Abstract
A lung ultrasound surface wave elastography (LUSWE) technique is developed to measure superficial lung tissue elastic properties. The purpose of this study was to translate LUSWE into clinical studies for assessing patients with interstitial lung disease (ILD) and present the pilot data from lung measurements on 10 healthy subjects and 10 patients with ILD. ILD includes multiple lung disorders in which the lung tissue is distorted and stiffened by tissue fibrosis. Chest radiography and computed tomography (CT) are the most commonly used techniques for assessing lung disease, but they are associated with radiation and cannot directly measure lung elastic properties. LUSWE provides a noninvasive and nonionizing technique to measure the elastic properties of superficial lung tissue. LUSWE was used to measure regions of both lungs through six intercostal spaces for patients and healthy subjects. The data are presented as wave speed at 100 Hz, 150 Hz, and 200 Hz at the six intercostal spaces. As an example, the surface wave speeds are, respectively, 1.88 ± 0.11 m/s at 100 Hz, 2.74 ± 0.26 m/s at 150 Hz, and 3.62 ± 0.13 m/s at 200 Hz for a healthy subject in the upper right lung; this is in comparison to measurements from an ILD patient of 3.3 ± 0.37 m/s at 100 Hz, 4.38 ± 0.33 m/s at 150 Hz, and 5.24 ± 0.44 m/s at 200 Hz in the same lung space. Significant differences in wave speed between healthy subjects and ILD patients were found. LUSWE is a safe and noninvasive technique which may be useful for assessing ILD.
Index Terms: surface wave elastography, lung, ultra-sound, wave speed, interstitial lung disease
I. Introduction
Surface wave elastography is a noninvasive technique to measure tissue elastic properties [1]. The purpose of this study was to translate the lung ultrasound surface wave elastography (LUSWE) technique into clinical studies for assessing patients with interstitial lung disease (ILD) based upon our previous feasibility studies.
Ultrasonography is not widely used in clinical practice for lung assessment. Lung tissue is normally filled with air and the difference in acoustic impedance between air and tissue is large. Most of the energy of the ultrasound wave is reflected from the lung surface. Ultrasonography evaluation of the thorax is therefore limited to evaluating structures outside of the lung such as pleural fluid and thoracic superficial masses or adenopathy [2]. For example, lung ultrasonography is excellent for diagnosing pleural diseases, and it is especially useful in the emergency and critical care settings [3, 4] for the detection of pleural effusions or guidance of procedures such as thoracentesis. Ultrasonography can be used effectively to evaluate the lung because more than 70% of the lung can be imaged through intercostal spaces [5]. Ultrasonography for lung assessment typically presents artifacts such as A-lines and B-lines and features such as lung sliding and lung point [4]. The A-line is a horizontal artifact indicating a normal lung surface. The B-line is a kind of comet-tail artifact indicating subpleural interstitial edema. These artifacts and features can be used to evaluate different lung disorders. Ultrasonic features of mild, moderate, and severe lung fibrosis have been identified [6]. Lung ultrasonography is an emerging technique for evaluating various disorders such as pneumothorax [7], peripheral lung lesions [8], lung consolidations [9], parasternal intercostal muscles [10], and diaphragm-related disorders [11].
With the goal of developing a noninvasive technique for measuring lung elastic property, we have developed an ultrasound-based LUSWE technique capable of measuring superficial lung tissue stiffness safely and quickly [12, 13]. In LUSWE, a harmonic vibration at a given low frequency is generated by the indenter of a handheld vibrator on the chest wall of a subject. The ultrasound probe is positioned about 5 mm away from the indenter in the same intercostal space to measure the generated surface wave propagation on the lung in that intercostal space. The speed of surface wave propagation on the lung is determined from the change in wave phase with distance.
We are evaluating LUSWE for assessing patients with ILD in a prospective clinical research. Many ILDs typically are distributed in the peripheral, subpleural regions of the lung [14, 15]. In ILD, the lung parenchyma becomes fibrotic and stiff leading to symptoms, especially dyspnea, and eventually to respiratory failure [16]. Diagnosis of lung fibrosis can be difficult, especially early in the disease course, because the symptoms are nonspecific (most commonly shortness of breath and a dry cough) [3, 6, 17]. Current diagnostic tools include medical history and physical examination, chest radiography, high-resolution computed tomography (HRCT), pulmonary function tests (PFTs) [18], and lung biopsy. The findings of physical examinations are usually nonspecific. ILD may be first suspected after an abnormal chest radiograph, but in most cases, the radiographs also show nonspecific or nondiagnostic findings. HRCT is the clinical standard for diagnosing lung fibrosis [19, 20], but it substantially increases radiation exposure for patients, even when using various techniques to reduce the dose [21]. Lung fibrosis results in stiffened lung tissue. However, current clinical techniques cannot measure lung elastic properties. For example, a spirometer, the main equipment used for PFTs, measures the volume of inspired and expired air [18, 22]. Various elastography [23] techniques have been developed to measure tissue stiffness. However, most techniques are unable to measure lung biomechanics in vivo adequately or safely. LUSWE may provide a noninvasive and safe method for measuring superficial lung tissues for assessing lung fibrosis.
II. Surface wave elastography technique
In a semi-infinite elastic medium, the surface wave speed can be related to the elastic modulus of the medium as [24]
| (1) |
where μ is the shear modulus and ρ is the density of the medium.
The surface wave speed is measured by the phase gradient method using cs(ω) = ωΔr/Δϕ, where Δr is the distance between two detection locations, and Δϕ is the phase change over that distance. The estimation of surface wave speed can be improved by measuring the phase change over multiple locations using a regression model Δϕ̂ = −αΔr + β, where Δϕ̂ denotes the regression value of multiple Δϕ measurements, α and β are regression parameters, and cs(ω) = ω/α.
In a typical surface wave elastography (SWE) system, a handheld shaker is made using an electromagnetic shaker (Model: FG-142, Labworks Inc., Costa Mesa, CA 92626). The handheld shaker applies a local excitation on the skin through an indenter with 3 mm diameter. The excitation is a 0.1 second harmonic vibration (for example, 10 cycles of 100 Hz signal). The resulting propagation of the tissue wave motion (typically a few micrometers) is detected using an ultrasound probe. SWE has been validated with various techniques [24–26]. The indentation technique measures the elasticity of a medium by indenting the medium and analyzing the relationship between the indentation displacement and the resulting force. We published a validation study between SWE and indentation on five gelatin samples with different concentrations (5%–15%) [26]. The surface wave speeds were measured in the three interfaces of a two layer gelatin phantom in water [27].
In this research, the surface wave speed on the lung is measured. The lung surface is between the intercostal muscle and the lung. Both the lung and the muscle contribute the surface wave speed on the lung. In lung testing, a direct vibration excitation on the lung surface is not possible. The surface wave propagation on the lung is produced by a vibration excitation on the chest wall. We previously demonstrated that the surface wave propagation on the lung can be generated by a vibration excitation on the surface of muscle in an ex vivo muscle-lung model [13]. In human studies, the indenter of the handheld shaker is placed on the chest wall in an intercostal space. The ultrasound probe is positioned about 5 mm away from the indenter in the same intercostal space to measure the surface wave propagation on the lung.
III. Human lung testing protocol
We finalized our clinical protocol for testing patients based upon our previous experience and clinical situations. Human lung studies were approved by the Mayo Clinic Institutional Review Board (IRB). Patients were tested in a sitting position. A 0.1-second harmonic vibration is generated by the indenter of the handheld shaker on the chest wall of the subject. The excitation force from the indenter is much less than 1 Newton and the subject only feels a small vibration on his/her chest wall. The measurement of surface wave speed on the lung is independent of the amplitude of excitation. A small tissue motion in tens of μm is enough for sensitive ultrasound detection of the generated tissue motion.
The Verasonics ultrasound system with an ultrasound probe of L11–4 with a central frequency of 6.4 MHz is used. In this study, the surface wave speed on the lung is measured at three frequencies of 100 Hz, 150 Hz and 200 Hz. The 100 Hz wave motion is stronger than the wave motion of higher frequency waves. The higher frequency waves have smaller wave length but decay rapidly over distance than the lower frequency waves. The frequency ranges chosen in this study consider the wave motion amplitude, spatial resolution and wave attenuation. The lung is tested at the total lung capacity when the subject takes a deep breath and hold for a few seconds. Both lungs of the subject are tested through six intercostal spaces. The upper anterior lungs are tested at the second intercostal space in the mid-clavicular line. The lower lateral lungs are tested at one intercostal space above the level of the diaphragm in the mid-axillary line. The lower posterior lungs are tested at one intercostal space above the level of the diaphragm in the mid-scapular line. Because of the expected variation of subjects’ anatomy, ultrasound imaging is used to identify the lungs and select appropriate intercostal spaces to measure the upper and lower lungs. Three measurements are performed at each location and at each frequency. Subject testing can be finished in about 40 minutes. Patients with ILD were enrolled in this research based on their clinical diagnosis. Healthy subjects were enrolled as controls if they were asymptomatic nonsmokers without any skin and lung fibrotic diseases.
We studied the differences of the surface wave speed measurements between 10 ILD patients and 10 healthy controls. In a previous study on assessing systemic sclerosis (SSc), we found that skin viscoelasticity were significantly higher in 10 patients with SSc than 10 healthy subjects [28]. All 10 patients (7 females and 3 males) had bibasilar, peripheral pulmonary fibrosis, which was scleroderma associated in 6, and the rest were 1 each with rheumatoid arthritis (RA), Sjogren’s syndrome, polymyositis, and idiopathic pulmonary fibrosis (IPF). The (Mean ± standard error of the mean (SEM)) age was 67.2±4.99 years. The total lung capacity (TLC) % predicted was 75.5±3.08 % for the ten patients; the diffusing capacity of the lungs for carbon monoxide (DLco) % predicted was moderately reduced at 53.0±3.65 % in 9 patients and could not be measured in one person with resting hypoxemia. Figure 1 shows representative CT images of two patients. The image is a cross section slice of the CT scan. Figure 1(a) shows mild bibasilar, mainly peripheral, ground glass abnormality and Figure 1(b) is from a patient with moderate bibasilar fibrosis, again with a mainly peripheral distribution.
Fig. 1.
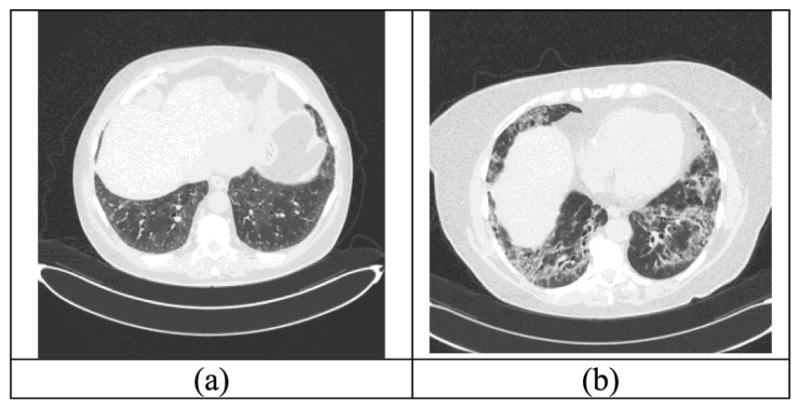
CT images for two patients with mild and moderate lung fibrosis. (a) mild bibasilar, mainly peripheral, ground glass abnormality; (b) moderate bibasilar fibrosis with mainly peripheral distribution.
Detection of in vivo human lung surface motion is guided by ultrasound imaging. Representative B-mode images of lung for a healthy control and a patient are shown in Figure 2. The lung surface of a healthy subject is typically smooth while a patient’s lung surface appears unsmooth. The normal component of the lung surface motion can be analyzed by cross-correlation analysis of the ultrasound tracking beams [12, 29]. In this study, eight locations over a length of approximately 6 mm on the lung surface were used to measure the normal component of the lung surface motion. The tissue motion is measured at these locations in response to a harmonic vibration on the chest wall. A high pulse repetition rate of 2000 pulse/s is used to detect tissue motion in response to the vibration excitation at 100, 150 and 200 Hz. A Verasonics ultrasound system (Verasonics, Inc; Kirkland, WA) is used which collects up to a few thousand imaging frames per second by using a plane-wave pulse transmission method.
Fig. 2.
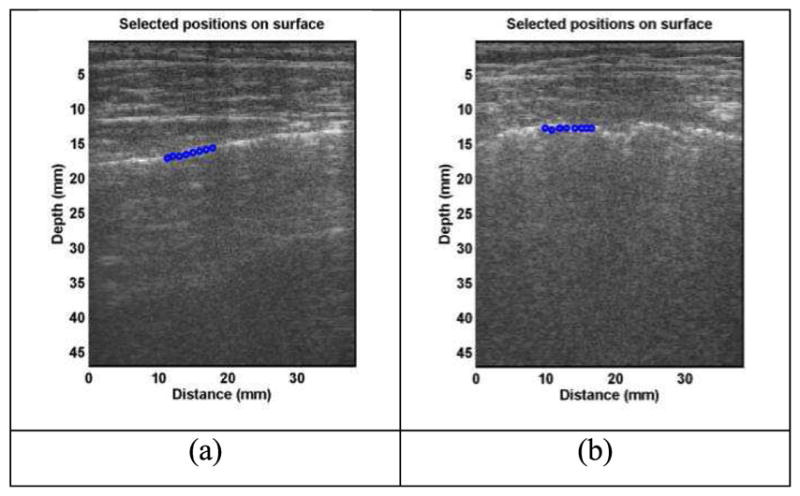
Representative B-mode images of lung for a healthy control (a) and for a patient (b). The lung surface of a healthy subject is typically smooth while a patient’s lung surface is relatively rough. Eight locations over a length of approximately 6 mm on the lung surface were used to measure the lung surface motion using ultrasound tracking beams.
IV. Results
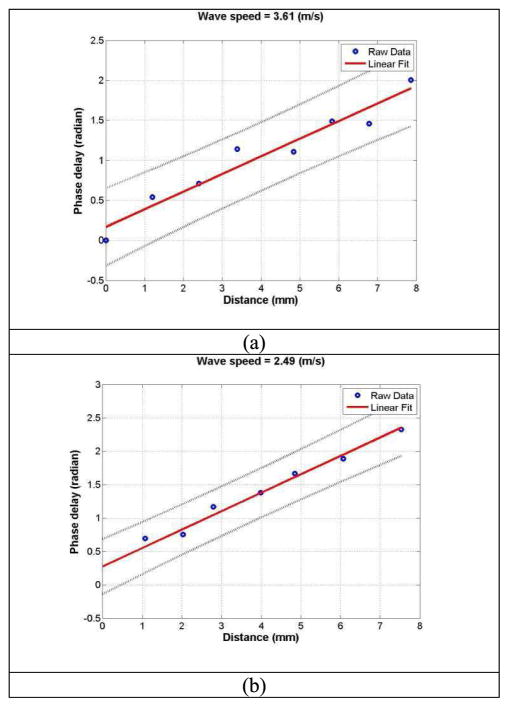
Surface wave speed on the lung is estimated by determining the change in wave phase with distance along the lung surface. The normal tissue motion at the first location on the lung is measured using an ultrasound tracking beam and is used as a reference. The wave phase delay of the tissue motions at the remaining locations on the lung, relative to the first location on the lung, is used to measure the surface wave speed on the lung. The surface wave speed is estimated by the phase changes simultaneously at the 8 locations. Figure 3 shows a representative wave speed at 150 Hz for a healthy subject and a patient in the right second intercostal space. The surface wave speeds were, respectively, 3.61 m/s and 2.49 m/s for the patient and the healthy subject. The wave speed on the lung surface is determined by analyzing ultrasound data directly from the lung. Therefore, the wave speed measurement is local and independent of the location and amplitude of excitation.
Fig. 3.
The wave phase delay of the remaining locations, relative to the first location, is used to measure the surface wave speed. Representative examples of wave speed at 150 Hz for a patient (a) and a healthy subject (b).
At each frequency, three measurements were made through each intercostal space. Table 1 shows representative measurements of surface wave speed for a healthy subject and a patient. The surface wave speed is shown in the format of mean ± SD for the three measurements at each location and each frequency. The three intercostal spaces are designated by a number from 1 to 3. The upper anterior lung is designated by 1. The lower lungs at the lateral and posterior positions are designated by 2 and 3, respectively. The right and left side of the lung are designated by a letter of R and L, respectively. Therefore, L1 represents the left anterior lung in the second intercostal space.
TABLE I.
Representative surface wave speeds of a healthy subject and a patient through different intercostal spaces at 100 Hz, 150 Hz and 200 Hz.
| healthy (m/s) | R1 | R2 | R3 | L1 | L2 | L3 |
|---|---|---|---|---|---|---|
| 100 | 1.88 ± 0.11 | 2.18 ± 0.36 | 2.32 ± 0.2 | 1.89 ± 0.22 | 1.83 ± 0.15 | 1.98 ± 0.15 |
| 150 | 2.74 ± 0.26 | 2.91 ± 0.19 | 3.05 ± 0.45 | 2.67 ± 0.17 | 2.55 ± 0.16 | 2.57 ± 0.13 |
| 200 | 3.62 ± 0.13 | 3.87 ± 0.21 | 3.15 ± 0.3 | 3.28 ± 0.13 | 3.3 ± 0.22 | 3.12 ± 0.2 |
|
| ||||||
| patient (m/s) | R1 | R2 | R3 | L1 | L2 | L3 |
| 100 | 3.3 ± 0.37 | 3.22 ± 0.4 | 3.1 ± 0.11 | 3.39 ± 0.18 | 2.65 ± 0.16 | 3.57 ± 0.13 |
| 150 | 4.38 ± 0.33 | 3.91 ± 0.05 | 4.47 ± 0.11 | 5.2 ± 0.35 | 2.91 ± 0.42 | 3.76 ± 0.31 |
| 200 | 5.24 ± 0.44 | 4.02 ± 0.4 | 6.19 ± 0.23 | 7.53 ± 0.35 | 4.87 ± 0.22 | 6.2 ± 0.64 |
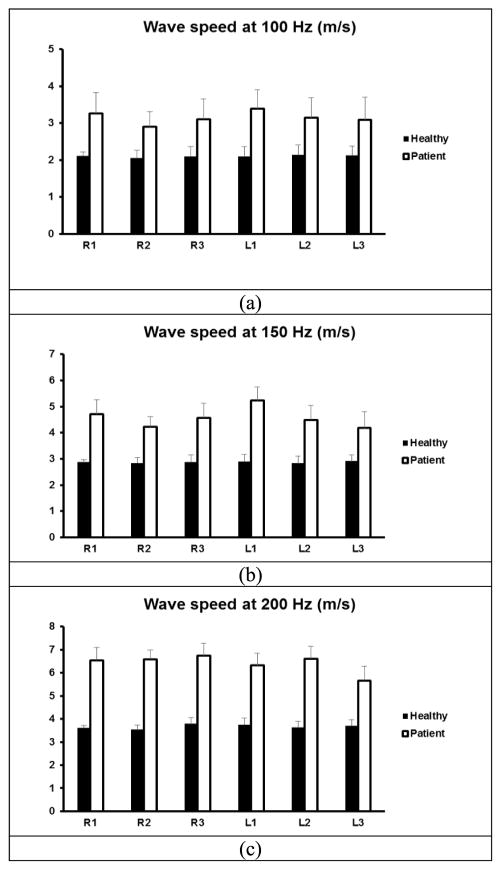
A comparison of wave speeds between 10 healthy subjects and 10 patients is shown in Figure 4 for 100 Hz, 150 Hz, and 200 Hz. An unpaired, two-tailed t-test between the healthy subjects and patients was conducted to compare the sample means. Differences in mean values were considered significant when p<0.05. The p-values for the t -test were less than 0.05 for all intercostal spaces at three frequencies, respectively.
Fig. 4.
Comparison of wave speeds between 10 healthy subjects and 10 patients through six intercostal spaces. Surface wave speed at (a) 100 Hz, (b) 150 Hz, (c) 200 Hz.
V. Discussion
Some lung diseases such as ILD are associated with changes in biomechanical properties of the lung. ILD consists of various lung disorders due to damage and fibrosis of the lung parenchyma. ILD can lead to other complications including pulmonary hypertension and respiratory failure. Diagnosis of lung fibrosis can be difficult because the symptoms are nonspecific at early stages [6] and global pulmonary function abnormalities cannot define the anatomical extent or local involvement of disease. High-resolution computed tomography (HRCT) is the clinical standard for diagnosing and characterizing lung fibrosis [19, 20]. HRCT is a special type of CT acquisition technique that uses 0.5–1 mm thick slices to produces high detail images of the parenchyma, pathological density changes, and architectural distortion that is associated with ILD. However, HRCT involves ionizing x-ray radiation exposure for patients. Lung fibrosis results in stiffened lung tissue. Although CT provides excellent anatomical imaging of the lung, it does not measure changes in lung elastic properties that occur with progressive fibrosis.
LUSWE is a noninvasive technique for measuring surface lung tissue elastic properties. Most ultrasound elastography methods use ultrasound radiation force (URF) to generate shear waves inside the tissue. To generate sufficient tissue motion using URF, a relatively high-intensity ultrasound field is needed. Although URF has been used in the liver and other tissues, URF should not be applied to lung tissue. In vivo animal lung studies demonstrated that the relatively high-intensity ultrasound field may cause alveolar hemorrhage or lung injury [30]. In LUSWE, the surface wave on the lung is safely generated by a local mechanical vibration on the chest. Diagnostic ultrasound is only used for detecting surface wave propagation on the lung. Therefore, the LUSWE technique is a safe method for lung testing and screening patients.
LUSWE uses the surface wave to evaluate superficial lung tissue. Many ILDs typically affect the lung periphery, close to the surface. LUSWE may be used to assess multiple lung disorders such as “wet” lungs when the alveoli are partly filled or filled with blood or fluids. In “wet” lungs, ultrasound may penetrate deeper in the diseased tissue than healthy lung tissue allowing imaging of deeper tissue [2]. Lung ultrasonography is also an emerging technique for evaluating lung disorders such as pneumothorax [7], peripheral lung lesions [8], and lung consolidations [9]. LUSWE provides both ultrasonography imaging of lung structures and measurements of lung stiffness, thereby, offering an additional tool for assessing lung disease.
Previously, we demonstrated that lung stiffness increases with the pulmonary pressure in ex vivo swine models [12]. In a recent study [13], a patient was tested at two lung volumes. Functional residual capacity (FRC) is at the end of a normal tidal expiration and total lung capacity (TLC) is at the end of a maximal inspiration. In the current study, we decide to test the patient’s lung only at the TLC for this large and longitudinal project as the TLC is not only an easier target to define but is also easy for a patient to perform reproducibly. Another consideration is that the current protocol takes about 40 minutes and adding FRC would double the testing time. We think that if the addition of FRC would be required for other clinical applications, a spirometer for pressure monitoring or a simplified testing protocol could be used in future.
The background foundation for this research is the Rayleigh surface wave propagation on a semi-infinite medium. The generated mechanical wave can propagate on the lung and inside the lung. However, detection of wave propagation using ultrasound is limited to the superficial lung tissue because of strong ultrasound beam attenuation in the lung tissue. The surface wave propagation is still in bulky lung tissue. If the surface wave propagates in a plate structure as a Lame wave, the wave speed will be dependent on the thickness of the plate and the mode of wave propagation.
There are some areas where we can improve the measurement of surface tissue motion on the lung. The detection of surface lung motion using ultrasound is limited to a thin layer of tissue. We use about a 1 mm thick tissue layer to analyze the tissue motion at a selected location. Further improvement may make it possible to use other novel techniques for measuring thin tissue layers [31]. The lung surface is not flat. A relatively flat surface of about 6mm length along the lung surface is used to estimate surface wave speed. Further improvement may consider the analysis of wave propagation along a curved surface. The measured surface wave speed on the lung should have some contributions from the intercostal muscle, because the lung surface is between the lung and the intercostal muscle. The intercostal muscle and the lung consist of a multilayer system. Finite element modelling (FEM) could be used to analyze the surface wave propagation along the interface between the muscle and the lung. Because we demonstrated in this report that the surface wave speed can separate healthy and ILD lungs, surface wave speed alone seems to have clinical use in assessing ILD.
VI. Conclusion
LUSWE provides a noninvasive and nonionizing technique to measure the elastic properties of superficial lung tissue. LUSWE was used to measure both lungs through six intercostal spaces for 10 healthy subjects and 10 patients with ILD. The data are presented as wave speed at 100 Hz, 150 Hz, and 200 Hz at the six intercostal spaces. Significant differences of the surface wave speed between healthy subjects and patients are found indicating that lung surface wave speed appears to be a biomarker for this disease. LUSWE may be useful for assessing ILD. Further work of LUSWE for lung testing will be improvement of accuracy and reproducibility of measurements on a thin lung tissue layer, consideration of a curved lung surface, and FEM simulation of surface wave propagation along the interface between the intercostal muscle and the lung.
Acknowledgments
This study is supported by NIH R01HL125234 from the National Heart, Lung, and Blood Institute. We thank Mrs. Jennifer Poston for editing this manuscript. The authors would like to thank the anonymous reviewers for their encouraging and constructive comments. We would also like to thank Dr. Scott Smith for invitation for this Special Issue of the Transactions-UFFC, and Dr. Jan D’hooge, associate editor, and Dr. Steven Freear, editor in chief, for their careful handling of the manuscript. With their help, we are able to improve this manuscript.
Biographies

Xiaoming Zhang received his bachelor degree, master degree and Ph.D. respectively in 1983, 1986 and 1990 from Huazhong University of Science and Technology (HUST), China. He then completed his postdoctoral training at the Department of Acoustics, Harbin Engineering University, China, and Institute of Sound and Vibration Research, University of Southampton, England. He was promoted to full professor in 1995 at HUST. He joined Mayo Clinic in 2001 and has been an Associate Professor of Biomedical Engineering since 2004. His research interests are in biomedical engineering, ultrasound, acoustics, elastography, instrumentation, and clinical translational research. He aims to develop non-invasive ultrasound based techniques to measure tissue elasticity and translate these techniques into clinic for assessing tissue diseases in a safe, quantitative, and cost-effective manner. He is a principal investigator of several NIH grants and multiple clinical studies.

Thomas Osborn, MD, received his bachelor’s degree in 1968 from Purdue University, West Lafayette, Indiana. He graduated from medical school in 1972 from Washington University in Saint Louis, Missouri, and did his internship there as well in Internal Medicine. He subsequently performed required United States Army service as a general practitioner in Alaska, finished his residency in the military, and served one additional year at the NSA in Fort Meade, Maryland, in the US Army. Following this, he did a fellowship in Rheumatology at Saint Louis University, Saint Louis, Missouri, and stayed on staff there as an associate professor, working on research, teaching, and clinical practice until approximately 1994. From 1994 until 1997, he was in private practice and subsequently did clinical care for Cleveland Clinic, South Florida, for five years. He then started on staff at Mayo Clinic in Rheumatology in 2003 to present as an associate professor. His primary interests have been scleroderma and scleroderma animal models. He also has published on Sjögren’s syndrome and a variety of other rheumatologic diseases. His most recent work has been with autoimmune pulmonary diseases, including pulmonary fibrosis. He has recently published and co-published articles related to research in this field. His present interest with Dr. Xiaoming Zhang has stemmed from work on scleroderma, skin, attempting to quantitate the amount of elasticity. He has previously reviewed grants for the National Institute of Health and the Rheumatology Research Foundation, which he continues.

Boran Zhou received the B.Sc degree from the Harbin Institute of Technology, Harbin, China in 2009, and the Ph.D degree from the Biomedical Engineering program in University of South Carolina, Columbia, South Carolina in 2014. In 2010, he joined the University of South Carolina, Columbia, SC, USA. During his Ph.D studies, he was also a teaching assistant for the course of Soft tissue biomechanics and a research assistant. He was a Postdoctoral Fellow in the University of South Carolina, Columbia, SC from 2015 to 2016. In 2016, he joined the Mayo Clinic, where he is currently a senior research fellow in the Department of Radiology. His research interests include ultrasound elastography, soft tissue computational and experimental modeling, biomedical engineering, instrumentation and clinical translational research.

Duane Meixner received his Associate in Science Degree in Diagnostic Medical Sonography in 1987 from Middlesex Community College in Bedford, Massachusetts. He joined the Mayo Clinic in 1987 and has been an Instructor of Radiology in the Mayo Clinic College of Medicine and Science since 2004. His research interests include ultrasound elastography, contrast-enhanced ultrasound, multimodality image fusion, volumetric ultrasound.

Randall R. Kinnick received the BME-EE degree in Mechanical and Electrical Engineering from General Motors Institute, Flint, MI, in 1976. Prior to coming to Mayo Clinic he spent eight years in the field of aerospace materials research. He is currently Lead Engineer in the Ultrasound Research Laboratory, Mayo Clinic College of Medicine, Rochester, MN. His present area of interest is the application of acoustic-elastic methods to invivo diagnostic use in humans. Mr. Kinnick has co-authored numerous articles in the field on a broad range of topics including imaging, diagnostic and therapeutic uses of ultrasound.

Dr. Bartholmai is an Associate Professor of Radiology and chair of the Division of Radiology Informatics at Mayo Clinic. He is established scientist and clinical radiologist with expertise in thoracic radiology and quantitative analysis of medical imaging data. Dr. Bartholmai has been principal investigator of multiple successful NIH, NHLBI, DOD and other extramurally funded projects related to basic science and clinical implementation/validation of quantitative analysis of lung HRCT images. Much of this work focuses on quantification of parenchymal lung disease (including emphysema, cystic lung disease and pulmonary fibrosis) tracking change over time, identifying and stratifying risk groups. His objectives include development of clinical tools that can serve as biomarkers and use of radiomics strategies to facilitate clinical decision support and predict disease outcomes from imaging data.

James F. Greenleaf (IEEE/M’73) received the B.S. degree in Electrical Engineering from the University of Utah, Salt Lake City, in 1964, the M.S. degree in Engineering Science from Purdue University, Lafayette, IN, in 1968, and the Ph.D. degree in Engineering Science from the Mayo Graduate School of Medicine, Rochester, MN, and Purdue University in 1970. He is currently Professor of Biomedical Engineering and Associate Professor of Medicine, Mayo Graduate School, and Consultant, Department of Physiology and Biomedical Engineering, and Internal Medicine, Division of Cardiovascular Diseases, Mayo Clinic Rochester. He has served on the IEEE Technical Committee for the Ultrasonics Symposium for ten years. He served on the IEEE Ultrasonics, Ferroelectrics, and Frequency Control Society (UFFC-S) Subcommittee on Ultrasonics in Medicine/IEEE Measurement Guide Editors, and on the IEEE Medical Ultrasound Committee. Doctor Greenleaf was President of the UFFC-S in 1992 and 1993. Doctor Greenleaf has 21 patents and is recipient of the 1986 J. Holmes Pioneer Award and the 1998 William J. Fry Memorial Lecture Award from the American Institute of Ultrasound in Medicine and is a Fellow of IEEE, American Institute of Ultrasound in Medicine, and American Institute for Medical and Biological Engineering, and the Acoustical Society of America. Doctor Greenleaf was the Distinguished Lecturer for IEEE Ultrasonics, Ferroelectrics, and Frequency Control Society (1990/1991) and recipient of the Rayleigh Award (2004) and the Purdue Distinguished Engineering Alumni Award (2017). His special field of interest is ultrasonic biomedical science, and he has published more than 450 articles and edited or authored five books in the field.

Sanjay Kalra received his MBBS degree from Christian Medical College, Vellore, India in 1981 and then completed residency, fellowship and research training at the Postgraduate Institute for Medical Education and Research, Chandigarh, India, Wythenshawe Hospital, Manchester, England, Royal University Hospital, Saskatoon, Canada and Mayo Clinic, Rochester, MN, USA, culminating in MD (India) and FRCP (UK) degrees and US board certification in Internal Medicine and Pulmonary Medicine. He has been on staff at Mayo Clinic, Rochester since 1997 where he is Consultant in the Division of Pulmonary & Critical Care Medicine and an Associate Professor of Medicine. His clinical and research interests are mainly focused on interstitial and airway related lung diseases.
References
- 1.Zhang X, Greenleaf JF, Pittelkow MR, Kinnick RR. System and method for non-invasively measuring tissue viscoelasticity using surface waves. 9044192, 06/2015. USA Patent. 2015
- 2.Volpicelli G. Lung sonography. J Ultrasound Med. 2013 Jan;32:165–71. doi: 10.7863/jum.2013.32.1.165. [DOI] [PubMed] [Google Scholar]
- 3.Mathis G, Lessnau KD. Atlas of Chest Sonography. Berlin: Springer-Verlag; 2008. [Google Scholar]
- 4.Hakimisefat B, Mayo PH. Lung ultrasonography. The Open Critical Care Medicine Journal. 2010;3:21–25. [Google Scholar]
- 5.Mayo PH, Doelken P. Pleural ultrasonography. Clin Chest Med. 2006 Jun;27:215–27. doi: 10.1016/j.ccm.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Sperandeo M, Varriale A, Sperandeo G, Filabozzi P, Piattelli ML, Carnevale V, et al. Transthoracic ultrasound in the evaluation of pulmonary fibrosis: our experience. Ultrasound Med Biol. 2009 May;35:723–9. doi: 10.1016/j.ultrasmedbio.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Noble VE. Think ultrasound when evaluating for pneumothorax. J Ultrasound Med. 2012;31:501–504. doi: 10.7863/jum.2012.31.3.501. [DOI] [PubMed] [Google Scholar]
- 8.Lie CH, Chao TY, Chung YH, Wang JL, Wang YH, Lin MC. New image characteristics in endobronchial ultrasonography for differentiating peripheral pulmonary lesions. Ultrasound Med Biol. 2009 Mar;35:376–81. doi: 10.1016/j.ultrasmedbio.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Barillari A, Franco FD, Colonna F. Chest ultrasound helps to diagnose pulmonary consolidations in pediatric patients. J Medical Ultrasound. 2011;19:27–31. [Google Scholar]
- 10.Cala SJ, Kenyon CM, Lee A, Watkin K, Macklem PT, Rochester DF. Respiratory ultrasonography of human parasternal intercostal muscle in vivo. Ultrasound Med Biol. 1998 Mar;24:313–26. doi: 10.1016/s0301-5629(97)00271-8. [DOI] [PubMed] [Google Scholar]
- 11.Gethin-Jones TL, Noble VE, Morse CR. Quantification of diaphragm function using ultrasound: evaluation of a novel technique. Ultrasound Med Biol. 2010 Nov;36:1965–9. doi: 10.1016/j.ultrasmedbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Qiang B, Hubmayr RD, Urban MW, Kinnick R, Greenleaf JF. Noninvasive ultrasound image guided surface wave method for measuring the wave speed and estimating the elasticity of lungs: A feasibility study. Ultrasonics. 2011;51:289–295. doi: 10.1016/j.ultras.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Osborn T, Kalra S. A noninvasive ultrasound elastography technique for measuring surface waves on the lung. Ultrasonics. 2016;71:183–8. doi: 10.1016/j.ultras.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells AU, Hansell DM, Rubens MB, Cullinan P, Black CM, du Bois RM. The predictive value of appearances on thin-section computed tomography in fibrosing alveolitis. Am Rev Respir Dis. 1993 Oct;148:1076–82. doi: 10.1164/ajrccm/148.4_Pt_1.1076. [DOI] [PubMed] [Google Scholar]
- 15.Desai SR, Veeraraghavan S, Hansell DM, Nikolakopolou A, Goh NS, Nicholson AG, et al. CT features of lung disease in patients with systemic sclerosis: comparison with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Radiology. 2004 Aug;232:560–7. doi: 10.1148/radiol.2322031223. [DOI] [PubMed] [Google Scholar]
- 16.Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med. 1994 Oct;150:967–72. doi: 10.1164/ajrccm.150.4.7921471. [DOI] [PubMed] [Google Scholar]
- 17.Loscalzo J, editor. Harrison’s Pulmonary and Critical Care Medicine. New York: Mc Graw Hill Medical; 2010. [Google Scholar]
- 18.Ruppel GL. Manual of Pulmonary Function Testing. St. Louis: Mosby Elsevier; 2009. [Google Scholar]
- 19.Mathieson JR, Mayo JR, Staples CA, Muller NL. Chronic diffuse infiltrative lung disease: comparison of diagnostic accuracy of CT and chest radiography. Radiology. 1989 Apr;171:111–6. doi: 10.1148/radiology.171.1.2928513. [DOI] [PubMed] [Google Scholar]
- 20.Verschakelen JA. The role of high-resolution computed tomography in the work-up of interstitial lung disease. Curr Opin Pulm Med. 2010 Sep;16:503–10. doi: 10.1097/MCP.0b013e32833cc997. [DOI] [PubMed] [Google Scholar]
- 21.Mayo JR. CT evaluation of diffuse infiltrative lung disease: dose considerations and optimal technique. J Thorac Imaging. 2009 Nov;24:252–9. doi: 10.1097/RTI.0b013e3181c227b2. [DOI] [PubMed] [Google Scholar]
- 22.Mulroney SE, Myers AK. Netter’s Essential Physiology. Philadephia: Saunders Elsevier; 2009. [Google Scholar]
- 23.Greenleaf JF, Fatemi M, Insana M. Selected methods for imaging elastic properties of biological tissues. Annu Rev Biomed Eng. 2003;5:57–78. doi: 10.1146/annurev.bioeng.5.040202.121623. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Greenleaf JF. Estimation of tissue’s elasticity with surface wave speed. J Acoust Soc Am. 2007 Nov;122:2522–5. doi: 10.1121/1.2785045. [DOI] [PubMed] [Google Scholar]
- 25.Qiang B, Greenleaf J, Oyen M, Zhang X. Estimating material elasticity by spherical indentation load-relaxation tests on viscoelastic samples of finite thickness. IEEE Trans Ultrason Ferroelectr Freq Control. 2011 Jul;58:1418–29. doi: 10.1109/TUFFC.2011.1961. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Qiang B, Greenleaf J. Comparison of the surface wave method and the indentation method for measuring the elasticity of gelatin phantoms of different concentrations. Ultrasonics. 2011 Feb;51:157–64. doi: 10.1016/j.ultras.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiang B, Zhang X, Greenleaf J. Measurement of surface wave speed in two-layered gelatin phantoms. 2010 IEEE International Ultrasonics Symposium; San Diego, USA. 2010. [Google Scholar]
- 28.Zhang X, Osborn TG, Pittelkow MR, Qiang, Kinnick RR, Greenleaf JF. Quantitative assessment of scleroderma by surface wave technique. Medical Engineering & Physics. 2011;33:31–37. doi: 10.1016/j.medengphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Hasegawa H, Kanai H. Improving accuracy in estimation of artery-wall displacement by referring to center frequency of RF echo. IEEE Trans Ultrason Ferroelectr Freq Control. 2006 Jan;53:52–63. doi: 10.1109/tuffc.2006.1588391. [DOI] [PubMed] [Google Scholar]
- 30.Zachary JF, Blue JP, Jr, Miller RJ, Ricconi BJ, Eden JG, O’Brien WD., Jr Lesions of ultrasound-induced lung hemorrhage are not consistent with thermal injury. Ultrasound Med Biol. 2006 Nov;32:1763–70. doi: 10.1016/j.ultrasmedbio.2006.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu D, Ebbini ES. Viscoelastic property measurement in thin tissue constructs using ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control. 2008 Feb;55:368–83. doi: 10.1109/TUFFC.2008.655. [DOI] [PMC free article] [PubMed] [Google Scholar]