Abstract
Background
Plasma insulin levels are predominantly the product of the morphological mass of insulin producing beta cells in the pancreatic islets of Langerhans and the functional status of each of these beta cells. Thus, deficiency in either beta cell mass or function, or both, can lead to insufficient levels of insulin, resulting in hyperglycemia and diabetes. Nonetheless, the precise contribution of beta cell mass and function to the pathogenesis of diabetes as well as the underlying mechanisms are still unclear. In the past, this was largely due to the restricted number of technologies suitable for studying the scarcely accessible human beta cells. However, in recent years, a number of new platforms have been established to expand the available techniques and to facilitate deeper insight into the role of human beta cell mass and function as cause for diabetes and as potential treatment targets.
Scope of Review
This review discusses the current knowledge about contribution of human beta cell mass and function to different stages of type 1 and type 2 diabetes pathogenesis. Furthermore, it highlights standard and newly developed technological platforms for the study of human beta cell biology, which can be used to increase our understanding of beta cell mass and function in human glucose homeostasis.
Major Conclusions
In contrast to early disease models, recent studies suggest that in type 1 and type 2 diabetes impairment of beta cell function is an early feature of disease pathogenesis while a substantial decrease in beta cell mass occurs more closely to clinical manifestation. This suggests that, in addition to beta cell mass replacement for late stage therapies, the development of novel strategies for protection and recovery of beta cell function could be most promising for successful diabetes treatment and prevention. The use of today's developing and wide range of technologies and platforms for the study of human beta cells will allow for a more detailed investigation of the underlying mechanisms and will facilitate development of treatment approaches to specifically target human beta cell mass and function.
Keywords: Diabetes, Human, Islet of Langerhans, Beta cell mass, Beta cell function, Pathogenesis, In vitro, In situ, In vivo
1. Introduction
In diabetes, uncontrolled and elevated blood glucose is the consequence of inadequate levels of plasma insulin, which are insufficient to effectively lower plasma glucose concentrations. Within a systemic environment, plasma insulin levels are usually the result of insulin clearance and, more importantly, insulin production and secretion by beta cells. Hereby, the total amount of released insulin depends on the absolute number of beta cells in the pancreatic islets of Langerhans (beta cell mass) and the output of each of these cells (beta cell function). For decades, the relative contribution of beta cell mass and function to the development of insufficient insulin levels and diabetes has been under debate. However, detailed knowledge on this aspect of diabetes pathogenesis will be crucial for the development of successful treatment approaches. Most of the currently available information on beta cell mass and function in diabetes stems from experiments on mouse models. Yet, many studies have demonstrated that human and mouse beta cells show vastly different characteristics, in particular when it comes to beta cell mass regulation. Thus, studies on human beta cells and islets are indispensable to develop therapies targeting beta cell mass, function, or both to treat diabetes.
The lack of studies on human beta cells is primarily related to limited availability of human samples and a shortage of technologies to comprehensively investigate human beta cell biology. However, in recent years the field has made great progress in the organized procurement of human tissue and the development of novel technologies. Utilizing these experimental platforms to study human beta cells will be necessary to enhance our current knowledge on human beta cell mass and function in diabetes development and bring us closer to effective diabetes therapies.
2. Human beta cell mass and function in diabetes pathogenesis
2.1. Type 1 diabetes
Type 1 diabetes (T1D) is a chronic autoimmune disorder in which the immune system attacks endogenous pancreatic beta cells resulting in insulin deficiency, chronic hyperglycemia, and long-term complications. Creating a successful cure for T1D will need to include stopping the self-damaging autoimmune process and restoring appropriate insulin release from beta cells. Addressing the latter requires detailed knowledge about alterations in beta cell mass and function in the asymptomatic prediabetic period of disease pathogenesis and the contribution of beta cell mass and function to clinical manifestation and the onset of hyperglycemia.
2.1.1. Beta cell mass and function during the prediabetic phase of T1D
Initially, progression of beta cell mass decline prior to the onset of hyperglycemia was thought to be linear [1]. However, in recent years, the model for prediabetic pathogenesis of T1D has been adjusted to reflect the relapsing and remitting progression of disease pathogenesis [2], [3], [4] and to acknowledge the heterogeneous progression time from seroconversion to diabetes which can range from weeks to over two decades [5]. Unfortunately, limited information on human beta cell mass is available for the asymptomatic time period [6]. However, it is thought that chronic insulitis reduces beta cell mass in the prediabetic phase by induced cell death via direct cell–cell contacts [7] or secreted proinflammatory cytokines [8]. This hypothesis was corroborated by a recent report measuring unmethylated INS DNA in human blood samples as an indicator for beta cell death [9]. The authors demonstrated that beta cell death is elevated in high risk T1D subjects and increases further towards clinical diagnosis, suggesting that the major reduction of beta cell mass occurs late during the prediabetic phase. This is in line with studies on human donor tissue which found no loss of beta cell mass in autoantibody positive subjects prior to diabetes in comparison to controls [10], [11], [12], while demonstrating a massive reduction in recent onset T1D patients [12], [13]. Besides changes in the intensity of the autoimmune attack, one explanation for a delayed decrease in beta cell mass despite ongoing cell death might be an increased beta cell proliferation in response to inflammation as seen in mice [14], [15]. This would also explain the twofold increased beta cell mass observed in nondiabetic autoantibody positive subjects with insulitis compared to autoantibody positive subjects without insulitis or controls [16].
Despite prolonged preservation of beta cell mass, plasma insulin levels indicate an altered insulin output already in the prediabetic phase, indicating changes in beta cell function. In several perspective, longitudinal studies in patients at risk beta cell performance was assessed by measuring C-peptide, glucose tolerance, and first phase insulin response in metabolic tests. While metabolic tests cannot reliably distinguish between the roles of cell mass and function (see also Section 3.3), these studies strongly contribute to our current understanding of the prediabetic phase in human T1D pathogenesis. An early study on 9 subjects observed no signs of elevated fasting or stimulated glucose levels in the prediabetic phase until immediately before disease onset, while demonstrating a progressive loss of the first phase insulin response [17]. Additional studies followed up on this topic in larger cohorts of patients at risk and showed that loss of first phase insulin release during the prediabetic phase is correlated with age and the number of autoantibody subtypes [18], [19], [20]. This is also confirmed by measurements of C-peptide demonstrating maintained fasting but decreasing stimulated C-peptide levels throughout the prediabetic phase [21]. Concentrating on the most recent studies, several groups have shown that a decline in first phase insulin release is already detectable 4–6 years before clinical onset [19], [20], [22], [23]. Taking into account that beta cell mass was found to be unchanged or even increased in the prediabetic phase [10], [11], [16], early changes in first phase insulin release are presumably the result of functional impairment. Closer to the emergence of hyperglycemia, the decline in first phase insulin release and stimulated C-peptide was observed to be further aggravated [21], [23], which might be due to combined beta cell dysfunction and increased beta cell apoptosis close to diabetes onset. Taken together these observations support the hypothesis that, especially in cases of a long prediabetic phase an early, slowly progressing functional impairment of beta cells precedes a late, rapidly advancing morphological destruction and functional decline of beta cells.
2.1.2. Beta cell mass and function at and after onset of hyperglycemia
Inferring from the observations of the prediabetic phase, onset of hyperglycemia is caused collectively by reduced beta cell mass and beta cell dysfunction. However, the extent of morphological and functional insufficiency varies between patients and contributes differently to the development of hyperglycemia. Conceivably, as a result from studying mostly severe and early onset cases, near total loss of beta cell mass (>80% reduction) at disease onset was a long held general paradigm for T1D pathogenesis [24], [25], [26], [27], [28]. However, this concept does not coincide with the residual beta cell function seen in subjects with onset in adolescence [29], [30], [31], suggesting a more preserved beta cell mass with up to 40% residual insulin-containing islets in older onset subjects [29], [32]. Hereby, the remaining beta cell mass and function at onset are determined by age [33], [34], degree, and cellular profile of insulitis [29]. In addition, evidence from us and others in mouse and human suggests that beta cell mass might be underestimated as a consequence of degranulation, an almost complete loss of insulin granules resulting in negative hormone staining of exhausted beta cells [14], [16], [35], [36]. After onset, beta cell destruction by the ongoing autoimmune infiltration continues and is additionally exacerbated by the increasing metabolic and glycemic overload causing ER stress and apoptosis [37]. In particular within the initial time period after diabetes onset, beta cell apoptosis is elevated [28], but seems to slow down in long-standing T1D [9], potentially as a result of the decreasing number of beta cells. Nevertheless, even after diabetes onset, compensatory mechanisms might operate against beta cell mass reduction. In a case report from 2006, Meier and colleagues showed enhanced beta cell proliferation in a recently diagnosed 89-year-old patient [38]. Likewise, Willcox et al. showed increased beta cell proliferation in a set of 10 patients with recent onset diabetes (<18 month) [39], which might have been induced by the inflammation itself [14] and/or the elevated glucose levels [40]. Alternatively, the occurrence of small beta cell clusters scattered throughout the exocrine tissue in long-standing T1D patients was interpreted as a cell population that evaded autoimmune destruction or a compensatory beta cell population of yet unknown origin [41], [42]. Hence, apoptosis and regeneration might occur simultaneously during disease progression and sustained apoptosis may be explained by continuous beta cell mass compensation through proliferation, transdifferentiation, or neogenesis [41], [43].
In addition to reduced cellular mass, beta cell function continues to exhibit an important role at diabetes onset and thereafter. Residual beta cells after onset and in long-standing diabetes show signs of functional exhaustion and degranulation [35], [36], while still expressing beta cell specific markers like glucose transporter 2 [44]. Nevertheless, despite a massive decline in beta cell mass and function by the time of onset, basal insulin secretion is preserved [21], [35], [45], [46], whereas stimulated C-peptide response is reduced in an onset age dependent manner [30], [46], [47], [48]. Within the first years following onset the deterioration of stimulated C-peptide levels continues rapidly [31], but slows down with disease duration [49], leading to marginal C-peptide levels in long-standing type 1 diabetic patients [49], [50], [51]. Support for a significant role of beta cell function in the onset of T1D is the observation that in a subset of recent-onset T1D patients, insulin treatment leads to a spontaneous partial or even full remission. This phenomenon, also known as the “honeymoon phase,” was initially described in the 1940s by Jackson and Brush [52], [53] and is characterized by a reduced or even absent insulin demand as well as an increase in C-peptide and pro-insulin levels [54], [55], [56]. Manifestation of the honeymoon phase is determined by the age at onset, disease severity, and, potentially, the number of islet autoantibodies [57], [58], [59], [60], [61]. Duration of the remission phase is known to be quite variable, ranging from several weeks up to years [57], [62]. As underlying mechanisms for the recovery of C-peptide and pro-insulin levels, an increase of beta cell mass, e.g. by proliferation, or the recovery of beta cell function are discussed. While beta cell proliferation during remission was shown in mice by our and other groups [14], [35], [36], human islets do not show mass regeneration in the same condition [36]. On the other hand, exhausted beta cells were found to regranulate and recover after intervention in parallel with stimulated insulin levels and normalization of metabolic parameters [36]. These observations substantiate the concept of functional beta cell recovery as cause of the honeymoon phase [35] and exemplify the important role of beta cell function in the onset and therapy of T1D.
2.2. Type 2 diabetes
Type 2 diabetes (T2D) is a progressive metabolic disorder characterized by insulin resistance and hyperglycemia. In T2D, insufficient levels of insulin fail to meet the elevated demand caused by an increased insulin resistance. Currently, most clinical treatments of T2D either target insulin resistance or aim to elevate insulin levels by increasing beta cell function. In light of a potential exhaustive effect on beta cells, more basic research turns to study mechanisms to regenerate beta cell mass or to preserve beta cell function. To that aim, a thorough understanding of the contribution and dynamics of beta cell mass and function to compensation of insulin resistance and progression to T2D are needed.
2.2.1. Beta cell compensation
While obesity and insulin resistance remain major risk factors for T2D [63], the compensatory capacity of beta cells is credited to prevent most obese and insulin resistant subjects from developing T2D [64], [65]. It is hypothesized that in these individuals, an increase in insulin output via enhanced secretion and/or expanded beta cell mass counteracts the development of hyperglycemia and glucose intolerance [65]. Evidence of human beta cell mass compensation in obesity was already reported in 1933, when Ogilvie described “an abnormally high percentage of islet tissue” within the pancreas of 19 cases of obesity in comparison to 19 lean subjects [66]. Further reports in the following decades observed similar increases in beta cell mass and more recent studies that analyzed beta cell mass in tissue from donor organs and surgical resections [67], [68] or islets after isolations [69], [70] revealed that, despite the strong variation between individuals, beta cell mass is generally increased by 50–90% in overweight or obese subjects. In 2003, Butler and colleagues published an elegant study based on a large pool of human autopsy tissue samples, providing additional convincing evidence of beta cell mass compensation [71]. In this study, tissue from 124 well-phenotyped subjects was analyzed and grouped according to BMI and glucose homeostasis status. The relative beta cell volume (the ratio of beta cell over total pancreas area) was found to be about 50% higher in obese nondiabetic subjects compared to lean controls. A less substantial increase in beta cell mass in obese subjects of about 20% was observed by Rahier and colleagues in a later study [72]. In search for the underlying mechanisms, Butler et al. did not detect significant numbers of proliferating beta cells but an increased frequency of insulin positive duct cells in the pancreas of obese subjects. Therefore, the authors concluded that neogenesis rather than proliferation leads to the observed beta cell mass increase [71]. In a later publication, the same group confirmed the 50% enlargement of beta cell mass in a different cohort of obese subjects, again without observing any increase in beta cell proliferation [73]. This is in line with other reports, which found neogenesis, and not proliferation, to be the underlying mechanism for beta cell mass increase in insulin resistant and impaired glucose tolerant subjects [74], [75]. Yet, a lack in the detection of beta cell proliferation might also be affected by a potential discrepancy in the time point of beta cell mass expansion and the assessment of indicators of proliferation.
In addition to an enlarged beta cell mass, plasticity of beta cell function contributes to the compensation of an increased insulin demand during obesity and insulin resistance. Normally, beta cell output in humans is measured by quantifying insulin or C-peptide plasma levels in response to metabolic tests. As discussed below, these tests cannot clearly distinguish between the contribution of beta cell function and mass. Nevertheless, correlating the compensatory increment in beta cell mass from histological studies with insulin plasma levels in response to metabolic tests during obesity can provide an estimate on the contribution of beta cell function to compensation of nondiabetic insulin resistance. In support of a major role of beta cell function in compensation, a number of different studies observed strongly elevated fasting plasma insulin levels and a several fold greater insulin response to stimulation in obese nondiabetic subjects [76], [77], [78], [79], [80], [81], clearly exceeding the reported 20–90% increase in beta cell mass. Importantly, despite the hypersecretion, beta cell release dynamics were mostly unchanged [79], [80]. Correspondingly, some studies found that isolated islets from obese subjects exhibit a two-to threefold elevated insulin secretion in comparison to islets from lean individuals [70], [82]. These studies suggest that beta cell functionality contributes to a large extent to increased insulin output in response to obesity and insulin resistance, multiplying the effect of beta cell mass expansion.
2.2.2. Beta cell deficiency
When the adaptive response of beta cells to increased insulin resistance is insufficient or fails hyperglycemia and T2D develop. Similar to compensation, relative insulin deficiency in T2D involves changes in both beta cell mass and function. By now, ample data is available that describes the occurrence of beta cell loss in T2D. Although a few reports did not detect any difference in the beta cell mass of T2D patients and nondiabetic controls [83], [84], most studies agree on a significant reduction in beta cell mass, ranging from 24% to 65% [67], [68], [71], [72], [85], [86], [87], [88]. This is additionally confirmed by a reduced yield and smaller size of islets isolated from organs of T2D versus nondiabetic donors [89]. The underlying mechanisms for the reduction of beta cell mass in T2D patients is most likely a dramatically increased rate of beta cell apoptosis [90], [91] and probably not related to differences in beta cell replication frequency or neogenesis [71]. Variations in the observed beta cell loss in the various studies could be caused by differences in the patient characteristics of the cohorts, e.g. age or BMI. In particular, differences in disease duration might influence the degree of beta cell loss. In the study of Rahier et al., mean mass deficit was 24% in patients within 5 years of overt diabetes onset whereas the deficit increased to 54% in those subjects with more than 15 years since diabetes onset [72]. However, this relationship of decline in beta cell mass and diabetes duration was not observed in a study by Yoon and colleagues [67].
Besides the reported reduction in beta cell mass, a substantial deficit in beta cell function is evident in T2D patients. Similar to the compensation phase, contribution of beta cell function to insulin insufficiency can be deduced from correlating the described loss of beta cell mass with glucose homeostasis and insulin release. As described above, beta cell mass reduction was observed to range from 24% to 65%. However, insulin secretion capacity in T2D was shown to be reduced 50%–97% [92], [93], [94], [95], [96], [97], demonstrating the contribution of beta cell function to insulin deficiency. In agreement with this, studies on living donors undergoing hemipancreatectomy have demonstrated that after removal of 50% of the pancreas, donors showed normal 24-hour glucose profiles even though insulin secretion was reduced [98]. Only in the presence of obesity and related insulin resistance, hemipancreatectomized patients showed an increased risk of developing T2D [99], revealing that a 24%–65% reduction of beta cell mass alone is unlikely to cause T2D. Another indication of beta cell dysfunction in T2D is the observed change in the dynamics of insulin release, which includes loss of first phase response [100] and disruption of regular oscillatory release patterns [101], [102]. These are more likely to be the result of functional alterations than changes in cell mass. Finally, the essential role of beta cell function in T2D is illustrated by the rapid recovery observed in several conditions, which occurs within a time frame that makes participation of beta cell mass as a means to increase insulin levels an unlikely scenario. For example, bariatric surgeries in obese T2D patients often lead to improvement in glucose control or even diabetes remission within days or weeks [103]. Fast recuperation of beta cell glucose sensitivity and early insulin response together with a normalization of insulin sensitivity are proposed mechanisms for this acute reversal [104], [105], [106]. A similar rapid recovery of beta cell function regarding glucose responsiveness and first phase secretion can also be observed during short-term caloric restriction of T2D patients [107], [108].
A recently proposed potential mechanism underlying beta cell deficits in T2D is beta cell dedifferentiation. It is suggested that beta cells in T2D do not die, rather they become silenced as a result of dedifferentiation [109]. Characteristics of beta cell dedifferentiation include reduced expression of key beta cell transcription factors like PDX1, Nkx 6.1, and MafA and re-expression of specific progenitor cell transcription factors including Neurogenin3, Oct4, Nanog, and L-Myc [110]. These dedifferentiated cells lose their beta cell identity, become insulin negative, and no longer contribute to the metabolic control of glucose homeostasis [109]. Interestingly, beta cell dedifferentiation has been demonstrated to be reversible by normalization of blood glucose with insulin therapy in a mouse study [111]. Thus, dedifferentiated cells appear as non-beta cells in insulin or C-peptide stainings and add to the impression of a decreased beta cell mass. However, retrieval of beta cell features and functional recovery, at least until a certain time point of disease pathogenesis seem possible. Nevertheless, the contribution of beta cell dedifferentiation to beta cell deficit in human T2D is still under debate [112], [113], [114] and requires further investigations to clarify the mechanism and significance of beta cell dedifferentiation in T2D pathogenesis. For instance, while a recent study could confirm the compromised expression of beta cell transcription factors like MAFA, PDX1, and NKX6.1 in human T2D beta cells, the authors did not detect differences in the expression levels of progenitor cell markers like Neurogenin3, Nanog, or MYCL1 [115].
Although beta cell dysfunction and beta cell loss is generally accepted in T2D pathogenesis, their kinetics during the progression to hyperglycemia is debated. Few studies address beta cell mass in subjects with signs of impaired glucose homeostasis prior to onset or in diabetic patients shortly after onset. Butler et al., using tissue samples of organ donors, observed a substantial reduction in relative beta cell area in nondiabetic subjects with impaired fasting blood glucose [71], whereas Meier et al., employing tissue samples from pancreatic surgeries, observed no significant reduction of fractional beta cell area in nondiabetic impaired glucose tolerant subjects [116]. Moreover, Rahier and colleagues observed a reduction of only 24% in beta cell mass in subjects within 5 years of diabetes onset, which exacerbated with disease duration [72]. In contrast, functional changes such as the acute insulin response to glucose have been described as an early predictor of the transition from normal glucose tolerance to impaired glucose tolerance [117]. Changes in insulin secretion dynamics are believed to lead to lower insulin signaling efficiency [118], [119], [120]. The resulting incomplete glucose clearance and subsequent elevated blood glucose, in concert with other cytotoxic factors, further deteriorate beta cell mass and function. This “vicious cycle” has been suggested to be responsible for total beta cell failure in T2D and clinical manifestations of the disease [121], [122]. These results point towards a rather moderate reduction of beta cell mass at diabetes onset and indicate that the observed more severe beta cell mass deficit from autopsy studies is the result of hyperglycemia. In line with this, beta cell function is suggested to be the prerequisite factor for the onset of diabetes [63], [123], [124], [125], [126], [127], being decreased by about 50–80% at the time of T2D diagnosis [63], [122], [127]. However, these observations do not rule out the possibility that a reduced inherent beta cell mass depicts a strong risk factor for the development of T2D. Various factors during fetal and postnatal life potentially influence the buildup of beta cell mass, resulting in a limited amount of beta cells to regulate glucose homeostasis throughout the organism's lifetime. This hypothesis is supported by the identification of T2D associated single-nucleotide polymorphisms (SNPs) in genes regulating beta cell replication, including CDKN2A and CDKN2B, which most likely limit compensatory proliferation and reduce beta cell mass expansion during development and maturation [128], [129], [130].
2.3. Summary – beta cell mass and function in diabetes
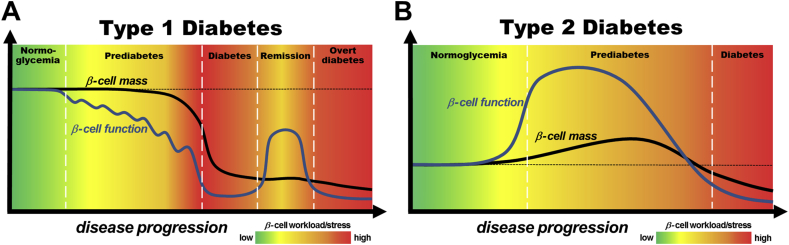
The current literature suggests that beta cell mass and function contribute in different ways to the development of T1D and T2D (Figure 1). In T1D (Figure 1A), the prediabetic phase seems to be characterized by a prolonged, gradual, functional impairment followed by a rapid decline in beta cell mass just before the onset of hyperglycemia. The amount of mass reduction at clinical manifestation is strongly heterogeneous and depends on factors like onset age; for example, there is a greater reduction in beta cell mass in early onset T1D. Nevertheless, functional exhaustion of beta cells is a major component in the development of hyperglycemia, as shown by the presence of exhausted cells and the transient remission after treatment start in some patients. Thus, immune intervention at T1D onset could be successful to recover at least partial control of glucose homeostasis. In contrast, T2D development (Figure 1B) is the result of a lack or cessation of morphological and functional beta cell compensation in response to an elevated insulin demand during insulin resistance. This typically successful compensation is mainly driven by functional alterations, which outperform mass adaptation in rate and extent. However, in certain situations, functional beta cell compensation seems to be insufficient or to deteriorate, increasing beta cell work load and stress and resulting in functional exhaustion, dedifferentiation, and finally beta cell death. Therefore, in T2D, the protection and recovery of beta cell function should be a main treatment and prevention target. Despite these findings, many aspects of the role of beta cell mass and function in diabetes pathogenesis are still unclear. Yet, we now have a broad spectrum of available technologies to study human beta cell biology, which should facilitate addressing unanswered questions.
Figure 1.
Models of the contribution of beta cell mass and function to pathogenesis of type 1 diabetes (A) and type 2 diabetes (B). (A): Beta cell mass and function in the development of type 1 diabetes. Initiation of islet autoimmunity by genetic and environmental factors leads to a relapsing-remitting decline of beta cell function, continuously increasing beta cell workload, and stress in the asymptomatic prediabetes phase. Shortly before clinical manifestation of diabetes the prolonged intensified beta cell workload and autoimmunity results in total cellular exhaustion and enhanced cell death leading to a massive decrease in beta cell mass and the onset of hyperglycemia. In some patients, initial insulin treatment induces temporary remission called the “honeymoon phase,” which is attributed to a moderate reduction in beta cell workload and antigenicity, resulting in functional recovery of residual beta cells. However, ongoing autoimmunity and elevated workload lead to recurrence of cellular exhaustion, cell death, and the development of overt diabetes. Black line: beta cell mass; Blue line: beta cell function. The color-coded background indicates the intensity of beta cell workload and stress caused by immune infiltration, metabolic demand and hyperglycemia. (B): Beta cell mass and function in the development of type 2 diabetes. In many individuals, genetic predisposition and unhealthy lifestyle lead to an increased insulin resistance, which is typically met by massive functional and moderate morphological compensation to maintain normoglycemia, thus increasing the workload of each beta cell. In some of these individuals, functional compensation halts, despite prolonged insulin resistance and results in a further escalation of beta cell workload and glucose intolerance. In this prediabetic phase, chronic glucose intolerance and elevated blood glucose levels continuously exacerbate beta cell workload and stress, culminating in cellular exhaustion, cell death, and clinical manifestation of hyperglycemia. Thereafter, uncontrolled hyperglycemia, often in concert with other cytotoxic factors, leads to accelerated beta cell mass loss and functional deterioration in overt diabetic patients. Black line: beta cell mass; Blue line: beta cell function. The color-coded background indicates the intensity of beta cell workload and stress caused by insulin resistance, metabolic demand, hyperglycemia and additional cytotoxic factors.
3. Technologies to study human beta cell mass and function
3.1. In vitro
In vitro approaches utilizing isolated human islets or dispersed beta cells are currently the method of choice for most investigators to study human beta cell biology. Although these preparations lack systemic and organ-level input and are affected by the isolation procedure as well as the following culture period, their use is highly beneficial for shedding light on diabetes pathogenesis and therapy. To a certain extent, islets isolated from subjects with different metabolic statuses can provide information on ongoing changes in beta cell mass. Whereas, the outcome of islet isolations depends on numerous factors and may not always reflect the actual beta cell mass, isolated islets can provide insight into changes in processes involved in the regulation of beta cell mass, like proliferation and apoptosis. For instance, islet size and beta cell number were observed to be increased in islets isolated from insulin resistant non-diabetic subjects [74], whereas increased apoptosis was observed in islets isolated from T2D patients [91]. Similarly, islets isolated from T1D patients were shown to exhibit elevated rates of apoptosis [131]. Furthermore, isolated islets serve as a good model for the investigation of hormone secretion in static and kinetic perifusion settings, with the possibility to test different protocols and compounds. For instance, use of isolated islets demonstrated the manifestation of functional compensation in obese and insulin resistant subjects [70], [82]. Similarly, islets isolated from T1D patients [131], [132], [133] revealed loss of beta cell function and recovery in culture. In addition, isolated islets can be employed to study different elements of the signal transduction pathway in beta cells, for example, by live cell imaging of cytosolic calcium [134], NAD(P)H [135] and exocytosis [136], by assessment of mitochondrial respiration and glycolysis [135], or by the use of the patch-clamp technique [137]. Finally, isolated islets are used to determine protein localization by immunohistochemistry [138] and whole islet protein expression by western blots [139]. Thus, in vitro studies enable detailed investigations on underlying mechanisms and potential treatment targets.
Among the new emerging in vitro technologies used on isolated human islets, transcriptomics seems to be a promising approach as it may help to resolve the roles of beta cell mass and function in diabetes. The first comprehensive transcriptomic analysis of major pancreatic cell types was reported in 2011; in this analysis, two novel beta cell specific transcriptional regulators (HOPX and HDAC9) were identified when the expression of 5038 genes were examined in dispersed human pancreatic cells [140]. Since then, different genes or pathways related to beta cell dysfunction, including the ones modified by free fatty acid (palmitate) [141], those involved in deregulated glucose metabolism [142] and alterations in ubiquitin–proteasome system (UPS) [143] have been discovered by transcriptomic analysis of whole human islets. Recent advances in next-generation sequencing approaches have now enabled such studies on the single cell level. Single cell analysis can help to target cell heterogeneity and, more importantly, to study cellular changes that would be diluted and undetectable when analyzed in whole islets or in sorted cell bulks [144]. Single cell transcriptomics analysis of dispersed human islets has identified cell type specific genes, transcription factors, and surface markers [145], [146] as well as rare cell types and subpopulations [144], [147], [148] and genes associated with obesity and type 2 diabetes [147], [148], [149]. In addition, a partially dedifferentiated status was observed in alpha and beta cells from T2D donors [144], supporting the contribution of dedifferentiation to beta cell deficit in diabetes. An interesting finding of these first studies was the downregulation of FXYD2 expression, which was found to be the most significant difference between beta cells from healthy and T2D individuals [147]. In mice, downregulation of FXYD2 expression leads to beta cell proliferation [150], suggesting an ongoing attempt to compensate for the reduced beta cell mass in human T2D.
Proteomics of isolated islets is another powerful, novel in vitro tool that can help disentangle changes in pathways involved in beta cell mass and function. For instance, quantitative protein profiling of islets from T2D and non-diabetic individuals revealed differential expression in twenty major pathways, which correlated with their insulin secretion profile [151]. Cell arrest, apoptosis, proliferation, and immune-response pathways were among the most differentially expressed pathways in T2D islets. Interestingly, employing mass cytometry technology with single cell analysis, a recent study could show that alpha cells exhibit the highest replication rate at basal conditions and after harmine stimulation [152].
Finally, although representing a very different approach, generation of beta cells from human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) represents a novel in vitro technology to study diabetes development and therapy. The last decade saw a rapid advancement in generation of so called beta-like cells derived from hESCs, leading to the first ongoing clinical trial [153]. Indeed, scalable differentiation protocols were developed to generate hESC-derived glucose-responsive beta cells in vitro that resemble human beta cells in gene expression patterns and ultrastructure and can ameliorate hyperglycemia in vivo [154], [155], [156]. Additionally, encapsulated in vitro generated beta-like cells were able to restore long-term glycemic control in immunocompetent mice without immunosuppression [157]. Besides its application as unlimited source of functional beta cells for drug screening and cell replacement therapy, in vitro generation of beta cells also holds great promise for disease modeling and the study of beta cell biology in diabetes. Recently, the first in vitro generation of hiPSC-derived beta cells from T1D diabetes patients has been reported [158]. In combination with advanced gene editing technologies like TALEN and CRISPR-Cas9, the progress in in vitro beta cell generation opens up numerous strategies to explore the role of specific beta cell genes in diabetes pathogenesis. A related and also rather new in vitro approach to study beta cells is the development and culture of islet and pancreas organoids. Starting from stem and progenitor cells, these structures can be grown into a similar three-dimensional organization as an organ. While application of this methodology for the pancreas is rather new, it has recently been successfully used to grow human pancreas organoids from pluripotent stem cells [159], [160]. The future development of this technique has great potential for studying developmental processes in particular, including the growth of beta cell mass and the formation of beta cell function. In addition, the use of iPSCs from adults has been shown useful for disease modeling of pancreatic cancer and cystic fibrosis [159], [161] and therefore could allow for studying aspects of beta cell mass and function in diabetes [162].
Thus, conventional and novel in vitro technologies have been and will be essential for identifying and confirming molecular mechanisms for beta cell demise and recovery. However, endogenous pancreatic islets are complex mini organs composed of different, interacting cell types, and their mass and function are regulated by multifaceted local and systemic signaling mechanisms. Thus, it is necessary to additionally employ more physiological in situ and in vivo approaches to validate in vitro findings and reflect the complex mechanisms of beta cell biology.
3.2. In situ
3.2.1. Histological analyses
The most common in situ approach for the study of human beta cells is the histological examination of fixed pancreas tissue of non-diabetic and diabetic deceased subjects or patients undergoing pancreatic surgeries. This approach allows for thorough morphometric analyses of pancreatic tissue and remains the gold standard for assessment of pathophysiological effects on islet cell mass and composition. Quantification of cell death and proliferation in such specimens is widely used in the field and has made significant contribution to our knowledge about diabetes pathogenesis. The identification of different subpopulations of endocrine cells, especially of beta cells [163], as well as the characterization of cell type specific markers that can be used in antibody stainings deepens our knowledge of islet cell plasticity and compensatory mechanisms like trans- or dedifferentiation [164], [165]. Electron microscopy of ultra-thin sections is another important tool for the determination of alterations on the intracellular level and is therefore also a widely used application for diabetes research. However, an important drawback of this technique is its limitation to fixed tissue, which is accompanied by a number of artifacts and does not allow assessment of function. In addition, procured tissue from diabetic patients often originates from deceased human donors with limited information on disease duration, health status, and therapy.
3.2.2. Pancreas tissue slices
More recently a novel in situ approach has been used to overcome some of the given limitations of employing fixed tissue sections or living single cells and isolated islets. To that aim, in 2003, Speier and Rupnik developed and published a tissue slice technique for mouse pancreas [166], which enabled investigations of pancreatic endocrine and exocrine cell biology in situ. Previously the tissue slice platform had proven to be a valuable tool for the study of cell biology of various organs under close to physiological conditions [167], [168], [169]. Applied to the pancreas, this technique allows for obtaining viable tissue while preserving organ structure and cellular surrounding of the islets. Importantly, in comparison to islet isolation, tissue slice preparation is performed in a considerably shorter time period, without enzymatic digestion or the need for resting culture. Slice thickness (usually between 120 and 150 μm) allows for three-dimensional analyses of intact islets in their natural surroundings, enabling assessment of islet composition and density within the exocrine tissue. In addition, mouse pancreas tissue slices demonstrated that formations and processes on the islet perimeter or within the surrounding exocrine component, like the neuroinsular complex [170] or the intrapancreatic innervation, can be investigated [171]. Besides enabling complex morphological analyses, another advantage of pancreas tissue slices is the investigation of cell function within viable pancreatic tissue, facilitating a plethora of functional assays close to the in vivo situation. Pancreas tissue slices have proven to be valuable for electrophysiological assessment of islet cell physiology, providing new insight into ion channel and gap junction characteristics under physiological conditions [172], [173], [174], [175], [176]. Also, intracellular Ca2+ dynamics alone or in combination with membrane potential and hormone secretion have been employed in tissue slices to obtain additional knowledge about exocrine and endocrine cell biology in an intact pancreatic tissue environment [177], [178], [179], [180].
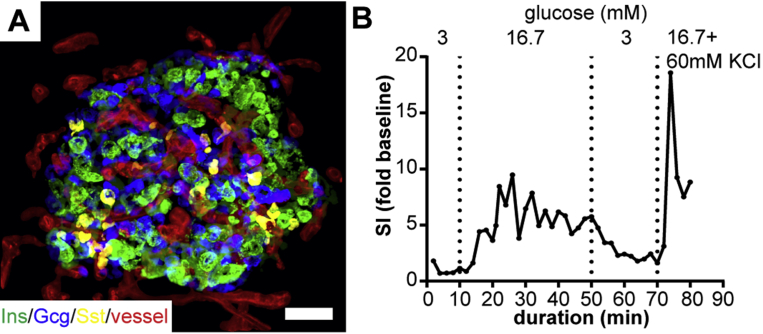
After successful use with animal models, the tissue slice approach has recently been adapted for the study of human endocrine and exocrine pancreas [181], [182], [183]. To that aim, human tissue is either obtained acutely from the pancreas of partially pancreatectomized patients [184] or from donor organs. The achieved preservation of additional morphological features in human tissue proved to facilitate a more complex spatial cellular analysis than obtained previously from isolated islets [185]. Thus, employing human pancreas tissue slices demonstrates that the three-dimensional vascular architecture of human islets differs significantly from that of mouse islets, facilitating specific cell–cell arrangements with substantial implications for cell interactions within the human islet (Figure 2A) [182]. Another important step was achieved with establishing the measurement of kinetic hormone release from perifused human tissue slices (Figure 2B) [183]. In combination with the above described techniques to investigate islet cell biology in tissue slices, this approach now holds great potential for gaining insight into human diabetes pathogenesis and potential treatment targets. Of particular interest is the use of tissue slices for the study of islet biology in T1D. Here, disease related islet destruction hampers the islet isolation process, biasing isolation outcome to predominantly less affected islets and limiting the ability to thoroughly investigate islet biology at different stages of the autoimmune attack. Tissue slices overcome this obstacle, allowing the determination of direct effects of immune cell infiltration on islet morphology and cell function. Furthermore, in tissue slices, scattered beta cell clusters within the exocrine tissue, which have been reported for both types of diabetes [41], [42], [112], can be investigated. Finally, establishing culture conditions that preserve human tissue slice viability and function for several days, as reported previously for pancreas tissue slices of the mouse [178], will increase the wide range of experimental opportunities for mid-term analyses of endocrine and exocrine tissue cell function. Taken together, pancreas tissue slices are a valuable tool to expand our knowledge on human beta cell biology under near physiological conditions by facilitating studies in an unperturbed in situ environment.
Figure 2.
Human pancreas tissue slices for the in situ study of human beta cell morphology and function. (A) Maximum intensity projection of a human islet in a pancreas tissue slice from a non-diabetic human patient that underwent partial pancreatectomy. Slices were stained for insulin (green), glucagon (blue), somatostatin (yellow), and fluorescently labeled lectin (red) following recently published protocols [182]. (B) Kinetic insulin release from 4 human pancreas tissue slices obtained from the same pancreatic tissue as used in (A). Insulin release is expressed as simulation index (SI) over mean basal secretion (0–10 min). Slices were perfused in a closed chamber with Krebs–Ringer bicarbonate HEPES buffer and indicated glucose concentrations at a flow rate of 200 μl/min using a perifusion system (Biorep). Insulin concentrations of the perfusate were assessed by ELISA. Human tissue was kindly provided by Michele Solimena and Jürgen Weitz (University Hospital Carl Gustav Carus, TU Dresden, Germany).
3.3. In vivo
The ultimate goal to understand and verify the contribution of beta cell mass and function to disease progression and therapy is in vivo assessment. In humans, in vivo beta cell capacity is usually evaluated by one of various metabolic tests, including fasting insulin, oral glucose tolerance test (OGTT), mixed meal tolerance test (MMTT), intravenous glucose tolerance test (IVGTT) with or without arginine stimulation, or the hyperglycemic clamp. The choice of test is influenced by the different technical complexity of the test and its ability to assess specific aspects of insulin secretion. Calculation of the homeostasis model assessment (HOMA and HOMA2) [186], [187] for beta cell output from fasting insulin and glucose plasma levels requires limited experimental experience, but only provides a measure of the basal state. In contrast, OGTT and IVGTT assess beta cell output in response to stimulation. Overall, the OGTT is rather easy to administer and includes the incretin effect on insulin secretion. The IVGTT, on the other hand, is more difficult to perform but allows assessment of first phase insulin release and bypasses the incretin axis, which is potentially modulated by input from the central nervous system [188]. A more reproducible technique that informs on glucose dependent insulin secretion is the hyperglycemic clamp technique. However, the high technical expertise demanded for its conductance limits its standard use to assess beta cell function.
Common to all metabolic tests is the difficulty distinguishing between the contribution of beta cell mass and function to the measured secretion. Several studies have reported good correlations between beta cell mass and metabolic tests in humans by relating the in vivo assessed beta cell response to isolated islet volume [189], the number of auto- or allotransplanted islets [190], [191], [192] or beta cell mass in tissue samples obtained from pancreatic surgery [116], [193]. However, these studies also showed a substantial variability between individuals of similar beta cell/islet mass. This is not surprising as beta cells demonstrate strong functional plasticity in response to physiological regulation [194], [195], [196] or treatment [197], [198], while beta cell mass changes in humans are comparatively moderate [73], [199]. Thus, changes in insulin or C-peptide secretion assessed in vivo within subjects during disease pathogenesis or therapy cannot be easily determined to be an alteration of beta cell mass or function.
Great hope has been put into the development of non-invasive in vivo imaging techniques that would allow assessment of beta cell mass in humans. In combination with metabolic tests, these techniques could represent the optimal approach to separate the roles of beta cell mass and function in glucose homeostasis. In this context, positron emission tomography (PET), single-photon emission computed tomography (SPECT), and magnetic resonance imaging (MRI) are currently considered the most promising imaging modalities to achieve this aim. These techniques share a large penetration depth into tissues, making them suitable for in vivo imaging in humans. But the approaches differ in resolution and sensitivity, with PET and SPECT showing higher sensitivity and MRI having better resolution. Central to successful in vivo imaging of beta cell mass with either of these techniques is the use of an optimal imaging probe. For in vivo application, this ligand has to be highly specific towards a stably expressed target on or in beta cells with a labeling specificity ratio for beta cells vs. exocrine cells of at least 1000:1 [200]. Furthermore, the probe should be non-toxic and exhibit beneficial pharmacokinetics. In numerous animal studies the use of PET, SPECT, and MRI in combination with various imaging probes for beta cell mass assessment has been investigated, but the translation to human has been limited so far. Clinical MRI in this field has been employed primarily to study the fate of transplanted islets in patients [201], [202], [203], avoiding the problem of probe specificity. To that aim isolated human islets are labeled with superparamagnetic iron oxide (SPIO) particles in vitro and infused into the liver of patients. In two of these studies no correlation between the number of transplanted islets and the MRI signal could be found [202], [203], whereas in the most recent study, the signal correlated with the in vitro labeling efficiency of the islet transplant [201]. Despite this limitation, all studies could confirm the safety of MRI monitoring of in vitro labeled islets after transplantation. Furthermore, the studies demonstrated the feasibility to follow early islet graft loss after transplantation, a known phenomenon mediated by an instant blood-mediated inflammatory reaction after islet infusion into the blood stream [204]. An interesting version of MRI for the imaging of endogenous beta cell mass is manganese-enhanced magnetic resonance imaging (MEMRI). To enhance MRI contrast for visualization of islets, this approach uses manganese, which is taken up into beta cells through voltage activated calcium channels [205]. In a retrospective analysis of whole-body MRI images from 143 patients, MEMRI could discriminate between non-diabetic and type 2 diabetic individuals [206], making it an interesting candidate for noninvasive beta cell imaging. Unfortunately, its dependence on the active opening of calcium channels is problematic as it does not allow distinction between beta cell mass and function.
In combination with computed tomography (CT), PET has been used to monitor human islets after transplantation to patients, either by pre-labeling the islets [207] or by in vivo injection of a radiotracer after islet engraftment [208]. Whereas the case report of Eich et al. [207] merely demonstrated the feasibility of PET imaging of pre-labeled transplanted islets in one subject, Eriksson and colleagues were able to correlate islet graft function in response to a MMTT with the PET signal in 8 patients [208]. However, the signal did not correlate either with the number of transplanted islets or with the longitudinal change of metabolic islet function. Therefore, a conclusion about the applicability of PET imaging for the outcome of islet transplantation will need further evaluation. Application of PET or SPECT for in vivo assessment of beta cell mass has focused mostly on imaging of the endogenous beta cells in the pancreas. A lot of effort has been put into the development of radiotracers for this purpose. Tracers to target the type 2 vesicular monoamine transporter (VMAT2) [209], [210], serotonin biosynthesis [211], and the glucagon-like peptide-1 receptor (GLP-1R) [212] were used to assess endogenous beta cell mass in control and type 1 diabetic patients. All of these studies demonstrated reduced PET/SPECT signals in the patient group, verifying the general feasibility of this technique to assess pancreatic beta cells in vivo. Nevertheless, tracer target specificity and modulation by beta cell function require further evaluation. Furthermore, most clinical in vivo imaging studies have observed the same high inter-subject variability in beta cell mass within the same subject group as previously observed in histological postmortem sampling [72]. Therefore, using cross-sectional studies of clinical in vivo imaging for diagnostic or therapeutic monitoring seems rather unlikely. Nonetheless, given that the sensitivity of tracer and imaging approach are high enough to assess relatively small changes in beta cell mass, clinical in vivo imaging might be a valuable approach to monitor beta cell mass during diabetes pathogenesis and therapy.
An alternative way to study human beta cell biology in vivo is the transplantation of isolated human islets from organ donors into animal models, e.g. mice. Taking the foreign environment into account, this approach enables studying human islets for prolonged periods of time in a controlled in vivo setting. In this context, human islets frequently have been transplanted under the kidney capsule of mice [213] but also into the liver, pancreas, lung, spleen [214], muscle [215], bone marrow [216], and the eye of mice [36]. Even more manifold than the transplantation sites used were the topics investigated in these studies, which include almost all aspects of human islet biology, including T1D [36], [217], T2D [218], [219] and islet transplantation [220]. Also, determinants of human beta cell mass, in particular beta cell proliferation [40], [221] and apoptosis [222], as well as modulators of beta cell function have been investigated intensely. Thus, human islet transplantation to mice often serves as a tool to verify findings obtained in animal models and is helpful for the development of therapies. The advantage of human islet transplantation models is the possibility to manipulate the systemic environment to study numerous aspects of disease pathogenesis and potential treatments. In most cases, the effects on human islet biology are assessed by immunohistochemical examination of the graft after termination of the experiment. Additionally, human beta cell function can be evaluated in this setting by measurement of systemic human insulin levels in the blood of the mouse [223]. Finally, human islet biology after transplantation to mice has also been assessed by in vivo imaging. In addition to an intense use of this approach for the study of the clinical imaging modalities, small animal transplantation models of human islets also allow for the use of molecular imaging techniques with reduced penetration depth, e.g. bioluminescence [224], optical coherence tomography [225], or laser scanning microscopy (LSM) [36]. Of particular interest is the recently established combination of cellular resolution LSM with transplantation of human islets into the anterior chamber of the eye, which enables, for the first time, longitudinal, noninvasive in vivo imaging of human islets at cellular resolution ([36], [226], [227], [228] for detailed review see the review by Leibiger and Berggren in this issue).
4. Conclusions
Our knowledge about the differential roles of beta cell mass and function in diabetes pathogenesis has increased tremendously during the last years, pointing towards a much more prominent role of beta cell function in disease development and therapy than previously believed. Nevertheless, many aspects regarding the kinetics of beta cell dysfunction and mass reduction as well as their underlying mechanisms remain unclear. Promise to solve the most imperative open questions in this field originates from the recent development of remarkable novel techniques and platforms to study human beta cells and islets (Table 1). Therefore, these new approaches expand the scope of routinely applied standard methods, now allowing detailed investigations of human islet cells from molecular mechanisms to in vivo performance. In particular, the combination of this broad range of available techniques will be central to the development of new treatment approaches for diabetes.
Table 1.
Methods for the assessment of beta cell mass and function.
| Samples | Methods | Readout | Refs | |
|---|---|---|---|---|
| In vitro | Single cells, isolated islets | Morphometric analyses (e.g. islet size, proliferation, apoptosis) | Beta cell mass | [74], [91], [131] |
| Hormone secretion | Beta cell function | [70], [82], [131], [132], [133] | ||
| Intracellular signaling (e.g. Ca2+, NADPH, exocytosis, mitochondria, electrophysiology) | Beta cell function | [134], [135], [136], [137] | ||
| Protein biochemistry | Beta cell function | [138], [139] | ||
| Omics | Beta cell function and identity | [140], [141], [142], [143], [144], [145], [146], [147], [148], [149], [150], [151], [152] | ||
| hESCs, hiPSCs, islet/pancreas organoids | In vitro differentiation | Beta cell mass and function in replacement therapy | [153], [154], [155], [156], [157], [158], [159], [160], [161], [162] | |
| In situ | Histological sections | Morphometric analyses (islet size, proliferation, apoptosis) | Beta cell mass | [163], [164], [165] |
| Pancreas tissue slices | Morphometric analyses | Beta cell mass | [182], [185] | |
| Hormone secretion | Beta cell function | [182], [185] | ||
| Intracellular signaling (e.g. Ca2+, electrophysiology) | [172], [173], [174], [175], [176], [177], [178], [179], [180] | |||
| In vivo | Metabolic tests | oGTT, MMTT, ivGTT | Sum of mass and function | [186], [187], [188] |
| Noninvasive imaging | PET, SPECT, MRI, bioluminescence, optical coherence tomography | Beta cell mass | [200], [201], [202], [203], [204], [205], [206], [224], [225] | |
| Transplantation into mice | Morphometric analyses (proliferation, apoptosis in islet grafts) | Beta cell mass | [40], [217], [218], [219], [220], [221], [222] | |
| Systemic hormone levels | Beta cell function | [40], [217], [218], [219], [220], [221], [222] | ||
| Longitudinal LSM imaging in the anterior chamber of the mouse eye | Beta cell mass | [36], [226], [227] | ||
| Beta cell function | [36], [226], [227] |
Acknowledgements
We would like to thank Michele Solimena (Paul Langerhans Institute Dresden) and Jürgen Weitz (University Clinic Carl Gustav Carus Dresden) for provision of human pancreatic tissues (informed consent was available from all patients). Work in the author's laboratory was supported with funds from the Paul Langerhans Institute Dresden (PLID) of Helmholtz Zentrum München at the University Clinic Carl Gustav Carus of Technische Universität Dresden, the German Ministry for Education and Research (BMBF) to the German Centre for Diabetes Research (DZD), the DFG-Research Center for Regenerative Therapies Dresden, Cluster of Excellence (CRTD), the DFG – SFB/Transregio 127, the European Foundation for the Study of Diabetes (EFSD)/Boehringer Ingelheim Basic Research Programme and the Helmsley Charitable Trust George S. Eisenbarth nPOD Award for Team Science.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Eisenbarth G.S. Type I diabetes mellitus. A chronic autoimmune disease. The New England Journal of Medicine. 1986;314(21):1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 2.Chatenoud L., Bluestone J.A. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nature Reviews Immunology. 2007;7(8):622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- 3.von Herrath M., Sanda S., Herold K. Type 1 diabetes as a relapsing-remitting disease? Nature Reviews Immunology. 2007;7(12):988–994. doi: 10.1038/nri2192. [DOI] [PubMed] [Google Scholar]
- 4.van Belle T.L., Coppieters K.T., von Herrath M.G. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiological Reviews. 2011;91(1):79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler A.G., Rewers M., Simell O., Simell T., Lempainen J., Steck A. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309(23):2473–2479. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pugliese A. Insulitis in the pathogenesis of type 1 diabetes. Pediatric Diabetes. 2016;17(Suppl 22):31–36. doi: 10.1111/pedi.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skowera A., Ellis R.J., Varela-Calvino R., Arif S., Huang G.C., Van-Krinks C. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. The Journal of Clinical Investigation. 2008;118(10):3390–3402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brozzi F., Nardelli T.R., Lopes M., Millard I., Barthson J., Igoillo-Esteve M. Cytokines induce endoplasmic reticulum stress in human, rat and mouse beta cells via different mechanisms. Diabetologia. 2015;58(10):2307–2316. doi: 10.1007/s00125-015-3669-6. [DOI] [PubMed] [Google Scholar]
- 9.Herold K.C., Usmani-Brown S., Ghazi T., Lebastchi J., Beam C.A., Bellin M.D. Beta cell death and dysfunction during type 1 diabetes development in at-risk individuals. The Journal of Clinical Investigation. 2015;125(3):1163–1173. doi: 10.1172/JCI78142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gianani R., Putnam A., Still T., Yu L., Miao D., Gill R.G. Initial results of screening of nondiabetic organ donors for expression of islet autoantibodies. Journal of Clinical Endocrinology & Metabolism. 2006;91(5):1855–1861. doi: 10.1210/jc.2005-1171. [DOI] [PubMed] [Google Scholar]
- 11.In't Veld P., Lievens D., De Grijse J., Ling Z., Van der Auwera B., Pipeleers-Marichal M. Screening for insulitis in adult autoantibody-positive organ donors. Diabetes. 2007;56(9):2400–2404. doi: 10.2337/db07-0416. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Calvo T., Zapardiel-Gonzalo J., Amirian N., Castillo E., Lajevardi Y., Krogvold L. Increase in pancreatic proinsulin and preservation of beta cell mass in autoantibody positive donors prior to type 1 diabetes onset. Diabetes. 2017 doi: 10.2337/db16-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diedisheim M., Mallone R., Boitard C., Larger E. Beta-cell mass in nondiabetic autoantibody-positive subjects: an analysis based on the network for pancreatic organ donors database. Journal of Clinical Endocrinology & Metabolism. 2016;101(4):1390–1397. doi: 10.1210/jc.2015-3756. [DOI] [PubMed] [Google Scholar]
- 14.Sherry N.A., Kushner J.A., Glandt M., Kitamura T., Brillantes A.M., Herold K.C. Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes. 2006;55(12):3238–3245. doi: 10.2337/db05-1034. [DOI] [PubMed] [Google Scholar]
- 15.Sreenan S., Pick A.J., Levisetti M., Baldwin A.C., Pugh W., Polonsky K.S. Increased beta-cell proliferation and reduced mass before diabetes onset in the nonobese diabetic mouse. Diabetes. 1999;48(5):989–996. doi: 10.2337/diabetes.48.5.989. [DOI] [PubMed] [Google Scholar]
- 16.Campbell-Thompson M., Fu A., Kaddis J.S., Wasserfall C., Schatz D.A., Pugliese A. Insulitis and beta-cell mass in the natural history of type 1 diabetes. Diabetes. 2016;65(3):719–731. doi: 10.2337/db15-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srikanta S., Ganda O.P., Gleason R.E., Jackson R.A., Soeldner J.S., Eisenbarth G.S. Pre-type I diabetes. Linear loss of beta cell response to intravenous glucose. Diabetes. 1984;33(8):717–720. doi: 10.2337/diab.33.8.717. [DOI] [PubMed] [Google Scholar]
- 18.Chase H.P., Cuthbertson D.D., Dolan L.M., Kaufman F., Krischer J.P., Schatz D.A. First-phase insulin release during the intravenous glucose tolerance test as a risk factor for type 1 diabetes. The Journal of Pediatrics. 2001;138(2):244–249. doi: 10.1067/mpd.2001.111274. [DOI] [PubMed] [Google Scholar]
- 19.Keskinen P., Korhonen S., Kupila A., Veijola R., Erkkila S., Savolainen H. First-phase insulin response in young healthy children at genetic and immunological risk for Type I diabetes. Diabetologia. 2002;45(12):1639–1648. doi: 10.1007/s00125-002-0981-8. [DOI] [PubMed] [Google Scholar]
- 20.Koskinen M.K., Helminen O., Matomaki J., Aspholm S., Mykkanen J., Makinen M. Reduced beta-cell function in early preclinical type 1 diabetes. European Journal of Endocrinology. 2016;174(3):251–259. doi: 10.1530/EJE-15-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sosenko J.M., Palmer J.P., Greenbaum C.J., Mahon J., Cowie C., Krischer J.P. Patterns of metabolic progression to type 1 diabetes in the diabetes prevention trial-type 1. Diabetes Care. 2006;29(3):643–649. doi: 10.2337/diacare.29.03.06.dc05-1006. [DOI] [PubMed] [Google Scholar]
- 22.Andersson C., Carlsson A., Cilio C., Cedervall E., Ivarsson S.A., Jonsdottir B. Glucose tolerance and beta-cell function in islet autoantibody-positive children recruited to a secondary prevention study. Pediatric Diabetes. 2013;14(5):341–349. doi: 10.1111/pedi.12023. [DOI] [PubMed] [Google Scholar]
- 23.Sosenko J.M., Skyler J.S., Beam C.A., Krischer J.P., Greenbaum C.J., Mahon J. Acceleration of the loss of the first-phase insulin response during the progression to type 1 diabetes in diabetes prevention trial-type 1 participants. Diabetes. 2013;62(12):4179–4183. doi: 10.2337/db13-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14(10):619–633. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- 25.Gepts W., De Mey J. Islet cell survival determined by morphology. An immunocytochemical study of the islets of Langerhans in juvenile diabetes mellitus. Diabetes. 1978;27(Suppl 1):251–261. doi: 10.2337/diab.27.1.s251. [DOI] [PubMed] [Google Scholar]
- 26.Junker K., Egeberg J., Kromann H., Nerup J. An autopsy study of the islets of Langerhans in acute-onset juvenile diabetes mellitus. Acta Pathologica et Microbiologica Scandinavica. Section A, Pathology. 1977;85(5):699–706. doi: 10.1111/j.1699-0463.1977.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 27.Kloppel G., Drenck C.R., Oberholzer M., Heitz P.U. Morphometric evidence for a striking B-cell reduction at the clinical onset of type 1 diabetes. Virchows Archiv A Pathological Anatomy and Histopathology. 1984;403(4):441–452. doi: 10.1007/BF00737292. [DOI] [PubMed] [Google Scholar]
- 28.Butler A.E., Galasso R., Meier J.J., Basu R., Rizza R.A., Butler P.C. Modestly increased beta cell apoptosis but no increased beta cell replication in recent-onset type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia. 2007;50(11):2323–2331. doi: 10.1007/s00125-007-0794-x. [DOI] [PubMed] [Google Scholar]
- 29.Leete P., Willcox A., Krogvold L., Dahl-Jorgensen K., Foulis A.K., Richardson S.J. Differential insulitic profiles determine the extent of beta-cell destruction and the age at onset of type 1 diabetes. Diabetes. 2016;65(5):1362–1369. doi: 10.2337/db15-1615. [DOI] [PubMed] [Google Scholar]
- 30.Sherry N.A., Tsai E.B., Herold K.C. Natural history of beta-cell function in type 1 diabetes. Diabetes. 2005;54(Suppl 2):S32–S39. doi: 10.2337/diabetes.54.suppl_2.s32. [DOI] [PubMed] [Google Scholar]
- 31.Steele C., Hagopian W.A., Gitelman S., Masharani U., Cavaghan M., Rother K.I. Insulin secretion in type 1 diabetes. Diabetes. 2004;53(2):426–433. doi: 10.2337/diabetes.53.2.426. [DOI] [PubMed] [Google Scholar]
- 32.Klinke D.J., 2nd Age-corrected beta cell mass following onset of type 1 diabetes mellitus correlates with plasma C-peptide in humans. PLoS One. 2011;6(11):e26873. doi: 10.1371/journal.pone.0026873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barker A., Lauria A., Schloot N., Hosszufalusi N., Ludvigsson J., Mathieu C. Age-dependent decline of beta-cell function in type 1 diabetes after diagnosis: a multi-centre longitudinal study. Diabetes, Obesity & Metabolism. 2014;16(3):262–267. doi: 10.1111/dom.12216. [DOI] [PubMed] [Google Scholar]
- 34.Dost A., Herbst A., Kintzel K., Haberland H., Roth C.L., Gortner L. Shorter remission period in young versus older children with diabetes mellitus type 1. Experimental and Clinical Endocrinology & Diabetes. 2007;115(1):33–37. doi: 10.1055/s-2007-948214. [DOI] [PubMed] [Google Scholar]
- 35.Akirav E., Kushner J.A., Herold K.C. Beta-cell mass and type 1 diabetes: going, going, gone? Diabetes. 2008;57(11):2883–2888. doi: 10.2337/db07-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chmelova H., Cohrs C.M., Chouinard J.A., Petzold C., Kuhn M., Chen C. Distinct roles of beta-cell mass and function during type 1 diabetes onset and remission. Diabetes. 2015;64(6):2148–2160. doi: 10.2337/db14-1055. [DOI] [PubMed] [Google Scholar]
- 37.Tersey S.A., Nishiki Y., Templin A.T., Cabrera S.M., Stull N.D., Colvin S.C. Islet beta-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes. 2012;61(4):818–827. doi: 10.2337/db11-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meier J.J., Lin J.C., Butler A.E., Galasso R., Martinez D.S., Butler P.C. Direct evidence of attempted beta cell regeneration in an 89-year-old patient with recent-onset type 1 diabetes. Diabetologia. 2006;49(8):1838–1844. doi: 10.1007/s00125-006-0308-2. [DOI] [PubMed] [Google Scholar]
- 39.Willcox A., Richardson S.J., Bone A.J., Foulis A.K., Morgan N.G. Evidence of increased islet cell proliferation in patients with recent-onset type 1 diabetes. Diabetologia. 2010;53(9):2020–2028. doi: 10.1007/s00125-010-1817-6. [DOI] [PubMed] [Google Scholar]
- 40.Levitt H.E., Cyphert T.J., Pascoe J.L., Hollern D.A., Abraham N., Lundell R.J. Glucose stimulates human beta cell replication in vivo in islets transplanted into NOD-severe combined immunodeficiency (SCID) mice. Diabetologia. 2011;54(3):572–582. doi: 10.1007/s00125-010-1919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meier J.J., Bhushan A., Butler A.E., Rizza R.A., Butler P.C. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia. 2005;48(11):2221–2228. doi: 10.1007/s00125-005-1949-2. [DOI] [PubMed] [Google Scholar]
- 42.Poudel A., Savari O., Striegel D.A., Periwal V., Taxy J., Millis J.M. Beta-cell destruction and preservation in childhood and adult onset type 1 diabetes. Endocrine. 2015;49(3):693–702. doi: 10.1007/s12020-015-0534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keenan H.A., Sun J.K., Levine J., Doria A., Aiello L.P., Eisenbarth G. Residual insulin production and pancreatic ss-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59(11):2846–2853. doi: 10.2337/db10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coppieters K.T., Wiberg A., Amirian N., Kay T.W., von Herrath M.G. Persistent glucose transporter expression on pancreatic beta cells from longstanding type 1 diabetic individuals. Diabetes/Metabolism Research and Reviews. 2011;27(8):746–754. doi: 10.1002/dmrr.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Meara N.M., Sturis J., Herold K.C., Ostrega D.M., Polonsky K.S. Alterations in the patterns of insulin secretion before and after diagnosis of IDDM. Diabetes Care. 1995;18(4):568–571. doi: 10.2337/diacare.18.4.568. [DOI] [PubMed] [Google Scholar]
- 46.Tsai E.B., Sherry N.A., Palmer J.P., Herold K.C. The rise and fall of insulin secretion in type 1 diabetes mellitus. Diabetologia. 2006;49(2):261–270. doi: 10.1007/s00125-005-0100-8. [DOI] [PubMed] [Google Scholar]
- 47.Greenbaum C.J., Anderson A.M., Dolan L.M., Mayer-Davis E.J., Dabelea D., Imperatore G. Preservation of beta-cell function in autoantibody-positive youth with diabetes. Diabetes Care. 2009;32(10):1839–1844. doi: 10.2337/dc08-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sherr J.L., Ghazi T., Wurtz A., Rink L., Herold K.C. Characterization of residual beta cell function in long-standing type 1 diabetes. Diabetes/Metabolism Research and Reviews. 2014;30(2):154–162. doi: 10.1002/dmrr.2478. [DOI] [PubMed] [Google Scholar]
- 49.Wang L., Lovejoy N.F., Faustman D.L. Persistence of prolonged C-peptide production in type 1 diabetes as measured with an ultrasensitive C-peptide assay. Diabetes Care. 2012;35(3):465–470. doi: 10.2337/dc11-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oram R.A., Jones A.G., Besser R.E., Knight B.A., Shields B.M., Brown R.J. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia. 2014;57(1):187–191. doi: 10.1007/s00125-013-3067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oram R.A., McDonald T.J., Shields B.M., Hudson M.M., Shepherd M.H., Hammersley S. Most people with long-duration type 1 diabetes in a large population-based study are insulin microsecretors. Diabetes Care. 2015;38(2):323–328. doi: 10.2337/dc14-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brush J.M. Initial stabilization of the diabetic child. American Journal of Diseases of Children. 1944;67(6):429–444. [Google Scholar]
- 53.Jackson R.L., Boyd J.D., Smith T.E. Stabilization of the diabetic child. American Journal of Diseases of Children. 1940;59(2):332–341. [Google Scholar]
- 54.Block M.B., Rosenfield R.L., Mako M.E., Steiner D.F., Rubenstein A.H. Sequential changes in beta-cell function in insulin-treated diabetic patients assessed by C-peptide immunoreactivity. The New England Journal of Medicine. 1973;288(22):1144–1148. doi: 10.1056/NEJM197305312882202. [DOI] [PubMed] [Google Scholar]
- 55.Kaas A., Andersen M.L., Fredheim S., Hougaard P., Buschard K., Petersen J.S. Proinsulin, GLP-1, and glucagon are associated with partial remission in children and adolescents with newly diagnosed type 1 diabetes. Pediatric Diabetes. 2012;13(1):51–58. doi: 10.1111/j.1399-5448.2011.00812.x. [DOI] [PubMed] [Google Scholar]
- 56.Scholin A., Nystrom L., Arnqvist H., Bolinder J., Bjork E., Berne C. Proinsulin/C-peptide ratio, glucagon and remission in new-onset Type 1 diabetes mellitus in young adults. Diabetic Medicine. 2011;28(2):156–161. doi: 10.1111/j.1464-5491.2010.03191.x. [DOI] [PubMed] [Google Scholar]
- 57.Abdul-Rasoul M., Habib H., Al-Khouly M. 'The honeymoon phase' in children with type 1 diabetes mellitus: frequency, duration, and influential factors. Pediatric Diabetes. 2006;7(2):101–107. doi: 10.1111/j.1399-543X.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 58.Chase H.P., MacKenzie T.A., Burdick J., Fiallo-Scharer R., Walravens P., Klingensmith G. Redefining the clinical remission period in children with type 1 diabetes. Pediatric Diabetes. 2004;5(1):16–19. doi: 10.1111/j.1399-543X.2004.00034.x. [DOI] [PubMed] [Google Scholar]
- 59.Muhammad B.J., Swift P.G., Raymond N.T., Botha J.L. Partial remission phase of diabetes in children younger than age 10 years. Archives of Disease in Childhood. 1999;80(4):367–369. doi: 10.1136/adc.80.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scholin A., Berne C., Schvarcz E., Karlsson F.A., Bjork E. Factors predicting clinical remission in adult patients with type 1 diabetes. Journal of Internal Medicine. 1999;245(2):155–162. doi: 10.1046/j.1365-2796.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- 61.Scholin A., Torn C., Nystrom L., Berne C., Arnqvist H., Blohme G. Normal weight promotes remission and low number of islet antibodies prolong the duration of remission in Type 1 diabetes. Diabetic Medicine. 2004;21(5):447–455. doi: 10.1111/j.1464-5491.2004.01175.x. [DOI] [PubMed] [Google Scholar]
- 62.Karges B., Durinovic-Bello I., Heinze E., Boehm B.O., Debatin K.M., Karges W. Complete long-term recovery of beta-cell function in autoimmune type 1 diabetes after insulin treatment. Diabetes Care. 2004;27(5):1207–1208. doi: 10.2337/diacare.27.5.1207. [DOI] [PubMed] [Google Scholar]
- 63.Kahn S.E., Hull R.L., Utzschneider K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 64.Meigs J.B., Wilson P.W., Fox C.S., Vasan R.S., Nathan D.M., Sullivan L.M. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. Journal of Clinical Endocrinology & Metabolism. 2006;91(8):2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 65.Kahn S.E., Prigeon R.L., McCulloch D.K., Boyko E.J., Bergman R.N., Schwartz M.W. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42(11):1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 66.Ogilvie R.F. The islands of langerhans in 19 cases of obesity. Journal of Pathology and Bacteriology. 1933;37(3):473–481. [Google Scholar]
- 67.Yoon K.H., Ko S.H., Cho J.H., Lee J.M., Ahn Y.B., Song K.H. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. Journal of Clinical Endocrinology & Metabolism. 2003;88(5):2300–2308. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- 68.Hanley S.C., Austin E., Assouline-Thomas B., Kapeluto J., Blaichman J., Moosavi M. {beta}-Cell mass dynamics and islet cell plasticity in human type 2 diabetes. Endocrinology. 2010;151(4):1462–1472. doi: 10.1210/en.2009-1277. [DOI] [PubMed] [Google Scholar]
- 69.Matsumoto I., Sawada T., Nakano M., Sakai T., Liu B., Ansite J.D. Improvement in islet yield from obese donors for human islet transplants. Transplantation. 2004;78(6):880–885. doi: 10.1097/01.tp.0000134396.03440.1e. [DOI] [PubMed] [Google Scholar]
- 70.Brandhorst H., Brandhorst D., Hering B.J., Federlin K., Bretzel R.G. Body mass index of pancreatic donors: a decisive factor for human islet isolation. Experimental and Clinical Endocrinology & Diabetes. 1995;103(Suppl 2):23–26. doi: 10.1055/s-0029-1211388. [DOI] [PubMed] [Google Scholar]
- 71.Butler A.E., Janson J., Bonner-Weir S., Ritzel R., Rizza R.A., Butler P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 72.Rahier J., Guiot Y., Goebbels R.M., Sempoux C., Henquin J.C. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes, Obesity & Metabolism. 2008;10(Suppl 4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 73.Saisho Y., Butler A.E., Manesso E., Elashoff D., Rizza R.A., Butler P.C. Beta-cell mass and turnover in humans: effects of obesity and aging. Diabetes Care. 2013;36(1):111–117. doi: 10.2337/dc12-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mezza T., Muscogiuri G., Sorice G.P., Clemente G., Hu J., Pontecorvi A. Insulin resistance alters islet morphology in nondiabetic humans. Diabetes. 2014;63(3):994–1007. doi: 10.2337/db13-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoneda S., Uno S., Iwahashi H., Fujita Y., Yoshikawa A., Kozawa J. Predominance of beta-cell neogenesis rather than replication in humans with an impaired glucose tolerance and newly diagnosed diabetes. Journal of Clinical Endocrinology & Metabolism. 2013;98(5):2053–2061. doi: 10.1210/jc.2012-3832. [DOI] [PubMed] [Google Scholar]
- 76.Ferrannini E., Natali A., Bell P., Cavallo-Perin P., Lalic N., Mingrone G. Insulin resistance and hypersecretion in obesity. European group for the Study of Insulin Resistance (EGIR) The Journal of Clinical Investigation. 1997;100(5):1166–1173. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seltzer H.S., Allen E.W., Herron A.L., Brennan M.T. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. The Journal of Clinical Investigation. 1967;46:323–335. doi: 10.1172/JCI105534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lillioja S., Mott D.M., Spraul M., Ferraro R., Foley J.E., Ravussin E. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. The New England Journal of Medicine. 1993;329(27):1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 79.Camastra S., Manco M., Mari A., Baldi S., Gastaldelli A., Greco A.V. beta-cell function in morbidly obese subjects during free living: long-term effects of weight loss. Diabetes. 2005;54(8):2382–2389. doi: 10.2337/diabetes.54.8.2382. [DOI] [PubMed] [Google Scholar]
- 80.Polonsky K.S., Given B.D., Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. The Journal of Clinical Investigation. 1988;81(2):442–448. doi: 10.1172/JCI113339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perley M., Kipnis D.M. Plasma insulin responses to glucose and tolbutamide of normal weight and obese diabetic and nondiabetic subjects. Diabetes. 1966;15(12):867–874. doi: 10.2337/diab.15.12.867. [DOI] [PubMed] [Google Scholar]
- 82.Mezghenna K., Pomies P., Chalancon A., Castex F., Leroy J., Niclauss N. Increased neuronal nitric oxide synthase dimerisation is involved in rat and human pancreatic beta cell hyperactivity in obesity. Diabetologia. 2011;54(11):2856–2866. doi: 10.1007/s00125-011-2264-8. [DOI] [PubMed] [Google Scholar]
- 83.Rahier J., Goebbels R.M., Henquin J.C. Cellular composition of the human diabetic pancreas. Diabetologia. 1983;24(5):366–371. doi: 10.1007/BF00251826. [DOI] [PubMed] [Google Scholar]
- 84.Guiot Y., Sempoux C., Moulin P., Rahier J. No decrease of the beta-cell mass in type 2 diabetic patients. Diabetes. 2001;50(Suppl 1):S188. doi: 10.2337/diabetes.50.2007.s188. [DOI] [PubMed] [Google Scholar]
- 85.Sakuraba H., Mizukami H., Yagihashi N., Wada R., Hanyu C., Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002;45(1):85–96. doi: 10.1007/s125-002-8248-z. [DOI] [PubMed] [Google Scholar]
- 86.Inaishi J., Saisho Y., Sato S., Kou K., Murakami R., Watanabe Y. Effects of Obesity and diabetes on alpha- and beta-cell mass in surgically resected human pancreas. Journal of Clinical Endocrinology & Metabolism. 2016;101(7):2874–2882. doi: 10.1210/jc.2016-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kloppel G., Lohr M., Habich K., Oberholzer M., Heitz P.U. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Survey and Synthesis of Pathology Research. 1985;4(2):110–125. doi: 10.1159/000156969. [DOI] [PubMed] [Google Scholar]
- 88.Clark A., Wells C.A., Buley I.D., Cruickshank J.K., Vanhegan R.I., Matthews D.R. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Research. 1988;9(4):151–159. [PubMed] [Google Scholar]
- 89.Deng S., Vatamaniuk M., Huang X., Doliba N., Lian M.M., Frank A. Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes. 2004;53(3):624–632. doi: 10.2337/diabetes.53.3.624. [DOI] [PubMed] [Google Scholar]
- 90.Marchetti P., Bugliani M., Lupi R., Marselli L., Masini M., Boggi U. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007;50(12):2486–2494. doi: 10.1007/s00125-007-0816-8. [DOI] [PubMed] [Google Scholar]
- 91.Marchetti P., Del Guerra S., Marselli L., Lupi R., Masini M., Pollera M. Pancreatic islets from type 2 diabetic patients have functional defects and increased apoptosis that are ameliorated by metformin. Journal of Clinical Endocrinology & Metabolism. 2004;89(11):5535–5541. doi: 10.1210/jc.2004-0150. [DOI] [PubMed] [Google Scholar]
- 92.Gastaldelli A., Ferrannini E., Miyazaki Y., Matsuda M., DeFronzo R.A., San Antonio metabolism study Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia. 2004;47(1):31–39. doi: 10.1007/s00125-003-1263-9. [DOI] [PubMed] [Google Scholar]
- 93.Holman R.R. Assessing the potential for alpha-glucosidase inhibitors in prediabetic states. Diabetes Research and Clinical Practice. 1998;40(Suppl):S21–S25. doi: 10.1016/s0168-8227(98)00038-2. [DOI] [PubMed] [Google Scholar]
- 94.Jensen C.C., Cnop M., Hull R.L., Fujimoto W.Y., Kahn S.E. Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes. 2002;51(7):2170–2178. doi: 10.2337/diabetes.51.7.2170. [DOI] [PubMed] [Google Scholar]
- 95.Ward W.K., Bolgiano D.C., McKnight B., Halter J.B., Porte D., Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. The Journal of Clinical Investigation. 1984;74(4):1318–1328. doi: 10.1172/JCI111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ferrannini E., Gastaldelli A., Miyazaki Y., Matsuda M., Mari A., DeFronzo R.A. Beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. Journal of Clinical Endocrinology & Metabolism. 2005;90(1):493–500. doi: 10.1210/jc.2004-1133. [DOI] [PubMed] [Google Scholar]
- 97.Osei K., Gaillard T., Schuster D.P. Pathogenetic mechanisms of impaired glucose tolerance and type II diabetes in African-Americans. The significance of insulin secretion, insulin sensitivity, and glucose effectiveness. Diabetes Care. 1997;20(3):396–404. doi: 10.2337/diacare.20.3.396. [DOI] [PubMed] [Google Scholar]
- 98.Kendall D.M., Sutherland D.E., Najarian J.S., Goetz F.C., Robertson R.P. Effects of hemipancreatectomy on insulin secretion and glucose tolerance in healthy humans. The New England Journal of Medicine. 1990;322(13):898–903. doi: 10.1056/NEJM199003293221305. [DOI] [PubMed] [Google Scholar]
- 99.Robertson R.P., Lanz K.J., Sutherland D.E., Seaquist E.R. Relationship between diabetes and obesity 9 to 18 years after hemipancreatectomy and transplantation in donors and recipients. Transplantation. 2002;73(5):736–741. doi: 10.1097/00007890-200203150-00013. [DOI] [PubMed] [Google Scholar]
- 100.Brunzell J.D., Robertson R.P., Lerner R.L., Hazzard W.R., Ensinck J.W., Bierman E.L. Relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests. Journal of Clinical Endocrinology & Metabolism. 1976;42(2):222–229. doi: 10.1210/jcem-42-2-222. [DOI] [PubMed] [Google Scholar]
- 101.Lang D.A., Matthews D.R., Burnett M., Turner R.C. Brief, irregular oscillations of basal plasma insulin and glucose concentrations in diabetic man. Diabetes. 1981;30(5):435–439. doi: 10.2337/diab.30.5.435. [DOI] [PubMed] [Google Scholar]
- 102.Polonsky K.S., Given B.D., Hirsch L.J., Tillil H., Shapiro E.T., Beebe C. Abnormal patterns of insulin secretion in non-insulin-dependent diabetes mellitus. The New England Journal of Medicine. 1988;318(19):1231–1239. doi: 10.1056/NEJM198805123181903. [DOI] [PubMed] [Google Scholar]
- 103.Schauer P.R., Mingrone G., Ikramuddin S., Wolfe B. Clinical Outcomes of metabolic surgery: efficacy of glycemic control, weight loss, and remission of diabetes. Diabetes Care. 2016;39(6):902–911. doi: 10.2337/dc16-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jorgensen N.B., Jacobsen S.H., Dirksen C., Bojsen-Moller K.N., Naver L., Hvolris L. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance. American Journal of Physiology. Endocrinology and Metabolism. 2012;303(1):E122–E131. doi: 10.1152/ajpendo.00073.2012. [DOI] [PubMed] [Google Scholar]
- 105.Guidone C., Manco M., Valera-Mora E., Iaconelli A., Gniuli D., Mari A. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55(7):2025–2031. doi: 10.2337/db06-0068. [DOI] [PubMed] [Google Scholar]
- 106.Polyzogopoulou E.V., Kalfarentzos F., Vagenakis A.G., Alexandrides T.K. Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes. 2003;52(5):1098–1103. doi: 10.2337/diabetes.52.5.1098. [DOI] [PubMed] [Google Scholar]
- 107.Malandrucco I., Pasqualetti P., Giordani I., Manfellotto D., De Marco F., Alegiani F. Very-low-calorie diet: a quick therapeutic tool to improve beta cell function in morbidly obese patients with type 2 diabetes. American Journal of Clinical Nutrition. 2012;95(3):609–613. doi: 10.3945/ajcn.111.023697. [DOI] [PubMed] [Google Scholar]
- 108.Lim E.L., Hollingsworth K.G., Aribisala B.S., Chen M.J., Mathers J.C., Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54(10):2506–2514. doi: 10.1007/s00125-011-2204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Accili D., Talchai S.C., Kim-Muller J.Y., Cinti F., Ishida E., Ordelheide A.M. When beta-cells fail: lessons from dedifferentiation. Diabetes, Obesity & Metabolism. 2016;18(Suppl 1):117–122. doi: 10.1111/dom.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Talchai C., Xuan S., Lin H.V., Sussel L., Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150(6):1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Z., York N.W., Nichols C.G., Remedi M.S. Pancreatic beta cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metabolism. 2014;19(5):872–882. doi: 10.1016/j.cmet.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Butler A.E., Dhawan S., Hoang J., Cory M., Zeng K., Fritsch H. Beta-cell deficit in obese type 2 diabetes, a minor role of beta-cell dedifferentiation and degranulation. Journal of Clinical Endocrinology & Metabolism. 2016;101(2):523–532. doi: 10.1210/jc.2015-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cinti F., Bouchi R., Kim-Muller J.Y., Ohmura Y., Sandoval P.R., Masini M. Evidence of beta-cell dedifferentiation in human type 2 diabetes. Journal of Clinical Endocrinology & Metabolism. 2016;101(3):1044–1054. doi: 10.1210/jc.2015-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Md Moin A.S., Dhawan S., Cory M., Butler P.C., Rizza R.A., Butler A.E. Increased frequency of hormone negative and polyhormonal endocrine cells in lean individuals with type 2 diabetes. Journal of Clinical Endocrinology & Metabolism. 2016;101(10):3628–3636. doi: 10.1210/jc.2016-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guo S., Dai C., Guo M., Taylor B., Harmon J.S., Sander M. Inactivation of specific beta cell transcription factors in type 2 diabetes. The Journal of Clinical Investigation. 2013;123(8):3305–3316. doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meier J.J., Menge B.A., Breuer T.G., Muller C.A., Tannapfel A., Uhl W. Functional assessment of pancreatic beta-cell area in humans. Diabetes. 2009;58(7):1595–1603. doi: 10.2337/db08-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weyer C., Bogardus C., Mott D.M., Pratley R.E. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. The Journal of Clinical Investigation. 1999;104(6):787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Porksen N. The in vivo regulation of pulsatile insulin secretion. Diabetologia. 2002;45(1):3–20. doi: 10.1007/s125-002-8240-x. [DOI] [PubMed] [Google Scholar]
- 119.Porksen N., Hollingdal M., Juhl C., Butler P., Veldhuis J.D., Schmitz O. Pulsatile insulin secretion: detection, regulation, and role in diabetes. Diabetes. 2002;51(Suppl 1):S245–S254. doi: 10.2337/diabetes.51.2007.s245. [DOI] [PubMed] [Google Scholar]
- 120.Matveyenko A.V., Liuwantara D., Gurlo T., Kirakossian D., Dalla Man C., Cobelli C. Pulsatile portal vein insulin delivery enhances hepatic insulin action and signaling. Diabetes. 2012;61(9):2269–2279. doi: 10.2337/db11-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Leahy J.L., Bonner-Weir S., Weir G.C. Beta-cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care. 1992;15(3):442–455. doi: 10.2337/diacare.15.3.442. [DOI] [PubMed] [Google Scholar]
- 122.Leahy J.L., Hirsch I.B., Peterson K.A., Schneider D. Targeting beta-cell function early in the course of therapy for type 2 diabetes mellitus. Journal of Clinical Endocrinology & Metabolism. 2010;95(9):4206–4216. doi: 10.1210/jc.2010-0668. [DOI] [PubMed] [Google Scholar]
- 123.Meier J.J., Bonadonna R.C. Role of reduced β-cell mass versus impaired β-cell function in the pathogenesis of type 2 diabetes. Diabetes Care. 2013;36(Suppl 2):S113–S119. doi: 10.2337/dcS13-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kahn S.E., Zraika S., Utzschneider K.M., Hull R.L. The beta cell lesion in type 2 diabetes: there has to be a primary functional abnormality. Diabetologia. 2009;52(6):1003–1012. doi: 10.1007/s00125-009-1321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pancreatic beta-cell mass or beta-cell function? That is the question!, (2008).
- 126.Matveyenko A.V., Butler P.C. Relationship between beta-cell mass and diabetes onset. Diabetes, Obesity & Metabolism. 2008;10(Suppl 4):23–31. doi: 10.1111/j.1463-1326.2008.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Defronzo R.A. Banting lecture. from the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saxena R., Voight B.F., Lyssenko V., Burtt N.P., de Bakker P.I., Chen H. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316(5829):1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 129.Scott L.J., Mohlke K.L., Bonnycastle L.L., Willer C.J., Li Y., Duren W.L. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316(5829):1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zeggini E., Weedon M.N., Lindgren C.M., Frayling T.M., Elliott K.S., Lango H. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316(5829):1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Krogvold L., Skog O., Sundstrom G., Edwin B., Buanes T., Hanssen K.F. Function of isolated pancreatic islets from patients at onset of type 1 diabetes: insulin secretion can be restored after some days in a nondiabetogenic environment in vitro: results from the DiViD Study. Diabetes. 2015;64(7):2506–2512. doi: 10.2337/db14-1911. [DOI] [PubMed] [Google Scholar]
- 132.Lupi R., Marselli L., Dionisi S., Del Guerra S., Boggi U., Del Chiaro M. Improved insulin secretory function and reduced chemotactic properties after tissue culture of islets from type 1 diabetic patients. Diabetes/Metabolism Research and Reviews. 2004;20(3):246–251. doi: 10.1002/dmrr.460. [DOI] [PubMed] [Google Scholar]
- 133.Conget I., Fernandez-Alvarez J., Ferrer J., Sarri Y., Novials A., Somoza N. Human pancreatic islet function at the onset of type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1993;36(4):358–360. doi: 10.1007/BF00400241. [DOI] [PubMed] [Google Scholar]
- 134.Johnson J.D., Misler S. Nicotinic acid-adenine dinucleotide phosphate-sensitive calcium stores initiate insulin signaling in human beta cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(22):14566–14571. doi: 10.1073/pnas.222099799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.De Marchi U., Thevenet J., Hermant A., Dioum E., Wiederkehr A. Calcium co-regulates oxidative metabolism and ATP synthase-dependent respiration in pancreatic beta cells. The Journal of Biological Chemistry. 2014;289(13):9182–9194. doi: 10.1074/jbc.M113.513184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dolai S., Xie L., Zhu D., Liang T., Qin T., Xie H. Synaptotagmin-7 functions to replenish insulin granules for exocytosis in human islet beta-cells. Diabetes. 2016;65(7):1962–1976. doi: 10.2337/db15-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Braun M., Ramracheya R., Bengtsson M., Zhang Q., Karanauskaite J., Partridge C. Voltage-gated ion channels in human pancreatic beta-cells: electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57(6):1618–1628. doi: 10.2337/db07-0991. [DOI] [PubMed] [Google Scholar]
- 138.Brissova M., Fowler M.J., Nicholson W.E., Chu A., Hirshberg B., Harlan D.M. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. The Journal of Histochemistry and Cytochemistry. 2005;53(9):1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 139.Arnush M., Heitmeier M.R., Scarim A.L., Marino M.H., Manning P.T., Corbett J.A. IL-1 produced and released endogenously within human islets inhibits beta cell function. The Journal of Clinical Investigation. 1998;102(3):516–526. doi: 10.1172/JCI844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dorrell C., Schug J., Lin C.F., Canaday P.S., Fox A.J., Smirnova O. Transcriptomes of the major human pancreatic cell types. Diabetologia. 2011;54(11):2832–2844. doi: 10.1007/s00125-011-2283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cnop M., Abdulkarim B., Bottu G., Cunha D.A., Igoillo-Esteve M., Masini M. RNA sequencing identifies dysregulation of the human pancreatic islet transcriptome by the saturated fatty acid palmitate. Diabetes. 2014;63(6):1978–1993. doi: 10.2337/db13-1383. [DOI] [PubMed] [Google Scholar]
- 142.Fadista J., Vikman P., Laakso E.O., Mollet I.G., Esguerra J.L., Taneera J. Global genomic and transcriptomic analysis of human pancreatic islets reveals novel genes influencing glucose metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(38):13924–13929. doi: 10.1073/pnas.1402665111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bugliani M., Liechti R., Cheon H., Suleiman M., Marselli L., Kirkpatrick C. Microarray analysis of isolated human islet transcriptome in type 2 diabetes and the role of the ubiquitin-proteasome system in pancreatic beta cell dysfunction. Molecular and Cellular Endocrinology. 2013;367(1–2):1–10. doi: 10.1016/j.mce.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 144.Wang Y.J., Schug J., Won K.J., Liu C., Naji A., Avrahami D. Single-cell transcriptomics of the human endocrine pancreas. Diabetes. 2016;65(10):3028–3038. doi: 10.2337/db16-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li J., Klughammer J., Farlik M., Penz T., Spittler A., Barbieux C. Single-cell transcriptomes reveal characteristic features of human pancreatic islet cell types. EMBO Reports. 2016;17(2):178–187. doi: 10.15252/embr.201540946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Muraro M.J., Dharmadhikari G., Grun D., Groen N., Dielen T., Jansen E. A single-cell transcriptome atlas of the human pancreas. Cell Systems. 2016;3(4) doi: 10.1016/j.cels.2016.09.002. 385–394 e383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Segerstolpe A., Palasantza A., Eliasson P., Andersson E.M., Andreasson A.C., Sun X. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metabolism. 2016;24(4):593–607. doi: 10.1016/j.cmet.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lawlor N., George J., Bolisetty M., Kursawe R., Sun L.V.S. Single cell transcriptomes identify human islet cell signatures and reveal cell-type-specific expression changes in type 2 diabetes. Genome Research. 2016 doi: 10.1101/gr.212720.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xin Y., Kim J., Okamoto H., Ni M., Wei Y., Adler C. RNA sequencing of single human islet cells reveals type 2 diabetes genes. Cell Metabolism. 2016;24(4):608–615. doi: 10.1016/j.cmet.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 150.Arystarkhova E., Liu Y.B., Salazar C., Stanojevic V., Clifford R.J., Kaplan J.H. Hyperplasia of pancreatic beta cells and improved glucose tolerance in mice deficient in the FXYD2 subunit of Na,K-ATPase. The Journal of Biological Chemistry. 2013;288(10):7077–7085. doi: 10.1074/jbc.M112.401190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nyblom H.K., Bugliani M., Fung E., Boggi U., Zubarev R., Marchetti P. Apoptotic, regenerative, and immune-related signaling in human islets from type 2 diabetes individuals. Journal of Proteome Research. 2009;8(12):5650–5656. doi: 10.1021/pr9006816. [DOI] [PubMed] [Google Scholar]
- 152.Wang Y.J., Golson M.L., Schug J., Traum D., Liu C., Vivek K. Single-cell mass cytometry analysis of the human endocrine pancreas. Cell Metabolism. 2016;24(4):616–626. doi: 10.1016/j.cmet.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Benthuysen J.R., Carrano A.C., Sander M. Advances in beta cell replacement and regeneration strategies for treating diabetes. The Journal of Clinical Investigation. 2016;126(10):3651–3660. doi: 10.1172/JCI87439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pagliuca F.W., Millman J.R., Gurtler M., Segel M., Van Dervort A., Ryu J.H. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159(2):428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rezania A., Bruin J.E., Arora P., Rubin A., Batushansky I., Asadi A. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nature Biotechnology. 2014;32(11):1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- 156.Russ H.A., Parent A.V., Ringler J.J., Hennings T.G., Nair G.G., Shveygert M. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. The EMBO Journal. 2015;34(13):1759–1772. doi: 10.15252/embj.201591058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vegas A.J., Veiseh O., Gurtler M., Millman J.R., Pagliuca F.W., Bader A.R. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nature Medicine. 2016;22(3):306–311. doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Millman J.R., Xie C., Van Dervort A., Gurtler M., Pagliuca F.W., Melton D.A. Generation of stem cell-derived beta-cells from patients with type 1 diabetes. Nature Communications. 2016;7:11463. doi: 10.1038/ncomms11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Boj S.F., Hwang C.I., Baker L.A., Chio, Engle D.D., Corbo V. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160(1–2):324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Huang L., Holtzinger A., Jagan I., BeGora M., Lohse I., Ngai N. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nature Medicine. 2015;21(11):1364–1371. doi: 10.1038/nm.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hohwieler M., Illing A., Hermann P.C., Mayer T., Stockmann M., Perkhofer L. Human pluripotent stem cell-derived acinar/ductal organoids generate human pancreas upon orthotopic transplantation and allow disease modelling. Gut. 2017;66(3):473–486. doi: 10.1136/gutjnl-2016-312423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Grapin-Botton A. Three-dimensional pancreas organogenesis models. Diabetes, Obesity & Metabolism. 2016;18(Suppl 1):33–40. doi: 10.1111/dom.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dorrell C., Schug J., Canaday P.S., Russ H.A., Tarlow B.D., Grompe M.T. Human islets contain four distinct subtypes of beta cells. Nature Communications. 2016;7:11756. doi: 10.1038/ncomms11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Arda H.E., Li L., Tsai J., Torre E.A., Rosli Y., Peiris H. Age-dependent pancreatic gene regulation reveals mechanisms governing human beta cell function. Cell Metabolism. 2016;23(5):909–920. doi: 10.1016/j.cmet.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Marselli L., Suleiman M., Masini M., Campani D., Bugliani M., Syed F. Are we overestimating the loss of beta cells in type 2 diabetes? Diabetologia. 2014;57(2):362–365. doi: 10.1007/s00125-013-3098-3. [DOI] [PubMed] [Google Scholar]
- 166.Speier S., Rupnik M. A novel approach to in situ characterization of pancreatic beta-cells. Pflugers Archiv. 2003;446(5):553–558. doi: 10.1007/s00424-003-1097-9. [DOI] [PubMed] [Google Scholar]
- 167.Poosti F., Pham B.T., Oosterhuis D., Poelstra K., van Goor H., Olinga P. Precision-cut kidney slices (PCKS) to study development of renal fibrosis and efficacy of drug targeting ex vivo. Disease Models & Mechanisms. 2015;8(10):1227–1236. doi: 10.1242/dmm.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.de Graaf I.A., Olinga P., de Jager M.H., Merema M.T., de Kanter R., van de Kerkhof E.G. Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nature Protocols. 2010;5(9):1540–1551. doi: 10.1038/nprot.2010.111. [DOI] [PubMed] [Google Scholar]
- 169.Wang K., Lee P., Mirams G.R., Sarathchandra P., Borg T.K., Gavaghan D.J. Cardiac tissue slices: preparation, handling, and successful optical mapping. American Journal of Physiology. Heart and Circulatory Physiology. 2015;308(9):H1112–H1125. doi: 10.1152/ajpheart.00556.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rodriguez-Diaz R., Speier S., Molano R.D., Formoso A., Gans I., Abdulreda M.H. Noninvasive in vivo model demonstrating the effects of autonomic innervation on pancreatic islet function. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:21456–21461. doi: 10.1073/pnas.1211659110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Meneghel-Rozzo T., Rozzo A., Poppi L., Rupnik M. In vivo and in vitro development of mouse pancreatic beta-cells in organotypic slices. Cell & Tissue Research. 2004;316(3):295–303. doi: 10.1007/s00441-004-0886-6. [DOI] [PubMed] [Google Scholar]
- 172.Rose T., Efendic S., Rupnik M. Ca2+-secretion coupling is impaired in diabetic Goto Kakizaki rats. The Journal of General Physiology. 2007;129(6):493–508. doi: 10.1085/jgp.200609604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Speier S., Gjinovci A., Charollais A., Meda P., Rupnik M. Cx36-mediated coupling reduces beta-cell heterogeneity, confines the stimulating glucose concentration range, and affects insulin release kinetics. Diabetes. 2007;56(4):1078–1086. doi: 10.2337/db06-0232. [DOI] [PubMed] [Google Scholar]
- 174.Speier S., Yang S.B., Sroka K., Rose T., Rupnik M. KATP-channels in beta-cells in tissue slices are directly modulated by millimolar ATP. Molecular and Cellular Endocrinology. 2005;230(1–2):51–58. doi: 10.1016/j.mce.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 175.Huang Y.C., Rupnik M., Gaisano H.Y. Unperturbed islet alpha-cell function examined in mouse pancreas tissue slices. Journal of Physiology. 2011;589(Pt 2):395–408. doi: 10.1113/jphysiol.2010.200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huang Y.C., Rupnik M.S., Karimian N., Herrera P.L., Gilon P., Feng Z.P. In situ electrophysiological examination of pancreatic alpha cells in the streptozotocin-induced diabetes model, revealing the cellular basis of glucagon hypersecretion. Diabetes. 2013;62(2):519–530. doi: 10.2337/db11-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dolensek J., Stozer A., Skelin Klemen M., Miller E.W., Slak Rupnik M. The relationship between membrane potential and calcium dynamics in glucose-stimulated beta cell syncytium in acute mouse pancreas tissue slices. PLoS One. 2013;8(12):e82374. doi: 10.1371/journal.pone.0082374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Marciniak A., Selck C., Friedrich B., Speier S. Mouse pancreas tissue slice culture facilitates long-term studies of exocrine and endocrine cell physiology in situ. PLoS One. 2013;8(11):e78706. doi: 10.1371/journal.pone.0078706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Stozer A., Dolensek J., Rupnik M.S. Glucose-stimulated calcium dynamics in islets of Langerhans in acute mouse pancreas tissue slices. PLoS One. 2013;8(1):e54638. doi: 10.1371/journal.pone.0054638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stozer A., Gosak M., Dolensek J., Perc M., Marhl M., Rupnik M.S. Functional connectivity in islets of Langerhans from mouse pancreas tissue slices. PLoS Computational Biology. 2013;9(2):e1002923. doi: 10.1371/journal.pcbi.1002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Marciniak A., Cohrs C.M., Tsata V., Chouinard J.A., Selck C., Stertmann J. Using pancreas tissue slices for in situ studies of islet of Langerhans and acinar cell biology. Nature Protocols. 2014;9(12):2809–2822. doi: 10.1038/nprot.2014.195. [DOI] [PubMed] [Google Scholar]
- 182.Cohrs C.M., Chen C., Jahn S.R., Stertmann J., Chmelova H., Weitz J. Vessel network architecture of adult human islets promotes distinct cell-cell interactions in situ and is altered after transplantation. Endocrinology. 2017 doi: 10.1210/en.2016-1184. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 183.Karimian N., Qin T., Liang T., Osundiji M., Huang Y., Teich T. Somatostatin receptor type 2 antagonism improves glucagon counterregulation in biobreeding diabetic rats. Diabetes. 2013;62(8):2968–2977. doi: 10.2337/db13-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ehehalt F., Sturm D., Rosler M., Distler M., Weitz J., Kersting S. Blood glucose homeostasis in the course of partial pancreatectomy–evidence for surgically reversible diabetes induced by cholestasis. PLoS One. 2015;10(8):e0134140. doi: 10.1371/journal.pone.0134140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kilimnik G., Jo J., Periwal V., Zielinski M.C., Hara M. Quantification of islet size and architecture. Islets. 2012;4(2):167–172. doi: 10.4161/isl.19256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Levy J.C., Matthews D.R., Hermans M.P. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 187.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 188.Osundiji M.A., Lam D.D., Shaw J., Yueh C.Y., Markkula S.P., Hurst P. Brain glucose sensors play a significant role in the regulation of pancreatic glucose-stimulated insulin secretion. Diabetes. 2012;61(2):321–328. doi: 10.2337/db11-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nano R., Melzi R., Mercalli A., Balzano G., Scavini M., Bonadonna R. Islet volume and indexes of beta-cell function in humans. Cell Transplantation. 2016;25(3):491–501. doi: 10.3727/096368915X688498. [DOI] [PubMed] [Google Scholar]
- 190.Robertson R.P., Bogachus L.D., Oseid E., Parazzoli S., Patti M.E., Rickels M.R. Assessment of beta-cell mass and alpha- and beta-cell survival and function by arginine stimulation in human autologous islet recipients. Diabetes. 2015;64(2):565–572. doi: 10.2337/db14-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ryan E.A., Lakey J.R., Paty B.W., Imes S., Korbutt G.S., Kneteman N.M. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51(7):2148–2157. doi: 10.2337/diabetes.51.7.2148. [DOI] [PubMed] [Google Scholar]
- 192.Teuscher A.U., Kendall D.M., Smets Y.F., Leone J.P., Sutherland D.E., Robertson R.P. Successful islet autotransplantation in humans: functional insulin secretory reserve as an estimate of surviving islet cell mass. Diabetes. 1998;47(3):324–330. doi: 10.2337/diabetes.47.3.324. [DOI] [PubMed] [Google Scholar]
- 193.Meier J.J., Breuer T.G., Bonadonna R.C., Tannapfel A., Uhl W., Schmidt W.E. Pancreatic diabetes manifests when beta cell area declines by approximately 65% in humans. Diabetologia. 2012;55(5):1346–1354. doi: 10.1007/s00125-012-2466-8. [DOI] [PubMed] [Google Scholar]
- 194.Brelje T.C., Scharp D.W., Lacy P.E., Ogren L., Talamantes F., Robertson M. Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology. 1993;132(2):879–887. doi: 10.1210/endo.132.2.8425500. [DOI] [PubMed] [Google Scholar]
- 195.Gravena C., Mathias P.C., Ashcroft S.J. Acute effects of fatty acids on insulin secretion from rat and human islets of Langerhans. Journal of Endocrinology. 2002;173(1):73–80. doi: 10.1677/joe.0.1730073. [DOI] [PubMed] [Google Scholar]
- 196.Henquin J.C., Dufrane D., Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes. 2006;55(12):3470–3477. doi: 10.2337/db06-0868. [DOI] [PubMed] [Google Scholar]
- 197.Farilla L., Bulotta A., Hirshberg B., Li Calzi S., Khoury N., Noushmehr H. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144(12):5149–5158. doi: 10.1210/en.2003-0323. [DOI] [PubMed] [Google Scholar]
- 198.Lupi R., Marchetti P., Giannarelli R., Coppelli A., Tellini C., Del Guerra S. Effects of glibenclamide and metformin (alone or in combination) on insulin release from isolated human pancreatic islets. Acta Diabetologica. 1997;34(1):46–48. doi: 10.1007/s005920050065. [DOI] [PubMed] [Google Scholar]
- 199.Butler A.E., Cao-Minh L., Galasso R., Rizza R.A., Corradin A., Cobelli C. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia. 2010;53(10):2167–2176. doi: 10.1007/s00125-010-1809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sweet I.R., Cook D.L., Lernmark A., Greenbaum C.J., Krohn K.A. Non-invasive imaging of beta cell mass: a quantitative analysis. Diabetes Technology & Therapeutics. 2004;6(5):652–659. doi: 10.1089/dia.2004.6.652. [DOI] [PubMed] [Google Scholar]
- 201.Malosio M.L., Esposito A., Brigatti C., Palmisano A., Piemonti L., Nano R. MR imaging monitoring of iron-labeled pancreatic islets in a small series of patients: islet fate in successful, unsuccessful, and autotransplantation. Cell Transplantation. 2015;24(11):2285–2296. doi: 10.3727/096368914X684060. [DOI] [PubMed] [Google Scholar]
- 202.Saudek F., Jirak D., Girman P., Herynek V., Dezortova M., Kriz J. Magnetic resonance imaging of pancreatic islets transplanted into the liver in humans. Transplantation. 2010;90(12):1602–1606. doi: 10.1097/tp.0b013e3181ffba5e. [DOI] [PubMed] [Google Scholar]
- 203.Toso C., Vallee J.P., Morel P., Ris F., Demuylder-Mischler S., Lepetit-Coiffe M. Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. American Journal of Transplantation. 2008;8(3):701–706. doi: 10.1111/j.1600-6143.2007.02120.x. [DOI] [PubMed] [Google Scholar]
- 204.Bennet W., Groth C.G., Larsson R., Nilsson B., Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Upsala Journal of Medical Sciences. 2000;105(2):125–133. doi: 10.1517/03009734000000059. [DOI] [PubMed] [Google Scholar]
- 205.Leoni L., Serai S.D., Haque M.E., Magin R.L., Roman B.B. Functional MRI characterization of isolated human islet activation. NMR in Biomedicine. 2010;23(10):1158–1165. doi: 10.1002/nbm.1542. [DOI] [PubMed] [Google Scholar]
- 206.Botsikas D., Terraz S., Vinet L., Lamprianou S., Becker C.D., Bosco D. Pancreatic magnetic resonance imaging after manganese injection distinguishes type 2 diabetic and normoglycemic patients. Islets. 2012;4(3):243–248. doi: 10.4161/isl.20857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Eich T., Eriksson O., Lundgren T. Visualization of early engraftment in clinical islet transplantation by positron-emission tomography. The New England Journal of Medicine. 2007;356(26):2754–2755. doi: 10.1056/NEJMc070201. [DOI] [PubMed] [Google Scholar]
- 208.Eriksson O., Selvaraju R., Eich T., Willny M., Brismar T.B., Carlbom L. Positron emission tomography to assess the outcome of intraportal islet transplantation. Diabetes. 2016;65(9):2482–2489. doi: 10.2337/db16-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Goland R., Freeby M., Parsey R., Saisho Y., Kumar D., Simpson N. 11C-dihydrotetrabenazine PET of the pancreas in subjects with long-standing type 1 diabetes and in healthy controls. Journal of Nuclear Medicine. 2009;50(3):382–389. doi: 10.2967/jnumed.108.054866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Normandin M.D., Petersen K.F., Ding Y.S., Lin S.F., Naik S., Fowles K. In vivo imaging of endogenous pancreatic beta-cell mass in healthy and type 1 diabetic subjects using 18F-fluoropropyl-dihydrotetrabenazine and PET. Journal of Nuclear Medicine. 2012;53(6):908–916. doi: 10.2967/jnumed.111.100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Eriksson O., Espes D., Selvaraju R.K., Jansson E., Antoni G., Sorensen J. Positron emission tomography ligand [11C]5-hydroxy-tryptophan can be used as a surrogate marker for the human endocrine pancreas. Diabetes. 2014;63(10):3428–3437. doi: 10.2337/db13-1877. [DOI] [PubMed] [Google Scholar]
- 212.Brom M., Woliner-van der Weg W., Joosten L., Frielink C., Bouckenooghe T., Rijken P. Non-invasive quantification of the beta cell mass by SPECT with (1)(1)(1)In-labelled exendin. Diabetologia. 2014;57(5):950–959. doi: 10.1007/s00125-014-3166-3. [DOI] [PubMed] [Google Scholar]
- 213.Mandel T.E., Hoffman L., Collier S., Carter W.M., Koulmanda M. Organ culture of fetal mouse and fetal human pancreatic islets for allografting. Diabetes. 1982;31(Suppl 4):39–47. doi: 10.2337/diab.31.4.s39. [DOI] [PubMed] [Google Scholar]
- 214.Hayek A., Beattie G.M. Experimental transplantation of human fetal and adult pancreatic islets. Journal of Clinical Endocrinology & Metabolism. 1997;82(8):2471–2475. doi: 10.1210/jcem.82.8.4151. [DOI] [PubMed] [Google Scholar]
- 215.Fransson M., Brannstrom J., Duprez I., Essand M., Le Blanc K., Korsgren O. Mesenchymal stromal cells support endothelial cell interactions in an intramuscular islet transplantation model. Regenerative Medicine Research. 2015;3:1. doi: 10.1186/s40340-015-0010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Meier R.P., Seebach J.D., Morel P., Mahou R., Borot S., Giovannoni L. Survival of free and encapsulated human and rat islet xenografts transplanted into the mouse bone marrow. PLoS One. 2014;9(3):e91268. doi: 10.1371/journal.pone.0091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zaldumbide A., Alkemade G., Carlotti F., Nikolic T., Abreu J.R., Engelse M.A. Genetically engineered human islets protected from CD8-mediated autoimmune destruction in vivo. Molecular Therapy. 2013;21(8):1592–1601. doi: 10.1038/mt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gargani S., Thevenet J., Yuan J.E., Lefebvre B., Delalleau N., Gmyr V. Adaptive changes of human islets to an obesogenic environment in the mouse. Diabetologia. 2013;56(2):350–358. doi: 10.1007/s00125-012-2775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Westermark P., Eizirik D.L., Pipeleers D.G., Hellerstrom C., Andersson A. Rapid deposition of amyloid in human islets transplanted into nude mice. Diabetologia. 1995;38(5):543–549. doi: 10.1007/BF00400722. [DOI] [PubMed] [Google Scholar]
- 220.Shiroki R., Poindexter N.J., Woodle E.S., Hussain M.S., Mohanakumar T., Scharp D.W. Human peripheral blood lymphocyte reconstituted severe combined immunodeficient (hu-PBL-SCID) mice. A model for human islet allograft rejection. Transplantation. 1994;57(11):1555–1562. [PubMed] [Google Scholar]
- 221.Wang P., Alvarez-Perez J.C., Felsenfeld D.P., Liu H., Sivendran S., Bender A. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nature Medicine. 2015;21(4):383–388. doi: 10.1038/nm.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Dai C., Kayton N.S., Shostak A., Poffenberger G., Cyphert H.A., Aramandla R. Stress-impaired transcription factor expression and insulin secretion in transplanted human islets. The Journal of Clinical Investigation. 2016;126(5):1857–1870. doi: 10.1172/JCI83657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Abdulreda M.H., Rodriguez-Diaz R., Caicedo A., Berggren P.O. Liraglutide compromises pancreatic beta cell function in a humanized mouse model. Cell Metabolism. 2016;23(3):541–546. doi: 10.1016/j.cmet.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fowler M., Virostko J., Chen Z., Poffenberger G., Radhika A., Brissova M. Assessment of pancreatic islet mass after islet transplantation using in vivo bioluminescence imaging. Transplantation. 2005;79(7):768–776. doi: 10.1097/01.tp.0000152798.03204.5c. [DOI] [PubMed] [Google Scholar]
- 225.Berclaz C., Schmidt-Christensen A., Szlag D., Extermann J., Hansen L., Bouwens A. Longitudinal three-dimensional visualisation of autoimmune diabetes by functional optical coherence imaging. Diabetologia. 2016;59(3):550–559. doi: 10.1007/s00125-015-3819-x. [DOI] [PubMed] [Google Scholar]
- 226.Chen C., Chmelova H., Cohrs C.M., Chouinard J.A., Jahn S.R., Stertmann J. Alterations in beta-cell calcium dynamics and efficacy outweigh islet mass adaptation in compensation of insulin resistance and prediabetes onset. Diabetes. 2016;65(9):2676–2685. doi: 10.2337/db15-1718. [DOI] [PubMed] [Google Scholar]
- 227.Speier S., Nyqvist D., Cabrera O., Yu J., Molano R.D., Pileggi A. Noninvasive in vivo imaging of pancreatic islet cell biology. Nature Medicine. 2008;14(5):574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Speier S. Experimental approaches for high-resolution in vivo imaging of islet of Langerhans biology. Current Diabetes Reports. 2011;11(5):420–425. doi: 10.1007/s11892-011-0207-x. [DOI] [PubMed] [Google Scholar]