Abstract
Alzheimer's disease (AD) is a chronic neurodegenerative disorder, in which multiple risk factors converge. Despite the complexity of the etiology of the disease, synaptic failure is the pathological basis of cognitive impairment, the cardinal sign of AD. Decreased synaptic density, compromised synaptic transmission, and defected synaptic plasticity are hallmark synaptic pathologies accompanying AD. However, the mechanisms by which synapses are injured in AD-related conditions have not been fully elucidated yet. Mitochondria are a critical organelle in neurons. The pivotal role of mitochondria in supporting synaptic function and the concomitant occurrence of mitochondrial dysfunction with synaptic stress in postmortem AD brains as well as AD animal models seem to lend the credibility to the hypothesis that mitochondrial defects underlie synaptic failure in AD. This concept is further strengthened by the protective effect of mitochondrial medicine on synaptic function against the toxicity of amyloid-β, a key player in the pathogenesis of AD. In this review, we focus on the association between mitochondrial dysfunction and synaptic transmission deficits in AD. Impaired mitochondrial energy production, deregulated mitochondrial calcium handling, excess mitochondrial reactive oxygen species generation, and release play a crucial role in mediating synaptic transmission deregulation in AD. The understanding of the role of mitochondrial dysfunction in synaptic stress may lead to novel therapeutic strategies for the treatment of AD through the protection of synaptic transmission by targeting to mitochondrial deficits.
Keywords: Alzheimer's disease, mitochondrial dysfunction, synaptic injury, synaptic mitochondria, synaptic transmission
Introduction
Characterized by progressive cognitive decline, Alzheimer's disease (AD) is the most common type of dementia attacking the aged population [1]. Among the known risk factors, age is the leading one in particular for the sporadic form of AD, with the incidence of this neurological disorder dramatically rising in an exponential pattern after 65 years of age [2–4]. The pathological hallmarks of AD include extracellular and intracellular deposition of amyloid beta (Aβ) [5–9], neurofibrillary tangles (NFTs) [10–12], synaptic failure [13–16], and neuronal loss [14, 17] in AD sensitive brain regions such as the hippocampus, neocortex, and nucleus basalis of Meynert [10, 18]. Clinical and pathological studies have shown a strong correlation between synaptic deficits and the degree of memory loss in AD [13–16]. Of note, discernible changes of synapses have been detected in the neocortex of patients, even in the very early stage of AD or with mild cognitive impairment (MCI), who have little or mild Aβ plaques and neurofibrillary tangles as well as neuronal death [19]. Such findings indicate that AD is a disease of synaptic dysfunction [20–25].
In the central nervous system (CNS), synapses are the contact sites of neurons to pass signals via synaptic transmission, also known as neurotransmission. Electric neurotransmission plays a critical role in the development of the nervous system, while the communications between CNS neurons in fully developed adults are predominantly dependent on the function of chemical synapses, at which neurotransmitters are released from the presynapses and target postsynaptic receptors to induce the downstream cascades related to synaptic activity [21, 26]. In recent years, increasing lines of evidence have implicated the essential and unique role of mitochondria in synaptic transmission. It is proposed that mitochondria are involved in each and every stage of neurotransmission including the synthesis and storage of neurotransmitters, the trafficking of synaptic vesicles (SVs), and the release of neurotransmitters from the presynapses, as well as the recycling of SVs [27–30]. It is generally accepted that mitochondria support synaptic transmission primarily via their functions in maintaining calcium homeostasis, providing energy, and regulating the production of reactive oxygen species (ROS), as well as synthesizing essential intermediates or final products of several neurotransmitters [27–30]. Conceivably, mitochondrial deficits impair synaptic activity at pathological states [30–33]. Indeed, the concomitant occurrence of synaptic stress and mitochondrial dysfunction has been repeatedly identified in many neurodegenerative diseases including AD [33, 34]. Furthermore, the protection of mitochondrial function preserves synaptic activity in AD-related pathophysiological settings in both basic and clinical platforms [35, 36], reinforcing the direct link between the two pathological processes accompanying AD. Thus, elucidating the relationship between mitochondrial abnormalities and synaptic injury in AD will foster us a better understanding of the pathogenesis of this neurodegenerative disease and hopefully lead to the development of therapeutic strategies for the protection of synaptic activity and the subsequent cognitive function.
Here, we focus on mitochondrial pathology in AD and discuss the link between mitochondrial dysfunction and chemical synaptic transmission impairment in this chronic neurological disorder.
Synaptic Transmission Changes in AD
It is a well-documented notion that synaptic activity is the basis of cognition and weakened synapses are closely associated with cognitive deficits [37]. In the past decades, histological studies have determined that synaptic alterations including synaptic loss, altered synaptic architecture, and compromised synaptic transmission as well as defected synaptic plasticity are characteristics of AD and the aging brains [21, 38–41]. Importantly, the degree of these synaptic changes correlates with the severity of cognitive decline in AD [21]. Although the molecular mechanisms causing synaptic failure in AD have not been elucidated in detail, Aβ toxicity, tau hyperphosphorylation, and mitochondrial dysfunction have been implicated as critical involving factors conjointly disrupting synapses [22, 25, 34, 42–44].
Alterations of neurotransmitters in AD
Neurotransmitters are a group of small molecules serve as the chemical messengers to transmit signals from one neuron to another or to other cell types. Ever since the identification of acetylcholine (ACh) and norepinephrine (NE) as neurotransmitters, currently there are more than 100 types of neurotransmitters on the list, which is still expanding [45]. The classic and very simplified definition of a neurotransmitter is a chemical that has been synthesized, then packaged and stored in SVs in presynapses, and ready to be released to synaptic clefts to regulate the excitatory state of other cells by acting on the receptors at postsynapses. By their influence on the excitatory state of the cells, neurotransmitters can be categorized into two clear-cut groups, excitatory and inhibitory [45]. It should be noted that some neurotransmitters such as ACh may have both excitatory and inhibitory effects depending on the types of receptors at the postsynapses [46, 47].
Owing to the progress in neurochemistry, neurotransmitter deregulation in AD brains has been intensively studied since the mid-twentieth century and still remains a target of great interest to date. The study on cholinergic system in AD dates back to the discovery of ACh [48–53]. Pathological studies of AD brains have found that severe lesions in nucleus basalis of Meynert, hippocampus, and neocortex are prominent in postmortem AD brains [54]. The hypothesis of cholinergic system deregulation in AD is built on the abundance and critical function of cholinergic neurons in these brain regions. This concept has been subsequently supported by the findings of severe degeneration of cholinergic neurons, reduced ACh synthesis and levels, decreased choline levels as well as down-regulated activity of choline acetyltransferase (ChAT), and the deregulations of ACh receptors in AD brains [47, 55–61]. Moreover, the concentration of ACh in the cerebrospinal fluid (CSF) extracted from AD patients is significantly reduced, and the levels of CSF ACh are proportionate to the severity of cognitive deficits in AD [62–64]. These observations have confirmed the perturbations of cholinergic system in the development of AD and further implicated the possibility of using CSF ACh as a diagnostic biomarker of AD. Indeed, efforts to rescue ACh deficits in AD by the application of acetylcholinesterase inhibitors such as donepezil, rivastigmine, and galantamine have demonstrated benefits for the treatment of AD [65, 66]. Despite their modest benefits and side-effects, acetylcholinesterase inhibitors are among the very few agents currently used as the first-line treatment for AD.
Another critical neurotransmitter associated with AD is glutamate, which is a member of amino-acid neurotransmitter family. Glutamate is the most plentiful excitatory signal messenger for a majority of synapses in neocortex and hippocampus [67, 68]. The ionotropic glutamate receptors, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) and n-methyl-D-aspartate receptor (NMDAR), play a key role in mediating synaptic transmission and synaptic plasticity [69, 70]. Although glutamate carries important physiological function, excess glutamatergic activation causes prolonged calcium overloading at postsynapses that perturbs mitochondrial function and some key intra-cellular signaling transduction pathways, eventually leading to severe synaptic injury. The devastating effects of glutamate excitotoxicity on synaptic activity and neuronal survival have been discerned in a variety of pathological scenarios including AD [71, 72]. A study using magnetic resonance spectroscopy measured glutamate plus glutamine (Gx) levels in bilateral posterior cingulate gyrus of AD and MCI patients as well as the age-matched non-AD controls, and found significant decreased Gx levels in AD brains; while the levels of glutamate in MCI brains were comparable to those in the control subjects [73]. However, another study showed no change in the levels of Gx in the frontal lobes from probable AD [74]. The results seem to suggest that the cerebral Gx levels are significantly changed at the late stage of AD. But it cannot be excluded that the changes of Gx in AD brains is region-specific. This needs further investigation. Interestingly, the studies on glutamate and glutamine in CSF from AD patients have found decreased glutamine [75] while significantly increased glutamate levels [76–79], which does not match the results collected from the AD brains. Although the mechanisms of this disparity are not clear, the aforementioned evidence at least suggests the dysmetabolism of glutamate/glutamine in AD-related pathological settings. Other than the changes of glutamate, alterations of glutamate receptor, NMDAR in particular, have also been observed in AD brains. Evidence has shown the hypersensitivity of NMDAR to glutamate in AD brains and the resultant glutamatergic toxicity leads to synaptic injury. Such NMDAR hypersensitivity might be the result of Aβ-induced intracellular calcium elevation and magnesium removal from the receptor [71, 80]. Apart from the direct role of Aβ in enhancing NMDAR sensitivity, Aβ may also impose indirect disruptive regulation on NMDAR activity, thus reducing the NMDAR-associated long-term potentiation. In addition, mitochondrial dysfunction-associated neuronal oxidative stress, calcium dysmetabolism, and ATP deficiency are as well proposed to contribute to the glutamate excitotoxicity of AD neurons [81–84].
Interestingly, a significant reduction of the density of NMDAR has been detected in AD neocortex and hippocampus, where Aβ deposition is heavy [85–87], which may underlie NMDAR hyposensitivity in the late stage of AD. In consistent with the influence of glutamatergic toxicity on synaptic function, memantine, a glutamate receptor antagonist has demonstrated some modest protection of memory loss in AD patients [88–90]. In addition to glutamate, gamma-aminobutyric acid (GABA) is another amino-acid neurotransmitter whose deregulation has been linked to synaptic stress in AD brains. Decreased levels of GABA, an inhibitory neurotransmitter, has been reported in patients with severe AD [91]. The deficits of GABAergic system are thought to contribute to synaptic perturbations through the interrupted crosstalk between GABAergic, cholinergic, and glutamatergic synapses due to the loss of inhibitory function of GABAergic neurons [92].
Alterations in monoamine neurotransmitters have also been reported in AD patients. The lesions in locus coeruleus, the major brain area for the synthesis of NE, and reduced brain NE levels have been implicated in memory loss and psychiatric symptoms in AD [93, 94]. The supplementation of NE has exhibited protection against Aβ-induced mitochondrial dysfunction and neuronal stress in cultured mouse neurons. Furthermore, the administration of adrenergic receptor agonists attenuates Aβ toxicity-associated cognitive impairment in mice; such protective effects are thought to be the result of enhanced NE release [95, 96]. Another example of deregulated monoamine neurotransmitter in AD is dopamine. Dopaminergic system defects, which are generally implicated in the pathogenesis of Parkinson's disease (PD), have as well been linked to AD [97, 98]. A previous study has compared the difference of dopaminergic system deficits in PD, AD, and AD with parkinsonism and found that although all of them have loss of presynaptic dopamine receptor D2 (DRD2) and decreased levels of tyrosine hydroxylase, these three groups demonstrated distinct patterns of lesions sites, which may explain the difference in extrapyramidal symptoms between each of them [99]. In addition, the dopaminergic changes were almost restricted to the nucleus accumbens in AD patients absent of PD-like symptoms, while the AD with parkinsonism group showed more severe damages in rostral caudate and putamen. In sharp contrast, the PD group had more vulnerability in the substantia nigra and ventral tegmental region [99]. Intriguingly, when they compared the levels of postsynaptic DRD2, DRD2 was preserved in the examined extrapyramidal system in AD groups but not the AD with parkinsonism group [100]. A more recent study has suggested that dopamine may mitigate cognitive impairment in AD at least in part through the interaction of dopaminergic and cholinergic systems [101]. However, although emerging evidence has highlighted the function of the dopaminergic system in the consolidation of memory [102, 103], the correlation between dopaminergic system deficits and AD cognitive impairment has not yet been comprehensively investigated.
Neurotransmitter alterations and their contribution to the development of AD are still a fast growing topic. Importantly, current pharmaceutical therapies for AD treatment are predominantly stemmed from the studies on neurotransmitters. With the identification of new neurotransmitters and the progress in AD research, in-depth studies on neurotransmitters in AD still hold promise for the future development of novel therapeutic strategies to protect synaptic function and memory in AD.
Deficits of synaptic vesicle cycling in AD
Neurotransmitter release and reuse are through SV cycling. Stepwise, SV cycling includes the loading of neurotransmitters into SVs, the SV trafficking to and docking at the active zone of presynapses, the releasing of neurotransmitters, and at last the endocytosis and recycling of SVs [104]. In addition to synaptic loss, dramatically decreased expression levels of SV proteins and their coding mRNAs have been discerned in vast areas of postmortem AD brains including most part of the neocortex, limbic system, basal ganglia, and cerebellum; and the changes have a strong correlation to cognitive impairment [19, 105–111]. It should be mentioned that the severity of these changes varies; and the relatively AD sensitive brain regions such as hippocampus, frontal, temporal, and parietal lobes, where there are more severe Aβ deposition, tau pathology, and mitochondrial damages, are early affected areas and demonstrate a greater loss of SV proteins and their coding mRNAs [108–111]. Another prominent change potentially leading to defected SV cycling in AD is the perturbations of signaling transduction pathways including cyclic-AMP-dependent protein kinase A (PKA), Ca2+/ calmodulin-dependent kinase II (CaMKII), and protein kinase C (PKC). Those signaling transduction pathways play critical roles in modulating the functional status of several SV and synaptic plasma membrane proteins via phosphorylation, thus potentiating presynaptic activity. In fact, deregulated activities of CaMKII [112–115], PKA [116, 117], and PKC [118, 119] have been repeatedly linked to compromised synaptic activity and neuronal death in AD. The dysfunction of these transduction pathways is at least in part associated with calcium dysmetabolism, ATP deficiency, and oxidative stress in AD neurons [112, 120].
Direct evidence of altered SV cycling in AD-relevant conditions was obtained predominantly in cultured neurons given the difficulty in recording SV exocytosis and endocytosis in an in vivo setting in AD patients or AD animal models. In a previous study, Aβ at nanomolar level induces severely impaired synaptic transmission in cultured mouse hippocampal neurons. With Aβ-induced reduction in the expression levels of SV proteins and postsynaptic receptors, the exposure of Aβ significantly disrupts SV depletion and recycling [121]. Furthermore, the effects of tau abnormalities on SV exo-and endocytosis through the damages on the integrity and functionality of microtubules have also been proposed to underlie SV mobilization in AD [122].
Therefore, a large number of direct and indirect evidence have pointed to the deregulation of neurotransmitters and SV exo- and endocytosis in AD-relevant pathological settings. To elucidate the mechanisms underlying the synaptic transmission deficits is of paramount importance for our understanding of synaptic injury and cognitive decline in AD.
Mitochondria and Synaptic Transmission
Mitochondria are a key organelle in eukaryotic cells. In addition to their role in energy metabolism, mitochondria have multiple traits of critical functions including maintaining appropriate regulating intracellular calcium homeostasis, intracellular redox balance, and mediating cell apoptosis and necrosis. Thus, mitochondria have a fundamental role in the life and death of cells. Of note, strong evidence has suggested the multiplicity of mitochondrial functions. Some mitochondrial functions are even cell-type specific. For example, mitochondria in steroidogenic cells carry the function of steroid hormone biosynthesis [123]; and mitochondria are essential sites for fatty acid synthesis in adipose and liver tissues [124–126]. Moreover, mitochondria have been found to be involved in RNA metabolism [127]. Chen and colleagues have reported the role of mitochondria in cell innate immune system mediating cellular anti-viral pathways [128]. It is therefore not surprising that more and more investigators have embraced the concept of mitochondrial heterogeneity. It is said that mitochondria are morphologically and functionally heterogeneous in different cell types as well as in different compartments of a single cell. Neurons represent a typical pattern of mitochondrial heterogeneity. Based on their physical position and functionality, neuronal mitochondria are categorized into several different subgroups, among which mitochondria at synapses or namely synaptic mitochondria are generally accepted to play an important role in supporting synaptic activity.
Synaptic mitochondria are a subpopulation of neuronal mitochondria specifically residing at synapses. Due to their extremely physical proximity to synapses, synaptic mitochondria are critical in assisting synaptic transmission.
Mitochondria and neurotransmitter synthesis and storage
The synthesis and packaging of some key neurotransmitters significantly rely on mitochondria. For example, ACh is synthesized from choline and acetyl co-enzyme A (Acetyl CoA); and the reaction is catalyzed by the enzyme called ChAT. Humans directly obtain choline from the diet; while Acetyl CoA is synthesized at mitochondria by using pyruvate from the glycolysis [129–136]. The synthesized ACh is subsequently transported into SVs through ACh transporter (VAChT) [137] via an ATP consuming process and then stored in SVs [138]. Another example is glutamate, which is critical for glutamatergic synapses. Due to the deficiency of pyruvate carboxylase in neurons [139–141], glutamate is synthesized in astrocytes [142–144] and then delivered into neurons by glutamate transporters [144, 145]. The synthesis of glutamate is at astrocyte mitochondria through multi-steps to convert oxaloacetate to α-ketoglutarate and eventually the final product, glutamate [142–144]. Other than their function in the synthesis of glutamate, the transport of glutamate from astrocytes into neurons and the packaging of glutamate into SVs are energy consuming processes significantly relying on the ATP producing function of mitochondria [144, 145]. In fact, neuronal mitochondria are also known to play a critical role in the de novo synthesis of several other key neurotransmitters such as NE [146–148], dopamine [148, 149], GABA [150, 151], and serotonin [151, 152]. Again, mitochondria are indispensable for the packaging of these neurotransmitters into SVs by providing ATP. In addition, mitochondria also have indirect influence on the production of non-classical neurotransmitters including adenosine, ATP, and nitric oxide via the function of mitochondria in the metabolism of these molecules. Therefore, mitochondria are extremely essential for the synthesis, packaging and storage of a number of neurotransmitters, the dysfunction of which are thought to be associated with AD.
Mitochondria and synaptic vesicle cycling
The process of synaptic transmission (that is, SV exocytosis) is initiated by calcium entry into presynapses via the voltage-gated calcium channels, which triggers the depolarization of the plasma membrane [153, 154]. By measuring the large synapses of the Calyx of Held, it is estimated that the depolarization-driven calcium transients last for around 400–500 μs [155]. The time course of calcium pulse may vary in different types of neurons depending on the size of the synapses. But in general the rise of calcium levels in the cytoplasm is transitory followed by a quick dissipation of the calcium sparks, which brings to an end of the neurotransmitter release [155, 156]. The aforementioned synaptic calcium handling mechanisms require the coordination of calcium influx and fast removal. The involving role of mitochondria in the two processes has received considerable attention. First, the activation of the voltage-gated calcium channel-forming Ca2+-ATPases predominantly relies on mitochondrial ATP provision [157, 158]. On the other hand, mitochondria are the major calcium sequestering unit at synapses and play a key role in maintaining the intra-synaptic calcium homeostasis [158–160]. Previous studies have shown that mitochondria at synapses buffer calcium following the depolarization-evoked calcium influx and dissipate intra-synaptic Ca2+ to resting levels in mouse retinal bipolar cells [158] and at the rat Calyx of Held [161] as well as the mouse motor nerve terminals [162]. Another important piece of evidence showing mitochondrial role in regulating calcium during synaptic transmission was collected from a study on mitochondrial conductance during the activation of the giant synapses in squid. Jonas and the colleagues found that the elevation of mitochondrial conductance is closely associated with the synaptic stimulation-driven calcium transients in the presynapses and lasts throughout the whole process of post-tetanic potentiation [163]. It could be argued that mitochondria are not the only calcium storing organelle at synapses and the calcium-retention capacity of endoplasmic reticulum (ER) should not be overlooked. Indeed, it has been suggested that mitochondria and ER vesicles near the active zone have similar extent of effect in regulating intra-synaptic calcium levels during synaptic activation [164]; while notably other studies indicated that the calcium uptake by mitochondria is more rapid than that by ER [165–167]. More than their role as the calcium bank, mitochondria and ER also release calcium, which has been identified to contribute to the production of miniature excitatory post synaptic currents in mouse hippocampal and neocortical neurons [168, 169] and the sustainment of prolonged SV exocytosis [165].
Of note, synapses have a high demand of ATP to energize the trafficking, exocytosis, and endocytosis of SVs. To meet the synaptic energy demand, mitochondria are delivered to dock at synapses, and these synaptic mitochondria serve as the in situ energy warehouse to fuel synapses [27, 170–173].
Furthermore, mitochondria are the major source of ROS in neurons. It has been suggested that superoxide at physiological levels may serve as a key regulator for synaptic transmission- and plasticity-related signaling molecules, receptors and channels, which are critical for long-term potentiation induction and memory formation. For example, scavenging superoxide through the overexpression of extracellular superoxide dismutase (SOD) in mice surprisingly inhibits long-term potentiation and long-term memory [174, 175]. Moreover, genetic mutation on or pharmaceutical inhibition of NADPH oxidase suppresses NMDAR activation-dependent ERK signaling transduction pathway in mouse CA1 region, which further implicates the critical role of ROS in synaptic activity [176]. However, the information of the physiological role of ROS in synaptic transmission and plasticity is still extremely limited, which is in sharp contrast to our knowledge on the disruptive effect of excess ROS on synaptic activity at pathological states.
In addition, it should not be neglected the indirect impacts of mitochondrial dysfunction on the functions of synaptic activity-modulating signaling pathways through ATP deficiency, calcium perturbations, and oxidative stress [112, 120].
Put together, mitochondrial function in ATP production, calcium homeostasis regulation, and redox balance maintenance is pivotal for the potentiation of synaptic transmission. Deficits in mitochondrial particularly synaptic mitochondrial function are detrimental to synaptic activity and strength.
Mitochondrial Dysfunction in AD: The Link To Synaptic Transmission Failure
The role of mitochondrial dysfunction in synaptic injury in AD has received considerable attention [177–180]. Mitochondrial dysfunction has been found in AD patients [181–185] and AD animal models [8, 9, 186–190] as well as in Aβ-insulted cells [191–193]. Importantly, despite the coincidence of mitochondrial dysfunction and synaptic stress in AD-related conditions, increasing evidence has shown that synaptic mitochondrial dysfunction occurs prior to severe synaptic injury in early stage AD [34, 194, 195] as well as in young AD animal models [187, 188, 196–199]. This further implicates the promoting role of mitochondrial deregulation in potentiating synaptic stress in AD.
Synaptic transmission has a very intensive energy demand and the majority of brain ATP is consumed to support synaptic activity [200]. Neuronal ATP is almost exclusively provided by mitochondria through oxidative phosphorylation (OXPHOS). It is noteworthy that mitochondrial OXPHOS deficits and ATP deficiency are hallmark pathologies in AD brains [201, 202]. Much work has been done on the defects of electron transfer chain (ETC) in AD mitochondria. Decreased activities of mitochondrial complexes I through IV have been detected in the neocortex and hippocampus from postmortem AD brains; while the deactivation of mitochondrial complex IV is more prominent with altered steady-state composition of this enzyme [203–207]. The deregulation of mitochondrial complex IV in neocortex has also been determined in AD animal models overexpressing human form Aβ [9, 187, 196, 208, 209]. Furthermore, mitochondrial ETC deficits are not just confined in AD sensitive brain regions. Decreased mitochondrial complex IV activity has been detected in platelets from AD patients [210, 211] and MCI subjects [211], suggesting mitochondrial ETC deficits are a systemic change in AD. In addition to the studies on mitochondrial complexes I to IV in AD, the functional status of mitochondrial complex V also known as F1Fo ATP synthase in AD has long been overlooked. This enzyme locates in the inner mitochondrial membrane and constitutes the primary site for OXPHOS [212]. In our recent study, we have found alterations of mitochondrial complex V in postmortem MCI and AD temporal lobes as well as in synaptic mitochondria from an AD mouse model [187]. The deregulation of mitochondrial complex V in AD-related conditions has a strong association with loss of its oligomycin sensitivity conferring protein (OSCP) subunit and the interplay of OSCP with Aβ. The deregulation of mitochondrial complex V leads to severe mitochondrial dysfunction and compromised synaptic transmission. Noteworthy, the restoration of OSCP rescues synaptic transmission from Aβ toxicity in cultured mouse neurons [187]. Put together, impaired mitochondrial ETC and defected mitochondrial OXPHOS are a primary pathology in AD, which results in insufficient ATP provision to fuel synaptic transmission.
Calcium influx is the initiative step of SV exocytosis. As discussed above (“Mitochondria and synaptic vesicle cycling”), normal synaptic mitochondrial calcium handling capacity is essential for synaptic transmission. Mitochondrial permeability transition pore (mPTP) is a non-selective trans-inner mitochondrial membrane pore. Over-activation of mPTP has been proposed to be a critical mechanism underlying compromised mitochondrial calcium buffering capacity in AD [208, 209, 213–217]. Excess mPTP leads to decreased mitochondrial calcium retention, collapsed mitochondrial membrane potential, reduced OXPHOS efficiency, and elevated ROS production and release, as well as ruptured mitochondrial membrane, eventually cell death [218, 219]. Although the exact molecular identity of mPTP still remains to be elucidated, a mitochondrial matrix protein called cyclophilin D(CypD) is a determined key regulator of mPTP formation [218, 219]. In our previous studies, we have found that the blockade of mPTP by genetic depletion of CypD confers protection against Aβ-mediated mitochondrial dysfunction as well as synaptic loss and synaptic transmission deficits in an AD mouse model [191, 197, 208], suggesting the deleterious impact of mPTP overactivation on synaptic transmission. This concept has further been supported by our recent study. Based on recent studies showing the role of mitochondrial F1Fo ATP synthase in the formation of mPTP [220, 221], we studied F1Fo ATP synthase (mitochondrial complex V) dysfunction in AD. We have determined the correlation of F1Fo ATP synthase deregulation and the activation of mPTP in AD-related conditions [187]. Of note, the restoration of F1Fo ATP synthase mitigates synaptic transmission injury in Aβ-insulted neurons [187]. Given the dual roles of F1Fo ATP synthase deregulation in ATP production and mPTP formation as well as the function of CypD, these studies suggest that defected synaptic transmission in AD-relevant conditions is associated, at least in part, with mPTP activation-induced mitochondrial calcium perturbations.
Impaired mitochondrial function results in excess ROS production and the resultant oxidative damages to neurons; and oxidative stress is characteristic of AD brains [222]. Several studies have found that lipid oxidation in presynaptic cytoplasm membrane obstructs the fusion pore opening, thus blocking the SV exocytosis leading to the abnormal detention of SVs in the active zone of presynapses [223, 224]. Moreover, oxidative stress induces hyperphosphorylation of tau through the inactivation of protein phosphatase 1 and 2A, causing abnormal tau aggregation [120], which blocks SV trafficking. Since mitochondria are known as the major source of ROS in neurons, previous attempts to scavenge mitochondrial ROS have shown significant protection against Aβ-induced synaptic injury. Dumont and the colleagues found that the overexpression of mitochondrial SOD significantly ameliorates mouse synaptic dysfunction and cognitive impairment in an AD mouse model expressing human form Aβ [225]. This is in agreement with Massaad and the colleagues' study [23]. These observations suggest the protective effect of reducing mitochondria-generated ROS on synaptic function in an AD mouse model and also serve as indirect evidence of the devastating role of excess ROS in mediating synaptic stress in AD-related pathological settings. Furthermore, similar protection has been reported by eliminating ROS using pharmaceutical approaches. The application of antioxidants, in particular mitochondria-targeted antioxidants such as mitoQ, SS peptides, mitochondrial SOD mimetic, and many others prevent synaptic degeneration from Aβ toxicity [226–228], which further confirms the contributing role of mitochondria-mediated oxidative stress in AD synaptic failure. Therefore, considering the physiological function of ROS in synaptic transmission and synaptic plasticity and the disruptive effect of excess ROS in particular mitochondrial ROS, it has been proposed that ROS act as a “double-edged sword” with respect to synaptic plasticity and memory consolidation [229]. To this end, how to eliminate excess mitochondrial ROS production while maintaining a physiological level of ROS would be an intriguing and critical scientific issue for the treatment of synaptic failure in AD.
To exert mitochondrial function in supporting synaptic transmission, it is a prerequisite that mitochondria accumulate at presynapses. Mitochondrial motility and dynamics are critical aspects of mitochondrial biology in neurons. Neurons are highly polarized cells and soma are the primary sites for mitochondrial generation. Newborn mitochondria are delivered to the terminal ends of neurites and anchor at synapses. During the transportation, mitochondria constantly change their morphology via the fission and fusion processes to accommodate their host cell's requirement. Indeed, deregulated mitochondrial motility and dynamics have deleterious impacts on synaptic transmission in diseases including AD [230] evidenced by the observations that suppressed neuronal mitochondrial movement and imbalanced mitochondrial fusion/fission are prominent in AD-related conditions. Zhu and the colleagues performed pioneer and comprehensive studies on alterations of neuronal mitochondrial dynamics in AD brains and Aβ-overexpressing cell lines [231]. They have found significantly deceased expression levels of optical atrophy 1, mitofusin 1 and 2, and dynamin-like protein 1 (Dlp1) with the concomitant elevation of mitochondrial fission 1 protein in the postmortem hippocampal tissues from AD patients. These results serve as solid evidence of neuronal mitochondrial fusion/fission imbalance toward increased mitochondrial fragmentation in AD brains. Importantly, they also observed increased mitochondrial accumulation in soma and lessened mitochondrial distribution in the neurites in AD hippocampal neurons, implicating the altered patterns of neuronal mitochondrial motility with increased mitochondrial anterograde transport in AD conditions [231]. These findings were soon verified in AD brains and AD animal models as well as Aβ-insulted cell lines by many other groups [42, 188, 191, 196, 232]. Notably, in an Aβ-rich environment, anterograde movement of axonal mitochondria is more vulnerable than retrograde movement [42, 191], which helps to interpret the evacuation of mitochondria from synapses and increased retrieval of axonal mitochondria to soma in AD neurons. In addition to the changes of expression levels of mitochondrial fusion and fission proteins, two other groups have found increased Dlp1 S-nitrosylation in AD [233], and Dlp1 interaction with Aβ [193], which may also play critical roles in promoting axonal mitochondrial fragmentation in AD-related environment. In our previous study, we have found the inhibition of mPTP by CypD depletion ameliorates intra-axonal calcium perturbations and oxidative stress in Aβ-treated neurons. The blockade of mPTP protects axonal mitochondrial motility and dynamics, and importantly preserves synaptic transmission from Aβ toxicity. In view of the critical role of normal axonal mitochondrial distribution and morphology in maintaining synaptic activity, the protection of synaptic transmission by mPTP inhibition has the implication to be associated with the ameliorated axonal mitochondrial motility and dynamics [191].
Conclusions and Perspectives
Synaptic deficits form the pathological basis of cognitive impairment, the cardinal sign of AD. Synaptic loss, synaptic transmission reduction, and synaptic plasticity impairment are early pathology in AD brains and exacerbate with the progress of cognitive decline in AD. Therefore, to understand the molecular basis of synaptic injury is of paramount importance for our understanding of the pathogenesis of AD as well as for the development of preventive and therapeutic strategies for the treatment. However, our knowledge is still limited for a full description of the mechanisms underlying synaptic failure in AD, given the complicated nature of the potentiation and regulation of synaptic activity and, more importantly, the many unknowns about the pathogenesis of AD.
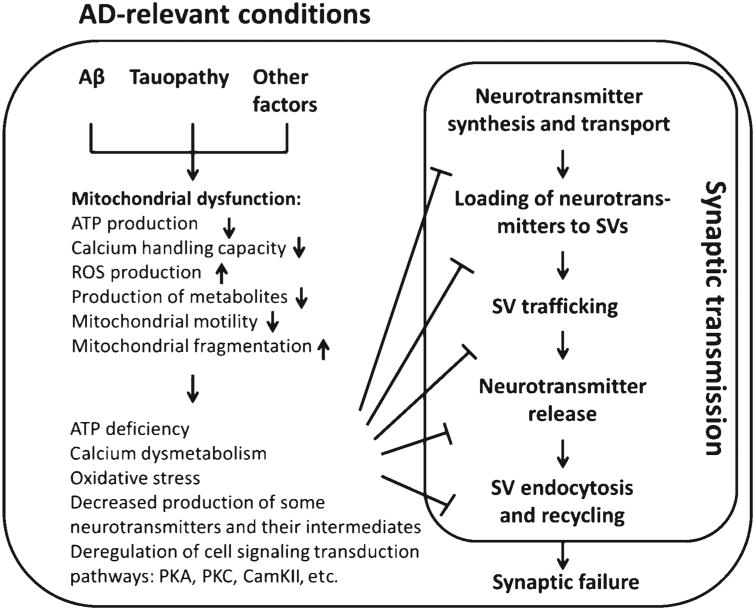
Indeed, despite the determined genetic risk factors for familial AD, the etiology of sporadic AD still remains enigmatic. Mitochondrial cascade hypothesis as an important supplementation to the prevailing Aβ cascade and tauopathy hypotheses has provided us an alternative avenue to understand the pathogenesis of AD, in particular its sporadic form [234]. Of note, the role of mitochondrial deficits in synaptic injury in AD has been firmly built on a large amount of, and still emerging, evidence. Mitochondria are actively involved in each and every step of synaptic transmission via their critical functions in providing energy, keeping intracellular redox balance, and maintaining intrasynaptic calcium homeostasis, as well as regulating key signaling transduction pathways, and synthesizing the intermediates and/or final products of several key neurotransmitters. The pivotal role of mitochondrial in synaptic transmission, the early mitochondrial dysfunction, and the concomitant mitochondrial and synaptic injury in postmortem AD brains and the AD animal models, as well as the protective effect of mitochondrial medicine [35, 227], have lent credibility to the hypothesis that mitochondrial deficits confer susceptibility to synaptic impairment in AD (Fig. 1). However, to fully address this concept, there are still many critical questions to be answered, e.g., which one happens first in AD, synaptic injury or mitochondrial dysfunction? What are the detailed mechanisms causing mitochondrial defects in AD-related conditions? Does synaptic injury in turn reinforces mitochondrial dysfunction in AD, thus forming a vicious cycle? The answers to these questions will foster a better understanding of synaptic failure and memory loss in AD. It is true that treatments targeting mitochondria are not likely to be the miraculous curetostop synaptic injury and restore cognitive function in AD, but protecting mitochondria still holds very high promise as of now, not only because of the critical role mitochondrial dysfunction plays in synaptic injury in AD, but also because of the lack of the definite cause of synaptic failure in this chronic neurodegenerative disorder.
Fig. 1.
Working hypothesis. In Alzheimer's disease (AD)-related conditions, the convergent effects of amyloid- β (Aβ), tauopathology, and other unknown factors induce mitochondrial dysfunction, thus leading to reduced ATP production, deregulated calcium homeostasis, and excess reactive oxygen species (ROS) generation, decreased production of some key neurotransmitters and their intermediates, as well as perturbed cell signaling cascades. These changes affect synaptic transmission, eventually causing synaptic failure in AD. PKA, protein kinase A; PKC, protein kinase C; CAMKII, Ca2+/ calmodulin-dependent kinase II; SV, synaptic vesicle.
Acknowledgments
This study is supported by research funding from NIH (R00AG037716, 1R01AG053588), NSFC (31271145, 81200847) and SDNSF (JQ201318). LG was supported by the Alzheimer's Association (NIRG-12-242803).
Footnotes
Authors' disclosures available online (http://j-alz.com/manuscript-disclosures/16-0702r1).
References
- 1.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Kidd PM. Alzheimer's disease, amnestic mild cognitive impairment, and age-associated memory impairment: Current understanding and progress toward integrative prevention. Altern Med Rev. 2008;13:85–115. [PubMed] [Google Scholar]
- 3.Swerdlow RH. Brain aging, Alzheimer's disease, and mitochondria. Biochim Biophys Acta. 2011;1812:1630–1639. doi: 10.1016/j.bbadis.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 5.Landon M, Kidd M. Amyloid in Alzheimer's disease. Biochem Soc Trans. 1989;17:69–72. doi: 10.1042/bst0170069. [DOI] [PubMed] [Google Scholar]
- 6.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 9.Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lust-bader JW, Xu HW, Stern D, McKhann G, Yan SD. Mitochondrial Abeta: A potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 10.Braak H, Del Trecidi K. Neuroanatomy and pathology of sporadic Alzheimer's disease. Adv Anat Embryol Cell Biol. 2015;215:1–162. [PubMed] [Google Scholar]
- 11.Alafuzoff I, Arzberger T, Al-Sarraj S, Bodi I, Bogdanovic N, Braak H, Bugiani O, Del-Tredici K, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Ince P, Kamphorst W, King A, Korkolopoulou P, Kovacs GG, Larionov S, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Seilhean D, Tagliavini F, Stadelmann C, Streichenberger N, Thal DR, Wharton SB, Kretzschmar H. Staging of neurofibrillary pathology in Alzheimer's disease: A study of the BrainNet Europe Consortium. Brain Pathol. 2008;18:484–496. doi: 10.1111/j.1750-3639.2008.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yen SH, Liu WK, Hall FL, Yan SD, Stern D, Dickson DW. Alzheimer neurofibrillary lesions: Molecular nature and potential roles of different components. Neurobiol Aging. 1995;16:381–387. doi: 10.1016/0197-4580(95)00022-7. [DOI] [PubMed] [Google Scholar]
- 13.Davies CA, Mann DM, Sumpter PQ, Yates PO. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer's disease. J Neurol Sci. 1987;78:151–164. doi: 10.1016/0022-510x(87)90057-8. [DOI] [PubMed] [Google Scholar]
- 14.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 15.Dickson DW, Crystal HA, Bevona C, Honer W, Vincent I, Davies P. Correlations of synaptic and pathological markers with cognition of the elderly. Neurobiol Aging. 1995;16:285–298. doi: 10.1016/0197-4580(95)00013-5. discussion 298-304. [DOI] [PubMed] [Google Scholar]
- 16.Sze CI, Troncoso JC, Kawas C, Mouton P, Price DL, Martin LJ. Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:933–944. doi: 10.1097/00005072-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Braak H, Del Tredici K, Schultz C, Braak E. Vulnerability of select neuronal types to Alzheimer's disease. Ann N Y Acad Sci. 2000;924:53–61. doi: 10.1111/j.1749-6632.2000.tb05560.x. [DOI] [PubMed] [Google Scholar]
- 18.Arendt T, Bigl V, Arendt A, Tennstedt A. Loss of neurons in the nucleus basalis of Meynert in Alzheimer's disease, paralysis agitans and Korsakoff's Disease. Acta Neuropathol. 1983;61:101–108. doi: 10.1007/BF00697388. [DOI] [PubMed] [Google Scholar]
- 19.Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Jr, Morris JC. Altered expression of synaptic proteins occurs early during progression of Alzheimer's disease. Neurology. 2001;56:127–129. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- 20.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 21.Sheng M, Sabatini BL, Sudhof TC. Synapses and Alzheimer's disease. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma T, Klann E. Amyloid beta: Linking synaptic plasticity failure to memory disruption in Alzheimer's disease. J Neurochem. 2012;120(Suppl 1):140–148. doi: 10.1111/j.1471-4159.2011.07506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massaad CA, Washington TM, Pautler RG, Klann E. Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106:13576–13581. doi: 10.1073/pnas.0902714106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 25.Rowan MJ, Klyubin I, Wang Q, Anwyl R. Synaptic plasticity disruption by amyloid beta protein: Modulation by potential Alzheimer's disease modifying therapies. Biochem Soc Trans. 2005;33:563–567. doi: 10.1042/BST0330563. [DOI] [PubMed] [Google Scholar]
- 26.Bennett MV. Electrical synapses, a personal perspective (or history) Brain Res Brain Res Rev. 2000;32:16–28. doi: 10.1016/s0165-0173(99)00065-x. [DOI] [PubMed] [Google Scholar]
- 27.Vos M, Lauwers E, Verstreken P. Synaptic mitochondria in synaptic transmission and organization of vesicle pools in health and disease. Front Synaptic Neurosci. 2010;2:139. doi: 10.3389/fnsyn.2010.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Storozhuk MV, Ivanova SY, Balaban PM, Kostyuk PG. Possible role of mitochondria in posttetanic potentiation of GABAergic synaptic transmission in rat neocortical cell cultures. Synapse. 2005;58:45–52. doi: 10.1002/syn.20186. [DOI] [PubMed] [Google Scholar]
- 29.Csordas G, Thomas AP, Hajnoczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. 1999;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeanneteau F, Arango-Lievano M. Linking mitochondria to synapses: New insights for stress-related neuropsychiatric disorders. Neural Plast. 2016;2016:3985063. doi: 10.1155/2016/3985063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okazawa H, Ikawa M, Tsujikawa T, Kiyono Y, Yoneda M. Brain imaging for oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Q J Nucl Med Mol Imaging. 2014;58:387–397. [PubMed] [Google Scholar]
- 32.Reddy PH. Misfolded proteins, mitochondrial dysfunction, and neurodegenerative diseases. Biochim Biophys Acta. 2014;1842:1167. doi: 10.1016/j.bbadis.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy PH, Manczak M, Mao P, Calkins MJ, Reddy AP, Shirendeb U. Amyloid-beta and mitochondria in aging and Alzheimer's disease: Implications for synaptic damage and cognitive decline. J Alzheimers Dis. 2010;20(Suppl 2):S499–512. doi: 10.3233/JAD-2010-100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du H, Guo L, Yan SS. Synaptic mitochondrial pathology in Alzheimer's disease. Antioxid Redox Signal. 2012;16:1467–1475. doi: 10.1089/ars.2011.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du H, Yan SS. Mitochondrial medicine for neurode-generative diseases. Int J Biochem Cell Biol. 2010;42:560–572. doi: 10.1016/j.biocel.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddy PH. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromolecular Med. 2008;10:291–315. doi: 10.1007/s12017-008-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrison JH, Baxter MG. The ageing cortical synapse: Hallmarks and implications for cognitive decline. Nat Rev Neurosci. 2012;13:240–250. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu W, Lu B. Synapses and dendritic spines as pathogenic targets in Alzheimer's disease. Neural Plast. 2012;2012:247150. doi: 10.1155/2012/247150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koffie RM, Hyman BT, Spires-Jones TL. Alzheimer's disease: Synapses gone cold. Mol Neurodegener. 2011;6:63. doi: 10.1186/1750-1326-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lippa CF, Hamos JE, Pulaski-Salo D, DeGennaro LJ, Drachman DA. Alzheimer's disease and aging: Effects on perforant pathway perikarya and synapses. Neurobiol Aging. 1992;13:405–411. doi: 10.1016/0197-4580(92)90115-e. [DOI] [PubMed] [Google Scholar]
- 41.Adams IM. Structural plasticity of synapses in Alzheimer's disease. Mol Neurobiol. 1991;5:411–419. doi: 10.1007/BF02935562. [DOI] [PubMed] [Google Scholar]
- 42.Calkins MJ, Reddy PH. Amyloid beta impairs mitochondrial anterograde transport and degenerates synapses in Alzheimer's disease neurons. Biochim Biophys Acta. 2011;1812:507–513. doi: 10.1016/j.bbadis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddy PH. Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria, and synaptic deprivation in Alzheimer's disease. Brain Res. 2011;1415:136–148. doi: 10.1016/j.brainres.2011.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: Implications for cognitive decline in aging and Alzheimer's disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rangel-Gomez M, Meeter M. Neurotransmitters and novelty: A systematic review. J Psychopharmacol. 2016;30:3–12. doi: 10.1177/0269881115612238. [DOI] [PubMed] [Google Scholar]
- 46.de Blas A, Mahler HR. Studies on nicotinic acetylcholine receptors in mammalian brain VI. Isolation of a membrane fraction enriched in receptor function for different neurotransmitters. Biochem Biophys Res Commun. 1976;72:24–32. doi: 10.1016/0006-291x(76)90955-4. [DOI] [PubMed] [Google Scholar]
- 47.Melancon BJ, Tarr JC, Panarese JD, Wood MR, Lindsley CW. Allosteric modulation of the M1 muscarinic acetylcholine receptor: Improving cognition and a potential treatment for schizophrenia and Alzheimer's disease. Drug Discov Today. 2013;18:1185–1199. doi: 10.1016/j.drudis.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bozzi R, Zubiani A. Use of acetylcholine administered intravenously refracta dosi in the treatment of presenile and senile psychoses. Riv Sper Freniatr Med Leg Alien Ment. 1952;76:263–268. [PubMed] [Google Scholar]
- 49.Spillane JA, Goodhart MJ, White P, Bowen DM, Davison AN. Choline in Alzheimer's disease. Lancet. 1977;2:826–827. doi: 10.1016/s0140-6736(77)90764-4. [DOI] [PubMed] [Google Scholar]
- 50.Renvoize EB, Jerram T. Choline in Alzheimer's disease. N Engl J Med. 1979;301:330. doi: 10.1056/NEJM197908093010614. [DOI] [PubMed] [Google Scholar]
- 51.Smith CM, Swash M, Exton-Smith AN, Phillips MJ, Over-stall PW, Piper ME, Bailey MR. Choline therapy in Alzheimer's disease. Lancet. 1978;2:318. doi: 10.1016/s0140-6736(78)91721-x. [DOI] [PubMed] [Google Scholar]
- 52.Ehrenstein G, Galdzicki Z, Lange GD. The choline-leakage hypothesis for the loss of acetylcholine in Alzheimer's disease. Biophys J. 1997;73:1276–1280. doi: 10.1016/S0006-3495(97)78160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar V, Giacobini E, Markwell S. CSF choline and acetylcholinesterase in early-onset vs. late-onset Alzheimer's disease patients. Acta Neurol Scand. 1989;80:461–466. doi: 10.1111/j.1600-0404.1989.tb03910.x. [DOI] [PubMed] [Google Scholar]
- 54.Palmer AM, Gershon S. Is the neuronal basis of Alzheimer's disease cholinergic or glutamatergic? FASEB J. 1990;4:2745–2752. doi: 10.1096/fasebj.4.10.2165009. [DOI] [PubMed] [Google Scholar]
- 55.Babic T. The cholinergic hypothesis of Alzheimer's disease: A review of progress. J Neurol Neurosurg Psychiatry. 1999;67:558. doi: 10.1136/jnnp.67.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer's disease: A review of progress. J Neurol Neurosurg Psychiatry. 1999;66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res. 2011;221:555–563. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 58.Strac DS, Muck-Seler D, Pivac N. Neurotransmitter measures in the cerebrospinal fluid of patients with Alzheimer's disease: A review. Psychiatr Danub. 2015;27:14–24. [PubMed] [Google Scholar]
- 59.Lombardo S, Maskos U. Role of the nicotinic acetylcholine receptor in Alzheimer's disease pathology and treatment. Neuropharmacology. 2015;96:255–262. doi: 10.1016/j.neuropharm.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 60.Jiang S, Li Y, Zhang C, Zhao Y, Bu G, Xu H, Zhang YW. M1 muscarinic acetylcholine receptor in Alzheimer's disease. Neurosci Bull. 2014;30:295–307. doi: 10.1007/s12264-013-1406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kantarci K, Weigand SD, Petersen RC, Boeve BF, Knopman DS, Gunter J, Reyes D, Shiung M, O'Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Jack CR., Jr Longitudinal 1H MRS changes in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2007;28:1330–1339. doi: 10.1016/j.neurobiolaging.2006.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohs RC, Davis BM, Greenwald BS, Mathe AA, Johns CA, Horvath TB, Davis KL. Clinical studies of the cholinergic deficit in Alzheimer's disease. II. Psychopharmacologic studies. J Am Geriatr Soc. 1985;33:749–757. doi: 10.1111/j.1532-5415.1985.tb04185.x. [DOI] [PubMed] [Google Scholar]
- 63.Davis BM, Mohs RC, Greenwald BS, Mathe AA, Johns CA, Horvath TB, Davis KL. Clinical studies of the cholinergic deficit in Alzheimer's disease. I. Neurochemical and neuroendocrine studies. J Am Geriatr Soc. 1985;33:741–748. doi: 10.1111/j.1532-5415.1985.tb04184.x. [DOI] [PubMed] [Google Scholar]
- 64.Tohgi H, Abe T, Hashiguchi K, Saheki M, Takahashi S. Remarkable reduction in acetylcholine concentration in the cerebrospinal fluid from patients with Alzheimer type dementia. Neurosci Lett. 1994;177:139–142. doi: 10.1016/0304-3940(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 65.Hashimoto M, Kazui H, Matsumoto K, Nakano Y, Yasuda M, Mori E. Does donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer's disease? Am J Psychiatry. 2005;162:676–682. doi: 10.1176/appi.ajp.162.4.676. [DOI] [PubMed] [Google Scholar]
- 66.Giacobini E. Do cholinesterase inhibitors have disease-modifying effects in Alzheimer's disease? CNS Drugs. 2001;15:85–91. doi: 10.2165/00023210-200115020-00001. [DOI] [PubMed] [Google Scholar]
- 67.Gruol DL, Netzeband JG, Parsons KL. Ca2+ signaling pathways linked to glutamate receptor activation in the somatic and dendritic regions of cultured cerebellar purkinje neurons. J Neurophysiol. 1996;76:3325–3340. doi: 10.1152/jn.1996.76.5.3325. [DOI] [PubMed] [Google Scholar]
- 68.Parsons CG, Danysz W, Quack G. Glutamate in CNS disorders as a target for drug development: An update. Drug News Perspect. 1998;11:523–569. doi: 10.1358/dnp.1998.11.9.863689. [DOI] [PubMed] [Google Scholar]
- 69.Liu M, Lewis LD, Shi R, Brown EN, Xu W. Differential requirement for NMDAR activity in SAP97beta-mediated regulation of the number and strength of glutamatergic AMPAR-containing synapses. J Neurophysiol. 2014;111:648–658. doi: 10.1152/jn.00262.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Faleiro LJ, Jones S, Kauer JA. Rapid AMPAR/ NMDAR response to amphetamine: A detectable increase in AMPAR/NMDAR ratios in the ventral tegmental area is detectable after amphetamine injection. Ann N Y Acad Sci. 2003;1003:391–394. doi: 10.1196/annals.1300.032. [DOI] [PubMed] [Google Scholar]
- 71.Butterfield DA, Pocernich CB. The glutamatergic system and Alzheimer's disease: Therapeutic implications. CNS Drugs. 2003;17:641–652. doi: 10.2165/00023210-200317090-00004. [DOI] [PubMed] [Google Scholar]
- 72.Cacabelos R, Takeda M, Winblad B. The glutamatergic system and neurodegeneration in dementia: Preventive strategies in Alzheimer's disease. Int J Geriatr Psychiatry. 1999;14:3–47. doi: 10.1002/(sici)1099-1166(199901)14:1<3::aid-gps897>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 73.Fayed N, Modrego PJ, Rojas-Salinas G, Aguilar K. Brain glutamate levels are decreased in Alzheimer's disease: A magnetic resonance spectroscopy study. Am J Alzheimers Dis Other Demen. 2011;26:450–456. doi: 10.1177/1533317511421780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ernst T, Chang L, Melchor R, Mehringer CM. Frontotemporal dementia and early Alzheimer disease: Differentiation with frontal lobe H-1 MR spectroscopy. Radiology. 1997;203:829–836. doi: 10.1148/radiology.203.3.9169712. [DOI] [PubMed] [Google Scholar]
- 75.Smith CC, Bowen DM, Francis PT, Snowden JS, Neary D. Putative amino acid transmitters in lumbar cerebrospinal fluid of patients with histologically verified Alzheimer's dementia. J Neurol Neurosurg Psychiatry. 1985;48:469–471. doi: 10.1136/jnnp.48.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jimenez-Jimenez FJ, Molina JA, Gomez P, Vargas C, de Bustos F, Benito-Leon J, Tallon-Barranco A, Orti-Pareja M, Gasalla T, Arenas J. Neurotransmitter amino acids in cerebrospinal fluid of patients with Alzheimer's disease. J Neural Transm (Vienna) 1998;105:269–277. doi: 10.1007/s007020050056. [DOI] [PubMed] [Google Scholar]
- 77.Jimenez-Jimenez FJ, Molina JA, Vargas C, Gomez P, Navarro JA, Benito-Leon J, Orti-Pareja M, Gasalla T, Cisneros E, Arenas J. Neurotransmitter amino acids in cerebrospinal fluid of patients with Parkinson's disease. J Neurol Sci. 1996;141:39–44. doi: 10.1016/0022-510x(96)00115-3. [DOI] [PubMed] [Google Scholar]
- 78.Kaiser E, Schoenknecht P, Kassner S, Hildebrandt W, Kinscherf R, Schroeder J. Cerebrospinal fluid concentrations of functionally important amino acids and metabolic compounds in patients with mild cognitive impairment and Alzheimer's disease. Neurodegener Dis. 2010;7:251–259. doi: 10.1159/000287953. [DOI] [PubMed] [Google Scholar]
- 79.Ferrarese C, Aliprandi A, Tremolizzo L, Stanzani L, De Micheli A, Dolara A, Frattola L. Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology. 2001;57:671–675. doi: 10.1212/wnl.57.4.671. [DOI] [PubMed] [Google Scholar]
- 80.Olivares D, Deshpande VK, Shi Y, Lahiri DK, Greig NH, Rogers JT, Huang X. N-methyl D-aspartate (NMDA) receptor antagonists and memantine treatment for Alzheimer's disease, vascular dementia and Parkinson's disease. Curr Alzheimer Res. 2012;9:746–758. doi: 10.2174/156720512801322564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gray CW, Patel AJ. Neurodegeneration mediated by glutamate and beta-amyloid peptide: A comparison and possible interaction. Brain Res. 1995;691:169–179. doi: 10.1016/0006-8993(95)00669-h. [DOI] [PubMed] [Google Scholar]
- 82.Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mattson MP, Pedersen WA, Duan W, Culmsee C, Camandola S. Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer's and Parkinson's diseases. Ann N Y Acad Sci. 1999;893:154–175. doi: 10.1111/j.1749-6632.1999.tb07824.x. [DOI] [PubMed] [Google Scholar]
- 84.Parameshwaran K, Dhanasekaran M, Suppiramaniam V. Amyloid beta peptides and glutamatergic synaptic dysregulation. Exp Neurol. 2008;210:7–13. doi: 10.1016/j.expneurol.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 85.Kravitz E, Gaisler-Salomon I, Biegon A. Hippocampal glutamate NMDA receptor loss tracks progression in Alzheimer's disease: Quantitative autoradiography in postmortem human brain. PLoS One. 2013;8:e81244. doi: 10.1371/journal.pone.0081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kurup P, Zhang Y, Xu J, Venkitaramani DV, Haroutunian V, Greengard P, Nairn AC, Lombroso PJ. Abeta-mediated NMDA receptor endocytosis in Alzheimer's disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci. 2010;30:5948–5957. doi: 10.1523/JNEUROSCI.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jacob CP, Koutsilieri E, Bartl J, Neuen-Jacob E, Arzberger T, Zander N, Ravid R, Roggendorf W, Riederer P, Grun-blatt E. Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer's disease. J Alzheimers Dis. 2007;11:97–116. doi: 10.3233/jad-2007-11113. [DOI] [PubMed] [Google Scholar]
- 88.Hu S, Yu X, Chen S, Clay E, Toumi M, Milea D. Memantine for treatment of moderate or severe Alzheimer's disease patients in urban China: Clinical and economic outcomes from a health economic model. Expert Rev Pharmacoecon Outcomes Res. 2015;15:565–578. doi: 10.1586/14737167.2015.1065734. [DOI] [PubMed] [Google Scholar]
- 89.Wilkinson D, Wirth Y, Goebel C. Memantine in patients with moderate to severe Alzheimer's disease: Meta-analyses using realistic definitions of response. Dement Geriatr Cogn Disord. 2014;37:71–85. doi: 10.1159/000353801. [DOI] [PubMed] [Google Scholar]
- 90.Nakamura Y, Kitamura S, Homma A, Shiosakai K, Matsui D. Efficacy and safety of memantine in patients with moderate-to-severe Alzheimer's disease: Results of a pooled analysis of two randomized, double-blind, placebo-controlled trials in Japan. Expert Opin Pharmacother. 2014;15:913–925. doi: 10.1517/14656566.2014.902446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mohr E, Bruno G, Foster N, Gillespie M, Cox C, Hare TA, Tamminga C, Fedio P, Chase TN. GABA-agonist therapy for Alzheimer's disease. Clin Neuropharmacol. 1986;9:257–263. doi: 10.1097/00002826-198606000-00004. [DOI] [PubMed] [Google Scholar]
- 92.Solas M, Puerta E, Ramirez MJ. Treatment options in Alzheimer's disease: The GABA Story. Curr Pharm Des. 2015;21:4960–4971. doi: 10.2174/1381612821666150914121149. [DOI] [PubMed] [Google Scholar]
- 93.German DC, Manaye KF, White CL, 3rd, Woodward DJ, McIntire DD, Smith WK, Kalaria RN, Mann DM. Disease-specific patterns of locus coeruleus cell loss. Ann Neurol. 1992;32:667–676. doi: 10.1002/ana.410320510. [DOI] [PubMed] [Google Scholar]
- 94.Yates CM, Simpson J, Gordon A, Maloney AF, Allison Y, Ritchie IM, Urquhart A. Catecholamines and cholinergic enzymes in pre-senile and senile Alzheimer-type dementia and Down's syndrome. Brain Res. 1983;280:119–126. doi: 10.1016/0006-8993(83)91179-4. [DOI] [PubMed] [Google Scholar]
- 95.Scullion GA, Kendall DA, Marsden CA, Sunter D, Pardon MC. Chronic treatment with the alpha2-adrenoceptor antagonist fluparoxan prevents age-related deficits in spatial working memory in APPxPS1 trans-genic mice without altering beta-amyloid plaque load or astrocytosis. Neuropharmacology. 2011;60:223–234. doi: 10.1016/j.neuropharm.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 96.Gibbs ME, Maksel D, Gibbs Z, Hou X, Summers RJ, Small DH. Memory loss caused by beta-amyloid protein is rescued by a beta(3)-adrenoceptor agonist. Neurobiol Aging. 2010;31:614–624. doi: 10.1016/j.neurobiolaging.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 97.Yates CM, Allison Y, Simpson J, Maloney AF, Gordon A. Dopamine in Alzheimer's disease and senile dementia. Lancet. 1979;2:851–852. doi: 10.1016/s0140-6736(79)92202-5. [DOI] [PubMed] [Google Scholar]
- 98.Martorana A, Koch G. Is dopamine involved in Alzheimer's disease? Front Aging Neurosci. 2014;6:252. doi: 10.3389/fnagi.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Murray AM, Weihmueller FB, Marshall JF, Hurtig HI, Gottleib GL, Joyce JN. Damage to dopamine systems differs between Parkinson's disease and Alzheimer's disease with parkinsonism. Ann Neurol. 1995;37:300–312. doi: 10.1002/ana.410370306. [DOI] [PubMed] [Google Scholar]
- 100.Joyce JN, Murray AM, Hurtig HI, Gottlieb GL, Trojanowski JQ. Loss of dopamine D2 receptors in Alzheimer's disease with parkinsonism but not Parkinson's or Alzheimer's disease. Neuropsychopharmacology. 1998;19:472–480. doi: 10.1016/S0893-133X(98)00044-X. [DOI] [PubMed] [Google Scholar]
- 101.Martorana A, Mori F, Esposito Z, Kusayanagi H, Monteleone F, Codeca C, Sancesario G, Bernardi G, Koch G. Dopamine modulates cholinergic cortical excitability in Alzheimer's disease patients. Neuropsychopharmacology. 2009;34:2323–2328. doi: 10.1038/npp.2009.60. [DOI] [PubMed] [Google Scholar]
- 102.Gelao B, Fazio L, Selvaggi P, Di Giorgio A, Taurisano P, Quarto T, Romano R, Porcelli A, Mancini M, Masellis R, Ursini G, DeSimeis G, Caforio G, Ferranti L, LoBianco L, Rampino A, Todarello O, Popolizio T, Blasi G, Bertolino A. DRD2 genotype predicts prefrontal activity during working memory after stimulation of D2 receptors with bromocriptine. Psychopharmacology (Berl) 2014;231:2361–2370. doi: 10.1007/s00213-013-3398-9. [DOI] [PubMed] [Google Scholar]
- 103.Lewis S. Learning and memory: Dopamine boosts ageing memories. Nat Rev Neurosci. 2012;13:812. doi: 10.1038/nrn3385. [DOI] [PubMed] [Google Scholar]
- 104.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 105.Baloyannis SJ, Manolidis SL, Manolidis LS. Synaptic alterations in the vestibulocerebellar system in Alzheimer's disease–a Golgi and electron microscope study. Acta Otolaryngol. 2000;120:247–250. doi: 10.1080/000164800750001026. [DOI] [PubMed] [Google Scholar]
- 106.Sze CI, Bi H, Kleinschmidt-DeMasters BK, Filley CM, Martin LJ. Selective regional loss of exocytotic presynaptic vesicle proteins inAlzheimer's disease brains. J Neurol Sci. 2000;175:81–90. doi: 10.1016/s0022-510x(00)00285-9. [DOI] [PubMed] [Google Scholar]
- 107.Lassmann H, Weiler R, Fischer P, Bancher C, Jellinger K, Floor E, Danielczyk W, Seitelberger F, Winkler H. Synaptic pathology in Alzheimer's disease: Immunological data for markers of synaptic and large dense-core vesicles. Neuroscience. 1992;46:1–8. doi: 10.1016/0306-4522(92)90003-k. [DOI] [PubMed] [Google Scholar]
- 108.Masliah E, Honer WG, Mallory M, Voigt M, Kushner P, Hansen L, Terry R. Topographical distribution of synaptic-associated proteins in the neuritic plaques of Alzheimer's disease hippocampus. Acta Neuropathol. 1994;87:135–142. doi: 10.1007/BF00296182. [DOI] [PubMed] [Google Scholar]
- 109.Masliah E, Terry RD, Alford M, DeTeresa R, Hansen LA. Cortical and subcortical patterns of synaptophysin-like immunoreactivity in Alzheimer's disease. AmJ Pathol. 1991;138:235–246. [PMC free article] [PubMed] [Google Scholar]
- 110.Masliah E, Terry RD, DeTeresa RM, Hansen LA. Immunohistochemical quantification of the synapse-related protein synaptophysin in Alzheimer disease. Neurosci Lett. 1989;103:234–239. doi: 10.1016/0304-3940(89)90582-x. [DOI] [PubMed] [Google Scholar]
- 111.Honer WG. Pathology of presynaptic proteins in Alzheimer's disease: More than simple loss of terminals. Neurobiol Aging. 2003;24:1047–1062. doi: 10.1016/j.neurobiolaging.2003.04.005. [DOI] [PubMed] [Google Scholar]
- 112.Ghosh A, Giese KP. Calcium/calmodulin-dependent kinase II and Alzheimer's disease. Mol Brain. 2015;8:78. doi: 10.1186/s13041-015-0166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang GR, Cheng XR, Zhou WX, Zhang YX. Age-related expression of calcium/calmodulin-dependent protein kinase II A in the hippocampus and cerebral cortex of senescence accelerated mouse prone/8 mice is modulated by anti-Alzheimer's disease drugs. Neuroscience. 2009;159:308–315. doi: 10.1016/j.neuroscience.2008.06.068. [DOI] [PubMed] [Google Scholar]
- 114.Wang YJ, Chen GH, Hu XY, Lu YP, Zhou JN, Liu RY. The expression of calcium/calmodulin-dependent protein kinase II-alpha in the hippocampus of patients with Alzheimer's disease and its links with AD-related pathology. Brain Res. 2005;1031:101–108. doi: 10.1016/j.brainres.2004.10.061. [DOI] [PubMed] [Google Scholar]
- 115.Simonian NA, Elvhage T, Czernik AJ, Greengard P, Hyman BT. Calcium/calmodulin-dependent protein kinase II immunostaining is preserved in Alzheimer's disease hippocampal neurons. Brain Res. 1994;657:294–299. doi: 10.1016/0006-8993(94)90979-2. [DOI] [PubMed] [Google Scholar]
- 116.Kim SH, Nairn AC, Cairns N, Lubec G. Decreased levels of ARPP-19 and PKA in brains of Down syndrome and Alzheimer's disease. J Neural Transm Suppl. 2001:263–272. doi: 10.1007/978-3-7091-6262-0_21. [DOI] [PubMed] [Google Scholar]
- 117.Blanchard BJ, devi Raghunandan R, Roder HM, Ingram VM. Hyperphosphorylation of human TAU by brain kinase PK40erk beyond phosphorylation by cAMP-dependent PKA: Relation to Alzheimer's disease. Biochem Biophys Res Commun. 1994;200:187–194. doi: 10.1006/bbrc.1994.1432. [DOI] [PubMed] [Google Scholar]
- 118.Talman V, Pascale A, Jantti M, Amadio M, Tuominen RK. PKC activation as a potential therapeutic strategy in Alzheimer's disease: Is there a role for ELAV-like proteins? Basic Clin Pharmacol Toxicol. 2016;119:149–160. doi: 10.1111/bcpt.12581. [DOI] [PubMed] [Google Scholar]
- 119.Hongpaisan J, Sun MK, Alkon DL. PKC epsilon activation prevents synaptic loss, Abeta elevation, and cognitive deficits in Alzheimer's disease transgenic mice. J Neurosci. 2011;31:630–643. doi: 10.1523/JNEUROSCI.5209-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kamat PK, Kalani A, Rai S, Swarnkar S, Tota S, Nath C, Tyagi N. Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer's disease: Understanding the therapeutics strategies. Mol Neurobiol. 2016;53:648–661. doi: 10.1007/s12035-014-9053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Parodi J, Sepulveda FJ, Roa J, Opazo C, Inestrosa NC, Aguayo LG. Beta-amyloid causes depletion of synaptic vesicles leading to neurotransmission failure. J Biol Chem. 2010;285:2506–2514. doi: 10.1074/jbc.M109.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Erez H, Shemesh OA, Spira ME. Rescue of tau-induced synaptic transmission pathology by paclitaxel. Front Cell Neurosci. 2014;8:34. doi: 10.3389/fncel.2014.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miller WL. Steroid hormone synthesis in mitochondria. Mol Cell Endocrinol. 2013;379:62–73. doi: 10.1016/j.mce.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 124.Fujiwara K, Hosaka H, Matsuda M, Okamura-Ikeda K, Motokawa Y, Suzuki M, Nakagawa A, Taniguchi H. Crystal structure of bovine lipoyltransferase in complex with lipoyl-AMP. J Mol Biol. 2007;371:222–234. doi: 10.1016/j.jmb.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 125.Howard CF., Jr Control of fatty acid synthesis in mitochondrial membranes. Biochim Biophys Acta. 1968;164:448–450. doi: 10.1016/0005-2760(68)90176-8. [DOI] [PubMed] [Google Scholar]
- 126.Donaldson WE, Wit-Peeters EM, Scholte HR. Fatty acid synthesis in rat liver: Relative contributions of the mitochondrial, microsomal and non-particulate systems. Biochim Biophys Acta. 1970;202:35–42. doi: 10.1016/0005-2760(70)90215-8. [DOI] [PubMed] [Google Scholar]
- 127.Hiltunen JK, Schonauer MS, Autio KJ, Mittelmeier TM, Kastaniotis AJ, Dieckmann CL. Mitochondrial fatty acid synthesis type II: More than just fatty acids. J Biol Chem. 2009;284:9011–9015. doi: 10.1074/jbc.R800068200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 129.Tatsumi H, Tsuji S, Anglade P, Motelica-Heino I, Soeda H, Katayama Y. Synthesis, storage and release of acetylcholine at and from growth cones of rat central cholinergic neurons in culture. Neurosci Lett. 1995;202:25–28. doi: 10.1016/0304-3940(95)12187-0. [DOI] [PubMed] [Google Scholar]
- 130.Collier B. Choline analogues: Their use in studies of acetylcholine synthesis, storage, and release. Can J Physiol Pharmacol. 1986;64:341–346. doi: 10.1139/y86-056. [DOI] [PubMed] [Google Scholar]
- 131.Hancox AJ, Scrimshire DA. Refinement and validation of a model describing synthesis storage and release of acetylcholine at the neuromuscular junction. J Biomed Eng. 1982;4:206–212. doi: 10.1016/0141-5425(82)90004-8. [DOI] [PubMed] [Google Scholar]
- 132.Hancox AJ, Scrimshire DA. A proposed model for the synthesis, storage and release of acetylcholine at the neuromuscular junction. J Biomed Eng. 1981;3:183–195. doi: 10.1016/0141-5425(81)90068-6. [DOI] [PubMed] [Google Scholar]
- 133.Gundersen CB. The effects of botulinum toxin on the synthesis, storage and release of acetylcholine. Prog Neurobiol. 1980;14:99–119. doi: 10.1016/0301-0082(80)90019-2. [DOI] [PubMed] [Google Scholar]
- 134.Greene LA, Rein G. Synthesis, storage and release of acetylcholine by a noradrenergic pheochromocytoma cell line. Nature. 1977;268:349–351. doi: 10.1038/268349a0. [DOI] [PubMed] [Google Scholar]
- 135.Walker RJ, Ramage AG, Woodruff GN. An electrophysiological study of the storage, synthesis and release of acetylcholine from an identifiable inhibitory synapse in the brain of Helix aspersa. Neuropharmacology. 1974;13:29–38. doi: 10.1016/0028-3908(74)90005-7. [DOI] [PubMed] [Google Scholar]
- 136.Potter LT. Synthesis, storage and release of [14C]acetylcholine in isolated rat diaphragm muscles. J Physiol. 1970;206:145–166. doi: 10.1113/jphysiol.1970.sp009003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Parsons SM, Bahr BA, Gracz LM, Kaufman R, Kornreich WD, Nilsson L, Rogers GA. Acetylcholine transport: Fundamental properties and effects of pharmacologic agents. Ann N Y Acad Sci. 1987;493:220–233. doi: 10.1111/j.1749-6632.1987.tb27203.x. [DOI] [PubMed] [Google Scholar]
- 138.Varoqui H, Erickson JD. Active transport of acetylcholine by the human vesicular acetylcholine transporter. J Biol Chem. 1996;271:27229–27232. doi: 10.1074/jbc.271.44.27229. [DOI] [PubMed] [Google Scholar]
- 139.Yu AC, Drejer J, Hertz L, Schousboe A. Pyruvate carboxylase activity in primary cultures of astrocytes and neurons. J Neurochem. 1983;41:1484–1487. doi: 10.1111/j.1471-4159.1983.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 140.Shank RP, Bennett GS, Freytag SO, Campbell GL. Pyruvate carboxylase: An astrocyte-specific enzyme implicated in the replenishment of amino acid neurotransmitter pools. Brain Res. 1985;329:364–367. doi: 10.1016/0006-8993(85)90552-9. [DOI] [PubMed] [Google Scholar]
- 141.Cesar M, Hamprecht B. Immunocytochemical examination of neural rat and mouse primary cultures using monoclonal antibodies raised against pyruvate carboxylase. J Neurochem. 1995;64:2312–2318. doi: 10.1046/j.1471-4159.1995.64052312.x. [DOI] [PubMed] [Google Scholar]
- 142.Waagepetersen HS, Qu H, Schousboe A, Sonnewald U. Elucidation of the quantitative significance of pyruvate carboxylation in cultured cerebellar neurons and astrocytes. J Neurosci Res. 2001;66:763–770. doi: 10.1002/jnr.10061. [DOI] [PubMed] [Google Scholar]
- 143.Patel MS. The effect of ketone bodies on pyruvate carboxylation by rat brain mitochondria. J Neurochem. 1974;23:865–867. doi: 10.1111/j.1471-4159.1974.tb04415.x. [DOI] [PubMed] [Google Scholar]
- 144.Pardo B, Contreras L, Satrustegui J. De novo synthesis of glial glutamate and glutamine in young mice requires aspartate provided by the neuronal mitochondrial aspartate-glutamate carrier Aralar/AGC1. Front Endocrinol (Lausanne) 2013;4:149. doi: 10.3389/fendo.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Grewer C, Gameiro A, Zhang Z, Tao Z, Braams S, Rauen T. Glutamate forward and reverse transport: From molecular mechanism to transporter-mediated release after ischemia. IUBMB Life. 2008;60:609–619. doi: 10.1002/iub.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nagatsu T, Levitt M, Udenfriend S. Tyrosine hydroxylase. The initial step in norepinephrine biosynthesis. J Biol Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- 147.Laduron P. Biosynthesis of catecholamines: New concepts. Actual Pharmacol (Paris) 1974;27:45–67. [PubMed] [Google Scholar]
- 148.Gasnier B. The loading of neurotransmitters into synaptic vesicles. Biochimie. 2000;82:327–337. doi: 10.1016/s0300-9084(00)00221-2. [DOI] [PubMed] [Google Scholar]
- 149.Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys. 2011;508:1–12. doi: 10.1016/j.abb.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Buddhala C, Hsu CC, Wu JY. A novel mechanism for GABA synthesis and packaging into synaptic vesicles. Neurochem Int. 2009;55:9–12. doi: 10.1016/j.neuint.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 151.Van der Kloot W. Loading and recycling of synaptic vesicles in the Torpedo electric organ and the vertebrate neuromuscular junction. Prog Neurobiol. 2003;71:269–303. doi: 10.1016/j.pneurobio.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 152.Rooke N, Li DJ, Li J, Keung WM. The mitochondrial monoamine oxidase-aldehyde dehydrogenase pathway: A potential site of action of daidzin. J Med Chem. 2000;43:4169–4179. doi: 10.1021/jm990614i. [DOI] [PubMed] [Google Scholar]
- 153.Li L, Chin LS. The molecular machinery of synaptic vesicle exocytosis. Cell Mol Life Sci. 2003;60:942–960. doi: 10.1007/s00018-003-2240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ghosh A, Greenberg ME. Calcium signaling in neurons: Molecular mechanisms and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- 155.Meinrenken CJ, Borst JG, Sakmann B. Calcium secretion coupling at calyx of Held governed by nonuniform channel-vesicle topography. J Neurosci. 2002;22:1648–1667. doi: 10.1523/JNEUROSCI.22-05-01648.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zucker RS. Calcium- and activity-dependent synaptic plasticity. Curr Opin Neurobiol. 1999;9:305–313. doi: 10.1016/s0959-4388(99)80045-2. [DOI] [PubMed] [Google Scholar]
- 157.Zenisek D, Matthews G. The role of mitochondria in presynaptic calcium handling at a ribbon synapse. Neuron. 2000;25:229–237. doi: 10.1016/s0896-6273(00)80885-5. [DOI] [PubMed] [Google Scholar]
- 158.Wan QF, Nixon E, Heidelberger R. Regulation of presynaptic calcium in a mammalian synaptic terminal. J Neurophysiol. 2012;108:3059–3067. doi: 10.1152/jn.00213.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Malli R, Graier WF. Mitochondrial Ca2+ channels: Great unknowns with important functions. FEBS Lett. 2010;584:1942–1947. doi: 10.1016/j.febslet.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gunter TE, Sheu SS. Characteristics and possible functions of mitochondrial Ca(2+) transport mechanisms. Biochim Biophys Acta. 2009;1787:1291–1308. doi: 10.1016/j.bbabio.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Billups B, Forsythe ID. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J Neurosci. 2002;22:5840–5847. doi: 10.1523/JNEUROSCI.22-14-05840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.David G, Barrett EF. Mitochondrial Ca2+ uptake prevents desynchronization of quantal release and minimizes depletion during repetitive stimulation of mouse motor nerve terminals. J Physiol. 2003;548:425–438. doi: 10.1113/jphysiol.2002.035196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jonas EA, Buchanan J, Kaczmarek LK. Prolonged activation of mitochondrial conductances during synaptic transmission. Science. 1999;286:1347–1350. doi: 10.1126/science.286.5443.1347. [DOI] [PubMed] [Google Scholar]
- 164.Mironov SL, Symonchuk N. ER vesicles and mitochondria move and communicate at synapses. J Cell Sci. 2006;119:4926–4934. doi: 10.1242/jcs.03254. [DOI] [PubMed] [Google Scholar]
- 165.Pivovarova NB, Pozzo-Miller LD, Hongpaisan J, Andrews SB. Correlated calcium uptake and release by mitochondria and endoplasmic reticulum of CA3 hippocampal dendrites after afferent synaptic stimulation. J Neurosci. 2002;22:10653–10661. doi: 10.1523/JNEUROSCI.22-24-10653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rizzuto R. Calcium mobilization from mitochondria in synaptic transmitter release. J Cell Biol. 2003;163:441–443. doi: 10.1083/jcb.200309111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rizzuto R, Bernardi P, Pozzan T. Mitochondria as all-round players of the calcium game. J Physiol. 2000;529(Pt 1):37–47. doi: 10.1111/j.1469-7793.2000.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Simkus CR, Stricker C. The contribution of intracellular calcium stores to mEPSCs recorded in layer II neurones of rat barrel cortex. J Physiol. 2002;545:521–535. doi: 10.1113/jphysiol.2002.022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Emptage NJ, Reid CA, Fine A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron. 2001;29:197–208. doi: 10.1016/s0896-6273(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 170.Dale N, Frenguelli BG. Release of adenosine and ATP during ischemia and epilepsy. Curr Neuropharmacol. 2009;7:160–179. doi: 10.2174/157015909789152146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 172.Hollenbeck PJ. Mitochondria and neurotransmission: Evacuating the synapse. Neuron. 2005;47:331–333. doi: 10.1016/j.neuron.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nguyen PV, Marin L, Atwood HL. Synaptic physiology and mitochondrial function in crayfish tonic and phasic motor neurons. J Neurophysiol. 1997;78:281–294. doi: 10.1152/jn.1997.78.1.281. [DOI] [PubMed] [Google Scholar]
- 174.Thiels E, Klann E. Hippocampal memory and plasticity in superoxide dismutase mutant mice. Physiol Behav. 2002;77:601–605. doi: 10.1016/s0031-9384(02)00900-9. [DOI] [PubMed] [Google Scholar]
- 175.Thiels E, Urban NN, Gonzalez-Burgos GR, Kanterewicz BI, Barrionuevo G, Chu CT, Oury TD, Klann E. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2000;20:7631–7639. doi: 10.1523/JNEUROSCI.20-20-07631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kishida KT, Pao M, Holland SM, Klann E. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. J Neurochem. 2005;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Reddy PH. Role of mitochondria in neurodegenerative diseases: Mitochondria as a therapeutic target in Alzheimer's disease. CNS Spectr. 2009;14:8–13. doi: 10.1017/s1092852900024901. discussion 16-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Reddy PH, Beal MF. Are mitochondria critical in the pathogenesis of Alzheimer's disease? Brain Res Brain Res Rev. 2005;49:618–632. doi: 10.1016/j.brainresrev.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 179.Lezi E, Swerdlow RH. Mitochondria in neurodegeneration. Adv Exp Med Biol. 2012;942:269–286. doi: 10.1007/978-94-007-2869-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Swerdlow RH, Kish SJ. Mitochondria in Alzheimer's disease. Int Rev Neurobiol. 2002;53:341–385. doi: 10.1016/s0074-7742(02)53013-0. [DOI] [PubMed] [Google Scholar]
- 181.Moreira PI, Siedlak SL, Wang X, Santos MS, Oliveira CR, Tabaton M, Nunomura A, Szweda LI, Aliev G, Smith MA, Zhu X, Perry G. Increased autophagic degradation of mitochondria in Alzheimer disease. Autophagy. 2007;3:614–615. doi: 10.4161/auto.4872. [DOI] [PubMed] [Google Scholar]
- 182.Moreira PI, Zhu X, Wang X, Lee HG, Nunomura A, Petersen RB, Perry G, Smith MA. Mitochondria: A therapeutic target in neurodegeneration. Biochim Biophys Acta. 2010;1802:212–220. doi: 10.1016/j.bbadis.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Moreira PI, Smith MA, Zhu X, Honda K, Lee HG, Aliev G, Perry G. Oxidative damage and Alzheimer's disease: Are antioxidant therapies useful? Drug News Perspect. 2005;18:13–19. doi: 10.1358/dnp.2005.18.1.877164. [DOI] [PubMed] [Google Scholar]
- 184.Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: Mechanistic implications. Ann Neurol. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- 185.Gibson GE, Sheu KF, Blass JP. Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm (Vienna) 1998;105:855–870. doi: 10.1007/s007020050099. [DOI] [PubMed] [Google Scholar]
- 186.Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer's disease. Hum Mol Genet. 2011;20:4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Beck SJ, Guo L, Phensy A, Tian J, Wang L, Tandon N, Gauba E, Lu L, Pascual JM, Kroener S, Du H. Deregulation of mitochondrial F1FO-ATP synthase via OSCP in Alzheimer's disease. Nat Commun. 2016;7:11483. doi: 10.1038/ncomms11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang L, Guo L, Lu L, Sun H, Shao M, Beck SJ, Li L, Ramachandran J, Du Y, Du H. Synaptosomal mitochondrial dysfunction in 5xFAD mouse model of Alzheimer's disease. PLoS One. 2016;11:e0150441. doi: 10.1371/journal.pone.0150441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lu L, Guo L, Gauba E, Tian J, Wang L, Tandon N, Shankar M, Beck SJ, Du Y, Du H. Transient cerebral ischemia promotes brain mitochondrial dysfunction and exacerbates cognitive impairments in young 5xFAD mice. PLoS One. 2015;10:e0144068. doi: 10.1371/journal.pone.0144068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: Implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 191.Guo L, Du H, Yan S, Wu X, McKhann GM, Chen JX, Yan SS. Cyclophilin D deficiency rescues axonal mitochondrial transport in Alzheimer's neurons. PLoS One. 2013;8:e54914. doi: 10.1371/journal.pone.0054914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Manczak M, Reddy PH. Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer's disease neurons: Implications for mitochondrial dysfunction and neuronal damage. Hum Mol Genet. 2012;21:2538–2547. doi: 10.1093/hmg/dds072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: Implications for neuronal damage. Hum Mol Genet. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang L, Trushin S, Christensen TA, Bachmeier BV, Gateno B, Schroeder A, Yao J, Itoh K, Sesaki H, Poon WW, Gylys KH, Patterson ER, Parisi JE, Diaz Brinton R, Salisbury JL, Trushina E. Altered brain energetics induces mitochondrial fission arrest in Alzheimer's Disease. Sci Rep. 2016;6:18725. doi: 10.1038/srep18725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proc Natl Acad Sci U S A. 2010;107:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Du H, Guo L, Wu X, Sosunov AA, McKhann GM, Chen JX, Yan SS. Cyclophilin D deficiency rescues Abeta-impaired PKA/CREB signaling and alleviates synaptic degeneration. Biochim Biophys Acta. 2014;1842:2517–2527. doi: 10.1016/j.bbadis.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Balietti M, Giorgetti B, Casoli T, Solazzi M, Tamagnini F, Burattini C, Aicardi G, Fattoretti P. Early selective vulnerability of synapses and synaptic mitochondria in the hippocampal CA1 region of the Tg2576 mouse model of Alzheimer's disease. J Alzheimers Dis. 2013;34:887–896. doi: 10.3233/JAD-121711. [DOI] [PubMed] [Google Scholar]
- 199.Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, Shirendeb UP, Calkins MJ, Reddy AP, Mao P, Manczak M. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer's disease: Implications to mitochondria-targeted antioxidant therapeutics. Biochim Biophys Acta. 2012;1822:639–649. doi: 10.1016/j.bbadis.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: Implications for neuroimaging. Trends Neurosci. 2004;27:489–495. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 201.Hoyer S. Oxidative metabolism deficiencies in brains of patients with Alzheimer's disease. Acta Neurol Scand Suppl. 1996;165:18–24. doi: 10.1111/j.1600-0404.1996.tb05868.x. [DOI] [PubMed] [Google Scholar]
- 202.Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, Castellano A, Pifferi F, Bocti C, Paquet N, Begdouri H, Bentourkia M, Turcotte E, Allard M, Barberger-Gateau P, Fulop T, Rapoport SI. Brain fuel metabolism, aging, and Alzheimer's disease. Nutrition. 2011;27:3–20. doi: 10.1016/j.nut.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Parker WD, Jr, Parks J, Filley CM, Kleinschmidt-DeMasters BK. Electron transport chain defects in Alzheimer's disease brain. Neurology. 1994;44:1090–1096. doi: 10.1212/wnl.44.6.1090. [DOI] [PubMed] [Google Scholar]
- 204.Parker WD, Jr, Parks JK. Cytochrome c oxidase in Alzheimer's disease brain: Purification and characterization. Neurology. 1995;45:482–486. doi: 10.1212/wnl.45.3.482. [DOI] [PubMed] [Google Scholar]
- 205.Kish SJ, Mastrogiacomo F, Guttman M, Furukawa Y, Taanman JW, Dozic S, Pandolfo M, Lamarche J, DiStefano L, Chang LJ. Decreased brain protein levels of cytochrome oxidase subunits in Alzheimer's disease and in hereditary spinocerebellar ataxia disorders: A nonspecific change? J Neurochem. 1999;72:700–707. doi: 10.1046/j.1471-4159.1999.0720700.x. [DOI] [PubMed] [Google Scholar]
- 206.Mutisya EM, Bowling AC, Beal MF. Cortical cytochrome oxidase activity is reduced in Alzheimer's disease. J Neurochem. 1994;63:2179–2184. doi: 10.1046/j.1471-4159.1994.63062179.x. [DOI] [PubMed] [Google Scholar]
- 207.Maurer I, Zierz S, Moller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol Aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- 208.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Du H, Guo L, Zhang W, Rydzewska M, Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol Aging. 2011;32:398–406. doi: 10.1016/j.neurobiolaging.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fisar Z, Hroudova J, Hansikova H, Spacilova J, Lelkova P, Wenchich L, Jirak R, Zverova M, Zeman J, Martasek P, Raboch J. Mitochondrial respiration in the platelets of patients with Alzheimer's disease. Curr Alzheimer Res. 2016;13:930–941. doi: 10.2174/1567205013666160314150856. [DOI] [PubMed] [Google Scholar]
- 211.Valla J, Schneider L, Niedzielko T, Coon KD, Caselli R, Sabbagh MN, Ahern GL, Baxter L, Alexander G, Walker DG, Reiman EM. Impaired platelet mitochondrial activity in Alzheimer's disease and mild cognitive impairment. Mitochondrion. 2006;6:323–330. doi: 10.1016/j.mito.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Carbajo RJ, Kellas FA, Yang JC, Runswick MJ, Montgomery MG, Walker JE, Neuhaus D. How the N-terminal domain of the OSCP subunit of bovine F1Fo-ATP synthase interacts with the N-terminal region of an alpha subunit. J Mol Biol. 2007;368:310–318. doi: 10.1016/j.jmb.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 213.Sanz-Blasco S, Valero RA, Rodriguez-Crespo I, Villalobos C, Nunez L. Mitochondrial Ca2+ overload underlies Abeta oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PLoS One. 2008;3:e2718. doi: 10.1371/journal.pone.0002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Moreira PI, Santos MS, Moreno A, Rego AC, Oliveira C. Effect of amyloid beta-peptide on permeability transition pore: A comparative study. J Neurosci Res. 2002;69:257–267. doi: 10.1002/jnr.10282. [DOI] [PubMed] [Google Scholar]
- 215.Moreira PI, Santos MS, Moreno A, Oliveira C. Amyloid beta-peptide promotes permeability transition pore in brain mitochondria. Biosci Rep. 2001;21:789–800. doi: 10.1023/a:1015536808304. [DOI] [PubMed] [Google Scholar]
- 216.Shevtzova EF, Kireeva EG, Bachurin SO. Effect of beta-amyloid peptide fragment 25-35 on nonselective permeability of mitochondria. Bull Exp Biol Med. 2001;132:1173–1176. doi: 10.1023/a:1014559331402. [DOI] [PubMed] [Google Scholar]
- 217.Manczak M, Reddy PH. Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer's disease. Hum Mol Genet. 2012;21:5131–5146. doi: 10.1093/hmg/dds360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 219.Gutierrez-Aguilar M, Baines CP. Structural mechanisms of cyclophilin D-dependent control of the mitochondrial permeability transition pore. Biochim Biophys Acta. 2015;1850:2041–2047. doi: 10.1016/j.bbagen.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA, Jr, Jonas EA. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci U S A. 2014;111:10580–10585. doi: 10.1073/pnas.1401591111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I, Lippe G, Bernardi P. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Reddy VP, Zhu X, Perry G, Smith MA. Oxidative stress in diabetes and Alzheimer's disease. J Alzheimers Dis. 2009;16:763–774. doi: 10.3233/JAD-2009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Arai M, Saito M, Takatsu H, Fukui K, Urano S. Dysfunction of the fusion of pre-synaptic plasma membranes and synaptic vesicles caused by oxidative stress, and its prevention by vitamin E. J Alzheimers Dis. 2011;24:759–766. doi: 10.3233/JAD-2011-101785. [DOI] [PubMed] [Google Scholar]
- 224.Omoi NO, Arai M, Saito M, Takatsu H, Shibata A, Fukuzawa K, Sato K, Abe K, Fukui K, Urano S. Influence of oxidative stress on fusion of pre-synaptic plasma membranes of the rat brain with phosphatidyl choline liposomes, and protective effect of vitamin E. J Nutr Sci Vitaminol (Tokyo) 2006;52:248–255. doi: 10.3177/jnsv.52.248. [DOI] [PubMed] [Google Scholar]
- 225.Dumont M, Wille E, Stack C, Calingasan NY, Beal MF, Lin MT. Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer's disease. FASEB J. 2009;23:2459–2466. doi: 10.1096/fj.09-132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer's disease neurons. J Alzheimers Dis. 2010;20(Suppl 2):S609–S631. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Calkins MJ, Manczak M, Reddy PH. Mitochondria-targeted antioxidant SS31 prevents amyloid beta-induced mitochondrial abnormalities and synaptic degeneration in Alzheimer's disease. Pharmaceuticals (Basel) 2012;5:1103–1119. doi: 10.3390/ph5101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ma T, Hoeffer CA, Wong H, Massaad CA, Zhou P, Iadecola C, Murphy MP, Pautler RG, Klann E. Amyloid beta-induced impairments in hippocampal synaptic plasticity are rescued by decreasing mitochondrial super-oxide. J Neurosci. 2011;31:5589–5595. doi: 10.1523/JNEUROSCI.6566-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 230.Cai Q, Davis ML, Sheng ZH. Regulation of axonal mitochondrial transport and its impact on synaptic transmission. Neurosci Res. 2011;70:9–15. doi: 10.1016/j.neures.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Decker H, Lo KY, Unger SM, Ferreira ST, Silverman MA. Amyloid-beta peptide oligomers disrupt axonal transport through an NMDA receptor-dependent mechanism that is mediated by glycogen synthase kinase 3beta in primary cultured hippocampal neurons. J Neurosci. 2010;30:9166–9171. doi: 10.1523/JNEUROSCI.1074-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Swerdlow RH, Burns JM, Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis: Progress and perspectives. Biochim Biophys Acta. 2014;1842:1219–1231. doi: 10.1016/j.bbadis.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]