Abstract
Acinetobacter baumannii represents nowadays an important nosocomial opportunistic pathogen whose reservoirs outside the clinical setting are obscure. Here, we traced the origins of the collection strain A. baumannii DSM30011 to an isolate first reported in 1944, obtained from the enriched microbiota responsible of the aerobic decomposition of the resinous desert shrub guayule. Whole-genome sequencing and phylogenetic analysis based on core genes confirmed DSM30011 affiliation to A. baumannii. Comparative studies with 32 complete A. baumannii genomes revealed the presence of 12 unique accessory chromosomal regions in DSM30011 including five encompassing phage-related genes, five containing toxin genes of the type-6 secretion system, and one with an atypical CRISPRs/cas cluster. No antimicrobial resistance islands were identified in DSM30011 agreeing with a general antimicrobial susceptibility phenotype including folate synthesis inhibitors. The marginal ampicillin resistance of DSM30011 most likely derived from chromosomal ADC-type ampC and blaOXA-51-type genes. Searching for catabolic pathways genes revealed several clusters involved in the degradation of plant defenses including woody tissues and a previously unreported atu locus responsible of aliphatic terpenes degradation, thus suggesting that resinous plants may provide an effective niche for this organism. DSM30011 also harbored most genes and regulatory mechanisms linked to persistence and virulence in pathogenic Acinetobacter species. This strain thus revealed important clues into the genomic diversity, virulence potential, and niche ranges of the preantibiotic era A. baumannii population, and may provide an useful tool for our understanding of the processes that led to the recent evolution of this species toward an opportunistic pathogen of humans.
Keywords: comparative genomics, preantibiotic era bacterium, virulence factors, CRISPRs/cas
Introduction
The genus Acinetobacter, family Moraxellaceae, class Gammaproteobacteria, is characterized by Gram-negative aerobic coccobacilli of ubiquitous environmental distribution and large metabolic capabilities (Bouvet and Grimont 1986). Among the genus, the phylogenetically closely-related species composing the Acinetobacter calcoaceticus–Acinetobacter baumannii (Acb) complex represent nowadays important opportunistic pathogens (Antunes etal. 2014). Infections due to A. baumannii in particular, rarely reported in healthcare settings before the 1970s, rapidly increased in importance with the global spread of a limited number of epidemic clonal complexes (CC) possessing multidrug-resistance (MDR) phenotypes (Diancourt etal. 2010; Antunes etal. 2014). Strains composing the CCs generally contain plasmids and chromosomally-located resistance islands (RI) and genomic islands (GI) encompassing different transposons and integrons which play pivotal roles in both antimicrobial and heavy metal resistance (Di Nocera etal. 2011; Nigro etal. 2011; Antunes etal. 2014; Touchon etal. 2014). Also, CC strains carry a large repertoire of insertion sequences (ISs) capable of mediating genome rearrangements, deletions, insertions, inversions, and gene overexpression with strong adaptive significances (Roca etal. 2012; Antunes etal. 2014; Touchon etal. 2014). The combination of the above factors, added to the intrinsic resistance of A. baumannii to desiccation and fever-associated temperatures, are considered main factors of persistence of pathogenic strains in the nosocomial environment (Roca etal. 2012; Antunes etal. 2014).
Whole-genome sequence (WGS) comparisons have become commonplace in examining strain-to-strain variability and in comparing pathogenic strains with environmental, less aggressive relatives in efforts to identify underlying genetic determinants and mechanisms responsible for phenotypic dissimilarities. When applied to A. baumannii, these approaches provided valuable information on both the present A. baumannii population structure and the mechanisms of acquisition and evolution of antimicrobial resistance (Di Nocera etal. 2011, Roca etal. 2012; Antunes etal. 2014; Touchon etal. 2014). It is now generally accepted that A. baumannii arose from other members of the Acb complex as the result of an ancient population bottleneck, followed by a recent population expansion from a few clinically-relevant clones endowed with a diverse arsenal of resistance genes (Diancourt etal. 2010; Antunes etal. 2014; Touchon etal. 2014). Still, the identification of A. baumannii virulence traits has remained elusive, and in fact large genetic variations have been found both between and within CC members suggesting a complex and even a multifactorial nature of mechanisms involved (Diancourt etal. 2010; Roca etal. 2012; Antunes etal. 2014). In this context it has recently been emphasized the importance of a deeper genomic study of non-clinical (“environmental”) isolates to clarify both the virulence potential of the aboriginal A. baumannii population and the evolutionary paths that led toward an opportunistic pathogen lifestyle (Antunes etal. 2014). However, and contrasting with the environmental ubiquity of most other members of the genus, the natural habitats and infection reservoirs of A. baumannii outside the clinical setting are still to be defined (Eveillard etal. 2013; Antunes etal. 2014). Although A. baumannii isolates have been obtained from non-clinical sources including domestic animals, human ectoparasites, vegetables, the plant rhizosphere, etc., whether they represent true environmental isolates or consequences of human waste contamination remains debated (Eveillard etal. 2013; Antunes etal. 2014).
Acinetobacter baumannii DSM30011 is a collection strain amenable for genetic manipulation (Wilharm etal. 2013). This organism is capable of killing Galleria mellonella larvae, outcompeting other clinically-relevant bacterial species in a type-6 secretion system (T6SS)-dependent manner, and forming biofilms and biopellicles (Repizo etal. 2015), that is, traits generally associated to bacterial persistence in different environments including the clinical setting (Roca etal. 2012; Longo etal. 2014; Nait Chabane etal. 2014). Here, we traced DSM30011 origins to a strain originally deposited at the NRRL in 1943 as Achromobacter lacticum 4-h (NRRL B-551, Agricultural Research Service Culture Collection, ARS, U.S. Department of Agriculture; see supplementary table S1, Supplementary Material online for details). This strain was isolated after enrichment of the resin-degrading natural microbiota responsible of the aerobic decomposition of guayule (Parthenium argentatum Gray), a resin-producing angiosperm of the Asteraceae family common to arid and semiarid areas of the south-western United States and north-central Mexico (Allen etal. 1944; Naghski etal. 1944). DSM30011 thus provided us with an environmental A. baumannii strain isolated by the middle of last century just before the onset of the antibiotic era (Antunes etal. 2014), and therefore well-differentiated both temporally and epidemiologically from the clinical strains predominant nowadays. Here, we conducted a comparative genomic analysis of DSM30011 with other Acinetobacter strains to obtain clues into the genomic diversity and virulence potential of the preantibiotic era A. baumannii population.
Materials and Methods
Strain Sources
The DSM30011 strain used for genome sequencing in this work was obtained from the DSMZ-German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany), and was kindly provided to us by Dr X. Charpentier (CIRI, Lyon, France). The original isolation and different denominations assigned to this strain by various collections are detailed below, under “Tracing DSM30011 origins” and in supplementary table S1, Supplementary Material online. Acinetobacter sp. strains NCIMB8208 and NCIMB8209 were obtained from the National Collection of Industrial Food and Marine Bacteria (NCIMB, Aberdeen, Scotland). The A. baumannii type strains ATCC19606 and ATCC17978 were part of the laboratory stock collection.
Genotypic Analysis
The evaluation of genomic differences between the different strains was done by a random amplification of polymorphic DNA assay using as primers the degenerate oligonucleotides 5′-GGTCGACYNGGRTC-3′ (#5314) and 5′-GGTCGACYTTNGYNGGRTC-3′ (#19) (Limansky and Viale 2002).
DSM30011 Genome Sequencing and Annotation
DSM30011 DNA was isolated using the DNeasy Blood and Tissue kit (Qiagen) following the manufacturer’s protocols. The genomic sequence was obtained using a paired-end (2 × 250 bp) strategy using the Illumina MiSeq method and following the protocols provided by the supplier. A total of 3,581, 922 × 2 reads were generated with an average length of 250 bp. Reads were subjected to quality assessment and further assembled using the Velvet version 1.2.10 (k-mer size used was 97). The de novo assembly process was optimized using the VelvetOptimiser script version 2.2.5. The resulting contigs were ordered and oriented with Mauve (Darling etal. 2010) using the A. baumannii ATCC17978 genome as reference. When necessary, the contigs were concatenated by including the sequence nnnnncacacacttaattaattaagtgtgtgnnnnn, that harbors stop codons in all six reading frames as was described in Repizo etal. (2014). The replication origin (oriC) was predicted by OriFinder (Gao and Zhang 2008). Assembled genome sequences were annotated using RAST (Aziz etal. 2008). Coding sequences (CDS) functions were manually curated using BLASTP searches (e value = 1 × e−10) of the NCBI nonredundant database. This whole genome shotgun project has been deposited at DDBJ/ENA/GenBank under the accession JJOC00000000. The version described in this paper is version JJOC02000000.
The presence of mobile genetic elements in the DSM30011 genome was investigated by the following online tools and/or open-access database and manual examinations: IslandViewer (http://pathogenomics.sfu.ca/islandviewer; Dhillon etal. 2015) for the GI regions and IS Finder (Siguier etal. 2006; https://www-is.biotoul.fr/) and ISSaga (Varani etal. 2011) for IS elements. CRISPRs were detected using the CRISPRs recognition tool v1.1 (Bland et al 2007), whereas putative prophage sequences were identified by PHAST and PHASTER analysis (Zhou etal. 2011; Arndt etal. 2016). Genes encoding antibiotic resistance were identified with ResFinder 2.1 (https://cge.cbs.dtu.dk/services/ResFinder/; Zankari etal. 2012) and RAST.
Phylogenetic and Shared Protein Families Analyses
To infer the relationships among the 64 Acinetobacter strains analyzed in this work which included 32 A. baumannii strains other than DSM30011, the corresponding proteomes were first retrieved from the NCBI and gathered into a local database together with the DSM30011 proteome. Homologous protein families were assembled with SILIX version 1.2.9 (Miele etal. 2011). More precisely, pairwise comparisons of proteins contained in the local database were performed using the BLASTP program version 2.2.26 with default parameters (Altschul etal. 1997). Proteins in a pair providing HSP (High-scoring Segment Pairs) with identity over 80% and covering at least 80% of the protein lengths were gathered together in the same family. This led to the assembly of 42,211 families. Association coefficients (SAB) were computed for each pair of strains as SAB = (100 × 2NAB)/(NA + NB), in which NA is the number of protein families present in strain A, NB is the number of protein families present in strain B and NAB is the number of protein families shared by strain A and strain B. This coefficient ranged from 0 when both strains do not share any gene family to 1 when all the families present in strain A were also present in strain B.
Among the 42,211 inferred protein families, 351 correspond to core gene families present in a single copy in all 64 Acinetobacter strains. For each family, the corresponding nucleotidic sequences were aligned with MUSCLE 3.8.31 (default parameters) on the basis of the aligned protein sequences, so as to preserve the codon integrity by avoiding introduction of incorrect frame shifts. The resulting 351 multiple alignments were then trimmed using BMGE1.1 with the CODON option (Criscuolo and Gribaldo 2010) and combined to build a large supermatrix (385, 926 nucleotide positions) that was used for phylogenetic inferences. A phylogenetic tree was then inferred with the Maximum Likelihood (ML) approach using IQTREE (Nguyen etal. 2015) with the GTR + R6 evolutionary model. The branch robustness of the ML tree was estimated with the nonparametric bootstrap procedure implemented in IQTREE (100 replicates of the original alignment).
Sequence Typing
Assignment of sequence types (ST) for DSM30011 was done using housekeeping genes: cpn60, gdh, gltA, gpi, gyrB, recA, and rpoD (Oxford scheme, Bartual etal. 2005) or cpn60, fusA, gltA, pyrG, recA, rplB, and rpoD (Pasteur scheme, Diancourt etal. 2010). For details see supplementary table S2, Supplementary Material online and the A. baumannii MLST Databases website (http://pubmlst.org/abaumannii/; Jolley and Maiden 2010).
Antimicrobial Susceptibility Testing
The general antimicrobial susceptibility of A. baumannii strains DSM30011, ATCC17978, and ATCC19606 was evaluated using the VITEK 2 System (bioMérieux) following the criteria recommended by the CLSI (Clinical and Laboratory Standards Institute, 2016, Performance standards for antimicrobial susceptibility testing. Document M100S, 26th ed., CLSI, Wayne, PA). Susceptibility tests to tetracycline, chloramphenicol, and macrolides such as azithromycin and erythromycin were done separately by disk assays on Mueller–Hinton agar (MHA) following CLSI protocols. In short, DSM30011 cells were grown overnight at 37 °C, resuspended in LB broth to a turbidity of 0.5 McFarland units, and spread on the surface of MHA-containing Petri plates. Antibiotic disks were then carefully deposited at the center of the agar surface, and the plates were incubated at 37 °C for 16 h before measuring the diameter of the corresponding growth inhibition zones.
Results and Discussion
Tracing DSM30011 Origins
This strain was isolated prior to 1,944 (see supplementary table S1, Supplementary Material online for details) from the natural microbiota enriched during the aerobic decomposition of defoliated guayule plants. This process, known as retting, was applied to reduce the content of terpene resins prior to the extraction of natural rubber for the industrial production of latex (Allen etal. 1944; Naghski etal. 1944). It involved a prior treatment of milled guayule shrubs in boiling water both to remove surface matter and to hydrate/soften the plant tissues, followed by the aerobic decomposition under high moisture of the resulting plant material by the prolific microbiota that survived the above treatment and which was located primarily within the bark (Naghski etal. 1944). This resulted in a marked disintegration of the woody tissues driven by a consortium of different fungal and bacterial groups, from which a number of isolates were isolated with the ability to degrade guayule resins. Among them group III-2b isolates (which fitted at the time the phenotypic description of A. lacticum) encompassed non-motile bacterial coccoid rods capable of growing up to 50 °C (Allen etal. 1944). Two of these isolates were deposited in 1943 at the Agricultural Research Service Culture Collection (ARS, NRRL, USDA) under the denominations A. lacticum B-551 and B-552, respectively, and were subsequently replicated in other collections under other designations including Acinetobacter sp. strains NCIMB8208 and NCIMB8209, respectively (see supplementary table S1, Supplementary Material online for details). A more comprehensive phenotypic analysis later conducted on these strains reassigned them to the newly described genus Acinetobacter Brisou and Prévot (Thornley 1967). More recently, and on the basis of rpoB sequence comparisons, the B-551 replica present at the Culture Collection of the Institute Pasteur (CIP68.38) was assigned to A. baumannii as a species (La Scola etal. 2006; Gundi etal. 2009). The phylogenetic analysis conducted here using core genes sequence comparisons (see below) allowed us to validate this assignment for the equivalent replica (DSM30011) present at the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany). We also verified the genomic identity between A. baumannii DSM30011 and NCIMB8208 by a random amplification PCR assay (supplementary fig. S1A, Supplementary Material online), which showed identical amplification profiles as expected from strains derived from the same isolate (Limansky and Viale 2002). In turn, the above profiles could be clearly differentiated from those obtained for other strains isolated during a similar time period including the clinical A. baumannii strains ATCC19606 and ATCC17978, and even from the companion strain NCIMB8209 (supplementary fig. S1A and table S1, Supplementary Material online), therefore indicating that they belong to different clonal lineages. It is also worth noting that DSM30011/NCIMB8208 can grow at 44 °C (supplementary fig. S1B, Supplementary Material online) in what represents a typical phenotype associated to A. baumannii but not to other Acinetobacter members (Bouvet and Grimont 1986; Nemec etal. 2011).
DSM30011 thus provided us with an A. baumannii strain originally isolated in the USA from a resinous desert plant >70 years ago, that is, on the verge of the massive introduction of antibiotic therapy to treat infections (Allen etal. 1944). Also, isolation of this strain preceded by a few years those of the A. baumannii clinical strains ATCC19606 (USA, 1948) and ATCC17978 (France, 1951; supplementary table S1, Supplementary Material online).
Genomic Features
DSM30011 genome sequencing (depth of coverage ∼350×) resulted in 13 contigs ranging from 309 to 1,346,914 bp in length, with an average size of 247,059 bp. The draft genome thus encompassed 3,949,168 bp with a G + C content of 38.7% (table 1), values that matched the averages reported for the genomes of the species composing the Acinetobacter genus (3.87 Mb and 39.6% respectively; Touchon etal. 2014). A total of 3,774 CDS and 62 structural RNAs (60 tRNAs and 2 rRNAs regions), were predicted by RAST (Aziz etal. 2008) in DSM30011 (table 1). The annotation covered 448 RAST subsystems (47%) with 1,746 CDS; 64 CDS being labeled as hypothetical proteins. On the other hand, 2,028 CDS (53%), from which 1,045 corresponded to hypothetical proteins, could not be assigned to any subsystem. No DNA sequences matching plasmid sequences deposited in GenBank were found, and no plasmids could be detected in DSM30011 using ordinary plasmid-extraction protocols (not shown).
Table 1.
General Features of the DSM30011 Genomea
| Feature | Value |
|---|---|
| Estimated size (Mbp) | 3.95 |
| GC content (%) | 38.7 |
| Number of CDSs | 3,774 |
| CDSs with predicted function (%) | 70.6 |
| Conserved hypothetical CDSs (%) | 29.4 |
| Number of tRNA genes | 60 |
| Number of rRNA operons | 2 |
| Phage regions | 5 |
| Complete ISsb | 0 |
| CRISPRs-cas clusters | 1 |
On the basis of RAST annotation.
IS1236-like and ISAba8-like remnants were detected (see supplementary table S7, Supplementary Material online).
Phylogenetic and MLST Analyses
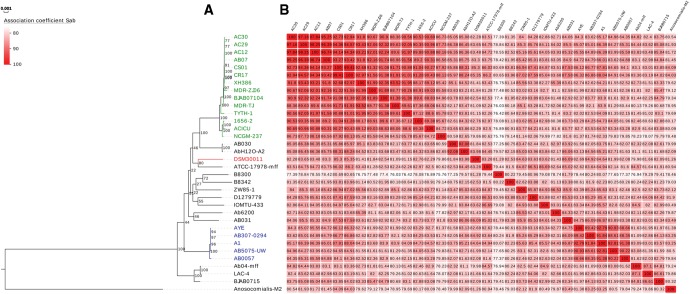
ML phylogenetic analysis based on the comparisons of the concatenated sequences of 351 core genes of 64 different Acinetobacter strains including species of the Acb complex (listed in supplementary table S3, Supplementary Material online) indicated a clear affiliation (bootstrap value (BV) = 100%) of DSM30011 to the A. baumannii cluster (fig. 1 and supplementary fig. S2, Supplementary Material online). Among this cluster, and with some exceptions such as the distinct subgroups formed by each the CCI and CCII strains, the relationships among A. baumannii strains (including DSM30011) were not well-resolved (most BV < 50%, fig. 1A). These observations agree with the lack of phylogenetic structure reported for the A. baumannii population (Diancourt etal. 2010). These authors found no evidence of phylogenetic structure within the clinical A. baumannii strain population analyzed, with the exception of a few tight terminal clusters corresponding to CCs of much more recent emergence (post1950) connected to the introduction of massive antimicrobial therapy.
Fig. 1.
—Maximum Likelihood phylogenetic analysis of Acinetobacter baumannii strains accompanied by the association coefficient for each pair of strains. (A) The ML phylogeny was computed based on 351 concatenated core gene sequences (full tree is shown in supplementary fig. S2, Supplementary Material online). Numbers at nodes correspond to bootstrap values (100 replicates of the original data set). The scale bar below corresponds to evolutionary distance (average number of the substitutions per site). CCI (in blue) and CCII (in green) correspond to subclusters formed by A. baumannii species assigned to epidemic clonal complexes CCI and CCII, respectively. (B) The table corresponds to the SAB association coefficient computed for each pair of strains as twice the number of shared gene families between the two strains divided by the number of gene families contained in the two strains, considering all the gene families (see Methods for details).
A. baumannii DSM30011 was assigned to novel sequence types on both MLST classification schemes currently in use: ST 1113 in the Oxford scheme and ST 738 in the Pasteur scheme (see supplementary table S2, Supplementary Material online). The fact that DSM30011 shared only 3 of 7 alleles (Oxford scheme) or 4 of 7 alleles (Pasteur scheme) with any closest matching strain (supplementary table S2, Supplementary Material online) prevented us to relate this strain to any of the different CC or ST presently defined (Karah etal. 2012).
Comparative Genomics of DSM30011
As indicated above, DSM30011 represents the earliest A. baumannii isolate reported to-date obtained from a non-clinical source. We therefore conducted a comparative genomic analysis of this strain in an effort to obtain clues on the diversity of the A. baumannii population existing before the antibiotic era (Antunes etal. 2014). We compared the proteomes of 64 Acinetobacter including the strain DSM30011 and 32 A. baumannii which allowed us to assemble 42,211 protein families. Regarding DSM30011 (3,774 predicted CDS), 3,725 families contain at least one sequence from this strain, and among these, 1.3% included >1. While 243 protein families were specific to DSM30011, 1,935 (51.9%) were shared by all A. baumannii strains. This number was close to recent estimations of the core genome content of this species (Peleg etal. 2012, Touchon etal. 2014). When core plus accessory genomes were compared, DSM30011 shared the highest number of CDSs (3,157 protein families, supplementary fig. S3, Supplementary Material online) with A. baumannii IOMTU-433, a MDR clinical strain recently isolated from a patient in Nepal (BioSample: SAMD00020223). On the other hand, DSM30011 shared the lowest number of CDSs with NCGM-237 (2,769 protein families), a MDR-strain isolated in Japan (Tada etal. 2014). However, these values could be biased by differences in strain proteome size. Indeed, based on association coefficients (SAB, see Methods), DSM30011 appeared more similar in terms of shared gene families to AB031 (SAB = 86.09, Fig. 1B), a tigecycline- and sulfamethoxazole/trimethoprim-resistant clinical isolate obtained from a bloodstream infection in Canada (Loewen etal. 2014). In addition, based on the same criterion DSM30011 was less similar to NCGM-237 (SAB = 79.86). Intermediate values were obtained for ATCC17978 (SAB = 83.28; 3,055 shared protein families), a clinical strain almost contemporary to DSM30011 (supplementary table S1, Supplementary Material online) which emerged in close proximity in the core genes phylogenetic tree of figure 1A (BV: 82%).
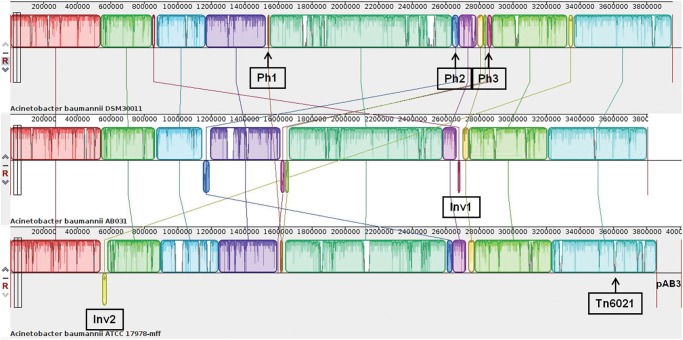
The genomes of strains AB031 and ATCC17978 were individually used as scaffolds to organize the contigs obtained for DSM30011 (fig. 2). Since a lower number of block rearrangements were required to organize the DSM30011 genome when ATCC17978 was used as reference, we adopted the genomic organization based on the latter strain for further analyses.
Fig. 2.
—Linear comparison of the genomes of A. baumannii strains DSM30011, AB031, and ATCC17978 inferred using Mauve. Each block corresponds to a DNA fragment of the chromosome distinctively colored for clarity. The degree of conservation is indicated by the vertical bars inside the blocks. Their position relative to the genome line denotes colinear and inverted regions. Putative prophage insertions (Ph1-3) are indicated below the DSM30011 genome (see supplementary table S6, Supplementary Material online for details). DNA inversions (Inv1-2) and the insertion of Tn6021 within comM in ATCC17978 are also shown (see text for details).
The inferred organization of the DSM30011 genome was compared with the whole genomes (chromosome and plasmids) of the two aforementioned A. baumannii strains. As seen in figure 2, a general shared synteny resulted between all of these genomes except for some worth-noting differences such as: 1) the presence, position and orientation of a 17 kbp-segment (Inv1) encompassing a GI conferring resistance to heavy metals (see also the immediate section below). This GI, which is notoriously missing in strain ATCC17978, is located in a different genomic locus and also in an inverted position in strain AB031 as compared with DSM30011; 2) several regions harboring putative prophages (Ph1-3) that will be described in detail below; 3) the presence, position, and orientation of a 33 kbp-segment (Inv2) encompassing a GI conferring resistance to heavy metals (see DSM30011 antimicrobial resistance). This GI is located in a different genomic locus and in an inverted position in strain ATCC17978 and is notoriously missing in strain AB031; 4) the interruption of the comM gene by Tn6021 in strain ATCC17978.
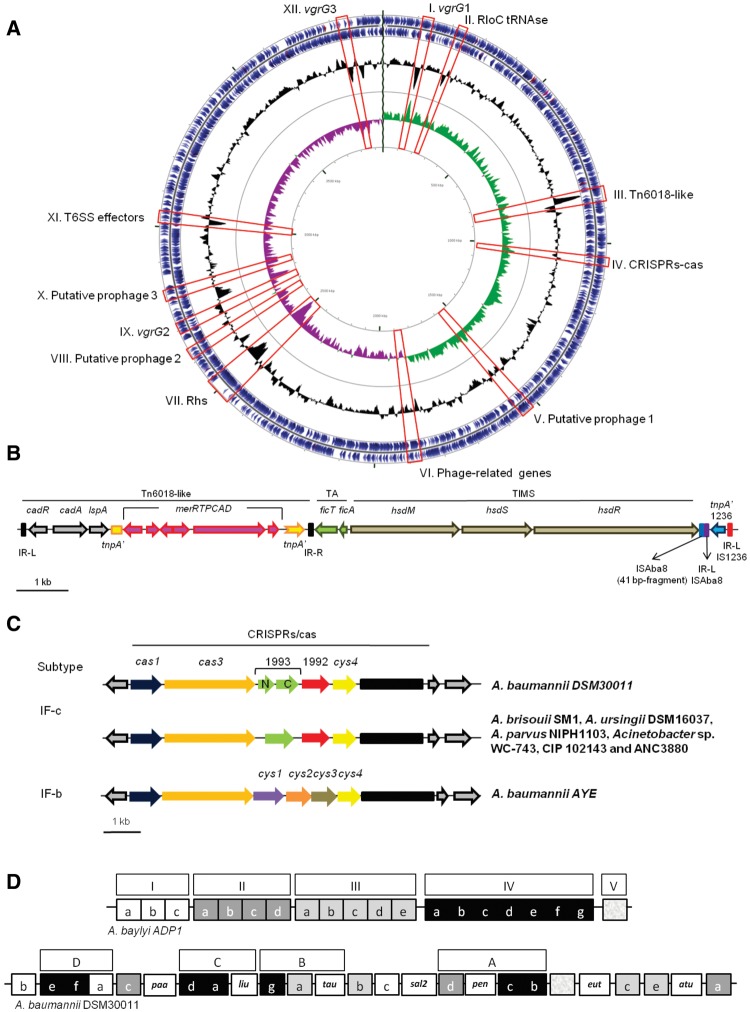
Two hundred and forty three proteins of the DSM30011 predicted proteome belong to protein families containing only DSM30011 CDS according to the parameters used to build the protein families with SILIX (see Methods). Subsequent BLAST sequence similarity-based comparison (e value cut-off 1 × e−4) of these 243 sequences against our local database (64 acinetobacter proteomes) revealed that 83 sequences have a hit in at least one other A. baumannii strain. Those sequences represent likely divergent homologues that could not be affiliated to any gene family because they did not fit the parameters used for protein family delineation by SILIX. one hundred thirty eight CDS (e value > 1 × e−4) presented no significant hits (false positives), and 22 CDS had no hits. Blasting the 243 CDS against a larger database containing >5,000 complete genomes from Archaea and major bacterial phyla (including 43 A. baumannii proteomes) revealed 89 CDS with significant best hits in A. baumannii; 52 CDS were affiliated with other gammaproteobacteria, 20 with other bacterial phyla, 69 (e value > 1 × e−4) provided no significant hits (false positives) and 13 showed no hits at all (supplementary table S4, Supplementary Material online). Among this group of 243 CDS, we distinguished 12 accessory gene clusters (AGC I–XII; table 2 and fig. 3A) from which eight were predicted as horizontally-transferred GIs (supplementary table S5, Supplementary Material online). Five AGCs (III, V, VI, VIII, and X), encompassed phage-related genes (table 2). In this regard, one incomplete and two intact prophages (supplementary table S6, Supplementary Material online) were identified in these DNA regions. All prophages shared significant sequence identities with the podoviral lytic bacteriophage YMC/09/02/B1251 ABA BP (NC_019541.1; Jeon etal. 2012). The intact prophages were 46.0 and 50.7 kbp long, contained a G + C of 39.2% and 37.9%, respectively, and harbored 66 and 69, respectively, open reading frames. Putative attL and attR recombination sites were detected in each case (supplementary table S6, Supplementary Material online).
Table 2.
Accessory Gene Clusters (AGC) Specific of DSM30011 as Compared with Other A. baumannii Genomes
| AGCa | GIb | Locus Tag (DSM30011_) | Locus Description |
|---|---|---|---|
| I | 1 | 00495–00510 | vgrG-associated region 1 |
| II | 2 | 00930–00940 | RloC-dependent phage defensive mechanism |
| III | 4 | 04065–04170 | Phage-related genes located next to Tn6018-like element |
| IV | – | 04925–04935 | CRISPRs-cas cluster |
| V | – | 07035–07240 | Putative prophage 1c |
| VI | – | 08780–08870 | Phage-related genes |
| VII | 7 | 11710–12025 | Region enriched in Rhs family protein-genes |
| VIII | – | 12710–13875 | Putative prophage 2c |
| IX | 8 | 13255–13320 | vgrG-associated region 2 |
| X | 9 | 13765–13660 | Putative prophage 3c |
| XI | 10 | 14490–14590 | T6SS putative effectors/Type I restriction-modification system |
| XII | 12 | 18385–18475 | vgrG-associated region 3 |
Refer to supplementary table S4, Supplementary Material online.
Refer to supplementary table S5, Supplementary Material online.
Refer to supplementary table S6, Supplementary Material online.
Fig. 3.
—Genomic features of A. baumannii DMS30011. (A) Genome map. The two outermost circles denote the positions of protein (blue) and RNA (red) coding sequences on the plus (circle 1) and minus (circle 2) strands. Circle 3 (black) indicates the GC content. Circle 4 denotes positive (green) and negative (purple) GC-skew. The different accessory gene clusters (AGC I–XII) characteristic of DSM30011 (see table 2 for details) are indicated by red boxes. The CGView software (Stothard and Wishart 2005) was used to construct the genome map. (B) Schematic representation of the genomic region encompassing Tn6018-like region and IS1236/ISAcsp3 mobile elements. (C) Schematic representation of the CRISPR-cas cluster. This locus (designated as subtype IF-c) is compared against a group of Acinetobacter strains bearing a locus with a similar genetic organization and also against A. baumannii AYE, the reference strain for the subtype IF-b. (D) Content and organization of catabolic loci in DSM30011 as compared with A. baylyi (Barbe etal. 2004). This figure follows and complements the schematical representation of catabolic loci elaborated by Di Nocera etal. (2011; see fig. 4 therein). Equivalent catabolic loci between the two strains are indicated by similar shades, and those present only in DSM30011 (liu, tau, pen, and atu) are indicated by open boxes. The composition of catabolic islands A–D in DSM30011 is indicated.
Interestingly, homologs of the error-prone DNA polymerase V subunits umuC (DSM30011_07275 and DSM30011_13575) and umuD (DSM30011_13570) were associated to or located within prophage regions 1 and 3 respectively (supplementary table S6, Supplementary Material online), a situation similar to that described for A. baumannii ATCC17978 (Hare etal. 2014). Furthermore, another umuD paralogue (DSM30011_10830) and genes encoding error-prone polymerases such as DinP (DSM30011_17910) and DnaE2 (DSM30011_10470; Norton etal. 2013) were found in the DSM30011 genome. The high frequency in Acinetobacter members of genes encoding these polymerases has already been noticed previously (Touchon etal. 2014), and proposed to play roles in the diversification of the genus including the mutational acquisition of resistance to toxins and antimicrobials.
RloC tRNAses have been proposed to be involved in resistance to phages (Davidov and Kaufmann 2008). A rloC homolog gene (DSM30011_00955) was identified next to AGC II (supplementary table S4, Supplementary Material online) in a region predicted to be part of GI 2 (supplementary table S5, Supplementary Material online) also present in A. baumannii ATCC19606.
AGCs I, IX, and XII encompass loci encoding T6SS-related VgrG proteins and associated antibacterial effectors (Repizo etal. 2015). Two additional gene clusters coding for putative T6SS-toxins (AGCs VII and XI) and cognate immunity proteins were also revealed by our analysis (supplementary table S4, Supplementary Material online). The diverse arsenal of T6SS-associated toxin genes encoded within GIs (supplementary table S5, Supplementary Material online) may possess adaptive significance, especially to compete with other microbes of the environment (Repizo etal. 2015).
A Mobile Elements Faulty Genome
Notably, and in sharp contrast to clinical A. baumannii strains (Peleg etal. 2012; Roca etal. 2012; Antunes etal. 2014; Touchon etal. 2014), an extremely low representation of transposable genetic elements such as IS or transposons was found in the DSM30011 genome. Nevertheless, defective versions of mobile genetic elements were found next to AGC III (supplementary tables S4 and S7, Supplementary Material online). In this region, we detected remnants of an IS1236/ISAcsp3 element (fig. 3B) of the IS3 family typical of the soil species Acinetobacter baylyi ADP1 (Gerischer and Ornston 1995), whose tnpA gene had been disrupted by an ISAba8-like element from which we located only the complete left inverted repeat (IR-L) and a short internal fragment of 41 bp (fig. 3B and supplementary fig. S4, Supplementary Material online). The latter fragment was preceded by the 3′ end of the hsdR gene belonging to a hsdMRS operon encoding a tripartite Type I restriction-modification (TIM) system and by two fic genes encoding a toxin–antitoxin (TA) system (fig. 3B). The association of TA and TIM systems in bacterial chromosomes in so-called defense islands (DI) against incoming DNA from viruses and plasmids has been previously reported, and their maintenance explained due to the deleterious effects resulting from the loss of the addictive elements that compose these systems (Makarova etal. 2011). Some DI are in turn associated to either active or inactivated mobile elements (Makarova etal. 2011), which seems the case for DSM30011. As seen in figure 3B, the TIM/TA arrangement is in turn preceded by a previously unreported Tn6018 variant (Tn6018-like) showing complete IR-L and IR-R repeats, but whose tnpA gene is interrupted by a merR-merTPCAD gene cluster coding for a complete Hg ion detoxification system (Boyd and Barkay, 2012). As seen in this figure this Tn6018 additionally carried cad genes encoding a Co/Zn/Cd ions tolerance system (Post etal. 2010). Tn6018 transposons are generally embedded within AbaR RI in clinical A. baumannii strains (Post etal. 2010), but this is clearly not the case of DSM30011 as discussed above. The particular assembly of metal detoxification systems in this Tn6018 variant may have provided DSM30011 cells with additional efflux capacity for different heavy metal ions, and represented an early stage in the formation of a RI toward toxic compounds increasingly found in the environment. Our search for similar assemblies in Acinetobacter genomes detected similar Tn6018 arrangements in the A. baumannii strains AB031, ATCC19606, 121738, 466760, MSP4-16 and in Acinetobacter sp. CIP 53.82.
An Atypical CRISPR-Cas Cluster in DSM30011
The genomic survey of DSM30011 identified a cluster of genes encoding a CRISPR-cas adaptive immune system (Makarova and Koonin 2015) located in AGC IV (supplementary table S8, Supplementary Material online). This cas cluster consists of six genes encoding a Cas1 endonuclease, a Cas3/Cas2 helicase/RNAse, a PBPRB1993-like N-terminal region, a PBPRB1993-like C-terminal region, a PBPRB1992-like protein, and a Csy4 protein, respectively (fig. 3C). Downstream of this locus it was found an array of 51 direct repeats of 28 bp, each separated by a 32 bp spacer (supplementary table S8, Supplementary Material online). A comparison between this system and the most common I-Fb CRISPR-cas subtype reported for A. baumannii (Touchon etal. 2014; Karah etal. 2015) indicated several differences (fig. 3C). The DSM30011 system could be assigned to the subtype I-F variant 2 of the classification of Makarova and Koonin (2015), in which PBPRB1992 and PBPRB1993 are in fact associated canonical proteins. A search for similar CRISPR-cas clusters identified analogous arrangements in two other A. baumannii strains: 869535 and 507_ABAU, in other Acinetobacter species such as Acinetobacter brisouii SM1, Acinetobacter ursingii DSM16037, and Acinetobacter parvus NIPH1103; and in Acinetobacter sp. isolates including WC-743, CIP 102143, and ANC3880 (fig. 3C and supplementary table S8, Supplementary Material online). Therefore, the CRISPR-cas cluster identified in DSM30011 and the above Acinetobacter strains represented a new subtype, for which we propose the designation I-Fc.
DSM30011 Antimicrobial Resistance
Conventional antimicrobial susceptibility assays indicated that DSM30011 showed clinical susceptibility to most antimicrobials tested except nitrofurantoin and, among β-lactams, ampicillin at MIC values just above CLSI recommended breakpoints (table 3). It is therefore not surprising that this strain lacks all previously characterized AbaR RI (Di Nocera etal. 2011; Roca etal. 2012). The ampicillin resistance of this strain most likely reflects the presence of β-lactamase genes (supplementary table S9, Supplementary Material online) including an ampC gene coding for an enzyme sharing 99.2% identity with cephalosporinase ADC-2 (GenBank accession WP_004746565.1) and a blaOXA-51-type gene encoding an enzyme identical to OXA-65 (GenBank accession AAW81337.1, Brown and Amyes 2005). The presence of these two genes in the chromosome of this environmental strain reinforces proposals that both provided for the intrinsic β-lactamase gene repertoire of A. baumannii (Turton etal. 2006; Hamouda etal. 2010, Roca etal. 2012; Evans and Amyes 2014).
Table 3.
DSM30011 Antimicrobial Susceptibility Phenotypes
| Method and Antimicrobials Tested | |||
|---|---|---|---|
| 1) Vitek-2 | MIC (µg/ml) |
||
| DSM30011 | ATCC17978 | ATCC19606 | |
| Ampicillin | 16 (R)a | 16 (R) | ≥32 (R) |
| Cefotaxime | 8 (S) | 8 (S) | 16 (R) |
| Ceftazidime | 4 (S) | 4 (S) | 16 (I) |
| Cefepime | 2 (S) | ≤1 (S) | 16 (I) |
| Imipenem | ≤0.25 (S) | ≤0.25 (S) | ≤0.25 (S) |
| Meropenem | ≤0.25 (S) | ≤0.25 (S) | 1 (S) |
| Amikacin | ≤2 (S) | ≤2 (S) | ≤2 (S) |
| Gentamicin | ≤1 (S) | ≤1 (S) | 8 (I) |
| Nalidixic acid | ≤2 (S) | 8 (S) | ≤2 (S) |
| Ciprofloxacin | ≤0.25 (S) | ≤0.25 (S) | 1 (S) |
| Nitrofurantoin | ≥512 (R) | ≥512 (R) | 256 (R) |
| Colistin | ≤0.5 (S) | ≤0.5 (S) | ≤0.5 (S) |
| Sulfamethoxazole/Trimetoprim (23.75/1.25) | ≤20 (S) | 160 (R) | ≥320 (R) |
| 2) Disk Diffusionb | Diameter Halo (mm) | ||
| Tetracyclin (30 µg) | 23 (S) | ||
| Azithromycin (15 µg) | 30 (n.s.c) | ||
| Erythromycin (15 µg) | 27 (n.s.) | ||
| Chloramphenicol (30 µg) | 15 (n.s.) | ||
Susceptibility interpretation (S: susceptible; R: resistant; I: intermediate) according to CLSI standards.
Tested only in DSM30011.
n.s.: not specified in the case of Acinetobacter spp., which are considered intrinsically resistant to these antibiotics. In the case of enterobacterial species such as Salmonella enterica serovar Typhi a 13 mm zone diameter for azithromycin (15 µg disk) is considered the limit separating susceptible from nonsusceptible isolates, and a 15 mm zone diameter for chloramphenicol (30 µg disk) corresponds to an isolate displaying intermediate resistance to this antibiotic.
DSM30011 also contains a carO (variant IV) homolog (DSM30011_04565) described only in A. baumannii (Mussi etal. 2011) and an oprD/occAB1 homolog (DSM30011_17845), two genes encoding outer membrane (OM) proteins proposed to participate in the permeation of carbapenems into the periplasm (Morán-Barrio etal. 2017). Resistance to cephalosporins and carbapenems in MDR strains of A. baumannii generally results, besides contributions of reduced OM permeability (Mussi etal. 2011; Morán-Barrio etal. 2017), from the overproduction of ADC-type and OXA-type enzymes mediated by IS insertions generating more efficient promoters upstream the corresponding β-lactamase genes (Brown and Amyes 2005; Ravasi etal. 2011; Roca etal. 2012; Evans and Amyes 2014). Although DSM30011 shows susceptibility to both cephalosporins and carbapenems (table 3), it is noteworthy that this strain isolated prior to 1,944 already contained the intrinsic genomic potentiality to evolve such resistances in a highly selective context.
DSM30011 also carries genes potentially providing resistance to aminoglycosides and chloramphenicol including aacA4, aadA, and catB2 (supplementary table S9, Supplementary Material online), indicating also a potentiality to evolve such resistances under selective pressure or, alternatively, to serve as reservoir of these resistance genes.
The susceptibility shown by DSM30011 to folate pathway inhibitors such as sulfamethoxazole/trimethoprim (table 3) is worth commenting. The synthetic sulfonamides were introduced for the treatment or prevention of bacterial infections before 1940, and represent the longest employed antimicrobials still in ample use in animal husbandry (Baran etal. 2011). Resistance among bacterial pathogens in the clinical setting emerged and disseminated rapidly after the introduction of sulfonamide therapy, a situation generally driven by mobile genetic elements carrying resistance genes including sulfonamide-resistant forms of dihydropteroate synthase (sul1, sul2) or different trimethoprim-resistant dihydrofolate reductase (dfr; Baran etal. 2011; Nigro and Hall 2011). The susceptibility of DSM30011 to sulfamethoxazole/trimethoprim (table 3) is thus compatible with the absence of sul or dfrA resistance genes in the genome (this work), and represents a notable exception when compared with clinical strains of A. baumannii (Post etal. 2010; Di Nocera etal. 2011; Nigro and Hall 2011; Roca etal. 2012; Loewen etal. 2014; Hamidian and Hall 2016). In this context, the A. baumannii clinical strains ATCC19606 and ATCC17978, which were isolated from human clinical samples as early as 1948 and 1951, respectively (supplementary table S1, Supplementary Material online), display sulfamethoxazole/trimethoprim resistance phenotypes (table 3, see also Nigro and Hall 2011; Hamidian and Hall 2016). Sulfonamide resistance in these strains most likely resulted from the lateral acquisition of GIsul2-like mobile elements, which were amply distributed among enterobacterial pathogens in the clinical setting worldwide during the first half of last century (Nigro and Hall 2011). The antimicrobial susceptibility shown by DSM30011 (table 3) is thus compatible with an original isolation of this strain in an environment still free of the selection pressure common to the clinical setting at that time.
Genes encoding components of the RND, DMT, MATE, MFS, and SMR efflux systems involved in clinical Acinetobacter strains in the extrusion of toxic compounds including some antimicrobials (Roca etal. 2012) were also found in the DSM30011 genome (supplementary table S9, Supplementary Material online). Thus, DSM30011 is also endowed with the capability to evolve reduced susceptibility to at least some of these antimicrobials due to the selection of particular mutations under the context of the corresponding selection pressures.
DSM30011 also contains many gene clusters encoding systems involved in the detoxification of noxious compounds (supplementary table S9, Supplementary Material online). Because all of the above loci are also present in both MDR and drug-susceptible A. baumannii strains, we regard them as part of the general detoxification mechanisms intrinsic to this species. In DSM30011 some of these clusters are scattered throughout the genome such as those for Hg and chromate ions (supplementary table S9, Supplementary Material online), while others are concentrated in a region of around 33 kbp constituting a GI (DSM30011_16215 to 16045) integrated next to the dusA gene (DSM30011_16220). The latter has been found to represent a common integration site for this kind of genetic elements in A. baumannii (Farrugia etal. 2015). This 33 kbp GI includes arsenate and heavy metal ion detoxification systems such as ars, czc, cop, and other genes putatively involved in Fe ions transport (feoAB).
Catabolic Potential
WGS analysis identified genes encoding many metabolic pathways involved in the utilization of a large variety of environmental compounds, including many substances produced by plants (supplementary table S10, Supplementary Material online). The presence and organization of different catabolic genes in DSM30011 as compared with the soil bacterium A. baylyi (Barbe etal. 2004) are shown in figure 3D and supplementary table S10, Supplementary Material online. In A. baylyi ADP1 many catabolic loci are clustered in the genome forming five major islands (I–V), which are in turn grouped in a so-called archipelago of catabolic diversity (Barbe etal. 2004). From the 20 loci forming this archipelago in A. baylyi, 18 equivalent clusters were detected in DSM30011 (fig. 3D and supplementary table S10, Supplementary Material online). Still, some differences were observed in both the organization and even the content of catabolic loci between these two Acinetobacter species. These differences included the absence in DSM30011 of loci IIb and IIId encoding the catabolisms of aryl esters and nitriles, a situation also reported for other A. baumannii strains (Di Nocera etal. 2011). In addition, seven other catabolic loci present in DSM30011 were not identified in A. baylyi including three (eut, pen, and tau) assigned to the use of ethanolamine, penicillin and taurine, respectively, in other A. baumannii strains (Vallenet etal. 2008). Other four catabolic loci also identified in DSM30011 but not in A. baylyi were atu, liu, sal2/gen, and paa (fig. 3D and supplementary table S10, Supplementary Material online). The atu locus in particular encodes a complete acyclic terpene degradation pathway (supplementary table S11, Supplementary Material online) homologous to that present in Pseudomonas aeruginosa PAO1 (Förster-Fromme etal. 2006). Similarly to P. aeruginosa, this pathway is most likely complemented with the liu (leucine/isovalerate degradation) cluster (Förster-Fromme etal. 2006) which was identified in DSM30011 in catabolic island C (fig. 3D, supplementary tables S10 and S11, Supplementary Material online). It is worth noting that acyclic monoterpenes are major components of isoprenoid oleoresins produced by many plants in response to insects and microbial pathogens (Franceschi etal. 2005; Zulak and Bohlmann 2010). In addition, a search among Acinetobacter genomes (supplementary table S3, Supplementary Material online) for this atu locus indicated its presence in all Acb complex strains, while on the contrary it was found in only 8 out of 23 non-Acb strains (Acinetobacter guillouiae CIP 63.46, Acinetobacter venetianus RAG-1 and VE_C3, Acinetobacter gerneri DSM 14967, Acinetobacter beijerinckii ANC 3835, Acinetobacter tjernbergiae DSM 14971, and the Acinetobacter sp. isolates ANC4105 and NIPH758).
Further attention was also given to the dca/pca/qui/pob/hca/van catabolic loci (Smith etal. 2003), from which the first five are located in island IV of the A. baylyi catabolic archipelago and van in island Ia (Barbe etal. 2004). The same loci were also located in the DSM30011 genome, but scattered among four different catabolic islands (fig. 3D and supplementary table S10, Supplementary Material online). These loci are involved in the degradation of long-chain dicarboxylic fatty acids and hydroxylated aromatic acids including hydroxycinnamic acids, all compounds constituting the hydrophobic heteropolymer suberin (Smith etal. 2003; Barbe etal. 2004). Suberin is located at the outermost layers of plant barks and functions to protect against the attack of insects and pathogens and also to prevent water loss from the tissues below (Franceschi etal. 2005; Graça 2015). The capability of DSM30011 to participate in the breaking down of many plant substances including chemical and physical plant defenses (see above), added to the ability to withstand elevated temperatures (supplementary fig. S1B, Supplementary Material online), are compatible with adaptive features selected to thrive in a particularly hostile niche provided by resin-producing desert plants (Allen etal. 1944).
Persistence and Virulence
To analyze the presence of genes potentially involved in persistence and virulence in DSM30011, we first constructed a list of all potential candidates and their regulatory genes (supplementary table S12, Supplementary Material online) described in Acinetobacter strains (Roca etal. 2012; Antunes etal. 2014; Eijkelkamp etal. 2014). The list included genes coding for the synthesis of the capsule and other exopolysaccharides, appendages, OM proteins and the T2SS; and also those involved in traits such as motility and iron scavenging. We excluded the superoxide dismutase sod gene (Heindorf etal. 2014) and the Tuf elongation factor tuf gene (Koenigs etal. 2015) because their ubiquity among the components of the Bacteria realm argues against any specific role of the corresponding gene products in virulence. The genes described in supplementary table S12, Supplementary Material online were thus used as queries to analyze for the presence of orthologs in the DSM30011 genome. As seen in the table 4, most genes encoding proteins with high sequence identity (>90%) to all potential virulence factors analyzed were found in DSM30011.
Table 4.
Presence in DSM30011 of Virulence and Environmental Persistence Factors Proposed for A. baumannii
| Virulence/Persistence Factor | Gene ID (DSM30011_) | Associated Genes | Homologous Loci in Other Acinetobacter Members | Reference |
|---|---|---|---|---|
| EPS | 18650–18570 | K locus | – | Hu etal. 2013 |
| 02660–02705 | OC locus | – | ||
| 01520–01525 | O-antigen ligases | M215_10480-75 | Harding etal. 2015 | |
| 06380–06395 | PNAG synthesis I | A1S_2162-60, A1S_3792 | Roca etal. 2012; Antunes etal. 2014 | |
| 14355–14365 | PNAG synthesis II | A1S_0940-38 | ||
| Appendages | 06115–06140 | Csu | A1S_2218-13 | Eijkelkamp etal. 2014 |
| 06750–06765 | Pilus 2 | A1S_2091-88 | ||
| 08905–08925 | P pilus | AB57_2007-03 | ||
| 10220–10235 | Pilus 3 | A1S_1510-07 | ||
| 01445–01465; 01565–01595; 03095–03110; 14670–14675; 17280–17290 | T4P | – | Eijkelkamp etal. 2011; Wilharm etal. 2013 | |
| 04375/15945 | ComEC/ComA | A1S_2610 | ||
| Outer membrane proteins | 03680–03690 | Bap system | ABBFA_RS03900 | De Gregorio etal. 2015 |
| 05060 | Bap-like | ABBFA_RS05040 | ||
| 00870 | Omp33 | A1S_3297 | Roca etal. 2012; Antunes etal. 2014 | |
| 02970 | OmpA | A1S_2840 | ||
| 12485 | OmpA like 1 | A1S_1193 | ||
| 14740 | OmpA like 2 | A1S_0884 | ||
| 13900 | Ata | A1S_1033-32 | ||
| 18275 | Pmt | A1S_0108 | ||
| Motility | 05005–05010 | DAP synthesis | A1S_2454-53 | Skiebe etal. 2012 |
| 18220–18255 | Biosurfactant synthesis | A1S_0119-12 | Clemmer etal. 2011 | |
| Iron scavenging | 05225–05310 | Acinetobactin | ACICU_02570-89 | Antunes etal. 2011; Mortensen and Skaar 2013 |
| 09010–09020 | Fur2-EntBA | ACICU_00267-65 | ||
| 09570–09625 | Hydroxamate | ACICU_01683-72 | ||
| 09785–09820 | Heme | ACICU_01639-32 | ||
| 17690–17700 | Ferrous ion | ACICU_00267-65 | ||
| T2SSb | 17575–17565; 05860–05865; 15765; 17280; 05690; 17080–17085; 09920–09935 | M215_01210-20; 03715-20; 07180; 08525; 08315-20; 14045-14060 | Harding etal. 2016 | |
| Other factors | 08955/02470/02730 | Pld1/2/3 | HMPREF0010_00607/03706/02731 | Stahl etal. 2015 |
| 06960/18680 | Plc1/2 | HMPREF0010_03297/00294 | Fiester etal. 2016 | |
| – | CpaA | M215_05100 | Tilley etal. 2014; Harding etal. 2016 | |
| 13925 | CipA | HMPREF0010_01565 | Koenigs etal. 2016 |
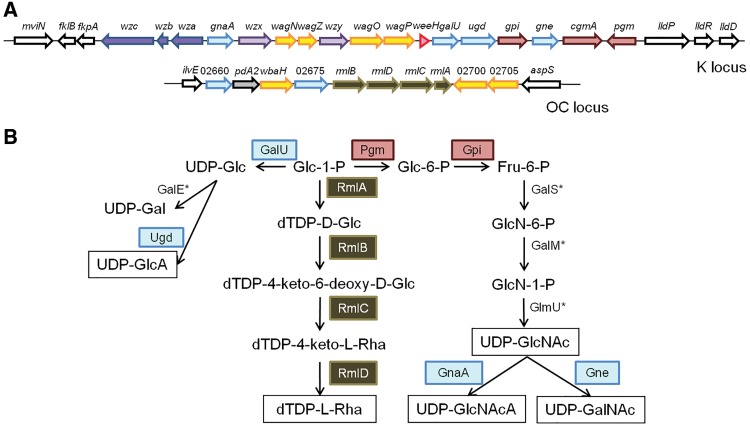
Among idiosyncratic features of proposed DSM30011 virulence genes worth remarking, we found differences in content and organization of genes linked to the production of K capsule-synthesizing proteins (Hu etal. 2013). The K locus identified in the DSM30011 genome (fig. 4, table 4 and supplementary table S12, Supplementary Material online) displayed a gene organization similar to that previously described for the polysaccharide gene cluster PSgc 20 reported for other A. baumannii strains (Hu etal. 2013). Still, the wagY glycosyl-transferase gene on the PSgc 20 cluster was found to be replaced by a wagN encoding the same function in DSM30011 (fig. 4A). Database searching identified the same arrangement in A. baumannii strains NIPH 601 and 299505, and in Acinetobacter sp. NIPH 1859, therefore indicating a previously unreported PSgc locus common to the above Acinetobacter strains.
Fig. 4.
—A. baumannii DSM30011 major polysaccharides. (A) Scheme showing the genetic organization of K and OC locus. Genes are colored according to the code used in supplementary table S12, Supplementary Material online. (B) Proposed biosynthetic pathways for sugars precursors of the main exopolysaccharides. Glc, d-glucose; GlcA, d-glucuronic acid; GlcN, 2-amino-2-deoxy-d-glucose; GlcNAc, 2-acetamido-2-deoxy-d-glucose; GlcNAcA, 2-acetamido-2-deoxy-d-glucuronic acid; Gal, d-galactose; GalA, d-galacturonic acid; GalNAc, 2-acetamido-2-deoxy-d-galactose. *The genes coding for these enzymes are located outside the K or OC gene clusters.
Furthermore, the gene locus involved in the synthesis of the outer core (OC) of the lipid A core moiety in DSM30011 includes the rmlBDAC cluster (supplementary table S12, Supplementary Material online) responsible of the biosynthesis of dTDP-l-rhamnose (fig. 4B; Russo etal. 2010, Hu etal. 2013). Rhamnose is a common constituent of Acinetobacter surface polysaccharides, and its presence in the OC has been reported to contribute to surface motility in Acinetobacter nosocomialis strain M2 (Clemmer etal. 2011).
Another feature worth noting in DSM30011 is the composition of the Bap protein (supplementary fig. S5, Supplementary Material online and table 4). This biofilm-associated OM protein has been shown to participate in biofilm maturation on different abiotic surfaces and in adherence to eukaryotic cells in some A. baumannii strains (Roca etal. 2012; Antunes etal. 2014). Bap is composed almost entirely of tandemly arranged amino acid repeats typical of adhesins (bacterial Ig-like 3 domains), and possess a type-1 secretion system target domain probably responsible for its export to the OM. Both the composition and number of tandem repeats among Bap proteins in the A. baumannii population are highly heterogeneous, and even the bap gene was found separated into different CDSs in some strains (De Gregorio etal. 2015). The latter was in fact the case for DSM30011, in which the bap gene was divided in two contiguous CDSs in a genomic locus that was conserved in other A. baumannii strains (supplementary table S12 and fig. S5, Supplementary Material online).
The A. baumannii cpaA gene encodes the metallopeptidase Cpa, an enzyme with the ability to cleave fibrinogen and deregulate blood coagulation (Tilley etal. 2014). CpaA, which was proposed to represent a virulence factor (Tilley etal. 2014), has been shown recently to represent a substrate of the Type II secretion system (Harding etal. 2016). Therefore, the conspicuous absence of cpaA in DSM30011 (table 4) and in the A. baumannii strains ATCC 17978 and ATCC 19606 isolated by the middle of last century (supplementary table S1, Supplementary Material online), supports proposals that this gene was recently acquired by A. baumannii in the clinical setting as the result of horizontal gene transfer events (Harding etal. 2016).
Conclusions
We traced in this work the origins of the A. baumannii DSM30011 collection strain to an isolate first reported in 1944 obtained from retting enrichment of the natural microbiota associated to a resin-producing desert shrub, guayule (Allen etal. 1944; Naghski etal. 1944). DSM30011 thus represents, to our knowledge, the earliest reported environmental A. baumannii strain isolated at the onset of the massive use of antibiotics starting by the middle of last century (Antunes etal. 2014). In concordance, DSM30011 showed general susceptibility to most clinically-employed antimicrobials including folate pathway inhibitors (table 3). Thus, this strain may certainly provide clues on the genomic content and diversity as well as niche ranges of the A. baumannii population that existed before the strong antimicrobial selection pressure associated to the antibiotic era (Antunes etal. 2014).
The genome analysis of DSM30011 revealed most of the traits that have been associated to Acinetobacter persistence and pathogenicity (Peleg etal. 2012; table 4 and supplementary table S12, Supplementary Material online). These include: 1) Two systems likely conferring the ability to endure in different environmental niches, such as a T6SS and associated gene clusters as well as a CRISPRs-cas system previously unreported in this species; 2) resistance genes against antimicrobials and other toxic compounds, including ADC-type AmpC and OXA-51-like β-lactamases and different efflux pumps; 3) systems driving genomic diversification such as different error-prone polymerases and Type IV pilus-mediated transformability; 4) genes encoding virulence mechanisms proposed for Acb clinical strains (table 4). On the contrary, DSM30011 lacks all RIs, ISs, GIs and plasmids typical of Acinetobacter clinical strains. This strongly suggests that these genetic elements of strong adaptive significance were recently acquired by infective A. baumannii lineages, most likely from other bacterial species co-existing in the clinical setting (Nigro and Hall 2011; Peleg etal. 2012; Antunes etal. 2014). This supports the notion that the highly selective conditions of the nosocomial environment have been crucial for both the adjustment of the expression of pathogenicity determinants and the acquisition of additional resistance traits that turned this species into a nosocomial pathogen (Antunes etal. 2014).
We also identified a previously unreported catabolic locus responsible of the complete degradation of acyclic monoterpenes, in addition to other catabolic pathways for many substances produced by plants including protective woody tissues (fig. 3D, supplementary tables S10 and S11, Supplementary Material online). The above features, added to the ability to endure high temperatures, are compatible with an organism thriving on resinous desert plants thus supporting such an environmental origin for DSM30011 (Allen etal. 1944). The presence of similar genomic features in all analyzed A. baumannii strains further suggests that resinous plants may provide environmental reservoirs for this species. In turn, it opens the possibility that certain phytophagous insects feeding in these plants (Morales-Jiménez etal. 2009; Keeling etal. 2013; Vilanova etal. 2014) represent vectors for the dissemination of A. baumannii in the environment.
Further studies on other A. baumannii strains of non-clinical origin are certainly required to elucidate the processes that led to the recent evolution of this species toward an opportunistic pathogen of humans.
Supplementary Material
Acknowledgments
We thank X. Charpentier (CIRI, Lyon, France) for the kind gift of the DSM30011 strain. We are indebted to J. Swezey (National Center for Agricultural Utilization Research, Agricultural Research Service, U.S. Department of Agriculture, Peoria, IL, USA) for providing us most valuable information concerning the NRRL collection strains B-551 and B-552. We are indebted to the curator teams of both the Institut Pasteur MLST system (Paris, France) and Oxford University (Oxford, UK) for their help in incorporating DSM30011 alleles and profiles at http://pubmlst.org/abaumannii/. We also thank P. Stothard for his generous help with the CGView software. This work was supported by a FINOVI Young Researcher Grant awarded to S.P.S.; by an Investissement d'Avenir (ANR-10-BINF-01-01) grant awarded to C.B.-A., and from grants to A.M.V. from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT); Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET-PIP1055); Ministerio de Ciencia, Tecnología e Innovación Productiva, Provincia de Santa Fe; Argentina. A.M.V., G.D.R., and M.E. are staff members of CONICET. C.B.-A. is member of the Institut Universitaire de France.
Supplementary Material
Supplementary data are available at Genome Biology and Evolution online.
Literature Cited
- Allen PJ, Naghski J, Hoover SR.. 1944. Decomposition of guayule resins by microorganisms. J Bacteriol. 47(6):559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes LC, Imperi F, Towner KJ, Visca P.. 2011. Genome-assisted identification of putative iron-utilization genes in Acinetobacter baumannii and their distribution among a genotypically diverse collection of clinical isolates. Res Microbiol. 162(3):279–284. [DOI] [PubMed] [Google Scholar]
- Antunes LCS, Visca P, Towner KJ.. 2014. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis. 71(3):292–301. [DOI] [PubMed] [Google Scholar]
- Arndt D, et al. 2016. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 44(W1):W16–W21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz RK, et al. 2008. The RAST server: rapid annotations using subsystems technology. BMC Genomics. 9:75.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran W, Adamek E, Ziemiańska J, Sobczak A.. 2011. Effects of the presence of sulfonamides in the environment and their influence on human health. J Hazard Mater. 196:1–15. [DOI] [PubMed] [Google Scholar]
- Barbe V, et al. 2004. Unique features revealed by the genome sequence of Acinetobacter sp. ADP1, a versatile and naturally transformation competent bacterium. Nucleic Acids Res. 32(19):5766–5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartual SG, et al. 2005. Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J Clin Microbiol. 43(9):4382–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland C, et al. 2007. CRISPR Recognition Tool (CRT): a tool for automatic detection of clustered regularly interspaced palindromic repeats. BMC Bioinformatics. 8:209.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvet PJM, Grimont PAD.. 1986. Taxonomy of the genus Acinetobacter with the recognition of Acinetobacter baumannii sp. nov., Acinetobacter haemolyticus sp. nov., Acinetobacter johnsonii sp. nov., and Acinetobacter junii sp. nov. and emended descriptions of Acinetobacter calcoaceticus and Acinetobacter lwoffii. Int J Syst Bacteriol. 36(2):228–240. [Google Scholar]
- Boyd E, Barkay T.. 2012. The mercury resistance operon: from an origin in a geothermal environment to an efficient detoxification machine. Front Microbiol. 3:349.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Amyes SG.. 2005. The sequences of seven class D beta-lactamases isolated from carbapenem-resistant Acinetobacter baumannii from four continents. Clin Microbiol Infect. 11(4):326–329. [DOI] [PubMed] [Google Scholar]
- Clemmer KM, Bonomo RA, Rather PN.. 2011. Genetic analysis of surface motility in Acinetobacter baumannii. Microbiology 157(Pt 9):2534–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscuolo A, Gribaldo S.. 2010. BMGE (Block Mapping and Gathering with Entropy): a new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol Biol. 10:210.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling AE, Mau B, Perna NT.. 2010. ProgressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE. 5(6):e11147.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidov E, Kaufmann G.. 2008. RloC: a wobble nucleotide-excising and zinc-responsive bacterial tRNase. Mol Microbiol. 69(6):1560–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio E, et al. 2015. Biofilm-associated proteins: news from Acinetobacter. BMC Genomics. 16:933.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon BK, et al. 2015. IslandViewer 3: more flexible, interactive genomic island discovery, visualization and analysis. Nucleic Acids Res. 43(W1):104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diancourt L, Passet V, Nemec A, Dijkshoorn L, Brisse S.. 2010. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS ONE. 5(4):e10034.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nocera PP, Rocco F, Giannouli M, Triassi M, Zarrilli R.. 2011. Genome organization of epidemic Acinetobacter baumannii strains. BMC Microbiol. 11:224.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp BA, et al. 2011. Adherence and motility characteristics of clinical Acinetobacter baumannii isolates. FEMS Microbiol Lett. 323(1):44–51. [DOI] [PubMed] [Google Scholar]
- Eijkelkamp BA, Stroeher UH, Hassan KA, Paulsen IT, Brown MH.. 2014. Comparative analysis of surface-exposed virulence factors of Acinetobacter baumannii. BMC Genomics. 15:1020.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BA, Amyes SJ.. 2014. OXA β-lactamases. Clin Microbiol Rev. 27(2):241–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eveillard M, Kempf M, Belmonte O, Pailhoriès H, Joly-Guillou ML.. 2013. Reservoirs of Acinetobacter baumannii outside the hospital and potential involvement in emerging human community-acquired infections. Int J Infect Dis. 17(10):e802–e805. [DOI] [PubMed] [Google Scholar]
- Farrugia DN, Elbourne LD, Mabbutt BC, Paulsen IT.. 2015. A novel family of integrases associated with prophages and genomic islands integrated within the tRNA-dihydrouridine synthase A (dusA) gene. Nucleic Acids Res. 43(9):4547–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiester SE, et al. 2016. Iron-regulated phospholipase c activity contributes to the cytolytic activity and virulence of Acinetobacter baumannii. PLoS ONE. 11(11):e0167068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster-Fromme K, et al. 2006. Identification of genes and proteins necessary for catabolism of acyclic terpenes and leucine/isovalerate in Pseudomonas aeruginosa. Appl Environ Microbiol. 72(7):4819–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi VR, Krokene P, Christiansen E, Krekling T.. 2005. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 167(2):353–375. [DOI] [PubMed] [Google Scholar]
- Gao F, Zhang C-T.. 2008. Ori-Finder: a web-based system for finding oriCs in unannotated bacterial genomes. BMC Bioinformatics. 9:79.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graça J. 2015. Suberin: the biopolyester at the frontier of plants. Front Chem. 3:62.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerischer U, Ornston LN.. 1995. Spontaneous mutations in pcaH and -G, structural genes for protocatechuate 3, 4-dioxygenase in Acinetobacter calcoaceticus. J Bacteriol. 177(5):1336–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundi VA, Dijkshoorn L, Burignat S, Raoult D, La Scola B.. 2009. Validation of partial rpoB gene sequence analysis for the identification of clinically important and emerging Acinetobacter species. Microbiology 155(Pt 7):2333–2341. [DOI] [PubMed] [Google Scholar]
- Hamidian M, Hall RM.. 2016. Acinetobacter baumannii ATCC 19606 carries GIsul2 in a genomic island located in the chromosome. Antimicrob Agents Chemother. 61(1):e01991–16. pii: e01991-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamouda A, Evans BA, Towner KJ, Amyes SGB.. 2010. Characterization of epidemiologically unrelated Acinetobacter baumannii isolates from four continents by use of multilocus sequence typing, pulsed-field gel electrophoresis, and sequence-based typing of blaOXA-51-like genes. J Clin Microbiol. 48(7):2476–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding CM, et al. 2015. Acinetobacter strains carry two functional oligosaccharyltransferases, one devoted exclusively to type IV pilin, and the other one dedicated to O-glycosylation of multiple proteins. Mol Microbiol. 96(5):1023–1041. [DOI] [PubMed] [Google Scholar]
- Harding CM, Kinsella RL, Palmer LD, Skaar EP, Feldman MF.. 2016. Medically relevant Acinetobacter species require a type II secretion system and specific membrane-associated chaperones for the export of multiple substrates and full virulence. PLoS Pathog. 12(1):e1005391.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare JM, Ferrell JC, Witkowski TA, Grice AN.. 2014. Prophage induction and differential RecA and UmuDAb transcriptome regulation in the DNA damage responses of Acinetobacter baumannii and Acinetobacter baylyi. PLoS ONE. 9(4):e93861.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindorf M, Kadari M, Heider C, Skiebe E, Wilharm G.. 2014. Impact of Acinetobacter baumannii superoxide dismutase on motility, virulence, oxidative stress resistance and susceptibility to antibiotics. PLoS ONE. 9(7):e101033.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Liu B, Dijkshoorn L, Wang L, Reeves PR.. 2013. Diversity in the major polysaccharide antigen of Acinetobacter baumannii assessed by DNA sequencing, and development of a molecular serotyping scheme. PLoS ONE. 8(7):e70329.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J, Kim J, Yong D, Lee K, Chong Y.. 2012. Complete genome sequence of the podoviral bacteriophage YMC/09/02/B1251 ABA BP, which causes the lysis of an OXA-23 producing carbapenem-resistant Acinetobacter baumannii isolate from a septic patient. J Virol. 86(22):12437–12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolley KA, Maiden MC.. 2010. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics. 11(1):595.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karah N, Sundsfjord A, Towner K, Samuelsen Ø.. 2012. Insights into the global molecular epidemiology of carbapenem non-susceptible clones of Acinetobacter baumannii. Drug Resist Update. 15(4):237–247. [DOI] [PubMed] [Google Scholar]
- Karah N, et al. 2015. CRISPR-cas subtype I-Fb in Acinetobacter baumannii: evolution and utilization for strain subtyping. PLoS ONE. 10(2):e0118205.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling CI, et al. 2013. Draft genome of the mountain pine beetle, Dendroctonus ponderosae Hopkins, a major forest pest. Genome Biol. 14:R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs A, Zipfel PF, Kraiczy P.. 2015. Translation elongation factor Tuf of Acinetobacter baumannii is a plasminogen-binding protein. PLoS ONE. 10(7):e0134418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs A, et al. 2016. CipA of Acinetobacter baumannii is a novel plasminogen binding and complement inhibitory protein. J Infect Dis. 213:1388–1399. [DOI] [PubMed] [Google Scholar]
- La Scola B, Gundi VA, Khamis A, Raoult D.. 2006. Sequencing of the rpoB gene and flanking spacers for molecular identification of Acinetobacter species. J Clin Microbiol. 44(3):827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limansky AS, Viale AM.. 2002. Can composition and structural features of oligonucleotides contribute to their wide-scale applicability as random PCR primers in mapping bacterial genome diversity? J Microbiol Methods. 50(3):291–297. [DOI] [PubMed] [Google Scholar]
- Loewen PC, Alsaadi Y, Fernando D, Kumar A.. 2014. Genome sequence of a tigecycline-resistant clinical isolate of Acinetobacter baumannii strain AB031 obtained from a bloodstream infection. Genome Announc. 2(5):e01036–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo F, Vuotto C, Donelli G.. 2014. Biofilm formation in Acinetobacter baumannii. New Microbiol. 37(2):119–127. [PubMed] [Google Scholar]
- Makarova KS, Wolf YI, Snir S, Koonin EV.. 2011. Defense islands in bacterial and archaeal genomes and prediction of novel defense systems. J Bacteriol. 193(21):6039–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova KS, Koonin EV.. 2015. Annotation and classification of CRISPR-Cas Systems In: Lundgren M, Charpentier E, Fineran PC, editors. CRISPR. Methods and protocols. Vol. 1311 New York: Springer; pp. 47–75. ISBN 978-1-4939-2686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele V, Penel S, Duret L.. 2011. Ultra-fast sequence clustering from similarity networks with SiLiX. BMC Bioinformatics. 12:116.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Jiménez J, Zúñiga G, Villa-Tanaca L, Hernández-Rodríguez C.. 2009. Bacterial community and nitrogen fixation in the red turpentine beetle, Dendroctonus valens LeConte (Coleoptera: Curculionidae: Scolytinae). Microb Ecol. 58(4):879–891. [DOI] [PubMed] [Google Scholar]
- Morán-Barrio J, et al. 2017. The Acinetobacter outer membrane contains multiple specific channels for carbapenem β-lactams as revealed by kinetic characterization analyses of imipenem permeation into Acinetobacter baylyi cells. Antimicrob Agents Chemother. 61(3):e01737–16.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen BL, Skaar EP.. 2013. The contribution of nutrient metal acquisition and metabolism to Acinetobacter baumannii survival within the host. Front Cell Infect Microbiol. 3:95.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussi MA, et al. 2011. Horizontal gene transfer and assortative recombination within the Acinetobacter baumannii clinical population provide genetic diversity at the single carO gene, encoding a major outer membrane protein channel. J Bacteriol. 193(18):4736–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghski J, White JW, Hoover SR.. 1944. Aerobic decomposition of Guayule Shrub (Parthenium argentatum Gray). J Bacteriol. 48(2):159–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nait Chabane Y, et al. 2014. Characterisation of pellicles formed by Acinetobacter baumannii at the air–liquid interface. PLoS ONE. 9(10):e111660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemec A, et al. 2011. Genotypic and phenotypic characterization of the Acinetobacter calcoaceticus–Acinetobacter baumannii complex with the proposal of Acinetobacter pittii sp. nov. (formerly Acinetobacter genomic species 3) and Acinetobacter nosocomialis sp. nov. (formerly Acinetobacter genomic species 13TU). Res Microbiol. 162(4):393–404. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ.. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro SJ, Hall RM.. 2011. GIsul2, a genomic island carrying the sul2 sulphonamide resistance gene and the small mobile element CR2 found in the Enterobacter cloacae subspecies cloacae type strain ATCC 13047 from 1890, Shigella flexneri ATCC 700930 from 1954 and Acinetobacter baumannii ATCC 17978 from 1951. J Antimicrob Chemother. 66(9):2175–2176. [DOI] [PubMed] [Google Scholar]
- Nigro SJ, Post V, Hall RM.. 2011. Aminoglycoside resistance in multiply antibiotic-resistant Acinetobacter baumannii belonging to global clone 2 from Australian hospitals. J Antimicrob Chemother. 66(7):1504–1509. [DOI] [PubMed] [Google Scholar]
- Norton MD, Spilkia AJ, Godoy VG.. 2013. Antibiotic resistance acquired through a DNA damage-inducible response in Acinetobacter baumannii. J Bacteriol. 195(6):1335–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg AY, et al. 2012. The success of Acinetobacter species; genetic, metabolic and virulence attributes. PLoS ONE. 7(10):e46984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post V, White PA, Hall RM.. 2010. Evolution of AbaR-type genomic resistance islands in multiply antibiotic-resistant Acinetobacter baumannii. J Antimicrob Chemother. 65(6):1162–1170. [DOI] [PubMed] [Google Scholar]
- Ravasi P, Limansky AS, Rodriguez RE, Viale AM, Mussi MA.. 2011. ISAba825, a functional insertion sequence modulating genomic plasticity and bla(OXA-58) expression in Acinetobacter baumannii. Antimicrob Agents Chemother. 55(2):917–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repizo GD, et al. 2014. Genomic comparative analysis of the environmental Enterococcus mundtii against enterococcal representative species. BMC Genomics. 15(1):489.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repizo GD, et al. 2015. Differential role of the T6SS in Acinetobacter baumannii virulence. PLoS ONE. 10(9):e0138265.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca I, Espinal P, Vila-Farrés X, Vila J.. 2012. The Acinetobacter baumannii oxymoron: commensal hospital dweller turned pan-drug-resistant menace. Front Microbiol. 3:148.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo T, et al. 2010. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect Immun. 78(9):3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M.. 2006. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 34(90001):D32–D36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiebe E, et al. 2012. Surface-associated motility, a common trait of clinical isolates of Acinetobacter baumannii, depends on 1, 3-diaminopropane. Int J Med Microbiol. 302(3):117–128. [DOI] [PubMed] [Google Scholar]
- Smith MA, Weaver VB, Young DM, Ornston LN.. 2003. Genes for chlorogenate and hydroxycinnamate catabolism (hca) are linked to functionally related genes in the dca-pca-qui-pob-hca chromosomal cluster of Acinetobacter sp. strain ADP1. Appl Environ Microbiol. 69(1):524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl J, Bergmann H, Göttig S, Ebersberger I, Averhoff B.. 2015. Acinetobacter baumannii virulence is mediated by the concerted action of three phospholipases D. PLoS ONE. 10(9):e0138360.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothard P, Wishart DS.. 2005. Circular genome visualization and exploration using CGView. Bioinformatics. 21(4):537–539. [DOI] [PubMed] [Google Scholar]
- Tada T, Miyoshi-Akiyama T, Shimada K, Shimojima M, Kirikae T.. 2014. Dissemination of 16S rRNA methylase armA-producing Acinetobacter baumannii and emergence of OXA-72 carbapenemase coproducers in Japan. Antimicrob Agents Chemother. 58(5):2916–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornley MJ. 1967. A taxonomic study of Acinetobacter and related genera. J Gen Microbiol. 49(2):211–257. [DOI] [PubMed] [Google Scholar]
- Tilley D, Law R, Warren S, Samis JA, Kumar A.. 2014. CpaA a novel protease from Acinetobacter baumannii clinical isolates deregulates blood coagulation. FEMS Microbiol Lett. 356(1):53–61. Epub 2014/06/10. [DOI] [PubMed] [Google Scholar]
- Touchon M, et al. 2014. The genomic diversification of the whole Acinetobacter genus: origins, mechanisms, and consequences. Genome Biol Evol. 6(10):2866–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton JF, et al. 2006. Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J Clin Microbiol. 44(8):2974–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallenet D, et al. 2008. Comparative analysis of Acinetobacters: three genomes for three lifestyles. PLoS ONE. 3(3):e1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani AM, Siguier P, Gourbeyre E, Charneau V, Chandler M.. 2011. ISsaga is an ensemble of web-based methods for high throughput identification and semi-automatic annotation of insertion sequences in prokaryotic genomes. Genome Biol. 12(3):R30.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilanova C, Marín M, Baixeras J, Latorre A, Porcar M.. 2014. Selecting microbial strains from pine tree resin: biotechnological applications from a terpene world. PLoS ONE. 9(6):e100740.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilharm G, Piesker J, Laue M, Skiebe E.. 2013. DNA Uptake by the nosocomial pathogen Acinetobacter baumannii occurs during movement along wet surfaces. J Bacteriol. 195(18):4146–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zankari E, et al. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 67(11):2640–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liang Y, Lynch KH, Dennis JJ, Wishart DS.. 2011. PHAST: a fast phage search tool. Nucleic Acids Res. 39(Web Server issue):W347–W352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulak KG, Bohlmann J.. 2010. Terpenoid biosynthesis and specialized vascular cells of conifer defense. J Integr Plant Biol. 52(1):86–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.