Abstract
Despite its importance in regulating emotion and mental wellbeing, the complex structure and function of the serotonergic system present formidable challenges toward understanding its mechanisms. In this paper, we review studies investigating the interactions between serotonergic and related brain systems and their behavior at multiple scales, with a focus on biologically-based computational modeling. We first discuss serotonergic intracellular signaling and neuronal excitability, followed by neuronal circuit and systems levels. At each level of organization, we will discuss the experimental work accompanied by related computational modeling work. We then suggest that a multiscale modeling approach that integrates the various levels of neurobiological organization could potentially transform the way we understand the complex functions associated with serotonin.
Keywords: Serotonin 5-HT, midbrain raphe nucleus, neural circuit, multiscale computational model
Introduction
Serotonin (5-hydroxytryptamine; 5-HT) neurons are clustered into rostral and caudal groups. The rostral group is located at the mesencephalon and rostral pons, including the midbrain raphe nuclei with major projections to the forebrain and are critical for the regulation of various functions, including emotion and decision-making (Müller and Jacobs, 2009). The caudal group is located from the caudal pons to the caudal portion of the medulla oblongata, with major projections to the brainstem and the spinal cord (Ding et al., 2003; Hornung, 2003). The effects of 5-HT on brain functions is mediated through its release and reuptake, and its 14 receptor subtypes throughout the brain (Barnes and Sharp, 1999). Genetic variation in 5-HT can affect emotional processing in animals and humans, and research has reported links between 5-HT and vulnerability to anxiety and depression (Müller and Jacobs, 2009). Although 5-HT-targeted drugs can have important therapeutic effects on various disorders (e.g. depression and post-traumatic stress disorders), such agents currently lack the efficacy and tolerability required, and further improvement is needed (Nichols and Nichols, 2008). This is largely due to the still unknown mechanisms that 5-HT exerts on brain systems.
Computational modeling and mathematical theories are useful tools to provide quantitative and conceptual understanding of observed experimental phenomena, while offering predictions for future tests (Abbott, 2008). Despite decades of research on the 5-HT system, its computational roles are still not completely known (Nakamura and Wong-Lin, 2014). Theories on the role of 5-HT in health and disease have been separately developed at different levels of biological descriptions, with efforts largely focused on either models with high biological details such as intracellular signaling mechanisms or those on cognitive and behavioral aspects such as decision-making (e.g. Doya, 2002; Zhou et al., 2014). With technological advancements, levels intermediate to these are more readily studied, revealing highly heterogeneous, complex and multifunctional aspects of the 5-HT system (e.g. Cohen et al., 2015; Muzerelle et al., 2016; Okaty et al., 2015). These experimental findings present challenges in modeling and understanding 5-HT functions.
In this paper, we will review existing experimental and computational modeling work on the 5-HT system at various levels of description, from intracellular through neuronal circuit to systems and behavioral levels (sections 2–5). Toward the end (section 6), we suggest, with some examples, that a multiscale computational modeling framework that integrates across multiple scales of 5-HT functions could potentially embrace the new types of complex data and further illuminate 5-HT functions. The paper will have a computational modeling focus, and biologically-based models will be emphasized as they have previously received less attention. We will not be able to cover the wide spectrum of studies on 5-HT system. For further information regarding detailed experimental work, we shall refer the readers to other comprehensive reviews on the 5-HT system (e.g. Jacobs and Azmitia, 1992; Barnes and Sharp, 1999; Celada et al., 2013; Müller and Jacobs, 2009; Smythies, 2005). For more extensive discussions on modeling neuromodulation and their effects on cognition, we refer the readers to insightful reviews, such as those by Fellous and Linster (1998), Doya (2002), Dayan (2012) and Marder (2012).
Intracellular signaling processes
5-HT presynaptic terminals
In the presynaptic terminals, 5-HT is synthesized and stored via a series of biochemical reactions beginning with the uptake of tryptophan. With sufficient presynaptic 5-HT terminal excitability and spiking activity, 5-HT can be released into the extracellular space. The released 5-HT may bind to postsynaptic 5-HT receptors (see below), be metabolized to 5-hydroxyindoleacetic acid (5-HIAA) via monoamine oxidase (MAO), removed through diffusion, or reabsorbed back to the presynaptic terminal via serotonin reuptake transporter (SERT) (Figure 1). Polymorphisms and alterations in the SERT gene have been linked to depression and mood disorders (Ansorge et al., 2004; Caspi, 2003; Heils et al., 1996; White et al., 2005). In addition, 5-HT1A autoreceptors can regulate presynaptic neuronal firing rates, while 5-HT1B autoreceptors decrease synthesis and release with increasing extracellular 5-HT concentration, with over-expression of 5-HT1A autoreceptors implicated in reducing serotonergic neurotransmission, and associated with major depression and suicide (Albert et al., 2011). In fact, 5-HT neurons’ unique identity arises from the co-expressing of genes including those directing 5-HT synthesis, reuptake, vesicular transport, autoreceptor signaling and metabolism, and alterations in the transcription regulatory networks governing these processes can lead to physiological and behavioral pathogenesis (Deneris and Wyler, 2012).
Figure 1.
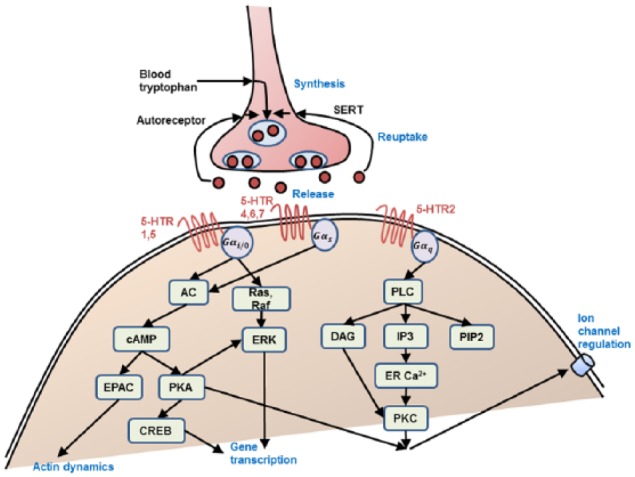
Schematic of the 5-HT presynaptic terminal processes and signal transduction pathways. Abbreviations as defined in main text. Only metabotropic 5-HT receptors are shown. Compared with Table 1.
5-HT: 5-hydroxytryptamine; SERT: serotonin reuptake transporter; Gαi/Gαo, Gαs, Gαq: isoforms of the α subunits of G protein-coupled receptors; AC: adenylyl cyclases; cAMP: cyclic adenosine monophosphate; EPAC: exchange proteins activated by cAMP; PKA: protein kinase A; CREB: cAMP response element-binding protein; Raf: rapidly accelerated fibrosarcoma kinase; ERK: extracellular signal regulated kinase; PLC: phospholipase C; IP3: inositol 1,4,5-trisphosphate; DAG: diacylglycerol; PIP2: phospholipid phosphatidylinositol 4,5-bisphosphate; ER: endoplasmic reticulum; PKC: protein kinase C.
Pharmacological drugs can directly affect these presynaptic signaling processes. For example, selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs) can block SERT on cell bodies, including presynaptic terminals, thus raising extracellular 5-HT concentration levels (Casanovas and Artigas, 1996; Gartside et al., 1995; Gillman, 2007; Tatsumi et al., 1997). Chronic treatments can lead to the desensitization of 5-HT1A autoreceptors (affecting 5-HT neuronal excitability) and downregulation of SERT mRNA (Adell et al., 2002; Benmansour et al., 2002; Mizra et al., 2007). Variations in SERT gene expression may be responsible for variability in antidepressant response (Porcelli et al., 2011). MAO inhibitors play a similar role with regard to increasing extracellular 5-HT by reducing the catabolism of 5-HT. However, TCAs and MAO inhibitors also affect other monoamines such as dopamine and norepinephrine and other receptor types, and thus their effects are more complex (Gillman, 2007; Shulman et al., 2013).
Models of 5-HT presynaptic terminals
Given the important direct relationships between intracellular signaling and drugs, it is imperative to understand their mechanisms more deeply, using in silico models. Currently, there are few computational models of 5-HT presynaptic signaling. Stoltenberg and Nag (2010) made use of control theory with differential and difference equations in developing a dynamical systems model. This model can simulate 5-HT neuronal firing rate due to tryptophan hydroxylase 2 (TPH2) and serotonin-transported-linked polymorphic region (5-HTTLPR) genotypes, and cerebrospinal fluid levels of the 5-HT metabolite 5-hydroxyin-doleacetic acid (CSF 5-HIAA). A biologically realistic mathematical model was developed by Best et al. (2010), based on previous work modeling the dopaminergic presynaptic terminal (Best et al., 2009). This model is constrained by experimental data, and consists of nine coupled nonlinear differential equations, describing the kinetic dynamics of the interacting substrates. Specific functions of the velocities could be derived via Michaelis–Menten kinetics. The model’s aim was to explore various hypotheses on 5-HT homeostasis and signaling. They include effects due to tryptophan (food intake), autoreceptor effects, acute dose of SSRIs, and polymorphisms of gene expressions.
Based on this model by Best et al. (2009), Flower and Wong-Lin (2014) used perturbation techniques to tease apart the relative dynamical timescales and relationship strengths among the substrates. This led to determining key relationships among the interacting substrates in the original full model, and allowed the reduction of the original model into simpler fast and slow versions. For example, the approximated reduced fast model could be described only by the relatively faster dynamics of the vesicular and extracellular 5-HT concentration levels, treating the rest of the substrates’ dynamics to be relatively constant. The fast reduced model, with only two differential equations to describe substrates’ dynamics, was able to substantially speed up the computational processing speed. This improvement becomes even more substantial when simulated with ~100,000 neurons, about the total number of 5-HT-containing neurons in the human brain (Flower and Wong-Lin, 2014).
While extracellular 5-HT concentration levels can be measured by traditional microdialysis methods, the temporal dynamics of the release and reuptake of extracellular 5-HT can be experimentally captured using voltammetry techniques (Bunin et al., 1998; Dankowski, and Wightman, 2013; Hashemi et al., 2011). Models based on such data have recently been developed, assuming the uptake kinetics of the extracellular 5-HT to follow the Michaelis–Menten equation, and fitted to the voltammetry data in tissue slice preparation (Bunin et al., 1998; Daws et al., 2005). The model has since been adopted into neural models in which neural firing can directly stimulate the increase in extracellular 5-HT level (Best et al., 2011; Flower and Wong-Lin, 2014; Joshi, 2014; Joshi et al., 2011, 2015, 2017). Using in vivo fast scan cyclic voltammetry, Wood et al. (2014) developed a more complex computational model to capture two distinct 5-HT reuptake mechanisms (one with high affinity and low efficiency and another with low affinity and high efficiency), and a rapid inhibitory autoreceptor control mechanism.
Computational models at the 5-HT presynaptic terminals at different levels of complexity have been developed. The generality of these models allow them to be applied to various brain areas innervated by 5-HT terminals. Importantly, there exist simpler models that are scalable (e.g. to the neuronal circuit level).
5-HT receptor signal transduction pathways
Rather early on, 5-HT was known to be an integral neuromodulator for learning and memory. In particular, due to its small nervous system, the marine mollusk Aplysia has been used as a powerful model system for nonassociative learning, such as habituation, dishabituation, and sensitization (Carew et al., 1971; Pinsker et al., 1970, 1973), and associative learning, such as classical, operant, and fear conditioning (Brembs et al., 2002; Carew et al., 1981, 1983; Lechner et al., 2000a, 2000b; Walters et al., 1981). Sensitization requires 5-HT-dependent synaptic plasticity from sensory to motor neurons (Brunelli et al., 1976; Castellucci and Kandel, 1976; MacKey et al., 1989; Marinesco and Carew, 2002). Specifically, the memory for sensitization exhibits distinct temporal phases: short-term, intermediate-term, and long-term sensitization (STS, ITS, LTS). ITS requires protein synthesis but not ribonucleic acid (RNA) synthesis, while LTS requires both protein and RNA synthesis.
These effects require knowledge of how the released 5-HT exerts its effects through a variety of membrane-bounded receptors, namely, ligand-gated ion channels and metabotropic 5-HT receptors (Maejima et al., 2013; Roth, 2006). The effects are complex and multifaceted, and hence deserve further attention. The 5-HT ligand-gated ion channels 5-HT3A and 5-HT3B are nonspecific cation channels and elicit fast excitatory postsynaptic potentials, while the metabotropic 5-HT receptors, with 7 transmembrane domains, are grouped into 6 families and 14 distinct subtypes with the heterotrimeric G-protein-coupled receptors (GPCRs) (Bockaert et al., 2006).
The heterotrimeric G-proteins are composed of the and the dimeric subunits. When the subunit is bound to guanosine diphosphate (GDP), and subunits will form the inactive heterotrimeric complex (Magali, 2012; McCudden et al., 2005; Siehler and Milligan, 2011). In the presence of 5-HT and the GPCRs activated, GDP will be replaced by guanosine triphosphate (GTP) and leads to the dissociation of G-proteins, not only activating the subunit but also liberating the subunit (Millan et al., 2008). The active subunit and subunit can relay information to different downstream signaling pathways (McCudden et al., 2005). There are several isoforms of the subunit associated with 5-HT: Gαi/Gαo, Gαs, and Gαq. For example, activated Gαi subunits inhibit the adenylyl cyclases (AC), the active Gαs subunits stimulate AC, activated Gαq subunits stimulate phospholipase Cβ (PLCβ), and activated Gαo subunits often lead to opening of K+ channels and closing of Ca2+ channels (Figure 1). The liberated dimeric subunits not only can regulate ion channels such as G-protein gated inward rectifier channels (GIRK or Kir 3 channels) as well as Ca2+ channels, but also regulate kinase and small G-proteins such as -mediated stimulation of extracellular signal regulated kinase (ERK) and mitogen activated protein kinases (MAPKs), Ras proteins and PLCβ (Berridge, 2014; McCudden et al., 2005).
AC, regulated by Gαs/Gαi, can convert ATP into the second messenger cyclic adenosine monophosphate (cAMP) using the catalytic regions on its larger cytoplasmic domains C1 and C2. The cAMP acts through three effector systems: protein kinase A (PKA), exchange proteins activated by cAMP (EPACs), and components of other signaling pathways. After cAMP binds to the regulatory subunits of PKA, the catalytic subunits of PKA can phosphorylate downstream target proteins to regulate specific cellular processes (Figure 1). The effects include: (i) enhancing gene transcription associated with formation of long-term memory regulated by cAMP response element-binding protein (CREB); (ii) increasing NMDA and AMPA receptor-mediated synaptic currents, and decreasing gamma-aminobutyric acid (GABA) receptor-mediated synaptic currents by phosphorylation of their subunits to control the receptors’ properties and their synaptic trafficking underlying plasticity; and (iii) modulating voltage-gated sodium, potassium, and calcium ion channels. EPACs bind to cAMP with high affinity and activate the Ras super family small GTPases Rap1 and Rap2, which can activate PLC, open the cyclic nucleotide-gated channels, and control the actin dynamics of cells (Cheng et al., 2008). The third kinds of effectors of cAMP are cyclic GMP phosphodiesterase (PDE) and Ca2+ channels Cav1.1 and Cav1.2. SSRIs can act on signal pathways such as cAMP on the postsynaptic neuronal cell, which can in turn release brain-derived neurotrophic factor that can enhance the growth and survival of neurons and synapses (Schwindinger and Robishaw, 2001).
PLCβ, stimulated by and subunits, can cleave the phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) into two kinds of second messenger: soluble inositol 1,4,5-trisphosphate (IP3) and membrane-adhering diacylglycerol (DAG). IP3 diffuses into the cytosol and releases Ca2+ from the endoplasmic reticulum. The DAG activates the enzyme protein kinase C (PKC), where the classic PKCs (PKCα, PKCβ and PKCγ) require both Ca2+ and DAG, whereas novel PKCs (PKCδ, PKCϵ and PKCη) are Ca2+-independent (Berridge, 2014; Turner et al., 2006).
To summarize, metabolic 5-HT receptors coupled to different isoforms of G-proteins can evoke different intracellular signaling pathways, modulating neuronal and synaptic activities. Table 1 provides some examples of the various complex modulatory effects on cells and synapses due to 5-HT receptor subtypes.
Table 1.
Some examples of serotonin (5-HT) modulatory effects on neurons and synapses due to various 5-HT receptor subtypes. Abbreviations as defined in main text. ↑ and ↓ denote an increase and decrease, respectively. Compared with Figure 1.
| Receptor subtype | Associated G-protein | Second messengers | Modulatory effects |
|---|---|---|---|
| 5-HT1 | Gαi/Gαo | cAMP,PKA | ↓cAMP, open K+ channels, ↓AMPA, ↓NMDA, ↓GABA, |
| 5-HT2 | Gαq | PLC,IP3, DAG, PKC | ↑cAMP, open Ca2+channel, ↑AMPA, ↑NMDA, ↓GABA |
| 5-HT3 | Ion channels | Ligand-gated Na+ and K+ channels | Fast excitatory postsynaptic potential |
| 5-HT4 | Gαs | cAMP,PKA | ↑cAMP |
| 5-HT5 | Gαi/Gαo | ↓cAMP | |
| 5-HT6 | Gαs | cAMP,PKA | ↑cAMP |
| 5-HT7 | Gαs | cAMP,PKA | ↑cAMP |
5-HT: 5-hydroxytryptamine; Gαi/Gαo, Gαs, Gαq: isoforms of the α subunits of G protein-coupled receptors; cAMP: cyclic adenosine monophosphate; PKA: protein kinase A; PKC: protein kinase C; DAG: diacylglycerol; PLC: phospholipase C; IP3: inositol 1,4,5-trisphosphate; AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; NMDA: N-Methyl-D-aspartic acid; GABA: gamma-Aminobutyric acid.
Models of signaling pathways and affected currents
The Michaelis–Menten kinetics formalism and the law of mass action approach are often used in modeling the biochemical signaling pathways (Keener and Sneyd, 2008; Michaelis and Menten, 1913). For example, to understand the interplay between 5-HT receptor subtypes on intracellular signaling pathway dynamics, a comprehensive mathematical model of 5-HT1A and 5-HT2A receptor-activated ERK pathways was developed by Chang et al. (2009). The transformation reactions were represented by the Michaelis–Menten formalism, and the dynamics of the reaction network formulated using the law of mass action. The results of this detailed mathematical model are in agreement with experimental data, showing the dominance of 5-HT2A receptor over 5-HT1A receptor in the MAPK signaling pathway, and the deleterious effects of regulator/enzymes affecting basal levels of ERK1/2. Hence, the model provides insights into the interplay of the two 5-HT receptor subtypes with potential applications in drug efficacy studies.
To more precisely formulate and test hypotheses of PKA and ERK activities in different memory learning/training protocols (short-term, intermediate-term, and long-term facilitations), Pettigrew et al. (2005) modeled 5-HT modulation on the Aplysia sensorimotor synapse, in which several parallel and feedback 5-HT-induced signaling pathways were simulated, including involvements of PKA, ERK and recombination-activing gene (REG). These led to intermediate- and long-term facilitations (ITF and LTF), which are correlated with ITS and LTS, respectively. Although this model was incomplete and did not include a gene regulatory step, it provided a qualitative representation of key biochemical processes essential for ITF and LTF of sensorimotor synapse. This model is subsequently modified with only the initial steps in the induction of LTF, plus a time delay in the phosphorylation the rapidly accelerated fibrosarcoma kinase and an inducer was added (Zhang et al., 2012). The modified version was used to search for a training protocol to maximize PKA–ERK interactions to enhance LTF. Later, to understand a previously unappreciated role for a PKC-dependent processes in the maintenance of STF, Zhou et al. (2014) modeled PKC signaling, in parallel with PKA mechanisms, to demonstrate that PKC was sufficient for STF at non-depressed synapses and the facilitation of depressed synapses.
In contrast with modeling the 5-HT metabotropic receptors, modeling the ligand-based 5-HT3 receptor largely comes from quantitative molecular analysis and homology modeling of the 5-HT3 receptor binding affinity, structure and dynamics (Menziani et al., 2001; Reeves et al., 2003; Schmidt and Peroutka, 1989). These modeling techniques are more complex, which required not only differential equations, but also other theories and methods in physics and chemistry (e.g. quantum mechanics).
The above examples show that computational models of 5-HT intracellular signaling via its various receptor subtypes can provide quantitative insights into the various effects caused by 5-HT receptors and the underlying biochemical pathways. Importantly, some of these pathways can be perturbed by drugs, and therefore, these models are highly useful for computer-aided drug discovery and development. However, the models have several parameters and complex pathway dynamics, and therefore remain a challenge in terms of scalability (e.g. bridging to the neuronal circuit level and beyond). In the next section, we will discuss how by directly modeling the effects (e.g. ion channel or current modulation), some of these complexities can be circumvented, providing a plausible solution.
Neuronal properties
Diversity of neuronal properties
5-HT neurons are largely found in the raphe nuclei of the brain (Müller and Jacobs, 2009). A major challenge of conventional single-unit recording is the identification of the chemical characteristics associated with the recorded neurons. For example, a substantial proportion of dorsal raphe nucleus (DRN) neurons are serotonergic: 30% in rats (Descarries et al., 1975), 70% of medium-sized DRN neurons in cats (Wiklund et al., 1981), and 70% in humans (Baker et al., 1991); however, the DRN also includes neurons containing other neurotransmitters, such as GABA, glutamate, dopamine, noradrenaline/norepinephrine and peptides (e.g. cholecystokinin, somatostatin, enkephalin, galanin, substance P, and neurotensin) (Fu et al., 2010; Jacobs and Azmitia, 1992; Kohler and Steinbusch, 1982; Michelsen et al., 2007). GABAergic neurons form the largest population of non-5-HT neurons in the dorsal raphe (Calizo et al., 2011; Celeda et al., 2001; Varga et al., 2001, 2003). However, there is little coexistence of GABA and 5-HT in the same cells (Fu et al., 2010; Shikanai et al., 2012). Dopamine, a monoamine like 5-HT, also appears to be present in DRN neurons, which are separate from 5-HT neurons (Descarries et al., 1986; Fu et al., 2010; Hökfelt et al., 1976; Yoshida et al., 1989).
The properties of neurons in the raphe nuclei are diverse and heterogeneous, including metabolism, anatomy, neurochemistry and physiology (Andrade and Haj-Dahmane, 2013; Beck et al., 2004; Fernandez et al., 2017; Gaspar and Lillesaar, 2012; Muzerelle et al., 2016; Okaty et al., 2015). Electrophysiologically, 5-HT and non-5-HT neurons are heterogeneous in terms of resting membrane potentials, input resistances, spike amplitudes and spike thresholds (Allers and Sharp, 2003; Kocsis et al., 2006; Li et al., 2001; Marinelli et al., 2004). There are also regional excitability differences among subnuclei within the DRN. For example, the lateral wings contain neurons with higher membrane excitability (Crawford et al., 2010; but see Shikanai et al., 2012). 5-HT neurons have been found to exhibit classical regular-spiking and bursting behavior (Cohen et al., 2015; Hajós et al., 1996, 2007; Hajós and Sharp, 1996; Kirby et al., 2003; Li et al., 2001) and are also associated with a characteristic slow after-hyperpolarization (AHP) (Kirby et al., 2003). The variety of 5-HT neuronal spiking behavior has been suggested to be due to the interplay among multiple ion channel currents (Aghajanian and Sanders-Bush, 2002).
Neuronal models
Despite the wealth of electrophysiological data accumulated over the decades, the membrane excitability and spike generation of 5-HT neurons have only recently been modeled (Penington and Tuckwell, 2012; Tuckwell, 2013; Tuckwell and Penington, 2014). In their latest model, based on voltage-clamp data, Tuckwell and Penington (2014) used a biophysical single-compartmental 5-HT neuronal model with 11 ion channel currents and calcium dynamics. Their conductance-based model includes fast sodium current, delayed rectifier potassium current, transient potassium current, slow non-inactivating potassium current, low-threshold calcium current, two high-threshold calcium currents, small- and large-conductance potassium currents, hyperpolarization-activated cation current, and leak currents. These currents are modeled using the Hodgkin–Huxley formalism (Hodgkin and Huxley, 1952). With this model, Tuckwell and Penington were able to account for a wide variety of electrophysiological properties of 5-HT neurons. For instance, the neuronal membrane potential of the model could exhibit spontaneous periodic spiking, spike doublets, and subthreshold humps or notches as found in experiments. The model also supported the competitive functions of the transient potassium current and the low-threshold calcium current on interspike intervals. The model also can mimic the excitatory effects of adrenoreceptor () effects by decreasing the potassium leak conductance and the resting membrane potential.
The neuronal spiking behavior of 5-HT neurons can also be modeled using different and simpler spiking neuronal models. In particular, Wong-Lin et al. (2011, 2012) have used the Izhikevich neuronal model (Izhikevich, 2003) that has only two coupled differential equations and four intrinsic neuronal parameters and hence is computationally efficient. Yet, it can replicate a repertoire of spiking and subthreshold membrane potential behaviors (e.g. slow periodic spiking, and spike doublets or triplets) as observed in 5-HT neurons without explicitly involving specific complex ion channel dynamics (Wong-Lin et al., 2011, 2012). This two-dimensional dynamical model is also highly conducive for rigorous mathematical (e.g. phase-plane) analysis, such that specific neuronal behavior can be easily selected without extensive brute-force model parameter searching (Izhikevich, 2007; Wong-Lin et al., 2011, 2012). A similar type of model, the adaptive exponential integrate-and-fire neuronal model (Brette and Gerstner, 2005), has also been adapted to model 5-HT neurons (Joshi, 2014; Joshi et al., 2015). Similarly, Tuckwell et al. (2015) subsequently also developed other reduced 5-HT neuronal models, namely the Fitzhugh–Nagumo (Fitzugh, 1961; Nagumo et al., 1962) and the reduced Hodgkin–Huxley model, both described by only two coupled differential equations. Despite some limitations (e.g. higher firing frequency), the general spiking behaviors are similar to those from experimental observations. Due to their lesser complexity, the models were also rigorously mathematically analyzed.
More generally, neuronal computational models can provide a platform to mimic the effects of 5-HT on the targeted areas, circumventing detailed modeling of signaling pathway processes. This can be done by directly simulating the affected conductances, currents or other neuronal properties. For example, based on voltage-clamp studies of sensory neurons in Aplysia, Baxter et al. (1999) used the Hodgkin–Huxley type model to describe the neuronal excitability of a sensory neuron modulated by 5-HT modulation. Specifically, the model was based on the knowledge that 5-HT induced elevation of cAMP modulated several membrane currents: (i) decreasing S current (IK,s) and a slow component of the Ca2+-activated K+ current (IK,Ca-s); (ii) decreasing the conductance and slowing the kinetics of a large, steeply voltage-dependent K+ current (IK-V); while (iii) enhancing a dihydropyridine-sensitive and slowly inactivating component of the Ca2+ current similar to the L-type Ca2+ current (ICa-L). Thus, Baxter et al. (1999) mimicked PKA and PKC effects by decreasing the conductances of IK,s, IK,Ca-s, while increasing that of ICa-L. They also simulated the actions of 5-HT on IK-V by increasing the time constants of the activating and inactivating variables. Despite the model not incorporating explicit intracellular signaling, the model could replicate experimental data of the PKA and PKC effects on membrane currents and action potential waveform. The model also predicted the important contribution of IK,Ca-s and ICa-L in the excitability of the sensory neurons, which had not been investigated prior to this work. Moreover, the model prompted further investigation of Na+ currents to fill the remaining discrepancy between the neuronal model behavior and experimental data.
Other models that mimicked the affected currents include Bertram (1993, 1994). Bertram (1993) modeled and mathematically analyzed how 5-HT could affect the burster R15 neuronal activity by modulating its subthreshold inward rectifier currents (IR) and negative-slope-region currents (INSR) that control the neuronal bursting mechanism. The model suggested that 5-HT increases the sensitivity of the burster neuron to synaptic perturbations due to the competition between various states (stationary, bursting and the beating attractor). Bertram (1994) further reduced the model for more detailed mathematical analysis of the model.
With a similar modeling approach, Cano-Colino et al. (2014) modeled 5-HT1A and 2A effects on prefrontal cortical neurons by modulating the 5-HT induced currents. Specifically, the 5-HT1A effects on prefrontal cortical (PFC) excitatory pyramidal cells were simulated by modeling the inhibitory 5-HT modulation on some GIRK currents (IGIRK). In contrast, 5-HT2A exerted excitatory effects on pyramidal cells via increased in intracellular Ca2+, which simultaneously inhibited Ca2+-activated afterhyperpolarization currents (IK,Ca) and activated an afterdepolarization current mediated by a Ca2+-dependent nonselective cation channel (ICan). In addition, their model also included 5-HT2A modulation of prefrontal inhibitory interneurons by decreasing the conductance of their leak current. This model will be further discussed in the next section.
To allow even higher scalability in the modeling, Joshi et al. (2017) fitted a nonlinear relationship between 5-HT concentration levels and the induced currents on targeted neurons based on experimental data from electrophysiology, pharmacology and voltammetry. By shifting the nonlinear input–output functions or changing the parameter values in the Michaelis–Menten relationships, the model can simulate drug (e.g. antidepressant) effects. The modeling framework proposed was sufficiently general to model the interactions of multiple drugs and neuromodulators on multiple brain regions. Simpler and more abstract models have been developed (Jalewa et al., 2014; Joshi et al., 2011); the modulated currents in Jalewa et al. (2014) were more abstract (i.e. based on a neural firing-rate type model (Wilson and Cowan, 1972)); and Joshi et al. (2011) directly obtained the nonlinear function from 5-HT to the neuronal population firing rate without introducing any affected currents. In contrast with 5-HT modulation via its metabotropic receptors, simplified more scalable model for 5-HT3 mediated currents has only recently been modeled (Jalewa et al., 2014). These models will be further discussed in the next section.
In summary, the computational models of 5-HT neurons can be rather complex with several ion channel currents involved. However, more recent modeling work has attempted to reduce the model to allow better scalability (e.g. neuronal circuit level). Models of neurons and neural populations innervated by 5-HT can also be simulated, circumventing the need to explicitly model the complex signaling transduction pathways. This is mainly achieved through direct modeling of the affected currents in the neuronal or neuronal population models.
Single neuronal functions and neuronal microcircuits
Circuits and functions of 5-HT neurons
A useful way to understand 5-HT function in the brain is to measure the activation pattern of single cells (‘units’) in the raphe nuclei of animals performing behavioral tasks. This can provide rich information about the context and events encoded by DRN neurons, at least some of which secrete 5-HT, and behavioral-pharmacological experiments can then examine how 5-HT is utilized at the projection sites. Due to its high spatiotemporal resolution, single-unit recording is also beneficial for analyzing trial-by-trial changes in activity related to particular aspects of behavioral tasks.
To date, several characteristics and heterogeneity of DRN neuronal activity have emerged from single-unit recording studies in awake animals (Cohen et al., 2015; Hayashi et al., 2015; Inaba et al., 2013; Li et al., 2013, 2016; Miyazaki et al., 2011; Nakamura et al., 2008; Ranade and Mainen, 2009). First, DRN neurons respond to various task events, including visual, olfactory, and somatosensory events, to incentive events such as rewards and punishments, to movement, and to delay, which indicates that there is a wide array of inputs to the DRN. Second, different activation patterns in terms of timescale were observed; very brief responses, sensory stimuli; brief responses well-aligned to the onset of tonic activity that may last across trials, which encodes appetitive and aversive contexts (Cohen et al., 2015; Hayashi et al., 2015). Third, responses with modulations in opposite directions were observed. For example, a group of neurons exhibited an increase while others exhibited a decrease in activity during the same sensory events, such as a tail pinch (Schweimer and Ungless, 2010) or air puff (Cohen et al., 2015). Task factors such as reward value also evoked opposite direction modulations in DRN neurons (Hayashi et al., 2015; Nakamura, 2013). It is unknown how and why such mirror-imaged modulation in neuronal activity is created and utilized downstream of the information processing. The characteristics of the DRN neuronal activity described above are in strong contrast to those observed in midbrain dopamine neurons which typically exhibit phasic, not tonic, and uniformly excitatory responses (Nakamura, 2013).
While the activities of DRN neurons are correlated with a variety of events (Ranade and Mainen, 2009), it appears that reward information is one of the most influential factors that modulate DRN neuronal activity (Bromberg-Martin et al., 2010; Cohen et al., 2015; Inaba et al., 2013; Luo et al., 2015; Miyazaki et al., 2011; Nakamura et al., 2008; Ranade and Mainen, 2009). One of the prevailing questions is whether the DRN is involved in appetitive or aversive information processing. To answer this question, Hayashi et al. (2015) measured the activities of DRN neurons while monkeys performed a Pavlovian conditioning task, in which visual cues predicted appetitive or aversive outcomes. In the ‘appetitive’ trial blocks, conditional visual stimuli which were associated with a liquid reward at different probabilities were presented. In the ‘aversive’ blocks, visual stimuli were associated with an aversive air puff at various probabilities. Note that, this separate presentation of appetitive and aversive conditional stimuli as a block created the appetitive and aversive context. Hayashi et al. (2015) found that single DRN neurons encode both appetitive and aversive information, but over differing time scales: a tonic and categorical activity to discriminate emotional (i.e. appetitive and aversive) contexts, and a relatively phasic, quantitative activity to encode rewarding events (i.e. the probability of outcomes) (Hayashi et al., 2015).
In terms of animal models of psychiatric disorders, recent work using acute pharmacogenetics perturbation on 5-HT neuronal activity of the dorsal and median raphe nuclei has demonstrated that these nuclei are causally linked to differential control on emotional behaviors (Teissier et al., 2015). Specifically, median raphe 5-HT hyperactivity in mice seems to encourage anxiety behavior while low dorsal/median raphe 5-HT activity increases depression-like behavior.
In addition to the raphe nuclei’s heterogeneous populations of 5-HT and non-5-HT neurons and diverse expression of neurotransmitters (and co-transmitters), these neurons also provide diffuse projection to multiple targets, thereby increasing their complexity. The mammalian DRN contains the majority of forebrain-projecting 5-HT neurons (Azmitia and Segal, 1978; Descarries et al., 1975; Jacobs and Azmitia, 1992; Moore et al., 1978; Vertes, 1991), which appears to be highly conserved across vertebrates (Azmitia, 2007). However, the projection of DRN 5-HT neurons is selective. Some 5-HT neurons make collaterals to multiple distal structures, but many appear to selectively target particular areas (Gagnon and Parent, 2014; Vasudeva et al., 2011; Waselus et al., 2011). Glutamatergic inputs appear to be nonuniformly distributed among DR subnuclei (Commons, 2009; Crawford et al., 2011), which could contribute to the diversity of neuronal activity in vivo. 5-HT neurons in different areas are highly interconnected (Bang et al., 2012), which may perhaps be important for autoregulation. Interestingly, GABAergic and glutamatergic inputs to DRN neurons can themselves be regulated by 5-HT (Lemos et al., 2006), which form larger regulatory circuits.
Many GABAergic neurons in the DRN are synaptically connected to 5-HT neurons (Bang and Commons, 2012; Weissbourd et al., 2014). DRN’s GABAergic neurons inhibit at least 20% of midline 5-HT neurons, and are likely important regulators of 5-HT function (Challis et al., 2013). For instance, the corticotropin-releasing factor, a stress-related hormone, can inhibit 5-HT neurons both directly and indirectly (via DRN’s GABAergic neurons) (Kirby et al., 2008; Lowry et al., 2000; Pernar et al., 2004). VGLUT3-expressing (glutamatergic) neurons also form a sizeable population in the DRN (Hioki et al., 2010). Recent studies have suggested a role in reward-based processes (Liu et al., 2014; McDevitt et al., 2014). Interestingly, there appears to be a substantial coexpression of 5-HT and glutamate in many DRN cells, with the potential to regulate targets across multiple timescales (Cohen et al., 2015; Johnson, 1994; Trudeau, 2004). Some of the dopaminergic neurons in DRN can also co-release glutamate and have been shown, with in vivo calcium imaging and optogenetics techniques, to be causally linked to social preference (following isolation) and place avoidance (Matthews et al., 2016).
Animal models with simpler organizations have also been useful to further illuminate the functions of 5-HT on targeted microcircuits (Marder, 2012). For instance, in zebrafish, the habenulo-raphe pathway is found to be necessary for active avoidance learning but not classical fear conditioning (Amo et al., 2014). In Cancer borealis crab, two mutually coupled neurons isolated from the gastric mill of the stomatogastric ganglion have been shown to be modulated by 5-HT by increasing the alternating bursting regime in parameter space and burst frequency (Grashow et al., 2009). In the nematode Caenorhabditis elegans, 5-HT could for example promote exploitation by speeding up foraging decision-making under complex environments (Iwanir et al., 2016) and transition from crawling to swimming (Vidal-Gadea et al., 2011), while in leeches and lampreys, 5-HT can modulate swimming behavior (Brodfuehrer et al., 1995; Harris-Warrick and Cohen, 1985).
Overall, the picture that has emerged is one of heterogeneity of cell type and connectivity, which has limited strong support for any particular theory of 5-HT function, though there may be some principles of afferent connectivity (Dorocic et al., 2014; Ogawa et al., 2014).
Neural circuit models
There are several computational models of 5-HT modulation of neural microcircuits. For instance, Meeter et al. (2006) had used a spiking neuronal network model of the hippocampus that included the entorhinal cortex, dentate gyrus, and fields CA1 and CA3, and demonstrated that 5-HT-mediated hyperpolarizing effect on principal cells could affect memory performance. Using simpler ‘firing-rate’-type network models, which included connectivity across the cortex, striatum, DRN, substantia nigra compacta (SNc) and thalamus, Reed et al. (2013) showed that long-range feedback connections in the circuit allows 5-HT to stabilize the network as dopamine neurons get depleted as in Parkinson’s disease. Given the known connections between DRN and the lateral hypothalamus (LHA), Joshi et al. (2011), Jalewa et al. (2014), Joshi (2014), and Joshi et al. (2017) had also developed similar firing-rate type models that described the interactions between the DRN and LHA. In the model in Jalewa et al. (2014), non-5-HT GABAergic neurons in the DRN and LHA were included to study the effects of direct and indirect connectivity on the DRN-LHA circuit dynamics. Using a spiking neuronal network model, which consisted of heterogeneous 5-HT and non-5-HT DRN neurons, Wong-Lin et al. (2011, 2012) accounted for the single-unit neuronal data from non-human primates performing rewarded and unrewarded tasks (Bromberg-Martin et al., 2010), as discussed above. After fitting the data, the model identified a potential DRN microcircuit model architecture that predicted the presence of fast inhibition from the non-5-HT to 5-HT neurons, and slow theta band oscillation in the network.
As the PFC is one of the most densely 5-HT modulated brain regions (Celada et al., 2013), it is important to model and understand the potential effects 5-HT has on the PFC functions. Given that dopamine is also known to strongly innervate the PFC, Wang and Wong-Lin (2013) used (‘mean-field’) firing-rate models to mathematically analyze how dopamine and 5-HT co-modulation on PFC neurons and synapses can affect PFC circuit dynamics. The PFC network model consisted of multiple pyramidal neuronal populations and fast spiking inhibitory interneurons, and NMDA-, AMPA- and GABA-mediated synapses. 5-HT1A, 5-HT2A, dopaminergic D1 and D2 receptor-mediated effects were implemented in the model, constrained by past experimental findings. The network model’s oscillatory behavior was found to be co-modulated in complex, non-intuitive ways, due to the different affinities and the PFC network connectivity. For example, the model showed that certain combination of dopamine and 5-HT receptors could lead to the robustness of beta and gamma band oscillations, or the existence of multiple discrete oscillatory regimes. The model also made predictions in terms of pharmacological (receptor agonist/antagonist) effects. For instance, the model could mimic the effects of 5-HT2A antagonists (by shifting of the input–output function) causing the reduction of beta band oscillation amplitude, which was consistent with 5-HT2 agonist (2,5-dimethoxy-4-iodoamphetamine; DOI) effects in the frontal cortex of anesthetized rats (Budzinska, 2009).
In another work, Cano-Colino et al. (2013, 2014) simulated the effects of 5-HT1A and 5-HT2A receptors on the PFC by incorporating simplified induced currents, IGIRK, and modulating membrane currents IK,Ca, and ICan and the leak currents, as discussed previously. This was then used to investigate the effects of 5-HT on spatial working memory (SWM). The PFC model, inspired by non-human primate studies (Williams et al., 2002), included inhibitory interneurons and pyramidal neurons, which were used to represent the maintenance of information about different target spatial locations. The model in Cano-Colino et al. (2014) showed that with increasing 5-HT concentration level, SWM performance of the network followed an inverted U-shape manner due to the differential effects of 5-HT1A and 5-HT2A receptors. The model suggested that SWM output errors due to low and high 5-HT levels are caused by the network’s dynamical instabilities. In Cano-Colino et al. (2013), further predictions of the same model were made. Specifically, the model suggested that excessive levels of 5-HT could cause SWM deficits that increased with delay duration, and higher vulnerability to distractors. Interestingly, the neuronal memory fields were predicted to be better tuned than the behavioral report for excessive 5-HT.
Maia and Cano-Colino (2015) also used a similar model to provide an explanation for reduced 5-HT levels leading to obsessive-compulsive disorder (OCD). Specifically, the model suggested that a decrease in 5-HT levels made the network more stable and difficult to get out of a stable steady (‘attractor’) state (arguably a neural substrate for obsessions and compulsions). Simulating the effects of a 5-HT2A blocker (mimicking the partial effects of second-generation antipsychotics) or a 5-HT1A agonist was effective in reducing the OCD state, through decreasing the overall network’s excitability.
Serotonergic influence on synapses has been modeled in a similar way. For example, Puzerey et al. (2014) modeled a cortical (layer 2/3) microcircuit model using excitatory and inhibitory neurons, bombarded by synaptic noise. The network model could mimic the effects of spontaneous epileptic seizures (‘fast runs’) by increasing the synaptic noise (to simulate 5-HT3R-mediated modulation) and excitatory coupling strength (to simulate 5-HT2R-mediated modulation). The inhibition of slow afterhyperpolarization current alone (due to 5-HT2R-mediated modulation) could also induce fast-run oscillation. By increasing the excitatory neuronal leak conductance (to simulate the postsynaptic effect of 5-HT1R activation in the presence of antidepressant fluoxetine) the epileptiform network dynamics can be suppressed, as observed in experiments. To capture a more systemic modulation, the abovementioned Wang and Wong-Lin (2013) work not only modeled the PFC neuronal excitability modulation by 5-HT, but also simultaneously simulated 5-HT modulation on the synapses by varying the excitatory and inhibitory synaptic coupling strengths.
The models discussed here showed how they could be used to systematically and quantitatively study 5-HT modulation on neuronal circuits within and out of the raphe nuclei. Importantly, this type of neural circuit models is promising in terms of mechanistically bridging from neuronal and synaptic physiological and pharmacological level to cognition and behavior.
Human cognition and behavior
Human studies
Similar to the animal studies discussed above, 5-HT in humans is known to play a role in a range of processes. They include learning and memory, aggression, sexual behavior, and sleep, among others (Harvey, 2003; Hull et al., 2004; Moresco et al., 2002). Dysfunctions in the 5-HT system have been linked to a range of brain disorders, including depression, anxiety disorders, impulsivity, eating disorders, schizophrenia, and addiction (Dalley et al., 2008, 2011; Maes and Meltzer, 1995; Tanaka et al., 2007; Young, 2013).
5-HT function in humans could be studied with various approaches. They include behavioral neurogenetics (the relationship between genes coding for 5-HT system and behavior), tryptophan depletion (which reduces 5-HT levels synthesized in the brain; see Figure 1), and psychopharmacological (the administration of 5-HT agonists and antagonists to healthy human subjects) studies. These can be combined with brain imaging (e.g. positron emission tomography and functional magnetic resonance imaging; fMRI) (Beliveau et al., 2015; Kumar and Mann, 2014; Spies et al., 2015) and genes related to 5-HT function, (e.g. associated with SERT and receptors) (Hariri and Holmes, 2006). In humans, the short allele of this gene has been shown to correlate with harm avoidance behavior compared with individuals with two copies of the long allele (Katsuragi et al., 1999; Lesch et al., 1996; Ricketts et al., 1998). Genetic variation of the 5-HT2A receptor gene is known to influence episodic memory in humans (de Quervain et al., 2003), while the SERT gene can affect risk-based decision-making (He et al., 2010; Kuhnen et al., 2013). Many tryptophan depletion studies investigated its role in learning as well as affective processes. For example, modulation in 5-HT levels by acute tryptophan depletion modulates the sensitivity to punishment (for review, Cools et al., 2011). Although controversial, several reports indicate acute tryptophan depletion induces inappropriate switching after probabilistic punishments (i.e. too sensitive to punishments) (Chamberlain, et al., 2006; Cools et al., 2008; Robinson et al., 2012). Psychopharmacological methods can investigate both decrease and increase in 5-HT levels on behavior. For example, 5-HT agonist psilocybin could reduce attentional performance in healthy human patients (Carter et al., 2005). At a more abstract level, 5-HT has also been found to promote prosocial behavior (Siegel and Crockett, 2013).
Cognitive models
Existing computational cognitive models focus on one or a few of 5-HT’s functions. Many of the models employ high-level reinforcement learning models such as temporal difference learning (Doya, 2002; Huys et al., 2016). There are models that suggest 5-HT plays an antagonistic function with respect to that of dopamine (Asher et al., 2010, 2013; Daw et al., 2002; Weng et al., 2013; Zaldivar et al., 2010). Although there are experimental supports to these studies (Hebart and Glascher, 2015), more recent experimental studies have shown that this theory is incomplete, as there are complex interactions between both neuromodulators (see above). In addition, some 5-HT receptors are found to inhibit dopamine release while others facilitate dopamine release (Alex and Pehek, 2007; Boureau and Dayan, 2011).
Other models have suggested that 5-HT plays more of a role in the scaling of future rewards (Doya, 2002; Schweighofer et al., 2008; Tanaka et al., 2007). Specifically, in these models, 5-HT controls the timing of reward prediction signals, which is represented by the discount factors in reinforcement learning models (Doya, 2002). Functional MRI (Tanaka et al., 2007) and dietary tryptophan depletion (Schweighofer et al., 2008) studies support the role of 5-HT in controlling the timescale of reward prediction as suggested by Doya (2002). A more recent computational modeling work has suggested that 5-HT projections to the striatum plays a role in risk computation, that is, computations related to reward variance (Balasubramani et al., 2014, 2015). These models were able to account for large behavioral datasets, including the role of 5-HT in reward, punishment, and risk-based decision-making, as reported in experimental studies (Homberg, 2012; Long et al., 2009; Murphy et al., 2009; Rogers, 2011).
Overall, cognitive models on 5-HT have been very successful in accounting for various animal and human neural data and decision-making behavior, and their changes due to drugs or psychiatric disorders. These models typically have fewer parameters than neuronal circuit models. However, a challenge would be to bridge it back to the more physiologically constrained neural circuit models.
Toward bridging across the scales
We have reviewed various experimental and computational approaches, from intracellular to human behavioral levels, and we have seen the complex functional roles played by 5-HT. A major contribution of this review is the synthesis and categorization of a wide range of computational and mathematical models of 5-HT systems. Table 2 provides a summary of the discussed models. Clearly, a number of them are limited to a single level of organization. Hence, an integrated view of 5-HT functions is currently lacking (Nakamura and Wong-Lin, 2014). To make better sense of its multifaceted roles, new approaches are needed.
Table 2.
Summary of the types of computational models. At each level of organization, models may span across multiple levels of organization. Italics: multiscale models.
| Common scale of description | Models | Range of scales | Mathematical/computational descriptions |
|---|---|---|---|
| Single neuronal electrophysiology (membrane potential dynamics) | Tuckwell et al. (2015); Tuckwell and Penington (2014) | Ion channels to neuronal membrane potential | Differential equations |
| Wong-Lin et al. (2011, 2012) | Neuronal membrane potential to behavior | ||
| Presynaptic terminal (5-HT synthesis, release and reuptake) | Best et al. (2010); Stoltenberg and Nag (2010) | Intracellular processes | Differential equations |
| Flower and Wong-Lin et al. (2012) | Intracellular processes and neuronal membrane potential | ||
| Bunin et al. (1998); Wood et al. (2014) | Release-reuptake | ||
| Joshi et al. (2011) | Release-reuptake to neural population dynamics | ||
| Signal transduction & modulated currents | Menziani et al. (2001); Reeves et al. (2003); Schmidt and Peroutka (1989) | Molecule/protein dynamics | Differential equations, and other techniques in physics and chemistry |
| Pettigrew et al. (2005); Zhang et al. (2012); Zhou et al. (2014) | Intracellular processes | Differential equations | |
| Bertram (1993, 1994) | Ion channel currents | ||
| Cano-Colino et al. (2014) | Ion channel currents to behavior | ||
| Joshi (2014); Joshi et al. (2011; 2017) | Release-reuptake to neural population | ||
| Neural circuit | Jalewa et al. (2014); Joshi et al. (2011); Puzerey et al. (2014); Wang and Wong-Lin (2013) | Microcircuit | Differential equations |
| Cano-Colino et al. (2013, 2014); Maia and Cano-Colino (2015); Meeter et al. (2006); Wong-Lin et al. (2011, 2012) | Microcircuit to behavior | ||
| Joshi et al. (2017); Reed et al. (2013) | Large-scale circuit | ||
| Cognition and behavior | Asher et al. (2013); Balasubramani et al. (2014); Daw et al. (2002); Doya (2002); Weng et al. (2013); Zaldivar et al. (2010) | Behavior | Algorithms, optimal/Bayesian, and difference/differential equations |
| Balasubramani et al. (2015) | Neural circuit and behavior |
5-HT: 5-hydroxytryptamine.
A potential direction would be to develop multiscale computational models that can bridge across multiple levels of descriptions. The models at different scales have to be linked in a consistent way so that the information from a lower scale can be carried into the simplified model at a higher scale. The advantages of such a computational approach are that they can integrate experimental data at various levels and from separate sources, shed insights into the mechanisms across different levels, and form predictions for future experiments.
As an example, suppose there was a research enquiry regarding how the (e.g. oscillatory) dynamics of a neural network is affected in a certain brain function disorder, say major depressive disorder. A biophysically detailed neural network model can reveal how specific intracellular or genetic processes can influence it. Reduced versions of the network model can be rigorously mathematically analyzed and predictions can be made regarding how specific cognition and behavior (e.g. memory) are altered. Multiscale modeling is already used in many fields of natural sciences and engineering (E, 2011; Horstemeyer, 2009) and in biology and systems medicine (Qu et al., 2011; Schnell et al., 2007). However, it has yet to be readily embraced in modeling neuromodulator systems.
Based on our review, we have already seen promising computational models that have bridged across multiple scales (italics in Table 2). For example, we have discussed 5-HT-based computational models that bridge from ion channels through neural networks to cognitive functions (e.g. Cano-Colino et al., 2013, 2014; Wang and Wong-Lin, 2013; Wong-Lin et al., 2011, 2012). Such models can also incorporate neuronal diversity in the DRN (e.g. Wong-Lin et al., 2011, 2012) or targeted brain regions (Cano-Colino et al., 2013, 2014; Wang and Wong-Lin, 2013). Other similar modeling attempts had been focused more on the effects of other monoaminergic systems, namely, dopaminergic and norepinephrine/noradrenaline modulation, especially on cortical computation (e.g. Brunel and Wang, 2001; Durstewitz and Seamans, 2002; Eckhoff et al., 2009, 2011).
One of the key ingredients, and indeed a major challenge when developing multiscale models, is to find essential components or behaviors at each scale that have to be retained and bridge to a higher scale. Otherwise, the overall model will become overwhelming with details, and a conceptual understanding of mechanisms will remain elusive. For instance, when there are multiple temporal scales co-existing, only certain biological variables (e.g. fast variables at the intracellular level of a 5-HT presynaptic terminal) may be important to produce certain behavior (e.g. neuronal activity dependent extracellular 5-HT level) at a higher scale, while leading to a lower dimensional model (e.g. Flower and Wong-Lin, 2014). However, in general it is not always clear cut what components need to be retained or ignored.
An advantage across many of the models discussed is their common mathematical ‘language’, in that they can generally be described by discrete (e.g. state transition algorithms in reinforcement learning) or more often, continuous (e.g. differential equations in neuronal circuit model) dynamical systems (Guckenheimer and Holmes, 1983; Strogatz, 2001). Thus, theories from dynamical systems can provide useful tools to model, analyze and unify various (e.g. emergent) phenomena and concepts in neurobiological and cognitive systems (Finkel, 2000; Freeman, 2007; Rabinovich et al., 2007).
Given this advantage, we may be able to make use of well-established dynamical model reductions methods, (e.g. center manifolds) (Guckenheimer and Holmes, 1983). For example, when certain compositions or dynamical variables of the system operate at relatively slower dynamics, the much faster dynamical variables can be assumed to have reached their quasi-steady states, or averaged out over time and spared the need to be updated over time (e.g. Ermentrout and Terman, 2010; Guckenheimer and Holmes, 1983; Izhikevich, 2007; Roxin and Ledberg, 2008; Wong and Wang, 2006). A simple but effective perturbation technique has been used to elucidate important timescales and strengths of relationships of substrates within the 5-HT presynaptic terminal model, which subsequently led to a reduced low-dimensional model, which can be incorporated into the next (neuronal circuit) scale (Flower and Wong-Lin, 2014). A recent similar technique has also been applied to dopaminergic presynaptic terminals (Cullen and Wong-Lin, 2014, 2015). Thus, well-justified approximations can reduce the number of model parameters and complexity to enhance understanding of the essentials while allowing scalability across multiple scales and with more efficient computations.
Further, ‘mean-field’ techniques, originating from statistical physics (to bridge atomic interactions to thermodynamical properties), have been successfully used for analyzing the emergent dynamical behavior of neural systems (Amit, 1992; Amit et al., 1985; Hopfield, 1982; Wilson and Cowan, 1972, 1973). Mean-field approaches are excellent approximations for understanding stationary states and linear (first-approximation) temporal responses of networks. Extended versions have now been used to investigate behaviors of sufficiently realistic noisy spiking neuronal circuits, providing a means to connect from an ion channel to cognitive models while allowing conceptual understanding via dynamical systems theory (e.g. Brunel and Wang, 2001; Burkitt, 2006a, 2006b; Eckhoff et al., 2011; Hasegawa, 2003; Renart et al., 2003). For example, Wang and Wong-Lin (2013) showed that it is possible to perform rigorous mathematical (stability) analysis of a system of heterogeneous populations of cortical neurons co-modulated differently by 5-HT and dopamine. In another example, Joshi et al. (2017) proposed a promising mean-field modeling framework to bridge to large-scale brain circuit dynamics, which can link pharmacological mechanisms to neuroimaging studies. However, more advanced mean-field approaches that embrace heterogeneity in the system (e.g. Harrison et al., 2015; Ly, 2015; Nichola and Campbell, 2013) would be required to model the complex and heterogeneous 5-HT system.
Crucial to the development of computational models and theoretical approaches are the availability of accurate experimental data. Despite decades of research linking 5-HT with its various functions such as emotional control, we still do not know what information 5-HT neurons signal nor how such signals are encoded. There are two reasons for such slow progress: (i) there is a lack of methods to record the activity of identified 5-HT neurons during behavior, and previous attempts are largely based on neuronal electrophysiological characteristics; and (ii) recent work has shown that the midbrain raphe is more complex than anticipated, with multiple 5-HT neuron subtypes. Interactions within the raphe microcircuitry also remain poorly understood.
To overcome the above issues, technical advances to tag neurons with chemical and anatomical specificity would lead to further understandings of the roles of specific types of neurons in the raphe (Cohen et al., 2015; Liu et al., 2014). Advanced data analytical (e.g. optogenetic) tools to manipulate serotonergic neurons would help refine current theories of their functions (Cohen et al., 2015; Fonseca et al., 2015; Liu et al., 2014; McDevitt et al., 2014; Miyazaki et al., 2014). Capturing the 5-HT system’s complexity will require large-scale recordings of identified neurons in behaving animals, and the generation of large datasets. Advanced analytical data (e.g. machine learning) techniques, modeling, and interpretation of such datasets will be required (Gao and Ganguli, 2015; Helmstaedter, 2015; Huys et al., 2016). In human studies, elucidating the connectivity changes due to 5-HT perturbations (e.g. drugs or gene alleles) could illuminate the whole-brain changes on behavior. Hence, effective/functional connectivity analysis of global brain activity such as dynamic causal modeling, Granger causal analysis and machine learning may prove useful (e.g. Beliveau et al, 2015; Youssofzadeh et al., 2015a, 2015b).
In summary, we have reviewed current experimental and computational work on 5-HT across multiple scales. We have also suggested that a more holistic understanding of the 5-HT’s complex functions may require an integrated multiscale modeling approach. With the availability of such multiscale models, one can rapidly test hypotheses, and provide model predictions that can be verified by future experiments. Such an approach could also illuminate future treatments related to brain disorders involving serotonergic dysfunctions, including anxiety, depression, schizophrenia and post-traumatic stress disorder. All of these will depend not only on advanced theoretical and computational techniques, but also on advanced experimental methods to generate better datasets and the systematic curation of the data.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: KFW-L was supported by BBSRC (BB/P003427/1), COST Action Open Multiscale Systems Medicine (OpenMultiMed) supported by COST (European Cooperation in Science and Technology), and Northern Ireland Functional Brain Mapping Facility (1303/101154803) funded by Invest NI and the University of Ulster. D-H W was supported by NSFC under grant 31671077, and 31511130066. KFW-L and D-HW were also jointly supported by The Royal Society - NSFC International Exchanges. KN was supported by a Grant-in-Aid for Scientific Research B, the Human Frontier Science program, Precursory Research for Embryonic Science and Technology, Strategic Research Program for Brain Sciences by the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Abbott LF. (2008) Theoretical neuroscience rising. Neuron 60: 489–495. [DOI] [PubMed] [Google Scholar]
- Adell A, Celada P, Abellán MT, et al. (2002) Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res Rev 39: 154–180. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Sanders-Bush E. (2002) Serotonin. In: David KL, Charney D, Coyle JT, et al. (eds) Neuropsychopharmacology: The Fifth Generation of Progress. New York: Raven Press, Ltd, pp. 15–46. [Google Scholar]
- Albert PR, Le François B, Millar AM. (2011) Transcriptional dysregulation of 5-HT1A autoreceptors in mental illness. Mol Brain 4: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alex KD, Pehek EA. (2007) Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther 113: 296–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allers KA, Sharp T. (2003) Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience 122: 193–204. [DOI] [PubMed] [Google Scholar]
- Amit DJ. (1992) Modeling Brain Function: The World of Attractor Neural Networks. Cambridge: Cambridge University Press. [Google Scholar]
- Amit DJ, Gutfreund H, Sompolinsky H. (1985) Spin glass models of neural networks. Phys Rev A 32: 1007–1018. [DOI] [PubMed] [Google Scholar]
- Amo R, Fredes F, Kinoshita M, et al. (2014) The habenulo-raphe serotonergic circuit encodes an aversive expectation value essential for adaptive active avoidance of danger. Neuron 84: 1034–1048. [DOI] [PubMed] [Google Scholar]
- Andrade R, Haj-Dahmane S. (2013) Serotonin neuron diversity in the dorsal raphe. ACS Chem Neurosci 4: 22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, et al. (2004) Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 306: 879–881. [DOI] [PubMed] [Google Scholar]
- Asher DE, Craig AB, Zaldivar A, et al. (2013) A dynamic, embodied paradigm to investigate the role of serotonin in decision-making. Front Integr Neurosci 7: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher DE, Zaldivar A, Krichmar JL. (2010) Effect of Neuromodulation on Performance in Game Playing: A Modeling Study. Paper presented at the Development and Learning (ICDL), 2010 IEEE 9th International Conference, University of Michigan, Ann Arbor, 18–21 August 2010. [Google Scholar]
- Azmitia EC. (2007) Serotonin and brain: Evolution, neuroplasticity, and homeostasis. Int Rev Neurobiol 77: 31–56. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. (1978) An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol 179: 641–668. [DOI] [PubMed] [Google Scholar]
- Baker KG, Halliday GM, Halasz P, et al. (1991) Cytoarchitecture of serotonin-synthesizing neurons in the pontine tegmentum of the human brain. Synapse 7: 301–320. [DOI] [PubMed] [Google Scholar]
- Balasubramani PP, Chakravarthy VS, Ali M, et al. (2015) Identifying the basal ganglia network model markers for medication-induced impulsivity in Parkinson’s disease patients. PLoS ONE 10: e0127542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramani PP, Chakravarthy VS, Ravindran B, et al. (2014) An extended reinforcement learning model of basal ganglia to understand the contributions of serotonin and dopamine in risk-based decision making, reward prediction, and punishment learning. Front Comput Neurosci 8: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang SJ, Commons KG. (2012) Forebrain GABAergic projections from the dorsal raphe nucleus identified by using GAD67-GFP knock-in mice. J Comp Neurol 520: 4157–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang SJ, Jensen P, Dymecki SM, et al. (2012) Projections and interconnections of genetically defined serotonin neurons in mice. Eur J Neurosci 35: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. (1999) A review of central 5-HT receptors and their function. Neuropharmacology 38: 1083–1152. [DOI] [PubMed] [Google Scholar]
- Baxter DA, Canavier CC, Clark JW, Jr, et al. (1999) Computational model of the serotonergic modulation of sensory neurons in Aplysia. J Neurophysiol 82: 2914–2935. [DOI] [PubMed] [Google Scholar]
- Beck SG, Pan YZ, Akanwa AC, et al. (2004) Median and dorsal raphe neurons are not electrophysiologically identical. J Neurophysiol 91: 994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beliveau V, Svarer C, Frokjaer VG, et al. (2015) Functional connectivity of the dorsal and median raphe nuclei at rest. Neuroimage 116: 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmansour S, Owens WA, Cecchi M, et al. (2002) Serotonin clearance in vivo is altered to a greater extent by antidepressant-induced downregulation of the serotonin transporter than by acute blockade of the transporter. J Neurosci 22: 6766–6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. (2014) Cell Signalling Pathways. London: Portland Press Limited. [Google Scholar]
- Bertram R. (1993) A computational study of the effects of serotonin on a molluscan burster neuron. Biol Cybern 69: 257–267. [DOI] [PubMed] [Google Scholar]
- Bertram R. (1994) Reduced-system analysis of the effects of serotonin on a molluscan burster neuron. Biol Cybern 70: 359–368. [DOI] [PubMed] [Google Scholar]
- Best JA, Nijhout HF, Reed MC. (2009) Homeostatic mechanisms in dopamine synthesis and release: A mathematical model. Theor Biol Med Model 6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best J, Nijhout HF, Reed M. (2010) Serotonin synthesis, release and reuptake in terminals: A mathematical model. Theor Biol Med Model 7: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best J, Nijhout HF, Reed M. (2011) Bursts and the efficacy of selective serotonin reuptake inhibitors. Pharmacopsychiatry 44: S76–S83. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Becamel C, et al. (2006) Neuronal 5-HT metabotropic receptors: Fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res 326: 353–372. [DOI] [PubMed] [Google Scholar]
- Boureau YL, Dayan P. (2011) Opponency revisited: Competition and cooperation between dopamine and serotonin. Neuropsychopharmacology 36: 74–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brembs B, Lorenzetti FD, Reyes FD, et al. (2002) Operant reward learning in Aplysia: Neuronal correlates and mechanisms. Science 296: 1706–1709. [DOI] [PubMed] [Google Scholar]
- Brette R, Gerstner W. (2005) Adaptive exponential integrate-and-fire model as an effective description of neuronal activity. J Neurophysiol 94: 3637–3642. [DOI] [PubMed] [Google Scholar]
- Brodfuehrer PD, Debski EA, O’Gara BA, et al. (1995) Neuronal control of leech swimming. J Neurobiol 27: 403–418. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Hikosaka O, Nakamura K. (2010) Coding of task reward value in the dorsal raphe nucleus. J Neurosci 30: 6262–6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunel N, Wang X-J. (2001) Effects of neuromodulation in a cortical network model of object working memory dominated by recurrent inhibition. J Comput Neurosci 11: 63–85. [DOI] [PubMed] [Google Scholar]
- Brunelli M, Castellucci VF, Kandel ER. (1976) Synaptic facilitation and behavioral sensitization in Aplysia: Possible role of serotonin and cyclic AMP. Science 194: 1178–1181. [DOI] [PubMed] [Google Scholar]
- Budzinska K. (2009) Serotonergic modulation of cortical and respiratory responses to episodic hypoxia. Eur J Med Res 14: 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunin MA, Prioleau C, Mailman RB, et al. (1998) Release of update rates of 5-hydroxytryptamine in the dorsal raphe and substantia nigra reticulata of the rat brain. J Neurochem 70: 1077–1087. [DOI] [PubMed] [Google Scholar]
- Burkitt AN. (2006. a) A review of the integrate-and-fire neuron model: I. Homogeneous synaptic input. Biol Cybern 95: 1–19. [DOI] [PubMed] [Google Scholar]
- Burkitt AN. (2006. b) A review of the integrate-and-fire neuron model: II. Inhomogeneous synaptic input and network properties. Biol Cybern 95: 97–112. [DOI] [PubMed] [Google Scholar]
- Calizo LH, Akanwa A, Ma X, et al. (2011) Raphe serotonin neurons are not homogeneous: Electrophysiological, morphological and neurochemical evidence. Neuropharmacology 61: 524–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Colino M, Almeida R, Compte A. (2013) Serotonergic modulation of spatial working memory: Predictions from a computational network model. Front Integr Neurosci 7: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Colino M, Almeida R, Gomez-Cabrero D, et al. (2014) Serotonin regulates performance nonmonotonically in a spatial working memory network. Cereb Cortex 24: 2449–2463. [DOI] [PubMed] [Google Scholar]
- Carew RD, Hawkins RD, Kandel ER. (1983) Differential classical conditioning of a defensive withdrawal reflex in Aplysia californica. Science 219: 397–400. [DOI] [PubMed] [Google Scholar]
- Carew TJ, Castellucci VF, Kandel ER. (1971) An analysis of dishabituation and sensitization of the gill-withdrawal reflex in Aplysia. Int J Neurosci 2: 79–98. [DOI] [PubMed] [Google Scholar]
- Carew TJ, Walters ET, Kandel ER. (1981) Associative learning in Aplysia: Cellular correlates supporting a conditioned fear hypothesis. Science 211: 501–504. [DOI] [PubMed] [Google Scholar]
- Carter OL, Burr DC, Pettigrew JD, et al. (2005) Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J Cogn Neurosci 17: 1497–1508. [DOI] [PubMed] [Google Scholar]
- Casanovas JM, Artigas F. (1996) Differential effects of ipsapirone on 5-hydroxytryptamine release in the dorsal and median Raphe neuronal pathways. J Neurochem 67: 1945–1952. [DOI] [PubMed] [Google Scholar]
- Caspi A. (2003) Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science 301: 386–389. [DOI] [PubMed] [Google Scholar]
- Castellucci VF, Kandel ER. (1976) Presynaptic facilitation as a mechanism for behavioral sensitization in Aplysia. Science 194: 1176–1178. [DOI] [PubMed] [Google Scholar]
- Celeda P, Puig MV, Casanovas JM, et al. (2001) Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABAA and glutamate receptors. J Neurosci 21: 9917–9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Artigas F. (2013) Serotonin modulation of cortical neurons and networks. Front Integr Neurosci 7: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis C, Boulden J, Veerakumar A, et al. (2013) Raphe GABAergic neurons mediate the acquisition of avoidance after social defeat. J Neurosci 33: 13978–13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Muller U, Blackwell AD, et al. (2006) Neurochemical modulation of response inhibition and probabilistic learning in humans. Science 311: 861–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CW, Poteet E, Schetz JA, et al. (2009) Towards a quantitative representation of the cell signaling mechanisms of hallucinogens: Measurement and mathematical modeling of 5-HT1A and 5-HT2A receptor-mediated ERK1/2 activation. Neuropharmacology 56: 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Ji Z, Tsalkova T, et al. (2008) Epac and PKA: A tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin (Shanghai) 40: 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Amoroso MW, Uchida N. (2015) Serotonergic neurons signal reward and punishment on multiple timescales. ELife 25: 4. doi: 10.7554/eLife.06346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG. (2009) Locally collateralizing glutamate neurons in the dorsal raphe nucleus responsive to substance P contain vesicular glutamate transporter 3 (VGLUT3). J Chem Neuroanat 38: 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Nakamura K, Daw ND. (2011) Serotonin and dopamine: Unifying affective, activational, and decision functions. Neuropsychopharmacology 36: 98–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Robinson OJ, Sahakian B. (2008) Acute tryptophan depletion in healthy volunteers enhances punishment prediction but does not affect reward prediction. Neuropsychopharmacology 33: 2291–2299. [DOI] [PubMed] [Google Scholar]
- Crawford LK, Craige CP, Beck SG. (2010) Increased intrinsic excitability of lateral wing serotonin neurons of the dorsal raphe: A mechanism for selective activation in stress circuits. J Neurophysiol 103: 2652–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford LK, Craige CP, Beck SG. (2011) Glutamatergic input is selectively increased in dorsal raphe subfield 5-HT neurons: Role of morphology, topography and selective innervation. Eur J Neurosci 34: 1794–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen M, Wong-Lin (2014) Analysis of a computational model of dopamine synthesis and release through perturbation. In: Bioinformatics and Biomedicine, 2014 IEEE International Conference, Belfast, UK, 2–5 November 2014, pp. 1–7. [Google Scholar]
- Cullen M, Wong-Lin (2015) An integrated dopaminergic neuronal model with reduced intracellular processes and inhibitory autoreceptors. IET Syst Biol 9: 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. (2011) Impulsivity, compulsivity, and top-down cognitive control. Neuron 69: 680–694. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, et al. (2008) Neurobehavioral mechanisms of impulsivity: Fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav 90: 250–260. [DOI] [PubMed] [Google Scholar]
- Dankowski EC, Wightman RM. (2013) Monitoring serotonin signaling on a subsecond time scale. Front Integr Neurosci 7: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Kakade S, Dayan P. (2002) Opponent interactions between serotonin and dopamine. Neural Netw 15: 603–616. [DOI] [PubMed] [Google Scholar]
- Daws LC, Montãnez S, Owens WA, et al. (2005) Transport mechanisms governing serotonin clearance in vivo revealed by high-speed chronoamperometry. J Neurosci Methods 143: 49–62. [DOI] [PubMed] [Google Scholar]
- Dayan P. (2012) Twenty-five lessons from computational neuromodulation. Neuron 76: 240–256. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Henke K, Aerni A, et al. (2003) A functional genetic variation of the 5-HT2a receptor affects human memory. Nat Neurosci 6: 1141–1142. [DOI] [PubMed] [Google Scholar]
- Deneris ES, Wyler SC. (2012) Serotonergic transcriptional networks and potential importance to mental health. Nat Neurosci 15: 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, Beaudet A, Watkins KC. (1975) Serotonin nerve terminals in adult rat neocortex. Brain Res 100: 563–588. [DOI] [PubMed] [Google Scholar]
- Descarries L, Berthelet F, Garcia S, et al. (1986) Dopaminergic projection from nucleus raphe dorsalis to neostriatum in the rat. J Comp Neurol 249: 511–520. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Marklund U, Yuan Q, et al. (2003) Lmx1b is essential for the development of serotonergic neurons. Nat Neurosci 6: 933–938. [DOI] [PubMed] [Google Scholar]
- Dorocic IP, Furth D, Xuan Y, et al. (2014) A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron 83: 663–678. [DOI] [PubMed] [Google Scholar]
- Doya K. (2002) Metalearning and neuromodulation. Neural Netw 15: 495–506. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. (2002) The computational role of dopamine D1 receptors in working memory. Neural Netw 15: 561–572. [DOI] [PubMed] [Google Scholar]
- E W. (2011) Principles of Multiscale Modeling. New York: Cambridge University Press. [Google Scholar]
- Eckhoff P, Wong-Lin KF, Holmes P. (2009) Optimality and robustness of a biophysical decision-making model under norepinephrine modulation. J Neurosci 29: 4301–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhoff P, Wong-Lin KF, Holmes P. (2011) Dimension reduction and dynamics of a spiking neural network model for decision making under neuromodulation. SIAM J Appl Dyn Syst 10: 148–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermentrout GB, Terman D. (2010) Mathematical Foundations of Neuroscience. New York: Springer-Verlag. [Google Scholar]
- Fellous JM, Linster C. (1998) Computational models of neuromodulation. Neural Comput 10: 771–805. [DOI] [PubMed] [Google Scholar]
- Fernandez SP, Muzerelle A, Scotto-Lomassese S, et al. (2017) Constitutive and acquired serotonin deficiency alters memory and hippocampal synaptic plasticity. Neuropsychopharmacology 42: 512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel LH. (2000) Neuroengineering models of brain disease. Annu Rev Biomed Eng 2: 577–606. [DOI] [PubMed] [Google Scholar]
- Fitzugh R. (1961) Impulses and physiological states in theoretical models of nerve membrane. Biophysical J 1: 445–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower G, Wong-Lin K. (2014) Reduced computational models of serotonin synthesis, release, and reuptake. IEEE Trans Biomed Eng 61: 1054–1061. [DOI] [PubMed] [Google Scholar]
- Fonseca MS, Murakami M, Mainen ZF. (2015) Activation of dorsal raphe serotonergic neurons promotes waiting but is not reinforcing. Curr Biol 25: 306–315. [DOI] [PubMed] [Google Scholar]
- Freeman WJ. (2007) The place of ‘codes’ in nonlinear neurodynamics. Prog Brain Res 165: 447–462. [DOI] [PubMed] [Google Scholar]
- Fu W, Le Maître E, Fabre V, et al. (2010) Chemical neuroanatomy of the dorsal raphe nucleus and adjacent structures of the mouse brain. J Comp Neurol 518: 3464–3494. [DOI] [PubMed] [Google Scholar]
- Gagnon D, Parent M. (2014) Distribution of VGLUT3 in highly collateralized axons from the rat dorsal raphe nucleus as revealed by single-neuron reconstructions. PLoS ONE 9: e87709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Ganguli S. (2015) On simplicity and complexity in the brave new world of large-scale neuroscience. Curr Opin Neurobiol 32: 148–155. [DOI] [PubMed] [Google Scholar]
- Gartside SE, Umbers V, Hajos M, et al. (1995) Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: Effects on 5-HT cell firing and extracellular 5-HT. Br J Pharmacol 115: 1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Lillesaar C. (2012) Probing the diversity of serotonin neurons. Philos Trans R Soc Lond B Biol Sci 367: 2382–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman PK. (2007) Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol 151: 737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashow R, Brookings T, Marder E. (2009) Reliable neuromodulation from circuits with variable underlying structure. Proc Natl Acad Sci 106: 11742–11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guckenheimer J, Holmes P. (1983) Nonlinear Oscillations, Dynamical Systems, and Bifurcations of Vector Fields. New York: Springer-Verlag. [Google Scholar]
- Hajós M, Allers KA, Jennings K, et al. (2007) Neurochemical identification of stereotypic burst-firing neurons in the rat dorsal raphe nucleus using juxtacellular labelling methods. Eur J Neurosci 25: 119–126. [DOI] [PubMed] [Google Scholar]
- Hajós M, Sharp T. (1996) Burst-firing activity of presumed 5-HT neurones of the rat dorsal raphe nucleus: Electrophysiological analysis by antidromic stimulation. Brain Res 740: 162–168. [DOI] [PubMed] [Google Scholar]
- Hajós M, Sharp T, Newberry NR. (1996) Intracellular recordings from burst-firing presumed serotonergic neurones in the rat dorsal raphe nucleus in vivo. Brain Res 737: 308–312. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. (2006) Genetics of emotional regulation: The role of the serotonin transporter in neural function. Trends Cogn Sci 10: 182–191. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM, Cohen AH. (1985) Serotonin modulates the central pattern generator for locomotion in the isolated lamprey spinal cord. J Exp Biol 116: 27–46. [DOI] [PubMed] [Google Scholar]
- Harrison PM, Badel L, Wall MJ, et al. (2015) Experimentally verified parameter sets for modelling heterogeneous neocortical pyramidal-cell populations. PLoS Comput. Biol 11: e1004165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey JA. (2003) Role of the serotonin 5-HT(2A) receptor in learning. Learn Mem 10: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H. (2003) Dynamical mean-field theory for noisy spiking neuron ensembles: Application to the Hodgkin-Huxley model. Phys Rev E 68: 041909. [DOI] [PubMed] [Google Scholar]
- Hashemi P, Dankowski EC, Wood KM, et al. (2011) In vivo electrochemical evidence for simultaneous 5-HT and histamine release in the rat substantia nigra pars reticulata following medial forebrain bundle stimulation. J Neurochem 118: 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Nakao K, Nakamura K. (2015) Appetitive and aversive information coding in the primate dorsal raphe nucleus. J Neurosci 35: 6195–6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Xue G, Chen C, et al. (2010) Serotonin transporter gene-linked polymorphic region (5-HTTLPR) influences decision making under ambiguity and risk in a large Chinese sample. Neuropharmacology 59: 518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebart MN, Glascher J. (2015) Serotonin and dopamine differentially affect appetitive and aversive general Pavlovian-to-instrumental transfer. Psychopharmacology (Berl) 232: 437–451. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, et al. (1996) Allelic variation of human serotonin transporter gene expression. J Neurochem 66: 2621–2624. [DOI] [PubMed] [Google Scholar]
- Helmstaedter M. (2015) The mutual inspirations of machine learning and neuroscience. Neuron 86: 25–28. [DOI] [PubMed] [Google Scholar]
- Hioki H, Nakamura H, Ma YF, et al. (2010) Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J Comp Neurol 518: 668–686. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117: 500–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T, Johansson O, Fuxe K, et al. (1976) Immunohistochemical studies on the localization and distribution of monoamine neuron systems in the rat brain. I. Tyrosine hydroxylase in the mes- and diencephalon. Med Biol 54: 427–453. [PubMed] [Google Scholar]
- Hopfield JJ. (1982) Neural networks and physical systems with emergent collective computational properties. Proc Natl Acad Sci USA 79: 2554–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR. (2012) Serotonin and decision making processes. Neurosci Biobehav Rev 36: 218–236. [DOI] [PubMed] [Google Scholar]
- Hornung JP. (2003) The human raphe nuclei and the serotonergic system. J Chem Neuroanat 26: 331–343. [DOI] [PubMed] [Google Scholar]
- Horstemeyer MF. (2009) Multiscale modeling: A review. In: Leszczyński J, Shukla MK. (eds) Practical Aspects of Computational Chemistry: Methods, Concepts and Applications. Netherlands: Springer, pp. 87–135. [Google Scholar]
- Hull EM, Muschamp JW, Sato S. (2004) Dopamine and serotonin: Influences on male sexual behavior. Physiol Behav 83: 291–307. [DOI] [PubMed] [Google Scholar]
- Huys QJ, Maia TV, Frank MJ. (2016) Computational psychiatry as a bridge from neuroscience to clinical applications. Nat Neurosci 19: 404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Mizuhiki T, Setogawa T, et al. (2013) Neurons in monkey dorsal raphe nucleus code beginning and progress of step-by-step schedule, reward expectation, and amount of reward outcome in the reward schedule task. J Neurosci 33: 3477–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanir S, Brown AS, Nagy S, et al. (2016) Serotonin promotes exploitation in complex environments by accelerating decision-making. BMC Biol 14: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhikevich EM. (2003) Simple model of spiking neurons. IEEE Trans Neural Netw 14: 1569–1572. [DOI] [PubMed] [Google Scholar]
- Izhikevich EM. (2007) Dynamical Systems in Neuroscience. Cambridge, MA: MIT Press. [Google Scholar]
- Jacobs BL, Azmitia EC. (1992) Structure and function of the brain serotonin system. Physiol Rev 72: 165–229. [DOI] [PubMed] [Google Scholar]
- Jalewa J, Joshi A, McGinnity TM, et al. (2014) Neural circuit interactions between the dorsal raphe nucleus and the lateral hypothalamus: an experimental and computational study. PLoS ONE 9: e88003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD. (1994) Synaptic glutamate release by postnatal rat serotonergic neurons in microculture. Neuron 12: 433–442. [DOI] [PubMed] [Google Scholar]
- Joshi A. (2014) Neural Circuit Modelling of the Orexin/Hypocretin System with Implications for Clinical Depression. PhD thesis. Londonderry, UK: University of Ulster. [Google Scholar]
- Joshi A, McGinnity TM, Prasad G, et al. (2015) Computational modelling of serotonin and orexin/hypocretin mediated GIRK and TRP-like currents. In: British Neuroscience Association (BNA) Meeting 2015, BNA0543 P2-E-005, Manchester, UK, 12–15 April 2015. [Google Scholar]
- Joshi A, Wong-Lin K, McGinnity TM, et al. (2011) A mathematical model to explore the interdependence between the serotonin and orexin/hypocretin systems. Conf Proc IEEE Eng Med Biol Soc 2011: 7270–7273. [DOI] [PubMed] [Google Scholar]
- Joshi A, Youssofzadeh V, Vemana V, et al. (2017) An integrated modelling framework for neural circuits with multiple neuromodulators. J R Soc Interface 14(126). doi: 10.1098/rsif.2016.0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuragi S, Kunugi H, Sano A, et al. (1999) Association between serotonin transporter gene polymorphism and anxiety-related traits. Biol Psychiatry 45: 368–370. [DOI] [PubMed] [Google Scholar]
- Keener J, Sneyd J. (2008) Mathematical Physiology I: Cellular Physiology. Interdisciplinary Applied Mathematics. 2nd edn. New York: Springer. [Google Scholar]
- Kirby LG, Freeman-Daniels E, Lemos JC, et al. (2008) Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J Neurosci 28: 12927–12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, et al. (2003) Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus: Electrophysiological and immunohistochemical studies. Neuroscience 116: 669–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B, Varga V, Dahan L, et al. (2006) Serotonergic neuron diversity: Identification of raphe neurons with discharges time-locked to the hippocampal theta rhythm. Proc Natl Acad Sci USA 103: 1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler C, Steinbusch H. (1982) Identification of serotonin and non-serotonin-containing neurons of the mid-brain raphe projecting to the entorhinal area and the hippocampal formation: A combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience 7: 951–975. [DOI] [PubMed] [Google Scholar]
- Kuhnen CM, Samanez-Larkin GR, Knutson B. (2013) Serotonergic genotypes, neuroticism, and financial choices. PLoS ONE 8: e54632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar JS, Mann JJ. (2014) PET tracers for serotonin receptors and their applications. Cent Nerv Syst Agents Med Chem 14: 96–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner HA, Baxter DA, Byrne JH. (2000. a) Classical conditioning of feeding in Aplysia: I. Behavioral analysis. J Neurosci 20: 3369–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner HA, Baxter DA, Byrne JH. (2000. b) Classical conditioning of feeding in Aplysia: II. Neurophysiological correlates. J Neurosci 20: 3377–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Pan Y, Ma X, et al. (2006) Selective 5-HT1B receptor inhibition of glutamatergic and GABAergic synaptic activity in the rat dorsal and median raphe. Eur J Neurosci 24: 3415–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, et al. (1996) Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274: 1527–1531. [DOI] [PubMed] [Google Scholar]
- Li Y, Dalphin N, Hyland BI. (2013) Association with reward negatively modulates short latency phasic conditioned responses of dorsal raphe nucleus neurons in freely moving rats. J Neurosci 33: 5065–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhong W, Wang D, et al. (2016) Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat Commun 7: 10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, Li H, Kaneko T, et al. (2001) Morphological features and electrophysiological properties of serotonergic and non-serotonergic projection neurons in the dorsal raphe nucleus: An intracellular recording and labeling study in rat brain slices. Brain Res 900: 110–118. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhou J, Li Y, et al. (2014) Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron 81: 1360–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long AB, Kuhn CM, Platt ML. (2009) Serotonin shapes risky decision making in monkeys. Soc Cogn Affect Neurosci 4: 346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Rodda JE, Lightman SL, et al. (2000) Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: Evidence for activation of a topographically organized mesolimbocortical serotonergic system. J Neurosci 20: 7728–7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Zhou J, Liu Z. (2015) Reward processing by the dorsal raphe nucleus: 5-HT and beyond. Learn Mem 22: 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly C. (2015) Firing rate dynamics in recurrent spiking neural networks with intrinsic and network heterogeneity. J Comput Neurosci 39: 311–327. [DOI] [PubMed] [Google Scholar]
- Mackey SL, Kandel ER, Hawkins RD. (1989) Identified serotonergic neurons LCB1 and RCB1 in the cerebral ganglia of Aplysia produce presynaptic facilitation of siphon sensory neurons. J Neurosci 9: 4227–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Masseck OA, Mark MD, et al. (2013) Modulation of firing and synaptic transmission of serotonergic neurons by intrinsic G protein-coupled receptors and ion channels. Front Integr Neurosci 7: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Meltzer HY. (1995) The serotonin hypothesis of major depression. In: Bloom FE, Kupfer DJ. (eds) Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press, 933–944. [Google Scholar]
- Magali W. (2012) GPCRs and G protein activation. In: Deniz Ekinci. (eds) Biochemistry. InTech, pp. 161–188. [Google Scholar]
- Maia TV, Cano-Colino M. (2015) The role of serotonin in orbitofrontal function and obsessive-compulsive disorder. Clin Psychol Sci 3: 460–482. [Google Scholar]
- Marder E. (2012) Neuromodulation of neuronal circuits: Back to the future. Neuron 76: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli S, Schnell SA, Hack SP, et al. (2004) Serotonergiv and nonserotonergic dorsal raphe neurons are pharmacologically and electrophysiologically heterogeneous. J Neurophysiol 92: 3532–3537. [DOI] [PubMed] [Google Scholar]
- Marinesco S, Carew TJ. (2002) Serotonin release evoked by tail nerve stimulation in the CNS of Aplysia: Characterization and relationship to heterosynaptic plasticity. J Neurosci 22: 2299–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews GA, Nieh EH, Vander Weele CM, et al. (2016) Dorsal raphe dopamine neurons represent the experience of social isolation. Cell 164: 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCudden CR, Hains MD, Kimple RJ, et al. (2005) G-protein signaling: Back to the future. Cell Mol Life Sci 62: 551–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt RA, Tiran-Cappello A, Shen H, et al. (2014) Serotonergic versus nonserotonergic dorsal raphe projection neurons: Differential participation in reward circuitry. Cell Rep 8: 1857–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeter M, Talamini L, Schmitt JA, et al. (2006) Effects of 5-HT on memory and the hippocampus: Model and data. Neuropsychopharmacology 31: 712–720. [DOI] [PubMed] [Google Scholar]
- Menziani MC, De Rienzo F, Cappelli A, et al. (2001) A computational model of the 5-HT3 receptor extracellular domain: Search for ligand binding sites. Theor Chem Acc 106: 98–104. [Google Scholar]
- Michaelis L, Menten ML. (1913) Die Kinetik der Invertinwirkung. Biochem Z 49: 333–369. [Google Scholar]
- Michelsen KA, Schmitz C, Steinbusch HW. (2007) The dorsal raphe nucleus – from silver stainings to a role in depression. Brain Res Rev 55: 329–342. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Marin P, Bockaert J, et al. (2008) Signaling at G-protein-coupled serotonin receptors: Recent advances and future research directions. Trends Pharmacol Sci 29: 454–464. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Miyazaki KW, Doya K. (2011) Activation of dorsal raphe serotonin neurons underlies waiting for delayed rewards. J Neurosci 31: 469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki KW, Miyzaki K, Tanaka KF, et al. (2014) Optogenetic activation of dorsal raphe serotonin neurons enhances patience for future rewards. Curr Biol 24: 2033–2040. [DOI] [PubMed] [Google Scholar]
- Mizra NR, Nielson EO, Troelsen KB. (2007) Serotonin transporter density and anxiolytic-like effects of antidepressants in mice. Prog Neuropsycho Biol Psych 31: 858–866. [DOI] [PubMed] [Google Scholar]
- Moore RY, Halaris AE, Jones BE. (1978) Serotonin neurons of the midbrain raphe: Ascending projections. J Comp Neurol 180: 417–438. [DOI] [PubMed] [Google Scholar]
- Moresco FM, Dieci M, Vita A, et al. (2002) In vivo serotonin 5HT(2A) receptor binding and personality traits in healthy subjects: A positron emission tomography study. Neuroimage 17: 1470–1478. [DOI] [PubMed] [Google Scholar]
- Müller CP, Jacobs BL. (2009) Handbook of the Behavioral Neurobiology of Serotonin. London: Academic Press. [Google Scholar]
- Murphy SE, Longhitano C, Ayres RE, et al. (2009) The role of serotonin in nonnormative risky choice: The effects of tryptophan supplements on the “reflection effect” in healthy adult volunteers. J Cogn Neurosci 21: 1709–1719. [DOI] [PubMed] [Google Scholar]
- Muzerelle A, Scotto-Lomassese S, Bernard JF, et al. (2016) Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5–B9) to the forebrain and brainstem. Brain Struct Funct 221: 535–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagumo J, Arimoto S, Yoshizawa S. (1962) An active pulse transmission line simulating nerve axon. Proc IRE 50: 2061–2070. [Google Scholar]
- Nakamura K. (2013) The role of the dorsal raphe nucleus in reward-seeking behavior. Front Integr Neurosci 7: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumoto M, Hikosaka O. (2008) Reward-dependent modulation of neuronal activity in the primate dorsal raphe nucleus. J Neurosci 28: 5331–5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Wong-Lin K. (2014) Functions and computational principles of serotonergic and related systems at multiple scales. Front Integr Neurosci 8: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichola W, Campbell SA. (2013) Mean-field models for heterogeneous networks of two-dimensional integrate and fire neurons. Front Comput Neurosci 7: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE, Nichols CD. (2008) Serotonin receptors. Chem Rev 108: 1614–1641. [DOI] [PubMed] [Google Scholar]
- Ogawa SK, Cohen JY, Hwang D, et al. (2014) Organization of monosynaptic inputs to the serotonin and dopamine neuromodulatory systems. Cell Rep 8: 1105–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okaty BW, Freret ME, Rood BD, et al. (2015) Multi-scale molecular deconstruction of the serotonin neuron system. Neuron 88: 774–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penington NJ, Tuckwell HC. (2012) Properties of I(A) in a neuron of the dorsal raphe nucleus. Brain Res 1449: 60–68. [DOI] [PubMed] [Google Scholar]
- Pernar L, Curtis AL, Vale WW, et al. (2004) Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J Neurosci 24: 1305–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew DB, Smolen P, Baxter DA, et al. (2005) Dynamic properties of regulatory motifs associated with induction of three temporal domains of memory in Aplysia. J Comput Neurosci 18: 163–181. [DOI] [PubMed] [Google Scholar]
- Pinsker HM, Hening WA, Carew TJ, et al. (1973) Long-term sensitization of a defensive withdrawal reflex in Aplysia. Science 182: 1039 –1042. [DOI] [PubMed] [Google Scholar]
- Pinsker HM, Kupfermann I, Castellucci VF, et al. (1970) Habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science 167: 1740–1742. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Drago A, Fabbri C, et al. (2011) Pharmacogenetics of antidepressant response. J Psychiatry Neurosci 36: 87–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzerey PA, Decker MJ, Galán RF. (2014) Elevated serotonergic signaling amplifies synaptic noise and facilitates the emergence of epileptiform network oscillations. J Neurophysiol 112: 2357–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Z, Garfinkel A, Weiss JN, et al. (2011) Multi-scale modeling in biology: How to bridge the gaps between scales? Prog Biophys Mol Biol 107: 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich MI, Varona P, Selverston AI, et al. (2007) Dynamical principles in neuroscience. Phys Rev Lett 98: 128106. [DOI] [PubMed] [Google Scholar]
- Ranade SP, Mainen ZF. (2009) Transient firing of dorsal raphe neurons encodes diverse and specific sensory, motor, and reward events. J Neurophysiol 102: 3026–3037. [DOI] [PubMed] [Google Scholar]
- Reed MC, Nijhout HF, Best J. (2013) Computational studies of the role of serotonin in the basal ganglia. Front Integr Neurosci 7: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves DC, Sayed MFR, Chau P-L, et al. (2003) Prediction of 5-HT3 receptor agonist-binding residues using homology modeling. Biophys J 84: 2338–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renart A, Brunel N, Wang X-J. (2003) Mean-field theory of recurrent cortical networks: From irregularly spiking neurons to working memory. In: Fend J. (ed.) Computational Neuroscience: A Comprehensive Approach. Boca Raton: CRC Press, pp. 431–490. [Google Scholar]
- Ricketts MH, Hamer RM, Sage JI, et al. (1998) Association of a serotonin transporter gene promoter polymorphism with harm avoidance behaviour in an elderly population. Psychiatr Genet 8: 41–44. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Cools R, Sahakian BJ. (2012) Tryptophan depletion disinhibits punishment but not reward prediction: Implications for resilience. Psychopharmacology (Berl) 219: 599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD. (2011) The roles of dopamine and serotonin in decision making: Evidence from pharmacological experiments in humans. Neuropsychopharmacology 36: 114–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL. (ed.) (2006) The Serotonin Receptors: From Molecular Pharmacology to Human Therapeutics. Totowa, NJ: Humana Press. [Google Scholar]
- Roxin A, Ledberg A. (2008) Neurobiological models of two-choice decision making can be reduced to a one-dimensional nonlinear diffusion equation. PLoS Comput Biol 4: e1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AW, Peroutka SJ. (1989) Three-dimensional steric molecular modeling of the 5-hydroxytrptamine3 receptor pharmacophore. Mol Pharmacol 36: 505–511. [PubMed] [Google Scholar]
- Schnell S, Grima R, Maini PK. (2007) Multiscale modeling in biology. Amer Sci 95: 134–142. [Google Scholar]
- Schweighofer N, Bertin M, Shishida K, et al. (2008) Low-serotonin levels increase delayed reward discounting in humans. J Neurosci 28: 4528–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweimer JV, Ungless MA. (2010) Phasic responses in dorsal raphe serotonin neurons to noxious stimuli. Neuroscience 171: 1209–1215. [DOI] [PubMed] [Google Scholar]
- Schwindinger WF, Robishaw JD. (2001) Heterotrimeric G-protein betagamma-dimers in growth and differentiation. Oncogene 20: 1653–1660. [DOI] [PubMed] [Google Scholar]
- Shikanai H, Yoshida T, Konno K, et al. (2012) Distinct neurochemical and functional properties of GAD67-containing 5-HT neurons in the rat dorsal raphe nucleus. J Neurosci 32: 14415–14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman KI, Herrmann N, Walker SE. (2013) Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs 27: 789–797. [DOI] [PubMed] [Google Scholar]
- Siegel JZ, Crockett MJ. (2003) How serotonin shapes moral judgement and behavior. Ann N Y Acad Sci 1299: 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siehler S, Milligan G. (2011) G Protein-Coupled Receptors: Structure, Signaling, and Physiology. New York: Cambridge University Press. [Google Scholar]
- Smythies J. (2005) Section V. Serotonin system. Int Rev Neurobiol 64: 217–268. [DOI] [PubMed] [Google Scholar]
- Spies M, Knudsen GM, Lanzenberger R, Kasper S. (2015) The serotonin transporter in psychiatric disorders: insights from PET imaging. Lancet Psychiatry 2: 743–755. [DOI] [PubMed] [Google Scholar]
- Stoltenberg SF, Nag G. (2010) Description and validation of a dynamical systems model of presynaptic serotonin function: Genetic variation, brain activation and impulsivity. Behav Genet 40: 262–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strogatz SH. (2001) Nonlinear Dynamics and Chaos: With Applications to Physics, Biology, Chemistry and Engineering. Cambridge, MA: Perseus Books Group. [Google Scholar]
- Tanaka SC, Schweighofer N, Asahi S, et al. (2007) Serotonin differentially regulates short- and long-term prediction of rewards in the ventral and dorsal striatum. PLoS One 2: e1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi M, Groshan K, Blakely RD, et al. (1997) Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol 340: 249–258. [DOI] [PubMed] [Google Scholar]
- Teissier A, Chemiakine A, Inbar B, et al. (2015) Activity of raphe serotonergic neurons controls emotional behaviors. Cell Rep 13: 1965–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau LE. (2004) Glutamate co-transmission as an emerging concept in monoamine neuron function. J Psychiatry Neuroci 29: 296–310. [PMC free article] [PubMed] [Google Scholar]
- Tuckwell HC. (2013) Biophysical properties and computational modeling of calcium spikes in serotonergic neurons of the dorsal raphe nucleus. BioSystems 112: 204–213. [DOI] [PubMed] [Google Scholar]
- Tuckwell HC, Penington NJ. (2014) Computational modeling of spike generation in serotonergic neurons of the dorsal raphe nucleus. Prog Neurobiol 118: 59–101. [DOI] [PubMed] [Google Scholar]
- Tuckwell HC, Zhou Y, Penington NJ. (2015) Simplified models of pacemaker spiking in raphe and locus coeruleus neurons. arXiv: 1508.05468 [q-bio.NC]. [Google Scholar]
- Turner JH, Gelasco AK, Ayiku HB, et al. (2006) 5-HT receptor signal transduction pathways. In: Roth BL. (ed.) From Molecular Pharmacology to Human Therapeutics. Totowa, NJ: Humana Press, pp. 143–206. [Google Scholar]
- Varga V, Kocsis B, Sharp T. (2003) Electrophysiological evidence for convergence of inputs from the medial prefrontal cortex and lateral habenula on single neurons in the dorsal raphe nucleus. Eur J Neurosci 17: 280–286. [DOI] [PubMed] [Google Scholar]
- Varga V, Székely AD, Csillag A, et al. (2001) Evidence for a role of GABA interneurones in the cortical modulation of midbrain 5-hydroxytryptamine neurones. Neuroscience 106: 783–792. [DOI] [PubMed] [Google Scholar]
- Vasudeva RK, Lin RC, Simpson KL, et al. (2011) Functional organization of the dorsal raphe efferent system with special consideration of nitrergic cell groups. J Chem Neuroanat 41: 281–293. [DOI] [PubMed] [Google Scholar]
- Vertes RP. (1991) A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol 313: 643–668. [DOI] [PubMed] [Google Scholar]
- Vidal-Gadea A, Topper S, Young L, et al. (2011) Caenorhabditis elegans selects distinct crawling and swimming gaits via dopamine and serotonin. Proc Natl Acad Sci USA 108: 17504–17509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters ET, Carew TJ, Kandel ER. (1981) Associative learning in Aplysia: Evidence for conditioned fear in an invertebrate. Science 211: 504–506. [DOI] [PubMed] [Google Scholar]
- Wang D-H, Wong-Lin K. (2013) Comodulation of dopamine and serotonin on prefrontal cortical rhythms: A theoretical study. Front Integr Neurosci 7: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Valentino RJ, Van Bockstaele EJ. (2011) Collateralized dorsal raphe nucleus projections: A mechanism for the integration of diverse functions during stress. J Chem Neuroanat 41: 266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbourd B, Ren J, DeLoach KE, et al. (2014) Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons. Neuron 83: 645–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J, Paslaski S, Daly J, et al. (2013) Modulation for emergent networks: Serotonin and dopamine. Neural Netw 41: 225–239. [DOI] [PubMed] [Google Scholar]
- White KJ, Walline CC, Barker EL. (2005) Serotonin transporters: Implications for antidepressant drug development. AAPS J 7: E421–E433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiklund L, Leger L, Persson M. (1981) Monoamine cell distribution in the cat brain stem: A fluorescence histochemical study with quantification of indolaminergic and locus coeruleus cell groups. J Comp Neurol 203: 613–647. [DOI] [PubMed] [Google Scholar]
- Williams GV, Rao SG, Goldman-Rakic PS. (2002) The physiological role of 5-HT2A receptors in working memory. J Neurosci 22: 2843–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HR, Cowan JD. (1972) Excitatory and inhibitory interactions in localized populations of model neurons. Biophys J 12: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HR, Cowan JD. (1973) A mathematical theory of the functional dynamics of cortical and thalamic nervous tissue. Kybernetik 13: 55–80. [DOI] [PubMed] [Google Scholar]
- Wong K-F, Wang X-J. (2006) A recurrent network mechanism of time integration in perceptual decisions. J Neurosci 26: 1314–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Lin K, Joshi A, Prasad G, et al. (2012) Network properties of a computational model of the dorsal raphe nucleus. Neural Netw 32: 15–25. [DOI] [PubMed] [Google Scholar]
- Wong-Lin K, Prasad G, McGinnity TM. (2011) A spiking neuronal network model of the dorsal raphe nucleus. In: Proceedings of 2011 International Joint Conference on Neural Networks, San Jose, CA, pp. 1591–1598. [Google Scholar]
- Wood KM, Zegja A, Nijhout HF, et al. (2014) Voltammetric and mathematical evidence for dual transport mediation of serotonin clearance in vivo. J Neurochem 130: 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Shirouzu M, Tanaka M, et al. (1989) Dopaminergic neurons in the nucleus raphe dorsalis innervate the prefrontal cortex in the rat: A combined retrograde tracing and immunohistochemical study using anti-dopamine serum. Brain Res 496: 373–376. [DOI] [PubMed] [Google Scholar]
- Young SN. (2013) Acute tryptophan depletion in humans: A review of theoretical, practical and ethical aspects. J Psychiatry Neurosci 38: 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssofzadeh V, Prasad G, Fagan AJ, et al. (2015. a) Signal propagation in the human visual pathways: An effective connectivity analysis. J Neurosci 35: 13501–13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssofzadeh V, Prasad G, Naeem N, et al. (2015. b) Temporal information of directed causal connectivity in multi-trial ERP data using partial Granger causality. Neuroinformatics 14: 99–120. [DOI] [PubMed] [Google Scholar]
- Zaldivar A, Asher DE, Krichmar JL. (2010) Simulation of how neuromodulation influences cooperative behavior. In: Doncieux S, Girard B, Guillot A, Hallam J, Meyer JA, Mouret JB. (eds) From Animals to Animats 11. SAB 2010. Lecture Notes in Computer Science, vol 6226 Berlin, Heidelberg: Springer. [Google Scholar]
- Zhang Y, Liu R-Y, Heberton GA, et al. (2012) Computational design of enhanced learning protocols. Nat Neurosci 15: 294–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Baxter DA, Byrne JH. (2014) Contribution of PKC to the maintenance of 5-HT-induced short-term facilitation at sensorimotor synapses of Aplysia. J Neurophysiol 112: 1936–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]


