Abstract
Background
The patho-physiological cross-talk between diabetes and obesity is well established. However, the choices of drugs suitable for combined treatment of diabetes and obesity are limited. Integration of complementary and alternative medicines (CAMs), like Ayurveda, with modern medicine would be a promising strategy to fill this gap. The diagnostic principles of Ayurveda define obesity as one of the predisposing factors of Madhumeha (correlated as diabetes) and recommends specific formulations for managing obese-diabetes. Lodhrasavam is one such poly-herbal formulation prescribed for obese-diabetic patients.
Objectives
The present study is an attempt to demonstrate the possible modes of action of Lodhrasavam, built on the hypothesis that the formulation can exert both anti-diabetic and anti-obesity actions.
Materials and methods
Lodhrasavam, following simulated gastro-intestinal digestion, was monitored for inhibition of α-amylase, α-glucosidase (key digestive enzyme targets of anti-diabetic drugs) and adipogenesis using standard in vitro model systems.
Results
Lodhrasavam digest inhibited α-amylase (90%) and α-glucosidase (78%) activity as well as reduced the differentiation of 3T3-L1 fibroblasts to adipocytes. Upon fractionation, the enzyme inhibitory activity and anti-adipogenic activity of the digest were found distributed in different solvent fractions. This partly indicates a potential pharmacological networking of chemically and functionally diverse bioactive molecules in Lodhrasavam.
Conclusion
The study provides a possible mode of action and an experimental support for the Ayurvedic use of Lodhrasavam for managing obese-diabetes. Generating scientific evidences and understanding the modes of action, in contemporary scientific language, would essentially help in expanding global acceptance of potentials of CAMs in the management of life style disorders.
Keywords: Ayurveda, Diabetes, Lodhrasavam, Digestive enzymes, Adipogenesis, In vitro digestion
1. Introduction
The link between diabetes and obesity is well established in both traditional and modern systems of medicines. The World Health Organization (WHO) estimates that 44% of the diabetes cases globally are attributable to overweight and obesity [1]. Though intensive lifestyle interventions are the foundation for managing weight gain in diabetes, studies have shown that pharmacotherapies are also necessary for patients to achieve long term weight loss [2]. Despite having an evident patho-physiological cross-talk between diabetes and obesity, the choices of drugs suitable for combined treatment of diabetes and obesity are limited in modern medicine [3]. Recent examples like glucagon-like peptide-1 receptor agonists show promising results in combating the dual burden, but their long term safety is question [4], [5]. Furthermore, the target based molecular drugs currently available for diabetes and obesity are associated with severe side effects such as insomnia, headache, constipation, hypoglycemia, weight gain, and renal complications [6], [7]. As an alternative, integrating complementary and alternative medicines (CAMs) with modern medicine would have promising applications in managing lifestyle disorders like diabetes and obesity. However, inadequate scientific evidence plus the use of indigenous languages and epistemologies in CAMs limit their global acceptance.
Ayurveda, an Indian traditional medicine system, prescribes several poly-herbal formulations for the treatment of diabetes and obesity. Studies have established the efficacy of some of these formulations, viz. Chandraprabha vati, Arogyavardhini vati, Naga bhasma, Shilajatu, and Nishamalaki using various in vivo models [8], [9], [10], [11], [12], [13]. Though the descriptions of diabetes in Ayurveda are not in terms of modern parameters such as blood glucose, serum insulin and insulin resistance, a striking relationship between obesity (Sthaulya) and diabetes has been emphasized [14]. Diabetes mellitus can be correlated to Madhumeha (sweet urine), one of the 20 types of Prameha (a set of clinical disorders manifested with excess and turbid urination) described in Ayurveda [15]. Sthaulya (obesity), caused from unwholesome diet and sedentary lifestyle, is considered as one of the predisposing factors of Madhumeha as well as Prameha. The etiology and pathophysiology of obese-diabetes (Sthula Prameha) is clearly demarcated from lean diabetes (Krusha Prameha) [16]. As a result, Ayurvedic therapeutics have come out with specific formulations and methodologies to treat obese-diabetic patients. Generating scientific evidence and understanding their modes of action in contemporary scientific language would essentially help in expanding their global acceptance as convincing complementary strategies for managing the dual burden of obesity and diabetes.
The present study attempts to understand the possible mode of action of Lodhrasavam, a fermented polyherbal formulation in Ayurveda, predominantly prescribed for obese-diabetic patients [17]. Lodhrasavam is a complex formulation prepared from 29 plant drugs, wherein Lodhra (Symplocos racemosa Roxb.) is considered as the major ingredient (Supplementary data – 1) [18]. Lodhra is referred as a medo-hara (anti-obesity) plant and is the prime member of Lodhradi-gana plants, a group of plants having medo-hara and kapha-hara properties [19]. Besides the anti-diabetic and anti-obesity applications, Lodhrasavam is also indicated for various other diseases such as anemia, skin diseases, anorexia, hemorrhoids [18]. Owing to its broad spectrum of therapeutic benefits and the inherent properties of the herbal ingredients present, Lodhrasavam could be deemed to be an Aushadha Rasayana (a curative and wellness product) in the management of diabetes. The present study, for the first time, demonstrates the anti-diabetic and anti-obesity potentials of Lodhrasavam using in vitro experimental models.
Being an accepted oral drug for obese diabetes, the possibility of Lodhrasavam inhibiting the digestive enzymes viz. alpha-amylase (α-A) and alpha-glucosidase (α-G), to exert their anti-diabetic effect, is focused in this study. Digestive enzyme inhibition is considered as one of the important therapeutic strategies for controlling postprandial hyperglycemia in diabetic patients, particularly in high carbohydrate consuming populations like Indians [20], [21]. Several digestive enzyme inhibitors are identified and reported from various medicinal plants [22], [23]. The anti-adipogenic potential of Lodhrasavam has been studied using 3T3-L1 pre-adipocytes, and a well standardized cell-line model for adipogenesis and anti-obesity research in vitro is established [24]. The use of a static in vitro digestion model, to simulate the gastro-intestinal digestion, adopted in the present work facilitated a realistic study of the formulation in total, than studying the individual herbal ingredients in part.
2. Materials and methods
2.1. Chemicals and reagents
Lodhrasavam, prepared in accordance with the Ayurveda text Ashtangahrudayam [18], was purchased from authentic commercial sources. 3T3-L1 fibroblasts were purchased from National Centre for Cell Sciences, Pune, India. All cell culture reagents used in the study were purchased from Gibco-BRL. The enzymes α-amylase (source – Aspergillus oryzae) and α-glucosidase (source – Saccharomyces cerevisiae) and other fine chemicals were purchased from Sigma–Aldrich. All other routine laboratory chemicals used in various assays were of analytical grade and purchased from SD-Fine chemicals.
2.2. Cell culture and differentiation
3T3-L1 fibroblasts were cultured in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin in an atmosphere of 5% CO2 at 37 °C. Differentiation of 3T3-L1 fibroblasts was induced by incubating two days post-confluent (day-0) plates in differentiation induction medium containing 500 μM 3-isobutyl-1-methylxanthine (IBMX), 250 nM dexamethasone and 100 nM insulin (MDI). On day-3, the medium was replaced with fresh culture medium and was replaced every alternate day till the cells attained complete adipocyte morphology.
2.3. In vitro digestion of Lodhrasavam
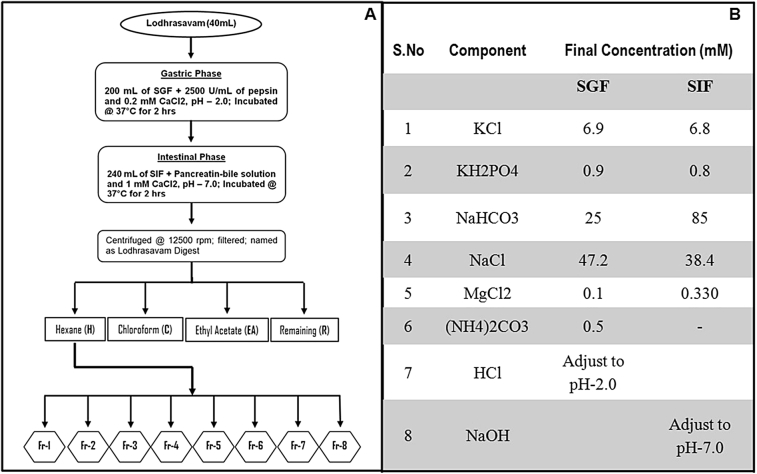
In vitro digestion of Lodhrasavam was carried out following published protocols [25] with modifications to suit the samples (Fig. 1A). The electrolyte solutions of simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) were prepared as shown in Fig. 1B. Oral phase of the digestion was omitted in the experiment as the starting material was in liquid form. For simulated gastric digestion, 40 mL of Lodhrasavam mixed with 250 mL of SGF containing 2500 U/mL pepsin and 0.16 mM CaCl2·2H2O (pH was adjusted to 2.0 by adding 6N HCl) was incubated at 37 °C for 2 h in a shaking water bath. Following gastric digestion, the gastric chyme mixed with 250 mL SIF containing pancreatin-bile solution (final concentration of 500 μg/mL pancreatin and 3 mg/mL bile) and 0.6 mM CaCl2·2H2O (pH adjusted to 7.0 by adding 5N NaOH) and incubated at 37 °C in shaking water bath for another 2 h to complete the digestion. The digest of Lodhrasavam (hereafter referred as LD) was filtered through cotton plug and the filtrate was collected and stored at −80 °C for further use.
Fig. 1.
(A) – Diagrammatic representation of methodology adopted for fractionation of Lodhrasavam digest, and (B) –Table showing the composition of SGF and SIF electrolytes.
2.4. Bioassay guided fractionation of Lodhrasavam digest
Fractionation of LD was done as illustrated in Fig. 1A. Initially, LD was successively extracted with hexane (H), chloroform (C) and ethyl acetate (EA) and the solvent was removed using a rotary evaporator at reduced temperature and pressure. The hexane extract of LD (LDH), obtained after sequential solvent extraction, was concentrated and fractionated by column chromatography. Briefly, the chromatographic column (18 × 300 mm size) packed with 25 gm silica in hexane was inoculated with 4 mL of LDH concentrate. Elution was started with 100% hexane and continued with 10% step-gradients of chloroform, ethyl acetate, methanol and water, till 100% water. The progress of separation was monitored by thin layer chromatography (TLC) and similar fractions were pooled together. Out of the 8 fractions obtained from the column, Fr-5 showed positive results in bioassays.
LD and all its subsequent fractions were estimated for total tannins following standard Folin - Ciocalteu method [26]. Briefly, 10 μL of digest/fraction mixed with 40 μL of water, 50 μL Folin's reagent and 100 μL of 3.5% Na2CO3 was incubated at room temperature for 30 min. A set of gallic acid standards (50, 25, 12.5, 6.25, 3.125 μg/mL) were prepared in the same manner. The absorbance was measured at 700 nm using plate reader (xMark Microplate Spectrophotometer, BioRad, USA). The experimental concentrations of test samples were expressed as ‘μg of gallic acid equivalent tannin (GAE)/mL of sample’.
2.5. α-Amylase and α-glucosidase inhibition assay
α-A and α-G assays were carried out following standard protocol [27] with modifications to suit to 96-well plate format. For α-A, all incubations were done at room temperature. Increasing concentrations of the samples were taken and the volume was made up to 50 μL using 0.02 M sodium phosphate buffer (pH 6.9). To this, 50 μL of α-A (0.5 mg/mL) was added and incubated for 30 min. Followed by this, 50 μL of 1% starch (soluble potato starch) was added as substrate and incubated for another 10 min. The reaction was stopped by adding 50 μL of 1% dinitrosalicylic acid (DNS) and the plate was heated at boiling water bath for 5 min till the development of an orange red color.
Similarly for α-G assay, different concentrations of the samples were taken and the volume was made up to 50 μL with 0.02 M sodium phosphate buffer (pH 6.9). To this, 50 μL of α-G (0.5 U/mL) was added and incubated for 10 min at room temperature, followed by the addition of 50 μL of 3.0 mM p-nitrophenyl glucopyranoside (pNPG) as substrate and incubation for 20 min at 37 °C. The reaction was stopped by adding 50 μL of 0.1 M Na2CO3.
For α-A and α-G the absorbance was read at 550 and 405 nm, respectively, using plate reader. For enzyme activity, 50 μL of buffer was used as control for both α-A and α-G and a set of test samples without enzyme was used to measure the basal level of reducing sugars present in the test samples. The absorbance was subtracted from the corresponding test readings. The percentage inhibition of enzyme activity for both α-A and α-G was calculated as follows.
2.6. Anti-adipogenesis assay and Oil-Red O staining
Anti-adipogenesis assay and Oil-Red O staining were done following published protocols with minor modifications [28]. Briefly, two days post confluent cells, grown in 48 well plates, were induced with MDI for three days in the presence of different concentrations of test samples (LD and its fractions). Following induction, the cells were replenished in normal culture medium, till the 6th day. Cells treated with MDI or vehicle alone were used as positive and negative control respectively. Oil Red O staining was done on the 6th day of differentiation. Cells were washed twice with phosphate buffer, and fixed with 4% formaldehyde for 1 h. After washing, cells were stained for 30 min in freshly diluted Oil Red O solution (0.5% Oil Red O in isopropanol diluted to 3:2 with H2O, and filtered) at 37 °C. Images of cells stained with Oil Red were captured using phase contrast microscope (Olympus-IX-71, Olympus America Inc, USA).
2.7. Statistical analysis
A one-way ANOVA test was performed to determine the significance of test samples compared to the controls and a value of p<0.05 was considered as significant.
3. Results and discussions
Ayurveda has very unique principles and procedures for designing and manufacturing therapeutic formulations [29]. Polyherbal formulations of Ayurveda are prepared through complex processes including boiling, extraction, and fermentation [14], [18]. Nevertheless, they are considered as rich repository of diverse bioactive molecules and are believed to exert their therapeutic effects through pharmacological-networking [30]. Studying individual component plants need not necessarily address all the possible post-digestive modifications and associated pharmacodynamic and pharmacokinetic differences that a formulation undergoes. Considering this, Lodhrasavam used in the present study was first subjected to a simulated in vitro gastro-intestinal digestion and the digest and its fractions were used for further studies (Fig. 1).
The component plants of Lodhrasavam for example, S. racemosa Roxb., Terminalia chebula Retz., Phylanthus emblica Roxb., Embelia ribes, Piper retrofractum Vahl., have been independently studied and reported using various in vitro and in vivo models for their potential anti-diabetic and hypolipidimic effects [31], [32], [33], [34]. But there are no scientific studies addressing the anti-diabetic and anti-adipogenic potentials of Lodhrasavam as a formulation. It is hypothesized that studying the formulation in its original form is more important because the various physico-chemical processes adopted for the preparation of Lodhrasavam would certainly alter its phytochemistry and bioactivity compared to the component plants. However, the present study is not comparing the biological effects of Lodhrasavam formulation with its component plants and it is a limitation.
3.1. LD and its fractions inhibit α-A and α-G activity
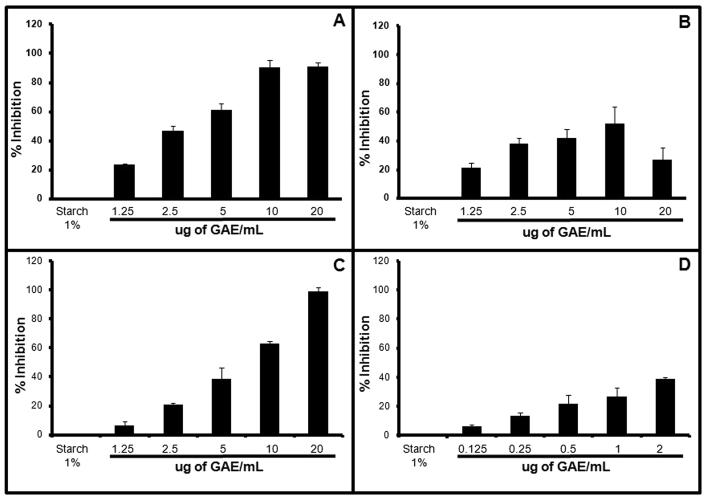
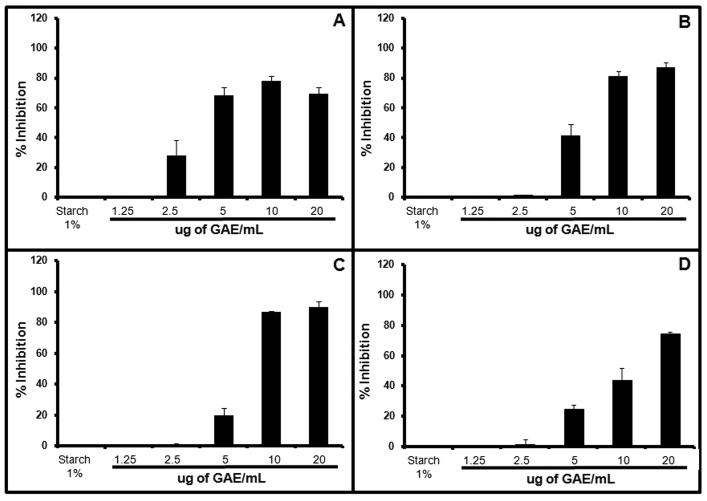
Digestive enzyme inhibition has shown to have promising therapeutic effects in the management of carbohydrate uptake related disorders like diabetes and obesity. LD, obtained from simulated in vitro digestion, was found to inhibit α-A and α-G activity in a concentration dependent manner. LD showed a maximum of 90 ± 3% inhibition of α-A activity at the highest concentration (20 μg of GAE/mL) of the digest tested in the assay (Fig. 2A). Similarly, it showed a concentration dependent inhibition of α-G with maximum of 78 ± 3% at 10 μg of GAE/mL concentration, beyond which the inhibition reduced (Fig. 3A). The observed inhibition of α-A and α-G by LD indicates its potential to control postprandial hyperglycemia through reducing carbohydrate digestion. Besides, it also supports the reported α-G inhibitory activity of some of the component plants in the formulation [35].
Fig. 2.
Graph showing the effect of Lodhrasavam digest and its fractions on α-A: Percentage inhibition of α-A by (A) LD, (B) LDH, (C) LDR and (D) Fr-5. Data are mean ± SEM of three independent experiments done in duplicates and the statistical significance was calculated with controls (p ≤ 0.05).
Fig. 3.
Graph showing the effect of Lodhrasavam digest and its fractions on α-G: Percentage inhibition of α-G by (A) LD, (B) LDC, (C) LDE and (D) LDR. Data are mean ± SEM of three independent experiments done in duplicates and the statistical significance was calculated with controls (p ≤ 0.05).
3.1.1. α-A and α-G inhibition by LD fractions
Polyherbal formulations are known to exert a multi-drug, multi-target mode of action which is different from the standard lock and key mechanism of action of molecular drugs [36]. As a preliminary step to understand the nature and distribution of bioactive molecules that inhibit digestive enzymes, LD was fractionated in a bioassay guided manner, as described in materials and methods (Fig. 1A), using α-A as the primary target. The hexane (LDH) and the remaining (LDR) fractions of LD, obtained from sequential solvent extraction, were found to retain α-A inhibitory activity wherein both LDH and LDR showed a concentration dependent α-A inhibition with a maximum of 51 ± 11% (10 μg of GAE/mL) and 98 ± 3% (20 μg of GAE/mL) respectively (Fig. 2B and C). The chloroform (LDC) and ethyl acetate (LDE) fractions obtained from LD inhibited α-G activity in a concentration dependent fashion with a maximum of 87 ± 3% and 90 ± 3% inhibition for LDC and LDE, respectively, at the highest concentration of 20 μg of GAE/mL used in the assay (Fig. 3B and C).
It is interesting to note that the LDH fraction did not inhibit α-G, as well as the LDC and LDE fractions did not inhibit α-A at any of the concentrations tested in the assay (unpublished observation). However, the LDR fraction was found to inhibit α-G, in addition to α-A, in a concentration dependent manner with a maximum of 74 ± 1% at 20 μg of GAE/mL (Fig. 3D). The observed results partially support the possibility of the presence of multiple bioactive molecules, chemically and functionally distinct, exerting a network action for the gross biological effect of Lodhrasavam as a digestive enzyme inhibitor. Solvent extraction or liquid–liquid partitioning is the method widely used for bioassay guided fractionation of compounds from a mixture based on their polarity and solubility. In the present study use of solvents like hexane, chloroform and ethyl acetate facilitated the fractionation of bioactive compounds from LD. Considering the principles of liquid–liquid partitioning as well as the differential responses of LD fractions in α-A and α-G inhibition, it is expected that the bioactive molecules in LD would have partitioned into different solvents based on their polarity and/or solubility. However, detailed chemical analysis needs to be done to precisely understand the nature, type and structure of bioactive compounds in different fractions.
3.2. Lodhrasavam inhibits fibroblast differentiation
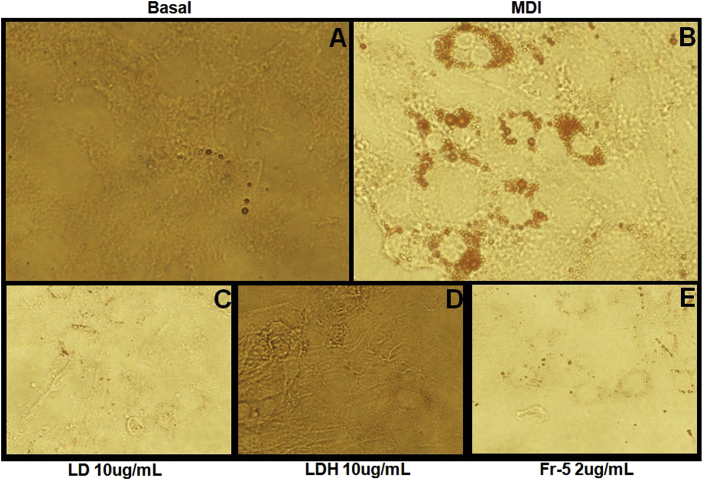
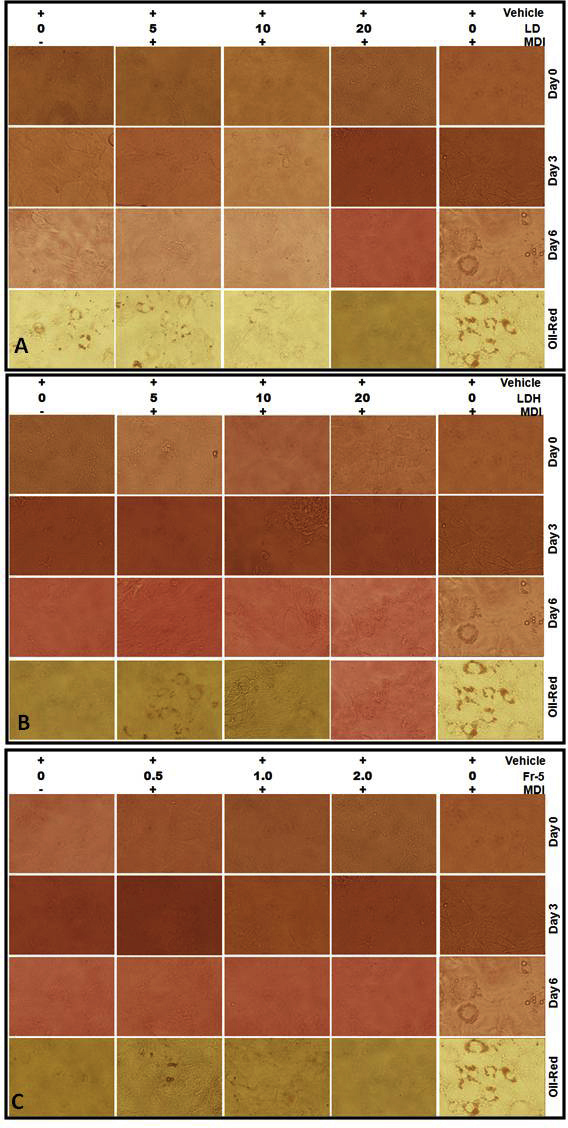
Being an Ayurvedic formulation of choice for treating obese-diabetes, the present study also investigated the modulatory effect of Lodhrasavam on adipogenesis using 3T3-L1 fibroblast model. Inhibition of MDI induced differentiation of fibroblasts to adipocytes is considered as one of the experimental strategies for evaluating the anti-obesity action of a formulation [37]. LD, when added along with MDI, was found to inhibit adipogenesis in a concentration dependent fashion. A complete inhibition of adipogenesis qualitatively observed as reduction of oil droplet formation was obtained at a concentration of 10 μg of GAE/mL (Fig. 4C). Fig. 4 is a representative image of Oil-Red O stained adipocytes upon treatment with test samples (details are given in Supplementary Data – 2).
Fig. 4.
Effect of Lodhrasavam digest and its fractions on adipogenesis: Oil Red O staining of 3T3-L1 fibroblasts differentiated with (A) Vehicle, (B) MDI, (C) MDI + LD, (D) MDI + LDH and (E) MDI + Fr-5 at different concentrations as indicated. Data presented are representative images of adipogenesis inhibition assay done in a concentration dependent manner. Images were captured using phase contrast microscope (Olympus-IX-71, Olympus America Inc, USA).
3.2.1. Modulation of adipogenesis by LD fractions
Similar to digestive enzyme inhibition the possibility of multiple bioactive compounds from Lodhrasavam modulating adipogenesis was taken into consideration and the fractions of LD were studied for adipogenesis inhibition. A concentration dependent inhibition of MDI mediated fibroblast differentiation was observed only with hexane (LDH) fraction (Fig. 4D). However at higher concentrations, above 10 μg of GAE/mL, all fractions including LDH were found to be cytotoxic.
The assay positive LDH fraction was further separated through column chromatography as described in materials and methods (Fig. 1A). Out of the eight fractions finally obtained, fraction 5 (Fr-5) was found to inhibit adipogenesis even at a very low concentration ranges from 0.5 to 2 μg of GAE/mL. Fr-5 showed a significant concentration dependent inhibition even at 1 μg of GAE/mL concentration (Fig. 4E). In addition, Fr-5 was also found to inhibit α-A in a concentration dependent manner. However, it showed a maximum of 38 ± 1% inhibition at the highest concentration (2 μg of GAE/mL) tested in the assay (Fig. 2D).
4. Conclusions
The study provides an experimental support for the use of Lodhrasavam for treating obese-diabetes. The observations from the current study indicate that Lodhrasavam mediated inhibition of digestive enzymes as well as adipocyte differentiation could potentially contribute to its anti-diabetic and anti-obesity properties. However, being a repository of multiple bioactive compounds, the possibility of Lodhrasavam modulating other anti-diabetic and anti-obesity targets cannot be ruled out. Additionally, our preliminary phytochemical studies indicate the possibility of a pharmacological networking of chemically and functionally diverse bioactive molecules contributing to the gross biological effects of Lodhrasavam. The study is significant in the context of diabetes and obesity as there are not many effective drugs available for managing the dual burden of obesity and diabetes. Nevertheless, the study needs to be expanded further for understanding the bioactive molecules and delineating the precise molecular mechanisms of action through which Lodhrasavam exerts its anti-diabetic and anti-obesity effects.
Conflict of interest
Authors declare no conflict of interest.
Sources of funding
The authors acknowledge the financial support of Department of Science and Technology-Science and Engineering Research Board, Govt. of India under the Fast-Track Young Scientist Project No: SB/YS/LS-264/2013. Authors also acknowledge the financial support for research assistant from ITDHST, Bangalore.
Acknowledgment
Authors acknowledge the support and guidance received from Prof. Padma Venkat, Advisor, School of Life Sciences, ITDHST for conducting the work.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jaim.2017.03.005.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Microscopic image showing a concentration dependent inhibition of adipogenesis by (A) LD, (B) LDH and (C) Fr-5.
References
- 1.http://www.who.int/features/factfiles/obesity/facts/en/index3.html. In.
- 2.Eckel R.H., Kahn S.E., Ferrannini E., Goldfine A.B., Nathan D.M., Schwartz M.W. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? J Clin Endocrinol Metabolism. 2011;96(6):1654–1663. doi: 10.1210/jc.2011-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Gaal L., Scheen A. Weight management in type 2 diabetes: current and emerging approaches to treatment. Diabetes Care. 2015;38(6):1161–1172. doi: 10.2337/dc14-1630. [DOI] [PubMed] [Google Scholar]
- 4.Kenkre J., Tan T., Bloom S. Treating the obese diabetic. Expert Rev Clin Pharmacol. 2013;6(2):171–183. doi: 10.1586/ecp.13.5. [DOI] [PubMed] [Google Scholar]
- 5.Scheen A.J., Van Gaal L.F. Combating the dual burden: therapeutic targeting of common pathways in obesity and type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):911–922. doi: 10.1016/S2213-8587(14)70004-X. [DOI] [PubMed] [Google Scholar]
- 6.Stein S.A., Lamos E.M., Davis S.N. A review of the efficacy and safety of oral antidiabetic drugs. Expert Opin Drug Saf. 2013;12(2):153–175. doi: 10.1517/14740338.2013.752813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang J.G., Park C.Y. Anti-obesity drugs: a review about their effects and safety. Diabetes Metabolism J. 2012;36(1):13–25. doi: 10.4093/dmj.2012.36.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wanjari M.M., Mishra S., Dey Y.N., Sharma D., Gaidhani S.N., Jadhav A.D. Antidiabetic activity of Chandraprabha vati - a classical Ayurvedic formulation. J Ayurveda Integr Med. 2016;7(3):144–150. doi: 10.1016/j.jaim.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar G., Srivastava A., Sharma S.K., Gupta Y.K. The hypolipidemic activity of Ayurvedic medicine, Arogyavardhini vati in Triton WR-1339-induced hyperlipidemic rats: a comparison with fenofibrate. J Ayurveda Integr Med. 2013;4(3):165–170. doi: 10.4103/0975-9476.118707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajput D., Patgiri B.J., Galib R., Prajapati P.K. Anti-diabetic formulations of Naga bhasma (lead calx): a brief review. Anc Sci Life. 2013;33(1):52–59. doi: 10.4103/0257-7941.134609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar S., Singh G., Pandey A.K., Singh R.H. A clinical study on the Naimittika Rasayana effect of Silajatu and Mamajjaka in type-2 diabetes mellitus. Ayu. 2014;35(4):404–410. doi: 10.4103/0974-8520.159000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma Rohit, Amin H., Ruknuddin G., Prajapati P.K. Efficacy of Ayurvedic remedies in type 2 diabetes: a review through works done at Gujarat Ayurved University, Jamnagar. J Med Nutr Nutraceuticals. 2015;4(2):63–69. [Google Scholar]
- 13.Yadav R.K., Mishra R., Chhipa R.P., Audichya K.C. Clinical trial of an indgenous compound drug nishaamalki in the management of madhumeha vis-a-vis diabetes mellitus. Anc Sci Life. 2001;21(1):18–24. [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma R.K., Dash V.B. vol. 3. Chowkhambha Sanskrit Series Office; Varanasi: 2007. (Agnivesa's Caraka-samhita: text with english translation & critical exposition based on Cakrapani Datta's ayurveda dipika). [Google Scholar]
- 15.Shastri A. 14 edn. Chaukhambha Publications; Varanasi, India: 2003. Sushruta Samhita, Ayurveda-Tattva-Samdipika commentary. [Google Scholar]
- 16.Sharma P.V. vol. 2. Chaukhambha visvabharati; Varanasi: 2000. (Susruta-Samhita, with English translation of text and Dalhana's commentary along with critical notes). [Google Scholar]
- 17.AFI . vol. I. Government of India, Ministry of Health and Family Welfare, Department of Indian Systems of Medicine & Homoeopathy; New Delhi: 2003. (The ayurvedic formulary of India, part I). [Google Scholar]
- 18.Murthy K.R.S. Chowkhambha Krishnadas Academy; Varanasi: 2014. Astanga hrudayam: text, english translation, notes, appendix & indices. [Google Scholar]
- 19.Kumari H., Pushpan R., Nishteswar K. Medohara and Lekhaniya dravyas (anti-obesity and hypolipidemic drugs) in Ayurvedic classics: a critical review. Ayu. 2013;34(1):11–16. doi: 10.4103/0974-8520.115437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Laar F.A., Lucassen P.L., Akkermans R.P., van de Lisdonk E.H., Rutten G.E., van Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28(1):154–163. doi: 10.2337/diacare.28.1.154. [DOI] [PubMed] [Google Scholar]
- 21.Tucci S.A., Boyland E.J., Halford J.C. The role of lipid and carbohydrate digestive enzyme inhibitors in the management of obesity: a review of current and emerging therapeutic agents. Diabetes Metab Syndr Obes. 2010;3:125–143. doi: 10.2147/dmsott.s7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H., Liu T., Huang D. Starch hydrolase inhibitors from edible plants. Adv Food Nutr Res. 2013;70:103–136. doi: 10.1016/B978-0-12-416555-7.00003-5. [DOI] [PubMed] [Google Scholar]
- 23.Sales P.M., Souza P.M., Simeoni L.A., Silveira D. alpha-Amylase inhibitors: a review of raw material and isolated compounds from plant source. J Pharm Pharm Sci. 2012;15(1):141–183. doi: 10.18433/j35s3k. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz-Ojeda F.J., Ruperez A.I., Gomez-Llorente C., Gil A., Aguilera C.M. Cell models and their application for studying adipogenic differentiation in relation to obesity: a review. Int J Mol Sci. 2016;17(7) doi: 10.3390/ijms17071040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minekus M., Alminger M., Alvito P., Ballance S., Bohn T., Bourlieu C. A standardised static in vitro digestion method suitable for food - an international consensus. Food Funct. 2014;5(6):1113–1124. doi: 10.1039/c3fo60702j. [DOI] [PubMed] [Google Scholar]
- 26.Ainsworth E.A., Gillespie K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc. 2007;2(4):875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- 27.Kazeem M.I., Adamson J.O., Ogunwande I.A. Modes of inhibition of alpha-amylase and alpha -glucosidase by aqueous extract of Morinda lucida Benth leaf. BioMed Res Int. 2013;2013:527570. doi: 10.1155/2013/527570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haridas Nidhina P.A., Poulose N., Gopalakrishnapillai A. Vanillin induces adipocyte differentiation in 3T3-L1 cells by activating extracellular signal regulated kinase 42/44. Life Sci. 2011;88(15–16):675–680. doi: 10.1016/j.lfs.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Parasuraman S., Thing G.S., Dhanaraj S.A. Polyherbal formulation: concept of ayurveda. Pharmacogn Rev. 2014;8(16):73–80. doi: 10.4103/0973-7847.134229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandran U., Mehendale N., Tillu G., Patwardhan B. Network pharmacology of ayurveda formulation Triphala with special reference to anti-cancer property. Comb Chem High Throughput Screen. 2015;18(9):846–854. doi: 10.2174/1386207318666151019093606. [DOI] [PubMed] [Google Scholar]
- 31.Venkidesh R., Pal D., Kumar C.K.A., Saravanakumar A., Mandal S.C. Studies on anti diabetic potential of symplocos racemosa roxb. Bark extract in streptozotocin induced diabetic rats. Int J Phytopharm Res. 2012;3(1):1–5. [Google Scholar]
- 32.Singh V.K., Reddy K. Management of Diabetes mellitus and its complications by Lodhra: a review. Int J Ayurvedic Med. 2015;6(4):305–309. [Google Scholar]
- 33.Durkar A.M., Patil R.R., Naik S.R. Hypolipidemic and antioxidant activity of ethanolic extract of Symplocos racemosa Roxb. in hyperlipidemic rats: an evidence of participation of oxidative stress in hyperlipidemia. Indian J Exp Biol. 2014;52(1):36–45. [PubMed] [Google Scholar]
- 34.Acharya N., Acharya S., Shah U., Shah R., Hingorani L. A comprehensive analysis on Symplocos racemosa Roxb.: traditional uses, botany, phytochemistry and pharmacological activities. J Ethnopharmacol. 2016;181:236–251. doi: 10.1016/j.jep.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 35.Bachhawat A., Shihabudeen M.S., Thirumurugan K. Screening of fifteen indian ayurvedic plants for alpha-glucosidase inhibitory activity and enzyme kinetics. Int J Pharm Pharm Sci. 2011;3:267–274. [Google Scholar]
- 36.Hopkins A.L. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4(11):682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 37.Roh C., Jung U. Screening of crude plant extracts with anti-obesity activity. Int J Mol Sci. 2012;13(2):1710–1719. doi: 10.3390/ijms13021710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.