Abstract
Background
Although the insulin-producing pancreatic β-cells are quite capable of adapting to both acute and chronic changes in metabolic demand, persistently high demand for insulin will ultimately lead to their progressive dysfunction and eventual loss. Recent and historical studies highlight the importance of ‘resting’ the β-cell as a means of preserving functional β-cell mass.
Scope of Review
We provide experimental evidence to highlight the remarkable plasticity for insulin production and secretion by the pancreatic β-cell alongside some clinical evidence that supports leveraging this unique ability to preserve β-cell function.
Major conclusions
Treatment strategies for type 2 diabetes mellitus (T2DM) targeted towards reducing the systemic metabolic burden, rather than demanding greater insulin production from an already beleaguered β-cell, should be emphasized to maintain endogenous insulin secretory function and delay the progression of T2DM.
Keywords: T2DM, Insulin production, β-cell rest
Abbreviations: ATF6, Activating Transcription Factor 6; CHOP, CCAAT/Enhancer-Binding Homologous Protein; EPAC, Exchange Factor Directly Activated by cAMP; EROβ1, ER-resident oxidoreductase β1; GIP, Gastric Inhibitory Polypeptide; GLP-1, Glucagon-like Peptide 1; GLUT2, Glucose Transporter 2; GSIS, Glucose Stimulated Insulin Secretion; IREα, Inositol Requiring Enzyme α; mTORC1, Mammalian Target of Rapamycin 1; NEFA, Non-esterified Fatty Acid; nH, Hill coefficient; PERK, Protein Kinase RNA-like Endoplasmic Reticulum Kinase; PKA, Protein Kinase A; PKC, Protein Kinase C; PLC, Phospholipase C; ROS, Reactive Oxygen Species; SNAP-25, Soluble NSF Attachment Protein 25; SNARE, Soluble NSF Attachment Protein Receptor; STZ, Streptozotocin; T2DM, Type 2 Diabetes Mellitus; TRP, Transient Receptor Potential; VAMP-2, Vehicle Associated Membrane Protein 2; VDCC, Voltage Dependent Calcium Channel
1. Homeostasis I: rapid intracellular changes meet metabolic demand
1.1. Nutrient sensing
Insulin-producing pancreatic β-cells are metabolically bound to the maintenance of physiological glycemic homeostasis. The acute response of the β-cell to elevations in circulating glucose concentrations following a meal has been extensively studied. However, the unique ability of the β-cell to adapt to chronic changes in glycemia conferred by a range of caloric extremes, from starvation to overnutrition, has been underappreciated yet reveals a remarkable adaptive plasticity of the β-cell to produce insulin depending on metabolic need. In this article, we examine the functional consequences of altered (pro)insulin biosynthesis and secretion in response to both acute and chronic changes in metabolic homeostasis.
The primary regulator of insulin secretion in β-cells is the circulating plasma glucose concentration [1]. The β-cell is fundamentally poised to sense glucose in the physiologically relevant range. It is equipped with the plasma membrane glucose transporter GLUT2 as well as glucokinase, which both have a Km for glucose in the millimolar range complementary to sensing circulating glucose concentrations. Once glucose enters the cell via GLUT2, it is phosphorylated by glucokinase to form glucose-6-phosphate in the rate limiting step of this process. The low expression of the high affinity forms of hexokinase in β-cells, which display end-product inhibition coupled with the sigmoidal enzyme kinetic behavior of glucokinase (nH = 1.7), ensures that at low glucose concentrations (≤2.5 mM) there is little substrate phosphorylation leading to greatly diminished glycolytic flux and reduced ATP/ADP ratio [2], [3]. Once formed, glucose-6-phosphate enters glycolysis to form pyruvate, which enters the mitochondrial Krebs' cycle to produce NADH and FADH2, which can then be oxidatively metabolized to produce ATP. β-cells have a unique metabolic inventory, not only geared toward ATP production but also for the generation of metabolic stimulus-coupling signals to regulate insulin production and secretion. For example, they lack both lactate dehydrogenase and the monocarboxylate transporter as an insurance policy against glucose-independent exercise-induced insulin secretion that could potentially result in fatal hypoglycemia [4]. Increased glycolytic flux in β-cells consequentially increases the ATP/ADP ratio that then causes plasma membrane ATP-sensitive K+ channels to close [5], leading to depolarization and subsequent opening of voltage-dependent L-type Ca2+channels (VDCCs) and rapid influx of Ca2+ into the cell. The rise in intracellular cytosolic Ca2+ is the primary mediator of insulin exocytosis [6], as it arbitrates the necessary events including insulin secretory granule (β-granule) traffic to the plasma membrane, priming, docking, and exocytotic regulated release via SNARE complexes.
Although glucose is the primary mediator of insulin secretion, a variety of other factors and macromolecules can contribute. Amino acids, fatty acids, incretins, some neurotransmitters, and certain pituitary hormones can influence insulin secretion. Growth hormone and prolactin both stimulate insulin secretion [7], the latter of which has also been shown to promote islet hypertrophy and hyperplasia during pregnancy. Interestingly, the prolactin inhibitor bromocriptine is part of the modern arsenal to combat T2DM; however, its anti-diabetic efficacy is more likely mediated by dopaminergic action rather than effects on prolactin-mediated insulin release [8]. Cortisol inhibits insulin secretion [9], while oxytocin was found to increase insulin secretion from isolated mouse islets in a glucose-independent manner [10]. Activation of membrane fatty acid receptors on the β-cell has contrasting effects on insulin secretion. The activation of the long chain fatty acid receptor GPCR40 has been shown to potentiate insulin secretion via phospholipase C activation [11], while activation of short chain fatty acid receptors FFAR2/FFAR3 may antagonize regulated insulin secretion [12], [13]. β-cell metabolism of endogenous fatty acids to long chain acyl-CoA can potentiate glucose stimulated insulin secretion, similar to malonyl-CoA [14], but the high glucose levels required for insulin secretion inhibit β-oxidation to some extent. Some amino acids, including the combination of l-glutamine and l-leucine, or l-arginine alone can contribute to insulin secretion by raising intracellular Ca2+ levels, albeit through different mechanisms [15], [16], [17], [18]. Beta-cells also contain neurotransmitter receptors on their cell surface, and considering the high degree of innervation present in pancreatic islets, it is not surprising that the nervous system has a profound effect on insulin secretion. For instance, the reduced fasting glycemia in splanchnicectomized dogs suggests an inhibitory role for the sympathetic nervous system on insulin secretion [19], which is further corroborated by the observed increase in basal insulin secretion observed in pancreatic transplant recipients [20]. Sympathetic signals such as epinephrine inhibit insulin secretion [21], while parasympathetic signals such as acetylcholine potentiate glucose stimulated insulin secretion [22]. The observation that orally delivered glucose leads to a greater insulin secretory response compared to intravenously administered glucose laid the foundation for the hypothesis that the gut may secrete an insulinotropic factor [23]. Indeed, both GLP-1 and GIP are gut hormones secreted by enteroendocrine cells in the intestine in response to macronutrients. In addition to potentiating glucose-stimulated insulin secretion, these peptides have garnered substantial pharmaceutical attention, particularly GLP-1 [24]. The mechanism underlying the potentiation of insulin secretion was found to be cAMP-dependent; both PKA and, to a lesser extent, EPAC activation contribute to the dose-dependent increase in glucose-stimulated secretion [25], [26]. However, chronic hyperglycemia has been implicated in PKA-mediated GLP-1 receptor downregulation, which may contribute to the blunted incretin effect observed during T2DM [27]. Recently, GLP-1 receptor agonism with physiological concentrations of GLP-1 (pmol/L) was found to activate PLC and, subsequently, the TRP family of channels leading to Na2+-mediated depolarization and insulin secretion [28]. Furthermore, GLP-1 has been shown to reduce β-cell apoptosis [29], delay gastric emptying [30], and inhibit food intake at the level of the arcuate nucleus [31]. The range of factors known to influence insulin secretion highlights the importance of the β-cell in maintaining organismal homeostasis by rapidly responding to metabolic changes. In this sense, the fate of insulin secretory granules (referred to as β-granules herein) within the β-cell is reflective of its external environment and should be considered when examining the plasticity of this endocrine cell.
1.2. Parallel (pro)insulin biosynthesis
Under normal circumstances, the β-cell retains a remarkable state in which insulin lost from intracellular stores by stimulated β-granule exocytosis is rapidly replenished by a parallel and specific upregulation of proinsulin biosynthesis (and other key β-granule proteins) [32]. One might assume that the regulation of insulin secretion and proinsulin biosynthesis are controlled similarly (although the molecular mechanisms are obviously different, i.e., regulated exocytosis versus protein synthesis translational control) [33]. However, there are some notable differences. The glucose threshold required for the secretion of insulin is between 4 and 6 mM, while the threshold for the biosynthesis of proinsulin is only 2–4 mM [34]. This bias assures (pro)insulin production occurs even in the absence of glucose-stimulated insulin secretion to ensure an optimal reservoir of insulin secretory stores. Glucose-induced proinsulin biosynthesis is Ca2+ independent and is regulated instead by alternative metabolic secondary signals produced from β-cell mitochondria in response to nutrient metabolism [35]. In particular, acute elevations in glucose following a meal induce rapid translation of preproinsulin mRNA. Other macronutrients, such as fatty acids, do not stimulate proinsulin biosynthesis despite being potent secretagogues for insulin secretion. Indeed, under conditions of chronic hyperlipidemia, excess fatty acids can lead to the depletion of intracellular insulin stores [36]. Similarly, the sulfonylurea class of drugs, including glibenclamide and glyburide, are powerful secretagogues but do not promote compensatory proinsulin biosynthesis [37]. Furthermore, they may accelerate β-cell loss, as ex vivo treatment of human islets with sulfonylureas led to a nearly 3-fold increase in apoptosis [38] The ADOPT study revealed ephemeral glycemic control in type 2 diabetics receiving glyburide monotherapy; glucose began to rebound only 3 months following treatment initiation [39]. As such, although sulfonylureas improve HbA1c in the short term, over time they are detrimental to β-cell function as they severely deplete insulin secretory capacity [40]. Acetylcholine does not appear to control insulin biosynthesis, but norepinephrine markedly inhibits both glucose-stimulated insulin secretion and production [22]. Finally, proinsulin biosynthesis is not regulated by the autocrine action of insulin [41]. A summary of factors with known effects on insulin secretion and (pro)insulin biosynthesis is provided in Table 1.
Table 1.
A summary of nutrients, peptide hormones, ions, neurotransmitters, and pharmaceuticals with known effects on β-cell proinsulin biosynthesis and insulin secretion.
| Effector | Proinsulin Biosynthesis | Insulin secretion | Remarks | Reference |
|---|---|---|---|---|
| Nutrients | ||||
| Glucose | Stimulates | Stimulates | Primary metabolic stimulus | [42], [43] |
| Mannose | Stimulates | Stimulates | Similar to glucose stimulation | [42], [43] |
| Mannoheptulose | Inhibits | Inhibits | Inhibits glucose stimulation by GK inhibition | [42], [43] |
| Fructose | No effect | Stimulates | Potentiates glucose stimulation | [42], [43], [44], [45] |
| Xylitol | No effect | No effect | [34], [46] | |
| Ribose | No effect | No effect | [46], [47] | |
| Galactose | No effect | No effect | Conflicting reports in literature | [43], [48] |
| N-acetylglucosamine | Slight stimulus | Stimulates | [43], [49] | |
| Other glucose stereoisomers | No effect | No effect | Includes allose, altrose, gulose, idose, talose | [50] |
| Other sugars | No effect | No effect | Includes sucrose, sorbitol, 2-deoxyglucose, 3-O-methylglucose, maltose, lactose | [34], [51] |
| Dihydroxyacetone | Slight stimulus | Stimulates | [42], [52] | |
| Glyceraldehyde | Slight stimulus | Stimulates | Potentiates glucose stimulation | [43], [47], [53], [54] |
| Pyruvate | Slight stimulus | Slight stimulus | Methyl-ester form | [32], [55] |
| Succinate | Stimulates | Stimulates | Methyl-ester form | [56], [57], [58] |
| Fumarate | No effect | No effect | Methyl-ester form | [56], [57] |
| Citrate | No effect | No effect | Methyl-ester form | [56], [57] |
| α-Ketoisocaproate | Stimulates | Stimulates | Potentiates glucose stimulation | [52], [53], [59] |
| Leucine | Stimulates | Stimulates | Only in the presence of glutamine | [52], [59] |
| Glutamine | No effect | No effect | [32], [60] | |
| Arginine | No effect | Stimulates | Possibly stimulates insulin secretion by depolarization | [16], [61], [62] |
| Inosine | Slight stimulus | Slight stimulus | [42], [47], [63] | |
| Guanosine | Slight stimulus | Slight stimulus | [47], [63] | |
| Adenosine | Slight stimulus | Slight stimulus | [63], [64] | |
| Ketone bodies | Slight stimulus | Stimulates | [52], [65], [66] | |
| Long Chain Fatty Acids | No Effect | Stimulates | [65], [67] | |
| Short Chain Fatty Acids | No Effect | Inhibit/Stimulate | Conflicting reports in the literature | [65], [68], [69], [70], [71], [72] |
| Peptide hormones | ||||
| ACTH | Stimulates | Stimulates | [73], [74] | |
| Corticosterone | Inhibits | Inhibits | [75], [76] | |
| Growth Hormone | Stimulates | Stimulates | [74], [77], [78] | |
| Glucagon | Stimulates | Stimulates | In in vitro studies - potentiates glucose stimulation | [79], [80] |
| GLP-1 (7–37) | Stimulates | Stimulates | Potentiates glucose stimulation | [81], [82] |
| GIP | Stimulates | Stimulates | Potentiates glucose stimulation | [83] |
| Somatostatin | No effect | Inhibits | Inhibits glucose stimulated insulin secretion | [84] |
| IAPP | No effect | No effect | [85], [86], [87] | |
| Type-1 interferons | Slight inhibition | No effect | Inhibits proinsulin biosynthesis at very high concentrations | [88], [89] |
| Inteleukin-1β | +/− | +/− | Concentration dependent | [90], [91], [92], [93] |
| Insulin | No effect | No effect | No autocrine direct effect | [94], [95] |
| Prolactin | No effect | Stimulates | Increases overall islet insulin synthesis via proliferative effect | [96], [97], [98] |
| Neurotransmitters | ||||
| Acetylcholine | No effect | Stimulates | Potentiates glucose stimulation | [99], [100] |
| Epinephrine | Inhibits | Inhibits | Inhibits glucose stimulation | [101], [102], [103] |
| Ions | ||||
| Ca2+ | Inhibits | Stimulates | Required for glucose stimulated insulin secretion. Inhibits general protein synthesis | [104], [105], [106], [107] |
| K+ | Stimulates | Stimulates | Stimulates insulin secretion by depolarization | [104], [108], [109] |
| Mg2+ | Required | No effect | Required for proinsulin biosynthesis | [104], [107] |
| Zn2+ | No effect | Inhibits | Involved in insulin crystal formation for storage in mature β-granules | [110], [111], [112] |
| Pharmacologic | ||||
| Sulfonylureas | No effect | Stimulates | [113], [114] | |
| Diazoxide | No effect | Inhibits | Inhibits glucose stimulated insulin secretion | [115], [116] |
| cAMP analogues | Stimulates | Stimulates | Potentiates glucose stimulation | [103], [117] |
| Phorbol esters | Slight stimulus | Stimulates | Slight potentiation of glucose-stimulated proinsulin biosynthesis reported | [118], [119], [120] |
| Trifluoperazine | No effect | Inhibits | CaM Kinase-II inhibition affects insulin secretion | [121] |
The regulation of proinsulin biosynthesis is predominately regulated at the translational level to allow a rapid and dynamic response to glucose that efficiently replenishes insulin secretory stores. This specific regulation of proinsulin biosynthesis by glucose also applies to ∼50 other β-cell proteins, all of which are β-granule proteins [122], [123]. As such, it is the principle mechanism for controlling β-granule biogenesis. This specific translational control was found to be orchestrated by a unique stem-loop in the 5′ UTR of preproinsulin mRNA [124], which is common to other β-granule protein mRNAs as well [118], [123], [125]. Translational control requires stimulus-coupled mitochondrial metabolism of glucose independent of β-cell depolarization and Ca2+ [126], [127]. Longer term (8 h+) glucose administration can stabilize preproinsulin mRNA through an UUGAA sequence and the PTB domain on the 3′ UTR [124]. Even longer term (18 h+) glucose exposure regulates preproinsulin gene expression [128], but these are relatively small effects and are not relevant under normal circumstances where fluctuations in glucose occur approximately 2 h post-prandially. Notwithstanding, the predominant translational control of proinsulin biosynthesis by glucose ensures that insulin secreted via exocytosis is rapidly replenished under normal circumstances to maintain intracellular insulin stores at optimal levels.
The production of insulin occurs in multiple, well-characterized steps. First, a preproinsulin precursor is translated, which contains an N-terminal signal sequence enabling the newly formed preproinsulin to enter the lumen of the rough endoplasmic reticulum (RER) to facilitate the proper folding of proinsulin, stabilized by three disulfide bonds [129]. The signal peptide of preproinsulin is rapidly cleaved, likely co-translationally, to form proinsulin. Proinsulin, the first prohormone to be discovered [130], is then trafficked from the RER through the Golgi apparatus continuum [131] and concentrates in restricted regions of the trans-Golgi network at sites where immature insulin granules form. Here, proinsulin to insulin and C-peptide processing begins by the action of two Ca2+-sensitive prohormone convertases, PCSK2 (PC2) and PCSK3 (PC1/3), with basic amino acid trimming of split-proinsulin intermediates by carboxypeptidase H/E [132], [133]. Consistent with the internal pH of newly forming β-granules, PCSK2/PCSK3 display optimal activity at pH 5.5 [134]. This acidic pH optimum and the influx of Ca2+ into an immature β-granule initiate proinsulin processing and retain insulin accumulation within the organelle where it is stored [135]. In addition, it has been proposed that PCSK2 and PCSK3 are also regulated by granin chaperones, including 7B2 (PCSK3) and proSAAS (PCSK2), but only the former has an appreciable effect on facilitating proinsulin processing [136]. As well as Ca2+ influx, and as immature β-granules mature, there is also an influx of Zn2+ via zinc transporters, most notably ZnT10, allowing hexameric crystallization of insulin comprising six insulin molecules to two Zn2+ cations [137]. It should also be noted that proinsulin processing is sequential, where PCSK3 catalyzes the first cleavage event to produce the intermediate des-31,32 proinsulin [138], although both endopeptidases are capable of cleaving either site [139]. This sequential proinsulin processing is reflected in that des-31,32 proinsulin is the predominant component of the hyperproinsulinemia found circulating in type 2 diabetes [140].
1.3. The fate of β-cell insulin stores
Normally, the β-cell maintains an optimal intracellular storage pool of insulin granules that is not only preserved by the balance between (pro)insulin production and insulin secretion but also by longer term β-granule/insulin degradation mediated by autophagic mechanisms [122], [141]. Like most neuroendocrine cells, β-cells have at least two secretory pathways, a regulated secretory pathway for the secretion of insulin in response to glucose, and a constitutive pathway that serves to renew membrane proteins and facilitate general non-regulated protein secretion and the delivery of newly synthesized plasma membrane proteins [142]. Radiolabeled pulse-chase experiments confirmed that (pro)insulin, regardless of whether it was newly synthesized or fully processed, is destined to the regulated pathway with 99% efficiency [143]. How proteins designated for secretion via the constitutive pathway or the regulated pathway are sorted at the trans-Golgi network remains unclear, as are the majority of the proteins that interact with (pro)insulin to direct its sequential synthesis, folding, trafficking, and packing. However, the rate-limiting step for proinsulin sorting is known to occur at the site of β-granule biogenesis in the trans-Golgi network, rather than the RER [144]. The specific proteins involved in (pro)insulin sorting at the Golgi likely involve a number of constituents. Key proteins found to be essential for insulin trafficking at the trans-Golgi have been elusive, although the PICK1/ICA69 heterodimeric complex as well as SORCS1 have emerged as candidates [145], [146]. The trafficking of new β-granules from the trans-Golgi requires kinesin-1, syntaxin-3, and TMEM24 [147], [148], [149], but non-protein components are also essential for regulated β-granule traffic. For example, Ca2+ is important for increasing β-granule transport to the plasma membrane in response to glucose, via Ca2+-dependent activation of protein phosphatase-2Bβ to dephosphorylate and increase kinesin mediated β-granule transport [150]. The transit time from the trans-Golgi network to the plasma membrane can occur in as little as 30–40 min, and newly synthesized (pro)insulin can be preferentially secreted in as little as one hour following synthesis [143]. However, full conversion of proinsulin to insulin requires ∼3 h [151], so during conditions of chronic hyperglycemia, there is an increased proportional secretion of incompletely processed insulin [140]. Also of note is that β-granules represent the richest sites of cholesterol accumulation in β-cells. Disruption of cholesterol biosynthesis leads to impaired membrane fusion and β-granule trafficking [152], while excessive cholesterol leads to grossly enlarged β-granules and disrupted trafficking [153]. As such, cholesterol mishandling of β-cells in type 2 diabetes could also contribute to the insulin secretory dysfunction.
Regulated insulin release is uniquely biphasic [154]. A few minutes following stimulation by glucose, the first phase of insulin secretion consists of a high amplitude but short duration burst of insulin exocytosis that largely consists of β-granules considered to be part of the readily releasable pool, that is, β-granules already docked and primed at the plasma membrane of the β-cell [155]. The reduced impedance of insulin secretion during the first phase is thought to be attributed to a number of proteins including gelsolin/syntaxin-4, which are involved in the unraveling of F-actin complexes near the plasma membrane, PKC dependent inactivation of the inhibitory Munc18/syntaxin-1 complex, and the synaptotagmin interaction with VAMP2 and SNAP-25 [156], [157], [158], [159], [160]. While most first phase insulin secretion consists of docked β-granules, some exocytosis of β-granules adjacent to the plasma membrane can also contribute. The trafficking of these β-granules is mediated by small GTPases, including Rab3A, Rab27, RaIA, and Rap1, all of which aid in the docking and priming of these granules to the plasma membrane, potentially in a cAMP-dependent manner via EPAC activity [161], [162]. The entire process of first phase insulin secretion is largely Ca2+ dependent [155], but the second phase of insulin secretion, which has a lower amplitude but longer duration [163], can contribute substantially to the total insulin output with prolonged stimulation [164]. The second phase of insulin release not only reflects exocytosis of primed and docked β-granules, but also reflects Ca2+-dependent transport β-granules to the plasma membrane prior to undergoing exocytosis [150], [163]. Often overlooked in the discussion of insulin exocytosis is the parallel endocytosis of the fused secretory granule membranes. If this did not occur, GSIS would lead to expanded β-cell size. While β-cell size does transiently increase (∼4 min) following GSIS [165], early studies using electron microscopy and horseradish-peroxidase confirmed that β-cell size is stable over time and that the rate of exocytosis is equal to that of endocytosis [166].
Not all insulin granules undergo exocytosis, and if retained for ∼5 days they are targeted for internal degradation by autophagy [122]. Normally, this system plays a janitorial role in maintaining optimal functional insulin secretory stores in the β-cell [167]. It has been presumed that this turnover occurs through macroautophagy (also referred to as crinophagy) [168], though β-granules are more commonly degraded by microautophagy, a process where aged β-granules are engulfed by autophagolysosomes [169], [170]. In fact, bulk autophagy may be inhibited by the degradation of β-granules, as the resultant amino acids released from insulin catabolism would activate mTORC1 that, in turn, suppress macroautophagy [171].
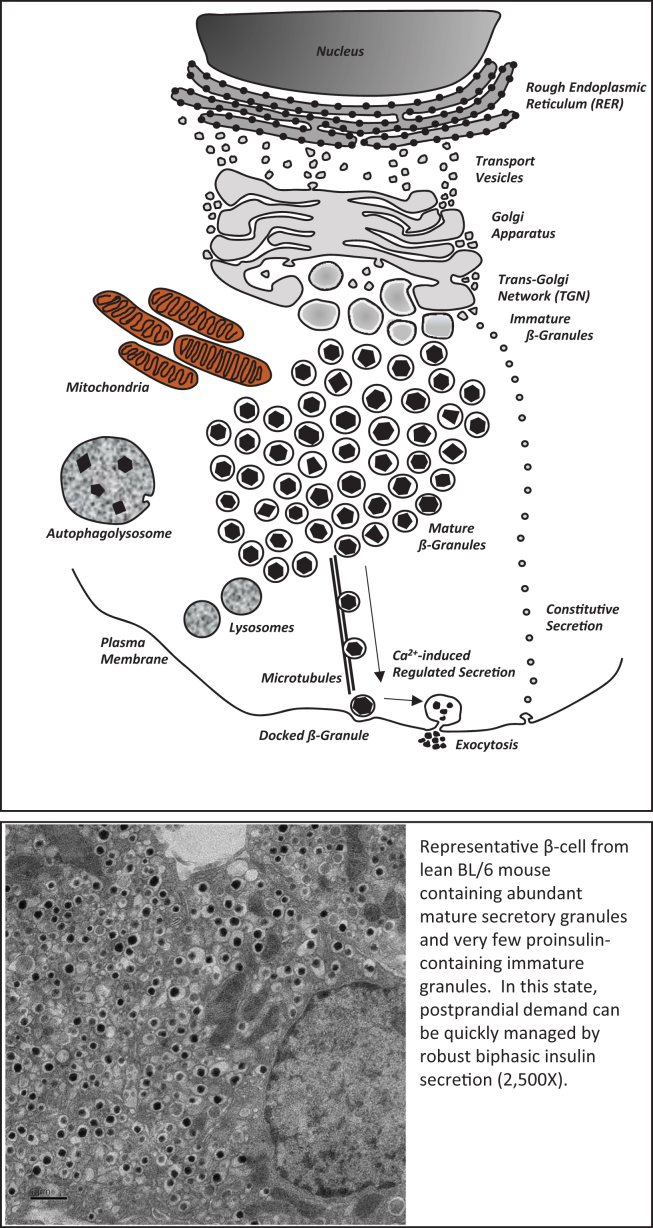
As depicted in Figure 1, under normal circumstances, a large and rather stable number of mature insulin granules characteristically mark normal β-cell biology, and this population is maintained via equilibrium between secretion, biosynthesis, and degradation. However, if this balance is disrupted, such as in obesity or during a prolonged fast, the β-cell will adapt in compensation to prevent failures associated with aberrant insulin secretion or biosynthesis. These latter physiological circumstances are outlined in the following section.
Figure 1.
A graphic representation and a transmission electron micrograph of a 12-week old lean C57/B6 β-cell demonstrating an abundance of mature insulin secretory granules and few immature proinsulin-containing granules. Magnification, 2500×.
2. Homeostasis II: altered metabolic demand challenges glycemic control
2.1. Response to starvation
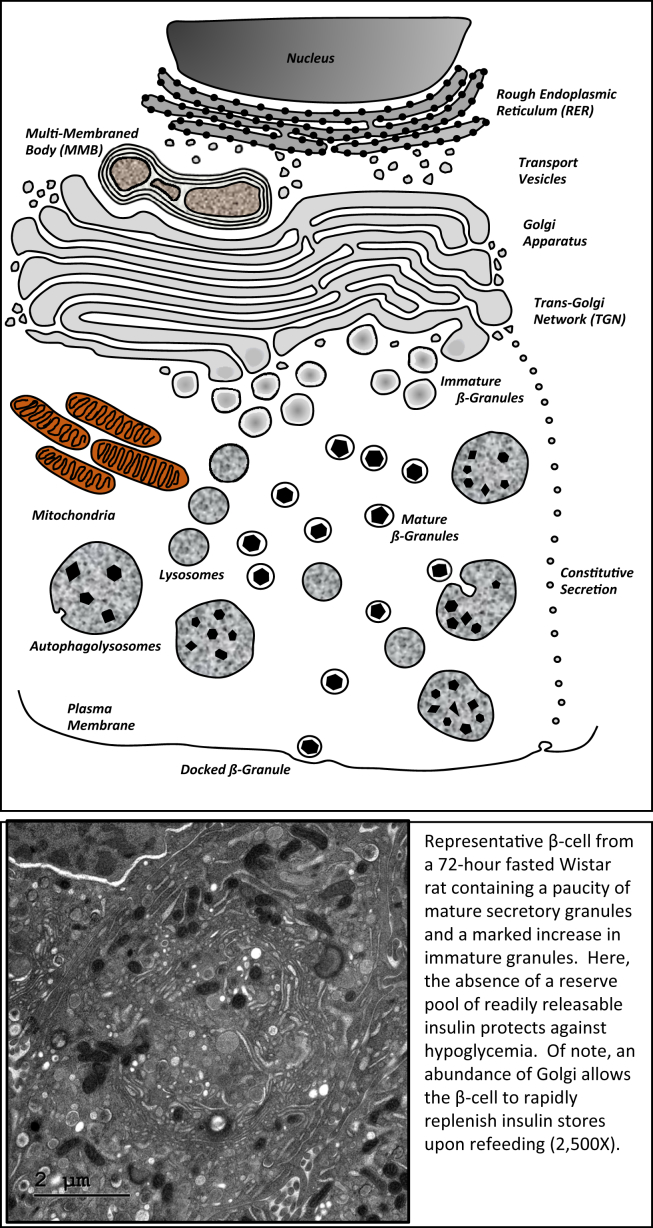
The modern human genome was likely selected during the late Paleolithic era, when humans sustained themselves primarily as hunter-gatherers [172]. Early humans thrived in an environment characterized by intermittent periods of fasting and feasting. The selection of regulatory metabolic genes that protected against starvation by prioritizing food intake and storage was essential [173] for our survival. Many biological systems remain wired to quickly adapt to oscillations between nutrient abundance and scarcity, and the pancreatic β-cell is no exception. Indeed, excessive insulin secretion during starvation could lead to detrimental hypoglycemia. Previous studies of β-cell ultrastructure provided clues as to how the β-cell adapts during starvation-refeeding. Prolonged starvation did not induce β-cell injury, but insulin stores become markedly reduced [174], [175]. In normal rats fasted for 3 days, electron micrographs of islet β-cells also reveal this massive insulin degranulation concurrent with increased numbers of autophagolysosomes and also an apparent expanded Golgi apparatus (unpublished data). This phenotype is portrayed in Figure 2, which shows the ‘poised’ state of the fasted β-cell, although depleted of mature β-granules to protect against hypoglycemia, the expanded Golgi stands ready for rapid (pro)insulin biosynthesis and maturation following a postprandial stimulus. Indeed, only 2–4 h after refeeding, β-cells replenish their stores of intracellular insulin and move towards a normal morphology (Figure 1), highlighting the ability of the entire cellular ultrastructure to rapidly and reversibly adjust to changes in metabolic demand (unpublished data). This remarkable adaptive plasticity is emphasized by insulinoma transplantation experiments in rats, where, in parallel to chronic hypoglycemia, endogenous β-cells ‘disappear’. However, following removal of the insulinoma, β-cells ‘reappear’ [176]. This finding cannot be explained by islet atrophy and subsequent regeneration but instead is the consequence of rapid endogenous β-cell degranulation in response to hypoglycemia (similar to that found in prolonged fasting), followed by prompt regranulation once circulating insulin levels are normalized and glucose homeostasis recovers.
Figure 2.
A graphic representation and a transmission electron micrograph of a 3-day fasted 12-week old Wistar rat β-cell showing degranulation of mature insulin secretory granules, a noticeable increase in immature granules, the presence of autophagolysosomes, and an expansion of the Golgi apparatus. Magnification, 2500×.
The β-cell adaptive plasticity can also be applied when the normal balance between insulin production and secretion becomes disrupted. An example is found in the lean Rab3a−/- transgenic mouse model. Here, β-cells have reduced insulin secretion caused by deletion of the GTPase, Rab3A, necessary for β-granule transport, but (pro)insulin production remains normal. However, a normal cytoplasmic pool of β-granules is maintained by upregulating autophagy to rebalance the excessive biosynthesis of (pro)insulin relative to decreased insulin secretion in this model [168].
2.2. Response to overnutrition
Obesity is frequently characterized by insulin resistance, a presumed state of insufficient insulin production, and insulin secretory dysfunction despite high metabolic demand. In these circumstances, the β-cell can adapt by increasing β-cell mass through a variety of means, including hypertrophy, β-cell proliferation, neogenesis, and inhibition of apoptosis, all of which lead to enhanced insulin secretory capacity [177]. However, although the β-cell is beautifully geared to adapt to fasting/refeeding states, it seems to struggle to adjust to chronic overnutrition. If glucose levels remain persistently high, the β-cell becomes dysfunctional and is ultimately unable to sufficiently compensate. In this case, a variety of stressors related to hyperglycemia and increased insulin demand, including oxidative stress, endoplasmic reticulum (ER) stress, hyperlipidemic stress, amyloidal stress, and inflammatory stress [178], [179], [180], [181], have been proposed to contribute to β-cell dysfunction and ultimately apoptosis (see Section 3.2). In addition, the hyperinsulinemia associated with obesity-linked T2DM can further drive β-cell dysfunction by promoting insulin-induced insulin resistance [182]. Also, in an insulin resistant state, the desensitization of CNS insulin receptors could lead to a feed-forward loop that drives further β-cell dysfunction [183]. The characteristic consequences of β-cell dysfunction during T2DM include diminished first phase insulin secretion, increased basal insulin secretion, impaired glucose-sensing of the β-cell, and an increased proportion of proinsulin secretion [184], [185]. However, it is unclear whether these manifestations of dysfunction contribute to the pathogenesis of T2DM, or if they are simply a consequence of β-cells striving to produce sufficient insulin to compensate for metabolic overload and an insulin resistant state [186], [187]. Observations of reduced pancreatic insulin content in parallel to insulin insufficiency have led to the notion that β-cells during T2DM have impaired secretory capacity [188]. Nearly half a century ago, ultrastructural studies of β-cells following infusion of high glucose or anti-insulin antisera revealed massive insulin degranulation, implying a presumed defective insulin secretion [189]. Importantly, the long standing assumption that insulin production declines during β-cell dysfunction [184] has led to many treatment strategies aimed at increasing β-cell insulin secretion, most notably sulfonylureas and GLP-1 receptor agonists [190], [191]. But long-term use of some of these, like sufonylureas, eventually accelerate β-cell demise and dysfunction by compromising endogenous insulin secretory capacity [38], [39], [191].
3. An unpaid debt: contributing factors to β-cell dysfunction
3.1. The consequence of chronic metabolic demand
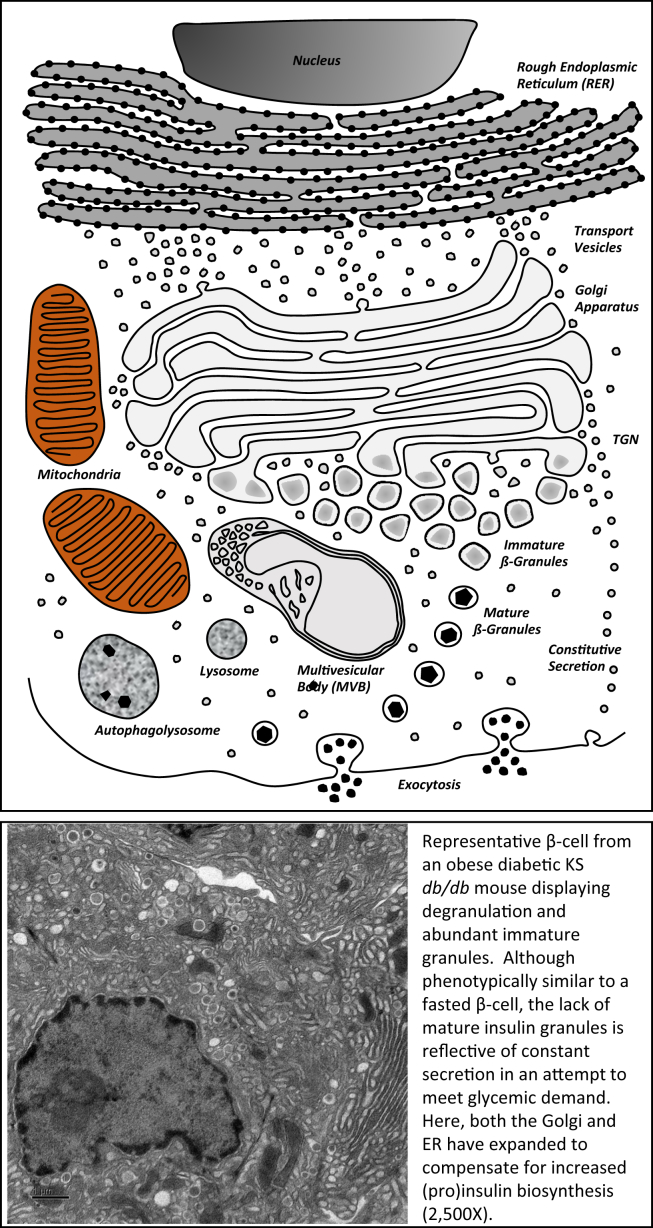
The observation that non-diabetic human obesity is associated with increased islet size has been known for 80 years [192]. Modern comparisons have confirmed that most obese non-diabetic donors have increased β-cell mass compared to lean controls, whereas pancreatic sections from obese T2DM, and even prediabetic subjects, have an apparent reduced β-cell mass [193], [194], [195], [196]. Interestingly, the ability to adapt to metabolic demand may be conferred during the prenatal period, as a low birth weight was associated with reduced β-cell mass and an increased likelihood of development diabetes later in life [197]. The primary driver of β-cell mass adaptation in obesity is insulin resistance, which increases the demand for insulin [198]. Tissue-specific knockout of the insulin receptor, particularly in the liver, began to parse out the role of these metabolic tissues in insulin resistance and the need for consequential β-cell compensation [199], [200], [201]. However, despite the obvious importance of insulin resistance during obesity and T2DM, around two-thirds of obese individuals do not develop frank diabetes, suggesting that the β-cell is capable of adapting to metabolic demand to a degree in a majority of individuals [202]. Although numerous genetic susceptibility variants have been identified for T2DM, their effect sizes are too small to account for either the prevalence of the disease or its relatively recent emergence (∼35 years) as a global epidemic [203], [204], [205], making it more likely that environmental factors such as diet and lifestyle are the predominant factors in the progression of T2DM. Once T2DM manifests in obese individuals, it is presumed that a stark decline in β-cell mass has been initiated. But this is difficult to demonstrate unequivocally, since there is currently no way to measure β-cell mass in living individuals, and it is estimated that most patients are not diagnosed until many years after the onset of T2DM [206]. This is indicated by there being significant β-cell loss in patients with impaired fasting glucose or prediabetes [195]. It is likely that rate of β-cell death via apoptosis as a consequence of stressors related to insulin resistance, inflammation, and hyperglycemia outweigh the rate of proliferation, as ex vivo studies of human islets revealed that glucolipotoxicity led to decreased markers of proliferation and increased apoptosis [207]. In this case, β-cell mass can become so depleted that a lifetime of supplementary insulin therapy would be the only recourse [208]. However, even as β-cell mass decreases, the remaining β-cells valiantly attempt to meet metabolic demand in the face of steadily increasing insulin resistance. Even in severely obese diabetic KSdb/db mice, that have an apparent decrease in β-cell mass, there is a marked increase in proinsulin biosynthesis in the remaining β-cells [209]. This upregulation of insulin production is facilitated by an expansion of the RER and Golgi apparatus, an increase in the number of immature β-granules but a stark reduction in mature β-granules (the cell biology of such obese diabetic β-cells is illustrated in Figure 3). Yet these animals remain markedly hyperinsulinemic (≥10-fold higher that lean KS+/+ control mice [209]). So, it seems that these β-cells are producing as much (pro)insulin as they possibly can and then rapidly secreting it in a persistent effort to manage the hyperglycemia and compensate for insulin resistance, futile though that might be with such decreased β-cell mass in this mouse model [209]. Although isolated islets from these animals display all the hallmarks of secretory dysfunction in T2DM, including elevated basal insulin secretion, blunted first phase insulin secretion, an increase in the ratio of secreted proinsulin to insulin, and left-shifted glucose sensing, these ‘flaws’ could in fact all be part of the compensatory mechanisms to secrete more insulin to meet the metabolic demand in the face of insulin resistance. In this sense, these β-cells may not be dysfunctional, but rather functionally adapted to produce continuous large quantities of insulin.
Figure 3.
A graphic representation and a transmission electron micrograph of a 12-week old obese diabetic KS db/db showing marked degranulation of mature insulin secretory granules, abundant immature granules, and an expansion of both the RER and Golgi apparatus. Magnification, 2500×.
3.2. A bombardment of stressors
A combination of many interrelated stressors causes β-cell loss in the pathogenesis of obese T2DM. As obesity develops, adipose tissue expands to provide a reservoir for the associated positive energy balance [210]. But, as a consequence, they release of a variety of adipokines and proinflammatory cytokines as well as NEFAs and glycerol as a result of increased lipolysis [211]. This is in addition to increased dietary lipid intake and hepatic lipogenesis. The deleterious effect of increased internal and external fatty acids on the β-cell is termed lipotoxicity. While fatty acids can potentiate insulin secretion, the absence of a complementary increase in proinsulin biosynthesis can deplete β-cells of mature insulin granules and contribute to dysfunction [166]. As obesity is accompanied by a chronic state of low-grade inflammation, enhanced secretion of proinflammatory cytokines, including TNF-α, IL-6, and IFN-γ, is another consequence of increased adiposity [212]. In adipose tissue, these cytokines can create a vicious cycle as they lead to macrophage recruitment, greater inflammation, and subsequent increased cytokine release. These can then have detrimental effects on β-cells that influence insulin secretion and survival [213].
Factors intrinsic to the β-cell may play a more prominent role. One notable factor in humans, islet amyloid polypeptide (amylin, or IAPP) is co-secreted with insulin and usually exists in a soluble form [214]. Like most other β-granule proteins, the biosynthesis of IAPP is coordinated with that of proinsulin [40]. Normally, IAPP functions centrally, where it contributes to postprandial satiety and gastric emptying. But when proinsulin, and proIAPP [40], biosynthesis are chronically increased in T2DM [209], IAPP can fold into cytotoxic aggregates, which have been implicated in β-cell apoptosis in primates [180]. IAPP aggregates form prior to the onset of frank T2DM during fasting hyperglycemia, implicating the onset of β-cell secretory dysfunction prior to IAPP aggregate deposition [215]. However, the occurrence of IAPP containing amyloid plaques is highly variable in islets from T2DM human cadavers (between 1% and 80%), suggesting that IAPP is not etiological in the pathogenesis of T2DM [216].
A frequently attributed factor in secretory dysfunction and apoptosis of β-cells is ER-stress. When protein translation is increased, cells can compensate by activating the unfolded protein response (UPR). This process is mediated by at least 3 proteins, IRE1α, PERK, and ATF6, which collaborate to slow protein translation, increase the production of protein-folding chaperones and enhance membrane biogenesis [217]. The UPR can also be upregulated when foreign or variant proteins are synthesized, and if persistent can lead to ER-stress, which occurs primarily through CHOP induction, RER dilation, and subsequent apoptosis [218]. An excellent example of this is the ‘Akita mouse’ in which a proinsulin variant is synthesized and inappropriately folded, inducing ER-stress, β-cell apoptosis, and subsequent lean diabetes [219]. It has been estimated that up to 20% of newly synthesized proinsulin is misfolded, and since proinsulin accounts for 30–50% of the total β-cell translational load, it is not unreasonable to assume that the β-cell routinely manages a substantial amount of protein misfolding [220]. However, this notion has been recently challenged [201]. In β-cells of obese diabetic KSdb/db mice, expanded RER and Golgi apparatus are observed to cater to increased proinsulin biosynthesis, but absolutely no evidence of a dilated RER or ER-stress was found [209]. The UPR is likely present to aid increased proinsulin production but not ER-stress, which would jeopardize this process [209]. Indeed, in light of massively upregulated insulin production in remaining β-cells in the pathogenesis of common obese T2DM, ER-stress is unlikely to play a significant role to β-cell loss.
Lipid peroxidation, protein oxidation, and DNA damage are all consequences of the excess generation of mitochondrial-derived products of oxidative phosphorylation. Oxygen is the final electron acceptor of respiration, the majority of which is reduced to H2O. However, a small percentage of the O2 is converted to free radicals, such as superoxide anion (•O2−), which are usually rapidly neutralized by a combination of cellular antioxidant enzymes including superoxide dismutase, glutathione peroxidase, and catalase [221]. Pancreatic β-cells are wired to have high rates of ATP production not only to provide energy but also for secondary stimulus-coupling signals to stimulate insulin production and secretion. However, they paradoxically have low levels of some antioxidant enzymes, such as glutathione peroxidase and catalase [222]. As a consequence, β-cells are deficient in H2O2 clearance. Oxygen radical production has been implicated in disrupted insulin secretion but is also a necessary byproduct of ATP generation and subsequent GSIS [223]. Uncoupling proteins facilitate the return of protons to the mitochondrial matrix. UCP-2 in particular is induced by •O2− and regulates the rate of respiration, mitochondrial membrane potential, ATP production, and insulin release [224]. Thus, it can protect β-cells from oxidative damage by slowing oxidative phosphorylation and attenuating GSIS [225]. However, this comes at the cost of reduced secretory capacity. Furthermore, ROS may disrupt insulin biosynthesis, as ROS exposed islets showed reduced insulin promoter activity and suppressed insulin mRNA activity [188], likely mediated by ROS-mediated activation of the c-Jun N-terminal kinase pathway (JNK) and subsequently reduced Pdx-1 binding to the insulin promoter [226]. As such, chronically elevated ROS production during obese T2DM in β-cells, likely driven by increased mitochondrial metabolism as a consequence of the chronic hyperglycemia and/or hyperlipidemia can cause oxidative stress leading to secretory dysfunction and apoptosis. The β-cells may be particularly susceptible to oxidative stress under such circumstances due to deficient expression of certain antioxidant enzymes [214].
3.3. Secretory dysfunction: beginning of the end?
In subjects with normal glucose tolerance and varying degrees of obesity, β-cell function was found to vary quantitatively with differences in insulin sensitivity [227]. In fact, studies in mice have revealed that β-cell secretory dysfunction is the primary driver of β-cell failure, rather than reduced β-cell mass, impaired glucose metabolism, or steatosis [228]. Several characteristic events mark the gradual loss of β-cell secretory dysfunction. Increased basal insulin secretion even in the absence of glucose stimulus is indicative of impaired glucose sensing [227]. Also, irregularity emerges in both the frequency and amplitude of the electrophysically-derived oscillations in insulin secretion [229]. In response to intravenous glucose administration, the amplitude of first phase insulin secretion is diminished, and, as the disease progresses, glucose-induced first phase insulin secretion is lost entirely [229], [230]. In parallel, there is a delayed and blunted secretory response to mixed meal consumption, underlying the impaired incretin response observed in insulin resistant subjects [231]. The mechanism of the diminished incretin effect in T2DM is likely due to chronic hyperglycemia that blunts GLP-1 receptor signaling in β-cells via a PKA-dependent mechanism [27]. Furthermore, there is a greater proportion of secreted proinsulin in response to a meal during secretory dysfunction in T2DM [232]. It could be argued that the hyperinsulinemia present during β-cell secretory dysfunction may be partly the consequence of non-regulated constitutive secretion of incompletely processed insulin. However, although the amount of proinsulin secreted is increased, leading to an increased ratio of proinsulin to insulin in the circulation, the absolute levels of circulating proinsulin remain a small proportion of the total circulating insulin, in part due to a greater degree of basal insulin secretion in the absence of appropriate stimulation [233]. Fortunately, β-cell dysfunction does not necessarily guarantee β-cell demise. By lightening the chronic barrage of insults directed at β-cells, most notably persistently elevated glucose, β-cells can then rapidly regain functional secretory capacity. Freshly isolated islets from either 6J or KSdb/db obese diabetic mice display hallmarks of secretory dysfunction and yet maintain a proinsulin biosynthetic rate that far exceeds that of their lean control counterparts. Further, when these ‘db/db islets’ are allowed euglycemic recovery for 8–12 h, dysfunction is reversed and normal insulin secretory capacity and function return [209]. As such, the concept of ‘β-cell rest’ is re-emerging as a means to preserve functional β-cell mass and delay the progression of T2DM.
4. Beating foreclosure: strategies to mitigate demand and retain secretory function
4.1. Evidence for in vivo β-cell rest
Perhaps the most efficacious means of halting the progression of obesity-linked T2DM and islet dysfunction in most individuals is by reducing caloric intake to the point at which energy storage is no longer heavily prioritized. This has been clearly established in rodent models of advanced diabetes, where dietary restriction of db/db mice to db/+ levels was found to preserve β-cell mass and function, in part, by reducing oxidative stress [234]. Likewise, in human individuals with T2DM, six-weeks of severe caloric restriction (>900 kcal/d) led to a doubling of their β-cell disposition index, which was likely a consequence of the 40% reduction in circulating insulin, indicative of reduced insulin demand [235]. In humans, lifestyle intervention including diet and exercise was found to be more effective than metformin for the prevention of T2DM [236]. Caloric restriction (CR) itself, particularly by alternating days of CR with ad libitum refeeding days, has shown potential to improve insulin sensitivity and reduce body weight. An 8-week study in obese individuals found that alternating days of 80% CR followed by ad libitum refeeding resulted in a 33% decrease in HOMA-IR and an 8% reduction in body weight [237]. All this might be viewed as in vivo evidence for taking effort off of the β-cell (i.e., ‘β-cell rest’) that then improves endogenous insulin secretory capacity. However, outside of an experimental setting, adherence to any form of caloric restriction for an extended period can be daunting for T2DM patients. Fortunately, a number of pharmaceutical strategies, particularly those that target multiple pathways independent of the β-cell, are attractive strategies to reduce the metabolic demand on the β-cell and preserve insulin secretory function.
More than 40 years ago, Greenwood et al. administered diazoxide (to inhibit endogenous insulin secretion and promote a degree of transient ‘β-cell rest’) to healthy volunteers and T2DM patients, previously untreated with insulin. β-cell function was assessed using a glucagon and tolbutamide stimulation test prior to and after treatment. The excursions in endogenous insulin secretion following stimulation tests increased over baseline levels by 7%, 70%, 280%, and 500% in T2DM placebo, healthy subjects, T2DM (low β-cell reserve) and T2DM (high β-cell reserve) treatment groups, respectively [238]. The authors concluded that poor insulin response in diabetics resulted, in part, from chronic overstimulation, and the observed improvements following diazoxide treatment reflected an increase in endogenous β-cell insulin stores. Note that diazoxide inhibits insulin secretion but has no effect on (pro)insulin biosynthesis (Table 1). Similar diazoxide studies in T2DM human islets have also shown that such treatment can restore normal pulsatile insulin secretion by recovering insulin secretory capacity [239].
Glaser et al. found similar effects on improved β-cell function following short-term (16.6 ± 1.5 days), continuous subcutaneous insulin infusion in T2DM patients uncontrolled by conventional oral therapies. Near euglycemia was achieved (<7.7 mM glucose) throughout the treatment period, resulting in improved glycemic control after cessation of insulin infusion. β-cell function tests (glucagon stimulation and IV glucose) conducted 48 h following cessation of insulin infusion resulted in marked improvements in maximal incremental C-peptide response to glucagon and peak insulin response. The authors conclude “that chronic hyperglycemia induces a further (reversible) impairment in the already reduced insulin secretory capacity of the diabetic [240].” Taken together, these early observational data provided evidence that short-term improvement of glycemic control can be beneficial to β-cell function by inducing β-cell rest and/or decreasing glucotoxicity, but its contribution and impact on achieving optimal metabolic control was speculative due to limitations in the size and duration of the treatment.
A Diabetes Outcome Progression Trail (ADOPT) evaluated rosiglitazone, metformin and glyburide as first line therapy for newly diagnosed T2DM patients in a double-blind, randomized, controlled clinical trial involving 4360 patients. The primary endpoint was monotherapy failure and the patients were treated for a median of 4 years [39]. Monotherapy failed in 143 patients on rosiglitazone (2.9 per 100 patient-years), 207 on metformin (4.3 per 100 patient-years), and 311 on glyburide (7.5 per 100 patient-years). At 4 years of evaluation, 40%, 36%, and 26% of the patients in the rosiglitazone, metformin and glyburide treatment groups, respectively, had %HbA1c < 7%. Rosiglitazone slowed the rate of β-cell dysfunction and improved insulin resistance to a greater extent compared to metformin and glyburide, which may explain the more durable effect on glycemic control. This also suggests that alleviating the demand on the β-cell by improving insulin sensitivity can induce a degree of β-cell rest that is beneficial to the treatment of T2DM. Conversely, the ADOPT study indicated that although glyburide had an initial beneficial effect, in the long run, it was detrimental to β-cell function and T2DM treatment [39]. This was likely due to accelerating reduced insulin secretory capacity of β-cells in T2DM, as although sulfonylureas increase insulin secretion, they have no effect on (pro)insulin production and deplete β-cell insulin stores (Table 1) [40].
4.2. Combination therapy and the case for early intervention
The ‘treat to failure approach’ may not be the optimal therapeutic strategy in slowing the progression of T2DM, and may do even less to retain residual β-cell function [241], [242]. Failure implies deterioration of glycemic control and necessitates more aggressive intervention—a cycle that if allowed to continue unabated can lead to a lifetime of insulin dependence. Fortunately, this perception is changing, and if %HbA1c target goals are not achieved after initiation of metformin and lifestyle intervention, combination therapies are recommended and progress rapidly, approximately every 3 months (dual, triple and injectable therapy) until target goals are achieved. In 2009, the LEAD-4 study showed that the GLP-1 agonist liraglutide on top of rosiglitazone and metformin therapies in T2DM individuals was not only well-tolerated but led to a 1.5 point drop in %HbA1c, weight loss, and a reduction in FBG by 40 mg/dL. Most notably, HOMA-β, a mathematical surrogate for β-cell function, improved by 5-fold in patients receiving liraglutide in addition to the other therapies [243]. A study of T2DM patients on metformin found that 4-week treatment cessation from the GLP-1 agonist exenatide following one year of combination metformin + exenatide therapy led to a massive decline in β-cell function back to pretreatment levels [244] that was comparable although slightly mitigated if treatment cessation occurred after 2 years of combination therapy [245]. Another study in 2013 utilized recently diagnosed T2DM patients and treated them with DPP-IV inhibition + TZD therapy or DPP-IV inhibition alone. Not only did the combination therapy show superior anti-diabetic efficacy in terms of weight loss, %HbA1c, and FBG, combination therapy induced robust improvements in β-cell function, as evidenced by improved β-cell glucose sensitivity and fasting secretory tone [246]. A meta-analysis of randomized controlled trials comparing SGLT-2 inhibition via canagliflozin in combination with metformin in T2DM patients found that HOMA2-%B was significantly improved by the addition of canagliflozin [247]. While all of these studies provide strong evidence for the efficacy of combination therapies in established T2DM patients, there is a scarcity of longitudinal data in patients at high risk of developing T2DM. Interestingly, a 10-year longitudinal study in 4106 participants with normal glucose tolerance found that impairment of β-cell function had a more profound effect on incident diabetes than did decreases in insulin sensitivity [248]. As such, these examples of clinical evidence are further supportive of the strategy to preserve endogenous β-cell functional mass for effective treatment of T2DM.
In 2017, the American Diabetes Association (ADA) suggested that prediabetes “…should not be viewed as a clinical entity in its own right but rather as an increased risk for diabetes [249].” As such, it is unsurprising that little clinical data exist on early pharmaceutical intervention in these at-risk individuals, even though a ‘non-diabetic’ HbA1c of 6.0% confers a 50% greater risk of developing diabetes than individuals with an HbA1c of 5.0% [250]. Considering that the β-cell is remarkably capable of restoring function in response to attenuated metabolic demand, an argument should be made for early intervention with a combination pharmaceutical approach to improve insulin sensitivity and/or reduce metabolic demand, that, in turn, creates less demand on the β-cell and preserves insulin secretory function to halt the onset or delay the progression of T2DM – thus following the aforementioned concept of β-cell rest. Key to this approach is early diagnosis of T2DM and early treatment to best maximize the return to normal function of what β-cell mass remains.
Conflict of interest
None declared.
References
- 1.Ohneda M., Johnson J.H., Inman L.R., Unger R.H. Glut-2 function in glucose-unresponsive beta-cells of dexamethasone-induced diabetes in rats. Journal of Clinical Investigation. 1993;92(4):1950–1956. doi: 10.1172/JCI116788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuit F.C., De Vos A., Moens K., Quartier E., Heimberg H. Glucose-induced B-cell recruitment and the expression of hexokinase isoenzymes. Advances in Experimental Medicine and Biology. 1997;426:259–266. doi: 10.1007/978-1-4899-1819-2_36. [DOI] [PubMed] [Google Scholar]
- 3.Detimary P., Gilon P., Henquin J.C. Interplay between cytoplasmic Ca2+ and the ATP/ADP ratio: a feedback control mechanism in mouse pancreatic islets. The Biochemical Journal. 1998;333(Pt 2):269–274. doi: 10.1042/bj3330269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sekine N., Cirulli V., Regazzi R., Brown L.J., Gine E., Tamaritrodriguez J. Low lactate-dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells – potential role in nutrient sensing. Journal of Biological Chemistry. 1994;269(7):4895–4902. [PubMed] [Google Scholar]
- 5.Inagaki N., Gonoi T., Clement J.P., Namba N., Inazawa J., Gonzalez G. Reconstitution of I-katp – an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270(5239):1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 6.Curry D.L., Bennett L.L., Grodsky G.M. Requirement for calcium ion in insulin secretion by perfused rat pancreas. American Journal of Physiology. 1968;214(1):174. doi: 10.1152/ajplegacy.1968.214.1.174. [DOI] [PubMed] [Google Scholar]
- 7.Engel F.L., Albertson T., Fredericks J., Lopez E. Evidence for stimulation of insulin secretion by growth hormone in the rat. Endocrinology. 1958;63(1):99–105. doi: 10.1210/endo-63-1-99. [DOI] [PubMed] [Google Scholar]
- 8.Defronzo R.A. Bromocriptine: a sympatholytic, D2-dopamine agonist for the treatment of type 2 diabetes. Diabetes Care. 2011;34(4):789–794. doi: 10.2337/dc11-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plat L., Byrne M.M., Sturis J., Polonsky K.S., Mockel J., Fery F. Effects of morning cortisol elevation on insulin secretion and glucose regulation in humans. American Journal of Physiology-endocrinology and Metabolism. 1996;270(1):E36–E42. doi: 10.1152/ajpendo.1996.270.1.E36. [DOI] [PubMed] [Google Scholar]
- 10.Gao Z.Y., Drews G., Henquin J.C. Mechanisms of the stimulation of insulin release by oxytocin in normal mouse islets. The Biochemical Journal. 1991;276(Pt 1):169–174. doi: 10.1042/bj2760169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro H., Shachar S., Sekler I., Hershfinkel M., Walker M.D. Role of GPR40 in fatty acid action on the beta cell line INS-1E. Biochemical and Biophysical Research Communications. 2005;335(1):97–104. doi: 10.1016/j.bbrc.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 12.Tang C., Ahmed K., Gille A., Lu S., Grone H.-J., Tunaru S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nature Medicine. 2015;21(2):173–177. doi: 10.1038/nm.3779. [DOI] [PubMed] [Google Scholar]
- 13.Layden B.T., Angueira A.R., Brodsky M., Durai V., Lowe W.L., Jr. Short chain fatty acids and their receptors: new metabolic targets. Translational Research. 2013;161(3):131–140. doi: 10.1016/j.trsl.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Prentki M., Vischer S., Glennon M.C., Regazzi R., Deeney J.T., Corkey B.E. Malonyl-coa and long-chain acyl-coa esters as metabolic coupling factors in nutrient-induced insulin-secretion. Journal of Biological Chemistry. 1992;267(9):5802–5810. [PubMed] [Google Scholar]
- 15.Heissig H., Urban K.A., Hastedt K., Zunkler B.J., Panten U. Mechanism of the insulin-releasing action of alpha-ketoisocaproate and related alpha-keto acid anions. Molecular Pharmacology. 2005;68(4):1097–1105. doi: 10.1124/mol.105.015388. [DOI] [PubMed] [Google Scholar]
- 16.Thams P., Capito K. L-Arginine stimulation of glucose-induced insulin secretion through membrane depolarization and independent of nitric oxide. European Journal of Endocrinology. 1999;140(1):87–93. doi: 10.1530/eje.0.1400087. [DOI] [PubMed] [Google Scholar]
- 17.Li C.H., Buettger C., Kwagh J., Matter A., Daikhin Y., Nissim I.B. A signaling role of glutamine in insulin secretion. Journal of Biological Chemistry. 2004;279(14):13393–13401. doi: 10.1074/jbc.M311502200. [DOI] [PubMed] [Google Scholar]
- 18.McClenaghan N.H., Barnett C.R., Oharte F.P.M., Flatt P.R. Mechanisms of amino acid-induced insulin secretion from the glucose-responsive BRIN-BD11 pancreatic B-cell line. Journal of Endocrinology. 1996;151(3):349–357. doi: 10.1677/joe.0.1510349. [DOI] [PubMed] [Google Scholar]
- 19.Hell N.S., de Aguiar Pupo A. Influence of the vagus and splanchnic nerves on insulin secretion and glycemia. Journal of the Autonomic Nervous System. 1979;1(1):93–101. doi: 10.1016/0165-1838(79)90008-0. [DOI] [PubMed] [Google Scholar]
- 20.Sonnenberg G.E., Hoffmann R.G., Johnson C.P., Kissebah A.H. Low- and high-frequency insulin secretion pulses in normal subjects and pancreas transplant recipients: role of extrinsic innervation. The Journal of Clinical Investigation. 1992;90(2):545–553. doi: 10.1172/JCI115893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller R.E., Waid T.H., Joyce M.P. Direct neural inhibition of insulin-secretion in response to systemic hypoglycemia. American Journal of Physiology. 1976;230(4):1090–1094. doi: 10.1152/ajplegacy.1976.230.4.1090. [DOI] [PubMed] [Google Scholar]
- 22.Gilon P., Henquin J.C. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocrine Reviews. 2001;22(5):565–604. doi: 10.1210/edrv.22.5.0440. [DOI] [PubMed] [Google Scholar]
- 23.McIntyre N., Holdsworth C.D., Turner D.S. Intestinal factors in control of insulin secretion. Journal of Clinical Endocrinology & Metabolism. 1965;25(10):1317. doi: 10.1210/jcem-25-10-1317. [DOI] [PubMed] [Google Scholar]
- 24.Drucker D.J., Nauck M.A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 25.Drucker D.J., Philippe J., Mojsov S., Chick W.L., Habener J.F. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(10):3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kashima Y., Miki T., Shibasaki T., Ozaki N., Miyazaki M., Yano H. Critical role of cAMP-GEFII–Rim2 complex in incretin-potentiated insulin secretion. The Journal of Biological Chemistry. 2001;276(49):46046–46053. doi: 10.1074/jbc.M108378200. [DOI] [PubMed] [Google Scholar]
- 27.Rajan S., Dickson L.M., Mathew E., Orr C.M.O., Ellenbroek J.H., Philipson L.H. Chronic hyperglycemia downregulates GLP-1 receptor signaling in pancreatic beta-cells via protein kinase A. Molecular Metabolism. 2015;4(4):265–276. doi: 10.1016/j.molmet.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shigeto M., Ramracheya R., Tarasov A.I., Cha C.Y., Chibalina M.V., Hastoy B. GLP-1 stimulates insulin secretion by PKC-dependent TRPM4 and TRPM5 activation. The Journal of Clinical Investigation. 2015;125(12):4714–4728. doi: 10.1172/JCI81975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maida A., Hansotia T., Longuet C., Seino Y., Drucker D.J. Differential importance of glucose-dependent insulinotropic polypeptide vs glucagon-like peptide 1 receptor signaling for beta cell survival in mice. Gastroenterology. 2009;137(6):2146–2157. doi: 10.1053/j.gastro.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Baggio L.L., Drucker D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 31.Secher A., Jelsing J., Baquero A.F., Hecksher-Sorensen J., Cowley M.A., Dalboge L.S. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. The Journal of Clinical Investigation. 2014;124(10):4473–4488. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skelly R.H., Bollheimer L.C., Wicksteed B.L., Corkey B.E., Rhodes C.J. A distinct difference in the metabolic stimulus-response coupling pathways for regulating proinsulin biosynthesis and insulin secretion that lies at the level of a requirement for fatty acyl moieties. The Biochemical Journal. 1998;331(Pt 2):553–561. doi: 10.1042/bj3310553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itoh M., Mandarino L., Gerich J.E. Antisomatostatin gamma-globulin augments secretion of both insulin and glucagon in vitro – evidence for a physiologic role for endogenous somatostatin in the regulation of pancreatic A-cell and B-cell function. Diabetes. 1980;29(9):693–696. doi: 10.2337/diab.29.9.693. [DOI] [PubMed] [Google Scholar]
- 34.Ashcroft S.J.H. Glucoreceptor mechanisms and the control of insulin release and biosynthesis. Diabetologia. 1980;18(1):5–15. doi: 10.1007/BF01228295. [DOI] [PubMed] [Google Scholar]
- 35.Leibowitz G., Khaldi M.Z., Shauer A., Parnes M., Oprescu A.I., Cerasi E. Mitochondrial regulation of insulin production in rat pancreatic islets. Diabetologia. 2005;48(8):1549–1559. doi: 10.1007/s00125-005-1811-6. [DOI] [PubMed] [Google Scholar]
- 36.Bollheimer L.C., Skelly R.H., Chester M.W., McGarry J.D., Rhodes C.J. Chronic exposure to free fatty acid reduces pancreatic beta cell insulin content by increasing basal insulin secretion that is not compensated for by a corresponding increase in proinsulin biosynthesis translation. Journal of Clinical Investigation. 1998;101(5):1094–1101. doi: 10.1172/JCI420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hakan Borg L.A., Andersson A. Long-term effects of glibenclamide on the insulin production, oxidative metabolism and quantitative ultrastructure of mouse pancreatic islets maintained in tissue culture at different glucose concentrations. Acta Diabetologica Latina. 1981;18(1):65–83. doi: 10.1007/BF02056108. [DOI] [PubMed] [Google Scholar]
- 38.Maedler K., Carr R.D., Bosco D., Zuellig R.A., Berney T., Donath M.Y. Sulfonylurea induced beta-cell apoptosis in cultured human islets. The Journal of Clinical Endocrinology and Metabolism. 2005;90(1):501–506. doi: 10.1210/jc.2004-0699. [DOI] [PubMed] [Google Scholar]
- 39.Kahn S.E., Haffner S.M., Heise M.A., Herman W.H., Holman R.R., Jones N.P. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. The New England Journal of Medicine. 2006;355(23):2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 40.Alarcon C., Verchere C.B., Rhodes C.J. Translational control of glucose-induced islet amyloid polypeptide production in pancreatic islets. Endocrinology. 2012;153(5):2082–2087. doi: 10.1210/en.2011-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wicksteed B., Alarcon C., Briaud I., Lingohr M.K., Rhodes C.J. Glucose-induced translational control of proinsulin biosynthesis is proportional to preproinsulin mRNA levels in islet beta-cells but not regulated via a positive feedback of secreted insulin. Journal of Biological Chemistry. 2003;278(43):42080–42090. doi: 10.1074/jbc.M303509200. [DOI] [PubMed] [Google Scholar]
- 42.Ashcroft S.J., Bunce J., Lowry M., Hansen S.E., Hedeskov C.J. The effect of sugars on (pro)insulin biosynthesis. The Biochemical Journal. 1978;174(2):517–526. doi: 10.1042/bj1740517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashcroft S.J., Crossley J.R. The effects of glucose, N-acetylglucosamine, glyceraldehyde and other sugars on insulin release in vivo. Diabetologia. 1975;11(4):279–284. doi: 10.1007/BF00422392. [DOI] [PubMed] [Google Scholar]
- 44.Hara E., Saito M. Impaired insulin secretion after oral sucrose and fructose in rats. Endocrinology. 1981;109(3):966–970. doi: 10.1210/endo-109-3-966. [DOI] [PubMed] [Google Scholar]
- 45.Kyriazis G.A., Soundarapandian M.M., Tyrberg B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(8):E524–E532. doi: 10.1073/pnas.1115183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ashcroft S.J., Weerasinghe L.C., Bassett J.M., Randle P.J. The pentose cycle and insulin release in mouse pancreatic islets. The Biochemical Journal. 1972;126(3):525–532. doi: 10.1042/bj1260525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain K., Logothetopoulos J. Stimulation of proinsulin biosynthesis by purine-ribonucleosides and D-ribose. Endocrinology. 1977;100(4):923–927. doi: 10.1210/endo-100-4-923. [DOI] [PubMed] [Google Scholar]
- 48.Parry D.G., Taylor K.W. The effects of sugars on incorporation of [3H)leucine into insulins. The Biochemical Journal. 1966;100(1):2C–4C. doi: 10.1042/bj1000002c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashcroft S.J., Crossley J.R., Crossley P.C. The effect of N-acylglucosamines on the biosynthesis and secretion of insulin in the rat. The Biochemical Journal. 1976;154(3):701–707. doi: 10.1042/bj1540701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ashcroft S.J., Lowry M. Beta-cell recognition of stereoisomers of D-glucose. Diabetologia. 1979;17(3):165–168. doi: 10.1007/BF01219744. [DOI] [PubMed] [Google Scholar]
- 51.Toyota T., Ando Y., Nishimura H., Hirata Y. Effects of maltose on insulin secretion in perfused rat pancreas. The Tohoku Journal of Experimental Medicine. 1971;104(4):325–330. doi: 10.1620/tjem.104.325. [DOI] [PubMed] [Google Scholar]
- 52.Grant A.M., Christie M.R., Ashcroft S.J. Insulin release from human pancreatic islets in vitro. Diabetologia. 1980;19(2):114–117. doi: 10.1007/BF00421856. [DOI] [PubMed] [Google Scholar]
- 53.Asanuma N., Aizawa T., Sato Y., Schermerhorn T., Komatsu M., Sharp G.W. Two signaling pathways, from the upper glycolytic flux and from the mitochondria, converge to potentiate insulin release. Endocrinology. 1997;138(2):751–755. doi: 10.1210/endo.138.2.4939. [DOI] [PubMed] [Google Scholar]
- 54.Malaisse W.J., Herchuelz A., Levy J., Sener A., Pipeleers D.G., Devis G. The stimulus-secretion coupling of glucose-induced insulin release XIX. The insulinotropic effect of glyceraldehyde. Molecular and Cellular Endocrinology. 1975;4(1):1–12. doi: 10.1016/0303-7207(76)90002-2. [DOI] [PubMed] [Google Scholar]
- 55.Sener A., Kawazu S., Hutton J.C., Boschero A.C., Devis G., Somers G. The stimulus-secretion coupling of glucose-induced insulin release. Effect of exogenous pyruvate on islet function. The Biochemical Journal. 1978;176(1):217–232. doi: 10.1042/bj1760217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacDonald M.J., Fahien L.A. Glyceraldehyde phosphate and methyl esters of succinic acid. Two “new” potent insulin secretagogues. Diabetes. 1988;37(7):997–999. doi: 10.2337/diab.37.7.997. [DOI] [PubMed] [Google Scholar]
- 57.Alarcon C., Wicksteed B., Prentki M., Corkey B.E., Rhodes C.J. Succinate is a preferential metabolic stimulus-coupling signal for glucose-induced proinsulin biosynthesis translation. Diabetes. 2002;51(8):2496–2504. doi: 10.2337/diabetes.51.8.2496. [DOI] [PubMed] [Google Scholar]
- 58.Malaisse W.J., Rasschaert J., Villanueva-Penacarrillo M.L., Valverde I. Respiratory, ionic, and functional effects of succinate esters in pancreatic islets. American Journal of Physiology. 1993;264(3 Pt 1):E428–E433. doi: 10.1152/ajpendo.1993.264.3.E428. [DOI] [PubMed] [Google Scholar]
- 59.Anderson A. Stimulaiton of insulin biosynthesis in isolated mouse islets by L-leucine, 2-aminonorbornane-2-carboxylic acid and alpha-ketoisocaproic acid. Biochimica et Biophysica Acta. 1976;437(2):345–353. doi: 10.1016/0304-4165(76)90004-0. [DOI] [PubMed] [Google Scholar]
- 60.Sener A., Hutton J.C., Malaisse W.J. The stimulus-secretion coupling of amino acid-induced insulin release. Synergistic effects of L-glutamine and 2-keto acids upon insulin secretion. Biochimica et Biophysica Acta. 1981;677(1):32–38. doi: 10.1016/0304-4165(81)90142-2. [DOI] [PubMed] [Google Scholar]
- 61.Basabe J.C., Lopez N.L., Viktora J.K., Wolff F.W. Insulin secretion studied in the perfused rat pancreas. I. Effect of tolbutamide, leucine and arginine; their interaction with diazoxide, and relation to glucose. Diabetes. 1971;20(7):449–456. doi: 10.2337/diab.20.7.449. [DOI] [PubMed] [Google Scholar]
- 62.Schatz H., Nierle C., Pfeiffer E.F. (Pro-) insulin biosynthesis and release of newly synthesized (pro-) insulin from isolated islets of rat pancreas in the presence of amino acids and sulphonylureas. European Journal of Clinical Investigation. 1975;5(6):477–485. doi: 10.1111/j.1365-2362.1975.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 63.Jain K., Logothetopoulos J. Metabolic signals produced by purine ribonucleosides stimulate proinsulin biosynthesis and insulin secretion. The Biochemical Journal. 1978;170(3):461–467. doi: 10.1042/bj1700461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andersson A. Opposite effects of starvation on oxidation of [14C]adenosine and adenosine-induced insulin release by isolated mouse pancreatic islets. The Biochemical Journal. 1978;176(2):619–621. doi: 10.1042/bj1760619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berne C. The effect of fatty acids and ketone bodies on the biosynthesis of insulin in isolated pancreatic islets of obese hyperglycemic mice. Hormone and Metabolic Research. 1975;7(5):385–389. doi: 10.1055/s-0028-1093733. [DOI] [PubMed] [Google Scholar]
- 66.Malaisse W.J., Lebrun P., Yaylali B., Camara J., Valverde I., Sener A. Ketone bodies and islet function: 45Ca handling, insulin synthesis, and release. The American Journal of Physiology. 1990;259(1 Pt 1):E117–E122. doi: 10.1152/ajpendo.1990.259.1.E117. [DOI] [PubMed] [Google Scholar]
- 67.Crespin S.R., Greenough W.B., 3rd, Steinberg D. Stimulation of insulin secretion by infusion of free fatty acids. The Journal of Clinical Investigation. 1969;48(10):1934–1943. doi: 10.1172/JCI106160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pingitore A., Chambers E.S., Hill T., Maldonado I.R., Liu B., Bewick G. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes, Obesity and Metabolism. 2016 doi: 10.1111/dom.12811. [DOI] [PubMed] [Google Scholar]
- 69.Veprik A., Laufer D., Weiss S., Rubins N., Walker M.D. GPR41 modulates insulin secretion and gene expression in pancreatic beta-cells and modifies metabolic homeostasis in fed and fasting states. FASEB Journal. 2016;30(11):3860–3869. doi: 10.1096/fj.201500030R. [DOI] [PubMed] [Google Scholar]
- 70.Priyadarshini M., Layden B.T. FFAR3 modulates insulin secretion and global gene expression in mouse islets. Islets. 2015;7(2):e1045182. doi: 10.1080/19382014.2015.1045182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ximenes H.M., Hirata A.E., Rocha M.S., Curi R., Carpinelli A.R. Propionate inhibits glucose-induced insulin secretion in isolated rat pancreatic islets. Cell Biochemistry and Function. 2007;25(2):173–178. doi: 10.1002/cbf.1297. [DOI] [PubMed] [Google Scholar]
- 72.Montague W., Taylor K.W. Regulation of insulin secretion by short chain fatty acids. Nature. 1968;217(5131):853. doi: 10.1038/217853a0. [DOI] [PubMed] [Google Scholar]
- 73.Genuth S., Lebovitz H.E. Stimulation of insulin release by coricotropin. Endocrinology. 1965;76:1093–1099. doi: 10.1210/endo-76-6-1093. [DOI] [PubMed] [Google Scholar]
- 74.Sieradzki J., Schatz H., Pfeiffer E.F. Hypophysis and function of pancreatic islets. IV. Effect of treatment with growth hormone and corticotrophin on insulin secretion and biosynthesis in isolated pancreatic islets of normal rats. Acta Endocrinologica (Copenh) 1977;86(4):813–819. [PubMed] [Google Scholar]
- 75.Billaudel B., Sutter B.C. Effect of corticosterone upon insulin biosynthesis and storage by isolated rat Langerhans islets. Diabetes & Metabolism. 1982;8(4):283–287. [PubMed] [Google Scholar]
- 76.Billaudel B., Sutter B.C.J. Direct effect of corticosterone upon insulin-secretion studied by 3 different techniques. Hormone and Metabolic Research. 1979;11(10):555–560. doi: 10.1055/s-0028-1092779. [DOI] [PubMed] [Google Scholar]
- 77.Kawabe T., Morgan C.R. Multiple effects of growth hormone on insulin release from isolated pancreatic islets. Metabolism. 1983;32(7):728–731. doi: 10.1016/0026-0495(83)90132-4. [DOI] [PubMed] [Google Scholar]
- 78.Pierluissi J., Pierluissi R., Ashcroft S.J. Effects of hypophysectomy and growth hormone on cultured islets of Langerhans of the rat. Diabetologia. 1982;22(2):134–137. doi: 10.1007/BF00254843. [DOI] [PubMed] [Google Scholar]
- 79.Samols E., Marri G., Marks V. Promotion of insulin secretion by glucagon. Lancet. 1965;2(7409):415. doi: 10.1016/s0140-6736(65)90761-0. [DOI] [PubMed] [Google Scholar]
- 80.Kawai K., Yokota C., Ohashi S., Watanabe Y., Yamashita K. Evidence that glucagon stimulates insulin secretion through its own receptor in rats. Diabetologia. 1995;38(3):274–276. doi: 10.1007/BF00400630. [DOI] [PubMed] [Google Scholar]
- 81.Fehmann H.C., Habener J.F. Insulinotropic hormone glucagon-like peptide-I(7-37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma beta TC-1 cells. Endocrinology. 1992;130(1):159–166. doi: 10.1210/endo.130.1.1309325. [DOI] [PubMed] [Google Scholar]
- 82.Mojsov S., Weir G.C., Habener J.F. Insulinotropin: glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. The Journal of Clinical Investigation. 1987;79(2):616–619. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schafer R., Schatz H. Stimulation of (Pro-)insulin biosynthesis and release by gastric inhibitory polypeptide in isolated islets of rat pancreas. Acta Endocrinologica (Copenh) 1979;91(3):493–500. doi: 10.1530/acta.0.0910493. [DOI] [PubMed] [Google Scholar]
- 84.Olsson S.E., Anderson A., Petersson B., Hellerstrom C. Effects of somatostatin on the biosynthesis and release of insulin from isolated pancreatic islets. Diabetes & Metabolism. 1976;2(4):199–202. [PubMed] [Google Scholar]
- 85.Nagamatsu S., Carroll R.J., Grodsky G.M., Steiner D.F. Lack of islet amyloid polypeptide regulation of insulin biosynthesis or secretion in normal rat islets. Diabetes. 1990;39(7):871–874. doi: 10.2337/diab.39.7.871. [DOI] [PubMed] [Google Scholar]
- 86.Nagamatsu S., Nishi M., Steiner D.F. Effects of islet amyloid polypeptide (IAPP) on insulin biosynthesis or secretion in rat islets and mouse beta TC3 cells. Biosynthesis of IAPP in mouse beta TC3 cells. Diabetes Research and Clinical Practice. 1992;15(1):49–55. doi: 10.1016/0168-8227(92)90067-2. [DOI] [PubMed] [Google Scholar]
- 87.O'Brien T.D., Westermark P., Johnson K.H. Islet amyloid polypeptide (IAPP) does not inhibit glucose-stimulated insulin secretion from isolated perfused rat pancreas. Biochemical and Biophysical Research Communications. 1990;170(3):1223–1228. doi: 10.1016/0006-291x(90)90524-q. [DOI] [PubMed] [Google Scholar]
- 88.Rhodes C.J., Taylor K.W. Effect of human lymphoblastoid interferon on insulin synthesis and secretion in isolated human pancreatic islets. Diabetologia. 1984;27(6):601–603. doi: 10.1007/BF00276977. [DOI] [PubMed] [Google Scholar]
- 89.Rhodes C.J., Taylor K.W. Effect of interferon and double-stranded RNA on B-cell function in mouse islets of Langerhans. The Biochemical Journal. 1985;228(1):87–94. doi: 10.1042/bj2280087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sandler S., Andersson A., Hellerstrom C. Inhibitory effects of interleukin 1 on insulin secretion, insulin biosynthesis, and oxidative metabolism of isolated rat pancreatic islets. Endocrinology. 1987;121(4):1424–1431. doi: 10.1210/endo-121-4-1424. [DOI] [PubMed] [Google Scholar]
- 91.Hansen B.S., Nielsen J.H., Linde S., Spinas G.A., Welinder B.S., Mandrup-Poulsen T. Effect of interleukin-1 on the biosynthesis of proinsulin and insulin in isolated rat pancreatic islets. Biomedica Biochimica Acta. 1988;47(4–5):305–309. [PubMed] [Google Scholar]
- 92.Spinas G.A., Hansen B.S., Linde S., Kastern W., Molvig J., Mandrup-Poulsen T. Interleukin 1 dose-dependently affects the biosynthesis of (pro)insulin in isolated rat islets of Langerhans. Diabetologia. 1987;30(7):474–480. doi: 10.1007/BF00279615. [DOI] [PubMed] [Google Scholar]
- 93.Spinas G.A., Mandrup-Poulsen T., Molvig J., Baek L., Bendtzen K., Dinarello C.A. Low concentrations of interleukin-1 stimulate and high concentrations inhibit insulin release from isolated rat islets of Langerhans. Acta Endocrinologica (Copenh) 1986;113(4):551–558. doi: 10.1530/acta.0.1130551. [DOI] [PubMed] [Google Scholar]
- 94.Leibowitz G., Oprescu A.I., Uckaya G., Gross D.J., Cerasi E., Kaiser N. Insulin does not mediate glucose stimulation of proinsulin biosynthesis. Diabetes. 2003;52(4):998–1003. doi: 10.2337/diabetes.52.4.998. [DOI] [PubMed] [Google Scholar]
- 95.Zawalich W.S., Zawalich K.C. Effects of glucose, exogenous insulin, and carbachol on C-peptide and insulin secretion from isolated perifused rat islets. The Journal of Biological Chemistry. 2002;277(29):26233–26237. doi: 10.1074/jbc.M202291200. [DOI] [PubMed] [Google Scholar]
- 96.Markoff E., Beattie G.M., Hayek A., Lewis U.J. Effects of prolactin and glycosylated prolactin on (pro)insulin synthesis and insulin release from cultured rat pancreatic islets. Pancreas. 1990;5(1):99–103. doi: 10.1097/00006676-199001000-00015. [DOI] [PubMed] [Google Scholar]
- 97.Nielsen J.H. Effects of growth hormone, prolactin, and placental lactogen on insulin content and release, and deoxyribonucleic acid synthesis in cultured pancreatic islets. Endocrinology. 1982;110(2):600–606. doi: 10.1210/endo-110-2-600. [DOI] [PubMed] [Google Scholar]
- 98.Johansson M., Mattsson G., Andersson A., Jansson L., Carlsson P.O. Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology. 2006;147(5):2315–2324. doi: 10.1210/en.2005-0997. [DOI] [PubMed] [Google Scholar]
- 99.Conaway H.H., Griffey M.A., Whitney J.E. Characterization of acetylcholine-induced insulin secretion in the isolated perfused dog pancreas. Proceedings of the Society for Experimental Biology and Medicine. 1975;150(2):308–312. doi: 10.3181/00379727-150-39025. [DOI] [PubMed] [Google Scholar]
- 100.Wollheim C.B., Sharp G.W. Regulation of insulin release by calcium. Physiological Reviews. 1981;61(4):914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]
- 101.Porte D., Jr., Graber A.L., Kuzuya T., Williams R.H. The effect of epinephrine on immunoreactive insulin levels in man. The Journal of Clinical Investigation. 1966;45(2):228–236. doi: 10.1172/JCI105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Malaisse W., Malaisse-Lagae F., Wright P.H., Ashmore J. Effects of adrenergic and cholinergic agents upon insulin secretion in vitro. Endocrinology. 1967;80(5):975–978. doi: 10.1210/endo-80-5-975. [DOI] [PubMed] [Google Scholar]
- 103.Malaisse W.J., Pipeleers D.G., Levy J. The stimulus-secretion coupling of glucose-induced insulin release. XVI. A glucose-like and calcium-independent effect of cyclic AMP. Biochimica et Biophysica Acta. 1974;362(1):121–128. doi: 10.1016/0304-4165(74)90033-6. [DOI] [PubMed] [Google Scholar]
- 104.Hansen S.E., Hedeskov C.J. Effect of cations on insulin biosynthesis in mouse pancreatic islets. Acta Biologica et Medica Germanica. 1981;40(1):9–13. [PubMed] [Google Scholar]
- 105.Yu X., Murao K., Sayo Y., Imachi H., Cao W.M., Ohtsuka S. The role of calcium/calmodulin-dependent protein kinase cascade in glucose upregulation of insulin gene expression. Diabetes. 2004;53(6):1475–1481. doi: 10.2337/diabetes.53.6.1475. [DOI] [PubMed] [Google Scholar]
- 106.Reddy D., Pollock A.S., Clark S.A., Sooy K., Vasavada R.C., Stewart A.F. Transfection and overexpression of the calcium binding protein calbindin-D28k results in a stimulatory effect on insulin synthesis in a rat beta cell line (RIN 1046-38) Proceedings of the National Academy of Sciences of the United States of America. 1997;94(5):1961–1966. doi: 10.1073/pnas.94.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bennett L.L., Curry D.L., Grodsky G.M. Calcium-magnesium antagonism in insulin secretion by the perfused rat pancreas. Endocrinology. 1969;85(3):594–596. doi: 10.1210/endo-85-3-594. [DOI] [PubMed] [Google Scholar]
- 108.Malaisse W.J., Zhang Y., Cancelas J., Prieto P.G., Olivares E., Villanueva-Penacarrillo M.L. Stimulation of proinsulin biosynthesis by K+ in rat pancreatic islets. Endocrine. 2004;23(1):51–57. doi: 10.1385/ENDO:23:1:51. [DOI] [PubMed] [Google Scholar]
- 109.Sener A., Malaisse W.J. The stimulus-secretion coupling of glucose-induced insulin release. XXXIX. Long term effects of K+ deprivation upon insulin biosynthesis and release. Endocrinology. 1980;106(3):778–785. doi: 10.1210/endo-106-3-778. [DOI] [PubMed] [Google Scholar]
- 110.Slepchenko K.G., James C.B., Li Y.V. Inhibitory effect of zinc on glucose-stimulated zinc/insulin secretion in an insulin-secreting beta-cell line. Experimental Physiology. 2013;98(8):1301–1311. doi: 10.1113/expphysiol.2013.072348. [DOI] [PubMed] [Google Scholar]
- 111.Slepchenko K.G., Daniels N.A., Guo A., Li Y.V. Autocrine effect of Zn(2)(+) on the glucose-stimulated insulin secretion. Endocrine. 2015;50(1):110–122. doi: 10.1007/s12020-015-0568-z. [DOI] [PubMed] [Google Scholar]
- 112.Wijesekara N., Dai F.F., Hardy A.B., Giglou P.R., Bhattacharjee A., Koshkin V. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia. 2010;53(8):1656–1668. doi: 10.1007/s00125-010-1733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Levy J., Malaisse W.J. The stimulus-secretion coupling of glucose-induced insulin release-XVII. Effects of sulfonylureas and diazoxide on insular biosynthetic activity. Biochemical Pharmacology. 1975;24(2):235–239. doi: 10.1016/0006-2952(75)90282-8. [DOI] [PubMed] [Google Scholar]
- 114.Foa P.P., Galansino G., Pozza G. Insulin secretion following carbutamide injections in normal dogs. Proceedings of the Society for Experimental Biology and Medicine. 1956;93(3):539–542. doi: 10.3181/00379727-93-22811. [DOI] [PubMed] [Google Scholar]
- 115.Howell S.L., Taylor K.W. Effects of diazoxide on insulin secretion in vitro. Lancet. 1966;1(7429):128–129. doi: 10.1016/s0140-6736(66)91263-3. [DOI] [PubMed] [Google Scholar]
- 116.Lin B.J., Haist R.E. Effects of some modifiers of insulin secretion on insulin biosynthesis. Endocrinology. 1973;92(3):735–742. doi: 10.1210/endo-92-3-735. [DOI] [PubMed] [Google Scholar]
- 117.Sussman K.E., Vaughan G.D. Insulin release after ACTH, glucagon and adenosine-3′-5′-phosphate (cyclic AMP) in the perfused isolated rat pancreas. Diabetes. 1967;16(7):449–454. doi: 10.2337/diab.16.7.449. [DOI] [PubMed] [Google Scholar]
- 118.Skelly R.H., Schuppin G.T., Ishihara H., Oka Y., Rhodes C.J. Glucose-regulated translational control of proinsulin biosynthesis with that of the proinsulin endopeptidases PC2 and PC3 in the insulin-producing MIN6 cell line. Diabetes. 1996;45(1):37–43. doi: 10.2337/diab.45.1.37. [DOI] [PubMed] [Google Scholar]
- 119.Gwilliam D.J., Jones P.M., Persaud S.J., Howell S.L. The role of protein kinase C in insulin biosynthesis. Acta Diabetologica. 1993;30(2):99–104. doi: 10.1007/BF00578222. [DOI] [PubMed] [Google Scholar]
- 120.Zawalich W., Brown C., Rasmussen H. Insulin secretion: combined effects of phorbol ester and A23187. Biochemical and Biophysical Research Communications. 1983;117(2):448–455. doi: 10.1016/0006-291x(83)91221-4. [DOI] [PubMed] [Google Scholar]
- 121.Schubart U.K., Fleischer N., Erlichman J. Ca2+-dependent protein phosphorylation and insulin release in intact hamster insulinoma cells. Inhibition by trifluoperazine. The Journal of Biological Chemistry. 1980;255(23):11063–11066. [PubMed] [Google Scholar]
- 122.Uchizono Y., Alarcon C., Wicksteed B.L., Marsh B.J., Rhodes C.J. The balance between proinsulin biosynthesis and insulin secretion: where can imbalance lead? Diabetes Obesity & Metabolism. 2007;9:56–66. doi: 10.1111/j.1463-1326.2007.00774.x. [DOI] [PubMed] [Google Scholar]
- 123.Guest P.C., Bailyes E.M., Rutherford N.G., Hutton J.C. Insulin secretory granule biogenesis – coordinate regulation of the biosynthesis of the majority of constituent proteins. Biochemical Journal. 1991;274:73–78. doi: 10.1042/bj2740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wicksteed B., Herbert T.P., Alarcon C., Lingohr M.K., Moss L.G., Rhodes C.J. Cooperativity between the preproinsulin mRNA untranslated regions is necessary for glucose-stimulated translation. The Journal of Biological Chemistry. 2001;276(25):22553–22558. doi: 10.1074/jbc.M011214200. [DOI] [PubMed] [Google Scholar]
- 125.Alarcon C., Lincoln B., Rhodes C.J. The biosynthesis of the subtilisin-related proprotein convertase PC3, but not that of the PC2 convertase, is regulated by glucose in parallel to proinsulin biosynthesis in rat pancreatic-islets. Journal of Biological Chemistry. 1993;268(6):4276–4280. [PubMed] [Google Scholar]
- 126.Prentki M., Matschinsky F.M. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiological Reviews. 1987;67(4):1185–1248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- 127.Pipeleers D.G., Marichal M., Malaisse W.J. The stimulus-secretion coupling of glucose-induced insulin release. XV. Participation of cations in the recognition of glucose by the beta-cell. Endocrinology. 1973;93(5):1012–1018. doi: 10.1210/endo-93-5-1012. [DOI] [PubMed] [Google Scholar]
- 128.Welsh M., Nielsen D.A., MacKrell A.J., Steiner D.F. Control of insulin gene expression in pancreatic beta-cells and in an insulin-producing cell line, RIN-5F cells. II. Regulation of insulin mRNA stability. The Journal of Biological Chemistry. 1985;260(25):13590–13594. [PubMed] [Google Scholar]
- 129.Steiner D.F., Terris S., Chan S.J., Rubenstein A.H. Chemical and biological aspects of insulin and proinsulin. Acta medica Scandinavica. Supplementum. 1976;601:55–107. [PubMed] [Google Scholar]
- 130.Steiner D.F., Cunningh D., Spigelma L., Aten B. Insulin biosynthesis – evidence for a precursor. Science. 1967;157(3789):697. doi: 10.1126/science.157.3789.697. [DOI] [PubMed] [Google Scholar]
- 131.Marsh B.J., Mastronarde D.N., Buttle K.F., Howell K.E., McIntosh J.R. Organellar relationships in the Golgi region of the pancreatic beta cell line, HIT-T15, visualized by high resolution electron tomography. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(5):2399–2406. doi: 10.1073/pnas.051631998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Steiner D.F. The proprotein convertases. Current Opinion in Chemical Biology. 1998;2(1):31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- 133.Davidson H.W. (Pro)Insulin processing: a historical perspective. Cell Biochemistry and Biophysics. 2004;40(3 Suppl):143–158. doi: 10.1385/cbb:40:3:143. [DOI] [PubMed] [Google Scholar]
- 134.Shennan K.I., Taylor N.A., Jermany J.L., Matthews G., Docherty K. Differences in pH optima and calcium requirements for maturation of the prohormone convertases PC2 and PC3 indicates different intracellular locations for these events. The Journal of Biological Chemistry. 1995;270(3):1402–1407. doi: 10.1074/jbc.270.3.1402. [DOI] [PubMed] [Google Scholar]
- 135.Davidson H.W., Rhodes C.J., Hutton J.C. Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic beta cell via two distinct site-specific endopeptidases. Nature. 1988;333(6168):93–96. doi: 10.1038/333093a0. [DOI] [PubMed] [Google Scholar]
- 136.Guest P.C., Abdel-Halim S.M., Gross D.J., Clark A., Poitout V., Amaria R. Proinsulin processing in the diabetic Goto-Kakizaki rat. The Journal of Endocrinology. 2002;175(3):637–647. doi: 10.1677/joe.0.1750637. [DOI] [PubMed] [Google Scholar]
- 137.Lemaire K., Chimienti F., Schuit F. Zinc transporters and their role in the pancreatic beta-cell. Journal of Diabetes Investigation. 2012;3(3):202–211. doi: 10.1111/j.2040-1124.2012.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rhodes C.J., Lincoln B., Shoelson S.E. Preferential cleavage of des-31,32-proinsulin over intact proinsulin by the insulin secretory granule type II endopeptidase. Implication of a favored route for prohormone processing. The Journal of Biological Chemistry. 1992;267(32):22719–22727. [PubMed] [Google Scholar]
- 139.Kaufmann J.E., Irminger J.C., Mungall J., Halban P.A. Proinsulin conversion in GH3 cells after coexpression of human proinsulin with the endoproteases PC2 and/or PC3. Diabetes. 1997;46(6):978–982. doi: 10.2337/diab.46.6.978. [DOI] [PubMed] [Google Scholar]
- 140.Davies M.J., Rayman G., Gray I.P., Day J.L., Hales C.N. Insulin deficiency and increased plasma concentration of intact and 32/33 split proinsulin in subjects with impaired glucose tolerance. Diabetic Medicine. 1993;10(4):313–320. doi: 10.1111/j.1464-5491.1993.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 141.Halban P.A. Structural domains and molecular lifestyles of insulin and its precursors in the pancreatic beta cell. Diabetologia. 1991;34(11):767–778. doi: 10.1007/BF00408349. [DOI] [PubMed] [Google Scholar]
- 142.Kelly R.B. Pathways of protein secretion in eukaryotes. Science. 1985;230(4721):25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- 143.Rhodes C.J., Halban P.A. Newly synthesized proinsulin insulin and stored insulin are released from pancreatic B-cells predominantly via a regulated, rather than a constitutive, pathway. Journal of Cell Biology. 1987;105(1):145–153. doi: 10.1083/jcb.105.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Haataja L., Snapp E., Wright J., Liu M., Hardy A.B., Wheeler M.B. Proinsulin intermolecular interactions during secretory trafficking in pancreatic beta cells. Journal of Biological Chemistry. 2013;288(3):1896–1906. doi: 10.1074/jbc.M112.420018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cao M., Mao Z., Kam C., Xiao N., Cao X., Shen C. PICK1 and ICA69 control insulin granule trafficking and their deficiencies lead to impaired glucose tolerance. Plos Biology. 2013;11(4) doi: 10.1371/journal.pbio.1001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kebede M.A., Oler A.T., Gregg T., Balloon A.J., Johnson A., Mitok K. SORCS1 is necessary for normal insulin secretory granule biogenesis in metabolically stressed beta cells. Journal of Clinical Investigation. 2014;124(10):4240–4256. doi: 10.1172/JCI74072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Heaslip A.T., Nelson S.R., Armstrong J.M., Lombardo A.T., Trybus K.M., Warshaw D.M. Involvement of myova and actin in insulin granule trafficking. Biophysical Journal. 2013;104(2):651A–A. [Google Scholar]
- 148.Zhu D., Koo E., Kwan E., Kang Y., Park S., Xie H. Syntaxin-3 regulates newcomer insulin granule exocytosis and compound fusion in pancreatic beta cells. Diabetologia. 2013;56(2):359–369. doi: 10.1007/s00125-012-2757-0. [DOI] [PubMed] [Google Scholar]
- 149.Pottekat A., Becker S., Spencer K.R., Yates J.R., III, Manning G., Itkin-Ansari P. Insulin biosynthetic interaction network component, TMEM24, facilitates insulin reserve pool release. Cell Reports. 2013;4(5):921–930. doi: 10.1016/j.celrep.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Donelan M.J., Morfini G., Julyan R., Sommers S., Hays L., Kajio H. Ca2+-dependent dephosphorylation of kinesin heavy chain on beta-granules in pancreatic beta-cells. Implications for regulated beta-granule transport and insulin exocytosis. The Journal of Biological Chemistry. 2002;277(27):24232–24242. doi: 10.1074/jbc.M203345200. [DOI] [PubMed] [Google Scholar]
- 151.Sizonenko S., Irminger J.C., Buhler L., Deng S., Morel P., Halban P.A. Kinetics of proinsulin conversion in human islets. Diabetes. 1993;42(6):933–936. doi: 10.2337/diab.42.6.933. [DOI] [PubMed] [Google Scholar]
- 152.Xia F., Xie L., Mihic A., Gao X., Chen Y., Gaisano H.Y. Inhibition of cholesterol biosynthesis impairs insulin secretion and voltage-gated calcium channel function in pancreatic beta-cells. Endocrinology. 2008;149(10):5136–5145. doi: 10.1210/en.2008-0161. [DOI] [PubMed] [Google Scholar]
- 153.Bogan J.S., Xu Y., Hao M. Cholesterol accumulation increases insulin granule size and impairs membrane trafficking. Traffic. 2012;13(11):1466–1480. doi: 10.1111/j.1600-0854.2012.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Waddell R.W., Beck J.C., Dupre J. Biphasic insulin secretory response to intravenous arginine in man. Diabetes. 1969;S 18:375. [Google Scholar]
- 155.Hou J.C., Min L., Pessin J.E. Insulin granule biogenesis, trafficking and exocytosis. In: Litwack G., editor. Vitamins and hormones insulin and IGFs. 2009. pp. 473–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Goody R.S., Mannherz H.G. The role of the cytoskeleton in transport and release of insulin-containing granules by pancreatic beta-cells. In: BoossBavnbek B., Klosgen B., Larsen J., Pociot F., Renstrom E., editors. Betasys: systems biology of regulated exocytosis in pancreatic beta-cells. 2011. pp. 83–95. [Google Scholar]
- 157.Tomas A., Yermen B., Min L., Pessin J.E., Halban P.A. Regulation of pancreatic beta-cell insulin secretion by actin cytoskeleton remodelling: role of gelsolin and cooperation with the MAPK signalling pathway. Journal of Cell Science. 2006;119(10):2156–2167. doi: 10.1242/jcs.02942. [DOI] [PubMed] [Google Scholar]
- 158.Zhang W., Efanov A., Yang S.N., Fried G., Kolare S., Brown H. Munc-18 associates with syntaxin and serves as a negative regulator of exocytosis in the pancreatic beta-cell. Journal of Biological Chemistry. 2000;275(52):41521–41527. doi: 10.1074/jbc.M005479200. [DOI] [PubMed] [Google Scholar]
- 159.Lang J., Fukuda M., Zhang H., Kiraly C., Mikoshiba K., Wollheim C.B. Subcellular localisation and role of synaptotagmin in insulin secreting pancreatic beta-cell lines. Diabetologia. 1996;39:432. [Google Scholar]
- 160.Wheeler M.B., Sheu L., Ghai M., Bouquillon A., Grondin G., Weller U. Characterization of SNARE protein expression in beta cell lines and pancreatic islets. Endocrinology. 1996;137(4):1340–1348. doi: 10.1210/endo.137.4.8625909. [DOI] [PubMed] [Google Scholar]
- 161.Branham M.T., Bustos M.A., De Blas G.A., Rehmann H., Zarelli V.E.P., Trevino C.L. Epac activates the small G proteins Rap1 and Rab3A to achieve exocytosis. Journal of Biological Chemistry. 2009;284(37):24825–24839. doi: 10.1074/jbc.M109.015362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xie L., He Y., Lopez J.A., James D.E., Gaisano H.Y. RaIA GTPase regulation of insulin exocytosis involves RaIA interactions with L- and R-type calcium channels. Diabetes. 2009;58:A415-A. [Google Scholar]
- 163.Rorsman P., Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46(8):1029–1045. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- 164.Curry D.L., Bennett L.L., Grodsky G.M. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology. 1968;83(3):572–584. doi: 10.1210/endo-83-3-572. [DOI] [PubMed] [Google Scholar]
- 165.Ma L., Bindokas V.P., Kuznetsov A., Rhodes C., Hays L., Edwardson J.M. Direct imaging shows that insulin granule exocytosis occurs by complete vesicle fusion. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(25):9266–9271. doi: 10.1073/pnas.0403201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Orci L., Malaisse F., Ravazzol M., Amherdt M., Renold A.E. Exocytosis-endocytosis coupling in pancreatic beta cell. Science. 1973;181(4099):561–562. doi: 10.1126/science.181.4099.561. [DOI] [PubMed] [Google Scholar]
- 167.Halban P.A., Wollheim C.B. Intracellular degradation of insulin stores by rat pancreatic-islets in vitro – an alternative pathway for homeostasis of pancreatic insulin content. Journal of Biological Chemistry. 1980;255(13):6003–6006. [PubMed] [Google Scholar]
- 168.Marsh B.J., Soden C., Alarcon C., Wicksteed B.L., Yaekura K., Costin A.J. Regulated autophagy controls hormone content in secretory-deficient pancreatic endocrine beta-cells. Molecular Endocrinology. 2007;21(9):2255–2269. doi: 10.1210/me.2007-0077. [DOI] [PubMed] [Google Scholar]
- 169.Glaumann H., Ahlberg J., Marzella L. In vitro uptake of particles by lysosomes by means of microautophagy. European Journal of Cell Biology. 1980;22(1):200. doi: 10.1016/0014-4827(80)90515-7. [DOI] [PubMed] [Google Scholar]
- 170.Marzella L., Ahlberg J., Glaumann H. Autophagy, heterophagy, microautophagy and crinophagy as the means for intracellular degradation. Virchows Archiv B-Cell Pathology Including Molecular Pathology. 1981;36(2–3):219–234. doi: 10.1007/BF02912068. [DOI] [PubMed] [Google Scholar]
- 171.Goginashvili A., Zhang Z., Erbs E., Spiegelhalter C., Kessler P., Mihlan M. Insulin secretory granules control autophagy in pancreatic beta cells. Science. 2015;347(6224):878–882. doi: 10.1126/science.aaa2628. [DOI] [PubMed] [Google Scholar]
- 172.Chakravarthy M.V., Booth F.W. Eating, exercise, and “thrifty” genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. Journal of Applied Physiology. 2004;96(1):3–10. doi: 10.1152/japplphysiol.00757.2003. [DOI] [PubMed] [Google Scholar]
- 173.Neel J.V. Diabetes mellitus – a thrifty genotype rendered detrimental by progress. American Journal of Human Genetics. 1962;14(4):353. [PMC free article] [PubMed] [Google Scholar]
- 174.Nerenberg S.T. Regranulation of beta-cells of islets of langerhans following insulin and starvation. American Journal of Clinical Pathology. 1953;23(4):340–342. doi: 10.1093/ajcp/23.4.340. [DOI] [PubMed] [Google Scholar]
- 175.Lever J.D., Findlay J.A. Specific granularity in pancreatic beta-cells of starved + free-fed Guinea-pig – quantitative assessment. Journal of Anatomy. 1964;98(1):55. [PMC free article] [PubMed] [Google Scholar]
- 176.Miyaura C., Chen L., Appel M., Alam T., Inman L., Hughes S.D. Expression of reg/PSP, a pancreatic exocrine gene: relationship to changes in islet beta-cell mass. Molecular Endocrinology. 1991;5(2):226–234. doi: 10.1210/mend-5-2-226. [DOI] [PubMed] [Google Scholar]
- 177.Rhodes C.J. Type 2 diabetes – a matter of beta-cell life and death? Science. 2005;307(5708):380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 178.Favre D., Niederhauser G., Fahmi D., Plaisance V., Brajkovic S., Beeler N. Role for inducible cAMP early repressor in promoting pancreatic beta cell dysfunction evoked by oxidative stress in human and rat islets. Diabetologia. 2011;54(9):2337–2346. doi: 10.1007/s00125-011-2165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang S., Kaufman R.J. The impact of the unfolded protein response on human disease. Journal of Cell Biology. 2012;197(7):857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Westermark G.T., Westermark P. Islet amyloid polypeptide and diabetes. Current Protein & Peptide Science. 2013;14(4):330–337. doi: 10.2174/13892037113149990050. [DOI] [PubMed] [Google Scholar]
- 181.Donath M.Y. Targeting inflammation in the treatment of type 2 diabetes. Diabetes Obesity & Metabolism. 2013;15:193–196. doi: 10.1111/dom.12172. [DOI] [PubMed] [Google Scholar]
- 182.Corkey B.E. Diabetes: have we got it all wrong? Insulin hypersecretion and food additives: cause of obesity and diabetes? Diabetes Care. 2012;35(12):2432–2437. doi: 10.2337/dc12-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bruning J.C., Gautam D., Burks D.J., Gillette J., Schubert M., Orban P.C. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289(5487):2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 184.Kahn S.E., Zraika S., Utzschneider K.M., Hull R.L. The beta cell lesion in type 2 diabetes: there has to be a primary functional abnormality. Diabetologia. 2009;52(6):1003–1012. doi: 10.1007/s00125-009-1321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Halban P.A., Polonsky K.S., Bowden D.W., Hawkins M.A., Ling C., Mather K.J. Beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care. 2014;37(6):1751–1758. doi: 10.2337/dc14-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Leahy J.L., Hirsch I.B., Peterson K.A., Schneider D. Targeting beta-cell function early in the course of therapy for type 2 diabetes mellitus. Journal of Clinical Endocrinology & Metabolism. 2010;95(9):4206–4216. doi: 10.1210/jc.2010-0668. [DOI] [PubMed] [Google Scholar]
- 187.Wajchenberg B.L. beta-cell failure in diabetes and preservation by clinical treatment. Endocrine Reviews. 2007;28(2):187–218. doi: 10.1210/10.1210/er.2006-0038. [DOI] [PubMed] [Google Scholar]
- 188.Poitout V., Robertson R.P. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocrine Reviews. 2008;29(3):351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Logothet J., Davidson J.K., Haist R.E., Best C.H. Degranulation of beta cells and loss of pancreatic insulin after infusions of insulin antibody or glucose. Diabetes. 1965;14(8):493. doi: 10.2337/diab.14.8.493. [DOI] [PubMed] [Google Scholar]
- 190.van Raalte D.H., Verchere C.B. Glucagon-Like Peptide-1 receptor agonists: beta-cell protection or exhaustion? Trends Endocrinol Metab. 2016;27(7):442–445. doi: 10.1016/j.tem.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 191.Alarcon C., Wicksteed B., Rhodes C.J. Exendin 4 controls insulin production in rat islet beta cells predominantly by potentiation of glucose-stimulated proinsulin biosynthesis at the translational level. Diabetologia. 2006;49(12):2920–2929. doi: 10.1007/s00125-006-0433-y. [DOI] [PubMed] [Google Scholar]
- 192.Ogilvie R.F. A quantitative estimation of the pancreatic islet tissue. Quarterly Journal of Medicine. 1937;6(23):287–300. [Google Scholar]
- 193.Hanley S.C., Austin E., Assouline-Thomas B., Kapeluto J., Blaichman J., Moosavi M. Beta-cell mass dynamics and islet cell plasticity in human type 2 diabetes. Endocrinology. 2010;151(4):1462–1472. doi: 10.1210/en.2009-1277. [DOI] [PubMed] [Google Scholar]
- 194.Maclean N., Ogilvie R.F. Quantitative estimation of the pancreatic islet tissue in diabetic subjects. Diabetes. 1955;4(5):367–376. doi: 10.2337/diab.4.5.367. [DOI] [PubMed] [Google Scholar]
- 195.Butler A.E., Janson J., Bonner-Weir S., Ritzel R., Rizza R.A., Butler P.C. beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 196.Kloppel G., Lohr M., Habich K., Oberholzer M., Heitz P.U. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Survey and Synthesis of Pathology Research. 1985;4(2):110–125. doi: 10.1159/000156969. [DOI] [PubMed] [Google Scholar]
- 197.Hales C.N., Barker D.J.P. The thrifty phenotype hypothesis. British Medical Bulletin. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 198.Biddinger S.B., Kahn C.R. From mice to men: insights into the insulin resistance syndromes. Annual Review of Physiology. 2006:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- 199.Michael M.D., Kulkarni R.N., Postic C., Previs S.F., Shulman G.I., Magnuson M.A. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Molecular Cell. 2000;6(1):87–97. [PubMed] [Google Scholar]
- 200.Bluher M., Michael M.D., Peroni O.D., Ueki K., Carter N., Kahn B.B. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Developmental Cell. 2002;3(1):25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- 201.Cariou B., Postic C., Boudou P., Burcelin R., Kahn C.R., Girard J. Cellular and molecular mechanisms of adipose tissue plasticity in muscle insulin receptor knockout mice. Endocrinology. 2004;145(4):1926–1932. doi: 10.1210/en.2003-0882. [DOI] [PubMed] [Google Scholar]
- 202.Meier J.J. Beta cell mass in diabetes: a realistic therapeutic target? Diabetologia. 2008;51(5):703–713. doi: 10.1007/s00125-008-0936-9. [DOI] [PubMed] [Google Scholar]
- 203.Saxena R., Voight B.F., Lyssenko V., Burtt N.P., de Bakker P.I.W., Chen H. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316(5829):1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 204.Sladek R., Rocheleau G., Rung J., Dina C., Shen L., Serre D. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445(7130):881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 205.Scott L.J., Mohlke K.L., Bonnycastle L.L., Willer C.J., Li Y., Duren W.L. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316(5829):1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Harris M.I. Epidemiologic studies on the pathogenesis of non-insulin-dependent diabetes mellitus (NIDDM) Clinical and Investigative Medicine. 1995;18(4):231–239. [PubMed] [Google Scholar]
- 207.Lupi R., Dotta F., Marselli L., Del Guerra S., Masini M., Santangelo C. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes. 2002;51(5):1437–1442. doi: 10.2337/diabetes.51.5.1437. [DOI] [PubMed] [Google Scholar]
- 208.Weir G.C., Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53:S16–S21. doi: 10.2337/diabetes.53.suppl_3.s16. [DOI] [PubMed] [Google Scholar]
- 209.Alarcon C., Boland B.B., Uchizono Y., Moore P.C., Peterson B., Rajan S. Pancreatic beta-cell adaptive plasticity in obesity increases insulin production but adversely affects secretory function. Diabetes. 2016;65(2):438–450. doi: 10.2337/db15-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rutkowski J.M., Stern J.H., Scherer P.E. The cell biology of fat expansion. The Journal of Cell Biology. 2015;208(5):501–512. doi: 10.1083/jcb.201409063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kahn S.E., Hull R.L., Utzschneider K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 212.Shoelson S.E., Lee J., Goldfine A.B. Inflammation and insulin resistance. The Journal of Clinical Investigation. 2006;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Agrawal N.K., Kant S. Targeting inflammation in diabetes: newer therapeutic options. World Journal of Diabetes. 2014;5(5):697–710. doi: 10.4239/wjd.v5.i5.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cao P., Marek P., Noor H., Patsalo V., Tu L.-H., Wang H. Islet amyloid: from fundamental biophysics to mechanisms of cytotoxicity. Febs Letters. 2013;587(8):1106–1118. doi: 10.1016/j.febslet.2013.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hull R.L., Westermark G.T., Westermark P., Kahn S.E. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. Journal of Clinical Endocrinology & Metabolism. 2004;89(8):3629–3643. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- 216.Clark A., Nilsson M.R. Islet amyloid: a complication of islet dysfunction or an aetiological factor in Type 2 diabetes? Diabetologia. 2004;47(2):157–169. doi: 10.1007/s00125-003-1304-4. [DOI] [PubMed] [Google Scholar]
- 217.Lee K.H., Tirasophon W., Okada T., Yoshida H., Mori K., Kaufman R.J. Cooperation of IRE1 alpha, ATF6, and XBP1 in the unfolded protein response in the endoplasmic reticulum (ER) Faseb Journal. 2002;16(4):A558–A. [Google Scholar]
- 218.Back S.H., Kaufman R.J. Endoplasmic reticulum stress and type 2 diabetes. Annual Review of Biochemistry. 2012;81:767–793. doi: 10.1146/annurev-biochem-072909-095555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Izumi T., Yokota-Hashimoto H., Zhao S., Wang J., Halban P.A., Takeuchi T. Dominant negative pathogenesis by mutant proinsulin in the Akita diabetic mouse. Diabetes. 2003;52(2):409–416. doi: 10.2337/diabetes.52.2.409. [DOI] [PubMed] [Google Scholar]
- 220.Sun J., Cui J., He Q., Chen Z., Arvan P., Liu M. Proinsulin misfolding and endoplasmic reticulum stress during the development and progression of diabetes. Molecular Aspects of Medicine. 2015;42:105–118. doi: 10.1016/j.mam.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sivitz W.I., Yorek M.A. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxidants & Redox Signaling. 2010;12(4):537–577. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sigfrid L.A., Cunningham J.M., Beeharry N., Borg L.A.H., Hernandez A.L.R., Carlsson C. Antioxidant enzyme activity and mRNA expression in the islets of Langerhans from the BB/S rat model of type 1 diabetes and an insulin-producing cell line. Journal of Molecular Medicine-JMM. 2004;82(5):325–335. doi: 10.1007/s00109-004-0533-4. [DOI] [PubMed] [Google Scholar]
- 223.Leloup C., Tourrel-Cuzin C., Magnan C., Karaca M., Castel J., Carneiro L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes. 2009;58(3):673–681. doi: 10.2337/db07-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Affourtit C., Brand M.D. Activity of uncoupling protein-2 in pancreatic beta cells. Biochimica Et Biophysica Acta-Bioenergetics. 2008;1777:S76–S77. doi: 10.1016/j.bbabio.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 225.Affourtit C., Jastroch M., Brand M.D. Uncoupling protein-2 attenuates glucose-stimulated insulin, secretion in INS-1E insulinoma cells by lowering mitochondrial reactive oxygen species. Free Radical Biology and Medicine. 2011;50(5):609–616. doi: 10.1016/j.freeradbiomed.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bashan N., Kovsan J., Kachko I., Ovadia H., Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiological Reviews. 2009;89(1):27–71. doi: 10.1152/physrev.00014.2008. [DOI] [PubMed] [Google Scholar]
- 227.Kahn S.E., Prigeon R.L., McCulloch D.K., Boyko E.J., Bergman R.N., Schwartz M.W. Quantification of the relationship between insulin sensitivity and beta-cell function in human-subjects – evidence for a hyperbolic function. Diabetes. 1993;42(11):1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 228.Peyot M.-L., Pepin E., Lamontagne J., Latour M.G., Zarrouki B., Lussier R. Beta-cell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes. 2010;59(9):2178–2187. doi: 10.2337/db09-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pfeifer M.A., Halter J.B., Porte D. Insulin-secretion in diabetes-mellitus. American Journal of Medicine. 1981;70(3):579–588. doi: 10.1016/0002-9343(81)90579-9. [DOI] [PubMed] [Google Scholar]
- 230.Ward W.K., Bolgiano D.C., McKnight B., Halter J.B., Porte D. Diminished B-cell secretory capacity in patients with noninsulin-dependent diabetes-mellitus. Journal of Clinical Investigation. 1984;74(4):1318–1328. doi: 10.1172/JCI111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rask E., Olsson T., Soderberg S., Johnson O., Seckl J., Holst J.J. Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care. 2001;24(9):1640–1645. doi: 10.2337/diacare.24.9.1640. [DOI] [PubMed] [Google Scholar]
- 232.Ward W.K., Lacava E.C., Paquette T.L., Beard J.C., Wallum B.J., Porte D. Disproportionate elevation of immunoreactive proinsulin in type-2 (non-insulin-dependent) diabetes-mellitus and in experimental insulin resistance. Diabetologia. 1987;30(9):698–702. doi: 10.1007/BF00296991. [DOI] [PubMed] [Google Scholar]
- 233.Polonsky K.S., Given B.D., Hirsch L.J., Tillil H., Shapiro E.T., Beebe C. Abnormal patterns of insulin-secretion in non-insulin-dependent diabetes-mellitus. New England Journal of Medicine. 1988;318(19):1231–1239. doi: 10.1056/NEJM198805123181903. [DOI] [PubMed] [Google Scholar]
- 234.Kanda Y., Hashiramoto M., Shimoda M., Hamamoto S., Tawaramoto K., Kimura T. Dietary restriction preserves the mass and function of pancreatic beta cells via cell kinetic regulation and suppression of oxidative/ER stress in diabetic mice. The Journal of Nutritional Biochemistry. 2015;26(3):219–226. doi: 10.1016/j.jnutbio.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 235.Sathananthan M., Shah M., Edens K.L., Grothe K.B., Piccinini F., Farrugia L.P. Six and 12 weeks of caloric restriction increases beta cell function and lowers fasting and postprandial glucose concentrations in people with type 2 diabetes. The Journal of Nutrition. 2015;145(9):2046–2051. doi: 10.3945/jn.115.210617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England Journal of Medicine. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Johnson J.B., Summer W., Cutler R.G., Martin B., Hyun D.H., Dixit V.D. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radical Biology and Medicine. 2007;42(5):665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Greenwood R.H., Mahler R.F., Hales C.N. Improvement in insulin secretion in diabetes after diazoxide. Lancet. 1976;1(7957):444–447. doi: 10.1016/s0140-6736(76)91473-2. [DOI] [PubMed] [Google Scholar]
- 239.Song S.H., Rhodes C.J., Veldhuis J.D., Butler P.C. Diazoxide attenuates glucose-induced defects in first-phase insulin release and pulsatile insulin secretion in human islets. Endocrinology. 2003;144(8):3399–3405. doi: 10.1210/en.2003-0056. [DOI] [PubMed] [Google Scholar]
- 240.Glaser B., Leibovich G., Nesher R., Hartling S., Binder C., Cerasi E. Improved beta-cell function after intensive insulin treatment in severe non-insulin-dependent diabetes. Acta Endocrinologica (Copenh) 1988;118(3):365–373. doi: 10.1530/acta.0.1180365. [DOI] [PubMed] [Google Scholar]
- 241.Defronzo R.A. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.DeFronzo R.A., Eldor R., Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care. 2013;36(Suppl 2):S127–S138. doi: 10.2337/dcS13-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zinman B., Gerich J., Buse J.B., Lewin A., Schwartz S., Raskin P. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD) Diabetes Care. 2009;32(7):1224–1230. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bunck M.C., Diamant M., Corner A., Eliasson B., Malloy J.L., Shaginian R.M. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2009;32(5):762–768. doi: 10.2337/dc08-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bunck M.C., Corner A., Eliasson B., Heine R.J., Shaginian R.M., Taskinen M.R. Effects of exenatide on measures of beta-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care. 2011;34(9):2041–2047. doi: 10.2337/dc11-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Van Raalte D.H., van Genugten R.E., Eliasson B., Moller-Goede D.L., Mari A., Tura A. The effect of alogliptin and pioglitazone combination therapy on various aspects of beta-cell function in patients with recent-onset type 2 diabetes. European Journal of Endocrinology. 2014;170(4):565–574. doi: 10.1530/EJE-13-0639. [DOI] [PubMed] [Google Scholar]
- 247.Yang T., Lu M., Ma L., Zhou Y., Cui Y. Efficacy and tolerability of canagliflozin as add-on to metformin in the treatment of type 2 diabetes mellitus: a meta-analysis. European Journal of Clinical Pharmacology. 2015;71(11):1325–1332. doi: 10.1007/s00228-015-1923-y. [DOI] [PubMed] [Google Scholar]
- 248.Ohn J.H., Kwak S.H., Cho Y.M., Lim S., Jang H.C., Park K.S. 10-year trajectory of beta-cell function and insulin sensitivity in the development of type 2 diabetes: a community-based prospective cohort study. Lancet Diabetes Endocrinol. 2016;4(1):27–34. doi: 10.1016/S2213-8587(15)00336-8. [DOI] [PubMed] [Google Scholar]
- 249.Marathe P.H., Gao H.X., Close K.L. American diabetes association standards of medical care in diabetes 2017. Journal of Diabetes. 2017;9(4):320–324. doi: 10.1111/1753-0407.12524. [DOI] [PubMed] [Google Scholar]
- 250.Zhang X., Gregg E.W., Williamson D.F., Barker L.E., Thomas W., Bullard K.M. A1C level and future risk of diabetes: a systematic review. Diabetes Care. 2010;33(7):1665–1673. doi: 10.2337/dc09-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]