Abstract
Background
Statins are effective in primary prevention of atherosclerotic cardiovascular disease. The 2013 American College of Cardiology/American Heart Association (ACC-AHA) guideline expands recommended statin use, but its cost-effectiveness has not been compared with other guidelines.
Methods
We used the Cardiovascular Disease (CVD) Policy Model to estimate the cost-effectiveness of the ACC-AHA, relative to current use, Adult Treatment Panel III (ATP III) guidelines, and universal statin use in all men age 45-74 years and women age 55-74 years over a 10-year horizon from 2016 to 2025. Sensitivity analyses varied costs, risks, and benefits. Main outcomes were incremental cost-effectiveness ratios (ICER) and numbers needed to treat for ten years per quality-adjusted life-year gained (NNT/QALY).
Results
Each approach produces substantial benefits and net cost savings relative to the status quo. Full adherence to the ATP III guideline would result in 8.8 million more statin users than the status quo, at an NNT/QALY of 35. The ACC-AHA guideline would potentially result in up to 12.3 million more statin users than the ATP III guideline, with a marginal NNT/QALY of 68. Moderate-intensity statin use in all men 45-74 and women 55-74 would result in 28.9 million more statin users than the ACC-AHA guideline, with a marginal NNT/QALY of 108. In all cases, benefits would be greater in men than women. Results vary moderately with different risk thresholds for instituting statins and statin toxicity estimates, but greatly depend on the disutility caused by daily medication use (pill burden).
Conclusions
At a population level, the ACC-AHA guideline for expanded statin use for primary prevention is projected to treat more people, save more lives, and cost less compared with ATP III, in both men and women. Whether individuals benefit from long-term statin use for primary prevention depends more on the disutility associated with pill burden than their degree of cardiovascular risk.
Keywords: statin, coronary heart disease, primary prevention, cost-effectiveness
Introduction
Coronary heart disease (CHD) is the leading cause of death worldwide.1 Inhibitors of HMG-CoA reductase, or statins, prevent CHD events and mortality in persons with known cardiovascular disease or risk equivalents2 and prevent CHD in asymptomatic low-risk persons.3 Given this potential benefit, many have suggested wide indications for their use, and availability without a prescription. This view has been reinforced by the decreasing cost of statins,4 evidence that toxicities may be less than previously believed,5, 6 and studies supporting benefits in lower-risk and moderate-risk middle-aged adults and younger elders.3,7
The Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III) had been the United States guideline for who should take statins for primary prevention of CHD, at what intensities, and to what low-density lipoprotein cholesterol (LDL-C) goals, from its release in 2002 until 2013.8
In 2013, an American College of Cardiology/American Heart Association (ACC-AHA) panel revisited this guideline and recommended that all persons with clinical atherosclerotic cardiovascular disease (ASCVD); all persons age 40-75 years with diabetes and LDL-C ≥ 70-190 mg/dL; and all persons over age 20 with LDL-C over 190 mg/dL should either start or continue statin use for the prevention of CHD and stroke.9 The panel also recommended statins for primary prevention for a fourth, lower risk group: those age 40-75 with 10 year atherosclerotic cardiovascular disease risk of 7.5% or greater. However, the ACC-AHA panel recommended that the latter group consider statins only after a clinician-patient risk discussion that included review of the potential for ASCVD reduction, other risk factors, lifestyle, adverse effects, drug-drug interactions, and patients' preferences. Assuming that these discussions end with the patient starting a statin, the ACC-AHA guideline would nearly double the population of individuals in the US taking statins, with considerable potential impact on atherosclerotic cardiovascular disease (ASCVD) prevention.10,11 The guideline has been controversial for its breadth of statin use,10 especially given recent evidence that daily medication use causes a non-trivial disutility for many individuals (also called “pill burden”);12,13 reviewers have also questioned its (largely LDL-C-independent) risk calculation method,14 and its abolition of LDL-C treatment goals.15 Recent research compared the cost-effectiveness of differing 10-year risk-based thresholds for statin use relative to ACC-AHA's 7.5% cutoff, finding that treatment remains cost-effective at a $100,000-per-QALY threshold using risk cutoffs lower than 7.5%.16,17 Subsequent work has demonstrated that, for persons with and without ASCVD and diabetes, full adherence to the ACC/AHA guideline could prevent more than 240,000 ASCVD events, chiefly in persons with high baseline risk.11 The population-level benefit of risk-based and LDL-C-based statin use thresholds in the primary prevention of ASCVD endpoints such as CHD and stroke has not been compared.
We used the CVD Policy Model (CVDPM),18 a Markov model of CHD and stroke in the US, to evaluate the efficacy and cost-effectiveness of the ACC-AHA guideline for statin use, relative to the ATP III guideline, in men 45-74 years and women 55-74 year without CHD, stroke, or diabetes, for prevention of ASCVD endpoints (CHD and stroke)and death. We compared these two strategies with current levels of statin use (as measured by national surveys), and with universal use of low dose statins in this age and sex-based cohort (men 45-74 and women 55-74).
Methods
Structure of the Model
The CVDPM is an established computer-simulated state-transition (Markov) cohort model of CVD (CHD and all-cause stroke) incidence, prevalence, mortality, and costs among persons 35-94 years in the US. The model predicts CHD and stroke incidence and mortality, as well as all-cause mortality among those with and without CVD, based on age (in 10-year increments), sex, systolic blood pressure, smoking status, high-density and low-density lipoprotein cholesterol level (HDL-C and LDL-C), diabetes mellitus, and statin use. The model does not currently incorporate race or ethnicity as a CHD or stroke risk factor.
The model has three components. First, a demographic-epidemiologic sub-model estimates the incidence of coronary heart disease and its sequelae (cardiac arrest, myocardial infarction (MI), angina, or CHD-related death); stroke; and death from other causes, based on the above risk factors. Second, a bridge sub-model characterizes all incident CHD and stroke events, and related events, over the following 30 days, with respect to their impact on subsequent CVD events and deaths. Third, a disease-history sub-model predicts the number of subsequent CHD and stroke events, revascularization procedures, and deaths among persons who have experienced such a CHD or stroke event, stratified by age, sex, and prior history of CHD or stroke events. Modifiable components of the model include population distributions, risk factor levels and distributions, risk factor (beta) coefficients, event rates, case fatality rates, costs, and disability adjustments.18 Risk factor levels, distributions, and beta-coefficients are derived from the National Health and Nutrition Examination Survey (NHANES) and the Framingham cohort study among other sources and reflect both an intrinsic increase in CVD events after diagnosis and the lifestyle factors that precipitated CVD.19,20 The model's predictive value for cardiac endpoints such as non-fatal myocardial infarction (MI) or death due to CHD has been validated against empiric data such as the West of Scotland Coronary Prevention Study (WOSCOPS).21 Each health state and event has an annual cost and quality-of-life adjustment. More details are published elsewhere.18,22,23
Data Sources
The CVDPM uses population size projections for 2010 through 2050 from the US Census24 and data from NHANES, 2007 to 2010, to estimate the prevalence and mean of CVD risk factors as well as the joint distribution of risk factors.19 The background prevalence of CVD is estimated from the National Health Interview Survey, 2009-2011,25 and CVD deaths, pre-hospital deaths from cardiac arrest, and non-CVD deaths from US Vital Statistics,26 on the basis of codes from the International Classification of Diseases, 10th revision (ICD-10).27 Case-fatality rates are derived from the National Hospital Discharge Survey (2010).28
The beta-coefficient for the effect of LDL-C lowering on CHD was derived from the Cholesterol Treatment Trialists' (CTT) 2012 meta-analysis from 27 randomized trials.3 The CTT report an independent relative risk reduction of 0.79 for any major coronary event per 1 mmol/L reduction in LDL-C from statins regardless of baseline LDL-C,3 and we modeled statin efficacy via this relationship. The beta-coefficients for the effect of LDL-C lowering on stroke was derived from Framingham Heart Study data.20 We derived the beta coefficient for the effect of statins on incident diabetes from a meta-analysis29 reporting one new case of diabetes for every 1020 person-years of statin use.
Our statin analysis estimated total health care costs through national data from the Centers for Medicare and Medicaid Services.30 We estimated the CHD cost component using California data,31 deflated using cost-to-charge ratios,32 and the ratio of the U.S. national average costs to the California average,33 and then inflated to 2016 dollars using the Bureau of Labor Statistics Consumer Price Index.34 We based quality-of-life weights on observational data from the Global Burden of Disease study,35 and discounted costs and QALYs at 3% per year. We derived the prevalence of statin use from NHANES survey data.19
Statin Therapy
We characterized statin doses that decrease LDL-C level by approximately 40% (e.g. pravastatin 40 mg) as moderate intensity, and 55% reduction (e.g. atorvastatin 80 mg) as high-intensity.9,36,37 We estimated medication cost for base case scenarios at $48 per year for moderate-intensity statins (Table 1), as found at Wal-Mart, Target, and other national pharmacy chains,38,39 and $148.30 per year for high-intensity statins, as found at Costco,40 and assumed stable prices over the 10 years of statin use modeled.
Table 1. Model Assumptions.
| Parameter | Base-Case Assumption | 95% CI for Monte Carlo | Reference |
|---|---|---|---|
| LDL-C lowering, %, moderate-intensity statin | 40 | 36-44 | 3,9 |
| Price, $/y | 48.67 | 10.57-438.00 | 38,39,52,53,54 |
| Myopathy, $/y | 0.01 | 0.00-0.02 | 9,44,45 |
| Liver panel, $/y | 1.17 | 0.59-1.76 | 44 |
| Lipid panel, $/y (ATP III scenario) | 19.00 | 9.50-28.50 | 44 |
| Physician visit, $/y | 7.30 | 3.65-10.95 | 45 |
| Hemorrhagic stroke, $/y | 1.50 | 0.38-3.38 | 9,20 |
| Diabetes, $/y | 7.75 | 1.16-19.75 | 29,46 |
| TOTAL | $66.39 | $16.34-473.85 | |
| LDL-C lowering, %, high-intensity statin | 54.77 | 50.77-58.77 | 3,9 |
| Statin Cost, $/y | 148.30 | 31.71-1217.00 | 40, 52,53,54 |
| Myopathy, $/y | 0.01 | 0.00-0.02 | 9,44,45 |
| Liver panel, $/y | 1.17 | 0.59-1.76 | 44 |
| Lipid panel, $/y (ATP III scenario) | 19.00 | 9.50-28.50 | 44 |
| Physician visit, $/y | 7.30 | 3.65-10.95 | 45 |
| Hemorrhagic stroke, $/y | 1.50 | 0.38-3.38 | 9,20 |
| Diabetes, $/y | 7.75 | 1.16-19.75 | 29,46 |
| TOTAL | $166.02 | $46.98-1281.35 | |
| Myopathy QALY penalty, per annum | 0.000001 | 0.00000-0.000002 | 3,9,35 |
| Hemorrhagic stroke QALY penalty, per annum | 0.00003 | 0.00001-0.00007 | 3,9,35 |
| Diabetes QALY penalty, per annum | 0.00007 | 0.00001-0.00019 | 31,35,42,43 |
| Unforeseen (toxicity, pill burden) QALY penalty, per annum | 0.0001 | 0.00001-0.001 | |
| Pill Burden QALY penalty, per annum | 0 | 0-0.00384 | 12,47 |
| TOTAL QALY penalty, per annum, with pill burden included | 0.000207 | 0.0000292-0.00510 | |
| TOTAL QALY penalty, per annum, with pill burden (0-0.00384) excluded | 0.000207 | 0.0000292-0.00126 | |
| Beta-coefficient, diabetes-statin use (any intensity) | 0.09 | 0.02-0.17 | 29 |
| Beta-coefficient, CHD-LDL-C level (per mmol/L) | 0.79 | 0.76-0.81 | 3 |
| Beta-coefficient, all-cause stroke-LDL-C level (per mmol/L) | 0.82 | 0.75-0.90 | 20 |
We derived side effect rates for incident myopathy, hemorrhagic (not ischemic) stroke, and diabetes due to statins from systematic reviews of statin trials.9,29,41 We based assumptions about patient monitoring requirements, and for the consequences of myopathy, hemorrhagic stroke, and diabetes on both clinical judgment and consensus disutility weights (Table 1).35,42,43 We presumed an additional penalty of 0.0001 QALYs per person-year to account for additional unforeseen toxicities (Table 1). We derived the costs of hospitalization for hemorrhagic stroke, laboratory testing, diabetes, and physician fees from Center for Medicare and Medicaid Services (CMS), the Medical Expenditure Panel Survey (MEPS), and hospital discharge data, among other sources.30,44,45,46
Given debate about whether randomized controlled trials under-report the toxicity associated with long-term statin use, we performed Monte Carlo probabilistic sensitivity analyses increasing the penalty from unforeseen toxicities to as high as 0.001 per year. Given the evidence of a decrement in quality of life associated with daily pill use,12 we concurrently performed probabilistic sensitivity analyses with a pill burden decrement of up to 0.00384 QALYs lost per year: the equivalent of losing 2 weeks of perfect health over one decade, a value derived from patient interviews.12,47 We did not assign a QALY penalty for statin use in base analyses. We assumed 100% uptake of therapy, with adherence rates comparable to those observed in intention to treat trials.3
Comparing Lipid-Lowering Strategies
We modeled four scenarios for primary prevention. In each scenario, we assumed universal use of high-intensity statins among those with CVD or diabetes. For persons without CVD or diabetes, we modeled:
Base case (status quo): current use of statin treatment among adults 35-94 years old, per NHANES 2007-2010.19
ATP III: statin treatment for the base-case population and for all men 45-74 and women 55-74 eligible by the ATP III algorithm (Table 2).
ACC-AHA: statin treatment for the base-case population and all men 45-74 and women 55-74 eligible by the ACC-AHA algorithm (assuming that all clinician-patient discussions in ASCVD-free persons with LDL <190 mg/dL and without diabetes result in a decision to treat).
Universal statin use based on age and sex criteria: statin treatment for the base-case population; all men 45-74 and women 55-74 eligible by the ACC-AHA algorithm, and all other men 45-74 and women 55-74.
Table 2. Intervention Target Groups for Primary Prevention.
| ATP III Criteria | ACC-AHA Criteria | Age/Sex-based Criteria |
|---|---|---|
| All men 45-74 and women 55-74 in the United States without CAD or diabetes who meet any of the following criteria: | All men 45-74 and women 55-74 in the United States without CAD or diabetes who are not on a statin and who meet any of the following criteria: | All men 45-74 and women 55-74 in the United States without CAD or diabetes who are not on a statin are started on one. |
| --Have LDL-C over 130, 2 or more CHD risk factors7, and a Framingham 10-year CHD risk over 10% | --Have LDL-C over 190, regardless of other risk factors | |
| --Have LDL-C over 160, 2 or more CHD risk factors7, and a Framingham 10-year CHD risk under 10% | --Have LDL-C under 190 but have an AHA 10-year CHD risk over 7.5%8 | |
| --Have LDL-C over 190 and <2 CHD risk factors |
Costs of intervention included the cost of the medication, one follow-up physician visit, and one liver panel per 10 years, and one annual lipid panel check in the ATP III and ACC-AHA scenarios. We also included toxicity costs and decrements as calculated above. A January 2014 US Food and Drug Administration Consumer Health Information Bulletin indicated that routine monitoring of liver enzymes among statin users is no longer necessary after an initial screen;48 however, the ACC-AHA guideline, like the ATP III guideline, recommends annual LDL-C testing to gauge adherence and response to therapy, despite its shift to a risk-based statin prescribing threshold.9 Therefore, we presumed only one liver panel over 10 years in all scenarios, and presumed annual LDL-C monitoring in both the ATP III and ACC-AHA scenarios (but not the universal age/sex-based scenario).
In the ATP III analyses, we modeled high-dose statin treatment in all persons with a 10-year Framingham CHD (not CVD) risk of 20% or more. If such persons had risk ≥20% despite a statin, we assumed prior moderate-dose statin use, and modeled switching to high-dose by modeling the incremental benefit and cost of switching from moderate- to high-dose statin. We modeled treating all persons with a calculated 10-year risk under 20% and no CHD or diabetes with a moderate-dose statin to decrease LDL-C below target (130 or 160 mg/dL).
In the ACC-AHA analyses, we modeled treating all persons with LDL-C of 190 mg/dL or above and not currently taking a statin with a high-dose statin (55% decrease in LDL-C), regardless of other risk factors.9 For persons with LDL-C under 190 mg/dL, we modeled treatment with a moderate dose statin among those with a 10-year CVD risk of ≥7.5%, calculated using the ACC-AHA Pooled Cohort Risk Equation.9,49
In the universal age/sex-based analysis, we modeled moderate-intensity or high-intensity statin use according to ACC-AHA criteria as above, and moderate-intensity for all other 45-74 year old men and 55-74 year old women, regardless of LDL-C level, lifestyle risk factors, or other exclusion criteria.
All interventions were modeled on a 10-year horizon in men 45-74 years of age and women 55-74 years of age. Women under age 55 were excluded due to the possible teratogenicity of statins50 and their relatively low risk of CVD. Men 35-45 were excluded due to the ACC/AHA's application of its 10-year risk guideline only to persons 40 and over.9 Persons 75 and over were excluded due to limited data on primary prevention in this group and the suggestion that their statin treatment guidelines should be individualized.9,22,51
Statistical Analyses
For each intervention, we calculated the difference in total persons started on statins, costs, incident CVD events, deaths, and QALYs between the baseline scenario of status quo statin treatment and the intervention scenario (e.g., complete adherence to ACC-AHA guidelines) over 10 years. We calculated the incremental cost-effectiveness ratio (ICER) as the quotient of the difference in total costs (numerator) over the difference in total QALYs (denominator). We also calculated the ICER relative to the next most extensive intervention. Lastly, we calculated the number needed to treat (NNT) for each scenario as the number of persons needing to undergo 10 years of treatment to save one QALY.
We performed deterministic analyses with single-variable changes, as well as probabilistic sensitivity analyses, in which multiple key inputs were repeatedly varied. To isolate the impact of unforeseen statin toxicities, we repeated the analysis with an additional penalty equivalent to 0.001 QALYs lost per person-year of statin use. To isolate the impact of differing treatment thresholds on the efficacy of the ACC-AHA strategy relative to ATP III, we repeated ACC-AHA analyses with the 10-year ACC-AHA ASCVD risk threshold for treatment adjusted from 7.5% to 5%, 10% (the threshold also employed by the European Society for Cardiology's separate calculator for first fatal ASCVD event),16 15%, and 20%.
We performed Monte Carlo probabilistic sensitivity analyses in which we varied the cost of statins, incidence and severity of statin toxicities, disutility associated with daily pill use, statin-related medical costs, and impact of statins on LDL-C (Table 1). We also varied the modeled relationship (beta coefficient) for the effect of LDL-C reduction on CHD incidence and the effect of statins on incident diabetes. These variations assumed a normal or logarithmic distribution derived from empiric data. For example, we varied the cost of moderate- and high-dose statins from $10.57 and $31.71 to $438 and $1217 per person-year respectively, based on data from Veterans Affairs wholesale prices and Consumer Reports non-generic drug price data.52,53,54 In the Monte Carlo simulations, we generated 95% uncertainty intervals (95% UIs) around our primary outcome measures for each intervention scenario. The 95% confidence intervals corresponding to the effect of each of these variations upon statin cost, toxicity, and other inputs are displayed in Table 1- for example, over 1,000 simulations, the total cost of annual statin use varied from $30.11 to $286.90, and the harms associated with statin use – from both medication toxicity and pill burden – varied from roughly 0.00003 to 0.005 QALYs per annum (Table 1). There were 1000 random draws from a standard normal distribution, scaled to the mean and confidence interval, for each varied parameter. The Monte Carlo program, written in Python, generates a new set of input parameters drawn from the distributions for each iteration, runs the given iteration baseline and intervention simulations with the new parameters, and stores the outcomes for each. The 95% uncertainty intervals for each outcome are then calculated using Microsoft Excel 2010. The work was approved by the Institutional Review Board of the University of California, San Francisco. Because all data were de-identified, informed consent was not required.
Results
In the baseline status quo scenario, we estimate that 13.9 million men age 45-75 and women age 55-75 without CVD or diabetes would be on statins in 2016 (22% of all in these groups). Full implementation of the ATP III guideline in this group would require de novo or intensified statin use among 8.8 million people (7.6 million started on statins de novo; 1.2 million with prior dose intensified), increasing the total treated by 23% to 22.7 million (Table 3). Alternatively, full implementation of the ACC-AHA criteria, assuming all clinician-patient risk discussions result in statin treatment, would raise the prevalence of statin use in this population to 55%, treating 12.3 million more than the maximum under ATP III (Table 3). Treatment of all men 45-74 and women 55-74 years would add 49.9 million statin users compared with the status quo, or 28.9 million relative to the ACC-AHA guideline. Neither the ACC-AHA nor the age/gender-based strategy intensified statin therapy among any persons already receiving statins in the base case.
Table 3. Comparative Cost-Effectiveness of Statin Use for Primary Prevention of Coronary Heart Disease (2016-2025), Relative to Status Quo.
| ATP III | ACC-AHA | Age/Sex-Based | |
|---|---|---|---|
| Total Additional Persons Acted On* | 8.76 m† | 21.0 m | 49.9 m |
| Total Cost | -$10.7 b | -14.6 b | -$26.0 b |
| Treatment costs ‡ | $8.29 b | $21.6 b | $35.0 b |
| Screening costs§ | $2.65 b | $6.36 b | $4.65 b |
| Savings from events averted | -$19.0 b | -$36.1 b | -$61.1 b |
| Incident CHD Events Averted | 341,000 | 578,000 | 999,000 |
| QALYs Saved | 253,000 | 436,000 | 705,000 |
| Lives Saved | 42,300 | 86,000 | 135,000 |
| Total Cost per QALY (ICER) | Cost-saving | Cost-saving | Cost-saving |
| NNT per QALY | 35 | 49 | 71 |
| NNT per Life Saved | 205 | 245 | 370 |
Total persons are relative to the status quo scenario.
7.6 million started on statins de novo; 1.2 with prior dose intensified.
All costs of treatment (including medication costs).
The cost of adverse outcomes; lipid and liver function tests; and physician visits associated with statin use.
The treatment costs associated with each scenario rise roughly in proportion to the number of persons treated. The total costs associated with each scenario, which incorporate costs from medication prices and toxicity as well as savings from CVD events averted and lower costs associated with chronic CVD, were negative in each of the three scenarios (including universal use in the age-sex cohort) relative to the status quo, indicating that each scenario was cost-saving for CVD compared with current treatment. Full adherence to ACC-AHA resulted in the greatest treatment cost of the three scenarios - $6.36 billion in screening costs (e.g. liver and lipid panel testing), including approximately $4.40 billion attributable to the cost of annual lipid panel testing not included in the age/sex universal scenario (Table 1, Table 3), and the remainder from physician visits, liver panels, and adverse outcomes.
The number of QALYs and lives saved rose as the number of persons on statins increased. However, increasingly broad criteria for statin use targeted ever-larger proportions of individuals with low CVD risk, such that the efficiency of additional statin use declined in terms of the NNT per additional QALY gained (Figure 1). The ACC-AHA guideline treats 2.4-fold more persons as ATP III, but would save 1.7-fold additional QALYs (Table 3). An age/sex-based guideline would increase the number of people treated by 2.4-fold compared to ACC-AHA, but would increase QALYs by a factor of 1.6 (Table 3). In both women and men, all strategies were cost-saving relative to the next-broadest strategy, but the NNT increases as the guideline broadened (35 for ATP III, 68 for ACC-AHA, and 108 for age/sex-based treatment).
Figure 1.
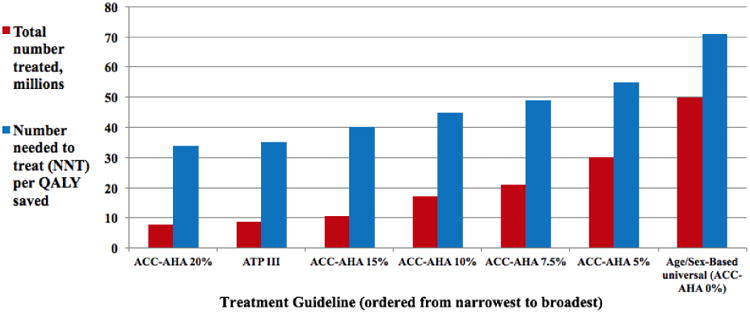
Comparative Treatment Coverage and Efficacy: ATP III, Age/sex based, and selected ACC-AHA scenarios for statin use (relative to status quo).
The impact of a broadening statin guideline differed between women and men (Table 4). All statin strategies were cost-saving for men relative to the next-broadest strategy, such that ACC-AHA dominated ATP III and the age/sex-based strategy dominated ACC-AHA in turn. However, in women the ACC-AHA strategy was not cost-saving relative to the ATP III strategy: its marginal cost per QALY was $3,400. The benefit of each strategy relative to the status quo and relative to the next-broadest strategy was substantially greater in men, overall and across all age subgroups (Table 4; Supplemental Tables 1; Supplemental Table 2). The marginal number needed to treat to save one QALY under the ACC-AHA strategy (relative to the ATP III strategy), for example, was 36 in men ages 65-74 but 69 in women ages 65-74. For the age/sex-based scenario, relative to ACC-AHA, the marginal NNT in men ages 55-64 was 73, but for women in the same age decile it rose to 156 (Supplemental Table 2). In all cases, older persons and males derived greater net benefit than younger persons and females.
Table 4. Comparative Cost-Effectiveness of Statin Use for Primary Prevention of Coronary Heart Disease Relative to Next-Broadest Strategy.
| ATP III | ACC-AHA | Age/Sex-Based | |
|---|---|---|---|
| Total Additional Persons Acted On | 8.76 m* | 12.3 m | 28.8 m |
| Total Cost | -$10.7 b | -$3.85 b | -$11.5 b |
| Treatment costs | $8.29 b | $13.3 b | $13.4 b |
| Screening costs | $2.65 b | $3.71 b | $2.69 b |
| Savings from events averted | -$19.0 b | -$17.2 b | -$24.9 b |
| Incident CHD Events Averted | 341,000 | 236,000 | 421,000 |
| QALYs Saved | 253,000 | 183,000 | 269,000 |
| Lives Saved | 42,300 | 43,100 | 49,000 |
| Total Cost per QALY (ICER) | Cost-saving | Cost-saving | Cost-saving |
| NNT per QALY | 35 | 68 | 108 |
| NNT per Life Saved | 205 | 285 | 589 |
7.6 million started on statins de novo; 1.2 with prior dose intensified.
Sensitivity Analyses
Results were sensitive to variations in the costs of statins; the extent of toxicity; and the disutility of taking a pill daily. However, results were robust to the change in treatment threshold under the ACC-AHA guideline based on 10-year cardiovascular risk, from 7.5% by default (Table 2) to other thresholds between 5% and 20% (Figure 1). Shifting the risk threshold for treatment under ACC-AHA from 7.5% to 5% would treat 8.6 million more people and save 21,600 more lives; this shift would change NNT per life saved, relative to the status quo, from 49 to 55 (Figure 1). Conversely, raising the ACC-AHA treatment threshold to 10% would treat 3.9 million fewer people and save 10,400 fewer lives, but lower the NNT to 45 from 49. At a threshold of 15%, the number treated under ACC-AHA drops by 10.5 million, but the NNT drops to 40, a result nearly on par with ATP III (Figure 1); at 20%, ACC-AHA treats 1.1 million fewer people than ATP III, but at a lower NNT of 34. Adding an overall QALY penalty of 0.001 per year to each scenario increased the marginal NNT for the three strategies from 35, 68, and 108 to 45, 217, and 559 for ATP III, ACC-AHA, and age/sex strategies respectively.
Probabilistic analyses demonstrated consistent results across a variety of cost and toxicity thresholds (Figure 2). To isolate the impact of pill burden on our results, we repeated these probabilistic analyses presuming zero pill burden (but preserving statin harms associated with medication toxicity). Figure 2 displays the impact of each of the 1000 simulations for each scenario on net QALYs saved (X axis) and net cost (Y axis) relative to the status quo. Without pill burden (top row), all of the simulations were cost-saving under ATP III; all but one under ACC-AHA, and all but one in the age/sex scenario; no simulations resulted in a negative NNT (indicating net harm) in any of the three scenarios. When pill burden was included in the simulations (bottom row), all of the simulations were cost-saving under ATP III, but a small number of simulations were no longer cost-saving under ACC-AHA and the age/sex-based scenario. Similarly, while none of the ATP III simulations resulted in net harm, five simulations in the ACC/AHA scenario and 34 in the age/sex-based scenario did result in net harm.
Figure 2. Monte Carlo Scatter Plots.
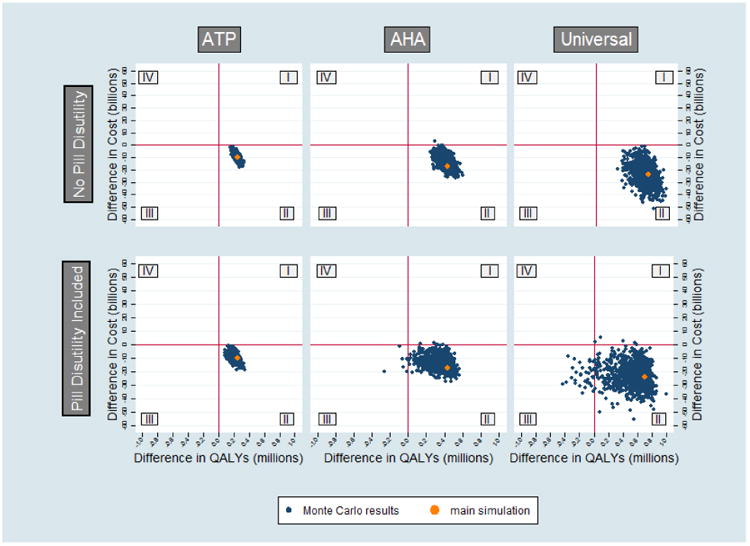
Points to the right of the Y axis (quadrants I and II) represent net benefit, and to the left of the Y axis (quadrants III and IV) represent net harm; points above the X axis (quadrants I and IV) represent a net positive cost, and points below the X axis (quadrants II and III) represent net cost-saving, such that points in the lower right quadrant (quadrant II) constitute net benefit with net cost-saving. Results presuming no pill burden are displayed in the top row and results presuming a nonzero pill burden are in the bottom row.
Discussion
We project that full adherence to the ACC-AHA guideline in the US among persons without CVD or diabetes, compared with their full adherence to ATP III, would avert thousands more CVD events and deaths over 10 years, while producing net cost savings for CVD in men and a cost-effective outcome ($3,400 per marginal QALY saved) in women. Full adherence to ATP III, ACC-AHA, and age/sex-based criteria are all beneficial and cost-saving compared with current rates of statin use for men and women combined. At a population level, this result is robust to large alterations of the 10-year risk threshold for treatment from 20% to 0% (treatment of all persons in this age-sex cohort). However, benefit is moderately sensitive to the estimated toxicity of statins, and highly sensitive to the variation in disutility associated with statin use. In other words, an individual patient's degree of benefit from long-term statin use depends strongly on their personal tolerance for pill burden and some on projected side effects – both factors more important than the patient's degree of cardiovascular risk.
We found that risk-based statin guidelines (such as ACC-AHA) and LDL-C-based strategies (such as ATP III) have nearly identical NNTs per QALY saved when directed at comparable sized populations of relatively equal baseline cardiovascular risk. However, because the incidence of statin toxicity is independent of cardiovascular risk, treatment of broader, lower-risk populations (for example, by lowering the ACC-AHA treatment threshold) may create scenarios in which the collective toxicity burden outweighs cardiovascular benefit. For example, when the ACC/AHA risk-based approach is applied at a 20% threshold, it has a similar NNT to ATP III relative to the status quo (34 versus 35) and treats a similar number of people (Figure 1). However, at its default threshold of 7.5% (per ACC-AHA guidelines), the ACC/AHA's threshold relative to the status quo rises to 49, and its marginal NNT to 68, as it treats 12.3 million more people. At a 5% treatment threshold, the ACC-AHA strategy's NNT rises to 55 relative to the status quo, with a marginal NNT of 72- as it treats 21.3 million persons more than ATP III. Similarly, inclusion of persons with CVD and diabetes would likely lower the NNT even further than applying a 20% risk threshold in this CVD/diabetes-free cohort. Egan et al recently reported a 10-year NNT of 18 for a similar ACC/AHA scenario including persons with ASCVD and diabetes, although this approach also presumed both more frequent use of high-intensity statins and a greater reduction in ASCVD risk per 1 mmol/L drop in LDL-C from statin treatment.11
Accounting for the known potential harms associated with statin use, plus adding an additional penalty to account for toxicities or harms yet to be identified, decreases their benefit, especially for the ACC/AHA and age/sex-based scenarios. However, all three strategies remain both beneficial (although with a high NNT) and cost-saving overall relative to the status quo. Assuming no pill burden, but varying incidence and severity of all known toxicities associated with statin use by 50 to 150% or more and the cost of statins by a factor of more than 20 also had minimal effect on all three strategies, with almost all of the 1000 Monte Carlo simulations for each strategy remaining efficacious and cost-saving (Figure 2, top row). This result suggests that even substantial variation in the estimated toxicity and price of statins produces little absolute difference in their overall toxicity and cost-effectiveness, because the absolute risk associated with these toxicities is low, and the cost-effectiveness of statins is substantive even at high-end prices.
However, accounting for even modest estimates of the potential pill burden associated with statins substantially diminishes statins' efficacy (Figure 2, bottom row). A pill disutility equivalent to two weeks' lost perfect health over a decade, a level identified by patient preference interviews,12,47 corresponds to an annual QALY penalty more than triple the high-end toxicity estimate in the probabilistic analyses above. When this penalty is added to these probabilistic analyses, full adherence to the ACC/AHA and age/sex-based strategies may result in net harm in the population relative to the status quo (Figure 2, bottom row, quadrants III and IV).
Although our results confirm that broad statin use for all persons, even those at low risk, can be cost-saving for CVD and result in net benefit, even a modest pill disutility may negate the benefit of statins in these individuals. Current estimates of pill disutility vary widely both between and within empiric studies12,47 likely reflecting real differences in medication disutility among specific targeted individuals.12 Given that the ACC/AHA guideline recommends a clinician-patient risk discussion prior to statin assignment our results suggest that physicians should also assess each patient's individual preference for daily pill use in making this patient-specific decision, particularly for those at lower risk.
Our study has several limitations. First, because the CVDPM is based in part on observational data, key assumptions (such as the impact of non-fatal cardiac events on later mortality) may be over- or under-estimates due to endogeneity or other factors. For example, the relationship between non-fatal cardiac events and later death may be confounded by lifestyle factors more prevalent in those who experience these events (e.g., dietary patterns), such that the measured relationship is an overestimate. Second, based on the ACC-AHA guidelines and substantial prior data,3 we modeled a constant percent LDL-C reduction for a given intensity of statin, and an age-independent effect of LDL-C-lowering on CVD events and mortality. Although randomized trials suggest a relatively constant level of benefit from statins,3,9 the existence of this benefit has not been directly proven in all low-risk groups,14,55 and the size of this benefit, when present, is uncertain3 and may vary across the cohorts we studied. For example, because the ACC/AHA guideline treats more older persons than ATP III at all thresholds, our finding of equivalent efficacy at comparable thresholds may not apply if statins' benefit attenuates with age within the study population (men 45-74 and women 55-74), as estimated by observational studies such as the Framingham Heart Study.20,56 Also, we assigned cohorts to treatment under the ACC/AHA risk threshold based on their status at the start of the 10-year simulation, but we did not change this status over that time; as a result, we may have underestimated treatment eligibility under the ACC/AHA guideline. Thirdly, our model incorporates CHD and stroke as elements of cardiovascular disease (CVD), but not peripheral artery disease, a third component of atherosclerotic cardiovascular disease (ASCVD) as defined by the ACC/AHA guideline. Fourthly, we assumed that all doctor-patient discussions of statin eligibility under ACC/AHA guidelines led to a decision to start a statin and further assumed 100% adherence, thereby likely overestimating the number of persons on statins in this scenario. Lastly, the CVDPM has several intrinsic limitations. It does not incorporate race expressly as a risk factor for ASCVD; nor does it incorporate certain non-traditional ASCVD risk factors, such as end-stage renal disease (ESRD); nor does it differentiate between ischemic stroke (against which statins are protective) and hemorrhagic stroke (for which statins increase risk); nor is it applicable in persons under 35. However, we incorporated race as a factor in the identification of populations eligible for statins via ACC-AHA's 7.5% 10-year ASCVD risk threshold,9,49 so that the ACC-AHA simulation reflects the impact of tailored therapy by race. We also added hemorrhagic stroke risk as a statin toxicity, partially offsetting statins' protective effect against all-cause (chiefly ischemic) stroke.9,20
We did not model toxicities attributed to statin use for which evidence is sparse or inconsistent, such as development of cataracts.57,58 However, in a scenario analysis, we included an additional QALY penalty of 0.001 (equivalent to the maximal disutility associated with all known statin toxicities, Table 1). In a separate analysis, we also included a QALY penalty of up to 0.00384 for pill disutility. The insight that emerges from these analyses is that at an individual level, the driver of benefit is not of the magnitude of statins' known and unknown medication toxicities but patient preference for taking a pill; a modest pill burden effect would have a greater effect on net QALYs compared with all known statin toxicities.
We project that full adherence to the ACC-AHA statin guideline for men 45-74 and women 55-74 would save thousands more lives over the decade than full adherence to ATP III or the status quo, with net cost savings at the population level. We also project that ACC-AHA risk-based guideline is substantively less efficient than ATP III's LDL-C-based approach at its current risk threshold, but the two approaches have similar efficiency when treating comparably high-risk pools. Importantly, statins remain cost-saving and efficacious in all three scenarios even after significant increases in medication toxicity – but a modest pill burden attributable to statins cause their harms to outweigh their benefit in some the ACC/AHA and age/sex-based scenarios, highlighting the sensitivity of our findings to individual patient preferences. More studies are therefore necessary not only to understand the direct harms from statins, but to gauge the true size of this pill burden and its degree of variation across individuals. Our analyses suggest that in populations at low risk of harms from statin use and unburdened by chronic pill use, broader use of statins would both avert substantive cardiovascular morbidity and prove cost-saving, even when baseline cardiovascular risk is low.
Supplementary Material
Table 5. Cost-Effectiveness relative to Next Broadest Strategy, by Sex.
| Males | ATP III | ACC-AHA | Age/Sex-Based |
|---|---|---|---|
| Total Additional Persons Acted On | 6.11 m | 7.37 m | 15.9 m |
| Total Cost | -$7.75 b | -$4.07 b | -$8.02 b |
| Treatment costs | $5.85 b | $7.33 b | $6.87 b |
| Screening costs | $1.85 b | $2.23 b | $1.48 b |
| Savings from events averted | -$13.6 b | -$11.4 b | -$14.9 b |
| Incident CHD Events Averted | 249,000 | 162,000 | 263,000 |
| QALYs Saved | 178,000 | 124,600 | 158,000 |
| Lives Saved | 42,900 | 28,400 | 27,700 |
| Total Cost per QALY (ICER) | Cost-saving | Cost-saving | Cost-saving |
| NNT per QALY | 35 | 60 | 101 |
| NNT per Life Saved | 204 | 260 | 574 |
|
| |||
| Females | ATP III | ACC-AHA | Age/Sex-Based |
|
| |||
| Total Additional Persons Acted On | 2.65 m | 4.91 m | 13.0 m |
| Total Cost | -$2.97 b | $219 m | -$3.43 b |
| Treatment costs | $2.44 b | $5.98 b | $6.58 b |
| Screening costs | $800 m | $1.48 b | $1.21 b |
| Savings from events averted | -$5.41 b | -$5.76 b | -$10.0 b |
| Incident CHD Events Averted | 92,100 | 74,500 | 158,000 |
| QALYs Saved | 74,900 | 58,600 | 110,000 |
| Lives Saved | 12,800 | 14,700 | 21,400 |
| Total Cost per QALY (ICER) | Cost-saving | $3,400 | Cost-saving |
| NNT per QALY | 36 | 84 | 118 |
| NNT per Life Saved | 206 | 334 | 609 |
Clinical Perspective.
What is new?
We compared three approaches to prescribing statins for primary prevention using simulation modeling and found all three (ATP III, ACC-AHA, and broader use of statins regardless of cardiovascular risk) to be effective and cost saving.
This result is highly sensitive to the perceived burden associated taking a daily medication.
What are the clinical implications?
Clinicians should offer statins for primary prevention of CVD events and associated mortality – both according to the current ACC/AHA guideline, and potentially for select patients outside this guideline.
A patient's preference for taking a daily pill is an important factor in assessing whether statin use results in net benefit.
Acknowledgments
The authors gratefully acknowledge the help of many colleagues at the Center for Vulnerable Populations at the University of California, San Francisco (CVP-UCSF), including Eugene Fairley, David Guzman, Antoinette Mason, and Lina Tieu among others.
Funding Sources: Dr. Heller's support comes from a T32 grant and John F. Fogarty grant award from the National Institutes of Health, the latter through the National Heart, Lung, and Blood Institute (NHLBI). Support for the Cardiovascular Disease Policy Model (CVDPM) comes from the Hypertension Guidelines R01 Award (R01HL107475-01), a K24 Award (K24DK103992) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), a K01 award (K01AG039387) from the National Institute on Aging (NIA), and also from NHLBI.
Footnotes
Disclosures: Kirsten Bibbins-Domingo is the Immediate Past Chair of the US Preventive Services Task Force. The ideas expressed in this manuscript are those of the authors and not official positions of the US Preventive Services Task Force.
References
- 1.Global Burden of Disease. World Health Organization; 2004. [Accessed on June 22, 2017]. Projections of mortality and burden of disease 2002–2030. Available from http://www.who.int/topics/global_burden_of_disease/en/ [Google Scholar]
- 2.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 3.Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–90. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engstrom A, Jacob J, Lundin D. Sharp drop in prices after the introduction of generic substitution. Swedish Pharmaceutical Benefits Board (LFN) for the World Health Organization Collaborating Centre for Pharmaceutical Pricing and Reimbursement Policy's Information Network (PPRI); Solna: 2006. Available from http://whocc.goeg.at/ [Google Scholar]
- 5.Chalasani N, Aljadhey H, Kesterson J, Murray M, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126:1287–92. doi: 10.1053/j.gastro.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Graham DJ, Staffa JA, Shatin D, Andrade SE, Schech SD, La Grenade L, Gurwitz JH, Chan KA, Goodman MJ, Platt R. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292:2585–2590. doi: 10.1001/jama.292.21.2585. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, Pais P, López-Jaramillo P, Leiter LA, Dans A, Avezum A. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374:2021–31. doi: 10.1056/NEJMoa1600176. [DOI] [PubMed] [Google Scholar]
- 8.Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 9.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the american college of cardiology/american heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Ioannidis JP. More than a billion people taking statins?: Potential implications of the new cardiovascular guidelines. JAMA. 2014;311:463–464. doi: 10.1001/jama.2013.284657. [DOI] [PubMed] [Google Scholar]
- 11.Egan BM, Li J, White K, Fleming DO, Connell K, Hernandez GT, Jones DW, Ferdinand KC, Sinopoli A. 2013 ACC/AHA Cholesterol Guideline and Implications for Healthy People 2020 Cardiovascular Disease Prevention Goals. J Am Heart Assoc. 2016;5:e003558. doi: 10.1161/JAHA.116.003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana M, Asaria P, Moraldo M, Finegold J, Hassanally K, Manisty CH, Francis DP. Patient-Accessible Tool for Shared Decision Making in Cardiovascular Primary Prevention: Balancing Longevity Benefits against Medication Disutility. Circulation. 2014;129:2539–2546. doi: 10.1161/CIRCULATIONAHA.113.007595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazar LD, Pletcher MJ, Coxson PG, Bibbins-Domingo K, Goldman L. Cost-effectiveness of statin therapy for primary prevention in a low-cost statin era. Circulation. 2011;124:146–153. doi: 10.1161/CIRCULATIONAHA.110.986349. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Wilson PW. A trial-based approach to statin guidelines. JAMA. 2013;310:1123–1124. doi: 10.1001/jama.2013.276529. [DOI] [PubMed] [Google Scholar]
- 15.Ray KK, Kastelein JJ, Boekholdt SM, Nicholls SJ, Khaw KT, Ballantyne CM, Catapano AL, Reiner Ž, Lüscher TF. The ACC/AHA 2013 guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: the good the bad and the uncertain: a comparison with ESC/EAS guidelines for the management of dyslipidaemias 2011. Eur Heart J. 2014;35:960–968. doi: 10.1093/eurheartj/ehu107. [DOI] [PubMed] [Google Scholar]
- 16.Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund I, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D European Association for Cardiovascular Prevention and Rehabilitation; ESC Committee for Practice Guidelines 2008-2010 and 2010-2012 Committees. ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 17.Pandya A, Sy S, Cho S, Weinstein MC, Gaziano TA. Cost-effectiveness of 10-Year Risk Thresholds for Initiation of Statin Therapy for Primary Prevention of Cardiovascular Disease. JAMA. 2015;314:142–150. doi: 10.1001/jama.2015.6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinstein MC, Coxson PG, Williams LW, Pass TM, Stason WB, Goldman L. Forecasting coronary heart disease incidence, mortality, and cost: the Coronary Heart Disease Policy Model. Am J Public Health. 1987;77:1417–26. doi: 10.2105/ajph.77.11.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Hyattsville, MD: 2012. [Accessed on June 22, 2017]. National Health and Nutrition Examination Survey. Available from: http://www.cdc.gov/nchs/data/databriefs/db103.htm. [Google Scholar]
- 20.Biologic Specimen and Data Repository Information Coordinating Center: National Heart, Lung, and Blood Institute; 2015. [Accessed on June 22, 2017]. Framingham Heart Study-Cohort (FHS-Cohort) data request site. Available from http://biolincc.nhlbi.nih.gov/studies/framcohort/?q=framingham. [Google Scholar]
- 21.West of Scotland Coronary Prevention Study Group. Influence of pravastatin and plasma lipids on clinical events in the West of Scotland Coronary Prevention Study (WOSCOPS) Circulation. 1998;97:1440–5. doi: 10.1161/01.cir.97.15.1440. [DOI] [PubMed] [Google Scholar]
- 22.Odden MC, Pletcher MJ, Coxson PG, Thekkethala D, Guzman D, Heller D, Goldman L, Bibbins-Domingo K. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med. 2015;162:533–41. doi: 10.7326/M14-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pletcher MJ, Lazar L, Bibbins-Domingo K, Moran A, Rodondi N, Coxson P, Lightwood J, Williams L, Goldman L. Comparing impact and cost-effectiveness of primary prevention strategies for lipid-lowering. Ann Internal Med. 2009;150:243–54. doi: 10.7326/0003-4819-150-4-200902170-00005. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Census Bureau. Washington, DC: 2013. U.S. interim projections by Age, Sex, Race, and Hispanic Origin. Available from https://www.census.gov/programs-surveys/popproj.html. [Google Scholar]
- 25.Center for Health Statistics. National Health Interview Survey 2009-2011. Available from ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Datasets/NHIS/
- 26.Centers for Disease Control and Prevention. National Center for Health Statistics. Underlying Cause of Death 1999-2010. [Accessed on June 22, 2017];CDC WONDER online database. 2012 Available from http://wonder.cdc.gov/ucd-icd10.html.
- 27.World Health Organization. Geneva: 2010. [Accessed on June 22, 2017]. International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) Available from: http://www.who.int/classifications/icd/ICD10Volume2_en_2010.pdf?ua=1. [Google Scholar]
- 28.Centers for Disease Control and Prevention. Hyattsville, MD: [Accessed on June 22, 2017]. National Hospital Discharge Survey. Available from: www.cdc.gov/nchs/about/major/hdasd/nhds.htm. [Google Scholar]
- 29.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Medicare & Medicaid Services. Baltimore: [Accessed on June 22, 2017]. National health expenditures by type of service and source of funds, CY 1960-2012. Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html. [Google Scholar]
- 31.California Office of Statewide Health Planning and Development. Public Version A-24 [CD-ROM] Sacramento: 2001. California patient discharge data, 2000. [Google Scholar]
- 32.California Office of Statewide Health Planning and Development. Hospital financial data for cost to charge ratio, inpatient discharge data hospital annual financial data, pivot profiles, 1999-2000. Sacramento: 2008. [Google Scholar]
- 33.U.S. Census Bureau. Average cost to community hospitals per patient, by state (Table 204) Washington, DC: Government Printing Office; 1998. Statistical Abstract of the United States. [Google Scholar]
- 34.Bureau of Labor Statistics. Washington, DC: 2008. [Accessed on June 22, 2017]. Consumer Price Index. Available from: http://data.bls.gov/cgi-bin/cpicalc.pl. [Google Scholar]
- 35.Institute for Health Metrics and Evaluation. Global burden of disease study 2010 Disability Weights. [Accessed on June 22, 2017]; Available from: http://ghdx.healthdata.org/record/global-burden-disease-study-2010-gbd-2010-disability-weights.
- 36.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 37.Kendrach MG, Kelly-Freeman M. Approximate equivalent rosuvastatin doses for temporary statin interchange programs. Ann Pharmacother. 2004;38:1286–92. doi: 10.1345/aph.1D391. [DOI] [PubMed] [Google Scholar]
- 38.Walmart formulary. [Accessed on June 22, 2017]; Available from http://i.walmart.com/i/if/hmp/fusion/genericdruglist.pdf.
- 39.Target formulary. [Accessed on June 22, 2017]; Available from https://tgtfiles.target.com/pharmacy/WCMP02-032536_RxGenericsList_NM10.pdf.
- 40.Costco formulary. [Accessed on June 22, 2017]; Available from https://www.costco.com/pharmacy/drug-results-details-price?storeId=10301&catalogId=10701&langId=-1&drugId=672&drugName=Atorvastatin&searchAlphabet=A&isPharmacy=true&encodedDrugName=Atorvastatin&drugSearch=alphaDrugSearch.
- 41.Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, Ward K, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:Cd004816. doi: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) National Diabetes Information Clearinghouse. Diabetic neuropathies: the nerve damage of diabetes. [Accessed on June 22, 2017]; Available from http://diabetes.niddk.nih.gov/dm/pubs/neuropathies/
- 43.The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) National Diabetes Information Clearinghouse. Prevent diabetes problems: keep your keep healthy. [Accessed on June 22, 2017]; Available from http://diabetes.niddk.nih.gov/dm/pubs/complications_feet/
- 44.Center for Medicare and Medicaid Services Fee Schedule Search. [Accessed on June 22, 2017]; Available from https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/FeeScheduleGenInfo/index.html.
- 45.Center for Medicare and Medicaid Services Physician fee schedule search. [Accessed on June 22, 2017]; Available from: https://www.cms.gov/apps/physician-fee-schedule/overview.aspx.
- 46.American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pletcher MJ, Tice JA, Pignone M, Browner WS. Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta-analysis. Arch Intern Med. 2004;164:1285–92. doi: 10.1001/archinte.164.12.1285. [DOI] [PubMed] [Google Scholar]
- 48.United States Food and Drug Administration. Consumer Update, January 2014. [Accessed on June 22, 2017]; Available from: http://www.consumermedsafety.org/latest-fda-medication-alerts/special-alerts/item/532-fda-expands-advice-on-statin-risks.
- 49.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Sorlie P, Stone NJ, Wilson PWF. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 50.Kazmin A, Garcia-Bournissen F, Koren G. Risks of Statin Use During Pregnancy: A Systematic Review. J Obstet Gynaecol Can. 2007;29:906–908. doi: 10.1016/S1701-2163(16)32656-1. [DOI] [PubMed] [Google Scholar]
- 51.Savarese G, Gotto AM, Paolillo S, D'Amore C, Losco T, Musella F, Scala O, Marciano C, Ruggiero D, Marsico F, De Luca G, Trimarco B, Perrone-Filardi P. Benefits of Statins in Elderly Subjects without Established Cardiovascular Disease: a Meta-Analysis. J Am Coll Cardiol. 2013;62:2090–2099. doi: 10.1016/j.jacc.2013.07.069. [DOI] [PubMed] [Google Scholar]
- 52.United States Department of Veterans Affairs. Pharmacy benefits management services. [Accessed on June 22, 2017]; Available from http://www.pbm.va.gov/PBM/PharmaceuticalPrices.asp.
- 53.Consumer Reports Health: Best Buy Drugs. Evaluating Statin Drugs to Treat High Cholesterol and Heart Disease: Comparing Effectiveness, Safety, and Price. [Accessed on June 22, 2017];Consumer Reports. 2012 Apr; Available from http://consumerhealthchoices.org/wp-content/uploads/2012/08/BBD-Statins-Full.pdf.
- 54.Consumer Reports Health: Best Buy Drugs. Statin Drugs to Treat High Cholesterol and Heart Disease: Comparing Effectiveness, Safety, and Price. [Accessed on June 22, 2017];Consumer Reports. 2014 Mar; Available from http://www.consumerreports.org/health/resources/pdf/best-buy-drugs/StatinsUpdate-FINAL.pdf.
- 55.Stone NJ. Preventing Atherosclerotic Cardiovascular Disease Using American College of Cardiology and American Heart Association Prevention Guidelines: Some Good News, But Caveats Remain. J Am Heart Assoc. 2016;5:e004197. doi: 10.1161/JAHA.116.004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Framingham Heart Study. [Accessed on June 22, 2017]; Available from https://www.framinghamheartstudy.org/risk-functions/cardiovascular-disease/10-year-risk.php.
- 57.Strandberg TE, Tarkkanen A. Cataracts and statin use: cause and effect not confirmed. JAMA Ophthalmol. 2014;132:365. doi: 10.1001/jamaophthalmol.2014.23. [DOI] [PubMed] [Google Scholar]
- 58.Kostis JB, Dobrzynski JM. Prevention of cataracts by statins: a meta-analysis. Journal Cardiovasc Pharmacol Ther. 2014;19:191–200. doi: 10.1177/1074248413511690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


