Significance
With training, stimulus detection or discrimination abilities can improve dramatically. This process, called perceptual learning, supports language acquisition, musical expertise, and professional judgments, such as the identification of abnormalities in X-rays. To explore neural mechanisms that support perceptual learning, we measured and manipulated auditory cortex activity as animals trained on an auditory task. We found improvements in neural sensitivity that correlated tightly with perceptual learning, both in absolute magnitude and time course, and depended strongly on task engagement. Disrupting auditory cortical function impaired learning while leaving perception largely intact. Our findings indicate that improvements in cortical sensitivity could plausibly explain perceptual learning, and suggest that plasticity within top-down networks may be a general mechanism for perceptual improvement.
Keywords: learning, plasticity, top-down, cortex, auditory
Abstract
Practice sharpens our perceptual judgments, a process known as perceptual learning. Although several brain regions and neural mechanisms have been proposed to support perceptual learning, formal tests of causality are lacking. Furthermore, the temporal relationship between neural and behavioral plasticity remains uncertain. To address these issues, we recorded the activity of auditory cortical neurons as gerbils trained on a sound detection task. Training led to improvements in cortical and behavioral sensitivity that were closely matched in terms of magnitude and time course. Surprisingly, the degree of neural improvement was behaviorally gated. During task performance, cortical improvements were large and predicted behavioral outcomes. In contrast, during nontask listening sessions, cortical improvements were weak and uncorrelated with perceptual performance. Targeted reduction of auditory cortical activity during training diminished perceptual learning while leaving psychometric performance largely unaffected. Collectively, our findings suggest that training facilitates perceptual learning by strengthening both bottom-up sensory encoding and top-down modulation of auditory cortex.
A broad range of sensory skills improve with practice during perceptual learning (PL), including language acquisition (1–3), musical abilities (4), and recognition of emotions (5). The neural bases for such perceptual improvement may vary widely. For example, training-based changes in neural activity have been identified in a number of brain regions, including early (6–13) and late (14, 15) sensory cortices, multisensory regions (16, 17), and downstream decision-making areas (18). Similarly, several neural mechanisms have been proposed, such as enhanced signal representation (19), reduction of external (20, 21) or internal (22, 23) noise, and improvement in sensory readout or decision making (13, 18, 24, 25).
The apparent divergence of loci and mechanisms associated with PL could be due, in part, to limitations of experimental design. For example, some neural changes associated with PL are transient (26–29), making it necessary to monitor neural activity throughout the duration of perceptual training, rather than making comparisons only after PL is complete (6–12, 14–16, 30). For similar reasons, it is critical to block the function of a specific candidate brain region during training to determine whether it plays a causal role in PL. Although some reports show that manipulating brain activity can influence PL (28, 31–34), there are no loss-of-function experiments to determine whether a particular region is required for behavioral improvement.
To address these unresolved issues, we recorded from auditory cortex (ACx) as animals improved on an auditory detection task and, in separate experiments, blocked ACx activity during the period of perceptual training. We found that neural and behavioral sensitivity improved in a nearly identical manner over the course of training, in terms of both absolute magnitude and kinetics. Furthermore, reversible down-regulation of ACx activity reduced learning without grossly impairing perception, suggesting that a critical amount of ACx activity is required for PL. Finally, the magnitude of ACx plasticity depended strongly on task performance. We propose an inclusive conceptual framework that acknowledges a role for plasticity within both the ascending sensory neuraxis and descending modulatory pathways.
Results
We trained Mongolian gerbils on an amplitude modulation (AM) detection task (Fig. 1A), a perceptual skill that displays significant improvement in humans (35). Animals were trained to drink from a water spout while in the presence of the “safe” stimulus (unmodulated noise), and to withdraw from the spout when the sound changed to the “warn” stimulus (0 dB relative to 100% depth, 5-Hz AM noise), to avoid an aversive shock. All animals learned this procedure quickly, reaching our performance criterion (d′ > 1.5) within four training sessions (2.1 ± 0.18 sessions, n = 16 animals across all experiments; see SI Appendix, Materials and Methods). To determine whether this auditory percept required ACx activity, we infused a high dose of muscimol (1 mg/mL; 1 μL per hemisphere; total dose of 2 μg) bilaterally in four animals (SI Appendix, Fig. S1A). At this concentration, muscimol significantly impaired AM detection (P = 0.0006; SI Appendix, Fig. S1B) by reducing hit rates (P < 0.0001; SI Appendix, Fig. S1C) without increasing false alarm rates (P = 0.63; SI Appendix, Fig. S1D).
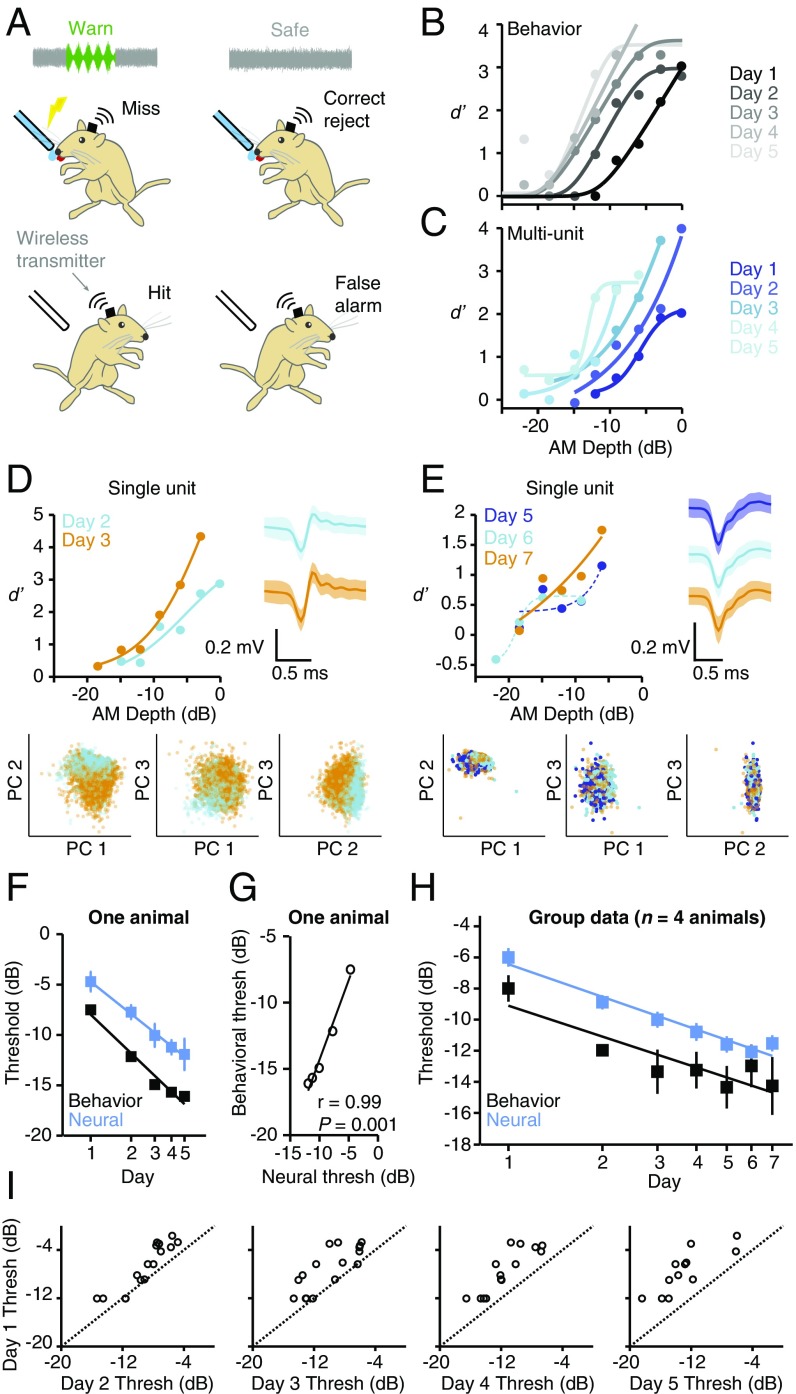
Fig. 1.
Cortical and behavioral improvements are similar in magnitude and time course. (A) Wireless recordings were made from ACx of animals as they performed an AM detection task. (B) Psychometric functions from one animal improved across days. Data from this animal are presented in C–G. (C) Neurometric functions from one multiunit recorded during task performance improved across days. (D) Neurometric performance for a single unit held across multiple sessions improved from day 2 (cyan) to day 3 (orange). Single-unit identity was confirmed by stable waveform shape [compare waveforms from day 2 (cyan) and day 3 (orange); Right]. Waveforms represent mean ± 2 SDs. Single-unit identity was also confirmed by the fact that waveforms from day 2 and day 3 clustered tightly together within principal component (PC) space (Bottom). (E) Data from another single unit held across multiple training sessions. Plot conventions are as in D. Dashed lines indicate fits that did not yield valid thresholds. (F) Mean ± SEM neural and behavioral sensitivity improve simultaneously in one animal (n = 30 sites; 4 to 7 per d). (G) Behavioral and neural thresholds of one animal are tightly correlated. (H) Mean ± SEM neural and behavioral thresholds improve simultaneously across all animals and units [neural: F6,224 = 16, P < 0.0001, n = 231 (range: 29 to 39 sites per d; SI Appendix, Table S1); behavior: F4,12 = 11, P = 0.0005, n = 4 animals]. (I) Day 1 vs. day 2 to 5 thresholds of units recorded over multiple days. See SI Appendix, Table S3 for statistics. The dashed lines are unity.
To verify that our task was well-suited to assess perceptual learning, we quantified behavioral performance across daily training sessions. During these sessions, we presented warn stimuli of varying AM depths (SI Appendix, Figs. S2 and S3) to obtain psychometric functions and derive AM detection thresholds (AM depth at d′ = 1; SI Appendix, Materials and Methods). AM depth detection improved during training, as shown for one representative animal in Fig. 1B. These improvements were due to increased hit rates (SI Appendix, Fig. S4A) rather than decreased false alarm rates (SI Appendix, Fig. S4B). Importantly, the hit rate for the largest AM depth tested (0 dB) was maximal on day 1 and remained steady on day 2, suggesting that the animals began perceptual training when already at perceptual asymptote for this stimulus value (SI Appendix, Fig. S4A). Additionally, the hit rates for shallow depths improved more gradually than for higher depths. These observations are consistent with the finding that PL progresses systematically from easy to difficult stimuli (36), and support the idea that our experimental paradigm specifically assessed PL, rather than associative or procedural learning.
To determine whether there is a temporal correlation between neural and behavioral improvement, we implanted animals with chronic electrode arrays and recorded single- and multiunit activity in left ACx as animals trained and improved on the AM detection task (n = 231 AM-responsive sites; n = 4 animals; SI Appendix, Materials and Methods and Table S1). We found that training improved neural performance, both at the multi- and single-unit level (Fig. 1 C–E). These improvements occurred in concert with behavior, such that the average firing rate (FR)-based neural sensitivity closely tracked psychometric sensitivity on a day-to-day basis (Fig. 1F). As illustrated in Fig. 1G for one representative animal, the majority (3/4) of our subjects showed a significant correlation between neural and behavioral thresholds (SI Appendix, Table S2). The distribution of correlation regression slopes did not differ significantly from a distribution centered around 1 (0.92 ± 0.10, t3 = −0.76, P = 0.50, n = 4), indicating that the ACx population threshold is a good predictor of perceptual sensitivity. At the group level, perceptual training had a significant effect on both neural (P < 0.0001) and behavioral thresholds (P = 0.0005), with improvement occurring at similar rates [neural, −7.0; behavioral, −6.6 dB/log(day); Fig. 1H]. In the one animal that we followed for 14 training days, neural improvement was maintained after perceptual performance reached asymptote (SI Appendix, Fig. S5).
In visual cortex, training-induced changes are often most pronounced in specific subpopulations of neurons (7, 14, 15). To determine whether a similarly selective mechanism operates in ACx, we examined thresholds for recording sites that were held across multiple training days. As shown in Fig. 1I, nearly all sites demonstrated significant training-induced improvement, regardless of starting threshold (day 1 vs. day 2 to 5 thresholds: all P < 0.001; see SI Appendix, Table S3 for details). This finding suggests that training may enhance sensitivity across the population of AM-responsive sites, rather than acting selectively on a restricted subset of units. It is important to note, however, that training could differentially affect units with different tuning properties, which were not systematically characterized here because many neurons failed to respond when animals were not engaged in the task.
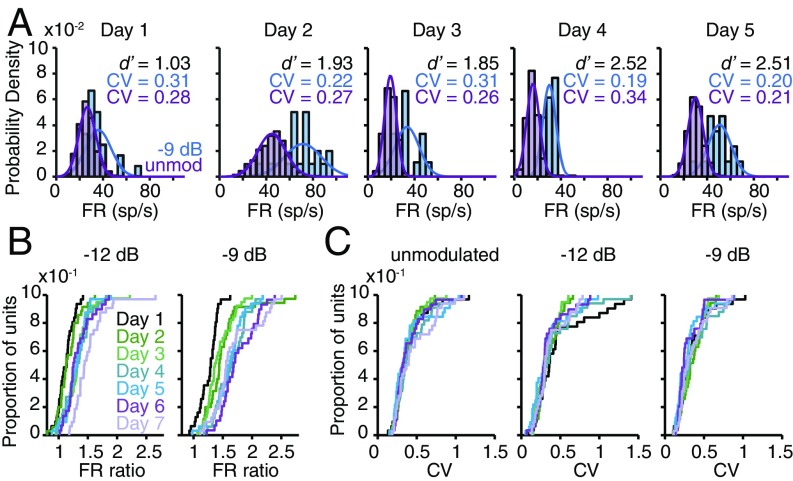
Improvements in neural sensitivity could be due to an increased separation of warn- and safe-evoked FR distributions (13), and/or a reduction in FR variability across training days (20–23, 37). As illustrated for one representative multiunit in Fig. 2A, warn and safe FR distributions gradually separated during training without a systematic change in the unit’s mean FR or trial-to-trial variability (measured by the coefficient of variation; CV). To quantify this effect across our population, we calculated an FR ratio (AM-evoked FR/unmodulated FR) for each unit on each training day. FR ratios steadily increased across days, leading to a larger separation between AM and unmodulated FRs (all P < 0.0001; Fig. 2B). In contrast, CVs remained stable throughout training (all P > 0.05; Fig. 2C), as did population FRs (all P > 0.05; see SI Appendix, Fig. S6 for a full explanation). These findings suggest that PL is supported by a gradual separation of the warn and safe FR distributions, rather than a reduction in response variability.
Fig. 2.
PL is supported by an increasing separation of the warn and safe FR distributions. (A) Warn (−9-dB AM) and safe (unmodulated) FR distributions from one representative multiunit show increasing separation during training. sp, spikes. (B) Perceptual training increases FR ratios across the population (−12 dB: H = 59.2, P < 0.0001; −9 dB: H = 65.8, P < 0.0001). (C) Perceptual training does not affect CV [unmodulated: H = 3.61, P = 0.73; −12 dB: H = 5.12, P = 0.53; −9 dB: H = 7.25, P = 0.30]. In B and C, n = 231 (range: 29 to 39 sites per d; SI Appendix, Table S1).
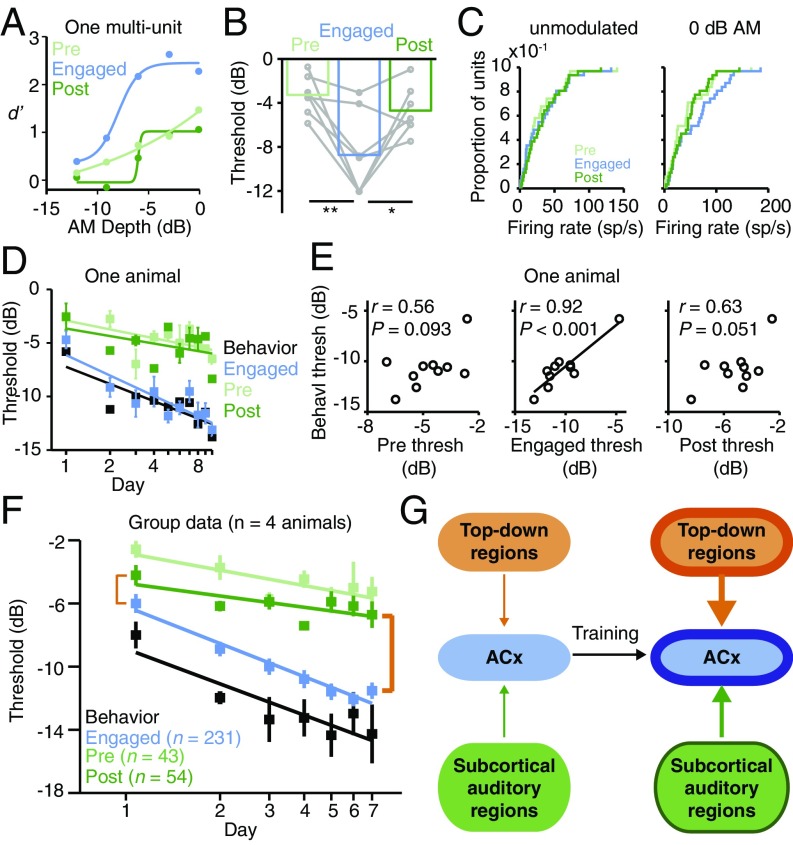
Behavioral evidence suggests that top-down processes, such as attention, arousal, and motivation, can facilitate or enable PL (12, 38–43). Here, we adopt the term “top-down” to mean the functional influence of a descending projection from one or more brain regions on neural activity in sensory cortex. A commonly used procedure to assess the magnitude of top-down mechanisms is to compare neural responsiveness during two different states of behavioral engagement (37, 44–49). Specifically, top-down inputs are thought to actively modulate ACx responses during task performance (or “engagement”) but not during nontask (“disengaged”) listening sessions (50, 51). Thus, the difference between engaged and disengaged sensitivity is a proxy for the strength of a top-down mechanism. We used this approach to examine whether training affects the magnitude of a top-down mechanism during PL. Specifically, we recorded ACx activity during disengaged listening sessions that occurred just before (“pre”) and just after (“post”) training sessions. During these disengaged sessions, the spout and metal floorplate were removed from the test cage; otherwise, the sound stimuli presented and the position of the recording electrodes were identical to behaviorally engaged sessions.
As training progressed, three outcomes were possible (SI Appendix, Fig. S7). If training does not affect the strength of a top-down mechanism, then the difference between engaged and disengaged AM sensitivity should remain constant, despite training-based improvement. Alternatively, if training weakens the strength of a top-down mechanism, the difference between engaged and disengaged AM sensitivity should become smaller. This scenario could occur if training allows sensory processing to become more automatic, as previously proposed (26, 52, 53). Finally, if training strengthens a top-down mechanism, such as attention (54, 55), the difference between engaged and disengaged AM sensitivity should become larger during PL.
Throughout training, a smaller proportion of units responded to AM during disengaged sessions compared with engaged sessions (all P < 0.001; SI Appendix, Tables S1 and S4), and those that did respond during disengaged sessions had poorer thresholds (P = 0.0022; Fig. 3 A and B). This weak sensitivity was due to reduced AM-evoked discharge rates during disengagement (P < 0.001; Fig. 3C and SI Appendix, Figs. S2 and S3). These state-dependent changes in AM sensitivity were not explained by recording instability, as FRs evoked by unmodulated noise did not differ significantly across conditions (P = 0.095; Fig. 3C).
Fig. 3.
Behaviorally gated neural plasticity increases during PL. (A) Day 1 performance of one multiunit. (B) Task engagement improves day 1 neural thresholds (F2,12 = 11, P = 0.0022; pre vs. engaged: t6 = 3.8, P = 0.0093; post vs. engaged: t6 = 2.7, P = 0.034; n = 7). Only units that responded under all conditions were included. (C) Task engagement does not affect day 1 unmodulated FRs [Χ2(2) = 4.71, P = 0.095] but increases AM-evoked FRs [Χ2(2) = 15.3, P < 0.001; pre vs. engaged: Z = −4.12, P < 0.001; post vs. engaged: Z = −3.67, P < 0.001]. All n = 31. (D) Mean ± SEM neural thresholds of one animal improve more quickly during task engagement than during disengaged listening [pre: n = 2 to 4 sites per d (32 total); engaged: n = 7 to 11 sites per d (86 total); post: n = 1 to 4 sites per d (23 total)]. (E) Neural thresholds correlate with behavioral thresholds in one animal (same subject as in D) only during task engagement. (F) Across all animals, mean ± SEM neural thresholds from disengaged listening sessions improve with training (pre: F6,37 = 4.3, P = 0.0021, n = 2 to 11 sites per d; post: F6,54 = 3.0, P = 0.014, n = 4 to 14 sites per d; SI Appendix, Table S1), but improvement is slower than during task engagement. The strength of top-down ACx modulation is reflected by the difference between engaged and disengaged thresholds (orange brackets). Engaged neural and behavioral data are replotted from Fig. 1H. (G) Model in which training induces plasticity in both bottom-up (thick green outline and arrow) and top-down (thick orange outline and arrow) pathways, leading to improved ACx sensory sensitivity (thick blue outline) that supports PL.
As training progressed, disengaged neural thresholds displayed a modest improvement but did not correlate with behavioral thresholds, as illustrated for a single animal in Fig. 3 D and E (group data in SI Appendix, Table S2; all P values are nonsignificant). Moreover, at the group level, the rate of disengaged improvement, while significant (all P < 0.05), was >50% slower than that observed during task engagement [pre, −3.2; engaged, −7.0; post, −2.4 dB/log(day); Fig. 3F]. As a result, the difference between engaged and disengaged neural thresholds grew larger as training progressed (compare beginning and ending orange brackets in Fig. 3F). Similar findings were observed using a timing-based analysis of AM-evoked activity (SI Appendix, Fig. S8). Collectively, these findings suggest that training strengthens both bottom-up inputs to and top-down modulation of ACx, which together give rise to PL (Fig. 3G).
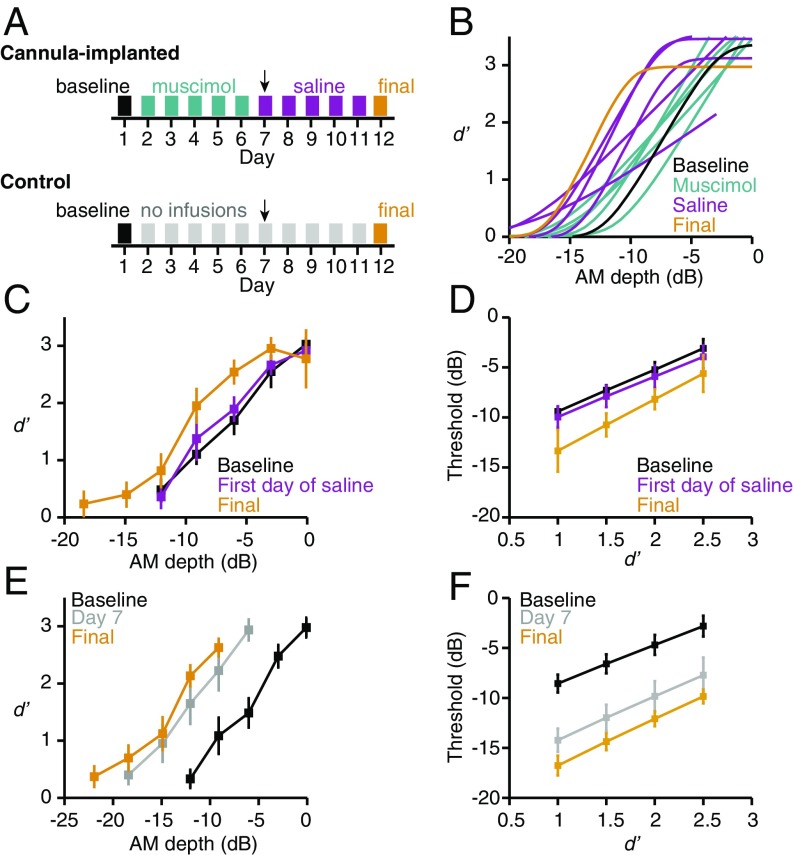
To determine whether ACx activity is required for PL, we assessed baseline behavioral performance in a separate group of animals (n = 6) and then paired perceptual training with bilateral ACx infusions of muscimol or saline (Fig. 4A and SI Appendix, Fig. S9). To distinguish the role of ACx in perceptual learning from its role in perception, it was important to identify a dose of muscimol that did not grossly perturb psychometric performance on the AM detection task. We found that 0.5 mg/mL (1 μL per hemisphere; total dose of 1 μg) allowed for excellent detection of 0-dB AM (SI Appendix, Fig. S10) and relatively unimpaired psychometric performance in the majority of our animals (SI Appendix, Fig. S11; P = 0.073). Furthermore, this dose had no effect on trial completion rates, false alarm rates, or reaction times (all P > 0.05; SI Appendix, Fig. S12), implying that motor processes and response biases were not impacted by the treatment. Thus, we used this dose to ask whether ACx activity is required for PL.
Fig. 4.
Reduced ACx activity disrupts PL. (A) Experimental timeline for cannula-implanted animals (Top) and unimplanted controls (Bottom). Arrows highlight the middle time point analyzed in C and D (first day of saline) and E and F (day 7). Muscimol dose used in these experiments was 1 μg (0.5 mg/mL; 1 μL per hemisphere). (B) Psychometric fits from one representative cannula-implanted animal during perceptual training. (C and D) Time point has a significant effect on (C) mean ± SEM d′ values (F2,10 = 7.2, P = 0.012) and (D) mean ± SEM thresholds extracted at different d′ values (F2,10 = 5.5, P = 0.025) from cannula-implanted animals (n = 6). (E and F) Time point has a significant effect on (E) mean ± SEM d′ values (F2,10 = 15, P = 0.001) and (F) mean ± SEM thresholds (F1.1,5.4 = 18, P = 0.006) from control animals (n = 6). Note that in C and E, we do not display points for which we had data from only a single animal.
As illustrated for one animal in Fig. 4B, behavioral performance remained stable throughout muscimol-paired sessions (also see SI Appendix, Fig. S11) but demonstrated steady improvement during subsequent sessions paired with saline. To quantify this observation at the group level, we compared behavioral performance at three time points: (i) baseline before perceptual training, (ii) the first day of saline infusion, and (iii) the final training session. If muscimol prevents PL, rather than simply impairing AM sensitivity, we would expect behavioral performance to be similar at baseline and the first day of saline infusion. As predicted, values obtained at baseline and on the first day of saline infusion largely overlapped. Final d′ values were higher, however, indicating improved perceptual performance (P = 0.012; Fig. 4C). To confirm that this effect was robust, we extracted AM depth thresholds for each animal at four different d′ values. As shown in Fig. 4D, final AM thresholds were lower than those obtained at baseline or during the first day of saline infusion (P = 0.025).
During muscimol infusion experiments, we controlled for the amount of daily practice by limiting animals to 50 warn trials per session (see SI Appendix, Materials and Methods for the rationale). Thus, it was possible that the lack of improvement between baseline and the first day of saline infusion was not due to a muscimol-induced learning impairment but instead to insufficient practice. To rule out this possibility, a separate group of control animals (n = 6) received perceptual training for 12 d with 50 warn trials per session, identical to the muscimol group (Fig. 4A). As shown in Fig. 4E, d′ values were higher on day 7 than at baseline, and approached those obtained on the final training day (P = 0.001). Similarly, thresholds were lower at day 7 and day 12 compared with baseline (P = 0.006; Fig. 4F). Collectively, these results show that reducing ACx activity during perceptual training prevents learning.
Discussion
PL is closely associated with long-term changes in sensory cortex activity (6–15, 56, 57), and these changes may contribute to learning (28, 31–34). However, no studies have tested whether sensory cortex is necessary for PL. Here, we found that bilateral ACx infusion of a low dose of muscimol could prevent practice-based improvement. We interpret this result to mean that the manipulation permitted enough ACx activity to allow for task performance across a range of AM depths but not enough to enable the plasticity mechanisms required for PL. This interpretation is consistent with a model of PL which posits that, in order for plasticity to occur, sensory-evoked activity must surpass a threshold for learning (58, 59). It is important to note, however, that muscimol disrupted psychometric performance to a greater degree in two of our six subjects (SI Appendix, Fig. S11). Thus, in these two animals, it is possible that a degraded sensory representation also contributed to impaired learning. Our findings are in line with previous manipulations that dissociated auditory processing from a learning mechanism. For example, song learning in juvenile zebra finches is diminished by administration of an NMDA receptor antagonist during a developmental sensory acquisition phase, even though the drug does not alter auditory brainstem responses (60) or song discrimination (61).
The results of our muscimol inactivation experiments indicate that proper ACx activity is required for PL. Consistent with this finding, we observed a tight correlation between ACx and behavioral plasticity throughout perceptual training, in terms of both magnitude and kinetics (Fig. 1H). The neural basis of PL has commonly been evaluated by focusing on two time points (pre- vs. posttraining) or groups (trained vs. untrained) (6–12, 14–16, 30). However, training-based perceptual improvement can be associated with transient phases of functional plasticity, even within a single network (26–29). Thus, studies that are restricted to one or two time points could fail to identify a temporary contribution of a particular brain region to PL. A handful of studies have recorded neural responses during the full time course of visual perceptual training; however, direct comparisons of neural and perceptual improvement either (i) were restricted to early and late time points (56, 57), (ii) revealed relatively weak neural improvements (13), or (iii) resulted in modest correlations between neural and behavioral plasticity (18). In contrast, we found that ACx neurons displayed substantial day-to-day improvements in neural sensitivity that were tightly correlated with, and could plausibly explain, PL.
AM-evoked responses in ACx are enhanced during task performance. This result is in line with an abundance of work demonstrating behaviorally gated modulations of ACx activity (37, 44–49, 62–66). Our observation that disengaged neural sensitivity is better if measured immediately after behavioral testing, rather than before (compare green lines in Fig. 3F), is consistent with an effect of task-specific plasticity that persists after the behavioral session has concluded. For example, Fritz et al. found that task-dependent spectrotemporal receptive field plasticity in ACx persists for minutes to hours after behavioral performance (47). Similarly, passive stimulus exposure has been found to facilitate PL if the exposure occurs within a short window following active practice (67), presumably when task-dependent neural enhancements are still operational (58, 59). It should be noted, however, that we cannot rule out the possibility that the enhancements we observed during behavioral performance were the result of non–task-specific arousal mechanisms (68, 69).
A distinguishing feature of our results is that the effect of task engagement increases in magnitude across perceptual training sessions. During task performance, neural improvements were pronounced and tightly correlated with perceptual abilities. During nontask listening sessions, however, neural improvements were present but weak, and were uncorrelated with behavior. These findings led us to propose an inclusive conceptual framework: Specifically, we posit that training induces plasticity within the top-down networks that modulate stimulus-driven activity during task performance, thereby augmenting bottom-up plasticity within sensory cortex.
Evidence for bottom-up plasticity derives from previous experiments in which animals were trained on an auditory detection or discrimination task and then anesthetized for extracellular recordings after reaching perceptual asymptote. These studies report that trained animals display altered auditory cortical responses, such as tonotopic reorganization (9, 12) and enhanced AM processing (10, 11). Similarly, Adab and colleagues recorded from V4 and posterior inferior temporal cortex neurons of awake monkeys during training on an orientation discrimination task (57, 70). The authors found that training enhanced the responses to the orientation gratings, and the enhancements were present both during task performance and during passive fixation. Together, these findings suggest that perceptual training can induce neural changes that are observable even when animals are anesthetized or when the task-relevant stimuli are unattended. Thus, we interpret the small, but significant, neural improvements we observed during task disengagement as reflecting plasticity within a bottom-up pathway.
It is important to recognize, however, that changes in bottom-up stimulus encoding do not explain all forms of PL (6, 56, 71, 72). For example, Gilbert and colleagues found that while perceptual training on an embedded contour detection task has little effect on basic response properties of monkey V1 neurons, it has a pronounced effect on how V1 neurons are modulated by stimulus context (6, 56, 71). This finding stands in contrast to those described above from V4 (57, 70) and primary ACx (9–12), where it was found that training modified stimulus-encoding properties. These apparent dissimilarities may reflect differences among functional networks, behavioral tasks, or species.
Our observation that neural improvements are more pronounced during task performance is consistent with the well-established finding that top-down processes known to modulate sensory cortex activity also facilitate and guide PL (12, 17, 38–43). For our purposes, top-down refers to the functional influence of a higher-order brain region on neural activity in ACx, brought about by task engagement. Several plausible candidate regions may mediate this top-down effect, either in isolation or in concert with one another, including frontal cortex (50, 51), nucleus basalis (73–75), locus coeruleus (76), ventral tegmental area (77, 78), and multisensory cortex (17).
Current models of PL suggest that top-down inputs act to restrict task-dependent plasticity to the appropriate neurons (79–83) or enhance stimulus signals above some threshold beyond which plasticity mechanisms are operational (58, 59). Our hypothesis is consistent with these models but posits that, rather than providing a static modulatory signal, top-down networks change throughout training, thereby contributing to improved ACx sensitivity and PL. This framework is similar to a computational model that has been proposed to explain visual brightness discrimination learning (84) and is consistent with human psychophysical and imaging evidence that training can improve visual attentional modulation (55, 85, 86) and general cognitive skills (23). Thus, training-induced plasticity in top-down modulatory processes may be a general mechanism that supports PL across sensory modalities.
Materials and Methods
Subjects.
Adult Mongolian gerbils (Meriones unguiculatus) were raised from commercially obtained breeding pairs (Charles River). Animals were housed on a 12-h light/12-h dark cycle and provided with ad libitum food and water unless otherwise noted. All procedures were approved by the Institutional Animal Care and Use Committee at New York University.
Behavior.
AM detection was assessed with an aversive conditioning paradigm as described previously (29, 87, 88). Psychometric functions were fit with cumulative Gaussians and transformed to the signal detection metric, d′ (89). Threshold was defined as the AM depth at which d′ = 1, unless otherwise stated.
Electrophysiology.
Extracellular single- and multiunit activity was recorded from left ACx as described previously (37, 90). Firing rates were transformed to d′ values and fit with logistic functions (37). Units were considered “responsive” if they generated a valid fit and threshold (at d′ = 1).
Infusions.
Guide cannulas (Plastics One) were implanted into bilateral ACx. Animals were anesthetized briefly with isoflurane, and infused with muscimol or saline 45 min before behavioral training.
Statistics.
Analyses were performed using JMP (SAS), PASW Statistics (IBM), or SPSS (IBM). When data were not normally distributed, nonparametric tests were used. All reported P values were Holm–Bonferroni–corrected for multiple comparisons.
For full methodological details, see SI Appendix. Data and analysis code can be found at https://nyu.box.com/v/caras-sanes-2017.
Supplementary Material
Acknowledgments
We thank Derek Wang and Stephen Young for assistance with data collection and histology, and other members of the D.H.S. laboratory for constructive feedback and discussions. Special thanks to Drs. Beverly Wright, Michael Long, Daniel Stolzberg, Jonathan Fritz, and Daniel Polley for helpful discussions and comments on earlier versions of this work. This research was supported by NIH R01DC014656 (to D.H.S.) and NIH K99DC016046 (to M.L.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Data and analysis code can be found at https://nyu.box.com/v/caras-sanes-2017.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1712305114/-/DCSupplemental.
References
- 1.Bradlow AR, Pisoni DB, Akahane-Yamada R, Tohkura Y. Training Japanese listeners to identify English /r/ and /l/: IV. Some effects of perceptual learning on speech production. J Acoust Soc Am. 1997;101:2299–2310. doi: 10.1121/1.418276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuhl PK, et al. Infants show a facilitation effect for native language phonetic perception between 6 and 12 months. Dev Sci. 2006;9:F13–F21. doi: 10.1111/j.1467-7687.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- 3.Sundara M, Polka L, Genesee F. Language-experience facilitates discrimination of /d-th/ in monolingual and bilingual acquisition of English. Cognition. 2006;100:369–388. doi: 10.1016/j.cognition.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 4.White EJ, Hutka SA, Williams LJ, Moreno S. Learning, neural plasticity and sensitive periods: Implications for language acquisition, music training and transfer across the lifespan. Front Syst Neurosci. 2013;7:90. doi: 10.3389/fnsys.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du Y, Zhang F, Wang Y, Bi T, Qiu J. Perceptual learning of facial expressions. Vision Res. 2016;128:19–29. doi: 10.1016/j.visres.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Crist RE, Li W, Gilbert CD. Learning to see: Experience and attention in primary visual cortex. Nat Neurosci. 2001;4:519–525. doi: 10.1038/87470. [DOI] [PubMed] [Google Scholar]
- 7.Schoups A, Vogels R, Qian N, Orban G. Practising orientation identification improves orientation coding in V1 neurons. Nature. 2001;412:549–553. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]
- 8.Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task. J Neurophysiol. 1992;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- 9.Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beitel RE, Schreiner CE, Cheung SW, Wang X, Merzenich MM. Reward-dependent plasticity in the primary auditory cortex of adult monkeys trained to discriminate temporally modulated signals. Proc Natl Acad Sci USA. 2003;100:11070–11075. doi: 10.1073/pnas.1334187100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao S, Chang EF, Woods J, Merzenich MM. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat Neurosci. 2004;7:974–981. doi: 10.1038/nn1293. [DOI] [PubMed] [Google Scholar]
- 12.Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan Y, et al. Perceptual training continuously refines neuronal population codes in primary visual cortex. Nat Neurosci. 2014;17:1380–1387. doi: 10.1038/nn.3805. [DOI] [PubMed] [Google Scholar]
- 14.Yang T, Maunsell JH. The effect of perceptual learning on neuronal responses in monkey visual area V4. J Neurosci. 2004;24:1617–1626. doi: 10.1523/JNEUROSCI.4442-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raiguel S, Vogels R, Mysore SG, Orban GA. Learning to see the difference specifically alters the most informative V4 neurons. J Neurosci. 2006;26:6589–6602. doi: 10.1523/JNEUROSCI.0457-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu Y, et al. Perceptual learning reduces interneuronal correlations in macaque visual cortex. Neuron. 2011;71:750–761. doi: 10.1016/j.neuron.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powers AR, III, Hevey MA, Wallace MT. Neural correlates of multisensory perceptual learning. J Neurosci. 2012;32:6263–6274. doi: 10.1523/JNEUROSCI.6138-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Law CT, Gold JI. Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nat Neurosci. 2008;11:505–513. doi: 10.1038/nn2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold J, Bennett PJ, Sekuler AB. Signal but not noise changes with perceptual learning. Nature. 1999;402:176–178. doi: 10.1038/46027. [DOI] [PubMed] [Google Scholar]
- 20.Dosher BA, Lu ZL. Perceptual learning reflects external noise filtering and internal noise reduction through channel reweighting. Proc Natl Acad Sci USA. 1998;95:13988–13993. doi: 10.1073/pnas.95.23.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dosher BA, Lu ZL. Mechanisms of perceptual learning. Vision Res. 1999;39:3197–3221. doi: 10.1016/s0042-6989(99)00059-0. [DOI] [PubMed] [Google Scholar]
- 22.Jones PR, Moore DR, Amitay S, Shub DE. Reduction of internal noise in auditory perceptual learning. J Acoust Soc Am. 2013;133:970–981. doi: 10.1121/1.4773864. [DOI] [PubMed] [Google Scholar]
- 23.Amitay S, Zhang YX, Jones PR, Moore DR. Perceptual learning: Top to bottom. Vision Res. 2014;99:69–77. doi: 10.1016/j.visres.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Petrov AA, Dosher BA, Lu ZL. The dynamics of perceptual learning: An incremental reweighting model. Psychol Rev. 2005;112:715–743. doi: 10.1037/0033-295X.112.4.715. [DOI] [PubMed] [Google Scholar]
- 25.Law CT, Gold JI. Reinforcement learning can account for associative and perceptual learning on a visual-decision task. Nat Neurosci. 2009;12:655–663. doi: 10.1038/nn.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sigman M, et al. Top-down reorganization of activity in the visual pathway after learning a shape identification task. Neuron. 2005;46:823–835. doi: 10.1016/j.neuron.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yotsumoto Y, Watanabe T, Sasaki Y. Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron. 2008;57:827–833. doi: 10.1016/j.neuron.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed A, et al. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron. 2011;70:121–131. doi: 10.1016/j.neuron.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 29.Sarro EC, von Trapp G, Mowery TM, Kotak VC, Sanes DH. Cortical synaptic inhibition declines during auditory learning. J Neurosci. 2015;35:6318–6325. doi: 10.1523/JNEUROSCI.4051-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghose GM, Yang T, Maunsell JH. Physiological correlates of perceptual learning in monkey V1 and V2. J Neurophysiol. 2002;87:1867–1888. doi: 10.1152/jn.00690.2001. [DOI] [PubMed] [Google Scholar]
- 31.Tegenthoff M, et al. Improvement of tactile discrimination performance and enlargement of cortical somatosensory maps after 5 Hz rTMS. PLoS Biol. 2005;3:e362. doi: 10.1371/journal.pbio.0030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karim AA, Schüler A, Hegner YL, Friedel E, Godde B. Facilitating effect of 15-Hz repetitive transcranial magnetic stimulation on tactile perceptual learning. J Cogn Neurosci. 2006;18:1577–1585. doi: 10.1162/jocn.2006.18.9.1577. [DOI] [PubMed] [Google Scholar]
- 33.Pleger B, et al. Repetitive transcranial magnetic stimulation-induced changes in sensorimotor coupling parallel improvements of somatosensation in humans. J Neurosci. 2006;26:1945–1952. doi: 10.1523/JNEUROSCI.4097-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shibata K, Watanabe T, Sasaki Y, Kawato M. Perceptual learning incepted by decoded fMRI neurofeedback without stimulus presentation. Science. 2011;334:1413–1415. doi: 10.1126/science.1212003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzgerald MB, Wright BA. Perceptual learning and generalization resulting from training on an auditory amplitude-modulation detection task. J Acoust Soc Am. 2011;129:898–906. doi: 10.1121/1.3531841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahissar M, Hochstein S. Task difficulty and the specificity of perceptual learning. Nature. 1997;387:401–406. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]
- 37.von Trapp G, Buran BN, Sen K, Semple MN, Sanes DH. A decline in response variability improves neural signal detection during auditory task performance. J Neurosci. 2016;36:11097–11106. doi: 10.1523/JNEUROSCI.1302-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiu LP, Pashler H. Improvement in line orientation discrimination is retinally local but dependent on cognitive set. Percept Psychophys. 1992;52:582–588. doi: 10.3758/bf03206720. [DOI] [PubMed] [Google Scholar]
- 39.Ahissar M, Hochstein S. Attentional control of early perceptual learning. Proc Natl Acad Sci USA. 1993;90:5718–5722. doi: 10.1073/pnas.90.12.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito M, Westheimer G, Gilbert CD. Attention and perceptual learning modulate contextual influences on visual perception. Neuron. 1998;20:1191–1197. doi: 10.1016/s0896-6273(00)80499-7. [DOI] [PubMed] [Google Scholar]
- 41.Seitz AR, Nanez JE, Sr, Holloway S, Tsushima Y, Watanabe T. Two cases requiring external reinforcement in perceptual learning. J Vis. 2006;6:966–973. doi: 10.1167/6.9.9. [DOI] [PubMed] [Google Scholar]
- 42.Seitz AR, Kim D, Watanabe T. Rewards evoke learning of unconsciously processed visual stimuli in adult humans. Neuron. 2009;61:700–707. doi: 10.1016/j.neuron.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukai I, Bahadur K, Kesavabhotla K, Ungerleider LG. Exogenous and endogenous attention during perceptual learning differentially affect post-training target thresholds. J Vis. 2011;11:25. doi: 10.1167/11.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller JM, et al. Single cell activity in the auditory cortex of rhesus monkeys: Behavioral dependency. Science. 1972;177:449–451. doi: 10.1126/science.177.4047.449. [DOI] [PubMed] [Google Scholar]
- 45.Benson DA, Hienz RD, Goldstein MH., Jr Single-unit activity in the auditory cortex of monkeys actively localizing sound sources: Spatial tuning and behavioral dependency. Brain Res. 1981;219:249–267. doi: 10.1016/0006-8993(81)90290-0. [DOI] [PubMed] [Google Scholar]
- 46.Ryan AF, Miller JM, Pfingst BE, Martin GK. Effects of reaction time performance on single-unit activity in the central auditory pathway of the rhesus macaque. J Neurosci. 1984;4:298–308. doi: 10.1523/JNEUROSCI.04-01-00298.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat Neurosci. 2003;6:1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- 48.Lee CC, Middlebrooks JC. Auditory cortex spatial sensitivity sharpens during task performance. Nat Neurosci. 2011;14:108–114. doi: 10.1038/nn.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niwa M, Johnson JS, O’Connor KN, Sutter ML. Active engagement improves primary auditory cortical neurons’ ability to discriminate temporal modulation. J Neurosci. 2012;32:9323–9334. doi: 10.1523/JNEUROSCI.5832-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winkowski DE, Bandyopadhyay S, Shamma SA, Kanold PO. Frontal cortex activation causes rapid plasticity of auditory cortical processing. J Neurosci. 2013;33:18134–18148. doi: 10.1523/JNEUROSCI.0180-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winkowski DE, et al. Orbitofrontal cortex neurons respond to sound and activate primary auditory cortex neurons. Cereb Cortex. January 8, 2017 doi: 10.1093/cercor/bhw409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaina LM, Belliveau JW, des Roziers EB, Zeffiro TA. Neural systems underlying learning and representation of global motion. Proc Natl Acad Sci USA. 1998;95:12657–12662. doi: 10.1073/pnas.95.21.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mukai I, et al. Activations in visual and attention-related areas predict and correlate with the degree of perceptual learning. J Neurosci. 2007;27:11401–11411. doi: 10.1523/JNEUROSCI.3002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amitay S, Irwin A, Moore DR. Discrimination learning induced by training with identical stimuli. Nat Neurosci. 2006;9:1446–1448. doi: 10.1038/nn1787. [DOI] [PubMed] [Google Scholar]
- 55.Byers A, Serences JT. Enhanced attentional gain as a mechanism for generalized perceptual learning in human visual cortex. J Neurophysiol. 2014;112:1217–1227. doi: 10.1152/jn.00353.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li W, Piëch V, Gilbert CD. Learning to link visual contours. Neuron. 2008;57:442–451. doi: 10.1016/j.neuron.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adab HZ, Vogels R. Practicing coarse orientation discrimination improves orientation signals in macaque cortical area V4. Curr Biol. 2011;21:1661–1666. doi: 10.1016/j.cub.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 58.Seitz AR, Dinse HR. A common framework for perceptual learning. Curr Opin Neurobiol. 2007;17:148–153. doi: 10.1016/j.conb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 59.Wright BA, Zhang Y. 2009. in The Cognitive Neurosciences, ed Gazzaniga MS (MIT Press, Cambridge, MA), pp 353–366.
- 60.Aamodt SM, Nordeen EJ, Nordeen KW. Blockade of NMDA receptors during song model exposure impairs song development in juvenile zebra finches. Neurobiol Learn Mem. 1996;65:91–98. doi: 10.1006/nlme.1996.0010. [DOI] [PubMed] [Google Scholar]
- 61.Basham ME, Nordeen EJ, Nordeen KW. Blockade of NMDA receptors in the anterior forebrain impairs sensory acquisition in the zebra finch (Poephila guttata) Neurobiol Learn Mem. 1996;66:295–304. doi: 10.1006/nlme.1996.0071. [DOI] [PubMed] [Google Scholar]
- 62.Fritz JB, Elhilali M, Shamma SA. Differential dynamic plasticity of A1 receptive fields during multiple spectral tasks. J Neurosci. 2005;25:7623–7635. doi: 10.1523/JNEUROSCI.1318-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fritz JB, Elhilali M, Shamma SA. Adaptive changes in cortical receptive fields induced by attention to complex sounds. J Neurophysiol. 2007;98:2337–2346. doi: 10.1152/jn.00552.2007. [DOI] [PubMed] [Google Scholar]
- 64.David SV, Fritz JB, Shamma SA. Task reward structure shapes rapid receptive field plasticity in auditory cortex. Proc Natl Acad Sci USA. 2012;109:2144–2149. doi: 10.1073/pnas.1117717109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin P, Fritz JB, Shamma SA. Rapid spectrotemporal plasticity in primary auditory cortex during behavior. J Neurosci. 2014;34:4396–4408. doi: 10.1523/JNEUROSCI.2799-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buran BN, et al. A sensitive period for the impact of hearing loss on auditory perception. J Neurosci. 2014;34:2276–2284. doi: 10.1523/JNEUROSCI.0647-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wright BA, Sabin AT, Zhang Y, Marrone N, Fitzgerald MB. Enhancing perceptual learning by combining practice with periods of additional sensory stimulation. J Neurosci. 2010;30:12868–12877. doi: 10.1523/JNEUROSCI.0487-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goris RL, Movshon JA, Simoncelli EP. Partitioning neuronal variability. Nat Neurosci. 2014;17:858–865. doi: 10.1038/nn.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McGinley MJ, David SV, McCormick DA. Cortical membrane potential signature of optimal states for sensory signal detection. Neuron. 2015;87:179–192. doi: 10.1016/j.neuron.2015.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adab HZ, Popivanov ID, Vanduffel W, Vogels R. Perceptual learning of simple stimuli modifies stimulus representations in posterior inferior temporal cortex. J Cogn Neurosci. 2014;26:2187–2200. doi: 10.1162/jocn_a_00641. [DOI] [PubMed] [Google Scholar]
- 71.Ramalingam N, McManus JN, Li W, Gilbert CD. Top-down modulation of lateral interactions in visual cortex. J Neurosci. 2013;33:1773–1789. doi: 10.1523/JNEUROSCI.3825-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Piëch V, Li W, Reeke GN, Gilbert CD. Network model of top-down influences on local gain and contextual interactions in visual cortex. Proc Natl Acad Sci USA. 2013;110:E4108–E4117. doi: 10.1073/pnas.1317019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- 74.Froemke RC, et al. Long-term modification of cortical synapses improves sensory perception. Nat Neurosci. 2013;16:79–88. doi: 10.1038/nn.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuchibhotla KV, et al. Parallel processing by cortical inhibition enables context-dependent behavior. Nat Neurosci. 2017;20:62–71. doi: 10.1038/nn.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martins AR, Froemke RC. Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat Neurosci. 2015;18:1483–1492. doi: 10.1038/nn.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stark H, Scheich H. Dopaminergic and serotonergic neurotransmission systems are differentially involved in auditory cortex learning: A long-term microdialysis study of metabolites. J Neurochem. 1997;68:691–697. doi: 10.1046/j.1471-4159.1997.68020691.x. [DOI] [PubMed] [Google Scholar]
- 78.Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- 79.Roelfsema PR, van Ooyen A. Attention-gated reinforcement learning of internal representations for classification. Neural Comput. 2005;17:2176–2214. doi: 10.1162/0899766054615699. [DOI] [PubMed] [Google Scholar]
- 80.Ahissar M, Hochstein S. The reverse hierarchy theory of visual perceptual learning. Trends Cogn Sci. 2004;8:457–464. doi: 10.1016/j.tics.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 81.Ahissar M, Nahum M, Nelken I, Hochstein S. Reverse hierarchies and sensory learning. Philos Trans R Soc Lond B Biol Sci. 2009;364:285–299. doi: 10.1098/rstb.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gilbert CD, Sigman M. Brain states: Top-down influences in sensory processing. Neuron. 2007;54:677–696. doi: 10.1016/j.neuron.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 83.Kim D, Seitz AR, Watanabe T. Visual perceptual learning by operant conditioning training follows rules of contingency. Vis Cogn. 2015;23:147–160. doi: 10.1080/13506285.2015.1015663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schäfer R, Vasilaki E, Senn W. Perceptual learning via modification of cortical top-down signals. PLoS Comput Biol. 2007;3:e165. doi: 10.1371/journal.pcbi.0030165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bartolucci M, Smith AT. Attentional modulation in visual cortex is modified during perceptual learning. Neuropsychologia. 2011;49:3898–3907. doi: 10.1016/j.neuropsychologia.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 86.Byers A, Serences JT. Exploring the relationship between perceptual learning and top-down attentional control. Vision Res. 2012;74:30–39. doi: 10.1016/j.visres.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heffner HE, Heffner RS. 1995. in Methods in Comparative Psychoacoustics, eds Klmup GM, Dooling RJ, Fay RR, Stebbins WC (Springer, Basel), pp 79–93.
- 88.Caras ML, Sanes DH. Sustained perceptual deficits from transient sensory deprivation. J Neurosci. 2015;35:10831–10842. doi: 10.1523/JNEUROSCI.0837-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Green DM, Swets JA. Signal Detection Theory and Psychophysics. Wiley; New York: 1966. [Google Scholar]
- 90.Buran BN, von Trapp G, Sanes DH. Behaviorally gated reduction of spontaneous discharge can improve detection thresholds in auditory cortex. J Neurosci. 2014;34:4076–4081. doi: 10.1523/JNEUROSCI.4825-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.