Abstract
Sensitization is defined as a process whereby repeated intermittent exposure to a given stimulus results in an enhanced response at subsequent exposures. Next to robust findings of an increased dopamine synthesis capacity in schizophrenia, empirical data and neuroimaging studies support the notion that the mesolimbic dopamine system of patients with schizophrenia is more reactive compared with healthy controls. These studies led to the conceptualization of schizophrenia as a state of endogenous sensitization, as stronger behavioral response and increased dopamine release after amphetamine administration or exposure to stress have been observed in patients with schizophrenia. These findings have also been integrated into the neurodevelopmental model of the disorder, which assumes that vulnerable neuronal circuits undergo progressive changes during puberty and young adulthood that lead to manifest psychosis. Rodent and human studies have made an attempt to identify the exact mechanisms of sensitization of the dopaminergic system and its association with psychosis. Doing so, several epigenetic and molecular alterations associated with dopamine release, neuroplasticity, and cellular energy metabolism have been discovered. Future research aims at targeting these key proteins associated with sensitization in schizophrenia to enhance the knowledge of the pathophysiology of the illness and pave the way for an improved treatment or even prevention of this severe psychiatric disorder.
Keywords: psychosis, positron emission tomography, amphetamine, dopamine, PHNO
Significance Statement
Sensitization denotes a neuro-behavioral process where repeated exposure to a stimulus leads to a progressive enhancement in the response to this stimulus. The underlying neurobiological processes are essential for reinforcement learning in animals and humans. However, sensitization occurs also with many drugs of abuse. The neurotransmitter dopamine plays a key role in mediating the amplification of neuronal and behavioural responses to environmental stimuli and drugs of abuse. Amphetamines increase the release of dopamine in the brain, a mechanism that is essential for inducing addictive behaviour. However, release of dopamine also induces psychotic symptoms in patients with schizophrenia. Patients with schizophrenia are particularly sensitive to amphetamines even if they have never before consumed the drug. Thus, schizophrenia is believed to be associated with a state of ‘natural sensitization’ towards amphetamines. This review highlights the importance of understanding sensitization for better understanding and treating schizophrenia.
Introduction
The Concept of Sensitization
Sensitization denotes a nonassociative learning process in which repeated exposure to a stimulus leads to a progressive amplification in the behavioral and neurochemical response. In a pharmacological context, sensitization is defined as an amplified response to a constant dose of a substance after repeated administration. Sensitization has been described for most drugs of abuse associated with addictive behavior, including amphetamines, cocaine, opiates, nicotine, Δ9-tetrahydrocannabinol, and alcohol. Sensitization is, so to say, the opposite of the more familiar concept of drug tolerance, the diminishing effect of a drug resulting from repeated administration. Sensitization is thus often referred to as reverse tolerance (see Figure 1). In animals, sensitization to cocaine or d-amphetamine typically presents as an increase in locomotor responses or stereotypies (Robinson and Becker, 1986). Humans sensitized with low-dose amphetamine report an increase in alertness, euphoria, or focus after amphetamine administration (Strakowski et al., 2001; Boileau et al., 2006).
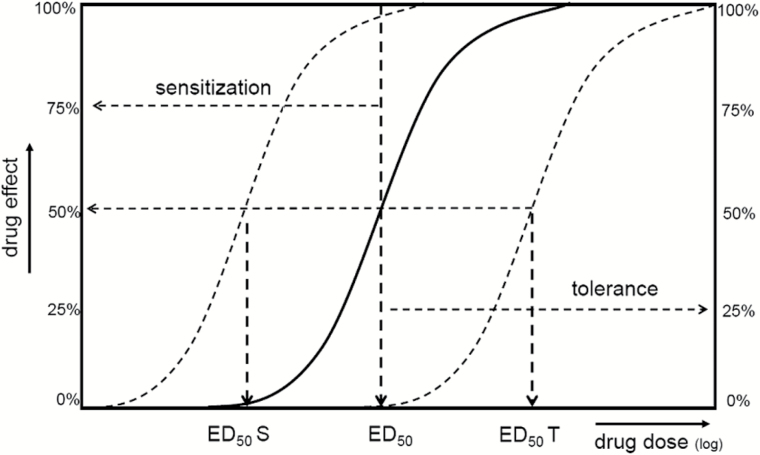
Figure 1.
Sensitization leads to an increase in drug effects. The dose of a drug producing half-maximal response (ED50) decreases with sensitization, so that a lower dose is needed to produce half-maximal drug effects (ED50S). Alternatively, the original ED50 will cause larger effects in the sensitized state. The opposite is found in tolerance, where the efficiency of a drug decreases and a higher dose (ED50T) is now needed to produce half-maximal effects, or where the original ED50 will now induce smaller effects.
A common mechanism of action of substances also serving as drugs of abuse is that they elicit a direct or indirect increase in brain extracellular dopamine levels immediately after drug administration. A release of dopamine is also a central element in the neurochemistry of behavioral learning (Keiflin and Janak, 2015), as dopamine release and specific patterns of activity in dopaminergic neurons are detected during conditioned (or Pavlovian) learning paradigms. In conditioned learning paradigms, a previously neutral environmental cue (the conditioned stimulus) is repeatedly presented in close temporal and spatial proximity to a primary or unconditioned stimulus such as food, sex, or an aversive pain stimulus. An animal’s learning rate in conditioning paradigms can be influenced by manipulating brain dopamine transmission: Higher levels of extracellular dopamine are associated with an increased response to the conditioned stimulus (better “learning”), and lower levels are associated with a decreased conditioned response. The connection between dopamine release induced by drugs of abuse and conditioned (or “cue”) learning is found across species using a broad variety of research methods, and it forms something like the core of the dopamine theory of addiction. As outlined later in this manuscript, there is substantial evidence that sensitization to psychostimulants is associated with a progressive increase in the amount of dopamine released in response to a given dose of the drug. As reflected in the term “drug learning” sometimes used when referring to psychostimulant sensitization, release of brain dopamine is a neurochemical mechanism common to learning and sensitization to psychostimulant drugs.
Amphetamines
Amphetamines constitute a group of chemically related synthetic derivatives of phenethylamine, a so-called trace amine that is naturally produced in catecholaminergic neurons by decarboxylation of the essential amino acid phenylalanine (Sitte and Freissmuth, 2015). Amphetamine itself is a synthetic compound first synthesized in 1887 (Edeleano, 1887) and is not known to occur naturally in any animals or plants. The main somatic effects of amphetamine include an increase in the activity of the sympathetic nervous system leading to mydriasis, bronchodilatation, and increased blood pressure and heart rate. The main psychophysiological effects are an increase in attention and vigilance, slight euphoria, increased drive, and an improvement in psychomotor speed and some cognitive domains related to attention and vigilance. However, amphetamine also increases errors in memory retrieval (Ballard et al., 2014). At higher doses, amphetamine induces stereotypies in animals and psychotic symptoms in humans.
The main sites of action of amphetamines are presynaptic monoamine-transmembrane transporters (the dopamine, norepinephrine, and serotonin reuptake transporters DAT, NET, and SERT) and the vesicular monoamine transporter 2 (VMAT2; Sulzer et al., 2005). The main physiological role of monoamine transporters is reuptake of monoamines into the presynaptic neuron immediately after they have been released into the synaptic cleft. Thereby, monoamine transporters regulate temporal and spatial spread of the monoamine signal. In contrast to pure transporter blockers such as cocaine or methylphenidate, amphetamines are also substrates of DAT, NET, and SERT and are thus transported in an ion gradient-dependent way into the neuron (Sitte and Freissmuth, 2015). There, amphetamines lead to neurotransmitter release from storage vesicles into the cytosol. Most importantly, however, amphetamines reverse the transport direction of monoamine transporters (Sitte and Freissmuth, 2015). By binding to the transporter from on the inside of the cell, amphetamines switch monoamine transporters from a reluctant to a willing state to perform outward transport and thus lead to a massive outflow of neurotransmitter into the synapse and surrounding extracellular space (Robertson et al., 2009).
Various amphetamines differ in their respective affinity to the 3 major monoamine transporters and have thus a distinct neuropharmacological profile. d-Amphetamine, the compound used in most animal and human studies, acts primarily at DAT and NET, and administration of d-amphetamine was shown to increase extracellular dopamine levels by several hundred percent over physiologic baseline levels (Zetterstrom et al., 1983; Laruelle et al., 1995; Breier et al., 1997).
Sensitization in Schizophrenia
Schizophrenia is a disorder with heterogeneous clinical manifestations characterized by a large variety of so-called positive (hallucinations, delusions, thought disorder, and motor symptoms) and negative symptoms (poverty of speech, apathy, social withdrawal). The presence of pronounced positive symptoms is what marks an acute psychotic episode of the illness, and generally, positive symptoms respond better to antipsychotic treatment. Negative symptoms are frequently found also outside of acute psychotic episodes and present a major therapeutic challenge in the treatment of schizophrenia (APA, 2013). The good responsiveness of positive symptoms to antipsychotic dopamine D2/3 receptor-blocking drugs is one of the reasons why psychosis has been associated with a hyper-dopaminergic state. Several complex genetic and environmental factors (birth in the winter months, urban upbringing, parental age, and others) have been shown to increase the risk for schizophrenia (Collip et al., 2008; van Winkel et al., 2008). As of yet it is unknown how these risk factors are related to each other and in which way they increase the illness risk. However, according to current interpretations (Laruelle, 2000; Kapur, 2003; Collip et al., 2008; van Winkel et al., 2008; Howes and Kapur, 2009; van Os and Kapur, 2009) of the dopamine theory of schizophrenia (van Rossum, 1966), all known risk factors seem to converge in a common final pathway, a hyper-dopaminergic state that causes psychotic symptoms in schizophrenia.
While sensitization is a concept that has substantial face value for explaining many behavioral patterns observed in patients with substance use disorders, its relationship to the phenomenologically diverse schizophrenia syndrome is not as self-evident. However, first descriptions of newly emerging psychotic states related to amphetamine intake date back to the first half of the last century (Young and Scoville, 1938). Since then, a link between amphetamine intake and psychotic symptoms has been described in many studies, some of them using escalating doses of amphetamine for prospective induction of psychotic symptoms in healthy volunteers (Ellinwood et al., 1973). Low doses of amphetamine induce mild euphoria, sometimes an increased sense of purpose, subjects feel awakened and notice an increase in the ability to focus their attention. In a plastic description, N. Richtand (Richtand 2006) notes that with increasing dose and frequency of intake, “symptoms following repetitive stimulant drug use evolve gradually from intense curiosity, progressing to intense exploration of the environment, which may be displayed in repetitive stereotyped searching, sorting, and examining behaviors. This curious ‘suspiciousness’ of the environment later evolves into paranoia and psychotic thought.” Moreover, experimentally administered amphetamine induces hallucination and classical Schneiderian first rank symptoms in healthy subjects (Janowsky and Risch, 1979). In these studies, symptoms usually resolved within hours after discontinuation of stimulant intake. In addition to positive symptoms of schizophrenia, an analysis of subjects suffering from methamphetamine-induced psychosis (Srisurapanont et al., 2011) showed considerable frequency and severity of negative symptoms. In summary, amphetamine is able to induce a reversible clinical picture resembling psychosis in schizophrenia in many aspects. However, to do so, amphetamines need to be consumed repeatedly and at high doses. Although studies specifically designed to measure behavioral and neurochemical effects of sensitization in humans used low amphetamine doses only, there is no reason to assume that neurochemical mechanisms of sensitization to higher stimulant doses differ completely from those to lower doses. In fact, as further discussed below, neurochemical findings in regular abusers of stimulants are in good agreement to what is found in prospectively sensitized animals.
Administering psychostimulants to patients with schizophrenia is one of the most frequently used challenge tests in psychiatric research. A classic 1987 review by Lieberman and colleagues (1987) lists 36 studies on the use of amphetamines or methylphenidate in patients with schizophrenia. Despite considerable heterogeneity of methods and design, the studies show that patients with schizophrenia exhibit greater responsiveness to psychostimulants than healthy subjects or patients with nonpsychotic illness. Generally, patients with full-blown psychosis showed larger changes in psychopathology upon psychostimulant administration, while patients with prominent negative symptoms showed no or little response. As described later in this review, today it is safe to say that hyper-responsiveness to stimulants in schizophrenia is due to an increased amount of dopamine released in response to the drug.
Competition and Blocking Experiments in Schizophrenia
So-called competition, displacement, or blocking paradigms use a decrease in dopamine D2/3 receptor radioligand binding after administration of a dopamine-releasing agent such as d-amphetamine as a proxy for the amount of dopamine released into the extracellular space (Laruelle, 2000; Ginovart, 2005). The effects of amphetamine-induced dopamine release on D2/3 radioligand binding have also been repeatedly studied in patients with schizophrenia. Using [123I]IBZM and single photon emission tomography, enhanced radioligand displacement in patients with schizophrenia and a positive correlation between displacement and the emergence or worsening of positive psychotic symptoms has been found (Laruelle et al., 1996). A study using [11C]raclopride and positron emission tomography (PET) (Breier et al., 1997) confirmed the finding in patients with schizophrenia and showed a clear relationship between amphetamine-induced displacement and the amount of dopamine released into the extracellular space in a cohort of nonhuman primates undergoing PET and in vivo microdialysis. Further studies confirmed and extended the finding by adding a dopamine-depletion paradigm that also elicited larger changes in radioligand binding in psychotic patients (Abi-Dargham et al., 1998; Abi-Dargham et al., 2009).
Presynaptic Dopamine Precursor Uptake and Storage in Schizophrenia
An increase in uptake and storage of the radiolabelled dopamine precursor [18F]FDOPA into the striatum is a robust and well-replicated finding in patients with schizophrenia (Fusar-Poli and Meyer-Lindenberg, 2013). The uptake of [18F]FDOPA shows a positive correlation with the severity of positive symptoms (Meyer-Lindenberg et al., 2002; McGowan et al., 2004; Kumakura and Cumming, 2009). This, together with evidence from the aforementioned competition studies where positive symptoms correlated with d-amphetamine-induced dopamine release, supports the close relationship between high dopamine levels and psychotic symptoms in schizophrenia. However, to our knowledge, there is no study directly relating increased uptake of [18F]FDOPA to enhanced d-amphetamine-induced dopamine release in sensitization or schizophrenia. A recently described key finding is that increased [18F]FDOPA uptake already occurs in prodromal stages of schizophrenia, where patients show no or almost no psychotic symptoms (Howes et al., 2009). This shows that dopamine overactivity predates the onset of full-blown psychosis and suggests that dopamine is causally involved in the pathogenesis of the illness.
In summary, several studies support a close link between the mechanism of action of psychostimulant drugs and psychotic symptoms in schizophrenia. The behavioral super-sensitivity of patients with schizophrenia towards substances increasing brain extracellular dopamine levels, together with an enhanced d-amphetamine-induced dopamine release suggest that schizophrenia is associated with a state of “natural sensitization” towards dopamine-releasing agents. We will further discuss below how the mechanisms of d-amphetamine sensitization might relate to the enhanced dopamine synthesis and storage capacity shown by [18F]FDOPA PET studies in schizophrenia.
The Neuropharmacology of Sensitization
Findings in Humans
On a neurochemical level, sensitization to amphetamines manifests as lasting hyper-responsiveness of mesencephalic dopaminergic pathways, paralleled by an increase in the amount of extracellular dopamine released from presynaptic terminals to a given dose of d-amphetamine in animals (Kalivas and Duffy, 1993; Wolf et al., 1993; Paulson and Robinson, 1995; Pierce and Kalivas, 1995; Robinson and Badiani, 1998; Robinson et al., 1998) and humans (Boileau et al., 2006; Booij et al., 2016). The literature reports only on a limited number of studies where humans were prospectively sensitized to stimulants (Strakowski et al., 2001; Farre et al., 2004; Boileau et al., 2006, 2016; O’Daly et al., 2011, 2014a, 2014b). An increase in d-amphetamine-induced reductions in striatal binding of the D2/3 receptor radioligand [11C]raclopride after repeated administration of a constant dose of d-amphetamine shows a progressive increase in the amount of dopamine released into the extracellular space in the striatum of sensitized individuals (Boileau et al., 2006; Booij et al., 2016). This increase is paralleled by an enhancement in behavioral measures, for example the eye-blink rate, ratings of alertness, euphoria or focus, and by enhanced amphetamine-induced plasma cortisol secretion (Strakowski et al., 2001; Farre et al., 2004).
Interestingly, dopamine release after a single amphetamine or ethanol administration seems to be greater in males (Munro et al., 2006; Urban et al., 2010), while behavioral effects of sensitization in animals have been found to be stronger in females (Becker et al., 2001; Strakowski et al., 2001; Cope et al., 2010; Chen et al., 2014). Sex differences are frequently attributed to the action of gonadal steroid hormones either during an early organizational period or in adulthood (Gillies et al., 2014). Castration of male rats facilitated the behavioral sensitization produced by either repeated amphetamine treatment or repeated restraint stress. In contrast, ovarectomy in female rats was without effect (Camp and Robinson, 1988). Neurochemical effects of sensitization to amphetamines in humans have so far been studied in males only (Boileau et al., 2006). Since schizophrenia, substance use disorders, and many other dopamine-related psychiatric disorders show a robust sexual dimorphism, there is a clear need for studies filling the gaps in our knowledge on the effects of sex on sensitization and its neurochemistry in humans.
Findings in Animals
Sensitization in research animals has been induced using several different protocols of repeated substance administration. The strength of sensitization is influenced by various factors, such as number and interval between treatments, dose, sex, age, and genetics (Post and Contel, 1983). Behavioral sensitization has been reported to occur in response to cocaine, amphetamine, morphine, ethanol, nicotine, and tetrahydrocannabinol (Joyce and Iversen, 1979; Robinson and Becker, 1986; Benwell and Balfour, 1992; Cunningham and Noble, 1992; Post et al., 1992; Cadoni et al., 2001). An important finding of several studies is the occurrence of cross-sensitization of the behavioral response between drugs. Cross-sensitization has been shown for amphetamine to morphine (Vezina and Stewart, 1989), amphetamine to cocaine (Santos et al., 2009), ethanol to cocaine and vice versa (Itzhak and Martin, 1999), and Δ9-tetrahydrocannabinol to morphine (Cadoni et al., 2001).
Molecular Mechanisms of Sensitization
As of yet, molecular mechanisms of sensitization of the dopamine system are only partially understood. Apparently, sensitization occurs in 2 stages, first by changes in VTA dopamine receptors, followed by a so-called expression phase, in which dopamine release is enhanced in the nucleus accumbens (Marinelli et al., 2003). Among others, a transient subsensitivity of dopamine autoreceptors in the ventral tegmental area has been suggested to play a role in the induction of sensitization (Wolf et al., 1993; Paulson and Robinson, 1995; Pierce and Kalivas, 1995; Calipari et al., 2015), as this change has been associated with increased basal activity of dopamine neurons (White and Wang, 1984; Henry et al., 1989). Long-lasting sensitization might also be accompanied by increases in the sensitivity of D1 dopamine receptors in the nucleus accumbens (Henry and White, 1991). This is supported by findings of blunted locomotor sensitization in D1-deficient mice (El-Ghundi et al., 2010) and the prevention of pertussis toxin-induced sensitization by D1 antagonists (Narayanan et al., 1996). For D2/3 autoreceptors, animal work points to decreased intracellular signal transmission via intracellular G-proteins after repeated amphetamine transmission (Sharpe et al., 2015) and changes of regulatory proteins of intracellular G-proteins such as Rgs9 (regulator of G-protein signalling) (Maple et al., 2007). These changes may occur already after a single stimulant administration specifically in the ventral tegmental area (Arora et al., 2011; Padgett et al., 2012).
Other cellular mechanisms involved in sensitization include altered activation of VMAT2, as a recent publication discovered that argon blocks sensitization by its antagonistic properties at the VMAT2. Chen et al. (2014) on the other hand found an important role of AKT1, a protein directly downstream of dopamine D2/3 receptors that interacts with beta-arrestin complex, a regulator of dopamine signalling cascades. Interestingly, male AKT1 knockout animals were less sensitive to methamphetamine-induced hyperlocomotion during methamphetamine challenge compared with wild-type controls and AKT1 knockout females (Chen et al., 2014). Another protein, the calmodulin kinase IIα (αCaMKII) is a transporter-interacting protein, which was found to regulate amphetamine-triggered reverse DAT transport. αCaMKII has been associated with development of sensitization, as mice depleted of this kinase showed blunted sensitization effects following repeated amphetamine exposure (Steinkellner et al., 2014). Furthermore, the authors speculate that subtle variations in the relative expression levels of DAT and of αCaMKII may contribute to inter-individual differences in the susceptibility to amphetamine addiction (Steinkellner et al., 2014).
A recent proteomics study (Wearne et al., 2015) examined brain protein expression in rats sensitized to methamphetamine. Expression of proteins previously implicated in the pathophysiology of schizophrenia was significantly altered in the prefrontal cortex of sensitized rats. These proteins are involved in mitochondrial function, cellular architecture, cell signalling, and synaptic plasticity, which, in turn, are tightly related to stress mechanisms on a cellular level. In accordance, cell adhesion molecules assessed peripherally were found to be altered in patients with schizophrenia and correlated with prefrontal grey matter volume (Piras et al., 2015).
The Role of Dopamine D3 Receptors
Cocaine- or methamphetamine-dependent subjects can safely be assumed to be sensitized to the action of psychostimulant drugs. Studies using the dopamine D2/3 receptor agonist PET radioligand [11C]-(+)-PHNO (Wilson et al., 2005) in humans addicted to cocaine or methamphetamine have consistently shown an increase in [11C]-(+)-PHNO binding in the substantia nigra/ventral tegmental area (Boileau et al., 2012; Matuskey et al., 2014; Payer et al., 2014). In these brain regions, the signal measured with [11C]-(+)-PHNO is predominantly due to binding to dopamine D3 receptors (see Figure 2; Graff-Guerrero et al., 2010). An increase in dopamine D3 receptor expression is also found in postmortem studies of cocaine addicted subjects (Staley and Mash, 1996; Segal et al., 1997). In view of these findings, dopamine D3 receptor antagonists have been proposed as a possible treatment for stimulant addiction. This notion is supported by rodent studies administering D3-preferring agonists, such as pramipexole, which enhanced opioid-conditioned reinforcement (Bertz et al., 2015). Vice versa, D3-preferring antagonists inhibited related rewarding effects of cocaine- and drug-seeking behavior (Xi et al., 2006; Song et al., 2012; John et al., 2015; Galaj et al., 2016).
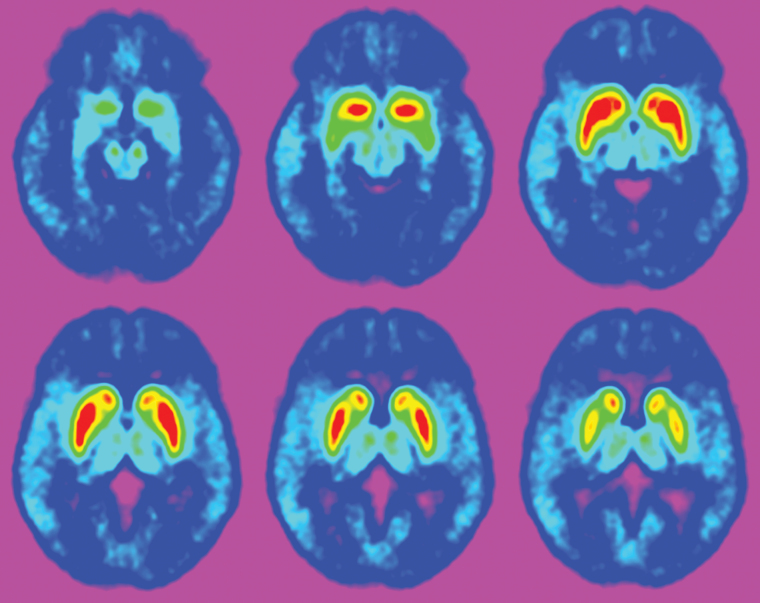
Figure 2.
Adjacent transversal slices of a positron-emission tomography image using the dopamine D2/3 receptor agonist radioligand [11C]-(+)-PHNO. Bright areas show binding to dopamine D2/3 receptors in the human striatum and brainstem substantia nigra/ventral tegmental area.
Still, this concept is somewhat at odds with a solid body of evidence showing that an increase in dopamine D3 receptor signalling in the ventral tegmental area actually acts as a brake on dopamine release in the striatum and reduces psychostimulant-induced behaviors in rodents (Damsma et al., 1993; Ahlenius and Salmi, 1994; Pugsley et al., 1995; Caine et al., 1997; Richtand et al., 2001). A pilot study on the administration of the D3-preferring agonist pramipexole to patients with schizophrenia (Kasper et al., 1997) showed beneficial effects against negative and positive symptoms. Since positive symptoms in schizophrenia correlate directly with amphetamine-induced dopamine release in the striatum of patients with schizophrenia, D3 agonism, although not directly shown in this or other studies so far, may have reduced striatal dopamine release in this collective.
There are various possible reasons for the discrepant findings. One is that most D2 or D3 receptor-preferring compounds are not entirely selective for either subtype. Therefore, it is hard to distinguish via which receptor behavioral or neurochemical effects are exerted, especially when opposing roles are suggested (Ellinwood et al., 2000). Another aspect is that D3-preferring compounds may act in a species-dependent way, and that their action may depend on the administration paradigm (cocaine self-administration vs conditioning/reinforcement) (Keck et al., 2015). Furthermore, studies suggest that individual D3 availability and the resulting occupancy or alternative D3 gene splicing might lead to differential effects when D3 ligands bind (Richtand, 2006; Mugnaini et al., 2013; Kim et al., 2014; John et al., 2015). Interestingly, brain-derived neurotrophic factor might influence the expression of D3 receptors and seems to be necessary for behavioral sensitization and co-occurring D3 overexpression (Guillin et al., 2001).
Although it would be intuitive to state that sensitization leads to increased D3 receptor expression and therefore that D3 should be antagonized in order to prevent sensitization, increased expression of D3 in its role as a regulator of DA release might be a compensatory mechanism in the development of sensitization, which, however, fails to fulfil its purpose.
In summary, the differential impact of dopamine D2 autoreceptors and D3 receptors in the substantia nigra/ventral tegmental area on induction and expression of sensitization appears insufficiently understood as of yet. An intriguing concept though is that sensitization to amphetamine, together with an increase in amphetamine-induced dopamine release into the striatum, are a consequence of tolerance in inhibitory systems induced by repeated exposure of brainstem dopamine receptors to high levels of dopamine itself (Richtand, 2006).
Stress, Sensitization, and Neurodevelopment
Several studies on the relationship between stress and psychostimulants in rodents were able to show cross-sensitization between repeated stress and repeated exposure to amphetamine (Antelman et al., 1980; Kalivas et al., 1986; Hamamura and Fibiger, 1993; Febo and Pira, 2011; Booij et al., 2016). It is known that stress preferentially stimulates dopamine release in mesolimbic projection (Horger and Roth, 1996) and frontal areas (Lataster et al., 2011) and that glucocorticoids modulate the sensitivity of mesencephalic dopaminergic neurons to drugs of abuse (Sorg and Kalivas, 1991; Deroche et al., 1995). In good agreement with animal studies, it was shown that exposure to stress is able to induce recurrence of psychotic symptoms in remitted patients with methamphetamine-induced psychosis (Yui et al., 2002). Patients with schizophrenia exhibit distinct alterations in the hypothalamic-pituitary-adrenal axis (Borges et al., 2013), and an increase in dopamine release and behavioral reactivity in response to stress has been observed in healthy subjects sensitized to amphetamine, in patients with schizophrenia (Mizrahi et al., 2012; Lataster et al., 2013), and in subjects at high risk for psychosis (Soliman et al., 2008; Mizrahi et al., 2014).
Lieberman and colleagues (1997) have proposed a framework extending the classical stress vulnerability model of psychosis to integrate the neurodevelopmental model of schizophrenia with findings linking stress to altered dopamine signalling: in early development, neuronal circuits are being formed which then experience neuroplastic changes during early adolescence (Feinberg, 1982; Keshavan et al., 1994). There, stress-related activity of mesolimbic dopamine neurons sets the vulnerability for sensitization. Sensitization finally leads to excessive dopamine release and progressive development of psychosis (Laruelle, 2000; Seeman 2011). Importantly, the view of schizophrenia as a degenerative disease has been substituted by the view of a disorder in which deficits in regenerative capacity are present (Falkai et al., 2015). These deficits may include the previously mentioned alterations of cell architecture and metabolism, higher oxidative stress, and the consequent changes of synaptic formations (Flatow et al., 2013; Wearne et al., 2015). In addition, as the prefrontal cortex is hypothesized to act as a brake on striatal dopamine release (Carlsson et al., 2001), a dysregulation of inhibitory feedback mechanisms involved in the regulation of ventral tegmental neuronal activity may result in a pathological potentiation of impulse-dependent phasic dopamine release. The association of frontal cortical thickness with amphetamine-induced dopamine release corroborates this notion (Casey et al., 2013). As of yet, the question is unresolved whether it is increased striatal dopamine transmission to cause altered activation of cortical networks (bottom-up) or whether insufficient frontal-cortical control of striatal neurons is leading to alterations in presynaptic dopamine release in psychosis (top-down).
Sensitization in Cells
In an in vitro examination, rat pheochromocytoma cells (PC-12), which are capable of dopamine release upon pharmacological stimulation but possess no dopamine receptors, were sensitized successfully by intermittent treatment with amphetamine for 5 consecutive days followed by a >6-day, drug-free interval. Sensitization of PC12 cells was marked by significantly increased dopamine release upon amphetamine stimulation in sensitized when compared with nonsensitized cells. This was not accompanied by increase in [H3]-dopamine uptake (Kantor et al., 2002). Intermittent amphetamine treatment also led to increased neurite outgrowth in PC-12 cells after an interval of more than 3 drug-free days (Park et al., 2002). Increased dopamine release and neurite outgrowth were dependent on protein kinase C and MAP kinase in vitro (Park et al., 2003). The fact that it is possible to sensitize cells to amphetamine holds the promise that it may be possible to identify drug targets enabling to target alterations in dopamine release already in presynaptic neurons.
What Can We Learn for Schizophrenia Research?
The observation of alterations in the dopaminergic system has led to the question how this effect might be related to clinical symptoms of schizophrenia. Notably, the dopaminergic changes are dependent on the illness phase (Laruelle et al., 1999), and the correlation of radioligand uptake and positive symptom severity (Meyer-Lindenberg et al., 2002; McGowan et al., 2004; Kumakura et al., 2007) suggests that the method does indeed capture a biologically meaningful signal closely connected to the pathogenesis of schizophrenia. There seems to be a differential effect of amphetamine on positive and negative symptoms of schizophrenia: positive symptoms and thought disorder intensify temporarily, while negative symptoms tend to improve. This suggests that, although positive and negative symptoms are likely to differ in their pathogenesis, dopamine plays a major role in both symptom groups. Patients in remission show comparably small responses to amphetamine (Lieberman et al., 1987), and amphetamine-induced dopamine release is larger during the acute phase of the illness (Laruelle, 2000). Although some authors consider the processes predisposing to psychosis in schizophrenia to be irreversible, especially those hypothesized to have a neurodevelopemental origin, and although the disorder has a progressive course in a considerable proportion of the patients, findings from imaging studies suggest that at least part of the dopamine-related pathogenetic alterations are reversible. In this context, antipsychotic medication has been proposed to contribute to the reversibility of sensitization by D2/3 blockade, although the exact mechanism is yet unknown. On the other hand, some observations suggest that chronic blockade of dopamine D2/3 receptors rather induces supersensitivity of postsynaptic receptor systems, leading to increased vulnerability for relapse once antipsychotic medication is discontinued. However, the possibly life-long persistence of a predisposition of the dopamine system to sensitize supports the necessity of long-term proactive measures to prevent psychosis in many patients.
In sum, an increase in amphetamine-induced dopamine release is common to amphetamine-induced sensitization in animals and humans, and the natural sensitization of the dopamine system observed in patients with schizophrenia is associated with increased amphetamine-induced dopamine release as well. The exact mechanisms and the association with increased dopamine synthesis observed in schizophrenia and preceding phases of the illness have yet to be elucidated, and amphetamine-induced sensitization might provide a suitable model for this purpose. Nevertheless, sensitization of the dopamine system is probably only one small part of the bigger picture of neurobiological changes occurring in schizophrenia. Different molecular changes are under intense investigation and might shed light on the development of sensitization in schizophrenia. Antipsychotics might reverse sensitization and restore the flow of information from frontal areas to cortico-striatal-thalamo-cortical loops, thereby leading to symptom relief. As of yet, schizophrenia is a disorder often associated with nonresponse or partial response to treatment, leading to high disability. Sensitization to amphetamine is a narrowly defined pharmaco-behavioral construct. Its neurochemical signature can be readily studied in humans and animals. One day, translational research on amphetamine-sensitization will hopefully help to identify new molecular drug targets allowing for improved therapeutic strategies in schizophrenia.
Sensitization
Increased response after repeated exposure to a stable dose of a substance
Cross-sensitization with stress described for most sensitizing substances
Close relationship to dopamine-mediated learning (e.g. conditioned learning)
Increased release of dopamine in response to a stable dose of amphetamine, cocaine, opiates, or methylphenidate
Increased release of dopamine in sensitized animals and humans
Sensitized dopamine response and psychiatric disorders
Behavioral sensitization towards amphetamines and methylphenidate in schizophrenia
Sensitization is believed to underlie habit-formation in addiction
Blunted dopamine release in substance use disorders
Dopaminergic changes in sensitization
Increased presynaptic release of dopamine
Increased quantal size
Increase in markers of dopamine synthesis
Increase in postsynaptic dopamine-D2/3 function
Sub-sensitivity of inhibitory auto-receptors
Altered dopamine D3 receptor binding and function
Acknowledgments
This work was supported in part by funds of the Austrian Science Fund P23585B09, the Oesterreichische Nationalbank (Oesterreichische Nationalbank, Anniversary Fund OENB16723), and the Vienna Science and Technology Fund CS15-033.
References
- Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M. (1998) Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. The American journal of psychiatry 155:761–767. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M. (2009) Baseline and amphetamine-stimulated dopamine activity are related in drug-naive schizophrenic subjects. Biological psychiatry 65:1091–1093. [DOI] [PubMed] [Google Scholar]
- Ahlenius S, Salmi P. (1994) Behavioral and biochemical effects of the dopamine D3 receptor-selective ligand, 7-OH-DPAT, in the normal and the reserpine-treated rat. Eur J Pharmacol 260:177–181. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Eichler AJ, Black CA, Kocan D. (1980) Interchangeability of stress and amphetamine in sensitization. Science 207:329–331. [DOI] [PubMed] [Google Scholar]
- APA (2013) Diagnostic and statistical manual of mental disorders: DSM-5, Fifth Edition Edition: American Psychiatric Association. [Google Scholar]
- Arora D, Hearing M, Haluk DM, Mirkovic K, Fajardo-Serrano A, Wessendorf MW, Watanabe M, Lujan R, Wickman K. (2011) Acute cocaine exposure weakens GABA(B) receptor-dependent G-protein-gated inwardly rectifying K+ signaling in dopamine neurons of the ventral tegmental area. The Journal of neuroscience: the official journal of the Society for Neuroscience 31:12251–12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ME, Gallo DA, de Wit H. (2014) Amphetamine increases errors during episodic memory retrieval. J Clin Psychopharmacol 34:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. (2001) Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Annals of the New York Academy of Sciences 937:172–187. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. (1992) The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol 105:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertz JW, Chen J, Woods JH. (2015) Effects of pramipexole on the acquisition of responding with opioid-conditioned reinforcement in the rat. Psychopharmacology (Berl) 232:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. (2006) Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Archives of general psychiatry 63:1386–1395. [DOI] [PubMed] [Google Scholar]
- Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J, Wilkins D, Selby P, George TP, Zack M, Furukawa Y, McCluskey T, Wilson AA, Kish SJ. (2012) Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. The Journal of neuroscience: the official journal of the Society for Neuroscience 32:1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij L, Welfeld K, Leyton M, Dagher A, Boileau I, Sibon I, Baker GB, Diksic M, Soucy JP, Pruessner JC, Cawley-Fiset E, Casey KF, Benkelfat C. (2016) Dopamine cross-sensitization between psychostimulant drugs and stress in healthy male volunteers. Transl Psychiatry 6:e740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges S, Gayer-Anderson C, Mondelli V. (2013) A systematic review of the activity of the hypothalamic-pituitary-adrenal axis in first episode psychosis. Psychoneuroendocrinology 38:603–611. [DOI] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D. (1997) Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proceedings of the National Academy of Sciences of the United States of America 94:2569–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, Pisanu A, Solinas M, Acquas E, Di Chiara G. (2001) Behavioural sensitization after repeated exposure to Delta 9-tetrahydrocannabinol and cross-sensitization with morphine. Psychopharmacology (Berl) 158:259–266. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF, Parsons LH, Everitt BJ, Schwartz JC, Sokoloff P. (1997) D3 receptor test in vitro predicts decreased cocaine self-administration in rats. Neuroreport 8:2373–2377. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Siciliano CA, Zimmer BA, Jones SR. (2015) Brief intermittent cocaine self-administration and abstinence sensitizes cocaine effects on the dopamine transporter and increases drug seeking. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 40:728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp DM, Robinson TE. (1988) Susceptibility to sensitization. I. Sex differences in the enduring effects of chronic D-amphetamine treatment on locomotion, stereotyped behavior and brain monoamines. Behav Brain Res 30:55–68. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. (2001) Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annual review of pharmacology and toxicology 41:237–260. [DOI] [PubMed] [Google Scholar]
- Casey KF, Cherkasova MV, Larcher K, Evans AC, Baker GB, Dagher A, Benkelfat C, Leyton M. (2013) Individual differences in frontal cortical thickness correlate with the d-amphetamine-induced striatal dopamine response in humans. The Journal of neuroscience: the official journal of the Society for Neuroscience 33:15285–15294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Kao HY, Min MY, Lai WS. (2014) A sex- and region-specific role of Akt1 in the modulation of methamphetamine-induced hyperlocomotion and striatal neuronal activity: implications in schizophrenia and methamphetamine-induced psychosis. Schizophr Bull 40:388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collip D, Myin-Germeys I, Van Os J. (2008) Does the concept of “sensitization” provide a plausible mechanism for the putative link between the environment and schizophrenia? Schizophr Bull 34:220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope ZA, Huggins KN, Sheppard AB, Noel DM, Roane DS, Brown RW. (2010) Neonatal quinpirole treatment enhances locomotor activation and dopamine release in the nucleus accumbens core in response to amphetamine treatment in adulthood. Synapse 64:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Noble D. (1992) Conditioned activation induced by ethanol: role in sensitization and conditioned place preference. Pharmacol Biochem Behav 43:307–313. [DOI] [PubMed] [Google Scholar]
- Damsma G, Bottema T, Westerink BH, Tepper PG, Dijkstra D, Pugsley TA, MacKenzie RG, Heffner TG, Wikstrom H. (1993) Pharmacological aspects of R-(+)-7-OH-DPAT, a putative dopamine D3 receptor ligand. Eur J Pharmacol 249:R9–10. [DOI] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Maccari S, Le Moal M, Simon H, Piazza PV. (1995) Stress-induced sensitization and glucocorticoids. I. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion. The Journal of neuroscience: the official journal of the Society for Neuroscience 15:7181–7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeleano L. (1887) Über einige Derivate der Phenylmethacrylsäure und der Phenylisobuttersäure. [Google Scholar]
- El-Ghundi MB, Fan T, Karasinska JM, Yeung J, Zhou M, O’Dowd BF, George SR. (2010) Restoration of amphetamine-induced locomotor sensitization in dopamine D1 receptor-deficient mice. Psychopharmacology (Berl) 207:599–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinwood EH, Jr, Sudilovsky A, Nelson LM. (1973) Evolving behavior in the clinical and experimental amphetamine (model) psychosis. The American journal of psychiatry 130:1088–1093. [DOI] [PubMed] [Google Scholar]
- Ellinwood EH, King GR, Davidson C, Lee TH. (2000) The dopamine D2/D3 antagonist DS121 potentiates the effect of cocaine on locomotion and reduces tolerance in cocaine tolerant rats. Behav Brain Res 116:169–175. [DOI] [PubMed] [Google Scholar]
- Falkai P, Rossner MJ, Schulze TG, Hasan A, Brzozka MM, Malchow B, Honer WG, Schmitt A. (2015) Kraepelin revisited: schizophrenia from degeneration to failed regeneration. Mol Psychiatry 20:671–676. [DOI] [PubMed] [Google Scholar]
- Farre M, de la Torre R, Mathuna BO, Roset PN, Peiro AM, Torrens M, Ortuno J, Pujadas M, Cami J. (2004) Repeated doses administration of MDMA in humans: pharmacological effects and pharmacokinetics. Psychopharmacology (Berl) 173:364–375. [DOI] [PubMed] [Google Scholar]
- Febo M, Pira AS. (2011) Increased BOLD activation to predator stressor in subiculum and midbrain of amphetamine-sensitized maternal rats. Brain research 1382:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I. (1982) Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res 17:319–334. [DOI] [PubMed] [Google Scholar]
- Flatow J, Buckley P, Miller BJ. (2013) Meta-analysis of oxidative stress in schizophrenia. Biological psychiatry 74:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Meyer-Lindenberg A. (2013) Striatal presynaptic dopamine in schizophrenia, part II: meta-analysis of [(18)F/(11)C]-DOPA PET studies. Schizophr Bull 39:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaj E, Haynes J, Nisanov R, Ananthan S, Ranaldi R. (2016) The dopamine D3 receptor antagonist, SR 21502, facilitates extinction of cocaine conditioned place preference. Drug Alcohol Depend 159:263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies GE, Virdee K, McArthur S, Dalley JW. (2014) Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: a molecular, cellular and behavioral analysis. Neuroscience 282C:69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginovart N. (2005) Imaging the dopamine system with in vivo [11c]raclopride displacement studies: understanding the true mechanism. Mol Imaging Biol 7:45–52. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Redden L, Abi-Saab W, Katz DA, Houle S, Barsoum P, Bhathena A, Palaparthy R, Saltarelli MD, Kapur S. (2010) Blockade of [11C](+)-PHNO binding in human subjects by the dopamine D3 receptor antagonist ABT-925. Int J Neuropsychopharmacol 13:273–287. [DOI] [PubMed] [Google Scholar]
- Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P. (2001) BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature 411:86–89. [DOI] [PubMed] [Google Scholar]
- Hamamura T, Fibiger HC. (1993) Enhanced stress-induced dopamine release in the prefrontal cortex of amphetamine-sensitized rats. Eur J Pharmacol 237:65–71. [DOI] [PubMed] [Google Scholar]
- Henry DJ, Greene MA, White FJ. (1989) Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: repeated administration. J Pharmacol Exp Ther 251:833–839. [PubMed] [Google Scholar]
- Henry DJ, White FJ. (1991) Repeated cocaine administration causes persistent enhancement of D1 dopamine receptor sensitivity within the rat nucleus accumbens. J Pharmacol Exp Ther 258:882–890. [PubMed] [Google Scholar]
- Horger BA, Roth RH. (1996) The role of mesoprefrontal dopamine neurons in stress. Crit Rev Neurobiol 10:395–418. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. (2009) The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull 35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, Bramon-Bosch E, Valmaggia L, Johns L, Broome M, McGuire PK, Grasby PM. (2009) Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Archives of general psychiatry 66:13–20. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL. (1999) Effects of cocaine, nicotine, dizocipline and alcohol on mice locomotor activity: cocaine-alcohol cross-sensitization involves upregulation of striatal dopamine transporter binding sites. Brain research 818:204–211. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, Risch C. (1979) Amphetamine psychosis and psychotic symptoms. Psychopharmacology (Berl) 65:73–77. [DOI] [PubMed] [Google Scholar]
- John WS, Newman AH, Nader MA. (2015) Differential effects of the dopamine D3 receptor antagonist PG01037 on cocaine and methamphetamine self-administration in rhesus monkeys. Neuropharmacology 92:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce EM, Iversen SD. (1979) The effect of morphine applied locally to mesencephalic dopamine cell bodies on spontaneous motor activity in the rat. Neurosci Lett 14:207–212. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. (1993) Time course of extracellular dopamine and behavioral sensitization to cocaine. II. Dopamine perikarya. The Journal of neuroscience: the official journal of the Society for Neuroscience 13:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Richardson-Carlson R, Van Orden G. (1986) Cross-sensitization between foot shock stress and enkephalin-induced motor activity. Biological psychiatry 21:939–950. [DOI] [PubMed] [Google Scholar]
- Kantor L, Park YH, Wang KK, Gnegy M. (2002) Enhanced amphetamine-mediated dopamine release develops in PC12 cells after repeated amphetamine treatment. Eur J Pharmacol 451:27–35. [DOI] [PubMed] [Google Scholar]
- Kapur S. (2003) Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. The American journal of psychiatry 160:13–23. [DOI] [PubMed] [Google Scholar]
- Kasper S, Barnas C, Heiden A, Volz HP, Laakmann G, Zeit H, Pfolz H. (1997) Pramipexole as adjunct to haloperidol in schizophrenia. Safety and efficacy. Eur Neuropsychopharmacol 7:65–70. [DOI] [PubMed] [Google Scholar]
- Keck TM, John WS, Czoty PW, Nader MA, Newman AH. (2015) Identifying Medication Targets for Psychostimulant Addiction: Unraveling the Dopamine D3 Receptor Hypothesis. Journal of medicinal chemistry 58:5361–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiflin R, Janak PH. (2015) Dopamine Prediction Errors in Reward Learning and Addiction: From Theory to Neural Circuitry. Neuron 88:247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Anderson S, Pettegrew JW. (1994) Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res 28:239–265. [DOI] [PubMed] [Google Scholar]
- Kim SW, Fowler JS, Skolnick P, Muench L, Kang Y, Shea C, Logan J, Kim D, Carter P, King P, Alexoff D, Volkow ND. (2014) Therapeutic doses of buspirone block D3 receptors in the living primate brain. Int J Neuropsychopharmacol 17:1257–1267. [DOI] [PubMed] [Google Scholar]
- Kumakura Y, Cumming P. (2009) PET studies of cerebral levodopa metabolism: a review of clinical findings and modeling approaches. Neuroscientist 15:635–650. [DOI] [PubMed] [Google Scholar]
- Kumakura Y, Cumming P, Vernaleken I, Buchholz HG, Siessmeier T, Heinz A, Kienast T, Bartenstein P, Grunder G. (2007) Elevated [18F]fluorodopamine turnover in brain of patients with schizophrenia: an [18F]fluorodopa/positron emission tomography study. The Journal of neuroscience: the official journal of the Society for Neuroscience 27:8080–8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M. (2000) The role of endogenous sensitization in the pathophysiology of schizophrenia: implications from recent brain imaging studies. Brain research Brain research reviews 31:371–384. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. (1999) Increased dopamine transmission in schizophrenia: relationship to illness phases. Biological psychiatry 46:56–72. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB. (1996) Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proceedings of the National Academy of Sciences of the United States of America 93:9235–9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Rosenblatt W, Zea-Ponce Y, Zoghbi SS, Baldwin RM, Charney DS, Hoffer PB, Kung HF, et al. (1995) SPECT imaging of striatal dopamine release after amphetamine challenge. Journal of nuclear medicine: official publication, Society of Nuclear Medicine 36:1182–1190. [PubMed] [Google Scholar]
- Lataster J, Collip D, Ceccarini J, Haas D, Booij L, van Os J, Pruessner J, Van Laere K, Myin-Germeys I. (2011) Psychosocial stress is associated with in vivo dopamine release in human ventromedial prefrontal cortex: a positron emission tomography study using [(1)(8)F]fallypride. Neuroimage 58:1081–1089. [DOI] [PubMed] [Google Scholar]
- Lataster T, Valmaggia L, Lardinois M, van Os J, Myin-Germeys I. (2013) Increased stress reactivity: a mechanism specifically associated with the positive symptoms of psychotic disorder. Psychol Med 43:1389–1400. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Kane JM, Alvir J. (1987) Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology (Berl) 91:415–433. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Sheitman BB, Kinon BJ. (1997) Neurochemical sensitization in the pathophysiology of schizophrenia: deficits and dysfunction in neuronal regulation and plasticity. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 17:205–229. [DOI] [PubMed] [Google Scholar]
- Maple AM, Perna MK, Parlaman JP, Stanwood GD, Brown RW. (2007) Ontogenetic quinpirole treatment produces long-lasting decreases in the expression of Rgs9, but increases Rgs17 in the striatum, nucleus accumbens and frontal cortex. Eur J Neurosci 26:2532–2538. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Cooper DC, Baker LK, White FJ. (2003) Impulse activity of midbrain dopamine neurons modulates drug-seeking behavior. Psychopharmacology (Berl) 168:84–98. [DOI] [PubMed] [Google Scholar]
- Matuskey D, Gallezot JD, Pittman B, Williams W, Wanyiri J, Gaiser E, Lee DE, Hannestad J, Lim K, Zheng MQ, Lin SF, Labaree D, Potenza MN, Carson RE, Malison RT, Ding YS. (2014) Dopamine D(3) receptor alterations in cocaine-dependent humans imaged with [(1)(1)C](+)PHNO. Drug Alcohol Depend 139:100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan S, Lawrence AD, Sales T, Quested D, Grasby P. (2004) Presynaptic dopaminergic dysfunction in schizophrenia: a positron emission tomographic [18F]fluorodopa study. Archives of general psychiatry 61:134–142. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, Weinberger DR, Berman KF. (2002) Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nature neuroscience 5:267–271. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Addington J, Rusjan PM, Suridjan I, Ng A, Boileau I, Pruessner JC, Remington G, Houle S, Wilson AA. (2012) Increased stress-induced dopamine release in psychosis. Biological psychiatry 71:561–567. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Kenk M, Suridjan I, Boileau I, George TP, McKenzie K, Wilson AA, Houle S, Rusjan P. (2014) Stress-induced dopamine response in subjects at clinical high risk for schizophrenia with and without concurrent cannabis use. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 39:1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnaini M, Iavarone L, Cavallini P, Griffante C, Oliosi B, Savoia C, Beaver J, Rabiner EA, Micheli F, Heidbreder C, Andorn A, Merlo Pich E, Bani M. (2013) Occupancy of brain dopamine D3 receptors and drug craving: a translational approach. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 38:302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, Wand GS. (2006) Sex differences in striatal dopamine release in healthy adults. Biological psychiatry 59:966–974. [DOI] [PubMed] [Google Scholar]
- Narayanan S, Wallace L, Uretsky N. (1996) Spontaneous and drug-stimulated locomotor activity after the administration of pertussis toxin into the ventral tegmental area. J Psychiatry Neurosci 21:172–180. [PMC free article] [PubMed] [Google Scholar]
- O’Daly OG, Joyce D, Stephan KE, Murray RM, Shergill SS. (2011) Functional magnetic resonance imaging investigation of the amphetamine sensitization model of schizophrenia in healthy male volunteers. Archives of general psychiatry 68:545–554. [DOI] [PubMed] [Google Scholar]
- O’Daly OG, Joyce D, Tracy DK, Stephan KE, Murray RM, Shergill S. (2014. a) Amphetamine sensitisation and memory in healthy human volunteers: a functional magnetic resonance imaging study. J Psychopharmacol 28:857–865. [DOI] [PubMed] [Google Scholar]
- O’Daly OG, Joyce D, Tracy DK, Azim A, Stephan KE, Murray RM, Shergill SS. (2014. b) Amphetamine sensitization alters reward processing in the human striatum and amygdala. PloS one 9:e93955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett CL, Lalive AL, Tan KR, Terunuma M, Munoz MB, Pangalos MN, Martinez-Hernandez J, Watanabe M, Moss SJ, Lujan R, Luscher C, Slesinger PA. (2012) Methamphetamine-evoked depression of GABA(B) receptor signaling in GABA neurons of the VTA. Neuron 73:978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YH, Kantor L, Wang KK, Gnegy ME. (2002) Repeated, intermittent treatment with amphetamine induces neurite outgrowth in rat pheochromocytoma cells (PC12 cells). Brain research 951:43–52. [DOI] [PubMed] [Google Scholar]
- Park YH, Kantor L, Guptaroy B, Zhang M, Wang KK, Gnegy ME. (2003) Repeated amphetamine treatment induces neurite outgrowth and enhanced amphetamine-stimulated dopamine release in rat pheochromocytoma cells (PC12 cells) via a protein kinase C- and mitogen activated protein kinase-dependent mechanism. J Neurochem 87:1546–1557. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. (1995) Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: a microdialysis study in behaving rats. Synapse 19:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer DE, Behzadi A, Kish SJ, Houle S, Wilson AA, Rusjan PM, Tong J, Selby P, George TP, McCluskey T, Boileau I. (2014) Heightened D3 dopamine receptor levels in cocaine dependence and contributions to the addiction behavioral phenotype: a positron emission tomography study with [11C]-+-PHNO. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 39:311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. (1995) Amphetamine produces sensitized increases in locomotion and extracellular dopamine preferentially in the nucleus accumbens shell of rats administered repeated cocaine. J Pharmacol Exp Ther 275:1019–1029. [PubMed] [Google Scholar]
- Piras F, Schiff M, Chiapponi C, Bossu P, Muhlenhoff M, Caltagirone C, Gerardy-Schahn R, Hildebrandt H, Spalletta G. (2015) Brain structure, cognition and negative symptoms in schizophrenia are associated with serum levels of polysialic acid-modified NCAM. Transl Psychiatry 5:e658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post R, Contel N. (1983) Human and animal studies of cocaine: Implications for development of behavioral pathology. In: Stimulants: Neurochemical, Behavioral and Clinical Perspectives. (C I, ed), pp 169–203. New York: Raven Press;. [Google Scholar]
- Post RM, Weiss SR, Fontana D, Pert A. (1992) Conditioned sensitization to the psychomotor stimulant cocaine. Annals of the New York Academy of Sciences 654:386–399. [DOI] [PubMed] [Google Scholar]
- Pugsley TA, Davis MD, Akunne HC, MacKenzie RG, Shih YH, Damsma G, Wikstrom H, Whetzel SZ, Georgic LM, Cooke LW, et al. (1995) Neurochemical and functional characterization of the preferentially selective dopamine D3 agonist PD 128907. J Pharmacol Exp Ther 275:1355–1366. [PubMed] [Google Scholar]
- Richtand NM. (2006) Behavioral sensitization, alternative splicing, and d3 dopamine receptor-mediated inhibitory function. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 31:2368–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richtand NM, Woods SC, Berger SP, Strakowski SM. (2001) D3 dopamine receptor, behavioral sensitization, and psychosis. Neurosci Biobehav Rev 25:427–443. [DOI] [PubMed] [Google Scholar]
- Robertson SD, Matthies HJ, Galli A. (2009) A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters. Mol Neurobiol 39:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Badiani A. (1998) Drug-induced adaptations in catecholamine systems: on the inevitability of sensitization. Adv Pharmacol 42:987–990. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. (1986) Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain research 396:157–198. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Browman KE, Crombag HS, Badiani A. (1998) Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev 22:347–354. [DOI] [PubMed] [Google Scholar]
- Santos GC, Marin MT, Cruz FC, Delucia R, Planeta CS. (2009) Amphetamine- and nicotine-induced cross-sensitization in adolescent rats persists until adulthood. Addict Biol 14:270–275. [DOI] [PubMed] [Google Scholar]
- Seeman P. (2011) All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2(high) receptors. CNS neuroscience & therapeutics 17:118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DM, Moraes CT, Mash DC. (1997) Up-regulation of D3 dopamine receptor mRNA in the nucleus accumbens of human cocaine fatalities. Brain Res Mol Brain Res 45:335–339. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Varela E, Bettinger L, Beckstead MJ. (2015) Methamphetamine self-administration in mice decreases GIRK channel-mediated currents in midbrain dopamine neurons. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitte HH, Freissmuth M. (2015) Amphetamines, new psychoactive drugs and the monoamine transporter cycle. Trends Pharmacol Sci 36:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman A, O’Driscoll GA, Pruessner J, Holahan AL, Boileau I, Gagnon D, Dagher A. (2008) Stress-induced dopamine release in humans at risk of psychosis: a [11C]raclopride PET study. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 33:2033–2041. [DOI] [PubMed] [Google Scholar]
- Song R, Yang RF, Wu N, Su RB, Li J, Peng XQ, Li X, Gaal J, Xi ZX, Gardner EL. (2012) YQA14: a novel dopamine D3 receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D3 receptor-knockout mice. Addict Biol 17:259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. (1991) Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain research 559:29–36. [DOI] [PubMed] [Google Scholar]
- Srisurapanont M, Arunpongpaisal S, Wada K, Marsden J, Ali R, Kongsakon R. (2011) Comparisons of methamphetamine psychotic and schizophrenic symptoms: a differential item functioning analysis. Prog Neuropsychopharmacol Biol Psychiatry 35:959–964. [DOI] [PubMed] [Google Scholar]
- Staley JK, Mash DC. (1996) Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. The Journal of neuroscience: the official journal of the Society for Neuroscience 16:6100–6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkellner T, Mus L, Eisenrauch B, Constantinescu A, Leo D, Konrad L, Rickhag M, Sorensen G, Efimova EV, Kong E, Willeit M, Sotnikova TD, Kudlacek O, Gether U, Freissmuth M, Pollak DD, Gainetdinov RR, Sitte HH. (2014) In vivo amphetamine action is contingent on alphaCaMKII. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 39:2681–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, Sax KW, Rosenberg HL, DelBello MP, Adler CM. (2001) Human response to repeated low-dose d-amphetamine: evidence for behavioral enhancement and tolerance. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 25:548–554. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. (2005) Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol 75:406–433. [DOI] [PubMed] [Google Scholar]
- Urban NB, Kegeles LS, Slifstein M, Xu X, Martinez D, Sakr E, Castillo F, Moadel T, O’Malley SS, Krystal JH, Abi-Dargham A. (2010) Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [(1)(1)C]raclopride. Biological psychiatry 68:689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Kapur S. (2009) Schizophrenia. Lancet 374:635–645. [DOI] [PubMed] [Google Scholar]
- van Rossum JM. (1966) The significance of dopamine-receptor blockade for the mechanism of action of neuroleptic drugs. Archives internationales de pharmacodynamie et de therapie 160:492–494. [PubMed] [Google Scholar]
- van Winkel R, Stefanis NC, Myin-Germeys I. (2008) Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophr Bull 34:1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Stewart J. (1989) The effect of dopamine receptor blockade on the development of sensitization to the locomotor activating effects of amphetamine and morphine. Brain research 499:108–120. [DOI] [PubMed] [Google Scholar]
- Wearne TA, Mirzaei M, Franklin JL, Goodchild AK, Haynes PA, Cornish JL. (2015) Methamphetamine-induced sensitization is associated with alterations to the proteome of the prefrontal cortex: implications for the maintenance of psychotic disorders. J Proteome Res 14:397–410. [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. (1984) Interactions of cholecystokinin octapeptide and dopamine on nucleus accumbens neurons. Brain research 300:161–166. [DOI] [PubMed] [Google Scholar]
- Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D, Houle S, Seeman P, Ginovart N. (2005) Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9-ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. Journal of medicinal chemistry 48:4153–4160. [DOI] [PubMed] [Google Scholar]
- Wolf ME, White FJ, Nassar R, Brooderson RJ, Khansa MR. (1993) Differential development of autoreceptor subsensitivity and enhanced dopamine release during amphetamine sensitization. J Pharmacol Exp Ther 264:249–255. [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Jr, Gitajn L, Gardner EL. (2006) The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 31:1393–1405. [DOI] [PubMed] [Google Scholar]
- Young D, Scoville W. (1938) Paranoid psychosis in narcolepsy and the possible danger of benzedrine treatment. Med Clin North Am 22:637–646. [Google Scholar]
- Yui K, Ikemoto S, Goto K. (2002) Factors for susceptibility to episode recurrence in spontaneous recurrence of methamphetamine psychosis. Annals of the New York Academy of Sciences 965:292–304. [DOI] [PubMed] [Google Scholar]
- Zetterstrom T, Sharp T, Marsden CA, Ungerstedt U. (1983) In vivo measurement of dopamine and its metabolites by intracerebral dialysis: changes after d-amphetamine. J Neurochem 41:1769–1773. [DOI] [PubMed] [Google Scholar]