Abstract
A concatenated dataset of LSU, SSU, ITS and tef1 DNA sequence data was analysed to investigate the taxonomic position and phylogenetic relationships of the genus Camarosporium in Pleosporineae (Dothideomycetes). Newly generated sequences from camarosporium-like taxa collected from Europe (Italy) and Russia form a well-supported monophyletic clade within Pleosporineae. A new genus Camarosporidiella and a new family Camarosporidiellaceae are established to accommodate these taxa. Four new species, Neocamarosporium korfii, N. lamiacearum, N. salicorniicola and N. salsolae, constitute a strongly supported clade with several known taxa for which the new family, Neocamarosporiaceae, is introduced. The genus Staurosphaeria based on S. lycii is resurrected and epitypified, and shown to accommodate the recently introduced genus Hazslinszkyomyces in Coniothyriaceae with significant statistical support. Camarosporium quaternatum, the type species of Camarosporium and Camarosporomyces flavigena cluster together in a monophyletic clade with significant statistical support and sister to the Leptosphaeriaceae. To better resolve interfamilial/intergeneric level relationships and improve taxonomic understanding within Pleosporineae, we validate Camarosporiaceae to accommodate Camarosporium and Camarosporomyces. The latter taxa along with other species are described in this study.
Key words: Multigene phylogeny, Muriformly septate, Pleomorphism, Pleosporales, Taxonomy
Taxonomic novelties: New families: Camarosporiaceae Wanas., K.D. Hyde & Crous; Camarosporidiellaceae Wanas., Wijayaw., Crous & K.D. Hyde; Neocamarosporiaceae Wanas., Wijayaw., Crous & K.D. Hyde
New genus: Camarosporidiella Wanas., Wijayaw. & K.D. Hyde
New species: Camarosporidiella elaeagnicola Wanas., Bulgakov & K.D. Hyde; Ca. eufemiana Wanas., Camporesi & K.D. Hyde; Ca. halimodendri Wanas., Bulgakov & K.D. Hyde; Ca. italica Wanas., Camporesi & K.D. Hyde; Ca. mackenziei Wanas., Bulgakov & K.D. Hyde; Ca. melnikii Wanas., Bulgakov & K.D. Hyde; Ca. mirabellensis Wanas., Camporesi & K.D. Hyde; Ca. premilcurensis Wanas., Camporesi & K.D. Hyde; Ca. schulzeri Wanas., Bulgakov & K.D. Hyde; Staurosphaeria rhamnicola Wanas., Yu. Sh. Gafforov & K.D. Hyde; Neocamarosporium korfii Wanas., E.B.G. Jones & K.D. Hyde; N. lamiacearum Dayar., E.B.G. Jones & K.D. Hyde; N. salicorniicola Dayarathne, E.B.G. Jones & K.D. Hyde; N. salsolae Wanas., Gafforov & K.D. Hyde
New combinations: Camarosporidiella caraganicola (Phukhams. et al.) Phukhams., Wanas. & K.D. Hyde; Ca. aborescentis (Phukhams. et al.) Phukhams., Wanas. & K.D. Hyde; Ca. arezzoensis (Tibpromma et al.) Wanas. & K.D. Hyde; Ca. celtidis (Shear) Thambugala, Wanas. & K.D. Hyde; Ca. clematidis (Wijayaw. et al.) Wijayaw., Wanas. & K.D. Hyde; Ca. elongata (Fr.) Wanas., Wijayaw. & K.D. Hyde; Ca. laburni (Pers.) Wanas., Bulgakov, Camporesi & K.D. Hyde; Ca. laburnicola (R.H. Perera et al.) Wanas. & K.D. Hyde; Ca. moricola (Chethana et al.) Wanas. & K.D. Hyde; Ca. robiniicola (Wijayaw. et al.) Wijayaw., Wanas. & K.D. Hyde; Ca. spartii (Trail) Wijayaw., Wanas. & K.D. Hyde; Neocamarosporium chenopodii (Ellis & Kellerm.) Wanas. & K.D. Hyde; N. obiones (Jaap) Wanas. & K.D. Hyde; Staurosphaeria aloes (Crous & M.J. Wingf.) Crous; Wanas. & K.D. Hyde
New name: Staurosphaeria lyciicola (Crous & R.K. Schumach.) Crous, Wanas. & K.D. Hyde
Epitypification (basionym): Staurosphaeria lycii Rabenh
Generic abbreviations: Camarosporidiella: Ca., Camarosporium: Cm., Camarosporomyces: Cs., Cucurbitaria: Cu
Introduction
Morphological characteristics, cultural studies and host-fungal association have been considered as important aspects in the traditional taxonomy of coelomycetous fungi (Sutton, 1980, Sivanesan, 1984, Nag Raj, 1993, Jeewon et al., 2002, Jeewon et al., 2003b, Jeewon et al., 2004, Wijayawardene et al., 2016). However, morphological plasticity of several coelomycetous genera led to poor generic and species delimitation, often resulting in incorrect taxonomic placement (Jeewon et al., 2003a, Shenoy et al., 2007, Wijayawardene et al., 2016). Proposing new genera (e.g. Vermisporium/Seimatosporium, fide Barber et al. 2011) and linking asexual genera with more than one sexual genus (e.g. Phoma and Camarosporium, fide Crous & Groenewald 2017) have resulted in taxonomic controversies among taxonomists and plant-pathologists (Wijayawardene et al., 2012a, Wijayawardene et al., 2012b, Hyde et al., 2013, Crous et al., 2015a). DNA-based sequence analyses have so far provided reliable evidence for more precise generic boundaries (e.g. Pestalotiopsis fide Jeewon et al., 2003b, Jeewon et al., 2004, Maharachchikumbura et al., 2012, Maharachchikumbura et al., 2014a, Maharachchikumbura et al., 2014b, Phoma fide De Gruyter et al., 2009, De Gruyter et al., 2012, Chen et al. 2015, Camarosporium fide Crous et al., 2013, Wijayawardene et al., 2014b, Wijayawardene et al., 2015, Wijayawardene et al., 2016, Coniothyrium fide Verkley et al., 2004, Verkley et al., 2014, Wijayawardene et al., 2016) and resolution of species complexes (e.g. Diplodia fide Phillips et al. 2008, Colletotrichum fide Damm et al., 2012, Damm et al., 2014).
The genus Camarosporium was introduced by Schulzer (1870) with Cm. quaternatum as the type species, and it is one of the largest coelomycetous genera, comprising over 500 epithets in Index Fungorum (2017). Several Camarosporium species have been reported as important plant pathogens with a worldwide distribution. Camarosporium pistaciae is known as a common pathogen responsible for blight of the shoots and panicles in pistachio production in Greece (Assimakopoulou & Elena 2010). Smith et al. (1988) listed Camarosporium dalmaticum, Cm. flaccidum, Cm. pistaciae, and Cm. strobilinum as plant pathogens in Europe. Camarosporium species are reported as causing damage in the cut-flower industry in the USA. (Taylor et al. 2001). Camarosporium species are also reported as common pathogens of deciduous trees in Europe and Cm. pini induces severe infection that can result in significant growth reduction to pine plantations (Ivanová & Bernadovičová 2010).
Sutton (1980) pointed out the heterogeneity of the genus, citing Camarosporium propinquum as an example. Sutton's (1980) prediction was confirmed by Wijayawardene et al. (2014c), who reported that Cm. propinquum should be accommodated in Didymosphaeriaceae. Camarosporium has been linked to Cucurbitaria (Kirk et al., 2008, Wijayawardene et al., 2012b, Doilom et al., 2013), Leptosphaeriaceae (Schoch et al. 2009) and Botryosphaeriales (Kirk et al., 2008, Liu et al., 2012, Wijayawardene et al., 2012b), although Crous et al. (2006) reported that Cm. quaternatum (based on CBS 134.97 culture, now described as Libertasomyces quercus; Crous & Groenewald 2017) does not belong to the Botryosphaeriales. Further evidence was provided that camarosporium-like taxa are polyphyletic within Pleosporales (Crous et al., 2014a, Crous et al., 2014b, Wanasinghe et al., 2014a, Wijayawardene et al., 2014a, Wijayawardene et al., 2014c, Wijayawardene et al., 2016, Crous and Groenewald, 2017), leading to more taxonomic confusion of Camarosporium and camarosporium-like taxa. In a recent study, Crous & Groenewald (2017) designated an epitype for Cm. quaternatum and treated Camarosporium s. str. in Coniothyriaceae, and reported this complex to have phoma-like synasexual morphs, and pleospora-like sexual morphs. To date there is DNA sequence data for only a small number of species, and the validity of taxonomic concepts and other species remains uncertain. Therefore, it has been necessary to recollect these taxa from type localities, isolate them in axenic culture, and analyse their DNA sequence data to better understand their morpho- and phylotaxonomy. Given the considerable taxonomic confusion among Camarosporium and its allies and its familial placement, this study was undertaken to answer the following questions: (i) Do camarosporium-like taxa represent a natural group?; (ii) What are the allied sexual and synasexual morphs of camarosporium-like taxa?; (iii) Where does Camarosporium quaternatum position itself within the Pleosporineae?
Materials and methods
Specimens and isolates
Fresh camarosporium-like specimens were collected in Europe (Russia and Italy) and Asia (Thailand and Uzbekistan) from various host plants. Uzbekistan specimens were loaned from Tashkent Mycological Herbarium (TASM), Tashkent. The specimens were examined following the methods described in Wanasinghe et al. (2014a). Axenic strains were established from single spores as described in Chomnunti et al. (2014), with a modification of the incubation temperature at 16 °C overnight in the dark. Germinated ascospores and conidia were observed with a Motic SMZ 168 Stereo Zoom microscope and transferred to potato dextrose agar (PDA; 39 g/L distilled water, Difco potato dextrose) for extraction of DNA, determination of growth rates and observation of cultural characteristics. The specimens are deposited at Mae Fah Luang University (MFLU) Herbarium, Chiang Rai, Thailand and the New Zealand Fungal Herbarium (PDD). Living cultures are deposited at the Culture Collection of Mae Fah Luang University (MFLUCC) and the Westerdijk Fungal Biodiversity Institute in Utrecht, the Netherlands (CBS)
Morphological classification
Digital images of the fruiting structures were captured with a Canon 450D digital camera fitted to a Nikon ECLIPSE 80i compound microscope. Squash mount preparations were prepared to determine micro-morphology, and free hand sections of sporocarps made to observe the shapes of ascomata/conidiomata and peridium structures. Measurements of morphological structures were taken from the widest part of each structure. Whenever possible, more than 30 measurements were made. The lengths and widths were measured using the Tarosoft (R) Image Frame Work program and images used for figures processed with Adobe Photoshop CS3 Extended v. 10.0 (Adobe®, San Jose, CA). Three sets of duplicate cultures of each isolate were measured to determine colony characteristics on PDA at 16 °C in the dark. Colony size was determined, and colour rated according to the colour charts of Rayner (1970) after 3 wk of incubation.
DNA extraction, PCR and sequencing
Isolates were grown on PDA for 3–4 wk at 16 °C and total genomic DNA was extracted from 50 to 100 mg of mycelium scraped from the edges of the growing cultures (Wu et al. 2001). Mycelium was ground to a fine powder in liquid nitrogen and DNA was extracted using the Biospin Fungus Genomic DNA Extraction Kit, BSC14S1 (BioFlux, P.R. China) following the instructions of the manufacturer. When fungi failed to germinate and grow in culture, DNA was extracted directly from ascomata using a DNA extraction kit (E.Z.N.A.® Forensic DNA kit, D3591-01, Omega Bio-Tek) following Telle & Thines (2008). DNA were stored at 4 °C for use in regular work and duplicated at −20 °C for long term storage. DNA sequence data was obtained from the partial sequences of four loci: the internal transcribed spacers (ITS1-5.8S nrDNA-ITS2, ITS), small subunit nrDNA (18S, SSU), large subunit nrDNA (28S, LSU) and translation elongation factor 1-alpha gene (tef1). Nuclear ITS regions were amplified using the primers ITS5 and ITS4 (White et al. 1990). The LSU was amplified using the primers LROR (Rehner & Samuels 1994) and LR5 (Vilgalys & Hester 1990), the SSU using the primers NS1 and NS4 (White et al. 1990), and tef1 using primers EF1-983F and EF1-2218R (Rehner & Buckley 2005). The polymerase chain reaction (PCR) was carried out with a final volume of 25 μL under the following protocol: 12.5 μL of 2 × Power Taq PCR MasterMix (a premix and ready to use solution, including 0.1 Units/μL Taq DNA Polymerase, 500 μM dNTP Mixture each (dATP, dCTP, dGTP, dTTP), 20 mM Tris-HCL pH 8.3, 100 mM KCl, 3 mM MgCl2, stabilizer and enhancer), 1 μL of each primer (10 μM), 1 μL genomic DNA extract and 9.5 μL deionised water. The reaction was then allowed to run for 35 cycles. The PCR profile was as follows: initial denaturation 95 °C for 5 min, 35 cycles of denaturation at 95 °C for 90 s, annealing for 90 s, elongation at 72 °C for 1 min, and final extension at 72 °C for 10 min. The annealing temperature was 55 °C for ITS, LSU, tef1 and 48 ºC for SSU. The amplified PCR fragments were sequenced by BGI, Ltd., Shenzhen, P.R. China. Sequences were deposited in GenBank (Table 1, Table 2).
Table 1.
Cultures and related GenBank accession numbers of Pleosporineae used in the phylogenetic analyses.
BCC: Belgian Coordinated Collections of Microorganisms; CBS: Culture Collection of the Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; CPC: Personal collection of P.W. Crous, Utrecht, the Netherlands; DAOM: Canadian Collection of Fungal Cultures, Ottawa, Canada; E.G.S.: Personal collection of Dr. E.G. Simmons; IBRC: Iranian Biological Resources Center, Academic Center for Education Culture and Research (ACECR), Tehran, Iran; JK: J. Kohlmeyer; MFLUCC/MFLU: Mae Fah Luang University Culture Collection, Chiang Rai, Thailand; NBRC: NITE Biological Resource Center, Department of Biotechnology, National Institute of Technology and Evaluation, Kisarazu, Chiba, Japan. *Strain designation from GenBank.
ITS: Internal transcribed spacers; LSU: partial 28S nrDNA; SSU: partial 18S nrDNA; tef1: translation elongation factor 1-alpha gene.
Table 2.
Cultures and related GenBank accession numbers of Pleosporineae obtained in this study.
| Taxon | Original no. | Culture no.1 | Specimen no.2 | Host or substrate | Country | GenBank accession no.3 |
|||
|---|---|---|---|---|---|---|---|---|---|
| ITS | LSU | SSU | tef1 | ||||||
| Camarosporidiella aborescentis | T-0477 | – | MFLU 15-2181 | Colutea orientalis | Russia | MF434115 | MF434202 | MF434290 | MF434378 |
| IT2674 | MFLUCC 17-0660 | MFLU 15-3630 | Colutea arborescens | Italy | MF434116 | MF434203 | MF434291 | MF434379 | |
| NK076 | MFLUCC 17-0738 | MFLU 16-2387 | Amorpha sp. | Russia | MF434117 | MF434204 | MF434292 | MF434380 | |
| Ca. arezzoensis | T-0009 | MFLUCC 14-0891 | MFLU 17-0455 | Amorpha fruticosa | Russia | MF434118 | MF434205 | MF434293 | MF434381 |
| T-0016 | MFLUCC 14-0899 = CBS 143102 | MFLU 17-0462 | Cytisus austriacus | Russia | MF434119 | MF434206 | MF434294 | MF434382 | |
| T-0064 | MFLUCC 14-0913 = CBS 143103 | MFLU 17-0475 | Cytisus borysthenicus | Russia | MF434120 | MF434207 | MF434295 | MF434383 | |
| T-0072 | MFLUCC 14-0916 = CBS 143104 | MFLU 17-0478 | Cytisus austriacus | Russia | MF434121 | MF434208 | MF434296 | MF434384 | |
| Ca. caraganicola | T-0005 | MFLUCC 14-0887 = CBS 143105 | MFLU 17-0453 | Caragana frutex | Russia | MF434122 | MF434209 | MF434297 | MF434385 |
| T-0013 | MFLUCC 14-0896 = CBS 143106 | MFLU 17-0459 | Caragana frutex | Russia | MF434123 | MF434210 | MF434298 | MF434386 | |
| T-0538 | MFLUCC 17-0697 = CBS 143107 | MFLU 15-2242 | Caragana frutex | Russia | MF434124 | MF434211 | MF434299 | MF434387 | |
| T-1488 | MFLUCC 17-0726 = CBS 143108 | MFLU 16-1782 | Caragana frutex | Russia | MF434125 | MF434212 | MF434300 | MF434388 | |
| Ca. celtidis | T-0193 | – | MFLU 15-1897 | Spiraea sp. | Russia | MF434126 | MF434214 | MF434302 | MF434389 |
| T-0332 | – | MFLU 15-2036 | Elymus repens | Russia | MF434127 | MF434215 | MF434303 | MF434390 | |
| T-0002 | MFLUCC 14-0884 = CBS 143109 | MFLU 17-0450 | Maclura pomifera | Russia | MF434128 | MF434216 | MF434304 | MF434391 | |
| T-0040 | MFLUCC 14-0904 = CBS 143110 | MFLU 17-0466 | Gleditsia triacanthos | Russia | MF434129 | MF434217 | MF434305 | MF434392 | |
| T-0358 | MFLUCC 17-556 | MFLU 15-2062 | Betula pendula | Russia | MF434130 | MF434218 | MF434306 | MF434393 | |
| T-0224 | MFLUCC 17-0676 = CBS 143111 | MFLU 15-1928 | Prunus padus | Russia | MF434131 | MF434219 | MF434307 | MF434394 | |
| T-0239 | MFLUCC 17-0679 | MFLU 15-1943 | Morus alba | Russia | MF434132 | MF434220 | MF434308 | MF434395 | |
| T-0767 | MFLUCC 17-0701 = CBS 143112 | MFLU 15-2912 | Ailanthus altissima | Russia | MF434133 | MF434221 | MF434309 | MF434396 | |
| NK041 | MFLUCC 17-0735 | MFLU 16-2358 | Robinia sp. | Russia | MF434134 | MF434222 | MF434310 | MF434397 | |
| Ca. elaeagnicola | T-0220 | – | MFLU 15-1924 | Artemisia santonicum | Russia | MF434135 | MF434223 | MF434311 | MF434398 |
| T-0511 | – | MFLU 15-2215 | Elaeagnus angustifolia | Russia | MF434136 | MF434224 | MF434312 | MF434399 | |
| T-0051 | MFLUCC 14-0908 = CBS 143113 | MFLU 17-0470 | Elaeagnus angustifolia | Russia | MF434137 | MF434225 | MF434313 | MF434400 | |
| T-0055 | MFLUCC 14-0911 = CBS 143114 | MFLU 17-0473 | Elaeagnus angustifolia | Russia | MF434138 | MF434226 | MF434314 | MF434401 | |
| T-0061 | MFLUCC 14-0912 = CBS 143115 | MFLU 17-0474 | Elaeagnus angustifolia | Russia | MF434139 | MF434227 | MF434315 | MF434402 | |
| T-0813 | MFLUCC 17-0705 | MFLU 15-2956 | Elaeagnus angustifolia | Russia | MF434140 | MF434228 | MF434316 | MF434403 | |
| T-0815 | MFLUCC 17-0706 | MFLU 15-2958 | Elaeagnus angustifolia | Russia | MF434141 | MF434229 | MF434317 | MF434404 | |
| T-0819 | MFLUCC 17-0707 | MFLU 15-2962 | Elaeagnus angustifolia | Russia | MF434142 | MF434230 | MF434318 | MF434405 | |
| T-1186 | MFLUCC 17-0712 | MFLU 16-1481 | Elaeagnus angustifolia | Russia | MF434143 | MF434231 | MF434319 | MF434406 | |
| NK067 | MFLUCC 17-0737 | MFLU 16-2382 | Elaeagnus angustifolia | Russia | MF434144 | MF434232 | MF434320 | MF434407 | |
| Ca. eufemiana | IT1621 | MFLUCC 17-0207 = CBS 143116 | MFLU 16-0182 | Cytisus sp. | Italy | MF434145 | MF434233 | MF434321 | MF434408 |
| Ca. halimodendri | T-0018 | MFLUCC 14-0901 = CBS 143117 | MFLU 17-0463 | Halimodendron halodendron | Russia | MF434146 | MF434234 | MF434322 | MF434409 |
| T-0041 | MFLUCC 14-0905 | MFLU 17-0467 | Halimodendron halodendron | Russia | MF434147 | MF434235 | MF434323 | MF434410 | |
| T-0050 | MFLUCC 14-0907 = CBS 143118 | MFLU 17-0469 | Caragana frutex | Russia | MF434148 | MF434236 | MF434324 | MF434411 | |
| T-0066 | MFLUCC 14-0914 | MFLU 17-0476 | Cytisus podolicus | Russia | MF434149 | MF434237 | MF434325 | MF434412 | |
| T-0419 | MFLUCC 17-0212 = CBS 143119 | MFLU 15-2123 | Lycium barbarum | Russia | MF434150 | MF434238 | MF434326 | MF434413 | |
| T-0468 | MFLUCC 17-0691 | MFLU 15-2172 | Halimodendron halodendron | Russia | MF434151 | MF434239 | MF434327 | MF434414 | |
| Ca. italica | IT1283 | MFLUCC 13-0547 | MFLU 17-0139 | Coronilla emerus | Italy | MF434152 | MF434240 | MF434328 | MF434415 |
| Ca. laburni | T-0003 | MFLUCC 14-0885 | MFLU 17-0451 | Laburnum anagyroides | Russia | MF434153 | MF434241 | MF434329 | MF434416 |
| IT83 | MFLUCC 14-0919 = CBS 143121 | MFLU 16-0094 | Laburnum anagyroides | Italy | MF434154 | MF434242 | MF434330 | MF434417 | |
| T-0811 | MFLUCC 17-0704 = CBS 143122 | MFLU 15-2954 | Laburnum anagyroides | Russia | MF434155 | MF434243 | MF434331 | MF434418 | |
| T-0838 | MFLUCC 17-0709 | MFLU 15-2981 | Laburnum sp. | Russia | MF434156 | MF434244 | MF434332 | MF434419 | |
| CR029 | MFLUCC 17-0751 = CBS 143120 | MFLU 17-1434 | Laburnum anagyroides | Russia | MF434157 | MF434245 | MF434333 | MF434420 | |
| CR032 | MFLUCC 17-0752 | MFLU 17-1435 | Laburnum anagyroides | Russia | MF434158 | MF434246 | MF434334 | MF434421 | |
| Ca. mackenziei | T-0001 | MFLUCC 14-0883 = CBS 143123 | MFLU 17-0449 | Caragana arborescens | Russia | MF434159 | MF434247 | MF434335 | MF434422 |
| T-0011 | MFLUCC 14-0893 = CBS 143124 | MFLU 17-0457 | Caragana arborescens | Russia | MF434160 | MF434248 | MF434336 | MF434423 | |
| T-0810 | MFLUCC 17-0703 | MFLU 15-2953 | Caragana sp. | Russia | MF434161 | MF434249 | MF434337 | MF434424 | |
| Ca. melnikii | T-0318 | MFLUCC 17-0684 | MFLU 15-2022 | Caragana frutex | Russia | MF434162 | MF434250 | MF434338 | MF434425 |
| Ca. mirabellensis | IT2139 | – | MFLU 17-228 | Robinia pseudoacacia | Russia | MF434163 | MF434251 | MF434339 | MF434426 |
| Ca. moricola | T-0232 | – | MFLU 15-1936 | Morus alba | Russia | MF434164 | MF434252 | MF434340 | MF434427 |
| T-0519 | – | MFLU 15-2223 | Morus alba | Russia | MF434165 | MF434253 | MF434341 | MF434428 | |
| T-0004 | MFLUCC 14-0886 | MFLU 17-0452 | Morus alba | Russia | MF434166 | MF434254 | MF434342 | MF434429 | |
| T-0015 | MFLUCC 14-0898 | MFLU 17-0461 | Morus alba | Russia | MF434167 | MF434255 | MF434343 | MF434430 | |
| T-0265 | MFLUCC 17-0680 | MFLU 15-1969 | Morus alba | Russia | MF434168 | MF434256 | MF434344 | MF434431 | |
| T-0371 | MFLUCC 17-0687 | MFLU 15-2075 | Morus alba | Russia | MF434169 | MF434257 | MF434345 | MF434432 | |
| T-0518 | MFLUCC 17-0694 | MFLU 15-2222 | Morus alba | Russia | MF434170 | MF434258 | MF434346 | MF434433 | |
| T-0856 | MFLUCC 17-0711 | MFLU 15-2999 | Morus alba | Russia | MF434171 | MF434259 | MF434347 | MF434434 | |
| T-01233 | MFLUCC 17-0714 = CBS 143125 | MFLU 16-1527 | Morus alba | Russia | MF434172 | MF434260 | MF434348 | MF434435 | |
| T-01332 | MFLUCC 17-0718 = CBS 143126 | MFLU 16-1626 | Morus alba | Russia | MF434173 | MF434261 | MF434349 | MF434436 | |
| T-01345 | MFLUCC 17-0719 | MFLU 16-1639 | Morus alba | Russia | MF434174 | MF434262 | MF434350 | MF434437 | |
| T-01476 | MFLUCC 17-0725 | MFLU 16-1770 | Morus alba | Russia | MF434175 | MF434263 | MF434351 | MF434438 | |
| Ca. premilcurensis | IT1681 | MFLUCC 17-0208 = CBS 143127 | MFLU 16-0185 | Cytisus sp. | Italy | MF434176 | MF434264 | MF434352 | MF434439 |
| Ca. robiniicola | T-0010 | MFLUCC 14-0892 = CBS 143128 | MFLU 17-0456 | Gleditsia triacanthos | Russia | MF434177 | MF434265 | MF434353 | MF434440 |
| T-0012 | MFLUCC 14-0894 = CBS 143129 | MFLU 17-0458 | Robinia neomexicana | Russia | MF434178 | MF434266 | MF434354 | MF434441 | |
| T-0042 | MFLUCC 14-0906 = CBS 143130 | MFLU 17-0468 | Gleditsia triacanthos | Russia | MF434179 | MF434267 | MF434355 | MF434442 | |
| T-0053 | MFLUCC 14-0909 = CBS 143131 | MFLU 17-0471 | Robinia pseudoacacia | Russia | MF434180 | MF434268 | MF434356 | MF434443 | |
| T-0403 | MFLUCC 17-0688 | MFLU 15-2104 | Robinia pseudoacacia | Russia | MF434181 | MF434269 | MF434357 | MF434444 | |
| T-1303 | MFLUCC 17-0716 = CBS 143132 | MFLU 16-1597 | Robinia sp. | Russia | MF434182 | MF434270 | MF434358 | MF434445 | |
| DL0004 | MFLUCC 17-0733 | MFLU 16-2300 | Robinia sp. | Russia | MF434183 | MF434271 | MF434359 | MF434446 | |
| Ca. schulzeri | T-0205 | – | MFLU 15-1909 | Gleditsia triacanthos | Russia | MF434184 | MF434272 | MF434360 | MF434447 |
| T-0014 | MFLUCC 14-0897 = CBS 143133 | MFLU 17-0460 | Elaeagnus angustifolia | Russia | MF434185 | MF434273 | MF434361 | MF434448 | |
| T-1305 | MFLUCC 17-0717 | MFLU 16-1599 | Robinia sp. | Russia | MF434186 | MF434274 | MF434362 | MF434449 | |
| T-1370 | MFLUCC 17-0722 | MFLU 16-1664 | Robinia sp. | Russia | MF434187 | MF434275 | MF434363 | MF434450 | |
| Ca. spartii | T-0070 | MFLUCC 14-0915 | MFLU 17-0477 | Cytisus ruthenicus | Russia | MF434188 | MF434276 | MF434364 | MF434451 |
| T-1189 | MFLUCC 17-0713 = CBS 143134 | MFLU 16-1484 | Bassia sp. | Russia | MF434189 | MF434277 | MF434365 | MF434452 | |
| Neocamarosporium korfii | CR006 | MFLUCC 17-0745 = CBS 143135 | MFLU 17-1436 | Bassia prostrata | Russia | MF434190 | MF434278 | MF434366 | MF434453 |
| N. lamiacearum | T-0846 | MFLUCC 17-0560 = CBS 143136 | MFLU 15-2989 | Lamiaceae sp. | Russia | MF434191 | MF434279 | MF434367 | MF434454 |
| CR-026 | MFLUCC 17-0750 = CBS 143137 | MFLU 17-1437 | Bassia sedoides | Russia | MF434192 | MF434280 | MF434368 | MF434455 | |
| N. salicorniicola | CHAM025 | MFLUCC 15-0957 | MFLU 15-0957 | Salicornia sp. | Thailand | MF434193 | MF434281 | MF434369 | – |
| N. salsolae | YG-S6-1 | MFLUCC 17-0826 | TASM 6099 | Salsola sp. | Uzbekistan | MF434194 | MF434282 | MF434370 | MF434456 |
| YG-S6-2 | MFLUCC 17-0827 | TASM 6100 | Salsola sp. | Uzbekistan | MF434195 | MF434283 | MF434371 | MF434457 | |
| Staurosphaeria lycii | T-0289 | MFLUCC 17-0210 = CBS 143140 | MFLU 15-1993 | Lycium barbarum | Russia | MF434196 | MF434284 | MF434372 | MF434458 |
| T-0418 | MFLUCC 17-0211 = CBS 143141 | MFLU 15-2122 | Lycium barbarum | Russia | MF434197 | MF434285 | MF434373 | MF434459 | |
| T-1346 | MFLUCC 17-0720 = CBS 143158 | MFLU 16-1640 | Lycium barbarum | Russia | MF434198 | MF434286 | MF434374 | MF434460 | |
| T-1347 | MFLUCC 17-0721 | MFLU 16-1641 | Lycium barbarum | Russia | MF434199 | MF434287 | MF434375 | MF434461 | |
| S. rhamnicola | YG-S4-5 | MFLUCC 17-0813 | TASM 6101 | Rhamnus sp. | Uzbekistan | MF434200 | MF434288 | MF434376 | MF434462 |
| YG-S4-4D | MFLUCC 17-0814 | TASM 6102 | Rhamnus sp. | Uzbekistan | MF434201 | MF434289 | MF434377 | MF434463 | |
Culture Collection of the Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Rai, Thailand.
MFLU: Mae Fah Luang University (MFLU) Herbarium, Chiang Rai, Thailand; TASM: Tashkent Mycological Herbarium, Institute of Botany and Zoology, Academy of Sciences of Uzbekistan, Tashkent, Uzbekistan.
ITS: Internal transcribed spacers; LSU: partial 28S nrDNA; SSU: partial 18S nrDNA; tef1: translation elongation factor 1-alpha gene.
Sequence alignment and phylogenetic analyses
Sequences generated from different primers of the four genes were analysed with other sequences retrieved from GenBank. Sequences with high similarity indices were determined from a BLAST search to find the closest matches with taxa in Pleosporineae, and from recently published data (Liu et al., 2015, Grum-Grzhimaylo et al., 2016, Crous and Groenewald, 2017, Tibpromma et al., 2017). The sequences were aligned in MAFFT v. 7 with the web server (http://mafft.cbrc.jp/alignment/server), using iterative refinements as E-INS-i method for ITS & tef1, and as G-INS-i method for LSU and SSU (Katoh & Standley 2013). The alignment was edited where necessary with BioEdit v. 7.0.5.2 (Hall 1999). The alignment properties for the individual genes are shown in the Table 3. The final alignment and tree were deposited in TreeBASE, submission ID: 21397 (http://www.treebase.org/).
Table 3.
Comparison of alignment properties of genes and nucleotide substitution models used in Pleosporineae phylogenetic analyses.
| LSU1 | SSU2 | ITS3 | tef14 | Combined LSU, SSU, ITS and tef1 | |
|---|---|---|---|---|---|
| Alignment strategy (MAFFT v. 7) | L-INS-i + manually | L-INS-i | E-INS-i | E-INS-i + manually | – |
| Number of characters included in analysis (including gaps) | 857 | 982 | 667 | 955 | 3461 |
| Number of constant characters | 671 | 877 | 358 | 677 | 2583 |
| Number of parsimony informative characters (%) | 158 (18%) | 75 (8%) | 286 (43%) | 226 (24%) | 745 (22%) |
| Number of uninformative and variable characters | 28 | 30 | 23 | 52 | 133 |
| Nucleotide substitution model | GTR + I + G | GTR + I + G | GTR + I + G | GTR + I + G | GTR + I + G |
LSU: partial 28S nrDNA.
SSU: partial 18S nrDNA.
ITS: Internal transcribed spacers.
tef1: translation elongation factor 1-alpha gene.
The final alignment (combined LSU, SSU, tef1 and ITS loci) included 212 strains, (representing 16 selected families within the Pleosporineae), the new taxa proposed in this study, and Cyclothyriella rubronotata (CBS 141486 & CBS 121892) as the outgroup taxon. Phylogenetic analyses of both individual and combined aligned data were based on Maximum Likelihood (ML), Maximum Parsimony (MP) and Bayesian analyses. The sequence alignments were converted to NEXUS file format (.nex) for maximum parsimony and Bayesian analyses using ClustalX2 v. 1.83 (Thompson et al. 1997). The NEXUS file was prepared for MrModeltest v. 2.2 after deleting the symbols =“ABCDEFGHIKLMNOPQRSTUVWXYZ” (Nylander 2004) in PAUP (Phylogenetic Analysis Using Parsimony) v. 4.0b10 (Swofford 2003). For the Randomized Accelerated Maximum Likelihood (RAxML) analysis, sequence alignments were converted to PHYLIP file format (.phy) using ALTER (alignment transformation environment: http://sing.ei.uvigo.es/ALTER/; 2017).
The MP bootstrap analysis was performed with PAUP, with 1000 bootstrap replicates using 10 rounds of heuristic search replicates with random addition of sequences and subsequent TBR branch swapping (MULTREES option in effect, steepest descent option not in effect) during each bootstrap replicate, with each replicate limited to 1 M rearrangements. All characters were unordered and given equal weight; gaps were treated as missing data; the COLLAPSE command was set to minbrlen. Descriptive tree statistics for parsimony were calculated for trees generated under different optimality criteria: Tree Length (TL), Consistency Index (CI), Retention Index (RI), Relative Consistency Index (RC) and Homoplasy Index (HI). The Kishino-Hasegawa tests (Kishino & Hasegawa 1989) were performed to determine whether trees were significantly different. Other details are outlined in Jeewon et al. (2003b) and Promputtha et al. (2007).
The evolutionary models for Bayesian analysis and ML were selected independently for each locus using MrModeltest v. 2.3 (Nylander 2004) under the Akaike Information Criterion (AIC) implemented in PAUP v. 4.0b10. The GTR + I + G model was selected as the best-fit model for each locus in both Bayesian and ML analyses.
The Bayesian analysis was performed in MrBayes v. 3.1.2 (Huelsenbeck & Ronqvist 2001) to evaluate Posterior probabilities (PP) (Rannala and Yang, 1996, Zhaxybayeva and Gogarten, 2002) by Markov Chain Monte Carlo sampling (BMCMC). Six simultaneous Markov chains were run for 5 M generations and trees were sampled every 500th generation. The distribution of log-likelihood scores was examined to determine the stationary phase for each search and to decide if extra runs were required to achieve convergence, using the program Tracer v. 1.5 (Rambaut & Drummond 2007). All sampled topologies beneath the asymptote (10 %) were discarded as part of a burn-in procedure; the remaining trees were used for calculating PP in the majority rule consensus tree.
The ML trees were generated using the RAxML-HPC2 on XSEDE (v. 8.2.8) (Stamatakis et al., 2008, Stamatakis, 2014) in the CIPRES Science Gateway platform (Miller et al. 2010) using a GTR + I + G model of evolution. Phylograms were visualised with FigTree v. 1.4.0 (Rambaut 2012) and annotated in Microsoft PowerPoint (2007) or Adobe Illustrator® CS5 (v. 15.0.0, Adobe®, San Jose, CA).
Results
Phylogenetic analyses
Topologies of trees (ML, MP and PP) for each gene dataset were compared and the overall tree topology was congruent to those obtained from the combined dataset.
The RAxML analysis of the combined dataset yielded a best scoring tree (Fig. 1) with a final ML optimisation likelihood value of −24419.107973. The matrix had 1 273 distinct alignment patterns, with 24.87 % of undetermined characters or gaps. Parameters for the GTR + I + G model of the combined LSU, SSU, tef1 and ITS were as follows: Estimated base frequencies; A = 0.24346, C = 0.239263, G = 0.26821, T = 0.249067; substitution rates AC = 1.464074, AG = 3.479937, AT = 2.089279, CG = 0.715575, CT = 7.524749, GT = 1.000; proportion of invariable sites I = 0.630738; gamma distribution shape parameter α = 0.492847. The maximum parsimonious dataset consisted of 3 461 characters, of which 2 583 were constant, 745 parsimony-informative and 133 parsimony-uninformative. The parsimony analysis of the data matrix resulted in the maximum of 1 000 equally most parsimonious trees with a length of 3 928 steps (CI = 0.341, RI = 0.793, RC = 0.27, HI = 0.659) in the first tree. The Bayesian analysis resulted in 10 000 trees after 5 M generations. The first 1 000 trees, representing the burn-in phase of the analyses, were discarded, while the remaining 9 000 trees were used or calculating posterior probabilities in the majority rule consensus tree.
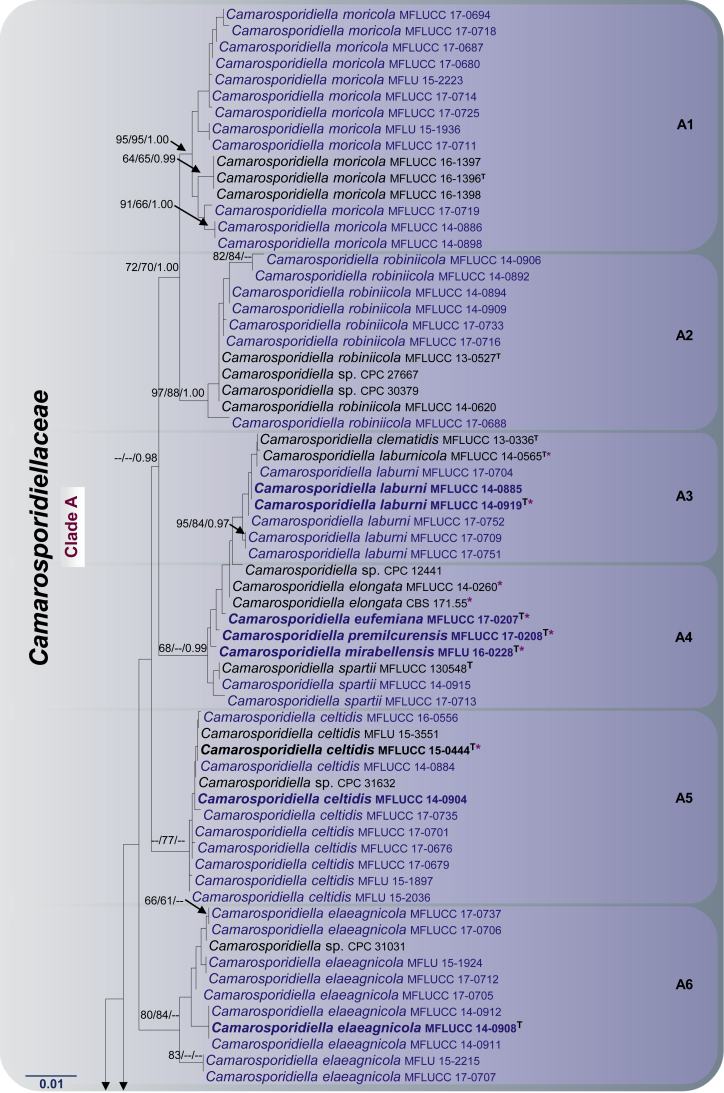
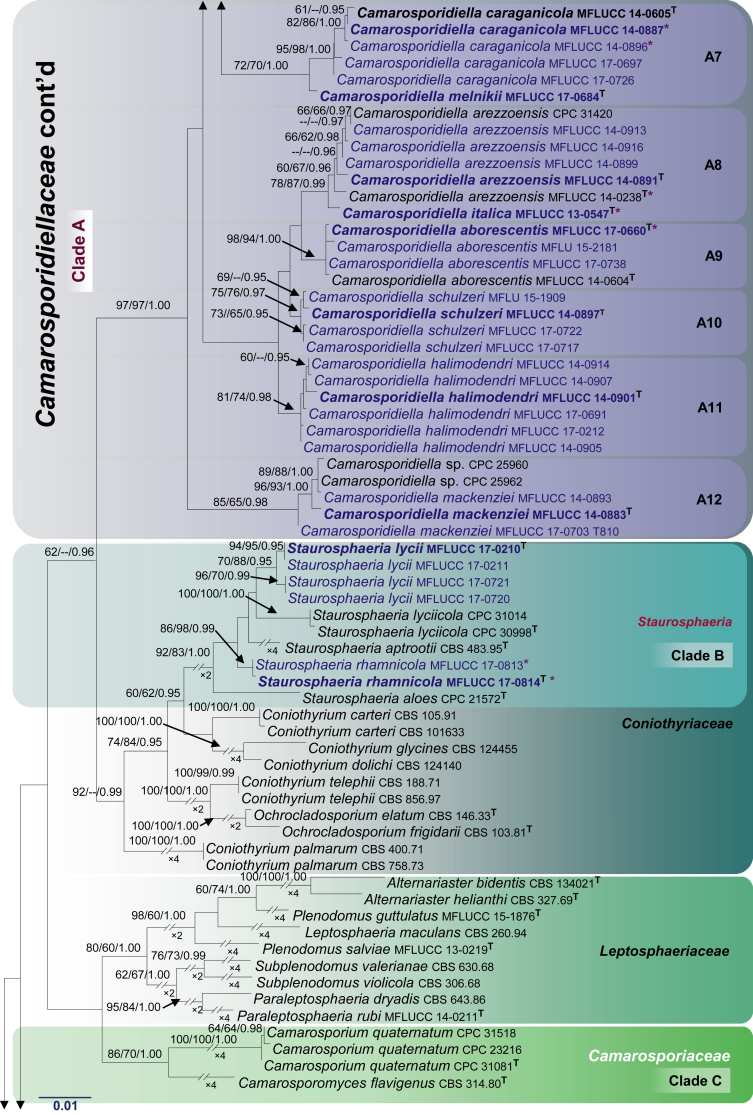
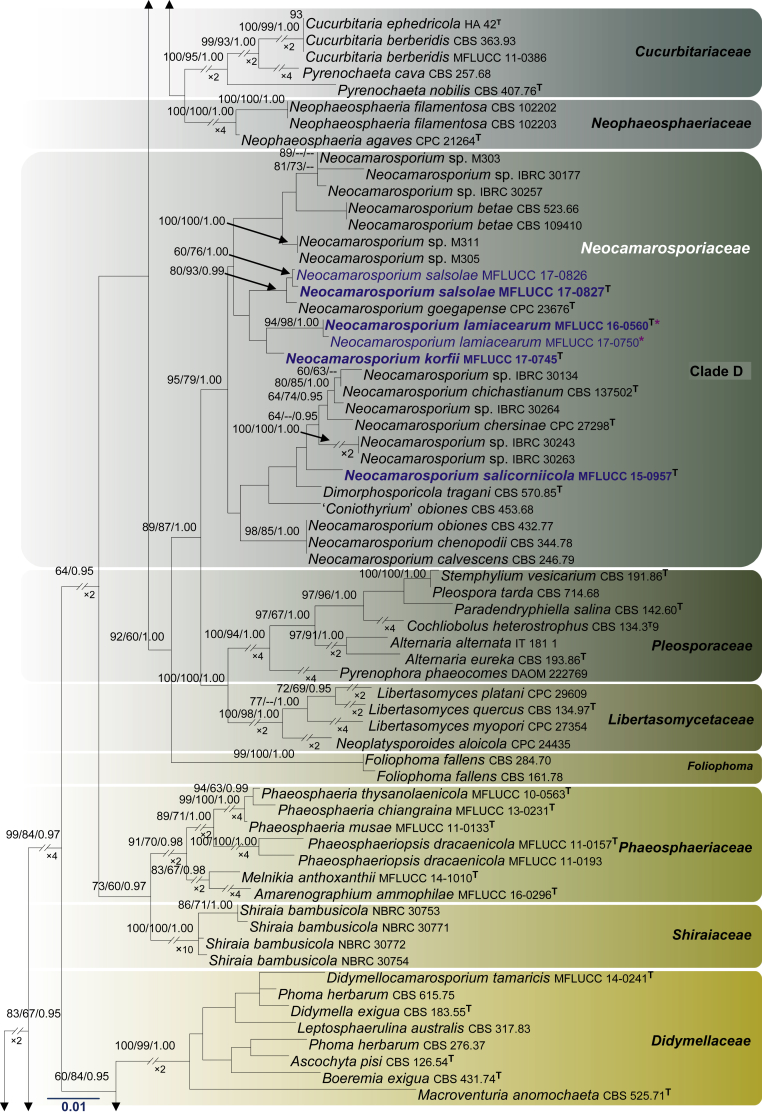
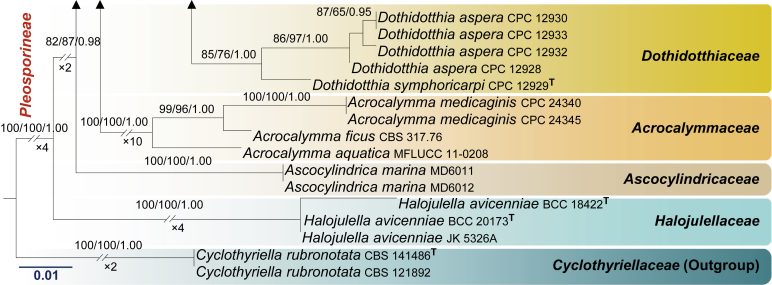
Fig. 1.
RAxML tree based on a combined dataset of LSU, SSU, tef1 and ITS partial sequences. Bootstrap support values for ML and MP equal to or greater than 60 %, Bayesian posterior probabilities (PP) equal to or greater than 0.95 are defined as ML/MP/PP above the nodes. Species used for morphological observation in this study are indicated in bold. Families, where known, and selected genera are indicated with coloured blocks. The tree is rooted to Cyclothyriella rubronotata (CBS 141486 & CBS 121892). The new isolates are in blue. Asterisk marks origin of isolates from single ascospore. The ex-type strains are noted with superscripted T. The scale bar represents the expected number of nucleotide substitutions per site.
Newly generated sequences from 75 isolates of camarosporium-like taxa grouped with Camarosporium aborescentis, Cm. arezzoensis, Cm. aureum, Cm. caraganicola, Cm. clematidis, Cm. elaeagnellum, Cm. elongata, Cm. laburnicola, Cm. moricola, Cm. robiniicola, Cm. spartii, Cm. uniseriatum and Cucurbitaria elongata (fide Wijayawardene et al., 2014a, Wijayawardene et al., 2014b, Wijayawardene et al., 2016, Tibpromma et al. 2015, Tibpromma et al., 2017, Thambugala et al., 2016, Crous and Groenewald, 2017). These taxa formed an isolated, well-supported clade (97 % ML, 97 % MP and 1.00 PP, Clade A, Fig. 1) within Pleosporineae which we formally introduce as Camarosporidiellaceae.
Six newly generated sequences, Staurosphaeria lycii (MFLUCC 17-0210, MFLUCC 17-0211, MFLUCC 17-0720 and MFLUCC 17-0721), S. rhamnicola (MFLUCC 17-0813 and MFLUCC 17-0814) grouped with Hazslinszkyomyces lycii (CPC 31014 and CPC 30998), the type species of Hazslinszkyomyces, H. aptrootii (CBS 483.95) and H. aloes (CPC 21572). These taxa form a monophyletic clade (Clade B) in Coniothyriaceae with significant statistical support (92 % ML, 83 % MP and 1.00 PP, Fig. 1) and hereby being referred to as the genus Staurosphaeria.
Camarosporium quaternatum and Camarosporomyces flavigenus always grouped together in a separate clade with high statistical support (86 % ML, 70 % MP and 1.00 PP, Clade C, Fig. 1). In different analyses, the placement of this clade is unstable and in this concatenated analysis, these taxa are basal to the Leptosphaeriaceae. We herein validate Camarosporiaceae to accommodate Camarosporium and Camarosporomyces.
Neocamarosporium korfii (MFLUCC 17-0745), N. lamiacearum (MFLUCC 17-560 and MFLUCC 17-0750), N. salicorniicola (MFLUCC 15-0957) and N. salsolae (MFLUCC 17-0826 and MFLUCC 17-0827) grouped with Coniothyrium obiones (CBS 453.68), Dimorphosporicola tragani (CBS 570.85), Neocamarosporium betae (CBS 109410 and CBS 523.66), N. calvescens (CBS 246.79), N. chenopodii (CBS 344.78), N. chersinae (CPC 27298), N. chichastianum (CBS 137502), N. goegapense (CPC 23676), N. obiones (CBS 432.77) and Neocamarosporium sp. (IBRC M 30134, IBRC M 30264, IBRC M 30177, IBRC M 30257, IBRC M 30243, IBRC M 30263, M303, M305 and M311) from a distinct clade (Clade D) with high bootstrap values (95 % and 79 % in ML and MP analyses respectively) and high PP value (1.00). Multi-gene phylogenetic analyses herein support the establishment of a new family, Neocamarosporiaceae fam. nov. for Clade D (Fig. 1).
Apart from establishing three new families, our multi-gene phylogeny, with relatively good support, this study also clarifies interfamilial relationships. In particular, we note that all the families herein are well supported monophyletic lineages. The Cucurbitariaceae was previously considered as a close ally to Coniothyriaceae (Hyde et al. 2013), Phaeosphaeriaceae (Wijayawardene et al. 2014a), Pleosporaceae (Doilom et al., 2013, Wanasinghe et al., 2014b), but this study demonstrates a sister relationship to Neophaeosphaeriaceae. It is also interesting to note that Neocamarosporiaceae shares close affinities to Pleosporaceae and Libertasomycetaceae. The affinities of Foliophoma, which is characterized by eustromatic conidiomata, uni- to multi-loculate with 1–3 ostioles and conidiogenous cells with periclinal thickening or percurrent proliferation at apex (Crous & Groenewald 2017), warrants further investigations as only one species has been described so far. Phaeosphaeriaceae and Shiraiaceae are closely related while Didymellaceae is sister family to Dothidotthiaceae. In other analyses (results not shown) the Microsphaeropsidaceae (introduced by Chen et al. 2015) clusters basal to the Didymellaceae. The other families viz. Acrocalymmaceae, Ascocylindricaceae and Halojulellaceae, with relatively few taxa are basal families of the Pleosporineae.
Camarosporiaceae Wanas., Wijayaw., K.D. Hyde & Crous, fam. nov. MycoBank MB821938; Facesoffungi number: FoF 03527.
Synonym: Camarosporiaceae Locq., Mycol. gén. struct. (Paris): 210 (1984); nom. inval., Art. 39.1 (Melbourne).
Saprobic, endophytic, pathogenic on leaves and wood in terrestrial habitats. Asexual morph: Conidiomata dimorphic, pycnidial, subcorticolous, single to gregarious, partly caespitose, globose, ostiole central, terete, short papillate. Conidiomata wall few-layered, consisting of a textura globulosa-angularis with red brown, thick-walled, and smooth cells. Conidiogenous cells formed from the inner cells of the pycnidial wall, doliiform, hyaline, thin-walled, annellidic. Conidia multicelled, muriformly septate, with one longitudinal or diagonal septum per cell and 1–2 per conidium, ellipsoidal, pyroid, clavate, straight to slightly curved, yellowish not brown, basal cell often paler or hyaline, wall golden. Synasexual morph: conidiomata separate, pycnidial, immersed to superficial, brown, globose, with 1–2 papillate ostioles, exuding a crystalline conidial mass. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lining the inner cavity, hyaline, smooth, ampulliform. Conidia solitary, hyaline, smooth, subcylindrical, straight, rarely curved, apex obtuse, base truncate. Sexual morph: Ascomata gregarious to solitary, immersed to erumpent, globose to subglobose, black, unilocular, ostiolate. Ostiole black, papillate. Peridium with several cell layers of textura angularis, with outer layer brown to reddish brown, inner layer hyaline to sub hyaline. Asci stipitate, cylindrical, bitunicate, (2–)4–8-spored. Ascospores uniseriate, ellipsoidal, medium brown, mostly with obtuse ends, muriform, 3–8 transverse septa, with 1–2 longitudinal septa, constricted at septa.
Type genus: Camarosporium Schulzer.
Notes: To better resolve interfamilial/intergeneric level relationships and improve taxonomic issues within Pleosporineae, we validate Camarosporiaceae (Camarosporiaceae Locq. 1984 was not validly published, Art. 39.1) to accommodate Camarosporium and Camarosporomyces. Wijayawardene et al. (2014b) also proposed Camarosporiaceae to accommodate Camarosporium s. str. but it was not formerly introduced.
Camarosporium Schulzer, Verh. K.K. Zool.-Bot. Ges. Wien 17: 717. 1870.
Description and illustration: Crous & Groenewald (2017).
Type species: Camarosporium quaternatum (Hazsl.) Schulzer.
Notes: Camarosporium morphologically resembles genera such as Camarographium, Camarosporiopsis, Camarosporula, Dichomera, Didymellocamarosporium, Hazslinszkyomyces, Libertasomyces, Magnicamarosporium, Melanocamarosporium, Melnikia, Murilentithecium, Neocamarosporium, Paracamarosporium, Phragmocamarosporium, Pseudocamarosporium, Pseudohendersonia, Suttonomyces and Xenocamarosporium in conidial shape and septation. However, these taxa are phylogenetically distinct and have subtle but specific morphological differences (Sutton, 1980, Butin, 1993, Crous et al., 2011, Crous et al., 2013, Crous et al., 2014b, Crous et al., 2015a, Crous et al., 2015b, Wijayawardene et al., 2014a, Wijayawardene et al., 2014b, Wijayawardene et al., 2014c, Wijayawardene et al., 2015, Wijayawardene et al., 2016, Tanaka et al., 2015, Tian et al., 2015, Crous and Groenewald, 2017).
Camarosporium quaternatum was introduced by Schulzer (1870) as the type species of Camarosporium. Schulzer (1870) did not provide any illustrations for Camarosporium quaternatum in his article and mentioned it is completely similar to Clinterium lycii, described in Hazslinszky (1865). The microfungal collections of F.A. Hazslinszky von Hazslin are preserved in the Hungarian Natural History Museum (BP), but the type of Cm. quaternatum has been lost. Therefore, in a recent study Crous & Groenewald (2017) designated the original illustrations as lectotypes, to facilitate epitypification.
Camarosporomyces Crous, IMA Fungus 8: 141. 2017.
Description and illustration: Crous & Groenewald (2017).
Type species: Camarosporomyces flavigenus (Constant. & Aa) Crous.
Notes: Camarosporomyces was introduced by Crous & Groenewald (2017) to accommodate Camarosporomyces flavigenus, a phoma-like fungus which was originally described as Phoma flavigena. In our molecular analyses, Camarosporomyces flavigenus is basal to other strains of Camarosporium quaternatum strains with good statistical support (Clade C, Fig. 1).
Camarosporidiellaceae Wanas., Wijayaw., Crous & K.D. Hyde, fam. nov. MycoBank MB821939; Facesoffungi number: FoF 03528.
Etymology: Referring to the name of the type genus.
Saprobic or endophytic or pathogenic on leaves and wood (Fig. 2). Asexual morph: Coelomycetous. Conidiomata pycnidial, immersed to sub-peridermal, globose, dark brown to black, unilocular. Conidiomata wall thick-walled, dark brown, composed of cells of textura angularis, inner layer with hyaline cells. Ostiole single, circular, central, papillate. Conidiogenous cells enteroblastic, annellidic, integrated to discrete, doliiform, lageniform or cylindrical, smooth, hyaline, formed from the inner cells of the pycnidial wall. Conidia medium brown to dark brown, phragmosporous to muriform, variable in shape, mostly ellipsoidal, curved to straight, truncate at the base, obtuse at the apex, continuous or constricted at the septa. Sexual morph: Ascomata gregarious to solitary, immersed to erumpent, globose to subglobose, black, unilocular, ostiolate. Ostiole black, papillate. Peridium with several cell layers of textura angularis, with outer layer brown to reddish-brown, inner layer hyaline to sub hyaline. Asci stipitate, cylindrical, bitunicate, (2–)4–8-spored. Ascospores uniseriate, ellipsoidal, medium brown, mostly with obtuse ends, muriform, 3–8 transverse septa, with 1–2 longitudinal septa, constricted at septa.
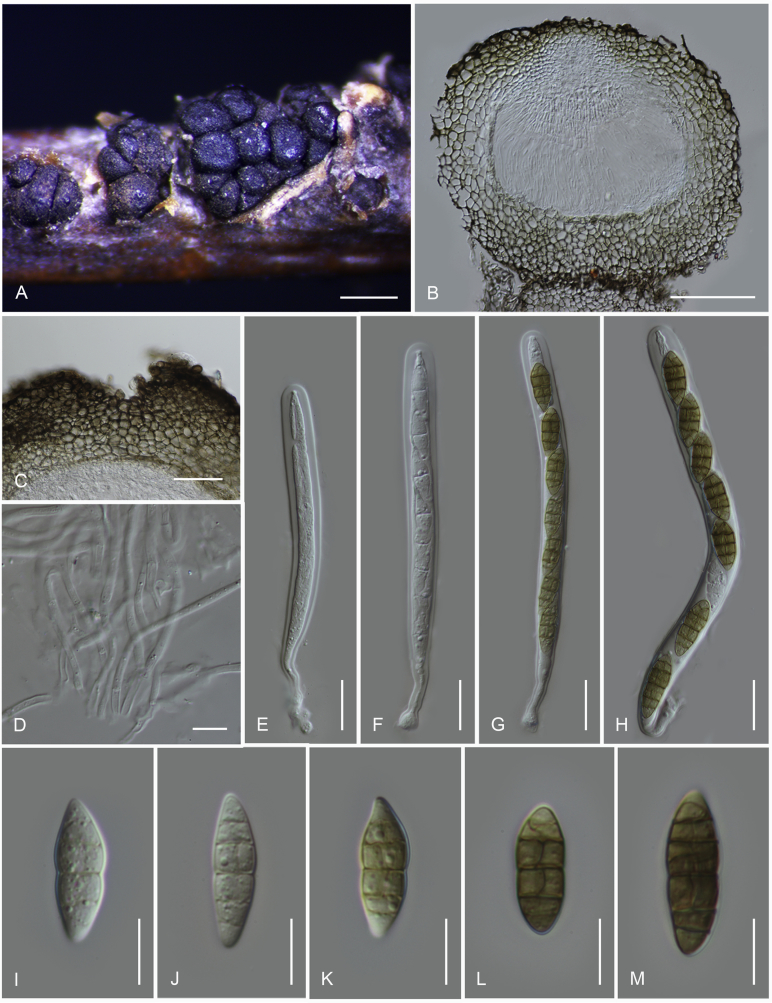
Fig. 2.
Camarosporidiella spp. on different hosts. A, B.Elaeagnus angustifolia. C.Caragana arborescens. D.Laburnum anagyroides. E, F.Morus alba.
Type genus: Camarosporidiella Wanas., Wijayaw. & K.D. Hyde.
Notes: Camarosporidiellaceae forms a highly-supported monophyletic lineage (97 %/97 %/1.00; Fig. 1, clade A) but lacks internal support. Morphological features are not informative for generic distinction within Clade A. The taxa studied here are treated below according to the phylogenetic clades (Subclades A1–A12, Fig. 1) as follows:
Camarosporidiella Wanas., Wijayaw. & K.D. Hyde, gen. nov. MycoBank MB821940; Facesoffungi number: FoF 03529.
Etymology: Resembling the genus Camarosporium.
Saprobic or endophytic or pathogenic on leaves and wood in terrestrial habitats. Asexual morph: Conidiomata pycnidial, immersed to sub-peridermal, globose, dark brown to black, unilocular. Conidiomata wall thick-walled, dark brown, composed of cells of textura angularis, inner layer with hyaline cells. Ostiole single, circular, centrally papillate. Macroconidiogenous cells enteroblastic, annellidic, integrated, indeterminate, doliiform, lageniform or cylindrical, smooth-walled, hyaline, formed from the inner cells of the pycnidial wall. Macroconidia medium brown to dark brown, phragmosporous to muriform, variable in shape, mostly ellipsoidal, curved to straight, truncate at base, obtuse at apex, continuous or constricted at the septa. Microconidiogenous cells present or absent in cultures, when present; intermingled with macroconidiogenous cells, hyaline, integrated, enteroblastic, percurrent annellidic, ampulliform to subcylindrical. Microconidia present or absent, when present; hyaline, round to oblong or ellipsoidal, with small guttules. Sexual morph: cucurbitaria-like. Ascomata black, superficial to semi-immersed, gregarious, confluent, sometimes scattered beneath the host periderm or on decorticated wood, fully or partly erumpent, globose, black, ostiolate. Ostiole central, short. Peridium composed of blackish to dark brown cells of textura angularis, cells towards the inside lighter, composed of thin-walled cells of textura angularis. Hamathecium comprising numerous, branched septate, pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical, short-pedicellate. Ascospores overlapping uniseriate, muriform, mostly ellipsoidal, 3–8-transversely septate, with 2–4 vertical septa, constricted at middle septum, initially hyaline, becoming brown at maturity, slightly paler, conical and narrow at the ends, not surrounded by a mucilaginous sheath.
Type species: Camarosporidiella caraganicola (Phukhams. et al.) Phukhams., Wanas. & K.D. Hyde.
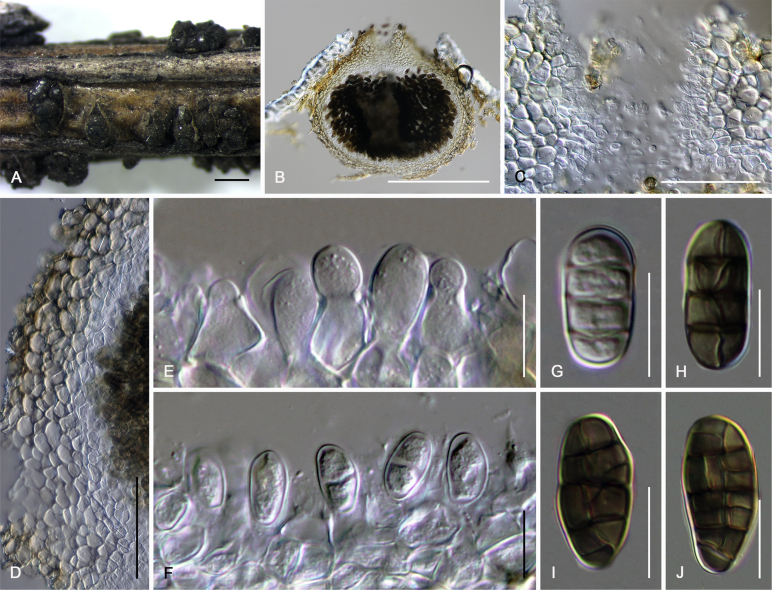
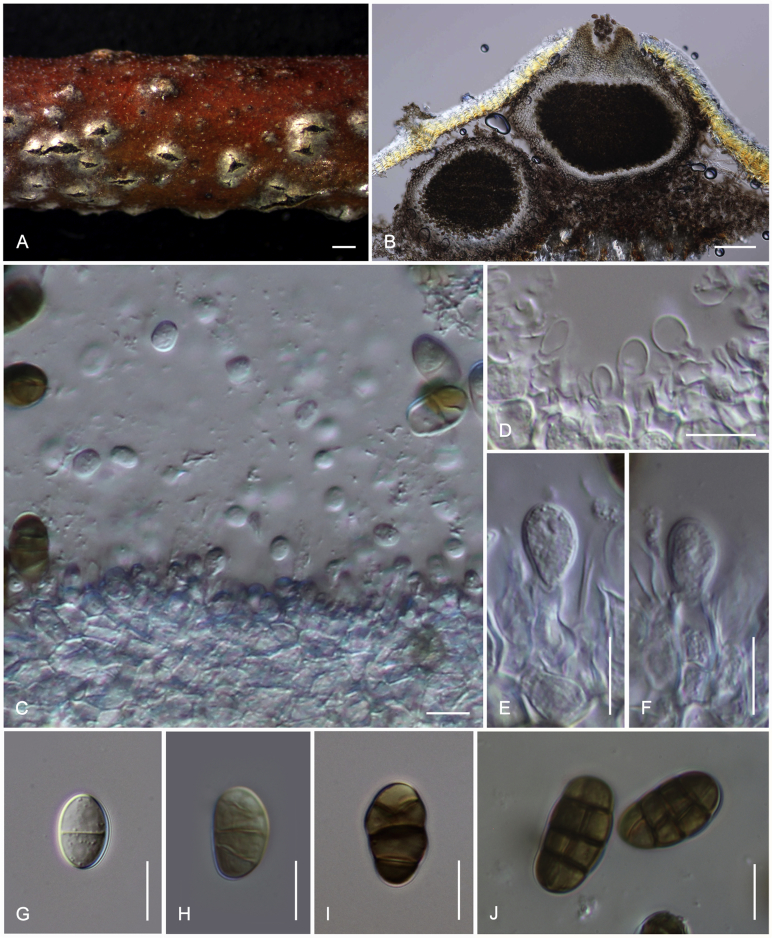
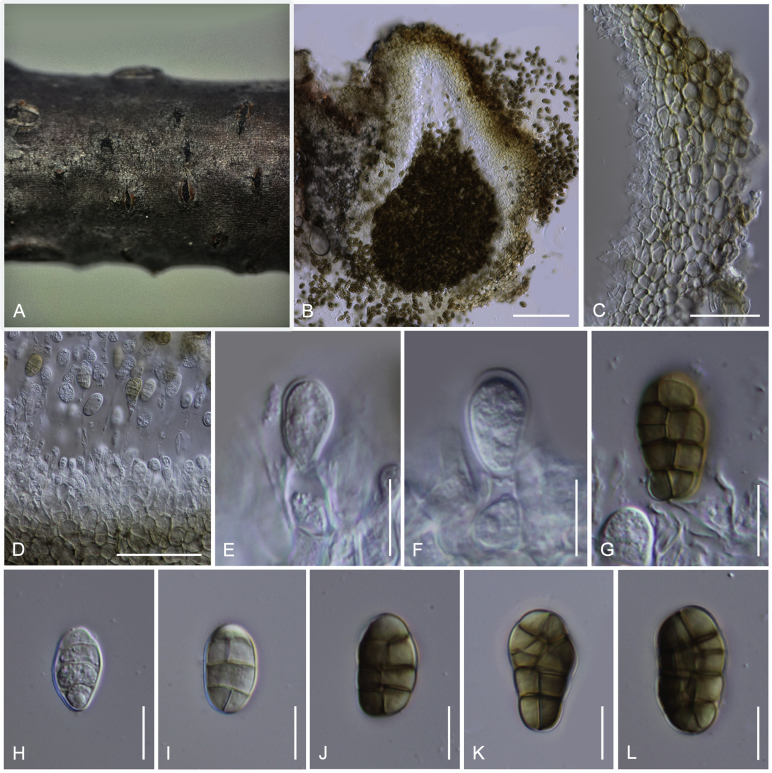
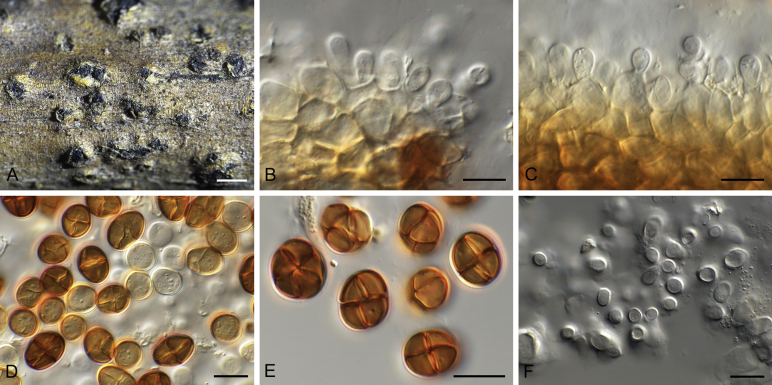
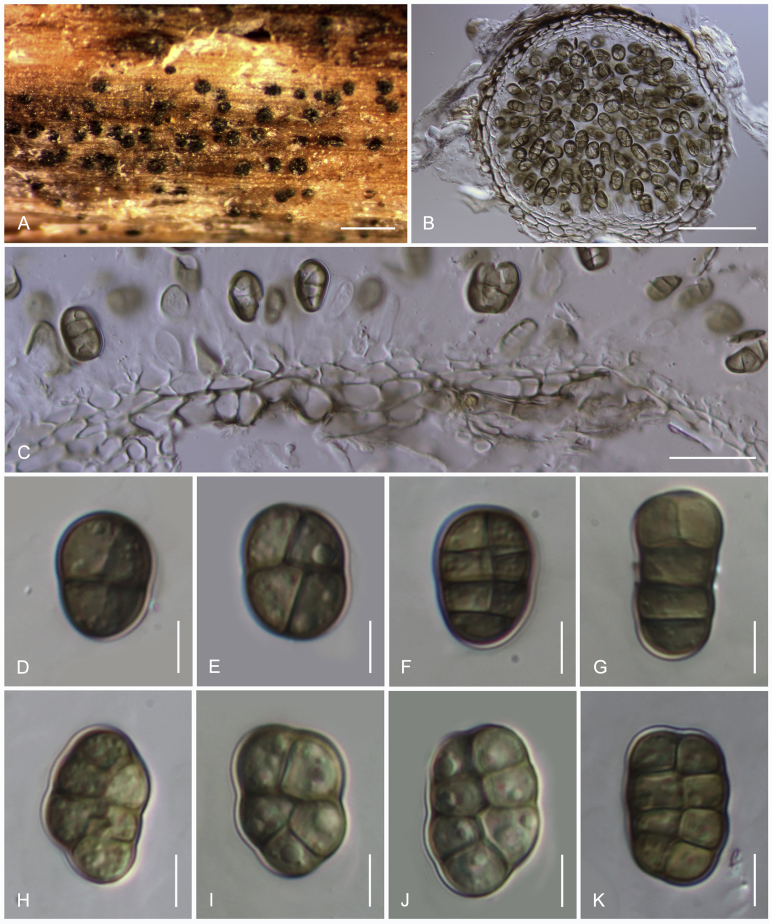
Camarosporidiella caraganicola (Phukhams. et al.) Phukhams., Wanas. & K.D. Hyde, comb. nov. MycoBank MB821941; Facesoffungi number: FoF 03530. Fig. 3, Fig. 4.
Fig. 3.
Asexual morph of Camarosporidiella caraganicola (MFLU 14-0794, holotype) A. Conidiomata on host surface. B. Vertical section through ostiole. C. Conidioma wall. D. Part of pycnidium wall. E, F. Conidiogenous cells and developing conidia. G–J. Conidia. Scale bars: A = 500 μm; B = 200 μm; C, D = 50 μm; E–J = 10 μm.
Fig. 4.
Sexual morph of Camarosporidiella caraganicola (MFLU 17-0453). A. Appearance of ascomata on host substrate. B. Section of ascoma. C. Close-up of ostiole. D. Pseudoparaphyses. E–H. Asci. I–M. Ascospores. Scale bars: A = 500 μm; B = 100 μm; C = 50 μm; D, I–M = 10 μm; E–H = 20 μm.
Basionym: Camarosporium caraganicola Phukhams. et al., Fungal Diversity 72: 156. 2015.
Saprobic on dead branches of Caragana frutex. Asexual morph: See Liu et al. (2015). Sexual morph: Ascomata 400–550 μm high, 450–500 μm diam ( = 436.2 × 457.8 μm, n = 10), black, superficial to semi-immersed, confluent, gregarious, sometimes scattered beneath the host periderm or on decorticated wood, fully or partly erumpent, globose, rough or hairy, ostiolate. Ostiole central, short, slightly sunken, minute, inconspicuous on surface, smooth, with ostiolar canal filled with hyaline cells. Peridium 60–80 μm wide at the base, 50–70 μm wide in sides, comprising 8–10 layers, with outer layer heavily pigmented, thick-walled, comprising blackish to dark brown cells of textura angularis, cells towards inside lighter, with inner layer composed 3–4 layers, hyaline, flattened, thin-walled cells of textura angularis. Hamathecium comprising numerous, 2.5–3 μm (n = 40) wide, filamentous, branched, septate, pseudoparaphyses. Asci 150–190 × 10–15 μm ( = 170.8 × 13.1 μm, n = 40), 8-spored, bitunicate, fissitunicate, cylindrical, short-pedicellate, rounded at apex with a minute ocular chamber. Ascospores 20–30 × 7–10 μm ( = 24.9 × 8.7 μm, n = 50), overlapping uniseriate, muriform, mostly ellipsoidal, 3–5-transversely septate, with 2–4 vertical septa, constricted at middle septum, initially hyaline, becoming brown at maturity, slightly paler, conical and narrow at the ends, not surrounded by a mucilaginous sheath.
Colonies on PDA: Slow growing, reaching 2 cm diam after 4 wk at 16 °C, later with dense mycelium, circular, rough margin white at first, greenish grey after 6 wk, reverse greenish grey, flat on the surface, without aerial mycelium.
Materials examined: Russia, Rostov region, Rostovna-Donu, BadiaTega, Botanical Garden of Southern Federal University, Systematic Arboretum, on dead twigs of Caragana frutex (Fabaceae), 26 Apr. 2014, T.S. Bulgakov T-046 (MFLU 14-0794, holotype; ex-type culture MFLUCC 14-0605; Rostov region, Rostov-na-Don city, Botanical garden of Southern Federal University, 47, 234635° N, 39, 656986° E, 3 Mar. 2014, T.S. Bulgakov T-005 (MFLU 17-0453, paratype, ex-paratype culture MFLUCC 14-0887 = CBS 143105); Rostov region, Oktyabrsky district, natural monument, 47, 5049392° N, 40, 1539564° E, 26 Apr. 2014, T.S. Bulgakov T-013, MFLU 17-0459, living culture MFLUCC 14-0896 = CBS 143106; Rostov region, Krasnosulinsky district, Donskoye forestry, Kabanya Balka, 47, 8643133° N, 40, 2421045° E, 28 Jun. 2015, T.S. Bulgakov T-538, MFLU 15–2242, living culture MFLUCC 17-0697 = CBS 143107, ibid. 18 Feb. 2016, T.S. Bulgakov T-1488, MFLU 16-1782, living culture MFLUCC 17-0726 = CBS 143108.
Notes: Camarosporidiella caraganicola (MFLUCC 14E-0605) is based on a strain derived from the asexual morph that was described by Liu et al. (2015). In this study, we have examined two specimens of the sexual morph of Camarosporidiella caraganicola (T-005 and T-013). These two taxa were collected from the same host (Caragana frutex) in the Rostov Region, Russia. By considering the identical host and statistical support, we conclude that these two taxa represent the holomorph of Camarosporidiella caraganicola. Also, we have observed another three specimens of the asexual morph of Ca. caraganicola (T-538 and T-1488). All strains of this species cluster together with significant statistical support of 95 % for ML, 98 % for MP and 1.00 for PP (Clade A7, Fig. 1).
Other accepted species
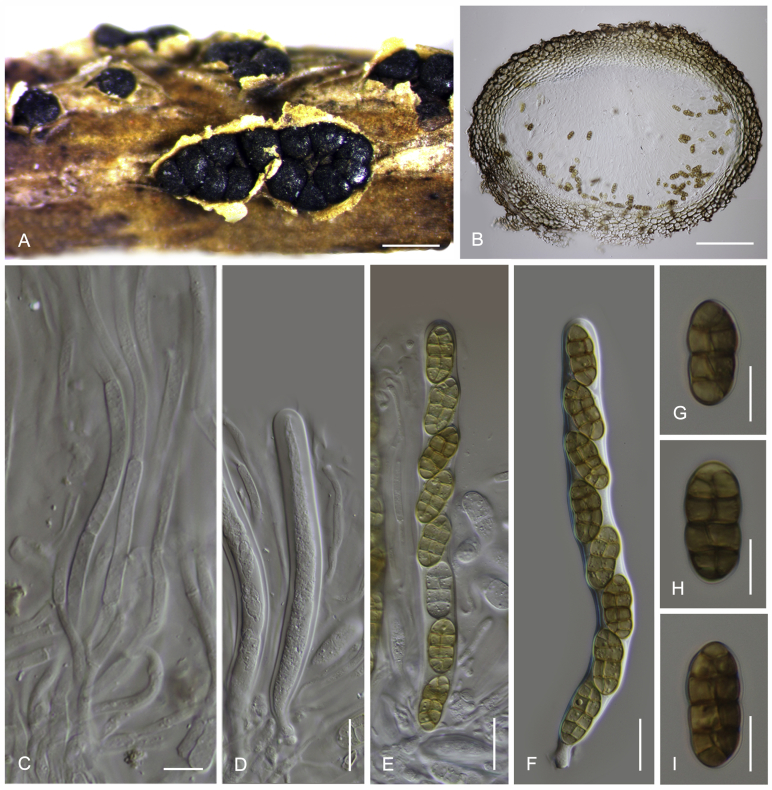
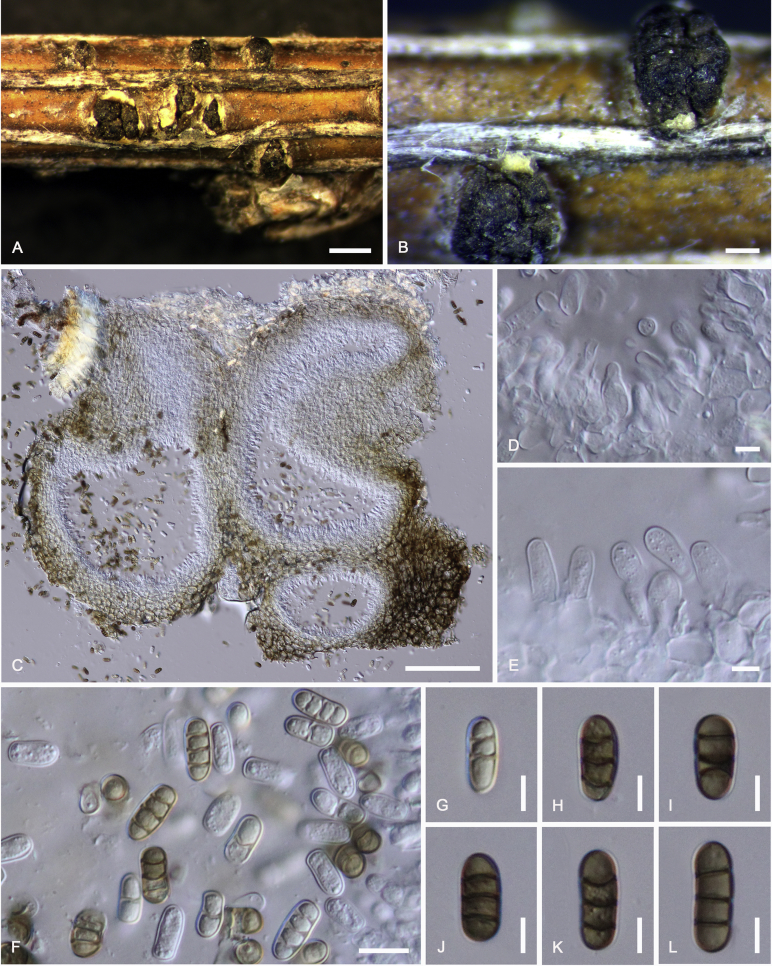
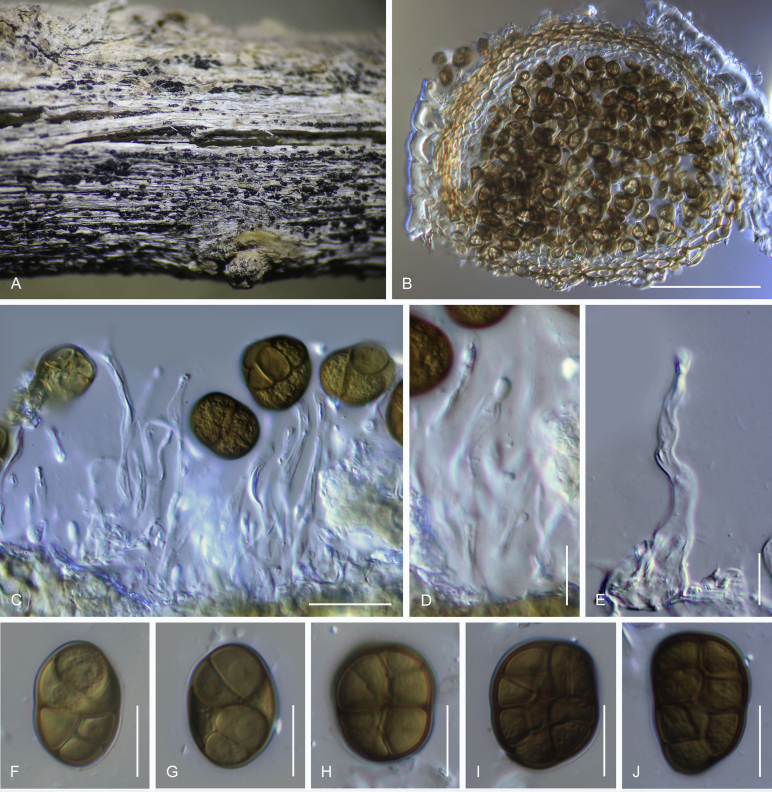
Camarosporidiella aborescentis (Phukhams. et al.) Phukhams., Wanas. & K.D. Hyde, comb. nov. MycoBank MB821942; Facesoffungi number: FoF 03531. Fig. 5.
Fig. 5.
Sexual morph of Camarosporidiella aborescentis (MFLU 15-3630). A. Appearance of ascomata on host substrate. B. Section of ascoma. C. Peridium. D. Pseudoparaphyses. E–H. Asci. I–N. Ascospores. Scale bars: B = 100 μm; C, D = 10 μm; E–H = 20 μm; I–N = 10 μm.
Basionym: Camarosporium aborescentis Phukhams. et al., in Liu et al., Fungal Diversity 72: 151. 2015.
Saprobic on woody branches. Asexual morph: See Liu et al. (2015) for illustrations. Sexual morph: Ascomata 350–450 μm high, 500–600 μm diam ( = 406.4 × 529.7 μm, n = 10), black, superficial to semi-immersed, confluent, gregarious, sometimes scattered beneath the host periderm or on decorticated wood, fully or partly erumpent, globose, rough or hairy, ostiolate. Ostiole central, short, slightly sunken, minute, inconspicuous on surface, smooth, with ostiolar canal filled with hyaline cells. Peridium 15–25 μm wide at the base, 25–50 μm wide in sides, comprising 6–10 layers, with outer layer heavily pigmented, thick-walled, comprising blackish to dark brown cells of textura angularis, cells towards inside lighter, with inner layer composed of 3–4 layers, hyaline, flattened, thin-walled cells of textura angularis. Hamathecium comprising numerous, 2–3 μm (n = 40) wide, filamentous, branched, septate, pseudoparaphyses. Asci 170–210 × 15–18 μm ( = 186.2 × 16.1 μm, n = 40), 8-spored, bitunicate, fissitunicate, cylindrical, short-pedicellate, rounded at apex with a minute ocular chamber. Ascospores 28–32 × 12–13 μm ( = 29.9 × 12.4 μm, n = 50), overlapping uniseriate, muriform, mostly ellipsoidal, 5–7-transversely septate, with 1–2 vertical septa, constricted at middle septum, initially hyaline, becoming brown at maturity, slightly paler, conical and narrow at the ends, not surrounded by a mucilaginous sheath.
Colonies on PDA: Slow growing, reaching 2 cm diam after 4 wk at 16 °C, later with dense mycelium, circular, rough margin, greenish-grey after 6 wk, reverse greenish-grey, flat on the surface, without aerial mycelium. Hyphae septate, branched, hyaline, thin-walled.
Materials examined: Italy, Forlì-Cesena Province, near Predappio, on dead branches of Colutea arborescens (Fabaceae), 25 Oct. 2015, E. Camporesi IT2674, MFLU 15–3630, living culture MFLUCC 17-0660. Russia, Rostov region, Rostov-on-Don city, Botanical Garden of Southern Federal University, Systematic Arboretum, parkland, 47,2350724° N, 39,6541643° E, on Colutea orientalis, 30 May 2015, T.S. Bulgakov T-477, MFLU 15-2181; on Amorpha sp., 14 Jun. 2016, T.S. Bulgakov NK076, MFLU 16-2387, living culture MFLUCC 17-0738.
Notes: Camarosporidiella aborescentis is morphologically similar to Camarosporium feurichii in having black conidiomata and brown, smooth-walled, oblong, 3-transversely septate conidia and usually with one longitudinal septum (Liu et al. 2015). In this study, we add another three strains to Camarosporidiella aborescentis from Italy and Russia. Altogether strains of this taxon cluster together with high statistical support of 98 % for ML, 94 % for MP and 1.00 for PP (Clade A9, Fig. 1).
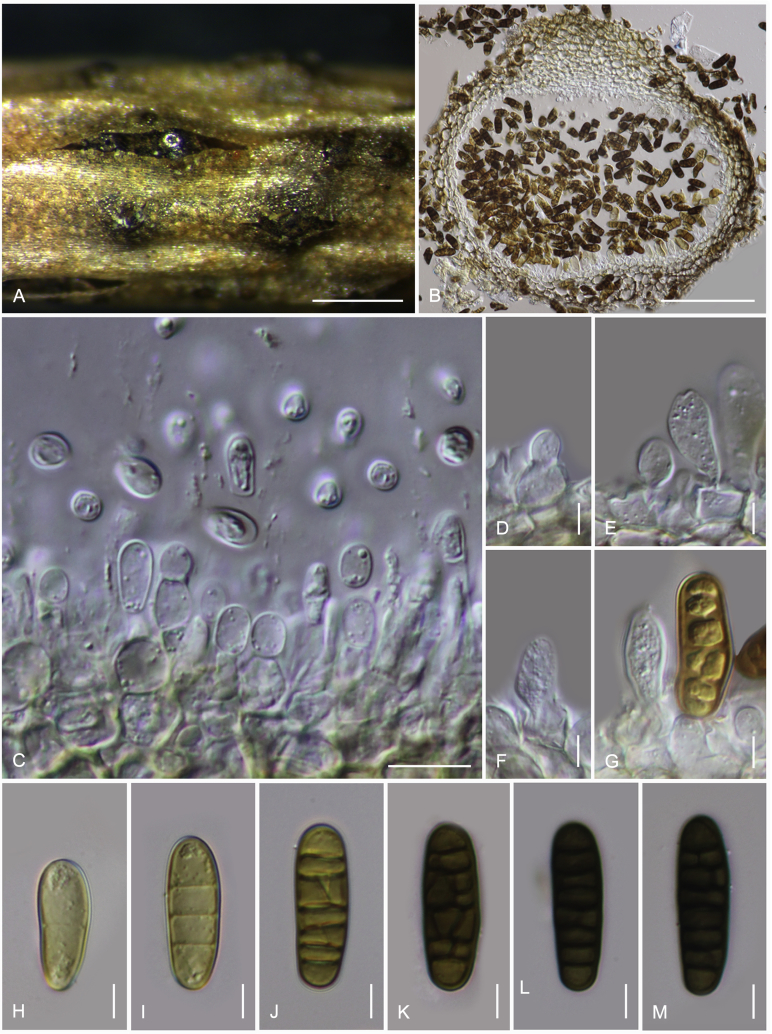
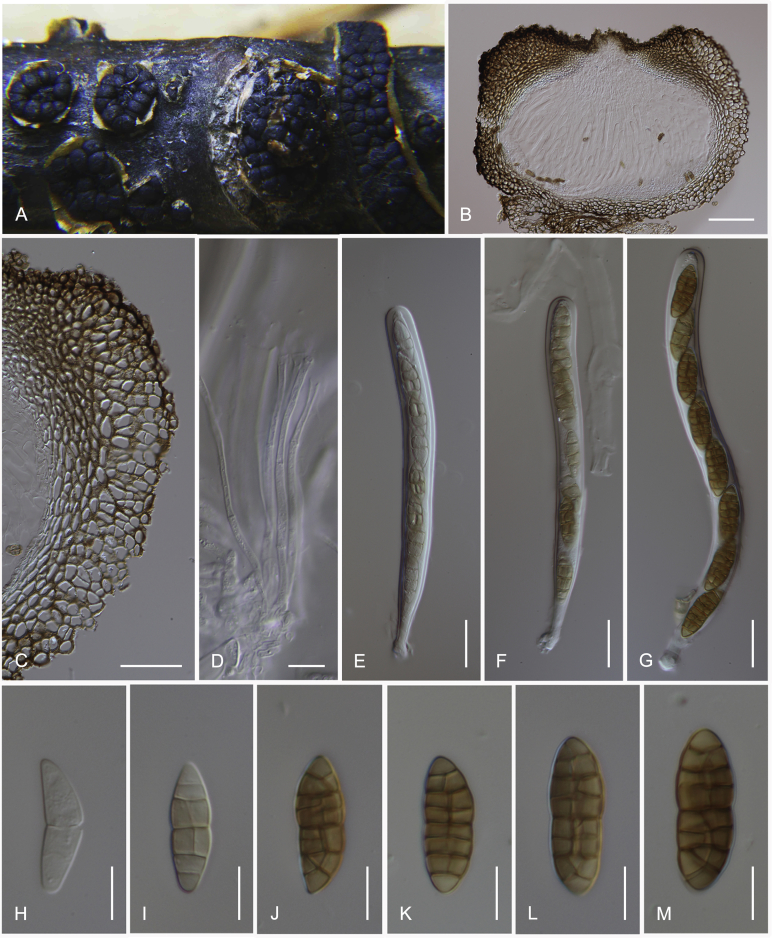
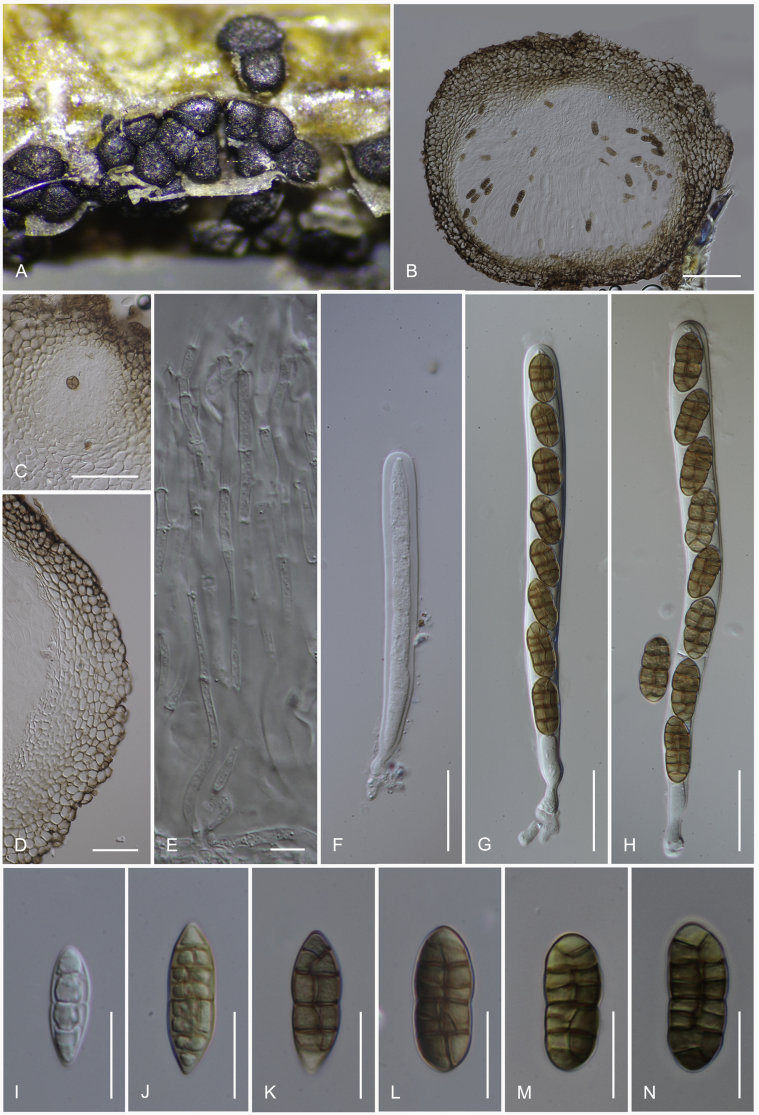
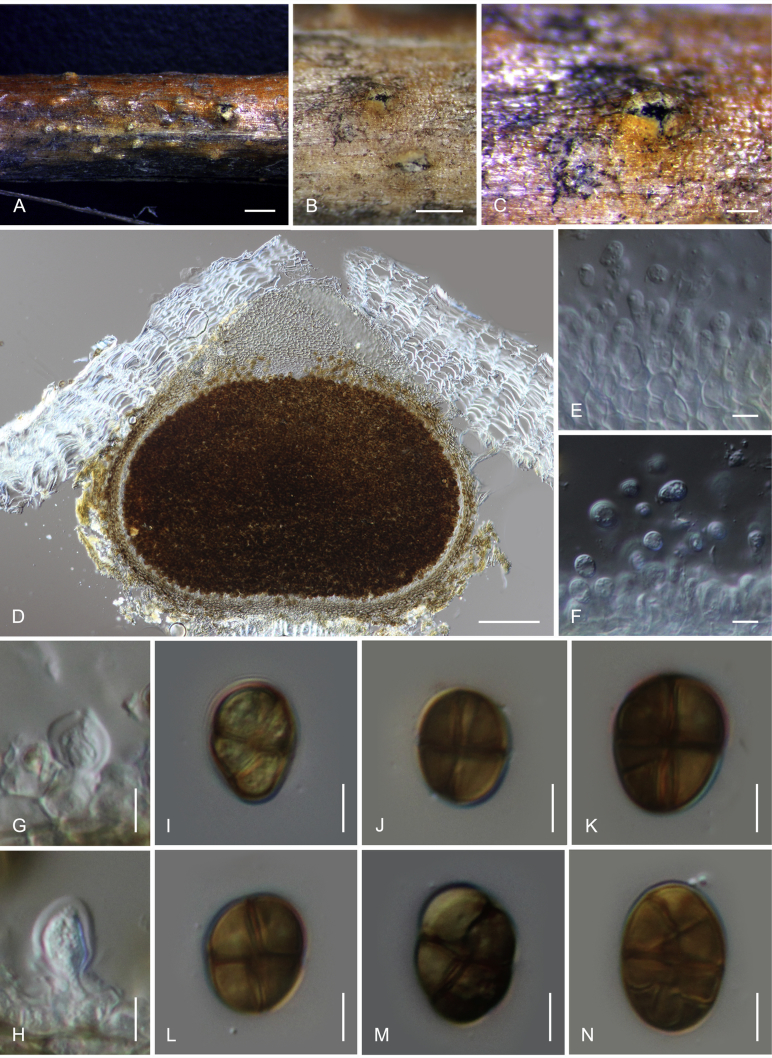
Camarosporidiella arezzoensis (Tibpromma et al.) Wanas. & K.D. Hyde, comb. nov. MycoBank MB821943; Facesoffungi number: FoF 03532. Fig. 6.
Fig. 6.
Asexual morph of Camarosporidiella arezzoensis (MFLU 17-0455). A. Conidiomata on host surface. B. Vertical section through conidioma. C. Microconidia. D–G. Conidiogenous cells and developing conidia. H–M. Macroconidia. Scale bars: A = 500 μm; B = 100 μm; C = 10 μm; D–M = 5 μm.
Basionym: Camarosporium arezzoensis Tibpromma et al., Saudi Journal of Biological Sciences 23: 2. 2016.
Saprobic or weakly necrotrophic on dead twigs and branches of Amorpha fruticosa. Asexual morph: Conidiomata pycnidial, 300–400 μm high, 300–350 μm diam ( = 347.9 × 324.5 μm, n = 10), solitary or gregarious, black, immersed, sometimes scattered beneath the host periderm or on decorticated wood, fully or partly erumpent, unilocular, with a papillate ostiolate. Ostiole 60–100 μm long, 100–150 μm diam ( = 82.2 × 115.4 μm, n = 6), central, long, smooth, ostiolar canal filled with hyaline or pale brown cells. Pycnidial wall multi-layered, 25–45 μm wide at the base, 25–35 μm wide in sides, thick, comprising 5–6 layers, outer layer heavily pigmented, thick-walled, comprising blackish or to dark reddish-brown cells of textura angularis, cells towards the inside lighter, inner layer composed of 1–2 layers, hyaline, thin-walled cells of textura angularis. Conidiophores reduced to conidiogenous cells. Macroconidiogenous cells enteroblastic, annellidic, doliiform, integrated, solitary, hyaline, smooth-walled, and formed from the inner layer of pycnidium wall. Macroconidia 20–28 × 6–9 μm ( = 24 × 7.8 μm; n = 40), cylindrical, straight to slightly curved, rounded at both ends, 4–7-transverse septate, with 1–2-longitudinal septa, muriform, smooth, brown to blackish-brown. Microconidiogenous cells intermingled with macro-conidiogenous cells, hyaline, discrete, enteroblastic with percurrent annellidic, ampulliform to subcylindrical. Microconidia 5–7.5 × 3.5–4.5 μm ( = 6.3 × 4 μm; n = 25), hyaline, round to oblong or ellipsoidal, with a few small guttules. Sexual morph: See Tibpromma et al. (2015).
Colonies on PDA: Slow growing, reaching 2 cm diam after 4 wk at 16 °C, later with dense mycelium, circular, rough margin, dirty white, reverse creamy grey, flat on the surface, without aerial mycelium. Hyphae septate, branched, hyaline, thin-walled.
Materials examined: Russia, Rostov Region, Rostov-on-Don city, Botanical garden of Southern Federal University, Lower Park, 47,2313935° N, 39,6648932° E, on Amorpha fruticosa (Fabaceae), 14 Apr. 2013, T.S. Bulgakov T-009 (MFLU 17-0455, living culture MFLUCC 14-0891); Azov district, Delta of Don river, sand dunes near Polushkin village, 47,1981111° N, 39,4148684° E, on Cytisus borysthenicus (Fabaceae), 8 May 2014, T.S. Bulgakov T-064, MFLU 17-0475, living culture MFLUCC 14-0913 = CBS 143103; Rostov-on-Don city, Botanical garden of Southern Federal University, Systematic Arboretum, 47,2360559° N, 39,6555591° E, on Cytisus austriacus (Fabaceae), 8 May 2014, T.S. Bulgakov T-072, MFLU 17-0478, living culture MFLUCC 14-0916 = CBS 143103; Rostov-on-Don city, Botanical garden of Southern Federal University, Systematic Arboretum, 47,2360559° N, 39,6555591° E, on Cytisus austriacus, 5 Mar. 2014, T.S. Bulgakov T-016, MFLU 17-0462, living culture MFLUCC 14-0899 = CBS 143102.
Notes: Camarosporidiella arezzoensis was reported as a sexual morph and is similar to Cucurbitaria species in having long cylindrical asci and narrowly fusiform, muriform ascospores, being 5–7-transversely septate, with 4–6 vertical septa (Tibpromma et al. 2015). An asexual morph was undetermined. In this study, we introduce the asexual morph of Ca. arezzoensis with four new collections from Russia on Amorpha fruticosa and Cytisus austriacus. Strains of Camarosporidiella arezzoensis cluster together with 60 % for ML, 67 % for MP and 0.96 for PP support (Clade A8, Fig. 1). Camarosporium amorphae (= Cucurbitaria amorphae) and Cm. amorphicola are also found on Amorpha fruticosa in Canada and Central Asia (Farr & Rossman 2017), but Cm. amorphae (20–24 × 9 μm, 4–5 transverse septa) has fewer transverse septa (Saccardo 1883) compared to the asexual morph of Camarosporidiella arezzoensis (20–28 × 6–9 μm, and 4–7 transverse septa). Records are lacking for comparison of Camarosporium amorphicola with our new taxon. Our collection differs from known other members in Camarosporidiella in having cylindrical conidia.
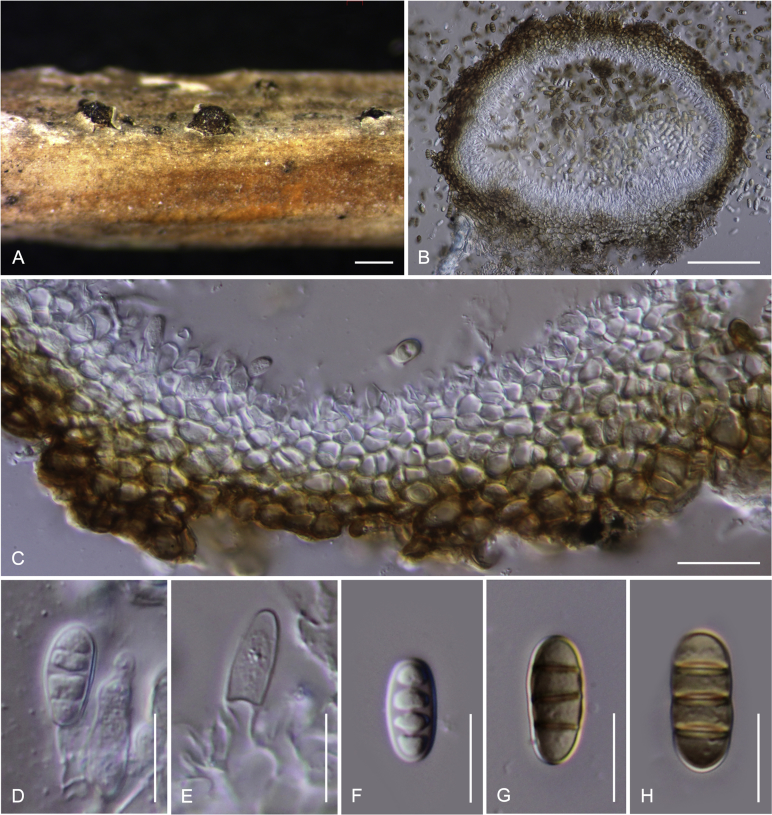
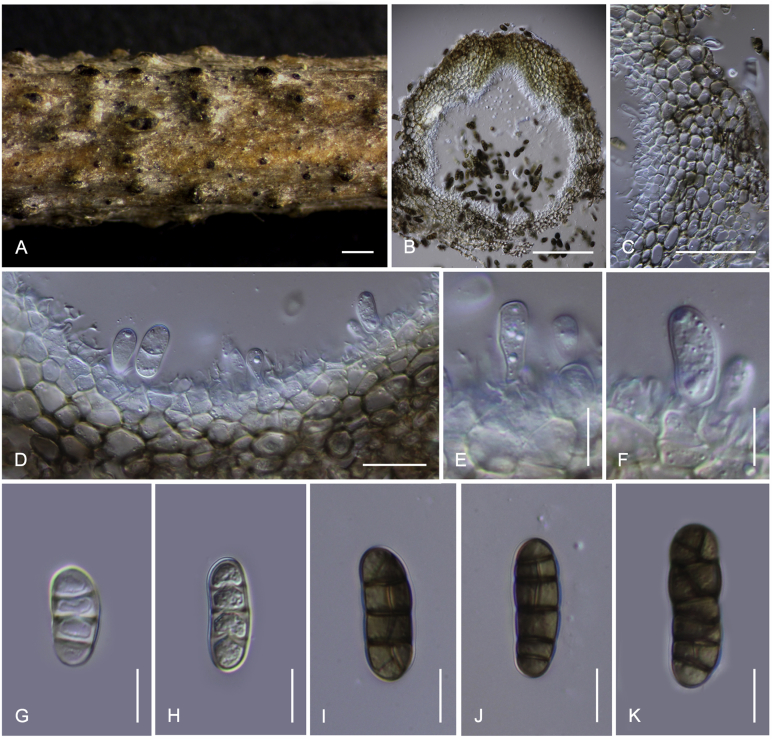
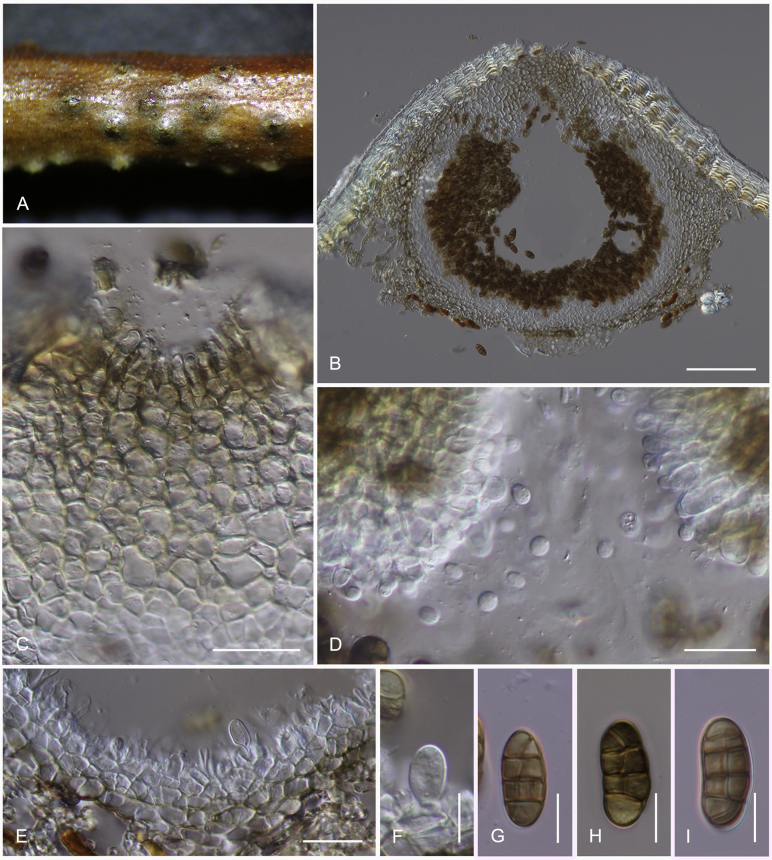
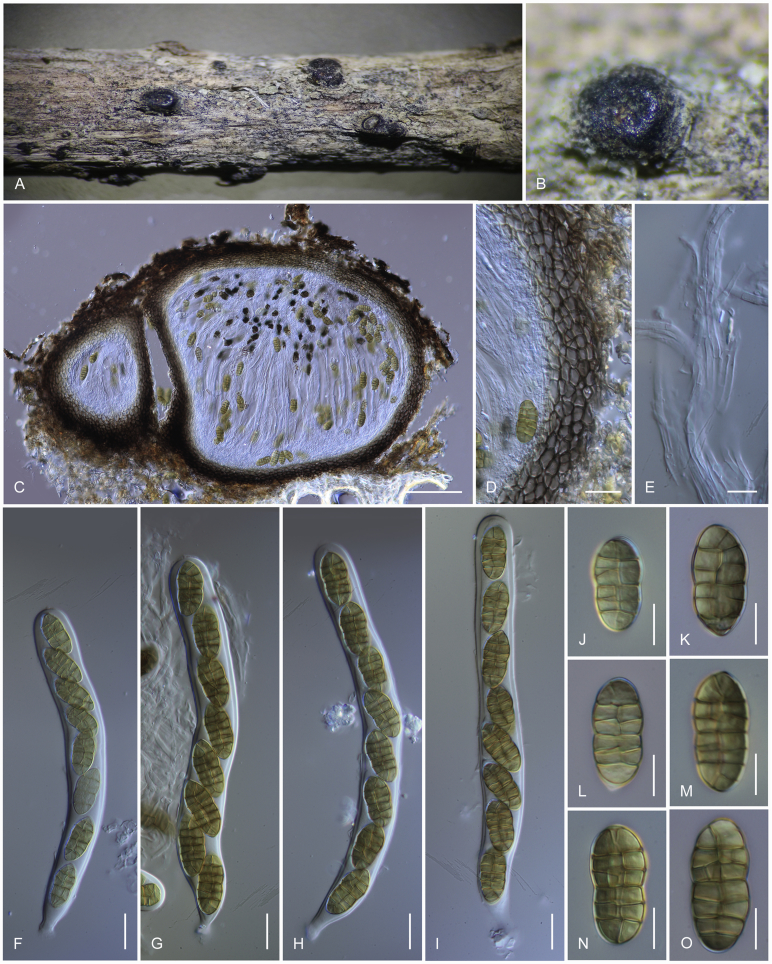
Camarosporidiella celtidis (Shear) Thambug., Wanas. & K.D. Hyde, comb. nov. MycoBank MB821945; Facesoffungi number: FoF 03533. Fig. 7, Fig. 8.
Fig. 7.
Sexual morph of Camarosporidiella celtidis (MFLU 17-469). A. Appearance of ascomata on host substrate. B. Section of ascoma. C. Pseudoparaphyses. D–E. Asci. F–I. Ascospores. Scale bars: B = 100 μm; C = 10 μm; D, E = 20 μm; F–I = 10 μm.
Fig. 8.
Asexual morph of Camarosporidiella celtidis (MFLU 17-0466). A. Conidiomata on host surface. B. Vertical section through conidioma. C. Conidiomata wall. D, E. Conidiogenous cells producing conidia. F–H. Conidia. Scale bars: A = 500 μm; B = 100 μm; C = 20 μm; D–H = 10 μm.
Basionym: Cucurbitaria celtidis Shear, Bull. Torrey bot. Club 29: 451. 1902.
Synonym: Camarosporium uniseriatum Thambug. et al., Stud. Fung. 1: 94. 2016.
Necrotrophic or saprobic on dead twigs and thin branches. Asexual morph: Conidiomata pycnidial, 300–350 μm high, 350–450 μm diam ( = 337.1 × 392.7 μm, n = 10), solitary or gregarious, black, immersed to semi-erumpent, unilocular. Pycnidial wall multi-layered, 20–25 μm wide at the base, 25–30 μm wide in sides, thick, comprising 3–4 layers, outer layer heavily pigmented, thick-walled, comprising blackish or to dark reddish-brown cells of textura angularis, cells towards the inside lighter, inner layer composed of 3–4 layers, hyaline, thick-walled cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells enteroblastic, annellidic, doliiform, integrated, solitary, hyaline, smooth-walled, and formed from the inner layer of pycnidium wall. Conidia 15–20 × 6–8 μm ( = 16.8 × 6.9 μm; n = 40), oblong, straight, rounded at both ends, sometimes narrowly rounded ends, 2–3-transversely septate, without longitudinal septa, smooth-walled, initially hyaline, becoming brown to dark brown at maturity. Sexual morph: Ascomata black, semi-immersed, becoming erumpent, scattered, solitary to gregarious, globose to subglobose, coriaceous, rough or hairy, ostiolate. Ostiole central, short, ostiolar canal filled with hyaline to lightly pigmented pseudoparenchymatous cells. Peridium comprising several layers, outer layers heavily pigmented, thick-walled, comprising blackish to dark brown cells of textura angularis, inner layers composed of hyaline, thin-walled cells of textura angularis. Hamathecium comprising 1–3 μm wide, numerous, filamentous, septate, pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical, short-pedicellate, apex rounded, with an ocular chamber. Ascospores uniseriate, slightly overlapping, initially hyaline, becoming dark brown at maturity, ellipsoid, oblong to fusoid, straight, muriform, with 3–5 transverse septa, and 1–2(–3) longitudinal septa, deeply constricted at the central septum, with rounded or acute ends, smooth-walled, without a mucilaginous sheath (Thambugala et al. 2016).
Materials examined: Russia, Rostov Region, Rostov-on-Don city, Botanical Garden of Southern Federal University, Higher Park, on twigs and branches of Celtis occidentalis (Cannabaceae), 5 Mar. 2014, T.S. Bulgakov T-037, MFLU 16-0469, reference specimen, living culture MFLUCC 15-0444 = ICMP 21250. ibid. on Gleditsia tracanthos (Fabaceae), 26 Mar. 2014, T.S. Bulgakov T-040, MFLU 17-0466, living culture MFLUCC 14-0904 = CBS 1431010; Shakhty city, Central Park, 47,7055886° N, 40,2059913° E, on Maclura pomifera (Moraceae), 12 Mar. 2013, T.S. Bulgakov T-002, MFLU 17-0450, living culture MFLUCC 14-0884 = CBS 143109; Rostov-on-Don city, Botanical Garden of Southern Federal University, 47, 2306722° N, 39, 6602583° E, on Spiraea sp. (Rosaceae), 15 Apr. 2015, T.S. Bulgakov T-193, MFLU 15-1897; Shakhty city, Central urban microdistrict, Central Park, 47, 7058052° N, 40,2065706° E, on Prunus padus, 9 Jul. 2015, T.S. Bulgakov T-224, MFLU 15-1928, living culture MFLUCC 17-0676 = CBS 143111; Shakhty city, Atyukhta River valley, Volchya Balka, 47,7122088° N, 40,1836753° E, on Morus alba, 5 Jul. 2015, T.S. Bulgakov T-239, MFLU 15-1943, living culture MFLUCC 17-0679; Shakhty city, Cotton Fabric urban microdistrict, Grushevka steppe slopes near Grushevsky pond, 47,7261179° N, 40,2587664° E, on Elymus repens, 12 May 2015, T.S. Bulgakov T-332, MFLU 15-2036; Shakhty city, Cotton Fabric urban microdistrict, Block park, 47,7122088° N, 40,1836753° E, on Betula pendula, 14 May 2015, T.S. Bulgakov T-358, MFLU 15-2062, living culture MFLUCC 16-0556; Shakhty city, Cotton Fabric urban microdistrict, Block park, 47,6922302° N, 40,0925446° E, on Ailanthus altissima, 21 Jul. 2015, T.S. Bulgakov T-767, MFLU 15-2912, living culture MFLUCC 17-0701 = CBS 143112.
Notes: Cucurbitaria celtidis was introduced by Shear (1902) from Celtis occidentalis. Thambugala et al. (2016) placed this species in the genus Camarosporium based on DNA sequence data from a fresh collection and introduced Cm. uniseriatum. However, in the present study, we accommodate Cucurbitaria celtidis in the new genus Camarosporidiella and the asexual morph of the species is described and illustrated (Fig. 7). Nine new isolates cluster in the Ca. celtidis clade (Subclade A5, Fig. 1), and they are differing from known other members in Camarosporidiella in having conidia without longitudinal septa. However, this subclade is only moderately supported ≤ 60 % ML & 77 % MP and ≤ 0.95 PP.
Camarosporidiella clematidis (Wijayaw. et al.) Wijayaw., Wanas. & K.D. Hyde, comb. nov. MycoBank MB821946; Facesoffungi number: FoF 03534.
Basionym: Camarosporium clematidis Wijayaw. et al., Phytotaxa 183: 19. 2014.
Illustrations: See Wijayawardene et al. (2014a).
Notes: Wijayawardene et al. (2014a) introduced this species from Clematis vitalba in Italy. The sexual morph has not been reported. In this study Camarosporidiella clematidis groups with Ca. laburnicola (Tibpromma et al. 2017), which was reported as the sexual morph. This subclade (Subclade A3, Fig. 1) is not supported and therefore the lifecycle link between these two taxa is ambiguous.
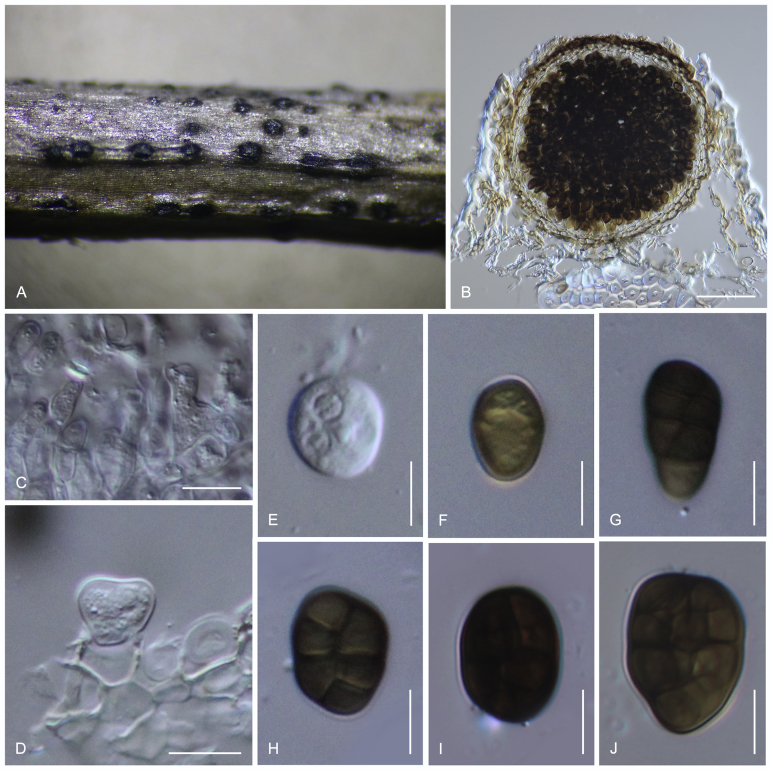
Camarosporidiella elaeagnicola Wanas., Bulgakov & K.D. Hyde sp. nov. MycoBank MB821947; Facesoffungi number: FoF 03535. Fig. 9.
Fig. 9.
Camarosporidiella elaeagnicola (MFLU 17-0470, holotype). A. Conidiomata on host surface. B. Vertical section through conidioma. C. Microconidia. D–F. Conidiogenous cells and developing conidia. G–J. Macroconidia. Scale bars: A = 500 μm; B = 100 μm; C–J = 10 μm.
Etymology: Named after the host genus from which it was collected, Elaeagnus.
Necrotrophic on dying branches of Elaeagnus angustifolia. Asexual morph: Conidiomata pycnidial, 300–500 μm high, 300–550 μm diam ( = 384.2 × 410.8 μm, n = 10), solitary or gregarious, black, immersed, uni- to multi-locular, with a papillate ostiole. Pycnidial wall multi-layered, 15–20 μm wide at the base, 30–40 μm wide in sides, comprising 5–8 layers, with heavily pigmented outer layer, thick-walled, comprising blackish to dark brown cells of textura angularis, with lighter cells towards the inside, with inner layer composed of 2–4 layers, hyaline, thin-walled cells of textura angularis. Macroconidiophores reduced to conidiogenous cells. Macroconidiogenous cells enteroblastic with percurrent annellations, doliiform, integrated, solitary, hyaline, smooth-walled, and formed from the inner layer of pycnidium wall. Macroconidia 18–25 × 9–13 μm ( = 19.5 × 10.8 μm; n = 30), oblong, straight to slightly curved, rounded at both ends, 2–3-transversely septate, with one longitudinal septum, muriform, smooth, pale to dark brown. Microconidiogenous cells intermingled with macroconidiogenous cells, hyaline, discrete, enteroblastic with percurrently annellidic, ampulliform to subcylindrical. Microconidia 5–6.5 × 3.5–4.5 μm ( = 5.9 × 4.1 μm; n = 25), hyaline, round to oblong or ellipsoidal, with a few small guttules. Sexual morph: Undetermined.
Colonies on PDA: Slow growing, reaching 2 cm diam after 4 wk at 16 °C, later with dense mycelium, circular, rough margin, white at the centre, greenish grey towards margin, reverse greenish-grey, flat on the surface, without aerial mycelium.
Materials examined: Russia, Rostov Region, Oktyabrsky District, Shakhty city, near Grushevsky pond, shelterbelt artificial forest, 47,7250642° N, 40,2564812° E, on Elaeagnus angustifolia (Elaeagnaceae), 18 May 2014, T.S. Bulgakov T-051 (MFLU 17-0470, holotype, ex-type culture MFLUCC 14-0908 = CBS 143113); Rostov-on-Don city, Botanical garden of Southern Federal University, Higher Park, 47,2360559° N, 39, 6555591° N, on Elaeagnus angustifolia, 26 Mar. 2014, T.S. Bulgakov T-055, MFLU 17-0473, living culture MFLUCC 14-0911 = CBS 143114; Azov disctrict, Delta of Don river, riverside bushes of channel near Obukhovka village, 47,60741° N, 39,4726807° E, on Elaeagnus angustifolia, 8 May 2014, T.S. Bulgakov T-061, MFLU 17-0474, living culture MFLUCC 14-0912 = CBS 143115; Rostov region, Shakhty city, 20th anniversary of Red Army microdistrict, Balka Solenaya, 47,7089819° N, 40,2637768° E, on Elaeagnus angustifolia, 1 May 2015, T.S. Bulgakov T-220, MFLU 15-1924; Krasnosulinsky district, Donskoye forestry, Kabanya Balka, 47,8672211° N, 40,247426° E, on Elaeagnus angustifolia, 18 Jun. 2015, T.S. Bulgakov T-511, MFLU 15-2215; Rostov-on-Don city, Botanical Garden of Southern Federal University, Systematic Arboretum, parkland (47,2350724° N, 39,6541643° E), on Elaeagnus angustifolia, 28 May 2015, T.S. Bulgakov T-813, MFLU 15-2956, living culture MFLUCC 17-0705; ibid. 30 May 2015 T-819, MFLU 15-2962, living culture MFLUCC 17-0707; ibid. 18 Feb. 2016 T-1186, MFLU 16-2382, living culture MFLUCC 17-0712; ibid. 14 Jun. 2016, T-NK067, MFLU 15-2962, living culture MFLUCC 17-0707.
Notes: In our phylogenetic analyses, 11 strains of Camarosporidiella elaeagnicola cluster together with 80 % ML and 84 % MP support (Subclade A6, Fig. 1). Ten of these isolations were collected on Elaeagnus angustifolia from Russia and one from Elaeagnus rhamnoides in Germany. Camarosporium elaeagnellum and Cm. elaeagni have also been found on Elaeagnus angustifolia from California, Canada and Ukraine (Farr & Rossman 2017). The relationship between these Camarosporium spp. with Camarosporidiella elaeagnicola cannot be investigated due to lack of morphological and molecular data for Camarosporium elaeagnellum and Cm. elaeagni. Thus, we introduce Camarosporidiella elaeagnicola as a new species.
Camarosporidiella elongata (Fr.) Wanas., Wijayaw. & K.D. Hyde, comb. nov. MycoBank MB821948; Facesoffungi number: FoF 03536.
Basionym: Sphaeria elongata Fr., Observationes mycologicae 1: 175. 1815.
Synonyms: Cucurbitaria elongata (Fr.) Grev., Scott. crypt. fl.: pl. 195. 1826.
Gibberidea elongata (Fr.) Kuntze, Revisio generum plantarum 3: 481. 1898.
Note: See Mirza (1968) for further details on Camarosporidiella elongata (= Cucurbitaria elongata).
Camarosporidiella eufemiana Wanas., Camporesi & K.D. Hyde, sp. nov. MycoBank MB821949; Facesoffungi number: FoF 03537. Fig. 10.
Fig. 10.
Camarosporidiella eufemiana (MFLU 16-0182, holotype) A. Appearance of ascomata on host substrate. B. Section of ascoma. C. Pseudoparaphyses. D–F. Asci. G–I. Ascospores. Scale bars: A = 500 μm; B = 100 μm; C = 5 μm; D–F = 20 μm; G–I = 10 μm.
Etymology: eufemiana, due to its occurrence in Santa Eufemia, Italy.
Saprobic on dead branches of Cytisus sp. Asexual morph: Undetermined. Sexual morph: Ascomata 350–400 μm high, 450–550 μm diam ( = 376.8 × 496.4 μm, n = 10), black, semi-erumpent to superficial, solitary or gregarious, globose, ostiolate. Ostiole short papillate central, slightly sunken, minute and inconspicuous at the surface, smooth, with ostiolar canal filled with hyaline to brown cells. Peridium 40–50 μm wide at the base, 40–70 μm wide in sides, thick, comprising 6–8 layers, with heavily pigmented outer layer, thick-walled, comprising blackish to dark brown elongated cells of textura angularis, cells towards the inside lighter, with inner layer composed 2–3 layers, hyaline, flattened, thin-walled cells of textura angularis. Hamathecium comprising numerous, 2.5–3.5 μm (n = 30) wide, filamentous, branched, septate, pseudoparaphyses. Asci 130–150 × 14–15 μm ( = 142.4 × 14.5 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical, short-pedicellate, rounded at apex with a minute ocular chamber. Ascospores 20–25 × 10–12 μm ( = 21.9 × 10.3 μm, n = 30), overlapping uniseriate or sometimes biseriate, muriform, mostly ellipsoidal, 3−5-transversely septate, with one longitudinal septum, deeply constricted at the middle septum, slightly constricted at remaining septa, initially hyaline, becoming brown at maturity, asymmetrical, upper part wider than lower part, conical and narrowly rounded at the ends, not surrounded by a mucilaginous sheath.
Colonies on PDA: Slow growing, reaching 3 cm diam after 4 wk at 16 °C, later with dense mycelium, circular, rough margin creamy to pale brown centre and dirty white towards the margin after 6 wk, reverse iron, flat on the surface, without aerial mycelium. Hyphae septate, branched, hyaline, thin-walled.
Material examined: Italy, Forlì-Cesena [FC], Premilcuore, Santa Eufemia, on dead aerial branches of Cytisus sp. (Fabaceae), 3 Jan. 2014, E. Camporesi IT1621 (MFLU 16-0182, holotype, ex-type culture MFLUCC 17-0207 = CBS 143116).
Notes: Camarosporidiella eufemiana morphologically resembles other sexual members in this genus in having similar asci and ascospore shapes. In the phylogenetic analyses, Ca. eufemiana groups as a sister taxon to Ca. elongata but with no statistical support (Subclade A4, Fig. 1). However, Ca. eufemiana is different from Ca. elongata in having longer asci (140–225 μm, Mirza 1968) with a long pedicel, while Ca. eufemiana has comparatively shorter asci (130–150 μm) with a short pedicel. Camarosporidiella laburni (= Cucurbitaria laburni) and Cucurbitaria spartii are also reported from Cytisus sp. (Mirza 1968). Camarosporidiella laburni (Subclade A3, Fig. 1) is phylogenetically distinct from Ca. eufemiana in this study. There is no molecular data available for Cucurbitaria spartii and its relationship to Camarosporidiella eufemiana cannot be resolved. However, Ca. eufemiana has shorter asci (130–150 μm) than Cucurbitaria spartii (150–240 μm, Mirza 1968).
Camarosporidiella halimodendri Wanas., Bulgakov & K.D. Hyde, sp. nov. MycoBank MB821950; Facesoffungi number: FoF 03538. Fig. 11.
Fig. 11.
Camarosporidiella halimodendri (MFLU 17-0463, holotype). A. Conidiomata on host surface. B. Vertical section through conidioma. C, D. Microconidia. E, F. Conidiogenous cells. G–L. Macroconidia. Scale bars: A = 500 μm; B = 100 μm; C = 50 μm; D–F = 10 μm; G = 20 μm; H–L = 10 μm.
Etymology: Named after the host genus from which it was collected, Halimodendron.
Saprobic or weakly pathogenic on dead branches of Halimodendron halodendron. Asexual morph: Conidiomata pycnidial, 500–600 μm high, 350–600 μm diam ( = 480.4 × 496.7 μm, n = 10), solitary or gregarious, black, immersed, sometimes scattered beneath the host periderm or on decorticated wood, fully or partly erumpent, unilocular, with a papillate ostiolate. Ostiole 100–200 μm long, 80–120 μm diam ( = 169.2 × 93.4 μm, n = 6), central, long, smooth, sometimes ostiolar canal filled with hyaline or pale brown cells. Pycnidial wall multi-layered, 25–35 μm wide at the base, 35–45 μm wide in sides, thick, comprising 5–6 layers, outer layer heavily pigmented, thick-walled, comprising blackish to dark reddish-brown cells of textura angularis, cells towards the inside lighter, inner layer composed of 1–2 layers, hyaline, thin-walled cells of textura angularis. Conidiophores reduced to conidiogenous cells. Macroconidiogenous cells enteroblastic, annellidic, doliiform, integrated, solitary, hyaline, smooth-walled, and formed from the inner layer of pycnidium wall. Macroconidia 18–25 × 8–12 μm ( = 21.5 × 10.7 μm; n = 40), oblong, straight to slightly curved, rounded at both ends, sometimes narrowly rounded ends, 4–6-transverse septate, with 1–2 longitudinal septa, with 2–4 oblique septa, muriform, smooth-walled, brown to dark brown. Microconidiogenous cells intermingled with macroconidiogenous cells, hyaline, discrete, enteroblastic with percurrent annellidic, ampulliform to subcylindrical. Microconidia 4.5–7.5 × 3.5–4.5 μm ( = 6.5 × 3.9 μm; n = 25), hyaline, round to oblong or ellipsoidal, with a few small guttules. Sexual morph: Undetermined.
Colonies on PDA: Slow growing, reaching 2 cm diam after 4 wk at 16 °C, later with dense mycelium, circular, rough margin, white, reverse cream-grey, flat on the surface, without aerial mycelium. Hyphae septate, branched, hyaline, thin-walled.
Materials examined: Russia, Rostov Region, Rostov-on-Don city, Botanical garden of Southern Federal University, Systematic Arboretum, 47,2360559° N, 39,6555591° E, on dying twigs and shrubs Halimodendron halodendron (Fabaceae), 8 May 2013, T.S. Bulgakov T-018 (MFLU 17-0463, holotype, ex-type culture, MFLUCC 14-0901 = CBS 143117); ibid. 26 Mar. 2014, T-041 (MFLU 17-0467, paratype, ex-paratype culture, MFLUCC 14-0905); Rostov Region, Shakhty city, near Grushevsky pond, stony steppe, 47,7237362° N, 39,2551937° E, on dead twigs of Caragana frutex (Fabaceae), 18 May 2014, T.S. Bulgakov T-050, MFLU 17-0469, living culture MFLUCC 14-0907 = CBS 143118; Rostov Region, Rostov-on-Don city, Botanical garden of Southern Federal University, Systematic Arboretum, 47,2360559° N, 39,6555591° E, on dead twigs of Cytisus podolicus (Fabaceae), 8 May 2014, T.S. Bulgakov T-066, MFLU 17-0476, living culture MFLUCC 14-0914; Rostov Region, Shakhty city, coal heap of former coal mine ‘Proletarian dictature’, 47,7104110° N, 40,2627254° E, on dead twigs of Lycium barbarum (Solanaceae), 21 May 2015, T.S. Bulgakov T-419, MFLU 15-2123, living culture MFLUCC 17-0212 = CBS 143119.
Notes: Camarosporium halimi (12–16 × 9–13 μm, 2–3 transverse septa) has also been found on Halimodendron halodendron from Iran (Farr & Rossman 2017), but it has smaller conidia with fewer transverse septa (Saccardo 1906) compared to Camarosporidiella halimodendri (8–25 × 8–12 μm, 4–6 transverse septa). In this study, we refer six strains to Ca. halimodendri (Subclade A11, Fig. 1), which group together with 81 % ML, 74 % MP, 0.98 PP statistical support and share similar morphologies.
Camarosporidiella italica Wanas., Camporesi & K.D. Hyde, sp. nov. MycoBank MB821951; Facesoffungi number: FoF 03539. Fig. 12.
Fig. 12.
Camarosporidiella italica (MFLU 16-0139, holotype). A. Appearance of ascomata on host substrate. B. Section of ascoma. C. Closeup of ostiole. D. Pseudoparaphyses. E. Peridium. F–H. Asci. I–N. Ascospores. Scale bars: B = 100 μm; C, E, F–H = 20 μm; D, I–N = 10 μm.
Etymology: italica, due to its occurrence in Italy.
Saprobic on dead branches of Coronilla emerus. Asexual morph: Undetermined. Sexual morph: Ascomata 400–450 μm high, 550–600 μm diam ( = 436.2 × 457.8 μm, n = 10), black, immersed to semi-erumpent, solitary or gregarious, globose, with an ostiole comprising greenish grey setae. Ostiole 60–90 μm long, 30–45 μm diam ( = 76.2 × 36.4 μm, n = 6) central, short, slightly sunken, minute and inconspicuous on the surface, smooth, ostiolar canal filled with hyaline to brown cells. Peridium 20–30 μm wide at the base, 40–50 μm wide in sides, thick, comprising 5–8 layers, outer layer heavily pigmented, thick-walled, comprising blackish to dark brown elongated cells of textura angularis, cells towards the inside lighter, inner layer composed of 2–3 layers, hyaline, flattened, thin-walled cells of textura angularis. Hamathecium comprising numerous, 3.5–4.5 μm (n = 40) wide, filamentous, branched, septate, pseudoparaphyses. Asci 150–180 × 15–20 μm ( = 164.7 × 18.4 μm, n = 30), 8-spored, bitunicate, fissitunicate, cylindrical, short-pedicellate, apex rounded with a minute ocular chamber. Ascospores 30–35 × 12–14 μm ( = 32.9 × 12.8 μm, n = 50), overlapping uniseriate or sometimes overlapping biseriate, muriform, mostly ellipsoidal, 6–8-transversely septate, with 2–3 longitudinal septa, deeply constricted at the middle septum, slightly constricted at remaining septa, initially hyaline, becoming brown at maturity, asymmetrical, upper part wider than lower part, slightly paler ends, conical and narrowly rounded at the ends, not surrounded by a mucilaginous sheath.
Colonies on PDA: Slow growing, reaching 3 cm diam after 4 wk at 16 °C, later with dense mycelium, circular, rough margin greenish grey after 6 wk, reverse greenish grey, flat on the surface, without aerial mycelium. Hyphae septate, branched, hyaline, thin.
Material examined: Italy, Forlì-Cesena [FC], Bagno di Romagna, Valgianna, on dead aerial twigs of Coronilla emerus (Fabaceae), 19 May 2013, E. Camporesi IT1283 (MFLU 16-0139, holotype, ex-type culture MFLUCC 13-0547).
Notes: Cucurbitaria coronillae, Cu. elongata and Cu. emeri are also recorded on Coronilla emerus (Munk, 1957, Mirza, 1968). These three species morphologically resemble Camarosporidiella italica with respect to their ascomata, peridium, asci and ascospore characters. Cucurbitaria emerus is different from Ca. italica in having diplodia-like uniseptate conidia. Cucurbitaria coronillae differs from Ca. italica in having much longer ascospores (> 30 μm) with 2–3 longitudinal septa while Cu. coronillae has comparatively shorter ascospores (< 27 μm) with one longitudinal septum. Cucurbitaria elongata differs from Ca. italica in having a prominently thicker perdium (100–180 μm) while Ca. italica has a peridium up to 50 μm wide.
Camarosporidiella laburni (Pers.) Wanas., Bulgakov, Camporesi & K.D. Hyde comb. nov. MycoBank MB821952; Facesoffungi number: FoF 03540. Fig. 13, Fig. 14.
Fig. 13.
Sexual morph of Camarosporidiella laburni (MFLU 16-0094). A. Appearance of ascomata on host substrate. B. Section of ascoma. C. Peridium. D. Pseudoparaphyses. E–G. Asci. H–M. Ascospores. Scale bars: B = 100 μm; C = 50 μm; D, H–M = 10 μm; E–G = 20 μm.
Fig. 14.
Asexual morph of Camarosporidiella laburni (MFLU 17-0451). A. Conidiomata on host surface. B. Vertical section through conidioma. C, D. Conidiomatal wall. E, F. Conidiogenous cells. G–K. Conidia. Scale bars: A = 500 μm; B = 100 μm; C = 50 μm; D = 20 μm; E–K = 10 μm.
Basionym: Sphaeria laburni Pers., Observ. mycol. 1: 68. 1796.
Synonyms: Cucurbitaria laburni (Pers.) De Not., Erb. critt. Ital., Ser. 1, fasc. 16: 875. 1862.
Gibberidea laburni (Pers.) Kuntze, Revisio generum plantarum 3: 481. 1898.
Camarosporium laburni Sacc. & Roum., Michelia 2: 630. 1882.
Saprobic on woody branches. Asexual morph: Conidiomata pycnidial, 300–350 μm high, 300–400 μm diam ( = 338.2 × 326.1 μm, n = 10), solitary to gregarious, black, immersed, unilocular, with an apapillate ostiole. Pycnidial wall multi-layered, 20–30 μm wide at the base, 40–50 μm wide in sides, thick, comprising 4–7 layers, outer layer heavily pigmented, thick-walled, comprising blackish to dark brown cells of textura angularis, cells towards the inside lighter, inner layer composed of 2–3 layers, hyaline, thin-walled cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells enteroblastic, annellidic, doliiform, integrated, solitary, hyaline, smooth-walled, and formed from the inner layer of pycnidium wall. Conidia 20–30 × 8–11 μm ( = 26.1 × 9.7 μm; n = 30), oblong, straight to slightly curved, rounded at both ends, 4–5-transversely septate, with 1–2 longitudinal septa, muriform, smooth-walled, initially hyaline, becoming blackish brown at maturity. Sexual morph: Ascomata 400–550 μm high, 500–600 μm diam ( = 462.4 × 559.9 μm, n = 10), black, superficial to semi-immersed, confluent, gregarious, sometimes scattered beneath the host periderm or on decorticated wood, fully or partly erumpent, globose, uniloculate, with an ostiole. Ostiole central, short, slightly sunken, minute and inconspicuous at the surface, smooth, ostiolar canal filled with hyaline cells. Peridium 40–60 μm wide at the base, 90–120 μm wide in sides, thick, comprising 10–12 layers, outermost layer heavily pigmented, thin-walled, comprising blackish to dark brown amorphous layer, middle layer heavily pigmented, thick-walled, comprising blackish to dark brown loosely packed cells of textura angularis, inner layer composed of 3–4 layers, reddish brown to hyaline, cells towards the inside lighter, flattened, thick-walled cells of textura angularis. Hamathecium comprising numerous, 2.5–3 μm (n = 40) wide, filamentous, branched septate, pseudoparaphyses. Asci 160–190 × 12–16 μm ( = 176.3 × 14.8 μm, n = 40), 8-spored, bitunicate, fissitunicate, cylindrical, short-pedicellate, apex rounded with a minute ocular chamber. Ascospores 27–32 × 10–12 μm ( = 30.4 × 11.1 μm, n = 50), overlapping uniseriate, muriform, mostly ellipsoidal, 6–7-transversely septate, with 1–2 longitudinal septa, deeply constricted at the middle septum, slightly constricted at remaining septa, initially hyaline, becoming pale brown at maturity, asymmetrical, slightly paler ends, conical and narrowly rounded at the ends, not surrounded by a mucilaginous sheath.
Colonies on PDA: Slow growing, reaching 2 cm diam after 4 wk at 16 °C, later with dense mycelium, circular, rough margin, greenish grey after 6 wk, reverse greenish grey, flat on the surface, without aerial mycelium.
Materials examined: Italy, Forlì-Cesena, Fiumicello di Premilcuore, on dead aerial branches of Laburnum anagyroides, 1 Jan. 2012, E. Camporesi, IT83, MFLU 16-0094, living culture MFLUCC 14−0919 = CBS 143121. Russia, Rostov Region, Rostov-on-Don city, Botanical garden of Southern Federal University, Systematic Arboretum, 47,2360559° N, 39,6555591° E, on dead twigs of Laburnum anagyroides (Fabaceae), 5 Mar. 2014, T.S. Bulgakov T-003, MFLU 17-0451, living culture MFLUCC 14-0885; Rostov-on-Don city, Botanical Garden of Southern Federal University, Systematic Arboretum, 47,2350733° N, 39,6541689° E, on Laburnum anagyroides, 30 May 2015, T.S. Bulgakov T-811, MFLU 15-2954, living culture MFLUCC 17-0704 = CBS 143122; on Laburnum sp., 28 May 2015, T.S. Bulgakov T-838, MFLU 15-2981, living culture MFLUCC 17-0709; Republic of Crimea, Feodosia city Municipality, Tepe-Oba ridge, artificial forest, 44,0108725° N, 35,3541327° E, on dead branches of Laburnum anagyroides, 23 Jun. 2016, T.S. Bulgakov CR029 (MFLU 17-1434, living culture MFLUCC 17-0751 = CBS 143120) ibid. CR032 (MFLU 17-1435, living culture MFLUCC 17-0752)
Notes: Camarosporidiella laburni (= Cucurbitaria laburni) was introduced by De Notaris (1862) for a collection from Italy. Our collection is also from Italy, which fits with the original description of Cm. laburni (De Notaris 1862) and the description of Mirza (1968). A comprehensive comparison of Cm. laburni with other species is given by Green (1931) and Mirza (1968), which is clearly different from the remaining described species in Cucurbitaria. By considering the continent, host and morphological evidence, we introduce DNA-based molecular data for Cm. laburni as Ca. laburni. Furthermore, in this study the strains (MFLUCC 14-0885, 17-0704, 17-0752, 17-0709, 17-0751, from conidia) cluster together with C. laburni (Subclade A3, Fig. 1) and we consider them belong to this species as the asexual morph. Camarosporidiella clematidis clusters in a subclade sister to Ca. laburni, but morphologically differs from Ca. laburni in having smaller conidia (10–17 × 7–9 μm) while Ca. laburni has comparatively larger conidia (20–30 × 8–11 μm).
Camarosporidiella laburnicola (R.H. Perera et al.) Wanas. & K.D. Hyde, comb. nov. MycoBank MB821953; Facesoffungi number: FoF 03541.
Basionym: Camarosporium laburnicola R.H. Perera et al., Fungal Diversity 83: 97. 2017.
Illustrations: See Tibpromma et al. (2017).
Notes: Camarosporidiella laburnicola (Subclade A3, Fig. 1) was isolated from Laburnum anagyroides and is morphologically similar to Ca. arezzoensis, Ca. elongatum and Ca. uniseriatum. However, Ca. laburnicola differs from these taxa in having smaller asci and ascospores with different numbers of longitudinal and transverse septa (Tibpromma et al. 2017). Camarosporidiella laburnicola is nested in between Ca. laburni and Ca. clematidis, but this relationship is not supported (≥ 60 ML & MP and ≥ 0.95 PP, Clade A3, Fig. 1). Camarosporidiella laburnicola is morphologically similar to Ca. laburni in ascomata, asci and ascospore characters. However, Camarosporidiella laburni differs from Ca. laburnicola in having much larger asci (160–190 × 12–16 μm) and ascospores (27–32 × 10–12 μm) while Ca. laburnicola has comparatively smaller asci (125–150 × 9–11 μm) and ascospores (15–21 × 6–8 μm).
Camarosporidiella mackenziei Wanas., Bulgakov & K.D. Hyde sp. nov. MycoBank MB821954; Facesoffungi number: FoF 03542. Fig. 15.
Fig. 15.
Camarosporidiella mackenziei (MFLU 17-0449, holotype). A. Conidiomata on host surface. B. Vertical section through conidioma. C. Conidiomatal wall. D. Microconidia. E–G. Conidiogenous cells forming conidia. H–L. Macro conidia. Scale bars: B = 100 μm; C, D = 50 μm; E–L = 10 μm.
Etymology: In honour of Dr. Eric Hugh Charles Mckenzie for his immense contribution to mycology.
Necrotrophic on dying branches of Caragana arborescens. Asexual morph: Conidiomata pycnidial, 450–550 μm high, 500–600 μm diam ( = 408.4 × 569.5 μm, n = 10), solitary or gregarious, black, immersed to semi-erumpent, unilocular, with a papillate ostiolate. Ostiole 120–160 μm long, 80–90 μm diam ( = 140.1 × 66.7 μm, n = 10), central, long, smooth, sometimes ostiolar canal filled with hyaline or pale brown cells. Pycnidial wall multi-layered, 40–50 μm wide at the base, 40–55 μm wide in sides, thick, comprising 4–6 layers, outer layer heavily pigmented, thick-walled, comprising blackish to dark reddish-brown cells of textura angularis, cells towards the inside lighter, inner layer composed of 1–2 layers, hyaline, thin-walled cells of textura angularis. Conidiophores reduced to conidiogenous cells. Macroconidiogenous cells enteroblastic, annellidic, doliiform, integrated, solitary, hyaline, smooth-walled, and formed from the inner layer of pycnidium wall. Macroconidia 17–25 × 9–13 μm ( = 19.6 × 11.1 μm; n = 50), oblong, straight to slightly curved, rounded at both ends, sometimes narrowly rounded ends, 3–4-transversely septate, with 1–2 longitudinal septa, muriform, smooth-walled, brown to dark brown. Microconidiogenous cells intermingled with macroconidiogenous cells, hyaline, integrated, enteroblastic with percurrent annellidic, ampulliform to subcylindrical. Microconidia 6.5–8 × 4–6 μm ( = 7.6 × 4.5 μm; n = 20), hyaline, round to oblong or ellipsoidal, with small guttules. Sexual morph: Undetermined.
Colonies on PDA: Slow growing, reaching 2 cm diam after 4 wk at 16 °C, later with dense mycelium, circular, rough margin, greenish grey, reverse greenish grey, flat on the surface, without aerial mycelium. Hyphae septate, branched, hyaline, thin-walled.
Materials examined: Russia, Rostov Region, Oktyabrsky district, natural sanctuary (Persianovskaya preserved steppe), shelterbelt artificial forest, 47,5036056° N, 40,1545572° E, on dying twigs and shrubs of Caragana arborescens (Fabaceae), 26 Apr. 2014, T.S. Bulgakov T-001 (MFLU 17-0449, holotype, ex-type culture, MFLUCC 14-0883 = CBS 143123); ibid. T-011 (MFLU 17-0457, paratype, ex-paratype culture, MFLUCC 14-0893 = CBS 143124). ibid. 47,2350724° N, 39,6541643° E, 28 May 2015, T-810, MFLU 15-2953, paratype, ex-paratype living culture MFLUCC 17-0703.
Notes: Camarosporidiella caraganicola has also been collected from Caragana spp. and resembles Ca. mackenziei in conidial dimensions (13–26 × 6–13 μm; Liu et al. 2015) and shape. However, they are phylogenetically apart (Subclades A7 and A12 respectively, Fig. 1).
The affiliation of Ca. mackenziei with Camarosporidiella sp. (CPC 25960, CPC 25962) cannot be compared as no details are available for these isolations (Subclade A12, Fig. 1). All the three strains of C. mackenziei in Subclade A12 (Fig. 1) are from the same locality and host. There are slight differences in their DNA sequence data and there could be a probability that they constitute a species complex. Perhaps more collections from different regions/hosts can further clarify their taxonomy in future studies. We reiterate, however, that morphologically they are similar and hence we consider them as one species.
Camarosporidiella melnikii Wanas., Bulgakov & K.D. Hyde, sp. nov. MycoBank MB821955; Facesoffungi number: FoF 03543. Fig. 16.
Fig. 16.
Camarosporidiella melnikii (MFLU 17-2022, holotype). A, B. Conidiomata on host surface. C. Vertical section through conidiomata. D, E. Conidiogenous cells forming conidia. F. Macro- and micro-conidia. G–L. Macroconidia. Scale bars: A = 500 μm; B = 200 μm; C = 100 μm; D–E = 5 μm; F = 10 μm; G–L = 5 μm.
Etymology: In honour of Vadim Alexandrovich Mel'nik (March 16, 1937 – April 10, 2017) for his immense contribution to mycology.
Necrotrophic on dying branches of Caragana frutex. Asexual morph: Conidiomata pycnidial, 350–550 μm high, 300–500 μm diam ( = 457.7 × 393.1 μm, n = 10), black, superficial to semi-immersed, confluent, gregarious, sometimes scattered beneath the host periderm or on decorticated wood, fully or partly erumpent, globose, ostiolate. Ostiole central, short, slightly sunken, minute, inconspicuous on surface, smooth, with ostiolar canal filled with hyaline cells. Ostiole 120–180 μm long, 70–90 μm diam ( = 150.1 × 82.7 μm, n = 10), central, long, smooth, sometimes ostiolar canal filled with hyaline or pale brown cells. Pycnidial wall multi-layered, 40–50 μm wide at the base, 40–75 μm wide in sides, thick, comprising 4–8 layers, outer layer heavily pigmented, thick-walled, comprising blackish to dark reddish-brown cells of textura angularis, cells towards the inside lighter, inner layer composed of 2–4 layers, hyaline, thin-walled cells of textura angularis. Conidiophores reduced to conidiogenous cells. Macroconidiogenous cells enteroblastic, annellidic, doliiform, integrated, solitary, hyaline, smooth-walled, and formed from the inner layer of pycnidium wall. Macroconidia 11–16 × 5–6 μm ( = 13.3 × 5.5 μm; n = 50), oblong, straight, rounded at both ends, sometimes narrowly rounded ends, 2–3-transversely septate, without longitudinal septa, smooth-walled, initially hyaline, becoming brown to dark brown at maturity. Microconidiogenous cells intermingled with macroconidiogenous cells, hyaline, integrated, enteroblastic with percurrent annellidic, ampulliform to subcylindrical. Microconidia 7–12 × 4–7 μm ( = 9.6 × μm; n = 20), hyaline, round to oblong or ellipsoidal, with small guttules. Sexual morph: Undetermined.
Colonies on PDA: Slow growing, reaching 2 cm diam after 4 wk at 16 °C, later with dense mycelium, circular, rough margin, creamy, reverse pale brown, flat on the surface, without aerial mycelium. Hyphae septate, branched, hyaline, thin-walled.
Material examined: Russia, Rostov region, Shakhty city, Cotton Fabric urban microdistrict, Grushevka steppe slopes near Grushevsky Pond, 47, 7234186° N, 40, 255065° E, on dead aerial branches of Caragana frutex (Fabaceae), 12 May 2015, T.S. Bulgakov T-318, MFLU 15-2022, holotype, ex-type living culture MFLUCC 17-0684).
Notes: Camarosporidiella melnikii is an independent taxon (sister to Ca. caraganicola isolates) and segregates from others with high statistical support (Subclade A7, Fig. 1). Ca. melnikii has smaller conidia (11–16 × 5–6 μm) without longitudinal septa, while Camarosporidiella caraganicola has comparatively larger conidia (13–26 × 6–13 μm; Liu et al. 2015) with longitudinal septa. Camarosporidiella melnikii also resembles Ca. celtidis in having 2–3-transversely septate conidia, without longitudinal septa, but phylogeny herein supports their distinction (Subclades A7 and A5 respectively, Fig. 1).
Camarosporidiella mirabellensis Wanas., Camporesi & K.D. Hyde, sp. nov. MycoBank MB821956; Facesoffungi number: FoF 03544. Fig. 17.
Fig. 17.
Camarosporidiella mirabellensis (MFLU 16-0228, holotype). A. Appearance of ascomata on host substrate. B. Section of ascoma. C. Peridium. D. Pseudoparaphyses. E–G. Asci. H–L. Ascospores. Scale bars: B = 100 μm; C = 50 μm; D = 5 μm; E–G = 20 μm; H–L = 10 μm.
Etymology: mirabellensis, due to its occurrence in Monte Mirabello, Italy.
Saprobic on woody branches. Asexual morph: Undetermined. Sexual morph: Ascomata 300–350 μm high, 500–550 μm diam ( = 323.9 × 523.3 μm, n = 10), black, immersed to semi-erumpent, solitary or gregarious, broadly oblong, cupulate when dry. Peridium 50–80 μm wide at the base, 60–90 μm wide in sides, thick, comprising 8–10 layers, outer layer heavily pigmented, thick-walled, comprising blackish to dark brown elongated cells of textura angularis, cells towards the inside lighter, inner layer composed of 2–3 layers, hyaline, flattened, thin-walled cells of textura angularis. Hamathecium comprising numerous, 1.5–2 μm (n = 30) wide, filamentous, branched, septate, pseudoparaphyses. Asci 140–170 × 12–16 μm ( = 158.8 × 13.7 μm, n = 30), 8-spored, bitunicate, fissitunicate, cylindrical, short-pedicellate, apex rounded with a minute ocular chamber. Ascospores 22–27 × 9–11 μm ( = 24.8 × 9.9 μm, n = 40), overlapping uniseriate or sometimes overlapping biseriate, muriform, mostly ellipsoidal, 3–5-transversely septate, with 1–2 longitudinal septa, deeply constricted at the middle septum, slightly constricted at remaining septa, initially hyaline, becoming brown at maturity, asymmetrical, slightly paler ends, conical and narrowed at the ends, not surrounded by a mucilaginous sheath.
Colonies on PDA: Slow growing, reaching 3 cm diam after 4 wk at 16 °C, later with dense mycelium, circular, rough margin dirty white after 6 wk, reverse creamy, flat on the surface, without aerial mycelium.
Material examined: Italy, Forlì-Cesena [FC], Predappio, Monte Mirabello, on dead aerial branches of Robinia pseudoacacia (Fabaceae), 3 Oct. 2014, E. Camporesi IT 2139 (MFLU 16-0228, holotype).
Notes: Unfortunately, we could not manage to maintain a living culture as subsequent attempts to subculture failed, and hence a living culture is unavailable. Camarosporidiella elongata and Ca. spartii have also been reported on Robinia pseudoacacia and morphologically resemble our new collection of Ca. mirabellensis in their ascomata, peridium, asci and ascospore characteristics (Mirza 1968). However, Ca. mirabellensis differs from Ca. elongata and Ca. spartii in having ascospores with fewer transverse septa (< 5). In multi-gene phylogenetic analyses, Ca. mirabellensis, Ca. eufemiana and Ca. premilcurensis are more closely related (Subclade A4, Fig. 1). We must point out that despite not having sufficient phylogenetic differences, we consider them morphologically different in terms of spore shape, structure and septation. While Ca. mirabellensis has pointed ends and more than one longitudinal septum, Ca. eufemiana has much more rounded ends with one longitudinal septum. Camarosporidiella premilcurensis, on the other hand, has larger asci, and more transverse septa. Therefore, we introduce Ca. mirabellensis as a novel species in order to minimize taxonomic ambiguity of Camarosporidiella.
Camarosporidiella moricola (Chethana et al.) Wanas. & K.D. Hyde, comb. nov. MycoBank MB821957; Facesoffungi number: FoF 03545.
Basionym: Camarosporium moricola Chethana et al., Fungal Diversity 83: 101. 2017.
Illustrations: See Tibpromma et al. (2017).
Materials examined: Russia, Rostov Region, Shakhty city, railroad artificial forest near Kazyonny pond, 47,7532324° N, 40,208931° E, on Morus alba (Moraceae), 26 Feb. 2014, T.S. Bulgakov T-004, MFLU 17-0452, living culture MFLUCC 14-0886; ibid. Rostov-on-Don city, Botanical garden of Southern Federal University, Higher Park, underwood, 47,2336592° N, 39,6593893° E, 26 Mar. 2014, T.S. Bulgakov T-015, MFLU 17-0461, living culture MFLUCC 14−0898; ibid. Shakhty city, Atyukhta River valley, Volchya Balka, 47,7122088° N, 40,1836753° E, 5 Jul. 2015, T.S. Bulgakov T-232, MFLU 15-1936; ibid. Shakhty city, Cotton Fabric urban microdistrict, Grushevka steppe slopes near Grushevsky Pond, 47,7234186° N, 40,255065° E, 30 Apr. 2015, T.S. Bulgakov T-265, MFLU 15-1969, living culture MFLUCC 17-0680; ibid. 14 May 2015, T.S. Bulgakov T-371, MFLU 15-2075, living culture MFLUCC 17-0687; ibid. Krasnosulinsky district, Donskoye forestry, Kabanya Balka, 47,8672211° N, 40,247426° E, 18 Jun. 2015, T.S. Bulgakov T-518, MFLU 15-2222, living culture MFLUCC 17-0694; ibid. Krasnosulinsky district, Donskoye forestry, artificial forest plantation, 47,8547249° N, 40,2318907° E, 18 Jun. 2015, T.S. Bulgakov T-856, MFLU 15-2999, living culture MFLUCC 17-0711; ibid., 1 Mar. 2016, T.S. Bulgakov T-1233, MFLU 16-1527, living culture MFLUCC 17-0714 = CBS 143125; ibid. 1 Mar. 2016, T.S. Bulgakov T-1332, MFLU 16-1626, living culture MFLUCC 17-0718 = CBS 143126; ibid. 24 Mar. 2016, T.S. Bulgakov T-1345, MFLU 16-1639, living culture MFLUCC 17-0719; ibid. 14 Mar. 2016, T.S. Bulgakov T-1476, MFLU 16-1770, living culture MFLUCC 17-0725.
Notes: Tibpromma et al. (2017) introduced Camarosporidiella moricola (= Camarosporium moricola) with three isolates, which were collected from Morus alba in Russia. In this study, we add another 12 strains to Camarosporidiella moricola (Subclade A1, Fig. 1) which were also collected from Morus alba in Russia. See more details in Tibpromma et al. (2017).
Camarosporidiella premilcurensis Wanas., Camporesi & K.D. Hyde, sp. nov. MycoBank MB821958; Facesoffungi number: FoF 03546. Fig. 18.
Fig. 18.
Camarosporidiella premilcurensis (MFLU 16-0185, holotype). A. Appearance of ascomata on host substrate. B. Section of ascoma. C. Close-up of ostiole. D. Peridium. E. Pseudoparaphyses. F–H. Asci. I–N. Ascospores. Scale bars: B = 100 μm; C, D = 50 μm; E = 5 μm; F–H = 20 μm; I–N = 10 μm.
Etymology: premilcurensis, due to its occurrence in Premilcuore, Italy.
Saprobic on Cytisus sp. Asexual morph: Undetermined. Sexual morph: Ascomata 400–450 μm high, 500–600 μm diam ( = 442.1 × 531.7 μm, n = 10), black, superficial to semi-immersed, confluent, gregarious, sometimes scattered beneath the host periderm or on decorticated wood, fully or partly erumpent, globose, uniloculate, with an apapillate ostiole. Ostiole central, short, slightly sunken, minute and inconspicuous at the surface, smooth, ostiolar canal filled with hyaline cells. Peridium 30–50 μm wide at the base, 60–90 μm wide in sides, thick, comprising 8–15 layers, outermost layer heavily pigmented, thin-walled, comprising blackish to dark brown amorphous layer, middle layer heavily pigmented, thick-walled, comprising blackish to dark brown loosely packed cells of textura angularis, inner layer composed of 3–4 layers, reddish brown to hyaline, cells towards the inside lighter, flattened, thick-walled cells of textura angularis. Hamathecium comprising numerous, 2.5–3.5 μm (n = 40) wide, filamentous, branched septate, pseudoparaphyses. Asci 160–210 × 14–16 μm ( = 181.1 × 15 μm, n = 40), 8-spored, bitunicate, fissitunicate, cylindrical, short-pedicellate, apex rounded with a minute ocular chamber. Ascospores 22–27 × 10–12 μm ( = 24.7 × 10.9 μm, n = 50), overlapping uniseriate, muriform, mostly ellipsoidal, 5–7-transversely septate, with 1–2 longitudinal septa, deeply constricted at the middle septum, slightly constricted at remaining septa, initially hyaline, becoming pale brown at maturity, asymmetrical, slightly paler, conical and narrowly rounded at the ends, not surrounded by a mucilaginous sheath.
Colonies on PDA: Slow growing, reaching 2 cm diam after 4 wk at 16 °C, later with dense mycelium, circular, rough margin, creamy after 6 wk, reverse greenish grey, flat on the surface, without aerial mycelium. Hyphae septate, branched, hyaline, thin-walled.
Material examined: Italy, Forlì-Cesena [FC], Premilcuore, Fantella, on dead aerial twigs of Cytisus sp. (Fabaceae), 28 Jan. 2014, E. Camporesi, IT1681 (MFLU 16-0185, holotype, ex-type culture MFLUCC 14−0939 = CBS 143127).
Notes: Camarosporidiella laburni has also been collected from the same host genus, Cytisus (Farr & Rossman 2017) and morphologically resembles Ca. premilcurensis in having similar ascomata, peridium, asci and ascospore characters. However, Ca. laburni has longer ascospores (> 27 μm) and a thicker peridium (> 100 μm), while Ca. premilcurensis has comparatively shorter ascospores (< 27 μm) and thinner peridium (> 90 μm). Camarosporidiella premilcurensis (Subclade A4, Fig. 1) is also phylogenetically distant from Ca. laburni and the latter appears to be more closely related to Ca. laburnicola and Ca. clematidis (Subclade A3, Fig. 1).
Camarosporidiella robiniicola (Wijayaw. et al.) Wijayaw., Wanas. & K.D. Hyde, comb. nov. MycoBank MB821959; Facesoffungi number: FoF 03547.
Basionym: Camarosporium robiniicola Wijayaw. et al., Phytotaxa 183: 21. 2014.
Synonym: Camarosporium aureum Norphanphoun et al., Fungal Diversity 72: 153. 2015.
Illustrations: See Wijayawardane et al. (2014a) and Liu et al. (2015).
Additional material examined: Russia, Rostov Region, Krasnosulinsky District, Donskoye forestry, artificial forest, 47,8621251° N, 40,2313757° E, on dead twigs of Gleditsia triacanthos (Fabaceae), 21 May 2013, T.S. Bulgakov T-042, MFLU 17-0468, living culture MFLUCC 14-0906 = CBS 143130; Rostov Region, Rostov-on-Don city, Botanical garden of Southern Federal University, Higher Park, 47, 2352837° N, 39, 6490788° E, on dead twigs of Gleditsia triacanthos, 14 May 2013, T.S. Bulgakov T-010, MFLU 17-0456, living culture MFLUCC 14-0892 = CBS 143128; Rostov region, Shakhty city, Atyukhta river valley, railroad artificial forest, 47,7113209° N, 40,1831603° E, on Robinia neomexicana (Fabaceae), 14 Mar. 2014, T.S. Bulgakov T-012, MFLU 17-0458, living culture MFLUCC 14-0894 = CBS 143129; Rostov-on-Don city, Botanical garden of Southern Federal University, Higher Park, 47,2389405° N, 39,6484137° E, on Robinia pseudoacacia (Fabaceae), 8 May 2014, T.S. Bulgakov T-053, MFLU 17-0471, living culture MFLUCC 14-0909 = CBS 143131; Shakhty city, 20th anniversary of Red Army microdistrict, Solyonaya Balka, 47,7104113° N, 340,2627254° E, on Robinia pseudoacacia, 21 May 2015, T.S. Bulgakov T-403, MFLU 15-2104, living culture MFLUCC 17-0688; ibid., on Robinia sp., 21 May 2016, T.S. Bulgakov T-1303, MFLU 16-1597, living culture MFLUCC 17-0688 = CBS 143132; ibid., on Robinia sp., 5 Jun. 2016, T.S. Bulgakov DL004, MFLU 16-2300, living culture MFLUCC 17-0733.
Notes: All strains of Camarosporidiella robiniicola (including the strain of Ca. aureum, MFLUCC 14-0620) cluster together with significant statistical support of 97 % for ML, 88 % for MP and 1.00 for PP (Clade A2, Fig. 1). Morphological comparison reveals identical morphs and our phylogeny strongly supports an association of Ca. aureum with other strains of Ca. robiniicola. Therefore, it would be taxonomically correct to treat them as conspecific. We herein synonymise Camarosporium aureum under Ca. robiniicola. See Liu et al. (2015) for more details on Camarosporium aureum.
Camarosporidiella schulzeri Wanas., Bulgakov & K.D. Hyde, sp. nov. MycoBank MB821960; Facesoffungi number: FoF 03548. Fig. 19.
Fig. 19.
Camarosporidiella schulzeri (MFLU 17-0460, holotype). A. Conidiomata on host surface. B. Vertical section through conidioma. C. Close-up of ostiole. D. Microconidia. E. Conidiogenous cells. F–I. Conidia. Scale bars: B = 100 μm; C = 50 μm; D, E = 20 μm; F–I = 10 μm.
Etymology: Named after Stephan V.M. Schulzer, who introduced the genus Camarosporium.
Necrotrophic on dying branches of Elaeagnus angustifolia. Asexual morph: Conidiomata pycnidial, 370–420 μm high, 380–460 μm diam ( = 366.4 × 420.7 μm, n = 10), solitary or gregarious, black, immersed or partly erumpent, unilocular, ostiolate. Ostiole 50–80 μm long, 50–70 μm diam ( = 67.2 × 60.4 μm, n = 6), central, long, smooth, sometimes ostiolar canal filled with hyaline or pale brown cells. Pycnidial wall multi-layered, 15–25 μm wide at the base, 25–35 μm wide in sides, thick, comprising 4–5 layers, with heavily pigmented outer layer, thick-walled, comprising blackish to dark reddish-brown cells of textura angularis, with lighter cells towards the inside, with inner layer composed of 1–2 layers, hyaline, thin-walled cells of textura angularis. Conidiophores reduced to conidiogenous cells. Macroconidiogenous cells enteroblastic, annellidic, doliiform, integrated, solitary, hyaline, smooth-walled, and formed from the inner layer of pycnidium wall. Macroconidia 15–21 × 8–12 μm ( = 18.9 × 10.1 μm; n = 40), oblong, straight to slightly curved, rounded at both ends, 2–3-transversely septate, with one longitudinal septum, muriform, smooth-walled, brown to dark brown. Microconidiogenous cells intermingled with macroconidiogenous cells, hyaline, integrated, enteroblastic, annellidic, ampulliform to subcylindrical. Microconidia 4.5–6.5 × 4.5–5.5 μm ( = 5.9 × 4.9 μm; n = 25), hyaline, round to oblong or ellipsoidal, with a few small guttules. Sexual morph: Undetermined.
Colonies on PDA: Slow growing, reaching 2 cm diam after 4 wk at 16 °C, later with dense mycelium, circular, rough margin, white, reverse cream-grey, flat on the surface, without aerial mycelium. Hyphae septate, branched, hyaline, thin-walled.
Materials examined: Russia, Rostov Region, Rostov-on-Don city, Botanical garden of Southern Federal University, Higher Park, underwood, 47,2360559° N, 39,6555591° E, on Elaeagnus angustifolia (Fabaceae), 26 Mar. 2014, T.S. Bulgakov T-014 (MFLU 17-0460, holotype, ex-type culture, MFLUCC 14-0897 = CBS 143133); Oktyabrsky district, south of Persianovsky settlement, Balka Khoruli (Khoruli gully), 47,5036926° N, 40,1241732° E, on Gleditsia triacanthos, 28 Apr. 2015, T.S. Bulgakov T-205, MFLU 15-1909; ibid., on Robinia sp., 14 Mar. 2016, T.S. Bulgakov T-1305, MFLU 16-1599, living culture MFLUCC 17-0722; ibid., on Robinia sp., 24 Mar. 2016, T.S. Bulgakov T-1370, MFLU 16-1664, living culture MFLUCC 17-0722.
Notes: The holotype of Camarosporidiella schulzeri was collected from Elaeagnus angustifolia, and Camarosporium caraganae (conidia 14–22 × 9–12 μm), Ca. elaeagnicola (21–23 × 8–10 μm), Camarosporidiella elaeagnicola (18–25 × 9–13 μm) and Ca. arezzoensis (22–30 × 8–10 μm) have also been reported from Elaeagnus (Farr & Rossman 2017, this study). Camarosporidiella elaeagnicola and Ca. arezzoensis are also positioned in different subclades (Subclades A6 and A8 respectively, Fig. 1) and this provides additional support to justify their species status. The relationship between Camarosporium caraganae and Ca. elaeagnicola with Camarosporidiella schulzeri cannot be investigated due to lack of molecular data for Camarosporium caraganae and Ca. elaeagnicola.
Camarosporidiella spartii (Trail) Wijayaw., Wanas. & K.D. Hyde, comb. nov. MycoBank MB821961; Facesoffungi number: FoF 03549.
Basionym: Camarosporium spartii Trail, Scott. Natural., N.S. 3 (“9”): 222. 1888.
Illustrations: See Wijayawardane et al. (2014a).
Note: This species is located basal in Subclade A4 (Fig. 1).
Coniothyriaceae W.B. Cooke, Revista de Biol. 12: 289. 1983 (“1980–1983”).
Type genus: Coniothyrium Corda.
Staurosphaeria Rabenh., Bot. Ztg. 16(40): 303. 1858. MycoBank MB5186; Facesoffungi number: FoF 03550.
Synonym: Hazslinszkyomyces Crous & R.K. Schumach, IMA Fungus 8: 143. 2017.
Necrotrophic or saprobic on dead branches. Asexual morph: Conidiomata solitary, globose, dark brown, immersed, erumpent, globose, with central ostiole; ostiolar canal filled with hyaline cells; conidiomatal wall of 6–8 layers of dark brown textura angularis. Conidiophores reduced to conidiogenous cells lining the inner cavity. Macroconidiogenous cells hyaline, smooth, doliiform, proliferating percurrently at apex. Macroconidia solitary, ellipsoid, smooth, red-brown, with central transverse septum, becoming muriformly septate. Microconidial cells intermingled with macroconidial cells, hyaline, integrated, proliferating percurrently at apex, subcylindrical. Microconidia hyaline, globose to ellipsoid, smooth, aseptate. Sexual morph: cucurbitaria-like. Ascomata black, superficial to semi-immersed, gregarious, confluent, sometimes scattered beneath the host periderm or on decorticated wood, fully or partly erumpent, globose, black, ostiolate. Ostiole central, short. Peridium composed of blackish to dark brown cells of textura angularis, cells towards the inside lighter, composed of thin-walled cells of textura angularis. Hamathecium comprising numerous, branched septate, pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical, short-pedicellate. Ascospores overlapping uniseriate, muriform, mostly ellipsoidal, 4–6-transversely septate, with 1–2 vertical septa, constricted at middle septum, initially hyaline, becoming brown at maturity, slightly paler, conical and narrow at the ends.
Type species: Staurosphaeria lycii Rabenh.
Staurosphaeria lycii Rabenh., Bot. Ztg. 16(40): 303. 1858. Facesoffungi number: FoF 03551. Fig. 20, Fig. 21.
Fig. 20.
Staurosphaeria lycii (HAL, lectotype). A. Conidiomata on host surface. B, C. Conidiogenous cells. D, E. Macroconidia. F. Microconidia. Scale bars: A = 500 μm, E–F = 10 μm.
Fig. 21.
Staurosphaeria lycii (MFLU 15-1993, epitype). A–C. Conidiomata on host surface. D. Vertical section through conidioma. E, F. Microconidiogenous cells and microconidia. G, H. Macroconidiogenous cells. I–N. Macro conidia. Scale bars: A = 1 mm, B = 500 μm; C, D = 100 μm; E–N = 5 μm.
Necrotrophic on dead branches of Lycium barbarum. Asexual morph: Conidiomata pycnidial, 500–600 μm high, 500–650 μm diam ( = 570.4 × 567.5 μm, n = 10), solitary, black, immersed or partly erumpent, unilocular, ostiolate. Ostiole 150–200 μm long, 200–250 μm diam ( = 170.2 × 235.4 μm, n = 6), central, long, smooth, ostiolar canal filled with hyaline cells. Pycnidial wall multi-layered, 20–25 μm wide at the base, 25–30 μm wide in sides, thick, comprising 4–5 layers, with heavily pigmented outer layer, thick-walled, comprising blackish to dark reddish-brown cells of textura angularis, with lighter cells towards the inside, with inner layer composed of 1–2 layers, hyaline, thin-walled cells of textura angularis. Conidiophores reduced to conidiogenous cells. Macroconidiogenous cells enteroblastic, annellidic, doliiform, integrated, solitary, hyaline, smooth-walled, and formed from the inner layer of pycnidium wall. Macroconidia 11–16 × 9–11 μm ( = 13.1 × 10.1 μm; n = 40), globose to oblong, rounded at both ends, 1–2-transversely septate, with one longitudinal septum, muriform, smooth-walled, brown to dark brown. Microconidiogenous cells intermingled with macroconidiogenous cells, hyaline, integrated, enteroblastic, annellidic, ampulliform to subcylindrical. Microconidia 3.5–6.5 × 3.5–4.5 μm ( = 5.1 × 4 μm; n = 25), hyaline, round to oblong or ellipsoidal, with a few small guttules. Sexual morph: Undetermined.
Colonies on PDA: Slow growing, reaching 2 cm diam after 4 wk at 16 °C, later with dense mycelium, circular, rough margin, white, reverse creamy grey, flat on the surface, without aerial mycelium. Hyphae septate, branched, hyaline, thin-walled.
Material examined: Germany, Dresden, on dry branches of Lycium barbarum (lectotype designated here, Rabenh., Klotzschii Herb. Viv. Mycol., Ed. nov., Ser. Prima, Cent. VIII, No. 736, in HAL). Russia, Rostov Region, Shakhty city, coal heap of former coal mine “Proletarian dictature”, 47,7104113° N, 40,2627254° E, on dying and dead twigs of Lycium barbarum (Solanaceae), 21 May 2015, T.S. Bulgakov T-289 (MFLU 15-1993, epitype designated here, MBT377706, ex-type culture, MFLUCC 17-0210 = CBS 143140); ibid., T-418 (MFLU 15-2122, living culture MFLUCC 17-0211 = CBS 143141).
Notes: The type species has very characteristic red-brown conidia, developing a transverse septum, and later vertical septa, dividing the conidium into four compartments. It is distinct from Camarosporium s. str. in that conidia in the latter are unevenly pigmented (pale brown at ends), and multi-septate, lacking a microconidial morph as observed in conidiomata of Staurosphaeria. It was in the past assumed that Staurosphaeria and Karstenula (Didymosphaeriaceae) are congeneric. However, the type species, K. rhodostoma, has been linked to the asexual morph Microdiplodia frangulae (Constantinescu 1993), so the generic synonymy with Staurosphaeria seems rather unlikely.
Other accepted species
Staurosphaeria aloes (Crous & M.J. Wingf.) Crous, Wanas. & K.D. Hyde, comb. nov. MycoBank MB821962; Facesoffungi number: FoF 03552.
Basionym: Camarosporium aloes Crous & M.J. Wingf., Persoonia 31: 247. 2013.
Synonym: Hazslinszkyomyces aloes (Crous & M.J. Wingf.) Crous, IMA Fungus 8: 143. 2017.
Illustrations and material examined: See Crous et al. (2013).
Staurosphaeria aptrootii (Crous & M.J. Wingf.) Crous, Wanas. & K.D. Hyde, comb. nov. MycoBank MB821963; Facesoffungi number: FoF 03553.
Basionym: Hazslinszkyomyces aptrootii Crous, IMA Fungus 8: 143. 2017.
Illustrations and material examined: See Crous & Groenewald (2017).
Staurosphaeria lyciicola (Crous & R.K. Schumach.) Crous, Wanas. & K.D. Hyde, nom. nov. MycoBank MB821964; Facesoffungi number: FoF 03554.
Basionym: Hazslinszkyomyces lycii Crous & R.K. Schumach., IMA Fungus 8: 144. 2017.
Illustrations and material examined: See Crous & Groenewald (2017).
Staurosphaeria rhamnicola Wanas., Gafforov & K.D. Hyde, sp. nov. MycoBank MB821965; Facesoffungi number: FoF 03555. Fig. 22.
Fig. 22.
Staurosphaeria rhamnicola (TASM 6102, holotype). A, B. Appearance of ascomata on host substrate. C. Section of ascoma. D. Peridium. E. Pseudoparaphyses. F–I. Asci. J–O. Ascospores. Scale bars: C = 100 μm; D, F–I = 20 μm, E, J–O = 10 μm.
Etymology: Named after the host genus from which it was collected, Rhamnus.
Saprobic on dead branches of Rhamnus sp. Asexual morph: Undetermined. Sexual morph: Ascomata 200–400 μm high, 250–350 μm diam ( = 320.4 × 296.8 μm, n = 10), black, superficial to semi-immersed, confluent, gregarious, sometimes scattered beneath the host periderm or on decorticated wood, fully or partly erumpent, globose, uniloculate, with an ostiole. Ostiole central, short, slightly sunken, minute and inconspicuous at the surface, smooth, ostiolar canal filled with hyaline cells. Peridium 25–40 μm wide at the base, 30–50 μm wide in sides, thick, comprising 8–12 layers, outermost layer heavily pigmented, thin-walled, comprising blackish to dark brown amorphous layer, middle layer heavily pigmented, thick-walled, comprising blackish to dark brown loosely packed cells of textura angularis, inner layer composed of 3–4 layers, reddish brown to hyaline, cells towards the inside lighter, flattened, thick-walled cells of textura angularis. Hamathecium comprising numerous, 2.5–3 μm (n = 40) wide, filamentous, branched septate, pseudoparaphyses. Asci 170–200 × 16–22 μm ( = 186.2 × 18.4 μm, n = 40), 8-spored, bitunicate, fissitunicate, cylindrical, short-pedicellate, apex rounded with a minute ocular chamber. Ascospores 26–32 × 12–14 μm ( = 30.4 × 13.1 μm, n = 50), overlapping uniseriate, muriform, mostly ellipsoidal, 5–6-transversely septate, with 1–2 longitudinal septa, deeply constricted at the middle septum, slightly constricted at remaining septa, initially hyaline, becoming pale brown at maturity, asymmetrical, slightly paler ends, conical and narrowly rounded at the ends, not surrounded by a mucilaginous sheath.
Colonies on PDA: Slow growing, reaching 2 cm diam after 4 wk at 16 °C, later with dense mycelium, circular, rough margin, creamy after 6 wk, reverse iron, flat on the surface, with aerial mycelium. Hyphae septate, branched, hyaline, thin-walled.
Material examined: Uzbekistan, Surxondaryo Province, Sherobod District, Oqtosh village on dead twigs of Rhamnus sp. (Rhamnaceae), 12 May 2016, Y. Gafforov, YG-S4-4D (TASM 6102, holotype); ibid., (MFLU 17-0183, isotype, ex-isotype culture MFLUCC 17-0814) ibid., YG-S4-5 (TASM 6101 = MFLU 17-0182, paratype, ex-paratype culture, MFLUCC 17-0813).
Notes: Staurosphaeria rhamnicola morphologically resembles Camarosporidiella arezzoensis, Ca. eufemiana, Ca. italica, Ca. laburni, Ca. mirabellensis, Ca. premilcurensis and Neocucurbitaria acerina in having similar ascomata, peridium, asci and ascospore characters. However, they are phylogenetically distant from Staurosphaeria rhamnicola (Clade B, Fig. 1).
Neocamarosporiaceae Wanas., Wijayaw., Crous & K.D. Hyde, fam. nov. MycoBank MB821966; Facesoffungi number: FoF 03556.
Etymology: Referring to the name of the type genus.
Saprobic on leaves and wood. Asexual morph: Conidiomata immersed, becoming erumpent, globose, brown to black, ostiolate. Ostiole papillate, central. Conidiomata wall composed of thin-walled, brown cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells proliferating several times percurrently near apex, ampulliform to doliiform, separate, hyaline, smooth-walled. Conidia solitary, initially hyaline, aseptate, developing initially a central septum and then becoming muriform, variable from globose to obovoid to ellipsoid, golden brown, finely roughened, thick-walled. Sexual morph: Ascomata superficial to semi-immersed, confluent, gregarious, fully or partly erumpent, globose, with an apapillate ostiole. Ostiole central, short, erect or slightly sunken, smooth, ostiolar canal filled with hyaline cells. Peridium thin, comprising blackish to brown loosely packed cells of textura angularis. Hamathecium comprising numerous, filamentous, branched septate, pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical-clavate to cylindrical, pedicellate, rounded at apex, with a minute ocular chamber. Ascospores uniseriately overlapping, muriform, mostly ellipsoidal, 5–7-transversely septate, with 1–2 longitudinal septa, deeply constricted at middle septum, slightly constricted at remaining septa, initially hyaline, becoming pale brown at maturity, rounded at both ends, surrounded by a mucilaginous sheath.
Type genus: Neocamarosporium Crous & M.J. Wingf.
Notes: Ariyawansa et al. (2015c) and Wijayawardene et al. (2016) treated Neocamarosporium as a genus in Pleosporaceae, but in our analyses four Neocamarosporium species, three new camarosporium-like taxa and a pleospora-like taxon also group with Coniothyrium obiones, Dimorphosporicola tragani, N. chersinae, N. chichastianum, N. goegapense, Pleospora chenopodii, P. halimiones, P. calvescens, P. betae and Phoma betae (which represent phoma-like and ascochyta-like asexual morphs fide de Gruyter et al. 2012) and reside in a distinct clade (Clade D, Fig. 1) in Pleosporineae with high bootstrap support (95 % and 79 % in ML and MP analyses respectively) and high PP value (1.00). This clade (Clade D) is distinct from Pleosporaceae sensu stricto which comprises Pleospora sensu stricto (= Stemphylium, see Woudenberg et al. 2017), the type genus. Therefore, Neocamarosporiaceae fam. nov. is introduced for Clade D based on morphology and multi-gene phylogeny.
The family Neocamarosporiaceae is somewhat similar to the Pleosporaceae, but differs in several respects. The characteristics of the ascomatal wall are distinctly different from each other. Pleosporaceae species have a thick peridium with several hyaline and pigmented cell layers, while Neocamarosporiaceae species have a thin peridium with only 2–3 pigmented cell layers and lack hyaline cell layers.
Ariyawansa et al. (2015c) mentioned that the asexual morphs of Pleosporaceae can be coelomycetous or hyphomycetous. Apart from Neocamarosporium (ascochyta-like, camarosporium-like and phoma-like) no other Pleosporaceae species produce a coelomycetous asexual morph. Therefore, it would appear that hyphomycetous asexual morphs are specific to Pleosporaceae. Therefore, by considering the sexual morph and asexual morph differences together with molecular support obtained herein with Neocamarosporiaceae in clade D, we believe that it would taxonomically be more appropriate to establish a new family to accommodate these species in Pleosporineae.
It is interesting to note that the species which were collected from marine to saline habitats, and produce muriform conidia, i.e. Neocamarosporium chersinae, N. chichastianum and N. salicorniicola cluster together as a subclade in clade D (Fig. 1), but could not be segregated from Dimorphosporicola tragani and Coniothyrium obiones based on our phylogenetic analyses. However, DNA sequence data of Coniothyrium obiones (CBS 453.68) analysed herein is an unverified sequence as it is not from the type material. Given that the relationship between the Coniothyrium obiones and other taxa is undetermined, we keep CBS 453.68 as “Coniothyrium” obiones for now. Dimorphosporicola tragani is different to other taxa in Neocamarosporium in conidial morphology and habitat. Consequently, it would appropriate to retain Dimorphosporicola as a separate genus in Neocamarosporiaceae. Furthermore, some of the Neocamarosporium spp. (e.g. N. chichastianum) have only ITS sequence data and it would be wise to consider or evaluate the utility of several other genes such as large subunit nrDNA (28S, LSU), small subunit nrDNA (18S, SSU), or translation elongation factor alpha 1 (tef1-α) to further elucidate phylogenetic relationships among this clade of fungi with more fresh collections.
Neocamarosporium Crous & M.J. Wingf., Persoonia 32: 273. 2014. emend.
Saprobic on leaves and wood. Asexual morph: See Crous et al. (2014b). Sexual morph: Ascomata superficial to semi-immersed, confluent, gregarious, fully or partly erumpent, globose, with an apapillate ostiole. Ostiole central, short, erect or slightly sunken, smooth, ostiolar canal filled with hyaline cells. Peridium thin, comprising blackish to brown loosely packed cells of textura angularis. Hamathecium comprising numerous, filamentous, branched septate, pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical-clavate to cylindrical, pedicellate, apex rounded with a minute ocular chamber. Ascospores overlapping uniseriate, muriform, mostly ellipsoidal, 5–7-transversely septate, with 1–2-longitudinal septa, deeply constricted at the middle septum, slightly constricted at remaining septa, initially hyaline, becoming pale brown at maturity, rounded at both ends, surrounded by a mucilaginous sheath.
Type species: Neocamarosporium goegapense Crous & M.J. Wingf.
Notes: The genus Neocamarosporium was introduced by Crous et al. (2014b) based on Neocamarosporium goegapense from South Africa, which is morphologically similar to the genus Camarosporium with its pycnidial conidiomata, hyaline, percurrently proliferating conidiogenous cells, and brown, muriform conidia (Crous et al. 2014b). Currently there are 25 strains that cluster in Neocamarosporium, representing 11 species (in this study). Also, a further three strains which was introduced by Grum-Grzhimaylo et al. (2016) and six strains which were collected from marine to saline habitats in Iran, group here as Neocamarosporium sp. In this study, we introduce Neocamarosporium korfii, N. lamiacearum (first sexual record), N. salicorniicola and N. salsolae as new species in Neocamarosporium.
Accepted species in Neocamarosporium
Neocamarosporium betae (Berl.) Ariyawansa & K.D. Hyde, Fungal Diversity 71: 119. 2015.
Basionym: Pyrenophora echinella var. betae Berl.: 208. 1888.
Synonyms: Phoma betae A.B. Frank, Z. Rübenzucker-Ind.: 905. 1892.
Pleospora betae Björl., Botaniska Notiser 1944: 218. 1944.
Neocamarosporium calvescens (Fr. ex Desm.) Ariyaw. & K.D. Hyde, Fungal Diversity 71: 120. 2015.
Basionym: Sphaeria calvescens Fr., Sclerom. Suec.: no. 401. 1822.
Synonyms: Pleospora calvescens (Fr. ex Desm.) Tul. & C. Tul., Selecta Fungorum Carpologia, Tomus Secundus. Xylariei - Valsei - Sphaeriei 2: 266. 1863.
Neocamarosporium chenopodii (Ellis & Kellerm.) Wanas. & K.D. Hyde, comb. nov. MycoBank MB821967; Facesoffungi number: FoF 03557.
Basionym: Phloeospora chenopodii Ellis & Kellerm., Journal of Mycology 4 (2–3): 26. 1888.
Synonyms: Pleospora chenopodii (Ellis & Kellerm.) Gruyter & Redhead, Index Fungorum 205: 1. 2014.
Neocamarosporium chersinae Crous, IMA Fungus 8: 146. 2017.
Illustrations and material examined: See Crous & Groenewald (2017).
Neocamarosporium goegapense Crous & M.J. Wingf., Persoonia 32: 273. 2014.
Illustrations and material examined: See Crous et al. (2014b).
Neocamarosporium obiones (Jaap) Wanas. & K.D. Hyde, comb. nov. MycoBank MB821968; Facesoffungi number: FoF 03558.
Basionym: Diplodina obiones Jaap, Verh. bot. Ver. Prov. Brandenb. 47: 96. 1905.
Synonyms: Ascochyta obiones (Jaap) P.K. Buchanan, Mycol. Pap. 156: 28. 1987.
Pleospora halimiones Gruyter & Verkley, Stud. Mycol. 75: 25. 2012.
Neocamarosporium korfii Wanas., E.B.G. Jones & K.D. Hyde, sp. nov. MycoBank MB821969; Facesoffungi number: FoF 03559. Fig. 23.
Fig. 23.
Neocamarosporium korfii (MFLU 17-1436, holotype). A. Appearance of conidiomata. B. Vertical section of conidioma. C, D. Conidiogenous cells. E–J. Conidia. Scale bars: B = 50 μm; C, D = 10 μm; E–J = 5 μm.
Etymology: In honour of Prof. Richard Paul “Dick” Korf (May 28, 1925 – August 20, 2016) for his immense contribution to mycology.
Saprobic on dead stems. Asexual morph: Conidiomata pycnidial, 130–200 μm high, 160–220 μm diam ( = 161.7 × 178.9 μm, n = 10), solitary or gregarious, black, superficial, unilocular. Ostiole inconspicuous. Pycnidial wall 12–20 μm wide, comprising 3–5 layers, outer layer heavily pigmented, thick-walled, comprising dark brown cells of textura angularis, cells towards the inside lighter, inner layer comprising 2–3 layers, hyaline, thin-walled cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells enteroblastic, annellidic, doliiform, integrated, solitary, hyaline, smooth-walled, and originated from the inner layer of pycnidium wall. Conidia 12–18 × 8–10 μm ( = 14.3 × 9.4 μm; n = 30), oblong, straight to slightly curved, rounded at both ends, 1–3-transversely septate, with 1–2 longitudinal septa, muriform, smooth-walled, dark brown, guttulated. Sexual morph: Undetermined.
Colonies on PDA: Slow growing, reaching 3 cm diam after 3 wk at 16 °C, powdery, circular, smooth margin, greenish brown, reverse dark brown.
Material examined: Russia, Republic of Crimea, Feodosia city Municipality, Karadag State Nature Reserve, 44,9145837°N, 35, 2025127°E, on dead branches of Bassia prostrata (Amaranthaceae), 23 Jun. 2016, T.S. Bulgakov CR006 (MFLU 17-1436 holotype, ex-type culture MFLUCC 17-0745 = CBS 143135).
Notes: Based on the multi-gene phylogenetic analyses (Fig. 1), our strain of Neocamarosporium korfii segregates from N. lamiacearum, but this subclade is not supported in the phylogenetic analyses (Clade D, Fig. 1). Neocamarosporium korfii is morphologically similar to N. salsolae and N. salicorniicola in conidiomatal characteristics and conidial shape. However, these species are phylogenetically distinct (Clade D, Fig. 1). Thus, in this paper we introduce N. korfii as a new species in Neocamarosporium.
Neocamarosporium lamiacearum Dayar., E.B.G. Jones & K.D. Hyde, sp. nov. MycoBank MB821970; Facesoffungi number: FoF 03560. Fig. 24.
Fig. 24.
Neocamarosporium lamiacearum (MFLU 15-2989, holotype). A, B. Appearance of ascomata on host substrate. C. Section of ascoma. D. Peridium. E. Pseudoparaphyses. F–H. Asci. I–L. Ascospores. Scale bars: A, B = 200 μm; C = 100 μm; D, E = 10 μm; F–H = 20 μm; I–L = 10 μm.
Etymology: Named after the host family from which it was collected, Lamiaceae.
Saprobic on dead stems of Lamiaceae sp. Asexual morph: Undetermined. Sexual morph: Ascomata 200–250 μm high, 180–250 μm diam ( = 232.1 × 217.4 μm, n = 10), black, superficial to semi-immersed, confluent, gregarious, cupulate when dry, globose, uniloculate, with an apapillate ostiole. Ostiole, 40–60 μm long, 40–60 μm diam, central, short, slightly sunken, minute and inconspicuous at the surface, smooth, ostiolar canal filled with hyaline cells. Peridium 10–15 μm wide at the base, 10–20 μm wide in sides, thin, comprising 2–3 layers, reddish brown to brown, cells towards the inside lighter, flattened, thin-walled cells of textura angularis. Hamathecium comprising numerous, 2.5–3 μm (n = 40) wide, filamentous, branched, septate, pseudoparaphyses. Asci 100–120 × 14–17 μm ( = 105.5 × 15 μm, n = 40), 8-spored, bitunicate, fissitunicate, cylindrical-clavate to cylindrical, pedicellate (10–20 μm long), apex rounded with a minute ocular chamber. Ascospores 14–20 × 8–11 μm ( = 15.9 × 9.4 μm, n = 50), overlapping uniseriate, muriform, mostly ellipsoidal, with 3–4-transversely septate, with one longitudinal septum, deeply constricted at the middle septum, slightly constricted at remaining septa, initially hyaline, becoming pale brown at maturity, upper part wider than lower part, slightly paler, rounded at both ends, conical at lower end, surrounded by a mucilaginous sheath.
Colonies on PDA: Slow growing, reaching 2 cm diam after 4 wk at 16 °C, later with dense mycelium, circular, rough margin, white, reverse cream-grey, flat on the surface, without aerial mycelium. Hyphae septate, branched, hyaline, thin-walled.
Materials examined: Russia, Rostov Region, Krasnosulinsky district, Donskoye forestry, 47,8547249°N, 40,2318907°E, steppe in gully, on dead stems of Lamiaceae plant (perhaps, Marrubium peregrinum or Phlomis herba-venti ssp. pungens), 28 Jun. 2015, T.S. Bulgakov T-846 (MFLU 15–2989, holotype, ex-type culture MFLUCC 16-0560 = CBS 143136); Russia, Republic of Crimea, Feodosia city Municipality, salt-march near Baraqol salty lake, 44, 9963682°N, 35, 2431965°E, on dead branches of Bassia sedoides (Amaranthaceae), 23 Jun. 2016, T.S. Bulgakov CR026 (MFLU 17-1437, paratype, ex-paratype culture, MFLUCC 17-0750 = CBS 143137).
Notes: Neocamarosporium lamiacearum is morphologically somewhat similar to taxa in Pleosporaceae in having cylindrical-clavate to cylindrical asci and muriform ascospores. However, the characteristics of the ascomatal wall in Neocamarosporium lamiacearum are noticeably different from Pleosporaceae taxa. Pleosporaceae species have a thick peridium with several hyaline and pigmented cell layers, while Neocamarosporiaceae lamiacearum has a thin peridium with only 2–3 pigmented cell layers and lack hyaline cell layers.
Neocamarosporium salicorniicola Dayarathne, E.B.G. Jones & K.D. Hyde, sp. nov. MycoBank MB821971; Facesoffungi number: FoF 03561. Fig. 25.
Fig. 25.
Neocamarosporium salicorniicola (MFLU 15-0957, holotype). A. Appearance of conidiomata on Salicornia sp. B. Vertical section of conidioma. C. Developing stages of conidia on conidiogenous cells. D–K. Conidia. Scale bars: B = 50 μm; C = 20 μm; D–K = 5 μm.
Etymology: Named after the host genus from which it was collected, Salicornia.
Saprobic on dead stems of Salicornia sp. Asexual morph: Conidiomata pycnidial, 75–110 μm high, 80–96 μm diam ( = 94.4 × 88 μm, n = 10), solitary or gregarious, black, superficial, unilocular. Ostiole inconspicuous. Pycnidial wall 7–11 μm wide, comprising 3–4 layers, outer layer heavily pigmented, thick-walled, comprising dark brown cells of textura angularis, cells towards the inside lighter, inner layer comprising 2–3 layers, hyaline, thin-walled cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells enteroblastic, annellidic, doliiform, integrated, solitary, hyaline, smooth-walled, and originated from the inner layer of pycnidium wall. Conidia 8–12 × 4–6 μm ( = 10.5 × 4.8 μm; n = 30), oblong, straight to slightly curved, rounded at both ends, 1–3-transversely septate, with one longitudinal septum, muriform, smooth-walled, dark brown, guttulated. Sexual morph: Undetermined.
Colonies on PDA: Slow growing, reaching 3 cm diam after 3 wk at 16 °C, powdery, circular, smooth margin, ash, reverse yellowish at margins and black at the middle.
Material examined: Thailand, Phetchaburi Province, Cha-Am, Chao Samran, on dead stem of Salicornia sp. (Amaranthaceae), 28 Jul. 2015, M. Dayarathne CHAM025 (MFLU 15-0957, holotype, ex-type culture MFLUCC 15-0957).
Notes: Based on the multi-gene phylogenetic analyses (Fig. 1), our strain of N. salicorniicola shares a sister relationship to N. jorjanensis and to Dimorphosporicola tragani but with no support (Clade D, Fig. 1). In this paper, we introduce Neocamarosporium salicorniicola as a new species in Neocamarosporium, based on its comparatively smaller and distinct dark, guttulate conidia, and its phylogenetic position.
Neocamarosporium salsolae Wanas., Gafforov & K.D. Hyde, sp. nov. MycoBank MB821972; Facesoffungi number: FoF 03562. Fig. 26.
Fig. 26.
Neocamarosporium salsolae (TASM 6100, holotype). A. Appearance of conidiomata on Salsola sp. B. Vertical section of conidioma. C–E. Conidiogenous cells and developing conidia. F–J. Conidia. Scale bars: B = 50 μm; C = 20 μm; D–J = 10 μm.
Etymology: Named after the host genus from which it was collected, Salsola.
Saprobic on dead stems of Salsola sp. Asexual morph: Conidiomata pycnidial, 120–150 μm high, 80–110 μm diam ( = 137.5 × 99.5 μm, n = 10), solitary or gregarious, black, superficial, unilocular. Ostiole inconspicuous. Pycnidial wall 6–10 μm wide, comprising 3–4 layers, outer layer heavily pigmented, thick-walled, comprising dark brown cells of textura angularis, cells towards the inside lighter, inner layer comprising 2–3 layers, hyaline, thin-walled cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells enteroblastic, annellidic, doliiform, integrated, solitary, hyaline, smooth-walled, and originated from the inner layer of pycnidium wall. Conidia 18–25 × 12–20 μm ( = 21.9 × 16.9 μm; n = 30), oblong, straight to slightly curved, rounded at both ends, 1–3-transversely septate, with 1–3 longitudinal septa, muriform, smooth-walled, dark brown. Sexual morph: Undetermined.
Colonies on PDA: Slow growing, reaching 3 cm diam after 3 wk at 16 °C, circular, smooth margin, dark green, reverse blackish green.
Materials examined: Uzbekistan, Surxondaryo Province, Sherobod District, Oqtosh village on dead stem of Salsola sp. (Amaranthaceae), 12 May 2016, Y. Gafforov YG-S6-(TASM 6100, holotype); ibid., (MFLU 17-0192, isotype, ex-isotype culture MFLUCC 17-0827) ibid., YG-S6-1 (TASM 6099 = MFLU 17-0191, paratype, ex-paratype culture, MFLUCC 17-0826).
Notes: Based on the multi-gene phylogenetic analyses (Fig. 1), our strains of Neocamarosporium salsolae cluster in a subclade sister to N. goegapense with significant statistical support (Clade D, Fig. 1).
Another accepted genus
Dimorphosporicola Crous, Fungal Biology 120: 1412.
Type species: Dimorphosporicola tragani Crous.
Dimorphosporicola tragani Crous, Fungal Biology 120: 1413.
Illustrations and material examined: See Crous & Groenewald (2016).
Notes: Dimorphosporicola was described by Crous & Groenewald (2017) to accommodate D. tragani, which is morphologically similar to Coleophoma species. But D. tragani is distinct from Coleophoma by having percurrently proliferate conidiogenous cells, and having dimorphic conidia. Dimorphosporicola and Neocamarosporium are different to each other in macro-conidial morphology.
Discussion
Our phylogenetic results indicate that camarosporium-like species which were treated as Camarosporium in previous papers (viz. Camarosporium arezzoensis, Cm. aborescentis, Cm. caraganicola, Cm. elaeagnellum, Cm. elongata, Cm. clematidis, Cm. spartii, Cm. robiniicola and Cm. aureum fide Wijayawardene et al., 2014a, Wijayawardene et al., 2014c, Liu et al., 2015, Tibpromma et al. 2016, Thambugala et al. 2016) are well positioned within the Pleosporineae and phylogenetically distinct from other genera. These species, which has been our focal group, constitute a strongly supported monophyletic lineage with 75 newly collected strains (Clade A). In contrast, Cm. quaternatum (CPC 23216, CPC 31081 & CPC 31518), the type species of Camarosporium, and other camarosporium-like taxa group in separate clades (Clade A, B, C and D, Fig. 1).
To discuss tree output (Fig. 1), we divided the taxa in the phylogram into 4 clades (A–D), and ingroup taxa in Clade A into 12 subclades (A1–A12). Generally, Camarosporidiella clustered separately from the rest with high support (Clade A, Fig. 1). All species in Clade A are distinct from other camarosporium-like taxa in Pleosporineae. Clade A comprises new strains and several other species were transferred to the new genus, Camarosporidiella (Fig. 1). With regards to conidial morphology, Camarosporidiella resembles Camarosporium sensu stricto and other camarosporium-like genera (Crous et al., 2014b, Crous et al., 2015a, Crous et al., 2015b, Wijayawardene et al., 2014c, Wijayawardene et al., 2015, Wijayawardene et al., 2016) but is phylogenetically distinct. Camarosporidiella arezzoensis, Ca. aborescentis, Ca. caraganicola, Ca. celtidis, Ca. eufemiana, Ca. elongata, Ca. italica, Ca. laburni, Ca. mirabellensis and Ca. premilcurensis represent the sexual morph of the genus. Thus, the holomorph of the genus comprises a camarosporium-like asexual morph and cucurbitaria-like sexual morph. With the addition of new specimens and species, updated morphological characterisation, DNA sequence data analyses with strong support, and the monophyletic status of Camarosporidiella species, a new family, Camarosporidiellaceae is established herein to circumscribe Camarosporidiella.
Clade B comprises two new collections which are morphologically similar to Staurosphaeria lycii and three other species i.e. Hazslinszkyomyces lycii, H. aptrootii and H. aloes in Coniothyriaceae with significant statistical support. By giving precedence to the oldest name, the genus Staurosphaeria is resurrected to accommodate this group. Clade C (Fig. 1), which comprises the type species of Camarosporium, is retained as Camarosporium sensu stricto and Camarosporomyces flavigenus. As in Wijayawardene et al. (2014b) and Crous & Groenewald (2017), Camarosporium sensu stricto groups with Coniothyriaceae, which comprises Coniothyrium sensu stricto and phoma-like species (de Gruyter et al. 2012). Recent studies reported that some camarosporium-like genera (such as Pseudocamarosporium and Paracamarosporium) have broader generic concepts (Crous et al., 2015a, Crous et al., 2015b). Wijayawardene et al. (2014c) introduced Pseudocamarosporium and Paracamarosporium to accommodate camarosporium-like taxa that reside in Didymosphaeriaceae. Crous et al. (2015b) showed that several coniothyrium-like species group with Pseudocamarosporium and Paracamarosporium in their phylogenetic study. Hence, both Pseudocamarosporium and Paracamarosporium exhibit camarosporium-like or coniothyrium-like conidial morphologies. Further phylogenetic investigations with broader taxon sampling of Camarosporium sensu stricto and Coniothyrium sensu stricto are warranted to better understand both the Camarosporium and Coniothyrium generic concepts.
Neocamarosporium goegapense and 10 other Neocamarosporium species cluster in a strongly supported clade (Clade D, Fig. 1). Based on morphology of the sexual and asexual morphs and multi-gene phylogeny, Neocamarosporiaceae was introduced. Previous studies and our results in this study demonstrate that naming of camarosporium-like taxa based only on morphology is inaccurate and hence there is a need to carry out DNA sequence-based studies. Wijayawardene et al. (2016) discussed the importance of re-collecting, and epitypifying of camarosporium-like taxa as currently it has more than 500 epithets in Index Fungorum (2017), and very few of these taxa are presently known from culture.
Acknowledgements
We thank the technical staff of Center of Excellence in Fungal Research, Sornram Sukpisit, Wilawan Punyaboon and Thatsanee Luangharn for their invaluable assistance. We are also grateful to Milan C. Samarakoon, Danushka Tennakoon, Indunil C. Senanayake, Asha J. Dissanayake, Qing Tian, Chuan-Gen Lin for their valuable assistance with the culture work, DNA isolation, amplification and sequencing. Dhanushka Wanasinghe is thankful to Hiran Ariyawansa for his valuable suggestions. Chayanard Phukhamsakda would like to thank Royal Golden Jubilee Ph. D. Program under Thailand Research Fund, for the award of a scholarship no. PHD/0020/2557 to study towards a PhD. Alan JL Phillips acknowledges the support from Biosystems and Integrative Sciences Institute (BioISI, FCT/UID/ Multi/04046/2013). R. Jeewon is grateful to University of Mauritius & Mae Fah Luang University for enabling research collaboration. K.D. Hyde thanks to National Research Council of Thailand (Mae Fah Luang University) for grants “Biodiversity, phylogeny and role of fungal endophytes of Pandanaceae” (Grant No: 592010200112) and Thailand Research Fund (TRF) grant no RSA5980068 entitled “Biodiversity, phylogeny and role of fungal endophytes on above parts of Rhizophora apiculata and Nypa fruticans”. National Research Council of Thailand (Mae Fah Luang University) grant no 60201000201 entitled “Diseases of mangrove trees and maintenance of good forestry practice”. Samantha C. Karunarathna thanks to Yunnan Provincial Department of Human Resources and Social Security funded postdoctoral project (number 179122). Kevin D. Hyde also thanks to the Chinese Academy of Sciences, project number 2013T2S0030, for the award of Visiting Professorship for Senior International Scientists at Kunming Institute of Botany. Y.S. Gafforov acknowledges the support from Committee for coordination science and technology development under the Cabinet of Ministers of Uzbekistan (Project No. P3-2014-0830174425).
Footnotes
Peer review under responsibility of Westerdijk Fungal Biodiversity Institute.
References
- Ariyawansa H.A., Hyde K.D., Jayasiri S.C. Fungal diversity notes 111–252 – taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity. 2015;75:27–274. [Google Scholar]
- Ariyawansa H.A., Phukhamsakda C., Thambugala K.M. Revision and phylogeny of Leptosphaeriaceae. Fungal Diversity. 2015;74:19–51. [Google Scholar]
- Ariyawansa H.A., Thambugala K.M., Manamgoda D.S. Towards a natural classification and backbone tree for Pleosporaceae. Fungal Diversity. 2015;71:85–139. [Google Scholar]
- Assimakopoulou Α., Elena K. Is there an influence of inorganic nutrition on the susceptibility of the pistachio to Camarosporium pistaciae? Options Mediterranéennes, Série A. 2010;94:181–185. [Google Scholar]
- Aveskamp M.M., de Gruyter J., Woudenberg J.H.C. Highlights of the Didymellaceae: a polyphasic approach to characterise Phoma and related pleosporalean genera. Studies in Mycology. 2010;65:1–60. doi: 10.3114/sim.2010.65.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveskamp M.M., Verkley G.J.M., de Gruyter J. DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia. 2009;101:363–382. doi: 10.3852/08-199. [DOI] [PubMed] [Google Scholar]
- Barber P.A., Crous P.W., Groenewald J.Z. Reassessing Vermisporium (Amphisphaeriaceae), a genus of foliar pathogens of Eucalypts. Persoonia. 2011;27:90–118. doi: 10.3767/003158511X617381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butin H. Morphological adaptation and spore pleomorphism in the form-complex Dichomera-Camarosporium and Fusicoccum-Dothiorella. Sydowia. 1993;45:161–166. [Google Scholar]
- Chen Q., Jiang J.R., Zhang G.Z. Resolving the Phoma enigma. Studies in Mycology. 2015;82:137–217. doi: 10.1016/j.simyco.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomnunti P., Hongsanan S., Hudson B.A. The sooty moulds. Fungal Diversity. 2014;66:1–36. [Google Scholar]
- Constantinescu O. Teleomorph-anamorph connections in ascomycetes: Microdiplodia anamorph of Karstenula rhodostoma. Mycological Research. 1993;97:377–380. [Google Scholar]
- Crous P.W., Braun U., Schubert K. Delimiting Cladosporium from morphologically similar genera. Studies in Mycology. 2007;58:33–56. doi: 10.3114/sim.2007.58.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Groenewald J.Z. They seldom occur alone. Fungal Biology. 2016;120:1392–1415. doi: 10.1016/j.funbio.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Crous P.W., Groenewald J.Z. The Genera of Fungi – G 4: Camarosporium and Dothiora. IMA Fungus. 2017;8:131–152. doi: 10.5598/imafungus.2017.08.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Hawksworth D.L., Wingfield M.J. Identifying and naming plant-pathogenic fungi: past, present, and future. Annual Review of Phytopathology. 2015;53:246–267. doi: 10.1146/annurev-phyto-080614-120245. [DOI] [PubMed] [Google Scholar]
- Crous P.W., Shivas R.G., Quaedvlieg W. Fungal Planet description sheets: 214–280. Persoonia. 2014;32:184–306. doi: 10.3767/003158514X682395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Slippers B., Wingfield M.J. Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology. 2006;55:235–253. doi: 10.3114/sim.55.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Summerell B.A., Shivas R.G. Fungal Planet description sheets 92–106. Persoonia. 2011;27:130–162. doi: 10.3767/003158511X617561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Guarro J. Fungal Planet description sheets: 154–213. Persoonia. 2013;31:188–296. doi: 10.3767/003158513X675925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Guarro J. Fungal Planet description sheets: 320–370. Persoonia. 2015;34:167–266. doi: 10.3767/003158515X688433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Schumacher R.K. Fungal Planet description sheets: 281–319. Persoonia. 2014;33:212–289. doi: 10.3767/003158514X685680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U., Cannon P.F., Woudenberg J.H.C. The Colletotrichum boninense species complex. Studies in Mycology. 2012;73:1–36. doi: 10.3114/sim0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U., O'Connell R.J., Groenewald J.Z. The Colletotrichum destructivum species complex – hemibiotrophic pathogens of forage and field crops. Studies in Mycology. 2014;79:49–84. doi: 10.1016/j.simyco.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gruyter J., Aveskamp M.M., Woudenberg J.H. Molecular phylogeny of Phoma and allied anamorph genera: towards a reclassification of the Phoma complex. Mycological Research. 2009;113:508–519. doi: 10.1016/j.mycres.2009.01.002. [DOI] [PubMed] [Google Scholar]
- De Gruyter J., Woudenberg J.H.C., Aveskamp M.M. Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia. 2010;102:1066–1081. doi: 10.3852/09-240. [DOI] [PubMed] [Google Scholar]
- De Gruyter J., Woudenberg J.H.C., Aveskamp M.M. Redisposition of phoma-like anamorphs in Pleosporales. Studies in Mycology. 2012;75:1–36. doi: 10.3114/sim0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Notaris Erbario Crittogamico Italiano. Fasc. 1862;16 no. 875. [Google Scholar]
- Doilom M., Liu J.K., Jaklitsch W.M. An outline of the family Cucurbitariaceae. Sydowia. 2013;65:167–192. [Google Scholar]
- Farr D.F., Rossman A.Y. 2017. Fungal databases, systematic mycology and microbiology laboratory, ARCS, USDA.http://nt.ars-grin.gov/fungaldatabases/ [Google Scholar]
- Green F.M. Observations on Cucurbitaria laburni. Transactions British Mycological Society. 1931;16:289–303. [Google Scholar]
- Grum-Grzhimaylo A.A., Georgieva M.L., Bondarenko S.A. On the diversity of fungi from soda soils. Fungal Diversity. 2016;76:27–74. [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Hazslinszky F.A. Beitrag zur Kentniss der Sphärien des Lyciums. Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft in Wien. 1865;15:447–452. [Google Scholar]
- Huelsenbeck J.P., Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Hyde K.D., Jones E.B.G., Liu J.K. Families of Dothideomycetes. Fungal Diversity. 2013;63:1–313. [Google Scholar]
- Ivanová H., Bernadovičová S. Species diversity of microscopic fungi on Austrian pines growing in urban greenery of Nitra town. Folia Oecologica. 2010;37:168–181. [Google Scholar]
- Jaklitsch W.M., Voglmayr H. Hidden diversity in Thyridaria and a new circumscription of the Thyridariaceae. Studies in Mycology. 2016;85:35–64. doi: 10.1016/j.simyco.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeewon R., Liew E.C.Y., Hyde K.D. Phylogenetic relationships of Pestalotiopsis and allied genera inferred from ribosomal DNA sequences and morphological characters. Molecular Phylogenetics and Evolution. 2002;25:378–392. doi: 10.1016/s1055-7903(02)00422-0. [DOI] [PubMed] [Google Scholar]
- Jeewon R., Liew E.C.Y., Hyde K.D. Molecular systematics of the Amphisphaeriaceae based on cladistic analyses of partial LSU rDNA gene sequences. Mycological Research. 2003;107:1392–1402. doi: 10.1017/s095375620300875x. [DOI] [PubMed] [Google Scholar]
- Jeewon R., Liew E.C.Y., Hyde K.D. Phylogenetic evaluation of species nomenclature of Pestalotiopsis in relation to host association. Fungal Diversity. 2004;17:39–55. [Google Scholar]
- Jeewon R., Liew E.C.Y., Simpson J.A. Phylogenetic significance of morphological characters in the taxonomy of Pestalotiopsis species. Molecular Phylogenetics and Evolution. 2003;27:372–383. doi: 10.1016/s1055-7903(03)00010-1. [DOI] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology & Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk P.M., Cannon P.F., Minter D.W. 10th ed. CABI; Wallingford: 2008. Ainsworth & Bisby's dictionary of the Fungi. [Google Scholar]
- Kishino H., Hasegawa M. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in hominoidea. Journal of Molecular Evolution. 1989;29:170–179. doi: 10.1007/BF02100115. [DOI] [PubMed] [Google Scholar]
- Liu J.K., Hyde K.D., Jones E.B.G. Fungal diversity notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Diversity. 2015;72:1–197. [Google Scholar]
- Liu J.K., Phookamsak R., Doilom M. Towards a natural classification of Botryosphaeriales. Fungal Diversity. 2012;57:149–210. [Google Scholar]
- Maharachchikumbura S.S.N., Guo L.D., Cai L. A multi-locus backbone tree for Pestalotiopsis, with a polyphasic characterization of 14 new species. Fungal Diversity. 2012;56:95–129. [Google Scholar]
- Maharachchikumbura S.S.N., Guo L.D., Chukeatirote E. Improving the backbone tree for the genus Pestalotiopsis; addition of P. steyaertii and P. magna sp. nov. Mycological Progress. 2014;3:617–624. [Google Scholar]
- Maharachchikumbura S.S.N., Hyde K.D., Groenewald J.Z. Pestalotiopsis revisited. Studies in Mycology. 2014;79:121–186. doi: 10.1016/j.simyco.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.A., Pfeiffer W., Schwartz T. Creating the CIPRES science gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE) 2010;1:1–8. [Google Scholar]
- Mirza F. Taxonomic investigations on the ascomycetous genus Cucurbitaria S.F. Gray. Nova Hedwigia. 1968;16:161–213. [Google Scholar]
- Morakotkarn D., Kawasaki H., Tanaka K. Taxonomic characterization of shiraia-like fungi isolated from bamboos in Japan. Mycoscience. 2008;49:258–265. [Google Scholar]
- Munk A. Danish Pyrenomycetes. A Preliminary Flora. Dansk botanisk Arkiv. 1957;17:1–491. [Google Scholar]
- Nag Raj T.R. Mycologue Publications; Vancouver, Canada: 1993. Coelomycetous anamorphs with appendage-bearing Conidia. [Google Scholar]
- Nylander J.A.A. Evolutionary Biology Centre, Uppsala University; 2004. MrModeltest 2.0. Program distributed by the author. [Google Scholar]
- Phillips A.J.L., Alves A., Pennycook S.R. Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia. 2008;21:29–55. doi: 10.3767/003158508X340742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phookamsak R., Liu J.K., McKenzie E.H.C. Revision of Phaeosphaeriaceae. Fungal Diversity. 2014;68:159–238. [Google Scholar]
- Promputtha I., Lumyong S., Vijaykrishna D. A phylogenetic evaluation of whether endophytes become saprotrophs at host senescence. Microbial Ecology. 2007;53:579–590. doi: 10.1007/s00248-006-9117-x. [DOI] [PubMed] [Google Scholar]
- Rambaut A. 2012. FigTree v. 1.4.0.http://tree.bio.ed.ac.uk/software/figtree/ Available at: [Google Scholar]
- Rambaut A., Drummond A.J. 2007. Tracer v. 1.4.http://beast.bio.ed.ac.uk/Tracer Available from: [Google Scholar]
- Rannala B., Yang Z. Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. Journal of Molecular Evolution. 1996;43:304–311. doi: 10.1007/BF02338839. [DOI] [PubMed] [Google Scholar]
- Rayner R.W. Commonwealth Mycological Institute; UK: 1970. A mycological colour chart. [Google Scholar]
- Rehner S.A., Buckley E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia. 2005;97:84–98. doi: 10.3852/mycologia.97.1.84. [DOI] [PubMed] [Google Scholar]
- Rehner S.A., Samuels G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research. 1994;98:625–634. [Google Scholar]
- Saccardo P.A. Sylloge Pyrenomycetum, Vol. II. Sylloge Fungorum. 1883;2:1–813. [Google Scholar]
- Saccardo P.A. Sylloge Fungorum. 1906;18:1–839. [Google Scholar]
- Schoch C.L., Crous P.W., Groenewald J.Z. A class–wide phylogenetic assessment of Dothideomycetes. Studies in Mycology. 2009;64:1–15. doi: 10.3114/sim.2009.64.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch C.L., Shoemaker R.A., Seifert K.A. A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia. 2006;98:1041–1052. doi: 10.3852/mycologia.98.6.1041. [DOI] [PubMed] [Google Scholar]
- Schulzer S. Mykologische Beiträge. Verhandlungen der Zoologisch-Botanischen Gesellschaft Wien. 1870;20:635–658. [Google Scholar]
- Shear C.L. Mycological notes and new species. Bulletin of the Torrey Botanical Club. 1902;29:449–457. [Google Scholar]
- Shenoy B.D., Jeewon R., Hyde K.D. Impact of DNA sequence-data on the taxonomy of anamorphic fungi. Fungal Diversity. 2007;26:1–54. [Google Scholar]
- Sivanesan A. J. Cramer; Vaduz: 1984. The bitunicate ascomycetes and their anamorphs. [Google Scholar]
- Smith I.M., Dunez J., Phillips D.H. Blackwell Scientific Publications; Oxford, UK: 1988. European handbook of plant diseases. 583 pp. [Google Scholar]
- Spatafora J.W., Sung G.H., Johnson D. A five-gene phylogeny of Pezizomycotina. Mycologia. 2006;98:1018–1028. doi: 10.3852/mycologia.98.6.1018. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A., Hoover P., Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Sutton B.C. Commonwealth Mycological Institute; Kew: 1980. The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, Massachusetts: 2003. PAUP* 4.0b10: phylogenetic analysis using parsimony (*and other methods) [Google Scholar]
- Tanaka K., Hirayama K., Yonezawa H. Revision of the Massarineae (Pleosporales, Dothideomycetes) Studies in Mycology. 2015;82:75–136. doi: 10.1016/j.simyco.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.E., Crous P.W., Swart L. Foliicolous and caulicolous fungi associated with Proteaceae cultivated in California. Mycotaxon. 2001;78:75–103. [Google Scholar]
- Telle S., Thines M. Amplification of cox2 (620 bp) from 2 mg of up to 129 years old herbarium specimens, comparing 19 extraction methods and 15 polymerases. PLoS One. 2008;3:3584. doi: 10.1371/journal.pone.0003584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thambugala K.M., Bulgakov T.S., Eungwanichayapant P.D. Camarosporium uniseriatum nom. nov., from Celtis occidentalis in European Russia. Studies in Fungi. 2016;1:90–98. [Google Scholar]
- Thompson J.D., Gibson T.J., Plewniak F. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;25:4876. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q., Hyde K.D., Liu J.K. Phylogenetic relationships and morphological reappraisal of Melanommataceae (Pleosporales) Fungal Diversity. 2015;74:267–324. [Google Scholar]
- Tibpromma S., Hyde K.D., Jeewon R. Fungal diversity notes 491–603: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity. 2017;83:1–261. [Google Scholar]
- Tibpromma S., Wijayawardene N.N., Manamgoda D.S. Camarosporium arezzoensis on Cytisus sp., an addition to sexual state of Camarosporium sensu stricto. Saudi Journal of Biological Sciences. 2016;23:1–8. doi: 10.1016/j.sjbs.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trakunyingcharoen T., Lombard L., Groenewald J.Z. Mycoparasitic species of Sphaerellopsis, and allied lichenicolous and other genera. IMA Fungus. 2014;5:391–414. doi: 10.5598/imafungus.2014.05.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkley G.J.M., Dukik K., Renfurm R. Novel genera and species of coniothyrium-like fungi in Montagnulaceae (Ascomycota) Persoonia. 2014;32:25–51. doi: 10.3767/003158514X679191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkley G.J.M., Starink-Willemse M., van Iperen A. Phylogenetic analyses of Septoria species based on the ITS and LSU–D2 regions of nuclear ribosomal DNA. Mycologia. 2004;96:558–571. [PubMed] [Google Scholar]
- Vilgalys R., Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanasinghe D.N., Jones E.B.G., Camporesi E. An exciting novel member of Lentitheciaceae in Italy from Clematis vitalba. Cryptogamie, Mycologie. 2014;35:323–337. [Google Scholar]
- Wanasinghe D.N., Jones E.B.G., Camporesi E. Dematiopleospora mariae gen. sp. nov., from Ononis Spinosa in Italy. Cryptogamy, Mycology. 2014;35:105–117. [Google Scholar]
- White T.J., Bruns T., Lee S. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenies. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR protocols: a guide to methods and applications. Academic Press; San Diego: 1990. pp. 315–322. [Google Scholar]
- Wijayawardene D.N.N., Mckenzie E.H.C., Chukeatirote E. Coelomycetes. Cryptogamie, Mycologie. 2012;33:215–244. [Google Scholar]
- Wijayawardene D.N.N., McKenzie E.H.C., Hyde K.D. Towards incorporating anamorphic fungi in a natural classification checklist and notes for 2011. Mycosphere. 2012;3:157–228. [Google Scholar]
- Wijayawardene N.N., Bhat D.J., Hyde K.D. Camarosporium sensu stricto in Pleosporineae, Pleosporales with two new species. Phytotaxa. 2014;183:16–26. [Google Scholar]
- Wijayawardene N.N., Crous P.W., Kirk P.M. Naming and outline of Dothideomycetes–2014 including proposals for the protection or suppression of generic names. Fungal Diversity. 2014;69:1–55. doi: 10.1007/s13225-014-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijayawardene N.N., Hyde K.D., Bhat D.J. Camarosporium-like species are polyphyletic in Pleosporales; introducing Paracamarosporium and Pseudocamarosporium gen. nov. in Montagnulaceae. Cryptogamie, Mycologie. 2014;35:177–198. [Google Scholar]
- Wijayawardene N.N., Hyde K.D., Camporesi E. Additions to brown spored coelomycetous taxa in Massarinae. Pleosporales: introducing Phragmocamarosporium gen. nov. and Suttonomyces gen. nov. Cryptogamie, Mycologie. 2015;36:213–224. [Google Scholar]
- Wijayawardene N.N., Hyde K.D., Wanasinghe D.N. Taxonomy and phylogeny of dematiaceous coelomycetes. Fungal Diversity. 2016;77(1):1–316. [Google Scholar]
- Woudenberg J.H.C., Groenewald J.Z., Binder M. Alternaria redefined. Studies in Mycology. 2013;75:171–212. doi: 10.3114/sim0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudenberg J.H.C., Hanse B., van Leeuwen G.C.M. Stemphylium revisited. Studies in Mycology. 2017;87:77–103. doi: 10.1016/j.simyco.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z.H., Wang T.H., Huang W. A simplified method for chromosome DNA preparation from filamentous fungi. Mycosystema. 2001;20:575–577. [Google Scholar]
- Zhang H., Hyde K.D., McKenzie E.H.C. Sequence data reveals phylogenetic affinities of Acrocalymma aquatica sp. nov., Aquasubmersa mircensis gen. et sp. nov. and Clohesyomyces aquaticus (freshwater coelomycetes) Cryptogamie, Mycologie. 2012;33:333–346. [Google Scholar]
- Zhaxybayeva O., Gogarten J.P. Bootstrap, Bayesian probability and maximum likelihood mapping: exploring new tools for comparative genome analyses. BMC Genomics. 2002;3:4. doi: 10.1186/1471-2164-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]