Supplemental Digital Content is Available in the Text.
Key Words: plate fixation, locked plating, dynamization, interfragmentary motion, stiffness, active plating, femur
Abstract
Background:
Decreasing the stiffness of locked plating constructs can promote natural fracture healing by controlled dynamization of the fracture. This biomechanical study compared the effect of 4 different stiffness reduction methods on interfragmentary motion by measuring axial motion and shear motion at the fracture site.
Methods:
Distal femur locking plates were applied to bridge a metadiaphyseal fracture in femur surrogates. A locked construct with a short-bridge span served as the nondynamized control group (LOCKED). Four different methods for stiffness reduction were evaluated: replacing diaphyseal locking screws with nonlocked screws (NONLOCKED); bridge dynamization (BRIDGE) with 2 empty screw holes proximal to the fracture; screw dynamization with far cortical locking (FCL) screws; and plate dynamization with active locking plates (ACTIVE). Construct stiffness, axial motion, and shear motion at the fracture site were measured to characterize each dynamization methods.
Results:
Compared with LOCKED control constructs, NONLOCKED constructs had a similar stiffness (P = 0.08), axial motion (P = 0.07), and shear motion (P = 0.97). BRIDGE constructs reduced stiffness by 45% compared with LOCKED constructs (P < 0.001), but interfragmentary motion was dominated by shear. Compared with LOCKED constructs, FCL and ACTIVE constructs reduced stiffness by 62% (P < 0.001) and 75% (P < 0.001), respectively, and significantly increased axial motion, but not shear motion.
Conclusions:
In a surrogate model of a distal femur fracture, replacing locked with nonlocked diaphyseal screws does not significantly decrease construct stiffness and does not enhance interfragmentary motion. A longer bridge span primarily increases shear motion, not axial motion. The use of FCL screws or active plating delivers axial dynamization without introducing shear motion.
INTRODUCTION
Angle-stable locked plating has become the standard treatment for most difficult fractures of the distal femur. Despite excellent early results, there is growing concern surrounding the relatively high nonunion rates after locked plate fixation of distal femur fractures. Most recent studies quote nonunion or fixation failure rates after locked plating of distal femur fractures of 10%–23%.1–6 There is abundant evidence that deficient fracture motion caused by overly stiff locking plates can suppress natural fracture healing, contributing to delayed unions, nonunions, and fixation failure.3,7–9 Conversely, research over the past 50 years has consistently demonstrated that controlled axial dynamization can improve the speed and strength of fracture healing by dynamically stimulating natural bone healing through callus formation.7,10–15
Two primary mechanical conditions critical for natural fracture healing have been identified: Callus formation is promoted by axial interfragmentary motion greater than 0.2 mm16,17; and fracture healing is inhibited when interfragmentary motion is dominated by shear displacement.18 Strategies aimed at altering the mechanical environment created by locked plating constructs and at promoting fracture healing by spontaneous callus formation were proposed as early as 2003.19
Four principal methods are currently promoted to reduce the stiffness of locked bridge plating constructs: diaphyseal fixation with nonlocking screws rather than locking screws; increasing the length of the bridge spanning the fracture zone19; screw dynamization with far cortical locking (FCL) screws8; and plate dynamization with active plates that have elastically suspended locking holes.20 It is not clear which of these 4 constructs provide the best mechanical environment to achieve the goal of early fracture dynamization to promote healing while minimizing any detrimental effects from motion.
This study measured construct stiffness as well as axial and shear motion at the fracture site to assess the efficacy by which each strategy can satisfy the basic conditions for mechanical stimulation of fracture healing. Specifically, this study tested the hypotheses that the 4 dynamization strategies will differ in their efficacy to decrease construct stiffness, to increase interfragmentary axial motion, and to prevent excessive shear motion.
MATERIAL AND METHODS
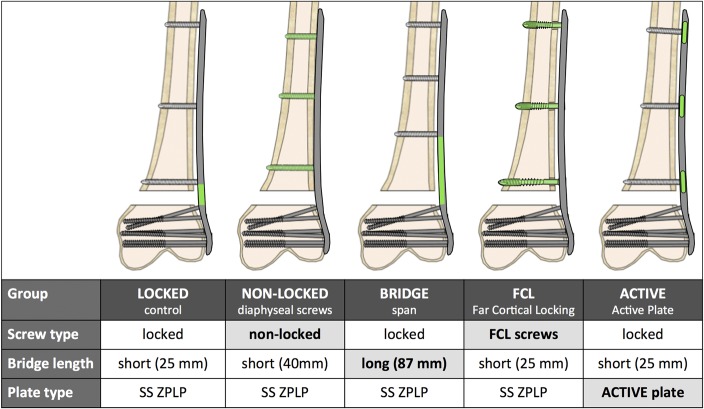
In a biomechanical bench-top study, periarticular locking plates were applied to bridge a metadiaphyseal fracture in femur surrogates. Construct stiffness was assessed under quasi-physiologic loading by measuring the resulting axial and shear motion at the fracture site. In the LOCKED control group, the periarticular plate was applied to the diaphysis with bicortical locking screws. The first locking screw was placed adjacent to the fracture to achieve a short-bridge span. Subsequently, 4 different strategies to decrease construct stiffness and to dynamize the fracture site were evaluated (Fig. 1): replacing diaphyseal locking screws with nonlocking screws (NONLOCKED group); bridge dynamization (BRIDGE group) by increasing the bridging span with locked screws in the diaphysis19; screw dynamization with FCL screws (FCL group)8; and plate dynamization with active locking plates (ACTIVE group).10,20 Construct stiffness was characterized by measuring the construct deformation in response to quasi-physiologic loading. Dynamization of the fracture site was characterized by measuring the interfragmentary motion in axial and shear direction. Finally, the stiffness and interfragmentary motion results of the 4 strategies for stiffness reduction were compared with the LOCKED control group to determine their effectiveness in dynamizing the fracture site.
FIGURE 1.
Strategies to dynamize a locked plating construct (LOCKED) for distal femur fractures. Editor's Note: A color image accompanies the online version of this article.
Specimens
Plating constructs were evaluated in fourth generation femur surrogate specimens made of fiber-reinforced epoxy composite (#3406, large size; Sawbones, Vashon, WA) to minimize interspecimen variability. An unstable distal femur fracture (AO/Orthopaedic Trauma Association 33-A3) was modeled by introducing a 10-mm gap osteotomy located 60 mm proximal to the intercondylar notch.21,22 This gap osteotomy simulated the biomechanical constraints of a comminuted fracture that relies on full-load transfer through the bridge plating construct because of a lack of bony apposition at the fracture site. In the LOCKED control group, this gap osteotomy was stabilized with a 286-mm long distal femur plate (ZPLP; Zimmer, Warsaw, IN) made of stainless steel. The plate had 13 holes for diaphyseal fixation, 7 of which were locking holes (Fig. 2A). The diaphyseal plate segment was applied using 3 evenly spaced 4.5-mm locking screws placed in the first, fourth, and seventh locking hole from the fracture site, resulting in a short-bridge span of 25 mm. A plate elevation of 1 mm over the proximal diaphysis was achieved with temporary spacers to simulate biological fixation with preservation of periosteal perfusion.23 The distal plate segment was applied to the metaphysis using six 5.5-mm cannulated locking screws in accordance with the manufacturer's technique guide. All screws were tightened to 4 Nm.
FIGURE 2.
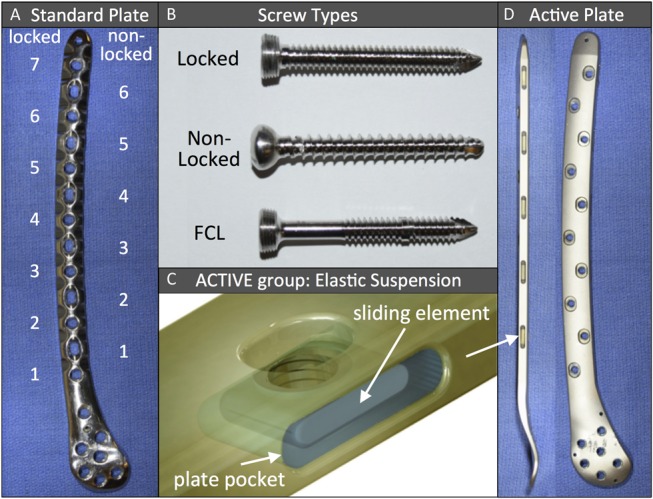
A, Distal femur plate with alternating locked and nonlocked holes; (B) 3 distinct screws used with standard femur plate; (C and D) active plate with screw holes located in elastically suspended sliding elements. Editor's Note: A color image accompanies the online version of this article.
Subsequently, 4 additional construct configurations were assembled by changing one variable of the LOCKED control group constructs at a time: For constructs of the NONLOCKED group, nonlocking screws were used in place of locking screws for diaphyseal fixation, using the first, fourth, and sixth nonlocking hole from the fracture site (Fig. 2B). Because of the alternating locking/nonlocking screw hole configuration of this plate, the nonlocking construct had an intermediate bridge span of 40 mm. BRIDGE group constructs used a longer bridge span (87 mm) than LOCKED control group constructs (25 mm) by placing diaphyseal locking screws in the third, fifth, and seventh locking hole from the fracture site. FCL group constructs replaced the 3 diaphyseal locking screws of LOCKED control group constructs with 3 FCL screws (4.5 mm MotionLoc; Zimmer) made of stainless steel. FCL screws rigidly lock into the plate and the far cortex, but they are not rigidly constrained in the near cortex underlying the plate. The elastic shaft of FCL screws can flex within the near cortex motion envelope to generate symmetric interfragmentary motion.8 ACTIVE group constructs had a screw configuration identical to that of the LOCKED control group, but used an active locking plate. Screw holes of active locking plates are integrated into individual sliding elements that are elastically suspended in a silicone envelope inside lateral plate pockets (Fig. 2C). Lateral pockets are arranged in an alternating pattern from both plate sides, resulting in a staggered locking hole configuration. The pocket geometry combined with the silicone suspension allows controlled axial translation, which enables up to 1.5 mm of axial motion across a fracture while providing stable fixation in response to bending and torsional loading.24 The silicone suspension consisted of long-term implantable medical-grade silicone elastomer. The active locking plate was made of stainless steel and was geometrically equivalent to the standard locking plate of the LOCKED control group (Fig. 2D). Five specimens of each of the 5 constructs were tested for reproducibility requiring a total of 25 construct tests.
Loading
For stiffness assessment, constructs were tested under quasi-physiological loading in a material test system according to an established loading protocol (see Figure, Supplemental Digital Content 1, http://links.lww.com/BOT/A993, describing specimen loading and outcome assessment).21,25 The femoral condyles were embedded in a mounting fixture using bone cement and were rigidly connected to the base of the test system (8874, Instron, Canton, MA). The metaphyseal plate segment was coated with soft clay to prevent nonphysiologic plate constraints. The femoral head was placed in a spherical recess of a polymer block that was attached to the test system actuator. This enabled axial load application while allowing unconstrained rotation of the femoral head. Load was induced along the mechanical axis of the femur, with the load vector intersecting the femoral head and the epicondylar center. Each construct was loaded in 50-N increments up to 700 N, corresponding to approximately one body weight.
Outcome Assessment
Constructs were characterized by determining their construct stiffness and interfragmentary motion using noncontact optical photogrammetry. For this purpose, an array of 4 active luminescent markers consisting of miniature light emitting diodes were glued to the osteotomy surfaces. An 18 megapixel digital camera (Canon EOS T6) captured the marker locations with a resolution of 0.01 mm after each incremental loading step. ImageJ quantitative image analysis software developed by the National Institute of Health (www.imagej.net) was used to extract marker displacement and to calculate the average axial motion dA and shear motion dS between osteotomy surfaces in response to incremental load steps. Because plate bending induces different amounts of axial motion at the near cortex and far cortex,26 axial motion dA was extracted individually for the near cortex (dA, NC) from markers 1 and 3, and for the far cortex (dA, FC) from markers 2 and 4, as depicted in Supplemental Digital Content 1 (see Figure, http://links.lww.com/BOT/A993). Construct stiffness SC was calculated by dividing the applied axial load by the axial motion dA at the midpoint between the near and far cortex, with dA = (dA, FC + dA, NC)/2.
Statistical Analysis
All results are reported as their mean and SD. Construct stiffness SC and interfragmentary motion parameters dS, dA, FC, and dA, FC of the 4 experimental groups was statistically compared with the LOCKED control group results using one-way analysis of variance testing including a post hoc Turkey honest significant difference (HSD) to identify significant differences. Each outcome parameter was analyzed individually, and a level of significance of α = 0.05 was used to detect significant differences.
RESULTS
Construct Stiffness
There was no significant difference between the stiffness of LOCKED control constructs (2998 ± 361 N/mm) and NONLOCKED constructs (2549 ± 355 N/mm, P = 0.08) (see Table, Supplemental Digital Content 2, http://links.lww.com/BOT/A994, summarizing construct stiffness and interfragmentary motion). However, compared with the LOCKED control group, BRIDGE group constructs had a 45% lower stiffness (P < 0.001), FCL group constructs had a 62% lower stiffness (P < 0.001), and ACTIVE group constructs had a 75% lower stiffness (P < 0.001) (Fig. 3).
FIGURE 3.
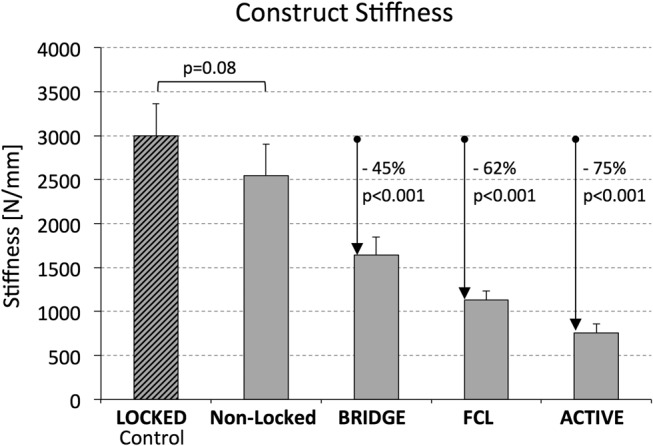
Construct stiffness achieved with the 4 strategies for plate dynamization, relative to the LOCKED control construct.
Axial Interfragmentary Motion
In each group, near cortex motion dA, NC was smaller than far cortex motion dA, FC (Fig. 4). Near cortex motion in response to one body weight loading (700 N) was the same for LOCKED control constructs and NONLOCKED constructs (0.10 ± 0.02 mm, P = 0.85) and remained below the 0.2-mm axial motion threshold required for callus stimulation. Compared with the LOCKED control group, dA, NC was 2 times greater in the BRIDGE group (P < 0.001), over 4 times greater in the FCL group (P < 0.001), and over 7 times greater in the ACTIVE group (P < 0.001). Similarly, far cortex motion was not significantly different between the LOCKED control group (0.37 ± 0.04 mm) and the NONLOCKED group (0.46 ± 0.08 mm) (P = 0.07). However, compared with the LOCKED control group, dA, FC was 73% greater in the BRIDGE group (P < 0.001), 105% greater in the FCL group (P < 0.001), and 303% greater in the ACTIVE group (P < 0.001).
FIGURE 4.
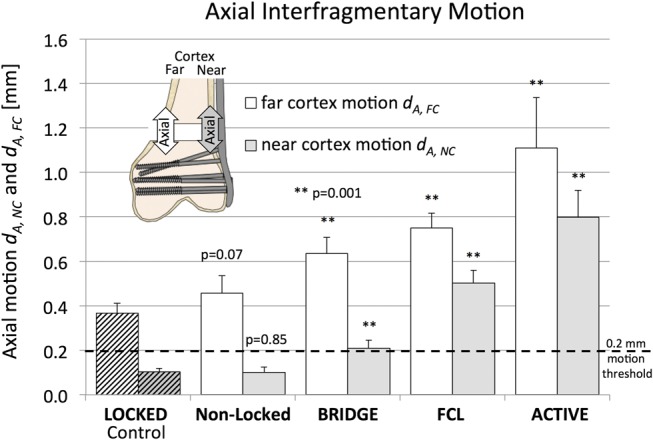
Axial motion at the near and far cortex, achieved with the 4 strategies for plate dynamization, relative to the LOCKED control construct. Editor's Note: A color image accompanies the online version of this article.
Shear Motion
Shear motion dS remained below 0.2 mm in all groups except in the BRIDGE group, which exhibited on average 0.96 ± 0.14 mm shear motion in response to one body weight loading (Fig. 5A). In BRIDGE constructs, this magnitude of shear motion was 50% greater than the corresponding far cortex motion and over 3 times greater than the near cortex motion. Using image analysis, shear-dominant motion in BRIDGE constructs was attributed to rotation of the femoral diaphysis around the proximal plate segment because of plate bending under axial loading, which caused the proximal osteotomy surface to be translated toward the locking plate (Fig. 5B).
FIGURE 5.
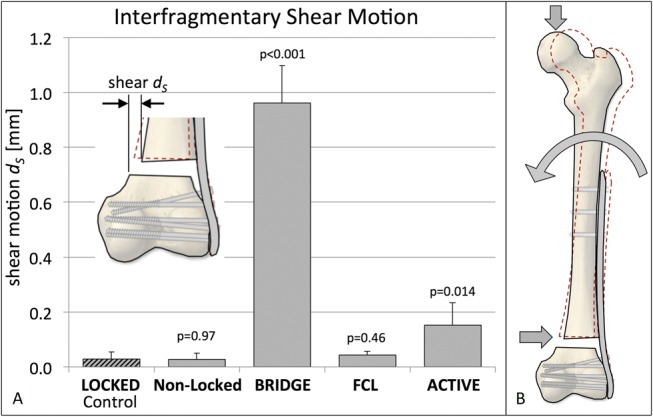
Resulting transverse shear, resulting from the 4 strategies for plate dynamization, relative to the LOCKED control construct. Editor's Note: A color image accompanies the online version of this article.
DISCUSSION
Results confirmed the study hypothesis by demonstrating that the 4 dynamization strategies yielded not only different amounts of construct stiffness and interfragmentary motion, but also different types of interfragmentary motion.
LOCKED group results confirmed that a locking plate with a short-bridge span results in asymmetric interfragmentary motion that is deficient for callus formation.3,13,16,26 Although one body weight loading may be excessive for early postoperative loading, it resulted only in 0.1-mm motion at the near cortex. This motion remained below the 0.2-mm motion threshold that has been established as the lower boundary for fracture motion required to promote callus formation.17 This result is supported by several in vivo and clinical studies that demonstrate suppression of callus formation and healing at the near cortex adjacent to a locking plate.3,7,9,20 The far cortex motion was greater than measured at the near cortex, likely secondary to plate bending. Clinically, the increased far cortex motion may allow callus to form, but the repetitive bending may also play a role in eventual fatigue failure of the plate before fracture healing occurs.
NONLOCKED constructs represent an intuitive response to the stiffness concern associated with locked plating by reverting to nonlocking screws in the diaphysis. However, substituting nonlocking diaphyseal fixation had no significant effect on construct stiffness or interfragmentary motion. This may be explained by the rigid compression of the plate onto the bone surface, which is required to retain stable fixation. Compressing the plate to the bone prevents any motion at the plate–bone interface, which is a prerequisite to induce symmetric interfragmentary motion.26 In contrast to locked plating constructs, the stiffness of a nonlocked construct will gradually decay as a result of dynamic loading.27 Although this can lead to increased fracture motion over time, the resulting uncontrolled motion is not a reliable strategy for dynamization. In addition, the natural fracture healing process responds with much more robust callus formation when exposed to early motion relative to delayed motion.28
BRIDGE constructs resembled the earliest and most widely proposed strategy to dynamize locked plating constructs. In a biomechanical study, Stoffel et al19 reported in 2003 that axial stiffness of locked plating constructs was mainly influenced by their bridge span. They recommended that one or 2 holes should be omitted on each side of the fracture to allow callus formation. They found that omitting 2 holes made the construct almost twice as flexible, but also 42% less strong. This study found a 45% stiffness reduction by omitting 2 screw holes. However, the greater flexibility of the longer bridge span increased motion primarily at the far cortex, whereas near cortex motion remained deficient. In addition, the longer bridge span induced up to 3 times more shear motion than axial motion. Although shear motion does not necessarily inhibit healing,29,30 several studies have shown that excessive or predominant shear motion will significantly delay healing.18,31 A recent study on the effect of bridge span on fracture motion also confirmed a disproportionate increase in shear motion.32 By analyzing 66 distal femur fractures stabilized with locking plates, they furthermore established a direct association between shear-dominated fracture motion and callus inhibition. Their findings, combined with the results of this study question the technique of increasing the bridge span to dynamize a locked construct, because this may weaken the construct and may cause asymmetric axial motion and excessive shear motion that inhibits fracture healing.
FCL and ACTIVE group constructs reduced stiffness compared with the LOCKED control group by 62%–75% to 1130 and 759 N/mm, respectively. Nevertheless, their stiffness remained substantially higher than the stiffness range of 50–400 N/mm reported for external fixators33,34 and Ilizarov frames.35 The fact that external fixators and Ilizarov frames are established clinical tools that promote fracture healing by callus formation suggests that the stiffness reduction of FCL and ACTIVE constructs is rather conservative and does not introduce excessive dynamization. FCL and ACTIVE constructs enhanced interfragmentary motion at the near and far cortex well above the 0.2-mm threshold needed to stimulate callus formation. The highest axial motion of 1.1 mm was observed at the far cortex of ACTIVE constructs, and remained at the lower limit of the 1–4 mm motion range reported for functional bracing.36 Most importantly, FCL and ACTIVE constructs delivered dynamization that was dominated by axial motion, not shear motion. These constructs allow a screw to be placed close to the fracture site without affecting stiffness, and therefore, limit the amount of shear motion possible. The controlled axial dynamization provided by FCL and ACTIVE constructs delivers faster and stronger healing. In an ovine fracture healing study, FCL constructs induced consistent and circumferential callus bridging and yielded 157% stronger healing compared with standard locked plating.7 Clinically, a prospective study of 31 consecutive distal femur fractures stabilized with FCL constructs reported no implant or fixation failure, an average time to union of 16 weeks and a nonunion rate of 3%.37 Similar to FCL constructs, ACTIVE plating induced 6 times more callus at 3 weeks postsurgery, and yielded 4 times stronger healing compared with rigid locked plating in an ovine fracture healing study.20 These in vivo and clinical studies of FCL and ACTIVE plating constructs demonstrated that controlled axial dynamization reliably promoted natural fracture healing.
Results of this study are limited by the use of femur surrogates. Validated surrogates were used to extract relative differences between constructs under highly reproducible test conditions.38 Because the surrogates represented a strong, nonosteoporotic femur, results may not be extrapolated to fracture fixation in the osteopenic femur. Moreover, this study only investigated construct stability in terms of stiffness and related interfragmentary motion, without loading constructs to failure to determine their strength. The strength of the tested constructs has been evaluated in previous studies, showing that increasing the bridge span will decrease construct strength,19 whereas the strength of FCL and active plating constructs is comparable with that of standard locked plating constructs.8,24 Testing was furthermore limited to static loading and did not investigate gradual loosening or fatigue of constructs under dynamic loading. Moreover, this study has been limited to a principal loading mode that combines axial compression and bending but not torsion. Only plates made of stainless steel were tested, which are approximately twice as stiff as geometrically equivalent plates made of Titanium alloy. Although results of this study raise concerns on the negative effect of shear-dominated interfragmentary motion on fracture healing, this concern should be formally investigated in a future in vivo study. Most importantly, emerging implant technologies that can provide controlled dynamization will require more clinical studies to document their effect on fracture healing, and to better define the range of interfragmentary motion that will promote healing of different fracture patterns at specific fracture locations.
In conclusion, results of this study indicate that intuitive technical tricks, such as reverting to nonlocking screws or using long plates to maximize the bridge span may not reliably achieve relative stability and adequate interfragmentary motion for promoting natural fracture healing. Conversely, engineered implant solutions in the form of FCL screws or active plates can reliably dynamize a locked plating construct to stimulate fracture healing. As such, results should encourage implant manufacturers to provide engineered solutions that reliably promote rather than potentially hinder fracture healing to avoid the need for and uncertainty of technical tricks intended to optimize construct stability.
Supplementary Material
Footnotes
Supported by the Research Foundation of the Legacy Health System. Implants have been provided by Zimmer Biomet. M. Bottlang, D. C. Fitzpatrick, and S. M. Madey receive royalties from Synthes and Zimmer Biomet and serve on the Zimmer speaker bureau.
M. Bottlang, D. C. Fitzpatrick, and S. M. Madey hold patents related to dynamization of osteosynthesis constructs, receive royalties from Synthes and Zimmer Biomet and serve on the Zimmer speaker bureau. The remaining authors report no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jorthotrauma.com).
REFERENCES
- 1.Henderson CE, Lujan TJ, Kuhl LL, et al. 2010 mid-America Orthopaedic Association Physician in Training Award: healing complications are common after locked plating for distal femur fractures. Clin Orthop Relat Res. 2011;469:1757–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann MF, Jones CB, Sietsema DL, et al. Outcome of periprosthetic distal femoral fractures following knee arthroplasty. Injury. 2012;43:1084–1089. [DOI] [PubMed] [Google Scholar]
- 3.Lujan TJ, Henderson CE, Madey SM, et al. Locked plating of distal femur fractures leads to inconsistent and asymmetric callus formation. J Orthop Trauma. 2010;24:156–162. [DOI] [PubMed] [Google Scholar]
- 4.Vallier HA, Immler W. Comparison of the 95-degree angled blade plate and the locking condylar plate for the treatment of distal femoral fractures. J Orthop Trauma. 2012;26:327–332. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez EK, Boulton C, Weaver MJ, et al. Predictive factors of distal femoral fracture nonunion after lateral locked plating: a retrospective multicenter case-control study of 283 fractures. Injury. 2014;45:554–559. [DOI] [PubMed] [Google Scholar]
- 6.Ricci WM, Streubel PN, Morshed S, et al. Risk factors for failure of locked plate fixation of distal femur fractures: an analysis of 335 cases. J Orthop Trauma. 2014;28:83–89. [DOI] [PubMed] [Google Scholar]
- 7.Bottlang M, Lesser M, Koerber J, et al. Far cortical locking can improve healing of fractures stabilized with locking plates. J Bone Joint Surg Am. 2010;92:1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottlang M, Doornink J, Fitzpatrick DC, et al. Far cortical locking can reduce stiffness of locked plating constructs while retaining construct strength. J Bone Joint Surg Am. 2009;91:1985–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roderer G, Gebhard F, Duerselen L, et al. Delayed bone healing following high tibial osteotomy related to increased implant stiffness in locked plating. Injury. 2014;45:1648–1652. [DOI] [PubMed] [Google Scholar]
- 10.Bottlang M, Tsai S, Bliven EK, et al. Dynamic stabilization of simple fractures with active plates delivers stronger healing than conventional compression plating. J Orthop Trauma. 2017;31:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foux A, Yeadon AJ, Uhthoff HK. Improved fracture healing with less rigid plates. A biomechanical study in dogs. Clin Orthop Relat Res. 1997:232–245. [DOI] [PubMed] [Google Scholar]
- 12.Panagiotopoulos E, Fortis AP, Lambiris E, et al. Rigid or sliding plate. A mechanical evaluation of osteotomy fixation in sheep. Clin Orthop Relat Res. 1999:244–249. [PubMed] [Google Scholar]
- 13.Uhthoff HK, Poitras P, Backman DS. Internal plate fixation of fractures: short history and recent developments. J Orthop Sci. 2006;11:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woo SL, Lothringer KS, Akeson WH, et al. Less rigid internal fixation plates: historical perspectives and new concepts. J Orthop Res. 1984;1:431–449. [DOI] [PubMed] [Google Scholar]
- 15.Goodship AE, Kenwright J. The influence of induced micromovement upon the healing of experimental tibial fractures. J Bone Joint Surg Br. 1985;67:650–655. [DOI] [PubMed] [Google Scholar]
- 16.Claes L. Biomechanical principles and mechanobiologic aspects of flexible and locked plating. J Orthop Trauma. 2011;25(suppl 1):S4–S7. [DOI] [PubMed] [Google Scholar]
- 17.Claes LE, Heigele CA, Neidlinger-Wilke C, et al. Effects of mechanical factors on the fracture healing process. Clin Orthop Relat Res. 1998;(355 suppl):S132–S147. [DOI] [PubMed] [Google Scholar]
- 18.Augat P, Burger J, Schorlemmer S, et al. Shear movement at the fracture site delays healing in a diaphyseal fracture model. J Orthop Res. 2003;21:1011–1017. [DOI] [PubMed] [Google Scholar]
- 19.Stoffel K, Dieter U, Stachowiak G, et al. Biomechanical testing of the LCP–how can stability in locked internal fixators be controlled? Injury. 2003;34(suppl 2):B11–B19. [DOI] [PubMed] [Google Scholar]
- 20.Bottlang M, Tsai S, Bliven EK, et al. Dynamic stabilization with active locking plates delivers faster, stronger, and more symmetric fracture-healing. J Bone Joint Surg Am. 2016;98:466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doornink J, Fitzpatrick DC, Madey SM, et al. Far cortical locking enables flexible fixation with periarticular locking plates. J Orthop Trauma. 2011;25(suppl 1):S29–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khalafi A, Curtiss S, Hazelwood S, et al. The effect of plate rotation on the stiffness of femoral LISS: a mechanical study. J Orthop Trauma. 2006;20:542–546. [DOI] [PubMed] [Google Scholar]
- 23.Stoffel K, Booth G, Rohrl SM, et al. A comparison of conventional versus locking plates in intraarticular calcaneus fractures: a biomechanical study in human cadavers. Clin Biomech (Bristol, Avon). 2007;22:100–105. [DOI] [PubMed] [Google Scholar]
- 24.Tsai S, Fitzpatrick DC, Madey SM, et al. Dynamic locking plates provide symmetric axial dynamization to stimulate fracture healing. J Orthop Res. 2015;33:1218–1225. [DOI] [PubMed] [Google Scholar]
- 25.Zlowodzki M, Williamson S, Cole PA, et al. Biomechanical evaluation of the less invasive stabilization system, angled blade plate, and retrograde intramedullary nail for the internal fixation of distal femur fractures. J Orthop Trauma. 2004;18:494–502. [DOI] [PubMed] [Google Scholar]
- 26.Bottlang M, Schemitsch CE, Nauth A, et al. Biomechanical concepts for fracture fixation. J Orthop Trauma. 2015;29(suppl 12):S28–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardner MJ, Brophy RH, Campbell D, et al. The mechanical behavior of locking compression plates compared with dynamic compression plates in a cadaver radius model. J Orthop Trauma. 2005;19:597–603. [DOI] [PubMed] [Google Scholar]
- 28.Kenwright J, Richardson JB, Goodship AE, et al. Effect of controlled axial micromovement on healing of tibial fractures. Lancet. 1986;2:1185–1187. [DOI] [PubMed] [Google Scholar]
- 29.Bishop NE, van Rhijn M, Tami I, et al. Shear does not necessarily inhibit bone healing. Clin Orthop Relat Res. 2006;443:307–314. [DOI] [PubMed] [Google Scholar]
- 30.Watson JT, Sauer P. The Effects of Fracture Healing Under Cyclic Shear Micromotion. 61st AAOS Annual Meeting; February 22, 1994; New Orleans, LA.
- 31.Duda GN, Sollmann M, Sporrer S, et al. Interfragmentary motion in tibial osteotomies stabilized with ring fixators. Clin Orthop Relat Res. 2002:163–172. [DOI] [PubMed] [Google Scholar]
- 32.Elkins J, Marsh JL, Lujan T, et al. Motion predicts clinical callus formation: construct-specific finite element analysis of supracondylar femoral fractures. J Bone Joint Surg Am. 2016;98:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finlay JB, Moroz TK, Rorabeck CH, et al. Stability of ten configurations of the Hoffmann external-fixation frame. J Bone Joint Surg Am. 1987;69:734–744. [PubMed] [Google Scholar]
- 34.Moroz TK, Finlay JB, Rorabeck CH, et al. External skeletal fixation: choosing a system based on biomechanical stability. J Orthop Trauma. 1988;2:284–296. [PubMed] [Google Scholar]
- 35.Caja V, Kim W, Larsson S, et al. Comparison of the mechanical performance of three types of external fixators: linear, circular and hybrid. Clin Biomech (Bristol, Avon). 1995;10:401–406. [DOI] [PubMed] [Google Scholar]
- 36.Sarmiento A, McKellop HA, Llinas A, et al. Effect of loading and fracture motions on diaphyseal tibial fractures. J Orthop Res. 1996;14:80–84. [DOI] [PubMed] [Google Scholar]
- 37.Bottlang M, Fitzpatrick DC, Sheerin D, et al. Dynamic fixation of distal femur fractures using far cortical locking screws: a prospective observational study. J Orthop Trauma. 2014;28:181–188. [DOI] [PubMed] [Google Scholar]
- 38.Chong AC, Miller F, Buxton M, et al. Fracture toughness and fatigue crack propagation rate of short fiber reinforced epoxy composites for analogue cortical bone. J Biomech Eng. 2007;129:487–493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.