Abstract
Purpose
Our previous experiments demonstrated that intravitreal injection of platelet-derived growth factor-AA (PDGF-AA) provides retinal ganglion cell (RGC) neuroprotection in a rodent model of glaucoma. Here we used PDGFRα-enhanced green fluorescent protein (EGFP) mice to identify retinal cells that may be essential for RGC protection by PDGF-AA.
Methods
PDGFRα-EGFP mice expressing nuclear-targeted EGFP under the control of the PDGFRα promoter were used. Localization of PDGFRα in the neural retina was investigated by confocal imaging of EGFP fluorescence and immunofluorescent labeling with a panel of antibodies recognizing different retinal cell types. Primary cultures of mouse RGCs were produced by immunopanning. Neurobiotin injection of amacrine cells in a flat-mounted retina was used for the identification of EGFP-positive amacrine cells in the inner nuclear layer.
Results
In the mouse neural retina, PDGFRα was preferentially localized in the ganglion cell and inner nuclear layers. Immunostaining of the retina demonstrated that astrocytes in the ganglion cell layer and a subpopulation of amacrine cells in the inner nuclear layer express PDGFRα, whereas RGCs (in vivo or in vitro) did not. PDGFRα-positive amacrine cells are likely to be Type 45 gamma-aminobutyric acidergic (GABAergic) wide-field amacrine cells.
Conclusions
These data indicate that the neuroprotective effect of PDGF-AA in a rodent model of glaucoma could be mediated by astrocytes and/or a subpopulation of amacrine cells. We suggest that after intravitreal injection of PDGF-AA, these cells secrete factors protecting RGCs.
Keywords: PDGF-AA, glaucoma, PDGFRα, neuroprotection, retina, retinal ganglion cells, astrocytes, amacrine cells
There are several risk factors associated with glaucoma, including elevated intraocular pressure (IOP), age, family history, ethnic origin, and certain medical conditions. The only clinically proven treatment of glaucoma is a pharmacologic or surgical reduction of IOP.1,2 However, IOP reduction does not always provide sufficient therapeutic effects, and there are multiple efforts to develop new neuroprotective therapies for glaucoma.3–5 Delivery of neurotrophic factors and inhibitors of apoptosis by intravitreal injection6,7 or viral vectors encoding different proteins,5,8–10 intravitreal injection of various types of stem cells,11–14 suppression of oxidative stress, and modulation of immune response have all been investigated.15
Intravitreal injection of mesenchymal stem cells (MSCs) isolated from bone marrow or umbilical cord blood has been tested by several laboratories.13,14,16,17 Such injections provided different degrees of retinal ganglion cell (RGC) neuroprotection in ocular hypertensive rodent glaucoma models14 as well as in an acute optic nerve damage model such as optic nerve crush (ONC).13,17,18 Neuroprotective effects of MSC injections were observed even without a direct contact of MSCs with the retina, implying that MSCs may secrete neuroprotective factors. Several such factors have been identified, and two of these factors, platelet-derived growth factor-AA (PDGF-AA) and PDGF-AB, were studied in greater detail using retinal explant cultures and an ocular hypertensive rat glaucoma model.19,20 In a rat glaucoma model, intravitreal injection of recombinant PDGF-AA or PDGF-AB efficiently blocked IOP-induced RGC death. However, the molecular mechanisms of the PDGF-mediated RGC neuroprotection remained elusive.
PDGF-AA and PDGF-AB are the ligands for PDGF receptors, which are members of the receptor tyrosine kinase family.21,22 In vertebrates, there are four PDGF genes encoding PDGF-A, PDGF-B, PDGF-C, and PDGF-D and two genes encoding PDGF receptors, PDGFRα and PDGFRβ.23 PDGF-A and PDGF-B form homo- or heterodimers. PDGF-AA is a specific ligand for PDGFRα, while PDGF-AB can interact with both PDGFRα and PDGFRβ.22 PDGF-AA/PDGFRα signaling affects a number of critical cellular functions including cell survival, proliferation, and differentiation.23
By using conventional and conditional PDGFRα knockout mice, the functions of PDGFRα in different tissues have been examined.24 Mice with a null mutation in PDGFRα die between embryonic day 8 (E8) and E16, displaying a variety of organ defects.25 The expression pattern of PDGFRα was investigated by in situ hybridization26 and immunostaining with corresponding antibodies.27–30 Information about the pattern of PFGFRα expression in the eye and especially in the retina is somewhat controversial, mainly due to the quality of PFGFRα antibodies used. The elucidation of the PDGFRα pattern of expression in the retina is critical for understanding the molecular mechanisms involved in RGC neuroprotection by PDGF-AA. Mice have been generated in which the histone H2B-enhance green fluorescent protein (EGFP) fusion protein reporter construct was knocked into the PDGFRα locus (αGFP).24 Although EGFP expression in the retina has not been analyzed in heterozygous αGFP/+ mice, EGFP expression faithfully reproduced the PDGFRα expression pattern in several analyzed tissues.24
In this report, we investigated the pattern of PDGFRα expression in the retina using αGFP/+ mice and wild-type (WT) mice. We identified cells expressing PDGFRα in the ganglion cell layer (GCL) as astrocytes, and in the inner nuclear layer (INL) as a subpopulation of amacrine cells. These data suggest an indirect mechanism of RGC neuroprotection by PFGF-AA in a rodent model of glaucoma.
Methods
Animals
Mice were maintained in accordance with guidelines set forth in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, using protocols approved by the National Eye Institute Committee on the Use and Care of Animals. PDGFRα-EGFP mice were purchased from The Jackson Laboratory (B6.129S4-PDGFRαtm11(EGFP)Sor/J, Stock #007669; Bar Harbor, ME, USA).
RGC Primary Cultures
Purification of RGCs was performed as described previously.31,32 Briefly, retinas were isolated from postnatal 1- to 10-day-old mice and dissociated with papain. Microglia cells were immunodepleted with anti-CD11b–conjugated Dynabeads (Life Technologies, Carlsbad, CA, USA). The suspension of retinal cells was immunopanned on plates preconjugated with anti-Thy1.2 antibody (Serotec, clone F7D5; Raleigh, NC, USA) and goat anti-mouse IgM (Jackson ImmunoResearch, West Grove, PA, USA) at room temperature. After extensive washing, RGCs were released from the plate by 0.025% trypsin, counted, and seeded at a density of 10,000 per well in 96-well plates or 50,000 cells per well in 24-well plates in the media composed of Neurobasal (Life Technologies), B27, N2 supplement, L-glutamine, forskolin, and penicillin/streptomycin. PDGF-AA (50 ng/mL), BDNF (50 ng/mL), and ciliary neurotrophic factor (CNTF) (50 ng/mL) or PDGF-AA, BDNF, and CNTF together were added to cultures where indicated. These concentrations of added proteins were selected following our previous studies.19 Cells were cultured in a CO2 incubator at 37° for 1 to 5 days.
RGC Viability Assay
RGC viability in culture was evaluated using a CellTiter-Glo assay kit (Promega, Madison, WI, USA). Briefly, primary RGCs were seeded onto a 96-well cell culture plate at a density of 10,000 cells/well. One to five days after seeding, cells were lysed with 50 μL 1× passive lysis buffer (Promega), and the luminescence was measured using a plate reader (1420 Multilabel Counter; Perkin Elmer, Shelton, CT, USA).
Western Blotting
Western blot analyses were performed as described previously.33 Briefly, isolated tissues or cells were homogenized in a lysis buffer (10 mM Tris-HCl, pH 7.5, 1 mM EDTA, 150 mM NaCl, 1% NP-40, 10% glycerol, and protease inhibitor cocktail) by repeated pipetting and then incubated for 20 minutes on ice. Following centrifugation, the soluble fraction was collected; 5 to 15 μg extracted proteins were separated on a 4% to 12% SDS-PAGE gel (Invitrogen, Carlsbad, CA, USA) and transferred to a polyvinylidene fluoride (PVDF) membrane (Invitrogen). Membranes were blocked with 5% Western Blocking Reagent/TBST (50 mM Tris, 150 mM NaCl, 0.05% Tween 20, pH 7.5) for 60 minutes and then incubated with indicated antibodies followed by incubation with anti-mouse or -rabbit IgG antibody conjugated to horseradish peroxidase (HRP) (1:10,000 dilution; GE Healthcare, Pittsburgh, PA, USA). The HRP signals were developed using a chemiluminescence detection kit (SuperSignal Femto Dura Extended Duration Substrate; Pierce, Rockford, IL, USA) and detected using FluorChem M (Protein Simple, San Jose, CA, USA).
Amacrine Cell Injection and Immunocytochemistry
Patch electrodes were pulled on a P-97 Micropipette Puller (Sutter, Novato, CA, USA) with a resistance of 12 to 18 MΩ. GFP-positive amacrine cells in the inner plexiform layer were targeted for recording and neurobiotin injection. The electrodes with solution contained 118 mM K-D-gluconate, 12 mM KCl, 0.5 mM MgCl2, 10 mM HEPES, 0.5 mM K-EGTA, 3 mM ATP, 1 mM GTP, and 1 mM neurobiotin (Vector Laboratories, Burlingame, CA, USA). pH of the media was adjusted to 7.4 with KOH. Slices were mounted on a Zeiss Examiner 1D microscope (Thornwood, NY, USA). They were superfused continuously with Ames' solution and bubbled continuously with 95%O2/5%CO2 at room temperature. Retina explants flat-mounted onto filter paper were sectioned into 220-μm-thick sections. Neurobiotin diffusion into single EGFP-positive amacrine cells was achieved by using the ZAP function in Axopatch 200B amplifiers (Molecular Devices, Sunnyvale, CA, USA). The electrode was left on the cell for approximately 5 minutes.
Retinal slices containing neurobiotin-injected amacrine cells were fixed for 20 minutes in 4% paraformaldehyde (PFA). The tissues were washed with standard solution (0.1 M sodium phosphate buffer plus 0.5% Triton X-100 and 0.1% NaN3, pH 7.4) at room temperature, blocked overnight with standard solution containing 4% normal donkey serum at 4°C. The tissues were then incubated with anti-Choline Acetyltransferase antibody (ChAT, 1:50 dilution; Millipore, Bedford, MA, USA) in the standard solution containing 1% donkey serum for 2 hours, followed by Cy5-conjugated donkey anti-goat (1:1000 dilution; Jackson ImmunoResearch). Neurobiotin was visualized with Alexa Fluor Cy3-conjugated streptavidin (1:1000 dilution; Molecular Probes, Eugene, OR, USA). Images were acquired with a Zeiss LSM-510 confocal microscope and contrast enhanced equally with Photoshop CS6 (Adobe Systems, San Jose, CA, USA).
Immunofluorescent Microscopy
Cells were washed with cold phosphate-buffered saline (PBS) and then fixed with 2% to 4% paraformaldehyde in PBS for 15 minutes at room temperature. After washing with PBS, cells were permeabilized with 0.25% Triton X-100 in PBS for 5 minutes at room temperature. Cells were incubated with a blocking solution (5% normal donkey serum/TBST) for 60 minutes at room temperature and then incubated with primary antibodies overnight at 4°C. After washing with PBS + Triton X-100 (PBST), cells were incubated with fluorescently labeled secondary antibodies according to the host specificity of primary antibodies and 4′,6-diamidino-2-phenylindole (DAPI) at room temperature for 1 hour. Specimens were washed with PBST and mounted in a slow-fade diamond reagent (Invitrogen). For whole-mount staining, retinas were dissected and fixed in 4% PFA for 20 minutes. Triton X-100 (0.4%) containing PBS was used for permeating the retina. Nonspecific binding sites in the tissue were blocked with 4% normal serum in PBST from the same host species as the labeled secondary antibody, and primary antibodies with appropriate dilution in the serum-containing PBST buffer were added. Whole-mount retinal tissues were incubated for 4 to 7 days and then washed with PBST at least five times for 20 minutes each time. Fluorescent secondary antibodies were diluted in PBST and added to retinal whole-mount tissues overnight. Then the tissues were washed in PBST and mounted. Samples were analyzed using a Zeiss LSM 780 confocal microscope and ZEN software. For whole-mount retinas, images were acquired from the four fields in the superior, inferior, temporal, and nasal quadrants.
For the quantification of RGCs, specimens were immunostained with antibodies against RNA binding protein with multiple splicing (RBPMS) (PhosphoSolutions, Aurora, CO, USA) or Brn3a (Millipore). For the quantification of the percentage of GFAP-positive/EGFP-positive cells in each layer, whole-mount retinal image stacks were obtained from three PDGFRα-EGFP mice. Images corresponding to GCL and INL were then separated and merged into one single image or separate images for GCL or INL (see Supplementary Figs. S1A, S1B). EGFP-positive cells in GCL and INL were counted first and then GFAP-positive cells were counted in the population of EGFP-positive cells to calculate the percentage of GFAP+/EGFP+ cells.
Open-Source Software and Statistical Analysis
All the imaging data were analyzed by ImageJ.34,35 Statistical analysis was performed using the R statistical computing language and its package tidyverse.36 Statistical comparisons of paired data were performed using Student's t-test. Multigroup data were analyzed using a 1-way analysis of variance (ANOVA) with subsequent post hoc pairwise t-test with Bonferroni's adjustment. Data are shown as mean ± standard error of the mean (SEM). P values < 0.01 were considered to be statistically significant.
Results
PDGFRα Expression in the Mouse Eye
To investigate the expression of PDGFRα in the mouse retina we used αGFP/+ mice. One of the advantages of this line is a nuclear localization of EGFP that allowed easy distinction between EGFP-positive cells and surrounding EGFP-negative cells. Although EGFP expression in the retina has not been investigated for this line, published results demonstrated that EGFP expression reproduced the expression pattern of PDGFRα in all tested tissues.24 In the adult mouse eye, EGFP-positive cells were observed in lens epithelial cells and differentiating lens fibers, corneal stroma, iris, drainage structures, retina, optic nerve, retinal pigmented epithelium, and sclera (Fig. 1).
Figure 1.
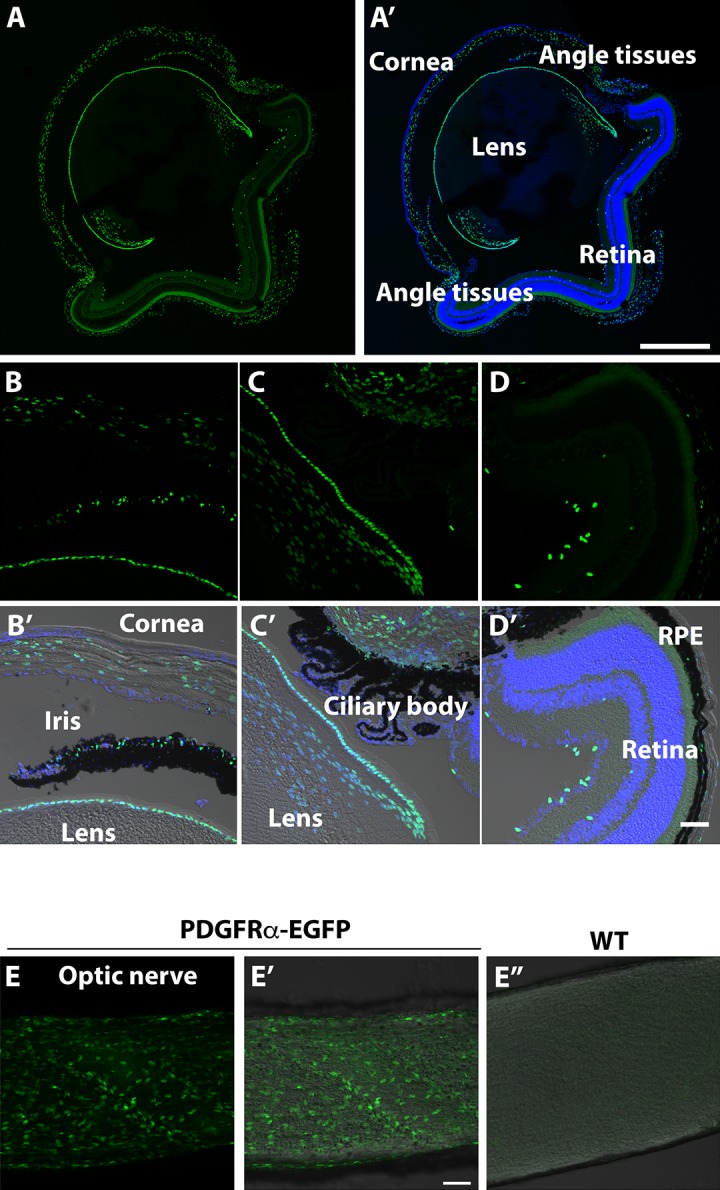
Expression pattern of αGFP in a mouse eye. (A, A') Transverse sections of 1-month-old mouse eye. (A') includes DAPI staining. (B–D, B'–D') Sections through the cornea, eye drainage structures (iris, trabecular meshwork and ciliary body), and retina. (B'–D') display images of the same areas shown in (B–D) obtained using differential interference contrast (DIC) microscopy for better visualization of the tissues. These include DAPI staining. RPE, retinal pigmented epithelium. (E–E”) Sections through the optic nerve. The wild-type optic nerve (E”) is shown for comparison. Scale bars: 500 μm (A, A'); 50 μm (B'–E”).
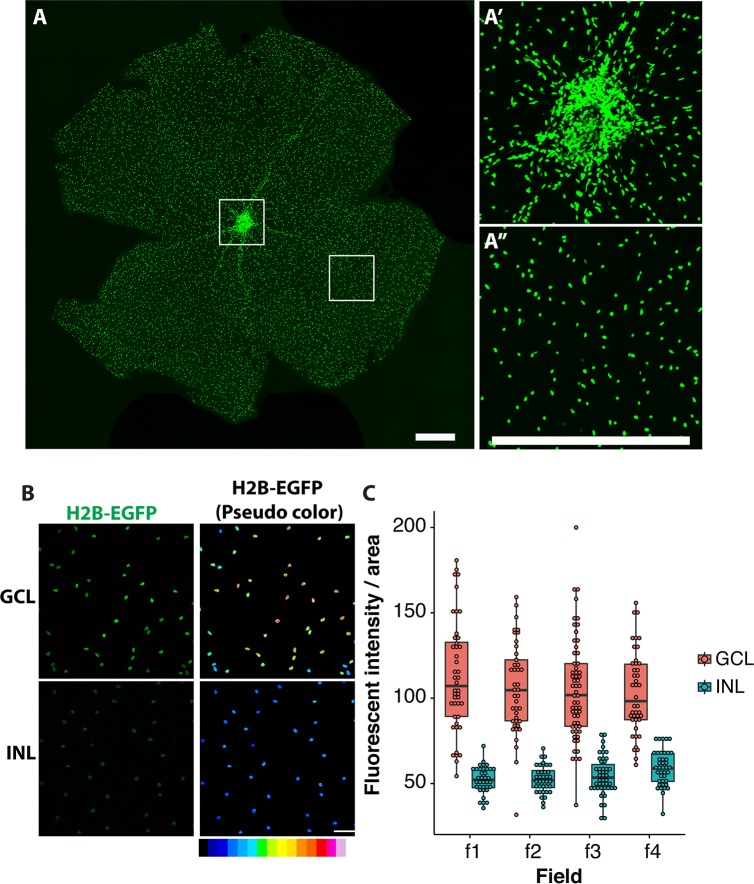
Expression pattern of PDGFRα in the retina was investigated in more detail. Analysis of the whole-mount retina demonstrated that the highest density of EGFP-positive cells was observed in the optic nerve head with a relatively even distribution through the rest of the retina (Figs. 2A–A”). EGFP-positive cells were located mainly in the GCL and INL with fluorescence intensity two times higher for the cells located in the GCL compared to the INL (Figs. 2B, 2C).
Figure 2.
Expression pattern of αGFP in the retina. (A–A”) Whole-mount retina from 1-month-old αGFP/+ mouse. Two boxed areas in (A) are shown at higher magnification at the right (A'–A”). (B, C) Relative intensity of EGFP fluorescence in the GCL and INL. Right images in (B) were pseudo-colored for better demonstration of differences in the intensity of fluorescence in the GCL and INL. Four fields were counted for each layer. Scale bars: 500 μm (A–A”); 50 μm (B).
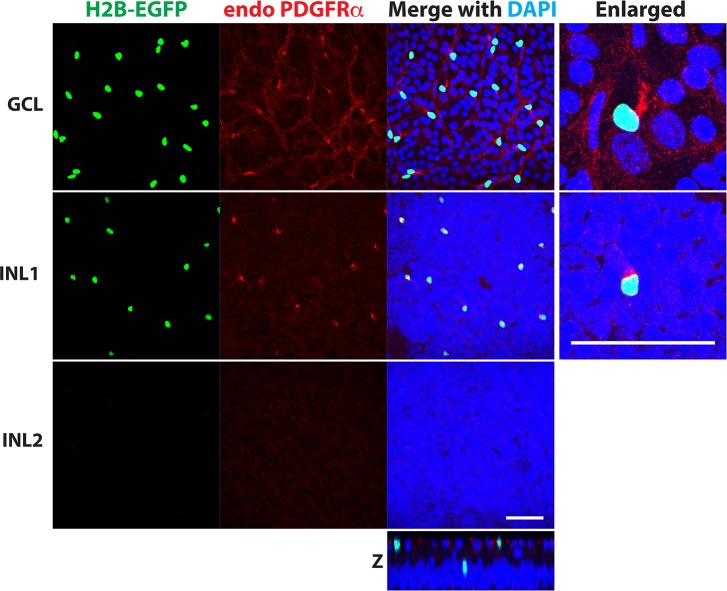
To confirm that EGFP expression in the retina, similar to other analyzed tissues,24 reproduced the expression pattern of PDGFRα, we tested several available PDGFRα antibodies and selected antibodies from Cell Signaling (Beverly, MA, USA) that were successfully used in previous publications dealing with mouse tissues.29,30 This antibody produced a strong staining in some cells in both GCL and INL, and these cells were EGFP positive. A weaker punctuated staining was also observed in some cells, and these cells were EGFP negative (Fig. 3). The pattern of weak punctuated staining in GCL did not reflect a typical distribution of RGCs.
Figure 3.
Colocalization of EGFP fluorescence and immunostaining with PDGFRα antibodies in the mouse retina. Whole-mount retina from 1-month-old αGFP/+ mouse was stained with antibodies against PDGFRα. Images were taken at different depths corresponding to the GCL and two positions in the INL (close to the inner plexiform layer and in the middle of INL: INL1 and INL2, respectively). Some faint signals were observed after staining with antibodies against PFGFRα, but the strongest PFGFRα signals were always in very close proximity with EGFP fluorescence. We did not observe EGFP-negative cells with strong PDGFRα signals. Scale bar: 50 μm. A reconstituted z-image from each confocal image also shown.
Identification of Retinal Cells Expressing PDGFRα
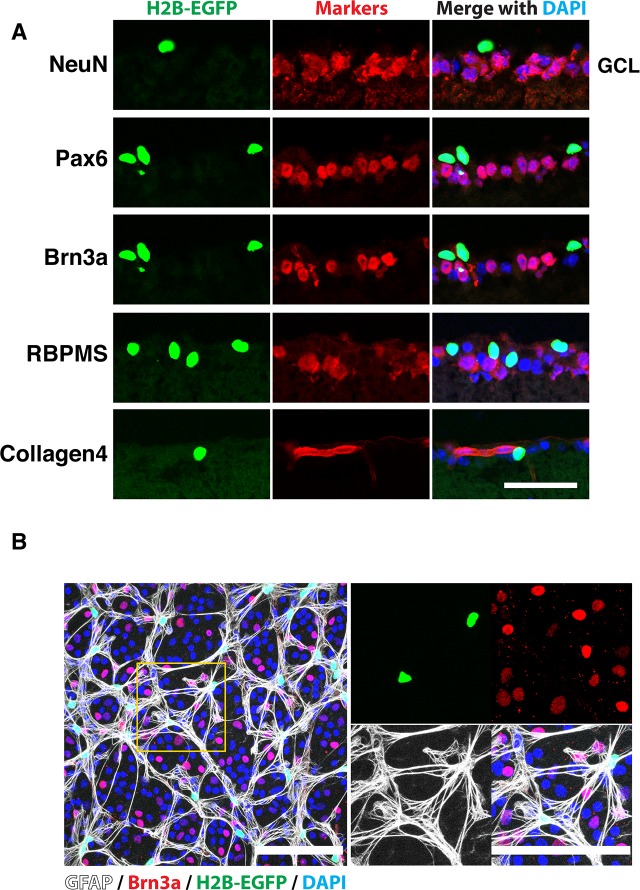
Several antibodies that recognize RGCs (Brn3a and RBPMS) or both RGCs and amacrine cells (Pax6 and NeuN) have been used to identify EGFP-positive cells in the GCL. Staining of transverse retinal sections demonstrated that EGFP-positive cells were not costained with any of these markers and that these cells were located outside of the collagen 4-positive blood vessels (Fig. 4A). Staining of the whole-mount retina confirmed that EGFP and Brn3a signals do not colocalize. Instead, 98 ± 1% of EGFP-positive cells in the GCL and 1.2 ± 1.2% of cells in the INL were costained with antibodies against GFAP, a known glial marker, as judged by counting of three independent samples (Fig. 4B, Supplementary Fig. S1). These data suggested that PDGFRα-expressing cells in the GCL are astrocytes.
Figure 4.
Colocalization of EGFP fluorescence with a glial marker in the GCL. (A) Transverse sections of 1-month-old αGFP eye were stained with indicated antibodies and DAPI. Only areas corresponding to the GCL are shown. Note the absence of colocalization of EGFP fluorescence with neuronal and blood vessel markers. (B) Whole-mount retina from 1-month-old αGFP/+ mouse was stained with indicated antibodies and DAPI. Right images are enlarged images of the boxed area on the left. Scale bars: 50 μm (A); 100 μm (B).
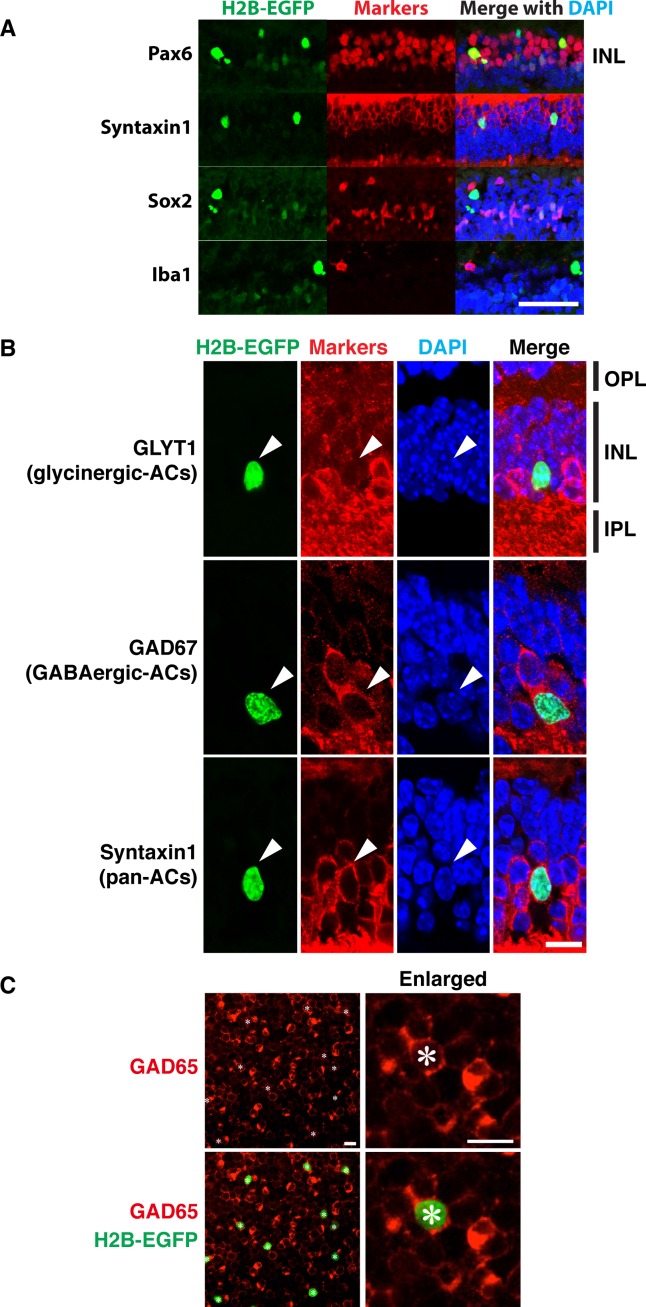
Several markers have been used to identify EGFP-positive cells in the INL. Transverse retinal sections were stained with antibodies against Pax6 and Syntaxin 1, markers that stain most of the identified subtypes of amacrine cells.37–40 Good colocalization of EGFP fluorescence and immunostaining has been observed for both markers (Fig. 5A). At the same time, no colocalization of EGFP fluorescence and immunostaining with antibodies against microglia/macrophage-specific protein Iba1 was observed (Fig. 5A). These data suggested that EGFP-positive cells in the INL represent one of the amacrine cell types. EGFP-positive cells in the INL were mainly observed in the sublayer of INL close to the GCL. This localization of EGFP-positive cells in the INL suggested that they do not belong to the non-gamma-Aminobutyric acidergic (non-GABAergic) non-glycinergic (nGnG) type of amacrine cell, since the nuclei of the nGnG type of amacrine cells are localized at the outermost edge of the amacrine cell zone, proximal to the region of bipolar cell nuclei.41 The absence of colocalization of EGFP fluorescence and immunostaining with antibodies against Sox2 and GLYT1, the known markers of cholinergic and glycinergic amacrine cells, respectively, suggested that EGFP-positive amacrine cells belong to one of the GABAergic types of amacrine cells (Figs. 5A, 5B). Staining of the whole-mount retina with GAD67 antibodies demonstrated that only a fraction of GABAergic types of amacrine cells express PDGFRα (Fig. 5C).
Figure 5.
Colocalization of EGFP fluorescence with amacrine cell markers in the INL. (A, B) Transverse sections of 1-month-old αGFP eye were stained with indicated antibodies and DAPI. Arrowheads mark the position of GFP-positive cells. IPL, inner plexiform layer; OPL, outer plexiform layer. (C) Whole-mount retina from αGFP/+ mouse was stained with antibodies against GAD67 and DAPI. Only GAD67 staining is shown on the right. Asterisk marks EGFP-positive cells. Scale bars: 50 μm (A); 10 μm (B, C).
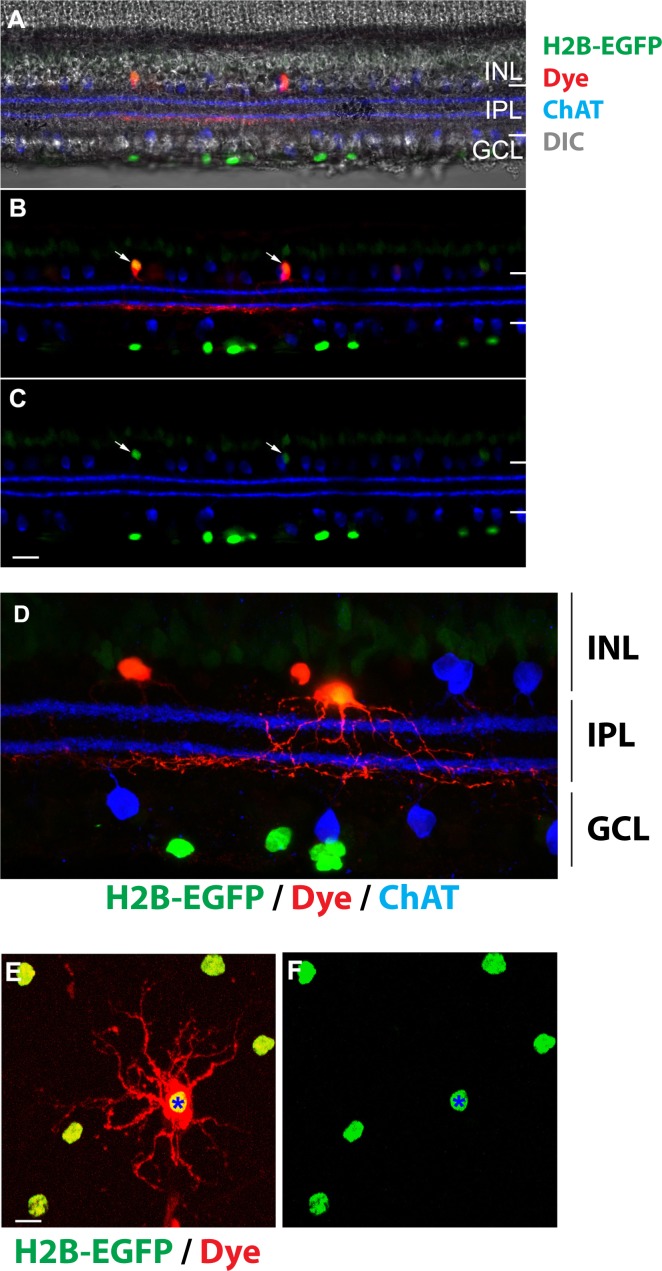
In mouse retina, GABAergic neurons compose approximately 40% of amacrine cells.42 To identify one or more subtypes of GABAergic amacrine cells expressing EGFP, we performed single-cell dye injections. We targeted EGFP-positive soma in the inner plexiform layer (IPL) for neurobiotin injection in freshly cut retinal slices or whole-mount pieces (n = 5, Fig. 6). We then fixed the tissues and processed them to visualize the morphology of the amacrine cells. The stratification of starburst amacrine cells (ChAT immunolabeling) has been used as a reference marker to localize the ramification of the dendrites from the GFP-positive amacrine cells. We found that these amacrine cells extend dendrites through IPL and narrowly ramify right beneath the ON ChAT band at approximately 65% depth of the IPL (Figs. 6B, 6D). The dendritic field of the analyzed amacrine cells is approximately 150 to 200 μm in diameter (Fig. 6D), consistent with being a type of GABAergic wide-field amacrine cell. According to the electron microscopy reconstruction results,43 the analyzed amacrine cells are likely to be Type 45. In addition, the amacrine cells appear to be coupled to each other as neurobiotin diffuses from the injected cell to neighboring cells that are also EGFP positive (Figs. 6B, 6C, 6E, 6F).
Figure 6.
Identification of amacrine cell type in the inner nuclear layer. (A–C) In a retinal slice, neurobiotin-injected amacrine cells (red) are EGFP positive (green). Starburst amacrine cells are labeled with a ChAT antibody (blue). Arrows point to the soma of amacrine cells. (D) A high-magnification image shows that the ramification of the amacrine cell dendrites (red) is beneath the ON ChAT band (blue). EGFP is shown in green. (E, F) In a piece of whole-mount retina, neurobiotin (red) injected into a EGFP-positive amacrine cell (asterisk) diffuses to neighboring EGFP-positive amacrine cells. Scale bars: 20 μm (A–C); 10 μm (D–F).
PDGF-AA Does Not Protect RGCs in Primary Cultures
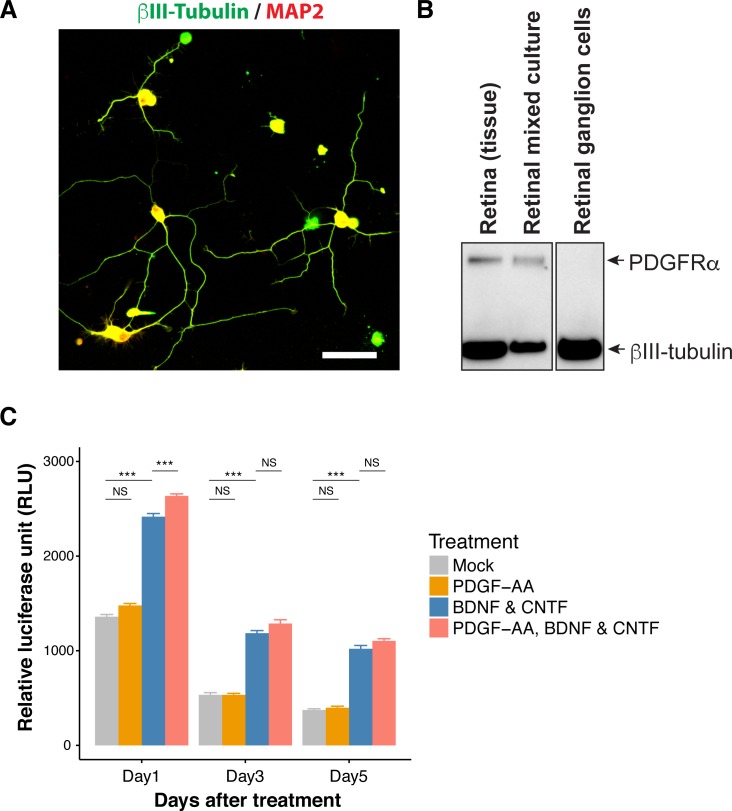
To confirm that PDGF-AA does not protect RGCs through direct interaction, we used RGC primary cultures. RGCs were isolated using a standard immunopanning protocol and showed typical neuronal morphology in culture (Fig. 7A). RGCs in culture, similar to RGCs in the retina, did not express PDGFRα as shown by Western blotting experiments (Fig. 7B). Addition of PDGF-AA to RGC cultures did not protect RGCs, while addition of a mixture of known neuroprotective factors, BDNF and CNTF, improved survival of RGCs (Fig. 7C). These results support the idea that PDGF-AA neuroprotective action on RGCs occurs through indirect mechanisms.
Figure 7.
PDGF-AA does not protect RGCs in culture. (A) RGCs isolated by immunopanning were stained with antibodies against acetylated tubulin and MAP2. Scale bar: 50 μm. (B) Western blot analysis of native retina, mixed retinal cultures, and isolated RGCs. (C) Survival of RGCs in culture in the presence of PDGF-AA (50 ng/mL), BDNF (50 ng/mL), and CNTF (50 ng/mL) or PDGF-AA, BDNF, and CNTF together. **P < 0.01; ***P < 0.001; NS, not statistically significant.
Discussion
The PDGF family of proteins and their receptors has multiple functions in embryonic development, cell growth, migration, differentiation, neurogenesis, osteogenesis tumor growth, and metastasis.44–46 PDGF-AA, one of the four family members, is mainly synthesized and secreted by epithelial cells, promoting mesenchyme expansion and angiogenesis. PDGF-AA/PDGFRα signaling affects a number of critical cellular functions including cell survival, proliferation, and differentiation.23
Previously, we identified PDGF-AA as a novel neuroprotective factor protecting RGCs in retinal explant cultures and in a laser-induced ocular hypertension model of glaucoma. The blockage of PDGFR signaling with a small-molecule inhibitor of PDGF receptor kinase, AG-1296, or with a small-molecule inhibitor of PI3 kinase that lies downstream in the PDGF receptor signaling cascade47 eliminated a neuroprotective effect of PDGF-AA.19 The nature of cells reacting to PDGF-AA in the retina was not elucidated in our previous study. A recent report demonstrated that intravitreal injections of PDGF-AB in rats with laser-induced elevation of IOP increased microglia and monocyte-derived macrophage population in the eye and protected intraretinal synapses from degeneration.20 Because PDGFRα is the only known receptor for PDGF-AA and because data about its expression in different types of retinal cells are controversial, here we reexamined PDGFRα expression in the retina to better understand possible mechanisms of its neuroprotective action. As a tool, we used PDGFRα-EGFP mice in which nuclear-targeted EGFP is expressed under the control of the PDGFRα promoter. Although cells showing the strongest immunostaining signals with antibodies against PDGFRα were also EGFP positive, some cells showing less intensive immunostaining were EGFP negative. We believe that this reflects not perfect quality of PDGFRα antibodies rather than restricted pattern of EGFP reporter expression that was knocked into the PDGFRα locus.
Analyzing the distribution of EGFP immunofluorescence in the retina, we demonstrated that PDGFRα is not expressed in RGCs in vivo or in vitro, implicating that PDGF-AA provides neuroprotection of RGCs not through direct interaction with these cells. We identified astrocytes in the GCL and a subpopulation of amacrine cells in the INL as cells expressing PDGFRα in the neural retina. These observations are supported by a single-cell RNA sequencing analysis.48 According to these data, PDGFRα is a marker of a distinct cluster of amacrine cells and is also expressed in astrocytes. Our present data suggest that retinal astrocytes in the GCL and/or subpopulation of amacrine cells in the INL play a role in the previously demonstrated RGC neuroprotective role of PDGF-AA in a rodent model of glaucoma.19 PDGF-AA–treated retinal astrocytes and/or subpopulation of amacrine cells may secrete factors that provide neuroprotection of RGCs. It is well documented that retinal astrocytes produce multiple factors under different normal and pathologic conditions.49–51 Some of these factors may enhance RGC survival, while others are efficient in the promotion of axon growth. One of such PDGF-AA–induced factors could be astrocyte-derived neurotropic factor that promotes retinal repair and regeneration.52 Astrocytes play a key role in synapse formation, maintenance, and function.53 It was shown that intravitreal PDGF-AB injection also protects intraretinal synapses and dendrites from degeneration in eyes with experimental IOP elevation.20 We believe that PDGF-AA interaction with PDGFRα in retinal astrocytes may contribute to synapse protection.
In conclusion, PDGFRα-EGFP mice represent a useful tool for isolation of cells expressing PDGFRα and elucidation of molecular changes induced in these cells after PDGF-AA treatment. The identification of factors secreted from activated astrocytes and/or subpopulation of amacrine cells after PDGF-AA treatment may lead to the development of appropriate RGC neuroprotection mechanisms.
Supplementary Material
Acknowledgments
The authors thank Bean Mead, PhD, for critical reading of the manuscript. We also thank Megan Kopera, Faazal Rehman, and all the animal care staff for the maintenance of animals.
Supported by the Intramural Research Programs of the National Eye Institute, National Institutes of Health.
Disclosure: S. Takahama, None; M.O. Adetunji, None; T. Zhao, None; S. Chen, None; W. Li, None; S.I. Tomarev, None
References
- 1. Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B;. for the BESs Study Group. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 2008; 115: 85– 93. [DOI] [PubMed] [Google Scholar]
- 2. Bagnis A, Papadia M, Scotto R, Traverso CE. . Current and emerging medical therapies in the treatment of glaucoma. Expert Opin Emerg Drugs. 2011; 16: 293– 307. [DOI] [PubMed] [Google Scholar]
- 3. Tian K, Shibata-Germanos S, Pahlitzsch M, Cordeiro MF. . Current perspective of neuroprotection and glaucoma. Clin Ophthalmol. 2015; 9: 2109– 2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nucci C, Russo R, Martucci A,et al. . New strategies for neuroprotection in glaucoma, a disease that affects the central nervous system. Eur J Pharmacol. 2016; 787: 119– 126. [DOI] [PubMed] [Google Scholar]
- 5. Wilson AM, Di Polo A. . Gene therapy for retinal ganglion cell neuroprotection in glaucoma. Gene Ther. 2011: 2; 127– 136. [DOI] [PubMed] [Google Scholar]
- 6. Ko ML, Hu DN, Ritch R, Sharma SC, Chen CF. . Patterns of retinal ganglion cell survival after brain-derived neurotrophic factor administration in hypertensive eyes of rats. Neurosci Lett. 2001; 305: 139– 142. [DOI] [PubMed] [Google Scholar]
- 7. Ji JZ, Elyaman W, Yip HK,et al. . CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: the possible involvement of stat3 pathway. Eur J Neurosci. 2004; 19: 265– 272. [DOI] [PubMed] [Google Scholar]
- 8. Martin KR, Quigley HA, Zack DJ,et al. . Gene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2003; 44: 4357– 4365. [DOI] [PubMed] [Google Scholar]
- 9. van Adel BA, Arnold JM, Phipps J, Doering LC, Ball AK. . Ciliary neurotrophic factor protects retinal ganglion cells from axotomy-induced apoptosis via modulation of retinal glia in vivo. J Neurobiol. 2005; 63: 215– 234. [DOI] [PubMed] [Google Scholar]
- 10. Yang L, Li S, Miao L,et al. . Rescue of glaucomatous neurodegeneration by differentially modulating neuronal endoplasmic reticulum stress molecules. J Neurosci. 2016; 36: 5891– 5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singhal S, Bhatia B, Jayaram H,et al. . Human muller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation. Stem Cells Transl Med. 2012; 1: 188– 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Venugopalan P, Wang Y, Nguyen T, Huang A, Muller KJ, Goldberg JL. . Transplanted neurons integrate into adult retinas and respond to light. Nat Commun. 2016; 7: 10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. . Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest Ophthalmol Vis Sci. 2013; 54: 7544– 7556. [DOI] [PubMed] [Google Scholar]
- 14. Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR. . Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010; 51: 2051– 2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hines-Beard J, Bond WS, Backstrom JR, Rex TS. . Virus-mediated EPOR76E gene therapy preserves vision in a glaucoma model by modulating neuroinflammation and decreasing oxidative stress. J Neuroinflammation. 2016; 13: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mead B, Hill LJ, Blanch RJ,et al. . Mesenchymal stromal cell-mediated neuroprotection and functional preservation of retinal ganglion cells in a rodent model of glaucoma. Cytotherapy. 2016; 18: 487– 496. [DOI] [PubMed] [Google Scholar]
- 17. Zwart I, Hill AJ, Al-Allaf F,et al. . Umbilical cord blood mesenchymal stromal cells are neuroprotective and promote regeneration in a rat optic tract model. Exp Neurol. 2009; 216: 439– 448. [DOI] [PubMed] [Google Scholar]
- 18. Junyi L, Na L, Yan J. . Mesenchymal stem cells secrete brain-derived neurotrophic factor and promote retinal ganglion cell survival after traumatic optic neuropathy. J Craniofacial Surg. 2015; 26: 548– 552. [DOI] [PubMed] [Google Scholar]
- 19. Johnson TV, DeKorver NW, Levasseur VA,et al. . Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain. 2014; 137: 503– 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chong RS, Osborne A, Conceicao R, Martin KR. . Platelet-derived growth factor preserves retinal synapses in a rat model of ocular hypertension. Invest Ophthalmol Vis Sci. 2016; 57: 842– 852. [DOI] [PubMed] [Google Scholar]
- 21. Heldin CH, Lennartsson J. . Structural and functional properties of platelet-derived growth factor and stem cell factor receptors. Cold Spring Harb Perspect Biol. 2013; 5: a009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li X, Eriksson U. . Novel PDGF family members: PDGF-C and PDGF-D. Cytokine Growth Factor Rev. 2003; 14: 91– 98. [DOI] [PubMed] [Google Scholar]
- 23. Heldin CH, Westermark B. . Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999; 79: 1283– 1316. [DOI] [PubMed] [Google Scholar]
- 24. Hamilton TG, Klinghoffer RA, Corrin PD, Soriano P. . Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Mol Cell Biol. 2003; 23: 4013– 4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soriano P. . The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development. 1997; 124: 2691– 2700. [DOI] [PubMed] [Google Scholar]
- 26. Schatteman GC, Morrison-Graham K, van Koppen A, Weston JA, Bowen-Pope DF. . Regulation and role of PGDF receptor alpha-subunit expression during embryogenesis. Development. 1992; 115: 123– 131. [DOI] [PubMed] [Google Scholar]
- 27. Kanamoto T, Rimayanti U, . Okumichi H, Kiuchi Y. Platelet-derived growth factor receptor alpha is associated with oxidative stress-induced retinal cell death. Curr Eye Res. 2011; 36: 336– 340. [DOI] [PubMed] [Google Scholar]
- 28. Robbins SG, Mixon RN, Wilson DJ,et al. . Platelet-derived growth factor ligands and receptors immunolocalized in proliferative retinal diseases. Invest Ophthalmol Vis Sci. 1994; 35: 3649– 3663. [PubMed] [Google Scholar]
- 29. Fusar Poli E, Zalfa C, D'Avanzo F,et al. . Murine neural stem cells model Hunter disease in vitro: glial cell-mediated neurodegeneration as a possible mechanism involved. Cell Death Dis. 2013; 4: e906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fredriksson L, Stevenson TK, Su EJ,et al. . Identification of a neurovascular signaling pathway regulating seizures in mice. Ann Clin Transl Neurol. 2015; 2: 722– 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barres BA, Silverstein BE, Corey DP, Chun LL. . Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988; 1: 791– 803. [DOI] [PubMed] [Google Scholar]
- 32. Welsbie DS, Yang Z, Ge Y,et al. . Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc Natl Acad Sci U S A. 2013; 110: 4045– 4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakaya N, Sultana A, Munasinghe J, Cheng A, Mattson MP, Tomarev SI. . Deletion in the N-terminal half of olfactomedin 1 modifies its interaction with synaptic proteins and causes brain dystrophy and abnormal behavior in mice. Exp Neurol. 2013; 250: 205– 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schneider CA, Rasband WS, Eliceiri KW. NIH. Image to ImageJ: 25 years of image analysis. Nat Methods. 2012; 9: 671– 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abràmoff MD, Magalhães PJ, Ram SJ. . Image processing with ImageJ. Biophotonics Int. 2004; 11: 36– 42. [Google Scholar]
- 36. Ihaka R, Gentleman R. R:. a language for data analysis and graphics. J Comput Graph Stat. 1996; 5: 299– 314. [Google Scholar]
- 37. Ding Q, Chen H, Xie X, Libby RT, Tian N, Gan L. . Barhl2 differentially regulates the development of retinal amacrine and ganglion neurons. J Neurosci. 2009; 29: 3992– 4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Melo J, Qiu X, Du G, Cristante L, Eisenstat DD. . Dlx1, Dlx2, Pax6, Brn3b, and Chx10 homeobox gene expression defines the retinal ganglion and inner nuclear layers of the developing and adult mouse retina. J Comp Neurol. 2003; 461: 187– 204. [DOI] [PubMed] [Google Scholar]
- 39. Sherry DM, Mitchell R, Standifer KM, du Plessis B. . Distribution of plasma membrane-associated syntaxins 1 through 4 indicates distinct trafficking functions in the synaptic layers of the mouse retina. BMC Neurosci. 2006; 7: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. . Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001; 105: 43– 55. [DOI] [PubMed] [Google Scholar]
- 41. Kay JN, Voinescu PE, Chu MW, Sanes JR. . Neurod6 expression defines new retinal amacrine cell subtypes and regulates their fate. Nat Neurosci. 2011; 14: 965– 972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mo Z, Li S, Yang X, Xiang M. . Role of the Barhl2 homeobox gene in the specification of glycinergic amacrine cells. Development. 2004; 131: 1607– 1618. [DOI] [PubMed] [Google Scholar]
- 43. Helmstaedter M, Briggman KL, Turaga SC, Jain V, Seung HS, Denk W. . Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature. 2013; 500: 168– 174. [DOI] [PubMed] [Google Scholar]
- 44. Cao Y. . Multifarious functions of PDGFs and PDGFRs in tumor growth and metastasis. Trends Mol Med. 2013; 19: 460– 473. [DOI] [PubMed] [Google Scholar]
- 45. Funa K, Sasahara M. . The roles of PDGF in development and during neurogenesis in the normal and diseased nervous system. J Neuroimmune Pharmacol. 2014; 9: 168– 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Donovan J, Abraham D, Norman J. . Platelet-derived growth factor signaling in mesenchymal cells. Front Biosci. 2013; 18: 106– 119. [DOI] [PubMed] [Google Scholar]
- 47. Fantl WJ, Escobedo JA, Martin GA,et al. . Distinct phosphotyrosines on a growth factor receptor bind to specific molecules that mediate different signaling pathways. Cell. 1992; 69: 413– 423. [DOI] [PubMed] [Google Scholar]
- 48. Macosko EZ, Basu A, Satija R,et al. . Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015; 161: 1202– 1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Howell GR, MacNicoll KH, Braine CE,et al. . Combinatorial targeting of early pathways profoundly inhibits neurodegeneration in a mouse model of glaucoma. Neurobiol Dis. 2014; 71: 44– 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Soto I, Howell GR. . The complex role of neuroinflammation in glaucoma. Cold Spring Harb Perspect Med. 2014; 4: a017269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang XT, Huang GH, Feng DF, Chen K. . Insight into astrocyte activation after optic nerve injury. J Neurosci Res. 2015; 93: 539– 548. [DOI] [PubMed] [Google Scholar]
- 52. Neves J, Zhu J, Sousa-Victor P, Konjikusic M,et al. . Immune modulation by MANF promotes tissue repair and regenerative success in the retina. Science. 2016; 353:aaf3646. [DOI] [PMC free article] [PubMed]
- 53. Sofroniew MV. . Reactive astrocytes in neural repair and protection. Neuroscientist. 2005; 11: 400– 407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.