Abstract
Purpose of review
PTSD in youth is common and debilitating. In contrast to adult PTSD, relatively little is known about the neurobiology of pediatric PTSD, nor how neurodevelopment may be altered. This review summarizes recent neuroimaging studies in pediatric PTSD and discusses implications for future study.
Recent findings
Pediatric PTSD is characterized by abnormal structure and function in neural circuitry supporting threat processing and emotion regulation. Furthermore, cross-sectional studies suggest that youth with PTSD have abnormal frontolimbic development compared to typically developing youth. Examples include declining hippocampal volume, increasing amygdala reactivity, and declining amygdala-prefrontal coupling with age.
Summary
Pediatric PTSD is characterized by both overt and developmental abnormalities in frontolimbic circuitry. Notably, abnormal frontolimbic development may contribute to increasing threat reactivity and weaker emotion regulation as youth age. Longitudinal studies of pediatric PTSD are needed to characterize individual outcomes and determine whether current treatments are capable of restoring healthy neurodevelopment.
Keywords: trauma, PTSD, neurodevelopment, children, adolescents, neuroimaging
Introduction
Approximately two-thirds of youth are exposed to trauma during childhood, and many develop PTSD as a result [1]. By age 18, roughly 8% of traumatized youth have met criteria for a diagnosis of PTSD, with numbers rising up to 40% in cases of sexual abuse and assault [1]. In addition to the psychological suffering imposed, PTSD is associated with lower academic achievement, and increasing incidence of depression, suicide attempts, and substance abuse into adulthood [2]. Childhood trauma and PTSD also pose a tremendous societal cost in terms of health care utilization and financial outlay. For example, the sequelae of childhood maltreatment, including PTSD, are estimated to cost the United States over $500 billion annually [3]. These sobering statistics highlight the need to elucidate neurodevelopmental disruptions in youth with PTSD, with the aim of mitigating its effects throughout the lifespan.
Neuroimaging studies in adult PTSD suggest structural and functional abnormalities in frontolimbic circuitry supporting threat processing and emotion regulation. Briefly, structural brain meta-analyses in adult PTSD show decreased gray matter volume in the dorsal anterior cingulate cortex (dACC) and ventromedial prefrontal cortex (vmPFC), as well as the hippocampus and temporal pole [4,5]. Functional brain meta-analyses in adult PTSD show hyperactivation of the amygdala, insula, and midACC, but hypoactivation of dACC and medial/lateral prefrontal regions particularly to threat-related stimuli [6–8]. Of note, many of these findings are unlikely to be specific to PTSD, and may instead represent common neural abnormalities across psychiatric disorders [9,10]. Functional connectivity findings in adult PTSD have been mixed, suggesting both greater [11–13] and lower [14] amygdala-mPFC connectivity to negative stimuli. Overall, frontolimbic models of adult PTSD posit hyperactivation of threat-promoting regions (amygdala, insula), and impaired function of contextual and emotion regulatory areas to negative stimuli [15,16]. In addition to the frontolimbic model, recent work also implicates dysfunction in larger scale brain networks in adult PTSD involved in salience detection, self-referential thought, and executive control. A more in depth discussion of these network abnormalities can found be found elsewhere in this special issue [17], though I briefly discuss relevant findings in pediatric PTSD later in this article.
Compared to adult PTSD, relatively few neuroimaging studies have been conducted in pediatric PTSD. Such studies are crucial to understanding the neurobiology of PTSD throughout development and for designing treatments that appropriately target developmental brain processes in youth. Recent studies have begun to shed light on the typical development of neural circuits supporting emotion processing and regulation, and provide a foundational backdrop for understanding abnormal neurodevelopment in pediatric PTSD. In this review, I will briefly summarize neuroimaging studies of frontolimbic circuitry in typically developing youth, and discuss the general impact of childhood trauma on frontolimbic development. Next, I will highlight recent studies from my lab and others which point to abnormal neurodevelopment in frontolimbic circuits in pediatric PTSD. While findings to date are based on cross-sectional age-related data, we are in the process of extending these findings in our longitudinal neuroimaging study of pediatric PTSD. Finally, I will discuss convergence of these findings with those in animal models and highlight some potential genetic mechanisms linking childhood trauma to impaired neurodevelopment and PTSD.
Typical development of neural circuits supporting negative emotion processing and regulation
Prevailing models of emotional development posit that emotional reactivity to negative content declines, while regulatory capacity increases with age in typically developing (TD) youth [18,19]. Indeed, negative emotions such as fear and anxiety tend to decrease with age in TD youth, albeit with shifts in content [20]. In the laboratory setting, affective ratings of both neutral and negative images decrease with age in TD youth [21]. Accordingly, TD youth also show increased ability to regulate negative emotion with age. For example, on automatic emotion regulation tasks (which use emotional distractors in the midst of cognitive demands) TD youth show increased performance with age [22–25]. Further evidence for age-related improvement in emotion regulation comes from studies of voluntary emotion regulation. These studies have primarily examined cognitive reappraisal of negative content, which is notable as a common target of cognitive-behavioral therapies in youth with affective disorders. Here, cross-sectional studies of TD youth show greater downregulation of negative affect with age [26–28] (though see [29,30]), and independent of general cognitive ability [27].
What are the neurodevelopmental changes which allow for age-related decline in emotional reactivity and improved emotion regulation? Current neural models suggest that increased emotional reactivity in youth (relative to adults) reflects relative delays in prefrontal vs. subcortical (e.g. amygdala) maturation [31,32]. For example, structural MRI studies show relatively early maturation of amygdala and other subcortical regions supporting threat reactivity (~age 5) [32], with much later maturation of emotion regulatory regions such as dorsal/lateral PFC (mid-20s) [31]. Functional MRI studies in TD youth also point to maturation in prefrontal-amygdala circuits which may allow for improved regulation of negative emotion with age. In cross-sectional studies of emotion processing and automatic regulation, amygdala reactivity to negative faces and images decreases with age [21,33–36], and is accompanied by greater structural and functional connectivity between the amygdala and mPFC/rACC [25,33,34,36]. In turn, amygdala-mPFC connectivity appears to partly mediate age-related decreases in normative anxiety [33]. On voluntary emotion regulation (reappraisal) tasks, TD youth show greater amygdala downregulation [28–30], increased lateral (l)PFC recruitment, and increased amygdala-lPFC coupling [26,28] with age. In summary, these studies suggest that age-related improvements in regulating negative emotion with age are reflected by declining amygdala reactivity to emotional stimuli, which in turn may be due to increasing recruitment and connectivity with prefrontal regulatory regions.
Impacts of child trauma exposure on neurodevelopment
Many studies now suggest that childhood trauma may influence the development of threat processing and emotion regulatory systems, contributing risk for the emergence of affective disorders. In a recent meta-analysis of structural MRI studies [37], childhood trauma was associated with reduced gray matter in the hippocampus and dorsolateral (dl)PFC, regions implicated in the contextual regulation of threat and emotion regulation, respectively. In functional MRI studies, we and others have shown that childhood trauma and adversity are associated with increased amygdala reactivity particularly to negative stimuli (e.g. [38–47]) as well as decreased resting functional coupling between the amygdala and vmPFC [48,49]. It is worth noting that such changes have been observed across healthy and psychiatric samples of youth. An intriguing possibility is that such changes allow for enhanced automatic detection of potential threats in the environment, thus serving an adaptive role for youth growing up in a dangerous environment. On the other hand, we have proposed that enhanced coupling between the amygdala and more dorsal/lateral prefrontal regions involved in higher level appraisal and emotion regulation may reduce the likelihood of psychopathology following childhood trauma [45]. Supporting this notion, childhood trauma is actually associated with increased prefrontal recruitment and amygdala-prefrontal coupling during emotion regulation in otherwise healthy youth or when adjusting for affective symptoms [40,45,46,50,51]. Conversely, recent neuroimaging studies of pediatric PTSD suggest a failure to upregulate prefrontal circuits, but nevertheless show increasing amygdala reactivity with age. These and other neuroimaging studies of pediatric PTSD are discussed further below.
Gray matter volume abnormalities in pediatric PTSD
To date, only three published studies have examined gray matter volume in pediatric PTSD using voxelwise approaches. In an initial report of youth with a spectrum of PTSD symptoms (~50% were threshold for PTSD), Carrion and colleagues found increased vmPFC gray matter volume in youth with PTSD symptoms compared to TD youth [52]. On the other hand, two recent studies, including our own, found decreased vmPFC volume in youth with PTSD relative to either TD or trauma-exposed youth without PTSD [53,54]. One possible reason for these inconsistences may be differences in illness severity among the study samples (i.e. whether including subthreshold PTSD or not). Interestingly, none of these studies found differences in average hippocampal volume between PTSD and TD youth. This is somewhat surprising given that reduced hippocampal volume is characteristic of both adult PTSD [4] and adults with a history of childhood trauma [37]. One possible reason for this discrepancy is a delayed developmental effect, whereby hippocampal volume either fails to increase with age or declines with age in youth with PTSD. To explore this possibility, we incorporated cross-sectional age-related differences (8–18 years) in our analysis of PTSD and TD youth. Here, we found a group by age interaction in right hippocampal volume, such that hippocampal volume increased with age in TD youth, but decreased with age in youth with PTSD [53]. Together, these findings would suggest that youth with PTSD show disrupted hippocampal development and reduced vmPFC volume, which may contribute to impaired regulation of threat via poor contextual gating and inhibition of threat responses, respectively.
Functional brain abnormalities during emotion processing in pediatric PTSD
Several published studies have examined functional brain activation during emotion processing in pediatric PTSD, using tasks involving presentation of emotional faces or images. A summary of differences in average frontolimbic activation or amygdala connectivity can be found in Table 1. In these studies, amygdala hyperactivation has been reported in only one study of pediatric PTSD [38], with no difference (negative studies) in three others [55–57]. Similar to the case for hippocampal volume, the general lack of average amygdala activation differences is curious given relatively consistent reports of amygdala hyperactivation in both adult PTSD [6–8] and adolescents/adults with a history of childhood trauma [47]. As in the case of hippocampal volume, this could represent a delayed developmental effect, whereby amygdala hyperactivation is not fully apparent until adulthood. To explore this possibility, we again incorporated cross-sectional age-related differences (8–18 years) in our analysis of PTSD and TD youth completing an emotional face processing task. Here, we found a group by age interaction on amygdala activation to both angry and happy faces. Specifically, TD youth showed the expected pattern of decreased amygdala activation with age, while youth with PTSD showed increased activation with age [35]. Interestingly, amygdala activation was actually lower in PTSD compared to TD youth at younger ages (<15 years), but showed hyperactivation by late adolescence. An intriguing possibility is that younger children with PTSD may actually show compensatory downregulation of the amygdala, which then becomes compromised as youth age. If this is the case, then one would expect that youth with PTSD may show compensatory engagement of prefrontal regulatory circuits particularly at younger ages. As discussed below, there is at least some evidence to suggest this is the case.
Table 1.
Summary of average frontolimbic activation or amygdala connectivity differences during emotion processing in pediatric PTSD compared to typically developing (TD) youth. Activation findings are summarized as increased (↑) in PTSD, decreased (↓) in PTSD, or with mixed findings (↑↓) in PTSD vs. TD youth. Negative studies refer to those in which no group differences in average activation were observed.
| Brain region/circuit | Activation (PTSD vs. TD youth) | Symptom relationship? | Reference | Negative studies |
|---|---|---|---|---|
| Amygdala | ↑ | No | [38] | [55–57] |
| vmPFC/rACC | ↑↓ | Hyperactivation positively correlated with symptoms | [38,56] | [55,57] |
| dACC | ↑ | Positively correlated with symptoms | [35,57] | [38,55,56] |
| dmPFC | ↑↓ | Hyperactivation positively correlated with symptoms | [35,55] | [38,56,57] |
| vlPFC | ↑↓ | No | [38,55,56] | [35,57] |
| dlPFC | ↓ | No | [38] | [35,55–57] |
| Amygdala-rACC/dmPFC | ↓* | Negatively correlated with symptoms | [35,57,59] | – |
specific to negative emotional stimuli.
Prefrontal-cingulate findings during emotion processing in pediatric PTSD have largely been mixed to date. With regard to mid/dACC activation, earlier studies found no differences in activation relative to TD youth [38,55,56]. On the other hand, we have found dACC hyperactivation to both threat pictures and emotional faces in our sample of youth with PTSD [35,57]. This is notable given that the dACC is hypoactive in adult PTSD [58]. The dACC has been implicated in the conscious appraisal of threat [58], and could conceivably represent an adaptive response at monitoring the environment in youth with PTSD. With regard to the PFC, published studies also show mixed activation findings in pediatric PTSD. Findings include increased [38] and decreased [56] vmPFC/rACC activation, increased [35] and decreased [55] dorsomedial (dm)PFC activation, increased [38,56] and decreased [55] ventrolateral (vl)PFC activation, and decreased dlPFC [38] activation in youth with PTSD (Table 1). There are many possible reasons for these discrepancies, including task differences, trauma-related factors (age at trauma, trauma type, trauma chronicity), and also potential sex differences [55] in youth with PTSD. Age-related differences also appear to factor in, supported by our finding of decreased dmPFC recruitment with age in youth with PTSD [57]. Furthermore, it is crucial to examine neural function in pediatric PTSD beyond brain activation, incorporating network level communication. To this end, we examined amygdala functional connectivity during emotion processing in our sample of youth with PTSD. Notably, we found that youth with PTSD exhibit reduced coupling between the amygdala and rACC/dmPFC, which was further linked to PTSD severity [35,57]. These findings also agree with studies showing an inverse relationship between PTSD symptoms and amygdala-rACC coupling in adolescent sexual assault victims [59]. We have also recently shown that the “failure” to upregulate amygdala-dmPFC coupling in the wake of childhood adversity is associated with anxiety and depressive symptoms by late adolescence. Thus, deficits in this key voluntary emotion regulation circuit [58,60] may play an important role in impaired regulation of negative emotion in pediatric PTSD.
Finally, we have also identified age-related abnormalities in amygdala-PFC coupling in pediatric PTSD. Specifically, we have shown that while TD youth exhibit increased amygdala-vmPFC coupling to negative images with age, youth with PTSD show decreased coupling with age [57]. Mirroring amygdala findings, however, younger youth with PTSD (<15 years) actually show greater amygdala-vmPFC coupling compared to TD youth, with the reverse pattern by late adolescence. Thus, younger children with PTSD may exhibit some degree of compensatory function in automatic emotion regulatory circuitry, with relative downregulation of the amygdala, but which becomes compromised as these youth age (Figure 1).
Figure 1.
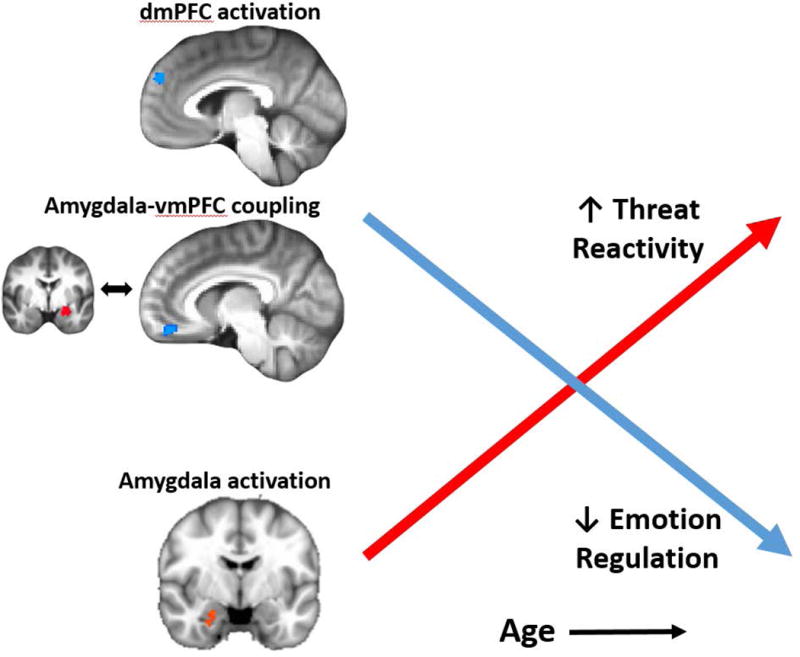
Age-related abnormalities in frontolimbic activation and amygdala-prefrontal connectivity in pediatric PTSD. Data from our cross-sectional studies [35,57] show that, in contrast to typically developing youth, youth with PTSD show increased amygdala activation with age, combined with decreased prefrontal recruitment and coupling with age. These findings suggest that youth with PTSD may have abnormal neurodevelopment in key frontolimbic circuits which could lead to increasing threat reactivity and weaker emotion regulation ability over time.
Frontolimbic development, age at traumatization, and implications for PTSD presentation in youth
How do the age-related findings in frontolimbic circuits relate to age of initial traumatization and sensitive periods in neurodevelopment? Childhood and adolescence are known vulnerable periods for the effects of trauma on neurodevelopment. For example, studies of adults with retrospective reports of childhood abuse suggest that abuse has the most prominent impact on hippocampal volume prior to age 14, amygdala volume between 10 to 11 years of age, and prefrontal volume between 14 to 16 years of age [61]. Notably, these stress-sensitive periods coincide with periods of maturation in these brain regions [31,32], though stress effects may not be fully apparent until adulthood. In support of this latter notion, our age-related findings in pediatric PTSD (e.g. increased amygdala activation, decreased amygdala-vmPFC coupling with age) were not accounted for by age at index trauma or duration of PTSD [35,53,57]. These findings suggest a more general and sustained pattern of abnormal neurodevelopment, further complicated by the possibility of early compensatory brain responses as outlined above. One likely possibility, then, is that youth with PTSD are subject to multiple and/or repeated traumas that interact with genetic vulnerability to alter neurodevelopment over the course of childhood and adolescence. Some of these potential mechanisms, including the glucocorticoid stress pathway, are discussed further below.
Next, what do the age-related findings and overall frontolimbic abnormalities suggest for the presentation of PTSD in youth? Of note, the DSM-5 introduced the preschool (< 6 years) subtype of PTSD, which requires fewer symptoms of avoidance and negative cognitions to meet the diagnostic threshold (1 avoidance and 2 cognition for adults compared to 1 avoidance or cognition for preschoolers) [62]. This subtype was introduced given evidence that PTSD is underdiagnosed in younger children using adult criteria, and could reflect immaturity in systems underlying appraisal and reporting of negative emotion. On the other hand, our findings might suggest that younger children with PTSD actually have compensatory frontolimbic development which could reduce the expression of certain PTSD symptoms such as negative emotional states and cognitions related to trauma. This intriguing possibility will require further study in these younger age groups to provide more definitive answers.
Finally, what implications do the extant neuroimaging findings in pediatric PTSD have for the presentation of dissociative symptoms in pediatric and adult PTSD? DSM-5 introduced the dissociative subtype of PTSD [62], recognizing an important variation in the PTSD presentation which requires more nuanced therapeutic approaches. Furthermore, early data in adults suggest potential neurobiological differences in dissociative PTSD [63]. Based on initial studies of dissociative PTSD, Lanius and colleagues have proposed a model of heightened prefrontal recruitment with associated dampening of amygdala-based threat responding [64]. Furthermore, recent studies suggest that dissociative PTSD patients show increased resting coupling between the amygdala and prefrontal cortex [65], and potentially greater anti-correlation between the default mode and executive networks [66]. Such findings are intriguing in the context of our findings in pediatric PTSD. While speculative, it is possible that trauma limited to earlier ages (e.g. < 15 years) could induce a sustained neural pattern characterized by increased amygdala-prefrontal connectivity and dampened amygdala reactivity, thereby contributing to a dissociative phenotype. However, further studies will be needed to test these hypothesis, ideally involving longitudinal imaging and larger sample sizes stratified by age at trauma exposure.
Large scale network abnormalities in pediatric PTSD
Complementing the frontolimbic model, recent work increasingly implicates abnormalities in larger brain networks in PTSD. For an in depth discussion of this topic in adult PTSD, I refer readers elsewhere in this special issue [17]. Briefly, numerous studies have identified a set of core, functionally connected networks in the brain. These networks include the default mode network (DMN), involved in self-referential thought and processing, the salience network (SN), involved in detection of relevant internal or external cues including threat, and the central executive network (CEN), involved in goal-directed behavior and emotion regulation [67]. Notably, the CEN and DMN are normally anti-correlated, reflecting functionally competing systems that switch between goal-directed behavior and internal processing [68]. As reviewed by Akiki and colleagues [17], studies of adult PTSD suggest increased SN activity, decreased DMN and CEN activity, and inefficient modulation between the CEN and DMN which could account for symptoms of hypervigilance, intrusive thought, and poor emotion regulation. The studies of pediatric PTSD reviewed above tend to agree with the notion of a hyperactive salience network (dACC, amygdala) particularly as youth age, as well as impaired recruitment of the CEN (dmPFC/dlPFC). To date, however, few studies have specifically examined large-scale network function in pediatric PTSD. In an initial study, we examined DMN function and its relationship to task positive networks (combining CEN/SN) in our sample of youth with PTSD [69]. In contrast to findings in adult PTSD, youth with PTSD showed increased connectivity within the DMN, combined with greater anti-correlation between the DMN and CEN/SN. CEN/SN strength, in turn, was associated with lower re-experiencing symptoms. These findings raise the intriguing possibility that pediatric PTSD may be characterized, in part, by compensatory recruitment of executive control systems to suppress trauma-related thought, although such compensatory recruitment could also conceivably contribute to dissociative symptoms as discussed above. However, further work is clearly needed to better characterize intrinsic network function in pediatric PTSD and how this changes over the course of development and into adulthood.
Neurobiological pathways to PTSD in youth: Insights from animal models
The studies reviewed above suggest neurodevelopmental abnormalities in frontolimbic circuits in pediatric PTSD which could contribute to deficits in threat discrimination and emotion regulation. However, in human studies, it is particularly difficult to establish clear environmental, genetic, and neural mechanisms causing PTSD. Extant studies have relied primarily on cross-sectional cohort designs, which may be influenced by other potential factors that differ between youth groups. For example, youth with PTSD may be more likely than TD youth to have chronic stressors (e.g. family financial stress, food scarcity), multiple trauma types, genetic vulnerability, and poor social support which cumulatively lead to the PTSD phenotype. As such, it is important to corroborate clinical research findings with those from animal models, which can more precisely control environmental exposures, genetic background, and developmental timing of trauma. On the other hand, creating an animal model of PTSD is inherently difficult given its syndromal nature and uniqueness to humans. Here, we may gain more traction by focusing on circuit-based abnormalities as well as specific behavioral constructs [70] found to be abnormal in PTSD, which can then be translated in animal models.
A notable area of research in rodent and non-human primate models involves the use of early life stress (ELS) such as abusive caregiving or stress hormone (glucocorticoid) exposure to simulate traumatic experiences and adversity during childhood. Studies of chronic stress or glucocorticoid exposure in adult animals show notable effects on frontolimbic circuitry including dendritic atrophy of the mPFC and hippocampus, and expansion of the basolateral amygdala [71]. While there are relatively fewer studies examining the effects of ELS on frontolimbic circuits in developing animals, initial studies suggest that ELS decreases hippocampal neuron proliferation [72], increases turnover of cortical dendritic spines [73], and increases amygdala volume [74] and reactivity [44]. Interestingly, rodent developmental studies of threat learning and extinction suggest that ELS induces early maturation of these processes, including enhanced threat learning and renewal [75], bearing similarity to enhanced threat bias in youth with ELS [44]. These findings, then, may be more indicative of the general effects of early life trauma and represent the brain’s attempt to adaptively detect threat, but at the potential cost of sustained hypervigilance and difficulty of inhibiting threat responses. Thus, neuroanatomical changes following childhood trauma may have both adaptation and vulnerability effects.
What is it then that differentiates so-called “resilient” and vulnerable youth from the effects of childhood abuse and adversity? In a recent study, we simultaneously examined the effects of childhood adversity and symptoms of internalizing (anxiety and depression) on prefrontal-amygdala function in a community sample of late adolescents [45]. Regardless of internalizing levels, all adolescents showed adversity-related increases in amygdala reactivity to negative images. However, differences emerged between low and high internalizing adolescents in prefrontal-amygdala coupling. Here, low internalizing adolescents showed adversity-related increases in prefrontal-amygdala coupling, while high internalizing adolescents did not. Remarkably, use of this same emotion processing task in our pediatric PTSD sample showed decreased amygdala connectivity to the same prefrontal region (rACC/dmPFC), which further correlated with PTSD severity [57]. Thus, early life adversity may generally induce neural changes geared towards improved automatic detection of threat (increased amygdala reactivity, age-related decline in amygdala-vmPFC coupling). On the other hand, emergence of trauma-related psychopathology may reflect a failure to enhance, or subsequently lose, higher level compensatory brain mechanisms involved in voluntary appraisal and emotion regulation. Further research, including longitudinal neuroimaging in youth starting prior to trauma exposure, will be needed to explore these possibilities.
What are some of the potential genetic and molecular mechanisms which may lead to PTSD and associated frontolimbic abnormalities in youth following trauma? A full review of such mechanisms is beyond the scope of this article; I refer readers elsewhere for further information on genetic mechanisms linking childhood adversity to mental and physical health [76,77], as well as the genetic and molecular mechanisms of PTSD reviewed in this special issue [78]. However, I highlight here some promising candidate pathways mediating the link between childhood trauma and psychopathology involving the glucocorticoid stress pathway. Studies across animals and humans point to altered methylation and expression of the glucocorticoid receptor (GR or NR3C1) and FKBP5, a GR chaperone protein which impedes GR signaling, following ELS [79–81]. In the case of NR3C1, ELS is associated with hypermethylation particularly in exon 1F, which in turn is associated with downregulation of GR in the brain and periphery [80,81]. In the case of FKBP5, a common variant of the gene’s enhancer region is associated with demethylation of GR response elements following ELS, leading to increased expression of FKBP5 and greater risk for psychopathology [76,77]. In both cases, the net effect is blunted glucocorticoid signaling and reduced negative feedback, leading to hyper-responsivity of the HPA stress response. Chronically elevated glucocorticoid levels, in turn, are known to induce remodeling of the hippocampus, amygdala, and mPFC [71] which may partially account for the neurodevelopmental abnormalities observed in pediatric PTSD. While no genetic/epigenetic studies have so far been reported in pediatric PTSD, several studies have now shown that ELS is associated with GR hypermethylation in youth, though findings for FKBP5 have so far been mixed [79]. Further research is clearly needed on the study of genetic and molecular mechanisms linking childhood trauma to PTSD and other psychopathology in youth. On the other hand, extant studies suggest potentially novel biological pathways which could be targeted in the prevention or treatment of pediatric PTSD.
Conclusions
In summary, the findings reviewed here suggest that pediatric PTSD shows some structural and functional brain abnormalities similar to adult PTSD. Examples include reduced vmPFC volume and impaired recruitment of lateral prefrontal cortex. Conversely, other features characteristic of adult PTSD, such as reduced hippocampal volume, and hyperactivity of the amygdala and insula, have not been consistently observed in pediatric PTSD. One reason for such discrepancies appears to be delayed neurodevelopmental effects in pediatric PTSD, including decreasing hippocampal volume, increasing amygdala reactivity, and decreasing amygdala-mPFC coupling with age. Thus, PTSD may differ in youth compared to adults both due to heightened stress sensitivity of developing neural systems, as well as delayed expression of the full effects of childhood trauma exposure. Additionally, recent studies implicate network level abnormalities in pediatric PTSD which may reflect effects of childhood trauma and the expression of PTSD, most notably for prefrontal-amygdala connectivity. In total, youth with PTSD exhibit both overt and developmental abnormalities in frontolimbic circuits which may contribute to increasing threat reactivity and declining emotion regulation capacity as youth age (Figure 1). Further work is needed, however, to advance our understanding of neurobiological mechanisms underlying pediatric PTSD. In particular, future studies are merited to examine longitudinal neurodevelopment, characterize larger scale intrinsic network function, explore genetic and molecular mechanisms of abnormal neurodevelopment, and determine to what extent current evidence-based treatments are capable of restoring “healthy” neurodevelopment. Studies such as these will be vital to mapping individual outcomes and developing novel, biologically based, and targeted treatments to alleviate the suffering of afflicted youth and their families.
Acknowledgments
Dr. Herringa’s work as reviewed here has been supported by the National Institute of Mental Health (K08 MH100267), the American Academy of Child & Adolescent Psychiatry, the Brain and Behavior Research Foundation, and the University of Wisconsin Institute for Clinical and Translational Research (NIH/NCATS UL1TR000427).
References
- 1.McLaughlin KA, Koenen KC, Hill ED, Petukhova M, Sampson NA, Zaslavsky AM, et al. Trauma exposure and posttraumatic stress disorder in a national sample of adolescents. J Am Acad Child Adolesc Psychiatry. 2013;52:815–830.e14. doi: 10.1016/j.jaac.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warshaw MG, Fierman E, Pratt L, Hunt M, Yonkers KA, Massion AO, et al. Quality of life and dissociation in anxiety disorder patients with histories of trauma or PTSD. Am J Psychiatry. 1993;150:1512–6. doi: 10.1176/ajp.150.10.1512. [DOI] [PubMed] [Google Scholar]
- 3.Fang X, Brown DS, Florence CS, Mercy JA. The economic burden of child maltreatment in the United States and implications for prevention. Child Abuse Negl. 2012;36:156–65. doi: 10.1016/j.chiabu.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuhn S, Gallinat J. Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol Psychiatry. 2013;73:70–4. doi: 10.1016/j.biopsych.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 5.O’Doherty DCM, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res. 2015;232:1–33. doi: 10.1016/j.pscychresns.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord. 2012;2:9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2012;36:2130–42. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 9•.Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–15. doi: 10.1001/jamapsychiatry.2014.2206. Meta-analysis of structural MRI studies across psychiatric diagnoses implicating common neural substrates of mental illness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of Common Neural Circuit Disruptions in Cognitive Control Across Psychiatric Disorders. AJP. 2017;174:676–85. doi: 10.1176/appi.ajp.2017.16040400. Meta-analysis of functional MRI studies across psychiatric diagnoses implicating common neural substrates of mental illness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol Psychiatry. 2010;68:433–41. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R, et al. Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biol Psychiatry. 2004;55:263–72. doi: 10.1016/j.biopsych.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 13.St Jacques PL, Botzung A, Miles A, Rubin DC. Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. J Psychiatr Res. 2011;45:630–7. doi: 10.1016/j.jpsychires.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, et al. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res. 2013;47:1469–78. doi: 10.1016/j.jpsychires.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–87. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–28. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akiki T, Averill C, Abdallah CG. A Network-Based Neurobiological Model of PTSD: Evidence from Structural and Functional Neuroimaging Studies. Current Psychiatry Reports. doi: 10.1007/s11920-017-0840-4. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Somerville LH, Jones RM, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–33. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casey B, Galván A, Somerville LH. Beyond simple models of adolescence to an integrated circuit-based account: A commentary. Developmental Cognitive Neuroscience. 2016;17:128–30. doi: 10.1016/j.dcn.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gullone E. The development of normal fear: A century of research. Clinical Psychology Review. 2000;20:429–51. doi: 10.1016/s0272-7358(99)00034-3. [DOI] [PubMed] [Google Scholar]
- 21.Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin R, et al. The transition from childhood to adolescence is marked by a general decrease in amygdala reactivity and an affect-specific ventral-to-dorsal shift in medial prefrontal recruitment. Dev Cogn Neurosci. 2016 doi: 10.1016/j.dcn.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grose-Fifer J, Rodrigues A, Hoover S, Zottoli T. Attentional capture by emotional faces in adolescence. Adv Cogn Psychol. 2013;9:81–91. doi: 10.2478/v10053-008-0134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–34. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen-Gilbert JE, Thomas KM. Inhibitory control during emotional distraction across adolescence and early adulthood. Child Dev. 2013;84:1954–66. doi: 10.1111/cdev.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heller AS, Cohen AO, Dreyfuss MFW, Casey BJ. Changes in cortico-subcortical and subcortico-subcortical connectivity impact cognitive control to emotional cues across development. Soc Cogn Affect Neurosci. 2016;11:1910–8. doi: 10.1093/scan/nsw097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, et al. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc Cogn Affect Neurosci. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silvers JA, McRae K, Gabrieli JDE, Gross JJ, Remy KA, Ochsner KN. Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion. 2012;12:1235–47. doi: 10.1037/a0028297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silvers JA, Shu J, Hubbard AD, Weber J, Ochsner KN. Concurrent and lasting effects of emotion regulation on amygdala response in adolescence and young adulthood. Dev Sci. 2015;18:771–84. doi: 10.1111/desc.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitskel NB, Bolling DZ, Kaiser MD, Crowley MJ, Pelphrey KA. How grossed out are you? The neural bases of emotion regulation from childhood to adolescence. Dev Cogn Neurosci. 2011;1:324–37. doi: 10.1016/j.dcn.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stephanou K, Davey CG, Kerestes R, Whittle S, Pujol J, Yücel M, et al. Brain functional correlates of emotion regulation across adolescence and young adulthood. Hum Brain Mapp. 2016;37:7–19. doi: 10.1002/hbm.22905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M, et al. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS ONE. 2012;7:e46970. doi: 10.1371/journal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, et al. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 2013;33:4584–93. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vink M, Derks JM, Hoogendam JM, Hillegers M, Kahn RS. Functional differences in emotion processing during adolescence and early adulthood. Neuroimage. 2014;91:70–6. doi: 10.1016/j.neuroimage.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 35•.Keding TJ, Herringa RJ. Paradoxical Prefrontal-Amygdala Recruitment to Angry and Happy Expressions in Pediatric Posttraumatic Stress Disorder. Neuropsychopharmacology. 2016;41:2903–12. doi: 10.1038/npp.2016.104. Functional MRI study showing cross-sectional increases in amygdala reactivity with age in pediatric PTSD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swartz JR, Carrasco M, Wiggins JL, Thomason ME, Monk CS. Age-related changes in the structure and function of prefrontal cortex-amygdala circuitry in children and adolescents: a multi-modal imaging approach. Neuroimage. 2014;86:212–20. doi: 10.1016/j.neuroimage.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paquola C, Bennett MR, Lagopoulos J. Understanding heterogeneity in grey matter research of adults with childhood maltreatment-A meta-analysis and review. Neurosci Biobehav Rev. 2016;69:299–312. doi: 10.1016/j.neubiorev.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Garrett AS, Carrion V, Kletter H, Karchemskiy A, Weems CF, Reiss A. Brain activation to facial expressions in youth with PTSD symptoms. Depress Anxiety. 2012;29:449–59. doi: 10.1002/da.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, et al. Heightened neural reactivity to threat in child victims of family violence. Curr Biol. 2011;21:R947–948. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 40•.McLaughlin KA, Peverill M, Gold AL, Alves S, Sheridan MA. Child Maltreatment and Neural Systems Underlying Emotion Regulation. J Am Acad Child Adolesc Psychiatry. 2015;54:753–62. doi: 10.1016/j.jaac.2015.06.010. Functional MRI study showing compensatory prefrontal recruitment during emotion regulation in maltreated adolescents when adjusting for psychiatric symptoms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012;71:286–93. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 42.Dannlowski U, Kugel H, Huber F, Stuhrmann A, Redlich R, Grotegerd D, et al. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum Brain Mapp. 2013;34:2899–909. doi: 10.1002/hbm.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki H, Luby JL, Botteron KN, Dietrich R, McAvoy MP, Barch DM. Early life stress and trauma and enhanced limbic activation to emotionally valenced faces in depressed and healthy children. J Am Acad Child Adolesc Psychiatry. 2014;53:800–813.e10. doi: 10.1016/j.jaac.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc Natl Acad Sci USA. 2013;110:18274–8. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Herringa RJ, Burghy CA, Stodola DE, Fox ME, Davidson RJ, Essex MJ. Enhanced prefrontal-amygdala connectivity following childhood adversity as a protective mechanism against internalizing in adolescence. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:326–34. doi: 10.1016/j.bpsc.2016.03.003. Functional MRI study in a longitudinal community sample of adolescents implicating enhanced amygdala-prefrontal coupling as a mechanism of stress resilience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marusak HA, Martin KR, Etkin A, Thomason ME. Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology. 2015;40:1250–8. doi: 10.1038/npp.2014.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hein TC, Monk CS. Research Review: Neural response to threat in children, adolescents, and adults after child maltreatment – a quantitative meta-analysis. J Child Psychol Psychiatr. 2017;58:222–30. doi: 10.1111/jcpp.12651. [DOI] [PubMed] [Google Scholar]
- 48.Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, et al. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci USA. 2013;110:19119–24. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Birn RM, Patriat R, Phillips ML, Germain A, Herringa RJ. Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depress Anxiety. 2014;31:880–92. doi: 10.1002/da.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elsey J, Coates A, Lacadie CM, McCrory EJ, Sinha R, Mayes LC, et al. Childhood trauma and neural responses to personalized stress, favorite-food and neutral-relaxing cues in adolescents. Neuropsychopharmacology. 2015;40:1580–9. doi: 10.1038/npp.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fonzo GA, Huemer J, Etkin A. History of childhood maltreatment augments dorsolateral prefrontal processing of emotional valence in PTSD. Journal of Psychiatric Research. 2016;74:45–54. doi: 10.1016/j.jpsychires.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carrion VG, Weems CF, Watson C, Eliez S, Menon V, Reiss AL. Converging evidence for abnormalities of the prefrontal cortex and evaluation of midsagittal structures in pediatric posttraumatic stress disorder: an MRI study. Psychiatry Res. 2009;172:226–34. doi: 10.1016/j.pscychresns.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Keding TJ, Herringa RJ. Abnormal structure of fear circuitry in pediatric post-traumatic stress disorder. Neuropsychopharmacology. 2015;40:537–45. doi: 10.1038/npp.2014.239. Structural MRI study showing cross-sectional decreases in hippocampal volume with age in pediatric PTSD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morey RA, Haswell CC, Hooper SR, De Bellis MD. Amygdala, Hippocampus, and Ventral Medial Prefrontal Cortex Volumes Differ in Maltreated Youth with and without Chronic Posttraumatic Stress Disorder. Neuropsychopharmacology. 2016;41:791–801. doi: 10.1038/npp.2015.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crozier JC, Wang L, Huettel SA, De Bellis MD. Neural correlates of cognitive and affective processing in maltreated youth with posttraumatic stress symptoms: does gender matter? Dev Psychopathol. 2014;26:491–513. doi: 10.1017/S095457941400008X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang P, Wu M-T, Hsu C-C, Ker J-H. Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: a functional MRI study. Neurosci Lett. 2004;370:13–8. doi: 10.1016/j.neulet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 57•.Wolf RC, Herringa RJ. Prefrontal-Amygdala Dysregulation to Threat in Pediatric Posttraumatic Stress Disorder. Neuropsychopharmacology. 2016;41:822–31. doi: 10.1038/npp.2015.209. Functional MRI study showing cross-sectional decreases in amygdala-vmPFC coupling with age in pediatric PTSD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalisch R, Gerlicher AMV. Making a mountain out of a molehill: On the role of the rostral dorsal anterior cingulate and dorsomedial prefrontal cortex in conscious threat appraisal, catastrophizing, and worrying. Neurosci Biobehav Rev. 2014;42:1–8. doi: 10.1016/j.neubiorev.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 59.Cisler JM, Scott Steele J, Smitherman S, Lenow JK, Kilts CD. Neural processing correlates of assaultive violence exposure and PTSD symptoms during implicit threat processing: A network-level analysis among adolescent girls. Psychiatry Res. 2013;214:238–46. doi: 10.1016/j.pscychresns.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee H, Heller AS, van Reekum CM, Nelson B, Davidson RJ. Amygdala-prefrontal coupling underlies individual differences in emotion regulation. Neuroimage. 2012;62:1575–81. doi: 10.1016/j.neuroimage.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016;17:652–66. doi: 10.1038/nrn.2016.111. Comprehensive review examining putative neural mechanisms linking childhood maltreatment to psychopathology, with consideration of both vulnerability and compensatory brain changes. [DOI] [PubMed] [Google Scholar]
- 62.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th. Washington, D.C: American Psychiatric Association; 2013. [Google Scholar]
- 63•.Krause-Utz A, Frost R, Winter D, Elzinga BM. Dissociation and Alterations in Brain Function and Structure: Implications for Borderline Personality Disorder. Curr Psychiatry Rep. 2017;19:6. doi: 10.1007/s11920-017-0757-y. Review of neuroimaging studies implicating potential brain substrates of dissociative symptoms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, et al. Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry. 2010;167:640–7. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nicholson AA, Densmore M, Frewen PA, Théberge J, Neufeld RW, McKinnon MC, et al. The Dissociative Subtype of Posttraumatic Stress Disorder: Unique Resting-State Functional Connectivity of Basolateral and Centromedial Amygdala Complexes. Neuropsychopharmacology. 2015;40:2317–26. doi: 10.1038/npp.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tursich M, Ros T, Frewen PA, Kluetsch RC, Calhoun VD, Lanius RA. Distinct intrinsic network connectivity patterns of post-traumatic stress disorder symptom clusters. Acta Psychiatr Scand. 2015;132:29–38. doi: 10.1111/acps.12387. [DOI] [PubMed] [Google Scholar]
- 67.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 68.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 69•.Patriat R, Birn RM, Keding TJ, Herringa RJ. Default-Mode Network Abnormalities in Pediatric Posttraumatic Stress Disorder. J Am Acad Child Adolesc Psychiatry. 2016;55:319–27. doi: 10.1016/j.jaac.2016.01.010. Functional MRI study of pediatric PTSD examining large scale intrinsic brain networks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liberzon I. Searching for Intermediate Phenotypes in Posttraumatic Stress Disorder. Biological Psychiatry [Internet] 2017 doi: 10.1016/j.biopsych.2017.06.005. [cited 2017 Jun 26];0. Available from: http://www.biologicalpsychiatryjournal.com/article/S0006-3223(17)31664-5/fulltext. [DOI] [PubMed]
- 71.McEwen BS, Nasca C, Gray JD. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanapat P, Galea LAm, Gould E. Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. International Journal of Developmental Neuroscience. 1998;16:235–9. doi: 10.1016/s0736-5748(98)00029-x. [DOI] [PubMed] [Google Scholar]
- 73.Liston C, Gan W-B. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. PNAS. 2011;108:16074–9. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Howell BR, Grand AP, McCormack KM, Shi Y, LaPrarie JL, Maestripieri D, et al. Early adverse experience increases emotional reactivity in juvenile rhesus macaques: Relation to amygdala volume. Dev Psychobiol. 2014;56:1735–46. doi: 10.1002/dev.21237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Callaghan BL, Richardson R. Early experiences and the development of emotional learning systems in rats. Biol Mood Anxiety Disord. 2013;3:8. doi: 10.1186/2045-5380-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cruceanu C, Matosin N, Binder EB. Interactions of early-life stress with the genome and epigenome: from prenatal stress to psychiatric disorders. Current Opinion in Behavioral Sciences. 2017;14:167–71. [Google Scholar]
- 77.Klengel T, Binder EB. Epigenetics of Stress-Related Psychiatric Disorders and Gene × Environment Interactions. Neuron. 2015;86:1343–57. doi: 10.1016/j.neuron.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 78.Girgenti M, Hare B, Ghosal S, Duman R. Molecular and Cellular Consequences of Stress: Implications in PTSD. Current Psychiatry Reports. doi: 10.1007/s11920-017-0841-3. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tyrka AR, Ridout KK, Parade SH. Childhood adversity and epigenetic regulation of glucocorticoid signaling genes: Associations in children and adults. Development and Psychopathology. 2016;28:1319–31. doi: 10.1017/S0954579416000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turecki G, Meaney MJ. Effects of the Social Environment and Stress on Glucocorticoid Receptor Gene Methylation: A Systematic Review. Biol Psychiatry. 2016;79:87–96. doi: 10.1016/j.biopsych.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Palma-Gudiel H, Córdova-Palomera A, Leza JC, Fañanás L. Glucocorticoid receptor gene (NR3C1) methylation processes as mediators of early adversity in stress-related disorders causality: A critical review. Neuroscience & Biobehavioral Reviews. 2015;55:520–35. doi: 10.1016/j.neubiorev.2015.05.016. [DOI] [PubMed] [Google Scholar]


