Significance
Complex life cycle evolution promotes phenotypic discontinuities across ontogeny, but adaptations for one stage may compromise evolution to another stage. Ancestral salamanders likely had a complex aquatic-to-terrestrial life cycle and a basic tetrapod body form. We show that permanent simplifications to aquatic-only or terrestrial-only life cycles have resulted in accelerated rates of salamander body form evolution. However, rate increases have been dramatically higher after loss of the terrestrial stage than after loss of the aquatic stage, suggesting that constraints can be stage-specific. This study provides an example of how long-term shifts in life cycle complexity can alter rates of evolution and play a significant role in shaping phenotypic distributions.
Keywords: Amphibia, direct development, paedomorphosis, metamorphosis, traits
Abstract
Metazoans display a tremendous diversity of developmental patterns, including complex life cycles composed of morphologically disparate stages. In this regard, the evolution of life cycle complexity promotes phenotypic diversity. However, correlations between life cycle stages can constrain the evolution of some structures and functions. Despite the potential macroevolutionary consequences, few studies have tested the impacts of life cycle evolution on broad-scale patterns of trait diversification. Here we show that larval and adult salamanders with a simple, aquatic-only (paedomorphic) life cycle had an increased rate of vertebral column and body form diversification compared to lineages with a complex, aquatic-terrestrial (biphasic) life cycle. These differences in life cycle complexity explain the variations in vertebral number and adult body form better than larval ecology. In addition, we found that lineages with a simple terrestrial-only (direct developing) life cycle also had a higher rate of adult body form evolution than biphasic lineages, but still 10-fold lower than aquatic-only lineages. Our analyses demonstrate that prominent shifts in phenotypic evolution can follow long-term transitions in life cycle complexity, which may reflect underlying stage-dependent constraints.
Complex life cycles allow organisms to exploit multiple environments across ontogeny (1, 2). Studies of complex life cycle evolution have largely focused on how metamorphic remodeling can “adaptively decouple” traits expressed at different stages (2). In essence, metamorphosis should minimize the negative consequences of stage-specific adaptations and facilitate the evolution of contrasting lifestyles within a single ontogeny (2–5). However, even when an organism endures a dramatic metamorphosis, some tissues may remain relatively unchanged or bear strong correlations among stages (3–5). This can present character conflicts that limit evolution by requiring that traits be simultaneously adapted to different ecological scenarios (3–12). In general, the loss of intraspecific complexity is predicted to promote trait diversification through “character release” (13). Despite the projected macroevolutionary consequences of maintaining intraspecific complexity, most quantitative analyses have been conducted within species (e.g., refs. 11 and 14).
The diverse developmental and reproductive modes of amphibians offer compelling systems for testing how ontogeny impacts phenotypic evolution (4, 15–17). Most amphibians exhibit a two-part (biphasic; bi) complex life cycle, with an aquatic larval stage followed by metamorphosis into a more terrestrial adult (4, 18). However, many lineages have deviated from this pattern to produce a wide range of developmental modes. Larval form paedomorphosis (pd) is a simplified life cycle that involves the retention of the ancestral aquatic larval morphology and ecology into adulthood (18), and has evolved many times in salamanders (19–21). Conversely, most species in the highly diverse family Plethodontidae fully transform in ovum (direct development; dd) and have an entirely terrestrial life cycle (4, 16, 22). Developmental diversity has been frequently implicated in shaping patterns of amphibian evolution (4, 15–17, 20, 23–25), but explicit phylogenetic tests of how life cycle complexity influences trait evolution have not been performed.
Here we used phylogenetic-based shape and continuous trait diversification (i.e., disparification; ref. 26) analyses to test whether salamander life cycle simplification influences rates of larval and adult body form evolution. If the maintenance of a complex life cycle constrains body form, then reducing life cycle complexity should increase the evolutionary rate of constituent traits. We tested these patterns against alternative models based on larval and adult ecologies. This study highlights the potential constraints of complex life cycles on the evolution of prominent morphological traits, and shows how responses to selection after simplification can be trait- and stage-dependent.
Results
Ancestral State Reconstructions.
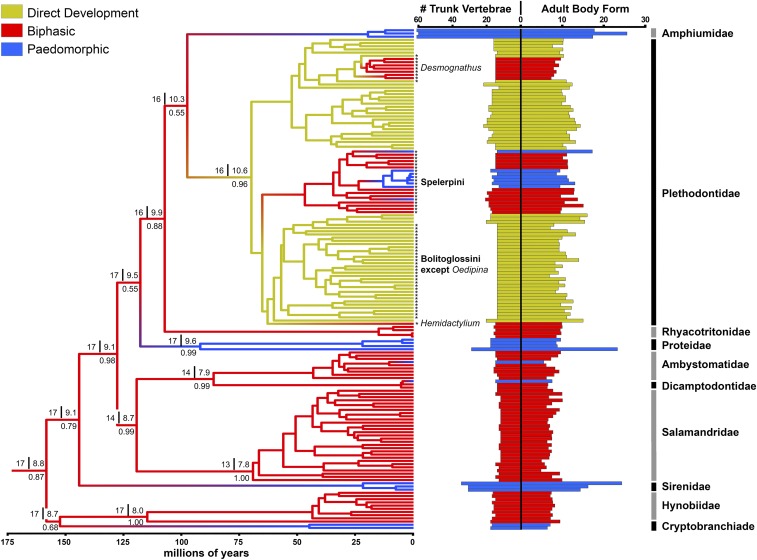
Bayesian ancestral state reconstruction (27) supports biphasic ancestors for basal salamander nodes, including the common ancestor of extant species (Fig. 1 and SI Appendix, Table S1). This indicates independent transitions to obligate paedomorphosis in cryptobranchids, sirenids, proteids,and amphiumids, as well as many recent transitions (Fig. 1). Our reconstruction also further supports a direct-developing ancestor for the Plethodontidae (21). This family appears to have re-evolved free-living aquatic larvae in Desmognathus, Hemidactylium, and the tribe Spelerpini (21, 28, 29), with several transitions to paedomorphosis in the latter clade (20, 21) (Fig. 1).
Fig. 1.
Bayesian ancestral state reconstructions of salamander life cycles, numbers of trunk vertebrae, and adult body forms (SI Appendix, Table S1, with comments on Mesozoic fossils). Branch colors indicate the most likely life cycle mode (highest proportional probabilities below branches). Above the branches are median numbers of trunk vertebrae (left of line) and adult body forms (right of line). Only taxa with both data types present (n = 157) are shown, but analyses were based on maximum data sets for each trait (SI Appendix, Table S9). Asterisks highlight select plethodontids referenced in the text.
We analyzed the evolution of two body form metrics: (i) “trunk vertebral number” (between the atlas and the sacrum; refs. 23 and 30), which is established during embryonic development and persistent across ontogeny (30, 31); and (ii) “adult body form” (adult body length divided by body width), which approximates the degree of elongation. Our Bayesian stable trait reconstructions (32) estimate that Mesozoic ancestors of modern salamander lineages had 13–17 trunk vertebrae (Fig. 1). This is consistent with Late Jurassic salamander fossils assigned to crown families (33–35), and demonstrates at least three major expansions in trunk vertebral numbers in paedomorphic lineages (amphiumids, sirenids, and the proteid genus Proteus). Ancestral salamanders likely had relatively stout adult body forms, similar to the estimated trait optimum for a biphasic life cycle (length-to-width ratio ∼8–10). Elongation was also independently derived multiple times in paedomorphic and direct-developing lineages (Fig. 1).
Increased Rates of Body Form Evolution in Paedomorphs.
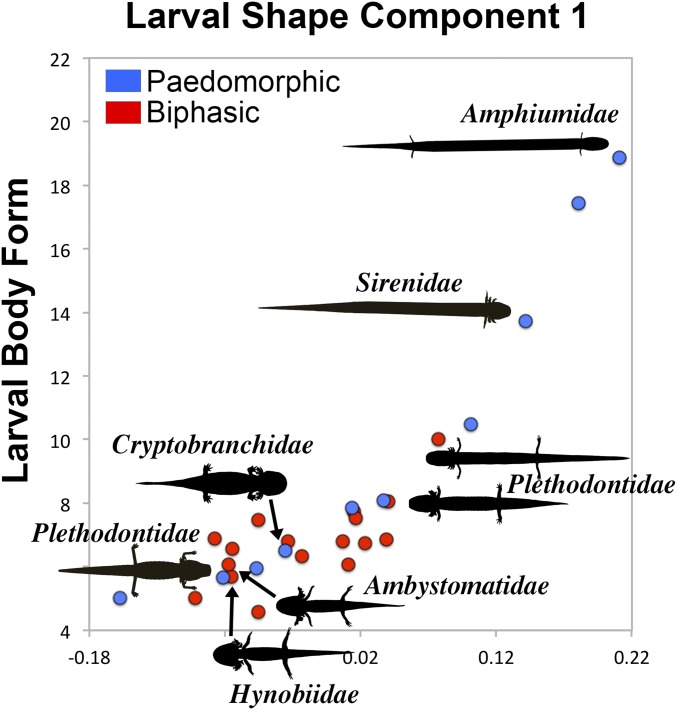
Body shapes of larval salamanders (n = 27 taxa from all 10 families) were analyzed using geometric morphometrics. Principal component 1 (PC1) explains 92% of the variation in larval body shape (Fig. 2) and is highly correlated with larval body length-to-body width ratio (“larval body form”; R2 = 0.707; P < 0.001), indicating that body elongation is a primary axis of shape variation in larval salamanders. Phylogenetic-based geometric morphometric analyses (36, 37) show that larvae of paedomorphic lineages have a >3.5-fold higher rate of body shape evolution than larvae of biphasic lineages (Sigma D ratio = 3.54; SI Appendix, Fig. S1). In comparison, lineages with lentic (pond-dwelling) larvae have a twofold higher rate of body shape evolution compared with lotic (stream-dwelling) lineages (Sigma D ratio = 1.99). In both cases, the differences in rate are significant compared with random groups (P < 0.0001), but life cycle has a higher rate ratio than larval ecology.
Fig. 2.
Larval salamander body shape variation. Plot of larval shape PC1 vs. larval body form. Silhouettes represent four morphologically disparate larvae of paedomorphs (left of points) and metamorphs (right of points).
Based on species with free-living larval forms, we compared the fit of variation in trunk vertebral number (n = 143 taxa) and adult body form (n = 113 taxa) across a sample of salamander phylogenies using Brownian motion (BM) and Ornstein–Uhlenbeck (OU) models (38). We tested whether the rates of evolution (σ2) and optima (θ) for body form metrics differ between life cycle modes (paedomorphic vs. biphasic) or larval ecologies (lentic vs. lotic). The best-fitting models for trunk vertebral number and adult body form allowed for different optima and rates of trait evolution between biphasic and paedomorphic lineages (Life Cycle BMθσ2 and OUθσ2; SI Appendix, Tables S2 and S3). For both metrics, the best-fit single rate models were substantially worse [delta Akaike Information Criterion (∆AIC) >130 and 80, respectively; AIC weights (wi) < 0.0001]. Trunk vertebral number is estimated to evolve at a ∼50-fold higher rate in paedomorphs than in biphasics. Paedomorphs also have a greater optimum number of trunk vertebrae (θ: bi, 15.7 ± 0.04; pd, 40.5 ± 0.55). Likewise, paedomorphs have a >18-fold higher rate of adult body form evolution compared with biphasics, and their optimal body forms are almost twice as elongate (θ: bi, 8.3 ± 0.01; pd, 15.6 ± 0.09). In stark contrast, models fit to larval ecology are substantially worse than those fit to life cycle (∆AIC >116 for trunk vertebral number and >83 for adult body form; wi < 0.0001).
Some salamanders can exhibit “facultative paedomorphosis” (39), and maintain the ability to metamorphose in nature. Throughout this study only obligate paedomorphs were coded as paedomorphic to evaluate the long-term consequences of life cycle simplification. Nevertheless, when we treat all species that can exhibit paedomorphosis (facultative and obligate) as a group, life cycle differences still best explain variation in trunk vertebral number and adult body form. However, the fit of the “facultative model” is significantly worse than when obligate paedomorphs are coded separately. This indicates that the vertebral columns and adult body forms of facultative paedomorphs evolve more similar to biphasics than to obligate paedomorphs (∆AIC >64 and 32, respectively). Our results are also robust whether ancestral salamanders were paedomorphic (40) or biphasic (SI Appendix, Tables S1–S3). Taken together, our analyses of vertebral columns and body forms of salamanders with aquatic larvae demonstrate that major disparities in rates are more likely a consequence of permanent shifts in life cycle than larval ecology.
Differential Impacts of Simplification on Body Form Evolution.
We further compared the fit of adult body form (n = 199 taxa) and trunk vertebral numbers (n = 265 taxa) with BM and OU models with that of phylogenies that also included direct developing species (one-part, terrestrial-only life cycle). We differentially coded direct developers to compare three different models: (i) Adult Ecology: terrestrial adults (direct developers and biphasics) compared with aquatic adults (paedomorphs), to test whether adult ecology influences rates of body form evolution; (ii) Two Life Cycles: one-part life cycle (direct developers and paedomorphs) compared with two-part life cycle (biphasics) to test whether life cycle simplification, regardless of the stage deleted, produced similar patterns of body form diversification; and (iii) Three Life Cycles: direct developers, biphasics, and paedomorphs, coded as three separate groups to evaluate whether each life cycle mode has a unique signature of trait diversification.
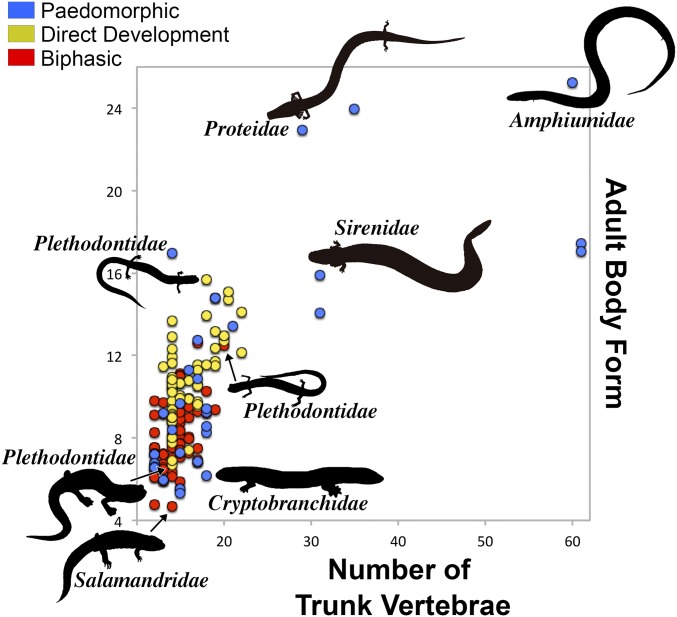
The Three Life Cycles OU model, which allows different optima and rates for paedomorphs, biphasics, and direct developers, was a substantially better fit for adult body form compared with the other models (∆AIC >6; wi > 0.95; Fig. 3 and SI Appendix, Table S4). Consistent with the analyses without direct developers, paedomorphs showed an ∼18-fold increase in the rate of adult body form evolution compared with biphasics. Rates of evolution for direct developers were also significantly higher than for biphasics, albeit to a lesser degree (∼1.6-fold). Likewise, the adult body form optimum for direct developers was higher than that for biphasics, but significantly lower than that for paedomorphs (θ: bi, 8.2 ± 0.02; dd, 10.6 ± 0.04; pd, 15.4 ± 0.08) (Fig. 3). For trunk vertebral data, BMθσ2 and OUθσ2 models for Adult Ecology and Three Life Cycles were fit somewhat equally, but substantially better than all models based on Two Life Cycles (∆AIC >130; SI Appendix, Table S5). In other words, trunk vertebral evolution is statistically similar among lineages with terrestrial adults, and >40-fold slower than in lineages with aquatic adults. The lack of difference in trunk vertebral number between direct developers and biphasics can be in part explained by neotropical plethodontids (24, 41), which can shift body shape independently of trunk vertebral number (discussed below). In summary, obligate paedomorphs show the most dramatic increase in optima and rates of trunk vertebral number and adult body form evolution compared with all other salamanders. Direct developers have significantly higher optima and rates of adult body form evolution compared with biphasics, but these parameters are still substantially lower than those of paedomorphs.
Fig. 3.
Adult salamander body shape and trunk vertebral number variation. Plot of adult body form vs. number of trunk vertebrae. Silhouettes represent morphologically disparate representatives of each life cycle mode.
In addition to a terrestrial adult ecology, direct developers and biphasics share the development of terrestrial morphology, which could entail developmental constraints (4, 15, 16). Two obligately paedomorphic families (Amphiumidae and Cryptobranchidae) exhibit partial metamorphosis, meaning that some structures transform (e.g., external gills), but others do not (e.g., internal gills). Body forms of partially metamorphic lineages are better fit with aquatic nontransforming lineages than with terrestrial transforming lineages (∆AIC > 38; SI Appendix, Table S4). This suggests that body form limitations of lineages with terrestrial adults and complete transformation do not extend to obligately aquatic lineages that only partially transform.
Body Form Evolution Takes Path of Least Resistance.
Neotropical plethodontids (tribe Bolitoglossini) include >300 species, all of which are direct developers. Remarkably, almost all species (except the genus Oedipina) have 14 trunk vertebrae (23, 24, 41) (Fig. 1). Using BM and OU models, we compared the fit of trunk vertebral number and “adult trunk form” (body length minus head length divided by body width) evolution between bolitoglossines (except Oedipina) and other direct developers (n = 62 taxa). Adult trunk form was used to capture the shape of the body that largely overlaps with trunk vertebrae. Bivariate evolutionary rate matrix analyses (under BM) show substantial differences in the rate and correlation of these two traits between the groups (∆AIC >37; wi > 0.99) (SI Appendix, Table S6). As expected, we found an extraordinarily low rate of evolution in the number of trunk vertebrate in bolitoglossines (minus Oedipina) compared with other direct developers. However, the rates of adult trunk form evolution are equivalent in the two groups, and there is only minimal support for differences in optima (SI Appendix, Tables S6–S8). Despite the seemingly high constraint on 14 vertebrae, the rate of trunk form evolution has not been significantly inhibited in bolitoglossines compared with other direct developers. This demonstrates how body form evolution can find alternative pathways to bypass apparently strong developmental constraints (24, 41–43).
Discussion
Life Cycle Constraints on Patterns of Trait Evolution.
Constraints shape phenotypic distributions by limiting trait variation (4, 24, 44–48). Therefore, the removal of constraints should provide opportunities for increased trait diversification, and may even permit evolution to novel reaches of phenotypic space (44, 47). Selection pressures can vary across ontogeny and present conflicts to adaptation (6–12). Such patterns are accentuated in organisms with complex life cycles, and while metamorphosis between stages can minimize conflicts (2), increased ontogenetic complexity should constrain evolutionary responses (12, 47). We analyzed trait evolution in a clade that exhibits extensive life cycle variation to test whether character conflicts inherent to complex life cycles limit phenotypic evolution over deep times scales. We found that multistage life cycles are associated with reduced evolution of salamander body forms. Ancestral salamanders likely had a biphasic life cycle (Fig. 1), and this strategy has resulted in relatively limited larval body shape, trunk vertebral number, and adult body form evolution over the last ∼160 My. In comparison, the numerous transitions to obligate paedomorphosis are associated with dramatic increases in evolutionary rates for these traits (Fig. 2 and SI Appendix, Tables S2–S5). Lineages with direct-developing life cycles also have elevated rates of adult body form evolution compared with biphasic lineages, but still substantially lower than those of paedomorphs (Fig. 3 and SI Appendix, Table S4). If the reduced body form variance in biphasic lineages is a product of constraint, then our results indicate that the impact of simplification on trait diversification is dependent on which stage is deleted from the life cycle.
Each life cycle stage potentially enforces a unique set of constraints that could limit variation at other stages (6–12). Therefore, addition or subtraction of stages could simultaneously alter the stringency of constraints on many traits and result in major shifts in phenotypic evolution. Obligate paedomorphosis entails deletion of the terrestrial stage and has produced the most dramatic effect on vertebral column and adult body form evolution (Fig. 3 and SI Appendix, Tables S4 and S5). In contrast, the loss of an aquatic larval stage through direct development has resulted in a relatively modest or no increase in rate for the same traits. However, it is important to note that the influences of constraints are likely stage- and trait-dependent. For example, in biphasic species, the larval hyobranchial apparatus is remodeled at metamorphosis, from a gape-and-suction feeding structure to a protrusible tongue skeleton (18, 49). In contrast, direct-developing species no longer require a functional larval throat skeleton and have highly derived tongues, attributed in part to loss of the aquatic larval stage (24, 49–51).
Life Cycle Shifts and the Evolution of Tetrapod Body Forms.
The anterior-to-posterior axis is a primary source of shape disparity across many vertebrate clades (30, 41, 52–56). Body elongation of tetrapods is largely a product of patterns of somitogenesis and specification of the sacrum during embryogenesis (30, 31). Therefore, trunk vertebral numbers and general axial shape are persistent across the ontogenies of most tetrapods, and are central to the functional morphology of locomotion on diverse substrates (52, 55). For example, trunk elongation of tetrapods often coincides with fossoriality (30, 41) or recolonization of aquatic environments (57). Species that undergo major ontogenetic transitions in habitat without a concomitant change in form (metamorphosis) may be constrained to a body shape that is sufficient for both environments. Biphasic salamanders require a general trunk shape that is effective in water as well as on land. Our reconstructions and several Late Jurassic species show that ancestral salamanders had relatively short trunks and trunk vertebral numbers similar to modern biphasic species (33–35) (Fig. 1). Since then, even some highly divergent biphasic species have remained similar in larval and adult shape (e.g., Ambystomatidae and Hynobiidae; divergence ∼160 Mya; Figs. 1 and 2). Over a shorter period, the independent shifts to an aquatic-only life cycle via paedomorphosis have given rise to salamanders with the most elongate body forms (e.g., Amphiumidae, Sirenidae, Proteus), and up to four times as many trunk vertebrae. Two extinct paedomorphic families (Batrachosauroididae and Scapherpetontidae) were also likely elongate (58, 59), further supporting an association between permanently aquatic life cycles and eel-like body forms.
Most actinopterygian eels use anguilliform locomotion, propelled by lateral undulation of the trunk and tail (60). Salamanders swim using the same regions of the axial skeleton (57), which may have provided a fitness advantage to eel-like body forms in several permanently aquatic salamanders. However, the divergent body forms of paedomorphs are a product of increasing both optimum and variance (rate). In fact, obligate paedomorphs have evolved a wide range of highly derived body forms. These include deep aquifer-dwelling paedomorphs (e.g., Eurycea rathbuni) that have lost trunk vertebrae and river-dwelling paedomorphs with dorsoventrally flattened bodies (e.g., cryptobranchids). Our analyses show that extensive salamander body form diversification follows simplification to a completely aquatic life cycle, perhaps facilitated by the removal of functional constraints of terrestriality.
Simplification to a terrestrial-only life cycle through direct development in the Plethodontidae has significantly increased rates and optima of adult body form evolution compared with biphasic lineages (Fig. 3 and SI Appendix, Table S4). Direct-developing plethodontids include species with terrestrial, arboreal, and fossorial ecologies (16, 30, 61, 62), with the greatest departures in body form by fossorial, “worm-like” taxa, such as Plethodon, Batrachoseps, Oedipina, and some Pseudoeurycea (23, 30, 41). There is generally a positive relationship between body elongation and numbers of vertebrae. Notable exceptions are the neotropical plethodontid salamanders (except Oedipina), which have remarkably conserved numbers of trunk vertebrae (14 for nearly all species; refs. 23 and 41). However, despite the seemingly canalized vertebral numbers, their rate of body form evolution has kept pace with that of other direct developers (SI Appendix, Tables S6–S8). These include species that evolved worm-like bodies without adding vertebrae (24, 41), similar to the elongation of cervical vertebrae in giraffes (63). Thus, the evolution of trunk shape is relatively independent of vertebral numbers in some clades.
Species can either change the length of individual vertebrae or patterns of postembryonic somatic growth (41–43), which provides multiple developmental pathways to convergent body forms. Neotropical plethodontids also demonstrate how functional evolution can circumvent constraints by following a path of least resistance (64). Ecologically, the evolution of direct development provided opportunities for salamanders to colonize diverse terrestrial habitats (16, 30, 41, 61, 62). Functionally, the loss of a free-living larval stage may have facilitated diversification by eliminating the compromise between optimal aquatic and terrestrial body forms.
Direct-developing and biphasic lineages share the development of a terrestrial morphology in addition to terrestriality itself. The process of metamorphosis (4, 15, 16) and the integration of typically postmetamorphic structures (9, 47) likely impart significant developmental constraints, but seem less likely to influence the body form parameters analyzed here. This is because (i) axial proportions of salamanders are primarily a product of early development, which occurs from months to years before metamorphosis; (ii) divergent body shapes, such as elongation, occur in lineages that metamorphose the fastest (direct developers) and slowest (paedomorphs); and (iii) families that partially metamorphose but are completely aquatic (e.g., amphiumids, cryptobranchids) also exhibit highly derived body forms (SI Appendix, Table S4). Traits directly involved in (or transformed during) metamorphosis are more likely to be constrained by this process (3, 4). The stage-dependent and differential effects of life cycle shifts and metamorphosis on trait evolution is a fertile area for future research at multiple levels of organization. Phylogenetic-based analyses of trait evolution can be a powerful tool for evaluating the role of life cycle evolution on phenotypic diversification.
Short- and Long-Term Life Cycle Transitions on Body Form Evolution.
Climatic variables (temperature and moisture) are correlated with vertebral counts in some salamanders (30, 65, 66), and temperature-dependent plasticity in axial variation is also well documented (30). However, developmental and evolutionary responses can be strikingly different among clades (reviewed in ref. 65). It has been suggested that shifts in the number of trunk vertebrae may reflect the proportion of life spent in the water vs. on land (or underground; refs. 30 and 65). Since both climatic patterns and the timing of life history events can be quite labile (39), it is likely that salamander body forms have been frequently reoptimized to match their contemporary conditions.
Our estimates of “selection strength” (α) on trunk vertebrae and adult body form were low. This suggests that recent patterns of selection (where α is best estimated; ref. 38) have had comparatively less impact on overall patterns of salamander body form diversification. The overarching patterns are best explained by increases in optima (θ) and rates (σ2) coinciding with long-term life cycle simplifications. Our findings are consistent with other macroevolutionary analyses that have demonstrated long-term stasis despite frequent phenotypic fluctuations over short time scales (67, 68). More pronounced and persistent changes in trait disparity are less frequent, and likely result from permanent shifts in adaptive zones (69–71). The body forms of biphasic salamanders (including those that exhibit facultative paedomorphosis) have not deviated significantly from ancestral patterns since the Mesozoic. Long-term life cycle simplifications correspond with distinct shifts to completely aquatic or completely terrestrial adaptive zones (23, 69), and have collectively produced a wide diversity of salamanders with disparate morphologies. This shows how major ontogenetic shifts, such as the deletion of a life cycle stage, may allow for less encumbered divergence in trait distributions and lead to a tremendous diversification of forms.
Materials and Methods
Phylogeny and Ancestral State Reconstructions.
We used BEAST 2.4 (72) to reconstruct a time-calibrated salamander phylogeny with 516 taxa based on three mitochondrial (Co1, Cytb, and ND2) and four nuclear (BDNF, Pomc, RAG1, and Slc8a3) protein-coding genes (totaling 7,401 character states; average of 3.3 genes and 3,047 bp per taxon; SI Appendix, Fig. S2 and Table S9). We used Partitionfinder 2.1 (73) to identify optimal substitution and site heterogeneity models. An uncorrelated lognormal molecular clock with node calibrations based on Shen et al. (74) and a Yule speciation prior were applied to the reconstruction (SI Appendix, Tables S10 and S11). Three separate analyses were run for a total of 30 million generations, and 1,000 chronograms were sampled from 20 million generations after stationarity of likelihood values (i.e., 1,000 post–burn-in trees). These chronograms (available on Dryad) are highly congruent with other recent phylogenies based in part on the same sequences.
Ancestral life cycle modes were reconstructed using a “Multistate” model and Markov chain Monte Carlo (MCMC) analysis in BayesTraits v. 2.0 (27). Life cycle was treated as an ordered, categorical trait with three states: direct development, biphasic, and paedomorphic. The life cycles and ecologies of most species are well established (SI Appendix, Table S9). Direct transitions were restricted to biphasic and direct development or biphasic and paedomorphic, but not between direct development and paedomorphic (transitions set to zero probability). The analysis was run for 4 million post–burn-in generations sampled at random across our 1,000 chronograms.
We compiled mode numbers of trunk vertebrae (between the atlas and the sacrum) from the literature and our own counts (SI Appendix, Table S9). The metrics for adult body form (snout-to vent length divided by body width) and adult trunk form (snout-to vent length minus head length divided by body width) were calculated from the measurements reported by Wiens and Hoverman (25). The purpose of using ratios was to capture relative elongation and remove size. We acknowledge that convergent patterns can be produced by changing length or width, in addition to the other limitations of analyzing ratios (75). Continuous trait analyses of trunk vertebral number and adult body form were performed under the Bayesian stable trait model in StableTraits v. 1.5 (32) and run for 1 million generations on a consensus of our Bayesian chronograms. The stable trait model allows for large changes in trait values among lineages, which we would anticipate given the strong differences in rates and optima among life cycle modes.
Rates of Larval Shape Evolution.
We used geometric morphometrics to test for differences in rates of larval shape evolution. These analyses were based on larvae from 27 species representing all 10 families of salamanders (SI Appendix, Table S9). All larvae were sampled from a point in ontogeny after four limbs were developed (except sirenids), but before the initiation of metamorphosis. Three to five specimens per taxon were imaged, and seven homologous landmarks were placed using tpsDig 2.16. Imaging and landmarking details are provided in SI Appendix, Fig. S3. Superimposition of homologous landmarks (76) and calculation of principal components were performed with the R (77) package geomorph v 3.0 (36). Mean body shapes were calculated for each taxon, and independent contrasts (78) in ape (79) were used to evaluate whether larval body shape PCs were correlated with univariate continuous metrics (e.g., larval body length-to-body width ratio). The function “compare.evol.rates” (37) in geomorph was used to test whether lineages with different life cycles or larval ecologies have different rates of body shape evolution. This function uses the aligned specimen coordinates to estimate multivariate evolutionary rates (σ2) for each group of taxa. The rank-ordered rate ratio (Sigma D) was used to compare the degree of difference in σ2 among groups of taxa. Observed rate ratios were compared with a simulated distribution of 9,999 rate ratios. These analyses were based on a pruned consensus of our 1,000 chronograms.
Rates and Optima of Vertebral Column and Adult Body Form Evolution.
We used OUwie v. 2.1 (38) to fit BM and OU models to the evolution of trunk vertebral numbers, adult body form, and adult trunk form. We compared models that estimated a single rate of trait evolution (σ2) and trait optimum (θ) across the phylogeny with models in which θ and/or σ2 could vary among different selective regimes (OUθ, BMθσ2, and OUθσ2). Alternative selective regimes (e.g., life cycles, ecologies) are described in Results. The “strength of selection parameter” (α) of OU models can be set to vary between groups (i.e., OUθα and OUθσ2α). However, we did not include these models, because α values were generally low (<0.01) (38) and did not show improvement in fit when separated by a selective regime. We fit all BM and OU analyses across 1,000 simmaps (80) per model on 1,000 pruned, post–burn-in Bayesian chronograms, and used ∆AIC scores and AIC weights (81) to compare the fit of models. Additional information on data transformations and outliers is provided in SI Appendix, Tables S2–S5. We estimated 95% confidence intervals (CIs) for parameters (σ2, θ, and α), and further evaluated the validity of the parameters through simulation using the “multiOU” function in phytools (80). We also used BM-based “evolvcv.lite” in phytools (80) to compare bivariate evolutionary rate matrices (82) between groups of direct developers.
Supplementary Material
Acknowledgments
We thank K. Irwin, B. Moon, S. Emel, P. Moler, M. Steffen, J. Phillips, D. Park, J. Civiello, J. Briggler, and numerous museums for providing live salamander larvae, images, or access to specimens. We also thank J. Beaulieu, M. Elliott, M. Linscott, H. O’Brien, and M. Zelditch for assistance with R codes and other tools, and M. Buchheim, T. Clay, A. Hess, N. Ledbetter, J. Phillips, R. Tovar, A. Trujano, two anonymous reviewers, and the Editor for comments on the manuscript. This research was supported by National Science Foundation Grants DEB 1050322 and Oklahoma EPSCoR IIA-1301789 (to R.M.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The phylogenetic tree set has been deposited in the Dryad Repository (dx.doi.org/10.5061/dryad.pn5kg).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1703877114/-/DCSupplemental.
References
- 1.Wilbur HM. Complex life cycles. Annu Rev Ecol Syst. 1980;11:67–93. [Google Scholar]
- 2.Moran NA. Adaptation and constraint in the complex life cycles of animals. Annu Rev Ecol Syst. 1994;25:573–600. [Google Scholar]
- 3.Raff RA. Constraint, flexibility, and phylogenetic history in the evolution of direct development in sea urchins. Dev Biol. 1987;119:6–19. doi: 10.1016/0012-1606(87)90201-6. [DOI] [PubMed] [Google Scholar]
- 4.Hanken J. Life history and morphological evolution. J Evol Biol. 1992;5:549–557. [Google Scholar]
- 5.Wray GA. The evolution of larval morphology during the post-Paleozoic radiation of echinoids. Paleobiology. 1992;18:258–287. [Google Scholar]
- 6.Lande R. A quantitative genetic theory of life history evolution. Ecology. 1982;63:607–615. [Google Scholar]
- 7.Cheverud JM, Rutledge JJ, Atchley WR. Quantitative genetics of development: Genetic correlations among age-specific trait values and the evolution of ontogeny. Evolution. 1983;37:895–905. doi: 10.1111/j.1558-5646.1983.tb05619.x. [DOI] [PubMed] [Google Scholar]
- 8.Schluter D, Price TD, Rowe L. Conflicting selection pressures and life-history trade-offs. Proc Biol Sci. 1991;246:11–17. [Google Scholar]
- 9.Zelditch ML, Bookstein FL, Lundrigan BL. The ontogenetic complexity of developmental constraints. J Evol Biol. 1993;6:121–141. [Google Scholar]
- 10.Marshall DJ, Morgan SG. Ecological and evolutionary consequences of linked life-history stages in the sea. Curr Biol. 2011;21:R718–R725. doi: 10.1016/j.cub.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Aguirre JD, Blows MW, Marshall DJ. The genetic covariance between life cycle stages separated by metamorphosis. Proc Biol Sci. 2014;281:20141091. doi: 10.1098/rspb.2014.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall DJ, Burgess SC, Connallon T. Global change, life-history complexity and the potential for evolutionary rescue. Evol Appl. 2016;9:1189–1201. doi: 10.1111/eva.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West-Eberhard MJ. Alternative adaptations, speciation, and phylogeny (a review) Proc Natl Acad Sci USA. 1986;83:1388–1392. doi: 10.1073/pnas.83.5.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corl A, Davis AR, Kuchta SR, Sinervo B. Selective loss of polymorphic mating types is associated with rapid phenotypic evolution during morphic speciation. Proc Natl Acad Sci USA. 2010;107:4254–4259. doi: 10.1073/pnas.0909480107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaffer HB. Evolution in a paedomorphic lineage, II: Allometry and form in the Mexican ambystomatid salamanders. Evolution. 1984;38:1207–1218. doi: 10.1111/j.1558-5646.1984.tb05644.x. [DOI] [PubMed] [Google Scholar]
- 16.Wake DB, Hanken J. Direct development in the lungless salamanders: What are the consequences for developmental biology, evolution and phylogenesis? Int J Dev Biol. 1996;40:859–869. [PubMed] [Google Scholar]
- 17.Wake MH. Reproductive modes, ontogenies, and the evolution of body form. Anim Biol. 2003;53:209–223. [Google Scholar]
- 18.Duellman WE, Trueb L. Biology of Amphibians. McGraw-Hill; New York: 1986. [Google Scholar]
- 19.Wiens J, Bonett R, Chippindale P. Ontogeny discombobulates phylogeny: Paedomorphosis and higher-level salamander relationships. Syst Biol. 2005;54:91–110. doi: 10.1080/10635150590906037. [DOI] [PubMed] [Google Scholar]
- 20.Bonett RM, Steffen MA, Lambert SM, Wiens JJ, Chippindale PT. Evolution of paedomorphosis in plethodontid salamanders: Ecological correlates and re-evolution of metamorphosis. Evolution. 2014;68:466–482. doi: 10.1111/evo.12274. [DOI] [PubMed] [Google Scholar]
- 21.Bonett RM, Steffen MA, Robison GA. Heterochrony repolarized: A phylogenetic analysis of developmental timing in plethodontid salamanders. Evodevo. 2014;5:27. doi: 10.1186/2041-9139-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerney RR, Blackburn DC, Müller H, Hanken J. Do larval traits re-evolve? Evidence from the embryogenesis of a direct-developing salamander, Plethodon cinereus. Evolution. 2012;66:252–262. doi: 10.1111/j.1558-5646.2011.01426.x. [DOI] [PubMed] [Google Scholar]
- 23.Wake DB. Comparative osteology and evolution of the lungless salamanders, family Plethodontidae. Mem So Cal Acad. 1966;4:1–111. [Google Scholar]
- 24.Wake DB. Homoplasy: The result of natural selection or evidence of design limitations? Am Nat. 1991;138:543–567. [Google Scholar]
- 25.Wiens JJ, Hoverman JT. Digit reduction, body size, and paedomorphosis in salamanders. Evol Dev. 2008;10:449–463. doi: 10.1111/j.1525-142X.2008.00256.x. [DOI] [PubMed] [Google Scholar]
- 26.Ackerly D. Conservatism and diversification of plant functional traits: Evolutionary rates versus phylogenetic signal. Proc Natl Acad Sci USA. 2009;106:19699–19706. doi: 10.1073/pnas.0901635106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagel M, Meade A. 2013 BayesTraits v. 2.0. (University of Reading, Reading). Available at www.evolution.rdg.ac.uk. Accessed December 21, 2016.
- 28.Mueller RL, Macey JR, Jaekel M, Wake DB, Boore JL. Morphological homoplasy, life history evolution, and historical biogeography of plethodontid salamanders inferred from complete mitochondrial genomes. Proc Natl Acad Sci USA. 2004;101:13820–13825. doi: 10.1073/pnas.0405785101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chippindale PT, Bonett RM, Baldwin AS, Wiens JJ. Phylogenetic evidence for a major reversal of life-history evolution in plethodontid salamanders. Evolution. 2004;58:2809–2822. doi: 10.1111/j.0014-3820.2004.tb01632.x. [DOI] [PubMed] [Google Scholar]
- 30.Jockusch EL. Geographic Variation and phenotypic plasticity of number of trunk vertebrae in slender salamanders, Batrachoseps (Caudata: Plethodontidae) Evolution. 1997;51:1966–1982. doi: 10.1111/j.1558-5646.1997.tb05118.x. [DOI] [PubMed] [Google Scholar]
- 31.Gomez C, et al. Control of segment number in vertebrate embryos. Nature. 2008;454:335–339. doi: 10.1038/nature07020. [DOI] [PubMed] [Google Scholar]
- 32.Elliot MG, Mooers AØ. Inferring ancestral states without assuming neutrality or gradualism using a stable model of continuous character evolution. BMC Evol Biol. 2014;14:226. doi: 10.1186/s12862-014-0226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao K-Q, Shubin NH. Earliest known crown-group salamanders. Nature. 2003;422:424–428. doi: 10.1038/nature01491. [DOI] [PubMed] [Google Scholar]
- 34.Gao K-Q, Shubin NH. Late Jurassic salamandroid from western Liaoning, China. Proc Natl Acad Sci USA. 2012;109:5767–5772. doi: 10.1073/pnas.1009828109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ascarrunz E, Rage J-C, Legreneur P, Laurin M. Triadobatrachus massinoti, the earliest known lissamphibian (Vertebrata: Tetrapoda) re-examined by μCT scan, and the evolution of trunk length in batrachians. Contrib Zool. 2016;85:201–234. [Google Scholar]
- 36.Adams DC, Otarola-Castillo E. Geomorph: An R package for the collection and analysis of geometric morphometric shape data. Methods Ecol Evol. 2013;4:393–399. [Google Scholar]
- 37.Adams DC. Quantifying and comparing phylogenetic evolutionary rates for shape and other high-dimensional phenotypic data. Syst Biol. 2014;63:166–177. doi: 10.1093/sysbio/syt105. [DOI] [PubMed] [Google Scholar]
- 38.Beaulieu JM, Jhwueng DC, Boettiger C, O’Meara BC. Modeling stabilizing selection: Expanding the Ornstein-Uhlenbeck model of adaptive evolution. Evolution. 2012;66:2369–2383. doi: 10.1111/j.1558-5646.2012.01619.x. [DOI] [PubMed] [Google Scholar]
- 39.Denoël M, Joly P, Whiteman HH. Evolutionary ecology of facultative paedomorphosis in newts and salamanders. Biol Rev Camb Philos Soc. 2005;80:663–671. doi: 10.1017/S1464793105006858. [DOI] [PubMed] [Google Scholar]
- 40.Skutschas P, Martin T. Cranial anatomy of the stem salamander Kokartus honorarius (Amphibia: Caudata) from the Middle Jurassic of Kyrgyzstan. Zool J Linnean Soc. 2011;161:816–838. doi: 10.1111/joa.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parra-Olea G, Wake DB. Extreme morphological and ecological homoplasy in tropical salamanders. Proc Natl Acad Sci USA. 2001;98:7888–7891. doi: 10.1073/pnas.131203598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Head JJ, Polly PD. Evolution of the snake body form reveals homoplasy in amniote Hox gene function. Nature. 2015;520:86–89. doi: 10.1038/nature14042. [DOI] [PubMed] [Google Scholar]
- 43.Reece JS, Mehta RS. Evolutionary history of elongation and maximum body length in moray eels (Anguilliformes: Muraenidae) Biol J Linn Soc Lond. 2013;109:861–875. [Google Scholar]
- 44.Alberch P. Ontogenesis and morphological diversification. Am Zool. 1980;20:653–667. [Google Scholar]
- 45.Maynard Smith J, et al. Developmental constraints and evolution. Q Rev Biol. 1985;60:265–287. [Google Scholar]
- 46.Arnold SJ. Constraints on phenotypic evolution. Am Nat. 1992;140:S85–S107. doi: 10.1086/285398. [DOI] [PubMed] [Google Scholar]
- 47.Raff A. The Shape of Life: Genes, Development, and the Evolution of Animal Form. Univ of Chicago Press; Chicago: 1996. [Google Scholar]
- 48.Schwenk K, Wagner GP. The relativism of constraints on phenotypic evolution. In: Pigliucci M, Preston K, editors. Phenotypic Integration: Studying the Ecology and Evolution of Complex Phenotypes. Oxford Univ Press; Oxford, UK: 2004. pp. 390–408. [Google Scholar]
- 49.Deban SM, Marks SB. Metamorphosis and evolution of feeding behaviour in salamanders of the family Plethodontidae. Zool J Linn Soc-Lond. 2002;134:375–400. [Google Scholar]
- 50.Wake DB. Functional and developmental constraints and opportunities in the evolution of feeding systems in urodeles. In: Mossakowski D, Roth G, editors. Environmental Adaptation and Evolution. Gustav Fischer; Stuttgart, Germany: 1982. pp. 51–66. [Google Scholar]
- 51.Wake DB, Blackburn DC, Lombard RE. Rampant homoplasy in complex characters: Repetitive convergent evolution of amphibian feeding structures. In: Dial KP, Shubin N, Brainerd EL, editors. Great Transformations in Vertebrate Evolution. Univ of Chicago Press; Chicago: 2015. pp. 395–405. [Google Scholar]
- 52.Gans C. Tetrapod limblessness: Evolution and functional corollaries. Am Zool. 1975;15:455–467. [Google Scholar]
- 53.Ward AB, Brainerd EL. Evolution of axial patterning in elongate fishes. Biol J Linn Soc Lond. 2007;90:97–116. [Google Scholar]
- 54.Brandley MC, Huelsenbeck JP, Wiens JJ. Rates and patterns in the evolution of snake-like body form in squamate reptiles: Evidence for repeated re-evolution of lost digits and long-term persistence of intermediate body forms. Evolution. 2008;62:2042–2064. doi: 10.1111/j.1558-5646.2008.00430.x. [DOI] [PubMed] [Google Scholar]
- 55.Siler CD, Brown RM. Evidence for repeated acquisition and loss of complex body-form characters in an insular clade of Southeast Asian semi-fossorial skinks. Evolution. 2011;65:2641–2663. doi: 10.1111/j.1558-5646.2011.01315.x. [DOI] [PubMed] [Google Scholar]
- 56.Claverie T, Wainwright PC. A morphospace for reef fishes: Elongation is the dominant axis of body shape evolution. PLoS One. 2014;9:e112732. doi: 10.1371/journal.pone.0112732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gillis G. Anguilliform locomotion in an elongate salamander (Siren intermedia): Effects of speed on axial undulatory movements. J Exp Biol. 1997;200:767–784. doi: 10.1242/jeb.200.4.767. [DOI] [PubMed] [Google Scholar]
- 58.Holman JA. Fossil Salamanders of North America. Indiana Univ Press; Bloomington, IN: 2006. [Google Scholar]
- 59.Bonett RM, Trujano-Alvarez AL, Williams MJ, Timpe EK. Biogeography and body size shuffling of aquatic salamander communities on a shifting refuge. Proc Biol Sci. 2013;280:20130200. doi: 10.1098/rspb.2013.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Breder CM. The locomotion of fishes. Zoologica. 1926;4:159–297. [Google Scholar]
- 61.Blankers T, Adams DC, Wiens JJ. Ecological radiation with limited morphological diversification in salamanders. J Evol Biol. 2012;25:634–646. doi: 10.1111/j.1420-9101.2012.02458.x. [DOI] [PubMed] [Google Scholar]
- 62.Rovito SM, Parra-Olea G, Hanken J, Bonett RM, Wake DB. Adaptive radiation in miniature: The minute salamanders of the Mexican highlands (Amphibia: Plethodontidae: Thorius) Biol J Linn Soc Lond. 2013;109:622–643. [Google Scholar]
- 63.van Sittert SJ, Skinner JD, Mitchell G. From fetus to adult: An allometric analysis of the giraffe vertebral column. J Exp Zoolog B Mol Dev Evol. 2010;314:469–479. doi: 10.1002/jez.b.21353. [DOI] [PubMed] [Google Scholar]
- 64.Schluter D. Adaptive radiation along genetic lines of least resistance. Evolution. 1996;50:1766–1774. doi: 10.1111/j.1558-5646.1996.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 65.Arntzen JW, Beukema W, Galis F, Ivanović A. Vertebral number is highly evolvable in salamanders and newts (family Salamandridae) and variably associated with climatic parameters. Contrib Zool. 2015;84:85–113. [Google Scholar]
- 66.Ficetola GF, et al. Morphological variation in salamanders and their potential response to climate change. Glob Change Biol. 2016;22:2013–2024. doi: 10.1111/gcb.13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eldredge N, et al. The dynamics of evolutionary stasis. Paleobiology. 2005;31:133–145. [Google Scholar]
- 68.Estes S, Arnold SJ. Resolving the paradox of stasis: Models with stabilizing selection explain evolutionary divergence on all timescales. Am Nat. 2007;169:227–244. doi: 10.1086/510633. [DOI] [PubMed] [Google Scholar]
- 69.Simpson GG. Tempo and Mode in Evolution. Columbia Univ Press; New York: 1944. [Google Scholar]
- 70.Uyeda JC, Hansen TF, Arnold SJ, Pienaar J. The million-year wait for macroevolutionary bursts. Proc Natl Acad Sci USA. 2011;108:15908–15913. doi: 10.1073/pnas.1014503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arnold SJ. Phenotypic evolution: The ongoing synthesis (American Society of Naturalists address) Am Nat. 2014;183:729–746. doi: 10.1086/675304. [DOI] [PubMed] [Google Scholar]
- 72.Bouckaert R, et al. BEAST 2: A software platform for Bayesian evolutionary analysis. PLOS Comput Biol. 2014;10:e1003537. doi: 10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lanfear R, Calcott B, Ho SY, Guindon S. Partitionfinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol. 2012;29:1695–1701. doi: 10.1093/molbev/mss020. [DOI] [PubMed] [Google Scholar]
- 74.Shen XX, et al. Enlarged multilocus data set provides surprisingly younger time of origin for the Plethodontidae, the largest family of salamanders. Syst Biol. 2016;65:66–81. doi: 10.1093/sysbio/syv061. [DOI] [PubMed] [Google Scholar]
- 75.Sokal RR, Rohlf FJ. Biometry: The Principles and Practices of Statistics in Biological Research. W.H. Freeman; San Francisco: 1981. [Google Scholar]
- 76.Rohlf FJ, Slice D. Extensions of Procrustes method for the optimal superimposition of landmarks. Syst Zool. 1990;39:40–59. [Google Scholar]
- 77.R Core Team 2017 R: A language and environment for statistical computing. Available at www.R-project.org/. Accessed June 3, 2017.
- 78.Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- 79.Paradis E, Claude J, Strimmer K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 80.Revell LJ. 2012 Phytools: Phylogenetic Tools for comparative biology (and other things). Available at faculty.umb.edu/liam.revell/phytools/. Accessed December 22, 2013.
- 81.Burnham KB, Anderson D. Model Selection and Multi-Model Inference: A Practical Information Theoretic Approach. 2nd Ed Springer; New York: 2002. [Google Scholar]
- 82.Revell LJ, Collar DC. Phylogenetic analysis of the evolutionary correlation using likelihood. Evolution. 2009;63:1090–1100. doi: 10.1111/j.1558-5646.2009.00616.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.