Abstract
We have recently demonstrated that mycobacterial ligands engage Toll like receptor 2 (TLR2) on CD4+ T cells and up-regulate T-cell receptor (TCR) triggered Th1 responses in vitro and in vivo. To better understand the role of T-cell expressed TLR2 on CD4+ T cell differentiation and function, we conducted a gene expression analysis of murine naïve CD4+ T-cells stimulated in the presence or absence of TLR2 co-stimulation. Unexpectedly, naïve CD4+ T- cells co-stimulated via TLR2 show a significant up-regulation of Il9 mRNA compared to cells co-stimulated via CD28. Under TH9 differentiation, we observed up regulation of TH9 differentiation, evidenced by increases in both percent of IL-9 secreting cells and IL-9 in culture supernatants in the presence of TLR2 agonist both in polyclonal and Ag85B cognate peptide specific stimulations. Human CD4+ T cells also showed increased in both IL9 mRNA expression and IL-9 cytokine secretion in response to TLR2 agonist under polarized condition. Under non-polarizing conditions, TLR2 engagement on CD4+ T-cells had minimal effect on IL-9 secretion and TH9 differentiation, likely due to a prominent effect of TLR2 signaling on IFN-γ secretion and TH1 differentiation. We also report that, TLR2 signaling in CD4+ T-cells increased expression of transcription factors BATF and PU.1, known to positively regulate TH9 differentiation. These results reveal a novel role of T-cell expressed TLR2 in enhancing the differentiation and function of TH9 T cells.
Keywords: TH9 T cells, CD4+ T cells, Toll like receptor 2
Introduction
CD4+ T cells are crucial for host defense against many pathogens, have pathogenic roles as drivers of autoimmune diseases and allergies, and are critical for suppression of immune reactivity [1]. After activation, CD4+ T cells can differentiate into diverse effector lineages, each one with distinct cytokine profiles, functions and roles in the immune response to foreign- and self-antigens. Currently known CD4+ T cell subsets are: T-helper 1 (TH1), T-helper 2 (TH2), T-helper 17 and 22 (TH17, TH22), follicular helper T cell (TFH), natural regulatory T cells (nT-regs), induced T-regulatory cells (iTreg), regulatory type 1 cells (Tr1) as well as T-helper 9 (TH9) cells (1, 2). Compared with the other TH cell subsets, TH9 cells, characterized by secretion of interleukin-9 (IL-9), and the factors that control their development and function are less well characterized.
Initial activation and differentiation of naïve CD4+ T cells results from TCR and CD4 interactions with peptide-MHC-II complexes presented on the surface of antigen presenting cells (APCs). Engagement of the TCR (signal 1) initiates intracellular signaling that, when combined with additional signal(s) from CD28 or other co-stimulatory receptors (signal 2) leads to naïve T-cell proliferation and differentiation into effector cells. Lineage-specific CD4+ T cell differentiation depends on the cytokine milieu, antigen concentration, type of APC and co-stimulatory molecules. In addition to CD28, other co-stimulatory receptors have been described on CD4+ T cells (3). Unlike CD28, which is constitutively expressed in both naïve and memory T cells, many co-stimulatory receptors are induced after activation. Toll-like receptor 2 (TLR2) has been recently recognized as a co-stimulatory receptor on CD4+, CD8+ T cells (4–7) and T-regs (8–10). TLR2 is unique among inducible co-stimulatory receptors in that it engages microbial ligands instead of receptors expressed by APCs. We previously reported on the ability of human CD4+T cells to directly recognize mycobacterial lipoproteins via TLR2 (11). In combination with TCR triggering, TLR2 engagement induced CD4+ T cell proliferation and secretion of IL-2 and IFN-γ in vitro (12). Furthermore, we demonstrated a role for TLR2 expressed on mouse CD4+ T cells in T-cell co-stimulation, TH1 differentiation, and protection to M. tuberculosis infection (12). Thus, our previous work indicated that in the absence of polarizing conditions (i.e. non polarizing) or under TH1 polarizing conditions, engagement of TLR2 on human or mouse CD4+T-cells enhances TH1 differentiation.
To extend our previous studies on the role of TLR2 in the regulation of T-cell function and differentiation, we compared the gene expression profile of mouse CD4+ T cells co-stimulated via CD28 or TLR2. Unlike CD28 co-stimulation, TLR2 co-engagement with CD3 triggered the regulation of a very discrete set of genes. Among the genes exclusively regulated in cells co-stimulated via TLR2, Il9 was the most significantly regulated gene. This led us to further analyze the effect of TLR2 engagement on CD4+ T cells in TH9 cell differentiation under TH9-polarizing or non-polarizing conditions. We showed that, in the presence of TGF-β and IL-4, TLR2 co-stimulation, both after polyclonal- and Ag-specific- activation, triggers IL-9 synthesis and secretion. These effects were mediated through regulation of the transcription factors BATF and PU.1. Thus simultaneous engagement of the TCR and TLR2 by microbial or endogenous ligands in the presence of TGF-β and IL-4 may contribute to the development of TH9 responses in infection, autoimmunity and/or allergy.
RESULTS
TLR2 co-stimulation induces discrete changes in the transcriptome of CD4+ T-cells
We have previously shown that TLR2 co-stimulation of CD4+ T cells increases TH1 differentiation. To further characterize the effects of TLR2 engagement on CD4+ T cells, we compared the transcriptional profiles of CD4+ T-cells stimulated with anti-CD3 in combination with either anti- CD28 or the TLR2 ligand P3CSK4. We first identified genes that were significantly regulated in response to either anti-CD28 or TLR2 co-stimulation compared to no co-stimulation (anti-CD3 alone). We then generated short lists of genes that were either regulated in both conditions or were exclusively regulated in one co-stimulatory condition (two fold change; p < 0.05). Twenty nine genes were found regulated by both in cells co-stimulated via CD28 and TLR2, 393 genes were exclusively regulated by anti-CD28 co-stimulation, and a small set of 5 genes were differentially regulated in response to TLR2 agonist (Fig.1A). The top 30 genes regulated by either anti-CD28 or TLR2 agonist are highlighted in Fig.1B. Il9, Tnfrsf8, Cytip, Satb1, AffyID: 10403825 (Tcrg-V1) were the 5 genes uniquely regulated in response to TLR2 agonist. The Il9 gene was identified as the most significantly regulated one in terms of the adjusted p value and the magnitude of expression (Fig. 1B). Up-regulation of Il9 gene expression in response to TLR2 co-stimulation required 48h of activation with anti-CD3 and TLR2 agonist, but was not observed 24h after stimulation (not shown). Induction of Il9 gene expression after TLR2 co-stimulation was confirmed by RT-PCR (Fig. 1C).
Figure 1.
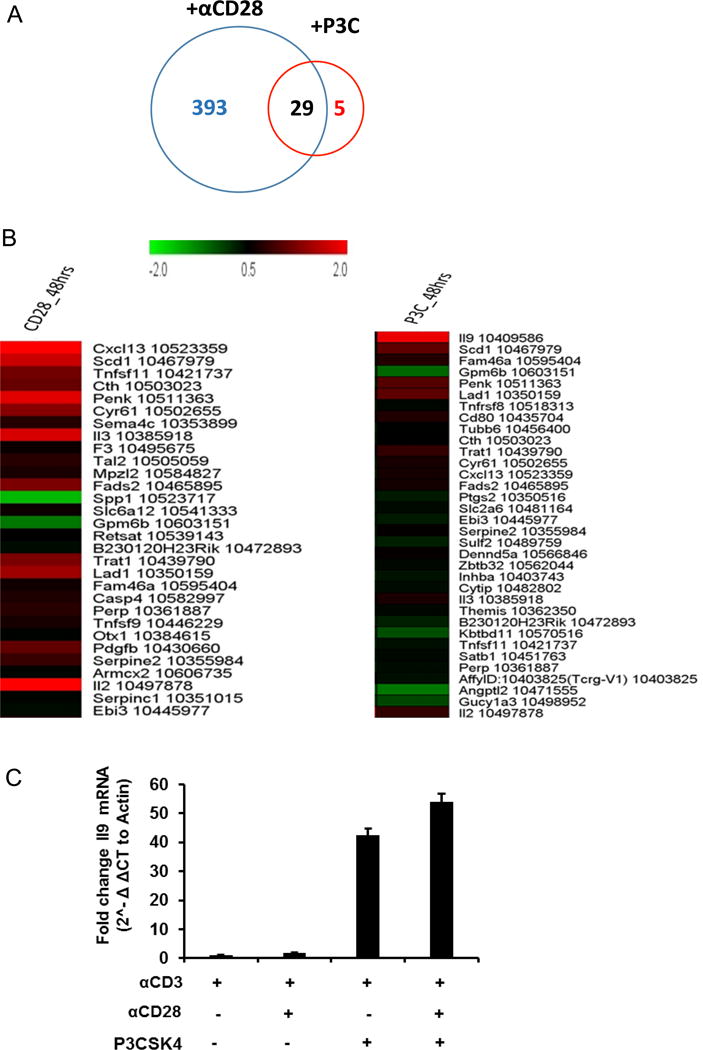
Transcriptional analysis of resting CD4+ T-cells co-stimulated via CD28 or TLR2. (A) Venn diagram representing genes expressed in response to anti-CD28− and/or TLR2− co-stimulation. (B) Top genes expressed in CD4+ T cells in response to antiCD28− or TLR2 co-stimulation. (C) Relative expression of Il9 gene after CD28− and/or TLR2 co-stimulation.
This comparative transcriptional profiling indicates that TLR2 signaling in CD4+ T-cells regulates a very discrete set of genes, among them Il9 is the most significantly regulated one. Furthermore, Il9 gene regulation seems to be specific of TLR2 co-stimulation since it was absent in cells co-stimulated via CD28. Our data suggest a new role for TLR2 signaling in the regulation of the Il9 gene expression and, possibly, TH9 cell differentiation in CD4+ T cells.
TLR2 engagement on CD4+ T cells upregulates TGF-β and IL-4 driven Il9 gene and TH9 differentiation
TH9 cells, characterized by the expression of IL9 mRNA and secretion of IL-9, represent a recently described CD4+ T cell effector subset. They develop from naïve cells in the presence of TGF-β and IL-4 (16, 17). Our microarray analysis identified the Il9 gene as a specific target of regulation by TLR2 co-stimulation. Therefore, we then investigated the role of TLR2 engagement on CD4+ T cells in TH9 development by activating CD4+ T-cells with anti-CD3 and anti-CD28 in absence of exogenous cytokines (non-polarizing condition) or presence of exogenous IL-4 and TGF-β (TH9 polarizing condition) as described (16, 18). As shown in Fig. 2A, we first confirmed that TLR2 stimulation increases Il9 mRNA expression in TH9 polarized CD4+ T cells. Next, we compared intracellular IL-9 expression and IL-9 secretion in CD4+ T cells stimulated under non-polarizing and polarizing conditions. Under non-polarizing conditions, the TLR2 agonist P3CSK4 had no effect on the percentage of IL-9+ cells (Fig. 2B) and on the levels of IL-9 in culture supernatants (Fig. 2C). On the other hand, and as shown before, both percentage of IFN-γ+ cells and amounts of IFN-γ in supernatants were increased in response to P3CSK4 under non-polarizing conditions (Fig. 2B, D). Under TH9 polarizing conditions P3CSK4 induced significant increases in both the percentage of IL-9+ cells (2-fold) and IL-9 secretion by CD4+ T cells (2-fold) (Fig. 2B, C) but had no effect on the percentage of IFN-γ+ cells or the levels of IFN-γ in supernatants (Fig. 2B, D). This effect was observed under both optimal (TGF-β 5 ng/ml; IL-4 10 ng/ml), and suboptimal (TGF-β 1ng/ml; IL-4 2ng/ml) TH9 polarizing conditions (Supplemental Fig. 1).
Figure 2.

TLR2 engagement on CD4+ T cells enhances IL9 mRNA and protein expression driven by polyclonal activation and TGF-β and IL-4. Naïve CD4+T cells from WT mice were stimulated with anti-CD3 and anti-CD28 mAbs and exogenous TGF-β (5ng/ml) and IL-4 (10ng/ml), with or without P3CSK4 (1μg/ml) for 48h. (A) Relative expression of Il9 mRNA under polarizing conditions with or without P3CSK4 (n=3).
(B) Flow cytometric analysis of CD4+ T cells labeled for intracellular IL-9 and IFN-γ.
(C) IL-9 in culture supernatants was determined by ELISA. (D) IFN-γ in culture supernatants was determined by ELISA.
Means ± SD of five independent experiments are shown. * p < 0.05, ** p < 0.01. NS denotes non-significant.
We next measured TLR2 expression under different stimulation conditions and confirmed that TLR2 expression in naïve CD4+T cells is induced by TCR activation, especially when combined with CD28 co-stimulation (Supplemental Fig. 2). Also, TLR2 expression was higher in CD4+T cells activated under TH9 polarizing conditions compared to those activated under non-polarizing conditions (Supplemental Fig. 2).
Altogether these results indicate that TLR2 engagement on CD4+ T cells reinforces TFG-β and IL-4 driven TH9 differentiation and function. Our data also demonstrate for the first time expression of TLR2 on CD4+ T-cells activated under TH9 polarizing conditions.
TLR2− but not other TLR ligands strengthen TH9 T cell differentiation in cells stimulated in presence of TGF-β and IL-4
In addition to TLR2, expression and function of several TLRs have been reported on various subsets of purified CD4+ T cells (9, 10, 12, 14, 19–22). Thus, we next investigated the effect of a panel of TLR ligands on IL-9 cytokine secretion by CD4+ T-cells activated under non-polarizing or TH9-polarizing conditions. We used IFN-γ secretion as a read out of the effects of TLR2 triggering under non-polarizing conditions where IFN-γ is predicted to be increased, and of TGF-β and IL-4 bioactivity where IFN-γ is predicted to be decreased. Under non-polarizing conditions the TLR1/2 ligand P3CSK4 and the TLR2/6 ligand P2CSK4 had no significant effect on IL-9 secretion in response to antiCD3/anti-CD28 stimulation (Fig. 3A). As expected, under these conditions the TLR2 ligands P3CSK4 and P2CSK4 enhanced anti-CD3/anti-CD28− induced IFN-γ response (Fig. 3B) [12]. On the other hand, under TH9 polarizing conditions, cells treated with both P3CSK4 and P2CSK4 secreted higher levels of IL-9, but not IFN-γ, in response to anti-CD3 and anti-CD28 compared to untreated cells. None of the other TLR ligands had any effect on IL-9 secretion under either non-polarizing or TH9 polarizing conditions (Fig.3A). Interestingly, LPS and Flagellin had minor inhibitory effect on IFN-γ secretion induced by anti-CD3/anti-CD28 under TH9 polarizing conditions (Fig. 3B).
Figure 3.

TLR2, but no other TLR ligands increase TGF-β and IL-4 driven IL-9 cytokine secretion. Naive CD4+ T cells were activated with anti-CD3 and anti-CD28 mAbs in the presence of different TLR ligands (1μg/ml) under non-polarizing and TH9 polarizing conditions for 48 h. IL-9 (A) and IFN-γ (B) ELISA were measured by culture supernatants. Means ± SD of three independent experiments are shown. * p < 0.05, ** p < 0.01, NS denotes non-significant.
To confirm that induction of IL-9 by P3CSK4 and P2CSK4 under TH9 polarizing conditions was dependent on TLR2, we used CD4+ T cells isolated from TLR2 knockout (KO) mice. As predicted, while the levels of IL-9 in response to stimulation were comparable between CD4+ T cells from TLR2 knockout- and WT mice, cells from TLR2KO mice failed to respond to P3CSK4 or P2CSK4 (Supplemental Fig. 3).
These results indicate that TLR2 is unique among TLRs in its ability to enhance TH9 polarization.
TLR2 engagement on CD4+ T cells enhances TGF-β and IL-4-driven TH9 differentiation of Ag-specific cells
To further test the role of T-cell-expressed TLR2 on TH9 T cell differentiation we used an Ag-specific system. To avoid confounding effects derived from the interaction of TLR2 ligands with TLR2 expressed on macrophages, we used BMDM isolated from TLR2 KO mice as APC. M. tuberculosis (MTB) Ag85B TCR transgenic CD4+ T cells were stimulated with Ag85B peptide-pulsed BMDM under non-polarizing or TH9 polarizing conditions in presence or absence of P3CSK4. While the percentage of Ag-specific IL-9+ T cells and the levels of IL-9 remained unchanged, the percentage of Ag-specific IFN-γ+ cells and the amount of IFN-γ secreted by these cells were significantly increased by P3CSK4 under non-polarizing conditions (Fig. 4A). On the other hand, when cells were activated in TH9 polarizing conditions, the percentage of Ag-specific IL-9+ T cells and the levels of IL-9 in culture supernatants almost tripled in cells treated with P3CSK4 compared to control (Fig. 4A, B). Under these conditions, the percentage of Ag-specific IFN-γ+ cells and the amount of IFN-γ secreted by these cells were very low and remained unchanged in cells treated with TLR2 ligand compared to control cells (Fig. 4 A, C).
Figure 4.

TLR2 engagement on Antigen85B specific CD4+ T cells increases TH9 differentiation driven by TGF-β and IL-4. Naive Ag85B transgenic CD4+ T cells were co-incubated with TLR2 KO BMDM pulsed with Ag85B peptide (1μg/ml) and co-stimulated with or without TLR2 ligand (P3CSK4) under non-polarizing and TH9 polarizing conditions for 48 h. (A) CD4+ T cells were permeabilized and labeled with mAbs to IL-9 and IFN-γ and percentages of IL-9+ or IFN-γ+ cells were determined by flow cytometry. (B, C) IL-9 and IFN-γ were measured in culture supernatants by ELISA. Means ± SD of five independent experiments are shown. * p < 0.05, ** p < 0.01. NS denote non- significant.
These results demonstrate that engagement of TLR2 on Ag-specific CD4+ T cells can strengthen TH9 differentiation in response to TCR engagement and exogenous TGF-β and IL-4. Also, our data reveals a dual role for TLR2 co-stimulation in CD4+ T cell subset differentiation that is dependent on the cytokine environment, with TLR2 co-stimulation enhancing TH1 differentiation under non-polarizing conditions and TH9 differentiation under the influence of TGF-β and IL-4.
TLR2 engagement on CD4+ T cells up-regulates BATF and PU.1
TH9 cell development depends on several transcription factors including PU.1, BATF and IRF4 (23–25). Thus, we assessed the effect of TLR2 triggering in CD4+ T cells on expression of Batf, Pu.1 and Irf4 genes after stimulation under non-polarizing or TH9 polarizing conditions. Tbx21 and Gata3 were used as controls for transcription factors that regulate TH1 and TH2 differentiation respectively (26, 27). As expected, Batf and Pu.1 were significantly increased in cells stimulated under TH9 polarizing conditions (Fig. 5 A, B). Furthermore, TLR2 co-stimulation enhanced Batf and Pu.1 under both non-polarizing and TH9 polarizing conditions (Fig 5 A, B). On the other hand, Irf4 mRNA was not significantly modulated by TLR2 co-stimulation (data not shown). As predicted, Tbx21 was significantly increased in cells co-stimulated via TLR2 compared to control under non-polarizing conditions but remained unchanged under TH9 polarizing conditions (Fig. 5C). Gata3, a TH2 transcription factor reported to play a supplementary role in the regulation of TH9 differentiation, was induced by TGF-β and IL-4 but remained unchanged in response to TLR2 co-stimulation (Fig. 5D).
Figure 5.

Effect of TLR2 engagement on the expression of TH9, TH1 and TH2 transcription factors under non-polarizing or TH9− polarizing conditions. Naïve CD4+ T cells were stimulated with anti-CD3 and anti-CD28 mAbs alone (non-polarizing) or combined with TGF-β and IL-4 (TH9− polarizing) and with or without P3CSK4. Batf (A), Pu.1 (B), Tbx21 (C) and Gata3 (D) mRNA expression was determined by RT-PCR and normalized to actin.
Bars represent means ± SD of three independent experiments. * p < 0.05, ** p < 0.01. NS denotes non-significant.
These data reveal that TLR2 engagement on CD4+ T cells up-regulates two of the three key transcription factors required for TH9 differentiation, i.e. BATF and PU.1. In addition, our results suggest that while both TH9 (BATF, PU.1) and TH1 (TBX21)- transcription factors are up-regulated by TLR2 under non-polarizing conditions, the net effect of these factors acting together is TH1 development. On the contrary, under the influence of TGF-β and IL-4, the presence of BATF, PU.1, GATA3 and absence of TBX21, leads to the development of IL-9 secreting cells. Thus, TLR2 co-stimulation regulates both TH1 and TH9 transcription factors, and depending on the cytokine environment contributes to either TH1 or TH9 differentiation.
TLR2 costimulation upregulates IL9 mRNA expression and IL-9 secretion in activated human CD4+ T cells polarized with TGF-β and IL-4
TGFb and IL4 driven TH9 differentiation were also reported in human CD4+ T cells (28). We next determined whether TLR2 costimulation of human CD4+ T cells induces IL9 mRNA expression under polarizing condition. CD4+ T cells from three donors were stimulated with plate-bound anti-CD3 and soluble anti-CD28 in the presence and absence of TLR2 agonist. This activation carried out both in the absence of exogenous cytokines (non-polarizing condition) or presence of exogenous IL-4 and TGF-β (TH9 polarizing condition) for 48 h, followed by RNA isolation and measurement of IL-9 levels in the culture supernatants. As shown (Fig. 6A), upregulation of IL9 mRNA expression in response to P3CSK4 stimulation under polarized condition was observed In all donors In agreement with the mRNA expression data, increased IL9 secretion in culture supernatants was observed in cells co-stimulated via TLR2 under polarized condition (Fig 6B). Under non-polarizing conditions, low IL9 mRNA expression was observed and no significant IL9 cytokine secretion was detected (Fig 6 A, B).
Figure 6.

TLR2 engagement on human CD4+ T cells enhances IL9 mRNA and protein expression driven by polyclonal activation and TGF-β and IL-4. Naïve CD4+ T cells from three human donors were stimulated with anti-CD3 (10ug/ml) and anti-CD28 mAbs (1ug/ml) and exogenous TGF-β (5ng/ml) and IL-4 (10ng/ml), with or without P3CSK4 (2μg/ml) for 48h. (A) Relative expression of Il9 mRNA under non-polarizing and polarizing conditions with or without P3CSK4 (n=3) is shown. (B) IL-9 cytokine in culture supernatants were determined by ELISA. Means ± SD of three technical replicates for each donor are shown.
Discussion
We previously demonstrated that TLR2 engagement on human and mouse CD4+ T cells triggers co-stimulatory signals that up-regulate TCR-driven activation and TH1 differentiation (12). To further understand the role of TLR2 co-stimulation on T cell differentiation and function, in the current study we performed a comparative gene expression analysis of CD4+ T cells co-stimulated via either CD28 or TLR2. As previously reported by other authors, co-stimulation via CD28 induced changes in expression of a wide array of genes (29, 30). On the other hand, our findings indicate that TLR2 co-stimulation triggers discrete changes in the transcriptome of CD4+ T cells, among which, the up-regulation of the Il9 gene was the most significant one. Furthermore, TLR2 co-stimulation of both mouse and human CD4+ T cells in the presence of TGF-β and IL-4 significantly increased Il9 gene expression and IL-9 secretion. These results suggest that TLR2 engagement on mouse and human CD4+ T cells does not merely increase T-cell activation and strengthen TH1 polarization, but also contributes to TH9 subset differentiation.
CD4+ T cells play major roles in the immune response through secretion of cytokines, activation of macrophages, B-cells and cytotoxic T cells, and also via suppression of immune reactions. After being activated, CD4+ T cells differentiate into different TH effector subsets. In addition to the classical TH1 and TH2 subsets, TH17, TH22, follicular helper T cells (TFH)-, Tregs-, Tr1- subsets as well as the new subset of Th9 cells have been described (1, 2). Generation of these different lineages is dependent on the complex interplay among TCR signals, co-stimulatory signals and importantly, the cytokine environment where T-cell activation takes place. Although TLRs are prototypic innate immune receptors, recent evidence demonstrates that they are also expressed on T lymphocytes, including CD4+, CD8+, and Tregs, where they have a role in co-stimulation and TH differentiation. TLR2, TLR5 and TLR 7/8 can provide a second signal for activation of T cells upon TCR engagement and promote development of TH1-, TH17- and Treg- cells (9, 10). While human CD4+ T-cells have been shown to express several TLRs (TLR2, TLR5, TLR7/8), there are considerable variations in evidence for TLR expressions on mouse CD4+ T cells (9, 21, 22). Here we demonstrated that, TLR2 engagement on mouse CD4+ T cells up-regulates Il9 gene expression and, when combined with known TH9-inducing cytokines such as TGF-β and IL-4, strengthens the development of IL-9-secreting T-cells. Interestingly, ligands for both TLR2/1 and TLR2/6 heterodimers increased the number of IL-9 producing cells, suggesting that both types of TLR2 heterodimers are expressed on mouse CD4+ T-cells and that their signals can synergize with those derived from TGF-β and IL-4 to modulate IL-9 secretion. On the other hand, ligands for other TLRs had no effect on IL-9 secretion. This is consistent with the lack of evidence for expression of most TLRs in mouse CD4+ T cells purified subsets. However, in addition to TLR2, mouse CD4+ T cells were reported to express TLR4 (31, 32). Thus, the lack of LPS effect on IL-9 secretion is likely due to the absence of synergy between signaling pathways downstream of TLR4 with those downstream of TGF-β and IL-4 rather than absence TLR4 expression. To our knowledge, this is the first report on the regulation of IL-9 secretion and TH9 differentiation by TLR2 expressed on CD4+ T cells.
TLR2 functions as co-stimulatory receptor on both human and mouse CD4+ T-cells (11, 12). TLR2 engagement by natural or synthetic ligands under non-polarizing conditions promoted secretion of IFN-γ and TH1 differentiation. Also, TLR2 engagement in polarized Th1 cells enhanced effector functions leading to immune protection against intracellular pathogens (12). Our current findings extend the regulatory role of TLR2 in CD4+ T cells, demonstrating that TLR2 engagement on CD4+ T cells has different effects depending on the cytokine environment where T-cell stimulation occurs. Under non-polarizing conditions TLR2 signaling enhances IFN-γ secretion and TH1 differentiation. In contrast, TLR2 triggering in combination with TGF-β and IL-4 signaling, leads to up-regulation of IL-9 secretion and increases the number of IL-9 secreting cells, thus favoring TH9 differentiation. These effects are independent of the mode of TCR engagement, i.e. through CD3 cross-linking or via cognate antigen recognition.
Previous studies have reported a role for IFN-γ in counter-regulation of TH9 T cell development (16, 33). This, along with our results suggests that TLR2 engagement in the absence of TGF-β and IL-4 likely primes T cells for IFN-γ secretion, and this in turn down-regulates IL-9 production. On the other hand, in the presence of TGF-β and IL-4, TLR2 signaling favors the STAT6/SMAD pathway that leads to enhanced Th9 differentiation (17). Of interest is the fact that TLR2 signaling in the absence of IL-4 and TGF-β increased Il9 gene expression, but had no or minimal effect on IL-9 secretion. This suggests that under non-polarizing conditions TLR2 signaling in T-cells can induce Il9 gene transduction; however for optimal cytokine production, which is the result of gene transduction, translation and protein stability (34), requires: a) additional signals initiated by IL-4 and TGF-β; and b) the absence of counter-regulation by IFN-γ.
TH cells are characterized not only by the cytokines they secrete but also by the lineage-specific transcription factors they express. PU.1 is an ETS family transcription factor that specifically stimulates the development of TH9 cells since it represses TH2 differentiation, and is considered a switch factor between these two subsets (35). BATF and IRF-4 are other members of the transcription factor network required for IL-9 production (24, 25). Our results demonstrate that TLR2 signaling in CD4+ T cells up-regulates both PU.1 and BATF, but not IRF-4, under both non-polarizing and Th9 polarizing conditions. On the other hand, TLR2 signaling had no effect on the TH2-stimulating transcription factor GATA3 under either condition. As expected, TLR2 signaling up-regulated the TH1-promoting factor TBX21 under non-polarizing conditions but had no effect on this factor in a Th9 polarizing cytokine environment. Thus, TLR2 engagement on CD4+ T cells up-regulates two of the three key transcription factors required for TH9 differentiation, i.e. BATF and PU.1. Furthermore, while both TH9 (BATF, PU.1)- and TH1 (TBX21)- transcription factors are up-regulated by TLR2 under non-polarizing conditions, the net effect of these factors acting together in TH1 development. On the other hand, under the influence of TGF-β, IL-4 and TLR2 signals, the presence of BATF, PU.1, GATA3 and absence of TBX21, leads to the development of IL-9 secreting cells. Thus, TLR2 co-stimulation regulates both TH1 and TH9 transcription factors, and depending on the cytokine environment, contributes to either TH1 or TH9 differentiation. Since we did not detect changes in IL-4 or TGF-β levels or IL-4R or TGF-βR1 expression after TLR2 stimulation (Supplemental Fig. 4), our data suggests that TLR2 synergizes with IL-4 and TGF-β at the signaling level to induce TH9 differentiation.
In summary, this study reveals a novel role for T-cell expressed TLR2 in enhancing the differentiation and function of TH9 T cells via up-regulation of PU.1 and BATF.
Materials and Methods
Antigens, Antibodies and Reagents
Mtb Ag85B encompasses the major epitope (aa 240–254) recognized by P25 TCR-Tg T cells (peptide 25). Peptide 25 (NH2-FQDAYNAAGGHNAVF-COOH) was purchased from Invitrogen. The following mAbs and isotype controls were purchased for analysis of receptor expression: anti-CD3-PE, anti-CD4-APC, and LIVE/DEAD violet and yellow cell stain (all from ebioscience); for intracellular staining: anti-mouse IL9-PE (RM9A4) and anti-mouse IFN-γ–FITC (XMG1.2) were purchased from Biolegend and Ebioscience respectively. Pam3Cys-SKKKK and Pam2Cys-SKKKK; EMC Microcollections GmbH, L2000; (P3CSK4 and P2CSK4 from here on in) was re-suspended in endotoxin-free PBS at 1 mg/ml prior to use. For T-cell activation, hamster anti-mouse CD3ε (145-2C11), anti-mouse CD28 (clone 37.51) from BD Biosciences were used. The following fluorescein isothiocyanate (FITC)-conjugated, phycoerythrin (PE)-conjugated, allophycocyanin-conjugated, phycoerythrin-Cy7-conjugated (PE-Cy7), and pacific Blue-conjugated, or eFluor450 (PB) Abs were purchased from the following vendors: BD Biosciences, San Jose, CA (FITC-CD8; 553031; FITC-CD14; 553739; FITC-CD45/B220; 553087; FITC-IA/IB, 553623); E-bioscience, San Diego, CA (FITC-NK1.1, 11-5941-82; FITC-CD40,11-0402-85; PE-Cy7-CD62L, 25-0621-82).
IL-2 was measured using a sandwich ELISA with the anti-IL-2 Ab pairs JES6-1A12 (eBioscience; 14-7022-85) and biotinylated JES6-5H4 (eBioscience; 13-7021-85). IFN-γ was measured by sandwich ELISA (R&D Systems; MIF00) and IL-9 ELISA was done using the DuoSet ELISA kit (R&D Systems; DY409) by following manufactured protocol.
Mice
Eight to twelve week-old C57BL/6J mice were purchased from Charles River Laboratories (Wilmington, MA). Mycobacterial Ag85B-specific TCR transgenic (P25 TCR-Tg) mice were generously provided by Kiyoshi Takatsu (University of Tokyo, Japan). P25TCR-Tg T cells recognize peptide (NH2-FQDAYNAAGGHNAVF-COOH) derived from MTB Ag85B in the context of MHC II I-Ab (13). Tlr-2 gene knockout mice in the C57BL/6J background (Tlr2−/−) were generously provided by O. Takeuchi and S. Akira (Osaka University, Osaka, Japan). Mice were housed under specific pathogen-free conditions. All experiments were performed in compliance with the U.S. Department of Health and Human Services Guide for the care and use of Laboratory Animals and were approved by the Institutional Animal care and Use Committee at Case Western Reserve University (protocol number: 2015-0030).
Isolation of mouse CD4+T cells
Mouse CD4+ T cells were isolated from spleens of 8- to 10-week old P25 TCR Tg mice or from the spleens of wild-type C57BL/6J mice. Tissues were dissociated, and RBC lysed in hypotonic lysis buffer (10 mM Tris-HCl and 0.83% ammonium chloride). Spleenocytes were plated in 100-mm tissue culture plates and allowed to adhere for 1 h at 37° C. Untouched CD4+ T cells were purified from non-adherent splenocytes using a CD4+ T-cell-negative isolation kit (Miltenyi Biotec) by following manufacturer’s instructions (purity > 95%). Highly purified naïve (CD25− CD44− CD62L+) CD4+ T cells were isolated from spleens using a combination of immune-MACS followed by FACS as described with some modification (12, 14). Briefly, Cells were first gated on the base of FSC and SSC parameters to select for small non-granular cells (lymphocyte gate) followed by an exclusion gate that eliminated contaminating cells (“dump FITC channel gate”). For enrichment of naïve CD4+ T cells, IMACS-CD4+ T cells were labeled with anti- CD62L-PE-Cy7 and sorted with a gate drawn around PE-Cy7 positive events in combination with the exclusion gate described above (“dump allophycocyanin channel gate”). After FACS, cells were labeled with anti- CD4, and anti- CD3 mAb and purity was determined in a BD FACS Aria flow cytometer. The average purity was 98 to 99% (range 99.6 ± 0.15% CD3+, 93.87± 1.93 CD4+).
Cell culture
All experiments were performed with purified CD4+ T cells cultured in complete media, i.e. D10F, consisting of DMEM (BioWhittaker, Walkersville, MD.) supplemented with fetal calf serum (10% v/v, Gemini-Bioproducts, West Sacramento, CA; 100–106), penicillin (100 IU/ml), streptomycin (100 μg/ml), HEPES (100mM), nonessential amino acids (100mM), L-glutamine (2 mM), and 2-mercaptoethanol (0.05 mM) at 37°C in 5% CO2.
Anti- CD3 and Anti-CD28 mAb stimulation
Mouse CD4+ T cells (105/well) were cultured in the presence of plate-bound anti-mouse CD3 mAb alone (BD, Franklin Lakes, NJ; 553058) and with anti mouse CD28 in presence of P3CSK4 or media alone. Cell-free culture supernatants (50 μl) were collected at 24 h (IL-2) and 100 μl at 48 h (IFN-γ, IL-9) for cytokine quantification by ELISA.
Isolation and activation of Human CD4+ T cells
Isolation and stimulation of human CD4+ T cells primary human Naïve CD4+ T cells from healthy donors were isolated as described previously (11, 14). Briefly, peripheral blood was obtained from healthy donors, and the T cells were isolated from the buffy coat using Ficoll-Hypaque (Amersham Biosciences) density gradient centrifugation, followed by isolation with EasySep Human Naïve CD4+ T cell isolation beads (STEM CELL Technology). Where required, flow sorting was used. T cells (>95% purity) were cultured/rested in complete ex vivo media (Life Technologies) until use. CD4+ T cells, were stimulated with plate-bound anti-CD3ε (10 mg/ml) and soluble anti-CD28 (1 mg/ml) in the presence and absence of TLR2 agonist (P3CSK4 2ug/ml). Cell-free culture supernatants (100 μl) were collected at 48h for IL9 cytokine quantification by ELISA (IL-9 Human ELISA Kit, Cat# BMS2081). All human cell studies were approved by the Case Western Reserve University Institutional Review Board and the National Institutes of Health (IRB number: 03-88-63). All adult subjects provided written informed consent, and a parent or guardian of any child participant provided informed consent on his/her behalf.
Microarray Analysis
Total RNA was isolated from naïve CD4+ T cells (from C57BL/6J mice using the RNeasy Plus Mini Kit (Qiagen, Germantown, MD; 74134), according to the manufacturer’s protocol. The quality of extracted RNA was assessed by the Agilent Bioanalyzer (Agilent Technologies). Total RNA from each sample was processed for hybridization onto Affymetrix Mouse Gene 1.0 ST Array which interrogates 28,853 genes with 770,317 distinct probes according to the manufacturer’s protocol and scanned. Normalized data were analyzed using Affymetrix Transcription Analysis Console (TAC) software based on the the FDR based t-test procedure within Significance Analysis of Microarrays software (version 3.09) to identify differentially expressed transcripts (15). The data discussed in this publication have been deposited and are accessible through GEO Series accession number GSE89513.
Ag85B specific stimulation
Bone marrow-derived macrophages (BMDM) from Tlr2−/− mice were generated by culturing bone marrows from C57BL/6J mice in complete DMEM containing 20% L929 (for MCSF) culture supernatant for 7 days. At day 8, BMDM were matured by treating with IFN-γ (4 ng/ml) for 48 h before use in assays. 2 ×106 BMM cells per well in 6-well plates or 1 ×105 BMM cells per well in 96-well plates were washed three times, and pulsed with or without Ag85B peptide for O/N at 37°C. BMDM were then co-cultured with naïve CD4+ T cells isolated from P25 TCR Tg mice with or without P3CSK4. Culture supernatants were taken 24 and 48 hours for cytokine measurements.
Assessment of intracellular cytokines by flow cytometry
Single-cell suspensions of tissues and sorted cells were counted and viability was assessed by trypan blue exclusion. A total of 2 × 106 cells were harvested for intracellular cytokine staining (ICS). CD4+ T cells were then washed and stained with Abs against cell surface markers and Live Dead dye, then treated with the Fix & Perm Cell Permeabilization Kit (Invitrogen, GAS004) according to manufacturer instructions and stained with correspondent intracellular Abs. Stained samples were acquired using a BD LSR II flow cytometer. Flow cytometry results were analyzed with FlowJo software (Tree Star, Inc., Ashland, OR).
RT-PCR
Total RNA was isolated from naïve CD4+ T cells from C57BL/6J mice and human donors using the RNeasy Plus Mini Kit (Qiagen, Germantown, MD; 74134), according to the manufacturer’s protocol. cDNA was generated using Superscript III First-Strand Synthesis System for RT-PCR and oligoDTs (Invitrogen; 18080051) or the QuantiTect® Reverse Transcription kit (Qiagen; 205310), according to the manufacturer’s protocol. Inventoried TaqMan gene expression Assay (Applied Biosystems; 4331182) and primers for TLR-2 (Invitrogen, Mm00442346_m1), Irf4 (Invitrogen, Mm01165979_m1), Spi1/Pu.1 (Invitrogen, Mm00488142_m1), Il9 (Invitrogen, Mm00434305_m1 for mouse and Hs00174125_m1 for Human), Batf (Invitrogen, Mm00479410_m1), Tbx21 (Invitrogen, Mm00450960_m1), Gata3 (Invitrogen, Mm00484683_m1 and the control housekeeping gene βactin (Invitrogen, Mm00607939_s1 s1 for Mouse and Hs99999903_m1 for Human) were used to perform real time-PCR. PCR and analysis was done using the StepOne Real-Time PCR Systems and Software (Applied Biosystems; 4376374). The comparative CT method (2ˆ−ΔΔCT) as described in the manufacturer’s guide was used to assess relative quantities of mRNA.
Statistical analysis
Microsoft excel was used to test for differences in means between specific treatment groups. The null hypothesis of no difference in means between specific treatment groups was tested using a Student’s t-statistic with a p-value <0.05 taken as evidence of a significant difference between groups (*p <0.05; **p <0.01).
Supplementary Material
Acknowledgments
A.F.K and R.E.R duly acknowledged the funding support from The American Association of Immunologist under the AAI career in Immunology Fellowship program. This work was supported by NIH grants AI099494 (to R.E.R), CFAR Developmental Award (CWRU/UH CFAR, P30 AI036219) (to R.E.R), AI027243-26 (to W.H.B). We also thank the Cytometry Facility of the Case Comprehensive Cancer Center (NIH P30 CA43703) for access to core facilities that supported this research. Microarray gene expression experiments were done in the Gene Expression & Genotyping Core Facility of the Case Comprehensive Cancer Center (P30 CA043703).
Abbreviations
- BMDM
Bone marrow-derived macrophages
- P3CSK4
Pam3Cys-KKKK
- TLR2
Toll like receptor 2
Footnotes
Conflict of Interest: The authors declare no financial or commercial conflict of interest
References
- 1.Luckheeram RV, Zhou R, Verma AD, Xia B. CD4(+)T cells: differentiation and functions. Clin Develop Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang S, Dong C. A complex issue on CD4+ T-cell subsets.pdf. Immunol Rev. 2013;252:5–11. doi: 10.1111/imr.12041. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Nat Acad Sci USA. 2004;101:3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imanishi T, Hara H, Suzuki S, Suzuki N, Akira S, Saito T. Cutting Edge: TLR2 Directly Triggers Th1 Effector Functions. J Immunol. 2007;178:6715–6719. doi: 10.4049/jimmunol.178.11.6715. [DOI] [PubMed] [Google Scholar]
- 6.Cottalorda A, Mercier BC, Mbitikon-Kobo FM, Arpin C, Teoh DY, McMichael A, Marvel J, Bonnefoy-Berard N. TLR2 engagement on memory CD8(+) T cells improves their cytokine-mediated proliferation and IFN-gamma secretion in the absence of Ag. Eur J Immunol. 2009;39:2673–2681. doi: 10.1002/eji.200939627. [DOI] [PubMed] [Google Scholar]
- 7.Mercier BC, Cottalorda A, Coupet CA, Marvel J, Bonnefoy-Berard N. TLR2 engagement on CD8 T cells enables generation of functional memory cells in response to a suboptimal TCR signal. J Immunol. 2009;182:1860–1867. doi: 10.4049/jimmunol.0801167. [DOI] [PubMed] [Google Scholar]
- 8.Macleod H, Wetzler LM. T Cell Activation by TLRs: A Role for TLRs in the Adaptive Immune Response. Sci STKE. 2007 doi: 10.1126/stke.4022007pe48. pe48. [DOI] [PubMed] [Google Scholar]
- 9.Jin B, Sun T, Yu XH, Yang YX, Yeo AE. The effects of TLR activation on T-cell development and differentiation. Clin Dev Immunol. 2012;2012:836485. doi: 10.1155/2012/836485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutmuller RP, Morgan ME, Netea MG, Grauer O, GJ A. Toll-like receptors on regulatory T cells: expanding immune regulation. Trends Immunol. 2006;27:387–393. doi: 10.1016/j.it.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Lancioni CL, Li Q, Thomas JJ, Ding X, Thiel B, Drage MG, Pecora ND, Ziady AG, Shank S, Harding CV, Boom WH, Rojas RE. Mycobacterium tuberculosis lipoproteins directly regulate human memory CD4(+) T cell activation via Toll-like receptors 1 and 2. Infect Immun. 2011;79:663–673. doi: 10.1128/IAI.00806-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reba SM, Li Q, Onwuzulike S, Ding X, Karim AF, Hernandez Y, Fulton SA, Harding CV, Lancioni CL, Nagy N, Rodriguez ME, Wearsch PA, Rojas RE. TLR2 engagement on CD4(+) T cells enhances effector functions and protective responses to Mycobacterium tuberculosis. Eur J Immunol. 2014;44:1410–1421. doi: 10.1002/eji.201344100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura T, Ariga H, Kinashi T, Uehara S, Kikuchi T, Nakada M, Tokunaga T, Xu W, Kariyone A, Saito T, Kitamura T, Maxwell G, Takaki S, Takatsu K. The role of antigenic peptide in CD4+ T helper phenotype development in a T cell receptor transgenic model. Int Immunol. 2004;16:1691–1699. doi: 10.1093/intimm/dxh170. [DOI] [PubMed] [Google Scholar]
- 14.Lancioni CL, Thomas JJ, Rojas RE. Activation requirements and responses to TLR ligands in human CD4+ T cells: Comparison of two T cell isolation techniques. J Immunol Methods. 2011;344:15–25. doi: 10.1016/j.jim.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Nat Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, Kühn R, Müller W, Palm N, Rüde E. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J Immunol. 1998;1:3989–3996. [PubMed] [Google Scholar]
- 17.Kaplan MH, Hufford MM, Olson MR. The development and in vivo function of T helper 9 cells. Nat Rev Immunol. 2015:295–307. doi: 10.1038/nri3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veldhoen M, Uyttenhove C, Snick JV, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor- ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9–producing subset. Nat Immunol. 2008:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 19.Rahman AH, Taylor DK, Turka LA. The contribution of direct TLR signaling to T cell responses. Immunol Res. 2009;45:25–36. doi: 10.1007/s12026-009-8113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds JM, Pappu BP, Peng J, Martinez GJ, Zhang Y, Chung Y, Ma L, Yang XO, Nurieva RI, Tian Q, Dong C. Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity. 2010;32:692–702. doi: 10.1016/j.immuni.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mansson A, Adner M, Cardell LO. Toll-like receptors in cellular subsets of human tonsil T cells: altered expression during recurrent tonsillitis. Respir Res. 2006;7:36. doi: 10.1186/1465-9921-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crellin NK, Garcia RV, Hadisfar O, Allan SE, Steiner TS, Levings MK. Human CD4+ T Cells Express TLR5 and Its Ligand Flagellin Enhances the Suppressive Capacity and Expression of FOXP3 in CD4+CD25+ T Regulatory Cells. J Immunol. 2005;175:8051–8059. doi: 10.4049/jimmunol.175.12.8051. [DOI] [PubMed] [Google Scholar]
- 23.Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, Jabeen R, McKinley C, Ahyi AN, Han L, Nguyen ET, Robertson MJ, Perumal NB, Tepper RS, Nutt SL, Kaplan MH. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jabeen R, Goswami R, Awe O, Kulkarni A, Nguyen ET, Attenasio A, Walsh D, Olson MR, Kim MH, Tepper RS, Sun J, Kim CH, Elizabeth J, Taparowsky EJ, Zhou B, Kaplan MH. Th9 cell development requires a BATF-regulated transcriptional network. J Clin Invest. 2013;123:4641–4653. doi: 10.1172/JCI69489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, Gerlitzki B, Hoffmann M, Ulges A, Taube C, Dehzad N, Becker M, Stassen M, Steinborn A, Lohoff M, Schild H, Schmitt E, Bopp T. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33:192–202. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A Novel Transcription Factor, T-bet, Directs Th1 Lineage Commitment.pdf. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 27.O’Garra A, Gabryšová L. Transcription Factors Directing Th2 Differentiation: Gata-3 Plays a Dominant Role. J Immunol. 2016;196:4423–4425. doi: 10.4049/jimmunol.1600646. [DOI] [PubMed] [Google Scholar]
- 28.Wong MT, Ye JJ, Alonso MN, Landrigan A, Cheung RK, Engleman E, Utz PL. Regulation of Human Th9 differentiation by type 1 Interfereons and IL-21. Nat Cell Bio. 2010;88:624–631. doi: 10.1038/icb.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riley JL, Mao M, Kobayashi S, Biery M, Burchard J, Cavet G, Gregson BP, June CH, Linsley PS. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Nat Acad Sci USA. 2002;99:11790–11795. doi: 10.1073/pnas.162359999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diehn M, Alizadeh AA, Rando OJ, Liu CL, Kryn Stankunas K, David Botstein D, Crabtree GR, Brown PO. Genomic expression programs and the integration of the CD28 costimulatory signal in T cell activation.pdf. Proc Nat Acad Sci USA. 2002;99:11796–11801. doi: 10.1073/pnas.092284399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gelman AE, Zhang J, Choi Y, Turka LA. Toll-Like Receptor Ligands Directly Promote Activated CD4+ T Cell Survival. J Immunol. 2004;172:6065–6073. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukata M, Breglio K, Chen A, Vamadevan AS, Goo T, Hsu D, Conduah D, Xu R, Abreu MT. The Myeloid Differentiation Factor 88 (MyD88) Is Required for CD4+ T Cell Effector Function in a Murine Model of Inflammatory Bowel Disease. J Immunol. 2008;180:1886–1894. doi: 10.4049/jimmunol.180.3.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murugaiyan G, Beynon V, Pires Da Cunha A, Joller N, Weiner HL. IFN-gamma limits Th9-mediated autoimmune inflammation through dendritic cell modulation of IL-27. J Immunol. 2012;189:5277–5283. doi: 10.4049/jimmunol.1200808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogel C, Abreu Rde S, Ko D, Le SY, Shapiro BA, Burns SC, Sandhu D, Boutz DR, Marcotte EM, Penalva LO. Sequence signatures and mRNA concentration can explain two-thirds of protein abundance variation in a human cell line. Mol Sys Biol. 2010;6:400. doi: 10.1038/msb.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goswami R, Kaplan MH. Gcn5 is required for PU.1-dependent IL-9 induction in Th9 cells. J Immunol. 2012;189:3026–3033. doi: 10.4049/jimmunol.1201496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


