed an effect of METH among HIV-negative participants but not in the HIV-positive group (Marquine et al., 2014). There is also some neuroimaging evidence that the combination of these two conditions mitigates brain changes that are seen in HIV infection or METH dependence alone (Archibald et al., 2012). Additionally, though not assessed in the current study, age may be relevant. Although some cognitive functions such as impulsivity and risky decision making may decrease with age in the general population (Deakin et al., 2004), a recent study (Iudicello et al., 2014) found that older (> 50 years) HIV+ individuals with a history of METH dependence did have substantially poorer functioning than younger (<40 years) participants, possibly suggesting increased vulnerability to combined risk factors in the context of aging. The average age of the current sample was just 40 years thus we could not examine the effects of aging. Studies are in progress to further investigate the impact of aging on cognition and functioning in METH and HIV.
An alternate reason that the dual-risk group does not appear to have worse everyday functioning than either risk group alone may be the fact that METH users who must manage their HIV disease face challenges of everyday living when compared to their METH–dependent, HIV-negative counterparts that force them to develop skills that would detected by the UPSA-2. For example, they must navigate the medical system in order to obtain and maintain healthcare—relevant tasks would include transporting themselves to medical appointments, managing extensive and sometimes complex medication regimens (single-tablet regimens have, however, improved adherence- see (Scott Sutton et al., 2016)), and other challenges that could negatively impact treatment adherence (Moore et al., 2012). Therefore, participants in this group may be relatively “practiced” at meeting the demands of everyday living. Social factors may be relevant; e.g., having an HIV-positive partner could result in the modeling of adaptive health behaviors such as medication adherence and drug abstinence (Carrico et al., 2014). A comparison of the demographic, HIV illness features, and METH use features of the current dually-affected cohort and the relevant cohort in the Blackstone et al. (2013) study suggests a somewhat more cognitively intact and healthier group in the current study with higher WRAT-4 scores, more education, higher CD4 counts, higher prevalence of ART use, and less METH use compared to the Blackstone et al. participants. Lower UPSA-2 scores were related to lower education and WRAT-4 scores both in this study and the Blackstone et al. report, but in contrast to that study, our HIV+/METH+ participants did not have lower education and WRAT-4 scores than the METH− groups, reflecting relatively intact premorbid intellectual functioning, or “cognitive reserve”, in the dually-affected participants. These cohort differences, and the observed relationship between cognitive reserve and everyday functioning, may help explain why prominent additive deficits of HIV and METH were seen in the Blackstone study but not the current one. Relatedly, the fact that the HIV−/METH+ participants in this study had higher, albeit non-significant, self-reported METH use than the HIV+/METH+ participants may also explain why dually-affected participants did not show more functional impairment than either singly-affected risk group. Self-report of more frequent and higher quantity of METH used was indeed related to lower UPSA-2 scores. We have previously observed a correlation between higher frequency of METH use and lower functioning scores in a smaller sample of HIV−/METH+ participants (Henry et al., 2010), a not-unexpected result given methamphetamine’s well-known neurotoxic effects especially in chronic users (Scott et al., 2007).
With respect to the association between UPSA-2 Medication Management score and nadir CD4 count, a similar relationship has been reported previously (Blackstone et al., 2012), supporting the premise that disease indicators such as immunosuppression may have implications for everyday functional ability. Nadir CD4 is a historical marker of past disease severity and represents a legacy of brain injury that is believed to manifest later as increased risk for neurobehavioral deficits (Heaton et al., 2011). More puzzling, however, were the findings that worse scores on the Medication Management subscale were related to lower viral load; relatedly participants with undetectable viral load had lower scores on this scale than those with a detectable viral load. These observations are in contrast to a previous report associating detectable viral load to poorer neurocognitive functioning in HIV-positive women (Giesbrecht et al., 2014). Our findings are challenging to explain but perhaps other factors besides the ability to organize and recall a medication regimen may be driving ART non-adherence and subsequent detectable viral load, e.g., depression, apathy, limited access to medications, etc.
Limitations of this study include lack of direct measures of ART adherence, which may have clouded our ability to understand the relationship between use of ART and everyday functioning in HIV-positive individuals. Additionally, there was a large variance in the METH use characteristics of the participants; for example in length of abstinence from the drug. These aspects of our study design likely precluded a refined analysis of the relationship between disease characteristics and functional ability. Furthermore, it can be argued that worse neurocognition (higher GDS) and more severe depressive symptomatology in the risk groups may have driven the observed functional impairment. It is challenging to assess the causal relationships among these highly interrelated deficits, but independent of the causal pathway it is clear that METH and HIV are each associated with everyday functioning problems that have implications for how these individuals navigate through the challenges of daily life.
5. Conclusion
While people with both HIV infection and chronic METH dependence show impairment on everyday functioning tasks and METH use patterns are related to task performance, dually-affected individuals do not appear to be more impaired than individuals with either condition alone. This finding adds to a complex body of literature that argues against a prominent additive effect of HIV and METH on some aspects of brain functioning and cognition, but the influences of aging and compensatory strategies need to be better understood, as do other factors not addressed here such as psychiatric symptomatology. Technological advances such as virtual reality and simulation of computer interfaces will increase the ecological validity of existing laboratory-based everyday functional assessments. Future research can also focus on the relationship of neurocognitive and everyday functioning deficits to “real-world” outcomes such as social behavior, which profoundly influences risk of acquiring and transmitting HIV and developing a substance use disorder.
Highlights.
Methamphetamine use disorder and HIV both impair “real-world” functioning
Heavier methamphetamine use was related to worse performance
Lack of additive deleterious effects of HIV and methamphetamine
Combined effects of HIV and methamphetamine may be moderated by other factors
Acknowledgments
The Translational Methamphetamine AIDS Research Center (TMARC) is supported by Center award P50DA026306 from the National Institute on Drug Abuse (NIDA). The authors thank Dustin Kreitner for her valuable contributions.
Role of Funding Source:
Nothing declared.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors:
AM oversaw the study, conducted the statistical analyses and wrote the manuscript. BL, JI, EM, SL, RH, and WP assisted with interpretation of the findings and write-up of the manuscript. All authors approved of the final manuscript before submission.
Conflict of Interest:
No conflict declared.
References
- Archibald SL, Jacobson MW, Fennema-Notestine C, Ogasawara M, Woods SP, Letendre S, Grant I, Jernigan TL. Functional interactions of HIV-infection and methamphetamine dependence during motor programming. Psychiatry Res. 2012;202:46–52. doi: 10.1016/j.pscychresns.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Turner BJ, Eggan F, Beckman R, Vitiello B, Morton SC, Orlando M, Bozzette SA, Ortiz-Barron L, Shapiro M. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58:721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- Blackstone K, Iudicello JE, Morgan EE, Weber E, Moore DJ, Franklin DR, Ellis RJ, Grant I, Woods SP. Human immunodeficiency virus infection heightens concurrent risk of functional dependence in persons with long-term methamphetamine use. J Addict Med. 2013;7:255–263. doi: 10.1097/ADM.0b013e318293653d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone K, Moore DJ, Heaton RK, Franklin DR, Jr, Woods SP, Clifford DB, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, Rivera-Mindt M, Deutsch R, Ellis RJ, Hampton Atkinson J, Grant I. Diagnosing symptomatic HIV–associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. J Int Neuropsychol Soc. 2012;18:79–88. doi: 10.1017/S135561771100141X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousman CA, Cherner M, Ake C, Letendre S, Atkinson JH, Patterson TL, Grant I, Everall IP. Negative mood and sexual behavior among non-monogamous men who have sex with men in the context of methamphetamine and HIV. J Affect Disorder. 2009;119:84–91. doi: 10.1016/j.jad.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchacz K, McFarland W, Kellogg TA, Loeb L, Holmberg SD, Dilley J, Klausner JD. Amphetamine use is associated with increased HIV incidence among men who have sex with men in San Francisco. AIDS. 2005;19:1423–1424. doi: 10.1097/01.aids.0000180794.27896.fb. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Carrico AW, Woolf-King SE, Neilands TB, Dilworth SE, Johnson MO. Stimulant use and HIV disease management among men in same-sex relationships. Drug Alcohol Depend. 2014;139:174–177. doi: 10.1016/j.drugalcdep.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattie JE, Doyle K, Weber E, Grant I, Woods SP. Planning deficits in HIV-associated neurocognitive disorders: component processes, cognitive correlates, and implications for everyday functioning. J Clin Exp Neuropsychol. 2012;34:906–918. doi: 10.1080/13803395.2012.692772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chana G, Everall IP, Crews L, Langford D, Adame A, Grant I, Cherner M, Lazzaretto D, Heaton R, Ellis R, Masliah E, Group, H. Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology. 2006;67:1486–1489. doi: 10.1212/01.wnl.0000240066.02404.e6. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry. 2005;162:361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Los Angeles, California. Life in the fast lane: HIV and meth. Science. 2012;337:176–177. doi: 10.1126/science.337.6091.176. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Brew BJ. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: A review. Neuropsychol Rev. 2009;19:169–185. doi: 10.1007/s11065-009-9092-3. [DOI] [PubMed] [Google Scholar]
- Deakin J, Aitken M, Robbins T, Sahakian BJ. Risk taking during decision-making in normal volunteers changes with age. J Int Neuropsychol Soc. 2004;10:590–598. doi: 10.1017/S1355617704104104. [DOI] [PubMed] [Google Scholar]
- Elliott RA, Goeman D, Beanland C, Koch S. Ability of older people with dementia or cognitive impairment to manage medicine regimens: a narrative review. Curr Clin Pharmacol. 2015;10:213–221. doi: 10.2174/1574884710666150812141525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesbrecht CJ, Thornton AE, Hall-Patch C, Maan EJ, Cote HC, Money DM, Murray M, Pick N. Select neurocognitive impairment in HIV-infected women: Associations with HIV viral load, hepatitis C virus, and depression, but not leukocyte telomere length. PloS one. 2014;9:e89556. doi: 10.1371/journal.pone.0089556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict Behav. 2010;35:593–598. doi: 10.1016/j.addbeh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iudicello JE, Morgan EE, Gongvatana A, Letendre SL, Grant I, Woods SP, Translational Methamphetamine A.R.C.G. Detrimental impact of remote methamphetamine dependence on neurocognitive and everyday functioning in older but not younger HIV+ adults: evidence for a legacy effect? J Neurovirol. 2014;20:85–98. doi: 10.1007/s13365-014-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiozis H, Gray S, Sacco J, Shapiro M, Kawas C. The Direct Assessment of Functional Abilities (DAFA): a comparison to an indirect measure of instrumental activities of daily living. Gerontologist. 1998;38:113–121. doi: 10.1093/geront/38.1.113. [DOI] [PubMed] [Google Scholar]
- Leifker FR, Patterson TL, Bowie CR, Mausbach BT, Harvey PD. Psychometric properties of performance-based measurements of functional capacity: test-retest reliability, practice effects, and potential sensitivity to change. Schizophr Res. 2010;119:246–252. doi: 10.1016/j.schres.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquine MJ, Iudicello JE, Morgan EE, Brown GG, Letendre SL, Ellis RJ, Deutsch R, Woods SP, Grant I, Heaton RK, Translational Methamphetamine A.R.C.G. Frontal systems behaviors in comorbid human immunodeficiency virus infection and methamphetamine dependency. Psychiatry Res. 2014;215:208–216. doi: 10.1016/j.psychres.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker RB, Asahchop E, Power C. The brain and HAART: collaborative and combative connections. Curr Opin HIV AIDS. 2014;9:579–584. doi: 10.1097/COH.0000000000000110. [DOI] [PubMed] [Google Scholar]
- Minassian A, Maihofer AX, Baker DG, Nievergelt CM, Geyer MA, Risbrough VB. Association of predeployment heart rate variability with risk of postdeployment posttraumatic stress disorder in active-duty marines. JAMA Psychiatry. 2015a;72:979–986. doi: 10.1001/jamapsychiatry.2015.0922. [DOI] [PubMed] [Google Scholar]
- Minassian A, Young JW, Cope ZA, Henry BL, Geyer MA, Perry W. Amphetamine increases activity but not exploration in humans and mice. Psychopharmacol. 2015b doi: 10.1007/s00213-015-4098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Blackstone K, Woods SP, Ellis RJ, Atkinson JH, Heaton RK, Grant I. Methamphetamine use and neuropsychiatric factors are associated with antiretroviral non-adherence. AIDS care. 2012;24:1504–1513. doi: 10.1080/09540121.2012.672718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Doyle KL, Minassian A, Henry BL, Perry W, Marcotte TD, Woods SP, Grant I. Elevated intraindividual variability in methamphetamine dependence is associated with poorer everyday functioning. Psychiatry Res. 2014;220:527–534. doi: 10.1016/j.psychres.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization, W.H. Composite International Diagnostic Interview, version 2.1. World Health Organization; Geneva, Switzerland: 1997. [Google Scholar]
- Patterson T, Goldman S. The UCSD Performance-Based Skills Assessment Administration Manual (UPSA-2) 2005 [Google Scholar]
- Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV. UCSD Performance-Based Skills Assessment: Development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull. 2001a;27:235–245. doi: 10.1093/oxfordjournals.schbul.a006870. [DOI] [PubMed] [Google Scholar]
- Patterson TL, Moscona S, McKibbin CL, Davidson K, Jeste DV. Social skills performance assessment among older patients with schizophrenia. Schizophr Res. 2001b;48:351–360. doi: 10.1016/s0920-9964(00)00109-2. [DOI] [PubMed] [Google Scholar]
- Rendell PG, Mazur M, Henry JD. Prospective memory impairment in former users of methamphetamine. Psychopharmacol. 2009;203:609–616. doi: 10.1007/s00213-008-1408-0. [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I, Group, H. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Sadek JR, Vigil O, Grant I, Heaton RK, Group, H. The impact of neuropsychological functioning and depressed mood on functional complaints in HIV-1 infection and methamphetamine dependence. J Clin Exp Neuropsychol. 2007;29:266–276. doi: 10.1080/13803390600659384. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Scott Sutton S, Magagnoli J, Hardin JW. Impact of pill burden on adherence, risk of hospitalization, and viral suppression in patients with HIV infection and AIDS receiving antiretroviral therapy. Pharmacother. 2016;36:385–401. doi: 10.1002/phar.1728. [DOI] [PubMed] [Google Scholar]
- Stevens J. Applied Multivariate Statistics For The Social Sciences. Lawrence Erlbaum Associates, Inc; New Jersey: 1992. [Google Scholar]
- Twamley EW, Doshi RR, Nayak GV, Palmer BW, Golshan S, Heaton RK, Patterson TL, Jeste DV. Generalized cognitive impairments, ability to perform everyday tasks, and level of independence in community living situations of older patients with psychosis. Am J Psychiatry. 2002;159:2013–2020. doi: 10.1176/appi.ajp.159.12.2013. [DOI] [PubMed] [Google Scholar]
- Vance DE, Wadley VG, Crowe MG, Raper JL, Ball KK. Cognitive and everyday functioning in older and younger adults with and without HIV. Clin Gerontol. 2011;34:413–426. doi: 10.1080/07317115.2011.588545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E, Montaner JS, Yip B, Tyndall MW, Schechter MT, O’Shaughnessy MV, Hogg RS. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. CMAJ. 2003;169:656–661. [PMC free article] [PubMed] [Google Scholar]
- Zweben JE, Cohen JB, Christian D, Galloway GP, Salinardi M, Parent D, Iguchi M. Psychiatric symptoms in methamphetamine users. Am J Addict. 2004;13:181–190. doi: 10.1080/10550490490436055. [DOI] [PubMed] [Google Scholar]


