Abstract
Stress is a major risk factor for psychiatric disorders, such as depression, posttraumatic stress disorder, and schizophrenia. Early life stress, such as maternal separation, can have long-term effects on the development of the central nervous system and pathogenesis of psychiatric disorders. In the present study, we found that maternal separation increased the susceptibility to stress in adolescent rats, increased the expression of Na+/K+/2Cl− cotransporter 1 (NKCC1) on postnatal day 14, and increased the expression of K+/2Cl− cotransporter 2 (KCC2) and γ-aminobutyric acid A (GABAA) receptor subunits on postnatal day 40 in the hippocampus. NKCC1 inhibition by the U.S. Food and Drug Administration-approved drug bumetanide during the first two postnatal weeks rescued the depressive- and anxiety-like behavior that was induced by maternal separation and decreased the expression of NKCC1, KCC2 and GABAA receptor α1 and β2,3 subunits in the hippocampus. Bumetanide treatment during early development did not adversely affect body weight or normal behaviors in naive rats, or affect serum osmolality in adult rats. These results suggest that bumetanide treatment during early development may prevent the maternal separation-induced susceptibility to stress and impairments in GABAergic transmission in the hippocampus.
Introduction
Stress refers to the effects of internal and external environmental factors that seriously threaten homeostasis1. Stressors have a major influence on mood, one’s sense of well-being, behavior, and health2. Individual responses to stress vary widely. Some individuals develop trauma-related mental illnesses, such as posttraumatic stress disorder and depression. Others develop mild to moderate psychological symptoms, whereas others do not present negative emotions3. Numerous studies suggest that the interaction between genetic factors and early-life environmental factors can affect individual epigenetic modifications, gene expression patterns, and brain development, which may lead to higher susceptibility to mental illness when exposed to environmental stress during adolescence or adulthood4–6. Therefore, it is considered that neuropsychiatric diseases are results of two (or more) stressors experienced over the lifespan, as stated in the Two/Multiple-Hit hypothesis7–10. Early life stress (ELS) is a major risk factor for mental health problems at all stages of life11. Previous studies have shown that ELS can lead to multiple behavioral abnormalities in adolescence and adulthood, such as depression and anxiety-like behavior12, learning and memory deficits13, and eating disorders14. Clinical and preclinical studies have found that ELS has a considerable influence on brain development, particularly hippocampal volume and synaptic plasticity15–17.
γ-Aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the central nervous system in adults. However, GABA can cause chloride ion (Cl−) efflux and excite immature neurons during early development18,19. The shift from excitatory to inhibitory neurotransmission mainly depends on a family of cation-chloride cotransporters (CCCs), particularly Na+/K+/2Cl− cotransporter 1 (NKCC1) and K+/2Cl− cotransporter 2 (KCC2). In immature neurons, the intracellular Cl− concentration ([Cl−]i) is mainly driven by NKCC1 while KCC2 expression is low. Therefore, neurons exhibit high [Cl−]i, causing Cl– efflux and postsynaptic depolarization when GABA acts on neurons. The greater expression of functional KCC2 during neuronal maturation results in lower [Cl−]i, causing Cl− influx when GABAA receptors are activated18. In the human neocortex, KCC2 expression begins to increase 40 weeks after birth, accompanied by a decrease in NKCC1 activity and expression, resulting in the conversion of GABA signals to hyperpolarization20. KCC2 is widely expressed in the central nervous system, specifically in neurons, and rarely in the peripheral nervous system or non-neuronal cell types21. NKCC1 is expressed in both neurons and glial cells in the central nervous system22.
Cation-chloride cotransporters play an important role in the development of synaptic formation and neuronal plasticity23,24. Derangements of CCCs have been implicated in the pathogenesis of seizures and neuropathic pain18 and also play a key role in many psychiatric disorders, including schizophrenia, autism, Down syndrome, and Fragile X syndrome25–28. The NKCC1 antagonist bumetanide is a U.S. Food and Drug Administration-approved loop diuretic that has proven to be useful for the treatment of epilepsy, autism, and schizophrenia29–31. Bumetanide treatment during early postnatal development could reverse neonatal exposure to anesthesia (sevoflurane)-induced long-term endocrine and neurobehavioral abnormalities32 and also rescue a genetic epilepsy in mice33, however, bumetanide had poor antiepileptic efficacy in the newborn babies with hypoxic ischemic encephalopathy (NEMO) trial, which was a Phase I/II trial that assessed the safety and optimal dose of bumetanide for the treatment of acute neonatal seizures34. This Phase I/II failure cannot necessarily be extrapolated to other types of seizures, and bumetanide might still be a therapeutic tool to attenuate several brain disorders35.
Although previous clinical and animal studies reported a role for CCCs in several neurological and psychiatric disorders, still unknown is whether CCCs modulate neural maladaptation after maternal separation (MS). In the present study, we used MS as the ELS and forced swimming to simulate low levels of stress during adolescence. We investigated the effects of MS on the expression of NKCC1 and KCC2 and explored whether bumetanide reverses the susceptibility to stress that is induced by MS and alterations of the GABAergic system in adolescent and adult rats.
Results
Maternal separation induced depressive- and anxiety-like behavior in adolescent rats
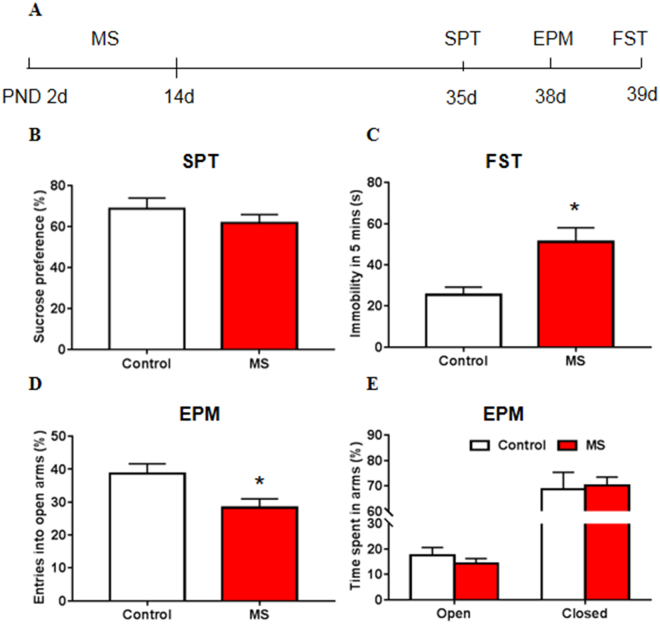
We first used different behavioral paradigms to detect whether depressive- and anxiety-like behaviors are observed in maternally separated adolescent rats. The statistical analysis of the behavioral data was performed using Student’s t-test. Maternal separation had no effect on sucrose preference in the sucrose preference test (SPT; t 31 = 1.068, p = 0.294; Fig. 1B) but significantly increased immobility time in the forced swim test (FST; t 28 = 3.244, p = 0.003; Fig. 1C). Maternal separation also led to anxiety-like behavior, reflected by significant change in the entries into the open arms (EPM; t 24 = 2.516, p = 0.019; Fig. 1D), with no significant change in the time spent in open arms (EPM; t 24 = 0.913, p = 0.370; Fig. 1E) or closed arms (EPM; t 24 = 0.229, p = 0.821; Fig. 1E). The body weight of rats showed no differences between maternal separation groups and control groups at P14 and P40 (p > 0.05; Fig. S2). The SPT and FST are well-established behavioral models of anhedonia and despair in rodents36,37. However, forced swimming during adolescence can also serve as a stressor and was additionally used to test the susceptibility to stress10. Altogether, these results suggest that maternal separation impaired emotional behavior in adolescent rats, reflected by depressive- and anxiety-like behavior and higher susceptibility to stress.
Figure 1.
Maternal separation led to depressive- and anxiety-like behavior in adolescent rats. (A) Experimental timeline of maternal separation (MS) and behavioral tests. (B) Sucrose preference in the sucrose preference test (SPT). (C) Immobility time in the forced swim test (FST). (D) Entries into open arms in the elevated plus maze (EPM). Entries into the open arms are shown as a percentage of all entries. (E) Time spent in arms in the EPM. Time spent in the open or closed arms is plotted as a percentage of the total observation time; time spent in the middle is not shown. The data are expressed as mean ± SEM. n = 16–17 per group (7–8 females, 8–10 males). *p < 0.05, compared with control group.
Maternal separation altered the expression of NKCC1, KCC2, and GABAA receptor subunits in the hippocampus
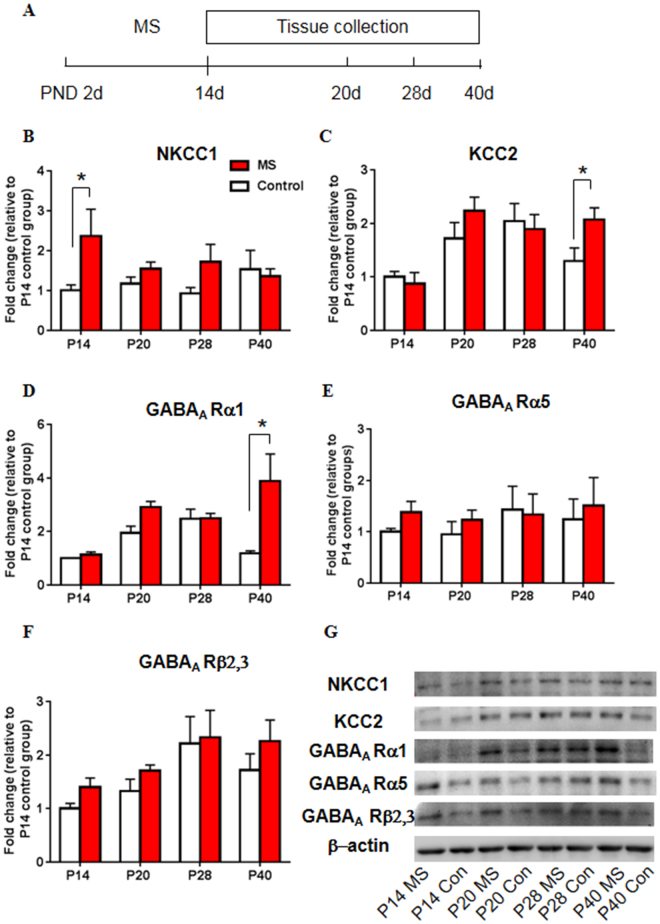
We next used Western blot to detect the expression of NKCC1 and KCC2 in maternally separated rats at different developmental stages (postnatal day 14 [P14], P20, P28, and P40). The two-way analysis of variance (ANOVA), with age (P14, P20, P28, P40) and stress (control and MS) as the between-subjects factors, followed by Tukey’s post hoc test, was used to analyze the Western blot data. A significant main effect of stress on the expression of NKCC1 was found (age: F 7,29 = 0.379, p = 0.769; stress: F 7,29 = 5.117, p = 0.031; age × stress: F 7,29 = 1.595, p = 0.212; Fig. 2B). The post hoc test revealed that MS significantly increased the expression of NKCC1 in the CA1 of the hippocampus on P14 (p = 0.015; Fig. 2B), with a tendency toward an increase in NKCC1 levels at other ages. The analysis revealed a significant main effect of age on the expression of KCC2 (age: F 7,31 = 6.986, p = 0.001; stress: F 7,31 = 1.985, p = 0.169; age × stress: F 7,31 = 1.676, p = 0.192; Fig. 2C). A significant difference in KCC2 expression was found between maternally separated rats and control rats on P40 (p = 0.036; Fig. 2C).
Figure 2.
Maternal separation altered the expression of NKCC1, KCC2, and GABAA receptor subunits in the hippocampus. (A) Experimental timeline of maternal separation and tissue collection. (B–F) NKCC1 (B), KCC2 (C), GABAA receptor α1 subunit (D), GABAA receptor α5 subunit (E), and GABAA receptor β2,3 subunit (F) levels in the CA1 area of the hippocampus on postnatal days 14, 20, 28, and 40. (G) Representative Western blots in the CA1. Full-length blots are presented in Supplementary Figure S4. n = 10 per group (4 females, 6 males). The data are expressed as mean ± SEM. *p < 0.05, compared with control group at corresponding ages.
Maternal separation also altered the expression of several GABAA receptor subunits. Significant main effects of age and stress on the expression of the GABAA receptor α1 subunit were found, with a significant age × stress interaction (age: F 7,27 = 4.010, p = 0.018; stress: F 7,27 = 8.420, p = 0.007; age × stress: F 7,27 = 3.780, p = 0.022; Fig. 2D). The post hoc test revealed that MS increased the levels of the GABAA receptor α1 subunit on P40 compared with the control group (p < 0.001; Fig. 2D). No significant main effects or interaction on the expression of the GABAA receptor α5 subunit were found (age: F 7,32 = 0.766, p = 0.522; stress: F 7,32 = 1.618, p = 0.213; age × stress: F 7,32 = 0.403, p = 0.752; Fig. 2E). A main effect of age on the expression of the GABAA receptor β2,3 subunit was found (age: F 7,31 = 3.998, p = 0.016; stress: F 7,31 = 2.287, p = 0.141; age × stress: F 7,31 = 0.143, p = 0.933; Fig. 2F). The post hoc test revealed no significant differences between the MS groups and control groups, but there was a tendency toward an increase in the levels of the GABAA receptor β2,3 subunit that was induced by MS. Additionally, we examined the levels of NKCC1 and KCC2 in the basolateral amygdala, but the results showed no significant changes (Fig. S1).
These results highlight the role of CCCs and GABAA receptors in ELS-induced behavioral changes and identify the hippocampus as a brain area that is vulnerable to ELS.
Systemic treatment with bumetanide during early development had no effect on body weight or normal behaviors
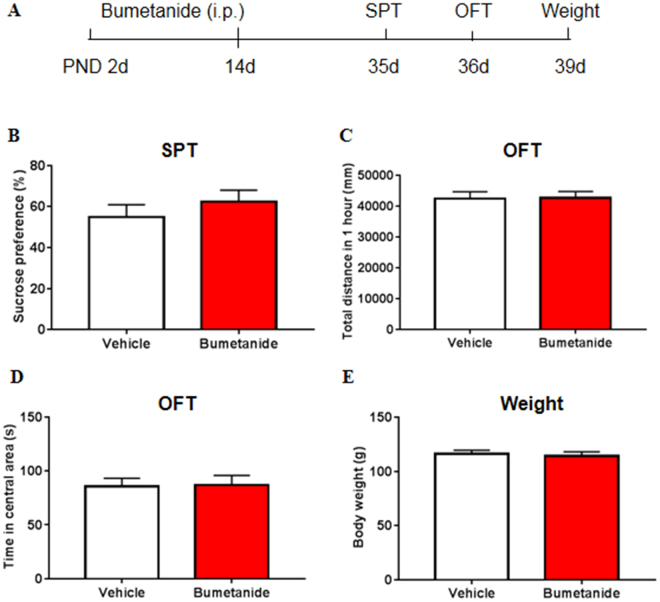
As maternal separation led to increased expression of NKCC1 at P14, and previous studies found bumetanide treatment during a vulnerable developmental period (the first two postnatal weeks) rescues a genetic epilepsy33, therefore, we investigated the effects of twice-daily bumetanide treatment during the first two postnatal weeks on body weight, locomotor activity, and emotional behaviors. Bumetanide had no effect on body weight (t 73 = 0.429, p = 0.669; Fig. 3E) or locomotor activity, with no difference in the total distance travelled in 1 h (t 72 = 0.066, p = 0.948; Fig. 3C). The time in the central area in the open field test (t 72 = 0.116, p = 0.908; Fig. 3D) and sucrose preference (t 49 = 0.906, p = 0.369; Fig. 3B) were not significantly different between the maternally separated group and control group, indicating that bumetanide did not lead to depressive- or anxiety-like behavior. These data highlight the safety of bumetanide treatment during early development.
Figure 3.
Systemic treatment with bumetanide during early development had no effect on body weight or normal behaviors. (A) Experimental timeline of bumetanide treatment and behavioral tests. (B) Sucrose preference in the SPT. (C) Total distance travelled in 1 h in the open field test (OFT). (D) Time spent in the central area in the OFT. (E) Body weight of bumetanide and vehicle rats. The data are expressed as mean ± SEM. n = 35–40 per group (13–15 females, 20–23 males).*p < 0.05, compared with vehicle group.
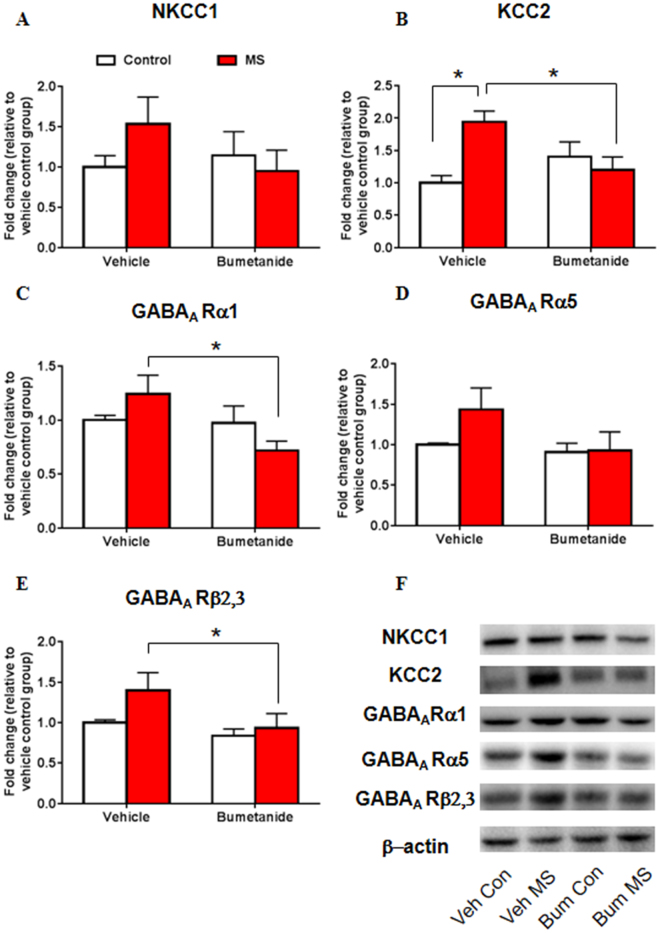
Systemic treatment with bumetanide during early development decreased NKCC1 and GABAA receptor subunit expression after maternal separation
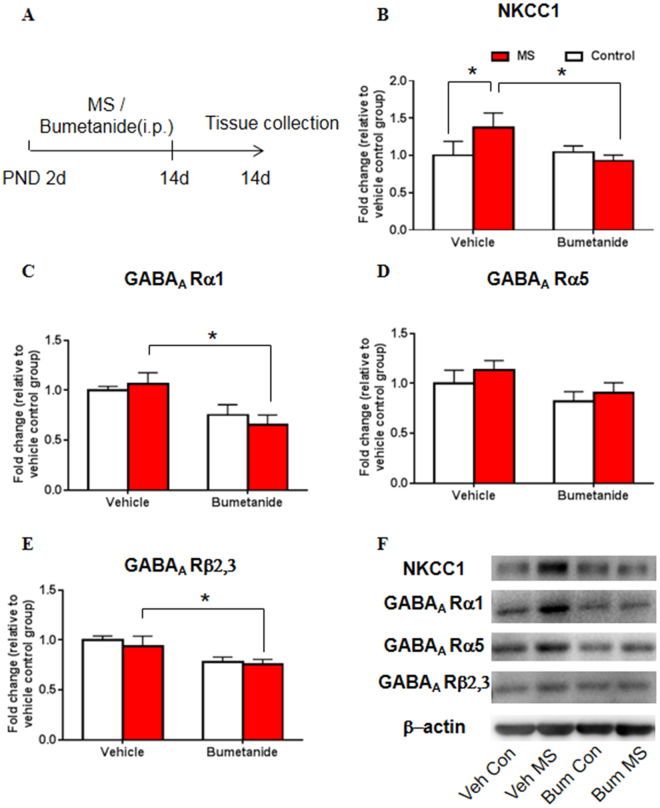
Then we examined the expression of NKCC1 and GABAA receptor subunits following MS and bumetanide treatment to determine whether bumetanide restores impairments in NKCC1 expression and the GABAergic system that are induced by MS. The two-way ANOVA, with drug treatment (vehicle and bumetanide) and stress (control and MS) as the between-subjects factors, was used to analyze the effects of NKCC1 inhibition on the expression of NKCC1 and GABAA receptor subunits. No significant main effects or interaction were found for the expression of NKCC1 (treatment: F 3,19 = 2.701, p = 0.117; stress: F 3,19 = 1.096, p = 0.308; treatment × stress: F 3,19 = 3.990, p = 0.060; Fig. 4B). A significant main effect of treatment on the expression of the GABAA receptor α1 subunit was found (treatment: F 3,19 = 11.808, p = 0.003; stress: F 3,19 = 0.033, p = 0.858; treatment × stress: F 3,19 = 0.676, p = 0.421; Fig. 4C). A significant effect of treatment on the expression of the GABAA receptor α5 subunit was found (treatment: F 3,19 = 5.230, p = 0.034; stress: F 3,19 = 1.539, p = 0.230; treatment × stress: F 3,19 = 0.058, p = 0.812; Fig. 4D). A significant effect of treatment on the expression of the GABAA receptor β2,3 subunit was also found (treatment: F 3,19 = 8.563, p = 0.009; stress: F 3,19 = 0.350, p = 0.561; treatment × stress: F 3,19 = 0.073, p = 0.791; Fig. 4E). The post hoc test showed that MS significantly upregulated the expression of NKCC1 (p = 0.049; Fig. 4B), which could be reversed by bumetanide treatment (p = 0.016; Fig. 4B). Bumetanide significantly reduced the expression of the GABAA receptor α1 subunit (p = 0.006; Fig. 4C) and β2,3 subunit (p = 0.040; Fig. 4E) in maternally separated rats compared with the vehicle group. These results suggest that bumetanide restored the MS-induced increase in NKCC1 expression and reduced GABAA receptor subunit expression in the hippocampus.
Figure 4.
Systemic treatment with bumetanide during early development altered NKCC1 and GABAA receptor subunit expression after maternal separation. (A) Experimental timeline of maternal separation, drug treatment, and tissue collection. (B–E) NKCC1 (B), GABAA receptor α1 subunit (C), GABAA receptor α5 subunit (D), and GABAA receptor β2,3 subunit (E) levels in the CA1 area of the hippocampus after maternal separation and bumetanide treatment. (F) Representative Western blots in the CA1. Full-length blots are presented in Supplementary Figure S5. n = 10 per group (4 females, 6 males). The data are expressed as mean ± SEM. *p < 0.05.
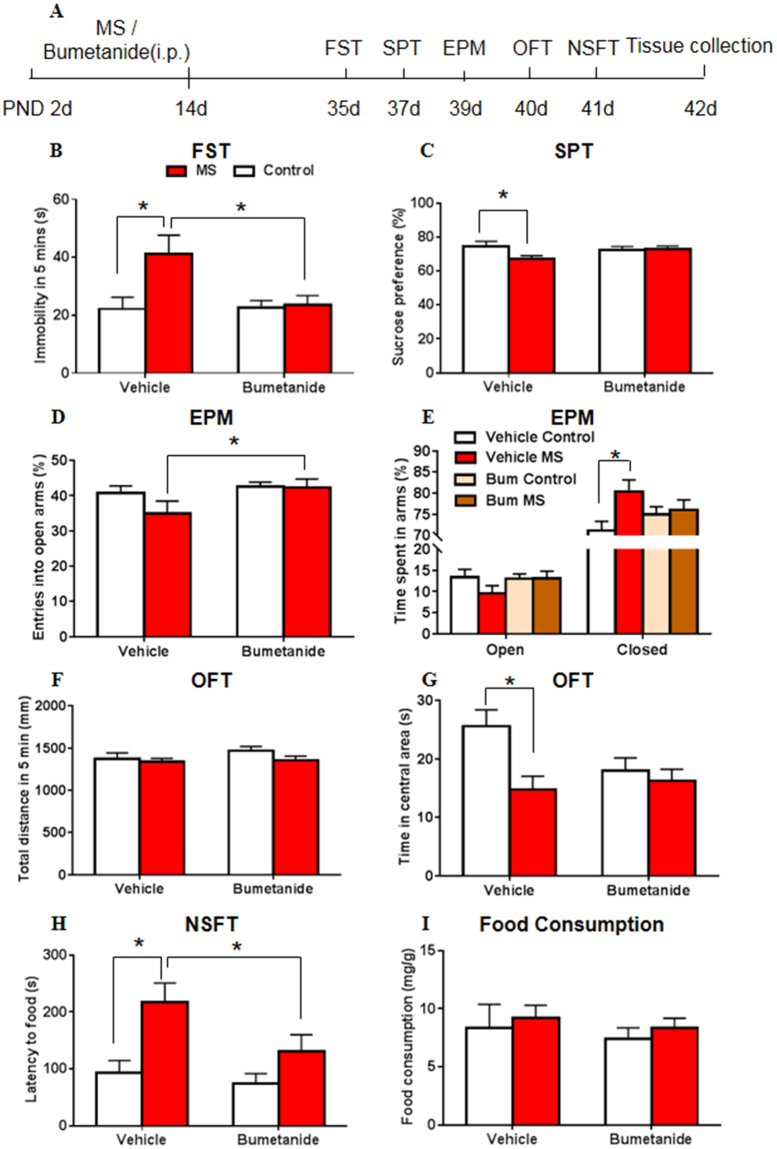
Systemic treatment with bumetanide during early development restored behavioral and molecular abnormalities in adolescent rats after maternal separation
Next, we investigated the effects of twice-daily bumetanide treatment on MS-induced depressive- and anxiety-like behavior. After maternal separation or bumetanide treatment from P2 to P14, each rat was subjected to the forced swimming as the stressor. We then conducted the SPT, EPM test, open field test (OFT), and novelty-suppressed feeding test (NSFT) to assess depressive- and anxiety-like behavior that was induced by MS and swim stress during adolescence. After the behavioral tests, the brain tissue were collected for western blotting to examine the expression of NKCC1, KCC2 and GABAA receptor subunits.
The two-way ANOVA, with drug treatment (vehicle and bumetanide) and stress (control and MS) as the between-subjects factors, was used to analyze the effects of NKCC1 inhibition on the behavioral measures. A significant main effect of stress on immobility time was found in the 5 min FST (treatment: F 3,73 = 3.275, p = 0.074; stress: F 3,73 = 4.537, p = 0.037; treatment × stress: F 3,73 = 3.617, p = 0.061; Fig. 5B). In the SPT, the analysis revealed no significant main effects on sucrose preference or interaction (treatment: F 3,67 = 0.673, p = 0.415; stress: F 3,67 = 2.635, p = 0.109; treatment × stress: F 3,67 = 2.988, p = 0.088; Fig. 5C). In the EPM, the analysis revealed no main effects or interaction for the percentage of entries into the open arms (treatment: F 3,71 = 3.081, p = 0.084; stress: F 3,71 = 1.299, p = 0.258; treatment × stress: F 3,71 = 1.101, p = 0.298; Fig. 5D), or for the time spent in open arms (treatment: F 3,71 = 0.818, p = 0.369; stress: F 3,71 = 1.120, p = 0.293; treatment × stress: F 3,71 = 1.265, p = 0.264; Fig. 5E), but a significant main effect of stress for the time spent in closed arms (treatment: F 3,71 = 0.009, p = 0.924; stress: F 3,71 = 4.271, p = 0.042; treatment × stress: F 3,71 = 2.647, p = 0.108; Fig. 5E). In the OFT, a significant main effect of stress on the time spent in the central area was found (treatment: F 3,71 = 1.453, p = 0.232; stress: F 3,71 = 6.224, p = 0.015; treatment × stress: F 3,71 = 3.271, p = 0.075; Fig. 5G). No main effects or interaction was found for the total distance travelled (treatment: F 3,71 = 0.988, p = 0.324; stress: F 3,71 = 1.581, p = 0.213; treatment × stress: F 3,71 = 0.449, p = 0.505; Fig. 5F). In the NSFT, a significant main effect of stress on the latency to feed was found (treatment: F 3,73 = 2.809, p = 0.098; stress: F 3,73 = 7.992, p = 0.006; treatment × stress: F 3,73 = 1.091, p = 0.300; Fig. 5H), with no effects or interactions for home cage food consumption (treatment: F 3,73 = 0.580, p = 0.449; stress: F 3,73 = 0.645, p = 0.425; treatment × stress: F 3,73 = 0.001, p = 0.974; Fig. 5I).
Figure 5.
Systemic treatment with bumetanide during early development restored behavioral abnormalities in adolescent rats after maternal separation. (A) Experimental timeline of maternal separation, drug treatment, and behavioral tests. (B) Immobility time in the FST. (C) Sucrose preference in the SPT. (D) Percentage of entries into the open arms in the EPM. (E) Time spent in arms in the EPM. Time spent in the open arms is plotted as a percentage of the total observation time. (F) Total distance travelled in 5 min in the OFT. (G) Time spent in the central area in the OFT. (H) Latency to feed in the novelty-suppressed feeding test (NSFT). (I) Food consumption in the homecage over 5 min in the NSFT. n = 16–20 per group (8–10 females, 8–10 males). The data are expressed as mean ± SEM. *p < 0.05.
The post hoc test revealed that MS in the vehicle-treated groups significantly decreased sucrose preference in the SPT (p = 0.044; Fig. 5C) and the time spent in the central area in the OFT (p = 0.011; Fig. 5G), while increased immobility time in the FST (p = 0.016; Fig. 5B), the time spent in closed arms (p = 0.025; Fig. 5E) in the EPM and the latency to food in the NSFT (p = 0.020; Fig. 5H). Bumetanide reversed the depressive- and anxiety-like behaviors that were induced by MS. Significant differences of immobility time in the FST (p = 0.003; Fig. 5B), entries into the open arms in the EPM (p = 0.031; Fig. 5D) and latency to food in the NSFT (p = 0.046; Fig. 5H) were found between bumetanide MS group and vehicle MS group. The post hoc test revealed no differences in total distance travelled (p > 0.05; Fig. 5F) in the OFT or home cage food consumption (p > 0.05; Fig. 5I) in the NSFT between groups.
The two-way ANOVA was also used to analyze the effects of NKCC1 inhibition on the expression of NKCC1, KCC2 and GABAA receptor subunits. No significant main effects or interaction were found for the expression of NKCC1 (treatment: F 3,16 = 0.727, p = 0.406; stress: F 3,16 = 0.437, p = 0.518; treatment × stress: F 3,16 = 2.008, p = 0.176; Fig. 6A). Significant main effect of stress and interaction were found for the expression of KCC2 (treatment: F 3,16 = 0.939, p = 0.347; stress: F 3,16 = 4.519, p = 0.049; treatment × stress: F 3,16 = 10.833, p = 0.005; Fig. 6B). A significant main effect of treatment on the expression of the GABAA receptor α1 subunit (treatment: F 3,16 = 4.803, p = 0.044; stress: F 3,16 = 0.003, p = 0.957; treatment × stress: F 3,16 = 3.940, p = 0.065; Fig. 6C) and GABAA receptor β2,3 subunit (treatment: F 3,16 = 4.523, p = 0.049; stress: F 3,16 = 2.814, p = 0.113; treatment × stress: F 3,16 = 1.035, p = 0.324; Fig. 6E) were found. No significant effects or interaction was found on the expression of the GABAA receptor α5 subunit (treatment: F 3,16 = 2.664, p = 0.122; stress: F 3,16 = 1.530, p = 0.234; treatment × stress: F 3,16 = 1.268, p = 0.277; Fig. 6D). The post hoc test showed that MS significantly upregulated the expression of KCC2 (p = 0.001; Fig. 6B), which could be reversed by bumetanide treatment (p = 0.008; Fig. 6B). Bumetanide significantly reduced the expression of the GABAA receptor α1 subunit (p = 0.009; Fig. 6C) and β2,3 subunit (p = 0.041; Fig. 6E) in maternally separated rats compared with the vehicle group.
Figure 6.
Systemic treatment with bumetanide during early development altered KCC2 and GABAA receptor subunit expression in adolescent rats after maternal separation. (A–E) NKCC1 (A), KCC2 (B), GABAA receptor α1 subunit (C), GABAA receptor α5 subunit (D), and GABAA receptor β2,3 subunit (E) levels in the CA1 area of the hippocampus after maternal separation and bumetanide treatment. (F) Representative Western blots in the CA1. Full-length blots are presented in Supplementary Figure S6. n = 12 per group (6 females, 6 males). The data are expressed as mean ± SEM. *p < 0.05.
These results suggest that bumetanide restored abnormalities in emotional behavior and also the increases in KCC2 expression and GABAA receptor subunit expression in adolescent rats that were exposed to MS and adolescent swim stress.
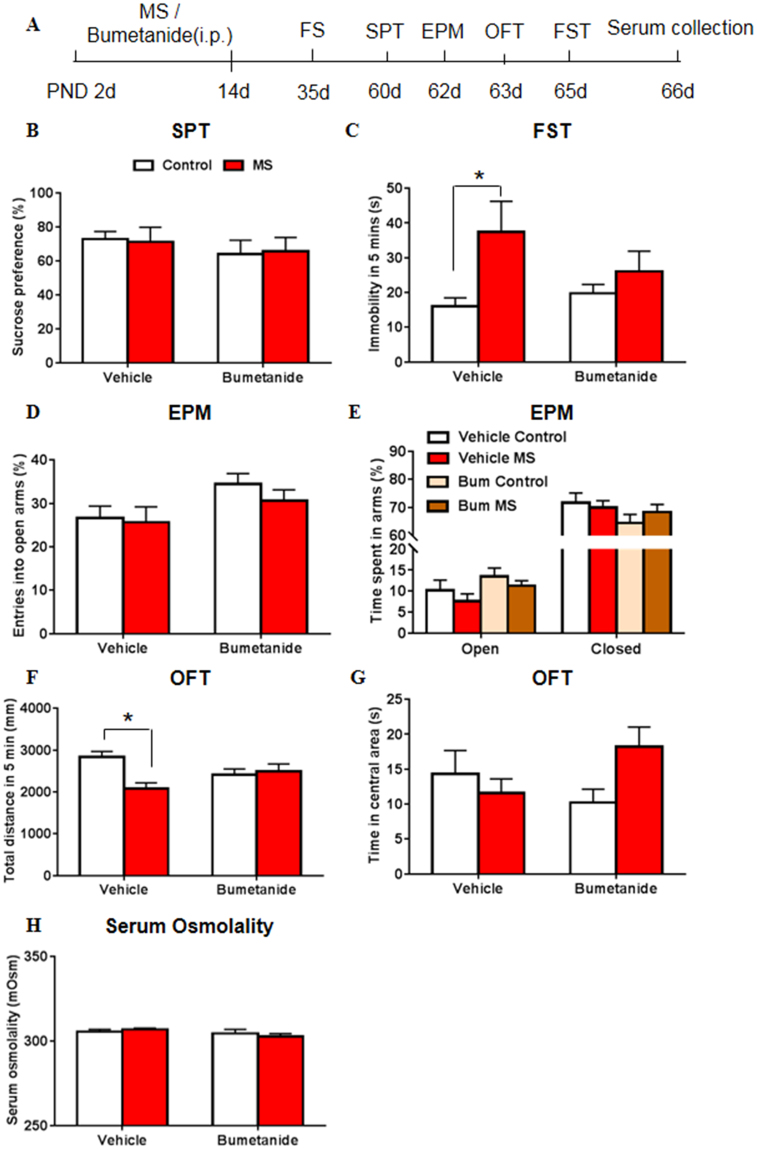
Systemic treatment with bumetanide during early development restored behavioral abnormalities in adult rats after maternal separation
Finally, we investigated the effects of twice-daily bumetanide treatment on MS-induced depressive- and anxiety-like behavior in adult rats. After maternal separation or bumetanide treatment from P2 to P14, each rat was subjected to the forced swimming as the stressor at P35. We then conducted the SPT, EPM test, open field test (OFT), and FST to assess depressive- and anxiety-like behavior that was induced by MS and swim stress during adulthood. After the behavioral tests, the serum of rats were collected for osmolality test.
The two-way ANOVA, with drug treatment (vehicle and bumetanide) and stress (control and MS) as the between-subjects factors, was used. The analysis revealed no significant main effects or interaction for sucrose preference in the SPT (treatment: F 3,35 = 0.876, p = 0.356; stress: F 3,35 = 0.001, p = 0.990; treatment × stress: F 3,35 = 0.048, p = 0.828; Fig. 7B), and for the time in open arms (treatment: F 3,39 = 3.398, p = 0.073; stress: F 3,39 = 1.580, p = 0.216; treatment × stress: F 3,39 = 0.011, p = 0.918; Fig. 7E) and closed arms (treatment: F 3,39 = 2.205, p = 0.146; stress: F 3,39 = 0.136, p = 0.715; treatment × stress: F 3,39 = 0.881, p = 0.354; Fig. 7E) in the EPM test. A significant main effect of stress on immobility time was found in the FST (treatment: F 3,42 = 0.0.488, p = 0.489; stress: F 3,42 = 6.364, p = 0.016; treatment × stress: F 3,42 = 1.874, p = 0.178; Fig. 7C). In the EPM, the analysis revealed a significant main effect of treatment for the percentage of entries into the open arms (treatment: F 3,39 = 4.894, p = 0.033; stress: F 3,39 = 0.703, p = 0.407; treatment × stress: F 3,39 = 0.249, p = 0.620; Fig. 7D). In the OFT, a significant main effect of stress and interaction on the total distance travelled were found (treatment: F 3,42 = 0.004, p = 0.952; stress: F 3,42 = 5.194, p = 0.028; treatment × stress: F 3,42 = 8.171, p = 0.007; Fig. 7F), with no significant main effects or interaction for the time spent in the central area (treatment: F 3,42 = 0.007, p = 0.932; stress: F 3,42 = 0.434, p = 0.513; treatment × stress: F 3,42 = 3.082, p = 0.086; Fig. 7G). The post hoc test revealed that MS in the vehicle-treated groups significantly increased immobility time in the FST (p = 0.009; Fig. 7C) and decreased total distance travelled in the OFT (p = 0.001; Fig. 7F). Bumetanide reversed the depressive- and anxiety-like behaviors that were induced by MS.
Figure 7.
Systemic treatment with bumetanide during early development restored behavioral abnormalities in adult rats after maternal separation. (A) Experimental timeline of maternal separation, drug treatment, and forced swimming (FS) stress during adolescence and behavioral tests in adult rats. (B) Sucrose preference in the SPT. (C) Immobility time in the FST. (D) Percentage of entries into the open arms in the EPM. (E) Time spent in arms in the EPM. Time spent in the open arms is plotted as a percentage of the total observation time. (F) Total distance travelled in 5 min in the OFT. (G) Time spent in the central area in the OFT. (H) Serum osmolality of each group. The standard curve of osmolality test was shown in Fig S3. n = 12 per group (6 females, 6 males).The data are expressed as mean ± SEM. *p < 0.05.
At last, we examined whether the effect of NKCC1 inhibition on behaviors was associated with serum osmolality. The two-way ANOVA, with drug treatment (vehicle and bumetanide) and stress (control and MS) as the between-subjects factors, was used. The analysis revealed no significant main effects or interaction for serum osmolality (treatment: F 3,41 = 2.470, p = 0.124; stress: F 3,41 = 0.029, p = 0.886; treatment × stress: F 3,41 = 0.894, p = 0.350; Fig. 7H). The post hoc test also revealed no differences (p > 0.05; Fig. 7H) between groups.
These results suggest that bumetanide reversed the depressive- and anxiety-like behaviors in the FST and OFT that were induced by MS in adult rats, and adolescence may be a more vulnerable time to MS-induced stress than adulthood.
Discussion
In the present study, we found that MS led to depressive- and anxiety-like behavior in adolescent rats and increased the expression of NKCC1, KCC2, and GABAA receptor subunits in the CA1 area of the hippocampus. Intraperitoneal injection of the NKCC1 inhibitor bumetanide during early development did not affect body weight, locomotor activity, or depressive- or anxiety-like behavior in naive rats. However, bumetanide reversed the behavioral abnormalities that were caused by MS and decreased NKCC1, KCC2, GABAA α1 and β2,3 subunit levels in the hippocampus. Moreover, bumetanide did not affect serum osmolality in adult rats. Altogether, our results suggest that NKCC1 in the hippocampus may play an important role in maintaining the function of GABAergic transmission and regulating the vulnerability to MS-induced stress.
Previous studies demonstrated that MS for 180 min per day from P2 to P14 or to P21 induced depression-like behaviors during adolescence38,39, and no significant difference was observed in the 15-min-per-day MS group39. Consistent with these studies, we found that MS increased the susceptibility to stress in adolescent rats. Differences in the MS protocol between the present study and previous studies include the duration of daily MS and total number of days of MS (i.e., separation for 15 or 180 min per day and from P2 to P14 or to P21), and these differences in MS intensity may lead to different behavioral outcomes. In the present study, we found a variety of behavioral changes observed in behavioral tests. Sucrose preference and immobility time in the FST are core symptoms of depression, which reflect the depression-like state, such as anhedonia and despair36,40. The EPM and NSFT are well-established behavioral model of anxiety40, and the OFT was used to measure the exploratory locomotor activity of animals41. Previous studies reported that maternal separation would not change basal sucrose preference in adolescent rats42 and adult rats43–45, which were consistent with our findings. Maternal separation reduced sucrose preference in adolescent rats which experienced forced swim stress but not in adult rats, and the differences may suggest adolescence may be a more vulnerable time to MS-induced stress than adulthood46. Previous studies also found that MS did not alter the time in the open arms in the EPM43 in adult rodents. Additionally, we found that MS increased immobility time in adolescent rats, whereas sucrose preference was unchanged. However, rats that experienced MS and forced swimming during adolescence showed decreased sucrose preference. This indicates that sucrose preference may reflect the depression-like state in rats that did not experience stress during adolescence, but forced swimming itself could act as a kind of stressor to detect stress vulnerability10,47. Previous studies also showed that rats experienced MS from P2 to P14 and restraint stress for 2 weeks during adolescence exhibited a decrease in sucrose preference, whereas no change was observed in rats that were not subjected to restraint stress43,44. Commons et al. recently discussed that the forced swim test measures coping strategy to an acute inescapable stress and thus provides unique insight into the neural limb of the stress response47. According to the Two-Hit hypothesis, the etiology of neuropsychiatric disorders usually involves multiple stressors experienced subsequently during different phases of life7,10. Based on these findings, we used forced swim stress during adolescence to measure the rats’ response to a second stress later in life10.
Growing evidence indicates that the hippocampus and amygdala are involved in ELS-related mental illness, such as depression15,16. Brain imaging studies found that the volume of the hippocampus in patients with depression is significantly smaller than normal control groups, whereas the volume of the left amygdala presents a tendency toward a decrease in patients with depression48. Both aged and adolescent patients with depression presented a reduction of effective connectivity from the amygdala to the hippocampus49,50. Repeated injections of corticosterone, which is an animal model of depression, led to alterations in GABAergic and glutamatergic activity, such as the expression of GAD67, GAD65, and several GABAA receptor subunits in the hippocampus and amygdala51. These results suggest that the hippocampus and amygdala play an important role in depression. Furthermore, a meta-analysis found that hippocampal volume was significantly reduced in children who suffered from childhood abuse, with no significant alterations in the volume of the amygdala52. These results may suggest that the hippocampus plays a more important role in stress vulnerability that is induced by ELS.
Cation-chloride cotransporters play an important role in neuronal development, including neuronal proliferation, migration, differentiation, and the wiring of neuronal networks through the modulation of GABA receptor-mediated synaptic transmission19,53. In the rodent cortex, the expression of KCC2 rapidly increases during development, consistent with intense synaptogenesis54. A greater proportion of KCC2 in cortical neurons is found in dendritic spines or their vicinity55, indicating that CCCs may be involved in the formation of dendritic spines. Additionally, a previous study found that interfering with the early depolarizing effect of GABA with the NKCC1 inhibitor bumetanide prolonged critical-period plasticity in visual cortical circuits. In the control group, the plasticity of the visual cortex was reduced at the end of the critical period of development (i.e., about 35 days after birth), whereas the visual cortex retained high plasticity at 35 days after birth in the bumetanide intervention group24. Therefore, CCCs may play a role in the depolarizing effect of GABA and selectively modulate cortical plasticity.
Derangements of CCCs are involved in various neuropsychiatric disorders, such as epilepsy, neuropathic pain, schizophrenia, autism, and Down’s syndrome18. The downregulation of KCC2 and upregulation of NKCC1 were found in the hippocampus in adult patients with temporal lobe epilepsy, leading to the depolarization of GABAA receptor-mediated activity in CA1 pyramidal cells56. In rodent models of autism, the GABA excitatory-inhibitory shift is abolished during delivery, leading to greater excitatory GABA transmission and autism-like behavior25. Stress can also lead to changes in the expression of CCCs. Chronic unpredictable mild stress led to an increase in NKCC1 expression in the hypothalamic paraventricular nucleus, resulting in the loss of GABA inhibition57. Similarly, higher expression of NKCC1 and lower expression of KCC2 in the hippocampus were observed in rats that were stressed by forced water administration using esophageal intubation58. Maternal restraint stress also resulted in an increase in the NKCC1/KCC2 ratio in the hippocampus at 14 and 28 days after birth59. In the present study, we found that MS increased NKCC1 expression in the CA1 area of the hippocampus on P14, which may be associated with greater susceptibility to stress in adolescent rats.
NKCC1 inhibition by bumetanide has been shown to be effective in the treatment of various neurological and psychiatric disorders29–31. Clinical studies have reported a therapeutic effect of bumetanide in temporal lobe epilepsy60 but not newborn seizures in a clinical trial35. Bumetanide treatment also attenuated seizures in a genetic animal model of epilepsy33 and neonatal rats after hypoxia-ischemia61. The synaptic plasticity and memory deficits in a mouse model of Down’s syndrome were restored by 1-week treatment with bumetanide26. A therapeutic effect of bumetanide was also found in an animal model of autism25, which was supported by a case report of childhood autism62. Although bumetanide inhibits both NKCC1 and NKCC2, NKCC2 mainly existed in kidney, and bumetanide could cross the brain-blood barrier63, we believe it was an optimal choice to selectively block NKCC1 in the brain. On the other hand, it is still unclear about the effects of NKCC1 antagonism on behavioral abnormalities that are induced by stress. In the present study, bumetanide treatment during early development restored the depressive- and anxiety-like behaviors that were induced by MS, which may extend the application of bumetanide to the field of stress vulnerability that is related to ELS.
The precise mechanisms that underlie the regulatory role of CCCs in the vulnerability to stress are unclear. We speculate that CCCs may be associated with GABAergic transmission. Alternations of NKCC1, KCC2, and GABAA receptor subunits have been observed in many brain diseases, such as experimental models of seizure64,65 and Fragile X syndrome27,66. Lower brain concentrations of GABA and alterations in GABAA receptor subunit expression were found in depressive patients67. A negative allosteric modulator of α5 subunit-containing GABAA receptors exerted a rapid and sustained antidepressant-like action in mice68. During early development, GABAA receptors that contain α5, β2, β3, and γ subunits are essential for the depolarizing action of GABA69. Early life stress may lead to long-term changes in GABAA receptors70, such as higher expression of the GABAA α4βδ subunit in the hippocampal CA1 region71. Previous studies found that prenatal stress altered GABAA receptor α1 and α5 subunit expression levels59. In the present study, MS increased the expression of the GABAA receptor α1 and β2,3 subunits in the hippocampus, and bumetanide treatment decreased the expression of GABAA receptor subunits compared with the vehicle groups. These findings indicate that stress influences the early depolarizing action of GABA, and these impairments can be restored by bumetanide. Additionally, manipulations of NKCC1 during early development led to the prolongation of cortical plasticity and lower density of perineuronal nets24, suggesting that the effect of CCCs on MS-induced stress vulnerability may be associated with neuronal plasticity and its regulators, such as brain-derived neurotrophic factor and perineuronal nets.
In summary, the present study shows that NKCC1 in the hippocampus plays an important role in regulating the stress response that is related to ELS. Maternal separation induced depressive- and anxiety-like behaviors in adolescent rats, which could be rescued by bumetanide administration during early development. The present findings suggest a new potential target for the treatment of ELS-related psychiatric disorders.
Methods
Animals and housing
Pregnant female Sprague-Dawley rats were purchased from the Center of Laboratory Animal Science, Peking University Health Science Center. The rats were pregnant for 13–15 days at the time of arrival in the laboratory. They were individually housed under a 12 h/12 h light/dark cycle with ad libitum access to water and food. All of the animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Biomedical Ethics Committee for Animal Use and Protection of Peking University.
Maternal separation
The day of delivery was denoted as postnatal day 0 (P0)72. The number of pups were limited to 10–12 pups per litter in order to eliminate confounding effects of litter size or differences in maternal care73 (5 litters per group). Maternal separation was applied from P2 to P14. Pups were removed from their home cage and isolated from their mothers in other cages for 3 h (9:00 AM–12:00 PM) during the dark period. For separation during the first week, the isolation boxes were placed in a humidified incubator at 32 °C. Control rats were reared together with their dam without disturbance74. For each experimental group, pups were separated from their mother at weaning age (P21). All the pups of one group was temporarily put into one cage, and then randomly divided into cages each containing up to five of the same gender per cage. The data presented in bars is comprised by male and female rat pooled data according to previous studies44,75,76.
Pharmacological treatment
From P2 to P14, the pups were injected i.p. twice daily with 0.2 mg/kg bumetanide (Sigma) or vehicle (2% dimethylsulfoxide in physiological saline) according to previous studies24,26,33,63. The final concentration of the drug is 0.1 mg/ml. Therefore, in our study the volume was less than 0.05 ml per injection using gauge 26 beveled needles during the first postnatal week. Apart from regular injection instructions, the needle was stayed for a while in case of drug leak. Bumetanide or vehicle was diluted in physiological saline immediately before the injection.
Serum collection and osmolarity measurement
Rats were decollated and blood was collected immediately. Samples were centrifuged and osmolarity of recovered serum was estimated by a Vapor pressure osmometer24.
Behavioral tests
Forced swim test
The forced swim test (FST) is a well-established behavioral model of despair37. Forced swimming during adolescence (P35) served as a second stressor47 and was also used to test for depressive-like behavior10. The FST was conducted during the dark period under dim light. Plastic cylinders (50 cm height, 24 cm diameter) contained tap water (25 °C ± 1 °C) to a depth of 35 cm. The animals were unable to touch the cylinder’s bottom with their hind paws or tail. The FST consisted of two sessions on two consecutive days (15 min pretest and 5 min test). The rats were individually placed in the cylinders for 15 min on the first day and then placed again in the cylinders 24 h later. The total duration of immobility (immobility time) was measured during a 5 min test. The behavior of each animal was simultaneously monitored by a video camera. Immobility was defined as the absence of any active movements other than those necessary to keep the head and nose above the water (e.g., when the rats floated in a vertical position). After each session, the rats were removed from the water, dried with a towel, placed in a warm enclosure, and then returned to their home cage. The videos were analyzed using EthoVision XT 10. The EthoVision XT 10 analyzed the behavior video based on manually set mobile and immobile thresholds. To find the correct threshold we follow the suggestion from engineer of EthoVision XT, and then we randomly chose two animals in each experiment and recorded the immobility time by two independent observers who were blind to the animal groups, and finely adjusted the immobile threshold based on the recorded time in each experiment.
Open field test
The open field test (OFT) was performed to assess exploratory locomotor activity in an unfamiliar environment as a rat model of depression, and this model mimics some aspects of human depression, including lower levels of activity, and lack of interest41. The test was conducted in a dark room. The apparatus consisted of a gray box that was open on top. The dimensions of the box were 100 cm × 100 cm × 40 cm. The floor was divided into 25 equal squares (20 cm × 20 cm). The arena was illuminated with a 40 W lamp in the center, 60 cm above the floor. Each rat was gently placed in the center square of the floor of the arena, and behavior was videotaped for 5 min or 1 h for locomotor activity assessment. After each rat was tested, the box was thoroughly cleaned to remove odor cues. Locomotor activity and the time spent in the central area were analyzed using EthoVision XT 10. We detected animal dwelling time in central area with the body of the rat was in the zone.
Sucrose preference test
The sucrose preference test (SPT) is considered an index of anhedonia77. The SPT was conducted during the dark period under dim red light according to previous studies40,41. The rats were habituated to a 1% sucrose solution for 48 h before the test day, during which water and 1% sucrose were offered in two identical bottles. The locations of these two bottles were interchanged every 24 h. The rats were deprived of food and water for 4 h, followed by a 1 h preference test, in which water and 1% sucrose were offered in two identical bottles. During the test, the locations of the bottles were interchanged every 30 min. The water and sucrose bottles were weighed to determine sucrose preference.
Novelty-suppressed feeding test
The novelty-suppressed feeding test (NSFT) was performed according to previously established protocols78. A more ‘anxious’ animal will take more time to begin eating in a novel environment79. The rats were deprived of food for 24 h and then placed in the corner of an open field arena (75 cm × 75 cm × 40 cm), and a pellet of food was placed on a white piece of paper (10 cm × 10 cm) in the center of the cage. Each test lasted 10 min, and the latency to approach the food and begin eating was recorded as the main test parameter. The latency to feed was recorded when the rat sat on the paper square and bit the pellet using its forepaws. Subsequent home cage food consumption over 5 min was the quantitative control measure for appetite.
Elevated plus maze
The EPM is based on a rat’s natural fear of open, unprotected, and elevated spaces and is considered an index of anxiety40. The EPM consisted of four arms (two open arms and two closed arms, each 50 cm long and 10 cm wide) that were arranged in a plus configuration and elevated 70 cm above the floor. The closed arms had 40 cm high walls. Illumination was 3 lux in the closed arms and 8 lux in the open arms. Each rat was first placed in the central zone of the EPM with its head facing an open arm. The rat was allowed to freely explore the maze for 5 min. The percentage of entries into the open arms and time spent on the open and closed arms were recorded from videos32,80 and analyzed using EthoVision XT 10. Previous studies counted all four paws into an arm as an entry81,82. Therefore, we detected animal dwelling time and entries into arms with the center point of rat was in the arms.
Western blot assays
The protocols for sample preparation and Western blot were based on our previous studies83–86. Bilateral tissue punches (12-gauge) were collected from the CA1 area of the hippocampus and basolateral amygdala. Each group was then divided into 5 or 6 samples based on the number of animals and each sample contained tissues from 2 rats44. And we did Western blotting on all samples of each group for every protein listed. And the brain tissues were then homogenized with RIPA lysis buffer (Applygen Technology, Beijing, China) with enzyme inhibitor cocktail (Applygen Technology, Beijing, China). The lysates were centrifuged at 12,000 × g for 15 min. The centrifugate was collected, and protein concentrations in all of the samples were quantified using the BCA assay kit (Applygen Technology, Beijing, China). Τhe samples were diluted with RIPA lysis buffer to equalize the protein concentrations. Equal amounts of samples (20 mg) underwent 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The primary antibodies were the following: anti-NKCC1 (1:1000; Cell Signaling Technology, catalog no. 14581 S), anti-KCC2 (1:1000; Abcam, catalog no. ab49917), anti-GABAA receptor α1 subunit (1:1000; Abcam, catalog no. ab33299), anti-GABAA receptor α5 subunit (1:1000; Abcam, catalog no. ab10098), anti-GABAA receptor β2,3 subunit (1:1000; Millipore, catalog no. MAB341), and anti-β-actin (1:1000; Sigma Aldrich, catalog no. A5316). Horseradish peroxidase-conjugated secondary antibody was used (1:2000; anti-rabbit and anti-mouse immunoglobulin G; Santa Cruz Biotechnology and Vector Laboratories, respectively). The levels of all proteins were normalized to the level of β-actin. Band intensities were quantified using Quantity One 4.0.3 (Bio-Rad, Hercules, CA, USA).
Statistical analysis
The data are expressed as mean±SEM. The statistical analysis of the behavioral and molecular data was performed using Student’s t-test and two-way ANOVA followed by Tukey’s post hoc test. Values of p < 0.05 were considered statistically significant.
Electronic supplementary material
Acknowledgements
This work was supported by the Natural Science Foundation of China (no. 81225009, U1402226, 31571099 and 81521063), the National Basic Research Program of China (Grants 2015CB553503 and 2015CB856400), and Beijing Municipal Science and Technology Commission (Grants Z161100002616006).
Author Contributions
D.H. and J.S. conceived the project and designed the experiments. D.H., Z.L.Y., and Y.Z. performed the experiments. Y.Z., Y.H., and W.Z. analyzed the data. D.H., Y.H., L.L., and J.S. wrote the paper with contributions from all of the other authors.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-12183-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Selye H. The Stress of Life–New Focal Point for Understanding Accidents. Industrial medicine & surgery. 1964;33:621–625. [PubMed] [Google Scholar]
- 2.Schneiderman N, Ironson G, Siegel SD. Stress and health: psychological, behavioral, and biological determinants. Annual review of clinical psychology. 2005;1:607–628. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Southwick SM, Charney DS. The science of resilience: implications for the prevention and treatment of depression. Science. 2012;338:79–82. doi: 10.1126/science.1222942. [DOI] [PubMed] [Google Scholar]
- 4.Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology. 2013;38:1858–1873. doi: 10.1016/j.psyneuen.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gattere, G. et al. Gene-environment interaction between the brain-derived neurotrophic factor Val66Met polymorphism, psychosocial stress and dietary intake in early psychosis. Early intervention in psychiatry, 10.1111/eip.12371 (2016). [DOI] [PubMed]
- 6.El Hage W, Powell JF, Surguladze SA. Vulnerability to depression: what is the role of stress genes in gene x environment interaction? Psychological medicine. 2009;39:1407–1411. doi: 10.1017/S0033291709005236. [DOI] [PubMed] [Google Scholar]
- 7.Pena CJ, et al. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science. 2017;356:1185–1188. doi: 10.1126/science.aan4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill RA, et al. Sex-specific disruptions in spatial memory and anhedonia in a “two hit” rat model correspond with alterations in hippocampal brain-derived neurotrophic factor expression and signaling. Hippocampus. 2014;24:1197–1211. doi: 10.1002/hipo.22302. [DOI] [PubMed] [Google Scholar]
- 9.Nederhof E, Schmidt MV. Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiology & behavior. 2012;106:691–700. doi: 10.1016/j.physbeh.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Lesse A, Rether K, Groger N, Braun K, Bock J. Chronic Postnatal Stress Induces Depressive-like Behavior in Male Mice and Programs second-Hit Stress-Induced Gene Expression Patterns of OxtR and AvpR1a in Adulthood. Molecular neurobiology. 2016 doi: 10.1007/s12035-016-0043-8. [DOI] [PubMed] [Google Scholar]
- 11.Nishi M, Horii-Hayashi N, Sasagawa T. Effects of early life adverse experiences on the brain: implications from maternal separation models in rodents. Frontiers in neuroscience. 2014;8:166. doi: 10.3389/fnins.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufman J, Charney D. Effects of early stress on brain structure and function: implications for understanding the relationship between child maltreatment and depression. Development and psychopathology. 2001;13:451–471. doi: 10.1017/S0954579401003030. [DOI] [PubMed] [Google Scholar]
- 13.Reincke SA, Hanganu-Opatz IL. Early-life stress impairs recognition memory and perturbs the functional maturation of prefrontal-hippocampal-perirhinal networks. Scientific reports. 2017;7:42042. doi: 10.1038/srep42042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahng JW. An animal model of eating disorders associated with stressful experience in early life. Hormones and behavior. 2011;59:213–220. doi: 10.1016/j.yhbeh.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Hanson JL, et al. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biological psychiatry. 2015;77:314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aust S, et al. Differential effects of early life stress on hippocampus and amygdala volume as a function of emotional abilities. Hippocampus. 2014;24:1094–1101. doi: 10.1002/hipo.22293. [DOI] [PubMed] [Google Scholar]
- 17.Derks NA, Krugers HJ, Hoogenraad CC, Joels M, Sarabdjitsingh RA. Effects of Early Life Stress on Synaptic Plasticity in the Developing Hippocampus of Male and Female Rats. PloS one. 2016;11:e0164551. doi: 10.1371/journal.pone.0164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nature reviews. Neuroscience. 2014;15:637–654. doi: 10.1038/nrn3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiological reviews. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- 20.Dzhala VI, et al. NKCC1 transporter facilitates seizures in the developing brain. Nature medicine. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- 21.Payne JA, Stevenson TJ, Donaldson LF. Molecular characterization of a putative K-Cl cotransporter in rat brain. A neuronal-specific isoform. The Journal of biological chemistry. 1996;271:16245–16252. doi: 10.1074/jbc.271.27.16245. [DOI] [PubMed] [Google Scholar]
- 22.Kanaka C, et al. The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience. 2001;104:933–946. doi: 10.1016/S0306-4522(01)00149-X. [DOI] [PubMed] [Google Scholar]
- 23.Wang DD, Kriegstein AR. Defining the role of GABA in cortical development. The Journal of physiology. 2009;587:1873–1879. doi: 10.1113/jphysiol.2008.167635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deidda G, et al. Early depolarizing GABA controls critical-period plasticity in the rat visual cortex. Nature neuroscience. 2015;18:87–96. doi: 10.1038/nn.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyzio R, et al. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343:675–679. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- 26.Deidda G, et al. Reversing excitatory GABAAR signaling restores synaptic plasticity and memory in a mouse model of Down syndrome. Nature medicine. 2015;21:318–326. doi: 10.1038/nm.3827. [DOI] [PubMed] [Google Scholar]
- 27.He Q, Nomura T, Xu J, Contractor A. The developmental switch in GABA polarity is delayed in fragile X mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:446–450. doi: 10.1523/JNEUROSCI.4447-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morita Y, et al. Characteristics of the cation cotransporter NKCC1 in human brain: alternate transcripts, expression in development, and potential relationships to brain function and schizophrenia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:4929–4940. doi: 10.1523/JNEUROSCI.1423-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemonnier E, et al. A randomised controlled trial of bumetanide in the treatment of autism in children. Translational psychiatry. 2012;2:e202. doi: 10.1038/tp.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eftekhari S, et al. Bumetanide reduces seizure frequency in patients with temporal lobe epilepsy. Epilepsia. 2013;54:e9–12. doi: 10.1111/j.1528-1167.2012.03654.x. [DOI] [PubMed] [Google Scholar]
- 31.Lemonnier E, Lazartigues A, Ben-Ari Y. Treating Schizophrenia With the Diuretic Bumetanide: A Case Report. Clinical neuropharmacology. 2016;39:115–117. doi: 10.1097/WNF.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 32.Xu C, et al. Anesthesia with sevoflurane in neonatal rats: Developmental neuroendocrine abnormalities and alleviating effects of the corticosteroid and Cl(-) importer antagonists. Psychoneuroendocrinology. 2015;60:173–181. doi: 10.1016/j.psyneuen.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marguet SL, et al. Treatment during a vulnerable developmental period rescues a genetic epilepsy. Nature medicine. 2015;21:1436–1444. doi: 10.1038/nm.3987. [DOI] [PubMed] [Google Scholar]
- 34.Pressler RM, et al. Bumetanide for the treatment of seizures in newborn babies with hypoxic ischaemic encephalopathy (NEMO): an open-label, dose finding, and feasibility phase 1/2 trial. The Lancet. Neurology. 2015;14:469–477. doi: 10.1016/S1474-4422(14)70303-5. [DOI] [PubMed] [Google Scholar]
- 35.Ben-Ari Y, Damier P, Lemonnier E. Failure of the Nemo Trial: Bumetanide Is a Promising Agent to Treat Many Brain Disorders but Not Newborn Seizures. Frontiers in cellular neuroscience. 2016;10:90. doi: 10.3389/fncel.2016.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warner-Schmidt JL, Duman RS. VEGF as a potential target for therapeutic intervention in depression. Current opinion in pharmacology. 2008;8:14–19. doi: 10.1016/j.coph.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. European journal of pharmacology. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 38.Yoo SB, et al. Adolescence fluoxetine increases serotonergic activity in the raphe-hippocampus axis and improves depression-like behaviors in female rats that experienced neonatal maternal separation. Psychoneuroendocrinology. 2013;38:777–788. doi: 10.1016/j.psyneuen.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 39.Bian Y, et al. Repeated Three-Hour Maternal Separation Induces Depression-Like Behavior and Affects the Expression of Hippocampal Plasticity-Related Proteins in C57BL/6N Mice. Neural plasticity. 2015;2015:627837. doi: 10.1155/2015/627837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suo L, et al. Predictable chronic mild stress in adolescence increases resilience in adulthood. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013;38:1387–1400. doi: 10.1038/npp.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu LZ, et al. Short photoperiod condition increases susceptibility to stress in adolescent male rats. Behavioural brain research. 2016;300:38–44. doi: 10.1016/j.bbr.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Hsiao YM, et al. Early life stress dampens stress responsiveness in adolescence: Evaluation of neuroendocrine reactivity and coping behavior. Psychoneuroendocrinology. 2016;67:86–99. doi: 10.1016/j.psyneuen.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Oines E, Murison R, Mrdalj J, Gronli J, Milde AM. Neonatal maternal separation in male rats increases intestinal permeability and affects behavior after chronic social stress. Physiology & behavior. 2012;105:1058–1066. doi: 10.1016/j.physbeh.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 44.Uchida S, et al. Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:15007–15018. doi: 10.1523/JNEUROSCI.1436-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shalev U, Kafkafi N. Repeated maternal separation does not alter sucrose-reinforced and open-field behaviors. Pharmacology, biochemistry, and behavior. 2002;73:115–122. doi: 10.1016/S0091-3057(02)00756-6. [DOI] [PubMed] [Google Scholar]
- 46.Holder MK, Blaustein JD. Puberty and adolescence as a time of vulnerability to stressors that alter neurobehavioral processes. Frontiers in neuroendocrinology. 2014;35:89–110. doi: 10.1016/j.yfrne.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Commons KG, Cholanians AB, Babb JA, Ehlinger DG. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS chemical neuroscience. 2017;8:955–960. doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caetano SC, et al. Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry research. 2004;132:141–147. doi: 10.1016/j.pscychresns.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Zheng, L. J. et al. Altered amygdala and hippocampus effective connectivity in mild cognitive impairment patients with depression: a resting-state functional MR imaging study with granger causality analysis. Oncotarget, 10.18632/oncotarget.15335 (2017). [DOI] [PMC free article] [PubMed]
- 50.Cullen KR, et al. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA psychiatry. 2014;71:1138–1147. doi: 10.1001/jamapsychiatry.2014.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lussier AL, Romay-Tallon R, Caruncho HJ, Kalynchuk LE. Altered GABAergic and glutamatergic activity within the rat hippocampus and amygdala in rats subjected to repeated corticosterone administration but not restraint stress. Neuroscience. 2013;231:38–48. doi: 10.1016/j.neuroscience.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 52.Calem M, Bromis K, McGuire P, Morgan C, Kempton MJ. Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. NeuroImage. Clinical. 2017;14:471–479. doi: 10.1016/j.nicl.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deidda G, Bozarth IF, Cancedda L. Modulation of GABAergic transmission in development and neurodevelopmental disorders: investigating physiology and pathology to gain therapeutic perspectives. Frontiers in cellular neuroscience. 2014;8:119. doi: 10.3389/fncel.2014.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fiumelli, H. et al. An ion transport-independent role for the cation-chloride cotransporter KCC2 in dendritic spinogenesis in vivo. Cerebral cortex(New York, N.Y.: 1991) 23, 378–388, 10.1093/cercor/bhs027 (2013). [DOI] [PubMed]
- 55.Kovacs K, Basu K, Rouiller I, Sik A. Regional differences in the expression of K(+)-Cl(−) 2 cotransporter in the developing rat cortex. Brain structure & function. 2014;219:527–538. doi: 10.1007/s00429-013-0515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 57.Gao Y, Zhou JJ, Zhu Y, Kosten T, Li DP. Chronic Unpredictable Mild Stress Induces Loss of GABA Inhibition in Corticotrophin-Releasing Hormone-Expressing Neurons through NKCC1 Upregulation. Neuroendocrinology. 2017;104:194–208. doi: 10.1159/000446114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsukahara T, Masuhara M, Iwai H, Sonomura T, Sato T. The effect of repeated stress on KCC2 and NKCC1 immunoreactivity in the hippocampus of female mice. Data in brief. 2016;6:521–525. doi: 10.1016/j.dib.2015.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veerawatananan B, Surakul P, Chutabhakdikul N. Maternal restraint stress delays maturation of cation-chloride cotransporters and GABAA receptor subunits in the hippocampus of rat pups at puberty. Neurobiology of stress. 2016;3:1–7. doi: 10.1016/j.ynstr.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huberfeld G, et al. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu JJ, et al. Bumetanide reduce the seizure susceptibility induced by pentylenetetrazol via inhibition of aberrant hippocampal neurogenesis in neonatal rats after hypoxia-ischemia. Brain research bulletin. 2017;130:188–199. doi: 10.1016/j.brainresbull.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 62.Lemonnier E, Ben-Ari Y. The diuretic bumetanide decreases autistic behaviour in five infants treated during 3 months with no side effects. Acta paediatrica. 2010;99:1885–1888. doi: 10.1111/j.1651-2227.2010.01933.x. [DOI] [PubMed] [Google Scholar]
- 63.Cleary RT, et al. Bumetanide enhances phenobarbital efficacy in a rat model of hypoxic neonatal seizures. PloS one. 2013;8:e57148. doi: 10.1371/journal.pone.0057148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reid AY, Pittman QJ, Teskey GC. A prolonged experimental febrile seizure results in motor map reorganization in adulthood. Neurobiology of disease. 2012;45:692–700. doi: 10.1016/j.nbd.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 65.Jansen LA, Peugh LD, Roden WH, Ojemann JG. Impaired maturation of cortical GABA(A) receptor expression in pediatric epilepsy. Epilepsia. 2010;51:1456–1467. doi: 10.1111/j.1528-1167.2009.02491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adusei DC, Pacey LK, Chen D, Hampson DR. Early developmental alterations in GABAergic protein expression in fragile X knockout mice. Neuropharmacology. 2010;59:167–171. doi: 10.1016/j.neuropharm.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 67.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Molecular psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zanos, P. et al. A Negative Allosteric Modulator for alpha5 Subunit-Containing GABA Receptors Exerts a Rapid and Persistent Antidepressant-Like Action without the Side Effects of the NMDA Receptor Antagonist Ketamine in Mice. eNeuro4, 10.1523/ENEURO.0285-16.2017 (2017). [DOI] [PMC free article] [PubMed]
- 69.Cellot G, Cherubini E. Functional role of ambient GABA in refining neuronal circuits early in postnatal development. Frontiers in neural circuits. 2013;7:136. doi: 10.3389/fncir.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skilbeck KJ, Johnston GA, Hinton T. Stress and GABA receptors. Journal of neurochemistry. 2010;112:1115–1130. doi: 10.1111/j.1471-4159.2009.06539.x. [DOI] [PubMed] [Google Scholar]
- 71.Smith SS. The influence of stress at puberty on mood and learning: role of the alpha4betadelta GABAA receptor. Neuroscience. 2013;249:192–213. doi: 10.1016/j.neuroscience.2012.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roceri M, Hendriks W, Racagni G, Ellenbroek BA, Riva MA. Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Molecular psychiatry. 2002;7:609–616. doi: 10.1038/sj.mp.4001036. [DOI] [PubMed] [Google Scholar]
- 73.Roque A, Ochoa-Zarzosa A, Torner L. Maternal separation activates microglial cells and induces an inflammatory response in the hippocampus of male rat pups, independently of hypothalamic and peripheral cytokine levels. Brain, behavior, and immunity. 2016;55:39–48. doi: 10.1016/j.bbi.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 74.Sousa VC, et al. Maternal separation impairs long term-potentiation in CA1-CA3 synapses and hippocampal-dependent memory in old rats. Neurobiology of aging. 2014;35:1680–1685. doi: 10.1016/j.neurobiolaging.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 75.Baldini S, et al. Enriched early life experiences reduce adult anxiety-like behavior in rats: a role for insulin-like growth factor 1. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:11715–11723. doi: 10.1523/JNEUROSCI.3541-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hennessy MB, Fitch C, Jacobs S, Deak T, Schiml PA. Behavioral effects of peripheral corticotropin-releasing factor during maternal separation may be mediated by proinflammatory activity. Psychoneuroendocrinology. 2011;36:996–1004. doi: 10.1016/j.psyneuen.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Delgado y, Palacios R, et al. Magnetic resonance imaging and spectroscopy reveal differential hippocampal changes in anhedonic and resilient subtypes of the chronic mild stress rat model. Biological psychiatry. 2011;70:449–457. doi: 10.1016/j.biopsych.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 78.Zhang RX, et al. EphB2 in the Medial Prefrontal Cortex Regulates Vulnerability to Stress. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2016;41:2541–2556. doi: 10.1038/npp.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology. 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- 80.Yan, L. et al. Neuregulin-2 ablation results in dopamine dysregulation and severe behavioral phenotypes relevant to psychiatric disorders. Molecular psychiatry, 10.1038/mp.2017.22 (2017). [DOI] [PMC free article] [PubMed]
- 81.Park JH, et al. Anxiolytic-like effects of ginseng in the elevated plus-maze model: comparison of red ginseng and sun ginseng. Progress in neuro-psychopharmacology & biological psychiatry. 2005;29:895–900. doi: 10.1016/j.pnpbp.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 82.Estanislau C, Morato S. Prenatal stress produces more behavioral alterations than maternal separation in the elevated plus-maze and in the elevated T-maze. Behavioural brain research. 2005;163:70–77. doi: 10.1016/j.bbr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 83.Han Y, et al. AMPK Signaling in the Dorsal Hippocampus Negatively Regulates Contextual Fear Memory Formation. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2016;41:1849–1864. doi: 10.1038/npp.2015.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jian M, et al. eIF2alpha dephosphorylation in basolateral amygdala mediates reconsolidation of drug memory. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:10010–10021. doi: 10.1523/JNEUROSCI.0934-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu, L. Z. et al. BDNF-GSK-3beta-beta-Catenin Pathway in the mPFC Is Involved in Antidepressant-Like Effects of Morinda officinalis Oligosaccharides in Rats. Int J Neuropsychopharmacol, 10.1093/ijnp/pyw088 (2016). [DOI] [PMC free article] [PubMed]
- 86.Luo YX, et al. A novel UCS memory retrieval-extinction procedure to inhibit relapse to drug seeking. Nat Commun. 2015;6:7675. doi: 10.1038/ncomms8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.