Abstract
The incidence of prostate cancer (PC) accompanying periodontal disease (PD) is anticipated to increase due to population aging. The aim of this study was to determine the association between PD and PC using data in the National Health Insurance Service-Health Examinee Cohort (NHIS-HEC). A random stratified sample of 187,934 South Koreans was collected from the NHIS database from 2002 to 2013. We assessed the relationship between PD and PC while adjusting for potential confounding factors (sex, age, household income, insurance status, residence area, hypertension, diabetes mellitus, cerebral infarction, angina pectoris, myocardial infarction, smoking status, alcohol intake, and regular exercise). The overall incidence of PC with PD among those aged 40 years and older was 0.28% (n = 531). In the multivariate Cox proportional-hazard regression analysis with adjustment for confounding factors, PD was associated with a 14% higher risk of PC (HR = 1.14, 95% CI = 1.01-1.31, P = 0.042). The findings of this study suggest that PD is significantly and positively associated with PC. Further studies are required to identify the mechanisms underlying the links between PD and PC.
Keywords: Cohort analysis, periodontal disease, periodontitis, prostate cancer.
Introduction
Periodontal disease (PD) is a chronic bacterial and inflammatory disease whose prevalence is reportedly as high as 90% 1, 2. PD can routinely injure or damage both oral soft and hard tissues such as gingiva, cementum, periodontal ligament, and supporting alveolar bone, and it is the primary risk factor for tooth loss in adults 3, 4. Recent studies have also found PD to be associated with cardiovascular disease, pregnancy disease, chronic respiratory disease, diabetes mellitus, and erectile dysfunction 5-8. Oral pathologic bacteria are not only a dominant risk factor for PD, but are also a risk factor for major systemic diseases due to several mechanisms involving modulation of the inflammatory pathway and systemic immunity 9.
Chronic inflammation caused by bacterial infection has been reported as one of the main factors underlying the development of cancer 10. Cumulative studies have demonstrated the relation between oral bacteria and initiation or progression of systemic cancer 11. In particular, oral pathologic bacteria such as Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, Tannerella forsythia, Treponema denticola, and Porphyromonas gingivalis are significantly positively associated with oral, pancreatic, and gastrointestinal cancers 12, 13. Oral pathologic bacteria can either up- or down-regulate proinflammatory cytokines and chemokines, including interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and C-reactive protein, resulting in oral pathologic bacteria being able to affect the oral and systemic immune systems of the body and also induce oncogenic responses 14, 15.
Prostate cancer (PC) is the most-common cancer in men in the United States and Europe. While PC is only the fifth-most-common diagnosed malignancy and the seventh leading cause of cancer death among men in South Korea, the rapid aging of the Korea population and the increasing prevalence of obesity have resulted in a steep increase in the incidence of PC recently. PD is apparently associated with oral bacterial dysbiosis and systemic immune responses, but the role of oral pathogenic bacteria in carcinogenesis remains unclear. Also, a direct correlation between PD and PC is still unclear and controversial. While numerous cross-sectional and cohort studies have identified associations between PD and facial cancers, especially those of the oral and neck, there have been very few well-designed studies with stratified longitudinal cohorts comparing PD and PC 16, 17. Our aim was therefore to determine whether there is a connection between PD and PC using data from a South Korean nationwide population based on National Health Insurance Service-Health Examinee Cohort (NHIS-HEC).
Materials and Methods
Study design and data collection
In South Korea, patients in the NHIS are required to pay approximately 30% of their total medical expenses when they use medical facilities, with the medical providers submitting claims for the remaining 70%. This has resulted in the NHIS having access to an enormous database (DB) of more than one trillion cases involving the entire Korean population of 51 million covering the past decade. This DB includes information on individual qualification criteria, insurance coverage and payouts, medical and dental records, health checkups, rare incurable diseases, systemic cancer, and long-term-care insurance services. The NHIS program is designed for the heads of households, household members aged 40 years or older, and workplace subscribers who receive regular health checkup examinations once every 2 years, which include a self-reported questionnaire on smoking, alcohol consumption, and exercise.
A random stratified sample of 514,866 South Koreans was collected from the NHIS DB by the NHIS Big Data Steering Department, which is conducting 1,476-stage simple sampling based on sex, age, and household income level for the 12 years from 2002 to 2013. After performing stratified sampling of the data for 2002, the data in the cohort DB are maintained to 2013 except when sample objects become ineligible due to death or emigration. To compensate for the natural reduction in the population data over time, neonatal samples are added yearly in order to maintain the sample size, making this a semidynamic cohort. All personal information and identification numbers were deleted prior to the analysis, and a computer-generated random number was allocated to each sample. This study conformed to the STROBE guidelines for reporting observational studies (www.strobe-statement.org) and was approved by the Institutional Review Board, Daejeon Dental Hospital, Wonkwang University (approval no. W1702/004-001).
Identification of sociodemographic factors
Potential confounding sociodemographic factors of PD and PC were assessed from the NHIS-HEC DB: sex, age (4 groups: those aged 40-69 years in 10-year intervals, and those aged ≥70 years), household income (5 groups: 41 groups were divided into 5 quintiles based on the insurance fee imposed on each household, with the Medical Aid Program [MAP] group corresponds to the first quintile), insurance status (3 groups: those in the MAP and those in the NHIS, where the NHIS group included both self-employed and employees), and residence area (2 groups: with a cutoff of 50,000 residents).
Identification of comorbidity
Patients who were diagnosed with hypertension, diabetes mellitus, and cardiovascular disease prior to 2002 were excluded, and the following lifestyle-related comorbidities present from 2002 to 2013 were diagnosed by medical doctors: hypertension (Korean Classification of Diseases, 6th revision [KCD-6] codes I10 and I15, corresponding to International Classification of Diseases, 10th revision [ICD-10-CM] codes I10 and I15), diabetes mellitus (KCD-6 codes E10-E14, corresponding to ICD-10-CM codes E10-E14), cerebral infarction (KCD-6 codes I63-I66, corresponding to ICD-10-CM codes I63-I66), angina pectoris (KCD-6 codes I20, corresponding to ICD-10-CM codes I20), and myocardial infarction (KCD-6 codes I21 and I22, corresponding to ICD-10-CM codes I21 and I22). To increase the validity of the assessment in comorbidities diagnoses, we only included patients with comorbidities who have been diagnosed at least twice during the period from 2002 to 2013.
Identification of patients with prostate cancer
97% of cancer patients are fully or partially covered by NHI and all cancer patients are registered in NHIS and Health Insurance Review and Assessment Service (HIRA) DB. Therefore, this study performed that PC diagnostic and prescription codes were obtained based on the NHIS and HIRA. Patients who were diagnosed with cancer prior to 2002 and aged <40 years were excluded according to self-reported questionnaire information in the NHIS regular health check-up examination. We selected patients who had been newly diagnosed with PC (KCD-6 codes C61; corresponding to ICD-10-CM codes C61) by medical oncologists and urologists from 2002 to 2013 and the date of which cancer was first diagnosed was assigned as the index date.
Identification of patients with periodontal disease
This study involved adult patients who were aged ≥40 years and diagnosed with acute periodontitis, chronic periodontitis, periodontosis, other PD, and unspecified PD (KCD-6 codes K05.2 to K05.6, corresponding to ICD-10-CM codes K05.2 to K05.6) prior to the index date between 2002 and 2013. The patients who were diagnosed as acute and chronic gingivitis (KCD-6 codes K05.0 to K05.1, corresponding to ICD-10-CM codes K05.0 to K05.1) were excluded as a reversible inflammatory PD. To increase the validity of the assessment of PD diagnoses, we only included patients with PD who have been diagnosed at least twice with a specific PD related-disorder during the period from 2002 through 2013. Clinical and radiographic diagnoses of PD were based on measurements of the amount of bleeding on probing, probing depth (measured between the gingival crest margin and the base of the apical lesion), clinical attachment level (measured between the cementoenamel junction and the base of the apical lesion), gingival inflammation, and radiographic alveolar bone loss according to criteria of the Centers for Disease Control and Prevention/American Academy of Periodontology case definitions by local dentists or periodontists 18, 19.
Statistical analysis
The chi-square test was used to identify significant differences in categorical cohort data for sociodemographic factors (sex, age, household income, insurance status, and residence area), comorbidities (hypertension, diabetes mellitus, cerebral infarction, angina pectoris, and myocardial infarction), self-reported questionnaire (smoking status, day's smoking, alcohol intake habits, one-time alcohol intake, and regular exercise), and incidence of PC with PD. We calculated hazard ratios (HRs) with 95% confidence intervals (CIs) using multivariate Cox proportional-hazards regression models while adjusting for sociodemographic factors, comorbidities, smoking status, alcohol intake, and regular exercise. The cumulative incidence of PC with/without PD was estimated using the Kaplan-Meier method and compared with the log-rank test. In all assessments performed in this study, P < 0.05 was considered statistically significant and statistical tests were two-sided. All cohort data used in this study were calculated using the Statistical Analysis System (version 9.2, SAS Institute, Cary, NC, USA) by the Department of Health Insurance Research, Ilsan Hospital, NHIS.
Results
Incidence of prostate cancer patients with periodontal disease
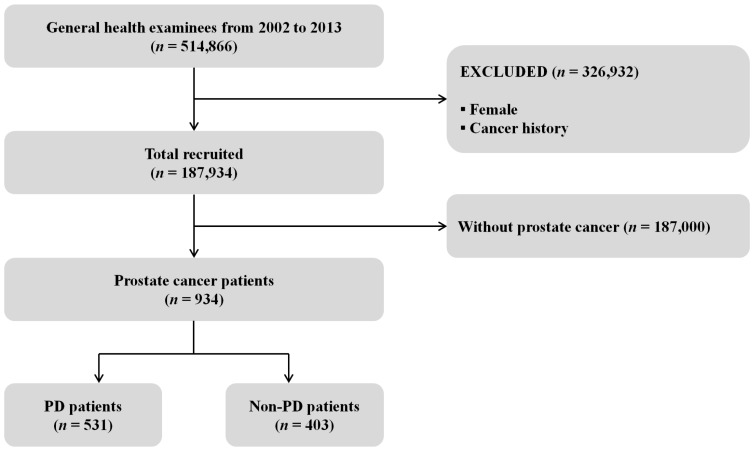
Fig. 1 shows a cohort flow chart for the inclusion and exclusion of the study participants. Among the total cohort of 514,866 people, 326,932 (Female and cancer history) were excluded from the study and 187,934 (18.3%) males and aged ≥40 years were recruited. We enrolled 934 subjects who were diagnosed with PC, comprising 531 PD patients (56.9%) and 403 Non-PD patients (43.1%). Those aged 60-69 years (n = 398) accounted for 42.6% of the surveyed subjects, while 361 (38.7%) were in the fifth quintile of household income, 927 (99.3%) were in the NHIS, and 782 (83.7%) lived in urban areas (Table 1).
Figure 1.
Flow chart of the inclusion and exclusion of participants in the National Health Insurance Service- Health Examinee Cohort during 2002-2013; PD: periodontal disease.
Table 1.
Baseline characteristics of the population included in the National Health Insurance Service-Health Examinee Cohort (NHIS-HEC) according to prostate cancer (PC) patients with/without periodontal disease (PD).
| Characteristics | PD (n, %) | Non-PD (n, %) | Pa |
|---|---|---|---|
| Total | 531 | 403 | |
| Age group (years) | |||
| 40-49 | 48 (9.0) | 45 (11.2) | <0.001 |
| 50-59 | 139 (26.2) | 98 (24.3) | |
| 60-69 | 246 (46.3) | 152 (37.7) | |
| ≥70 | 98 (18.5) | 108 (26.8) | |
| Household incomeb | |||
| First quintile | 49 (9.2) | 70 (17.4) | <0.001 |
| Second quintile | 66 (12.4) | 56 (13.9) | |
| Third quintile | 87 (16.4) | 68 (16.9) | |
| Fourth quintile | 110 (20.7) | 67 (16.6) | |
| Fifth quintile | 219 (41.3) | 142 (35.2) | |
| Insurance status | |||
| MAP | 4 (0.8) | 3 (0.7) | <0.001 |
| NHIS (self-employed) | 293 (55.2) | 206 (51.1) | |
| NHIS (employees) | 234 (44.0) | 194 (48.2) | |
| Residence areac | |||
| Urban | 466 (87.8) | 316 (78.4) | <0.001 |
| Rural | 65 (12.2) | 87 (21.6) |
Abbreviations: MAP: Medical Aid Program.
aP values were calculated using the chi-square test; boldface denotes statistical significance (P < 0.05).
bDivided into five quintiles based on the insurance fee imposed on each household, with the MAP group classed into the first quintile.
cClassified with a cutoff of 50,000 residents.
Results of comorbidity and regular health check-up examination
Although comorbid disease (hypertension, diabetes mellitus, cerebral infarction, angina pectoris, and myocardial infarction) showed higher incidence of PD patients compared with Non-PD patients, only myocardial infarction was statistically significantly higher in PD patients compared with Non-PD patients (P < 0.001). In the questionnaire survey, smoking and exercise status were only statistically significant between PD patients and Non-PD patients (P < 0.001; Table 2).
Table 2.
Results of comorbidity and regular health check-up examination
| Characteristics | PD (n, %) | Non-PD (n, %) | Pa |
|---|---|---|---|
| Comorbid disease | |||
| Hypertension | 329 (62.0%) | 246 (61.0%) | 0.776 |
| Diabetes mellitus | 194 (36.5%) | 133 (33.0%) | 0.262 |
| Cerebral infarction | 102 (19.2%) | 61 (15.1%) | 0.104 |
| Angina pectoris | 22 (4.1%) | 13 (3.2%) | 0.465 |
| Myocardial infarction | 142 (26.7%) | 67 (16.6%) | <0.001 |
| Self-reported questionnaire | |||
| Smoking statusb | |||
| Yes | 371 (69.9%) | 133 (33.0%) | <0.001 |
| No | 160 (30.1%) | 270 (67.0%) | |
| Day's smoking | |||
| <20 piece | 334 (90.0%) | 112 (84.2%) | 0.072 |
| ≥20 piece | 37 (10.0%) | 21 (15.8%) | |
| Alcohol intake habits | |||
| No drinking | 259 (48.8%) | 207 (51.4%) | 0.153 |
| 1-4 times/week | 237 (44.6%) | 159 (39.5%) | |
| Almost every day | 35 (6.6%) | 37 (9.1%) | |
| One-time alcohol intake | |||
| <1 bottle of beer (360ml) | 237 (87.1%) | 170 (86.7%) | 0.900 |
| ≥1 bottle of beer (360ml) | 35 (12.9%) | 26 (13.3%) | |
| Regular exercise | |||
| No | 244 (46.0%) | 229 (56.8%) | <0.001 |
| 1-4 times/week | 209 (39.4%) | 108 (26.8%) | |
| ≥5 times/week | 78 (14.6%) | 66 (16.4%) | |
Abbreviations: PD: periodontal disease
aP values were calculated using the chi-square test; boldface denotes statistical significance (P < 0.05).
bSubjects were currently not smoking, or had smoked <100 cigarettes were classified as non-smokers, while the other subjects were classified as smokers.
Association of prostate cancer with periodontal disease
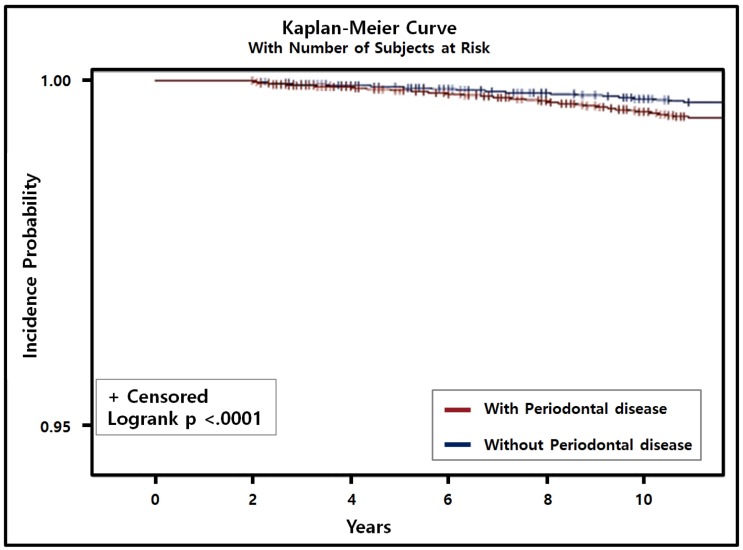
In the multivariate Cox regression analysis with adjustment for sociodemographic factors, comorbidities, smoking status, alcohol intake, and regular exercise, PD was associated with a 14% higher risk of PC (HR = 1.14, 95% CI = 1.01-1.31, P = 0.042). In addition, using the adjusted Kaplan-Meier curve with the number of subjects at risk, we found that the incidence of PC with PD was significantly higher than that of PC without PD (P < 0.001; Fig. 2). The presence of both PC and PD was significantly positively correlated with current smoking (HR = 1.68, 95% CI = 1.52-1.85, P < 0.001), smoking ≥20 cigarettes a day (HR = 1.20, 95% CI = 1.04-1.40, P = 0.015), consuming alcohol every day (HR = 1.17, 95% CI = 1.09-1.25, P < 0.001), and consuming ≥1 bottle of beer (360 ml) daily (HR = 1.08, 95% CI = 1.03-1.13, P < 0.001; Table 3).
Figure 2.
Cumulative incidence of prostate cancer with/without periodontal disease (PD) was estimated using the Kaplan-Meier method and compared using the log-rank test. Incidence of prostate cancer patients with PD was higher and significantly different from prostate cancer patients without PD (P < 0.001).
Table 3.
Association of PC patients with PD in multivariate Cox proportional-hazard regression analysis with adjustment for confounding factors.
| Characteristics | HR | 95% CI | Pa |
|---|---|---|---|
| Age group (ref: 40-49 years) | |||
| 50-59 | 2.21 | 1.92-2.55 | <0.001 |
| 60-69 | 5.86 | 5.14-6.68 | <0.001 |
| ≥70 | 14.31 | 12.57-16.30 | <0.001 |
| Household income (ref: First quintile) | |||
| Second quintile | 1.33 | 1.11-1.58 | <0.001 |
| Third quintile | 1.16 | 0.99-1.35 | 0.066 |
| Fourth quintile | 1.12 | 0.99-1.28 | 0.069 |
| Fifth quintile | 1.13 | 0.97-1.31 | 0.118 |
| Insurance status (ref: MAP) | |||
| NHIS (self-employed) | 0.42 | 0.19-0.91 | 0.026 |
| NHIS (employees) | 0.46 | 0.21-0.99 | 0.045 |
| Residence area (ref: Urban) | |||
| Rural | 0.97 | 0.80-1.16 | 0.713 |
| Smoking status (ref: No) | |||
| Yes | 1.68 | 1.52-1.85 | <0.001 |
| Day's smoking (ref: <20 piece) | |||
| ≥20 piece | 1.20 | 1.04-1.40 | 0.015 |
| Alcohol intake habits (ref: No) | |||
| 1-4 times/week | 0.94 | 0.91-0.97 | <0.001 |
| Almost every day | 1.17 | 1.09-1.25 | <0.001 |
| 1-time alcohol (ref: <1 bottle) | |||
| ≥1 bottle (360ml) | 1.08 | 1.03-1.13 | 0.001 |
| Regular exercise (ref: No) | |||
| 1-4 times/week | 1.00 | 0.97-1.03 | 0.858 |
| ≥5 times/week | 0.83 | 0.78-0.89 | <0.001 |
| Periodontal disease | 1.14 | 1.01-1.31 | 0.042 |
Abbreviations: PD: periodontal disease; HR: hazard ratio; CI: confidence interval.
aBoldface denotes statistical significance (P < 0.05).
Multivariate regression analysis adjusted for sex, age, household income, insurance status, residence area, hypertension, diabetes mellitus, cerebral infarction, angina pectoris, myocardial infarction, smoking status, alcohol intake, and regular exercise.
Discussion
PD is the most-common inflammatory disease, and many of its known risk factors - sex, age, smoking, obesity, hypertension, and diabetes mellitus - are also considered to be risk factors for systemic cancer 20. We observed that the risk of PC was significantly positively correlated with age ≥70 years, lower income, being registered with the MAP, myocardial infarction, heavy smoking, high alcohol consumption, and less exercise. The data was not statistically significant except second quintile, but the lower the household income, the higher the risk for PC. This can be explained by the reduced accessibility to healthcare services and limitations of the current study involving PC patients who only visited clinics for the cancer but not for the PD. We also found that the incidence of PD - which is well known to be related to oral cancer - was also significantly positive associated with PC in a multivariate analysis.
Few studies have investigated the association of PC with PD 21-23. In one prospective cohort based on the Health Professionals Follow-Up Study it was found that PD was correlated with a slight but significant increase in the risk of systemic cancer, but PD was also inversely correlated with PC (HR = 0.99, 95% CI = 0.61-1.59) and tooth loss (HR = 0.70, 95% CI = 0.50-0.97) 21. Hiraki et al. 22 conducted a large-scale case-control study of Japanese patients, and reported that increased tooth loss was correlated with a decreased risk of PC after adjusting for potential confounding factors (odds ratio [OR] = 0.49, 95% CI = 0.19-1.26, P = 0.049). In particular, the risk of PC was higher when more teeth were remaining, and this may have been due not only to the sociodemographic and economic characteristics of the studied subjects, but also to the number of teeth affected by PD. Unlike these studies, the NHANES I Epidemiologic Follow-up Study found that PD was associated with an elevated risk of PC (HR = 1.81, 95% CI = 0.76-4.34) 23. However, that study was hampered by poor statistical power due to the smallness of the sample and also potentially by the smallness of any real effects.
Both the bivariate analysis (HR = 1.60, 95% CI = 1.40-1.83, P < 0.001; data not shown) and multivariate analysis (HR = 1.14, 95% CI = 1.01-1.31, P = 0.042) performed in the present study showed that PD was positively and significantly associated with PC. Both diseases produce generalized inflammation and infection in the body, and especially chronic PD has been shown to increase the level of prostate-specific antigen (PSA) that is produced primarily by epithelial prostate cells and is used to diagnose PC 24. The PSA level is significantly higher in chronic prostatitis patients with PD characterized by a gingival clinical attachment level of ≥ 2.7 mm (10.8±7.0 ng/ml) than in those without such PD (5.6±3.7 ng/ml, P = 0.05) 24. In addition, Alwithanani et al. 25 reported that the mean PSA level was significantly higher in PD patients regardless of the presence or absence of PC and the prostate symptom score, and that the PSA level might decrease after treating PD. There is accumulating evidence of proinflammatory cytokines such as IL-6, IL-8, IL-18, TNF-α, and C-reactive protein being associated with the pathogenesis of PC 24. These observations may indicate the similarity of the etiopathogenesis of PD and PC.
A recent systematic review found a significant positive relationship between PD and erectile dysfunction 26. Both conditions share chronic inflammation and systemic endothelial dysfunction, which are also key mechanisms of carcinogenesis 27. The invasion of oral pathological bacteria, especially Porphyromonas gingivalis, may induce traumatic injury and irritation of the epithelium and mucosa, and play a role in subsequent cancer progression 28, 29. A nationwide population-based cohort study found that the risk of cancer was 1.42-fold higher (95% CI = 1.03-2.09, P = 0.039) in patients with erectile dysfunction 30. In our previous study we found a strong association between PD and vasculogenic erectile dysfunction in a nationwide population-based NHIS-HEC (OR = 1.53, 95% CI = 1.41-1.65, P < 0.001) 31. Although the evidence is not definitive, these results suggest that a diagnosis of PD is associated with a higher risk of PC.
Androgen deprivation therapy (ADT) is the most-common standard treatment for advanced PC, and its application is increasing rapidly 32. However, many studies have suggested that ADT is associated with cardiovascular disease, diabetes mellitus, and Alzheimer's disease, with in particular it being the main cause of male osteoporosis since it can affect almost any bone structure of the body 33, 34. Male osteoporosis can result from various causes, including bone resorption, bone loss, and bone fracture, and several studies have found that PD can be exacerbated by systemic bone loss such as osteoporosis or rheumatoid arthritis 35-37. Famili et al. 38 considered that patients with PC receiving ADT are more likely to develop PD or experience worsening of PD than are patients who are not on ADT. Although it is difficult to conclude that ADT for PC can induce or exacerbate PD, this observation should be considered when deciding whether or not to apply ADT to PC patients.
This study was subject to several limitations. Firstly, selection bias may have been present due to its retrospective design and only including patients who visited dental clinics and hospitals and who were diagnosed with PD and PC 39. Secondly, this study did not analyze medical and dental records, which restricted the ability to diagnose the severity and the follow-up period of PD and PC in the DB of this cohort. Therefore, future cohort studies should consider the severity and the follow-up period of PD and PC in the design. Finally, because the data were acquired from a diverse group of medical and dental specialties, inconsistent diagnostic criteria were likely to have been applied. Nevertheless, the results from this nationwide population-based longitudinal cohort study in South Korea are considered to provide evidence for a link between PD and PC. PD is clearly intimately associated with systemic inflammation and immune dysregulation, which are both direct and indirect etiological factors in systemic cancer 40, 41. However, the etiological and bidirectional associations between PD and PC remain unclear, and little is known about their underlying mechanisms. Therefore, further investigations of the biological plausibility and the gathering of clinical and epidemiological evidence are needed to both strengthen and understand the association between PD and PC.
Conclusion
To our knowledge, this study is the first to investigate the association of PC with PD using a retrospective analysis of a nationwide population-based NHIS-HEC. In contrast to some previous studies, the present cohort study has shown that patients with PD have a significantly but slightly positive in patients with PC. Further studies are required to strengthen this hypothesis and focus on identifying the mechanisms underlying the links between PD and PC. If confirmed, this observation could have clinical and public health implications given the increasing prevalence of PD worldwide.
Acknowledgments
This study used a national sample cohort data of the National Health Insurance Service (NHIS-2017-2-377) and was supported by Wonkwang University in 2017. The authors report no conflicts of interest related to this study.
Abbreviations
- PC
prostate cancer
- PD
periodontal disease
- NHIS-HEC
National Health Insurance Service-Health Examinee Cohort
- IL
interleukin
- TNF
tumor necrosis factor
- DB
database
- HIRA
Health Insurance Review and Assessment Service
- PSA
prostate-specific antigen
- ADT
Androgen deprivation therapy.
References
- 1.Burt B, Research S, Therapy Committee of the American Academy of P. Position paper: epidemiology of periodontal diseases. J Periodontol. 2005;76:1406–19. doi: 10.1902/jop.2005.76.8.1406. [DOI] [PubMed] [Google Scholar]
- 2.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–20. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 3.Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004;34:9–21. doi: 10.1046/j.0906-6713.2002.003421.x. [DOI] [PubMed] [Google Scholar]
- 4.Highfield J. Diagnosis and classification of periodontal disease. Aust Dent J. 2009;54(Suppl 1):S11–26. doi: 10.1111/j.1834-7819.2009.01140.x. [DOI] [PubMed] [Google Scholar]
- 5.Beck JD, Offenbacher S. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol. 2005;76:2089–100. doi: 10.1902/jop.2005.76.11-S.2089. [DOI] [PubMed] [Google Scholar]
- 6.Wang Q, Kang J, Cai X, Wu Y, Zhao L. The association between chronic periodontitis and vasculogenic erectile dysfunction: a systematic review and meta-analysis. J Clin Periodontol. 2016;43:206–15. doi: 10.1111/jcpe.12512. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Choi JK, Kim SH, Cho KH, Kim YT, Choi SH. et al. Association between periodontal flap surgery for periodontitis and vasculogenic erectile dysfunction in Koreans. J Periodontal Implant Sci. 2017;47:96–105. doi: 10.5051/jpis.2017.47.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JH, Oh JY, Youk TM, Jeong SN, Kim YT, Choi SH. Association between periodontal disease and non-communicable diseases: A 12-year longitudinal health-examinee cohort study in South Korea. Medicine (Baltimore) 2017;96:e7398. doi: 10.1097/MD.0000000000007398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsen I, Yilmaz O. Modulation of inflammasome activity by Porphyromonas gingivalis in periodontitis and associated systemic diseases. J Oral Microbiol. 2016;8:30385. doi: 10.3402/jom.v8.30385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakoff-Nahoum S. Why cancer and inflammation? Yale J Biol Med. 2006;79:123–30. [PMC free article] [PubMed] [Google Scholar]
- 11.Kudo Y, Tada H, Fujiwara N, Tada Y, Tsunematsu T, Miyake Y. et al. Oral environment and cancer. Genes Environ. 2016;38:13. doi: 10.1186/s41021-016-0042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michaud DS, Izard J, Wilhelm-Benartzi CS, You DH, Grote VA, Tjonneland A. et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut. 2013;62:1764–70. doi: 10.1136/gutjnl-2012-303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang JS, Tsai CR, Chen LT, Shan YS. Investigating the Association Between Periodontal Disease and Risk of Pancreatic Cancer. Pancreas. 2016;45:134–41. doi: 10.1097/MPA.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 14.Meurman JH. Oral microbiota and cancer. J Oral Microbiol; 2010. p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B, Khalaf H, Sirsjo A, Bengtsson T. Gingipains from the Periodontal Pathogen Porphyromonas gingivalis Play a Significant Role in Regulation of Angiopoietin 1 and Angiopoietin 2 in Human Aortic Smooth Muscle Cells. Infect Immun. 2015;83:4256–65. doi: 10.1128/IAI.00498-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen BW, Tsai CS, Lin CL, Chang YJ, Lee CF, Hsu CH. et al. Cancer risk among gingivitis and periodontitis patients: a nationwide cohort study. QJM. 2014;107:283–90. doi: 10.1093/qjmed/hct248. [DOI] [PubMed] [Google Scholar]
- 17.Zeng XT, Deng AP, Li C, Xia LY, Niu YM, Leng WD. Periodontal disease and risk of head and neck cancer: a meta-analysis of observational studies. PLoS One. 2013;8:e79017. doi: 10.1371/journal.pone.0079017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Page RC, Eke PI. Case definitions for use in population - Based surveillance of periodontitis. J Periodontol. 2007;78:1387–99. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 20.Michaud DS, Kelsey KT, Papathanasiou E, Genco CA, Giovannucci E. Periodontal disease and risk of all cancers among male never smokers: an updated analysis of the Health Professionals Follow-up Study. Ann Oncol. 2016;27:941–7. doi: 10.1093/annonc/mdw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michaud DS, Liu Y, Meyer M, Giovannucci E, Joshipura K. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. 2008;9:550–8. doi: 10.1016/S1470-2045(08)70106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiraki A, Matsuo K, Suzuki T, Kawase T, Tajima K. Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol Biomarkers Prev. 2008;17:1222–7. doi: 10.1158/1055-9965.EPI-07-2761. [DOI] [PubMed] [Google Scholar]
- 23.Hujoel PP, Drangsholt M, Spiekerman C, Weiss NS. An exploration of the periodontitis-cancer association. Ann Epidemiol. 2003;13:312–6. doi: 10.1016/s1047-2797(02)00425-8. [DOI] [PubMed] [Google Scholar]
- 24.Joshi N, Bissada NF, Bodner D, Maclennan GT, Narendran S, Jurevic R. et al. Association between periodontal disease and prostate-specific antigen levels in chronic prostatitis patients. J Periodontol. 2010;81:864–9. doi: 10.1902/jop.2010.090646. [DOI] [PubMed] [Google Scholar]
- 25.Alwithanani N, Bissada NF, Joshi N, Bodner D, Demko C, Maclennan GT. et al. Periodontal Treatment Improves Prostate Symptoms and Lowers Serum PSA in Men with High PSA and Chronic Periodontitis. Dentistry. 2015;5:284. [Google Scholar]
- 26.Kellesarian SV, Kellesarian TV, Ros Malignaggi V, Al-Askar M, Ghanem A, Malmstrom H, Association Between Periodontal Disease and Erectile Dysfunction: A Systematic Review. Am J Mens Health; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zambon JP, de Mendonca RR, Wroclawski ML, Karam A, Santos RD, de Carvalho JAM. et al. Cardiovascular and metabolic syndrome risk among men with and without erectile dysfunction: case-control study. Sao Paulo Med J. 2010;128:137–40. doi: 10.1590/S1516-31802010000300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer MS, Joshipura K, Giovannucci E, Michaud DS. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control. 2008;19:895–907. doi: 10.1007/s10552-008-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng XT, Xia LY, Zhang YG, Li S, Leng WD, Kwong JS. Periodontal Disease and Incident Lung Cancer Risk: A Meta-Analysis of Cohort Studies. J Periodontol; 2016. pp. 1–13. [DOI] [PubMed] [Google Scholar]
- 30.Chung SD, Kang JH, Liao CH, Chiu KM, Lin HC. Increased risk for cancer following erectile dysfunction: a nationwide population-based follow-up study. J Sex Med. 2011;8:1513–20. doi: 10.1111/j.1743-6109.2010.02076.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee JH, Lee JS, Park JY, Choi JK, Kim DW, Kim YT. et al. Association of Lifestyle-Related Comorbidities With Periodontitis: A Nationwide Cohort Study in Korea. Medicine (Baltimore) 2015;94:e1567. doi: 10.1097/MD.0000000000001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–44. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 33.Alibhai SM, Duong-Hua M, Sutradhar R, Fleshner NE, Warde P, Cheung AM. et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009;27:3452–8. doi: 10.1200/JCO.2008.20.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bagcchi S. ADT in prostate cancer and risk of Alzheimer's disease. Lancet Oncol. 2016;17:e12. doi: 10.1016/S1470-2045(15)00578-1. [DOI] [PubMed] [Google Scholar]
- 35.Higano CS. Management of bone loss in men with prostate cancer. J Urol. 2003;170:S59–63. doi: 10.1097/01.ju.0000097351.48848.1f. discussion S4. [DOI] [PubMed] [Google Scholar]
- 36.Rees TD. A profile of the patient with periodontal disease? Periodontol 2000. 2003;32:9–10. doi: 10.1046/j.0906-6713.2002.03201.x. [DOI] [PubMed] [Google Scholar]
- 37.Van Dyke TE, Sheilesh D. Risk factors for periodontitis. J Int Acad Periodontol. 2005;7:3–7. [PMC free article] [PubMed] [Google Scholar]
- 38.Famili P, Cauley JA, Greenspan SL. The effect of androgen deprivation therapy on periodontal disease in men with prostate cancer. J Urol. 2007;177:921–4. doi: 10.1016/j.juro.2006.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JH, Lee JS, Choi JK, Kweon HI, Kim YT, Choi SH. National dental policies and socio-demographic factors affecting changes in the incidence of periodontal treatments in Korean: A nationwide population-based retrospective cohort study from 2002-2013. BMC Oral Health. 2016;16:118. doi: 10.1186/s12903-016-0310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carneiro FS, Webb RC, Tostes RC. Emerging role for TNF-alpha in erectile dysfunction. J Sex Med. 2010;7:3823–34. doi: 10.1111/j.1743-6109.2010.01762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sahingur SE, Yeudall WA. Chemokine function in periodontal disease and oral cavity cancer. Front Immunol. 2015;6:214. doi: 10.3389/fimmu.2015.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]