Abstract
To investigate the relationship between gonadotroph function and ultrastructure, we have compared, in parallel in female mice, the effects of several different mutations that perturb the hypothalamic-pituitary-gonadal axis. Specifically, serum and pituitary gonadotrophin concentrations, gonadotrophin gene expression, gonadotroph structure and number were measured. Follicle-stimulating hormone β knockout (FSHβKO), follicle-stimulating hormone receptor knockout (FSHRKO), luteinising hormone receptor knockout (LuRKO), hypogonadal (hpg) and ovariectomised mice were compared with control wild-type or heterozygote female mice. Serum levels of LH were elevated in FSHβKO and FSHRKO compared to heterozygote females, reflecting the likely decreased oestrogen production in KO females, as demonstrated by the threadlike uteri and acyclicity. As expected, there was no detectable FSH in the serum or pituitary and an absence of expression of the FSHβ subunit gene in FSHβKO mice. However, there was a significant increase in expression of the FSHβ and LHβ subunit genes in FSHRKO female mice. The morphology of FSHβKO and FSHRKO gonadotrophs was not significantly different from the control, except that secretory granules in FSHRKO gonadotrophs were larger in diameter. In LuRKO and ovariectomised mice, stimulation of LHβ and FSHβ mRNA, as well as serum protein concentrations, were reflected in subcellular changes in gonadotroph morphology, including more dilated rough endoplasmic reticula and fewer, larger secretory granules. In the gonadotophin-releasing hormone deficient hpg mouse, gonadotrophin mRNA and protein levels were significantly lower than in control mice and gonadotrophs were correspondingly smaller with less abundant endoplasmic reticula and reduced numbers of secretory granules. In summary, major differences in pituitary content and serum concentrations of the gonadotrophins LH and FSH were found between control and mutant female mice. These changes were associated with changes in expression of the gonadotrophin subunit genes and were reflected in the cellular structure and secretory granule appearance within the gonadotroph cells.
Keywords: gonadotrophs, follicle-stimulating hormone, luteinising hormone, anterior pituitary, ultrastructure
The gonadotrophins, luteinising hormone (LH) and follicle-stimulating hormone (FSH), have a key role in the differentiation, maturation and function of the mammalian reproductive system. The main functions of LH and FSH in female physiology are well known: FSH stimulates follicular maturation and granulosa cell oestrogen production, whereas LH stimulates theca cell androgen production in the ovary (the substrate for granulosa cell oestrogen production), triggers ovulation and maintains the progesterone production of the corpus luteum. LH and FSH are synthesised within a single cell type in the anterior pituitary, the gonadotroph, and both consist of a common α subunit and a specific β subunit responsible for conferring biological activity on the heterodimer (1). Cyclical changes in gonadotrophin production and release drive the female reproductive cycle. The regulation of the pulsatile secretion of LH and of tonic secretion of FSH is complex and involves integration of gonadotophin-releasing hormone (GnRH), activin, inhibin and ovarian-derived steroid signalling (2, 3). Gonadotrophs can be identified by their distinctive secretory granules of variable size and electrondensity (3). LH secretion occurs in discrete pulses in response to pulsatile GnRH, whereas FSH secretion appears to be released independent of pulsatile GnRH (2). Because both LH and FSH are produced and released from the same gonadotrophs via constitutive and regulated secretory pathways, differential packaging of LH and FSH with the granins, secretoganin II and chromagranin A, respectively, in gonadotroph secretory granules has been proposed to underlie the different molecular pathways of secretion (4, 5).
Mutations in gonadotrophin and gonadotrophin receptor genes severely affect fertility and mouse models exist for many human reproductive abnormalities, with genetic alterations in gonadotrophin secretion or action (6, 7). Knowledge of reproductive mutant models has corroborated information from human mutations and provides useful tools for understanding infertility and its treatment. In a recent study, we compared the effect of mutations, both naturally occurring and genetically engineered, which perturb the hypothalamic-pituitary-gonadal axis in male mice (8). We now report the effects of the same mutations on reproductive function in female mice. Specifically, we have compared FSHβ knockout (FSHβKO), FSH receptor knockout (FSHRKO), LH receptor knockout (LuRKO), hypogonadal (hpg) and ovariectomised mice.
The FSHβKO mouse has a genetically engineered deletion of exons 1, 2 and 3 in the gene encoding the FSHβ subunit. As a result, there is no production of biologically active FSH within the pituitary but, in contrast to male mice carrying the same mutation, female mice are infertile (9). Ovaries are significantly reduced in size compared to normal litter mates, and uteri are thin and atrophic. Follicular development appears normal up to the pre-antral stage but mature follicles and corpora lutea are not seen within the ovaries, indicating that normal oestrous cycles do not occur. Ovulation in FSHβKO mice can be induced by the administration of pregnant mare’s serum gonadotrophin and human chorionic gonadotrophin (9).
The FSHRKO mouse carries a genetically engineered deletion in the gene encoding the FSH receptor. By contrast to male mice, the resulting inability to respond to circulating FSH results in infertility in female mice (10). As in adult FSHβKO females, ovaries are significantly reduced in size relative to normal mice, with follicular development arrested at the pre-antral stage (11). The uterus is atrophic and the vagina imperforate and the mice do not undergo oestrous cycles. By contrast to FSHβKO females, there is no response to exogenous FSH (10).
The LuRKO mouse has a genetically engineered deletion of exon 11 of the LH receptor. Consequently, mice are unable to respond to circulating LH and both males and females are infertile (12). In adult females, follicular development progresses to the antral stage but no mature follicles or corpora lutea are found and the mice are acyclic. Uteri are thin and atrophic and vaginal opening is delayed (12).
The hpg mouse is a naturally occurring mutant with a deletion in the gene encoding the hypothalamic GnRH (13). Adult female mice homozygous for the deletion present with under-development of the reproductive tract. The ovaries are very small and follicular development does not proceed beyond the pre-antral stage. The uterus is atrophic and the vagina imperforate. hpg mice are deficient in gonadotrophic and ovarian steroid hormones (14, 15) and peptides, although ovarian and uterine development can be restored by the administration of exogenous GnRH (16) or gonadotrophins (17).
The present study aimed to investigate for the first time pituitary synthesis and regulation of LH and FSH and to relate to gonadotroph ultrastructure in several mutant female mice in parallel in a single study (8). Throughout the study, we refer to these mice in the order described above, namely FSH mutants, LH receptor mutant, hpg and ovariectomised mice, because this reflects the severity of the consequences of the mutations on female gonadal axis function.
Materials and methods
Mutant mice
Breeding colonies of FSH receptor deficient, FSHRKO mice, FSHβ deficient (10), FSHβKO mice (9) and LH receptor deficient, LuRKO mice (12) were established in our laboratory. Because both FSHRKO and FSHβKO females are infertile but males are fertile, breeding pairs of KO males and heterozygous females were used to generate heterozygous and KO offspring in a 1 : 1 ratio. Heterozygous females were used as controls for these two lines. The hpg mutation was identified by polymerase chain reaction (PCR) analysis of tail DNA, as described previously (18). FSHR, FSHβ and LHR mutations were identified as previously described (8) Hypogonadal (hpg) mice with a deletion in the gene encoding the GnRH gene (16) from the original colony discovered at the MRC Laboratories, Harwell, Oxford, were bred within our department. The hpg mice were on a C3H/HeH-101/H genetic background and the knockout mice were on a mixed C57B16/129 background. All procedures were carried out in accordance with the Animals (Scientific Procedures) Act 1986 and with the approval of a local ethical review committee.
Serum and tissue collection
All procedures were carried out under anaesthesia [Rompun: Ketaset: 0.1 ml/kg of a 20 : 4% (v/v) solution; Veterinary Supplies, University of Oxford, Oxford, UK] in 8-week-old mice. For the analysis of gonadotrophin hormones, blood was collected from the jugular sinus and serum separated and frozen at −20 °C for assays. For the analysis of gonadotrophin subunit mRNA or gonadotrophin hormone, pituitaries were dissected out, snap frozen in liquid nitrogen and stored at −70 °C until assayed. Ovaries and uteri were dissected out and weighed.
Ovariectomy
Animals were anaesthetised as described above. An incision of 0.5 cm was made in the dorsal skin and abdominal wall of the right flank, and the ovary, oviduct and distal region of the uterine horn were exposed. The ovarian artery was located and clamped, and the ovary was dissected free from the surrounding fat and oviduct and removed. Abdominal wall and skin were sutured and the procedure repeated on the left side. Mice were killed 1 month post ovariectomy and comparisons were made with unoperated control mice.
Hormone assays
Serum and pituitary levels of FSH and LH were measured using in-house immunofluorimetric assays (Delfia; Wallac OY, Turku, Finland) as described previously (8).
RNA extraction
Total RNA was extracted from individual pituitaries with Trizol (Life Technologies, Paisley, UK) and residual genomic DNA was removed by DNAse treatment (DNA-free; Ambion Inc., Austin, TX, USA). DNAse-treated RNA was quantified by spectrophotometric measurement at λ 260 nm. In total, 1 µg of RNA was reverse transcribed using random hexamers (Ambion Inc.) and Moloney murine leukaemia virus reverse transcriptase (Life Technologies).
Quantitative real-time PCR
PCR experiments were carried out in a 25-µl volume using a 96-well plate format. Primers and probes were designed using Primer Express (Applied Biosystems, Warrington, UK) and probes were synthesised with FAM 5′ and TAMRA 3′ (Taqman®; Applied Biosystems). Primers were used at a final concentration of 300 nm and probes at a concentration of 150 nm in ABI universal master mix (Applied Biosystems). Primers and probes were selected from sequences generated using Primer Express (Applied Biosystems). Primer and probe sequences are listed in Table 1. To our knowledge, alteration of gonadotrophin expression would be unlikely to affect pituitary thyrotrophs and so thyrotrophin-stimulating hormone (TSH)β subunit gene activity was measured as a negative control for comparison with gonadotroph β subunit activity. Fluorescence was detected on an ABI 7700 system (Applied Biosystems). No reverse transcription controls for each sample were screened to check for the presence of residual genomic DNA.
Table 1.
Sequences of Mouse Primer and Probe Sets for Gonadotrophin Subunit Genes.
| Subunit gene |
Primer | Sequence 5′ to 3′ | ID Number |
|---|---|---|---|
| Common α | Forward | CTGTTGCTTCTCCAGGGCATA | NM009889 |
| Reverse | TTCTTTGGAACCAGCATTGTCTT | ||
| Probe | CCCACTCCCGCCAGGTCCAA | ||
| LHβ | Forward | TGGCCGCAGAGAATGAGTTC | MM25145 |
| Reverse | CTCGGACCATGCTAGGACAGTAG | ||
| Probe | CCCAGTCTGCATCACCTTCACCACC | ||
| FSHβ | Forward | GGAGAGCAATCTGCTGCCATA | MM12932 |
| Reverse | GCAGAAACGGCACTCTTCCT | ||
| Probe | CTGTGAATTGACCAACATCACCATC TCAGTAGA | ||
| TSHβ | Forward | ACTTCATCTACAGAACGGTGGAAAT | MMTSHB1 |
| Reverse | GCGACAGGGAAGGAGAAATAAG | ||
| Probe | CCAGGATGCCCGCACCATGTTACT |
Probes are dual labelled FAM 5′ and TAMRA 3′ (Taqman®). LH, luteinising hormone; FSH, follicle-stimulating hormone; TSH, thyrotrophin-stimulating hormone.
To measure cDNA levels, a threshold cycle (Ct) was selected within the exponential phase of the amplification for all standards and samples. Arbitrary standards were generated by serial dilutions of a cDNA pool from normal adult male pituitaries. A standard curve was generated by plotting standards against Ct values, sample values were read from this standard curve and mRNA levels were normalised relative to an endogenous control mGapdh mRNA (Applied Biosystems) to allow comparison of different mRNAs between samples. A comparison between mGapdh and wbscr (33) found that mGapdh showed a more stable pattern of expression across pituitary glands from normal and mutant mice and was therefore selected as the housekeeping gene of choice.
Tissue processing and electron microscopy
Pituitary glands for electron microscopy analysis were processed and analysed as described previously (8). Briefly, the tissue was contrasted with uranyl acetate [2% (w/v) in distilled water], dehydrated in ethanol and embedded in LR Gold resin (Agar Scientific, Stansted, UK). Ultrathin sections (50–80 nm) were prepared using a Reichart-Jung ultracut microtome and mounted on nickel grids (Agar Scientific). For identification of bi-hormonal gonadotroph secretory granules, sections were labelled for LH, and FSH as described previously (8). Specificity of antibody labelling was confirmed in negative control sections in which the primary antibody was replaced with non-immune serum. Finally, sections were counterstained with lead citrate and uranyl acetate and examined on a JOEL 1010 transmission electron microscope (JOEL USA Inc., Peabody, MA, USA). For each pituitary (n = 4), four randomly orientated sections, each containing ten grid squares of intact tissue, were counted for individual gonadotrophs identified by immunogold labelling for LH and FSH. The total secretory cell population was determined by counting the total number of nucleated pituitary (granulated and folliculo-stellate) cells per grid. Gonadotroph number was expressed as a percentage of the total secretory cell population. Immunogold identification of somatotrophs using a rabbit anti-mouse growth hormone primary antibody (dilution 1 : 4000; NHPP) was also carried to quantify the percentage of somatotrophs. This was intended as a control for changes in the size of the gonadotroph population because changes in the somatotroph population were not expected in the mutant mice investigated (19). Cell morphology was analysed digitally using AXIOVISON, version 4.5 (Zeiss, Oberkochen, Germany) (8). The parameters measured were: cytoplasmic, nuclear and total cell areas; granule area, granule density, granule diameter, percentage of secretory granules located in a 300-nm depth of the plasma membrane and rough endoplasmic reticulum (rough ER). The methodology is described elsewhere (8).
Statistical analysis
Normal and heterozygous mice from the hpg and LuR colonies were initially analysed separately. Where there was no significant difference, data from normal mice and heterozygous mice were combined and expressed as control heterozygous/normal (H/N). Heterozygous females were used as normal controls in FSHR and FSHβ colonies. Each mutant line was compared with its own control, comparisons were not made across different lines. Means were compared by one-way ANOVA. Where a significant overall difference was detected between groups, differences between individual means were assessed by the Bonferroni test and an unpaired t-test. P < 0.05 was considered statistically different.
Results
Pituitary and serum gonadotrophin concentrations and pituitary gene expression
Figure 1 shows pituitary and serum gonadotrophin concentrations and Fig. 2 shows gonadotrophin subunit gene expression for each mutant and control. Table 2 provides a summary of changes measured for each mutant and control.
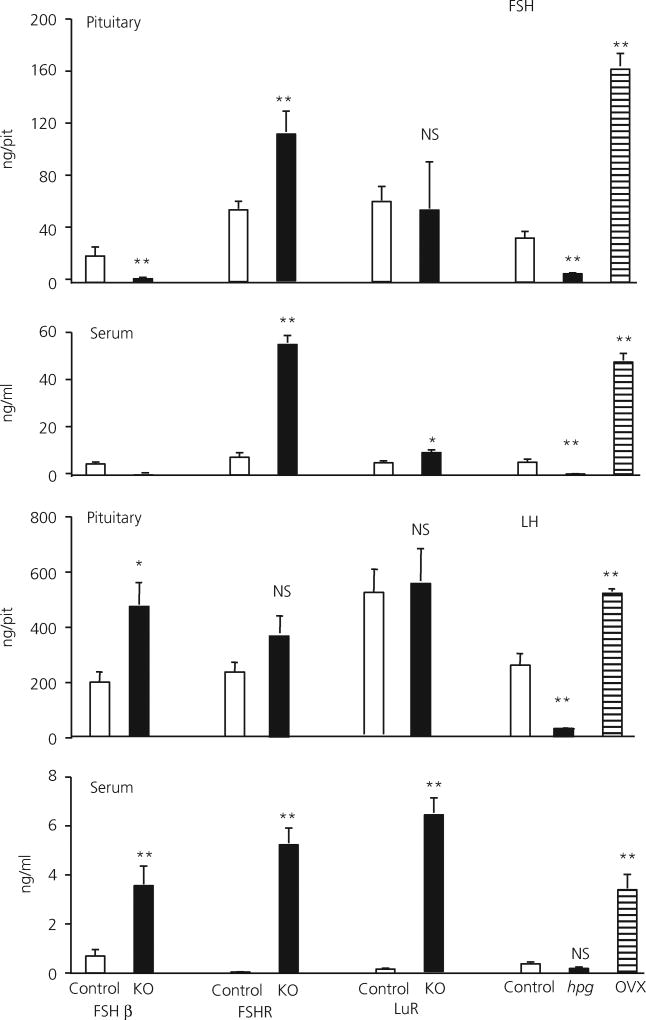
Fig. 1.
Pituitary and serum luteinising hormone (LH) and follicle-stimulating hormone (FSH) levels in adult FSHβ heterozygote control (11), FSHβ knockout (KO) (10), FSH receptor (FSHR) heterozygote control (7), FSHRKO (13), LH receptor (LuR) heterozygote/normal control (25), LuRKO (10), hypogonadal (hpg) heterozygote/normal control (18), hpg (13) and ovariectomised female mice (9). The number of mice in each group is shown in brackets. Results are expressed as the mean ± SEM. **P < 0.01 versus respective strain control. Open columns, control mice; filled columns, mutant mice, knockout (KO) or hpg; striped columns, ovariectomised (OVX) mice. NS, not significant.
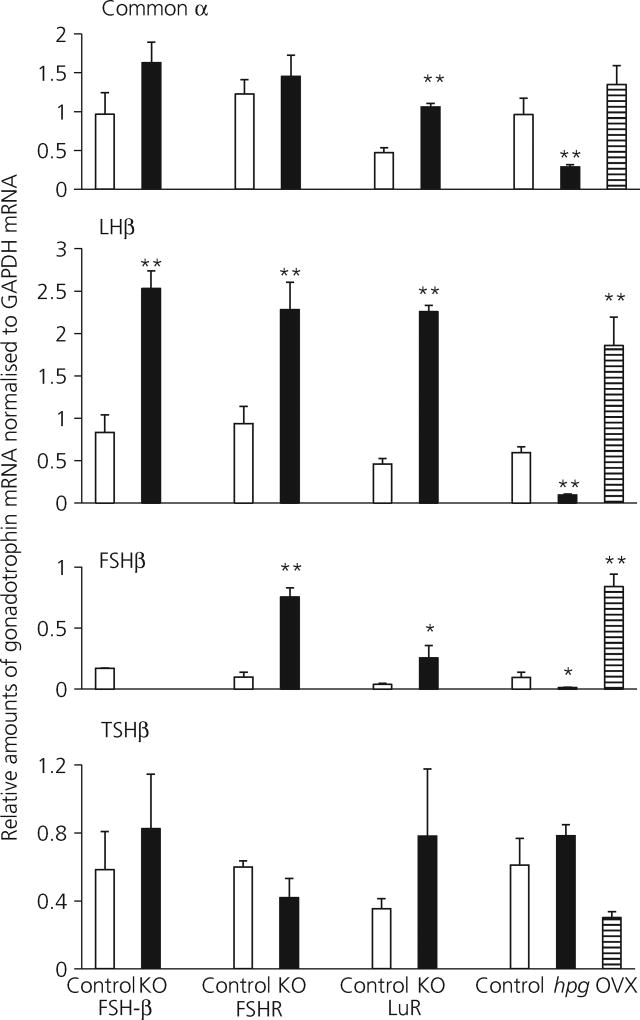
Fig. 2.
Gonadotroph common α, luteinising hormone (LHβ), follicle-stimulating hormone (FSHβ) and thyroid-stimulating hormone (TSHβ) subunit mRNA levels, normalised to mGapdh mRNA in adult control, FSHβKO FSHRKO, LuRKO, hpg and ovariectomised female mice. Results are expressed as the mean ± SEM. **P < 0.01 versus respective strain control, n = 4 mice per group. Open columns, control mice; filled columns, mutant mice, knockout (KO) or hpg; striped columns, ovariectomised (OVX) mice. hpg, hypogonadal; KO, knockout; LuR, LH receptor; R, receptor.
Table 2.
Summary of the Changes in Pituitary and Serum Follicle-stimulating Hormone (FSH) and Luteinising Hormone (LH) Concentration and mRNA Levels in Mouse Mutant Lines.
| FSHβKO | FSHRKO | LuRKO | hpg | OVX | ||
|---|---|---|---|---|---|---|
| Pituitary | FSH | Absent | ↑ | ↔ | ↓ | ↑ |
| Serum | FSH | Absent | ↑ | ↑ | ↓ | ↑ |
| Pituitary mRNA | FSHβ | Absent | ↑ | ↔ | ↓ | ↑ |
| Pituitary mRNA | Common α | ↔ | ↔ | ↑ | ↓ | ↑ |
| Pituitary | LH | ↑ | ↔ | ↔ | ↓ | ↑ |
| Serum | LH | ↑ | ↑ | ↑ | ↔ | ↑ |
| Pituitary mRNA | LHβ | ↑ | ↑ | ↑ | ↓ | ↑ |
| Pituitary mRNA | TSHβ | ↔ | ↔ | ↔ | ↔ | ↓ |
Direction of arrows indicate direction of change, horizontal arrows indicate no overall change. LuR, LH receptor; KO knockout; OVX, ovariectomised; hpg hypogonadal; TSH, thyrotrophin-stimulating hormone.
In FSHβKO adult females, pituitary and serum levels of FSH were at, or below the limit of detection of the FSH assay throughout. Furthermore, no expression of the FSHβ gene was detected, confirming the absence of synthesis of FSH in this mutant. In control female mice, serum FSH was 22% of the pituitary content of FSH. Serum LH levels were 0.3% of pituitary content in control FSHβH females and 0.7% in FSHβKO females. mRNA levels of both common α and TSHβ subunit genes were not significantly different in FSHβKO female mice compared to control mice, whereas LHβ mRNA was significantly (P < 0.01) increased.
In FSHRKO females, serum and pituitary concentrations of FSH were significantly higher compared to control females and serum concentrations of LH were significantly higher, reflecting the higher expression of FSHβ and LHβ subunit genes in the FSHRKO females relative to heterozygote females. There was no significant difference in the expression of common α or TSHβ subunit genes between KO and heterozygous females in FSHR females (Fig. 2).
By contrast, in LuRKO mice, serum concentrations of FSH were significantly (P < 0.01) higher, approximately doubled, compared with concentrations in control females (Fig. 1) but pituitary content of FSH was not significantly different. A similar pattern was measured for LH; in LuRKO females, serum LH was significantly higher, being increased by approximately 30-fold (P < 0.01) compared to control females, although there was no significant difference in LH pituitary content. mRNA levels of common α (P < 0.01), LHβ (P < 0.01) and FSHβ (P < 0.05) were significantly higher in LuRKO females relative to LuRH/N females, whereas there was no significant difference in amount of TSHβ mRNA measured.
In hpg females, the pituitary content of FSH and LH was significantly reduced (P < 0.01) compared to normal/heterozygous females. Serum levels of FSH and LH were also significantly lower compared to control mice, (P < 0.01). mRNA levels of common α, LHβ and FSHβ genes were significantly lower in hpg females compared to control mice but the amount of TSHβ mRNA was not significantly different. As expected, in ovariectomised mice, both pituitary and serum LH and FSH were significantly increased (P < 0.01) compared to intact controls. mRNA levels of common α, LHβ, FSHβ genes were all also significantly increased. The amount of TSHβ mRNA was reduced in ovariectomised mice but did not reach significance.
Gonadotroph morphology
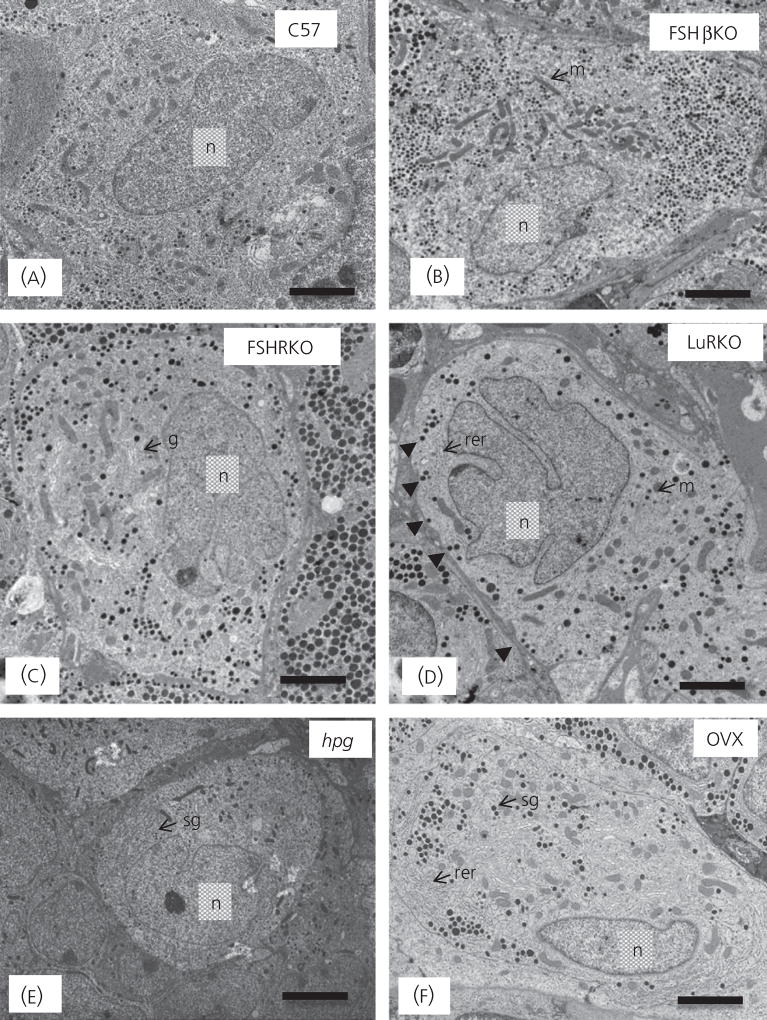
Figure 3 shows representative electron micrographs of gonadotrophs in control and transgenic mice and Table 3 shows the quantitative analysis of gonadotrophs and their organelles. Gonadotrophs in rodents and sheep can be identified by their distinctive population of secretory granules, which have a variable size and electron density (3). In control mice, secretory granules were mainly moderately electron-dense of variable size (100–250 nm). There was an absence of the distinctive subpopulation of granules that display an electron-dense core and a relatively electron-lucent ‘halo’. Cell and cytoplasmic areas showed no significant difference between controls (Fig. 3a) and FSHβKO (Fig. 3b), FSHRKO (Fig. 3c) and LuRKO (Fig. 3d) mice. However, gonadotrophs in hpg female mice (Fig. 3e) were significantly (P < 0.01) smaller than in control mice. Gonadotrophs in ovariectomised mice (Fig. 3f) were similar to control gonadotrophs in size. No significant difference was measured in nuclear area between control and mutant mice, with the exception of significantly (P < 0.01) smaller nuclei in hpg gonadotrophs (Table 3).
Fig. 3.
Representative electron micrographs of gonadotrophs in normal and female mutant mice: (a) C57 Normal, (b) FSHβKO, (c) FSHRKO, (d) LuRKO, (e) hpg and (f) ovariectomised (OVX). Scale bar = 2 µm. Representative organelles are labelled: m, mitochondria; rer, rough endoplasmic reticulum; sg secretory granule; n, nucleus; g, Golgi apparatus. (D) Arrowheads indicate granules at the periphery of the gonadotroph. FSH, follicle-stimulating hormone; hpg, hypogonadal; KO, knockout; LuR, luteinising hormone receptor; R, receptor.
Table 3.
Subcellular Morphology of Gonadotrophs in Female 8-Week-Old Female Normal and Mutant Mice.
| Control | FSHβKO | FSHRKO | LuRKO | hpg | Ovariectomised | |
|---|---|---|---|---|---|---|
| Cell area (µm2) | 98 ± 10 | 98 ± 9 | 110 ± 10 | 100 ± 9 | 60 ± 4a | 101 ± 8 |
| Cytoplasm area (µm2) | 70 ± 5 | 77 ± 6 | 85 ± 6 | 79 ± 5 | 45 ± 2a | 80 ± 7 |
| Nuclear area (µm2) | 28 ± 2 | 21 ± 3 | 25 ± 2 | 21 ± 2 | 15 ± 1a | 21 ±2 |
| Granule diameter (nm) | 140 ± 10 | 148 ± 8 | 175 ± 9b | 170 ± 9b | 112 ± 6a | 175 ± 6b |
| Granule density (/µm2) | 0.1 ± 0.007 | 0.082 ± 0.006 | 0.09 ± 0.005 | 0.05 ± 0.008c | 0.06 ± 0.006a | 0.055 ± 0.006b |
| Rough endoplasmic reticulum (units) | 2.1 ± 0.1 | 2.0 ± 0.2 | 2.2 ± 0.2 | 3.5 ± 0.3b | 1.1 ± 0.1a | 3.0 ± 0.2c |
| % Granules at perimeter | 51 ± 5 | 49 ± 5 | 46 ± 8 | 65 ± 5c | 45 ± 3 | 52 ± 5 |
P< 0.01 versus Normal C3H;
P<0.01;
P<0.05 versus control.
Values are expressed as the mean ± SEM (n = 4 animals). Three groups of controls were independently assessed C57BL6/129 (transgenic mouse background), C3H (hpg background) and LuRH (transgenic heterozygote). No significant differences were found for each of the parameters measured and, for simplicity, the control data shown are from the C57BL6/129 mice. FSH, follicle-stimulating hormone; hpg, hypogonadal; KO, knockout; LuR, luteinising hormone receptor; R, receptor.
Secretory granule numerical density represents the balance between gonadotrophin synthesis and release (20). Secretory granule density and distribution were not significantly different in FSHβKO and FSHRKO gonadotrophs compared to control mice (Table 3). However, granule density was significantly reduced compared to control mice in hpg (P < 0.01), LuRKO (P < 0.05) and ovariectomised (P < 0.01) gonadotrophs. Secretory granule diameter was significantly increased in LuRKO (P < 0.01; Fig. 3d), FSHRKO (P < 0.05; Fig. 3c) and ovariectomised (P < 0.01; Figs 3f and 4b) gonadotrophs compared to control mice but was significantly reduced (P < 0.01) in hpg (Fig. 3e) mice. Rough ER expansion reflects increased secretory protein synthesis. Rough ER was significantly (P < 0.01) reduced in the hpg (Fig. 3e) but significantly increased in the LuRKO (P < 0.01; Fig. 3d) and ovariectomised (P < 0.05; Fig. 3f) mice. The percentage of granules at the perimeter of the cell (which reflects the readily releasable pool of granules) was significantly (P < 0.05) increased in the LuRKO gonadotrophs but was not significantly different in the other mutant and ovariectomised mice (Table 3). No changes were seen in the thyrotroph population for any of the morphology parameters measured (data not shown).
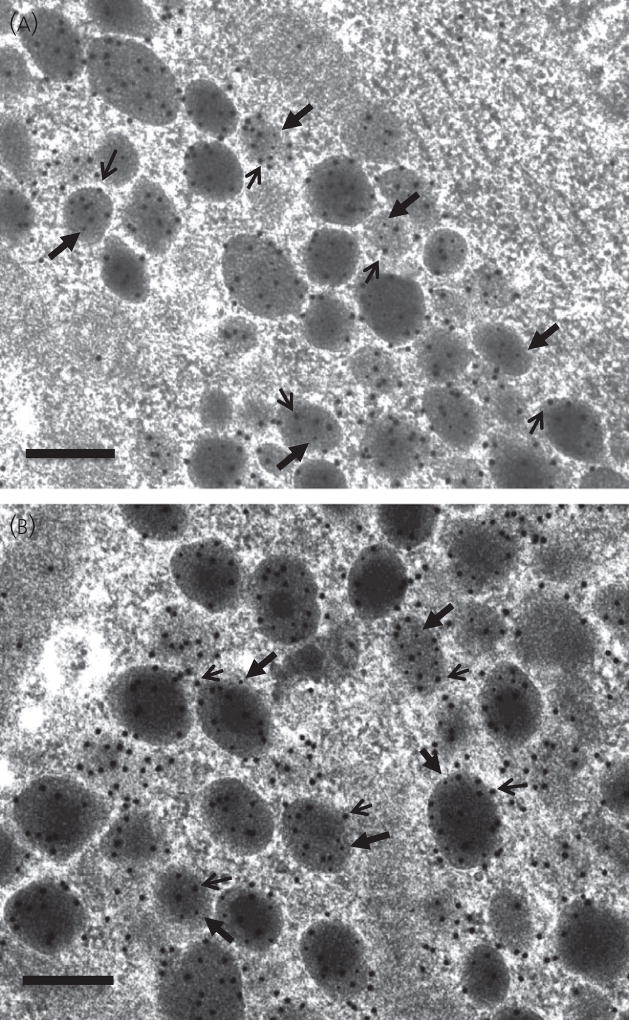
Fig. 4.
Representative electron micrographs to show the morphological appearance of gonadotroph secretory granules in female (a) C57 Normal; (b) ovariectomised mice. Scale bar = 200 nm. Secretory granules analogous to the characteristic granules distinctive of male gonadotrophs comprising a dense core and electron ‘lucent’ halo were observed in ovariectomised gonadotrophs only. The cells were immunolabelled for luteinising hormone (LH) detected with 15 nm immunogold particles (indicated by arrows) and follicle-stimulating hormone detected with 5 nm immunogold particles (indicated by filled arrowheads).
Figure 4 shows the distribution of immunogold labelling of LH and FSH on secretory granules in control C57 mice (Fig. 4a) and ovariectomised mice (Fig. 4b). LH immunolabel detected with 15 nm immunogold particles and FSH immunolabel detected with 5 nm immunogold particles were distributed together in the majority of secretory granules and no particular distribution was observed in relation to granules of a particular electron density.
Gonadotroph number
No significant difference in the proportion of LH/FSH-immunoreactive gonadotrophs (as a percentage of total secretory cell number) was measured between control, FSHβKO, FSHRKO, LuRKO and ovariectomised mice (Table 4). However, gonadotroph number was significantly reduced (P < 0.05) in hpg females compared to control mice.
Table 4.
Percentage of Luteinising Hormone (LH) and Follicle-Stimulating Hormone (FSH)-positive cells (Gonadotrophs) and Growth Hormone (GH)-Positive Cells (Somatotrophs) in Wild-Type and Mutant Female Mice as a Percentage of Total Anterior Pituitary Secretory Cell Number.
| Control | FSHβKO | FSHRKO | LuRKO | hpg | Ovariectomised | |
|---|---|---|---|---|---|---|
| % LH and FSH positive cells | 14 ± 2 | 16 ± 3 | 18 ± 2 | 18 ± 2 | 9 ± 1* | 12 ± 1 |
| % GH positive cells | 55 ± 3 | 55 ± 2 | 57 ± 2 | 50 ± 2 | 53 ± 4 | 51 ± 2 |
Values are expressed as the mean ± SEM (n = 4 animals). hpg, hypogonadal; KO, knockout; LuR, luteinising hormone receptor; R, receptor.
P < 0.05 versus control.
Ovary and uterine weights and histology
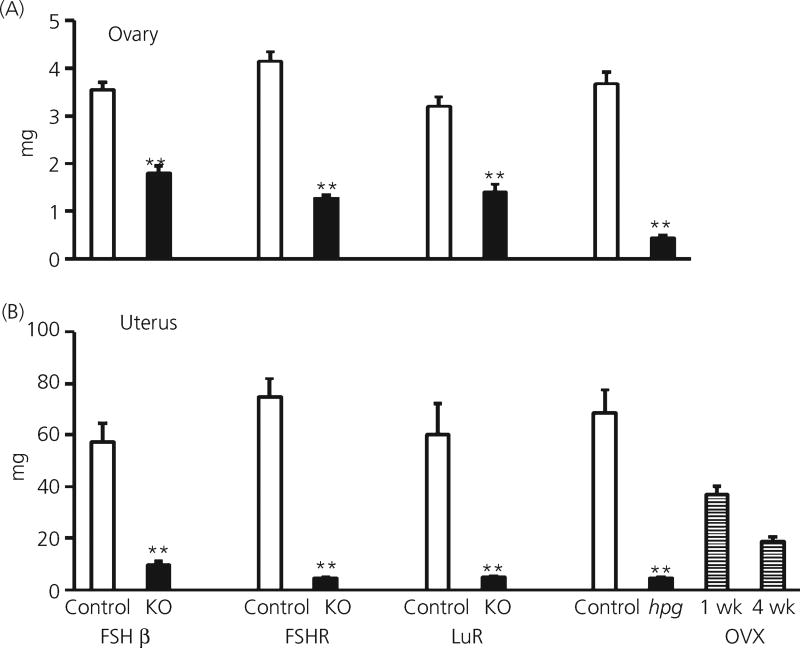
Ovarian weights in adult FSHβKO, FSHRKO and LuRKO mice were significantly (P < 0.01) reduced to 30–50% of the corresponding heterozygote control females (Fig. 5a). In hpg mice, ovarian weights were 14% of control weights (Fig. 5). Uterine weights in all mutant mice were significantly (P < 0.01) reduced to approximately 7% of the weight of heterozygote controls (Fig. 5b). The vagina remained imperforate in all mutant mice at 8 weeks of age.
Fig. 5.
Ovarian and uterine weights in adult control (18) FSHβKO (15), FSHRKO (33), LuRKO (6) and hpg (9) and ovariectomised (5) female mice. Open columns: control mice; Filled columns: KO, knockout mice; hpg, naturally occurring mutant. Striped columns: OVX, ovariectomised 1 week and 4 weeks. Results are expressed as the mean ± SEM, **P < 0.01 versus respective strain control. The number of mice in each group is shown in brackets. FSH, follicle-stimulating hormone; hpg, hypogonadal; KO, knockout; LuR, luteinising hormone receptor; R, receptor.
Discussion
The present study compared the effects of mutations that perturb the hypothalamic-pituitary-gonadal axis in female mice to advance understanding of the structure and function of gonadotrophs in vivo under conditions of altered GnRH, LH, FSH and ovarian steroid and peptide signalling. Pituitary cells produce large amounts of protein hormones, packaged into dense-cored secretory granules so that large amounts of hormone are rapidly available when required (21). Granules must translocate and dock at the plasma membrane and undergo a series of priming events for granule fusion to occur and granules adjacent to the plasma membrane are hence described as the ‘readily-releasable’ pool (22). Real-time imaging studies in adrenal chromaffin and β-cells have shown that there is preferential secretion of newly-formed secretory granules that move within seconds to minutes to the plasma membrane, whereas older granules if not secreted move away from the plasma membrane to within the cell (23). Therefore, secretory granules located in proximity to the plasma membrane in gonadotrophs may similarly represent release-competent younger granules. Secretory granule size in adrenal chromaffin cells has been shown to correlate with the size of quanta of catecholamine released (24) and so it is possible that gonadotroph granule size similarly reflects the amount of gonadotrophin content. Although there have been few dynamic studies of gonadotrophs aiming to understand how granule volume and number are regulated, the combined study of gonadotroph ultrastructure and hormone measurements allows inferences to be made between secretory pathway function and structure.
At the ultrastructural level, gonadotrophs are distinctive for the varied size and electron density of the secretory granules contained compared to other pituitary secretory cell types. In rat and sheep gonadotrophs, the different secretory storage granules have been categorised into three populations, distinguished by differential packaging with granin proteins, namely small, electron-dense, LH and secretogranin II (SgII) positive granules; large, electronlucent, FSH and chromogranin A (CgA) positive granules and intermediatesized granules containing an electron dense LH and SgII positive core and an electron-lucent FSH and CgA-positive ‘halo’ outer region (3, 25). These granule populations are particularly distinctive in the male rat gonadotroph but less so in the female rat (25). Consistent with these observations, in the present study, gonadotrophs in female control mice show a less extreme variation in secretory granule appearance compared to male mice, granule diameter was overall smaller, and there was no obvious compartmentalisation of FSH to larger, electron-lucent secretory granules (8). Interestingly, granules were relatively more marginated to the plasma membrane in control female mice than in male mice, possibly as a result of differences in feedback control mechanisms. Although changes in gonadotroph structure with the oestrous cycle have been reported in the rat (26), we did not detect any significant differences in gonadotroph morphology across the mouse oestrous cycle (data not shown).
As seen in male FSHβKO and FSHRKO mice, the female FSH mutants present with the same phenotype but, in contrast to male mice, significantly higher concentrations of FSH (FSHRKO) and LH (both mutants) were measured in both the serum and pituitary, indicating a loss of feedback regulation of these hormones at the level of the pituitary and hypothalamus. The high concentrations of these gonadotrophins were associated with significant increases in mRNA levels of the FSHβ subunit gene in FSHRKO females and LHβ subunit gene in both mutant females. In control mice, cyclic changes in ovarian steroid production regulate gonadotrophin secretion and release throughout the oestrus cycle (27, 28). It is likely that the high levels of FSH and LH measured are associated with the absence of biologically active oestrogen in these mutant mice as demonstrated by the atrophic uteri and imperforate vaginae. Consistent with these observations, female oestrogen receptor ERα KO mice are also infertile with increased serum concentrations of gonadotrophins (29, 30). The early stages of ovarian follicle development do not require gonadotrophin input (31, 32) but FSH is required to progress beyond the pre-antral stage (33, 34). In the absence of FSH stimulation, follicles do not acquire LH receptors and are unable to convert thecal supplies of androgens to oestrogen. Ovarian concentrations of inhibins A and B have been found to be significantly lower in FSHβKO and FSHRKO mice compared to normal female mice (35) and, in turn, feedback regulation of FSH is impaired.
The ultrastructure of gonadotrophs in FSHβKO female mice, however, was not significantly different in any of the parameters measured despite the loss of FSH stores, increased pituitary LH content and increased LH serum concentrations. With respect to secretory granule morphology, increased LH content was not reflected in an increase in granule diameter, although this may have been counteracted by the absence of FSH stores. In male FSHβKO mice, a similar profile of morphology was evident, except that secretory granule diameter was reduced (8), probably reflecting the consequence of the loss of the significantly larger FSH stores in the male (8). In female FSHRKO mice gonadotrophs, the only difference measured was an increase in secretory granule diameter compared to control gonadotrophs, which may reflect the approximate doubling of pituitary FSH content.
As observed in male LuRKO mice, serum levels of both FSH and LH were significantly increased above control levels in LuRKO females but, in contrast to male LuRKO mice, the pituitary content of FSH and LH were maintained in the female despite the increased release of both hormones from the pituitary (8). The increased synthesis and release of FSH and LH in LuRKO females was associated with significantly higher mRNA levels for all three gonadotrophin subunit genes. The increase in the production of the normally abundant common α subunit contrasted with the unchanged level of common α subunit in FSHβKO and FSHRKO females. A further difference between FSHβKO, FSHRKO and LuRKO females relates to inhibin production within the ovary (35, 36). High levels of ovarian and serum inhibin B in the LuRKO may account for the more moderate rise in FSHβ mRNA levels and serum FSH levels compared to the marked rise in serum LH in LuRKO females. Analysis of gonadotroph ultrastructure in female LuRKO mice revealed fewer but larger diameter secretory granules, possibly maintaining pituitary gonadotrophin stores, which were marginalised to a greater degree towards the plasma membrane compared to control mice. The greater number of secretory granules distributed towards the plasma membrane and raised concentrations of serum LH and FSH are consistent with increased granule release. In addition, rough ER was more dilated than control, likely indicating increased protein synthesis because protein synthesis inhibitors have been shown to reverse dilated rough ER in other systems (37).
In adult hpg female mice, despite the absence of steroid or peptide feedback to the pituitary, there was no increase in either FSH or LH relative to control females and subunit gene mRNA levels remained at or below control levels, emphasising the primary role of hypothalamic GnRH in facilitating gonadotrophin synthesis within the pituitary. In agreement with previous studies (20, 38) hpg gonadotrophs were smaller than controls, with less prominent rough ER, reduced secretory granule area and smaller secretory granules. The reduced size of hpg gonadotrophs correlated with the profound lack of synthesis and secretion of FSH and LH (although increased gonadotrophin synthesis and secretion, as in the LuRKO female, did not correlate with an increase in cell area).
By contrast, in ovariectomised female mice, where all ovarian regulatory factors have been physiologically removed, a significant increase was measured in FSHβ and LHβ subunit gene mRNA levels, in addition to a significant increase in the serum and pituitary content of FSH and LH in the present study and as reported previously (39, 40). At the subcellular level, many correlates of hyper-stimulated gonadotrophin production and secretion were detected in ovariectomised mice. Dilated rough ER correlated with the increased protein synthesis and glycosylation associated with increased production of the gonadotrophins. The reduction in granule area per cytoplasmic profile was consistent with increased release of granules. In ovariectomised female mice, secretory granules increased in size and granules with an electron-dense core and electron-lucent halo, frequently seen in male mice and rats, were observed for the first time in the present study. Thus, a high pituitary FSH content appears to be associated with the presence of larger granules with electron-lucent ‘halos’ (8). This suggests that ovariectomy in female mice induces sufficiently high FSH production and storage for formation of granule halos during granule production. Interestingly the pituitary content of FSH in male mice 1 month post ovariectomy was reduced to levels seen in hpg mice (8), suggesting that the removal of testicular feedback to the pituitary results in the depletion of pituitary stores and the release of all newly-synthesised hormone, indicating a fundamental difference in gonadotrophin feedback control between male and female mice.
Because oestrogen has been shown to negatively regulate both GnRH at the hypothalamus and to moderate release of LH and FSH from the pituitary, the removal of oestrogen will facilitate both increased release of gonadotrophins from the pituitary and allow increased stimulation of gonadotrophin synthesis through increased GnRH activity. This difference in regulation between male and female mice would allow for tight control of LH under normal conditions and for rapid release and replacement of LH following an ovulatory surge in normal female mice. Because FSH is under the joint control of inhibin and oestrogen produced from the follicular granulose cells at different stages of maturity, this may allow the different pattern of FSH increase seen throughout the follicular phase of the cycle. However, in the absence of oestrogen regulation, the over-riding effect is GnRH stimulation of increased synthesis of FSH within the pituitary.
In summary, although the genetic mutations carried by the female mice in the present study all result in infertility, a different pattern of hormonal changes was found in each mutant, which was reflected in gonadotrophin subunit gene expression and gonadotroph ultrastructure. Consistent between the female mutants are the observations that increased gonadotrophin synthesis and release corresponded with increased rough ER density, a decrease in granule numerical density and an increase in granule diameter relating to increased FSH production. The present study investigated the single time point of 8 weeks of age. Further studies are investigating changes at earlier developmental stages to determine when differences in gonadotroph structure and function appear.
Acknowledgments
We thank Vivienne Wilkins and Lynne Scott for technical assistance and the Staff of the Biological Services Unit, Oxford, for care of the animals.
References
- 1.Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Ann Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- 2.Farnworth PG. Gonadotrophin secretion revisited. How many ways can FSH leave a gonadotroph? J Endocrinol. 1995;145:387–395. doi: 10.1677/joe.0.1450387. [DOI] [PubMed] [Google Scholar]
- 3.McNeilly AS, Crawford JL, Taragnat C, Nicol L, McNeilly JR. The differential secretion of FSH and LH: regulation through genes, feedback and packaging. Reproduction Suppl. 2003;61:463–476. [PubMed] [Google Scholar]
- 4.Nicol L, McNeilly JR, Stridsberg M, McNeilly AS. Differential secretion of gonadotrophins: investigation of the role of secretogranin II and chromogranin A in the release of LH and FSH in LbetaT2 cells. J Mol Endocrinol. 2004;32:467–480. doi: 10.1677/jme.0.0320467. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe T, Uchiyama Y, Grube D. Topology of chromogranin A and secretogranin II in the rat anterior pituitary: potential marker proteins for distinct secretory pathways in gonadotrophs. Histochemistry. 1991;96:285–293. doi: 10.1007/BF00271348. [DOI] [PubMed] [Google Scholar]
- 6.Rulli SB, Huhtaniemi I. What have gonadotrophin overexpressing transgenic mice taught us about gonadal function? Reproduction. 2005;130:283–291. doi: 10.1530/rep.1.00661. [DOI] [PubMed] [Google Scholar]
- 7.Peltoketo H, Rivero-Müuller A, Ahtiainen P, Poutanen M, Huhtaniemi I. Consequences of genetic manipulations of gonadotrophins and gonado-trophin receptors in mice. Ann Endocrinol (Paris) 2010;71:170–176. doi: 10.1016/j.ando.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Abel MH, Charlton HM, Huhtaniemi I, Pakarinen P, Kumar TR, Christian HC. An investigation into pituitary gonadotrophic hormone synthesis, secretion, subunit gene expression and cell structure in normal and mutant male mice. J Neuroendocrinol. 2013;25:863–875. doi: 10.1111/jne.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 10.Abel MH, Wootton N, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology. 2000;141:1795–1803. doi: 10.1210/endo.141.5.7456. [DOI] [PubMed] [Google Scholar]
- 11.Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15:172–183. doi: 10.1210/mend.15.1.0582. [DOI] [PubMed] [Google Scholar]
- 13.Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G. Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature. 1977;269:338–340. doi: 10.1038/269338a0. [DOI] [PubMed] [Google Scholar]
- 14.Mannan MA, O’Shaughnessy PJ. Ovarian steroid metabolism during postnatal development in the normal mouse and in the adult hypogonadal (hpg) mouse. J Reprod Fertil. 1988;82:727–734. doi: 10.1530/jrf.0.0820727. [DOI] [PubMed] [Google Scholar]
- 15.O’Shaughnessy PJ, Mannan MA. Development of cytochrome P-450 side chain cleavage mRNA levels in neonatal ovaries of normal and hypogonadal (hpg) mice. Mol Cell Endocrinol. 1994;104:133–138. doi: 10.1016/0303-7207(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 16.Charlton HM, Halpin DM, Iddon C, Rosie R, Levy G, McDowell IF. The effects of daily administration of single and multiple injections of gonadotropin-releasing hormone on pituitary and gonadal function in the hypogonadal (hpg) mouse. Endocrinology. 1983;113:535–544. doi: 10.1210/endo-113-2-535. [DOI] [PubMed] [Google Scholar]
- 17.Halpin DM, Charlton HM. Effects of short-term injection of gonadotrophins on ovarian follicle development in hypogonadal (hpg) mice. J Reprod Fertil. 1988;82:393–400. doi: 10.1530/jrf.0.0820393. [DOI] [PubMed] [Google Scholar]
- 18.Lang J. Assay for deletion in the GnRH (hpg) locus using PCR. Mouse Genome. 1991;89:857. [Google Scholar]
- 19.Scully KM, Gleiberman AS, Lindzey J, Lubahn DB, Korach KS, Rosenfeld MG. Role of estrogen receptor-alpha in the anterior pituitary gland. Mol Endocrinol. 1997;11:674–681. doi: 10.1210/mend.11.6.0019. [DOI] [PubMed] [Google Scholar]
- 20.Lewis CE, Morris JF, Fink G, Johnson M. Changes in the granule population of gonadotrophs of hypogonadal (hpg) and normal female mice associated with the priming effect of LH-releasing hormone in vitro. J Endocrinol. 1986;109:35–44. doi: 10.1677/joe.0.1090035. [DOI] [PubMed] [Google Scholar]
- 21.Dannies PS. Protein hormone storage in secretory granules: mechanisms for concentration and sorting. Endocr Rev. 1999;20:3–21. doi: 10.1210/edrv.20.1.0354. [DOI] [PubMed] [Google Scholar]
- 22.Michael DJ, Cai H, Xiong W, Ouyang J, Chow RH. Mechanisms of peptide hormone secretion. Trends Endocrinol Metab. 2006;17:408–415. doi: 10.1016/j.tem.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Duncan RR, Greaves J, Wiegand UK, Matskevich I, Bodammer G, Apps DK, Shipston MJ, Chow RH. Functional and spatial segregation of secretory vesicle pools according to vesicle age. Nature. 2003;422:176–180. doi: 10.1038/nature01389. [DOI] [PubMed] [Google Scholar]
- 24.Grabner CP, Price SD, Lysakowski A, Fox AP. Mouse chromaffin cells have two populations of dense core vesicles. J Neurophysiol. 2005;94:2093–2104. doi: 10.1152/jn.00316.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe T, Azuma T, Banno T, Jeziorowski T, Ohsawa Y, Waguri S, Grube D, Uchiyama Y. Immunocytochemical localization of chromogranin A and secretogranin II in female rat gonadotropes. Arch Histol Cytol. 1998;61:99–113. doi: 10.1679/aohc.61.99. [DOI] [PubMed] [Google Scholar]
- 26.Blake CA. Correlative study of changes in the morphology of the LH gonadotroph and anterior pituitary gland LH secretion during the 4-day rat estrous cycle. Biol Reprod. 1980;23:1097–1108. doi: 10.1095/biolreprod23.5.1097. [DOI] [PubMed] [Google Scholar]
- 27.Bingel AS, Schwartz NB. Pituitary LH content and reproductive tract changes during the mouse oestrous cycle. J Reprod Fertil. 1969;19:215–222. doi: 10.1530/jrf.0.0190215. [DOI] [PubMed] [Google Scholar]
- 28.Murr SM, Geschwind II, Bradford GE. Plasma LH and FSH during different oestrous cycle conditions in mice. J Reprod Fertil. 1973;32:221–230. doi: 10.1530/jrf.0.0320221. [DOI] [PubMed] [Google Scholar]
- 29.Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröone H-J, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorling AA, Todman MG, Korach KS, Herbison AE. Critical role for estrogen receptor alpha in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroen-docrinology. 2003;78:204–209. doi: 10.1159/000073703. [DOI] [PubMed] [Google Scholar]
- 31.Kendall SK, Saunders TL, Jin L, Lloyd RV, Glode LM, Nett TM, Camper SA. Targeted ablation of pituitary gonadotropes in transgenic mice. Mol Endocrinol. 1991;5:2025–2036. doi: 10.1210/mend-5-12-2025. [DOI] [PubMed] [Google Scholar]
- 32.Kendall SK, Samuelson LC, Saunders TL, Wood RI, Camper SA. Targeted disruption of the pituitary glycoprotein hormone alpha-subunit produces hypogonadal and hypothyroid mice. Genes Dev. 1995;9:2007–2019. doi: 10.1101/gad.9.16.2007. [DOI] [PubMed] [Google Scholar]
- 33.Abel MH, Huhtaniemi I, Pakarinen P, Kumar TR, Charlton HM. Age-related uterine and ovarian hypertrophy in FSH receptor knockout and FSHβ subunit knockout mice. Reproduction. 2003;125:165–173. doi: 10.1530/rep.0.1250165. [DOI] [PubMed] [Google Scholar]
- 34.Burns KH, Yan C, Kumar TR, Matzuk MM. Analysis of ovarian gene expression in follicle-stimulating hormone beta knockout mice. Endocrinology. 2001;142:2742–2751. doi: 10.1210/endo.142.7.8279. [DOI] [PubMed] [Google Scholar]
- 35.Hirst RC, Abel MH, Wilkins V, Simpson C, Knight PG, Zhang F-P, Huhta-niemi I, Kumar TR, Charlton HM. Influence of mutations affecting gonadotropin production or responsiveness on expression of inhibin subunit mRNA and protein in the mouse ovary. Reproduction. 2004;128:43–52. doi: 10.1530/rep.1.00176. [DOI] [PubMed] [Google Scholar]
- 36.Woodruff TK, Besecke LM, Groome N, Draper LB, Schwartz NB, Weiss J. Inhibin A and inhibin B are inversely correlated to follicle-stimulating hormone, yet are discordant during the follicular phase of the rat estrous cycle, and inhibin A is expressed in a sexually dimorphic manner. Endocrinology. 1996;137:5463–5467. doi: 10.1210/endo.137.12.8940372. [DOI] [PubMed] [Google Scholar]
- 37.Boissy RE, Beato KE, Nordlund JJ. Dilated rough endoplasmic reticulum and premature death in melanocytes cultured from the vitiligo mouse. Am J Pathol. 1991;138:1511–1525. [PMC free article] [PubMed] [Google Scholar]
- 38.McDowell IF, Morris JF, Charlton HM. Characterization of the pituitary gonadotroph cells of hypogonadal (hpg) male mice: comparison with normal mice. J Endocrinol. 1982;95:321–330. doi: 10.1677/joe.0.0950321. [DOI] [PubMed] [Google Scholar]
- 39.Parkening TA, Collins TJ, Smith ER. Plasma and pituitary concentrations of luteinizing hormone, follicle-stimulating hormone and prolactin in aged, ovariectomized CD-1 and C57BL/6 mice. Exp Gerontol. 1982;17:437–443. doi: 10.1016/s0531-5565(82)80005-3. [DOI] [PubMed] [Google Scholar]
- 40.Kovacic N, Parlow AF. Alterations in serum FSH-LH ratios in relation to the estrous cycle, pseudopregnancy, and gonadectomy in the mouse. Endocrinology. 1972;91:910–915. doi: 10.1210/endo-91-4-910. [DOI] [PubMed] [Google Scholar]